Abstract
With the aim of persistence property analysis and ecotoxicological impact of veterinary pharmaceuticals on different terrestrial species, different classes of veterinary pharmaceuticals (n = 37) with soil degradation property (DT50) were gathered and subjected to QSAR and q-RASAR model development. The models were developed from 2D descriptors under organization for economic cooperation and development guidelines with the application of multiple linear regressions along with genetic algorithm. All developed QSAR and q-RASAR were statistically significant (Internal = R2adj: 0.721–0.861, Q2LOO: 0.609–0.757, and external = Q2Fn = 0.597–0.933, MAEext = 0.174–0.260). Further, the leverage approach of applicability domain assured the model’s reliability. The veterinary pharmaceuticals with no experimental values were classified based on their persistence level. Further, the terrestrial toxicity analysis of persistent veterinary pharmaceuticals was done using toxicity prediction by computer assisted technology and in-house built quantitative structure toxicity relationship models to prioritize the toxic and persistent veterinary pharmaceuticals. This study will be helpful in estimation of persistence and toxicity of existing and upcoming veterinary pharmaceuticals.
Keywords: Veterinary pharmaceuticals, QSPR, q-RASAR, MLR, Genetic algorithm, Applicability domain
Introduction
Soil quality is one of the essential factors that ensure plant health, but in the recent era, soil pollution is becoming a greater hazard with increased anthropogenic activity and chemical use, including pharmaceuticals. Pharmaceuticals are essential for the treatment and prevention of disease in both humans and animals.1 Pharmaceuticals are physiologically active compounds that are applied in human and veterinary healthcare.2 Veterinary pharmaceuticals (VPs) are used to treat illnesses and safeguard the health of animals all over the world. These compounds are used in various ways, depending on both the animal field and industry.3 VPs are given to the animals through medicated feed, injections, or external application. The discharge of urine and faces from medicated animals and the application of contaminated manure to agricultural land are two significant routes via which VPs infiltrate into the environment easily.4,5 Further, the topical VPs are applied by spraying, by which the droplets of the VPs drift out into the surrounding environments. The higher dose of the VPs compared to human pharmaceuticals and the lack of specific areas of discharge of VPs by the animals leads to more spreadability and toxicity of VPs. They enter into the environment as a parent, conjugates, oxidized or hydrolysed products of the parent compound depending on the chemical and animal type.6 They are currently of increasing concern since residues have been discovered in agricultural soils, groundwater, and surface water.7 The fate of VPs is included in the risk assessment to forecast concentrations in the relevant environmental compartments.8 While certain active compounds may degrade during storage, a significant portion of the active compounds might still reach the soil environment through manure application.9 Several regulation and regulatory bodies of different country require the environmental safety profile of the VPs during the registration like in India Central Drugs Standard Control Organization (CDSCO), in Zambia, Zambia Medicines Regulatory Authority, in Malaysia, National Pharmaceutical Control Bureau Ministry of Health, Malaysia, in US, International Cooperation on Harmonization of Technical Requirements for Registration of Veterinary Medicinal Products, and Europe, REACH and etc. (https://cdsco.gov.in/opencms/opencms/en/Home/;https://www.vichsec.org/en/about/what-is-vich.html;https://faolex.fao.org/docs/pdf/zam196366.pdf; https://www.npra.gov.my/images/Guidelines_Central/Guidelines_on_Veterinary/REGOVP_JULY2014_191015.pdf.). These overall prompted the authors to select the VPs in this study. The pictorial representation of the entry of VPs on the environment and their harmful effects was represented in Fig. 1.
Fig. 1.
Environmental and ecosystem-wide impacts of applied and industrial VPs.
Many VPs are ionizable chemicals, with pKa values within natural soil pH ranges. As a result, depending on the ambient circumstances, they might appear in the environment as negative, neutral, zwitterionic, or positively charged species. Diverse chemical characteristics result in different sorption interactions with soil or sediment for these species. The majority of soil surfaces have a negative charge. Cationic species can therefore be electrostatically attracted, but anionic species are often rejected. Anions and zwitterions can absorb to negative soil surfaces via electrostatic attraction or more specialized surface-bridging processes if they form complexes with divalent and trivalent cations.10 Additionally, VPs with polar functional groups can bind to mineral and organic components of the soil by polar interactions, such as hydrogen bonding.11 Also, neutral species might accumulate by interacting hydrophobically with the organic material in the soil.12 This way, the discharged VPs, and their metabolites can affect the soil and other organisms. The existence of these chemicals in soil compartment can be analyzed by the analysis of soil degradation of the chemicals. The soil degradation of chemicals can be defined as the time required for the chemical concentration under defined conditions to decline in the amount at application which is denoted by DTX(DTX is the time required for the chemical concentration under defined conditions to decline to X% of the amount at application.). The soil degradation indicates the persistence property of any chemical in soil. The USEPA classified the chemicals based on their persistence level (DT50 = 0–30 days = Non-persistent; DT50 = 30–100 days = Persistent in a moderate way; DT50 = 100–365 days = Persistent; and above 365 days = extremely tenacious) (http://sitem.herts.ac.uk/aeru/ppdb/).
The OECD guideline 307 (OECD guideline for testing chemicals, aerobic and anaerobic transformation in soil) describes the procedure for testing soil degradation, selection of chemicals, soil selection, etc. (OECD 307). According to OECD guideline 307, the soil degradation test is applicable for slightly volatile, non-volatile, water-soluble, or water-insoluble compounds. The test should not be applied to highly volatile chemicals from soil (e.g. fumigants, organic solvents) and thus cannot be kept in soil under the experimental conditions of this test.
Once the highly persistent chemicals are released into the environment, they travel as such from one compartment to another and exhibit a variety of detrimental effects on various organisms including plants found in the soil compartment. Many veterinary drugs include data on their toxicity to a variety of species. This is because data on the toxicity of these compounds to fish, daphnids, algae, bacteria, earthworms, plants, and occasionally dung invertebrates is often required throughout the risk assessment process.4 The chemical toxicity to the plants and microbes present in soil (especially terrestrials) is directly correlated with the persistence level of the chemicals as many of the chemicals can stay as such in the soil for a long period; for example, DDT can stay in the soil for years and shows many harmful effects.13–15 Antibiotics in the soil, even at very low concentrations [below the minimum inhibitory concentration (MIC)], cause genetic changes in bacterial genomes and the transmission of antibiotic resistance genes (ARGs) and associated mobile genetic material (MGEs), such as plasmids, transposons, and genomic islands, between and among microbial species.16 Hence, the ecotoxicological evaluation of chemicals is gaining much attention in recent days along with their persistence property analysis.
Of various plants in environment onion and lettuce protagonist significantly in human an animal’s life due to their rich source of various phytonutrients. These phytonutrients have both applications food and the therapeutic in treating several disease conditions such as cancer, diabetes, neurological diseases, etc. Apart from their phytonutritional value, they play a significant role as bioindicator in environment. These prompted us to select these two plant species in this study for the phytotoxicity analysis of persistent VPs. Further OECD (OECD Guideline no 208) also recommended these two species for phytotoxicity study.
Further, among various ecotoxicological endpoints on terrestrial species the rodent acute oral toxicity is of interest in recent days due to their highly genetic linkage between human and rat. Acute toxicity defines the occurrence of adverse effects in a short period of time.
The persistence property estimation of chemicals in soil and toxicity estimation in different species through wet lab practice is very costly in terms of time and money. Hence, the available in-silico tools such as the quantitative structure activity relationship (QSAR) and q-RASAR approach and their application help to estimate the persistence property and toxicity of chemicals and fill the data gap. Many QSAR models reported with the persistence and degradation property of the chemicals.14,15,17–24 Mainly the reported models are based on aquatic compartment. This is the first in silico report on the persistence and toxicity of VPs. In these regards, the objectives of this research were categorized into two parts:
(1) QSAR and q-RASAR modeling of VPs for soil degradation analysis to identify/prioritize persistent VPs on soil and the application of models to untested VPs.
(2) Finally, toxicity estimation of persistent VPs by the application of available in silico models.
Materials and methods
Experimental data and structures
The starting point of the research was the collection of experimental data of VPs. Initially, 383 VPs with soil degradation quality indicated as DT50 (the time required for the chemical concentration under defined conditions to decline to 50% of the amount at application.) were gathered from Veterinary Substances Database (VSDB) (http://sitem.herts.ac.uk/aeru/vsdb/index.htm). Following the dataset collection, the data curation was carried out which involved the deletion of duplicates, salts, and metal-containing VPs. This left 306 VPs (39 with experimental value and 267 without experimental value) for further analysis. The final dataset was reported in supporting information (Table S1). Among the whole collected compounds (n = 306), 142, 54 compounds are used as human pharmaceuticals and as agrochemicals respectively along with VPs (Table S1). Finally, the structures of the final dataset were downloaded from the ChemSpider database (http://www.chemspider.com/), and each structure was checked for its correctness. Finally, the ChemDraw tool was used for cleaning the structures (leading to optimization of abnormal bond distances/angles, etc.).
Descriptors calculation
In the present work, a total of 1,444 1D and 2D (topological, physicochemical, and structure indices) descriptors were calculated using PaDEL descriptor software (V2.21).25 Finally, pretreatment of the descriptors was done by using the algorithm available in QSARINS software to remove the constant (>80%), zero, non-informative, and high inter-correlated (>85%) descriptors. Finally, a total of 502 descriptors were used for further analysis.
QSPR models development and validation
In the initial stage of the model development and validation, the influential VPs were identified and omitted from the dataset. Hence, the dataset reduced from 39 VPs to 37 VPs. We have conducted a retrospective study to identify compounds that demonstrate abnormal behavior in their potential activity cliffs as reported by the Banerjee and Roy 2023.26 This analysis was based on the similarity concept, and we computed two similarity-based coefficients, sm1 and sm2, for this purpose. Compound 187 exhibited higher MaxNeg and NegAvgSim values, suggesting it’s inactive. Compound 262 exhibited characteristics that were on the borderline, and its predictive capabilities were not as strong as reported in supporting information (Table S3). In fact, the models that included two compounds performed poorly in terms of statistics, as mentioned in the supporting information (Table S4). Therefore, we have excluded two compounds from the dataset. Finally, 37 VPs were used for the further analysis. Before the descriptor selection and model development, the selection of training and test sets is one of the important steps for QSPR model development.27 The whole data set was divided into training set and test set, using structure-based and response-based splitting techniques with the approximate ratio of 70:30 for training and test set compounds, respectively. The training and test set compounds were reported in supporting information (Table S1).
For the QSPR model development, Multiple Linear Regression (MLR) using the Ordinary Least Squares (OLS) with genetic algorithm for feature selection was applied as a chemometric tool. Several models were developed for both splitting using training set compounds. Initially, the top 50 models were selected based on the value of cross-validation correlation among the several models for each splitting and further, the developed models were applied to test set compounds to judge the predictive power and for the external validation of the developed models. Finally, the best model was selected for further analysis in each splitting based on the values of different internal and external validation parameters. The model development was done in accord with OECD principles for QSAR validation. The complete analysis, from dataset splitting to model development, was done using QSARINS software under the Windows operating system.28
Different statistical parameters were used to assure the internal stability of the developed QSPR models. The coefficient of determination R2 was used as a measure of the goodness-of-fit,29 while internal robustness was verified by the cross-validation coefficient Q2LOO (leave-one-out). Additional care was taken to reduce the data co-linearity by applying of QUIK rule.30 Additionally, Y randomization Y-scrambling analysis [2,000 iteration] was done to make evident that the models were justifiable, not by chance. External validation of the models were measured by different external validation parameters such as r2mavg, Q2F1, Q2F2, and Q2F3, CCCext, (concordance correlation coefficient)31–33 and the root mean absolute error of prediction (MAEext).34 The prediction reliability of the models was also verified. Finally, assumptions of MLR models such as residuals normality, residuals independency and the models homoscedasticity analysis were analyzed by different methods.
The Durbin Watson (DW) test was performed to check the residual independency for the both models. The Durbin Watson statistics always assume a value of 0 and 4. When the value of the DW is equal to 2, there is no autocorrelation in the data. The value of DW <2 indicates the positive autocorrelation while >2, negative autocorrelation. To test for positive autocorrelation at significance level α (alpha), the test statistic DW is compared to lower and upper critical values:
If DW < Lower critical value: There is statistical evidence that the data is positively autocorrelated.
If DW > Upper critical value: There is no statistical evidence that the data is positively correlated.
If DW is in between the lower and upper critical values: The test is inconclusive.
To test for negative autocorrelation at significance level α (alpha), the test statistic 4-DW is compared to lower and upper critical values:
If 4-DW < Lower critical value: There is statistical evidence that the data is negatively autocorrelated.
If 4-DW > Upper critical value: There is no statistical evidence that the data is negatively correlated.
If 4-DW is in between the lower and upper critical values: The test is inconclusive.
Applicability domain and prediction reliability indicator analysis
The applicability domain (ad) for QSAR models is the boundary of the structure and the responses depending on the chemical environment of the data used for model development. The QSAR model cannot be considered reliable, only based on statistical parameters; molecules present in the training set and their chemical location also plays an essential role in validating models. All models have limitations or operate according to their chemical variability and reaction values. It is thought that if the chemical falls within the function of the ``domain'' or ``space'' their predictions are naturally reliable, but if we look at them without AD, they are not reliable predictions. HAT value (h) measures the effect the structure of certain chemicals has on the model. Compounds are called other than AD if it has a higher value (h) than the prescribed value. The h* value can be calculated as 3p/n, where p is the number of model variables plus one, and n is the number of objects used to calculate the model. The outside AD compounds can be represented in William’s plot, which is a plot between HAT value (X axis) and standard residual (Y axis). In William’s plot if compounds fall outside of the defined h* value the compounds are called structural outlier while the compounds fall outside of the standards residual value of (± 3) they are called response outlier. Insubria Graphs were considered for the study of ad of the unknown compounds. Apart from the AD, the prediction reliability indicator was also calculated for all the models to assure the prediction quality of the models.35
Read across
Read Across (RA) is an advanced predictive technology that utilizes structural similarities to evaluate the potential toxicity or activity of chemicals. Recognized and approved by regulatory bodies like REACH and USEPA, RA offers advantages over traditional QSAR models, particularly in scenarios with limited datasets.36 The fundamental principle of RA is based on the assumption that substances with similar chemical structures exhibit comparable toxicological effects. Even in the absence of comprehensive experimental data, RA can predict potential effects by analyzing the known toxicity of a reference chemical compound. This approach is especially valuable for small datasets where traditional QSAR models may struggle. Unlike traditional dataset division methods, RA does not follow specific rules for data splitting. Instead, a similar data division approach as used in QSAR model development is employed. RA's predictive accuracy relies on optimizing hyperparameter such as sigma value, gamma value, the number of similar compounds, and threshold values for distance and similarity. Thorough optimization enhances the accuracy and reliability of RA predictions.37
For RA predictions, the Read-Across-v4.2 tool developed by DTC lab is utilized.38 This tool predicts toxicity using various similarity-based variables, and its predictions are validated through parameters such as Q2F1, Q2F2, MAE, and RMSE. Q2F1 and Q2F2 represent cross-validated squared correlation coefficients for the test sets, indicating the model's ability to predict within its training data and generalize to new data. Lower MAE signifies better prediction accuracy, and RMSE assesses overall predictive performance. In dataset preparation, RA requires two input files for training and test sets. Optimization of hyperparameters ensures accurate predictions. The RA tool processes both datasets with optimized parameters, using Euclidean, Gaussian, and Laplacian values.39 The selection of the prediction method is based on external validation criteria, ensuring good agreement with validation metrics. Introducing a novel prediction technique, q-RASAR combines RA and QSAR, resulting in more robust models compared to QSAR alone. q-RASAR models offer easy interpretation, identify quantitative contributions of chemical properties, and present an intriguing strategy for designing expert systems in pharmaceuticals and organic chemical toxicity and ecotoxicity predictions.40,41
Results and discussion
QSPR model development to estimate persistence property of VPs
In the first step of QSPR model development, the whole dataset was divided into training and test set compounds using two splitting techniques, as reported in materials and methods section. A bunch of GA-MLR models has been developed by using training set compounds for each splitting. Among the several models, the best model was selected initially, based on internal validation parameters and variable reflection for each splitting and further externally validated the selected models on respective test set compounds. All the selected models were sufficiently strong both internally and externally. Models 1 and 2 explain 72.9% and 72.1% of the variance (adjusted coefficient of variation (R2adj)), respectively (Table1). The leave-one-out predicted variance was 62.5% for model1 and 60.9% for the model2 (Table 1). When models 1 & 2 were applied to test set compounds, the predictive R2 value for the test set was found to be statistically significant (Q2Fn = 0.597–0.933) (Table 1). Further, a minimal value of rout mean square error (RMSE) (0.04–0.192) for both internal and external also indicated the less prediction errors and goodness of each developed QSPR model. The scattered plots of the developed models were represented in Fig. 2, which shows the experimental (X axis) vs. estimated (Y axis) activity value. The same combination of variables appeared in both the selected models with only a difference in the regression coefficient. The variables were written in the descending order of their contribution reflected based on standardized coefficient value (Equations (1) and (2)).
Table 1.
Statistical parameters of the developed models indicating robustness of models.
| Equation Number | Splitting | R2 | R2adj | Q2Loo | RMSETr | MAETr | Q2Fn | R2mavg | CCCExt | MAEExt | RMSEext | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Q2F1 | Q2F2 | Q2F3 | |||||||||||
| (1) | Response-based | 0.761 | 0.728 | 0.624 | 0.412 | 0.343 | 0.748 | 0.604 | 0.871 | 0.582 | 0.844 | 0.260 | 0.303 |
| (2) | Structure-based | 0.754 | 0.721 | 0.609 | 0.423 | 0.365 | 0.791 | 0.760 | 0.926 | 0.696 | 0.872 | 0.174 | 0.231 |
| (3) | Structure-based (q-RASAR) | 0.860 | 0.835 | 0.757 | 0.318 | 0.255 | 0.768 | 0.733 | 0.918 | 0.487 | 0.811 | 0.194 | 0.244 |
Fig. 2.
Scattered plots illustrating the linearity of experimental vs. predicted activity with R2 of 0.761 and 0.754 for model1 (A) and model2 (B).
Variable GATS8c was the most important among all three variables, with the highest coefficient value. GATS8c is one of the autocorrelation descriptors in which the Geary algorithm calculates autocorrelation. The lag 8 weighted by charge indicated the same charged atom or group in 8 topological distances. The second important variable for both models was MATS8p, the Moran autocorrelation descriptor of lag 8 and weighted by polarizability. Variable GATS4S was the least influential variable. It is also one of the Geary autocorrelation variables of lag 4 weighted by I-state. A positive contribution was observed for all the variables. Values of important variables, the variables' meaning, and the variables' correlation matrix were reported in supporting information (Table S1).
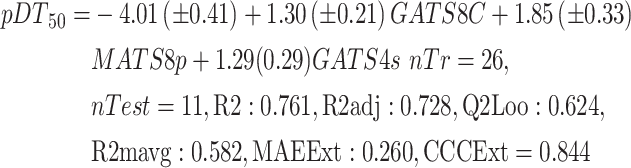 |
(1) |
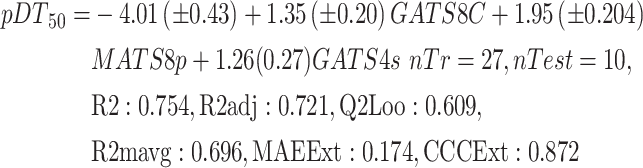 |
(2) |
Additionally, residuals normality, residuals independency and the models homoscedasticity analysis was done to fulfill the assumptions of MLR models. The quantile–quantile (Q-Q graph) graph demonstrates that the residuals meet normality assumptions of linear regression. As is shown Fig. 3 most of the data points fall on the diagonal line of the Q-Q plots. The scattered plots of residual vs predicted endpoints shows that the points are randomly equally scattered below and above the zero and they do not present patterns or heteroscedastic trends (Fig. 4). Finally, to check the residual independency, we have performed the DW test for the both models. The DW value of our test demonstrate that the residuals are independent (Table 2).
Fig. 3.
Q-Q graph displaying the fall of residuals data points on the diagonal line satisfying the normality hypothesis of MLR models (A = Model1, B = Model2).
Fig. 4.
The residual vs. predicted endpoints scattered graphs highlights equal scattering of points within the positive and negative zone (A = Model1, B = Model2).
Table 2.
Durbin Watson analysis.
| Models | N/K | DW | DL | DU |
|---|---|---|---|---|
| Model1 | 26/3 | 1.67681 | 1.143 | 1.652 |
| Model2 | 27/3 | 1.89697 | 1.162 | 1.651 |
Note: N = Number of training set compounds, K = Number of independent variables
Applicability domain and prediction reliability analysis of developed models
However, the statistical value of different validation parameters suggests the model’s robustness; further, we calculated the applicability domain to assure the reliability of models to veterinary pharmaceuticals. The compounds are reliable when they come inside the AD, as they are interpolated into the training set data. Compounds beyond the AD are less dependable since they are extrapolated. By using the leverage approach, the applicability domain was estimated for each model. The AD analysis of the models suggested only one compound (Compound 81) was structural outside the AD, as it crosses the cutoff HAT value (h* = 0.46 and 0.44 for model 1 and 2 respectively) (Fig. 5). Further, along with AD, prediction reliability indicator also assured the prediction reliability of test set compounds. This overall analysis indicates the good quality and performance of the developed models.
Fig. 5.
William's plots illustrate a chemical which exceeds the HAT limit (0.46 and 0.44) for model1 (A) and 2 (B).
Development of q-RASAR model
The structural and physicochemical descriptors of the training and test sets used for the QSAR and subsequent q-RASAR analysis have been provided in a spreadsheet of Supplementary Materials SI-1. The training set consists of 27 compounds which were used for the q-RASAR model development while the test set consists of 10 compounds which were used for the validation of the q-RASAR model.
After conducting the optimization process for best QSAR models (Model 2) in the read across tool, we have successfully predicted the toxicity values for datasets. The quantitative read across tool offers three different prediction methods: Euclidean distance-based similarity function (ED), Laplacian kernel function similarity estimation (LK), and Gaussian kernel function similarity estimation (GK). After careful evaluation of the external validation metrics, we selected the prediction method that surpasses the external validation criteria, ensuring that it demonstrates superior performance in accurately predicting toxicity values across the datasets. The external validation parameter for selected prediction method from RA was reported in Table S6.
The RASAR descriptors were generated based on the chosen descriptors in the final QSAR model. The q-RASAR model for VPs is selected which was robust on the basis of their internal and external parameters. The validation parameters for all the developed models along with their variables were written in the descending order of their contribution reflected based on standardized coefficient value given in Equation (3). We now assess the efficacy of the q-RASAR model in comparison to previously established QSAR models. While the internal validation metrics, specifically R2 and Q2(LOO), showed similar values for both the QSAR and q-RASAR models (R2 = 0.754, Q2(LOO) = 0.609 for QSAR, and R2 = 0.860, Q2(LOO) = 0.757 for q-RASAR), the external validation metrics for the QSAR model, namely Q2F1, Q2 F2, and Q2 F2, are notably lower. In contrast, the q-RASAR models exhibit superior predictive performance. In addition, it was observed that the developed QSAR models consisted of three descriptors, while the q-RASAR model implemented four descriptors. The intercorrelation matrix of the variables is provided in supporting information Table S5. The scatter plots of all the q-RASAR models have been provided in supporting information Fig. S1. 99% of the compounds in q-RASAR model 3 was found inside the ad.
 |
(3) |
Prediction of untested VPs from the developed models to find their persistence level
In addition to the modeling dataset, we gathered 267 VPs with no experimental values. After confirmation of the model’s quality based on the values of different statistical parameters, predictive power, and reliability of models by the leverage approach of ad; the developed models were applied to these untested VPs to get predictions and classify them. The outside ad, VPs were not considered for the classification. Outside ad VPs were represented in Insubria plots (Fig. 6). Similar to QSAR models are used, the set of corresponding q-RASAR models were systematically utilized to evaluate a range of unidentified compounds. When q-RASAR Model 3 was applied to VPs, it was confirmed that a significant 96.62% of the compounds were found to be within the specified ad. Insubria plot for q-RASAR model is reported in supporting information Fig. S2. Based on the prediction and ad analysis the VPs were classified as non-persistent (DT50 = 0–30 days), persistent in a moderate way (DT50 = 30–100 days) persistent (DT50 = 100–365 days) and extremely tenacious (DT50 = above 365 days) according to USEPA classification (http://sitem.herts.ac.uk/aeru/ppdb/en/docs/5_2.pdf) for QSAR and q-RASAR (Table 3). The prediction reliability indicator was also calculated for untested VPs. The results revealed that all the untested VPs showed good or moderate prediction quality. The detailed analysis of prediction, prediction quality and classification of untested VPs was reported in supporting information (Table S1 and S7).
Fig. 6.
Insubria plots for model 1 (A) and 2 (B) demonstrating AD of untested compounds.
Table 3.
Classification of untested VPs based on their persistent level by applying QSAR and q-RASAR.
| Models | Outside ad VPs | Non-persistent (DT50 = 0–30 days) |
Persistent in a moderate way (DT50 = 30–100 days) |
Persistent (DT50 = 100–365 days) |
Extremely tenacious (DT50 = above 365 days |
|---|---|---|---|---|---|
| QSAR (Consensus) |
6 | 133 | 80 | 31 | 17 |
| q-RASAR | 9 | 148 | 89 | 18 | 3 |
Application of TOPKAT and in-house models for the toxicity prediction of persistent VPs on terrestrial species
After the prioritization of the persistent, persistent in a moderate way, and extremely tenacious VPs, their toxicity analysis was done. In this regard, in this study Lactuca sativa and Allium cepa models (Equations (4)–(6) for Lactuca sativa and Equations (7) and (8) for Allium cepa) were developed by our research group42,43 for phytotoxicity, and the TOPKAT model available in discovery studio client (DSC 4.5) software for rat acute oral toxicity estimation was selected. A significant number of persistent and highly tenacious compounds were included in the QSAR model covering the compounds of q-RASAR. We have chosen QSAR classified compounds to predict the toxicity of persistent VPs on terrestrial species. The summary of the analysis was reported in Table 4. The detailed analysis was given in supporting information (Table S2), and the Insubria plots were shown in Fig. S3, indicating the outside ad VPs. The high percentage of the inside ad and the prediction reliability indicator assured the reliable applicability of in house built and TOPKAT models.
Table 4.
Toxicity of persistent VPs on one animal species (rat), and two plant species (Lactuca sativa, Allium Cepa).
| Persistent Level | Total number of Compound | Toxicity | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Rat Oral | Lactuca sativa | Allium Cepa | ||||||||
| In AD | T | NT | In AD | T | NT | In AD | T | NT | ||
| Persistent | 31 | 27 | 24 | 3 | 25 | 23 | 2 | 28 | 5 | 23 |
| Persistent in a moderate way | 80 | 69 | 53 | 16 | 57 | 52 | 5 | 76 | 18 | 58 |
| Extremely tenacious | 17 | 17 | 14 | 3 | 13 | 11 | 2 | 16 | 3 | 13 |
| Total | 128 | 113 | 91 | 22 | 95 | 86 | 9 | 120 | 26 | 94 |
Note: T = Toxic, NT = Non-toxic
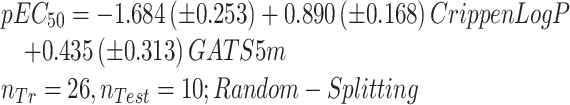 |
(4) |
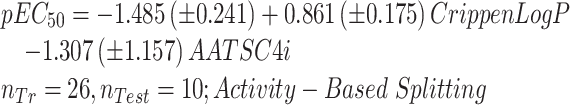 |
(5) |
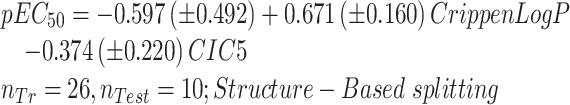 |
(6) |
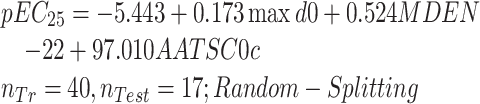 |
(7) |
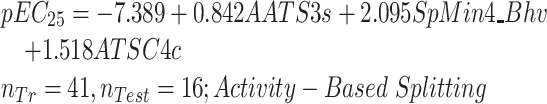 |
(8) |
Overview and conclusion
With the aim of identification of persistence property of VPs and their toxic impact mainly on terrestrial species, the QSAR and q-RASAR modeling was approached in this study. In this regard VPs with DT50 values were collected from VSDB database and subjected to QSAR and q-RASAR model development. Different statistical matrices assured the robustness, reliability, and applicability of the developed models.
After the assurance of models quality, the models were applied to the untested compounds for their persistence property prediction and classification. Further, the toxicity analysis of the persistent VPs was done by using in-house-built QSTR and TOPKAT models on three terrestrial species. The classification of untested VPs indicates a high number of compounds were under the three persistent level (persistent in a moderate way, persistent and extremely tenacious) and showed toxicity toward the Lactuca sativa, rat acute oral toxicity and for Allium Cepa to some extent. Proposed results show the significant utility in the reliable prioritization of persistent and toxic compounds in terrestrial compartment. The overall results obtained from this research work will be helpful for society and the scientific community to estimate the persistence property and toxicity of the existing and upcoming VPs.
Supplementary Material
Acknowledgments
The authors acknowledge Prof. Paola Gramatica for the free license of QSARINS. One of the authors PB (PhD Registration no.185261502) thanks to DST-SERB Govt. of India, New Delhi (File No. EMR/2017/004497) for the financial support in the form of fellowship and infrastructural support provided by host university.
Contributor Information
Purusottam Banjare, Department of Pharmacy, Guru Ghasidas Vishwavidyalaya (A Central University), Bilaspur 495009, Chhattisgarh, India; Department of Pharmaceutical Chemistry, Apollo College of Pharmacy, Anjora, Durg 491001, Chhattisgarh, India.
Rekha Singh, Department of Pharmacy, Guru Ghasidas Vishwavidyalaya (A Central University), Bilaspur 495009, Chhattisgarh, India.
Nilesh Kumar Pandey, Department of Pharmacy, Guru Ghasidas Vishwavidyalaya (A Central University), Bilaspur 495009, Chhattisgarh, India.
Balaji Wamanrao Matore, Department of Pharmacy, Guru Ghasidas Vishwavidyalaya (A Central University), Bilaspur 495009, Chhattisgarh, India.
Anjali Murmu, Department of Pharmacy, Guru Ghasidas Vishwavidyalaya (A Central University), Bilaspur 495009, Chhattisgarh, India.
Jagadish Singh, Department of Pharmacy, Guru Ghasidas Vishwavidyalaya (A Central University), Bilaspur 495009, Chhattisgarh, India.
Partha Pratim Roy, Department of Pharmacy, Guru Ghasidas Vishwavidyalaya (A Central University), Bilaspur 495009, Chhattisgarh, India.
Author contributions
Purusottam Banjare: Methodology, Validation, Investigation, Writing—original draft, reviewing, and editing. Rekha Singh: Data collection and curation, Writing—original draft, reviewing, and editing. Nilesh Kumar Pandey: Data collection and Curation, Writing—original draft, reviewing, and editing. Balaji Wamanrao Matore: Writing—original draft, reviewing, and editing. Anjali Murmu: Writing—original draft, reviewing, and editing. Jagadish Singh: Formal analysis, reviewing, and editing. Partha Pratim Roy: Conceptualization, Supervision, reviewing, and editing.
Conflict of interest statement: There is no conflict of interest.
Funding
This work was supported by the Science & Engineering Research Board (SERB) Department of Science and Technology (DST), Govt. of India, New Delhi (File No. EMR/2017/004497), received by Dr. Partha Pratim Roy.
Data availability
The data for the chemicals is already reported in the supporting material and is also available in Footprint Database (http://sitem.herts.ac.uk/aeru/vsdb/index.htm).
References
- 1. Boxall ABA, Johnson P, Smith EJ, Sinclair CJ, Stutt E, Levy LS. Uptake of veterinary medicines from soils into plants. J Agric Food Chem. 2006:54(6):2288–2297. [DOI] [PubMed] [Google Scholar]
- 2. Gworek B, Kijeńska M, Wrzosek J, Graniewska M. Pharmaceuticals in the soil and plant environment: a review. Water Air Soil Pollut. 2021:232(4):145. [Google Scholar]
- 3. Berendsen RL, Vismans G, Yu K, Song Y, Jonge R, Burgman WP, Burmølle M, Herschend J, Bakker PAHM, Pieterse CMJ. Disease-induced assemblage of a plant-beneficial bacterial consortium. ISME J. 2018:12(6):1496–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Boxall ABA, Fogg LA, Blackwell PA, Blackwell P, Kay P, Pemberton EJ, Croxford A. Veterinary medicines in the environment. Rev Environ Contam Toxicol. 2004, pp. 1–91. [DOI] [PubMed]
- 5. Boxall A, Barrett K, Crane M. Exposure assessment of veterinary medicines in terrestrial systems. In: Veterinary medicines in the environment. CRC Press; 2008, pp. 129–153 [Google Scholar]
- 6. Tolls J. Sorption of veterinary pharmaceuticals in soils: a review. Environ Sci Technol. 2001:35(17):3397–3406. [DOI] [PubMed] [Google Scholar]
- 7. Hamscher G, Sczesny S, Höper H, Nau H. Determination of persistent tetracycline residues in soil fertilized with liquid manure by high-performance liquid chromatography with electrospray ionization tandem mass spectrometry. Anal Chem. 2002:74(7):1509–1518. [DOI] [PubMed] [Google Scholar]
- 8. Winckler C, Grafe A. Use of veterinary drugs in intensive animal production. J Soils Sediments. 2001:1(2):66–70. [Google Scholar]
- 9. Ingerslev F, Toräng L, Loke M-L, Halling-Sørensen B, Nyholm N. Primary biodegradation of veterinary antibiotics in aerobic and anaerobic surface water simulation systems. Chemosphere. 2001:44(4):865–872. [DOI] [PubMed] [Google Scholar]
- 10. terLaak TL, Gebbink WA, Tolls J. The effect of pH and ionic strength on the sorption of sulfachloropyridazine, tylosin, and oxytetracycline to soil. Environ Toxicol Chem. 2006:25(4):904–911. [DOI] [PubMed] [Google Scholar]
- 11. Neal RH, Sposito G. Selenate adsorption on alluvial soils. Soil Sci Soc Am J. 1989:53(1):70–74. [Google Scholar]
- 12. Koleva YK, Cronin MTD, Madden JC, Schwöbel JAH. Modelling acute oral mammalian toxicity. 1. Definition of a quantifiable baseline effect. Toxicol in Vitro. 2011:25(7):1281–1293. [DOI] [PubMed] [Google Scholar]
- 13. Banjare P, Matore B, Singh J, Roy PP. In silico local QSAR modeling of bioconcentration factor of organophosphate pesticides. In Silico Pharmacology. 2021:9(1):28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ye T, Wei Z, Spinney R, Dionysiou DD, Luo S, Chai L, Yang Z, Xiao R. Quantitative structure–activity relationship for the apparent rate constants of aromatic contaminants oxidized by ferrate (VI). Chem Eng J. 2017:317:258–266. [Google Scholar]
- 15. Yang Z, Luo S, Wei Z, Ye T, Spinney R, Chen D, Xiao R. Rate constants of hydroxyl radical oxidation of polychlorinated biphenyls in the gas phase: a single−descriptor based QSAR and DFT study. Environ Pollut. 2016:211:157–164. [DOI] [PubMed] [Google Scholar]
- 16. Cycoń M, Mrozik A, Piotrowska-Seget Z. Antibiotics in the soil environment-degradation and their impact on microbial activity and diversity. Front Microbiol. 2019:10. 10.3389/fmicb.2019.00338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Moon J, Lee B, Ra J-S, Kim K-T. Predicting PBT and CMR properties of substances of very high concern (SVHCs) using QSAR models, and application for K-REACH. Toxicol Rep. 2020:7:995–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lombardo A, Manganaro A, Arning J, Benfenati E. Development of new QSAR models for water, sediment, and soil half-life. Sci Total Environ. 2022:838(Pt 1):156004. [DOI] [PubMed] [Google Scholar]
- 19. Maeder V, Escher BI, Scheringer M, Hungerbühler K. Toxic ratio as an indicator of the intrinsic toxicity in the assessment of persistent, bioaccumulative, and toxic chemicals. Environ Sci Technol. 2004:38(13):3659–3666. [DOI] [PubMed] [Google Scholar]
- 20. Puzyn T, Haranczyk M, Suzuki N, Sakurai T. Estimating persistence of brominated and chlorinated organic pollutants in air, water, soil, and sediments with the QSPR-based classification scheme. Mol Divers. 2011:15(1):173–188. [DOI] [PubMed] [Google Scholar]
- 21. Dimitrov S, Breton R, MacDonald D, Walker JD, Mekenyan O. Quantitative prediction of biodegradability, metabolite distribution and toxicity of stable metabolites. SAR QSAR Environ Res. 2002:13(3–4):445–455. [DOI] [PubMed] [Google Scholar]
- 22. Gramatica P, Cassani S, Sangion A. Are some “safer alternatives” hazardous as PBTs? The case study of new flame retardants. J Hazard Mater. 2016:306:237–246. [DOI] [PubMed] [Google Scholar]
- 23. Papa E, Gramatica P. QSPR as a support for the EU REACH regulation and rational design of environmentally safer chemicals: PBT identification from molecular structure. Green Chem. 2010:12(5):836. [Google Scholar]
- 24. Sangion A, Gramatica P. PBT assessment and prioritization of contaminants of emerging concern: pharmaceuticals. Environ Res. 2016:147:297–306. [DOI] [PubMed] [Google Scholar]
- 25. Yap CW. PaDEL‐descriptor: an open source software to calculate molecular descriptors and fingerprints. J Comput Chem. 2011:32(7):1466–1474. [DOI] [PubMed] [Google Scholar]
- 26. Banerjee A, Roy K. Prediction-inspired intelligent training for the development of classification read-across structure–activity relationship (c-RASAR) models for organic skin sensitizers: assessment of classification error rate from novel similarity coefficients. Chem Res Toxicol. 2023:36(9):1518–1531. [DOI] [PubMed] [Google Scholar]
- 27. Roy PP, Roy K. On some aspects of variable selection for partial least squares regression models. QSAR Comb Sci. 2008:27(3):302–313. [Google Scholar]
- 28. Gramatica P, Chirico N, Papa E, Cassani S, Kovarich S. QSARINS: a new software for the development, analysis, and validation of QSAR MLR models. J Comput Chem. 2013:34(24):2121–2132. [Google Scholar]
- 29. Snedecor CW, Cochran GW. Oxford and IBH. Delhi; 1967, pp. 381–418. [Google Scholar]
- 30. Todeschini R, Consonni V, Maiocchi A. The K correlation index: theory development and its application in chemometrics. Chemom Intell Lab Syst. 1999:46(1):13–29. [Google Scholar]
- 31. Gramatica P. Principles of QSAR modeling. Int J Quant Struct Prop Relationsh. 2020:5(3):61–97. [Google Scholar]
- 32. Chirico N, Gramatica P. Real external predictivity of QSAR models. Part 2. New intercomparable thresholds for different validation criteria and the need for scatter plot inspection. J Chem Inf Model. 2012:52(8):2044–2058. [DOI] [PubMed] [Google Scholar]
- 33. Chirico N, Gramatica P. Real external predictivity of QSAR models: how to evaluate it? Comparison of different validation criteria and proposal of using the concordance correlation coefficient. J Chem Inf Model. 2011:51(9):2320–2335. [DOI] [PubMed] [Google Scholar]
- 34. Chai T, Draxler RR. Root mean square error (RMSE) or mean absolute error (MAE)?—Arguments against avoiding RMSE in the literature. Geosci Model Dev. 2014:7(3):1247–1250. [Google Scholar]
- 35. Roy K, Ambure P, Kar S. How precise are our quantitative structure–activity relationship derived predictions for new query chemicals? ACS Omega. 2018:3(9):11392–11406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rovida C, Barton-Maclaren T, Benfenati E, Caloni F, Chandrasekera PC, Chesné C, Cronin MTD, de Knecht J, Dietrich DR, Escher SE, et al. Internationalization of read-across as a validated new approach method (NAM) for regulatory toxicology. ALTEX. 2020:37(4):579–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pandey NK, Murmu A, Banjare P, Matore BW, Singh J, Roy PP. Integrated predictive QSAR, Read Across, and q-RASAR analysis for diverse agrochemical phytotoxicity in oat and corn: a consensus-based approach for risk assessment and prioritization. Environ Sci Pollut Res. 2024:1–16. [DOI] [PubMed] [Google Scholar]
- 38. Banerjee A, Chatterjee M, De P, Roy K. Quantitative predictions from chemical read-across and their confidence measures. Chemom Intell Lab Syst. 2022:227:1–9. [Google Scholar]
- 39. Banerjee A, Roy K. Machine-learning-based similarity meets traditional QSAR: “q-RASAR” for the enhancement of the external predictivity and detection of prediction confidence outliers in an hERG toxicity dataset. Chemom Intell Lab Syst. 2023:237:1–13. [Google Scholar]
- 40. Chatterjee M, Banerjee A, De P, Gajewicz-Skretna A, Roy K. A novel quantitative read-across tool designed purposefully to fill the existing gaps in nanosafety data. Environmental Science Nano. 2022:9(1):189–203. [Google Scholar]
- 41. Banerjee A, Roy K. On some novel similarity-based functions used in the ML-based q-RASAR approach for efficient quantitative predictions of selected toxicity end points. Chem Res Toxicol. 2023:36(3):446–464. [DOI] [PubMed] [Google Scholar]
- 42. Murmu A, Banjare P, Singh J, Roy PP. First QSTR report on allium Cepa Phytotoxicity of pesticides. Int J Quant Struct Prop Relationsh. 2021:7(2):1–28. [Google Scholar]
- 43. Vishvkarma A, Banjare P, Singh J, Roy PP. In silico predictive phytotoxicity modeling of Lactuca sativa of personal care product ingredients. Int J Quant Struct Prop Relationsh. 2021:6(1):25–44. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data for the chemicals is already reported in the supporting material and is also available in Footprint Database (http://sitem.herts.ac.uk/aeru/vsdb/index.htm).








