Abstract
Aims
The adaptive immune response plays an important role in atherosclerosis. In response to a high-fat/high-cholesterol (HF/HC) diet, marginal zone B (MZB) cells activate an atheroprotective programme by regulating the differentiation and accumulation of ‘poorly differentiated’ T follicular helper (Tfh) cells. On the other hand, Tfh cells activate the germinal centre response, which promotes atherosclerosis through the production of class-switched high-affinity antibodies. We therefore investigated the direct role of Tfh cells and the role of IL18 in Tfh differentiation in atherosclerosis.
Methods and results
We generated atherosclerotic mouse models with selective genetic deletion of Tfh cells, MZB cells, or IL18 signalling in Tfh cells. Surprisingly, mice lacking Tfh cells had increased atherosclerosis. Lack of Tfh not only reduced class-switched IgG antibodies against oxidation-specific epitopes (OSEs) but also reduced atheroprotective natural IgM-type anti-phosphorylcholine (PC) antibodies, despite no alteration of natural B1 cells. Moreover, the absence of Tfh cells was associated with an accumulation of MZB cells with substantially reduced ability to secrete antibodies. In the same manner, MZB cell deficiency in Ldlr−/− mice was associated with a significant decrease in atheroprotective IgM antibodies, including natural anti-PC IgM antibodies. In humans, we found a positive correlation between circulating MZB-like cells and anti-OSE IgM antibodies. Finally, we identified an important role for IL18 signalling in HF/HC diet–induced Tfh.
Conclusion
Our findings reveal a previously unsuspected role of MZB cells in regulating atheroprotective ‘natural’ IgM antibody production in a Tfh-dependent manner, which could have important pathophysiological and therapeutic implications.
Keywords: B cells, T cells, Atherosclerosis, Antibodies, Interleukin-18
Graphical Abstract
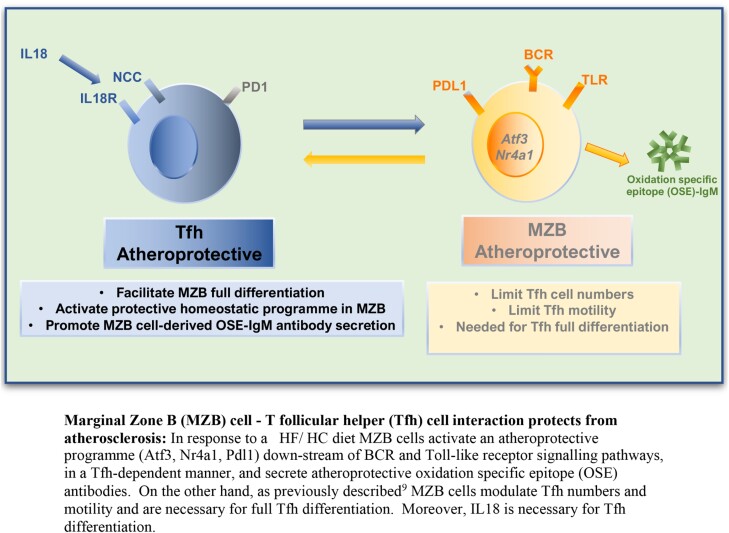
Graphical Abstract.
Time of primary review: 19 days
1. Introduction
Atherosclerosis is an arterial pathology with multiple genetic and environmental risk factors, initiated in response to trapping of low-density lipoproteins (LDLs) in the intima and their acquisition of inflammatory and immunogenic properties. The subsequent immune response involves interactions between vascular and circulating cells and mediators. Broad evidence supports the inflammatory theory of atherosclerosis, and innate and adaptive immune cells have been shown to participate in all stages of the disease.1,2
T follicular helper (Tfh) cells are specialized T cells that can be distinguished from other T helper populations for the expression of CXCR5, PD1 and ICOS surface markers, and their signature transcription factor, B cell lymphoma 6 (BCL6). They are the key orchestrators of the germinal centre (GC) reaction through their support of follicular B cell proliferation, somatic hypermutation, and class switch recombination, leading to the secretion of high-affinity antibodies and the formation of long-lived plasma and memory B cells.3 They are also involved in the extrafollicular plasma B cell response.4 Both the role of GC B cells5,6 and the role of Tfh cells7–10 remain controversial arguing for context-dependent properties of these cells in atherosclerosis. We have previously shown that Ldlr−/− mice with genetic deletion of marginal zone B (MZB) cells,9 which accumulate high numbers of ‘poorly differentiated’ Tfh cells resembling pre-Tfh,9 promote atherosclerosis. Using anti-ICOS-L antibodies to deplete Tfh in both ApoE−/− 8 and Ldlr−/− 9 mice did not impact atherosclerosis, but using the same strategy to reduce Tfh in mouse models with exacerbated Tfh/pre-Tfh cells was associated with decreased atherosclerosis. Thus, the role of the ‘normal’ Tfh response in atherosclerosis remains unclear.
Furthermore, previous RNA-seq from our ‘poorly differentiated’ Tfh vs. ‘normal’ Tfh cells identified Il18r as a potential driver of Tfh differentiation.9 IL18 is a pro-atherogenic cytokine11 that belongs to the IL1 family, and after the successful CANTOS trial results demonstrating the benefit of targeting IL1β in coronary artery disease (CAD), IL18 has been postulated as an alternative target.12 Its pro-atherogenic role in mice so far was attributed to its effect on Th cell differentiation and IFNγ production,13 but its role in Tfh differentiation has not been studied before.
2. Methods
2.1. Animals
All experiments were approved by the Home Office, UK (PPL PP9485757). The following strains of mice were used: CD4Cre/+ 14 and Rag2−/− mice (Jackson Lab); C57Bl6 and Ldlr −/− (Charles River); Bcl6flox/flox 15 (a gift to AS from Dr Harker); Il18r−/−; NCC−/− 16; Cd79aCre/+; and Rbpjkflox/flox.17,18
For atherosclerosis studies, recipients were lethally irradiated and injected 107 bone marrow (BM) cells i.v. After 4 weeks recovery,19 they were fed a high-fat/high-cholesterol (HF/HC; 21% fat, 0.15% cholesterol) diet for 8 or 16 weeks.
For IL18 studies, mice received i.p. IL18 (Biotechne) in PBS (2 μg/mouse/day) or PBS for 8 days. For immunization, 2 × 109 sheep red blood cell (SRBC) was injected i.p.
At the end of the study, mice were euthanized by rising concentrations of CO2 inhalation in their cage (CO2 flow rate of 2 L/min for 5 min).
2.2. Flow cytometry
Single-cell suspensions of spleen, BM, para-aortic lymph node (PALN), and peritoneal lavage were stained with fluorophore-conjugated antibodies (see Supplementary material online, Table S1) and analysed using LSRII Fortessa (BD) flow cytometer. Dead cells were excluded based on FSc and SSc. Cell subsets were then identified as previously described9,20 (see Supplementary material online, Table S2). Examples of flow gating strategies are found in Supplementary material online, Figure S1.
2.3. Extent and composition of atherosclerotic lesions
The lesions in the root of the aorta beneath all three-valve leaflets were analysed with Masson’s trichrome or immunofluorescence as previously described.9,20 The antibodies to detect are macrophages (Mac-3, 1:200, Santa Cruz) and T cells (CD3, 1:100, Dako). Whole aortas were used in an en face preparation for oil red O staining. All was quantified with ImageJ as previously described.9,20
2.4. Determination of circulating antibodies
Specific antibody titres in plasma were determined by chemiluminescent ELISA as previously described.21,22
2.5. Determination of serum lipid levels
Total cholesterol, HDL-C, and triglycerides were measured using an enzymatic method in a Siemens Dimension RxL analyser.
2.6. Purification of MZB
For splenic MZB cell purification, first B cell–enriched populations were separated by EasySep B cell purification kit (StemCell). B cells were stained with anti-CD23-PE, anti-CD21-FITC, and 7-AAD for cell viability. MZBs (CD21hiCD23low) were sorted using Aria III Cell Sorter (BD). Purity was >95%.
2.7. Ex vivo MZB cell IgM production stimulation assay
A total of 250 000 sorted MZB cells/well were cultured for 3 days with CpG ODN (2 μM tlrl-1826, InvivoGen). IgM antibody levels in supernatants were measured using ELISA.
2.8. RNA sequencing
Splenic MZB cells were directly sorted in RLT Plus Micro RNA buffer for RNA extraction (Qiagen). RNA integrity number (RIN) values for all samples were >7. RNA (2.5 ng) was whole transcriptome amplified using Ovation RNA-seq System V2 (NuGEN). Two micrograms per sample of the amplified cDNA was used to generate sequencing library using Ovation Rapid DR Library System (NuGEN). Sequencing was performed on an Illumina HiSeq 2500 (CRUK, Cambridge). Bioinformatic analysis was performed as explained in Supplementary material online, Methods.
2.9. Quantitative RT-PCR
RNA from sorted MZB cells and splenic cells from fresh frozen optimal cutting temperature (OCT) compound embedded spleen sections were isolated using RNAeasy Plus micro kit (Qiagen). RT-PCR was performed using QuantiTect Reverse Transcription Kit (Qiagen) or SMART-Seq v4 (Takara Bio). Primer sequences in Supplementary material online, Table S3.
2.10. Human samples
Blood samples were analysed from participants with atherosclerosis enrolled in the following studies: (i) Residual Inflammation and Plaque Progression Long-Term Evaluation (RIPPLE, NCT04073810; n = 16); (ii) Rituximab in Patients With Acute ST-elevation MI Study (RITA-MI, NCT0307219923; n = 21); and (iii) Coronary Assessment in Virginia cohort (CAVA, n = 20; see Supplementary material online, Table S4). Participants from RIPPLE (2 weeks) and RITA-MI (48 h) had recent myocardial infarction (MI). Participants in the CAVA study had stable CAD. Participants gave written consent in accordance with the protocol approved by the local research ethics committee in the UK (19/EE/0043; 16/EE/0241) or the institutional review board at the University of Virginia, USA (IRB HSR #15328), in accordance with the Declaration of Helsinki and UK Human Tissue Act 2004.
2.11. Statistical analysis
Values are expressed as means ± SEM. Where data sets passed normality tests, differences between values were examined using Student’s t-test or two-way analysis of variance (ANOVA). For experiments with four or less replicates, non-parametric Mann–Whitney test was used. For correlations, Spearman’s non-parametric test was applied. For EnrichR top canonical pathways and genes in the RNA-seq, Fisher’s exact test was used. In all figures, *P < 0.05, **P < 0.01, and ***P < 0.001.
3. Results
3.1. Absence of Tfh increases early atherosclerosis
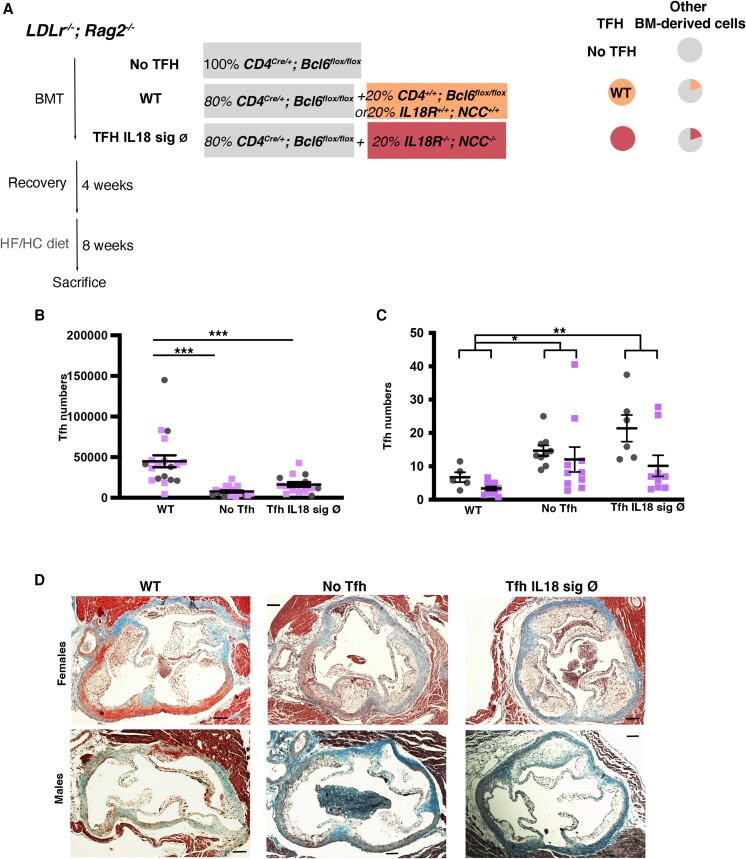
To study the role of Tfh in atherosclerosis, we first followed a BM reconstitution methodology in Ldlr−/− mice, described in a previously published report on the role of Tfh cells in atherosclerosis.7 We generated mice with T cell–specific conditional deletion (CD4Cre/+) of the Tfh lineage transcription factor Bcl6 (Bcl6flox/flox). We then reconstituted lethally irradiated Ldlr−/− mice with a BM containing 100% cells from CD4Cre/+; Bcl6flox/flox (that are unable to generate Tfh). Our control mice received a BM containing 80% cells from CD4Cre/+; Bcl6flox/flox + 20% cells from CD4+/+; Bcl6flox/flox (which reconstitute CD4+/+; Bcl6flox/flox WT Tfh). After recovery, mice were put on a HF/HC western diet for 8 weeks (see Supplementary material online, Figure S2A). This time point was chosen based on the fact that in mouse models of HF/HC-induced atherosclerosis, adaptive immunity plays a major role in the early phase of the disease but becomes less important in the late phase with extended duration of HF/HC diet and severe hypercholesterolaemia.24 Ldlr−/− mice transplanted with CD4Cre/+; Bcl6flox/flox mice BM showed only a partial (50%) reduction in Tfh (see Supplementary material online, Figure S2B), suggesting that, despite ‘lethal’ irradiation, the recipient host (which is competent for Tfh generation) was able to reconstitute a large number of Tfh. The presence of recipient T cell progenitor cells resistant to irradiation has been previously shown.25 Surprisingly, despite a partial depletion of Tfh cells, these mice showed an unexpected significant increase of atherosclerotic plaque development in the aortic roots (see Supplementary material online, Figure S2C), suggesting that Tfh may have a protective, not detrimental, role in atherosclerosis.
To track the specific Tfh reconstitution in lethally irradiated mice, we decided to use the CD45 (cluster of differentiation 45) congenic lineage tracing system. Lethally irradiated CD45.2+ CD45.1− CD4Cre/+; Bcl6flox/flox (No Tfh) and CD45.2+ CD45.1− CD4+/+; Bcl6flox/flox (WT) recipient mice were reconstituted with 80% CD45.2+ CD45.1− CD4Cre/+; Bcl6flox/flox + 20% CD45.2− CD45.1+ BM. After recovery, mice were injected with SRBCs to induce the activation of the Tfh-GC response26 (see Supplementary material online, Figure S2D). Specific Tfh reconstitution from the BM (80% of CD4+ CD44+ CXCR5+ PD1+ Tfh coming from the CD45.2− CD45.1+ donor) was successful only when the recipient was deficient for Tfh (CD45.2+ CD45.1− CD4Cre/+; Bcl6flox/flox). The BM was unable to successfully reconstitute Tfh in WT recipients (CD45.2+ CD45.1− CD4+/+; Bcl6flox/flox). Most of the reconstituted Tfh in the latter mice were still from the recipient host (see Supplementary material online, Figure S2E). Our data are in agreement with the published literature that T cells are unlikely to reconstitute fully from the donor BM if the recipient is T cell competent.25
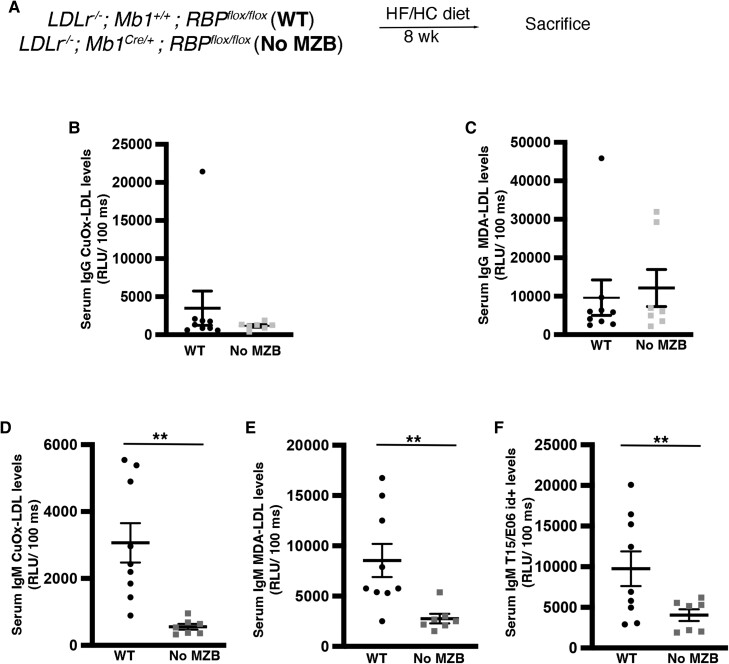
To obtain an atherosclerotic mouse model with a specific deletion of Tfh, we reconstituted lethally irradiated Ldlr−/−; Rag2−/− mice (no T cells and no B cells, to avoid reconstitution with irradiation-resistant recipient T cells) with a BM containing 100% CD4Cre/+; Bcl6flox/flox (No Tfh group) or a mixed BM chimaera containing 80% CD4Cre/+; Bcl6flox/flox + 20% CD4+/+; Bcl6flox/flox (WT Tfh group; Figure 1A). Additional lethally irradiated Ldlr−/−; Rag2−/− mice were reconstituted with 80% CD4Cre/+; Bcl6flox/flox + 20% Il18r−/−; NCC−/− (Tfh Il18 sign Ø) or 20% Il18r+/+; NCC+/+ (WT group) to study the role of the IL18 signalling pathway in Tfh (see the last chapter of Results). After recovery, mice were put on a HF/HC diet for 8 weeks. There was almost a full depletion of splenic (Figure 1B; Supplementary material online, Figure S3A) and PALN (see Supplementary material online, Figure S3B) Tfh cells in the No Tfh group. All the other T cell subsets, including splenic T effector memory (TEM), T regulatory (Treg), and T follicular regulatory (Tfr), and both splenic and PALN Th17 and Th1 cells were not significantly altered in WT vs. No Tfh mice (see Supplementary material online, Figure S3C–I). Numbers of splenic neutrophils, macrophages, Ly6Chi and Ly6Clo monocytes, and eosinophils were not different between both groups (see Supplementary material online, Figure S4A–E).
Figure 1.
Tfh cell deficiency and the absence of IL18 signalling in Tfh cells accelerate the development of atherosclerosis in mice. Data from males and females Ldlr−/−; Rag2−/− after BM transplant with 100% CD4Cre/+; Bcl6flox/flox (No Tfh); 80% CD4Cre/+; Bcl6flox/flox + 20% CD4+/+; Bcl6flox/flox; or 20% Il18r+/+; NCC+/+ (WT) and 80% CD4Cre/+; Bcl6flox/flox + 20% Il18r−/−; NCC−/− (Tfh Il18 sign Ø) fed a HF/HC diet for 8 weeks. Represented data from five experiments. WT group includes mice that are either CD4+/+; Bcl6flox/flox (littermates to the No TFH group) or Il18r+/+; NCC+/+ WT (littermates to Il18r−/−; NCC−/− mice) and were combined to reduce the number of animals used in these experiments (3Rs). (A) Schematic diagram of the experiment. (B) Total splenic Tfh cells (CD4+ CD44hi CXCR5+ PD1+; n = 6–10 mice/group). (C) Quantification of atherosclerotic plaque area in aortic roots. (D) Representative images of Masson’s trichrome staining (original magnification ×10; scale bars: 200 μm). Each symbol represents an individual mouse; horizontal bars denote mean ± SEM. (B) Student’s t-test and (C) two-way ANOVA. *P < 0.05, **P < 0.01, and ***P < 0.001.
Lack of Tfh led to a striking acceleration of atherosclerosis in aortic roots (Figure 1C and D) and aortic arches (see Supplementary material online, Figure S5A), confirming an unsuspected atheroprotective role for Tfh. The substantial increase in atherosclerosis could not be explained by changes in serum lipid levels (see Supplementary material online, Figure S5B–D). Plaque macrophage content was not significantly different between WT and No Tfh mice (see Supplementary material online, Figure S6A–C). In No Tfh mice, there was a significant increase of CD3+ T cells in both intima and adventitia compared with WT mice (see Supplementary material online, Figure S6D–F). Plaque collagen content and necrotic core size were significantly increased in the No Tfh group compared to the WT group (see Supplementary material online, Figure S6G and H).
The difference in plaque size between both groups was smaller after extended duration (16 weeks) of HF/HC diet (see Supplementary material online, Figure S7A–D), consistent with the predominant role of adaptive immunity in the early stages of atherosclerosis in these murine models.24
3.2. Tfh are necessary for the secretion of IgM natural antibodies
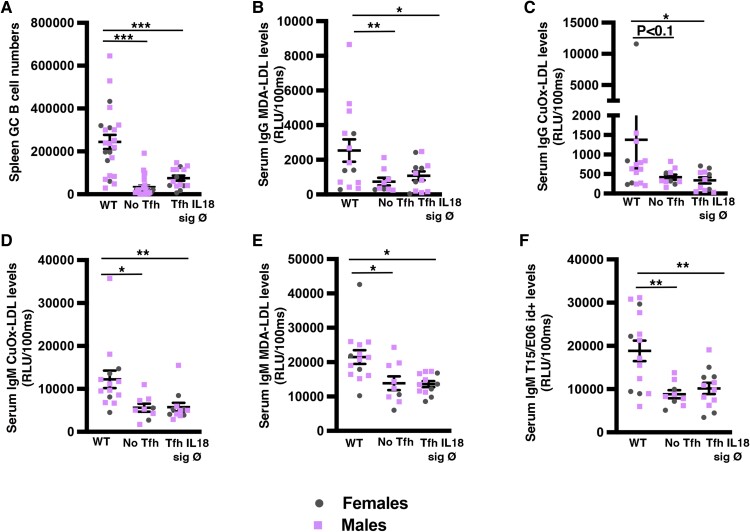
As expected, splenic and PALN GC B cells were profoundly reduced in No Tfh compared with WT Tfh mice (Figure 2A; see Supplementary material online, Figure S8A and B), which was associated with a reduction of plasma cells in the BM (see Supplementary material online, Figure S8C). Concomitantly, there was a significant reduction of serum IgG antibodies against malondialdehyde (MDA)-LDL and almost significant for IgG anti–copper-oxidized (CuOx)-LDL (Figure 2B and C). MDA and CuOx-LDL IgM antibodies were also significantly reduced in No Tfh vs. WT Tfh mice (Figure 2D and E). Surprisingly, mice with No Tfh also had significantly lower levels of the previously defined B1 cell–derived natural IgM T15/E06 idiotype+ (id+) antibody directed against the oxidation-specific epitope (OSE) phosphorylcholine (PC; Figure 2F) while levels of B1a and B1b peritoneal cells were similar between the two groups (see Supplementary material online, Figure S8D). Other reported positive regulators of IgM levels in atherosclerosis like IL5 and BAFF were significantly upregulated in spleens of No Tfh compared to WT Tfh mice (see Supplementary material online, Figure S8E–H). These data suggest that at least part of the ‘natural’ IgM anti-PC antibody production is dependent on Tfh activation and may explain the acceleration of early atherosclerosis in the absence of Tfh.
Figure 2.
Lack of Tfh cells and absence of IL18 signalling in Tfh lead to a profound reduction of anti-OSE IgG and IgM antibodies, including ‘natural’ IgM antibodies. Data from the same experimental groups as in Figure 1. (A) Total numbers of splenic GC B cells (B220+ Gl7hi CD95hi; n = 6–11 mice/group). (B–F) Graphs showing total serum subtypes of IgG (B, C) and IgM (D–F) levels. Student’s t-test. *P < 0.05, **P < 0.01, and ***P < 0.001.
3.3. The absence of Tfh leads to ‘aberrant’ MZB cells
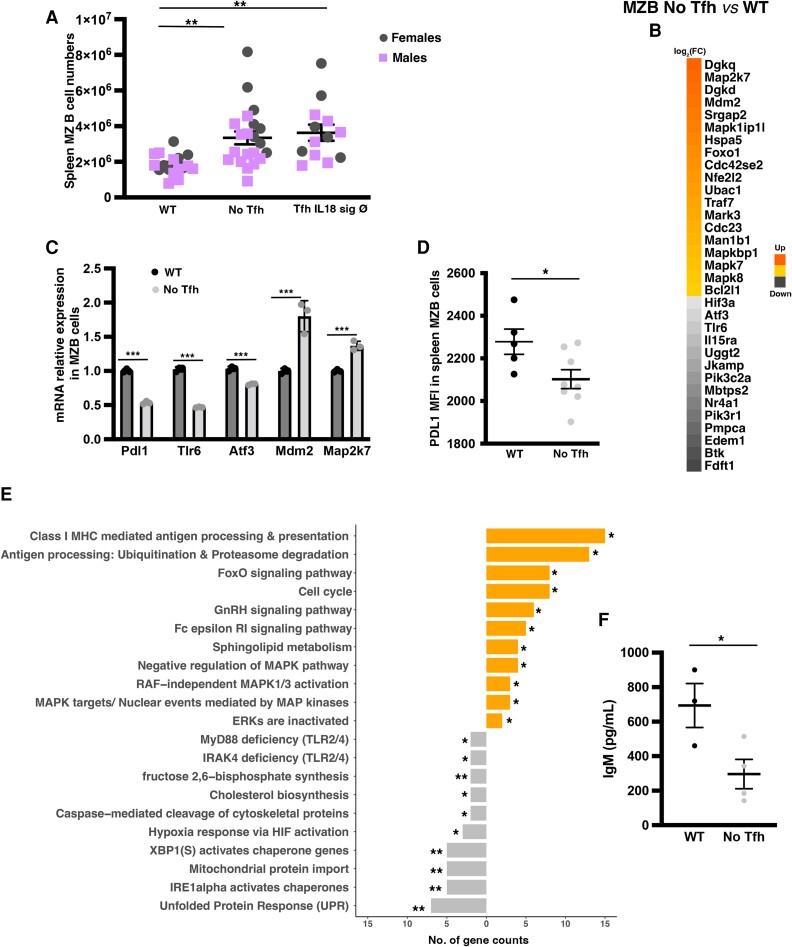
Given our previous work demonstrating direct interaction between (pre-)Tfh and MZB cells,9 which are innate-like B cells and the main producers of IgM antibodies,27 we examined changes in MZB cells. Splenic total and follicular B cell numbers were similar between the two groups (see Supplementary material online, Figure S8H and I), but lack of Tfh was associated with a significant increase in MZB cell numbers (Figure 3A). Intrigued by this finding and to gain additional mechanistic insight, we performed RNA-seq on sorted splenic MZB cells after 8 weeks of HF/HC diet in WT vs. No Tfh groups. We first focused on a subset of genes that we have shown previously to be required for the activation of an atheroprotective programme in MZB cells in response to HF/HC diet.9,20 MZB cells from No Tfh mice had a significant decrease of transcription factors Atf3 and Nr4a1, and the surface protein PDL1, as well as their upstream regulators, including the B cell receptor and Toll-like receptor (Btk, Tlr6) signalling pathways (Figure 3B–D).
Figure 3.
Lack of Tfh cells leads to accumulation of ‘aberrant’ MZB cells. Data from the same experimental No Tfh and WT groups. (A) Total splenic MZB cells (n = 12–15 mice/group). (B, C, and E) MZB cell RNA-seq from No Tfh and WT groups (n = 5–6 mice/group). (B) Clustered heat map of 33 genes that were differentially expressed. (C) qRT-PCR for Pdl1 (n = 3 mice/group). (D) PDL1 surface expression by flow cytometry. (E) Selected significantly enriched GSEA pathways. Each bar represents the number of significantly expressed genes in each pathway. Orange denotes up- and grey downregulated in MZB cells from No Tfh vs. WT. (F) IgM levels in the supernatants of sorted MZB cells (n = 6–9 mice/group) after culture with CpG. Representative plot from two independent experiments with similar results. P < 0.05 (Student’s t-test) and P < 0.1 (Mann–Whitney). (A, C, D, and F) Student’s t-test and (E) Fisher’s exact test. *P < 0.05, **P < 0.01, and ***P < 0.001.
Further analysis of the RNA-seq data revealed a downregulation in the expression of the master regulators necessary for the formation and activation of antibody-secreting plasma B cells28 (Figure 3E). There was a significant decrease in the unfolded protein response (e.g. Edem1, Atf3, and Ppp2r5b), endoplasmic-inducing stress (e.g. IRE1 alpha activated chaperones: Ddx11 and Extl3), and XBP1 signalling pathways (e.g. Srpr, Edem1, and Add1), providing a plausible explanation for the significant decrease of IgM antibody production in mice with No Tfh. On the other hand, MZB cells from No Tfh showed significant upregulation of genes related to proliferation (e.g. Mapk8 and Mapk7) and cell cycle (e.g. Cdc23, Cdkn2, and E2f2) pathways, which might be activated independently of BCR signalling pathway, causing the accumulation of these ‘aberrant’ MZB cells. Thus, lack of Tfh leads to the accumulation of ‘aberrant’ MZB cells that are unable to activate their atheroprotective programme and form antibody-secreting cells.
To test this hypothesis, sorted splenic MZB cells from WT Tfh vs. No Tfh groups after 8 weeks on HF/HC diet were cultured with CpG (to stimulate IgM production). MZB cells from No Tfh mice secrete significantly less IgM antibodies than those from WT mice (Figure 3F), and this was associated with decrease PDL1 expression (see Supplementary material online, Figure S9A and B) confirming the in vivo phenotype. Furthermore, MZB cells from SRBC immunized Cd4Cre/+; Bcl6flox/flox (No Tfh) mice also showed significant reduced IgM secretion compared to MZB cells from Cd4+/+; Bcl6flox/flox (WT) mice (see Supplementary material online, Figure S9C), supporting a Tfh-dependent MZB cell antibody secretion in different clinical contexts.
3.4. MZB cells produce atheroprotective IgM antibodies
To address the role of MZB cells in the production of IgM antibodies in atherosclerosis, we created a new atherosclerotic mouse model with No MZB cells from birth: Ldlr−/−; Cd79aCre/+; RBPflox/flox (No MZB) and Ldlr−/−; Cd79a+/+; RBPflox/flox (WT; Figure 4A). As previously shown,9,29 Notch signalling disruption selectively in B cells led to mice with No MZB (see Supplementary material online, Figure S10A), which, as expected,9 significantly increased Tfh cells after 8 weeks of a HF/HC diet (see Supplementary material online, Figure S10B). Tfh from Ldlr−/− mice with No MZB showed reduced CXCR5 and PD1 expression compared with WT mice (see Supplementary material online, Figure S10C and D), corroborating our previous finding that lack of MZB cells leads to the accumulation of ‘poorly differentiated’ Tfh.9 There was a tendency to increased GC levels (see Supplementary material online, Figure S10E) but IgG-CuOx-LDL and IgG-MDA-LDL were not different between the two groups (Figure 4B and C). However, absence of MZB cells led to a dramatic decrease of IgM anti-CuOx-LDL, IgM anti-MDA-LDL, and IgM T15/E06 id+ titres (Figure 4D and F). Thus, MZB cells substantially contribute to the production of atheroprotective ‘natural’ anti-OSE IgM antibodies.
Figure 4.
MZB cells are necessary for the formation of IgM natural antibodies. Males and females Ldlr−/−; Cd79aCre/+; RBPflox/flox and Ldlr−/−; Cd79a+/+; RBPflox/flox (WT) were fed a HF/HC diet for 8 weeks (A–F). (A) Schematic diagram of the experimental procedure. (B–F) Graphs showing total serum subtypes of IgG (B–D) and IgM (E–G) levels. Student’s t-test. **P < 0.01.
3.5. Circulating human MZ-like B cells positively correlate with anti-OSE IgM antibody levels
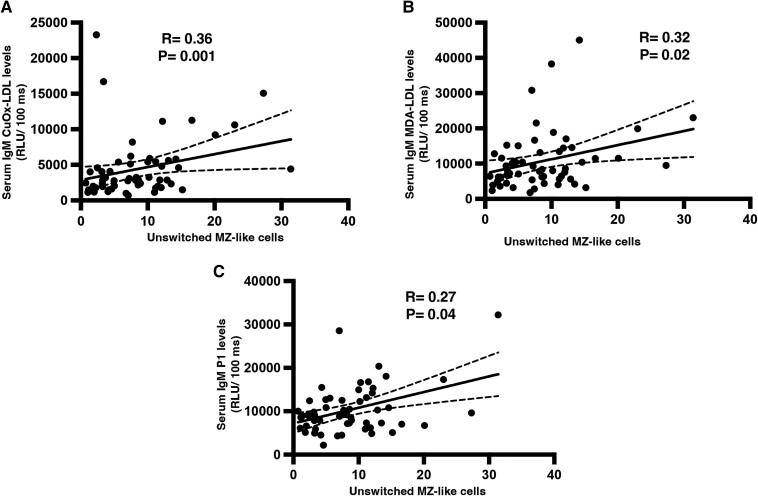
To explore the association between MZB cells and the levels of atheroprotective anti-OSE IgM antibodies in humans, we measured blood MZB-like cells and IgM antibodies in 57 patients with established CAD with or without a recent MI (see Supplementary material online, Table S4). Circulating MZB-like cells were defined as unswitched CD27+ IgD+ memory B cells9,30 (see Supplementary material online, Table S2 and Figure S1). We found a significant positive correlation between circulating MZB-like cells and IgM antibodies against CuOx-LDL, MDA-LDL, and the P1 peptide mimotope of MDA-LDL (Figure 5A–C). MZ-like cells did not correlate with IgG levels for any of the OSE specificities checked (data not shown). This result further suggests a potential role of MZB cells as producers of anti-OSE IgM antibodies in humans.
Figure 5.
Circulating human MZ-like B cells positively correlate with anti-OSE IgM levels. PBMCs and serum were collected from patients of the RIPPLE (2 weeks post-MI), RITA-MI (2 days post-MI), and CAVA (stable CAD) populations (n = 57; A–C). Correlations between unswitched MZ-like B cells and (A) IgM CuOx-LDL, (B) IgM MDA-LDL, and (C) IgM-P1 antibodies. Spearman’s rank order correlation.
3.6. The absence of IL18 signalling pathway in Tfh decreases their numbers and increases atherosclerosis
Re-analysis of our previous RNA-seq data on ‘poorly differentiated’ Tfh in the absence of MZB cells identified Il18r as one of the genes that were differentially expressed in these Tfh cells9 (see Supplementary material online, Figure S11A). Interestingly, both Tfh and IL18 serum levels were significantly increased in Ldlr−/− mice fed a HF/HCD compared with mice fed a control chow diet (see Supplementary material online, Figure S11B and C). Administration of IL18 to C57Bl/6 mice significantly increased Tfh cells, including in a widely validated model of SRBC-induced Tfh-GC formation (see Supplementary material online, Figure S11D). Therefore, we decided to address the role of IL18 in Tfh cell formation in atherosclerosis.
IL18 has been shown to bind to both IL18R and Na-Cl co-transporter (NCC also known as SLC12A3).16 Therefore, we reconstituted lethally irradiated Ldlr−/−; Rag2−/− mice with 80% CD4Cre/+; Bcl6flox/flox + 20% Il18r−/−; NCC−/− (Tfh Il18 sign Ø) or 20% Il18r+/+; NCC+/+ (WT group). Lack of IL18 signalling in Tfh led to >50% reduction of Tfh numbers (Figure 1B) and was associated with a significant increase in the development of atherosclerotic plaques in the aortic roots (Figure 1C and D) and aortic arches (see Supplementary material online, Figure S5A). There were no significant changes in serum lipid levels in both groups (see Supplementary material online, Figure S5B–D). Similar to the No Tfh group, abrogation of IL18 signalling in Tfh was associated with a decrease in GC B cells, IgG MDA-LDL, IgM-CuOx-LDL, IgM-MDA-LDL, and IgM T15/E06 id+ antibodies (Figure 2A–F), as well as an accumulation of MZB cells (Figure 3A), further supporting a role for Tfh–MZB cell interaction in regulating the humoral response during HF/HC diet–induced atherosclerosis.
4. Discussion
Tfh play essential roles in the differentiation of antigen-specific memory B cells and antibody-producing plasma cells, and dysregulation of Tfh is involved in several disease settings, including response to infectious agents, cancer, and autoimmunity,31,32 but little is known about the potential role of Tfh in atherosclerosis.
Clement et al. 8 showed that lack of Qa-1–restricted CD8+ Treg cells in Apoe−/− mice led to the accumulation of Tfh cells and acceleration of atherosclerosis, which was prevented by administration of anti-ICOSL antibody. Similarly, anti-ICOSL treatment prevented the increased accumulation of Tfh cells in MZB cell–deficient Ldlr−/− mice and the acceleration of atherosclerosis.9 While Clement et al. 8 did not perform any characterization of the accumulated Tfh in their model, we showed that the accumulated Tfh cells in MZB-deficient mice were poorly differentiated.9 Thus, it was still unclear whether normal Tfh cell development in hyperlipidaemic mice was critical for atherosclerosis development. Anti-ICOSL administration did not alter atherosclerosis development in LDLr−/− and ApoE−/− probably due to effects on other immune cells expressing ICOS. Addressing the role of Tfh cells in atherosclerosis required the use of a more selective genetic model, and this was done by Gaddis et al.7 who used a model of genetic deficiency of Tfh. They reported a slight but significant decrease in atherosclerosis in mice with No Tfh compared to controls. The apparent discrepancy between their data and ours could in part be related to the use of littermate controls in our case to control for Cre toxicity, compared to the use of C57Bl6 mice that were not littermates of CD4Cre/+; Bcl6flox/flox mice in their case.
In our mouse model, we have depleted all Tfh cells. Emerging evidence suggests that there are different Tfh subsets (Tfh1, Tfh2, and Tfh17). These subsets appear to have different differentiation pathways33 and functions.34 In the future, it will be interesting to develop new mouse models to interrogate the specific roles of each of these subsets in atherosclerosis.
What could be the mechanism of early atherosclerosis acceleration in the absence of Tfh cells? This cannot be explained by the profound reduction of GC B cells given that complete genetic deficiency of GC B cells was shown to be atheroprotective.5 Moreover, while genetic deficiency of GC B cells substantially reduces IgG antibodies, it does not affect the production of (anti-OSE) IgM antibodies.5 Martos-Folgado et al. 6 using a mouse with specific deletion of GC-derived plasma cells demonstrated that a substantial proportion of these IgM atheroprotective antibodies come from extrafollicular B cells. Therefore, the dramatic reduction of both IgG and IgM anti-OSE antibodies in mice with genetic Tfh deficiency strongly suggests a role for Tfh-dependent extrafollicular antibody responses in atherosclerosis, in a similar manner as in experimental immunization models using CD4Cre/+; Bcl6flox/flox mice have demonstrated that Tfh cells that do not enter the GC significantly contribute to extrafollicular responses.4 MZB cells are the prototypical B cells that engage in extrafollicular responses and are the major producers of IgM antibodies.27,35,36 Our data using MZB cell–deficient mice indicate that a substantial proportion of atheroprotective anti-OSE IgM21,22,37,38 arise from MZB cells during atherosclerosis, pointing to a determinant role of (pre-)Tfh–MZB cell interactions in this process. Indeed, we show that deletion of Tfh impairs MZB cell properties, leading to the accumulation of MZB cells with substantially altered function and antibody-secreting machinery.
Our work also identifies a previously unexplored role of MZB cells in the production of natural OSE-IgM antibodies such as the anti-PC E06/T15 antibody, which plays an important atheroprotective role.39 Its production has so far been associated only with B1 cells,40,41 but the contribution of MZB cells was overlooked. Here, we unequivocally demonstrate the important role of MZB cells in the production of anti-PC E06/T15 IgM antibody during atherosclerosis in mice. Moreover, a substantial level of this antibody production is Tfh dependent.
In our moue model, lack of Tfh leads to accumulation of ‘aberrant’ MZB cells unable to secrete anti-OSE IgM antibodies. This phenotype could result from the absence of Tfh-derived IL21, which is required for optimal antibody production by extrafollicular B cells4 or from co-stimulatory/inhibitory signalling pathways (i.e.PD1-PDL1). Further studies are needed to elucidate the exact mechanisms orchestrating this MZB–Tfh interaction that could be targeted to modulate the antibody secretion capability of MZB cells during atherosclerosis.
Currently, the hunt to target the inflammatory response in atherosclerosis has accelerated after the positive results of the CANTOS trial, which tested the use of canakinumab (neutralizing anti-IL1β) to treat high-risk atherosclerotic patients with prior MI. A consecutive study found that the residual inflammatory risk in patients with high cardiovascular risk and treated with canakinumab was positively correlated with IL18 levels and suggested that anti-IL18 inhibitors should be considered as potential future anti-inflammatory drugs to treat atherothrombosis.12 Our group was the first to show that IL18 expression was associated with human plaque instability and that IL18 inhibition was effective in reducing experimental atherosclerosis.11 However, our present experiments suggest that the role of IL18 in Tfh cells may limit the extent of atheroprotection associated with systemic IL18 inhibition.
Our current data show significant correlation between circulating human MZ-like B cells and levels of anti-OSE IgM antibodies, supporting a potential role for human MZB cells in the production of atheroprotective anti-OSE IgM antibodies. Human and mouse Tfh share many characteristics3 but circulating Tfh-like cells (cTfh) have only been described in humans and they are lacking in mouse. The role and significance of cTfh still need to be better defined.42,43 Despite initial reports that cTfh may resemble GC Tfh of secondary lymphoid organs,3 the vast majority of the current data suggest that cTfh are those that have never entered the GC and, thus, may more closely resemble pre-Tfh. The clinical cardiovascular relevance of Tfh–MZB cell interaction merits further investigation.
In conclusion, this work reveals new roles for Tfh and MZB cells regulating HF/HC-induced atherosclerosis. Tfh cell deficiency accelerates atherosclerosis, and this is associated with an altered differentiation phenotype and anti-OSE IgM antibody-producing capacity of MZB cells. We also uncover the important role of MZB cells in the production of the atheroprotective anti-OSE IgM antibodies opening a new quest to find ways we could modulate these cells to promote IgM secretion. Our work fills an important gap in our understanding of the role of these immune cell subsets in atherosclerosis and opens new lines of investigation to target Tfh–MZB cell interactions that will have important therapeutic consequences.
Our work also finds an important role of IL18 in Tfh differentiation, suggesting that the use of systemic strategies to block IL18 may increase the risk of fatal infection due to the risk of reduced Tfh cells and antibody production.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Supplementary Material
Acknowledgements
We acknowledge Ana Petrunkina, Natalia Savynkh, and Nika Romashova in the Phenotyping Hub of the Department of Medicine (University of Cambridge) for their help in flow cytometry and sorting. We acknowledge measurements of lipids, BAFF, and IL5 in blood by Keith Burling and Peter Baker and the Core Biochemical Assay Laboratory (CBAL).
Contributor Information
James Harrison, Department of Medicine, University of Cambridge, Cambridge Biomedical Campus, Cambridge, UK.
Stephen A Newland, Department of Medicine, University of Cambridge, Cambridge Biomedical Campus, Cambridge, UK.
Wei Jiang, Department of Medicine, University of Cambridge, Cambridge Biomedical Campus, Cambridge, UK.
Despoina Giakomidi, Department of Medicine, University of Cambridge, Cambridge Biomedical Campus, Cambridge, UK.
Xiaohui Zhao, Department of Medicine, University of Cambridge, Cambridge Biomedical Campus, Cambridge, UK.
Marc Clement, Department of Medicine, University of Cambridge, Cambridge Biomedical Campus, Cambridge, UK; Laboratory for Vascular Translational Sciences (LVTS), Université de Paris, INSERM U1148, Paris, France.
Leanne Masters, Department of Medicine, University of Cambridge, Cambridge Biomedical Campus, Cambridge, UK.
Andrej Corovic, Department of Medicine, University of Cambridge, Cambridge Biomedical Campus, Cambridge, UK.
Xian Zhang, Department of Medicine, Brigham and Woman’s Hospital, Harvard Medical School, Boston, MA, USA.
Fabrizio Drago, Division of Cardiovascular Medicine, Department of Medicine, University of Virginia, Charlottesville, VA, USA.
Marcella Ma, Wellcome-MRC Institute of Metabolic Science and Medical Research Council Metabolic Diseases Unit, University of Cambridge, UK.
Maria Ozsvar Kozma, Department of Laboratory Medicine, Medical University of Vienna, Vienna, Austria.
Froher Yasin, Department of Medicine, University of Cambridge, Cambridge Biomedical Campus, Cambridge, UK.
Yuta Saady, Department of Medicine, University of Cambridge, Cambridge Biomedical Campus, Cambridge, UK.
Hema Kothari, Division of Cardiovascular Medicine, Department of Medicine, University of Virginia, Charlottesville, VA, USA.
Tian X Zhao, Department of Medicine, University of Cambridge, Cambridge Biomedical Campus, Cambridge, UK.
Guo-Ping Shi, Department of Medicine, Brigham and Woman’s Hospital, Harvard Medical School, Boston, MA, USA.
Coleen A McNamara, Division of Cardiovascular Medicine, Department of Medicine, University of Virginia, Charlottesville, VA, USA.
Christoph J Binder, Department of Laboratory Medicine, Medical University of Vienna, Vienna, Austria.
Andrew P Sage, Department of Medicine, University of Cambridge, Cambridge Biomedical Campus, Cambridge, UK.
Jason M Tarkin, Department of Medicine, University of Cambridge, Cambridge Biomedical Campus, Cambridge, UK.
Ziad Mallat, Department of Medicine, University of Cambridge, Cambridge Biomedical Campus, Cambridge, UK; PARCC Inserm U970, Universite de Paris, Paris, France.
Meritxell Nus, Department of Medicine, University of Cambridge, Cambridge Biomedical Campus, Cambridge, UK.
Funding
This work was supported by the British Heart Foundation by an Intermediate Basic Science Research Fellowship (FS/20/23/34784) to M.N. and the British Heart Foundation with Project Grants (PG/17/73/33251 and PG/22/10898) to M.N. and Z.M. Z.M. is supported by the British Heart Foundation through a Chair of Cardiovascular Medicine (G101517). This work was also supported by Fondation Leducq to Z.M., C.A.M., and C.B. (G107743) and RITA-MI project funded through Horizon 2020 Framework Programme to Z.M. (G106938). The RIPPLE study was supported by the Wellcome Trust (211100/Z/18/Z) and the British Heart Foundation (FS/CRTF/20/24035) to J.M.T.
Data availability
Most of the data underlying this work are included in the manuscript and Supplementary material. The rest of the data will be shared by the corresponding author upon reasonable request.
Translational perspective.
We have shown that natural IgM antibodies protect from atherosclerosis, and they are produced by MZB cells in a Tfh-dependent manner. IL18 is necessary for Tfh differentiation and consequently in the production of IgG and IgM antibodies. Thus, clinical trials targeting B cells and IL18 would have to take into consideration its role on the Tfh–MZB cell interaction.
References
- 1. Mallat Z, Binder CJ. The why and how of adaptive immune responses in ischemic cardiovascular disease. Nat Cardiovasc Res 2022;1:431–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Soehnlein O, Libby P. Targeting inflammation in atherosclerosis—from experimental insights to the clinic. Nat Rev Drug Discov 2021;20:589–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Crotty S. T follicular helper cell biology: a decade of discovery and diseases. Immunity 2019;50:1132–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lee SK, Rigby RJ, Zotos D, Tsai LM, Kawamoto S, Marshall JL, Ramiscal RR, Chan TD, Gatto D, Brink R, Yu D, Fagarasan S, Tarlinton DM, Cunningham AF, Vinuesa CG. B cell priming for extrafollicular antibody responses requires Bcl-6 expression by T cells. J Exp Med 2011;208:1377–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Centa M, Jin H, Hofste L, Hellberg S, Busch A, Baumgartner R, Verzaal NJ, Lind Enoksson S, Perisic Matic L, Boddul SV, Atzler D, Li DY, Sun C, Hansson GK, Ketelhuth DFJ, Hedin U, Wermeling F, Lutgens E, Binder CJ, Maegdesfessel L, Malin SG. Germinal center-derived antibodies promote atherosclerosis plaque size and stability. Circulation 2019;139:2466–2482. [DOI] [PubMed] [Google Scholar]
- 6. Martos-Folgado I, del Monte-Monge A, Lorenzo C, Busse CE, Delgado P, Mur SM, Cobos-Figueroa L, Escolà-Gil JC, Martín-Ventura JL, Wardemann H, Ramiro AR. MDA-LDL vaccination induces athero-protective germinal-center-derived antibody responses. Cell Rep 2022;41:111468. [DOI] [PubMed] [Google Scholar]
- 7. Gaddis DE, Padgett LE, Wu R, McSkimming C, Romines V, Taylor AM, McNamara CA, Kronenberg M, Crotty S, Thomas MJ, Sorci-Thomas MG, Hedrick CC. Apolipoprotein AI prevents regulatory to follicular helper T cell switching during atherosclerosis. Nat Commun 2018;9:1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Clement M, Guedj K, Andreata F, Morvan M, Bey L, Khallou-Laschet J, Gaston A-T, Delbosc S, Alsac J-M, Bruneval P, Deschildre C, Le Borgne M, Castier Y, Kim H-J, Cantor H, Michel J-B, Caligiuri G, Nicoletti A. Control of the T follicular helper-germinal center B-cell axis by CD8(+) regulatory T cells limits atherosclerosis and tertiary lymphoid organ development. Circulation 2015;131:560–570. [DOI] [PubMed] [Google Scholar]
- 9. Nus M, Sage AP, Lu Y, Masters L, Lam BYH, Newland S, Weller S, Tsiantoulas D, Raffort J, Marcus D, Finigan A, Kitt L, Figg N, Schirmbeck R, Kneilling M, Yeo GSH, Binder CJ, de la Pompa PJ, Mallat Z. Marginal zone B cells control the response of follicular helper T cells to a high-cholesterol diet. Nat Med 2017;23:601–610. [DOI] [PubMed] [Google Scholar]
- 10. Douna H, de Mol J, Amersfoort J, Schaftenaar FH, Kiss MG, Suur BE, Kroner MJ, Binder CJ, Bot I, van Puijvelde GHM, Kuiper J, Foks AC. IFNγ-stimulated B cells inhibit T follicular helper cells and protect against atherosclerosis. Front Cardiovasc Med 2022;9:781436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mallat Z, Corbaz A, Scoazec A, Graber P, Alouani S, Esposito B, Humbert Y, Chvatchko Y, Tedgui A. Interleukin-18/interleukin-18 binding protein signaling modulates atherosclerotic lesion development and stability. Circ Res 2001;89:E41–E45. [DOI] [PubMed] [Google Scholar]
- 12. Ridker PM, MacFadyen JG, Thuren T, Libby P. Residual inflammatory risk associated with interleukin-18 and interleukin-6 after successful interleukin-1β inhibition with canakinumab: further rationale for the development of targeted anti-cytokine therapies for the treatment of atherothrombosis. Eur Heart J 2020;41:2153–2163. [DOI] [PubMed] [Google Scholar]
- 13. Whitman SC, Ravisankar P, Daugherty A. Interleukin-18 enhances atherosclerosis in apolipoprotein E(-/-) mice through release of interferon-gamma. Circ Res 2002;90:E34–E38. [DOI] [PubMed] [Google Scholar]
- 14. Lee PP, Fitzpatrick DR, Beard C, Jessup HK, Lehar S, Makar KW, Pérez-Melgosa M, Sweetser MT, Schlissel MS, Nguyen S, Cherry SR, Tsai JH, Tucker SM, Weaver WM, Kelso A, Jaenisch R, Wilson CB. A critical role for Dnmt1 and DNA methylation in T cell development, function, and survival. Immunity 2001;15:763–774. [DOI] [PubMed] [Google Scholar]
- 15. Johnston RJ, Poholek AC, DiToro D, Yusuf I, Eto D, Barnett B, Dent AL, Craft J, Crotty S. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science 2009;325:1006–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang J, Sun C, Gerdes N, Liu C, Liao M, Liu J, Shi MA, He A, Zhou Y, Sukhova GK, Chen H, Cheng XW, Kuzuya M, Murohara T, Zhang J, Cheng X, Jiang M, Shull GE, Rogers S, Yang C-L, Ke Q, Jelen S, Bindels R, Ellison DH, Jarolim P, Libby P, Shi G-P. Interleukin 18 function in atherosclerosis is mediated by the interleukin 18 receptor and the Na-Cl co-transporter. Nat Med 2015;21:820–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hobeika E, Thiemann S, Storch B, Jumaa H, Nielsen PJ, Pelanda R, Reth M. Testing gene function early in the B cell lineage in mb1-cre mice. Proc Natl Acad Sci U S A 2006;103:13789–13794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tanigaki K, Han H, Yamamoto N, Tashiro K, Ikegawa M, Kuroda K, Suzuki A, Nakano T, Honjo T. Notch-RBP-J signaling is involved in cell fate determination of marginal zone B cells. Nat Immunol 2002;3:443–450. [DOI] [PubMed] [Google Scholar]
- 19. Frasca D, Guidi F, Arbitrio M, Pioli C, Poccia F, Cicconi R, Doria G. Hematopoietic reconstitution after lethal irradiation and bone marrow transplantation: effects of different hematopoietic cytokines on the recovery of thymus, spleen and blood cells. Bone Marrow Transplant 2000;25:427–433. [DOI] [PubMed] [Google Scholar]
- 20. Nus M, Basatemur G, Galan M, Cros-Brunsó L, Zhao TX, Masters L, Harrison J, Figg N, Tsiantoulas D, Geissmann F, Binder CJ, Sage AP, Mallat Z. NR4A1 deletion in marginal zone B cells exacerbates atherosclerosis in mice-brief report. Arterioscler Thromb Vasc Biol 2020;40:2598–2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Binder CJ, Hörkkö S, Dewan A, Chang M-K, Kieu EP, Goodyear CS, Shaw PX, Palinski W, Witztum JL, Silverman GJ. Pneumococcal vaccination decreases atherosclerotic lesion formation: molecular mimicry between Streptococcus pneumoniae and oxidized LDL. Nat Med 2003;9:736–743. [DOI] [PubMed] [Google Scholar]
- 22. Chou MY, Fogelstrand L, Hartvigsen K, Hansen LF, Woelkers D, Shaw PX, Choi J, Perkmann T, Bäckhed F, Miller YI, Hörkkö S, Corr M, Witztum JL, Binder CJ. Oxidation-specific epitopes are dominant targets of innate natural antibodies in mice and humans. J Clin Invest 2009;119:1335–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhao TX, Aetesam-Ur-Rahman M, Sage AP, Victor S, Kurian R, Fielding S, Ait-Oufella H, Chiu Y-D, Binder CJ, Mckie M, Hoole SP, Mallat Z. Rituximab in patients with acute ST-elevation myocardial infarction: an experimental medicine safety study. Cardiovasc Res 2022;118:872–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Song L, Leung C, Schindler C. Lymphocytes are important in early atherosclerosis. J Clin Invest 2001;108:251–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bosco N, Swee LK, Bénard A, Ceredig R, Rolink A. Auto-reconstitution of the T-cell compartment by radioresistant hematopoietic cells following lethal irradiation and bone marrow transplantation. Exp Hematol 2010;38:222–232.e2. [DOI] [PubMed] [Google Scholar]
- 26. Linterman MA, Beaton L, Yu D, Ramiscal RR, Srivastava M, Hogan JJ, Verma NK, Smyth MJ, Rigby RJ, Vinuesa CG. IL-21 acts directly on B cells to regulate Bcl-6 expression and germinal center responses. J Exp Med 2010;207:353–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cerutti A, Cols M, Puga I. Marginal zone B cells: virtues of innate-like antibody-producing lymphocytes. Nat Rev Immunol 2013;13:118–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Iwakoshi NN, Lee A-H, Vallabhajosyula P, Otipoby KL, Rajewsky K, Glimcher LH. Plasma cell differentiation and the unfolded protein response intersect at the transcription factor XBP-1. Nat Immunol 2003;4:321–329. [DOI] [PubMed] [Google Scholar]
- 29. Oka C, Nakano T, Wakeham A, de la Pompa JL, Mori C, Sakai T, Okazaki S, Kawaichi M, Shiota K, Mak TW, Honjo T. Disruption of the mouse RBP-J kappa gene results in early embryonic death. Development 1995;121:3291–3301. [DOI] [PubMed] [Google Scholar]
- 30. Weller S, Braun MC, Tan BK, Rosenwald A, Cordier C, Conley ME, Plebani A, Kumararatne DS, Bonnet D, Tournilhac O, Tchernia G, Steiniger B, Staudt LM, Casanova J-L, Reynaud C-A, Weill J-C. Human blood IgM ‘memory’ B cells are circulating splenic marginal zone B cells harboring a prediversified immunoglobulin repertoire. Blood 2004;104:3647–3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tangye SG, Ma CS, Brink R, Deenick EK. The good, the bad and the ugly—TFH cells in human health and disease. Nat Rev Immunol 2013;13:412–426. [DOI] [PubMed] [Google Scholar]
- 32. Walker LSK. The link between circulating follicular helper T cells and autoimmunity. Nat Rev Immunol 2022;22:567–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ma X, Nakayamada S. Multi-source pathways of T follicular helper cell differentiation. Front Immunol 2021;12:621105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Seth A, Craft J. Spatial and functional heterogeneity of follicular helper T cells in autoimmunity. Curr Opin Immunol 2019;61:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Oliver AM, Martin F, Gartland GL, Carter RH, Kearney JF. Marginal zone B cells exhibit unique activation, proliferative and immunoglobulin secretory responses. Eur J Immunol 1997;27:2366–2374. [DOI] [PubMed] [Google Scholar]
- 36. Appelgren D, Eriksson P, Ernerudh J, Segelmark M. Marginal-zone B-cells are main producers of IgM in humans, and are reduced in patients with autoimmune vasculitis. Front Immunol 2018:9:2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tsiantoulas D, Bot I, Ozsvar-Kozma M, Goderle L, Perkmann T, Hartvigsen K, Conrad DH, Kuiper J, Mallat Z, Binder CJ. Increased plasma IgE accelerate atherosclerosis in secreted IgM deficiency. Circ Res 2017;120:78–84. [DOI] [PubMed] [Google Scholar]
- 38. van den Berg VJ, Vroegindewey MM, Kardys I, Boersma E, Haskard D, Hartley A, Khamis R. Anti-oxidized LDL antibodies and coronary artery disease: a systematic review. Antioxidants (Basel) 2019;8:484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Que X, Hung M-Y, Yeang C, Gonen A, Prohaska TA, Sun X, Diehl C, Määttä A, Gaddis DE, Bowden K, Pattison J, MacDonald JG, Ylä-Herttuala S, Mellon PL, Hedrick CC, Ley K, Miller YI, Glass CK, Peterson KL, Binder CJ, Tsimikas S, Witztum JL. Oxidized phospholipids are proinflammatory and proatherogenic in hypercholesterolaemic mice. Nature 2018;558:301–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kyaw T, Tay C, Krishnamurthi S, Kanellakis P, Agrotis A, Tipping P, Bobik A, Toh BH. B1a B lymphocytes are atheroprotective by secreting natural IgM that increases IgM deposits and reduces necrotic cores in atherosclerotic lesions. Circ Res 2011;109:830–840. [DOI] [PubMed] [Google Scholar]
- 41. Rosenfeld SM, Perry HM, Gonen A, Prohaska TA, Srikakulapu P, Grewal S, Das D, McSkimming C, Taylor AM, Tsimikas S, Bender TP, Witztum JL, McNamara CA. B-1b cells secrete atheroprotective IgM and attenuate atherosclerosis. Circ Res 2015;117:e28–e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang Y, Liu Z, Wu J, Li F, Li G, Dong N. Profiling circulating T follicular helper cells and their effects on B cells in post-cardiac transplant recipients. Ann Transl Med 2020;8:1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ghamar Talepoor A, Khosropanah S, Doroudchi M. Functional subsets of circulating follicular helper T cells in patients with atherosclerosis. Physiol Rep 2020;8:e14637. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Most of the data underlying this work are included in the manuscript and Supplementary material. The rest of the data will be shared by the corresponding author upon reasonable request.