Abstract
Objective
Effective treatment options are available for chronic venous insufficiency associated with superficial venous reflux. Although many patients with C2 and C3 disease based on the CEAP (Clinical-Etiological-Anatomical-Pathophysiological) classification have combined great saphenous vein (GSV) and saphenofemoral junction (SFJ) reflux, some may not have concomitant SFJ reflux. Several payors have determined that symptom severity in patients without SFJ reflux does not warrant treatment. In patients planned for venous ablation, we tested whether Venous Clinical Severity Scores (VCSS) are equivalent in those with GSV reflux alone compared with those with both GSV and SFJ reflux.
Methods
This cross-sectional study was conducted at 10 centers. Inclusion criteria were: candidate for endovenous ablation as determined by treating physician; 18 to 80 years of age; GSV reflux with or without SFJ reflux on ultrasound; and C2 or C3 disease. Exclusion criteria were prior deep vein thrombosis; prior vein ablation on the index limb; ilio-caval obstruction; and renal, hepatic, or heart failure requiring prior hospitalization. An a priori sample size was calculated. We used multiple linear regression (adjusted for patient characteristics) to compare differences in VCSS scores of the two groups at baseline, and to test whether scores were equivalent using a priori equivalence boundaries of +1 and −1. In secondary analyses, we tested differences in VCSS scores in patients with C2 and C3 disease separately.
Results
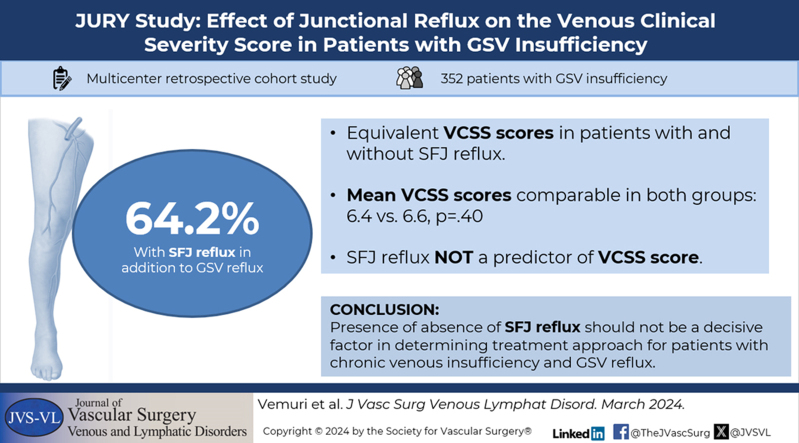
A total of 352 patients were enrolled; 64.2% (n = 226) had SFJ reflux, and 35.8% (n = 126) did not. The two groups did not differ by major clinical characteristics. The mean age of the cohort was 53.9 ± 14.3 years; women comprised 74.2%; White patients 85.8%; and body mass index was 27.8 ± 6.1 kg/m2. The VCSS scores in patients with and without SFJ reflux were found to be equivalent; SFJ reflux was not a significant predictor of VCSS score; and mean VCSS scores did not differ significantly (6.4 vs 6.6, respectively, P = .40). In secondary subset analyses, VCSS scores were equivalent between C2 patients with and without SFJ reflux, and VCSS scores of C3 patients with SFJ reflux were lower than those without SFJ reflux.
Conclusions
Symptom severity is equivalent in patients with GSV reflux with or without SFJ reflux. The absence of SFJ reflux alone should not determine the treatment paradigm in patients with symptomatic chronic venous insufficiency. Patients with GSV reflux who meet clinical criteria for treatment should have equivalent treatment regardless of whether or not they have SFJ reflux.
Keywords: Ablation, Great saphenous vein, Reflux, Saphenofemoral junction, Venous
Graphical abstract
Article Highlights.
-
•
Type of Research: Multicenter cohort study
-
•
Key Findings: The study found that there was no significant difference in Venous Clinical Severity Scores (VCSS) between patients with and without saphenofemoral junction (SFJ) reflux. SFJ reflux did not predict VCSS scores, and the mean VCSS scores were comparable in both groups (6.4 vs 6.6; P = .40).
-
•
Take Home Message: The presence or absence of SFJ reflux should not be a decisive factor in determining the treatment approach for patients with chronic venous insufficiency and great saphenous vein reflux. Patients with great saphenous vein reflux who meet clinical criteria for treatment should have equivalent treatment regardless of whether or not they have SFJ reflux.
Chronic venous insufficiency (CVI) affects more than 25 million people in the United States (U.S.), and approximately 6 million present with advanced disease including ulcerations. CVI has a significant impact on quality of life and on health care costs, with an estimated $3 billion spent in the U.S. per year.1, 2, 3, 4
Safe and effective treatment options are currently available for CVI associated with superficial venous reflux. Venous ablation is indicated in patients with severe disease or in those with persistent symptoms after a course of conservative therapy, the duration of which is typically set by each state or region’s insurance carriers. Although many patients with symptomatic C2 and C3 disease based on the CEAP (Clinical-Etiological-Anatomical-Pathophysiological) classification have great saphenous vein (GSV) reflux with involvement of the saphenofemoral junction (SFJ), some patients may, in fact, not have concomitant SFJ reflux. Although GSV reflux has been an inclusion criterion in most clinical trials that examine the efficacy of venous ablation, concomitant SFJ reflux was not typically a prerequisite for enrollment and was not recorded routinely. Therefore, information is limited on symptom severity in this subset of patients with GSV but without SFJ reflux. As a result, several insurance agencies have determined that the symptom severity of patients without SFJ reflux does not rise to the level of requiring or receiving treatment.5
The historical belief that SFJ reflux is the cause of CVI is based on the antegrade theory of venous reflux. The theory presumes that loss of valve function at the SFJ creates venous hypertension that is transmitted to valves below the junction, which in turn, causes further progressively distal valve dysfunction.6 This theory has been challenged by numerous investigations. Labropoulos et al have demonstrated that the most common location for the development of GSV reflux is in the below-the-knee segment of the GSV.7 Additional research has found that superficial venous disease is secondary to degradative vein wall processes, resulting in valvular dysfunction and not secondary to venous hypertension-induced valvular reflux.4 Therefore, provided the patient has severe enough CVI, the requirement of SFJ reflux as a pre-requisite for GSV ablation is not based on current physiologic evidence.
The aim of our study was to test whether the scores obtained from a metric of the signs and symptoms of CVI, the modified Venous Clinical Severity Score (VCSS),8 are equivalent in patients who have GSV reflux alone, compared with patients who have both GSV and SFJ reflux.
Methods
Study design and setting
We performed a cross-sectional study of patients undergoing GSV ablation at 10 participating centers (Englewood Medical Center, NJ; Center for Vein Restoration, NJ; New York University Langone Health, NY; Mount Sinai, NY; Stony Brook Medicine, NY; University of Michigan, MI; Lake Washington Vascular, Bellevue, WA; Northwell Health, NY; McLaren Health, MI; and Washington University, MO). This manuscript was prepared based on STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) reporting standards.9 Sponsors did not have access to the data, and all analyses were performed independently at the Center for Vascular Research of the University of Maryland. Our study was approved by the Institutional Review Boards of the University of Maryland and of the participating clinical centers.
Participants
To be eligible for the study, patients had to meet 4 inclusion criteria: (1) being 18 to 80 years of age; (2) having reflux (≥0.5 seconds) in the GSV (demonstrated by ultrasound in at least two contiguous segments above the knee) with or without reflux at the SFJ (demonstrated by ultrasound); (3) being considered candidates for endovascular treatment of the GSV by the treating physician; and (4) having varicose veins or edema (defined as a C2 or C3 classification on the CEAP clinical scoring system). Exclusion criteria were: (1) history of, or stigmata on ultrasound of, deep vein thrombosis (DVT); (2) prior vein ablation on the index lower extremity; (3) concomitant obstruction of the ilio-caval system; and (4) known end-stage renal disease, or hepatic disease, or congestive heart failure requiring prior hospitalization. A web-based data capture system was used by the centers to enter study data directly into electronic case report forms. In patients with bilateral disease, the leg being evaluated for potential treatment was enrolled, and if both were being evaluated for treatment, only one leg with the more severe C class was enrolled.
Variables
Our primary outcome measure was the difference in modified VCSS scores between the two groups of patients—those with GSV and SFJ reflux vs those with GSV reflux alone—after adjusting for potential confounders. The modified VCSS evaluates 10 hallmarks of chronic venous disease, each on a scale of 0 to 3: pain (none, occasional, daily, daily limiting); varicose veins (none, few, calf or thigh, calf and thigh); venous edema (none, foot and ankle, above ankle below knee, to the knee or above); skin hyperpigmentation (none, perimalleolar, diffuse lower 1/3 of calf, wider above lower 1/3 of calf); inflammation (none, perimalleolar, diffuse, lower 1/3 or calf, wider above lower 1/3 of calf); induration; number of ulcers (none, 1 ulcer, 2, 3); size of ulcers (not applicable = 0, diameter less than 2 centimeter = 1, diameter 2 to 6 centimeter = 2, diameter greater than 6 centimeter = 3); durations of ulcers (not applicable = 0, less than 3 months = 1, greater than 3 months but less than 1 year = 2, Not healed for greater than 1 year = 3); and compliance with compression therapy (none, intermittent, most days, fully compliant). Possible VCSS scores range from 0 to 30.8
Other data collected included age (in years), sex, race (American Indian or Alaska Native, Asian, Black or African American, Native Hawaiian or Other Pacific Islander, White, and multiracial), ethnicity (Hispanic or Latino and not Hispanic or Latino), body mass index (BMI, kg/m2), largest GSV vein diameter (mm), and Chronic Venous Insufficiency Quality of Life Questionnaire (CIVIQ) score.
Sample size estimations
We performed an a priori sample size calculation before any data were collected. We calculated sample size for a two-sample equivalence test of VCSS scores using the Two-One-Sided t-Tests (TOST) procedure with an equivalence margin of +1 or −1, 80% power, and 95% significance. Passman et al reported a mean total VCSS score of 2.83 (standard deviation [SD], 0.47) among 2907 patients.10 To be conservative, we used SD = 1 in our sample size estimates. The estimated sample size was 300 patients. After enrolling 100 patients, we calculated the mean VCSS score and its SDs in our cohort (without unblinding the statisticians to group identity) and recalculated sample sizes. Based on the new sample size estimates, we adjusted our target enrollment to 340.
Statistical methods
Comparison of characteristics of the two groups was performed using the Pearson χ2 or the Fisher exact test for categorical data, and the Student t-tests or Wilcoxon Rank Sum tests for continuous data. Comparisons of mean scores of individual components of the VCSS between the two groups are presented as histograms.
We first computed crude differences in VCSS scores between the two groups (those with SFJ reflux vs those without SFJ reflux). We next used multiple linear regression (adjusted for group, age, sex, race [non-white vs white], ethnicity [Hispanic vs non-Hispanic], and BMI) to generate adjusted difference of VCSS scores in our two groups. Graphical displays of the estimates of the difference between the two groups and their 95% confidence intervals were used to determine if the differences in mean VCSS scores were equivalent. VCSS scores from patients with and without SFJ reflux were considered equivalent if the 95% confidence limit of the primary outcome (the difference in VCSS score in patients with vs without SFJ reflux) did not include the a priori equivalence boundaries of +1 and −1. In two separate secondary analyses, we computed crude and adjusted differences in VCSS scores between patients with vs without SFJ reflux in those with C2 disease and in those with C3 disease.
All statistical analyses were two-tailed. A P-value of < .05 was used to denote significance. Statistical analyses were performed using SAS version 9.4 (SAS Institute Inc) and were repeated in R version 4.2.2 (R Core Team [2023]) by two distinct statisticians. Results were compared to ensure reliability and reproducibility of the results.
Results
Participants
A total of 352 patients were enrolled in the study. Their ablation procedures were performed between January 1, 2015, and December 31, 2022. The number of patients enrolled by site was: Center for Vein Restoration, n = 56; NYU Langone Health, n = 49; Mount Sinai, n = 11; Stony Brook Medicine, n = 42; University of Michigan, n = 28; Lake Washington Vascular, n = 115; Northwell Health, n = 41; and McLaren Health, n = 10. Within the cohort, 64.2% (n = 226) had SFJ reflux, and 35.8% (n = 126) did not have SFJ reflux.
Descriptive data
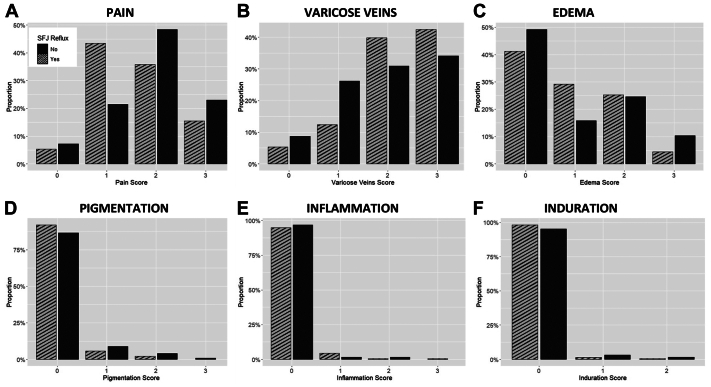
Clinical characteristics of the cohort, and by group, are presented in Table I. The mean age of the patients was 53.9 ± 14.3 years. There was no difference in age between the two groups (P = .18). The population was composed largely of women (74.2%; n = 262). The sex distribution did not differ by group (P = .84). White patients comprised 85.8% (n = 302) of the cohort. Distribution by race did not differ by reflux group (P = .55). Among the patients with SFJ reflux, 93.3% (n = 209) were not Hispanic or Latino compared with 75.6% (n = 93) patients without SFJ reflux (P < .001). There was no difference in BMI between the groups, (28.1 vs 27.9 kg/m2; P < .10). The mean VCSS scores of patients with vs without SFJ reflux also did not differ significantly (6.4 vs 6.6, respectively; P = .40). The scores for individual VCSS questions were generally similarly distributed between groups or followed no specific pattern of distribution (Fig 1). The two exceptions were: a larger proportion of patients with SFJ reflux scored higher for varicose veins, whereas a larger proportion of patients without SFJ reflux scored higher for pain.
Table I.
Characteristics of patients
| Variables | All (N = 352) | SFJ reflux, yes (n = 226; 64.2%) | SFJ reflux, no (n = 126; 35.8%) | P value |
|---|---|---|---|---|
| Age, years | 53.9 (14.3) | 53.1 (14.4) | 55.3 (14.1) | .184 |
| Sex | .842 | |||
| Female | 262 (74.2) | 169 (74.8) | 93 (73.8) | |
| Male | 90 (25.5) | 57 (25.2) | 33 (26.2) | |
| Race | .548 | |||
| White | 302 (85.8) | 191 (84.9) | 111 (88.8) | |
| Black | 15 (4.3) | 10 (14.4) | 5 (4.0) | |
| Other race | 33 (9.3) | 24 (10.7) | 9 (7.2) | |
| Ethnicity | <.001 | |||
| Hispanic or Latino | 45 (12.8) | 15 (6.7) | 30 (24.4) | |
| Not Hispanic or Latino | 302 (85.8) | 209 (93.3) | 93 (75.6) | |
| BMI, kg/m2 | 27.8 (6.1) | 28.1 (6.0) | 27.3 (6.3) | .094 |
| VCSS score | 6.5 (2.4) | 6.4 (2.1) | 6.6 (2.7) | .397 |
BMI, Body mass index; SFJ, saphenofemoral junction; VCSS, venous clinical severity scores.
Data are presented as number (%) or mean (standard deviation).
Fig 1.
Distribution of scores obtained on individual components of the venous clinical severity score (VCSS) among patients undergoing venous ablation with isolated reflux in the great saphenous vein (GSV) vs those with combined reflux in the GSV and the saphenofemoral junction (SFJ). A, Pain; B, varicose veins; C, edema; D, pigmentation; E, inflammation; and F, induration scores. Note: The scores for pain, varicose veins, edema, pigmentation, and inflammation range from 0 to 3, while the scores for induration range from 0 to 2.
Primary outcome
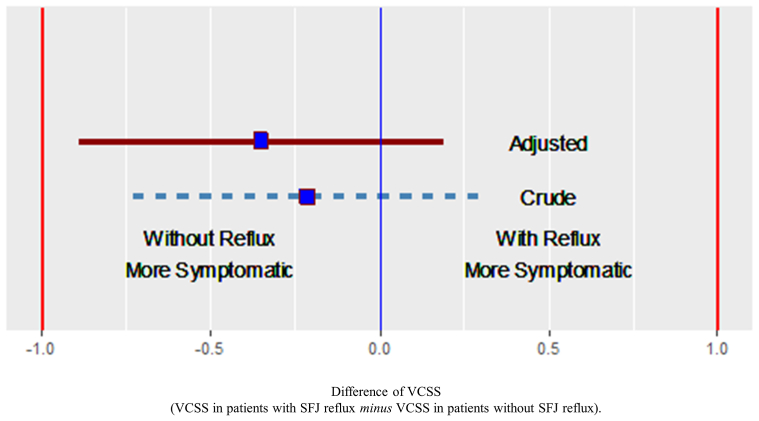
In the entire cohort, SFJ reflux was not a significant predictor of VCSS score in crude unadjusted analyses (β ± SE, −0.21 ± 0.26; P = .42) or in analyses adjusted for age, sex, race, ethnicity, and BMI (0.35 ± 0.28; P = .20) (Table II). In crude and adjusted analyses, using −1 and +1 as equivalence boundaries, the VCSS scores in patients with and without SFJ reflux were found to be equivalent (Fig 2).
Table II.
Difference in Venous Clinical Severity Scores (VCSS) between patients with combined reflux in the great saphenous vein (GSV) and the saphenofemoral junction (SFJ) vs those with isolated reflux in the GSV
| Analysis | Number of patients | Difference in VCSS (with GSV + SFJ reflux vs GSV reflux alone) |
95% confidence interval |
P-value | Interpretation |
|||
|---|---|---|---|---|---|---|---|---|
| Difference | SE | Low | High | Differencec | Equivalentd | |||
| Crudea | 352 | −0.21 | 0.26 | −0.73 | 0.30 | .42 | Not significant | Yes |
| Adjustedb | 346 | −0.35 | 0.28 | −0.89 | 0.19 | .20 | Not significant | Yes |
Crude: VCSS score = f(SFJ reflux).
Adjusted: VCSS score = f(SFJ reflux, age, sex, race, ethnicity, body mass index).
A difference is significant if the confidence interval excludes 0 (ie, P < .05).
A difference is equivalent if the 95% confidence interval excludes both −1 and +1.
Fig 2.
Difference in venous clinical severity scores (VCSS) between patients with combined reflux in the great saphenous vein (GSV) and the saphenofemoral junction (SFJ) vs those with isolated reflux in the GSV. The boxes are the mean scores; the lines delimit the 95% confidence interval around the mean scores. Note: adjusted analysis included adjustments for age, sex, race, ethnicity, and body mass index (BMI).
Secondary analyses
In subset analyses of our cohort, the mean VCSS score in patients with C2 disease was 5.57 ± 2.11 (no SFJ reflux, 5.77 ± 2.43 and with SFJ reflux, 5.41 ± 1.78). SFJ reflux was not a significant predictor of VCSS score (−0.55 ± 0.33; P = .10). After adjusting for age, sex, race, ethnicity, and BMI, and using the same −1 and +1 as equivalence boundaries, the VCSS scores were equivalent between C2 patients with and without SFJ reflux.
In another subset analysis, the mean VCSS score in patients with C3 disease was 7.50 ± 2.24 (no SFJ reflux, 8.43 ± 2.44 and with SFJ reflux, 7.20 ± 2.09). SFJ reflux was a predictor of VCSS score in patients with C3 disease (−1.27 ± 0.40; P < .002). In an adjusted analysis, using −1 and +1 as equivalence boundaries, the VCSS scores of C3 patients with SFJ reflux were not equivalent to those without reflux, and were, in fact lower than those without SFJ reflux.
Discussion
The JURY (Effect of JUnctional Reflux on the Venous Clinical SeveritY Score) study found that in patients with GSV reflux selected for vein ablation procedures, VCSS scores were equivalent between those with and those without accompanying SFJ reflux. A partnership with the American Venous Forum ensured the participation of a broad variety of high-volume, high-quality, clinical centers in this effort. This approach permitted the efficient collection of essential data such as the CEAP scores, VCSS, and detailed ultrasound study reports in a real-world setting to achieve appropriate sample size and power. Therefore, patients with C2 or C3 CVI (with GSV reflux with or without SFJ reflux) have similar disease severity, and one group (those without SFJ reflux) must not be denied consideration for ablative treatment based solely on this difference.
The characteristics of the 352 patients enrolled in our study were consistent with patients typically seen in high-volume venous practices, and included an overall mean age of 53.9 years, 74.2% females, 85.8% Caucasian, and a mean BMI of 27.8 kg/m2. The enrolled patient population was consistent with other large studies evaluating early CEAP-stage CVI and venous ablation procedures. In one representative study, the mean age of participants was 52.4 years, 75.6% were female, >90% were Caucasian, and mean BMI was 27.7 kg/m2.11 In our study, a comparison of the 126 patients without SFJ reflux and 226 patients with SFJ reflux showed that the mean VCSS score of patients without SFJ reflux was 6.6 ± 2.7 and not significantly different from those with SFJ reflux (6.4 ± 2.1; P = .40). Our VCSS scores were also consistent with pre-procedural scores of patients with early stage CVI planned for endovenous ablation in other studies. Brown et al reported a median VCSS score of 7 (interquartile range [IQR], 6-10)11; Shaidakov et al reported a median of 5.94 (IQR, 6.25)12; and Conway et al reported a mean of 5.38 (SD, 2.04).13
Our findings are reliable. Previous studies have found no statistical difference in VCSS scores between patients with and without SFJ reflux. However, the absence of a statistical difference alone does not confirm the absence of a difference, because the finding may also be a function of an inadequate sample size and power (type II error). We therefore determined our sample size a priori to ensure that our analyses had appropriate power. Our results are clinically relevant. We used an equivalence analysis guided by clinically informed yet stringent VCSS score boundaries. This ensured that our results identify as equivalent patients that are viewed as having equivalent disease severity in clinical practice. Our results are generalizable. We recruited from multiple clinical centers, located in different geographical locations within the U.S., and receiving treatment at a mixture of academic- and community-based venous practices. Our sample size and analysis accommodated the variance expected from observations made at multiple clinical centers as opposed to reports from single-center experiences. Finally, our results have a reduced probability of being confounded by differences in patient characteristics among groups. We planned our sample size to permit analyses adjusted for common sources of confounding such as age, sex, BMI, and race/ethnicity; and excluded patients with prior DVT, severe medical comorbidities, or venous ablation in the index limb.
The correlation between VCSS scores and the CEAP classification has been reported to be modest at best. In one of the largest epidemiologic studies conducted to evaluate this relationship, 5814 limbs in 2907 screening participants demonstrated a mean VCSS score of 2.83 (SD, 0.47) and mean C score of 1.4 (SD, 1.2) with only moderate correlation between them (rs = 0.49). Venous reflux has been found to occur in fewer segments in patients with early C stage, compared with higher C stage, CVI. In one report segmental reflux was more common in C0-3 disease, whereas SFJ and more proximal reflux was more common in C4-6 disease.14 In our study, VCSS scores in C2 patients were numerically lower, and in C3 patients were statistically lower in those with SFJ reflux compared with those without SFJ reflux. Our results confirm that disease severity is not necessarily driven by the presence or absence of SFJ reflux.
Multi-society appropriate use criteria recommend venous ablation in patients with symptomatic CVI and truncal incompetence to improve their symptoms and quality of life without consideration for the presence or absence of SFJ reflux as a requirement.15 The results of our study provide real-world evidence to support that recommendation. Payor restrictions on treatment coverage contingent on inappropriate anatomic criteria are barriers to access to appropriate care in symptomatic patients. These restrictions are without merit and are not based on clinical evidence. In a study of 1882 limbs with varicose veins, 29.4% of limbs with GSV reflux had a competent terminal valve.16 Therefore, such restrictions are potentially preventing almost one-third of patients with CVI from receiving appropriate care. The results of this investigation negate the erroneous presumption that treatment must be guided by the presence or absence of SFJ reflux. The results of this work must encourage changes in coverage policies that allow more equitable care for patients with venous disease.
The JURY study demonstrates how an involved academic society can engage with its membership to form an effective team to address the needs of the patients they serve in a real-world setting. Members of major academic societies offer expertise, knowledge, and access to patients through their active clinics. Academic societies have the ability to empower their membership by providing the requisite infrastructure to initiate and accomplish clinical investigations in real-world settings. This study may provide a roadmap for future larger collaborative intra- and inter-societal endeavors to create high-quality data that will inform and support best practices in the care of patients with venous disease.
Limitations
The study recruited some patients through a retrospective chart review and some prospectively. Therefore, the data are subject to a selection bias. This bias was minimized by systematically collecting information on all consecutive patients for whom complete information was available on their clinical features, inclusion-exclusion criteria, C score, and VCSS scores. The diagnosis of the location of reflux was made based on ultrasound testing performed at individual clinical sites and could be subject to interoperator variability. However, the goal of the study was to analyze real-world data, and hence, it reflects actual clinical practices in busy venous clinics. The determination of the modified VCSS score can be rater-dependent. However, the variance of our scores was not different, and certainly not larger than those reported from single centers or from large clinical trials. A majority of patients had C2 disease, and few clinical comorbidities were noted in our cohort. This, again, is a reflection of the relatively lower age of patients presenting with venous disease as compared with arterial disease in general clinical practices. A more focused evaluation in patients with more advanced venous disease and/or those with significant comorbidities may help to characterize the importance of SFJ reflux in this smaller group of patients. Other limitations may be that we did not require the specific reflux time to be recorded. We did require it to be >500 ms, based on data showing no correlation between reflux time and the VCSS.17 Further, because we did not require vein diameter to be entered, we do not have complete data to be able to perform a reliable statistical analysis. We think these data could be important when considering treatment effect, although literature suggests there is not a strong correlation between size and VCSS.18 A difference in mean GSV diameter has been shown in those patients with and without reflux,19 but as all our patients had reflux, we did not require those data for this study. Finally, the study was designed to assess differences in clinical presentation by VCSS. Another study is being planned with post-procedural mid- and long-term follow-up, which will help assess treatment efficacy in both groups of patients.
Conclusions
This multi-center study confirms that symptom severity is equivalent in patients with GSV reflux with or without SFJ reflux. VCSS scores were equivalent in both crude and adjusted analyses of the entire cohort and in patients with C2 disease. Interestingly, in patients with C3 disease, VCSS scores of patients with SFJ reflux were significantly lower than those without SFJ reflux. The absence of SFJ reflux alone should not determine the treatment paradigm in patients with symptomatic CVI. Patients with GSV reflux who meet clinical criteria for treatment should have equivalent treatment coverage regardless of whether or not they have SFJ reflux.
Author Contributions
Conception and design: CV, BL
Analysis and interpretation: CV, KG, PP, MS, WT, AO, NM, YE, AG, TM, SS, JS, BL
Data collection: CV, KG, PP, MS, WT, AO, NM, YE, AG
Writing the article: CV, BL
Critical revision of the article: KG, PP, MS, WT, AO, NM, YE, AG, TM, SS, JS, BL
Final approval of the article: CV, KG, PP, MS, WT, AO, NM, YE, AG, TM, SS, JS, BL
Statistical analysis: SS, JS
Obtained funding: BL
Overall responsibility: BL
Disclosures
K.D.G. reports advisory board and research grant recipient for Medtronic and Boston Scientific. M.S. reports advisory board for Boston Scientific. W.T. reports consultant and research grant recipient for Boston Scientific. A.T.O. reports Medtronic global advisory board; and Medtronic and Surmodics PI preclinical studies. N.J.M. reports consultant for Inari Medical and Boston Scientific. Y.E. report consultant for Cook and member of Boston Scientific venous medical advisory board. A.P.G. reports consultant/speaker for: Boston Scientific, Medtronic, Bard, Tactile Medical, and Philips.
Footnotes
Funding: The study sponsor is the American Venous Forum (AVF) with funding through a research grant from Boston Scientific. The funding source had no access to data, and was not involved in collecting, monitoring, or analyzing study data. Investigators also had funding from the National Institutes of Health (NIH) Awards NS080168, NS097876 and AG000513 and Veterans Affairs AwardsHSRDC19-20-407, RRDRX000995 and CSRDCX001621 (B.K.L.); NIH Award AG028747 and Baltimore VA Medical Centre GRECC (J.D.S.).
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
Appendix
The CME exam for this article can be accessed athttp://www.jvsvenous.org/cme/home.
References
- 1.Rabe E., Guex J.J., Puskas A., Scuderi A., Fernandez Quesada F., VCP Coordinators Epidemiology of chronic venous disorders in geographically diverse populations: results from the Vein Consult Program. Int Angiol. 2012;31:105–115. [PubMed] [Google Scholar]
- 2.Beebe-Dimmer J.L., Pfeifer J.R., Engle J.S., Schottenfeld D. The epidemiology of chronic venous insufficiency and varicose veins. Ann Epidemiol. 2005;15:175–184. doi: 10.1016/j.annepidem.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 3.Kim Y., Png C.Y.M., Sumpio B.J., DeCarlo C.S., Dua A. Defining the human and health care costs of chronic venous insufficiency. Semin Vasc Surg. 2021;34:59–64. doi: 10.1053/j.semvascsurg.2021.02.007. [DOI] [PubMed] [Google Scholar]
- 4.Eberhardt R.T., Raffetto J.D. Chronic venous insufficiency. Circulation. 2014;130:333–346. doi: 10.1161/CIRCULATIONAHA.113.006898. [DOI] [PubMed] [Google Scholar]
- 5.Welch H.J., Schul M.W., Monahan D.L., Iafrati M.D., Health Policy Committees of the American Venous Forum and the American Venous and Lymphatic Society Private payers' varicose vein policies are inaccurate, disparate, and not evidence based, which mandates a proposal for a reasonable and responsible policy for the treatment of venous disease. J Vasc Surg Venous Lymphat Disord. 2021;9:820–832. doi: 10.1016/j.jvsv.2020.12.076. [DOI] [PubMed] [Google Scholar]
- 6.Kistner R.L. Primary venous valve incompetence of the leg. Am J Surg. 1980;140:218–224. doi: 10.1016/0002-9610(80)90010-0. [DOI] [PubMed] [Google Scholar]
- 7.Labropoulos N., Leon M., Nicolaides A.N., Giannoukas A.D., Volteas N., Chan P. Superficial venous insufficiency: correlation of anatomic extent of reflux with clinical symptoms and signs. J Vasc Surg. 1994;20:953–958. doi: 10.1016/0741-5214(94)90233-x. [DOI] [PubMed] [Google Scholar]
- 8.Vasquez M.A., Rabe E., McLafferty R.B., Shortell C.K., Marston W.A., Gillespie D., et al. American Venous Forum Ad Hoc Outcomes Working Group Revision of the venous clinical severity score: venous outcomes consensus statement: special communication of the American venous Forum Ad Hoc outcomes working group. J Vasc Surg. 2010;52:1387–1396. doi: 10.1016/j.jvs.2010.06.161. [DOI] [PubMed] [Google Scholar]
- 9.Ghaferi A.A., Schwartz T.A., Pawlik T.M. STROBE reporting guidelines for observational studies. JAMA Surg. 2021;156:577–578. doi: 10.1001/jamasurg.2021.0528. [DOI] [PubMed] [Google Scholar]
- 10.Passman M.A., McLafferty R.B., Lentz M.F., Nagre S.B., Iafrati M.D., Bohannon W.T., et al. Validation of venous clinical severity score (VCSS) with other venous severity assessment tools from the American venous Forum, national venous screening program. J Vasc Surg. 2011;54(6 Suppl):2S–9S. doi: 10.1016/j.jvs.2011.05.117. [DOI] [PubMed] [Google Scholar]
- 11.Brown C.S., Osborne N.H., Kim G.Y., Sutzko D.C., Wakefield T.W., Obi A.T., et al. Effect of concomitant deep venous reflux on truncal endovenous ablation outcomes in the Vascular Quality Initiative. J Vasc Surg Venous Lymphat Disord. 2021;9:361–368.e3. doi: 10.1016/j.jvsv.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shaidakov E.V., Grigoryan A.G., Ilyukhin E.A., Bulatov V.L., Rosukhovskiy D.A. Radiofrequency ablation or stripping of large-diameter incompetent great saphenous varicose veins with C2 or C3 disease. J Vasc Surg Venous Lymphat Disord. 2016;4:45–50. doi: 10.1016/j.jvsv.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 13.Conway R.G., Almeida J.I., Kabnick L., Wakefield T.W., Buchwald A.G., Lal B.K. Clinical response to combination therapy in the treatment of varicose veins. J Vasc Surg Venous Lymphat Disord. 2020;8:216–223. doi: 10.1016/j.jvsv.2019.10.015. [DOI] [PubMed] [Google Scholar]
- 14.Engelhorn C.A., Engelhorn A.L., Cassou M.F., Zanoni C.C., Gosalan C.J., Ribas E. Classificação anátomo-funcional da insuficiência das veias safenas baseada no eco-Doppler colorido, dirigida para o planejamento da cirurgia de varizes. J Vasc Bras. 2004;3:13–19. [Google Scholar]
- 15.Masuda E., Ozsvath K., Vossler J., Woo K., Kistner R., Lurie F., et al. The 2020 appropriate use criteria for chronic lower extremity venous disease of the American venous Forum, the society for vascular surgery, the American vein and lymphatic society, and the society of interventional radiology. J Vasc Surg Venous Lymphat Disord. 2020;8:505–525.e4. doi: 10.1016/j.jvsv.2020.02.001. [DOI] [PubMed] [Google Scholar]
- 16.Chastanet S., Pittaluga P. Patterns of reflux in the great saphenous vein system. Phlebology. 2013;28(Suppl 1):39–46. doi: 10.1177/0268355513477021. [DOI] [PubMed] [Google Scholar]
- 17.Kokkinidis D.G., Ochoa Chaar C.I., Mena-Hurtado C.I., Attaran R.R. Correlation between reflux time and venous clinical severity score in patients undergoing saphenous vein ablation: a prospective study. Phlebology. 2023;38:62–66. doi: 10.1177/02683555221146730. [DOI] [PubMed] [Google Scholar]
- 18.Attaran R.R., Bhalla A., Mena-Hurtado C.I., Ochoa Chaar C.I. Correlation between great saphenous length of treatment zone and diameter with improvement in symptoms after ablation. J Vasc Surg Venous Lymphat Disord. 2021;9:1443–1450. doi: 10.1016/j.jvsv.2021.02.013. [DOI] [PubMed] [Google Scholar]
- 19.Myoung J.K., Pyeong J.P., Bum H.K., Seung G.L., Geon Y.B., Sung R.L. Association between venous reflux and diameter of great saphenous vein in lower thigh. J Vasc Surg Venous Lymphat Disord. 2020;8:100–105. doi: 10.1016/j.jvsv.2019.04.016. [DOI] [PubMed] [Google Scholar]