Abstract
Background:
Most genetic studies of asthma and allergy have focused on common variation in individuals primarily of European ancestry. Studying the role of rare variation in quantitative phenotypes and in asthma phenotypes in populations of diverse ancestries can provide additional, important insights into the development of these traits. Objective: We sought to examine the contribution of rare variants to different asthma- or allergy-associated quantitative traits in children with diverse ancestries and explore their role in asthma phenotypes.
Methods:
We examined whole-genome sequencing data from children participants in longitudinal studies of asthma (n = 1035; parent-identified as 67% Black and 25% Hispanic) to identify rare variants (minor allele frequency < 0.01). We assigned variants to genes and tested for associations using an omnibus variant-set test between each of 24,902 genes and 8 asthma-associated quantitative traits. On combining our results with external data on predicted gene expression in humans and mouse knockout studies, we identified 3 candidate genes. A burden of rare variants in each gene and in a combined 3-gene score was tested for its associations with clinical phenotypes of asthma. Finally, published single-cell gene expression data in lower airway mucosal cells after allergen challenge were used to assess transcriptional responses to allergen.
Results:
Rare variants in USF1 were significantly associated with blood neutrophil count (P = 2.18 ×10−7); rare variants in TNFRSF21 with total IgE (P = 6.47 ×10−6) and PIK3R6 with eosinophil count (P = 4.10 ×10−5) reached suggestive significance. These 3 findings were supported by independent data from human and mouse studies. A burden of rare variants in TNFRSF21 and in a 3-gene score was associated with allergy-related phenotypes in cohorts of children with mild and severe asthma. Furthermore, TNFRSF21 was significantly upregulated in bronchial basal epithelial cells from adults with allergic asthma but not in adults with allergies (but not asthma) after allergen challenge.
Conclusions:
We report novel associations between rare variants in genes and allergic and inflammatory phenotypes in children with diverse ancestries, highlighting TNFRSF21 as contributing to the development of allergic asthma.
Keywords: Whole-genome sequencing, total IgE, neutrophils, eosinophils
Genome-wide association studies (GWASs) have identified thousands of associations between common variants and hundreds of complex traits.1–3 However, rare variants, which comprise the bulk of human genetic variation,4 have been largely overlooked because they have not been well represented by the variants included on commonly used genotyping arrays and because most studies are underpowered to detect individual rare variant associations. However, the increased availability of whole-genome sequencing and the development of variant-set tests that jointly test the association of multiple variants in a gene allow for a more comprehensive study of the role of genome-wide rare variation in complex traits. Although these variants do not necessarily explain a significant proportion of heritability,5 rare variant studies can more directly identify causal genes and mechanisms, as well as novel therapeutic targets.6
Previous studies have explored the contribution of rare variants in asthma and allergic diseases and have implicated genes harboring rare variants in asthma using various study designs across diverse ancestries.5,7–16 Among the more notable findings are associations between asthma and rare variants in the IL339,16 and filaggrin11,17 genes. Indeed, the discovery of a rare loss-of-function variant in IL33 that conferred protection from asthma by disrupting binding to its receptor, ST2, led to the identification of astegolimab, an ST2 inhibitor, as an effective therapy for reducing exacerbations in individuals with hard-to-treat asthma.18
Other studies have explored the role of rare variation in asthma-associated traits, including allergic, inflammatory, and pulmonary traits, some in individuals with diverse ancestries.9,10,19–25 However, these studies most often examined just 1 or a limited number of traits, and most used targeted sequencing9,10,24 or exonic variants,16,19,22,23 or performed GWASs of individual rare variants.20,21 Capturing all coding and noncoding variation across the entire genome through whole-genome sequencing and using a gene-based test may be a more effective approach for studying the genetic architecture of asthma-associated quantitative phenotypes and clinical phenotypes of asthma.
In this study, we explored the contributions of rare variants (minor allele frequency [MAF] < 0.01) to 8 asthma- and allergy-associated quantitative traits in 1035 children with diverse ancestries from 2 longitudinal studies: the Asthma Phenotypes in the Inner City (APIC) study26,27 and the URban Environment and Childhood Asthma (URECA) birth cohort study.28 Three of the top gene associations that we identified were further supported by external data, and single-cell sequencing data from lower airway mucosal cells before and after allergen challenge highlighted a potentially important role of TNSRSF21 in the development of allergic asthma (AA), and as a possible therapeutic target for AA.
METHODS
Study populations
These studies included children from 2 cohorts that were component studies of the Inner-City Asthma Consortium29 (for study overview, see Fig E1 in this article’s Online Repository at www.jacionline.org). APIC26,27 was a 1-year longitudinal study of children and adolescents (ages 6–17 years) with asthma living in low-income areas of 9 US cities (Baltimore, Md; Boston, Mass; Chicago, Ill; Cincinnati, Ohio; Dallas, Tex; Denver, Colo; Detroit, Mich; New York, NY; and Washington, DC). All APIC participants had a physician’s diagnosis for asthma and at least 2 asthma episodes requiring bronchodilator administration in the previous year.27 URECA is a prospective birth cohort study of children living in low-income areas of 4 US cities (Baltimore, Md; Boston, Mass; New York, NY; and St Louis, Mo).28 Mothers were enrolled into this study during pregnancy; at least 1 parent had a history of asthma or an allergic disease28; asthma in the child was defined by physician’s diagnosis at age 7 or 10 years, lung function criteria, and/or reported symptoms.30
Data for both cohorts were obtained following written informed consent from a parent and assent from the child. The clinical studies in URECA were approved by a central institutional review board (IRB) at the University of Wisconsin and Western Institutional Review Board (IRB # 20142570). The clinical studies in APIC were approved at each recruiting site: Johns Hopkins (IRB #5), Boston University Medical Campus (Blue Panel IRB), Children’s Memorial Medical Center (IRB #2011–14581), Cincinnati Children’s Hospital IRB, University of Texas Southwestern Medical School (IRB #8843), National Jewish Health IRB, Henry Ford Health System (IRB #6782), Columbia University Medical Center (IRB #1), and Children’s National Medical Center IRB. The studies described in this article were approved by the University of Chicago (IRB 19–0046).
Asthma-associated quantitative phenotypes
Eight quantitative traits that reflect component features of asthma were available for both cohorts and were the focus of this study. We included 2 quantitative measures of allergic sensitization: total serum IgE (IU) concentration and the number of sensitizations to 15 common inhaled and food allergens (mouse, dog, house dust mite [×2], cat, roach, mold [×2], ragweed, maple, oak, timothy grass, peanut, egg, and milk), according to serum allergen-specific IgE concentration (positive is ≥0.35 kU/L). Immune and inflammatory phenotypes included blood cell counts (eosinophils and neutrophils, both in cells/mm3) and fractional exhaled nitric oxide in parts per billion, which was measured following American Thoracic Society guidelines.31 Lung function measures included percent predicted FEV1, FEV1/forced vital capacity (using the normalized z score), and bronchodilator response (percentage change from baseline in FEV1 following 4 inhalations of albuterol).26,28 The Mann-Whitney U test was used to assess differences in traits between individuals with and without asthma, and the chi-square test was used to assess differences in self-reported race and sex ratios (% female).
Quantitative trait normalization
We performed trait normalization for the 8 quantitative traits. We adjusted for potential confounding factors by fitting a linear mixed model accounting for asthma status, age at which the trait was measured, sex, the first 10 principal components of ancestry (from common variants32), and genetic relatedness between individuals modeled as a random effect. Asthma was included as a covariate to account for disease-induced phenotypic differences; analyses for the most significant results were repeated without including asthma as a covariate and in individuals with asthma only to exclude the possibility of collider bias effects.33 We applied a quasi-Poisson linear mixed model for the count data (blood neutrophil count, blood eosinophil count, and allergen sensitization) and a gaussian linear mixed model for all other traits. Total IgE and blood neutrophil count were log transformed, and blood eosinophil count was square-root transformed before regression fitting. For neutrophil count, we additionally corrected for the common variant within Duffy blood group gene, ACKR1/ DARC (rs2814778; 1 for CC, 0 for CT, TT), because the homozygous CC genotype is associated with decreased neutrophil counts in individuals with African ancestry.34 We also repeated analyses without including rs2814778 as a covariate for comparison. For bronchodilator response, we additionally corrected for FEV1 % predicted and FEV1.2 Residuals from all regressions were rank-based, inverse-normal transformed,35 and used as the outcome variables in further analyses.
Whole-genome sequencing and variant calling
Whole-genome sequencing was performed and variant calls were generated as described in Dapas et al.32 Briefly, whole-genome sequencing was performed using the NovaSEQ6000 (Il-lumina, Inc, San Diego, Calif), generating 150bp paired-end reads. Sequences were processed according to Genome Analysis Toolkit best practices, and reads were aligned to the GRCh38 human reference genome.36 Aligned reads underwent duplicate removal and base quality score recalibration against known sites in the Genome Analysis Toolkit resource bundle.37 Sample swaps were assessed using VerifyBamID.38 See Dapas et al32 for more details.
To isolate rare variants, we first selected the 21,073,226 variants with MAF less than 0.01 in the combined APIC and URECA children (see Fig E2 in this article’s Online Repository at www.jacionline.org). We then excluded variants that were common (MAF≥ 0.01) in any of the 1000 Genomes Project super populations (African, American, East Asian, European, and South Asian) because variants with deleterious effects are unlikely to be found at common frequencies in any population.4 This removed 4.56% of variants in our data set, leaving a final set of 20,093,812 variants for downstream analysis.
Association tests
To group variants for gene-based association testing, we binned all coding and noncoding rare variants located between the 3′UTR and 5kb upstream of the 5′UTR for each gene, including both protein-coding genes and ncRNAs. We used STAAR (variant-Set Test for Association using Annotation infoRmation)39 for gene-based association testing with the 8 quantitative phenotypes described above. STAAR incorporates 3 variant-set tests: the burden test,40–43 the sequence kernel association test,44 and the aggregated Cauchy association test.45 We did not weigh the variants by their frequency due to the narrow range of MAFs (0.00097–0.099) in this sample. We excluded genes with fewer than 5 rare variants, resulting in 24,902 genes for analysis. We used a stringent Bonferroni correction of 0.05/(24,902 × 8 phenotypes) less than 2.51 ×10−7 and considered a P-value threshold of less than 1 ×10−5for suggestive significance. For the top gene associations with each trait, we further examined associations stratified by coding and noncoding variants in the gene set. When a subset of variants assigned to one gene set resided in the exon of another nearby gene, we repeated analyses after excluding those variants from the gene set. For all genes with evidence of association, we also examined associations for each variant within the gene set through linear regression using the same covariates used in the gene-based tests.
External gene and variant validation
To further evaluate the associations with the most significant gene for each of the 8 traits, we first assessed whether predicted gene expression was associated with the same or related trait in the phenomeXcan database,46 which reports associations between predicted gene expression based on genetic variation and specific phenotypes (primarily from the UK Biobank47). We then evaluated publicly available mouse knockout studies of the associated genes to see whether the resulting phenotypes included traits related to those in our study.48 We next examined which variants resided in active enhancer and transcriptional start sites in blood and epithelial cells from ROADMAP.49
Burden of rare variants and asthma phenotypes
For each of the 3 candidate genes prioritized using external evidence, we defined the number of rare variants in that gene as its “burden score” for each individual. The total of rare variants across all 3 genes for each individual was used in a “3-gene score.” These burden scores were tested for association with asthma and allergy phenotypes that were previously defined within the APIC and URECA cohorts, as described.27,50
We performed Mann-Whitney U tests to determine whether the individual or 3-gene scores were associated with clinical phenotypes. The APIC cohort was composed of children with a doctor’s diagnosis of asthma. Using predefined phenotypes in APIC,27 we compared phenotype A (minimally symptomatic asthma and rhinitis, low allergy/inflammation, and normal pulmonary physiology during the study) to each of the more severe phenotypes B to E (B: lower allergy and inflammation, intermediate rhinitis symptoms, and mildly impaired pulmonary physiology; C: minimally symptomatic asthma and rhinitis with an intermediate degree of allergy and allergic inflammation; D: symptomatic rhinitis and higher levels of allergy and allergic inflammation, with intermediate impairment of pulmonary physiology; E: highest levels of symptomatic asthma and rhinitis and highest degree of allergy and allergic inflammation, with most impaired pulmonary physiology). The URECA cohort includes children with and without asthma, with the former comprised largely of children with mild asthma. Using predefined phenotypes in URECA, we compared children with little to no allergy or asthma (phenotype low wheeze/low atopy) to each of 5 other phenotypes: low wheeze/high atopy (LW-HA), transient wheeze/low atopy, high wheeze/low atopy, high wheeze/high atopy, and high wheeze/ high atopy/low lung function. The comparisons within each phenotype were not completely independent (all compared against the same reference group), so Bonferroni corrections would have been overly conservative.
Patterns of gene expression and responses to allergen challenge
To gain further insight into the potential functional or clinical effects of the prioritized genes, we used publicly available single-cell RNA-sequencing data from lower airway mucosal cells from 4 individuals with AA and 4 individuals with allergies alone (no asthma) at baseline and 24 hours after segmental allergen challenge from Alladina et al.51 Using data on differentially expressed genes (Data File S6 in the published manuscript) and their interactive website (https://villani.mgh.harvard.edu/allergy-asthma/), we examined the expression patterns and differential expression between individuals with AA and individuals with allergy only at baseline and after allergen challenge for the 3 prioritized genes.
RESULTS
Sample composition
We examined rare variation and allergy- and asthma-associated phenotypes from 1035 children in APIC (n = 508) and URECA (n = 527). The parent-reported racial and ethnic composition of their children was 67% Black non-Hispanic, 25% Hispanic, and 8% other (White, mixed, unknown) in APIC and 72% Black non-Hispanic, 20% Hispanic, and 8% other (White, mixed, unknown) in URECA. The demographic and clinical characteristics of these children are presented in Table I and Table E1; a principal-component analysis plot of the genetic ancestries of these children is shown in Fig E3 (in the Online Repository available at www.jacionline.org). In this sample, there were fewer females and fewer parent-reported Black and more parent-reported Hispanic individuals among children with asthma compared with children without asthma, but neither proportion of self-reported race and ethnicity nor % female was significantly different after correcting for 11 comparisons (Pcorrected < .0045). Except for blood neutrophil count, measurements of all the clinical phenotypes significantly differed between children with and without asthma after multiple testing correction (Table I). The correlations between phenotypes are shown in Fig E4 (in the Online Repository available at www.jacionline.org).
TABLE I.
Sample composition
| Characteristic | All | Asthma | Nonasthma | P value (asthma vs nonasthma) |
|---|---|---|---|---|
| Sample size | 1035 | 681 | 226 | — |
| Mean age (y) | 9.99 | 10.56 | 9.37 | 1.51 × 10−7 |
| % Female | 46.18 | 43.86 | 52.65 | .025 |
| % Self-reported race and ethnicity | ||||
| Black | 67.25 | 65.20 | 75.22 | .015 |
| Hispanic | 24.93 | 27.50 | 16.81 | |
| White | 1.35 | 1.03 | 1.33 | |
| Other/mixed/unknown | 6.47 | 6.27 | 6.64 | |
| Traits, median (IQR) | ||||
| Allergen sensitization (positive test results) | 3.00 (7) | 4.67 (7.67) | 1.00 (3.33) | 7.6 × 10−21 |
| Total IgE concentration (IU/mL) | 158 (463) | 243 (655) | 70 (123) | 7.67 × 10−21 |
| Blood eosinophil count (cells/mm3) | 200 (300) | 300 (340) | 200 (200) | 3.13 × 10−12 |
| FENO (ppb) | 17.00 (25.54) | 19.50 (29) | 8.00 (11) | 2.77 × 10−16 |
| Blood neutrophil count (cells/mm3) | 2700 (1800) | 2700 (1900) | 2500 (1700) | .01 |
| FEV1 % predicted | 95.50 (20.37) | 93.70 (21.54) | 100.75 (16.61) | 1.20 × 10−8 |
| FEV1/FVC (z score) | −1.06 (1.60) | −1.27 (1.67) | −0.52 (1.20) | 1.49 × 10−17 |
| Bronchodilator response (% change from baseline) | 8.40 (10.82) | 9.80 (11.84) | 5.60 (8.06) | 7.83 × 10−14 |
Results are reported separately by asthma status for the combined APIC and URECA samples. The ages correspond to the year at which total IgE was measured (all other ages listed in Table E1). The medians and IQRs (in parentheses) are listed for all quantitative traits. Missing data were not included in calculations.
FVC, Forced vital capacity; FENO, fractional exhaled nitric oxide; IQR, interquartile range; IU, international units; ppb, parts per billion.
Overview of associations
We performed gene-based variant-set tests for 8 quantitative traits in participants from the APIC and URECA cohorts. We assigned rare variants to 25,605 genes, and required gene sets to have at least 5 variants, resulting in 24,902 genes examined. The mean number of variants in each variant set was 508 (median number, 207; range, 5–17,174; see Fig E5 in this article’s Online Repository at www.jacionline.org). The gene-based association test results are presented in Table E2 (in the Online Repository available at www.jacionline.org); the most significant gene for each of the 8 traits is listed in Table II.
TABLE II.
Results of rare-variant, gene-based association studies
| Trait | Gene* | Location | # Var | Coding P value | Noncoding P value | P value |
|---|---|---|---|---|---|---|
| Blood neutrophil count | USF1 | 1q23.3 | 79 | .98 | 1.15 × 10−9 | 2.18 × 10−7 |
| Total IgE | TNFRSF21 | 6p12.3 | 794 | .24 | 5.90 × 10−6 | 6.47 × 10−6 |
| Feno | CTBP1-AS | 4p16.3 | 151 | .72 | 3.26 × 10−5 | 2.34 × 10−5 |
| FEV1/FVC | VRK3 | 19q13.33 | 547 | .04 | 3.59 × 10−5 | 3.15 × 10−5 |
| Bronchodilator response | MRPL44 | 2q36.1 | 129 | 1.68 × 10−3 | 3.68 × 10−5 | 3.44 × 10−5 |
| Blood eosinophil count | PIK3R6 | 17p13.1 | 608 | 8.93 × 10−3 | 5.12 × 10−5 | 4.10 × 10−5 |
| FEV1 % predicted | TEX36-AS1 | 10q26.13 | 93 | NA | 8.61 × 10−5 | 6.08 × 10−5 |
| Allergen sensitization | VAMP3 | 1p36.23 | 124 | 0.75 | 3.96 × 10−5 | 6.42 × 10−5 |
The most significant gene association for each trait is given. For each quantitative trait, the gene, location, number of variants in the gene set (# Var), P value in analyses considering just coding or just noncoding variants in the variant set, and the overall P value are given. One association (USF1 with blood neutrophil count, P = 2.18 ×10−7) met the Bonferroni-adjusted significance threshold of 2.51 × 10−7. The full results are presented in Table E2. The 3 bolded associations are the results with support from external sources and are described in further detail.
FENO, Fractional exhaled nitric oxide; FVC, forced vital capacity.
CTBP1-AS, C-terminal binding protein 1 antisense RNA; VRK3, vaccinia-related kinase 3; MRPL44, mitochondrial ribosomal protein L44; TEX36-AS1, testis expressed protein 36 antisense RNA 1.
All associations remained nominally significant (P <.05) in analyses that did not include asthma as a covariate in the trait normalization, and all associations except the association of VAMP3 (vesicle-associated membrane protein 3) with allergic sensitization remained nominally significant in the analyses including only children with asthma (see Table E3 in this article’s Online Repository at www.jacionline.org). For 2 genes, VAMP3 and USF1 (upstream transcription factor 1), the variants that were within 5kb upstream of their 5′UTR included some that were in the exons of neighboring genes and were therefore designated as coding variants. Nine single nucleotide variants in the VAMP3 set were coding variants for CAMTA1 (calmodulin binding transcription activator 1; see Fig E6 in this article’s Online Repository at www.jacionline.org); when these single nucleotide variants were excluded, the P values for VAMP3 were largely unchanged (5.13 × 10−5 for the set and P =.85 for coding variation). The USF1 results are further discussed in the next section.
We next used publicly available resources to investigate additional evidence supporting the most significant gene associations for each of the 8 phenotypes (see Tables E4 and E5 in this article’s Online Repository at www.jacionline.org). Three associations were supported by orthogonal data: USF1 with blood neutrophil count, TNFRSF21 (tumor necrosis factor receptor superfamily member 21) with total IgE, and PIK3R6 (phosphoinositide-3-kinase regulatory subunit) with blood eosinophil count. These are discussed below and presented in bold font in Table II. The remaining associations did not have corroborating support from these external resources and were not further investigated.
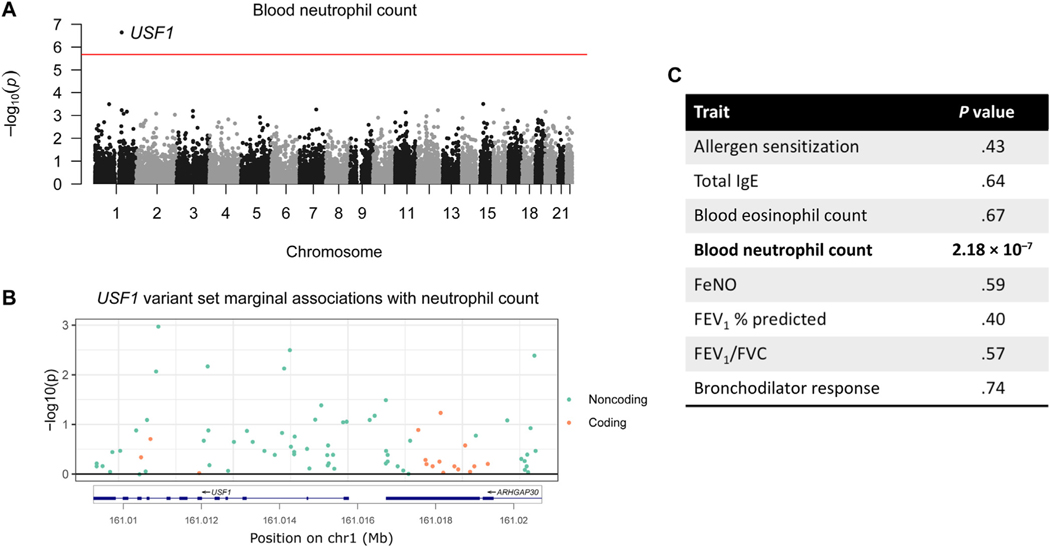
USF1 is associated with blood neutrophil count
The most significant gene-trait pair was USF1 with blood neutrophil count (Table II and Fig 1, A). This association was also significant (P = 1.97 × 10−8) in a secondary analysis that did not correct for the genotype at the Duffy blood group gene (see Methods). The association was more significant when only the 63 noncoding variants were included in the set (P = 1.15 × 10−9); the set comprised only of the 16 coding variants showed no evidence of associations (P =.98) (Table II). No single variant contributed disproportionately to the association (Fig 1, B). The variant set included 13 SNVs in the coding region of the nearby gene (ARHGAP30). When we removed these variants, the association for USF1 was more significant (P = 1.55 × 10−9 vs 2.18 ×10−7). This variant set was not associated with any of the other 7 traits (Fig 1, C).
FIG 1.
USF1 and neutrophil count. A, Manhattan plot for gene set associations with blood neutrophil count. Each point represents a gene. The red line is the Bonferroni significance threshold. B, Marginal associations and locations of variants in the USF1 variant set. Variants are color coded green if they are noncoding and orange if coding in either USF1 or its neighboring gene, ARHGAP30. C, USF1 association with other traits. FENO, Fractional exhaled nitric oxide; FVC, forced vital capacity.
We evaluated the USF1 gene association further using publicly available mouse knockout data and phenomeXcan, a resource that reports associations between predicted gene expression from multiple tissues and GWAS traits.46 PhenomeXcan reported that predicted USF1 expression across 33 tissues (see Table E6 in this article’s Online Repository at www.jacionline.org) was significantly associated with both neutrophil percentage (P = 4.66 × 10−4) and neutrophil count (P = 8.02 ×10−3), with lower predicted USF1 expression associated with higher values of both measures. Furthermore, mice with Usf1 knocked out in bone marrow cells had increased blood neutrophil counts,52 which is consistent with the direction of effect from phenomeXcan. According to epithelial cell annotations in ROADMAP,49 28% of rare variants we identified for USF1 were located in the transcription start site (TSS) and none were in enhancers. Using the blood cell annotations, 19% were located in the TSS and 34% were in enhancers (see Fig E7 in this article’s Online Repository at www.jacionline.org). These orthogonal data based on gene expression and mouse knockout studies support our results, validating the association between USF1 and neutrophil counts, and further suggesting that rare variants in USF1 impact neutrophilia via their effects on gene expression.
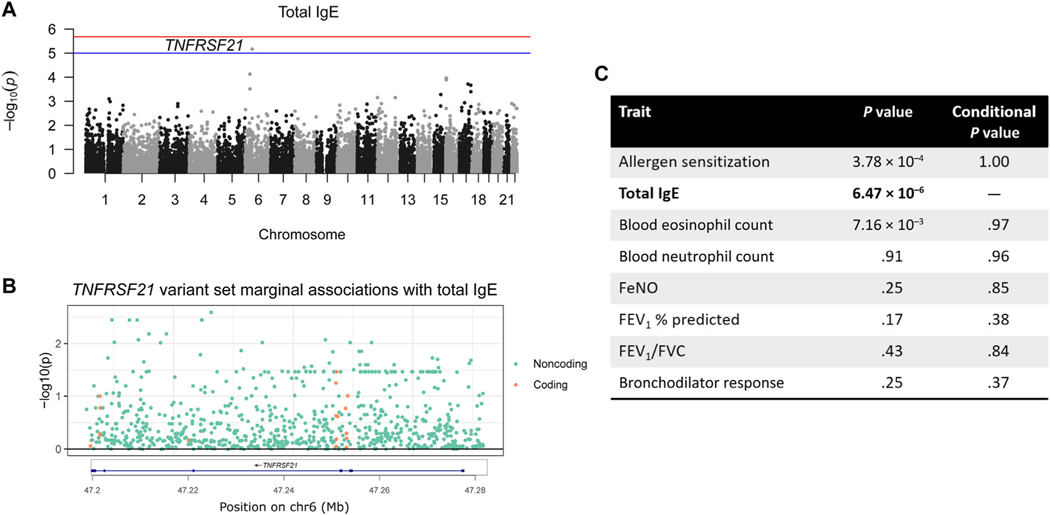
TNFRSF21 is associated with total IgE
The second most significant association, which reached suggestive significance (P = 6.47 × 10−6), was between TNFRSF21 and total IgE levels (Fig 2, A). Similar to USF1, no single variant contributed disproportionately to the association (Fig 2, B). TNFRSF21 was also nominally associated with both allergen sensitization and blood eosinophil count (Fig 2, C). However, these 3 traits were highly correlated (Fig E4), and when we repeated the associations including total IgE levels as a covariate in the model, the associations with allergen sensitization and blood eosinophil count were no longer significant (Fig 2, C). This conditional analysis suggests that this gene may be a marker of more generalized atopy or type 2 inflammation. In addition, the association with TNFRSF21 was slightly more significant when considering the 776 noncoding variants (P = 5.90 × 10−6 vs 6.47 × 10−6), whereas the association with the 18 coding variants was not significant (P =.24; Table II).
FIG 2.
TNFRSF21 and total IgE. A, Manhattan plot for total IgE levels. Red line is Bonferroni significance and blue is suggestive significance (1.00 × 10−5). B, Marginal associations of variants in the TNFRSF21 variant set. C, TNFRSF21 association with the other traits before and after conditioning on total IgE. FENO, Fractional exhaled nitric oxide; FVC, forced vital capacity.
Total IgE was not available in the phenomeXcan data set, and predicted TNFRSF21 expression was not associated with the available allergic traits in the UK Biobank (food allergy, allergic rhinitis, and eczema). However, the Mouse Genome Informatics resource reported that Tnfrsf21 mouse knockouts had significantly increased IgE levels in response to protein challenge,48,53 supporting a relationship between TNFRSF21 and total IgE. ROADMAP epithelial cell annotations indicated that 3% of rare variants were located in the TSS and 4% were in enhancers. According to blood cell annotations, 0.1% were located in the TSS and 4% were in enhancers (Fig E7). Although more limited, these results support the association between TNFRSF21 and total IgE and suggest that rare variants in this gene may influence IgE levels or type 2 inflammation via their impact on gene expression.
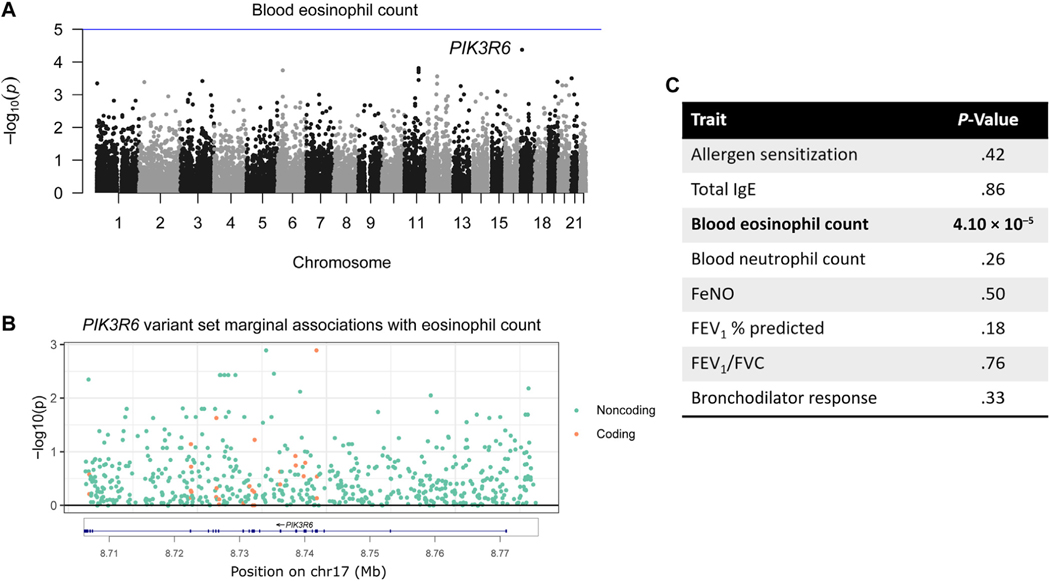
PIK3R6 is associated with blood eosinophil count
The most significant association for blood eosinophil counts was with PIK3R6 with P = 4.10 × 10−5 (Fig 3, A). In contrast to the previous 2 gene-trait pairs, the P value for the associations between PIK3R6 and eosinophil count was slightly less significant when analyzing just the 581 noncoding variants (P = 5.19 × 10−5) and retained nominal significance (P = 3.90 × 10−3) when considering only the 27 coding variants. This indicates that both sets of variants contributed to the association. No single variant was responsible for the entire signal (Fig 3, B), and PIK3R6 was not associated with any other trait (Fig 3, C).
FIG 3.
PIK3R6 and eosinophil count. A, Manhattan plot for blood eosinophil count. B, Marginal associations of variants in variant set. C, PIK3R6 association with the other traits.
The association was validated by phenomeXcan: the most significant association for predicted PIK3R6 expression was with eosinophil percentage (P = 3.96 × 10−6) across 26 tissues (Table E6). It was also associated with eosinophil count (P = 1.85 × 10−4). Furthermore, Pik3r6 knockout mice reported decreased granulocyte numbers.48,54 Although the type of granulocyte was not specified, these measures would have included eosinophils. According to epithelial cell annotations in ROADMAP, 5% of the rare variants were located in enhancers and none were in the TSS. Similarly, using the blood cell annotations, 16% were located in enhancers and none were in the TSS (Fig E7). Taken together, these results support a possible association between PIK3R6 variants and eosinophil counts.
TNFRSF21 and a 3-gene score is associated with respiratory phenotypes
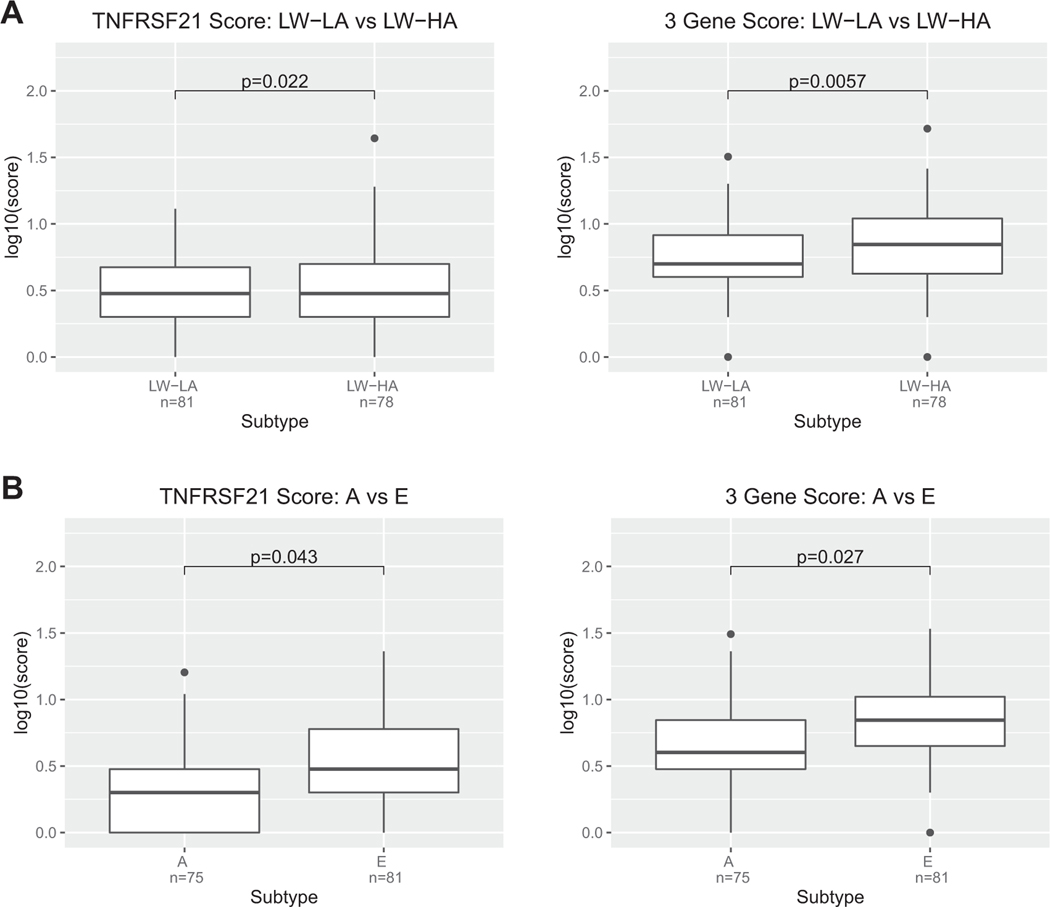
We next asked whether rare variant gene scores, reflecting the burden of rare variants for each individual, for each of the 3 candidate genes (USF1, PIK3R6, TNFRSF21) or a composite 3-gene score was associated with respiratory phenotypes in the URECA and APIC cohorts. In these analyses, we assigned a score to each individual that reflected the number of rare variants in each gene carried by that individual, or the number of rare variants across all 3 genes carried by that individual. We used phenotype groupings previously defined in these children.27,50 In URECA,
composed mostly of children without asthma or with mild asthma, the TNFRSF21 score was nominally associated with the LW-HA phenotype compared with the low wheeze, low atopy group (P = 2.25 × 10−2) (Fig 4, A; see Fig E8 and Table E7 in the Online Repository available at www.jacionline.org). The gene scores for USF1 and PIK3R6 were not associated with any phenotypes in the URECA children. However, the 3-gene score was also associated with the LW-HA phenotype and was more significant than the association with TNFRSF21 alone (P = 5.69 ×10−3). These results in URECA children with allergic sensitization indicated a positive association between more rare variants and more atopy in the absence of wheeze.
FIG 4.
TNFRSF21 and 3-gene score with URECA and APIC phenotypes. Boxplots show the log10-transformed total number of rare variants in (A) TNFRSF21 and (B) the 3-gene score for each individual in URECA phenotypes LW-LA (low wheeze/low atopy) vs LW-HA (low wheeze/high atopy) and for APIC phenotypes A (minimally symptomatic asthma and rhinitis; low allergy/inflammation) vs E (highest levels of symptomatic asthma and rhinitis; highest degree of allergy and allergic inflammation). For all results, see Fig E8 and Table E7. Sample sizes for each phenotype are also shown.
In APIC, composed of children at all levels of asthma severity, the only nominally significant association with a single gene score was also between TNFRSF21 and phenotype E compared with the mildest group (phenotype A) (P = 4.2 × 10−2). Phenotype E describes children with symptomatic asthma and the highest IgE levels, eosinophil counts, and allergen sensitizations compared with the other phenotype groups, consistent with this gene showing at least modest associations with these 3 phenotypes in the gene-based association tests. The 3-gene score also had a stronger association (P = 2.69 × 10−2) with phenotype E than did the TNFRSF21 score alone, possibly reflecting the contribution of rare variants in all 3 genes in this phenotype of severe asthma. There were no other nominally significant associations between the other gene scores and phenotypes in the APIC children (Fig 4, B, Fig E8, and Table E7). These results in APIC further indicated a positive association between more rare variants in these genes and more severe disease.
TNFRSF21 is highly expressed in basal epithelial cells and responsive to allergen challenge
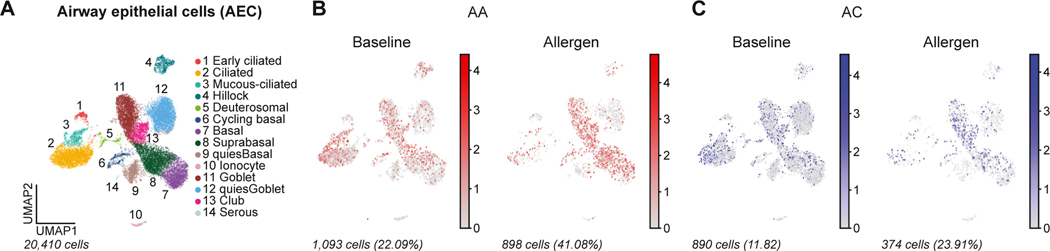
Finally, to gain further insight into the expression patterns and transcriptional regulation of the prioritized genes in airway cells, we used publicly available single-cell RNA-sequencing data in lower airway mucosal cells from individuals with AA and allergic (nonasthma) controls (AC) measured at baseline and after allergen challenge.51 USF1 was most highly expressed in lower airway mononuclear phagocytes compared with all other cells (P = 3.3 × 10−4; false-discovery rate = 1.2 × 10−3), and PIK3R6 was most highly expressed in lower airway mast cells compared with all other cells (P = 1.9 × 10−9; false-discovery rate = 9.3 × 10−8), but the expression of these genes did not differ between the AA and AC groups at baseline or after allergen challenge (data not shown). In contrast, TNFRSF21 was most highly expressed in bronchial epithelial cells compared with all other cell types (P = 3.7 × 10−8; false-discovery rate = 1.8 × 10−7) (see Fig E9 in this article’s Online Repository at www.jacionline.org). Although expression levels did not differ significantly at baseline between AAs and ACs in any cell type, expression of TNFRSF21 in basal epithelial cells showed a significant response to allergen challenge in the AA group compared with the AC group (Group × Treatment interaction: P = 2.1 × 10−3; Padjusted = 6.8 × 10−2), but not in the AC group (P = 3.1 × 10−1; Padjusted ~1) (Fig 5; see Fig E10 in this article’s Online Repository at www.jacionline.org).
FIG 5.
TNFRSF21 expression in airway epithelial cells (AECs) in individuals with AA and AC. Uniform manifold approximation and projection (UMAP) of 20,410 AECs from a publicly available database in (A) with 14 AEC subclusters obtained from endobronchial brush sampling of third-to fourth-generation airway segments from patients with AA and ACs before and 24 hours after segmental allergen challenge. Feature plots in (B) AA (red) and (C) AC (blue) using pseudocoloring to depict TNFRSF21 expression at baseline and after allergen challenge. Cell number and percentages (%) represent gene expression across all AEC subclusters and scaled gene expression in log(CPM).
DISCUSSION
In this study, we examined the contribution of rare variants to allergy- and asthma-associated quantitative traits using a gene-based approach. We included a broad panel of allergic, immune/ inflammatory, and pulmonary traits measured in children with diverse ancestries. Overall, our studies suggested that rare non-coding variation within or just upstream of genes contribute more to variation in these traits than do rare coding variation (Table II), further supporting other findings that complex traits are primarily driven by both common and rare noncoding variation that have effects on gene expression rather than on protein function.55–57 Indeed, 3 of the associations were supported by orthogonal evidence derived from independent data sets based on predicted gene expression and from mouse knockout studies. All 3 of these genes represent novel associations with immune, atopic, and inflammatory phenotypes.
One association was significant after multiple test correction for 24,902 genes. The gene set for USF1 was associated with blood neutrophil count in the APIC and URECA children, and predicted USF1 expression was associated with neutrophil counts in adults in the UK Biobank. Moreover, a mouse model with Usf1 knocked out had increased neutrophil counts.52 Both the phenomeXcan and mouse knockout results indicated that decreased expression of this gene is associated with elevated levels of circulating neutrophils. USF1 encodes a transcription factor belonging to the basic helix-loop-helix leucine zipper family of proteins that can regulate expression through E-box motifs.58 This gene is also located within 2Mb of the Duffy blood group gene (ACKR1/ DARC34), but because this association remained significant when conditioning on the Duffy variant, the association between USF1 and neutrophil counts was not due to linkage disequilibrium with the Duffy null allele (see Methods). Several studies have reported a relationship between USF1 and immune-related traits,52,59–62 and the locus containing this gene has been significantly associated with both white blood cell count63 and granulocyte percentage of myeloid white cells64 in GWASs. As a transcription factor, USF1 may be regulating expression of genes important in inflammatory processes and possibly even asthma: several studies have reported that asthma risk alleles at the loci encoding the MUC5AC and ORMDL3/GSDMB genes affect binding of USF1.65–67 Although our studies did not support a role for rare variants in this gene contributing to asthma or atopy susceptibility in children, these combined data strongly support a role for this gene in determining circulating neutrophil levels and possibly in other inflammatory conditions.59–62
We also report an association between PIK3R6 and eosinophil count. This was further supported by both phenomeXcan and Pik3r6 knockout mice.54 PIK3R6 encodes a lipid kinase that acts as a regulatory subunit for the PI3K gamma complex and is primarily activated by G protein–coupled receptors.68 Notably, several studies in human cohorts have implicated PIK3R6 expression in other eosinophilic or allergic diseases. For example, PIK3R6 was among the most significantly differentially expressed genes in peripheral blood cells between eosinophilic and noneosinophilic chronic obstructive pulmonary disease,69 between patients with AA and nonasthma/nonallergic controls,70 and in a meta-analysis of atopy in white blood cells.71 Together, these studies point to a potentially important role of this gene in eosinophilic-related traits.
The second most significant association was between TNFRSF21 and total IgE. Measures of IgE were not available in phenomeXcan, but Tnfrsf21-deficient mice had increased IgE levels in response to protein challenge.53 TNFRSF21 encodes the Death Receptor 6 protein and is a member of the tumor necrosis factor superfamily and signals through the NF-κB pathway.72,73 Studies in mice have also implicated this gene in the TH2 response,53,74,75 specifically through activating the Jun amino terminal kinase (JNK) pathway in regulating TH2-cell differentiation.53 TH2 inflammation is a hallmark of allergic disease, but evidence supporting a role of TNFRSF21 in this pathway has come largely from mouse studies. Our results implicate this gene more directly in the development of AA in humans.
Studies of quantitative traits are more powerful for identifying genetic associations than are studies of binary traits, particularly in small samples,76 but the goal of these studies is generally to identify variants that ultimately affect disease risk or outcomes. Therefore, we used quantitative trait mapping to identify 3 candidate genes that may impact risk for asthma or allergic disease. Analyses of these 3 genes with clinical phenotypes of allergy and asthma in the APIC and URECA cohorts converged on a role of rare variants in TNFRSF21 and the 3-gene score with AA phenotypes. In the URECA cohort, both scores were associated with the LW-HA phenotype. In the APIC cohort, TNFRSF21 and the 3-gene score were associated with the most severe asthma phenotype (symptomatic asthma, high IgE, eosinophilia, and sensitization to multiple allergens). The different associations between the 2 cohorts likely reflect the different ascertainment of children in each. URECA is a birth cohort in which a family member had asthma or allergic disease. As a result, this cohort includes children without asthma and with generally mild asthma among affected children.30,50 In contrast, APIC is a 1-year longitudinal study of children with asthma, of whom nearly half were classified as “difficult to treat.”26,27 Despite these differences, both studies of urban children highlight TNFRSF21 and the 3-gene score in the development of allergy and asthma phenotypes.
A role for TNFRSF21 in AA was additionally supported by the observation of greater increase in TNFRSF21 expression in basal epithelial cells in adults with AA after allergen challenge compared with adults with allergy but not asthma and establishes a role for this gene in T2 inflammatory responses in human airways. Our genetic studies further suggest that rare variants in this gene contribute to this response and to the development of allergic disease and asthma in children.
Strengths of the study include the use of 2 study cohorts of children with whole-genome sequencing and comprehensive longitudinal phenotyping. Each study defined distinct groups of children on the basis of multiple measurements conducted over 1 (APIC) or over 10 (URECA) years, thereby adding precision beyond that achieved in cross-sectional observations. In addition, the study populations include a high percentage of Black and Hispanic children with high rates of allergy and asthma, populations that have been underrepresented in genetic studies.77 Despite these strengths, there are limitations. First, because of the modest sample size (n = 1035), we had low power to detect associations with individual rare variants and we were not well powered to detect genome-wide associations with asthma per se or with clinical subtypes of asthma. The lack of true (general population) controls also reduced power to detect associations with asthma or atopy. As a result, we had to limit our studies of asthma and atopy to 3 candidate genes that were put forward from our genome-wide studies of quantitative phenotypes. Second, the rare variant method we used (STAAR) does not report effect estimates or SEs, which limits interpretation of our results. Third, not all gene candidates identified in our studies (CTPB1-AS, TEX36-AS1) were available in phenomeXcan or the Mouse Genome Informatics resource, and not all phenotypes (total IgE, bronchodilator response, FEV1/forced vital capacity) were available in phenomeXcan, so it was not possible to validate all associations using these databases. Fourth, the genetic findings of this study have not been replicated in an independent cohort with whole-genome sequencing and similar quantitative phenotypes. Nonetheless, we were able to validate 3 of the most significant associations using orthogonal data from phenomeXcan and/or mouse knockout studies. Finally, the associations with AA phenotypes in APIC and URECA were only nominally significant. However, despite the small sample sizes, results in 2 independent cohorts highlighted the TNFRSF21 gene. A role for this gene in AA was further supported as an allergen-responsive gene in bronchial epithelial cells among adults with AA compared with adults with allergy but not asthma.51 These combined data underscore the robustness of the associations across biological contexts and provide convergent support for expression levels of TNFRSF21 impacting asthma-associated quantitative phenotypes and AA.
In summary, we identified rare variants in genes associated with allergic and inflammatory phenotypes using whole-genome sequencing data from children with diverse ancestries. We further validated 3 associations through external data sources, which converged on a role for rare variants in these 3 genes and, particularly in TNFRSF21, with asthma and atopy. Our study in well-characterized cohorts of children highlights the importance of rare variation in the development of asthma-associated quantitative phenotypes and AA phenotypes and identifies novel candidate genes that may serve as therapeutic targets.
Supplementary Material
Acknowledgments
We acknowledge Dr Hae Kyung (Haky) Im for her advice and helpful discussions, the University of Chicago Center for Research Informatics for its cluster resources, and the coordinators and participants in all the included studies.
DISCLOSURE STATEMENT
This work was supported by the National Institutes of Health (NIH) (grant nos. U19 AI62310, R01 HL104608, UG3/UH3 OD023282, HHSN272200900052C, HHSN272201000052I, UM1 AI114271, UM1 AI160040, DP2 CA247831, and UH2 AI4434); the Department of Defense (grant no. PR150903/W81XWH-16-1-0493), and funding from Sanofi and Regeneron. Site data collection was supported by the following NIH grants: RR00052 and UL1 TR001079 (Baltimore); M01 RR00533, UL1 RR025771, and 1 UL1 TR001430 (Boston); UL1 TR000150 (Chicago); UL1 TR000451 and UL1 TR001105 (Dallas); Ul1 RR025780 (Denver); UL1 TR000040, M01 RR00071, and UL1 TR001873 (New York); UL1 TR000075 (DC); and UL1 TR000077 (Cincinnati). S.M.C. was supported by NIH grant number T32 GM007197. J.A. was supported by NIH grant number KL2 TR002542. M.D. was supported by NIH grant numbers TL1 TR002388 and T32 HL007605. Single-cell sequencing studies were supported by the NIH (grant no. KL2 TR002452 to J.A., grant no. DP2 CA247831 to A.-C.V., and grant no. UH2 AI44434 to J.L.C.), and a DOD grant PR150903/W81XWH-16-1-0493 and Sanofi iAward (B.D.M).
Disclosure of potential conflict of interest:
All authors, with the exception of P. J. Gergen, report grants from the National Institutes of Health (NIH)/National Institute of Allergy and Infectious Diseases during the conduct of study. S. Clay, J. Alladina, N. P. Smith, C. M. Visness, M. Dapas, M. Kattan, P. J. Gergen, and J. L. Cho have nothing to disclose outside the submitted work. W. W. Busse reports consulting fees from Novartis, GlaxoSmithKline, Genentech, Sanofi, AstraZeneca, and Regeneron and royalties from Elsevier outside the submitted work. M. A. Gill reports an honorarium for and support for travel to the 2017 AAAAI meeting during the conduct of study and monetary compensation from the American Academy of Pediatrics for her work teaching the biannual Pediatrics board review course, PREP The Course. G. K. K. Hershey reports grants from Adare during the conduct of the study. D. J. Jackson reports personal fees from Novartis, Pfizer, Regeneron, AstraZeneca, Sanofi, and Vifor Pharma, grants and personal fees from GlaxoSmithKline, and grants from NIH/National Heart, Lung, and Blood Institute, outside the submitted work. R. S. Gruchalla reports government employment from the Center for Biologics Evaluation and Research as well as personal fees from Consulting Massachusetts Medical Society, outside the submitted work. A. H. Liu reports personal fees from Phadia ThermoFisher as consulting honoraria, grants and nonfinancial support from ResMed/Propeller Health, nonfinancial support from Revenio, grants and personal fees from Avillion, and personal fees from Labcorp, outside the submitted work. L. B. Bacharier reports book royalties from Elsevier, consulting fees from Sanofi, Regeneron, Genentech, GlaxoSmithKline, DBV Technologies, Teva, Medscape, Kinaset, OM Pharma, and AstraZeneca, honoraria from Sanofi, Regeneron, and GlaxoSmithKline, participation in advisory board for DBV Technologies, AstraZeneca, and Vertex, leadership role in the American Academy of Allergy, Asthma & Immunology and the American Board of Allergy and Immunology, and medical writing services for Sanofi/Regeneron. J. E. Gern reports consulting fees from AstraZeneca and Meissa Vaccines and 2 patents related to the methods to enhance the production of rhinoviruses and stock options with Meissa Vaccines. C. M. Kercsmar reports royalties from UpToDate. R. A. Wood reports grants from DBV, Aimmune, Regeneron, Genentech, Novartis, Food Allergy Research & Education (FARE), and Genentech and royalties from UpToDate. R. G. Robison received grant support from DBV Technologies and Aimmune Therapeutics. A.-C. Villani has a financial interest in 10X Genomics; the company designs and manufactures gene sequencing technology for use in research, and such technology is being used in this research. Dr Villani’s interests were reviewed by The Massachusetts General Hospital and Mass General Brigham in accordance with their institutional policies. B. D. Medoff receives research funding from and served as a consultant for Sanofi and Regeneron. C. C. Ober reports personal fees from the American Association of Asthma, Allergy and Immunology.
Abbreviations
- AA
Allergic asthma
- AC
Allergic (nonasthma) control
- APIC
Asthma Phenotypes in the Inner City
- GWAS
Genome-wide association study
- LW-HA
Low wheeze/high atopy
- MAF
Minor allele frequency
- PIK3R6
Phosphoinositide-3-kinase regulatory subunit
- ppb
Parts per billion
- STAAR
Variant-Set Test for Association using Annotation infoRmation
- TNFRSF21
Tumor necrosis factor receptor superfamily member 21
- TSS
Transcription start site
- URECA
URban Environment and Childhood Asthma
- USF1
Upstream transcription factor 1
- VAMP3
Vesicle-associated membrane protein 3
REFERENCES
- 1.MacArthur J, Bowler E, Cerezo M, Gil L, Hall P, Hastings E, et al. The new NHGRI-EBI Catalog of published genome-wide association studies (GWAS Catalog). Nucleic Acids Res 2017;45:D896–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim KW, Ober C. Lessons learned from GWAS of asthma. Allergy Asthma Immunol Res 2019;11:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vicente CT, Revez JA, Ferreira MAR. Lessons from ten years of genome-wide association studies of asthma. Clin Transl Immunol 2017;6:e165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Auton A, Abecasis GR, Altshuler DM, Durbin RM, Bentley DR, Chakravarti A, et al. A global reference for human genetic variation. Nature 2015;526:68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Igartua C, Myers RA, Mathias RA, Pino-Yanes M, Eng C, Graves PE, et al. Ethnic-specific associations of rare and low-frequency DNA sequence variants with asthma. Nat Commun 2015;6:5965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Momozawa Y, Mizukami K. Unique roles of rare variants in the genetics of complex diseases in humans. J Hum Genet 2021;66:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeWan AT, Egan KB, Hellenbrand K, Sorrentino K, Pizzoferrato N, Walsh KM, et al. Whole-exome sequencing of a pedigree segregating asthma. BMC Med Genet 2012;13:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taliun D, Harris DN, Kessler MD, Carlson J, Szpiech ZA, Torres R, et al. Sequencing of 53,831 diverse genomes from the NHLBI TOPMed Program. Nature 2021;590:290–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith D, Helgason H, Sulem P, Bjornsdottir US, Lim AC, Sveinbjornsson G, et al. A rare IL33 loss-of-function mutation reduces blood eosinophil counts and protects from asthma. PLoS Genet 2017;13:e1006659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morin A, Madore AM, Kwan T, Ban M, Partanen J, R€onnblom L, et al. Exploring rare and low-frequency variants in the Saguenay–Lac-Saint-Jean population identified genes associated with asthma and allergy traits. Eur J Hum Genet 2019;27: 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cameron-Christie S, Mackay A, Wang Q, Olsson H, Angermann B, Lassi G, et al. A broad exome study of the genetic architecture of asthma reveals novel patient subgroups. bioRxiv 2020;12:10.419663. 10.1101/2020.12.10.419663. [DOI] [Google Scholar]
- 12.Chang D, Hunkapiller J, Bhangale T, Reeder J, Mukhyala K, Tom J, et al. A whole genome sequencing study of moderate to severe asthma identifies a lung function locus associated with asthma risk. Sci Rep 2022;12:5574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Campbell CD, Mohajeri K, Malig M, Hormozdiari F, Nelson B, Du G, et al. Whole-genome sequencing of individuals from a founder population identifies candidate genes for asthma. PLoS One 2014;9:e104396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chakraborty S, Dakle P, Sinha A, Vishweswaraiah S, Nagori A, Salimath S, et al. Genetic variations in olfactory receptor gene OR2AG2 in a large multigenerational family with asthma. Sci Rep 2019;9:19029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bogari NM, Amin AA, Rayes HH, Abdelmotelb A, Taher MM, Al-Allaf FA, et al. Next generation exome sequencing of pediatric asthma identifies rare and novel variants in candidate genes. Dis Markers 2021;2021:8884229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Backman JD, Li AH, Marcketta A, Sun D, Mbatchou J, Kessler MD, et al. Exome sequencing and analysis of 454,787 UK Biobank participants. Nature 2021;599: 628–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu J, Deng Y, Yu B, Mo B, Luo L, Yang J, et al. Targeted resequencing showing novel common and rare genetic variants increases the risk of asthma in the Chinese Han population. J Clin Lab Anal 2021;35:e23813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kelsen SG, Agache IO, Soong W, Israel E, Chupp GL, Cheung DS, et al. Astegolimab (anti-ST2) efficacy and safety in adults with severe asthma: a randomized clinical trial. J Allergy Clin Immunol 2021;148:790–8. [DOI] [PubMed] [Google Scholar]
- 19.Donkel SJ, Portilla Fernández E, Ahmad S, Rivadeneira F, van Rooij FJA, Ikram MA, et al. Common and rare variants genetic association analysis of circulating neutrophil extracellular traps. Front Immunol 2021;12:615527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamada M, Motoike IN, Kojima K, Fuse N, Hozawa A, Kuriyama S, et al. Genetic loci for lung function in Japanese adults with adjustment for exhaled nitric oxide levels as airway inflammation indicator. Commun Biol 2021;4:1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Drake KA, Torgerson DG, Gignoux CR, Galanter JM, Roth LA, Huntsman S, et al. A genome-wide association study of bronchodilator response in Latinos implicates rare variants. J Allergy Clin Immunol 2014;133:370–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mousas A, Ntritsos G, Chen MH, Song C, Huffman JE, Tzoulaki I, et al. Rare coding variants pinpoint genes that control human hematological traits. PLoS Genet 2017;13:e1006925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang T, Jackson VE, Smith AV, Chen H, Bartz TM, Sitlani CM, et al. Rare and low-frequency exonic variants and gene-by-smoking interactions in pulmonary function. Sci Rep 2021;11:19365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ortega VE, Li X, O’Neal WK, Lackey L, Ampleford E, Hawkins GA, et al. The effects of rare SERPINA1 variants on lung function and emphysema in SPIRO-MICS. Am J Respir Crit Care Med 2020;201:540–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee EY, Mak ACY, Hu D, Sajuthi S, White MJ, Keys KL, et al. Whole-genome sequencing identifies novel functional loci associated with lung function in Puerto Rican youth. Am J Respir Crit Care Med 2020;202:962–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pongracic JA, Krouse RZ, Babineau DC, Zoratti EM, Cohen RT, Wood RA, et al. Distinguishing characteristics of difficult-to-control asthma in inner-city children and adolescents. J Allergy Clin Immunol 2016;138:1030–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zoratti EM, Krouse RZ, Babineau DC, Pongracic JA, O’Connor GT, Wood RA, et al. Asthma phenotypes in inner-city children. J Allergy Clin Immunol 2016; 138:1016–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gern JE, Visness CM, Gergen PJ, Wood RA, Bloomberg GR, O’Connor GT, et al. The Urban Environment and Childhood Asthma (URECA) birth cohort study: design, methods, and study population. BMC Pulm Med 2009;9:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gergen PJ, Teach SJ, Togias A, Busse WW. Reducing exacerbations in the inner city: lessons from the Inner-City Asthma Consortium (ICAC). J Allergy Clin Immunol Pract 2016;4:22–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O’Connor GT, Lynch SV, Bloomberg GR, Kattan M, Wood RA, Gergen PJ, et al. Early-life home environment and risk of asthma among inner-city children. J Allergy Clin Immunol 2018;141:1468–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Recommendations for standardized procedures for the on-line and off-line measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide in adults and children-1999. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. Am J Respir Crit Care Med 1999;160:2104–17. [DOI] [PubMed] [Google Scholar]
- 32.Dapas M, Thompson EE, Wentworth-Sheilds W, Clay S, Visness CM, Calatroni A, et al. Multi-omic association study identifies DNA methylation-mediated genotype and smoking exposure effects on lung function in children living in urban settings. PLoS Genet 2023;19:e1010594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Munafò MR, Tilling K, Taylor AE, Evans DM, Smith GD. Collider scope: when selection bias can substantially influence observed associations. Int J Epidemiol 2018;47:226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reich D, Nalls MA, Kao WHL, Akylbekova EL, Tandon A, Patterson N, et al. Reduced neutrophil count in people of African descent is due to a regulatory variant in the duffy antigen receptor for chemokines gene. PLOS Genet 2009;5: e1000360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Auer PL, Reiner AP, Leal SM. The effect of phenotypic outliers and non-normality on rare-variant association testing. Eur J Hum Genet 2016;24:1188–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 2009;25:1754–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van der Auwera GA, Carneiro MO, Hartl C, Poplin R, del Angel G, Levy-Moon-shine A, et al. From fastQ data to high-confidence variant calls: the genome analysis toolkit best practices pipeline. Curr Protoc Bioinforma 2013;43:11.10. 1–11.10.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jun G, Flickinger M, Hetrick KN, Romm JM, Doheny KF, Abecasis GR, et al. Detecting and estimating contamination of human DNA samples in sequencing and array-based genotype data. Am J Hum Genet 2012;91:839–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li X, Li Z, Zhou H, Gaynor SM, Liu Y, Chen H, et al. Dynamic incorporation of multiple in silico functional annotations empowers rare variant association analysis of large whole-genome sequencing studies at scale. Nat Genet 2020;35:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morgenthaler S, Thilly WG. A strategy to discover genes that carry multi-allelic or mono-allelic risk for common diseases: a cohort allelic sums test (CAST). Mutat Res 2007;615:28–56. [DOI] [PubMed] [Google Scholar]
- 41.Madsen BE, Browning SR. A groupwise association test for rare mutations using a weighted sum statistic. PLoS Genet 2009;5:e1000384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morris AP, Zeggini E. An evaluation of statistical approaches to rare variant analysis in genetic association studies. Genet Epidemiol 2010;34:188–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li B, Leal SM. Methods for detecting associations with rare variants for common diseases: application to analysis of sequence data. Am J Hum Genet 2008;83: 311–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu MC, Lee S, Cai T, Li Y, Boehnke M, Lin X. Rare-variant association testing for sequencing data with the sequence kernel association test. Am J Hum Genet 2011; 89:82–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu Y, Chen S, Li Z, Morrison AC, Boerwinkle E, Lin X. ACAT: a fast and powerful p value combination method for rare-variant analysis in sequencing studies. Am J Hum Genet 2019;104:410–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pividori M, Rajagopal PS, Barbeira A, Liang Y, Melia O, Bastarache L, et al. PhenomeXcan: mapping the genome to the phenome through the transcriptome. Sci Adv 2020;6:eaba2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bycroft C, Freeman C, Petkova D, Band G, Elliott LT, Sharp K, et al. The UK Biobank resource with deep phenotyping and genomic data. Nature 2018;562:203–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bult CJ, Blake JA, Smith CL, Kadin JA, Richardson JE, Anagnostopoulos A, et al. Mouse Genome Database (MGD) 2019. Nucleic Acids Res 2019;47:D801–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roadmap Epigenomics Consortium, Kundaje A, Meuleman W, Ernst J, Bilenky M, Yen A, et al. Integrative analysis of 111 reference human epigenomes. Nature 2015;518:317–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bacharier LB, Beigelman A, Calatroni A, Jackson DJ, Gergen PJ, O’Connor GT, et al. Longitudinal phenotypes of respiratory health in a high-risk urban birth cohort. Am J Respir Crit Care Med 2019;199:71–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alladina J, Smith NP, Kooistra T, Slowikowski K, Kernin IJ, Deguine J, et al. A human model of asthma exacerbation reveals transcriptional programs and cell circuits specific to allergic asthma. Sci Immunol 2023;8:eabq6352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hoekstra M, Ren B, Laurila PP, Hildebrand RB, Soronen J, Frodermann V, et al. Hematopoietic upstream stimulating factor 1 deficiency is associated with increased atherosclerosis susceptibility in LDL receptor knockout mice. Sci Rep 2021;11:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhao H, Yan M, Wang H, Erickson S, Grewal IS, Dixit VM. Impaired c-Jun amino terminal kinase activity and T cell differentiation in death receptor 6-deficient mice. J Exp Med 2001;194:1441–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Deladeriere A, Gambardella L, Pan D, Anderson KE, Hawkins PT, Stephens LR. The regulatory subunits of PI3Kg control distinct neutrophil responses. Sci Signal 2015;8:ra8. [DOI] [PubMed] [Google Scholar]
- 55.Ma M, Ru Y, Chuang LS, Hsu NY, Shi LS, Hakenberg J, et al. Disease-associated variants in different categories of disease located in distinct regulatory elements. BMC Genomics 2015;16:S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Boyle EA, Li YI, Pritchard JK. An expanded view of complex traits: from polygenic to omnigenic. Cell 2017;169:1177–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang F, Lupski JR. Non-coding genetic variants in human disease. Hum Mol Genet 2015;24:R102–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.National Library of Medicine, USF1 upstream transcription factor 1 [Homo sapiens (human)]. [cited 2022 Jul 1]. Available at: https://www.ncbi.nlm.nih.gov/gene/7391. [Google Scholar]
- 59.Ruuth M, Soronen J, Kaiharju E, Merikanto K, Perttil€a J, Metso J, et al. USF1 deficiency alleviates inflammation, enhances cholesterol efflux and prevents cholesterol accumulation in macrophages. Lipids Health Dis 2018;17:285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fan YM, Hernesniemi J, Oksala N, Levula M, Raitoharju E, Collings A, et al. Upstream transcription factor 1 (USF1) allelic variants regulate lipoprotein metabolism in women and USF1 expression in atherosclerotic plaque. Sci Rep 2014;4:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kristiansson K, Ilveskoski E, Lehtim€aki T, Peltonen L, Perola M, Karhunen PJ. Association analysis of allelic variants of USF1 in coronary atherosclerosis. Arterioscler Thromb Vasc Biol 2008;28:983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Reiner AP, Carlson CS, Jenny NS, Durda JP, Siscovick DS, Nickerson DA, et al. USF1 gene variants, cardiovascular risk, and mortality in European Americans: analysis of two US cohort studies. Arterioscler Thromb Vasc Biol 2007;27: 2736–42. [DOI] [PubMed] [Google Scholar]
- 63.Wojcik GL, Graff M, Nishimura KK, Tao R, Haessler J, Gignoux CR, et al. Genetic analyses of diverse populations improves discovery for complex traits. Nature 2019;570:514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Astle WJ, Elding H, Jiang T, Allen D, Ruklisa D, Mann AL, et al. The allelic landscape of human blood cell trait variation and links to common complex disease. Cell 2016;167:1415–29.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Popa SC, Shin JA. The intrinsically disordered loop in the USF1 bHLHZ domain modulates its DNA-binding sequence specificity in hereditary asthma. J Phys Chem B 2019;123:9862–71. [DOI] [PubMed] [Google Scholar]
- 66.Shrine N, Portelli MA, John C, Soler Artigas M, Bennett N, Hall R, et al. Moderate-to-severe asthma in individuals of European ancestry: a genome-wide association study. Lancet Respir Med 2019;7:20–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schedel M, Michel S, Gaertner VD, Toncheva AA, Depner M, Binia A, et al. Polymorphisms related to ORMDL3 are associated with asthma susceptibility, alterations in transcriptional regulation of ORMDL3, and changes in TH2 cytokine levels. J Allergy Clin Immunol 2015;136:893–903.e14. [DOI] [PubMed] [Google Scholar]
- 68.National Library of Medicine, PIK3R6 phosphoinositide-3-kinase regulatory subunit 6 [Homo sapiens (human)]. [cited 2022 Jul 1]. Available at: https://www.ncbi.nlm.nih.gov/gene/146850. [Google Scholar]
- 69.Yun JH, Chase R, Parker MM, Saferali A, Castaldi PJ, Silverman EK, et al. Peripheral blood gene expression signatures of eosinophilic chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol 2019;61:398–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Elena-Pérez S, Heredero-Jung DH, García-Sánchez A, Estravís M, Martin MJ, Ramos-González J, et al. Molecular analysis of IL-5 receptor subunit alpha as a possible pharmacogenetic biomarker in asthma. Front Med 2021;7:1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jiang Y, Gruzieva O, Wang T, Forno E, Boutaoui N, Sun T, et al. Transcriptomics of atopy and atopic asthma in white blood cells from children and adolescents. Eur Respir J 2019;53:1900102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.National Library of Medicine, TNFRSF21 TNF receptor superfamily member 21 [Homo sapiens (human)]. [cited 2022 Jul 1]. Available at: https://www.ncbi.nlm.nih.gov/gene/27242. [Google Scholar]
- 73.Pan G, Bauer JH, Haridas V, Wang S, Liu D, Yu G, et al. Identification and functional characterization of DR6, a novel death domain-containing TNF receptor. FEBS Lett 1998;431:351–6. [DOI] [PubMed] [Google Scholar]
- 74.Venkataraman C, Justen K, Zhao J, Galbreath E, Na S. Death receptor-6 regulates the development of pulmonary eosinophilia and airway inflammation in a mouse model of asthma. Immunol Lett 2006;106:42–7. [DOI] [PubMed] [Google Scholar]
- 75.Liu J, Na S, Glasebrook A, Fox N, Solenberg PJ, Zhang Q, et al. Enhanced CD4+ T cell proliferation and Th2 cytokine production in DR6-deficient mice. Immunity 2001;15:23–34. [DOI] [PubMed] [Google Scholar]
- 76.Yang J, Wray NR, Visscher PM. Comparing apples and oranges: equating the power of case-control and quantitative trait association studies. Genet Epidemiol 2009. Available at: www.interscience.wiley.com. Accessed July 30, 2023. [DOI] [PubMed] [Google Scholar]
- 77.Popejoy AB, Fullerton SM. Genomics is failing on diversity. Nature 2016;538: 161–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.