Abstract
Background:
In randomized trials, one primary outcome is typically chosen to evaluate the consequences of an intervention while other important outcomes are relegated to secondary outcomes. This issue is amplified for many obstetric trials in which an intervention may have consequences for both the pregnant person and the child. In contrast, desirability of outcome ranking (DOOR), a paradigm shift for the design and analysis of clinical trials based on patient-centric evaluation, allows multiple outcomes – including from more than one individual – to be considered concurrently.
Objective:
Our aim is to describe DOOR methodology tailored for obstetric trials and apply the methodology to maternal-perinatal paired (dyadic) outcomes in which both individuals may be affected by an intervention but may experience discordant outcomes (e.g., an obstetric intervention may improve perinatal but worsen maternal outcomes).
Study Design:
This secondary analysis applies the DOOR methodology to data from the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network ARRIVE trial. The original analysis showed no statistical difference in the primary (perinatal composite) outcome, but a decreased risk of the secondary outcome of cesarean delivery with elective induction at 39 weeks. In the present DOOR analysis, dyadic outcomes ranging from spontaneous vaginal delivery without severe neonatal complication (most desirable) to cesarean delivery with perinatal death (least desirable) were classified into eight categories ranked by overall desirability by experienced investigators. Distributions of the DOOR were compared by estimating the probability of having a more desirable dyadic outcome with elective induction at 39 weeks of gestation than with expectant management. To account for various perspectives on these outcomes, a complementary analysis, called the partial credit strategy, was used in order to grade outcomes on a 100-point scale and estimate the difference in overall treatment scores between groups using a t-test.
Results:
All 6096 participants from the trial were included. The probability of a better dyadic outcome for a randomly selected patient who was randomized to elective induction was 53% (95% CI: 51 – 54%) implying that elective induction led to a better overall outcome for the dyad when taking multiple outcomes into account concurrently. Furthermore, the DOOR probability of averting cesarean with elective induction was 52% (95% CI: 51 – 53%), which was not at the expense of an operative vaginal delivery or a poorer outcome for the perinate (i.e., survival with a severe neonatal complication or perinatal death). Randomization to elective induction also was advantageous in the majority of the partial credit score scenarios.
Conclusion:
DOOR methodology is a useful tool for obstetric trials as it provides a concurrent view of the effect of an intervention on multiple dyadic outcomes, potentially allowing for better translation of data for decision-making and person-centered care.
Keywords: desirability of outcome ranking, dyadic outcome, induction of labor
INTRODUCTION
Well-designed clinical trials in obstetrics are conducted with the hope that recommendations based on the study findings are subsequently put into practice1–2. An obstetric trial generally evaluates the impact of one treatment or intervention on at least two individuals (i.e. the pregnant person and at least one perinate). Moreover, any given intervention may have beneficial effects on some outcomes but deleterious effects on others. Thus, even when clinical trials in obstetrics are pragmatic, the results may not easily translate to or be useful in decision making since the overall benefit on multiple meaningful outcomes for both the pregnant person and child may not be clear.
Even though outcomes from both the birthing person and the perinate are weighed in clinical decision making, there are no common methodologies by which the patterns of multiple outcomes for both are concurrently considered and translated into a single primary outcome to evaluate the effects of a trial’s intervention. Traditionally, separate analyses to evaluate the effects on each maternal and perinatal outcome are performed3–4. Patients and clinicians then must make qualitative determinations based on these multiple results to determine practice guidelines and clinical decision. However, these separate analyses may lead to difficulty in translating study findings into clinical practice recommendations, particularly if the effect of an intervention on maternal and perinatal outcomes is discordant, such as in the Term Breech trial5. Occasionally, post-hoc quantitative attempts are made to combine these multiple outcomes in benefit-risk analyses with the assumption that such analyses inform the totality of effects. However, this is a suboptimal approach, because summing marginal analyses of each maternal or perinatal outcome does not effectively characterize the total effect of the intervention on the pregnancy. In such an approach, the associations between the exposure of interest and the cumulative nature of the component outcomes of the pregnancy are not captured, and competing risk challenges further impair the interpretation6. Also, sometimes a single “composite” outcome may be used, in which an outcome is considered to occur if any of its component outcomes have occurred; consequently, those with one component outcome are considered equivalent to those with more than one of those component outcomes, even though clinical circumstances of the two (i.e., one component versus more than one component outcome) may be dramatically different.
An alternative approach for the design, monitoring, analysis, and reporting of clinical trials – that is able to take into account not only multiple outcomes but multiple individuals at once and that allows the incorporation of patient preferences regarding different outcomes – is the desirability of outcome ranking (DOOR) approach, which was originally presented by Evans et al.7. The DOOR approach synthesizes the results of multiple outcomes to produce a single probability that a patient will have a better overall outcome when randomized to the treatment versus the control group. Thus, the DOOR probability allows one to directly and intuitively interpret holistic maternal and perinatal evidence, and to more easily translate and communicate data for clinical decision-making.
Although DOOR was originally used to evaluate both the benefits and harms to an individual patient, it can be extended to evaluate the benefits and harms for both the pregnant person and the perinate. In this context, the DOOR probability would represent the probability that the pregnant individual and the perinate (i.e. the dyad) in the treatment group have overall better outcomes than those in the control group. Furthermore, a complementary analysis called partial credit analyses allows the opportunity to examine the robustness of the clinical trial results by incorporating various patient or clinician perspectives on the desirability of the outcomes.
Our objective is to use DOOR methodology and the partial credit strategy in the context of an obstetric trial8 in order to demonstrate its use, the approach to interpretation, and the potential value of its a priori incorporation into future obstetric studies.
MATERIALS AND METHODS
Desirability Of Outcome Ranking (DOOR):
The Desirability Of Outcome Ranking (DOOR) is a paradigm for the design, monitoring, analysis, and reporting of clinical trials based on patient-centric benefit and risk. It involves categorizing responses of all participants with respect to their overall outcome, where the overall outcome is based on each participant’s experience during the course of the clinical trial7. These overall outcomes are mutually exclusive and collectively exhaustive. In obstetric clinical trials, this overall outcome can be defined as the paired maternal-perinatal (dyadic) outcome, which incorporates outcomes from both the pregnant individual and perinate experienced during pregnancy through the postpartum and neonatal periods. A combined maternal-perinatal outcome provides a comprehensive patient-centric description of the trial results, which is particularly useful when maternal and perinatal outcomes are discordant (e.g., cesarean delivery may improve neonatal outcome but worsen maternal outcome). The construction of these dyadic outcomes would typically be performed prior to the analysis of trial results. Deliberation to synthesize outcomes can be achieved using multiple methods such as blinded adjudication committees to provide an overall ranking for each outcome or using the Delphi method9, a structured, iterative procedure in which a group of experts reach a consensus opinion.
Application of the DOOR begins with defining the dyadic outcomes of the DOOR and ranking each dyadic outcome based on desirability. Each dyadic outcome in the clinical trial is categorized into each level of the DOOR. During analyses, all possible pairwise comparisons of the dyadic outcomes in the treatment group versus the dyadic outcomes in the control group are made. For each comparison, the dyadic outcome on treatment is either more desirable, less desirable, or has a tied desirability. The analysis consists of estimating the DOOR probability, which is the probability (with accompanying confidence intervals) that a dyad assigned to a new treatment has a more desirable overall dyadic outcome than a dyad assigned to a control. To calculate the probability, the total number of pairwise comparisons that were more desirable plus half of the tied comparisons (numerator) is divided by the total number of possible pairwise comparisons (denominator). A DOOR probability of 50% resembles flipping a fair coin and implies no treatment difference in the desirability of the dyadic outcomes (confidence interval includes 50%). If there is a difference and the treatment provides an advantage with respect to the outcomes, then the probability will be greater than 50% (and the lower bound of the confidence interval will be greater than 50%). A higher DOOR probability means that there is a greater likelihood that the treatment leads to a better overall dyadic outcome than the control. The confidence interval for the DOOR probability is calculated based on the method presented by Halperin et al.10. The p-value is from a Wilcoxon-Mann-Whitney U test using a normal approximation without continuity correction.
Partial Credit:
To aide in clinical decision-making and tailoring management, the partial credit strategy is used to evaluate the overall robustness of the trial results by incorporating various perspectives on the importance and severity of outcomes6. While DOOR evaluates a treatment based on ranked dyadic outcomes, the partial credit strategy evaluates the treatment based on dyadic outcomes graded as a continuous measure. This strategy involves scoring or ‘grading’ each ordinal level of the DOOR from zero (least desirable) to 100 (most desirable). For example, suppose that the overall dyadic outcomes of the DOOR are the following:
No adverse event (AE) for either the birthing person or perinate (most desirable)
No AE for the birthing person, but an AE for the perinate
An AE for the birthing person, but not for the perinate
AEs for both the birthing person and perinate (least desirable)
In this simple example, there are four dyadic outcomes where the most and least desirable dyadic outcomes would be assigned the highest and lowest grades, respectively. With the partial credit strategy, credits (ranging from zero to 100) could be assigned to the two overall dyadic outcomes with discordant maternal-perinatal outcomes (#2 and #3). Assigning a partial credit grade of 100 would mean that the dyadic outcome is as desirable as the most desirable dyadic outcome (i.e., no AE for either the birthing person or perinate). Assigning a partial credit grade of zero would mean that the overall dyadic outcome was as desirable as the least desirable outcome (i.e., both the birthing person and perinate experienced AEs).
These grades are then used to calculate the average grade for each treatment group (ranging from zero to 100), which is a continuous measure of the overall desirability for the treatment (i.e., a higher grade means treatment leads to a more desirable outcome). The difference in grades between groups is then evaluated using Welch’s t-test. Given a set of partial credit grades, if the treatment has no advantage with respect to the outcomes, then the grade difference will be near zero. If the treatment has an advantage with respect to the outcomes, then the difference will be greater than zero.
Assessing the robustness of the trial’s results is the evaluation of all treatment grade differences for all possible combinations of partial credit grades (perspectives). This determines the level of favorability of the treatment relative to all possible perspectives on these outcomes.
Illustration and Application to the ARRIVE Trial:
We illustrate the DOOR methodology using results from the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units (MFMU) Network ARRIVE trial (A Randomized Trial of Induction Versus Expectant Management). This was a randomized, controlled, parallel-group trial to test the hypothesis that elective induction of labor (IOL) at 39 weeks’ gestation among low-risk nulliparous individuals with a singleton gestation would result in a lower risk of a composite outcome of perinatal death or severe neonatal complications compared with expectant management (EM). A complete description of the study design, methodology, and results has been previously published8. The original analysis concluded no statistical difference in the frequency of the perinatal composite outcome but a decrease in the frequency of cesarean delivery (the major secondary outcome of the trial) with planned elective IOL at 39 weeks’ gestation. Eight dyadic outcomes from the ARRIVE trial were considered (listed from most to least desirable; Table 1):
Table 1.
DOOR distribution and outcomes by treatment group from the ARRIVE trial
| Maternal-Perinatal Paired Outcomes (listed from most desirable to least desirable) | Induction of Labor (n=3059) | Expectant Management (n=3037) | Loss (−) or Gain (+) per 1000 dyads† | ||
|---|---|---|---|---|---|
| Maternal | Perinatal | Difference | Cumulative Difference | ||
| Spontaneous vaginal delivery | No perinatal event | 2188 (71.5) | 2020 (66.5) | +50 | +50 |
| Operative vaginal delivery | No perinatal event | 210 (6.9) | 237 (7.8) | −9 | +41 |
| Cesarean delivery | No perinatal event | 529 (17.3) | 616 (20.3) | −30 | +11 |
| Spontaneous vaginal delivery | Survived with severe neonatal complication(s)* | 79 (2.6) | 84 (2.8) | −2 | +9 |
| Operative vaginal delivery | Survived with severe neonatal complication(s)* | 11 (0.4) | 21 (0.7) | −3 | +6 |
| Cesarean delivery | Survived with severe neonatal complication(s)* | 40 (1.3) | 56 (1.8) | −5 | 0 |
| Vaginal delivery‡ | Perinatal death | 2 (0.1) | 1 (0.03) | 0 | +1 |
| Cesarean delivery | Perinatal death | 0 | 2 (0.1) | −1 | 0 |
Data presented as n (%), unless otherwise noted.
One or more of the following during the antepartum or intrapartum period or during the delivery hospitalization: respiratory support within 72 hours after birth, 5-minute Apgar score ≤ 3, hypoxic–ischemic encephalopathy, seizure, infection (confirmed sepsis or pneumonia), meconium aspiration syndrome, birth trauma (bone fracture, neurologic injury, or retinal hemorrhage), intracranial or subgaleal hemorrhage, or hypotension requiring vasopressor support.
Expected loss (−) or gain (+) is the percent difference in induction vs. expectant management per 1000 participants.
Spontaneous and operative vaginal delivery combined with perinatal death due to low frequencies.
Spontaneous vaginal delivery without severe neonatal complication (most desirable)
Operative vaginal delivery without severe neonatal complication
Cesarean delivery without severe neonatal complication
Spontaneous vaginal delivery and neonate survived with severe neonatal complication(s)
Operative vaginal delivery and neonate survived with severe neonatal complication(s)
Cesarean delivery and neonate survived with severe neonatal complication(s)
Vaginal delivery with perinatal death
Cesarean delivery with perinatal death (least desirable)
DOOR analyses were then applied as previously described above using these dyadic outcomes. In addition, the partial credit strategy was applied to demonstrate the robustness of the clinical trial results. To ease illustration of the partial credit strategy, levels of the DOOR were combined such that four dyadic outcomes with two partial credit dyadic outcomes are considered (#2 and #3; Table 2):
Table 2.
Partial credit grading for DOOR outcome from the ARRIVE trial
| DOOR | Partial Credit Grading Key | |
|---|---|---|
| MFMU Network* | Individual Provided Grade | |
| Vaginal delivery with no perinatal event | 100 | 100 |
| Cesarean with no perinatal event | 84 | X1 |
| Any event with severe neonatal complication | 42 | X2 |
| Any event involving perinatal death | 0 | 0 |
Partial credit grading for dyadic outcomes ranged from 0 (least desirable) to 100 (most desirable).
X1, partial credit grade for cesarean delivery with no perinatal event; X2, any mode of delivery with severe neonatal complication.
Average grades surveyed from the principal investigators (n=14) and nurse coordinators (n=12) of the MFMU Network.
Vaginal delivery with no perinatal event (score 100)
Cesarean delivery with no perinatal event (score X1)
Any mode of delivery with severe neonatal complication (score X2)
Any mode of delivery involving perinatal death (score 0)
In this example, X1 and X2 are the partial credit grades with X1 ≥ X2. Applying different values for X1 and X2 corresponds to evaluating various perspectives on these outcomes. To examine the robustness of the clinical trial results for all possible perspectives on these outcomes, treatment grade differences for combinations of values for X1 ≥ X2 were evaluated. To illustrate the MFMU’s perspective on these dyadic outcomes, we surveyed the principal investigators (n=14) and nurse coordinators (n=12) of the MFMU Network. Each investigator or coordinator was asked to grade each possible dyadic outcome. Then, X1 and X2 was calculated as the average grade for that particular dyadic outcome. Using these values for X1 and X2, the grade for each treatment was determined and treatment grades were compared.
Analyses were performed using the “DOOR Analyses: Standard Edition” online R shiny application (https://methods.bsc.gwu.edu/), which calculates the DOOR probability and confidence intervals, and performs the partial credit analyses based on user input. Frequencies for each dyadic outcome (input for the online application) were obtained using SAS statistical software (version 9.4). All tests were two-sided and p-values less than 0.05 were considered statistically significant.
RESULTS
All 6096 participants from the ARRIVE trial were included in this secondary analysis. A summary of the DOOR distribution of the maternal-perinatal paired (dyadic) outcomes for each treatment group are displayed in Table 1. With respect to these ranked dyadic outcomes, IOL had a cumulative positive number of dyads who experienced more desirable outcomes than those in the EM group. There were more dyads who had a spontaneous vaginal delivery without a perinatal event (most desirable outcome) among those who underwent IOL compared with EM (71.5% vs. 66.5%; Risk Difference (RD): 50 per 1000 participants, 95% CI: 27–73 per 1000 participants). This suggests that IOL was associated with a benefit towards more desirable outcomes and that participants who underwent IOL likely had a desirable dyadic outcome for the pregnancy.
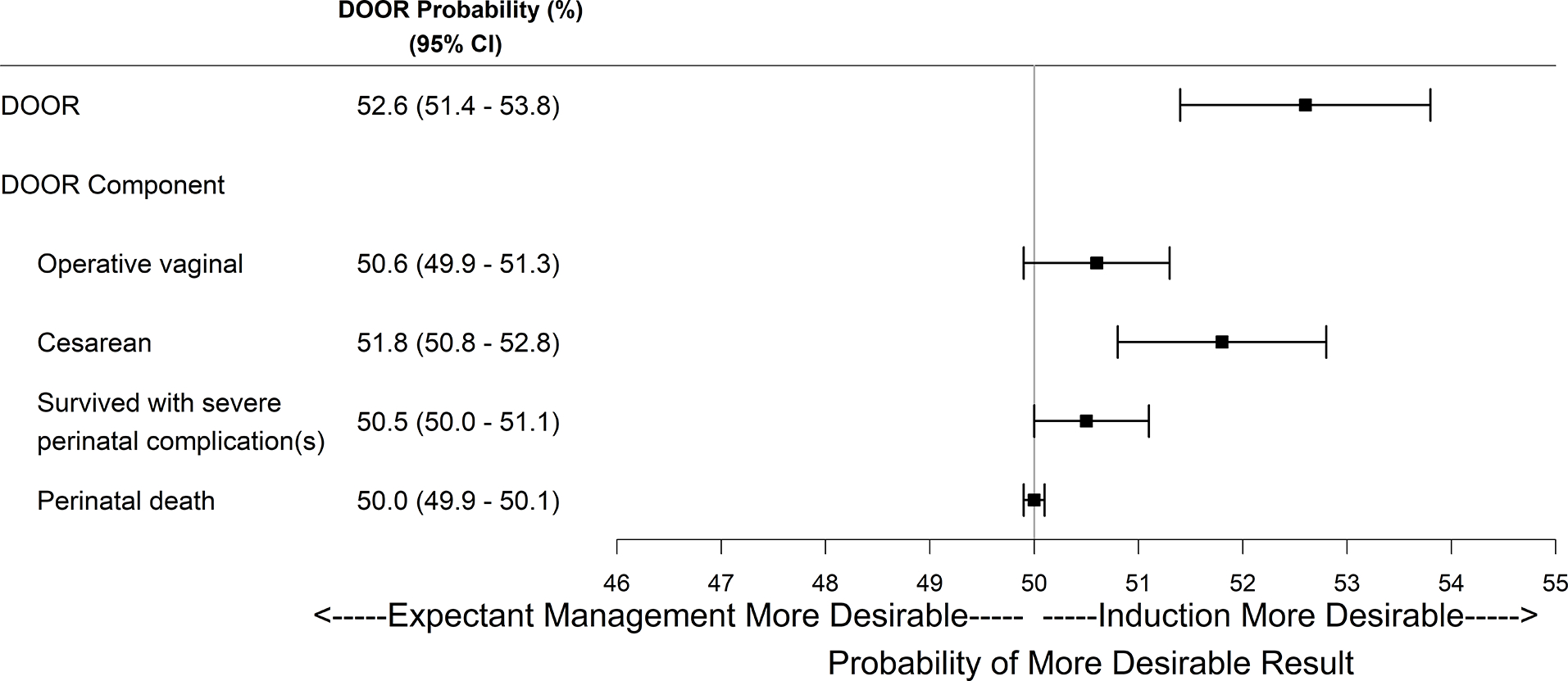
The DOOR probability for a randomly selected participant who underwent IOL versus EM was 52.6% (95% CI 51.4 – 53.8%), which implies that undergoing IOL led to a better overall outcome. The DOOR probability of averting cesarean with IOL was 51.8% (95% CI 50.8 – 52.8%), which is not at the expense of a greater risk of operative vaginal delivery (50.6%, 95% CI: 49.9 – 51.3%) or a poorer outcome for the perinate (survived with severe neonatal complication: 50.5%, 95% CI: 50.0 – 51.1%; perinatal death: 50.0%, 95% CI: 49.9 – 50.1%) (Figure 1). In summary, these results (with 95% confidence) indicate that those randomized to induction had better overall outcomes without incurring any individual less preferred outcome such as operative vaginal delivery or poor perinatal outcome.
Figure 1. DOOR probability forest plot for induction versus expectant management from the ARRIVE trial.
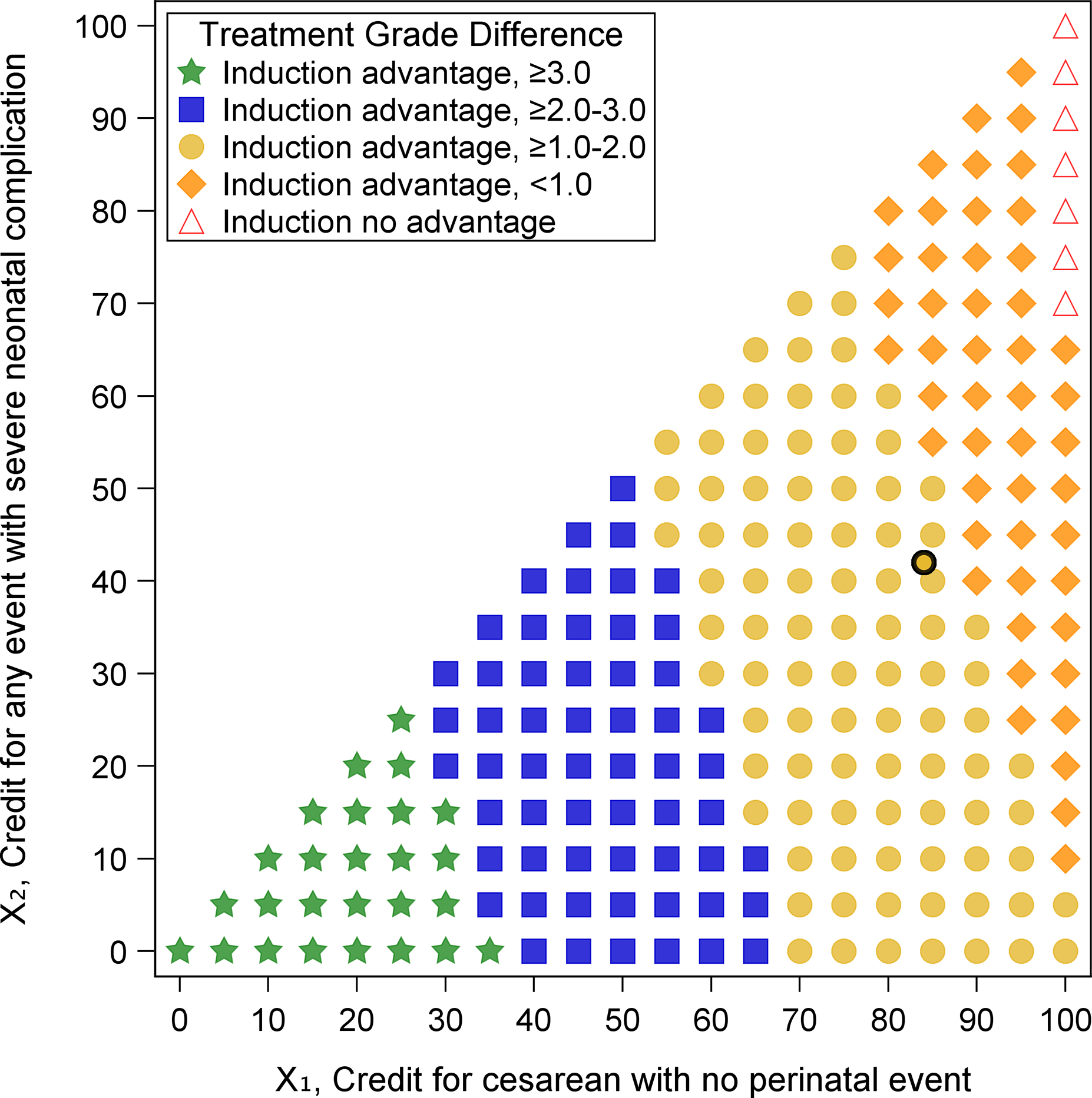
Figure 2 depicts score differences between treatment groups for all pairs of partial credit values of cesarean delivery with no perinatal event (X1) and any mode of delivery with severe neonatal complication (X2), where X1 ≥ X2. For each pair of values (X1, X2), the score difference was evaluated. IOL was considered as having an advantage towards more desirable outcomes when the grade difference was greater than zero (statistical significance depicted as orange diamonds (difference greater than zero to less than 1.0), yellow circles (difference at least 1.0 to less than 2.0), blue squares (difference at least 2.0 to less than 3.0), green stars (difference at least 3.0), and no statistical significance depicted as red triangles in Figure 2). Those in the IOL group had a higher grade (more desirable) than those in the EM group for nearly all pairs of values (grade difference range: 0.24 to 4.07 on the 100-point scale; all p<0.05) which suggests that the results from the ARRIVE trial are relatively robust to all possible perspectives on these outcomes. IOL had no advantage when X1=100 and X2≥70; an unlikely perspective scenario in which an individual simultaneously views a cesarean delivery without a perinatal event as the most desirable outcome (X1=100) and delivering a perinate with severe neonatal complication(s) as a fair outcome (X2≥70). Grades surveyed from MFMU investigators and coordinators determined average partial credit grades for these dyadic outcomes of X1=84 and X2=42 (depicted as a black circle in Figure 2). Thus, from a survey of obstetricians and research nurses, undergoing IOL yields more favorable outcomes than EM (grade difference: 1.1, 95% CI: 0.4 – 1.8 on a 100-point scale) (Figure 2). A comprehensive set of DOOR results for the ARRIVE trial are provided in the Supplementary Materials.
Figure 2. Treatment effects for various partial credit grades from the ARRIVE trial.
IOL, Induction of Labor; EM, Expectant Management.
Difference = Grade for IOL – Grade for EM.
The difference in treatment grades was evaluated using a t-test for each pair of values (X1, X2).
Induction advantage means that the grades for IOL and EM were significantly different (green stars, blue squares, yellow circles, orange diamonds).
Induction no advantage means that the grades for IOL and EM were not significantly different (red triangles).
Black circle corresponds to the average grades surveyed from the principal investigators and nurse coordinators of the MFMU Network (X1=84, X2=42)
COMMENT
Principal Findings:
In this secondary analysis, DOOR methodology was applied to existing data from the ARRIVE trial. The analysis revealed that those randomized to induction of labor at 39 weeks were significantly more likely to have a better outcome overall, when both maternal and perinatal outcomes were considered concurrently. This example illustrates how, in the obstetrics context, DOOR provides for a comprehensive assessment of pregnancy interventions as it is able to incorporate in a single outcome whether there is an overall difference in multiple maternal and perinatal outcomes related to an intervention. Partial credit analyses, which can be performed as part of the DOOR analysis, further help with translation of trial results to clinical value, as it provides insight into the robustness of a trial’s results and can incorporate patient or clinician input into the meaningfulness of and preference for different outcomes.
Results in the Context of What is Known:
The original results from the ARRIVE trial and these current analyses fundamentally agree; however, the conclusions from DOOR may provide a more translatable interpretation of these study results to allow better person-centered decision making and clinical guidance with regard to best practice.
Clinical Implications:
While DOOR methodology may provide additional insight into the results of clinical trials, the ARRIVE trial illustrates particularly well how it may ease clinical translation. In ARRIVE, although there was no statistically significant difference in the primary outcome (i.e., a composite neonatal outcome), there was a significant reduction for some individual neonatal outcomes, as well as in the cesarean delivery and preeclampsia rates among those randomized to the elective induction of labor (IOL) group. Consequently, some professional organizations recommended offering patients elective IOL at 39 weeks’ gestation. However, the statistical nuances of the study’s primary outcome, and the subtleties of counseling patients regarding benefits with respect to other health outcomes that the intervention of labor induction affects, have made translation and implementation of the results more challenging11. In this manuscript, we demonstrate how the DOOR analysis can be used as an approach to overcome these limitations: compared to expectant management, elective IOL at 39 weeks’ gestation is more likely to achieve desirable overall outcomes for both the pregnant patient and their baby. The interpretation of the results from these DOOR analyses are likely to be more intuitive to patients than counseling patients based on a series of individual results that does not account for how those results are associated with one another with respect to individual participants within the study.
Research Implications:
Future trials in obstetrics with multiple outcomes that have relevance to decision making, and in particular when there is the potential for discordant maternal and perinatal outcomes, may benefit from the DOOR method in the trial design where DOOR outcomes can be defined as the primary endpoint, or alternatively, as an a priori planned sensitivity analysis. Incorporating DOOR into the trial design could include both provider ranking as well as patient ranking of outcomes. The trial can then be planned with a sample size based upon a superiority test to evaluate whether the DOOR probability is greater than 50% (i.e., the threshold for significance) in one group versus the other. In defining DOOR outcomes, continuous outcomes (e.g., length of stay) may also be used, which would provide more granular levels of dyadic responses. DOOR outcomes do not require combining maternal and perinatal outcomes, but if appropriate could be limited to outcomes exclusively from the pregnant person or perinate. Lastly, DOOR methodology in conjunction with inverse probability of treatment weighting (ITPW) methods to address confounding can be applied for treatment comparison analyses in observational obstetric studies.
Strengths and Limitations:
A strength of the DOOR approach is that it allows insight into the effect of an intervention on multiple outcomes among more than one individual concurrently, and also accounts for the pattern of outcomes among participants (i.e., an individual with two adverse outcomes has a worse overall outcome than an individual with one adverse outcome). A limitation to applying DOOR methodology is the need to rank numerous outcomes for complex studies with complicated outcomes; in this circumstance, forming a predetermined ordinal ranking could be difficult or challenging. A limitation of this secondary analysis is that we did not evaluate patient grades for which the partial credit analyses would be most beneficial. Nevertheless, this analysis was meant to be illustrative, and we incorporated all possible combinations of partial credit grades. In future studies, these analyses can be extended and patient input elicited for a broader perspective.
Conclusions:
A review of risk information in the American College of Obstetricians and Gynecologists (ACOG) Practice Bulletins revealed that most recommendations do not present numerical data in a way that allows optimal communication with and counseling of patients12. Considering that obstetric trials often have multiple outcomes for both a pregnant person and child, which need to be holistically synthesized in order to generate clinical recommendations, and that individual preferences are essential to these recommendations, it is essential to use analytic methods that optimize management and improve the quality of decision making13–15. The DOOR method is an alternative and complementary approach to the analysis and design of obstetric trials that provides a comprehensive assessment and perspective on clinical trial results that may assist in better communication of results from research studies.
Supplementary Material
Condensation:
Desirability of outcome ranking (DOOR) provides an opportunity to more comprehensively and concurrently capture maternal and perinatal outcomes in obstetric clinical trials.
Tweetable Statement:
Desirability of outcome ranking (DOOR) provides an opportunity to more comprehensively and concurrently capture maternal and perinatal outcomes in obstetric clinical trials.
AJOG at a Glance:
A. Why was this study conducted?
Obstetric trial results may not easily translate into knowing the best approach to clinical practice since the overall effect of an intervention on the birthing person and perinate may differ.
Desirability of outcome ranking (DOOR) is a method for the design and analysis of trials that considers multiple maternal and perinatal outcomes concurrently.
B. What are the key findings?
In a secondary analysis of the ARRIVE trial data, DOOR analysis reveals that those randomized to induction of labor at 39 weeks were significantly more likely to have a better outcome overall, when both maternal and perinatal outcomes were considered concurrently.
While the DOOR analysis provides an interpretation that is overall consistent with the original results the conclusions from DOOR may provide more insight, for both patients and clinicians, into the best approach to clinical practice.
C. What does this study add to what is already known?
The DOOR method provides an alternative and complementary approach to the design and analysis of obstetric trials that aides in translating trial results into the best approach to clinical care for patients and clinicians.
ACKNOWLEDGMENTS
The authors thank the ARRIVE trial study personnel Gail Mallett, R.N., M.S., C.C.R.C. and Kim Hill, R.N., B.S.N. for protocol development and coordination between clinical research centers; Lindsay Doherty, Ph.D. for protocol and data management; Elizabeth A. Thom, Ph.D., Robert M. Silver, M.D., and Yasser Y. El-Sayed, M.D. for protocol development and oversight; and Trisha M. Boekhoudt, M.P.H. for coordination of the survey data.
Funding:
Supported by grants (HD40512, U10 HD36801, HD27869, HD34208, HD68268, HD40485, HD40500, HD53097, HD40560, HD40545, HD27915, HD40544, HD34116, HD68282, HD87192, HD68258, HD87230) from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Center for Advancing Translational Sciences (UL1TR001873). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclosure: The authors report no commercial conflicts of interest related to the subject matter of the manuscript.
Poster Presentation Information: The 43rd Annual Pregnancy Meeting, Society for Maternal-Fetal Medicine, February 6–11, 2023, San Francisco, California
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Grecio J. SANDOVAL, George Washington University Biostatistics Center, Washington, DC.
William A. GROBMAN, Departments of Obstetrics and Gynecology of Northwestern University, Chicago, IL.
Scott R. EVANS, George Washington University Biostatistics Center, Washington, DC.
Madeline M. RICE, George Washington University Biostatistics Center, Washington, DC.
Rebecca G. CLIFTON, George Washington University Biostatistics Center, Washington, DC.
Suneet P. CHAUHAN, University of Texas Health Science Center at Houston, Children’s Memorial Hermann Hospital, Houston, TX.
Maged M. COSTANTINE, Ohio State University, Columbus, OH.
Kelly S. GIBSON, MetroHealth Medical Center, Case Western Reserve University, Cleveland, OH.
Monica LONGO, Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, MD.
Torri D. METZ, University of Utah Health, Salt Lake City, UT.
Emily S. MILLER, Departments of Obstetrics and Gynecology of Northwestern University, Chicago, IL.
Samuel PARRY, University of Pennsylvania, Philadelphia, PA.
Uma M. REDDY, Columbia University, New York, NY.
Dwight J. ROUSE, Brown University, Providence, RI.
Hyagriv N. SIMHAN, University of Pittsburgh, Pittsburgh, PA.
John M. THORP, Jr, University of North Carolina at Chapel Hill, Chapel Hill, NC.
Alan T.N. TITA, University of Alabama at Birmingham, Birmingham, AL.
George R. SAADE, University of Texas Medical Branch, Galveston, TX.
REFERENCES
- 1.Mol BW, Ruifrok AE. Global alignment, coordination and collaboration in perinatal research: the Global Obstetrics Network (GONet) Initiative. Am J Perinatol. 2013. Mar;30(3):163–6. 10.1055/s-0032-1322512. [DOI] [PubMed] [Google Scholar]
- 2.Patsopoulos NA. A pragmatic view on pragmatic trials. Dialogues Clin Neurosci. 2011;13(2):217–24. 10.31887/dcns.2011.13.2/npatsopoulos. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thom EA, Rouse DJ. What we have learned about conducting randomized controlled trials in the NICHD MFMU network. Semin Perinatol. 2003. Jun;27(3):253–60. 10.1016/s0146-0005(03)00023-5. [DOI] [PubMed] [Google Scholar]
- 4.Thom EA, Rice MM, Saade GR, Reddy UM. What we have learned about the design of randomized trials in pregnancy. Semin Perinatol. 2016. Aug;40(5):328–34. 10.1053/j.semperi.2016.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hannah ME, Hannah WJ, Hewson SA, Hodnett ED, Saigal S, Willan AR. Planned caesarean section versus planned vaginal birth for breech presentation at term: a randomised multicentre trial. Lancet. 2000. Oct 1;356(9239):1375–83. 10.1016/s0140-6736(00)02840-3. [DOI] [PubMed] [Google Scholar]
- 6.Evans SR, Follmann D. Using outcomes to analyze patients rather than patients to analyze outcomes: a step toward pragmatism in benefit: risk evaluation. Stat Biopharm Res. 2016. Oct 1;8(4):386–93. 10.1080/19466315.2016.1207561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Evans SR, Rubin D, Follmann D, Pennello G, Huskins WC, Powers JH, Schoenfeld D, Chuang-Stein C, Cosgrove SE, Fowler VG Jr, Lautenbach E. Desirability of outcome ranking (DOOR) and response adjusted for duration of antibiotic risk (RADAR). Clin Infect Dis. 2015. Sep 1;61(5):800–6. 10.1093/cid/civ495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grobman WA, Rice MM, Reddy UM, Tita AT, Silver RM, Mallett G, Hill K, Thom EA, El-Sayed YY, Perez-Delboy A, Rouse DJ, et al. Labor induction versus expectant management in low-risk nulliparous women. N Engl J Med. 2018. Aug 9;379(6):513–23. 10.1056/nejmoa1800566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sinha IP, Smyth RL, Williamson PR. Using the Delphi technique to determine which outcomes to measure in clinical trials: recommendations for the future based on a systematic review of existing studies. PLoS Med. 2011. Jan 25;8(1):e1000393. 10.1371/journal.pmed.1000393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Halperin M, Hamdy MI, Thall PF. Distribution-free confidence intervals for a parameter of Wilcoxon-Mann-Whitney type for ordered categories and progressive censoring. Biometrics. 1989. Jun;45(2):509–21. [PubMed] [Google Scholar]
- 11.Migliorelli F, De Oliveira SS, de Tejada BM. The ARRIVE Trial: Towards a universal recommendation of induction of labour at 39 weeks?. Eur J Obstet Gynecol Reprod Biol. 2020. Jan 1;244:192–5. 10.1016/j.ejogrb.2019.10.034. [DOI] [PubMed] [Google Scholar]
- 12.Foggin H, Hutcheon JA, Liauw J. Making sense of harms and benefits: Assessing the numeric presentation of risk information in ACOG obstetrical clinical practice guidelines. Patient Educ Couns. 2022. May; 105(5):1216–1223. 10.1016/j.pec.2021.08.030. [DOI] [PubMed] [Google Scholar]
- 13.Downe S, Finlayson K, Oladapo O, Bonet M, Gülmezoglu AM. What matters to women during childbirth: A systematic qualitative review. PLoS One. 2018. Apr 17;13(4): e1094906. 10.1371/journal.pone.0194906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kennedy HP, Cheyney M, Dahlen HG, Downe S, Foureur MJ, Homer CSE, Jefford E, McFadden A, Michel-Schuldt M, Sandall J, Soltani H, Speciale AM, Stevens J, Vedam S, Renfrew MJ. Asking different questions: A call to action for research to improve the quality of care for every woman, every child. Birth. 2018. Sep;45(3): 222–231. 10.1111/birt.12361. [DOI] [PubMed] [Google Scholar]
- 15.Society for Maternal-Fetal Medicine. SMFM statement on elective induction of labor in low-risk nulliparous women at term: the ARRIVE trial. Am J Obstet Gynecol. 2019. Jul;221(1): B2–B4. 10.1016/j.ajog.2018.08.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


