Abstract
Coronaviruses are assembled by budding into a pre-Golgi compartment from which they are transported along the secretory pathway to leave the cell. In cultured epithelial cells, they are released in a polarized fashion; depending on the virus and cell type, they are sorted preferentially either to the apical domain or to the basolateral plasma membrane domain. In this study, we investigated the role of the coronavirus spike protein, because of its prominent position in the virion the prime sorting candidate, in the directionality of virus release. Three independent approaches were taken. (i) The inhibition of N glycosylation by tunicamycin resulted in the synthesis of spikeless virions. The absence of spikes, however, did not influence the polarity in the release of virions. Thus, murine hepatitis virus strain A59 (MHV-A59) was still secreted from the basolateral membranes of mTAL and LMR cells and from the apical sides of MDCKMHVR cells, whereas transmissible gastroenteritis virus (TGEV) was still released from the apical surfaces of LMR cells. (ii) Spikeless virions were also studied by using the MHV-A59 temperature-sensitive mutant Albany 18. When these virions were produced in infected LMR and MDCKMHVR cells at the nonpermissive temperature, they were again preferentially released from basolateral and apical membranes, respectively. (iii) We recently demonstrated that coronavirus-like particles resembling normal virions were assembled and released when the envelope proteins M and E were coexpressed in cells (H. Vennema, G.-J. Godeke, J. W. A. Rossen, W. F. Voorhout, M. C. Horzinek, D.-J. E. Opstelten, and P. J. M. Rottier, EMBO J. 15:2020–2028, 1996). The spikeless particles produced in mTAL cells by using recombinant Semliki Forest viruses to express these two genes of MHV-A59 were specifically released from basolateral membranes, i.e., with the same polarity as that of wild-type MHV-A59. Our results thus consistently demonstrate that the spike protein is not involved in the directional sorting of coronaviruses in epithelial cells. In addition, our observations with tunicamycin show that contrary to the results with some secretory proteins, the N-linked oligosaccharides present on the viral M proteins of coronaviruses such as TGEV also play no role in viral sorting. The implications of these conclusions are discussed.
Coronaviruses are enveloped, positive-strand RNA viruses and cause a wide spectrum of diseases in humans and animals. They have a marked tropism for epithelial cells, resulting most often in enteric and/or respiratory infections, although some of these viruses do spread systemically (16, 25). Transmissible gastroenteritis virus (TGEV), for example, infects intestinal epithelial cells, causing an enteric disease in pigs (7, 30, 31), whereas mouse hepatitis virus strain A59 (MHV-A59) replicates in the upper respiratory mucosa before being disseminated to other organs (reference 5 and references therein).
The plasma membrane of an epithelial cell is divided into an apical domain and a basolateral domain, which are separated by tight junctions; their compositions differ due to selective transport of proteins and lipids. Protein transport in cells is generally signal mediated. Signals for basolateral targeting have been found in the cytoplasmic tails of membrane proteins, but little is known about the sorting signals involved in apical targeting. Some observations suggest that they reside in the luminal domain of the protein; the removal of membrane anchors from apical proteins resulted in their apical secretion (for recent reviews, see references 8, 23, 24, and 26). For some proteins, the presence of N-glycans was found to be an absolute prerequisite for apical delivery (18, 47) (for a review, see reference 9).
The release of many viruses from epithelial cells is restricted to a specific membrane domain (for reviews, see references 4 and 46). This is also the case for coronaviruses, as we have shown recently. TGEV was secreted through the apical surface in studies with porcine epithelial kidney (LLC-PK1) cells (36), whereas MHV-A59 preferentially emerged from the basolateral surfaces of these cells as well as of human colon carcinoma (Caco-2) and murine epithelial kidney (mTAL) cells (35, 37, 38). However, the mouse virus was almost exclusively released from the apical membranes of MDCK cells (37).
For viruses that bud at the plasma membrane, polarized release was found to be a consequence of the directional transport of viral membrane proteins to a specific membrane surface (for reviews, see references 4 and 46). Coronaviruses, however, are assembled at intracellular membranes of the intermediate compartment (19, 20, 44) and are transported in vesicles by the constitutive secretory pathway out of the cell (45). Nothing is known about the mechanisms which underlie the targeted release of intracellularly budding viruses from epithelial cells, but it seems quite likely that viral particles contain sorting signals that direct them into vesicles destined for either the apical or basolateral membrane. Of all the structural elements that constitute a coronavirus particle, the spike (S) protein is the most likely sorting determinant for several reasons. First, it is exposed on the virion, thus presenting itself favorably to the cellular export machinery. Second, it has a large ectodomain that could easily accommodate one or more targeting structures. In contrast, only small parts of the other envelope proteins (M and E) are exposed; in addition, they may be shielded by the bulky S structures. Third, the S protein already has a targeting function in virus entry by binding to the receptor at the cell surface.
In this study, we focused on the possible role of the S protein in viral targeting. In addition, we wanted to establish whether N-linked oligosaccharides present on the S protein of MHV-A59 and on the M and S proteins of TGEV contribute to targeting. By using the antibiotic tunicamycin (TM), an MHV-A59 temperature-sensitive (ts) mutant, and an expression system for the production of coronavirus-like particles, we found that neither the S protein nor N-linked oligosaccharides were required for the polarized release of MHV-A59 and TGEV from epithelial cells.
MATERIALS AND METHODS
Cells, viruses, and antisera.
The preparation of LLC-PK1 (35) and MDCK (11) cells stably expressing the MHV receptor gene, designated LMR and MDCKMHVR cells, respectively, has been reported previously. mTAL cells were maintained as previously described (38). The preparation of polarized cell monolayers on filters (pore size, 0.45 μm; 4.5 cm2) (Transwell inserts; Costar Corp., Cambridge, Mass.) was also described earlier (35, 37, 38). Infections were done with the Purdue strain of TGEV, MHV-A59, and Albany 18, the ts mutant of MHV-A59 (33). The production of rabbit polyclonal antiserum to MHV-A59 has previously been reported (42). Monoclonal antibodies (MAb), J7.6 and J1.3, to the S and M proteins of MHV strain JHM (10), respectively, were kindly provided by John Fleming (Department of Neurology, University of Wisconsin, Madison). Polyclonal antiserum against TGEV was a kind gift of Ines Anton and Luis Enjuanes (Centro Nacional de Biotecnologia, CSIC, Universidad Autónoma, Canto Blanco, Madrid, Spain), and MAb against the TGEV spike protein (995) was kindly provided by Rob Meloen (ID-DLO, Lelystad, The Netherlands).
Construction of recombinant SFVs.
The BamHI fragment of vector pJCE1 (41) containing the MHV-A59 membrane (M) protein gene was cloned into the BamHI site of vector pSFV1 (GIBCO BRL, Life Technologies, Inc.) behind the SP6 promoter, resulting in plasmid pS1mM. Oligonucleotides 469 (5′-GGATTAGATATCATCCACCTCTA-3′; reverse complement of nucleotides 651 to 673 [2]) and 471 (5′-TTAAGGCATTGTCCAGGCATATG-3′; idential to nucleotides 68 to 90 [2]) were used to amplify the cDNA fragment of plasmid pRG68, which contains MHV-A59 gene 5 open reading frames (ORFs) 5a and 5b (1, 48), with the latter encoding the E protein. cDNA was amplified by PCR as previously described (17). The PCR fragment was purified from the gel, blunt ended, phosphorylated, and ligated into pNoTA/T7 (5 Prime→3 Prime, Inc.) according to the manufacturer’s instructions, resulting in plasmid pNoTA/T7m5. To obtain pS1m5, the BamHI fragment of pNoTA/T7m5 containing MHV-A59 ORFs 5a and 5b was ligated into the BamHI site of vector pSFV1. The MHV-A59 ORF 5a-5b segment was also cut out of plasmid pNoTa/T7m5 as a PmeI fragment, which was then cloned into the SmaI site of plasmid pS1mM to obtain plasmid pS1mM5. RNAs were transcribed from pS1mM, pS1m5, pS1mM5, and the pSFV helper plasmid (a kind gift of Peter Bredenbeek, Department of Virology, Leiden University, Leiden, The Netherlands) by in vitro RNA transcription according to the manufacturer’s (Pharmacia) instructions. Subsequently, recombinant Semliki Forest viruses (SFVs) were prepared by coelectroporation of RNAs encoding MHV-A59 proteins and helper RNAs encoding SFV structural proteins into BHK-21 cells by the method of Liljeström and Garoff (21). Recombinant viruses were harvested at 24 h after electroporation and titrated on BHK-21 cells by an indirect immunofluorescence assay.
Virus infections.
Epithelial cells grown on filters were rinsed with prewarmed Dulbecco’s modified Eagle’s medium (DMEM) at 16 h postseeding (p.s.) and inoculated from the apical side with MHV-A59 or TGEV at a multiplicity of infection of 10 or from the basolateral side with recombinant SFVs diluted in DMEM. Infections were done at 37°C, except for infections with MHV Albany 18, which were done at 33°C. In the latter case, cells were incubated at 33°C (permissive temperature) or 39°C (restrictive temperature) after the 1-h inoculation period. Basolateral inoculations were done by placing filters on a 75-μl droplet of inoculum on Parafilm; apical inoculation was achieved by replacing the apical culture medium with 500 μl of inoculum. After 1 h, the inoculum was removed, filters were rinsed three times with DMEM, and cells were further incubated in DMEM containing 10% fetal calf serum. When indicated, TM (Boehringer Mannheim Biochemicals) was added to a final concentration of 0.5 or 2 μg per ml.
Metabolic labeling, immunoprecipitation, and immunoisolation.
Infected epithelial cells, grown on filter supports, were labeled from 4.5 to 7.5 h postinfection (p.i.; LMR cells), from 6 to 9 h p.i. (MDCKMHVR and mTAL cells), or from 8.5 to 11.5 h p.i. (MHV Albany 18 infections) by replacing apical and basolateral media with minimal essential medium lacking methionine, followed by the addition of 200 μCi of l-35S in vitro labeling mix (Amersham) to the basolateral medium and, when indicated, the addition of 0.5 or 2 μg of TM per ml to both media. After the labeling period, apical and basolateral media were harvested and cells were rinsed with ice-cold phosphate-buffered saline and solubilized in 300 μl of TES lysis buffer (20 mM Tris-HCl [pH 7.5], 1 mM EDTA, 100 mM NaCl) containing 1% Triton X-100, 1 μg of aprotinin per ml, 1 μg of pepstatin per ml, and 100 μg of phenylmethylsulfonyl fluoride per ml. Nuclei were removed from cell lysates by centrifugation at 12,000 × g for 10 min at 4°C. For immunoprecipitation of viral proteins a 50-μl aliquot of the lysate was taken and diluted further with 450 μl of TES. To detect virus release, culture media were harvested and cleared by centrifugation for 10 min at 1,500 × g at 4°C. For immunoprecipitation of viral proteins, 0.25 volume of a 5×-concentrated stock solution of lysis buffer was added to supernatants, followed by 10 μl of anti-MHV serum or 5 μl of anti-TGEV serum, and samples were incubated overnight at 4°C. Immune complexes were adsorbed to formalin-fixed Staphylococcus aureus cells (Pansorbin; Calbiochem) with 75 μl of a 10% (wt/vol) suspension. After a 30-min incubation period at 4°C, the immune complexes were collected by centrifugation at 12,000 × g and washed three times with radioimmunoprecipitation assay buffer (20 mM Tris-HCl [pH 7.5], 150 mM NaCl, 5 mM EDTA, 1% Triton X-100, 0.1% sodium dodecyl sulfate [SDS], and 1% deoxycholate) and once with TES. The final pellets were resuspended in 30 or 60 μl of Laemmli sample buffer (62.5 mM Tris-HCl [pH 6.8], 2% SDS, 10% glycerol, and 5% mercaptoethanol), incubated for 10 min at room temperature, and heated for 2 min at 95°C. Samples were analyzed by electrophoresis on an SDS–10% polyacrylamide gel. TGEV and MHV-A59 particle release into the medium was also analyzed by immunoisolation. The procedure used was similar to the immunoprecipitation procedure except that MAb 995 (10 μl of a diluted [1:100] stock solution), anti-TGEV serum (5 μl), anti-MHV serum (10 μl), MAb J7.6 (20 μl), or MAb J1.3 (20 μl) was added directly to the cleared medium and that all washes of bacteria were done with TES.
RESULTS
Effect of TM on TGEV and MHV-A59 release from LMR cells.
TM affects N-glycosylation in a concentration-dependent manner which varies in different cell types. Initial experiments showed that the use of TM at a concentration of 2 (LMR and mTAL cells) or 0.5 (MDCKMHVR cells) μg per ml was sufficient to completely block the N-glycosylation of MHV S and TGEV S and M proteins (data not shown). As was observed earlier (40), these treatments were at the expense of overall protein synthesis, leading to a significant decrease in viral proteins also. To examine the possible effect of this glycosylation inhibitor on the release of MHV and TGEV, polarized LMR cells were infected with these viruses and labeled with 35S labeling mix and each culture medium was harvested and analyzed for the presence of viral proteins by an immunoprecipitation assay. As observed previously, without the drug, TGEV and MHV-A59 proteins were released preferentially through the apical and basolateral plasma membranes, respectively. Treatment with TM did not affect the polarity of viral-protein secretion; proteins were still shed from the same surfaces (Fig. 1). Note that in spite of the drastic decrease in total protein synthesis caused by TM (data not shown), the amounts of MHV-A59 N and M proteins released were not greatly affected (Fig. 1), in contrast to those of the TGEV proteins.
FIG. 1.
Release of TGEV and MHV-A59 from TM-treated LMR cells. Parallel cultures of LMR cells grown on filters were infected with TGEV or MHV-A59 from the apical side at 16 h p.s. To some cultures, 2 μg of TM per ml was added at 1 h p.i., and these drug concentrations were maintained throughout the experiment. Cells were labeled with 35S labeling mix from 4.5 to 7.5 h p.i., and each culture medium was harvested and analyzed. Viral proteins were immunoprecipitated from the apical (lanes A) and basolateral (lanes B) medium with anti-MHV or anti-TGEV serum. Indicated on the left are the positions of TGEV nucleocapsid (N) and S proteins and of glycosylated and unglycosylated forms of the membrane protein (M and M′, respectively). Indicated on the right are the positions of the cleaved form of the spike protein (S1/S2) and the N and M proteins of MHV-A59. Note that the high-molecular-mass protein (∼250 kDa) detected in the basolateral medium is an unidentified cellular protein nonspecifically coimmunoprecipitated only from the basolateral medium of LMR cells (36).
Consistent with previous data (15, 27, 40), the viral material secreted in the presence of TM did not contain the S protein. For TGEV, only the unglycosylated precursor of the M protein and the N protein (the latter was visible only after longer exposures of the gel to film) (data not shown) were released; neither the glycosylated nor unglycosylated form of the TGEV S protein was secreted. Similarly, no MHV-A59 S protein was shed from TM-treated cells. We also checked the effect of TM treatment on infectious-virus production and found that the amounts of infectious TGEV and MHV-A59 particles released were reduced 105-fold to 1,000 and 10 50% tissue culture infective doses/ml, respectively.
Additional evidence that only spikeless viral particles were produced in the presence of TM and that they were secreted with the same polarity as that of wild-type virus came from an experiment with TGEV in which particles were immunoisolated from the culture medium with MAb, 995, to the TGEV S protein and with anti-TGEV serum. Cell lysates and culture media from TM-treated and untreated 35S-labeled cells were divided into three equal parts. To different aliquots, MAb 995, anti-TGEV serum, or no antibodies (as a negative control) were added, and all samples were further processed similarly in parallel. Analyses of cell-bound viral proteins show the inhibitory effects of TM on N-glycosylation and protein synthesis (Fig. 2A and B). More importantly, they also demonstrate that this MAb recognizes the unglycosylated form of the S protein very well compared to the amount of this protein precipitated by the polyclonal antiserum (Fig. 2B). Affinity purification of viral particles from the culture medium worked very efficiently, which is clear from the coprecipitation of N and M proteins with the S protein when this MAb was used (Fig. 2C). Particles were detected only in the apical medium, not only for control cells but also for cells treated with TM. However, in the latter case, these particles apparently lacked S protein since they could not be affinity purified with the MAb (Fig. 2D).
FIG. 2.
Immunoisolation of TGEV particles from the medium of TM-treated LMR cells. LMR cells grown on filters were infected with TGEV from the apical side at 16 h p.s. In some cultures, 2 μg of TM per ml (B and D) was present from 1 h p.i. onward. Cells were labeled with 35S labeling mix from 4.5 to 7.5 h p.i. and lysed, and viral proteins were immunoprecipitated from lysates (A and B). (C and D) Viral particles were immunoisolated from the apical (lanes A) or basolateral (lanes B) medium with a MAb to the TGEV spike protein (αS) or anti-TGEV serum (αT); a control sample was processed without antibodies (−). The high-molecular-mass protein (∼250 kDa) found in the basolateral medium in panel D (and in longer exposures of panel C; not shown) is an unidentified cellular protein nonspecifically coimmunoprecipitated only from the basolateral medium of LMR cells (36). The exposure times of the gels in panels A through D were 7, 84, 3, and 84 h, respectively. Indicated on the left are the positions of the glycosylated and unglycosylated forms of the spike (S and S′, respectively) and membrane (M and M′, respectively) proteins and the nucleocapsid (N) protein. Molecular mass markers (in kilodaltons) are indicated on the right.
Effect of TM on the release of MHV-A59 from MDCKMHVR cells.
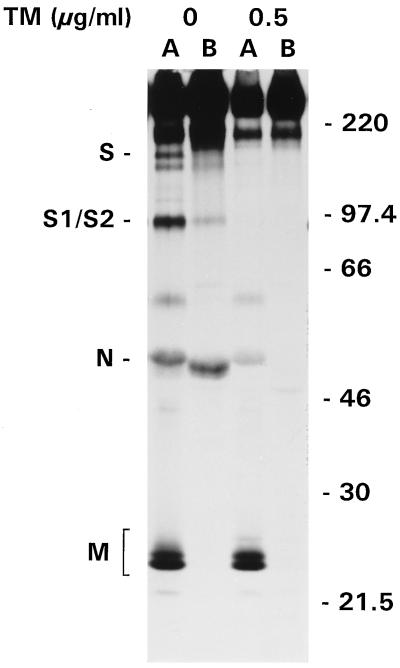
In contrast to its basolateral release from mTAL and LMR cells, MHV-A59 is released from apical surfaces of MDCKMHVR cells. Therefore, we also investigated the effect of TM on the release of MHV-A59 from these cells. As shown in Fig. 3, TM did not affect the direction of viral release from MDCKMHVR cells; the virus was still secreted almost exclusively from the apical surface. Again, only spikeless particles were released and the amounts of particles shed from treated and untreated cells were of the same order of magnitude, despite the decrease in overall protein synthesis observed in the analysis of cell lysates (results not shown).
FIG. 3.
Release of MHV-A59 from TM-treated MDCKMHVR cells. Filter-grown MDCKMHVR cells were infected with MHV-A59 from the apical side at 16 h p.s. In some cultures, 0.5 μg of TM per ml was present from 1 h p.i. onward. Cells were labeled with 35S labeling mix from 6 to 9 h p.i., and viral proteins were immunoprecipitated from apical (lanes A) and basolateral (lanes B) media with anti-MHV serum. Indicated on the left are the positions of the uncleaved (S) and cleaved (S1/S2) forms of the spike protein and the membrane (M) and nucleocapsid (N) proteins. Molecular mass markers (in kilodaltons) are indicated on the right.
Release of MHV-A59 ts mutant Albany 18 from LMR and MDCKMHVR cells.
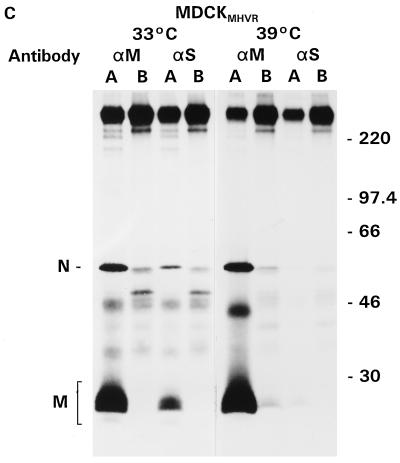
In another approach for studying the release of spikeless coronavirus particles, we used MHV-A59 ts mutant Albany 18. Due to a mutation in the S gene, this virus assembles virions that lack spikes at the nonpermissive temperature (39°C) (33). Initial experiments showed that the S proteins of virions released from LMR and MDCKMHVR cells infected with Albany 18 at the permissive temperature could be clearly visualized only when cells were labeled late in infection. However, by that time, the epithelial cell monolayer had lost its intactness and, consequently, the necessary tight barrier between apical and basolateral compartments. We therefore applied the more sensitive approach of immunoisolation, which allowed analyses at earlier time points in infection. Intact viral particles were isolated from the culture medium with MAb against the S and M proteins. Since it is essential in this assay that the anti-S antibodies used recognize the S protein at both the permissive and restrictive temperatures, viral proteins were also immunoprecipitated from infected-cell lysates. This is shown for LMR cells in Fig. 4A, where we used MAb J7.6 and J1.3 and (as positive and negative controls) polyclonal antisera against MHV and vesicular stomatitis virus, respectively. Clearly, the anti-S MAb specifically recognized the spike protein not only at the permissive temperature but also at the restrictive temperature; the anti-M MAb specifically precipitated only the M protein. The medium of these cells was then used for the immunoisolation of released viral particles. The results (Fig. 4B) demonstrate that at the permissive temperature (33°C), ts mutant virions were secreted exclusively into the basolateral medium, similar to wild-type MHV-A59 secretion (35). Interestingly, released particles were isolated with about the same efficiency by either antibody, although the presence of radiolabeled S protein was detectable only after very long exposure times (not shown). Virus was still released preferentially through the basolateral membrane at the restrictive temperature, as shown by immunoisolation with anti-M antibodies. These particles were indeed devoid of spikes, as the anti-S MAb did not mediate their purification, showing again that the S protein is not involved in the polarized sorting of MHV-A59.
FIG. 4.
Release of MHV-A59 ts mutant Albany 18 from LMR cells and MDCKMHVR cells. Filter-grown LMR (A and B) and MDCKMHVR (C) cells were infected with MHV-A59 ts mutant Albany 18 from the apical side at 16 h p.s. After the 1-h inoculation period, cells were further incubated at 33°C (permissive temperature) or 39°C (nonpermissive temperature), as indicated. Cells were labeled with 35S labeling mix from 8.5 to 11.5 h p.i., and viral proteins were immunoprecipitated from cell lysates (A) or immunoisolated in the absence of any detergent from the apical (lanes A) or basolateral (lanes B) medium (B and C) with MAb against S (αS) and M (αM) proteins and polyclonal antisera against MHV and vesicular stomatitis virus (vsv). Indicated on the left are the positions of the 150-kDa form of the spike protein (S/gp150) and the membrane (M) and nucleocapsid (N) proteins. Molecular mass markers (in kilodaltons) are indicated on the right. Note that the exposure times for the gels of experiments performed at 33°C were about three times as long as those for the gels of experiments done at 39°C, except for panel A, where only half the amount of sample was loaded for the experiment performed at 39°C compared to that for the experiment performed at 33°C.
Because the directionality of MHV-A59 release from polarized MDCKMHVR cells is the opposite of that from all other cells tested so far (37), we also analyzed the behavior of the MHV-A59 ts mutant in these cells. As Fig. 4C shows, this virus was indeed secreted into the apical medium at both the permissive and restrictive temperatures. Viral particles produced at the latter temperature did not carry spikes, as they could be immunoisolated only with the anti-M MAb, not with S-specific antibodies.
Release of MHV-A59-like particles from mTAL cells.
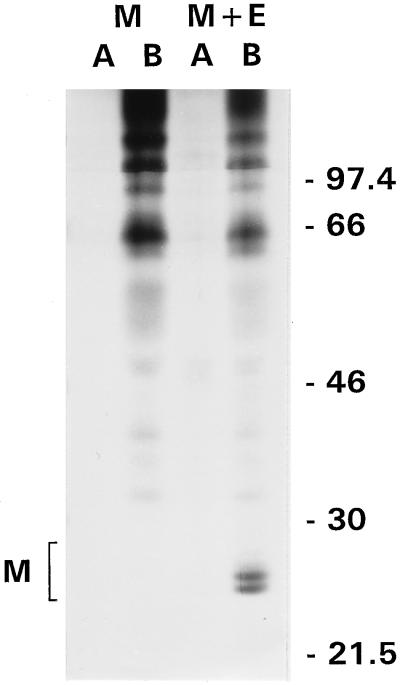
We wanted to independently confirm our finding that the S protein has no role in the targeted release of coronavirions. To this end, we exploited our recent finding that virus-like particles are assembled and released when the M and E proteins are coexpressed in cells (49). However, the vaccinia virus expression system used in those studies did not appear to be applicable for our purposes because of the cytopathic effects of the vector virus, which destroyed the integrity of our epithelial monolayers. We therefore adopted another vector, SFV, and prepared recombinant viruses expressing the M or E protein alone or together. Attempts to apply these vectors to LMR or MDCKMHVR cells were unsuccessful because these cells did not appear to be susceptible to SFV infection. Fortunately, mTAL cells were infectable and expressed the coronavirus genes. We analyzed the direction of release of virus-like particles generated from the M and E proteins. As shown in Fig. 5, these particles were secreted exclusively into the basolateral medium. M protein, which remained fully intracellular on its own, was released into the lower medium when E protein was cosynthesized. Since MHV-A59 was released from mTAL cells with the same polarity, the results again indicate that the S protein is not required for targeting to a specific membrane.
FIG. 5.
Release of MHV-A59-like particles from mTAL cells. To express the M and E genes of MHV-A59 in mTAL cells, filter-grown cells were infected at 16 h p.i. with recombinant SFVs, vS1mM and vS1mM5, expressing the MHV-A59 M gene (M) and the MHV-A59 M and E genes (M + E), respectively. Cells were labeled with 35S labeling mix from 6 to 9 h p.i., and viral proteins were immunoprecipitated from the apical (lanes A) and basolateral (lanes B) media with anti-MHV serum. The positions of M proteins are bracketed on the left. Molecular mass markers (in kilodaltons) are indicated on the right.
DISCUSSION
In this study, we investigated the possible role of the S protein in the targeted release of coronaviruses from epithelial cells. In an inhibitor approach, we used TM, which had been shown earlier to lead to the assembly of virions lacking S protein (15, 27, 40). In addition, we used MHV-A59 ts mutant Albany 18, which also produces spikeless viruses at the restrictive temperature. Another design used the synthesis of spikeless virus-like particles from coexpressed M and E genes. Our results allow the conclusion that the S protein is not required for the directional secretion of coronaviruses from polarized epithelial cells. In addition, they show that the N-linked sugars present on viral envelope proteins are not essential for virion sorting.
In every cell-virus combination tested, spikeless coronavirions were released from TM-treated epithelial cells in a polar fashion and their route was invariably the same as that taken by intact virions (35, 37, 38): spikeless MHV-A59 particles were secreted from the basolateral sides of LMR and mTAL cells but from the apical sides of MDCKMHVR cells; TGEV particles were shed from the apical surfaces of LMR cells. The data obtained with spikeless virus-like particles produced by the coexpression of MHV-A59 M and E genes were consistent. Particles generated in this way in mTAL cells were secreted only from the basolateral surface. Unfortunately, these studies could not be extended to LMR and MDCKMHVR cells due to limitations inherent to the expression systems.
After intracellular budding, coronaviruses accumulate in the lumen of the intermediate compartment or the endoplasmic reticulum. They are transported by vesicular carriers through the Golgi complex to the plasma membrane to be released by exocytosis (45). The sorting of coronavirus particles may therefore be compared to that of cellular secretory proteins. N-linked sugars have previously been proposed to play a role in the directional release of secretory proteins from epithelial cells, but the published data are contradictory. Whereas N-glycans were not involved in the apical secretion of human corticosteroid-binding globulin (28) or hepatitis B virus surface antigen (14, 22), they were an absolute prerequisite for the apical sorting of a cellular glycoprotein complex (gp80) (47) and erythropoietin (18) in MDCK cells. In addition, the nonglycosylated rat growth hormone was released from both sides of MDCK cells, whereas the insertion of a N-glycosylation site led to the secretion of a glycosylated protein through the apical surface (43). For gp80, it was shown that simple core glycosylation was sufficient for its apical transport; the modifications of oligosaccharides that normally occur during intracellular transport were not required (29, 50). They suggested that the core sugars do not act as a direct sorting signal but impose and stabilize a secondary structure on the polypeptide chain which is necessary to interact with sorting receptors. The importance of N-glycans for proper folding of a polypeptide probably varies between different proteins. This may explain why some proteins are dependent on N-glycosylation for correct targeting to the apical membrane, whereas others are not.
Our TM experiments indicate that N-glycosylation does not play a role in the targeted release of coronaviruses. First, the absence of the S protein with its many N-linked oligosaccharides from viral particles did not affect the direction of their transport. Second, for TGEV also, the M protein is N glycosylated; unglycosylated and spikeless TGEV particles produced in the presence of TM were still secreted apically.
A side effect of TM was an apparent decrease in total protein synthesis. However, the amounts of MHV-A59 found in the extracellular medium of LMR and MDCKMHVR cells was not greatly affected by this drug. In contrast, MHV-A59 release from mTAL cells was significantly decreased upon TM treatment, a phenomenon that may have been caused by the MHV receptor. Recently, it was shown for MHV (3, 12) and TGEV (6) that high-level expression of the viral receptor inhibited virus production, possibly by the binding of S protein to intracellular receptor molecules. LMR and MDCKMHVR cells express the MHV receptor glycoprotein at a higher level than do mTAL cells; therefore, such an interaction may occur in the first two cell lines but not (or to a lesser extent) in the last. Because the S protein is not incorporated into virions in TM-treated cells, the particles cannot bind to receptor molecules. More virions may therefore be released from TM-treated cells than from untreated cells.
As indicated above, the S protein is not required for the polarized sorting of coronaviruses. If we maintain that any sorting signal(s) is exposed on the exterior of viral particles, we are left with the M and E proteins. Little is known about the small membrane E protein, of which only a few molecules per virion are incorporated (13, 49, 51). We have indications that very little, if any, of this protein protrudes from the virion surface (32). The M protein is the most abundant virion protein. It spans the lipid bilayer three times, leaving a short NH2-terminal domain and possibly a small loop between the second and third transmembrane domains outside the virion (39). For TGEV, it was claimed that the COOH-terminal domain also protrudes at the outside (34); therefore, a sorting signal may be present in any of these domains. Intracellular transport of the coronavirus M protein differs from that of most other viral glycoproteins, including the S protein. Whereas viral membrane proteins are generally targeted to the cell surface, the migration of the M protein is limited to the perinuclear region. This does not exclude, however, the possibility that when it is incorporated into a virion, the M protein contains the sorting signal responsible for polarized virus release.
An interesting inference from our study is that the coronavirus receptor glycoprotein most likely is not involved in the targeting of viral particles. Earlier, we assumed such a role; we thought it might in some way mediate virion transport via its interaction with the S protein. Having ruled out the involvement of the latter, this idea can no longer be upheld. However, we do not exclude the possibility that some cellular receptor specifically recognizes some component on the virion to effect sorting. The identity of this receptor remains elusive, as does that of the virion component to which it binds, although the number of candidates in each case has decreased by one.
ACKNOWLEDGMENTS
We are very grateful to Paul Masters for providing MHV-A59 ts mutant Albany 18. We thank Raoul de Groot for helpful discussions. Ingrid Rossen-de Vaan is thanked for her help with preparation of the figures.
Footnotes
This paper is dedicated to Tommy Rossen.
REFERENCES
- 1.Bredenbeek P J, Charité J, Noten J F H, Luytjes W, Horzinek M C, van der Zeijst B A M, Spaan W J M. Sequences involved in the replication of coronaviruses. Adv Exp Med Biol. 1987;218:65–72. doi: 10.1007/978-1-4684-1280-2_8. [DOI] [PubMed] [Google Scholar]
- 2.Budzilowicz C J, Weiss S R. In vitro synthesis of two polypeptides from a nonstructural gene of coronavirus mouse hepatitis virus strain A59. Virology. 1987;157:509–515. doi: 10.1016/0042-6822(87)90293-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen W, Madden V J, Bagnell C R, Jr, Baric R S. Host-derived intracellular immunization against mouse hepatitis virus infection. Virology. 1997;228:318–332. doi: 10.1006/viro.1996.8402. [DOI] [PubMed] [Google Scholar]
- 4.Compans R W. Virus entry and release in polarized epithelial cells. Curr Top Microbiol Immunol. 1995;202:209–219. doi: 10.1007/978-3-642-79657-9_14. [DOI] [PubMed] [Google Scholar]
- 5.Compton S R, Barthold S W, Smith A L. The cellular and molecular pathogenesis of coronaviruses. Lab Anim Sci. 1993;43:15–26. [PubMed] [Google Scholar]
- 6.Delmas B, Kut E, Gelfi J, Laude H. Overexpression of TGEV cell receptor impairs the production of virus particles. Adv Exp Med Biol. 1995;380:379–385. doi: 10.1007/978-1-4615-1899-0_62. [DOI] [PubMed] [Google Scholar]
- 7.Doyle L P, Hutchings L M A. A transmissible gastroenteritis in pigs. J Am Vet Med Assoc. 1946;108:257–259. [PubMed] [Google Scholar]
- 8.Eaton S, Simons K. Apical, basal, and lateral cues for epithelial polarization. Cell. 1995;82:5–8. doi: 10.1016/0092-8674(95)90045-4. [DOI] [PubMed] [Google Scholar]
- 9.Fiedler K, Simons K. The role of N-glycans in the secretory pathway. Cell. 1995;81:309–312. doi: 10.1016/0092-8674(95)90380-1. [DOI] [PubMed] [Google Scholar]
- 10.Fleming J O, Stohlman S A, Harmon R C, Lai M M, Frelinger J A, Weiner L P. Antigenic relationships of murine coronaviruses: analysis using monoclonal antibodies to JHM (MHV-4) virus. Virology. 1983;131:296–307. doi: 10.1016/0042-6822(83)90498-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gagneten S, Gout O, Dubois Dalcq M, Rottier P J M, Rossen J W A, Holmes K V. Interaction of mouse hepatitis virus (MHV) spike glycoprotein with receptor glycoprotein MHVR is required for infection with an MHV strain that expresses the hemagglutinin-esterase glycoprotein. J Virol. 1995;69:889–895. doi: 10.1128/jvi.69.2.889-895.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gallagher T M. Overexpression of the MHV receptor. Effect on progeny virus secretion. Adv Exp Med Biol. 1995;380:331–336. [PubMed] [Google Scholar]
- 13.Godet M, L’Haridon R, Vautherot J F, Laude H. TGEV corona virus ORF4 encodes a membrane protein that is incorporated into virions. Virology. 1992;188:666–675. doi: 10.1016/0042-6822(92)90521-P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gonzáles A, Nicovani S, Juica F. Apical secretion of hepatitis B surface antigen from transfected Madin-Darby canine kidney cells. J Biol Chem. 1993;268:6662–6667. [PubMed] [Google Scholar]
- 15.Holmes K V, Doller E W, Sturman L S. Tunicamycin resistant glycosylation of coronavirus glycoprotein: demonstration of a novel type of viral glycoprotein. Virology. 1981;115:334–344. doi: 10.1016/0042-6822(81)90115-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holmes K V, Lai M M C. Coronaviridae: the viruses and their replication. In: Fields B N, Knipe D M, Howley P M, Chanock R M, Melnick J L, Monath T P, Roizman B, Straus S E, editors. Virology. Vol. 1. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 1075–1093. [Google Scholar]
- 17.Kawasaki E S, Wang A M. Detection of gene expression. In: Erlich H A, editor. PCR technology: principles and applications for DNA amplification. New York, N.Y: Stockton Press; 1989. pp. 89–97. [Google Scholar]
- 18.Kitagawa Y, Sano Y, Ueda M, Higashio K, Narita H, Okano M, Matsumoto S-I, Sasaki R. N-glycosylation of erythropoietin is critical for apical secretion by Madin-Darby canine kidney cells. Exp Cell Res. 1994;213:449–457. doi: 10.1006/excr.1994.1222. [DOI] [PubMed] [Google Scholar]
- 19.Klumperman J, Krijnse Locker J, Meijer A, Horzinek M C, Geuze H J, Rottier P J M. Coronavirus M proteins accumulate in the Golgi complex beyond the site of virion budding. J Virol. 1994;68:6523–6534. doi: 10.1128/jvi.68.10.6523-6534.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krijnse Locker J, Ericsson M, Rottier P J M, Griffiths G. Characterization of the budding compartment of mouse hepatitis virus: evidence that transport from the RER to the Golgi complex requires only one vesicular transport step. J Cell Biol. 1994;124:55–70. doi: 10.1083/jcb.124.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liljeström P, Garoff H. A new generation of animal cell expression vectors based on the Semliki Forest virus replicon. Bio/Technology. 1991;9:1356–1361. doi: 10.1038/nbt1291-1356. [DOI] [PubMed] [Google Scholar]
- 22.Marzolo, M. P., and A. Gonzáles. 1995. Glycosylation is not required for apical secretion of the hepatitis B surface antigen by MDCK and Fisher polarized epithelial cells. Mol. Biol. Cell 6(Suppl.):400a.
- 23.Matter K, Yamamoto E M, Mellman I. Structural requirements and sequence motifs for polarized sorting and endocytosis of LDL and Fc receptors in MDCK cells. J Cell Biol. 1994;126:991–1004. doi: 10.1083/jcb.126.4.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mays R W, Beck K A, Nelson W J. Organization and function of the cytoskeleton in polarized epithelial cells: a component of the protein sorting machinery. Curr Opin Cell Biol. 1994;6:16–24. doi: 10.1016/0955-0674(94)90111-2. [DOI] [PubMed] [Google Scholar]
- 25.McIntosh K. Coronaviruses. In: Fields B N, Knipe D M, Howley P M, Chanock R M, Melnick J L, Monath T P, Roizman B, Straus S E, editors. Virology. Vol. 1. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 1095–1103. [Google Scholar]
- 26.Mostov K E, Cardone M H. Regulation of protein traffic in polarized epithelial cells. Bioessays. 1995;17:129–138. doi: 10.1002/bies.950170208. [DOI] [PubMed] [Google Scholar]
- 27.Mounir S, Talbot P J. Sequence analysis of the membrane protein gene of human coronavirus OC43 and evidence for O-glycosylation. J Gen Virol. 1992;73:2731–2736. doi: 10.1099/0022-1317-73-10-2731. [DOI] [PubMed] [Google Scholar]
- 28.Musto N A. Polarized secretion of human corticosteroid binding globulin by MDCK and BeWo cells. Exp Cell Res. 1993;209:271–276. doi: 10.1006/excr.1993.1311. [DOI] [PubMed] [Google Scholar]
- 29.Parczyk K, Koch-Brandt C. The role of carbohydrates in vectorial exocytosis: the secretion of the gp 80 glycoprotein complex in a ricin-resistant mutant of MDCK cells. FEBS Lett. 1991;278:267–270. doi: 10.1016/0014-5793(91)80132-m. [DOI] [PubMed] [Google Scholar]
- 30.Pensaert M, Haelterman E O, Burnstein T. Transmissible gastroenteritis of swine: virus-intestinal cell interactions. 1. Immunofluorescence, histopathology and virus production in the small intestine through the course of infection. Arch Gesamte Virusforsh. 1970;31:321–334. doi: 10.1007/BF01253767. [DOI] [PubMed] [Google Scholar]
- 31.Pensaert M, Haelterman E O, Hinsman E J. Transmissible gastroenteritis of swine: virus-intestinal cell interactions. 2. Electron microscopy of the epithelium in isolated jejunal loops. Arch Gesamte Virusforsh. 1970;31:335–351. doi: 10.1007/BF01253768. [DOI] [PubMed] [Google Scholar]
- 32.Raamsman, M. J. B., and P. J. M. Rottier. Unpublished data.
- 33.Ricard C S, Koetzner C A, Sturman L S, Masters P S. A conditional-lethal murine coronavirus mutant that fails to incorporate the spike glycoprotein into assembled virions. Virus Res. 1995;39:261–276. doi: 10.1016/0168-1702(95)00100-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Risco C, Antón I M, Suñé C, Pedregosa A M, Martín-Alonso J M, Parra F, Carrascosa J L, Enjuanes L. Membrane protein molecules of transmissible gastroenteritis coronavirus also expose the carboxy-terminal region on the external surface of the virion. J Virol. 1995;69:5269–5277. doi: 10.1128/jvi.69.9.5269-5277.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rossen J W A, Bekker C P J, Strous G J A M, Horzinek M C, Dveksler G S, Holmes K V, Rottier P J M. A murine and a porcine coronavirus are released from opposite surfaces of the same epithelial cells. Virology. 1996;224:345–351. doi: 10.1006/viro.1996.0540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rossen J W A, Bekker C P J, Voorhout W F, Strous G J A M, van der Ende A, Rottier P J M. Entry and release of transmissible gastroenteritis coronavirus are restricted to apical surfaces of polarized epithelial cells. J Virol. 1994;68:7966–7973. doi: 10.1128/jvi.68.12.7966-7973.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rossen J W A, Strous G J A M, Horzinek M C, Rottier P J M. Mouse hepatitis virus strain A59 is released from opposite sides of different epithelial cell types. J Gen Virol. 1997;78:61–69. doi: 10.1099/0022-1317-78-1-61. [DOI] [PubMed] [Google Scholar]
- 38.Rossen J W A, Voorhout W F, Horzinek M C, van der Ende A, Strous G J A M, Rottier P J M. MHV-A59 enters polarized murine epithelial cells through the apical surface but is released basolaterally. Virology. 1995;210:54–66. doi: 10.1006/viro.1995.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rottier P J M. The coronavirus membrane protein. In: Siddell S G, editor. The Coronaviridae. New York, N.Y: Plenum Press; 1995. pp. 115–139. [Google Scholar]
- 40.Rottier P J M, Horzinek M C, van der Zeijst B A M. Viral protein synthesis in mouse hepatitis virus strain A59-infected cells: effect of tunicamycin. J Virol. 1981;40:350–357. doi: 10.1128/jvi.40.2.350-357.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rottier P J M, Rose J K. Coronavirus E1 glycoprotein expressed from cloned cDNA localizes in the Golgi region. J Virol. 1987;61:2042–2045. doi: 10.1128/jvi.61.6.2042-2045.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rottier P J M, Spaan W J M, Horzinek M C, van der Zeijst B A M. Translation of three mouse hepatitis virus strain A59 subgenomic RNAs in Xenopus laevis oocytes. J Virol. 1981;38:20–26. doi: 10.1128/jvi.38.1.20-26.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scheiffele P, Peränen J, Simons K. N-glycans as apical sorting signals in epithelial cells. Nature. 1995;378:96–98. doi: 10.1038/378096a0. [DOI] [PubMed] [Google Scholar]
- 44.Tooze J, Tooze S, Warren G. Replication of coronavirus MHV-A59 in sac cells: determination of the first site of budding of progeny virions. J Clin Microbiol. 1984;19:388–393. [PubMed] [Google Scholar]
- 45.Tooze J, Tooze S A, Fuller S D. Sorting of progeny coronavirus from condensed secretory proteins at the exit from the trans-Golgi network of AtT20 cells. J Cell Biol. 1987;105:1215–1226. doi: 10.1083/jcb.105.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tucker S P, Compans R W. Virus infection of polarized epithelial cells. Adv Virus Res. 1993;42:187–247. doi: 10.1016/S0065-3527(08)60086-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Urban J, Parczyk K, Leutz A, Kayne M, Konder-Koch C. Constitutive apical secretion of an 80-kDa sulfated glycoprotein complex in the polarized epithelial Madin-Darby canine kidney cell line. J Cell Biol. 1987;105:2735–2743. doi: 10.1083/jcb.105.6.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Van der Most R G, Bredenbeek P J, Spaan W J M. A domain at the 3′ end of the polymerase gene is essential for encapsidation of coronavirus defective interfering RNAs. J Virol. 1991;65:3219–3226. doi: 10.1128/jvi.65.6.3219-3226.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vennema H, Godeke G-J, Rossen J W A, Voorhout W F, Horzinek M C, Opstelten D-J E, Rottier P J M. Nucleocapsid-independent assembly of coronavirus-like particles by co-expression of viral envelope protein genes. EMBO J. 1996;15:2020–2028. doi: 10.1002/j.1460-2075.1996.tb00553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wagner M, Morgans C, Koch-Brandt C. The oligosaccharides have an essential but indirect role in sorting gp80 (clusterin, TRPM-2) to the apical surface of MDCK cells. Eur J Cell Biol. 1995;67:84–88. doi: 10.1016/0022-2313(95)00112-3. [DOI] [PubMed] [Google Scholar]
- 51.Yu X, Bi W, Weiss S R, Leibowitz J L. Mouse hepatitis virus gene 5b protein is a new virion envelope protein. Virology. 1994;202:1018–1023. doi: 10.1006/viro.1994.1430. [DOI] [PubMed] [Google Scholar]