Abstract
Deaths from cancer are mostly due to metastatic disease that becomes resistant to therapy. A mainstay treatment for many cancers is chemotherapy for which the dosing strategy is primarily limited by patient toxicity. While this Maximum Tolerated Dose (MTD) approach builds upon the intuitively appealing principle that maximum therapeutic benefit is achieved by killing the largest possible number of cancer cells, there is increasing evidence that moderation might allow host-specific features to contribute to success. We believe that a ‘Goldilocks Window’ of sub-maximal chemotherapy will yield improved overall outcomes. This window combines the complex interplay of cancer cell death, immune activity, emergence of chemoresistance, and metastatic dissemination. These multiple activities driven by chemotherapy have tradeoffs that depend on the specific agents used as well as their dosing levels and schedule. Here we present evidence supporting the idea that MTD may not always be the best approach and offer suggestions towards a more personalized treatment regime which integrates insights into patient-specific eco-evolutionary dynamics.
Keywords: Maximum tolerable dose, adaptive therapy, metastatic risk, chemotherapy dosing, chemoresistance
Finding the Goldilocks Window
Cancer remains a devastating disease and, justifiably, elicits a visceral desire in both patients and doctors to treat it with the greatest effort (1). In the metastatic setting, cytotoxic chemotherapies remain a staple of oncology treatment even in the face of more recently developed targeted therapies and immunotherapies. These ‘traditional’ chemotherapeutics, unlike targeted agents, do not specifically differentiate between normal and cancer cells. Rather, they target dividing cells and gain a therapeutic advantage by exploiting key growth dynamic differences between normal and cancer cells. Since cancer cells proliferate more rapidly than normal cells (with some exceptions), tumors are more susceptible to multiple types of chemotherapeutic drugs which induce cell death during cell cycling (2). In addition, cancer cells often have dysfunctional DNA-repair mechanisms that confer increased vulnerability to chemotherapeutics compared to normal cells (3).
Traditionally, chemotherapies are administered at Maximum Tolerated Dose (MTD) based on an implicit assumption that the optimal therapeutic dose must kill as many cancer cells as possible (4). Initial (Phase 1) clinical trials for new chemotherapeutic agents are designed to determine the MTD, which then becomes the typical drug dose (5). Alternative strategies using less than the MTD have been proposed (e.g. minimum effective dose, metronomic therapy, and optimal biologic dose of targeted therapies (6). Dose intensive regimes giving the maximum tolerated dose over the shortest possible period of time have also been investigated. However, MTD generally remains the standard of care strategy for most cancer treatment agents and is the most common clinical strategy ((7,8)).
While MTD treatment is successful in curing some cancers (e.g. acute lymphoblastic leukemia and testicular cancer), it rarely produces complete cancer cell eradication in most common metastatic cancers (e.g. mixed lineage leukemias, sarcomas, and breast, pancreatic and lung cancers (9,10)). However, even when not curative, many MTD treatments for common metastatic cancers are effective in the sense that they frequently reduce tumor burden.
A fundamental barrier to cure is the pretreatment presence of tumor subpopulations that are, or can rapidly become, resistant to treatment. In general, less-responsive tumors maintain a cancer population that is more genetically and phenotypically diverse and exhibit complex ecologies containing stromal cells, hematologic and lymphatic vasculature, and diverse immune cell types ((9,11)). Heterogeneity in cell cycling also plays an important role because most tumors contain quiescent cancer cells, which are less sensitive to chemotherapeutics (12) These cells may remain dormant during treatment but emerge to cause recurrence weeks, months or years following treatment (12). In contrast, rapidly cycling populations respond more readily and may rapidly decline (13). Importantly, normal cells also have different cycling fractions so that dose-limiting toxicity is typically dependent on the most rapidly cycling normal tissue (e.g. bone marrow or GI mucosal cells). This includes highly proliferating immune cells which may play a hitherto unrecognized but significant role in tumor control.
These diverse tumor-host interactions form a complex spatially and temporally dynamic ecosystem ((14,15)). Perturbing such a system elicits complicated, non-linear dynamics in both tumor and host tissues that are difficult to intuitively predict in individual patients. Here, we discuss alternative chemotherapy regimens that move away from standard MTD in favor of dosing schedules that consider the whole tumor-ecosystem and its dynamic changes over time.
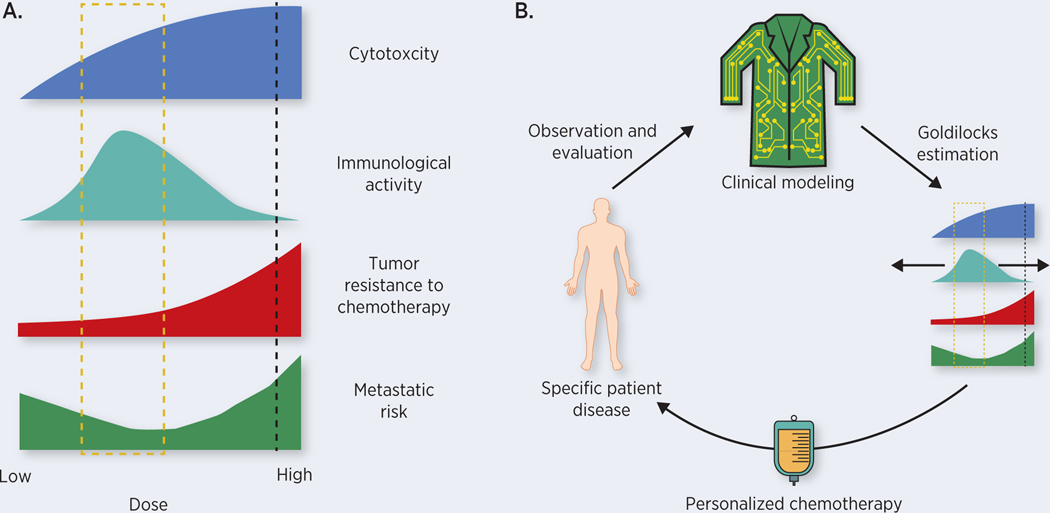
Optimal administration of chemotherapy requires balancing often opposing effects on different components of the tumor-ecosystem. We propose that such a treatment strategy, coined the ‘Goldilocks Window’ (Figure 1), can be identified using mathematical methods that balance multiple goals: maximizing cancer cell killing under the constraints of minimizing patient toxicity, increasing the immune response to the tumor, decreasing the metastatic risk, and delaying the proliferation of resistant phenotypes. We propose that the Goldilocks Window, namely the range of treatment doses that leads to the greatest treatment success, may not always include MTD regimens (Figure 1A). Furthermore, the window is not fixed and will change as both the tumor and host are altered by treatment (Figure 1B). This novel perspective on chemotherapy dosing is built upon a quantitative understanding of the ecological and evolutionary dynamics of cancer before, during, and after therapy.
Figure 1:
The Goldilocks Window. A) Current MTD strategies (black dashed line) increase chemotherapy doses to limits of patient tolerability. This dosing chases diminishing returns in direct cytotoxic effects due to saturation dynamics mediated by cell-intrinsic and microenvironmental protections. Dose reduction may decrease direct toxic effects on the tumor at the same time increasing patient immune involvement by preventing depletion of important immune populations. On a longer timescale, dosing in this Goldilocks Window (gold box) can also prevent or delay the emergence of chemoresistance as well as decreasing metastatic risk. B) Precision Chemotherapy. The Goldilocks Window is likely to differ for individual patients and may shift during the course of individual patient treatment. Clinical models based on eco-evolutionary modeling of chemotherapy’s tradeoffs can be continuously updated with new patient data to aid the clinician in estimating how the Window changes. This presents clinicians with a process by which they can offer personalized chemotherapy despite the high degree of variation across patients and disease trajectories.
Preserving immune activity to maximize cell kill
The MTD strategy is designed to maximize tumor cell killing by applying the largest dose the patient can tolerate. The implicit assumption is that the only benefit of the drug is its cytotoxicity towards cancer cells. However, this ignores the potential role of host defenses, particularly the immune system, that simultaneously contribute to tumor suppression. The immune system plays a complicated role in counteracting tumor development and aiding therapeutic responses ((16,17)). Immunoediting and pro-growth inflammatory responses shape the tumor ecosystem as well as host immunity (18). These two interacting systems both antagonize and support each other so that strong perturbations may promote immune responses to tumor cells or facilitate immune escape by the cancer cells. The therapeutic window could be increased by targeting drugs so that anti-tumor immunity is promoted while maintaining tumor cytotoxicity. Antibody drug conjugates, for example, take advantage of the potential for activating specific immune responses while also maintaining the benefits against cancer cells (19).
Immune cells, particularly those in the proliferation phase, are sensitive to the majority of chemotherapies, leading to systemic immune-cell depletion ((20,21)). Interestingly, however, some chemotherapy regimens appear to be immune-stimulating by increasing the cytotoxic effects of lymphocytes through induction of immunogenic cell death (ICD), which leads to the release of tumor antigens (16). This results in myeloid cell activation and effector T-cell recruitment ((22),(23)). Furthermore, chemotherapy agents that alter DNA replication, including alkylating agents, antimetabolites, and topoisomerase inhibitors, induce cellular stress pathways that increase expression of NKG2D ligands on tumor cells ((24)) activating adaptive and innate lymphocytes including natural killer (NK) cells, NKT cells, γδ T cells, and CD8+ αβ T cells (25).
Importantly, the immune effects of chemotherapy are often dose-dependent. Low doses of cyclophosphamide, for example, preferentially eliminate regulatory T cells ((26),(27)) and low concentrations of 5-fluorouracil (5-FU) deplete myeloid-derived suppressor cells (28). While these shifts result in greater tumor cell kill by activating host defenses, they also reduce immune cell-derived growth promoting factors including pro-angiogenic factors (28). These changes likely have clinical consequences as studies of homeostatic proliferation after chemotherapeutic depletion of lymphocytes show renewed, albeit transient, host immune response to tumor cells (29).
Recently, mathematical models have demonstrated that transient increases in immune responsiveness can be harnessed to improve tumor control by using a Goldilocks Window approach to chemotherapy dosing (30). Moderation is key: with an overly conservative chemotherapy dose, there is insufficient direct drug-mediated cytotoxicity as well as insufficient depletion of tolerized T cells, resulting in tumor growth. However, a large dose (i.e. MTD), while increasing drug-induced cytotoxicity, depletes proliferating T-cell and NK cell populations and reduces immune-mediated tumor cell killing. Over successive cycles, the patient’s immune system becomes too depleted to mount effective anti-tumor activity, and any tumour cells that escape the chemotherapy will also escape immune detection and regrow following the last cycle. The optimal range of chemotherapy, the Goldilocks Window, resides in a middle ground which has direct cytotoxic effects on cancer populations while also avoiding immune over-depletion but still transiently breaking immune tolerance.
Since MTD is standard clinical practice, studies examining lower doses are rare. However, the model predictions of a Goldilocks Window are supported by some studies. The efficacy of anti-HER2 therapy in breast cancer is dependent, in part, on NK-cell mediated antibody dependent cellular cytotoxicity (31). This effect was significantly reduced when it was combined with high doses of paclitaxel in vivo (32). In fact, the addition of high-dose docetaxel and paclitaxel to anti-HER2 treatment was less effective than the anti-HER2 antibody alone. High-dose paclitaxel was also seen to lead to poorer immune memory generation, and caused mice to be more susceptible to tumor rechallenge after initial tumor regression (32). Similarly, paclitaxel adjuvant chemotherapy following radiotherapy abrogated radiotherapy-induced T-cell responses and led to greater tumor colony formation in vivo (33).
This tradeoff arising from chemotherapy’s impact on the patient’s immune system can be exploited through strategic changes in both dosing and drug types and potential inclusion of more targeted chemotherapy regimens to minimize off-target effects. The impact of chemotherapies on the immune response to cancer cells is diverse (more extensively reviewed in (34)) and must eventually be integrated into patient-specific and drug-specific mathematical models. Our recent work suggests that lowering the chemotherapeutic dose, even slightly, can lead to net benefits in tumor control by allowing greater T-cell responses to accumulate over multiple injections (30). This mirrors predictions in the importance of preserving broader immune populations from depletion by treatment (20).
Evolutionary Tradeoffs: Resistance and Adaptive Therapy
A significant barrier to tumor control and eradication is chemoresistance, leading to relapse and disease progression in cancers (35). One rationale for MTD and dose-dense treatment strategies is the assumption that eliminating as many tumor cells as quickly as possible minimizes the probability of developing a resistant population. This paradigm works well in genetically homogenous cancers (e.g. testicular cancer), however, in more heterogeneous tumors, resistance frequently emerges during MTD treatment. In these cases, high chemotherapy dosing may actually accelerate the development of resistance, because MTD applies a strong selection pressure that maximally increases the fitness disparity between sensitive and resistant phenotypes ((36–38)). This accelerates the proliferation of resistant clones in the tumor, often using space and resources made available by elimination of chemosensitive cells ((39),(40)). Furthermore, MTD applies the greatest selection pressure during initial administration when the tumor microenvironment is intact. The undisrupted mesenchymal components of the tumor-host ecosystem can protect cells that are nominally sensitive to the treatment. This cell-cell protection allows time for the intrinsically sensitive cells to acquire resistance mechanisms ((41–43)) at which point these transformed chemoresistant cells could proliferate independently of the host mesenchyma.
These selection pressures suggest that chemotherapy scheduling has a significant effect on the strength and timing of the emergence of resistance in patients (9,44). Recently, there have been efforts to incorporate ecological and evolutionary theory into cancer treatment ((45,46)) that are similar to strategies for controlling antibiotic resistance and managing agricultural pests (47). One such eco-evolutionary treatment approach is ‘adaptive therapy’, which exploits the fitness costs and competition dynamics of resistance to maintain a drug-sensitive population within the tumor (45). Resistant clones have a clear fitness advantage during treatment; however resistance is often associated with a cost in the absence of drug ((38)). While there is limited direct evidence of this cost in cancer (see (48–51)) it has long been recognized and exploited in domains outside cancer treatment. In malaria, drug resistance imparts a fitness cost due to the increased metabolic requirements of resistance mechanisms. Fitness differences between wildtype and resistant pathogens is small but the effect is significantly modified when there is competition between the two populations (38). In efforts to manage antibiotic resistance in bacteria, a proposed strategy has been to suspend antibiotic use until resistant bacteria are outcompeted by sensitive clones (52). (53). Similarly, in treatment with cyclin-dependent kinase inhibitors (CDKi), populations of resistant cells were shown to proliferate more slowly than populations of treatment-sensitive phenotypes (54). Adaptive therapy has sought to exploit this dynamic by applying therapy only for limited time periods, thereby having sensitive cells outcompete the resistant cells in check between doses of the therapy. For this to occur, limited treatment is necessary to maintain a residual population of sensitive cells that can then use their proliferative advantage to suppress growth of treatment-resistant phenotypes. This approach has been shown to be successful in metastatic prostate cancer patients in an early pilot trial ((45), (55)) as well as in in vivo experiments (56).
An alternative ecology-based treatment strategy for avoiding resistance, targets elements of the tumor ecosystem that produce ‘public goods’ necessary for cancer cell proliferation ((9,39,57–59)). This strategy is designed to block production of key microenvironmental factors such as diffusible growth factors, protease inhibitors, pro-angiogenic factors (57) as well metabolites such as lactate that acidify the microenvironment and suppress local anti-tumour immunity (60). By altering or destroying the tumor-supportive immunosuppressive niche, this approach inhibits the collective growth of the tumor population while not directly applying selection pressure for resistance on individual cells. Of interest here, chemotherapy has heterogeneous effects on public goods and often in a dose-dependent manner. For example, studies utilizing metronomic schedules (i.e., a low dose given frequently) often demonstrate anti-angiogenic effects (61). In another study, mouse models of low-dose metronomic 5-FU contained fewer cancer-associated fibroblasts and higher drug sensitivity compared to standard dosing (44). Multidrug-resistant cells are often more glycolytic than their sensitive counterpart (62). These cells acidify the surrounding environment and support tumor growth via acid-mediated invasion and immune suppression ((63–65)). Thus, modifying chemotherapy doses can alter these supportive features that contribute to therapy resistance and cancer cell proliferation.
Goldilocks dosing and metastatic risk
A key issue in the treatment of cancers is the risk of metastatic dissemination. Efforts to delay or prevent metastasis are central to almost all treatment regimens ((66),(67)). Metastatic dissemination is a complex, multistep process ((68), (69)), but in general both tumor size and the amount of time that the cancer is present are both likely to affect the probabilities of metastasis based on cumulative risk principles. For example, larger tumors may shed more tumor cells into circulation than smaller tumors (Figure 2A), and similarly the total numbers of cells shed will increase the longer a tumor persists in the body. Additionally, having greater numbers of tumour cells and longer time periods increase the potential that local cells acquire the phenotype necessary to avoid immune detection and become a successful disseminated metastasis (55). This cumulative risk principle motivates cancer treatment towards a goal of ‘maximum kill, fast kill’.
Figure 2.
Differential trade-offs based on chemotherapy dosing. A) Without treatment the tumor remains immunosuppressive and grows unchecked, but will have low resistance to treatment and fewer cells with high metastatic potential. B) Goldilocks dose. The optimal treatment dose seeks to balance removal of immune suppression (red pentagons) and control of resistant (green circles) and metastatic phenotypes (yellow stars) while reducing tumor burden. C) Maximum tolerated dose induces the greatest initial tumor reduction but at the cost of increasing the proportion of chemoresistant cells, reducing immune activity and potentially promoting greater proportional dispersal of metastatic cells.
However, the dynamics of metastasis – and its risk – may be more complex than previously recognized ((70), (71)). For example, the previously outlined assumption about the risk of metastasis and tumor volume may not be correct in all settings. In breast cancer, a retrospective analysis found that tumor volume and lymph node metastases do not linearly increase, with metastatic lymph node involvement rates rapidly plateauing in larger tumors (72). Furthermore, under some circumstances, chemotherapy may increase the metastatic potential of cancer populations through interconnected phenotypic, microenvironmental, and immunologic changes ((73–75)).
Phenotypic Selection
The heavy selection pressures associated with MTD may select for highly metastatic clones. Improvements in characterizing circulating tumor cells (CTCs), shed from the primary tumor into circulation, have reinforced the model that successful engraftment and metastasis is a challenging task for disseminated cells (76). Cellular mechanisms for chemoresistance, however, may be pleiotropic and lead to direct or indirect advantages in metastasis. For example, the epithelial-to-mesenchymal transition (EMT) is an important step in the metastatic process. Recent studies in lung (77) and pancreatic (78) cancers have shown that EMT phenotypes are more chemoresistant due to a decreased cell cycling rate. This can then improve the net growth of metastatic sites due to a persistent, chemoresistant population. In an in vivo model of colon cancer (79), long-term evolutionary selection in the presence of 5-FU led to resistant cells which metastasized at a much higher rate than the parental chemosensitive cells. Further investigation showed that chemoresistant (5-FU and oxaliplatin) cells had greater migratory potential in a scratch wound assay (80). More generally, the increase in stem cell phenotype associated with EMT confers chemoresistance due to decreases in cancer cell growth rates (81,82).
The potential overlap between chemoresistant and metastatic phenotypes raises questions about the evolutionary consequences of MTD. High dose chemotherapy’s goal of rapid and maximal tumor reduction may achieve immediate but temporary disease control at the cost of greater metastatic risk. The prospect of a successful cellular strategy for one challenge (chemotherapy) also being advantageous for another challenge (metastasis) is not uncommon within diverse evolving populations. Cross-resistance, where cancer cell resistance to one drug also yields resistance to a previously unencountered drug, has been a long-recognized challenge to combination chemotherapy (83). This is a more specific case of synergistic pleiotropy, where genetic changes may improve fitness in multiple dimensions (84). In other words, quickly reducing the tumor size to reduce the total number of cells is of little value if the remaining cells are highly metastatic (Figure 2C).
In general, the metastatic cascade is highly plastic in terms of phenotypic behavior ((69,71,73,74)). Tumors that are in the process of metastasizing are most likely to be heterogeneous and therefore the application of strong selection pressures such as chemotherapy at MTD has a significant chance to select for aggressive cell types that will lead to rapid treatment failure. We propose that Goldilocks approaches (Figure 2B) will be most beneficial in these types of cancers, where the opposing costs and benefits to each cellular phenotype are likely to create optimal windows of therapeutic opportunity. However, the mechanisms of generating this plastic heterogeneity need to be better understood, particularly from the point of view of temporal progression under therapy.
Microenvironmental Remodeling and Exploitation
In addition to phenotypic selection, MTD chemotherapy has the potential to increase metastatic potential by remodeling tissue microenvironments more favorably for disseminating cancer cells. Cancer cells do not grow in isolation and instead exploit tissue microenvironments for chemoprotection and growth advantages during metastasis (85–87). In a murine model of breast cancer, paclitaxel was shown to delay primary tumor growth, but increase the abundance of microanotomical structures which allow cancer cell dissemination through the vasculature (88). Other studies have also demonstrated the role of chemotherapy in inducing pro-metastatic stromal changes in the tumor microenvironment through angiogenesis and inflammation (reviewed more extensively in (89)). Of note, C-X-C Motif Chemokine Ligand 1 (CXCL1) expression in breast cancer cells is associated with lung metastases and is also a key mechanism of chemoresistance in multiple cancers (90–93). However, both in vivo studies and an analysis of patient histology showed that neoadjuvant chemotherapy could induce an increase in TNF-α secretion from stromal cells (90). TNF-α leads to an upregulation of CXCL1 and greater metastatic potential. This microenvironmental change was observed in the neoadjuvant setting for breast cancer where chemotherapy is applied at high doses to explicitly achieve a maximum tumor reduction before surgery. In contrast, dose reductions have been indicated to potentially prevent this microenvironmental remodeling in favor of metastasis. Metronomic low-dose therapy in a murine model was shown to prevent stromal cell signaling necessary for the induction of tumor-initiating cells (94). Within the “seed and soil” model of tumor metastasis, MTD chemotherapy may be inadvertently tilling the soil for later metastatic growth (60).
Immunologic changes
The immune system inhibits the growth of metastatic lesions in a variety of clinical and preclinical studies ((95,96)).T-cell depletion promoted the growth of metastatic lesions in a spontaneous murine melanoma model (97). Natural killer (NK) cells are cytotoxic lymphoid cells whose activation is regulated by a varying cascade of activating and inhibiting receptors ((60)). NK cells recognize foreign, stressed, and malignant cells and are important regulators of metastatic growth ((98)). NK-cell activity is linked to better outcomes in patients with high metastatic risk (95) and murine models show an increase incidence of metastasis when NK-cell function is inhibited (99). Furthermore, epithelial to mesenchymal transition in cancer cells (EMT), a key step in the metastatic cascade, is associated with the upregulation of NK-cell activating ligands, Cell adhesion molecule-1 (CADM1), UL16 binding proteins (ULBPs), and MHC class I polypeptide-related sequence A (MICA) and a reduction in NK cell inhibiting ligand E-cadherin (98,100). These changes cause metastatic tumor cells to be more susceptible to NK-mediated killing. However, this immunoprotective effect is impaired by lymphodepletion, lymphocyte anergy or induction of checkpoints. As noted above, high-dose chemotherapy has systemic effects on the immune system, and the recovery of chemo-induced immune suppression can be slow, causing an increase in the potential for metastatic growth (101).
These observations have implications for the Goldilocks Window: if the development of chemoresistance also confers greater metastatic risk, then minimum residual disease induced by MTD may also represent the most metastatically dangerous population of cancer cells. This challenges the current model that assumes rapid, maximal elimination of cancer cells is the most effective way to reduce metastatic risk for the patient. In contrast, lower-dose Goldilocks Window strategies, while allowing for larger tumor sizes, may have a net reduction in metastatic potential. This may mean that even for patients who are responding well to chemotherapeutics, drug-dose reductions may lead to better long-term outcomes than MTD. Such a decision will understandably be likely to increased clinician and patient unease since it seems paradoxical to discontinue or reduce successful therapy while the tumor is shrinking or even undetectable. However, the use of MTD in the great majority of cases is based on intuition rather than empirical evidence that it is the best approach. Indeed, in other fields such as pest management where the paradigm of MTD has been challenged, it has been found inferior when treatment resistance is a factor.
Clearly, the precise dynamics leading to the evolution of migration and dissemination in proliferating populations remains an open question, and more investigation is warranted for studies with altered dosing regimens. A common emerging model is that of evolutionary and ecological trade-offs associated with the emergence of drug resistance. In this regard, mathematical models are uniquely suited to bringing quantitative estimates of the relative strengths and impacts of these trade-offs. Recent work has suggested that selection for a migratory phenotype and the attendant risks of metastasis is influenced by both the trade-offs between migration and proliferation as well as the microenvironmental changes that occur in the tumor niche (102). The so called ‘go or grow’ trade-off has been studied extensively by the mathematical modeling community despite limited experimental evidence to support it ((102–104)). Recent work in glioma has shown that anti-proliferative treatments select for more migratory tumor cells whereas anti-migratory treatments select for more proliferative cells (105,106) We propose that murine models be developed in conjunction with mathematical models with the goal of examining how the metastatic potential of a cancer changes with different dosing strategies. This would allow understanding the magnitude of trade-off necessary for migration promotion. Similarly, these mathematical modeling frameworks could then be expanded to examine how trade-offs change at different stages of disease.
Conclusions
Chemotherapy agents remain integral components of treatment strategies for primary and metastatic cancers. Similar to treatment of infectious agents, the most intuitively appealing goal for chemotherapy favors application of maximally lethal force to eradicate as many cancer cells as possible. In this setting, calling for restraint would seem paradoxical and even dangerous. However, increasing recognition of the complexity in tumor-host interactions and heterogeneity in tumor and immune response to chemotherapy has led to questioning the longstanding convention of MTD treatment.
Here, we have highlighted three tradeoffs that critically influence chemotherapy outcomes based on tumor-host eco-evolutionary dynamics: 1) preserving and promoting the immune system to maximize tumor cell kill, 2) avoiding chemoresistance, and 3) reducing metastatic risk (Figure 2). We propose application chemotherapy should focus on defining a “Goldilocks Window” balances the benefits of cancer cell death against costs including adverse effects on the patient’s immune state and increased cancer cell resistance and metastatic potential (Figure 2B). Furthermore, these tradeoffs are not constant over the course of treatment.
Maintaining treatment in “the Goldilocks Window” will likely require development of mathematical models that capture the complex often non-linear dynamics of these tumor-host interactions. Once a patient-specific model has been developed, periodic re-evaluation, model calibration and re-optimization will be necessary throughout the course of treatment. This approach will also require a new generation of clinical data focused not just on measurements of tumor volume but on details of its ecological and evolutionary state to parameterize and constantly update mathematical models for continuous optimization of treatment. This will require a transformation in how patient disease is approached from a monolithic cancer population to a dynamic intra-patient ecosystem. To quantitatively characterize this more complex view of patient disease, these data will require routine measurement of circulating tumor cells and macromolecules, immune cell populations and immune biomarkers, as well as application of new image analytic methods to routine clinical MRI and CT scans. Through continuous interactions between model development and new data acquisition from novel technologies, we anticipate that the quality and quantity of patient data analytics will continue to improve resulting in new more successful patient-specific treatment strategies.
Acknowledgements
DSP was supported by a Marshall Scholarship from the Marshall Aid Commemoration Commission. COF was supported by grants from the Health Research Board of Ireland (RP 2008/189 and EIA-2017-013) and a Science Foundation Ireland Investigator Award (12/IA/1667). ARA, DSP, KAL, MRT and RAG gratefully acknowledge funding from both the Cancer Systems Biology Consortium and the Physical Sciences Oncology Network at the National Cancer Institute, through grants U01CA232382 and U54CA193489, as well as support from the Moffitt Center of Excellence for Evolutionary Therapy.
Footnotes
Conflict of interest:
None declared
References
- 1.Penson RT, Schapira L, Daniels KJ, Chabner BA, Lynch TJ. Cancer as metaphor. Oncologist. 2004;9:708–16. [DOI] [PubMed] [Google Scholar]
- 2.Hanahan D, Weinberg RA. The Hallmarks of Cancer. Cell. 2000;100:57–70. [DOI] [PubMed] [Google Scholar]
- 3.Torgovnick A, Schumacher B. DNA repair mechanisms in cancer development and therapy. Front Genet. 2015;6:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hryniuk WM. More is better. J Clin Oncol. 1988;6:1365–7. [DOI] [PubMed] [Google Scholar]
- 5.Toloi D de A, Jardim DLF, Hoff PMG, Riechelmann RSP. Phase I trials of antitumour agents: fundamental concepts. Ecancermedicalscience. 2015;9:501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Banys-Paluchowski M, Schütz F, Ruckhäberle E, Krawczyk N, Fehm T. Metronomic Chemotherapy for Metastatic Breast Cancer - a Systematic Review of the Literature. Geburtshilfe Frauenheilkd. 2016;76:525–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crompton S. Whatever happened to the minimum effective dose? Cancer World. 2018;84. [Google Scholar]
- 8.Takimoto CH. Maximum tolerated dose: clinical endpoint for a bygone era? Target Oncol. 2009;4:143–7. [DOI] [PubMed] [Google Scholar]
- 9.Kareva I, Waxman DJ, Lakka Klement G. Metronomic chemotherapy: an attractive alternative to maximum tolerated dose therapy that can activate anti-tumor immunity and minimize therapeutic resistance. Cancer Lett. 2015;358:100–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dingli D, Ailawadhi S, Bergsagel PL, Buadi FK, Dispenzieri A, Fonseca R, et al. Therapy for Relapsed Multiple Myeloma: Guidelines From the Mayo Stratification for Myeloma and Risk-Adapted Therapy. Mayo Clin Proc. 2017;92:578–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ledzewicz U, Schättler H, Gahrooi MR, Dehkordi SM. On the MTD paradigm and optimal control for multi-drug cancer chemotherapy. Math Biosci Eng. 2013;10:803–19. [DOI] [PubMed] [Google Scholar]
- 12.Endo H, Inoue M. Dormancy in cancer. Cancer Sci. 2019;110:474–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garcia-del-Muro X, Maroto P, Gumà J, Sastre J, López Brea M, Arranz JA, et al. Chemotherapy As an Alternative to Radiotherapy in the Treatment of Stage IIA and IIB Testicular Seminoma: A Spanish Germ Cell Cancer Group Study. J Clin Oncol. 2008;26:5416–21. [DOI] [PubMed] [Google Scholar]
- 14.Pienta KJ, McGregor N, Axelrod R, Axelrod DE. Ecological therapy for cancer: defining tumors using an ecosystem paradigm suggests new opportunities for novel cancer treatments. Transl Oncol. 2008;1:158–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Basanta D, Anderson ARA. Homeostasis Back and Forth: An Ecoevolutionary Perspective of Cancer. Cold Spring Harb Perspect Med. 2017;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galluzzi L, Zitvogel L, Kroemer G. Immunological Mechanisms Underneath the Efficacy of Cancer Therapy. Cancer Immunol Res. 2016;4:895–902. [DOI] [PubMed] [Google Scholar]
- 17.Binnewies M, Roberts EW, Kersten K, Chan V, Fearon DF, Merad M, et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med. 2018;24:541–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–8. [DOI] [PubMed] [Google Scholar]
- 19.Alley SC, Okeley NM, Senter PD. Antibody–drug conjugates: targeted drug delivery for cancer. Curr Opin Chem Biol. 2010;14:529–37. [DOI] [PubMed] [Google Scholar]
- 20.Wu J, Waxman DJ. Immunogenic chemotherapy: Dose and schedule dependence and combination with immunotherapy. Cancer Lett. 2018;419:210–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahlmann M, Hempel G. The effect of cyclophosphamide on the immune system: implications for clinical cancer therapy. Cancer Chemother Pharmacol. 2016;78:661–71. [DOI] [PubMed] [Google Scholar]
- 22.Kersten K, Salvagno C, de Visser KE. Exploiting the Immunomodulatory Properties of Chemotherapeutic Drugs to Improve the Success of Cancer Immunotherapy. Front Immunol. 2015;6:516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown JS, Sundar R, Lopez J. Combining DNA damaging therapeutics with immunotherapy: more haste, less speed. Br J Cancer. 2018;118:312–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galluzzi L, Bravo-San Pedro JM, Vitale I, Aaronson SA, Abrams JM, Adam D, et al. Essential versus accessory aspects of cell death: recommendations of the NCCD 2015. Cell Death Differ. 2015;22:58–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Pillis LG, Radunskaya AE, Wiseman CL. A Validated Mathematical Model of Cell-Mediated Immune Response to Tumor Growth. Cancer Res. 2005;65:7950–8. [DOI] [PubMed] [Google Scholar]
- 26.Lutsiak MEC, Semnani RT, De Pascalis R, Kashmiri SVS, Schlom J, Sabzevari H. Inhibition of CD4(+)25+ T regulatory cell function implicated in enhanced immune response by low-dose cyclophosphamide. Blood. 2005;105:2862–8. [DOI] [PubMed] [Google Scholar]
- 27.Scurr M, Pembroke T, Bloom A, Roberts D, Thomson A, Smart K, et al. Low-Dose Cyclophosphamide Induces Antitumor T-Cell Responses, which Associate with Survival in Metastatic Colorectal Cancer. Clin Cancer Res. 2017;23:6771–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vincent J, Mignot G, Chalmin F, Ladoire S, Bruchard M, Chevriaux A, et al. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res. 2010;70:3052–61. [DOI] [PubMed] [Google Scholar]
- 29.Gameiro SR, Caballero JA, Higgins JP, Apelian D, Hodge JW. Exploitation of differential homeostatic proliferation of T-cell subsets following chemotherapy to enhance the efficacy of vaccine-mediated antitumor responses. Cancer Immunol Immunother. 2011;60:1227–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park DS, Robertson-Tessi M, Luddy KA, Maini PK, Bonsall MB, Gatenby RA, et al. The Goldilocks Window of Personalized Chemotherapy: Getting the Immune Response Just Right. Cancer Res. 2019;79:5302–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Griguolo G, Pascual T, Dieci MV, Guarneri V, Prat A. Interaction of host immunity with HER2-targeted treatment and tumor heterogeneity in HER2-positive breast cancer. J Immunother cancer. 2019;7:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park S, Jiang Z, Mortenson ED, Deng L, Radkevich-Brown O, Yang X, et al. The therapeutic effect of anti-HER2/neu antibody depends on both innate and adaptive immunity. Cancer Cell. 2010;18:160–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee Y, Auh SL, Wang YY, Burnette B, Wang YY, Meng Y, et al. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood. 2009;114:589–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bracci L, Schiavoni G, Sistigu A, Belardelli F. Immune-based mechanisms of cytotoxic chemotherapy: implications for the design of novel and rationale-based combined treatments against cancer. Cell Death Differ. 2014;21:15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Niero EL, Rocha-Sales B, Lauand C, Cortez BA, de Souza MM, Rezende-Teixeira P, et al. The multiple facets of drug resistance: one history, different approaches. J Exp Clin Cancer Res. 2014;33:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gatenby RA, Silva AS, Gillies RJ, Frieden BR. Adaptive therapy. Cancer Res. 2009;69:4894–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chowell D, Napier J, Gupta R, Anderson KS, Maley CC, Sayres MAW. Modeling the Subclonal Evolution of Cancer Cell Populations. Cancer Res. 2018;78:830–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Read AF, Day T, Huijben S. The evolution of drug resistance and the curious orthodoxy of aggressive chemotherapy. Proc Natl Acad Sci. 2011;108:10871–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aktipis CA, Maley CC, Pepper JW. Dispersal Evolution in Neoplasms: The Role of Disregulated Metabolism in the Evolution of Cell Motility. Cancer Prev Res. 2012;5:266–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aktipis CA, Nesse RM. Evolutionary foundations for cancer biology. Evol Appl. 2013;6:144–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marusyk A, Tabassum DP, Janiszewska M, Place AE, Trinh A, Rozhok AI, et al. Spatial Proximity to Fibroblasts Impacts Molecular Features and Therapeutic Sensitivity of Breast Cancer Cells Influencing Clinical Outcomes. Cancer Res. 2016;76:6495–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu T, Zhou L, Li D, Andl T, Zhang Y. Cancer-Associated Fibroblasts Build and Secure the Tumor Microenvironment. Front cell Dev Biol. 2019;7:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pérez-Velázquez J, Gevertz JL, Karolak A, Rejniak KA. Microenvironmental Niches and Sanctuaries: A Route to Acquired Resistance. Adv Exp Med Biol. 2016;936:149–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ma Y, Wang YY, Xu Z, Wang YY, Fallon JK, Liu F. Extreme low dose of 5-fluorouracil reverses MDR in cancer by sensitizing cancer associated fibroblasts and down-regulating P-gp. PLoS One. 2017;12:e0180023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang J, Cunningham JJ, Brown JS, Gatenby RA. Integrating evolutionary dynamics into treatment of metastatic castrate-resistant prostate cancer. Nat Commun. 2017;8:1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gatenby RA, Silva AS, Gillies RJ, Frieden BR. Adaptive therapy. Cancer Res. 2009;69:4894–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thomas F, Donnadieu E, Charriere GM, Jacqueline C, Tasiemski A, Pujol P, et al. Is adaptive therapy natural? PLoS Biol. 2018;16:e2007066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gallaher JA, Enriquez-Navas PM, Luddy KA, Gatenby RA, Anderson ARA. Spatial Heterogeneity and Evolutionary Dynamics Modulate Time to Recurrence in Continuous and Adaptive Cancer Therapies. Cancer Res. 2018;78:2127–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.West J, You L, Zhang J, Gatenby RA, Brown JS, Newton PK, et al. Towards Multidrug Adaptive Therapy. Cancer Res. 2020;80:1578–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Silva AS, Kam Y, Khin ZP, Minton SE, Gillies RJ, Gatenby RA. Evolutionary Approaches to Prolong Progression-Free Survival in Breast Cancer. Cancer Res. 2012;72:6362–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Strobl M, West J, Viossat Y, Damaghi M, Robertson-Tessi M, Brown J, et al. Turnover modulates the need for a cost of resistance in adaptive therapy. bioRxiv. 2020;2020.01.22.914366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lenski RE. The cost of antibiotic resistance--from the perspective of a bacterium. Ciba Found Symp. 1997;207:131–40; discussion 141–51. [DOI] [PubMed] [Google Scholar]
- 53.Melnyk AH, Wong A, Kassen R. The fitness costs of antibiotic resistance mutations. Evol Appl. 2015;8:273–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bacevic K, Noble R, Soffar A, Wael Ammar O, Boszonyik B, Prieto S, et al. Spatial competition constrains resistance to targeted cancer therapy. Nat Commun. 2017;8:1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.West J, Robertson-Tessi M, Luddy K, Park DS, Williamson DFK, Harmon C, et al. The Immune Checkpoint Kick Start: Optimization of Neoadjuvant Combination Therapy Using Game Theory. JCO Clin Cancer Informatics. 2019;1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Enriquez-Navas PM, Kam Y, Das T, Hassan S, Silva A, Foroutan P, et al. Exploiting evolutionary principles to prolong tumor control in preclinical models of breast cancer. Sci Transl Med. 2016;8:327ra24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pepper JW. Drugs that target pathogen public goods are robust against evolved drug resistance. Evol Appl. 2012;5:757–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Basanta D, Gatenby RA, Anderson ARA. Exploiting evolution to treat drug resistance: combination therapy and the double bind. Mol Pharm. 2012;9:914–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Archetti M, Ferraro DA, Christofori G. Heterogeneity for IGF-II production maintained by public goods dynamics in neuroendocrine pancreatic cancer. Proc Natl Acad Sci. 2015;112:1833–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Piñeiro Fernández J, Luddy KA, Harmon C, O’Farrelly C, O’Farrelly C. Hepatic Tumor Microenvironments and Effects on NK Cell Phenotype and Function. Int J Mol Sci. 2019;20:4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Laquente B, Viñals F, Germà JR. Metronomic chemotherapy: an antiangiogenic scheduling. Clin Transl Oncol. 2007;9:93–8. [DOI] [PubMed] [Google Scholar]
- 62.Xu R-H, Pelicano H, Zhou Y, Carew JS, Feng L, Bhalla KN, et al. Inhibition of glycolysis in cancer cells: a novel strategy to overcome drug resistance associated with mitochondrial respiratory defect and hypoxia. Cancer Res. 2005;65:613–21. [PubMed] [Google Scholar]
- 63.Gillies RJ, Gatenby RA. Metabolism and its sequelae in cancer evolution and therapy. Cancer J. 21:88–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer. 2004;4:891–9. [DOI] [PubMed] [Google Scholar]
- 65.Robertson-Tessi M, El-Kareh A, Goriely A. A model for effects of adaptive immunity on tumor response to chemotherapy and chemoimmunotherapy. J Theor Biol. 2015;380:569–84. [DOI] [PubMed] [Google Scholar]
- 66.Xie W, Regan MM, Buyse M, Halabi S, Kantoff PW, Sartor O, et al. Metastasis-Free Survival Is a Strong Surrogate of Overall Survival in Localized Prostate Cancer. J Clin Oncol. 2017;35:3097–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Caswell-Jin JL, Plevritis SK, Tian L, Cadham CJ, Xu C, Stout NK, et al. Change in Survival in Metastatic Breast Cancer with Treatment Advances: Meta-Analysis and Systematic Review. JNCI cancer Spectr. 2018;2:pky062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.van der Toom EE, Verdone JE, Pienta KJ. Disseminated tumor cells and dormancy in prostate cancer metastasis. Curr Opin Biotechnol. 2016;40:9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Popper HH. Progression and metastasis of lung cancer. Cancer Metastasis Rev. 2016;35:75–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Friberg S, Nyström A. Cancer Metastases: Early Dissemination and Late Recurrences. Cancer Growth Metastasis. 2015;8:43–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hunter KW, Crawford NPS, Alsarraj J. Mechanisms of metastasis. Breast Cancer Res. 2008;10 Suppl 1:S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sopik V, Narod SA. The relationship between tumour size, nodal status and distant metastases: on the origins of breast cancer. Breast Cancer Res Treat. 2018;170:647–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Karagiannis GS, Condeelis JS, Oktay MH. Chemotherapy-Induced Metastasis: Molecular Mechanisms, Clinical Manifestations, Therapeutic Interventions. Cancer Res. 2019;79:4567–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Keklikoglou I, Cianciaruso C, Güç E, Squadrito ML, Spring LM, Tazzyman S, et al. Chemotherapy elicits pro-metastatic extracellular vesicles in breast cancer models. Nat Cell Biol. 2019;21:190–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lee HH, Bellat V, Law B. Chemotherapy induces adaptive drug resistance and metastatic potentials via phenotypic CXCR4-expressing cell state transition in ovarian cancer. PLoS One. 2017;12:e0171044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Giuliano M, Shaikh A, Lo HC, Arpino G, De Placido S, Zhang XH, et al. Perspective on Circulating Tumor Cell Clusters: Why It Takes a Village to Metastasize. Cancer Res. 2018;78:845–52. [DOI] [PubMed] [Google Scholar]
- 77.Fischer KR, Durrans A, Lee S, Sheng J, Li F, Wong STC, et al. Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance. Nature. 2015;527:472–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zheng X, Carstens JL, Kim J, Scheible M, Kaye J, Sugimoto H, et al. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature. 2015;527:525–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Durinikova E, Kozovska Z, Poturnajova M, Plava J, Cierna Z, Babelova A, et al. ALDH1A3 upregulation and spontaneous metastasis formation is associated with acquired chemoresistance in colorectal cancer cells. BMC Cancer. 2018;18:848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Huang W-S, Hsieh M-C, Huang C-Y, Kuo Y-H, Tung S-Y, Shen C-H, et al. The Association of CXC Receptor 4 Mediated Signaling Pathway with Oxaliplatin-Resistant Human Colorectal Cancer Cells. PLoS One. 11:e0159927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shibue T, Weinberg RA. EMT, CSCs, and drug resistance: the mechanistic link and clinical implications. Nat Rev Clin Oncol. 2017;14:611–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shang Y, Cai X, Fan D. Roles of epithelial-mesenchymal transition in cancer drug resistance. Curr Cancer Drug Targets. 2013;13:915–29. [DOI] [PubMed] [Google Scholar]
- 83.Garraway LA, Jänne PA. Circumventing Cancer Drug Resistance in the Era of Personalized Medicine. Cancer Discov. 2012;2:214–26. [DOI] [PubMed] [Google Scholar]
- 84.McGee LW, Sackman AM, Morrison AJ, Pierce J, Anisman J, Rokyta DR. Synergistic Pleiotropy Overrides the Costs of Complexity in Viral Adaptation. Genetics. 2016;202:285–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Meads MB, Gatenby RA, Dalton WS. Environment-mediated drug resistance: a major contributor to minimal residual disease. Nat Rev Cancer. 2009;9:665–74. [DOI] [PubMed] [Google Scholar]
- 86.Gomis RR, Gawrzak S. Tumor cell dormancy. Mol Oncol. 2017;11:62–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Linde N, Fluegen G, Aguirre-Ghiso JA. The Relationship Between Dormant Cancer Cells and Their Microenvironment. Adv Cancer Res. 2016;132:45–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Karagiannis GS, Pastoriza JM, Wang Y, Harney AS, Entenberg D, Pignatelli J, et al. Neoadjuvant chemotherapy induces breast cancer metastasis through a TMEM-mediated mechanism. Sci Transl Med. 2017;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Perelmuter VM, Tashireva LA, Savelieva OE, Denisov EV, Kaigorodova EV, Zavyalova MV, et al. Mechanisms behind prometastatic changes induced by neoadjuvant chemotherapy in the breast cancer microenvironment. Breast cancer (Dove Med Press. 2019;11:209–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Acharyya S, Oskarsson T, Vanharanta S, Malladi S, Kim J, Morris PG, et al. A CXCL1 paracrine network links cancer chemoresistance and metastasis. Cell. 2012;150:165–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yuan M, Zhu H, Xu J, Zheng Y, Cao X, Liu Q. Tumor-Derived CXCL1 Promotes Lung Cancer Growth via Recruitment of Tumor-Associated Neutrophils. J Immunol Res. 2016;2016:6530410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang D, Sun H, Wei J, Cen B, DuBois RN. CXCL1 Is Critical for Premetastatic Niche Formation and Metastasis in Colorectal Cancer. Cancer Res. 2017;77:3655–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang N, Liu W, Zheng Y, Wang S, Yang B, Li M, et al. CXCL1 derived from tumor-associated macrophages promotes breast cancer metastasis via activating NF-κB/SOX4 signaling. Cell Death Dis. 2018;9:880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chan T-S, Hsu C-C, Pai VC, Liao W-Y, Huang S-S, Tan K-T, et al. Metronomic chemotherapy prevents therapy-induced stromal activation and induction of tumor-initiating cells. J Exp Med. 2016;213:2967–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Blomberg OS, Spagnuolo L, de Visser KE. Immune regulation of metastasis: mechanistic insights and therapeutic opportunities. Dis Model Mech. 2018;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Adler FR, Gordon DM. Cancer ecology and evolution: positive interactions and system vulnerability. Curr Opin Syst Biol. 2019;17:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Eyles J, Puaux A-L, Wang X, Toh B, Prakash C, Hong M, et al. Tumor cells disseminate early, but immunosurveillance limits metastatic outgrowth, in a mouse model of melanoma. J Clin Invest. 2010;120:2030–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.López-Soto A, Gonzalez S, Smyth MJ, Galluzzi L. Control of Metastasis by NK Cells. Cancer Cell. 2017;32:135–54. [DOI] [PubMed] [Google Scholar]
- 99.Takeda K, Hayakawa Y, Smyth MJ, Kayagaki N, Yamaguchi N, Kakuta S, et al. Involvement of tumor necrosis factor-related apoptosis-inducing ligand in surveillance of tumor metastasis by liver natural killer cells. Nat Med. 2001;7:94–100. [DOI] [PubMed] [Google Scholar]
- 100.Chockley PJ, Chen J, Chen G, Beer DG, Standiford TJ, Keshamouni VG. Epithelial-mesenchymal transition leads to NK cell-mediated metastasis-specific immunosurveillance in lung cancer. J Clin Invest. 2018;128:1384–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kang D-H, Weaver MT, Park N-J, Smith B, McArdle T, Carpenter J. Significant impairment in immune recovery after cancer treatment. Nurs Res. 58:105–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gallaher JA, Brown JS, Anderson ARA. The impact of proliferation-migration tradeoffs on phenotypic evolution in cancer. Sci Rep. 2019;9:2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Boddy AM, Huang W, Aktipis A. Life History Trade-Offs in Tumors. Curr Pathobiol Rep. 2018;6:201–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gerlee P, Nelander S. The Impact of Phenotypic Switching on Glioblastoma Growth and Invasion. Alber MS, editor. PLoS Comput Biol. 2012;8:e1002556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Picariello HS, Kenchappa RS, Rai V, Crish JF, Dovas A, Pogoda K, et al. Myosin IIA suppresses glioblastoma development in a mechanically sensitive manner. Proc Natl Acad Sci U S A. 2019;116:15550–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gallaher JA, Massey SC, Hawkins-Daarud A, Noticewala SS, Rockne RC, Johnston SK, et al. From cells to tissue: How cell scale heterogeneity impacts glioblastoma growth and treatment response. Finley S, editor. PLOS Comput Biol. 2020;16:e1007672. [DOI] [PMC free article] [PubMed] [Google Scholar]