Inadvertent misplacement or migration of a “Viatorr” stent graft (W.L. Gore & Associates, Inc. Flagstaff, AR) during transjugular intrahepatic portosystemic shunt (TIPS) creation can occur either during stent deployment or subsequent manipulation of balloons or catheters within the stent. 1 2 3 Minor degrees of stent migration in the cephalad or caudal direction often have no clinical consequence. However, major cephalad migration of the stent graft can result in outflow obstruction of the inferior vena cava or problems during hepatic venous anastomosis of the transplanted liver. 4 The reported techniques for the removal of a centrally migrated stent graft involve using a loop snare to capture the cephalad portion of the stent and retrieving it into a large caliber sheath, 2 using forceps-like device for holding the proximal end to allow subsequent passage of a snare, 5 or forceful pulling of the stent into the sheath and surgical removal of the stent graft. 3
Caudal migration of the stent graft can result in occlusion of the major portal vein branch. Major caudal migration can result in diverting the entire portal flow into the TIPS. It can also interfere with portal vein anastomosis during liver transplantation. Removal of caudally migrated TIPS stent is often difficult as the cranial segment of the stent graft is within the parenchymal tract, limiting the ability to snare the hepatic venous end of the stent graft for retrieval. Balloon-assisted repositioning of the stent graft is rarely successful. In this case, we report successful removal of a caudally migrated TIPS stent with the “involute” technique.
Case Report
A 55-year-old male patient with cirrhosis and severe refractory variceal bleeding was referred for TIPS ( Fig. 1a ). TIPS was created between the middle hepatic vein and the left portal vein, and a 10 mm × 7 cm Viatorr stent graft was deployed ( Figs. 1b , 2a ). A post-TIPS portal venography demonstrated continued flow into the esophageal varices through the left gastric vein ( Fig. 1c ). The 10-Fr sheath was advanced through the TIPS to facilitate cannulation of the left gastric vein with a 5-Fr catheter. During this process, the Viatorr stent graft migrated caudally, with its bare portion laying at the splenoportal confluence and the cephalad portion of the stent within the parenchymal tract ( Figs. 3a , 2b ).
Fig. 1.

Portal venography after accessing the left portal vein from the middle hepatic vein ( a ) shows a patent portal vein (open black arrow) and enlarged esophageal varices (closed black arrow). The additional sheath in the inferior vena cava/middle hepatic vein (white arrow) housed an intravascular ultrasound for guidance during the TIPS. Post-TIPS portal venography ( b and c ) shows a patent portal vein with accurately positioned TIPS (arrowhead) and persistent esophageal varices (closed black arrow).
Fig. 2.

Schematic demonstration of procedural steps. ( a ) The TIPS between the middle hepatic vein and left portal vein. ( b ) Caudal migration of Viatorr stent graft after a guide catheter (red) was advanced through the TIPS. ( c ) The 10-Fr sheath was exchanged for 16- and 10-Fr sheaths. An additional guide wire (buddy wire) (blue) was advanced by the side of the stent graft. ( d ) A Teflon-coated angled guide wire (purple) advanced from the lumen to the outside of the stent graft through the interstices of the bare portion of the stent graft. ( e ) A snare system (pink) was advanced through the 16-Fr sheath and snared by the Teflon-coated wire, forming a loop through a segment of the bare portion of the stent graft. ( f ) The looped wire was pulled out through the venous access sheath, “involuting” the stent graft in the process. ( g ) The involuted stent graft was removed via the venous access sheath. ( h ) Cross-sectional view of the “involute” technique where a loop is formed around a segment of the bare portion of the stent graft with the Teflon-coated guide wire (purple) and snare system (pink).
Fig. 3.
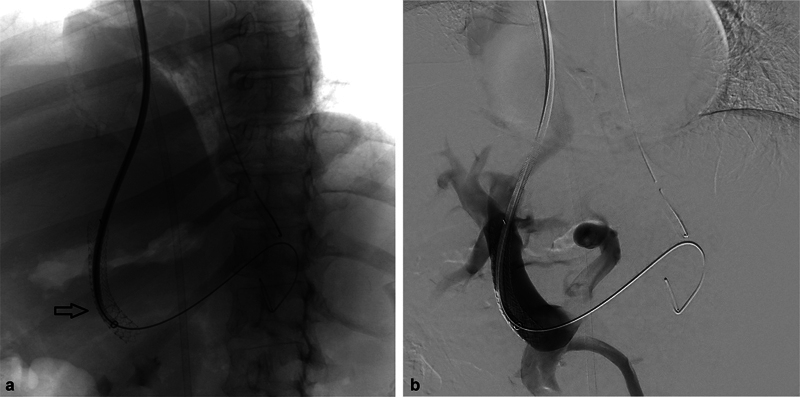
Portal venography ( a ) and fluoroscopic image ( b ) show caudal migration of the stent graft into the main portal vein.
Two Amplatz wires were advanced into the splenic vein, and the 10-Fr sheath was exchanged for a 16-Fr sheath and 10-Fr sheath that were positioned within the TIPS parenchymal tract. The guide wire within the 10-Fr sheath was then navigated outside the stent graft and placed as a buddy wire in the splenic vein ( Figs. 4 , 2c ). The guide wire of the 16-Fr sheath was exchanged for a stiff hydrophilic guide wire, which was advanced from the lumen of the stent graft to outside the stent graft through the interstices of the bare portion of the stent graft ( Fig. 2d ). The guide wire was then manipulated into the splenic vein. Subsequently, a snare system was advanced through the 16-Fr sheath and into the splenic vein, and the hydrophilic guide wire was snared from the splenic vein, thus forming a loop through a segment of the bare portion of the stent graft ( Figs. 5a , 2e ). The looped wire was pulled out while keeping the sheath intact within the parenchymal tract. During this process, the stent graft “involuted” and pulled into the sheath ( Figs. 5b , 6 , and 2f ). The system was removed once the entire stent graft was within the 16-Fr sheath ( Fig. 2g ).
Fig. 4.

Fluoroscopic image showing the deployment of the second sheath and buddy wire.
Fig. 5.

Fluoroscopic image showing Teflon-coated angled guide wire and snare system deployment ( 5a ) and then retraction of the stent graft into the sheath ( 5b ).
Fig. 6.

Image of retrieved stent graft.
The 10-Fr sheath was advanced into the main portal vein, and another Viatorr stent graft was deployed in the correct position ( Fig. 7 ). The varices were subsequently embolized without any other complication. A follow-up clinic visit and duplex ultrasound of the TIPS at 6 weeks showed good clinical outcomes with no recurrent variceal bleeding and a patent TIPS.
Fig. 7.

Portal venography showing the final placement of TIPS stent graft.
Discussion
There are many techniques for the removal of intravascular foreign bodies: Curry snare, 6 loop snare, 7 pigtail through snare, 8 and forceps use. 9 The Curry snare was first described in 1969 and involves “folding” a guide wire around a foreign body and then retracting it. 6 This technique is useful for removing foreign bodies from large vessels, such as the aorta or vena cava. In this technique, the loop of the snare can be quite variable and can be modified at the will of the operator. The technique is makeshift, using a long guide wire that is folded and inserted through a larger diameter guiding catheter or sheath. Subsequently, commercially available snares with pre-shaped snare loop configurations and guide catheters became available (e.g., the goose neck snare, Ensnare, Dormia basket). The loop snare technique was initially described in 2007 to remove an inferior vena cava filter tilted against the vessel wall. 7 In cases with no accessible free ends for snaring a foreign body, a pigtail catheter or a reverse curve catheter can be used to loop around the mid portion of the foreign body and create a free end to be grabbed by a snare loop catheter. 8 Grasping forceps were described as a technique for the removal of an inferior vena cava filter that was embedded in the vessel walls where a snare technique was not possible. 9 Forceps are preferred over snares in cases where it is not possible to reposition the foreign body to a more favorable position or if it is incorporated into the vessel. However, forceps could damage the foreign body and cause vessel perforation. Therefore, selecting a technique for intravascular foreign body removal necessitates balancing feasibility and safety.
The loop-snare technique to capture the free end of a stent or stent graft for its removal is well described in the literature. 10 However, this technique was not possible in our case as the proximal end was embedded in the liver parenchymal portion of TIPS tract, and the distal end was opposed to the portal vein wall. Forceps were not considered due to the risk of peeling off of the PTFE from the cranial end of the TIPS stent graft and the risk of liver parenchymal injury. The current description of Viatorr stent graft retrieval used a modification of the previously described technique for removing an intravascular foreign body without access to a free end. In this technique, a wire is looped around the intravascular foreign body using a reverse curve catheter, and the free end of the wire is snared out to create a loop around the foreign body ( Fig. 2 ). The loop acts as a fulcrum and folds the foreign body to allow its retrieval. Similarly, we used a guide wire to create a loop around a segment of the bare portion of the Viatorr stent graft by passing it through the stent interstices and snaring the distal end. This technique is simple and allows easy retrieval of the stent graft.
This was our first case of caudal migration of a Viatorr stent graft in the last 10 years (during which 650 TIPS were performed with the stent graft). This caudal migration occurred with the new “controlled expansion” TIPS endoprosthesis. It may be related to the additional constrictive sleeve placed around the stent graft to limit the complete expansion of the stent. We suggest more caution while catheterizing the new Viatorr-controlled expansion stent graft in the immediate post-deployment phase.
Conclusion
Removal of caudally migrated TIPS stent is uniquely difficult as the cranial segment of the stent graft is within the parenchymal tract, limiting the ability to snare the hepatic venous end of the stent graft for retrieval. The current case demonstrates the feasibility of the “involute” technique of retrieving a caudally migrated TIPS.
Funding Statement
Funding This study was not supported by any funding.
Conflict of Interest None related to the manuscript.
Other Conflicts of Interest
S.P.K. reports grants from NIH, BD, Black Swan, and Trisalus for Institution; reports royalties from Elsevier, Springer, and Thieme for himself; reports consulting fees from Penumbra, Okami Medical, Boston Scientific, Medtronic, Instylla, and BD for himself; reports stock from Biogen Inc, Clover Health Investments Corp, Inovio Pharmaceuticals, Moderna Inc, Pfizer Inc, Novavax Inc., Orphazyme, Cassava Sciences Inc., Vivos Therapeutics Inc., Ardelyx Inc., Althea Health, Sarepta Therapeutics, Clover Health Investments Corp., CureVac BV, Immunoprecise Antibodies Ltd., Infinity Pharmaceuticals Inc., Zymergen Inc., BioNTech SE, Trillium Therapeutics Inc., Theravance Biopharma Inc., Doximity Inc., Eargo Inc., Allogent Therapeutics Inc., NRx Pharmaceuticals Inc., Atea pharmaceuticals Inc., for himself and spouse.
References
- 1.Ferguson E, Cwikiel W. Percutaneous removal of two self-expanding stent grafts following failed deployment. Acta Radiol. 2006;47(07):667–671. doi: 10.1080/02841850600791534. [DOI] [PubMed] [Google Scholar]
- 2.Ray M J, Savage C, Klintmalm G B, Rees C R. Endovascular caudal retraction of the cranial end of a misplaced Viatorr TIPS prior to liver transplantation. Proc Bayl Univ Med Cent. 2012;25(04):341–343. doi: 10.1080/08998280.2012.11928871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cwikiel W, Bergenfeldt M, Keussen I. Endovascular removal of the Viatorr stent-grafts. Report of two cases. Pol J Radiol. 2015;80:277–280. doi: 10.12659/PJR.893642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suhocki P V, Lungren M P, Kapoor B, Kim C Y. Transjugular intrahepatic portosystemic shunt complications: prevention and management. Semin Intervent Radiol. 2015;32(02):123–132. doi: 10.1055/s-0035-1549376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vu J, Kim S K. Percutaneous retrieval of a misplaced transjugular intrahepatic portosystemic shunt stent using the rigid endobronchial forceps. Int J Gastrointest Interv. 2016;5:156–158. [Google Scholar]
- 6.Curry J L. Recovery of detached intravascular catheter or guide wire fragments. A proposed method. Am J Roentgenol Radium Ther Nucl Med. 1969;105(04):894–896. doi: 10.2214/ajr.105.4.894. [DOI] [PubMed] [Google Scholar]
- 7.Rubenstein L, Chun A K, Chew M, Binkert C A. Loop-snare technique for difficult inferior vena cava filter retrievals. J Vasc Interv Radiol. 2007;18(10):1315–1318. doi: 10.1016/j.jvir.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 8.Greenfield D H, McMullan G K, Parisi A F, Askenazi J. Snare retrieval of a catheter fragment with inaccessible ends from the pulmonary artery. Cathet Cardiovasc Diagn. 1978;4(01):87–90. doi: 10.1002/ccd.1810040112. [DOI] [PubMed] [Google Scholar]
- 9.Burke C T, Dixon R G, Stavas J M. Use of rigid bronchoscopic forceps in the difficult retrieval of the Günther Tulip inferior vena cava filter. J Vasc Interv Radiol. 2007;18(10):1319–1323. doi: 10.1016/j.jvir.2007.06.033. [DOI] [PubMed] [Google Scholar]
- 10.Egglin T K, Dickey K W, Rosenblatt M, Pollak J S. Retrieval of intravascular foreign bodies: experience in 32 cases. AJR Am J Roentgenol. 1995;164(05):1259–1264. doi: 10.2214/ajr.164.5.7717243. [DOI] [PubMed] [Google Scholar]


