Abstract
Senescence is a condition of cell cycle arrest that increases inflammation and contributes to the development of chronic diseases in the aging human body. While several compounds described as senolytics and senomorphics produce health benefits by reducing the burden of senescence, less attention has been devoted to lifestyle interventions that produce similar effects. We describe here the effects of exercise, nutrition, caloric restriction, intermittent fasting, phytochemicals from natural products, prebiotics and probiotics, and adequate sleep on senescence in model organisms and humans. These interventions can be integrated within a healthy lifestyle to reduce senescence and inflammation and delay the consequences of aging.
Keywords: Caloric restriction, Exercise, Metformin, Nutrition, Phytochemicals, Senescence
1. Introduction
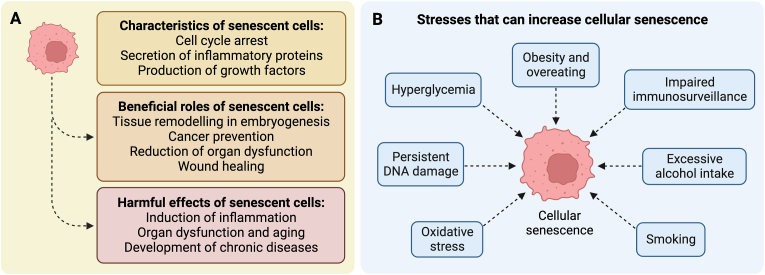
Cellular senescence is a condition of cell cycle arrest in which cells cease to proliferate despite favorable growth conditions [1] (Fig. 1A). Various endogenous and exogenous forms of stress can induce the development of senescent cells, including telomere dysfunction [1], persistent DNA damage [2], hyperglycemia [3], obesity [4], and smoking [5], among others (Fig. 1B). Cellular senescence is involved in embryogenesis, wound healing, and cancer prevention [1,2,6] (Fig. 1A). During embryogenesis, senescent cells play a role in embryonic growth and tissue patterning [7]. When the body sustains a wound, senescent cells release growth factors that contribute to cell growth and tissue healing [8]. Pro-inflammatory cytokines released by senescent cells also serve as signals for activation of stem cells and recruitment of immune cells into the wound [6,9]. Upon persistent DNA damage, cells may also enter senescence, which can decrease organ dysfunction and the risk of cancer [2] (Fig. 1A).
Fig. 1.
Characteristics of senescent cells and examples of stresses that increase senescence in the human body. (A) Characteristics and roles of senescent cells. (B) Stresses and lifestyle habits that increase the senescence burden.
The beneficial role of senescent cells early in life can become detrimental in later years. The accumulation of senescent cells with time reduces the capacity of the body to regenerate and induces chronic inflammation via the senescence-associated secretory phenotype (SASP), which contributes to the condition of inflammaging during the aging process and especially in older adults [10,11] (Fig. 1A). The SASP consists of a complex mixture of cytokines, chemokines, growth factors, proteases and reactive oxygen species (ROS) that may induce senescence in surrounding cells [12]. Evidence that the accumulation of senescent cells contributes to the development of chronic diseases during aging (Fig. 1A) comes from depletion studies in model organisms. Accordingly, treatment with compounds termed “senolytics” that can kill senescent cells have been shown to produce many health benefits. For instance, intermittent treatment of mice with the senolytic compounds dasatinib and quercetin decreased physical dysfunction and extended lifespan by 36 % in aged mice [13]. Similarly, dasatinib and quercetin improved cardiac function and physical performance in old mice by reducing the senescent cell burden [14]. The same cocktail also reduced intestinal senescence, inflammation and dysbiosis in mice [15]. In addition, senomorphic compounds such as apigenin, 4,4′-dimethoxychalcone, glucosamine, and metformin—which can inhibit senescent cell phenotype and the SASP—have shown promising effects in model organisms [6].
Preliminary clinical studies suggest that treatment with senolytics may also produce beneficial effects in pulmonary fibrosis [16] and kidney disease [17]. While senotherapies have emerged as potential treatments for chronic diseases, less attention has been devoted to the effects of lifestyle interventions that are widely available, easy to implement, and safe when used as recommended.
1.1. Effects of exercise on senescence
Exercise is widely recognized to produce beneficial effects on health of animals and humans. Several preclinical studies indicate that exercise can reduce the number of senescent cells in various organs including the heart, liver, muscles, kidneys, and adipose tissues [18,19] (Fig. 2). For instance, wheel running for three weeks reduced the senescence marker and cyclin-dependent kinase inhibitor p16 in the heart of mice [20]. Aerobic treadmill exercise for 15–60 min daily, five times per week for six weeks reduced levels of senescence-associated beta-galactosidase in the kidneys of aged mice [21]. Similarly, a three-month swimming program reduced senescence markers and the pro-inflammatory cytokine interleukin-6 (IL-6) in the liver of rodents treated with d-galactose to induce aging [22]. However, the high heterogeneity of exercise regimens used in these animal models and the sole reliance on senescence markers limit our understanding of the mechanisms underlying the effects of exercise on senescence.
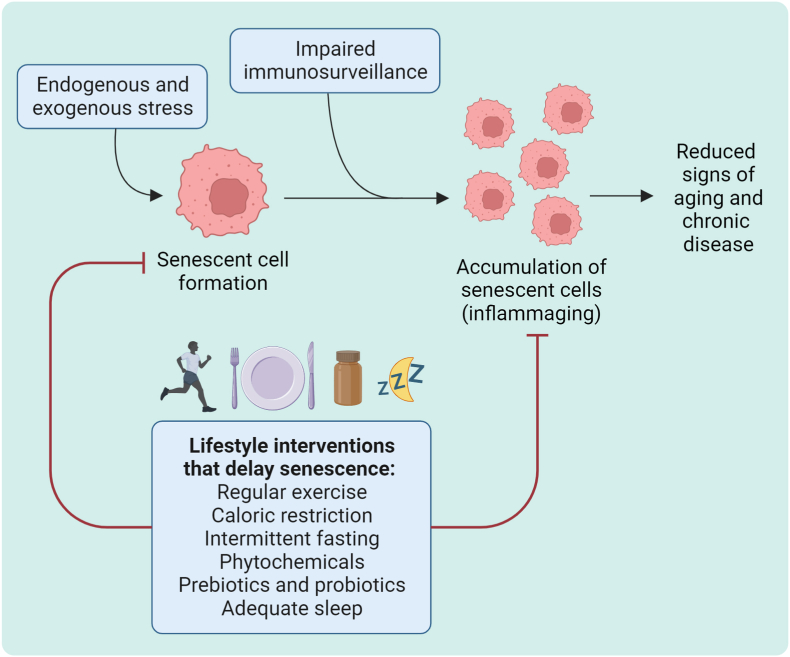
Fig. 2.
Lifestyle interventions to delay senescence. The formation and accumulation of senescent cells can be reduced by various lifestyle interventions that can improve health and longevity, mainly by reducing inflammation.
Another important concept that is poorly recognized is that the relationship between exercise and senescence is not straightforward. For instance, exercise-induced senescence of fibro-adipogenic progenitor cells is beneficial for inducing regeneration of muscle cells following exercise [23]. Furthermore, exercise at extremely-high intensity can be detrimental for the body. For instance, swimming at extreme intensity that induces exhaustion can produce senescence in the hippocampus and impair memory in rats [24]. This observation is a tell-tale sign of a hormesis response to stress, showing that exercise induces health benefits at low or moderate intensity, whereas excessive intensity and overtraining can produce detrimental effects [25]. These results indicate that exercise can reduce the number of senescent cells, but several factors including exercise intensity and the health condition of the host can affect the outcome.
In humans, regular physical activity is associated with reduced levels of p16INK4 in T lymphocytes [26]. A five-month training program reduced the number of p16INK4-positive senescent cells in thigh adipose tissues of older overweight women [27]. Expression of p16 and IL-6 was elevated in the colonic mucosa of middle-aged and older overweight men compared to young sedentary men, whereas this elevation was blunted in age-matched endurance runners with several years of experience [28]. Similarly, the increase of senescent endothelial cells and impaired vascular endothelial function observed in brachial arteries of older sedentary individuals was absent in older exercising subjects [29]. In another study, leukocyte infiltration of skeletal muscles increased during resistance training in healthy and active young men, which may have induced the clearance of senescent cells [30]. Furthermore, a high-protein diet produced greater muscle gain and better senescent cell clearance [30], indicating that diet influences these processes.
1.2. Effects of diet on senescence
Various components found in the human diet including proteins, carbohydrates, fatty acids, vitamins and polyphenols have been shown to modulate the development of cellular senescence [31]. As noted above, hyperglycemia is a factor that can induce senescence [3] (Fig. 1B). High glycemic diets can promote production of advanced glycation end products (AGEs) that result from cooking foods that are high in carbohydrates, fats and proteins. Glycated collagen, a form of AGEs, can induce vascular senescence in rats [32]. While a high glycemic diet, overeating and disrupted glucose metabolism may increase the senescence burden, caloric restriction (CR) and intermittent fasting may reduce the number of senescent cells (Fig. 2). CR consists of reducing energy intake from food without resulting in malnutrition. CR has been shown to reduce the signs of aging and extend lifespan in animal models by activating autophagy, damage repair and expression of antioxidant enzymes and by reducing the production of growth factors and pro-inflammatory cytokines [33,34]. Fontana et al. showed that CR can reduce the number of senescent cells in the large intestine of both mice and humans [35]. Intermittent fasting for 30 days (17–19 h daily) had a tendency to reduce expression of the senescence markers p16INK4A and p21 in the blood of healthy men, although the results did not reach statistical significance [36]. Supplementation with ß-hydroxybutyrate—a ketone body that increases in the blood of people who consumed a ketogenic diet—reduced vascular senescence in aged mice [37]. A fasting-mimicking diet for four days followed bi-monthly rejuvenated the immune system in mice [38], which may reduce accumulation of senescent cells by improving immunosurveillance. The possibility of improving immunosurveillance using a fasting-mimicking diet may be especially beneficial as disruption of the immune system during aging leads to reduced immunosurveillance and accumulation of senescent cells in the aging body [39].
A high-protein diet can be beneficial for inducing muscle gain and reduce inflammation following exercise [30]. Conversely, a low-protein diet can improve the health span and longevity in animals that are fed ad libitum [40]. These observations suggest that individuals hoping to improve exercise performance may make choices that affect health and lifespan. Adults aged 50–65 who consume a high-protein diet (>20 % of calories from proteins) had a 78 % higher overall mortality and a four-time higher risk of cancer mortality, while this trend was reversed in people over 65 years of age who showed reduced overall mortality and cancer mortality on a high-protein diet [41]. The phenomenon of higher mortality in adults who consumed a high-protein diet was attenuated to some extent if the proteins were from plants instead of animals [41]. In mice, consumption of a high-protein diet for three months increased expression of pro-inflammatory genes and the senescence markers p16 and p21 in the liver, with an increase of fat further enhancing the levels of pro-inflammatory markers [42].
A high-fat diet induced accumulation of senescent cells in the pancreas and adipose tissues of mice, contributing to inflammation and insulin resistance [43,44]. A high-fat diet also resulted in oxidative stress and senescent cell accumulation in the murine brain, which could be partially abrogated by treatment with the ginseng-derived compound ginsenoside [45]. While high-fat diets have been used to induce obesity and are widely believed to be detrimental to health, it appears that these effects are at least partly due to a lack of dietary fibers and intake of a large excess of calories [46].
Various phytochemicals such as quercetin, piperlongumine and fisetin also possess senolytic properties [6] (Fig. 2). The tea-derived polyphenol epigallogatechin gallate (EGCG) reduces inflammation, gut dysbiosis, and the accumulation of senescent cells in the adipose and intestinal tissues of mice [47]. A polyphenol-rich herbal extract of milk thistle produced senolytic effects in human skin cells in vitro [48]. Berberine also reduced senescence by inhibiting the mTOR signalling pathway [49]. Diets that are rich in phytochemicals such as the Mediterranean diet, which emphasizes plant-based foods and healthy fats, are likely to reduce the accumulation of senescent cells. Accordingly, cultivating endothelial cells with the serum of elderly subjects who had consumed a Mediterranean diet for four weeks led to reduced intracellular reactive oxygen species and apoptosis and a lower number of cells with short telomeres [50].
1.3. A cautionary note on the anti-diabetic compound metformin
Metformin is a synthetic derivative of French lilac, a herbal plant that has been traditionally used in Europe for diabetes treatment. Metformin has been shown to produce senomorphic effects in mice [[51], [52], [53]]. This compound is currently being studied as part of the TAME (Targeting Aging with MEtformin) study as an anti-aging nutraceutical compound to reduce symptoms of aging-related chronic diseases in healthy humans [54].
Metformin is also used to treat polycystic ovary syndrome (PCOS), a condition associated with elevated levels of testosterone in women. Similarly, metformin reduces testosterone levels in men with type 2 diabetes [55,56]. This reduction of testosterone in diabetic men taking metformin is associated with erectile dysfunction [55], which is an adverse side effect that has not been adequately considered so far in aging-related studies. Furthermore, metformin abrogated the beneficial effects of exercise on insulin sensitivity and mitochondrial respiration in older subjects [57], suggesting that a combination of hormetic stresses such as metformin and exercise may produce detrimental additive effects in older individuals. Given the possibility that a large number of healthy aged people may soon start using metformin to delay aging, these potential adverse effects require further attention in clinical trials and post-approval drug safety surveillance.
1.4. The gut microbiota: effect of prebiotics and probiotics on senescence
Expanding research shows that the gut microbiome plays an important role in maintaining health and preventing chronic diseases, with diet playing a major role in modulating the gut microbiota composition [58,59]. The gut microbiota varies considerably during aging and is associated with a reduced diversity and lower level of beneficial commensals [60]. We showed earlier that polysaccharides derived from the medicinal mushrooms Ganoderma lucidum and Hirsutella sinensis, acting as prebiotics, can reduce inflammation, insulin resistance and obesity in high-fat diet-fed mice by modulating the composition of the gut microbiota [61,62]. It is now recognized that the gut microbiota mediates several of the beneficial effects of dietary components and pharmaceutical compounds, including dietary fibers [63] and metformin [64].
Flavonoids such as resveratrol and quercetin can modulate the composition of the gut microbiota in animal models [65,66]. The cocktail of dasatinib and quercetin reduced senescent cells in the intestines and the inflammatory burden in aged mice [15]. In addition, the cocktail modulated the gut microbiota, increasing beneficial commensals such as Akkermansia muciniphila, Ruminococcus spp., and Butyrivibrio spp. Notably, these changes were observed in aged mice but not in young mice [15], indicating that the prebiotics may reverse aging-associated dysbiosis. It thus appears plausible that many of the effects of this senolytic cocktail may be mediated by the gut microbiota. Alternatively, this may apply only to quercetin which is known to act as a prebiotic.
Probiotics can also produce beneficial effects by preventing the formation of senescent cells [67] (Fig. 2). For instance, two-week supplementation with fermented milk containing Lactobacillus and Streptococcus probiotics improved macrophage function and natural killer cell activity in aged mice [68]. Supplementation with culture supernatants of Lactobacillus fermentum reduced stress-induced senescence in murine preadipocytes by decreasing DNA damage, expression of p16INK4A and p21, SASP markers, and NF-kB activation [69]. Oral administration of Lactobacillus to aged mice reduced pro-inflammatory markers, enhanced intestinal tight junctions, and reduced the accumulation of senescent cells in the colon [70].
1.5. Effects of sleep deprivation on senescence
Sleep is also widely recognized to produce health benefits in animal models and humans. Sleep fragmentation, sleep loss and insomnia can increase inflammation and senescence, possibly contributing to the development of aging-related diseases [71]. Chronic sleep deprivation during a period of 20 weeks induced vascular endothelial dysfunction including mild hypertension, inflammation, and increase of the senescence marker p16INK4A in the arteries of mice [72]. Women participating in the Women's Health Initiative study who reported sleep disturbance and insomnia had higher levels of late differentiated T cells [73], which are thought to represent senescent or near senescent cells. A single night of partial sleep deprivation (in which sleep was allowed only from 3 a.m. to 7 a.m.) was sufficient to induce gene expression related to the DNA damage response, pro-inflammatory SASP factors, and the senescence marker p16INK4A in peripheral blood mononuclear cells (PBMCs) of older adults (aged 61 to 86) [74]. While these preliminary results appear consistent, more research is needed to examine the impact of chronic sleep disturbance on human aging and disease development.
2. Conclusions
Different lifestyle interventions including exercise, nutrition, intermittent fasting, and consumption of phytochemicals, prebiotics and probiotics, and adequate sleep can produce anti-senescence effects in model organisms and humans. Given the widespread beneficial effects of these lifestyle interventions, the findings described here are perhaps not surprising—except that the reduction of senescent cells represents a new mechanism of action to explain the effects of these interventions. The effects of lifestyle factors on senescence are quite complex and can easily be neutralized or become detrimental depending on their intensity and frequency. Moreover, an unhealthy lifestyle involving sedentarity, consumption of excess alcohol, smoking, lack of sleep and sunlight exposure and chronic stress may offset some of the beneficial effects of other interventions on senescence. More attention should therefore be given to the modalities that produce beneficial effects and their interactions with anti-senescence compounds and other lifestyle habits.
Disclaimer Statement
J.D.Y. is Chairman of the Board of Chang Gung Biotechnology. J.M., D.M.O., and J.D.Y. are named on patents held jointly by Chang Gung University and Chang Gung Biotechnology related to the preparation and use of dietary supplements.
Acknowledgments
The authors' work is supported by Primordia Institute of New Sciences and Medicine.
Footnotes
Peer review under responsibility of Chang Gung University.
References
- 1.Di Micco R, Krizhanovsky V, Baker D, d’Adda di Fagagna F. Cellular senescence in ageing: from mechanisms to therapeutic opportunities. Nat Rev Mol Cell Biol. 2021;22(2):75–95. doi: 10.1038/s41580-020-00314-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Campisi J, d’Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8(9) doi: 10.1038/nrm2233. 729–40. [DOI] [PubMed] [Google Scholar]
- 3.Maeda M, Hayashi T, Mizuno N, Hattori Y, Kuzuya M. Intermittent high glucose implements stress-induced senescence in human vascular endothelial cells: role of superoxide production by NADPH oxidase. PLoS One. 2015;10(4) doi: 10.1371/journal.pone.0123169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ogrodnik M, Zhu Y, Langhi LGP, Tchkonia T, Kruger P, Fielder E, et al. Obesity-induced cellular senescence drives anxiety and impairs neurogenesis. Cell Metabol. 2019;29(5) doi: 10.1016/j.cmet.2019.01.013. 1061–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nyunoya T, Monick MM, Klingelhutz A, Yarovinsky TO, Cagley JR, Hunninghake G.W. Cigarette smoke induces cellular senescence. Am J Respir Cell Mol Biol. 2006;35(6) doi: 10.1165/rcmb.2006-0169OC. 681–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martel J, Ojcius DM, Wu CY, Peng HH, Voisin L, Perfettini JL, et al. Emerging use of senolytics and senomorphics against aging and chronic diseases. Med Res Rev. 2020;40(6) doi: 10.1002/med.21702. 2114-31. [DOI] [PubMed] [Google Scholar]
- 7.Storer M, Mas A, Robert-Moreno A, Pecoraro M, Ortells MC, Di Giacomo V, et al. Senescence is a developmental mechanism that contributes to embryonic growth and patterning. Cell. 2013;155(5) doi: 10.1016/j.cell.2013.10.041. 1119–30. [DOI] [PubMed] [Google Scholar]
- 8.Wilkinson HN, Hardman MJ. Senescence in wound repair: emerging strategies to target chronic healing wounds. Front Cell Dev Biol. 2020;8:773. doi: 10.3389/fcell.2020.00773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiche A, Le Roux I, von Joest M, Sakai H, Aguin SB, Cazin C, et al. Injury-induced senescence enables in vivo reprogramming in skeletal muscle. Cell Stem Cell. 2017;20(3) doi: 10.1016/j.stem.2016.11.020. 407–14. [DOI] [PubMed] [Google Scholar]
- 10.Franceschi C, Garagnani P, Vitale G, Capri M, Salvioli S. Inflammaging and ‘garb-aging’. Trends Endocrinol Metabol. 2017;28(3):199–212. doi: 10.1016/j.tem.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 11.Franceschi C, Garagnani P, Parini P, Giuliani C, Santoro A. Inflammaging: a new immune–metabolic viewpoint for age-related diseases. Nat Rev Endocrinol. 2018;14(10) doi: 10.1038/s41574-018-0059-4. 576–90. [DOI] [PubMed] [Google Scholar]
- 12.Borodkina AV, Deryabin PI, Giukova AA, Nikolsky NN. “Social life” of senescent cells: what is SASP and why study it? Acta Naturae. 2018;10(1):4–14. [PMC free article] [PubMed] [Google Scholar]
- 13.Xu M, Pirtskhalava T, Farr JN, Weigand BM, Palmer AK, Weivoda MM, et al. Senolytics improve physical function and increase lifespan in old age. Nat Med. 2018;24(8) doi: 10.1038/s41591-018-0092-9. 1246–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu Y, Tchkonia T, Pirtskhalava T, Gower AC, Ding H, Giorgadze N, et al. The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell. 2015;14(4) doi: 10.1111/acel.12344. 644–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saccon TD, Nagpal R, Yadav H, Cavalcante MB, Nunes ADC, Schneider A, et al. Senolytic combination of dasatinib and quercetin alleviates intestinal senescence and inflammation and modulates the gut microbiome in aged mice. J. Gerontol. A. Biol. Sci. Med. Sci. 2021;76(11) doi: 10.1093/gerona/glab002. 1895–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Justice JN, Nambiar AM, Tchkonia T, LeBrasseur NK, Pascual R, Hashmi SK, et al. Senolytics in idiopathic pulmonary fibrosis: results from a first-in-human, open-label, pilot study. EBioMedicine. 2019;40 doi: 10.1016/j.ebiom.2018.12.052. 554–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hickson LJ, Langhi Prata LGP, Bobart SA, Evans TK, Giorgadze N, Hashmi SK, et al. Senolytics decrease senescent cells in humans: preliminary report from a clinical trial of Dasatinib plus Quercetin in individuals with diabetic kidney disease. EBioMedicine. 2019;47 doi: 10.1016/j.ebiom.2019.08.069. 446–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen XK, Yi ZN, Wong GT, Hasan KMM, Kwan JS, Ma AC, et al. Is exercise a senolytic medicine? A systematic review. Aging Cell. 2021;20(1) doi: 10.1111/acel.13294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang X, Englund DA, Aversa Z, Jachim SK, White TA, LeBrasseur NK. Exercise counters the age-related accumulation of senescent cells. Exerc Sport Sci Rev. 2022;50(4):213–221. doi: 10.1249/JES.0000000000000302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Werner C, Hanhoun M, Widmann T, Kazakov A, Semenov A, Poss J, et al. Effects of physical exercise on myocardial telomere-regulating proteins, survival pathways, and apoptosis. J Am Coll Cardiol. 2008;52(6) doi: 10.1016/j.jacc.2008.04.034. 470–82. [DOI] [PubMed] [Google Scholar]
- 21.Bao C, Yang Z, Li Q, Cai Q, Li H, Shu B. Aerobic endurance exercise ameliorates renal vascular sclerosis in aged mice by regulating PI3K/AKT/mTOR signaling pathway. DNA Cell Biol. 2020;39(2) doi: 10.1089/dna.2019.4966. 310–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang CC, Chiang WD, Huang WC, Huang CY, Hsu MC, Lin WT. Hepatoprotective effects of swimming exercise against D-galactose-induced senescence rat model. Evid Based Complement Alternat Med. 2013;2013 doi: 10.1155/2013/275431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saito Y, Chikenji TS, Matsumura T, Nakano M, Fujimiya M. Exercise enhances skeletal muscle regeneration by promoting senescence in fibro-adipogenic progenitors. Nat Commun. 2020;11(1):889. doi: 10.1038/s41467-020-14734-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu B, Liu W, Liu P, Liu X, Song X, Hayashi T, et al. Silibinin alleviates the learning and memory defects in overtrained rats accompanying reduced neuronal apoptosis and senescence. Neurochem Res. 2019;44(8) doi: 10.1007/s11064-019-02816-2. 1818–29. [DOI] [PubMed] [Google Scholar]
- 25.Mattson MP. Hormesis defined. Ageing Res Rev. 2008;7(1):1–7. doi: 10.1016/j.arr.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Y, Sanoff HK, Cho H, Burd CE, Torrice C, Ibrahim JG, et al. Expression of p16(INK4a) in peripheral blood T-cells is a biomarker of human aging. Aging Cell. 2009;8(4) doi: 10.1111/j.1474-9726.2009.00489.x. 439–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Justice JN, Gregory H, Tchkonia T, LeBrasseur NK, Kirkland JL, Kritchevsky SB, et al. Cellular senescence biomarker p16INK4a+ cell burden in thigh adipose is associated with poor physical function in older women. J Gerontol A Biol Sci Med Sci. 2018;73(7) doi: 10.1093/gerona/glx134. 939–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Demaria M, Bertozzi B, Veronese N, Spelta F, Cava E, Tosti V, et al. Long-term intensive endurance exercise training is associated to reduced markers of cellular senescence in the colon mucosa of older adults. NPJ Aging. 2023;9(1):3. doi: 10.1038/s41514-023-00100-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rossman MJ, Kaplon RE, Hill SD, McNamara MN, Santos-Parker JR, Pierce GL, et al. Endothelial cell senescence with aging in healthy humans: prevention by habitual exercise and relation to vascular endothelial function. Am J Physiol Heart Circ Physiol. 2017;313(5):H890. doi: 10.1152/ajpheart.00416.2017. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang C, Jiao Y, Wei B, Yang Z, Wu JF, Jensen J, et al. Aged cells in human skeletal muscle after resistance exercise. Aging (Albany NY) 2018;10(6) doi: 10.18632/aging.101472. 1356-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Diwan B, Sharma R. Nutritional components as mitigators of cellular senescence in organismal aging: a comprehensive review. Food Sci Biotechnol. 2022;31(9) doi: 10.1007/s10068-022-01114-y. 1089–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen J, Brodsky SV, Goligorsky DM, Hampel DJ, Li H, Gross SS, et al. Glycated collagen I induces premature senescence-like phenotypic changes in endothelial cells. Circ Res. 2002;90(12) doi: 10.1161/01.res.0000022161.42655.98. 1290–8. [DOI] [PubMed] [Google Scholar]
- 33.Fontana L, Partridge L, Longo VD. Extending healthy life span--from yeast to humans. Science. 2010;328(5976) doi: 10.1126/science.1172539. 321–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Longo VD, Anderson RM. Nutrition, longevity and disease: from molecular mechanisms to interventions. Cell. 2022;185(9) doi: 10.1016/j.cell.2022.04.002. 1455–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fontana L, Mitchell SE, Wang B, Tosti V, van Vliet T, Veronese N, et al. The effects of graded caloric restriction: XII. Comparison of mouse to human impact on cellular senescence in the colon. Aging Cell. 2018;17(3) doi: 10.1111/acel.12746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Erlangga Z, Ghashang SK, Hamdan I, Melk A, Gutenbrunner C, Nugraha B. The effect of prolonged intermittent fasting on autophagy, inflammasome and senescence genes expressions: an exploratory study in healthy young males. Hum Nutr Metab. 2023;32 [Google Scholar]
- 37.Han YM, Bedarida T, Ding Y, Somba BK, Lu Q, Wang Q, et al. beta-Hydroxybutyrate prevents vascular senescence through hnRNP A1-mediated upregulation of Oct4. Mol Cell. 2018;71(6) doi: 10.1016/j.molcel.2018.07.036. 1064–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brandhorst S, Choi IY, Wei M, Cheng CW, Sedrakyan S, Navarrete G, et al. A periodic diet that mimics fasting promotes multi-system regeneration, enhanced cognitive performance, and healthspan. Cell Metabol. 2015;22(1):86–99. doi: 10.1016/j.cmet.2015.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kale A, Sharma A, Stolzing A, Desprez PY, Campisi J. Role of immune cells in the removal of deleterious senescent cells. Immun Ageing. 2020;17:16. doi: 10.1186/s12979-020-00187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Le Couteur DG, Solon-Biet S, Cogger VC, Mitchell SJ, Senior A, de Cabo R, et al. The impact of low-protein high-carbohydrate diets on aging and lifespan. Cell Mol Life Sci. 2016;73(6) doi: 10.1007/s00018-015-2120-y. 1237–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Levine ME, Suarez JA, Brandhorst S, Balasubramanian P, Cheng CW, Madia F, et al. Low protein intake is associated with a major reduction in IGF-1, cancer, and overall mortality in the 65 and younger but not older population. Cell Metabol. 2014;19(3) doi: 10.1016/j.cmet.2014.02.006. 407–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nehme J, Yang D, Altulea A, Varela-Eirin M, Wang L, Hu S, et al. High dietary protein and fat contents exacerbate hepatic senescence and SASP in mice. FEBS J. 2023;290(5) doi: 10.1111/febs.16292. 1340–7. [DOI] [PubMed] [Google Scholar]
- 43.Sone H, Kagawa Y. Pancreatic beta cell senescence contributes to the pathogenesis of type 2 diabetes in high-fat diet-induced diabetic mice. Diabetologia. 2005;48(1):58–67. doi: 10.1007/s00125-004-1605-2. [DOI] [PubMed] [Google Scholar]
- 44.Minamino T, Orimo M, Shimizu I, Kunieda T, Yokoyama M, Ito T, et al. A crucial role for adipose tissue p53 in the regulation of insulin resistance. Nat Med. 2009;15(9) doi: 10.1038/nm.2014. 1082–7. [DOI] [PubMed] [Google Scholar]
- 45.Hou J, Jeon B, Baek J, Yun Y, Kim D, Chang B, et al. High fat diet-induced brain damaging effects through autophagy-mediated senescence, inflammation and apoptosis mitigated by ginsenoside F1-enhanced mixture. J Ginseng Res. 2022;46(1):79–90. doi: 10.1016/j.jgr.2021.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.San-Cristobal R, Navas-Carretero S, Martinez-Gonzalez MA, Ordovas JM, Martinez JA. Contribution of macronutrients to obesity: implications for precision nutrition. Nat Rev Endocrinol. 2020;16(6) doi: 10.1038/s41574-020-0346-8. 305–20. [DOI] [PubMed] [Google Scholar]
- 47.Sharma R, Kumar R, Sharma A, Goel A, Padwad Y. Long-term consumption of green tea EGCG enhances murine health span by mitigating multiple aspects of cellular senescence in mitotic and post-mitotic tissues, gut dysbiosis, and immunosenescence. J Nutr Biochem. 2022;107 doi: 10.1016/j.jnutbio.2022.109068. [DOI] [PubMed] [Google Scholar]
- 48.Woo J, Shin S, Cho E, Ryu D, Garandeau D, Chajra H, et al. Senotherapeutic-like effect of Silybum marianum flower extract revealed on human skin cells. PLoS One. 2021;16(12) doi: 10.1371/journal.pone.0260545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao H, Halicka HD, Li J, Darzynkiewicz Z. Berberine suppresses gero-conversion from cell cycle arrest to senescence. Aging (Albany NY) 2013;5(8) doi: 10.18632/aging.100593. 623–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marin C, Delgado-Lista J, Ramirez R, Carracedo J, Caballero J, Perez-Martinez P, et al. Mediterranean diet reduces senescence-associated stress in endothelial cells. Age (Dordr) 2012;34(6) doi: 10.1007/s11357-011-9305-6. 1309–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moiseeva O, Deschenes-Simard X, St-Germain E, Igelmann S, Huot G, Cadar AE, et al. Metformin inhibits the senescence-associated secretory phenotype by interfering with IKK/NF-kappaB activation. Aging Cell. 2013;12(3) doi: 10.1111/acel.12075. 489–98. [DOI] [PubMed] [Google Scholar]
- 52.Smieszek A, Strek Z, Kornicka K, Grzesiak J, Weiss C, Marycz K. Antioxidant and anti-senescence effect of metformin on mouse olfactory ensheathing cells (mOECs) may be associated with increased brain-derived neurotrophic factor levels-an ex vivo study. Int J Mol Sci. 2017;18(4):872. doi: 10.3390/ijms18040872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fielder E, Wan T, Alimohammadiha G, Ishaq A, Low E, Weigand BM, et al. Short senolytic or senostatic interventions rescue progression of radiation-induced frailty and premature ageing in mice. Elife. 2022:11:e75492. doi: 10.7554/eLife.75492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barzilai N, Crandall JP, Kritchevsky SB, Espeland MA. Metformin as a tool to target aging. Cell Metabol. 2016;23(6) doi: 10.1016/j.cmet.2016.05.011. 1060–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Al-Kuraishy HM, Al-Gareeb AI. Erectile dysfunction and low sex drive in men with type 2 DM: the potential role of diabetic pharmacotherapy. J Clin Diagn Res. 2016;10(12):FC21–6. doi: 10.7860/JCDR/2016/19971.8996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cai T, Hu Y, Ding B, Yan R, Liu B, Cai L, et al. Effect of metformin on testosterone levels in male patients with type 2 diabetes mellitus treated with insulin. Front Endocrinol. 2021;12 doi: 10.3389/fendo.2021.813067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Konopka AR, Laurin JL, Schoenberg HM, Reid JJ, Castor WM, Wolff CA, et al. Metformin inhibits mitochondrial adaptations to aerobic exercise training in older adults. Aging Cell. 2019;18(1) doi: 10.1111/acel.12880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lin CS, Chang CJ, Lu CC, Martel J, Ojcius DM, Ko YF, et al. Impact of the gut microbiota, prebiotics, and probiotics on human health and disease. Biomed J. 2014;37(5) doi: 10.4103/2319-4170.138314. 259–68. [DOI] [PubMed] [Google Scholar]
- 59.Valdes AM, Walter J, Segal E, Spector TD. Role of the gut microbiota in nutrition and health. BMJ. 2018;361:k2179. doi: 10.1136/bmj.k2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nagpal R, Mainali R, Ahmadi S, Wang S, Singh R, Kavanagh K, et al. Gut microbiome and aging: physiological and mechanistic insights. Nutr Healthy Aging. 2018;4(4) doi: 10.3233/NHA-170030. 267–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chang CJ, Lin CS, Lu CC, Martel J, Ko YF, Ojcius DM, et al. Ganoderma lucidum reduces obesity in mice by modulating the composition of the gut microbiota. Nat Commun. 2015;6:7489. doi: 10.1038/ncomms8489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wu TR, Lin CS, Chang CJ, Lin TL, Martel J, Ko YF, et al. Gut commensal Parabacteroides goldsteinii plays a predominant role in the anti-obesity effects of polysaccharides isolated from Hirsutella sinensis. Gut. 2019;68(2) doi: 10.1136/gutjnl-2017-315458. 248–62. [DOI] [PubMed] [Google Scholar]
- 63.Schachter J, Martel J, Lin CS, Chang CJ, Wu TR, Lu CC, et al. Effects of obesity on depression: a role for inflammation and the gut microbiota. Brain Behav Immun. 2018;69:1–8. doi: 10.1016/j.bbi.2017.08.026. [DOI] [PubMed] [Google Scholar]
- 64.Zhang Q, Hu N. Effects of metformin on the gut microbiota in obesity and type 2 diabetes mellitus. Diabetes Metab Syndr Obes. 2020;13 doi: 10.2147/DMSO.S286430. 5003–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sung MM, Kim TT, Denou E, Soltys CM, Hamza SM, Byrne NJ, et al. Improved glucose homeostasis in obese mice treated with resveratrol is associated with alterations in the gut microbiome. Diabetes. 2017;66(2) doi: 10.2337/db16-0680. 418–25. [DOI] [PubMed] [Google Scholar]
- 66.Tamura M, Hoshi C, Kobori M, Takahashi S, Tomita J, Nishimura M, et al. Quercetin metabolism by fecal microbiota from healthy elderly human subjects. PLoS One. 2017;12(11) doi: 10.1371/journal.pone.0188271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Boyajian JL, Ghebretatios M, Schaly S, Islam P, Prakash S. Microbiome and human aging: probiotic and prebiotic potentials in longevity, skin health and cellular senescence. Nutrients. 2021;13(12):4550. doi: 10.3390/nu13124550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hunsche C, Cruces J, De la Fuente M. Improvement of redox state and functions of immune cells as well as of behavioral response in aged mice after two-week supplementation of fermented milk with probiotics. Curr Microbiol. 2019;76(11) doi: 10.1007/s00284-019-01759-9. 1278–89. [DOI] [PubMed] [Google Scholar]
- 69.Kumar R, Sharma A, Gupta M, Padwad Y, Sharma R. Cell-free culture supernatant of probiotic Lactobacillus fermentum protects against H(2)O(2)-induced premature senescence by suppressing ROS-Akt-mTOR axis in murine preadipocytes. Probiotics Antimicrob Proteins. 2020;12(2) doi: 10.1007/s12602-019-09576-z. 563–76. [DOI] [PubMed] [Google Scholar]
- 70.Jeong JJ, Kim KA, Jang SE, Woo JY, Han MJ, Kim DH. Orally administrated Lactobacillus pentosus var. plantarum C29 ameliorates age-dependent colitis by inhibiting the nuclear factor-kappa B signaling pathway via the regulation of lipopolysaccharide production by gut microbiota. PLoS One. 2015;10(2) doi: 10.1371/journal.pone.0116533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Carroll JE, Prather AA. Sleep and biological aging: a short review. Curr Opin Endocr Metab Res. 2021;18 doi: 10.1016/j.coemr.2021.03.021. 159–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Carreras A, Zhang SX, Peris E, Qiao Z, Gileles-Hillel A, Li RC, et al. Chronic sleep fragmentation induces endothelial dysfunction and structural vascular changes in mice. Sleep. 2014;37(11) doi: 10.5665/sleep.4178. 1817–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Carroll JE, Irwin MR, Levine M, Seeman TE, Absher D, Assimes T, et al. Epigenetic aging and immune senescence in women with insomnia symptoms: findings from the Women’s Health Initiative study. Biol Psychiatry. 2017;81(2) doi: 10.1016/j.biopsych.2016.07.008. 136–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Carroll JE, Cole SW, Seeman TE, Breen EC, Witarama T, Arevalo JMG, et al. Partial sleep deprivation activates the DNA damage response (DDR) and the senescence-associated secretory phenotype (SASP) in aged adult humans. Brain Behav Immun. 2016;51 doi: 10.1016/j.bbi.2015.08.024. 223-9. [DOI] [PMC free article] [PubMed] [Google Scholar]