Abstract
Hemoptysis is a common clinical symptom in emergency patients. It is characterized by the discharge of bloody sputum, which originates from the lower respiratory tract. In the majority of cases, this event is self-limiting, and only in less than 5% of cases, it is massive. Mitral valve stenosis is an uncommon cause of hemoptysis, with a prevalence of 4.2%. In rare cases of this condition, massive and sudden hemoptysis occurs, which is called pulmonary apoplexy. Here, a 35-year-old woman with a history of mitral valve stenosis is introduced who was referred to the hospital with a complaint of massive hemoptysis and sudden shortness of breath. According to the history of mitral valve stenosis, the patient was diagnosed with pulmonary apoplexy. After treatment, both the imaging findings and the patient's symptoms resolved within a short period of time. Even though pulmonary apoplexy is often severe, it can still respond well to conservative treatments and may indicate a need for immediate attention to the stenosis of the mitral valve.
Keywords: Pulmonary apoplexy, Mitral stenosis, Hemoptysis, Bloody cough
1. Introduction
Hemoptysis is a frequent symptom in emergency patients, and it refers to the coughing up of blood originating from the lower respiratory tract [1]. This condition is generally self-limiting and resolves on its own in most cases. However, less than 5% of cases can be severe, requiring immediate medical attention [2]. Managing hemoptysis can be difficult as it can be caused by various medical conditions and is associated with a high mortality rate in cases of massive hemoptysis [3]. Although there is no universally accepted specific volume of blood to define massive hemoptysis, it is generally characterized by the presence of bloody sputum amounting to approximately 150 ml in 24 hours or bleeding at a rate of ≥100 ml per hour. This condition can be life-threatening when accompanied by significant airway obstruction, impaired oxygen exchange, or hemodynamic disturbances [4].
MS is a heart valve disease that occurs when the mitral valve narrows to less than 2 cm in diameter. This condition is primarily caused by rheumatic mitral stenosis in young people from underdeveloped countries and degenerative mitral stenosis in older adults from industrialized countries [5]. Hemoptysis due to MS is a relatively uncommon phenomenon. However, in rare cases of MS, patients may experience a sudden and massive hemoptysis, which is medically referred to as pulmonary apoplexy [6,7].
We report a case of pulmonary apoplexy in a patient with MS who responded well to conservative therapy despite massive hemoptysis.
2. Case presentation
The patient is a 35-year-old woman who was referred to the hospital with the complaint of bloody sputum discharge and sudden shortness of breath about an hour before the visit. Her hemoptysis occurred five times, and each time contained about 30–35 cc of gross blood without clots. She also complained of MMRC IV shortness of breath, bilateral chest pain, and nausea, which started at the same time as hemoptysis. She had a history of cholecystectomy surgery a year ago. During the cardiology consultation before surgery, she was diagnosed with severe mitral stenosis (MS). Despite the recommendation for percutaneous mitral balloon commissurotomy (PMBC), appropriate measures were not taken. Additionally, she was admitted to the heart center one month ago due to shortness of breath and pain in the left hemithorax. She underwent coronary angiography and was diagnosed with one-vessel disease. However, the angioplasty had to be delayed because the patient did not give consent for the procedure.
In the physical examination, the patient's blood pressure was measured at 115/70 mmHg, pulse rate was 150 beats per minute, respiratory rate was 26 breaths per minute, body temperature was normal at 36.8 °C, and her oxygen saturation was 84% at the time of admission, which improved to 96% with the use of oxygen. The patient's chest movements were symmetrical. However, diffuse crackle sounds were heard in the lung during auscultation. In the heart examination, it was noticed that the first heart sound (S1) was louder than usual and had a delay. The second heart sound (S2) was also louder than usual, with its two components being closer together, and P2 was louder than A2. An opening snap (split) was also heard during exhalation at the apex. A small interval existed between the A2 and the opening snap (OS). In the lying position on the left side, following OS, a low-pitched diastolic murmur with a rumbling quality was heard at the tip of the heart. The patient's jugular vein pressure was not prominent, and the abdominal examination was normal. Fig. 1 shows the patient's electrocardiogram.
Fig. 1.
The electrocardiogram of the patient. In lead II, the P waves are tall and peaked, indicating a right atrial abnormality (blue arrow). The first half of the right precordial leads denotes right atrial depolarization (red arrow), while the second half denotes left atrial depolarization (green arrow). Due to mitral stenosis, the patient has left atrial abnormality, which has caused a double-peaked P wave in leads V2 and V3 (yellow arrow).
The patient was admitted to the intensive care unit and was prescribed tablets of Acetaminophen Codeine three times a day, 30 cc of Dextromethorphan syrup three times a day, 20mg of Furosemide ampules twice a day, 1gr of Meropenem ampules three times a day, 1gr of Vancomycin twice a day, and 500mg of Tranexamic Acid three times a day. Blood tests, Mycobacterium tuberculosis smear and culture, D-dimer, connective tissue diseases specific tests, PCR test for COVID-19, and influenza were performed for the patient. The laboratory results are presented in Table 1.
Table 1.
The laboratory results of the patient during her admission.
| Lab tests | Reference range | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 |
|---|---|---|---|---|---|---|---|---|
| WBC, count/mm | 4000–10000 | 8000 | 9000 | 9200 | 9000 | 8800 | 8600 | 9000 |
| Hb, mg/dl | 12–15 | 13.9 | 13.3 | 14.3 | 13.8 | 13.2 | 13.3 | 13 |
| MCV, fl | 80–96 | 83 | 83 | 80 | 81 | 80 | 82 | 80 |
| Platelet, count/mm | 150000–450000 | 138000 | 143000 | 150000 | 181000 | 172000 | 213000 | 215000 |
| ESR, mm/hr | >22 | 22 | 12 | |||||
| CRP | Neg | Neg. | ||||||
| BUN, mg/dl | 15–45 | 23 | 22 | 40 | 24 | |||
| Cr, mg/dl | 0.5–1.4 | 0.8 | 0.9 | 1.1 | 0.8 | |||
| INR | 1–1.4 | 1.1 | 1.2 | |||||
| Troponin, ng/mL | 0–0.04 | 0.008 | ||||||
| Ca, mg/dl | 8.5–10.5 | 7.7 | 7.8 | 8.3 | 8.8 | 9 | ||
| P, mg/dl | 3–4.5 | 3.6 | 4.4 | 4.7 | ||||
| TSH, micIU/ml | 0.35–4.94 | 1.2 | 2.3 | |||||
| AST, IU/l | <41 | 23 | 8 | 23 | ||||
| ALT, IU/l | <41 | 10 | 18 | 47 | ||||
| ALP, IU/l | <306 | 109 | 92 | 171 | ||||
| APS tests | Neg. | Neg. | ||||||
| Anti dsDNA | Neg. | Neg. | ||||||
| ANA | <0.5 | 0.4 | ||||||
| P-ANCA | Neg. | Neg. | ||||||
| C-ANCA | Neg. | Neg. |
WBC: White blood cells; Hb: Hemoglobin MCV: Mean corpuscular volume; LDH: Lactate dehydrogenase; Cr: Creatinine; CRP: C-reactive protein; ESR: Erythrocyte sedimentation rate; INR: International normalized ratio; PTT: Partial thromboplastin time; BUN: Blood urea nitrogen; Ca: Calcium; P: Phosphorus; TSH: Thyroid-stimulating hormone; AST: Aspartate aminotransferase; ALT: Alanine aminotransferase; ALP: Alkaline phosphatase; APS tests: Lupus anticoagulant, Anticardiolipin antibody, Beta-2 glycoprotein; CPK: Creatinine phosphokinase; ANA: Anti-nuclear antibody; ANCA: Antineutrophil cytoplasmic antibodies; ds-DNA: Double stranded deoxyribonucleic acid.
An emergency cardiac consultation was requested for the patient due to the history of MS. The echocardiographic examination (Fig. 2) showed evidence of severe MS (Mitral valve area = 0.954 cm2, mean pressure gradient = 9 mmHg (moderate), total Wilkin's score = 8–9 (leaflet calcification = Grade 2, leaflet mobility = Grade 2–3, leaflet thickness = Grade 2, impairment of the subvalvular apparatus = Grade 2), left ventricle ejection fraction (LVEF) of 55%, mild pulmonary hypertension (PH) (systolic pulmonary artery pressure (PAP) = 45 mmHg), mild aorta insufficiency (AI), mild tricuspid regurgitation (TR), normal left ventricular size and systolic function, normal right ventricular size and systolic function, Bi-atrial enlargement. Also, the cardiologist recommended taking bisoprolol 2.5mg tablets twice a day to regulate heart rate between 60 and 70 bpm, continuing diuretic, and undergoing PMBC as soon as possible.
Fig. 2.
The patient's echocardiography. (a) determination of the mean mitral gradient from Doppler diastolic mitral flow. (b) apical four-chamber viewing shows the severe stenosis of the mitral valve's leaflets (yellow arrow) and the dilation of the left atrium (LA). (c) the pulmonary artery systolic pressure estimation using TR velocity and gradient in a short axis view. (d) calculation of the mitral valve area (red arrow).
The lung CT scan without contrast enhancement showed bilateral patchy central consolidation and air bronchogram evidence (Fig. 3).
Fig. 3.
Lung CT scan. (a, b) diffuse central patchy consolidation (red arrow). (c, d) complete resolution of the imaging findings on the sixth day of hospitalization. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
On the second day of hospitalization, her hemoptysis showed signs of improvement and decreased to twice a day. By the third day, it had stopped entirely. Her shortness of breath also gradually improved; on the third day, her oxygen saturation level reached 96% without supplemental oxygen. The results of the PCR test for influenza and COVID-19 were negative. In the CT scan of the lungs, which was performed on the sixth day of hospitalization, complete resolution of imaging findings was observed (Fig. 3). All symptoms improved on the seventh day of hospitalization, and she was in good general condition. The oxygen saturation level reached 97%, and the shortness of breath and hemoptysis were alleviated completely. She was discharged with a prescription of Furosemide 40 mg daily and Bisoprolol 2.5 mg daily. Two months later, the patient underwent PMBC surgery.
3. Discussion
Hemoptysis, the condition characterized by bleeding from small or large blood vessels in the lungs, is typically caused by a range of factors [8]. These may include immune, vascular, cardiovascular, and coagulation issues in the case of small vessel bleeding, while large vessel bleeding can be attributed to infectious, cardiovascular, congenital, neoplastic, and vasculitis factors. Specifically, MS is one of the known causes of hemoptysis from small vessels [1,9]. However, the prevalence of hemoptysis cases due to MS is relatively low, accounting for only 4.2%, as determined by a study conducted by Ittrich H et al. [7,10].
MS is a heart valve disease that occurs when the mitral valve narrows to less than 2 cm in diameter. This condition is primarily caused by rheumatic mitral stenosis in young people from underdeveloped countries and degenerative mitral stenosis in older adults from industrialized countries. According to the latest classification presented by the American Heart Association (AHA) in 2020, severe MS is characterized by an MVA of ≤1.5 cm2 and a transmitral mean gradient of >5–10 mm Hg in normal heart rate [5]. Other causes that may lead to severe MS include mitral annular calcification, radiation-related valve disease, Fabry disease, Whipple disease, mucopolysaccharidosis, methysergide therapy, carcinoid valve disease, endomyocardial fibrosis, and systemic autoimmune diseases such as lupus and rheumatoid arthritis [[11], [12], [13]]. MS can disrupt the blood flow to the ventricle, increasing pressure on the left atrium wall. This pressure, in turn, can increase the pulmonary vein pressure, causing reverse blood flow from the pulmonary veins to the bronchial venous network. This leads to congestion and increased pressure in the bronchial vessels, which can result in the rupture of these vessels and hemoptysis originating from the pulmonary and bronchial arteries [11,14]. Hemoptysis following MS may manifest as sudden bleeding (pulmonary apoplexy), bloody sputum after severe coughing accompanied by shortness of breath and nocturnal bronchitis, or pink, foamy sputum due to left heart involvement leading to pulmonary edema [13]. Pulmonary apoplexy was first described by Wood in 1954 as a severe and sudden onset of bleeding from the lungs following MS [6]. This used to be considered an early complication of MS, and it was believed that the walls of the bronchial veins thicken over time, preventing their rupture. Furthermore, increased pressure in the right ventricle and right atrium due to pulmonary hypertension may decrease the degree of shunting from the pulmonary veins to the bronchus [15].
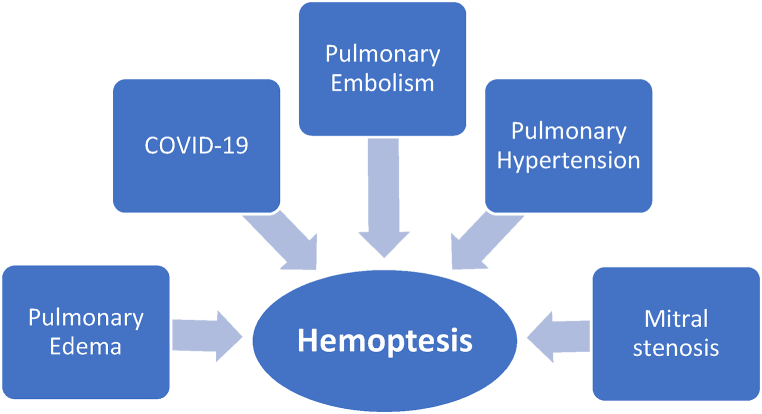
Our patient had severe MS, as classified by the AHA [5]. She experienced massive hemoptysis, with a cumulative volume of approximately 150 ml of excreted blood and a drop in her oxygen saturation level to 84%. Due to the abrupt onset of the hemoptysis, it is considered pulmonary apoplexy. In 50% of cases of massive hemoptysis, there is a risk of suffocation due to the filling of the airways with blood, leading to cardiorespiratory arrest and death. However, cases of pulmonary apoplexy similar to our case can often be treated conservatively with positive outcomes [15,16]. Differential diagnoses in the presented case can be seen in Fig 4.
Fig. 4.
Differential diagnosis of hemoptysis in the presented case.
Hemoptysis is a rare symptom in cases of COVID-19 that may occur in a minimal number of patients in the form of massive hemoptysis [17]. As our patient's PCR test was negative, no fever was detected, and the lung imaging findings have completely improved after three days of treatment, it is less likely that the symptoms are due to COVID-19 infection [18]. It also was unlikely that connective tissue diseases caused the symptoms, as their specific tests were all negative. Additionally, the patient's negative D-dimer test results suggest that the possibility of pulmonary embolism is low, as this test has a high diagnostic sensitivity [19]. One of the conditions that can result in similar imaging findings is pulmonary edema following heart failure, which can be ruled out because of the patient's EF of 55%, the absence of pink secretions, and the presence of gross hemoptysis.
Furthermore, the patient's echocardiography showed signs of pulmonary hypertension (PH), which is another possible cause of hemoptysis. PH is typically characterized by a mean pulmonary artery pressure (mPAP) of ≥25 mmHg and a mean pulmonary capillary wedge pressure (mPAWP) of >15 mmHg [20]. The risk factors for hemoptysis in patients with PH are impaired hemodynamics, congenital heart diseases, and prolonged bronchial artery hypertrophy [21,22]. Since our patient lacked any risk factors, PH was ruled out as the primary cause of symptoms. In our case, PMBC was recommended because successful mitral valve intervention prevents the recurrence of hemoptysis and alleviates MS-induced PH [11,23].
4. Conclusion
Pulmonary apoplexy is a rare occurrence in the field of MS. Despite causing massive hemoptysis; it can be effectively managed with timely diagnosis and supportive treatment. It should be considered a warning sign that urgent attention is needed to manage MS effectively.
Financial/nonfinancial disclosures
None declared.
Funding
This article was prepared without any support or funding
Ethics statements
Written informed consent was obtained from the patient to publish this case report. A copy of the written consent is available for review by the Editor‐in‐Chief of this journal.
Ethics approval
Not applicable.
CRediT authorship contribution statement
Ali Hossein Samadi Takaldani: Visualization, Validation, Supervision, Conceptualization. Nima Javanshir: Writing – original draft. Amirpasha Mansour: Writing – original draft, Investigation. Asma Salmani: Resources, Investigation. Mohammad Negaresh: Writing – review & editing, Writing – original draft, Visualization, Supervision.
Declaration of competing interest
The authors hereby declare that they have no competing interests to disclose. I have reviewed the phrase you provided and found it suitable for the manuscript. Please be advised that I appreciate your attention to detail and your considerable efforts in ensuring that the manuscript meets the highest standards of quality and integrity.
Acknowledgments
Not applicable.
Contributor Information
Ali Hossein Samadi Takaldani, Email: dr.ah.samadi@gmail.com.
Nima Javanshir, Email: nimajvn95@gmail.com.
Amirpasha Mansour, Email: dr.pasha.m@gmail.com.
Asma Salmani, Email: salmaniasma1000@gmail.com.
Mohammad Negaresh, Email: mohamad.negaresh@gmail.com.
References
- 1.Jeudy J., Khan A.R., Mohammed T.-L., Amorosa J.K., Brown K., Dyer D.S., et al. ACR appropriateness criteria® hemoptysis. J. Thorac. Imag. 2010;25(3):W67–W69. doi: 10.1097/RTI.0b013e3181e35b0c. [DOI] [PubMed] [Google Scholar]
- 2.Lordan J., Gascoigne A., Corris P. The pulmonary physician in critical care• Illustrative case 7: Assessment and management of massive haemoptysis. Thorax. 2003;58(9):814–819. doi: 10.1136/thorax.58.9.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seedat U.F., Seedat F. Post-primary pulmonary TB haemoptysis–When there is more than meets the eye. Respiratory medicine case reports. 2018;25:96–99. doi: 10.1016/j.rmcr.2018.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ibrahim W.H. Massive haemoptysis: the definition should be revised. Eur. Respir. J. 2008;32(4):1131–1132. doi: 10.1183/09031936.00080108. [DOI] [PubMed] [Google Scholar]
- 5.Otto C.M., Nishimura R.A., Bonow R.O., Carabello B.A., Erwin J.P., 3rd, Gentile F., et al. ACC/AHA guideline for the management of patients with Valvular heart disease: a report of the American College of cardiology/American heart association joint committee on clinical practice guidelines. Circulation. 2020;143(5):e72–e227. doi: 10.1161/CIR.0000000000000923. 2021. [DOI] [PubMed] [Google Scholar]
- 6.Wood P. An appreciation of mitral stenosis. I. Clinical features. Br. Med. J. 1954;1(4870):1051–1063. doi: 10.1136/bmj.1.4870.1051. ; contd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ittrich H., Bockhorn M., Klose H., Simon M. The diagnosis and treatment of hemoptysis. Deutsches Ärzteblatt international. 2017;114(21):371. doi: 10.3238/arztebl.2017.0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sirajuddin A., Mohammed T. A 44-year-old man with hemoptysis: a review of pertinent imaging studies and radiographic interventions. Cleve. Clin. J. Med. 2008;75(8):601–607. doi: 10.3949/ccjm.75.8.601. [DOI] [PubMed] [Google Scholar]
- 9.Yoon W., Kim J.K., Kim Y.H., Chung T.W., Kang H.K. Bronchial and nonbronchial systemic artery embolization for life-threatening hemoptysis: a comprehensive review. Radiographics. 2002;22(6):1395–1409. doi: 10.1148/rg.226015180. [DOI] [PubMed] [Google Scholar]
- 10.Munoz R., Morell V., da Cruz E.M., Vetterly C., da Silva J.P. Springer; 2010. Critical Care of Children with Heart Disease: Basic Medical and Surgical Concepts. [Google Scholar]
- 11.Botezatu B., Kakar S., Ren M., Shirke M., Masharani K., Pillai K., et al. Mitral valve disease: a view on pathophysiology and management of the most common valve disease in the world. Authorea Preprints. 2020 [Google Scholar]
- 12.Harky A., Botezatu B., Kakar S., Ren M., Shirke M.M., Pullan M. Mitral valve diseases: pathophysiology and interventions. Prog. Cardiovasc. Dis. 2021;67:98–104. doi: 10.1016/j.pcad.2021.03.008. [DOI] [PubMed] [Google Scholar]
- 13.Korzan S., Jones E., Mutneja R., Grover P. Mitral stenosis due to rheumatic heart disease - a rare cause of massive hemoptysis. Respir Med Case Rep. 2018;24:35–39. doi: 10.1016/j.rmcr.2018.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leung W.H., Lau C.P., Wong C.K., Cheng C.H. Fatal massive pulmonary hemorrhage complicating mitral stenosis. Clin. Cardiol. 1990;13(2):136–138. doi: 10.1002/clc.4960130214. [DOI] [PubMed] [Google Scholar]
- 15.Remetz M.S., Cleman M.W., Cabin H.S. Pulmonary and pleural complications of cardiac disease. Clin. Chest Med. 1989;10(4):545–592. [PubMed] [Google Scholar]
- 16.Chun J.-Y., Morgan R., Belli A.-M. Radiological management of hemoptysis: a comprehensive review of diagnostic imaging and bronchial arterial embolization. Cardiovascular and interventional radiology. 2010;33:240–250. doi: 10.1007/s00270-009-9788-z. [DOI] [PubMed] [Google Scholar]
- 17.Barış S.A., Coşkun İ.S., Selvi G., Boyacı H., Başyiğit İ. Case series of COVID-19 presenting with massive hemoptysis. Turkish Thoracic Journal. 2020;21(6):454. doi: 10.5152/TurkThoracJ.2020.20150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nicola M., O'Neill N., Sohrabi C., Khan M., Agha M., Agha R. Evidence based management guideline for the COVID-19 pandemic-Review article. Int. J. Surg. 2020;77:206–216. doi: 10.1016/j.ijsu.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Group JJW Guidelines for the diagnosis, treatment and prevention of pulmonary thromboembolism and deep vein thrombosis (JCS 2009)–digest version. Circ. J. 2011;75(5):1258–1281. doi: 10.1253/circj.cj-88-0010. [DOI] [PubMed] [Google Scholar]
- 20.Galiè N., Humbert M., Vachiery J.-L., Gibbs S., Lang I., Torbicki A., et al. ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the joint task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT) Eur. Heart J. 2015;37(1):67–119. doi: 10.1093/eurheartj/ehv317. 2016. [DOI] [PubMed] [Google Scholar]
- 21.Landzberg M.J. Congenital heart disease associated pulmonary arterial hypertension. Clin. Chest Med. 2007;28(1):243–253. doi: 10.1016/j.ccm.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 22.Tio D., Leter E., Boerrigter B., Boonstra A., Vonk-Noordegraaf A., Bogaard H.J. Risk factors for hemoptysis in idiopathic and hereditary pulmonary arterial hypertension. PLoS One. 2013;8(10) doi: 10.1371/journal.pone.0078132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fawzy M.E., Hassan W., Stefadouros M., Moursi M., El Shaer F., Chaudhary M.A. Prevalence and fate of severe pulmonary hypertension in 559 consecutive patients with severe rheumatic mitral stenosis undergoing mitral balloon valvotomy. J. Heart Valve Dis. 2004;13(6):942–948. [PubMed] [Google Scholar]