Abstract
Background
Sentinel lymph node biopsy (SLNB) is commonly used in the surgical management of male breast cancer. Contrary to female breast cancer, limited data exist about its performance in male breast cancer. The objective of this systematic review and meta-analysis was to evaluate the SLNB accuracy in male breast cancer.
Methods
MEDLINE, EMBASE, Web of Science and The Cochrane Library were searched from January 1995 to April 2023 for studies evaluating the SLNB identification rate and false-negative rate in male breast cancer with negative preoperative axillary evaluation and primary surgery. For SLNB false-negative rate, the gold standard was the histology of axillary lymph node dissection (ALDN). Methodological quality was assessed by using the QUADAS-2 tool. Pooled estimates of the SLNB identification rate and false-negative rate were calculated. Heterogeneity of the pooled studies was evaluated using I2 index.
Results
A total of 12 retrospective studies were included. The 12 studies that reported the SLNB identification rate gathered a total of 164 patients; the 5 studies that reported the SLNB false-negative rate gathered a total of 50 patients with a systematic ALND. The pooled estimate of the SLNB identification rate was 99.0%. The SLNB false-negative rates were 0% in the 5 included studies and consequently so as the pooled estimate of the false-negative rate with no heterogeneity.
Conclusion
SLNB for male breast cancer, following negative preoperative axillary assessment and primary surgery, appears feasible, consistent, and effective. Our research supports conducting immediate SLNB histological evaluation to facilitate prompt ALND in case of positive results.
Keywords: Male breast cancer, Sentinel lymph node biopsy, Accuracy, Identification rate, False-negative rate, Systematic review, Meta-analysis
Highlights
-
•
Male breast cancer often involves axillary node invasion.
-
•
High sentinel lymph node detection rates are observed.
-
•
Sentinel node detection typically has a very low false-negative rate.
Abbreviations
- MBC
male breast cancer
- SLNB
sentinel lymph node biopsy
- ALND
axillary lymph node dissection
- ASCO
American Society of Clinical Oncology
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
1. Introduction
Male breast cancer (MBC) is a rare disease representing only 1% of all breast cancers and accounting in 2022 in the United States for 2710 new cases and 530 deaths with a lifetime risk of 1/1000 [1,2]. However, data from the Surveillance Epidemiology and End Results showed that its incidence has increased by 40% in the United States from 1975 to 2015 [3]. Due to a delayed diagnosis, MBC more often presents axillary lymph nodes metastases at diagnosis than female breast cancer (42% and 33% respectively) [[4], [5], [6]] resulting in more advanced stages [7,8] and worst prognosis.
In 2019, the American Society of Clinical Oncology (ASCO) published guidelines about management of the axilla in early stage-breast cancer [9]; for patients age <70 years without significant competing comorbidities, SLNB should be considered for axillary staging. The ASCO supports the generalization of those guidelines for early-stage MBC because the Panel believes that it is unlikely that SLNB will be less accurate in men than it is in women (recommendation, acceptable; level of evidence, limited). In 2022, the National Comprehensive Cancer Network (NCCN) also published breast cancer guidelines with special considerations for MBC [10]; concerning axillary lymph node surgery, SLNB should be performed in the setting of MBC with a clinically node-negative axilla (if necessary confirmed by ultrasound) [11,12]. Nevertheless, the NCCN specifies that few males have been included in breast cancer trials [13], and that those recommendations are extrapolated from findings of clinical trials focusing on breast cancer in females.
The objective of this systematic review and meta-analysis was to evaluate the SLNB accuracy in MBC with negative preoperative axillary evaluation and following primary surgery.
2. Materials and methods
2.1. Protocol and registration
This systematic review and meta-analysis was conducted according to the PRISMA guidelines (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) [14]. The protocol was registered in the PROSPERO international prospective register of systematic reviews and information about it is available at http://www.crd.york.ac.uk/prospero/, no CRD42021241080. No ethical approval or written, informed consent was required.
2.2. Data sources and search strategy
A comprehensive search of MEDLINE, EMBASE, Web of Science and The Cochrane Library looked for relevant published articles about the accuracy of SLNB in MBC. We restricted our search to the period from January 1995 to April 2023 and studies in English, French and Spanish. Reference lists and citation sections of the retrieved articles were also screened for additional eligible studies. The search equation was: (breast cancer [Title/Abstract] OR breast tumour[Title/Abstract] OR breast tumor[Title/Abstract] OR breast carcinoma[Title/Abstract]) AND (male[Title/Abstract] OR men[Title/Abstract] OR man[Title/Abstract]) AND (sentinel lymph node[Title/Abstract] OR sentinel node[Title/Abstract]).
2.3. Selection criteria
The following inclusion criteria were used to select articles for this study: (1) original studies evaluating the SLNB accuracy in MBC; (2) with negative preoperative axillary evaluation (3) and primary surgery. Studies were excluded if they focused on men with recurrent breast cancer.
2.4. Study selection
Two reviewers (GP and CM) independently selected the studies. After removal of duplicates, the reviewers began by reading titles and abstracts. If the study appeared relevant, the full text was then assessed for eligibility based on our inclusion and exclusion criteria. Finally, a selection of articles for the review was made. Disagreements, if any, were planned to be resolved by consensus by a third reviewer (CH).
2.5. Data extraction and Quality Assessment
One reviewer (GP), using a standardized data extraction form, recorded data from each selected study, and a second reviewer (CM) checked them. The following data were extracted.
-
-
author, year and country of publication, study characteristics.
-
-
patients mean age, clinical presentation, tumor size, tumor histology and immunochemistry, preoperative axillary evaluation, TNM stage, type of surgery, adjuvant treatment, axillary or metastatic relapse, median follow-up.
-
-
SLNB detection protocol and characteristics.
-
-
axillary lymph node dissection (ALND) characteristics.
-
-
and accuracy: SLNB identification rate and SLNB false-negative rate
Two reviewers (GP and CM) independently assessed for each study the risk of bias and applicability concerns for four domains (patient selection, index test, reference standard, flow and timing) using the QUADAS-2 tool (Quality Assessment of Diagnostic Accuracy Studies) [15]. QUADAS-2 was performed with Review Manager 5.3.
2.6. Statistical analysis
The outcomes of this meta-analysis were the SLNB identification rate and the SLNB false-negative rate. The SLNB identification rate was calculated as the number of patients in whom SLNB was successfully identified divided by the total number of patients who underwent SLNB. The SLNB false-negative rate could be obtain only if a systematic ALND was performed. Indeed, it was calculated as (the number of false-negative patients)/(number of false-negative patients + the number of true-positive patients). A false-negative was defined as a negative SLNB with a positive lymph node in the ALDN, and a true-positive patient was defined as a positive SLNB with or without a positive ALDN.
Pooled estimates of the SLNB identification rate and the SLNB false-negative rate were calculated using random-effects model [16]. The precision of an effect size and the pooled estimates were calculated with 95% confidence interval. Heterogeneity of the pooled studies was evaluated using the I2 index (I2 > 50% was considered substantial heterogeneity) [17]. Forests plots were used to visually display the results of the individual studies and the pooled estimates.
Values were considered statistically significant when the p-value was less than 0.05 (p < 0.05). The “Metaprop” module for meta-analysis of diagnostic accuracy studies was used in STATA version 18 (College Station, TX, USA).
3. Results
3.1. Study selection
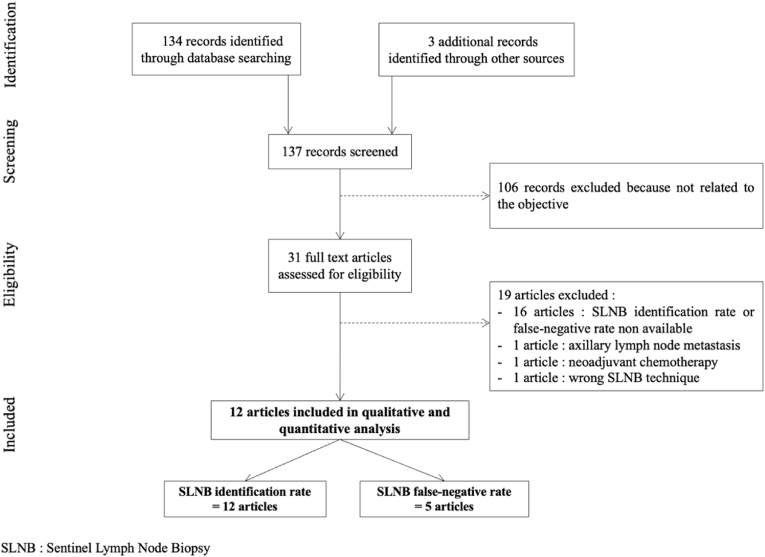
Fig. 1 presents the flow chart of the study selection process. The initial search result generated 137 articles. After screening, based on title and abstract review, 31 articles were assessed for eligibility [[18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47]]. After reading full-text articles, 12 articles were included in the systematic review and meta-analysis [25,33,[36], [37], [38], [39], [40], [41],[43], [44], [45], [46]]. All those articles reported on the SLNB identification rate, and 5 articles reported on the SLNB false-negative rate [38,[43], [44], [45], [46]].
Fig. 1.
PRISMA flow chart of the study selection process.
Nineteen articles were excluded. Sixteen articles did not report SLNB identification rate or SLNB false-negative rate [[18], [19], [20], [21], [22], [23], [24],[26], [27], [28], [29], [30], [31], [32],42,48]; one article included patients with preoperative axillary lymph node metastasis [47]; one article included patients with neoadjuvant chemotherapy [35]; one article was excluded because of a wrong SLNB technique [34].
3.2. Study description
Characteristics of studies and participants included in the systematic review and meta-analysis are summarized in Table 1. All the 12 studies were retrospective, published between 2003 and 2018, in English except for one in Spanish [33]. Preoperative axillary evaluation was performed in 8 studies with clinical examination only [[36], [37], [38], [39], [40], [41],44,46] and in 3 studies with clinical examination and ultrasound [33,43,45]. For one study, preoperative axillary evaluation was not described [25]. Primary surgery with SLNB±systematic ALND was performed in all studies. The 12 studies that reported the SLNB identification rate gathered a total of 153 patients; the 5 studies that reported the SLNB false-negative rate gathered a total of gathered a total of 50 patients with a systematic ALND. Patient's mean age varied between 57.2 and 70.0 years. One hundred and thirty-six patients (88.9%) had a mastectomy, and 17 patients (11.1%) had a lumpectomy. For 89 patients, adjuvant treatments were indicated: 23 patients (25.8%) had chemotherapy, 26 patients (29.2%) had radiotherapy, and 74 patients (83.1%) had Tamoxifen. Medians follow-up varied between 18 and 67 months. Three patients presented a metastatic relapse.
Table 1.
Characteristics of studies and participants.
| Study Year |
Study design | Inclusion period | Patient's number (n | Patient's mean age (years) | Preoperative axillary evaluation | Primary surgery with SLNB | ALND | Type of breast surgery (n | Adjuvant treatment (n) | Median follow-up (months) |
|---|---|---|---|---|---|---|---|---|---|---|
|
Albo 2003 |
Retrospective | 1999–2000 | 7 | 61.1 (44–76) | Clinical (100.0%) +/− Ultrasound (100.0%) |
Yes | Systematic in 4 out of 7 patients | Mastectomy | – | – |
|
Cimmino 2004 |
Retrospective | 1998–2002 | 6 | 59.8 (51–67) | Clinical (100.0 %) | Yes | Only if SLNB + | Mastectomy | – | – |
|
De Cicco 2004 |
Retrospective | 1999–2003 | 18 | 59 (46–80) | Clinical (100.0%) | Yes | Only if SLNB + | Mastectomy | HT (18) | 26.5 (6–48) |
|
Gentilini 2007 |
Retrospective | 1999–2005 | 32 | 58 (33–80) | Clinical (100.0%) | Yes | Only if SLNB + | Mastectomy | CT (5) RT (14) HT (25) |
30 (1–63) |
|
Golshan 2007 |
Retrospective | 1996–2006 | 6 | 61 (38–86) | Clinical (100.0%) | Yes | Only if SLNB + | Lumpectomy | CT (3) HT (6) |
67 (36–97) |
|
Goyal 2004 |
Retrospective | 1998–2003 | 9 | 70 (26–79) | Clinical (100.0%) +/− Ultrasound (100.0%) |
Yes | Systematic in 5 out of 9 patients | Mastectomy | – | – |
|
Koukouras 2012 |
Retrospective | 2000–2010 | 11 | 66.1 (34–84) | Clinical (100.0 %) | Yes | Systematic | Mastectomy (9) Lumpectomy (2) |
– | – |
|
Martin 2017 |
Retrospective | 2008–2016 | 15 | 68.2 (45–83) | Clinical (100.0%) +/− Ultrasound (100.0%) |
Yes | Only if SLNB + | Mastectomy (13) Lumpectomy (2) |
– | 18 |
|
Port 2001 |
Retrospective | 1996–1999 | 16 | 57.2 (36–70) | Clinical (100.0%) | Yes | Systematic in 11 out of 15 patients | Mastectomy (15) Lumpectomy (1) |
– | – |
|
Rusby 2006 |
Retrospective | 1996–2006 | 30 | 62 (24–86) | Clinical (100.0%) | Yes | Systematic in 19 out of 31 patients | Mastectomy (25) Lumpectomy (6) |
CT (14) RT (12) HT (22) |
58 |
|
Simsek 2018 |
Retrospective | 2009–2012 | 10 | – | – | Yes | Only if SLNB + | – | – | – |
|
Suehiro 2015 |
Retrospective | – | 3 | 65.7 (64–69) | Clinical (100.0%) | Yes | Only if SLNB + | Mastectomy | CT (1) HT (3) |
56 (10–80) |
CT: Chemotherapy; RT: Radiotherapy; HT: Hormonotherapy; ALND: Axillary Lymph Node Dissection; SLNB: Sentinel Lymph Node Biopsy; -: data not available.
MBC clinical and histological characteristics are presented in Table 2. Patients had mostly a breast mass (83.0%–100.0%) and less often a nipple discharge (6.7%–16.7%). Mean clinical tumor size varied between 1.3 and 2.2 cm. Invasive ductal carcinoma was found in 127 patients (82.4%), intracystic papillary carcinoma in 12 patients (7.8%), ductal carcinoma in situ in 8 patients (5.2 %), invasive lobular carcinoma in 1 patient (0.6%), mixed invasive lobular and ductal carcinoma in 1 patient (0.6%), mucoepidermoid in 1 patient (0.6%), mucinous carcinoma in 1 patient (0.6%), and papillary invasive carcinoma in 1 patient (0.6%). Estrogen and progesterone receptors were found positive in 123 patients (97.6%) and in 89 patients (80.2%) respectively.
Table 2.
MBC clinical and histological characteristics.
| Study Year |
Clinical presentation | Mean tumor size (cm | T stage (TNM) (n) |
Tumor histology (n) | Tumor grade (n | Lymphovascular invasion (n | Positive ER (n)- | Positive PR (n) | Positive Her2 (n | Ki67 > 20% (n |
|---|---|---|---|---|---|---|---|---|---|---|
|
Albo 2003 |
Mass (85.7 %) Nipple discharge (14.3 %) |
1.9 (0.5–3.8) | T1 to T2 | IDC (7) | Grade 2 (5) Grade 3 (2) |
– | 7 (100.0 %) | 1 (14.3 %) | 3 (42.9 %) | – |
|
Cimmino 2004 |
Mass (83.3 %) Nipple discharge (16.7 %) |
1.6 (0.7–2.8) | T1 to T2 | IDC (4) IPC (1) DCIS (1) |
Grade 1 (1) Grade 2 (3) Grade 3 (1) |
2 (33.3 %) | 4 (66.7 %) | – | – | – |
|
De Cicco 2004 |
– | – | Tis (2) T1 (10) T2 (3) |
IDC (11) IPC (5) DCIS (2) |
Grade 1 (1) Grade 2 (9) Grade 3 (2) |
– | 18 (100.0 %) | 12 (66.7 %) | – | 7 (38.9 %) |
|
Gentilini 2007 |
– | – | T1 (23) T2 (3) |
IDC (23) IPC (3) DCIS (3) Other (3) |
Grade 1 (11) Grade 2/3 (21) |
– | 31 (96.9 %) | 25 (78.1 %) | 5 (15.6 %) | 17 (53.1 %) |
|
Golshan 2007 |
Mass (83.3 %) | 1.7 (0.8–3.0) | – | IDC (6) | Grade 2 (4) Grade 3 (2) |
– | 6 (100.0 %) | 5 (83.3 %) | 2 (33.3 %) | – |
|
Goyal 2004 |
– | – | T1 (4) T2 (5) |
IDC (9) | Grade 1 (1) Grade 2 (8) |
– | – | – | – | – |
|
Koukouras 2012 |
– | – | – | IDC (10) Mucoepidermoid (1) |
– | – | – | – | – | – |
|
Martin 2017 |
– | 2.2 (5–40) | T1 to T2 | IDC (12) IPC (2) DCIS (1) |
Grade 1 (2) Grade 2 (12) Grade 3 (1) |
– | 15 (100.0 %) | 15 (100.0 %) | 2 (13.3 %) | 2 (13.3 %) |
|
Port 2001 |
Mass (83.3 %) Nipple discharge (6.3 %) |
1.3 (0.1–1.3) | T1 to T2 | IDC (14) IPC (1) DCIS (1) |
Grade 1 (0) Grade 2 (6) Grade 3 (5) |
5 (31.3 %) | 15 (93.8 %) | – | – | – |
|
Rusby 2006 |
Mass (83.0 %) | 1.8 (0.1–4) | T1 (19) T2 (12) |
IDC (28) ILC (1) IDC/ILC (1) Mucinous (1) |
Grade 1 (2) Grade 2 (18) Grade 3 (11) |
– | 29 (93.5 %) | 28 (90.3 %) | 5 (16.1 %) | 17 (54.8 %) |
|
Simsek 2018 |
– | – | – | – | – | – | – | – | – | – |
|
Suehiro 2015 |
Mass (100.0 %) | 1.7 (1.0–2.5) | – | IDC (3) | Grade 1 (2) Grade 3 (2) |
– | 3 (100.0 %) | 3 (100.0 %) | 0 (0.0 %) | 1 (33.3 %) |
IDC: Invasive Ductal Carcinoma; ILC: Invasive Lobular Carcinoma; IP: Intracystic Papillary Carcinoma; DCIS: Ductal Carcinoma in Situ; ER: Estrogen Receptor; PR: Progesterone Receptor; -: data not available.
3.3. Quality Assessment
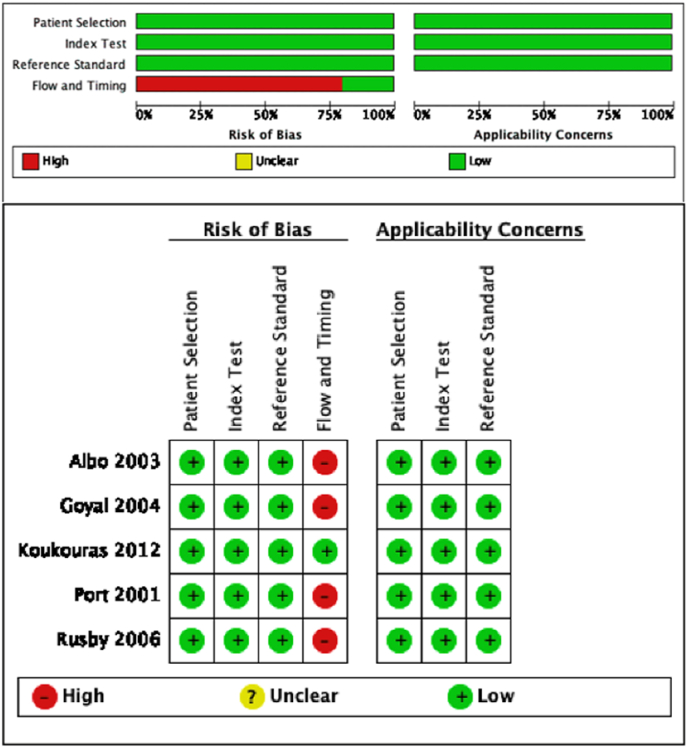
The methodological quality of the 5 studies that evaluated SLNB diagnostic accuracy with the SLNB false-negative rate is illustrated in Fig. 2. The reference standard was the ALND histology. The quality of the included studies was high for 3 out of 4 domains. The principal risk of bias interested “flow and timing” in 4 studies [38,[43], [44], [45]]. Indeed, in those articles, only a part of the included patients presented a systematic ALND in which the false-negative rate was calculated.
Fig. 2.
QUADAS-2 risk of bias and applicability concerns.
3.4. SLNB accuracy
-
-
SLNB detection technique
SLNB detection technique is presented in Table 3. Most studies used dual mapping techniques with Tc99 m and methylene blue (78 patients, 53.1%) [25,33,37,38,38,39,[43], [44], [45]]. The two other mapping techniques were: Tc99 m alone (60 patients, 40.8%) [36,40] or methylene blue alone (9 patients, 6.1%) [46]. Preoperative lymphoscintigraphies were performed in 113 patients (92.9%) with a positive detection in 98 patients (86.7%).
-
-
SLNB identification rate and SLNB false-negative rates
Table 3.
SLNB detection technique.
| Study | SLNB detection technique (n) | Preoperative lymphoscintigraphy (n) | Positive lymphoscintigram (n) | Mean positive SLN lymphoscintigram |
|---|---|---|---|---|
| Albo | Tc99 m + MB (7) | 7 (100.0%) | 5 (71.4%) | 2.0 (0–5) |
| Cimmino | Tc99 m + MB (6) | 5 (83.3%) | 5 (100.0%) | – |
| De Cicco | Tc99 m (18) | 18 (100.0%) | 18 (100.0%) | 1.3 (−) |
| Gentilini | Tc99 m (32) | 32 (100.0%) | 32 (100.0%) | 1.3 (−) |
| Golshan | – | – | – | – |
| Goyal | Tc99 m + MB (9) | 9 (100.0%) | 9 (100.0%) | – |
| Koukouras | MB (11) | 0 (0.0%) | – | – |
| Martin | Tc99 m + MB (15) | 15 (100.0%) | 15 (100.0%) | – |
| Port | Tc99 m + MB (16) | 16 (100.0%) | 11 (68.8%) | – |
| Rusby | Tc99 m + MB (16) Tc99 m (10) MB (5) |
8 (25.8%) | – | – |
| Simsek | Tc99 m + MB (6) MB (4) |
– | – | – |
| Suehiro | Tc99 + MB (3) | 3 (100.0%) | 3 (100.0%) | – |
SLNB: Sentinel Lymph Node Biopsy; SLN: Sentinel Lymph Node; MB: Methylene Blue; Tc99 m: 99 m Technetium-labeled sulphur Colloid; -: data not available.
The forest plot of SLNB identification rate is presented Fig. 3. The SLNB identification rates ranged from 80.0% to 100.0% in the 12 included studies. For 9 studies the SLNB identification rate was 100% [33,36,37,[39], [40], [41],43,45,46]. Simsek et al., Rusby et al. and Port et al. respectively reported a SLNB identification rate of 80.0%, 90.0% and 94.0% [25,38,44]. The studies of Simsek et al. and Rusby et al. with the lowest SLNB identification rates were the only to use methylene blue alone as mapping technique for respectively 4 out of 10 patients and 5 out of 31 patients [25,44]. The pooled estimate of the SLNB identification rate was 99.0% (95% CI 97.1%–101.0%) with no heterogeneity (I2 = 0.0%, p = 0.85). The SLNB false-negative rates were 0% in the 5 included studies and consequently so as the pooled estimate of the false-negative rate.
-
-
SLNB and ALND results
Fig. 3.
Forest plot of the SLNB identification rate. Identification rates are reported with 95% confidence intervals. The pooled estimate rate was 99.0% (95% CI 97.1%–101.0%). The heterogeneity score (I2) was 0.00% (p = 0.85).
SLNB and ALND results are presented in Table 4. Mean number of lymph nodes removed per biopsy varied between 1.1 and 3.0. SLNB was found positive in 70 patients (44.8%). Seventy-nine ALND were performed (51.3%). In 53.2 % of cases, ALND was performed in the presence of macrometastases on SLNB extemporaneous histologic examination. In 41.8 % of cases, ALND was systematically performed. ALND was performed for SLNB failure in 2.5% of cases (2 patients). If micrometastases were found on post-operative immunohistochemical analysis, ALND was delayed (2.5% of cases).
-
-
Characteristics of MBC with positive SLNB
Table 4.
SLNB and ALND results.
| Study Year |
Mean SLN removed (n)< | Type of metastases | Patients with positive SLN (n | Patient with non SLN positive additional lymph node (n |
ALND performed (n) | Mean ALN removed (n | Mean positive ALN (n) |
|---|---|---|---|---|---|---|---|
|
Albo 2003 |
2.6 (1–7) | – | 1 (14.3%) | – | 4 (57.1%) | 18.5 (10–27) | 0.75 (0–3) |
|
Cimmino 2004 |
2.3 (1–4) | Macrometastases (75.0 %) Micrometastases (25.0 %) |
4 (66.7%) | 1 (25.0%) | 4 (100.0%) | – | – |
|
De Cicco 2004 |
1.1 (1–2) | Macrometastases (50.0 %) Micrometastases (50.0 %) |
6 (33.3%) | 1 (16.7%) | 6 (100.0%) | 23.6 (15–33) | 0.01 (0–1) |
|
Gentilini 2007 |
1.5 (1–3) | Macrometastases (66.6 %) Micrometastases (33.3 %) |
6 (18.8%) | 2 (6.2%) | 6 | – | – |
|
Golshan 2007 |
– | Macrometastases (100.0 %) | 5 (83.3%) | 1 (20.0%) | 5 (100.0%) | 25 | – |
|
Goyal 2004 |
2.4 (1–6) | – | 5 (55.6%) | 3 (60.0%) | 5 (55.5%) | – | – |
|
Koukouras 2012 |
1.5 (1–2) | – | 7 (63.3%) | – | 11 (100.0%) | – | – |
|
Martin 2017 |
2.1 (1–5) | – | 9 (60.0%) | – | 9 (100.0%) | 15 (5–24) | 5.8 (0–24) |
|
Port 2001 |
2.8 (1–5) | Macrometastases (60.0%) Micrometastases (40.0 %) |
5 (31.3%) | 1 (20.0%) | 5 (33.3%) | – | – |
|
Rusby 2006 |
2.3 | – | 17 (54.8%) | 6 (35.3%) | 19 (100.0%) | 20 | – |
|
Simsek 2018 |
2.1 | Macrometastases (66.7.0%) Micrometastases (33.3 %) |
3 (30.0%) | – | 4 (40.0%) | – | 0 |
|
Suehiro 2015 |
3.0 (2–4) | Macrometastases (100.0 %) | 1 (33.3%) | – | 1 (100.0%) | 15.0 | 2 |
SLNB: Sentinel Lymph Node Biopsy; SLN: Sentinel Lymph Node; ALN D: Axillary Lymph Node Dissection; ALN: Axillary Lymph Node; -: data not available.
Tree studies with a total of 15 patients described characteristics of MBC with positive SLNB (Table 5) [33,36,43]. Mean tumor size was 1.7–2.4 cm. Invasive ductal carcinoma was mostly represented (14 patients). Tumors were grade 1 (3 patients), 2 (10 patients) and 3 (2 patients). All tumors expressed estrogen receptors. Fourteen tumors presented a Ki67 > 25%.
Table 5.
Characteristics of MBC with positive SLNB.
| Study Year |
Mean tumor size (cm) | T stage (TNM | Tumor histology | Tumor grade | Positive ER | Positive PR | Positive Her2 | Ki67 <25% |
Mean SLN removed (n) | Mean ALN removed (n | Mean positive ALN (n) |
|---|---|---|---|---|---|---|---|---|---|---|---|
|
Albo 2003 |
1.7 | – | IDC (1) | Grade 2 (1) | 1 | 0 | 0 | – | 1 | 17 | 3 |
|
De Cicco 2004 |
– | T1 (6) | IDC (5) DCIS (1) |
Grade 1 (1) Grade 2 (3) Grade 3 (1) |
5 | 5 | – | 3 | 1 (1) | 23.6 (15–33) | 0.007 (0–1) |
|
Martin 2017 |
2.4 (5–4.0) | – | IDC (8) DCIS (1) |
Grade 1 (2) Grade 2 (6) Grade 3 (1) |
9 | 9 | 1 | 8 | 2.1 (1–5) | 15 (5–24) | 5.8 (0–24) |
IDC: Invasive Ductal Carcinoma; DCIS: Ductal Carcinoma in Situ; ER: Estrogen Receptor; PR: Progesterone Receptor; SLN: Sentinel Lymph Node; ALN: Axillary Lymph Node; -: data not available.
4. Discussion
We conducted the first systematic review and meta-analysis that evaluated SLNB accuracy in MBC with negative preoperative axillary evaluation and primary surgery. Our quantitative analysis included 12 studies with 153 patients for the SLNB identification rate and 5 studies with 50 patients for the SLNB false-negative rate. We observed a very high pooled estimate SLNB identification rate of 99.0% (95% CI 97.1%–101.0%) with no heterogeneity (I2 = 0.0%, p = 0.85). The average of lymph nodes removed was 1.1–3.0 and 44.8% of them were metastatic. Importantly, the SLNB false-negative rates were 0% in the 5 included studies.
This study has several strengths. First, we followed a standardized protocol with a comprehensive search strategy, study selection, and data extraction. The quality of the 5 included studies that evaluated SLNB diagnostic accuracy with the SLNB false-negative rate was high for 3 out of 4 domains; in particular, the reference standard was the ALND histology and enabled a misclassification bias to be ruled out. Bivariate random-effects model was used to evaluate the estimate pooled SLNB identification rate: the patient samples were pooled so that the findings of this meta-analysis are more robust than any of the individual studies. Then, the included patients were homogenous, mainly consisting of T1 and T2 invasive ductal carcinomas without palpable axillary adenopathy, aligning with the demographics of the largest retrospective MBC cohorts to date [49,50]. The SLNB detection technique included Tc99 m combined for half the patients with methylene blue, which is the most effective detection technique [51,52]. Finally, our results are powerful because none of the patients included had received neoadjuvant treatment or had preoperative N+ status. Indeed, the meta-analysis of Lin et al. found a SLNB similar pooled identification rate of 97.4% (95% CI 95.3–99.5) and a high SLNB pooled false-negative rate of 7.4% (95% CI -0.9-15.8) but included studies with patients presenting preoperative N+ status and/or undergoing neoadjuvant chemotherapy [53].
However, this meta-analysis has limitations. First, only 12 studies were included because the literature is sparse in relation to the rarity of this cancer. Most of these studies were retrospective, single-center. Our strict inclusion criteria allowed us to include a total of 153 patients, which represents a small cohort and should lead to precautions in the interpretation of the results. Second, the absence of ultrasound in the preoperative axillary evaluation for half of the patients could potentially overestimate SLNB eligibility and so increase the SLNB positivity rate [54].
Despite these challenges, our meta-analysis corroborates the high rate of positive SLNB in MBC found in extensive database reviews [55]. For instance, Greif et al. found a 41.9% rate of axillary nodal involvement among 13 457 MBC [50]. While the ACOSOG Z0011 trial has established the non-inferiority of SLNB alone compared to ALND in women with certain early-stage breast cancers (with 1 or 2 metastatic sentinel lymph nodes) [56]. The recent sub-analysis of the SINODAR-ONE trial focusing on the omission of complete axillary lymph node dissection in cases of T1-2 female breast cancer with 1–2 sentinel lymph node macrometastasis conclude that the overall survival and recurrence-free survival rates of patients treated with SLNB only were not inferior to those treated with ALND [57]. The increased SLNB positivity rate in MBC warrants careful consideration before extrapolating these results to the male population. Recently, Chung et al., retrospectively compared MBC with clinically axillary node-negative, T1 and T2 breast cancer and 1 or 2 positive sentinel lymph nodes who underwent SLNB (61.1%) or ALND (38.9%) alone. After propensity score matching, ALND was significantly associated with superior survival compared with SLNB (5-year overall survival of 83.8% vs 76.0%) [58]. This underscores the importance of systematic SLNB extemporaneous histologic examination followed by immediate ALND if metastases are detected in MBC.
It is to be noted that there are no data in the literature concerning SLNB after neoadjuvant chemotherapy in MBC. Recent recommendations, based on women breast cancer, suggest that for patients who become clinically node-negative following neoadjuvant chemotherapy, SLNB may be performed on condition that at least 3 nodes, including those initially positive, are removed [59,60]. Further studies in MBC specifically seems to be crucial to determine whether these guidelines are or not applicable to men.
5. Conclusion
Sentinel lymph node biopsy in MBC with negative preoperative axillary evaluation and primary surgery is feasible, reproducible, and efficient. Contrary to woman breast cancer and due to the high rate of positive SLNB and to the significantly superior survival of ALND compared to SLNB alone in this case, our study encourages the realization of systematic SLNB extemporaneous histologic examination in MBC to perform immediate ALND if positive, but that management must be validated with prospective studies.
Declarations of interest
none.
CRediT authorship contribution statement
Guillaume Parpex: Writing – review & editing, Writing – original draft, Validation, Methodology, Data curation, Conceptualization. Marie Ottaviani: Writing – review & editing, Methodology, Data curation. Henri Lorphelin: Writing – review & editing. Matthieu Mezzadri: Writing – review & editing. Eva Marchand: Writing – review & editing. Laurence Cahen-Doidy: Writing – review & editing. Jean Louis Benifla: Writing – review & editing. Cyrille Huchon: Writing – review & editing, Validation, Conceptualization. Camille Mimoun: Writing – review & editing, Writing – original draft, Validation, Supervision, Methodology, Formal analysis, Data curation, Conceptualization.
Footnotes
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Mj H., Mr S., Er B., F C., Kj K., Dc K., et al. Management of male breast cancer: ASCO guideline. J Clin Oncol : Official Journal of the American Society of Clinical Oncology. 2020;38 doi: 10.1200/JCO.19.03120. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer statistics. CA Cancer J Clin. 2022;72:7–33. doi: 10.3322/caac.21708. 2022. [DOI] [PubMed] [Google Scholar]
- 3.Cancer Statistics Review, 1975-2015 - Previous Version - SEER Cancer Statistics Review.SEER n.d. https://seer.cancer.gov/archive/csr/1975_2015/index.html (accessed January 31, 2022).
- 4.Borgen P.I., Wong G.Y., Vlamis V., Potter C., Hoffmann B., Kinne D.W., et al. Current management of male breast cancer. A review of 104 cases. Ann Surg. 1992;215:451–457. doi: 10.1097/00000658-199205000-00007. ; discussion 457-459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Veronesi U., Paganelli G., Galimberti V., Viale G., Zurrida S., Bedoni M., et al. Sentinel-node biopsy to avoid axillary dissection in breast cancer with clinically negative lymph-nodes. Lancet. 1997;349:1864–1867. doi: 10.1016/S0140-6736(97)01004-0. [DOI] [PubMed] [Google Scholar]
- 6.Giordano S.H. Breast cancer in men. N Engl J Med. 2018;378:2311–2320. doi: 10.1056/NEJMra1707939. [DOI] [PubMed] [Google Scholar]
- 7.Anderson W.F., Jatoi I., Tse J., Rosenberg P.S. Male breast cancer: a population-based comparison with female breast cancer. J Clin Oncol. 2010;28:232–239. doi: 10.1200/JCO.2009.23.8162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miao H., Verkooijen H.M., Chia K.-S., Bouchardy C., Pukkala E., Larønningen S., et al. Incidence and outcome of male breast cancer: an international population-based study. J Clin Oncol. 2011;29:4381–4386. doi: 10.1200/JCO.2011.36.8902. [DOI] [PubMed] [Google Scholar]
- 9.Brackstone M., Baldassarre F.G., Perera F.E., Cil T., Chavez Mac Gregor M., Dayes I.S., et al. Management of the axilla in early-stage breast cancer: Ontario health (cancer care Ontario) and ASCO guideline. J Clin Oncol. 2021;39:3056–3082. doi: 10.1200/JCO.21.00934. [DOI] [PubMed] [Google Scholar]
- 10.Gradishar W.J., Moran M.S., Abraham J., Aft R., Agnese D., Allison K.H., et al. Breast cancer, version 3.2022, NCCN clinical practice guidelines in Oncology. J Natl Compr Canc Netw. 2022;20:691–722. doi: 10.6004/jnccn.2022.0030. [DOI] [PubMed] [Google Scholar]
- 11.Gao Y., Goldberg J.E., Young T.K., Babb J.S., Moy L., Heller S.L. Breast cancer screening in high-risk men: a 12-year longitudinal observational study of male breast imaging utilization and outcomes. Radiology. 2019;293:282–291. doi: 10.1148/radiol.2019190971. [DOI] [PubMed] [Google Scholar]
- 12.Cloyd J.M., Hernandez-Boussard T., Wapnir I.L. Outcomes of partial mastectomy in male breast cancer patients: analysis of SEER, 1983-2009. Ann Surg Oncol. 2013;20:1545–1550. doi: 10.1245/s10434-013-2918-5. [DOI] [PubMed] [Google Scholar]
- 13.Duma N., Hoversten K.P., Ruddy K.J. Exclusion of male patients in breast cancer clinical trials. JNCI Cancer Spectr. 2018;2:pky018. doi: 10.1093/jncics/pky018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gøtzsche P.C., Ioannidis J.P.A., et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62:e1–e34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 15.Whiting P.F., Rutjes A.W.S., Westwood M.E., Mallett S., Deeks J.J., Reitsma J.B., et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529–536. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 16.Reitsma J.B., Glas A.S., Rutjes A.W.S., Scholten R.J.P.M., Bossuyt P.M., Zwinderman A.H. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol. 2005;58:982–990. doi: 10.1016/j.jclinepi.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 17.Higgins J.P.T., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 18.Kiluk J.V., Lee M.C., Park C.K., Meade T., Minton S., Harris E., et al. Male breast cancer: management and follow-up recommendations. Breast J. 2011;17:503–509. doi: 10.1111/j.1524-4741.2011.01148.x. [DOI] [PubMed] [Google Scholar]
- 19.Fentiman I.S. Surgical options for male breast cancer. Breast Cancer Res Treat. 2018;172:539–544. doi: 10.1007/s10549-018-4952-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cardoso F., Bartlett J.M.S., Slaets L., van Deurzen C.H.M., van Leeuwen-Stok E., Porter P., et al. Characterization of male breast cancer: results of the EORTC 10085/TBCRC/BIG/NABCG international male breast cancer program. Ann Oncol. 2018;29:405–417. doi: 10.1093/annonc/mdx651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Co M., Lee A., Kwong A. Delayed presentation, diagnosis, and psychosocial aspects of male breast cancer. Cancer Med. 2020;9:3305–3309. doi: 10.1002/cam4.2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gómez-Raposo C., Zambrana Tévar F., Sereno Moyano M., López Gómez M., Casado E. Male breast cancer. Cancer Treat Rev. 2010;36:451–457. doi: 10.1016/j.ctrv.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 23.Flaherty D.C., Bawa R., Burton C., Goldfarb M. Breast cancer in male adolescents and young adults. Ann Surg Oncol. 2017;24:84–90. doi: 10.1245/s10434-016-5586-4. [DOI] [PubMed] [Google Scholar]
- 24.De La Cruz L.M., Thiruchelvam P.T.R., Shivani J., Trina J., Blankenship S.A., Fisher C.S. Saving the male breast: a systematic literature review of breast-conservation surgery for male breast cancer. Ann Surg Oncol. 2019;26:3939–3944. doi: 10.1245/s10434-019-07588-1. [DOI] [PubMed] [Google Scholar]
- 25.Simsek O., Belli A.K., Aydogan F., Karatas A., Canbay E., Kepil N., et al. Combination technique is superior to dye alone in identification of the sentinel lymph node in male breast cancer. Am Surg. 2018;84:1957–1960. [PubMed] [Google Scholar]
- 26.Moncayo V.M., Aarsvold J.N., Grant S.F., Bartley S.C., Alazraki N.P. Status of sentinel lymph node for breast cancer. Semin Nucl Med. 2013;43:281–293. doi: 10.1053/j.semnuclmed.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 27.Cutuli B., Le-Nir C.C.-S., Serin D., Kirova Y., Gaci Z., Lemanski C., et al. Male breast cancer. Evolution of treatment and prognostic factors. Analysis of 489 cases. Crit Rev Oncol Hematol. 2010;73:246–254. doi: 10.1016/j.critrevonc.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 28.Liukkonen S., Saarto T., Mäenpää H., Sjöström-Mattson J. Male breast cancer: a survey at the Helsinki university central hospital during 1981-2006. Acta Oncol. 2010;49:322–327. doi: 10.3109/02841861003591723. [DOI] [PubMed] [Google Scholar]
- 29.Wang J., Kollias J., Marsh C., Maddern G. Are males with early breast cancer treated differently from females with early breast cancer in Australia and New Zealand? Breast. 2009;18:378–381. doi: 10.1016/j.breast.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 30.Eryilmaz M.A., Igci A., Muslumanoglu M., Ozmen V., Koc M. Male breast cancer: a retrospective study of 15 years. J BUON. 2012;17:51–56. [PubMed] [Google Scholar]
- 31.Cao X., Wang C., Liu Y., Qiu P., Cong B., Wang Y. Axillary and internal mammary sentinel lymph node biopsy in male breast cancer patients: case series and review. OncoTargets Ther. 2015;8:1499–1502. doi: 10.2147/OTT.S84659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luo H., Meng K., He J., Hu Z., Yang O., Lan T., et al. Intracystic papillary carcinoma of the breast in males: three case reports. Medicine (Baltim) 2020;99 doi: 10.1097/MD.0000000000020278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martin-Marcuartu J.J., Alvarez-Perez R.M., Sousa Vaquero J.M., Jimenez-Hoyuela García J.M. Selective sentinel lymph node biopsy in male breast cancer. Rev Esp Med Nucl Imagen Mol (Engl Ed) 2018;37:146–150. doi: 10.1016/j.remn.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 34.Maráz R., Boross G., Pap-Szekeres J., Markó L., Rajtár M., Ambrózay É., et al. The role of sentinel node biopsy in male breast cancer. Breast Cancer. 2016;23:85–91. doi: 10.1007/s12282-014-0535-1. [DOI] [PubMed] [Google Scholar]
- 35.Flynn L.W., Park J., Patil S.M., Cody H.S., Port E.R. Sentinel lymph node biopsy is successful and accurate in male breast carcinoma. J Am Coll Surg. 2008;206:616–621. doi: 10.1016/j.jamcollsurg.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 36.De Cicco C., Baio S.M., Veronesi P., Trifirò G., Ciprian A., Vento A., et al. Sentinel node biopsy in male breast cancer. Nucl Med Commun. 2004;25:139–143. doi: 10.1097/00006231-200402000-00008. [DOI] [PubMed] [Google Scholar]
- 37.Suehiro S., Abe M., Takumi Y., Hashimoto T., Kamei M., Osoegawa A., et al. The clinical manifestations and treatment of male breast cancer: a report of three cases. Surg Case Rep. 2015;1:92. doi: 10.1186/s40792-015-0103-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Port E.R., Fey J.V., Cody H.S., Borgen P.I. Sentinel lymph node biopsy in patients with male breast carcinoma. Cancer. 2001;91:319–323. [PubMed] [Google Scholar]
- 39.Cimmino V.M., Degnim A.C., Sabel M.S., Diehl K.M., Newman L.A., Chang A.E. Efficacy of sentinel lymph node biopsy in male breast cancer. J Surg Oncol. 2004;86:74–77. doi: 10.1002/jso.20045. [DOI] [PubMed] [Google Scholar]
- 40.Gentilini O., Chagas E., Zurrida S., Intra M., De Cicco C., Gatti G., et al. Sentinel lymph node biopsy in male patients with early breast cancer. Oncol. 2007;12:512–515. doi: 10.1634/theoncologist.12-5-512. [DOI] [PubMed] [Google Scholar]
- 41.Golshan M., Rusby J., Dominguez F., Smith B.L. Breast conservation for male breast carcinoma. Breast. 2007;16:653–656. doi: 10.1016/j.breast.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 42.Bratman S.V., Kapp D.S., Horst K.C. Evolving trends in the initial locoregional management of male breast cancer. Breast. 2012;21:296–302. doi: 10.1016/j.breast.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 43.Albo D., Ames F.C., Hunt K.K., Ross M.I., Singletary S.E., Kuerer H.M. Evaluation of lymph node status in male breast cancer patients: a role for sentinel lymph node biopsy. Breast Cancer Res Treat. 2003;77:9–14. doi: 10.1023/a:1021173902253. [DOI] [PubMed] [Google Scholar]
- 44.Rusby J.E., Smith B.L., Dominguez F.J., Golshan M. Sentinel lymph node biopsy in men with breast cancer: a report of 31 consecutive procedures and review of the literature. Clin Breast Cancer. 2006;7:406–410. doi: 10.3816/CBC.2006.n.058. [DOI] [PubMed] [Google Scholar]
- 45.Goyal A., Horgan K., Kissin M., Yiangou C., Sibbering M., Lansdown M., et al. Sentinel lymph node biopsy in male breast cancer patients. Eur J Surg Oncol. 2004;30:480–483. doi: 10.1016/j.ejso.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 46.Koukouras D., Spyropoulos C., Zygomalas A., Tzoracoleftherakis E. Sentinel node biopsy in male breast carcinoma: is the “female” approach justified? Eur J Gynaecol Oncol. 2012;33:255–256. [PubMed] [Google Scholar]
- 47.Boughey J.C., Bedrosian I., Meric-Bernstam F., Ross M.I., Kuerer H.M., Akins J.S., et al. Comparative analysis of sentinel lymph node operation in male and female breast cancer patients. J Am Coll Surg. 2006;203:475–480. doi: 10.1016/j.jamcollsurg.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 48.Gennari R., Renne G., Travaini L., Bassi F., Zurrida S. Sentinel node biopsy in male breast cancer: future standard treatment? Eur J Surg. 2001;167:461–462. doi: 10.1080/110241501750243833. [DOI] [PubMed] [Google Scholar]
- 49.Giordano S.H., Cohen D.S., Buzdar A.U., Perkins G., Hortobagyi G.N. Breast carcinoma in men: a population-based study. Cancer. 2004;101:51–57. doi: 10.1002/cncr.20312. [DOI] [PubMed] [Google Scholar]
- 50.Greif J.M., Pezzi C.M., Klimberg V.S., Bailey L., Zuraek M. Gender differences in breast cancer: analysis of 13,000 breast cancers in men from the National Cancer Data Base. Ann Surg Oncol. 2012;19:3199–3204. doi: 10.1245/s10434-012-2479-z. [DOI] [PubMed] [Google Scholar]
- 51.Canavese G., Gipponi M., Catturich A., Di Somma C., Vecchio C., Rosato F., et al. Sentinel lymph node mapping in early-stage breast cancer: technical issues and results with vital blue dye mapping and radioguided surgery. J Surg Oncol. 2000;74:61–68. doi: 10.1002/1096-9098(200005)74:1<61::aid-jso14>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 52.OʼReilly E.A., Prichard R.S., Al Azawi D., Aucharaz N., Kelly G., Evoy D., et al. The value of isosulfan blue dye in addition to isotope scanning in the identification of the sentinel lymph node in breast cancer patients with a positive lymphoscintigraphy: a randomized controlled trial (ISRCTN98849733) Ann Surg. 2015;262:243–248. doi: 10.1097/SLA.0000000000001213. [DOI] [PubMed] [Google Scholar]
- 53.Lin A.P., Huang T.-W., Tam K.-W. Treatment of male breast cancer: meta-analysis of real-world evidence. Br J Surg. 2021;108:1034–1042. doi: 10.1093/bjs/znab279. [DOI] [PubMed] [Google Scholar]
- 54.Houssami N., Ciatto S., Turner R.M., Cody H.S., Macaskill P. Preoperative ultrasound-guided needle biopsy of axillary nodes in invasive breast cancer: meta-analysis of its accuracy and utility in staging the axilla. Ann Surg. 2011;254:243–251. doi: 10.1097/SLA.0b013e31821f1564. [DOI] [PubMed] [Google Scholar]
- 55.Carter M., Reyna C., Shaughnessy E., Hanseman D., Meier T., Barrord M., et al. Trends and outcomes associated with axillary management of males with clinical N0 breast cancer-an NCDB analysis. J Surg Res. 2021;268:97–104. doi: 10.1016/j.jss.2021.06.041. [DOI] [PubMed] [Google Scholar]
- 56.Giuliano A.E., Ballman K.V., McCall L., Beitsch P.D., Brennan M.B., Kelemen P.R., et al. Effect of axillary dissection vs No axillary dissection on 10-year overall survival among women with invasive breast cancer and sentinel node metastasis: the ACOSOG Z0011 (alliance) randomized clinical trial. JAMA. 2017;318:918–926. doi: 10.1001/jama.2017.11470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tinterri C., Canavese G., Gatzemeier W., Barbieri E., Bottini A., Sagona A., et al. Sentinel lymph node biopsy versus axillary lymph node dissection in breast cancer patients undergoing mastectomy with one to two metastatic sentinel lymph nodes: sub-analysis of the SINODAR-ONE multicentre randomized clinical trial and reopening of enrolment. Br J Surg. 2023;110:1143–1152. doi: 10.1093/bjs/znad215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chung S.H., de Geus S.W.L., Shewmaker G., Romatoski K.S., Drake F.T., Ko N.Y., et al. Axillary lymph node dissection is associated with improved survival among men with invasive breast cancer and sentinel node metastasis. Ann Surg Oncol. 2023;30:5610–5618. doi: 10.1245/s10434-023-13475-7. [DOI] [PubMed] [Google Scholar]
- 59.Cardoso F., Kyriakides S., Ohno S., Penault-Llorca F., Poortmans P., Rubio I.T., et al. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30:1674. doi: 10.1093/annonc/mdz189. [DOI] [PubMed] [Google Scholar]
- 60.Gradishar W.J., Moran M.S., Abraham J., Aft R., Agnese D., Allison K.H., et al. Breast cancer, version 3.2022, NCCN clinical practice guidelines in Oncology. J Natl Compr Canc Netw. 2022;20:691–722. doi: 10.6004/jnccn.2022.0030. [DOI] [PubMed] [Google Scholar]