Abstract
Very-long-chain PUFAs (VLC-PUFAs) are a group of lipids with chain lengths >24 carbons, and the ELOVL4 (elongation of very-long-chain FA-4) enzyme is responsible for vertebrate VLC-PUFA biosynthesis. Studies on the role of VLC-PUFAs in vision have been hindered because of the need for adequate animal models to capture the global loss of VLC-PUFAs. Since homozygous Elovl4 ablation is lethal in neonatal mice because of catastrophic drying from the loss of their protective skin barrier, we established a zebrafish (Danio rerio) model of Elovl4 ablation. We generated Elovl4b KO zebrafish by creating a 56-bp deletion mutation in exon 2 of the Elovl4b gene using CRISPR-Cas9. We used GC-MS and LC-MS/MS to analyze the VLC-PUFA and lipid profiles from wild-type and Elovl4b KO fish eyes. We also performed histology and visual-behavioral tests. We found that heterozygous and homozygous Elovl4b KO zebrafish eyes had altered lipid profiles and a significantly lower C30 to C36 VLC-PUFA abundance than wild-type fish. Moreover, Elovl4b+/− and Elovl4b−/− KO larvae had significantly lower motor activity in response to light-dark cycles than their age-matched controls. Elovl4b−/− adult fish showed no obvious differences in gross retinal morphology and lamination compared with wild type, except for the presence of lipid droplets within the retinal pigment epithelial cell layer of Elovl4b−/− fish. Our data indicate that the loss of Elovl4b in zebrafish changes ocular lipid profiles and leads to visual abnormalities and subtle retinal changes. These findings highlight the use of zebrafish as a model for VLC-PUFA depletion and ELOVL4-related dysfunction.
Supplementary key words: zebrafish, ELOVL4, lipids, very-long-chain PUFAs, retinal degeneration
Very-long-chain PUFAs (VLC-PUFAs) comprise <2% of FAs in the retina and are enriched in photoreceptor outer segment disks. VLC-PUFAs are unique not only because of their chain length but also because they have a saturated FA-like structure near the carboxylic acid proximal group and a distal PUFA tail group, consistent with a bifunctional role in maintaining photoreceptor structural integrity and membrane fluidity (1). They are not present in significant amounts in typical diets. Thus, humans and most animals rely on de novo biosynthesis by ELOVL4 (elongation of very long-chain FA-4) from long-chain PUFA (LC-PUFA) dietary precursors, such as linoleic acid, α-linolenic acid, EPA, DHA, and arachidonic acid (2). VLC-PUFAs are not currently available as dietary supplements, but fish oils enriched in C26 and C28 VLC-PUFAs have been prepared, and we have developed organic synthetic pathways to prepare ≥C30 VLC-PUFAs in gram quantities using organozinc reactions (3). The study of VLC-PUFAs has become prominent because they are implicated in widespread retinal disorders such as diabetic retinopathy and age-related macular degeneration (AMD), for which there currently are no cures (4).
Very-long-chain FAs (VLC-FAs), including VLC-PUFAs, are synthesized by ELOVL4, which is a fatty acyl elongase (or 3-keto acyl-CoA synthase) expressed in the retina, testes, skin, and brain that is responsible for the initial rate-limiting step and subsequent elongation steps in the biosynthesis of VLC-FAs (5). Patients with Stargardt 3 disease (STGD3), an early-onset macular dystrophy, have C-terminal ELOVL4 mutations that lead to a premature stop in ELOVL4 protein translation, creating a truncated transmembrane protein without an endoplasmic reticulum dilysine retention signal (KXKXX). The three identified mutations associated with autosomal dominant STDG3 occur in exon 6 of ELOVL4 (2). These mutations occur downstream of the dideoxy iron-binding motif (HXXHH), which is in the catalytic domain of the elongase. Although these mutant forms of ELOVL4 are reported not to be catalytically active, cell culture studies have shown that mutant proteins also mislocalize and aggregate in the cytoplasm, causing cellular stress and photoreceptor cell death (5, 6). The combined effects of protein mislocalization and reduced levels of retinal VLC-PUFAs because of haploinsufficiency are thought to underlie the retinal pathology seen in patients with STGD3. In addition, age-related declines in ELOVL4 and ELOVL2 function may contribute to low levels of retinal VLC-PUFAs associated with AMD (2, 7).
The retinal effects of homozygous ELOVL4 KO mutations in humans are poorly characterized because they usually result in early death. Less severe recessive mutations in ELOVL4 in humans are typically toward the N-terminus of the protein. These N-terminal mutations have been associated with nervous system disorders (spinocerebellar ataxia), skin disorders (ichthyosis), and reproductive disorders (8, 9, 10). Recent work indicates that these mutations predominantly affect the enzyme’s ability to make very-long-chain saturated FAs (VLC-SFAs) rather than VLC-PUFAs; so, unsurprisingly, these individuals have not been reported to have a retinal phenotype.
Various mouse models have been developed to study STDG3 mutations in vivo. Still, the complete ablation of Elovl4 is lethal in neonatal mice because of the loss of VLC-SFA-containing ceramides in the skin, which leads to subsequent dehydration (11). Elovl4 KO mice whose skin phenotype was rescued by the expression of an Elovl4 transgene in their skin suffered from severe seizures and died in the first month of life before retinal degeneration phenotypes became apparent (9). In addition, a separate study showed a dose-dependent relationship in the observed severity of disease phenotypes because of the overexpression of the STGD3-specific Elovl4 mutation in mouse photoreceptors (12). So far, the conditional knock-in of human STGD3 mutations and the conditional KO of Elovl4 in mouse ocular tissue present with varying phenotypes and take a relatively long time to deplete VLC-PUFAs and manifest disease progression (13, 14). Thus, with the current rodent models, it is still difficult to fully recapitulate the human disease phenotype, and it is unclear if VLC-PUFA depletion alone is sufficient to cause significant retinal pathology.
Zebrafish (Danio rerio) have gained popularity as tools for lipid metabolism and retinal degeneration research as they are genetically similar to mammals, robust, and have quick generation times. They are a class of bony fish (teleosts) that live in freshwater in the tropical subregion of India. They have retinal structures, cell types, and cone-rich retinas reminiscent of the cone-dense macula in higher-order primates. Moreover, many genes relevant to human disease research are evolutionarily conserved and duplicated in the zebrafish genome. Interestingly, zebrafish gene homologs for many members of the ELOVL elongase family have been identified (10, 15). Elovl4a, which catalyzes the formation of VLC-SFAs, is expressed in most zebrafish tissues, including the brain, other neuronal tissue, skin, and liver. Meanwhile, Elovl4b, which primarily catalyzes the production of VLC-PUFAs, is restricted to the gonads, pineal gland, and retinal photoreceptor layer (10). In addition, the VLC-PUFA biosynthetic pathway is relatively conserved between fish and humans (Fig. 1). As in mammals, VLC-PUFAs greater than 26 carbons in chain length are thought to be exclusively synthesized by Elovl4b on a similar biosynthetic pathway consisting of other elongases, β-oxidases, and FA desaturases. Moreover, their retinal cell types and structure are conserved and comparable to humans. Although zebrafish, like rodents, do not possess maculas, their cone-rich retinas provide a valuable tool for understanding human pathologies.
Fig. 1.
VLC-PUFA biosynthetic pathways in mammals and zebrafish. Comparison of the VLC-PUFA biosynthetic pathways from linoleic (18:2n-6) and α-linolenic (18:3n-3) acid precursors of zebrafish and mammalian vertebrates (adapted from Refs. (10) and (13)). Large dashed black arrows represent the desaturation steps, small dashed arrows represent the β-oxidation steps, and solid arrows represent the elongation steps. AA, arachidonic acid; ALA, α-linolenic acid; LA, linoleic acid. Color key: red text: mammalian (human and murine) orthologues, blue text: zebrafish orthologues, and brown text and arrows: pathway presumed but not confirmed in zebrafish.
Until now, the effects of complete or partial loss of VLC-PUFAs on visual behavior and retinal morphology were unclear in the absence of confounding effects of protein mislocalization and aggregation. This study investigates the impact of homozygous and heterozygous germline deletion mutations of Elovl4b on ocular lipids, VLC-PUFAs, visual function, and ocular morphology in adult and larval zebrafish.
Materials and methods
Zebrafish husbandry
AB strain zebrafish (Zebrafish International Research Center, Eugene, OR) were used as wild-type experimental controls and to generate deletion mutants. Adult zebrafish were maintained by the University of Utah’s Centralized Zebrafish Core Facility under a controlled 14 h light/10 h dark cycle. They were fed a commercial diet of 3–5% body weight per day of Zeigler® zebrafish granules and SPAROS® zebrafish feed containing essential amino acids, vitamins, minerals, FAs, and marine phospholipids twice daily in a recirculating aquarium system. The Institutional Animal Care and Use Committee of the University of Utah approved the breeding and experimental procedures used.
Zebrafish Elovl4b ablation and stable line generation
To prepare for CRISPR-Cas9 ribonucleoprotein complex microinjection into zebrafish embryos, CHOPCHOP (https://chopchop.cbu.uib.no) was used to determine guide RNA sequences targeting zebrafish Elovl4b with minimal predicted off-target effects (16). Two Elovl4b-targeting guide RNA sequences in exon 2 were selected, 5′ TCTCCTTACCTGCTATGGTAAGG 3′ and 5′ CAGGTGAACGACCGTCTCCATGG 3′. Single guide RNA (sgRNA) oligonucleotides and Cas9 nuclease (Alt-R® S.p. Cas9 Nuclease V3, 100 μg [catalog no.: 1081058]) were purchased from Integrated DNA Technologies, Coralville, IA. The CRISPR-Cas9 ribonucleoprotein complex was assembled by mixing 2.5 μM of each sgRNA complex solution with 5 μM Cas9 protein solution diluted in Cas9 working buffer (20 mM Hepes-NaOH [pH 7.5], 350 mM KCl, and 20.5% glycerol), and nuclease-free water, after which the mixture was incubated at 37°C for 5 min and returned to room temperature shortly before microinjection into one-cell stage zebrafish embryos.
Genotyping PCR
Genomic DNA was extracted from caudal fin clips of adult zebrafish. Fin tissue was incubated in 75 μl lysis buffer (40 mM NaOH, 0.2 mM EDTA) for 1 h at 98°C. The lysed samples were diluted with 75 μl of 40 mM Tris-HCl (pH 5.5) and centrifuged for 1 min. Then 2 μl of the supernatant was incubated with 0.5 μM forward (5′ CTGCTTCTGTTGTGTTTTCTGC 3′) and reverse (5′ ACACAAATGCTTGGCACAATTA 3′) primers, 7 μl ddH20, and 10 μl 2× M-PCR OPTI™ Mix (Dye Plus) (Bimake; catalog no.: B45012) in a total volume of 20 μl. Samples were denatured at 94°C for 5 min, followed by 28 cycles of amplification consisting of 30 s at 94°C, 30 s at 60.1°C, and 30 s at 72°C, followed by a final primer extension of 7 min at 72°C. Deletion mutations were identified by gel electrophoresis.
Establishment of an Elovl4b−/− zebrafish line
F0 mosaic fish were outcrossed with wild-type AB fish to confirm germline transmission of the deletion mutation and to screen out and minimize nonspecific off-target mutations. Heterozygous mutants were mated to generate homozygous Elovl4b−/− mutants that were crossed for subsequent generations. This stable line was established following protocols outlined by Moravec and Pelegri (17). Genomic DNA from Elovl4b−/− fish was extracted and purified by gel electrophoresis to sequence the deletion mutation. DNA extraction was performed using the DNeasy® Blood & Tissue Kit from Qiagen, Inc. For PCR, samples were denatured at 94°C for 5 min, followed by 30 cycles of amplification consisting of 2 min at 94°C, 30 s at 62.4°C, and 30 s at 72°C, followed by a final primer extension of 7 min at 72°C. The primers used to amplify the region for sequencing were forward 5′ TGTCATTGGGAGATGAGCAA 3′ and reverse 5′ GTAAAGGCCCATCTCACTGG 3′. PCR was performed to amplify the sequence around the region of interest with primers spanning 627 bp around the target site for the deletion mutation. PCR products were purified by gel electrophoresis, extracted with QIAquick® Gel Extraction Kit, suspended in 30 μl Buffer EB, and sequenced by the University of Utah’s Sequencing Core facility.
RT-PCR
Fish were euthanized by ice-chilled water immersion. After death, brain and retinal tissue were isolated through microdissection and homogenized using Fisherbrand™ RNase-free disposable pellet pestles. They were stored in liquid nitrogen until RNA extraction. Total RNA from brains and retinal tissue of age- and sex-matched wild-type, heterozygous, and homozygous Elovl4b KO fish was isolated using a Qiagen RNeasy mini kit following the manufacturer’s protocol. RNA was quantified using a NanoDrop spectrophotometer (Thermo Scientific). For the determination of mRNA expression levels and to validate germline mutagenesis, total RNA was reverse transcribed with a Verso cDNA Synthesis Kit (ThermoFisher) according to the manufacturer’s protocols using gene-targeting primers synthesized by the University of Utah DNA Peptide Synthesis core facility. The primer sets used are detailed in supplemental Table S1. The resultant RT-PCR products were resolved by gel electrophoresis on 2.5% agarose and imaged using an Invitrogen iBright™ CL750 Imager (Carlsbad, CA).
Retinal histology
To assess gross retinal morphology, 1-year-old zebrafish heads were fixed in 10% neutral-buffered formalin and shipped to HistoWiz (https://home.histowiz.com) for gross processing, paraffin wax embedding, sectioning, and staining with toluidine blue. Pathogenic subretinal pigment epithelium (RPE) lipid deposition is a characteristic of retinal degenerative diseases like AMD (18, 19). We assessed changes in retinal morphology and lipid deposition of retinal sections stained with oil red O (ORO). Whole eyes were isolated from 13-month-old male zebrafish euthanized by ice water immersion. Right eyes were fixed in 4% paraformaldehyde overnight and then incubated in 15% sucrose and 30% sucrose overnight at 4oC. The lenses were removed, and the eye cups oriented in cryomolds with Tissue-Tek® optimum cutting temperature compound, Sakura® Finetek (VWR; catalog no.: 25608-930), with the nasal sides of the eye cups directed into the mold. The eye cups in optimum cutting temperature compound were then flash frozen with isopentane before storage at −80oC prior to sectioning. A Leica cryostat was used to make ∼10–12 μm thick sections, which were placed on SuperFrost Plus microscope slides (catalog no.: 12-550-15; Fisher Scientific, Pittsburgh, PA). Sections were stained according to protocols used by Noel et al. (20). Briefly, sections were brought to room temperature before being stained with 0.3% ORO in 60% isopropanol solution, rinsed with 60% isopropanol, and then counterstained with hematoxylin. Slides were mounted in Fluoromount-G® mounting medium (catalog no.: 0100-01; SouthernBiotech, Birmingham, AL) before imaging at 40× with a Zeiss AxioScan slide scanner.
Visual motor response assay and behavioral tests
A detailed description of the visual motor response (VMR) assay and behavioral tests can be found in the Supplemental data.
GC-MS and LC-MS/MS analyses
Omega-3 (n-3) and omega-6 (n-6) VLC-PUFAs were analyzed using GC-MS and previously published protocols (2). For total lipid analyses, we adapted the protocol used by Harkiewicz et al. (21) for lipid extraction from zebrafish tissue and LC-MS/MS. A detailed description of the sample preparation and MS conditions of VLC-PUFA and lipidomics analyses can be found in the Supplemental materials and methods.
Results
Elovl4b KO zebrafish are fertile and produce viable offspring
ELOVL4 is responsible for the rate-limiting step in vertebrate VLC-PUFA biosynthesis. Zebrafish Elovl4a and Elovl4b are conserved between zebrafish and their mammalian orthologs. A discussion of the conserved motifs can be found in the Supplemental data and supplemental Fig. S1. We targeted exon 2 of Elovl4b to test the effect of VLC-PUFA depletion in the absence of protein formation because exon 2 is far upstream of the STGD3 mutations on exon 6 of human ELOVL4—which cause C-terminal truncation and mislocalization of ELOVL4. The deletion mutation on exon 2 was near to and downstream of the start codon to improve the chances of a missense mutation, which would minimize the possibility of functional protein formation (22). To generate Elovl4b KO fish, we injected Cas9 nuclease duplexed with two sgRNA oligonucleotides targeting exon 2 of Elovl4b to create a 56-bp deletion mutation in Elovl4b for simpler screening via PCR and gel electrophoresis. Surviving embryos were raised to adulthood and screened for germline mutations by outcrossing with wild-type AB strain zebrafish. The F1 generation of Elovl4b KO fish with our desired germline mutation was then crossed to produce F2 fish with wild-type, heterozygous, and homozygous Elovl4b deletion mutations (Fig. 2A). To confirm Elovl4b deletion, we sequenced the Elovl4b KO mutant and identified a 56-base pair deletion mutation and premature termination codon at amino acid position 5 of Elovl4b′s 303 amino acid residues (Fig. 2B). This deletion generates a fish with the mutation: Elovl4b c.187_242del p.T3SfsX3. We also performed RT-PCR analyses and found that the signal for Elovl4b gene expression was reduced in the brain and retinas of heterozygous Elovl4b mutant fish and undetectable in homozygous Elovl4b mutants (Fig. 3). Although ELOVL4 is expressed in the testes of mammalian vertebrates, Elovl4b ablation did not affect the fertility of the fish, as male and female Elovl4b−/− zebrafish appeared normal and produced clutch sizes comparable to wild type when mated.
Fig. 2.
Sequencing and PCR analysis. A: Gel electrophoresis showing wild-type, heterozygous, and homozygous Elovl4b KO zebrafish. DNA was extracted from caudal fin clips and amplified using PCR. The PCR product was subsequently run on 2% agarose gel at 110 V. This image was captured using an iBright CL750 imaging system (Invitrogen, Carlsbad, CA). B: Nucleotide sequence alignment between wild-type and homozygous Elovl4b mutant fish shows that a 56 bp deletion mutation at exon 2 of zebrafish Elolv4b leads to a premature termination codon at position 5 of the protein-coding sequence. The start codons are highlighted in yellow, and the premature termination codon is highlighted in green.
Fig. 3.
RT-PCR analysis of brain and ocular tissue from Elovl4b KO fish. Gel electrophoresis of RT-PCR products at their expected molecular weight. Elovl4b expression is reduced in brain and retina lysates in heterozygous and homozygous Elovl4b KO fish compared with WT controls.
Elovl4b ablation leads to the loss of C30-C36 VLC-PUFAs and changes in major lipid groups
Mammalian ELOVL4 is known to be responsible for the synthesis of VLC-PUFAs >24 carbons in chain length. To investigate the effect of Elovl4b ablation on ocular VLC-PUFAs, we compared the levels of ocular C24–C36 VLC-PUFAs in wild-type and homozygous Elovl4b KO fish eyes. We found via GC-MS analysis that the deletion of Elovl4b increased the levels of C24 to C28 VLC-PUFAs relative to wild type and diminished the levels of C30 to C-36 n-3 and n-6 VLC-PUFAs in their eyes to nearly undetectable levels. Moreover, fish heterozygous for our Elovl4b mutation showed variable intermediate levels of VLC-PUFAs (Fig. 4A–G).
Fig. 4.
Lipid profile of C24-C36 N-3 and N-6 VLC-PUFAs in Elovl4b+/− (blue), Elovl4b−/− (orange), and wild-type Elovl4b+/+ (gray) zebrafish eyes. A: C24-C36, (B) C26, (C) C28, (D) C30, (E) C32, (F) C34, and (G) C36 n-3 and n-6 PUFAs. VLC-PUFA analysis was done using gas chromatography and mass spectrometry. Elovl4b+/−n = 3, Elovl4b−/−n = 3, and Elovl4b+/+n = 2. Lipid peak areas were normalized to the internal standard. Error bars represent the standard error of the mean. P values between groups were obtained using a one-tailed t-test with unequal variance. ∗P ≤ 0.05, ∗∗P ≤ 0.01, and ∗∗∗P ≤ 0.001.
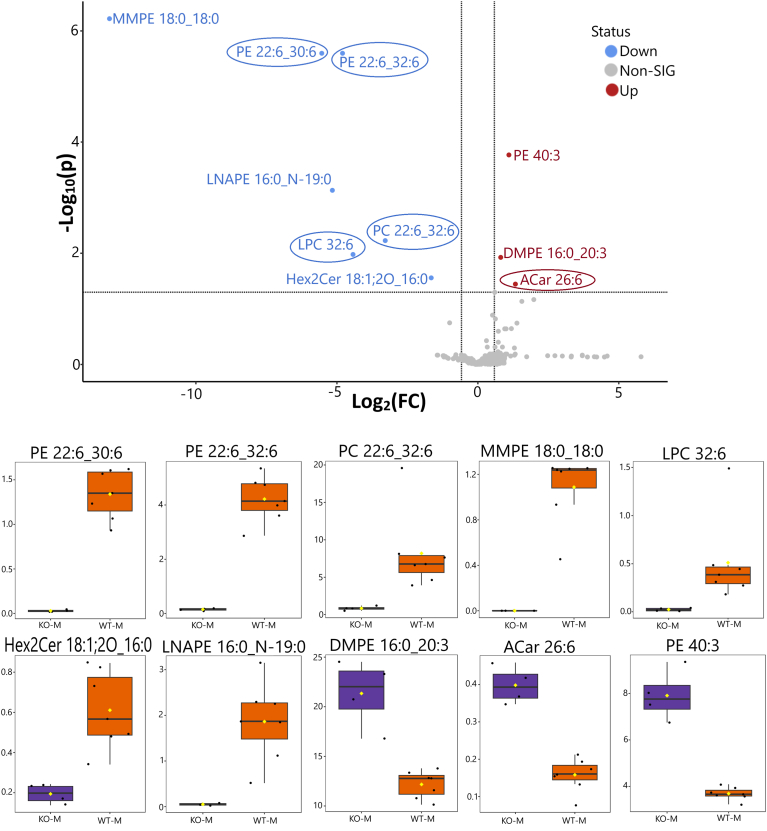
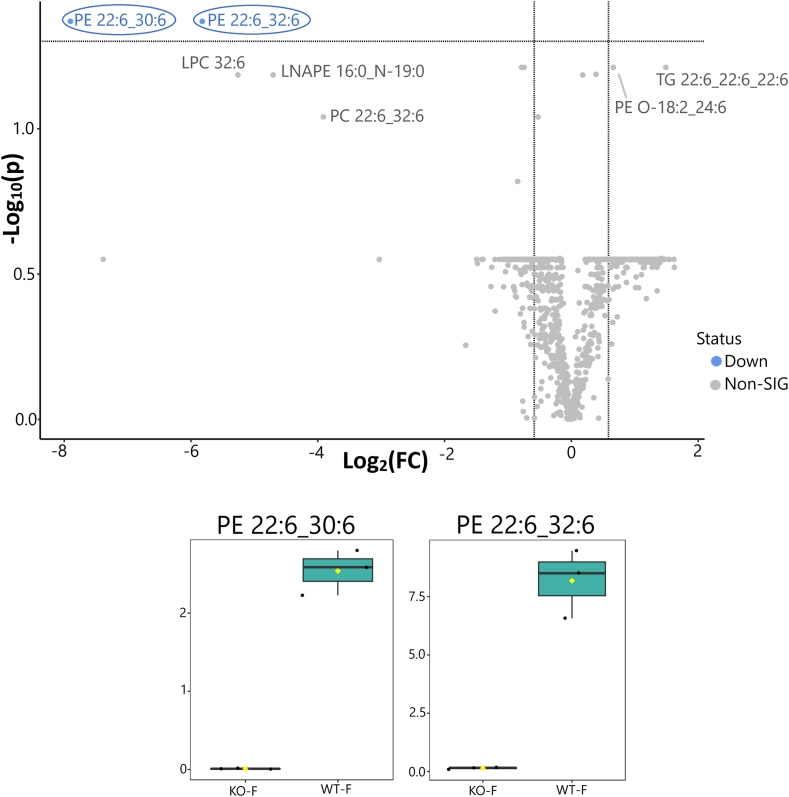
An untargeted lipidomic analysis also revealed significant decreases in the relative abundance of major lipid classes between wild-type control and homozygous Elovl4b KO fish. We evaluated the lipid profiles by genotype and sex. We found 10 compounds to be significantly different between the male wild-type and homozygous Elovl4b mutant fish (Fig. 5), whereas only two of these compounds were significantly different between these genotypes among the female fish (P < 0.05; fold change >1.5; false discovery rate, adjusted) (Fig. 6).
Fig. 5.
Volcano and box plot analyses of ocular lipid profiles of male Elovl4b KO and WT zebrafish normalized to tissue weight using a one-way ANOVA test. Volcano plot shows that seven compounds were significantly downregulated, whereas three compounds were significantly upregulated in ocular lipid comparisons between homozygous Elovl4b KO fish and WT age- and sex-matched controls (n ≥ 4 each). Blue dots represent significantly downregulated lipid species; red dots represent significantly upregulated lipid species; and gray dots represent lipid groups that were not significant in the comparison using our parameters. VLC-PUFA-containing species are circled in volcano plot comparisons. The box plots show the relative peak intensities of significantly altered lipids. Male WT (WT-M; orange) and homozygous Elovl4b KO fish (KO-M; dark purple) (n ≥ 4 each). The y-axes represent the relative peak intensities obtained through LC-MS/MS analysis. Significance was determined using the following parameters: P < 0.05, fold change >1.5, and false discovery rate adjusted.
Fig. 6.
Volcano and box plot analyses of ocular lipid profiles of female Elovl4b KO and WT zebrafish normalized to tissue weight using a one-way ANOVA test. Volcano plot analysis showing two compounds significantly downregulated in female homozygous Elovl4b KO fish compared with WT age- and sex-matched controls (n = 3 each). Blue dots represent significantly downregulated lipid species; and gray dots represent lipid groups that were not significant in the comparison using our parameters. VLC-PUFA-containing species are circled in volcano plot comparisons. The box plots show the relative peak intensities of significantly altered lipids. Female WT (WT-F; teal green) and homozygous Elovl4b KO fish (KO-F; tan brown) (n = 3 each). The y-axes represent the relative peak intensities obtained through LC-MS/MS analysis. Significance was determined using the following parameters: P < 0.05, fold change >1.5, and false discovery rate adjusted.
C30 and C32 VLC-PUFA-containing phosphatidylethanolamines (PE 22:6_30:6 and PE 22:6_32:6) were significantly reduced in female and male Elovl4b−/− fish compared with their wild-type counterparts. Moreover, male Elovl4b−/− fish had reduced levels of C32 VLC-PUFA-containing PCs (PC 22:6_32:6 and lysophosphatidylcholine 32:6), in addition to other lipid groups like monomethyl-phosphatidylethanolamine 18:0_18:0 and lyso-N-acyl-phosphatidylethanolamine (LNAPE 16:0_N-19:0), which have not previously been associated with ELOVL4 activity. LNAPEs are low-abundance intermediates in the biosynthesis of N-acylethanolamines (NAEs). NAEs are lipid mediators involved in regulating homeostatic processes, including energy balance, metabolism, nociception, and inflammation (23). Low levels of LNAPEs (which are NAE intermediates) may make Elovl4b−/− zebrafish more susceptible to inflammation.
In contrast, as a possible compensatory measure, the levels of C26 VLC-PUFA containing acylcarnitine (ACar 26:6) increased in male Elovl4b−/− fish compared with wild type, as well as PE 40:3 and 1,2-dimyristoyl-sn-glycero-3-phosphoethanolamine (DMPE) 16:0_20:3. PE 40:3 and DMPE 16:0_20:3 contain LC-PUFAs but have never been linked with ELOVL4 inactivity. Increased concentrations of the DMPE phospholipid headgroup have been linked to a lower conformational stability of rhodopsin (an important receptor for phototransduction) in dodecyltrimethylammonium bromide and octylglucoside detergent systems (24).
Elovl4b ablation does not affect gross morphology but leads to the abnormal deposition of neutral lipids in the RPE
We found no difference in the gross morphology and lamination of the toluidine blue-stained retinas of adult homozygous Elovl4b KO fish compared with wild-type fish (Fig. 7A, B). However, we found ORO-stained lipid droplets situated in the basal RPE of the Elovl4b−/− mutant fish that were not present in wild-type adults (Fig. 7C).
Fig. 7.
Gross histology and ORO stain. Toluidine blue-stained retinal sections of 1-year-old wild-type and homozygous Elovl4b KO fish. We observed no significant differences in the gross morphology and lamination of the retinas from both groups. Images were taken at 4× (A) and 40× (B) magnification. Cryosections of 1-year-old age-matched male wild-type and homozygous Elovl4b KO zebrafish retinas, treated with ORO to stain lipids (red) and counterstained with hematoxylin (blue) (C). The red arrows point to lipid-rich ORO-stained puncta localizing in the basal RPE of homozygous Elovl4b KO mutants. Images were taken at 40× with a Zeiss AxioScan slide scanner. GCL, retinal ganglion cell layer; INL, inner nuclear layer; IPL, inner plexiform layer; IS, inner segment; ONL, outer nuclear layer; OPL, outer plexiform layer; OS, outer segment; RPE, retinal pigment epithelium.
Elovl4b ablation disrupts visual behavior in larval zebrafish
To assess visual behavioral function in Elovl4b deletion mutant fish, we performed VMR experiments at 5 days post-fertilization (dpf). The VMR assay measures the larval zebrafish’s activity level in response to light stimulus. It is a well-characterized larval response showing a spike in activity in response to light onset (VMR-ON) and light offset (VMR-OFF) with a return to baseline activity levels (25, 26). We observed a marked decrease in activity levels of the Elovl4−/− larvae in the light-to-dark phases compared with heterozygous mutants and wild-type controls (Fig. 8A). This reduction in activity is most clearly observed under dark conditions. As a control, we tested their response to different frequency sounds (Fig. 8B) with the same set of fish and found no significant difference among the groups.
Fig. 8.
Elovl4b KO larvae show a diminished visual-motor response. A: Activity plot of zebrafish larvae response to light stimulus in alternating light-dark cycles. Homozygous and heterozygous Elovl4b KO fish show reduced activity in response to light-dark cycles compared with WT controls. B: Activity plot of control experiments showing no significant difference in larval response to different sound frequencies. Shaded region = dark. Data plotted as mean ± SEM, N = 32. AU, arbitrary unit; D2L, dark to light transition; Het, heterozygous Elovl4b KO mutant; Homo, homozygous Elovl4b KO mutant zebrafish larvae; L2D, light to dark transition.
We investigated the VMR-ON and VMR-OFF responses of 6 dpf larvae in a separate experiment and found that the activity of each group peaked within 2–3 s after light-off but returned to baseline within 10–15 s after peak activity (Supplemental Fig. S2A,B). However, Elovl4−/− larvae had a muted response in the VMR-OFF test (Supplemental Fig. S2B). This result shows that Elovl4b ablation distinctly affects the VMR-OFF response in 6 dpf zebrafish larvae.
Discussion
Understanding the implications of retinal VLC-PUFA depletion in the absence of confounding factors induced by protein mislocalization is critical for defining the roles of ELOVL4 and VLC-PUFAs in maintaining retinal health. This study provides the first in vivo animal model of targeted, complete VLC-PUFA depletion, where we demonstrate a direct link between Elovl4b loss, lipid profile changes, VLC-PUFA depletion, and poor visual outcomes.
We used CRISPR-Cas9 to generate a 56-bp deletion mutation in exon 2 of zebrafish Elovl4b. Fortunately, despite the expression of Elovl4b in critical organs like the gonads and pineal gland (10), homozygous Elovl4b KO mutant fish are fertile and appear normal. This is likely because of zebrafish’s duplicate genome in which two copies of Elovl4 exist with differing expression patterns and functions. Although Elovl4b′s gene duplicate, Elovl4a cannot synthesize VLC-PUFAs, it can make VLC-SFAs to ensure normal function in diverse extraocular tissues, especially the nervous system, the integument, and possibly the reproductive organs. The divergent function and tissue distribution of the Elovl4 gene duplicates is not unique in zebrafish. For example, zebrafish transducin is encoded by the duplicate genes Gnb1a and Gnb1b, which are also reported to have differing functions and expression patterns in the retina (27).
The deletion mutation caused a corresponding reduction in mRNA levels of Elovl4b in brain, retina, and RPE homogenates of Elovl4b+/− and Elovl4b−/− fish. The low levels of RT-PCR product from Elovl4b in the wild-type brain were likely because of Elov4b′s expression in the pineal gland, whereas the Elovl4b RT-PCR signal in the RPE may be attributed to endogenous expression of Elovl4b in RPE cells.
The near complete loss of C30-C36 VLC-PUFAs in homozygous Elovl4b mutant zebrafish was accompanied by an accumulation of C24-28 VLC-PUFAs in lipid extracts from their whole eyes (Fig. 4A–C). It was previously established that when heterologously expressed in Saccharomyces cerevisiae, Elovl4b synthesizes C26 to C36 n-3 and n-6 VLC-PUFAs (Fig. 1) (10). While this may be the case, we still found C26 and C28 n-3 and n-6 VLC-PUFAs in the eyes of Elovl4b KO fish. Likely, other ELOVLs (including perhaps Elovl4b′s gene duplicate, Elovl4a) can elongate to C28 but no further, or the trace amounts of C28 VLC-PUFAs in their feed may have been incorporated into eye tissue (supplemental Fig. S3). Serrano et al. (28) determined that the levels of LC-PUFAs in feed formulations and the mode of rearing can impact the levels of VLC-PUFAs in the eyes and other tissues of gilthead sea bream. Although the biosynthetic pathways of certain VLC-PUFAs may be redundant in zebrafish, this study clarifies that loss of VLC-PUFA >C28 is associated with a retinal and functional phenotype. This suggests that various strategies to increase retinal levels of these VLC-PUFAs through oral supplementation, gene augmentation, and other methods could have beneficial retinal health effects.
Our lipidomics data revealed differences in the lipid profiles of male and female wild-type and Elovl4b−/− zebrafish eyes. There were significant reductions in VLC-PUFA-containing phospholipids in male and female Elovl4b−/− zebrafish eyes as well as the substantial changes in a few additional lipid groups in the male Elovl4b−/− zebrafish (Fig. 5). The relative absence of pleiotropy in the altered lipid species (≤10 in total) between the Elovl4b KO and wild-type groups suggests a narrowed function of Elovl4b in fish and the specificity of the gene-deletion induction strategy. We likewise detected sex-specific differences in the regulation of some lipid classes between the wild-type and Elovl4b−/− mutant groups. For instance, more lipid groups, like lysolipids and VLC-PUFA-containing PCs, were significantly downregulated in Elovl4b−/− mutant male tissue but not in their female counterparts (Fig. 6). In addition, there was a significant increase in the levels of a VLC-PUFA-containing ACar 26:6 in male Elovl4b−/− fish eyes compared with wild type (Fig. 5). Increased levels of ACars are associated with FA oxidation disorders such as diabetic retinopathy (29). Differences in ocular lipid profile changes between male and female fish may influence the manifestation and severity of Elovl4b deficiency in these fish, but the mechanisms underlying these intriguing sex differences and their physiological significance remain to be explored.
We further evaluated the lipidomics data using the lipid ontology (LiON) database, which can rank and group enriched lipids in group comparisons according to various functional, cellular component, physical or chemical property, and lipid classification subcategories (30). The LiON enrichment analysis showed that glycerolipids, lipids with neutral head groups, lipids associated with lipid storage, droplet formation, and FAs <18 carbons in chain length, amongst others, were enriched in male and female Elovl4b−/− fish eyes compared with sex-matched controls (supplemental Fig. S4). Moreover, some lipids with intrinsic negative curvatures that are upregulated in Elovl4b KO eyes (e.g., ceramides, PEs, and diacylglycerols) might have implications in photoreceptor membrane stability and membrane dynamics where the photoreceptor disks and lamellae rely on distinct curvature patterns. We performed lipid analyses on whole eyes because of the miniature size of zebrafish eyes—which need to be pooled for sufficient lipid extraction and detection. In the future, it would be interesting to evaluate retinal- and RPE-specific changes in the lipid profile of these groups.
Elovl4b−/− fish displayed normal gross morphology and lamination in corroboration with previous studies by Dasyani et al. (31) on larval Elovl2 knockdown crispants. Given that zebrafish regenerate neurons, it is not remiss to speculate that this intrinsic ability would mask any evidence of retinal degeneration. However, our Elovl4b−/− fish had evidence of lipid accumulation within the basal RPE layer, a morphological correlate with the changes in C24 to C28 VLC-PUFAs and shorter-chain FAs observed in our GC-MS and lipid ontology studies (Fig. 4 and supplemental Fig. S4). The lipid abnormalities observed in the RPE of Elovl4b KO zebrafish are consistent with research on retinal degenerations in humans (32) and Rp1l1 mutant zebrafish (20). Moreover, incubating fetal bovine RPE cells with high concentrations of phytanic acid (a shorter chain branched FA) elicited the formation of abnormal lipid-containing vacuoles (33)—which suggests that high levels of shorter-chain FAs can contribute to pathogenic lipid deposits in RPE cells. Nevertheless, further investigation is needed to evaluate other abnormalities like photoreceptor swelling, abnormal cell proliferation, vascular endothelial growth factor upregulation, changes in metabolism, signaling, energetics, or any other ultrastructural changes of the retinal and RPE cells, which may be deleterious to the Elovl4b KO fish.
Our VMR studies revealed that Elovl4b−/− larvae had significantly reduced activity levels in response to the light-dark cycles compared with wild-type controls, whereas heterozygous Elovl4b KO larvae had an intermediate activity level response (Fig. 8A). By the fourth iteration of the dark-to-light cycle, the homozygous and heterozygous fish had similar activity levels in the dark, possibly indicating an adaptation to the light stimulation cycles (Fig. 8A). In agreement with Dasyani et al. (31), we observed significant changes in the VMR-OFF response of our 6 dpf larvae (supplemental Fig. S2B).
ELOVL4 dysfunction is involved in other disorders ranging from ichthyosis to spinocerebellar ataxia. We did not fully assess these fish’s motor or neurological function, but our Elovl4b heterozygous and homozygous mutant fish look and behave otherwise normally. VMR-ON responses are thought to reflect cone function, whereas VMR-OFF responses are associated with rod responses (31, 34). However, more time-course experiments, possibly involving electroretinograms and optokinetic and optomotor response tests—throughout the life span of the fish—from larvae to adulthood can be used to determine if Elovl4 dysfunction results in primarily a rod or cone dystrophy, or both.
Our studies demonstrate that Elovl4 haploinsufficiency leads to decreased levels of ocular VLC-PUFAs and altered VMR responses in heterozygous Elovl4b KO fish. This finding has significant implications for AMD and STGD3 disease, where ocular VLC-PUFA depletion is incomplete. Although clinical studies have shown that diet can affect the severity of STGD3 disease (35), supplementing a regular diet with fish oil did little to improve the visual outcomes of patients with STGD3 in a cohort study (36). Conversely, wild-type and Elovl4 conditional-KO mice gavage-fed C32:6n-3 VLC-PUFA had improved visual acuity and electroretinogram measurements from baseline (14). Perhaps a more direct approach with specific VLC-PUFAs, administered in adequate concentrations, would yield positive patient results.
In conclusion, we have successfully generated homozygous Elovl4b KO zebrafish using CRISPR-Cas9. We found that Elovl4b ablation causes VLC-PUFA depletion and significant alterations in the ocular lipid profiles of homozygous Elovl4b mutant zebrafish, whereas haploinsufficient heterozygous mutant fish present an intermediate phenotype. In addition, we demonstrated that the global loss of Elovl4b and consequent loss of VLC-PUFAs >C28 affect larval visual behavior significantly and generate subtle retinal morphological changes, including oil droplets in the RPE. This exciting finding sheds new light on the biosynthetic pathway of VLC-PUFAs in zebrafish and elucidates the effect of C30-C36 VLC-PUFA depletion on ocular function and retinal morphology. This zebrafish model is the first long-lived in vivo animal model of global VLC-PUFA depletion, and the data generated from these models would be useful in developing future therapies against VLC-PUFA-mediated pathologies. In the future, it will be interesting to compare these VLC-PUFA-depleted fish with fish having known STGD3 mutations and to attempt rescue with dietary VLC-PUFA supplements.
Data availability
The data supporting this study’s findings are referenced or contained within the article.
Supplemental data
This article contains supplemental data 37, 38, 39, 40, 41, 42, 43, 44, 45, 46.
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Acknowledgments
The authors acknowledge the Centralized Zebrafish Animal Resource at the University of Utah for providing zebrafish husbandry, laboratory space, and equipment to carry out portions of this research. Lipidomic analysis was performed at the Metabolomics Core Facility at the University of Utah. Mass spectrometry equipment was obtained through NCRR Shared Instrumentation grants 1S10OD016232-01, 1S10OD018210-01A1, and 1S10OD021505-01. The authors thank Dan Cuthbertson of Agilent Technologies for assistance in implementing iterative exclusion in the tandem mass spectrometry experiments. Sequencing was performed at the DNA Sequencing Core Facility, and imaging with the Zeiss AxioScan slide scanner was performed at the Health Sciences Center Cell Imaging Core, University of Utah. This work was supported by the National Institutes of Health grants EY034497 and EY014800, the Foundation for Fighting Blindness, and an unrestricted grant from Research to Prevent Blindness, New York, NY, to the Department of Ophthalmology and Visual Sciences, University of Utah.
Author contributions
U. N. and P. S. B. conceptualization; U. N., S. P., and J. A. M. methodology; U. N., S. P., and J. A. M. formal analysis; U. N., S. P., and J. A. M. investigation; P. S. B. resources; U. N. and J. A. M. writing–original draft; U. N., S. P., J. A. M., and P. S. B. writing–review & editing; U. N., S. P., and J. A. M. visualization; P. S. B. supervision; P. S. B. project administration; P. S. B. funding acquisition.
Funding and additional information
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Supplemental data
References
- 1.Nwagbo U., Bernstein P.S. Understanding the roles of very-long-chain polyunsaturated fatty acids (VLC-PUFAs) in eye health. Nutrients. 2023;15:3096. doi: 10.3390/nu15143096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu A., Chang J., Lin Y., Shen Z., Bernstein P.S. Long-chain and very long-chain polyunsaturated fatty acids in ocular aging and age-related macular degeneration. J. Lipid Res. 2010;51:3217–3229. doi: 10.1194/jlr.M007518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wade A., Rallabandi R., Lucas S., Oberg C., Gorusupudi A., Bernstein P.S., et al. The synthesis of the very long chain polyunsaturated fatty acid (VLC-PUFA) 32:6 n-3. Org. Biomol. Chem. 2021;19:5563–5566. doi: 10.1039/d1ob00491c. [DOI] [PubMed] [Google Scholar]
- 4.Gorusupudi A., Chang F.Y., Nelson K., Hageman G.S., Bernstein P.S. n-3 PUFA supplementation alters retinal very-long-chain-PUFA levels and ratios in diabetic animal models. Mol. Nutr. Food Res. 2019;63 doi: 10.1002/mnfr.201801058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agbaga M.P., Tam B.M., Wong J.S., Yang L.L., Anderson R.E., Moritz O.L. Mutant ELOVL4 that causes autosomal dominant stargardt-3 macular dystrophy is misrouted to rod outer segment disks. Invest. Ophthalmol. Vis. Sci. 2014;55:3669–3680. doi: 10.1167/iovs.13-13099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chertemps T., Duportets L., Labeur C., Ueda R., Takahashi K., Saigo K., et al. A female-biased expressed elongase involved in long-chain hydrocarbon biosynthesis and courtship behavior in Drosophila melanogaster. Proc. Natl. Acad. Sci. U. S. A. 2007;104:4273–4278. doi: 10.1073/pnas.0608142104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen D., Chao D.L., Rocha L., Kolar M., Nguyen Huu V.A., Krawczyk M., et al. The lipid elongation enzyme ELOVL2 is a molecular regulator of aging in the retina. Aging Cell. 2020;19 doi: 10.1111/acel.13100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aldahmesh M.A., Mohamed J.Y., Alkuraya H.S., Verma I.C., Puri R.D., Alaiya A.A., et al. Recessive mutations in ELOVL4 cause ichthyosis, intellectual disability, and spastic quadriplegia. Am. J. Hum. Genet. 2011;89:745–750. doi: 10.1016/j.ajhg.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hopiavuori B.R., Deák F., Wilkerson J.L., Brush R.S., Rocha-Hopiavuori N.A., Hopiavuori A.R., et al. Homozygous expression of mutant ELOVL4 leads to seizures and death in a novel animal model of very long-chain fatty acid deficiency. Mol. Neurobiol. 2018;55:1795–1813. doi: 10.1007/s12035-017-0824-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Monroig O., Rotllant J., Cerdá-Reverter J.M., Dick J.R., Figueras A., Tocher D.R. Expression and role of Elovl4 elongases in biosynthesis of very long-chain fatty acids during zebrafish Danio rerio early embryonic development. Biochim. Biophys. Acta. 2010;1801:1145–1154. doi: 10.1016/j.bbalip.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 11.Li W., Sandhoff R., Kono M., Zerfas P., Hoffmann V., Ding B.C.H., et al. Depletion of ceramides with very long chain fatty acids causes defective skin permeability barrier function, and neonatal lethality in ELOVL4 deficient mice. Int. J. Biol. Sci. 2007;3:120–128. doi: 10.7150/ijbs.3.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karan G., Lillo C., Yang Z., Cameron D.J., Locke K.G., Zhao Y., et al. Lipofuscin accumulation, abnormal electrophysiology, and photoreceptor degeneration in mutant ELOVL4 transgenic mice: a model for macular degeneration. Proc. Natl. Acad. Sci. U. S. A. 2005;102:4164–4169. doi: 10.1073/pnas.0407698102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hopiavuori B.R., Anderson R.E., Agbaga M.P. ELOVL4: very long-chain fatty acids serve an eclectic role in mammalian health and function. Prog. Retin. Eye Res. 2019;69:137–158. doi: 10.1016/j.preteyeres.2018.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gorusupudi A., Rallabandi R., Li B., Arunkumar R., Blount J.D., Rognon G.T., et al. Retinal bioavailability and functional effects of a synthetic very-long-chain polyunsaturated fatty acid in mice. Proc. Natl. Acad. Sci. U. S. A. 2021;118 doi: 10.1073/pnas.2017739118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carmona-Antoñanzas G., Monroig Ó., Dick J.R., Davie A., Tocher D.R. Biosynthesis of very long-chain fatty acids (C > 24) in Atlantic salmon: cloning, functional characterisation, and tissue distribution of an Elovl4 elongase. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2011;159:122–129. doi: 10.1016/j.cbpb.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 16.Labun K., Montague T.G., Krause M., Torres Cleuren Y.N., Tjeldnes H., Valen E. CHOPCHOP v3: expanding the CRISPR web toolbox beyond genome editing. Nucleic Acids Res. 2019;47:W171–W174. doi: 10.1093/nar/gkz365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moravec C.E., Pelegri F.J. Humana Press Inc.; Totowa, NJ: 2019. An Accessible Protocol for the Generation of CRISPR-Cas9 Knockouts Using INDELs in Zebrafish. Methods in Molecular Biology. 1920; pp. 377–392. [DOI] [PubMed] [Google Scholar]
- 18.Apte R.S. Targeting tissue lipids in age-related macular degeneration. EBioMedicine. 2016;5:26–27. doi: 10.1016/j.ebiom.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Curcio C.A., Presley J.B., Malek G., Medeiros N.E., Avery D.V., Kruth H.S. Esterified and unesterified cholesterol in drusen and basal deposits of eyes with age-related maculopathy. Exp. Eye Res. 2005;81:731–741. doi: 10.1016/j.exer.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 20.Noel N.C.L., Nadolski N.J., Hocking J.C., MacDonald I.M., Allison W.T. Progressive photoreceptor dysfunction and age-related macular degeneration-like features in rp1l1 mutant zebrafish. Cells. 2020;9:2214. doi: 10.3390/cells9102214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harkewicz R., Du H.J., Tong Z.Z., Alkuraya H., Bedell M., Sun W.O., et al. Essential role of ELOVL4 protein in very long chain fatty acid synthesis and retinal function. J. Biol. Chem. 2012;287:11469–11480. doi: 10.1074/jbc.M111.256073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uehara H., Zhang X., Pereira F., Narendran S., Choi S., Bhuvanagiri S., et al. Start codon disruption with CRISPR/Cas9 prevents murine Fuchs’ endothelial corneal dystrophy. Elife. 2021;10 doi: 10.7554/eLife.55637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ventura G., Calvano C.D., Bianco M., Castellaneta A., Losito I., Cataldi T.R.I. PE, or not PE, that is the question: the case of overlooked lyso-N-acylphosphatidylethanolamines. Rapid Commun. Mass Spectrom. 2023;37 doi: 10.1002/rcm.9527. [DOI] [PubMed] [Google Scholar]
- 24.Fischer T.H., Williams T.P. The effect of phospholipid structure on the thermal-stability of rhodopsin. Biochim. Biophys. Acta. 1982;707:273–279. doi: 10.1016/0167-4838(82)90361-2. [DOI] [PubMed] [Google Scholar]
- 25.Liu Y.W., Ma P., Cassidy P.A., Carmer R., Zhang G.N., Venkatraman P., et al. Statistical analysis of zebrafish locomotor behaviour by generalized linear mixed models. Sci. Rep. 2017;7:2937. doi: 10.1038/s41598-017-02822-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ganzen L., Venkatraman P., Pang C.P., Leung Y.F., Zhang M.Z. Utilizing zebrafish visual behaviors in drug screening for retinal degeneration. Int. J. Mol. Sci. 2017;18:1185. doi: 10.3390/ijms18061185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lagman D., Callado-Pérez A., Franzén I.E., Larhammar D., Abalo X.M. Transducin duplicates in the zebrafish retina and pineal complex: differential specialisation after the teleost tetraploidisation. PLoS One. 2015;10 doi: 10.1371/journal.pone.0121330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Serrano R., Navarro J.C., Sales C., Portoles T., Monroig O., Beltran J., et al. Determination of very long-chain polyunsaturated fatty acids from 24 to 44 carbons in eye, brain and gonads of wild and cultured gilthead sea bream (Sparus aurata) Sci. Rep. 2022;12:10112. doi: 10.1038/s41598-022-14361-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fort P.E., Rajendiran T.M., Soni T., Byun J., Shan Y., Looker H.C., et al. Diminished retinal complex lipid synthesis and impaired fatty acid β-oxidation associated with human diabetic retinopathy. JCI Insight. 2021;6 doi: 10.1172/jci.insight.152109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Molenaar M.R., Jeucken A., Wassenaar T.A., van de Lest C.H.A., Brouwers J.F., Helms J.B. LION/web: a web-based ontology enrichment tool for lipidomic data analysis. Gigascience. 2019;8 doi: 10.1093/gigascience/giz061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dasyani M., Gao F., Xu Q., Van Fossan D., Zhang E., Pinto A F.M., et al. Elovl2 is required for robust visual function in zebrafish. Cells. 2020;9:2583. doi: 10.3390/cells9122583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jun S., Datta S., Wang L., Pegany R., Cano M., Handa J.T. The impact of lipids, lipid oxidation, and inflammation on AMD, and the potential role of miRNAs on lipid metabolism in the RPE. Exp. Eye Res. 2019;181:346–355. doi: 10.1016/j.exer.2018.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bernstein P.S., Lloyd M.B., Oday W.T., Bok D. Effect of phytanic acid on cultured retinal-pigment epithelium - an invitro model for refsums disease. Exp. Eye Res. 1992;55:869–878. doi: 10.1016/0014-4835(92)90013-i. [DOI] [PubMed] [Google Scholar]
- 34.Zhuang Y.Y., Xiang L., Wen X.R., Shen R.J., Zhao N., Zheng S.S., et al. Slc7a14 is indispensable in zebrafish retinas. Front. Cell Dev. Biol. 2019;7:333. doi: 10.3389/fcell.2019.00333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hubbard A.F., Askew E.W., Singh N., Leppert M., Bernstein P.S. Association of adipose and red blood cell lipids with severity of dominant Stargardt macular dystrophy (STGD3) secondary to an ELOVL4 mutation. Arch. Ophthalmol. 2006;124:257–263. doi: 10.1001/archopht.124.2.257. [DOI] [PubMed] [Google Scholar]
- 36.Choi R., Gorusupudi A., Bernstein P.S. Long-term follow-up of autosomal dominant Stargardt macular dystrophy (STGD3) subjects enrolled in a fish oil supplement interventional trial. Ophthalmic Genet. 2018;39:307–313. doi: 10.1080/13816810.2018.1430240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu A., Terry R., Lin Y., Nelson K., Bernstein P.S. Comprehensive and sensitive quantification of long-chain and very long-chain polyunsaturated fatty acids in small samples of human and mouse retina. J. Chromatogr. A. 2013;1307:191–200. doi: 10.1016/j.chroma.2013.07.103. [DOI] [PubMed] [Google Scholar]
- 38.Koelmel J.P., Kroeger N.M., Ulmer C.Z., Bowden J.A., Patterson R.E., Cochran J.A., et al. LipidMatch: an automated workflow for rule-based lipid identification using untargeted high-resolution tandem mass spectrometry data. BMC Bioinformatics. 2017;18:331. doi: 10.1186/s12859-017-1744-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xia J., Sinelnikov I.V., Han B., Wishart D.S. MetaboAnalyst 3.0--making metabolomics more meaningful. Nucleic Acids Res. 2015;43:W251–W257. doi: 10.1093/nar/gkv380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Messchaert M., Dona M., Broekman S., Peters T.A., Corral-Serrano J.C., Slijkerman R.W.N., et al. Eyes shut homolog is important for the maintenance of photoreceptor morphology and visual function in zebrafish. PLoS One. 2018;13 doi: 10.1371/journal.pone.0200789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lagali P.S., Liu J., Ambasudhan R., Kakuk L.E., Bernstein S.L., Seigel G.M., et al. Evolutionarily conserved ELOVL4 gene expression in the vertebrate retina. Invest. Ophthalmol. Vis. Sci. 2003;44:2841–2850. doi: 10.1167/iovs.02-0991. [DOI] [PubMed] [Google Scholar]
- 42.Goujon M., McWilliam H., Li W.Z., Valentin F., Squizzato S., Paern J., et al. A new bioinformatics analysis tools framework at EMBL-EBI. Nucleic Acids Res. 2010;38:W695–W699. doi: 10.1093/nar/gkq313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Molday R.S., Zhang K. Defective lipid transport and biosynthesis in recessive and dominant Stargardt macular degeneration. Prog. Lipid Res. 2010;49:476–492. doi: 10.1016/j.plipres.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hirokawa T., Boon-Chieng S., Mitaku S. SOSUI: classification and secondary structure prediction system for membrane proteins. Bioinformatics. 1998;14:378–379. doi: 10.1093/bioinformatics/14.4.378. [DOI] [PubMed] [Google Scholar]
- 45.Lang X., Wang L., Zhang Z. Stability evaluation of reference genes for real-time PCR in zebrafish (Danio rerio) exposed to cadmium chloride and subsequently infected by bacteria Aeromonas hydrophila. Aquat. Toxicol. 2016;170:240–250. doi: 10.1016/j.aquatox.2015.11.029. [DOI] [PubMed] [Google Scholar]
- 46.Betancor M.B., Oboh A., Ortega A., Mourente G., Navarro J.C., de la Gandara F., et al. Molecular and functional characterisation of a putative elovl4 gene and its expression in response to dietary fatty acid profile in Atlantic bluefin tuna (Thunnus thynnus) Comp. Biochem. Phys. B Biochem. Mol. Biol. 2020;240 doi: 10.1016/j.cbpb.2019.110372. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting this study’s findings are referenced or contained within the article.