Abstract
Background
Maternal history of trauma is a risk factor for distress during pregnancy. The purpose of this paper was to examine the theorized differential impact of a cognitive behavioral intervention (Mothers and Babies Personalized; MB-P) on maternal distress and emotional regulation for those with ≥ 1 adverse childhood experiences (ACEs; vs no ACEs) from pregnancy to 3 months postpartum.
Methods
Between August 2019 and August 2021, eligible pregnant individuals aged ≥ 18 years, < 22 weeks’ gestation, and English-speaking were recruited from 6 university-affiliated prenatal clinics. Participants (N = 100) were randomized to MB-P (n = 49) or control (n = 51). Analyzable data were collected for 95 participants. Analyses tested progression of change (slope) and at individual timepoints (panel analysis) for perinatal mental health outcomes.
Results
The majority of participants (n = 68, 71%) reported experiencing > 1 ACE (median = 1, range: 0–11). Participants demonstrated significant differential effects for depressive symptoms in absence of ACEs (standardized mean differences [SMD] = 0.82; 95% confidence interval [CI] = [0.13-1.51]) vs in presence of ACEs (SMD = 0.39; 95% CI = [−0.20 to 0.97]) and perceived stress in absence of ACEs (SMD = 0.92; 95% CI = [0.23-1.62]) vs in presence of ACEs (SMD = −0.05; 95% CI = [−0.63 to 0.53]). A panel analysis showed significantly reduced depressive symptoms postintervention and increased negative mood regulation at 3 months postpartum for individuals with ACEs.
Conclusions
Findings support effectiveness of the MB-P intervention to reduce prenatal distress for all pregnant individuals. Preliminary exploration suggests the possibility that individuals with ACEs may benefit from enhanced trauma-informed content to optimize the effects of a perinatal intervention.
Keywords: primary prevention, perinatal intervention, maternal distress, emotional regulation, adverse childhood experiences, randomized controlled trial
Introduction
A history of trauma, including adverse childhood experiences (ACEs: abuse, neglect, household challenges) are an important root contributor of negative outcomes across the life span.1 In particular, these adverse experiences are associated with increased risk for distress (anxiety, depression, and stress) during the perinatal period.2,3 Research on prevalence of ACEs reported by pregnant people varies greatly, with past studies indicating between 41% and 84% of pregnant individuals experience ≥ 1 ACE.4,5 The high prevalence of ACEs among pregnant people and the possible implications of these experiences for maternal and child health underscore the need to include information on individuals’ history of childhood adversity in prenatal care.6,7
Emotional dysregulation is a common, yet often overlooked consequence of early childhood adversity.8 Difficulties in emotional regulation associated with ACEs are linked to poor mental health outcomes (eg, depression, pregnancy-specific anxiety, overall psychological distress9) and mediate the relationship between ACEs and adult psychological distress.10 Furthermore, prenatal exposure to distress indirectly confers risk from maternal ACEs to poor perinatal and birth outcomes,4 including preterm birth, low birth weight in the offspring, and antenatal and postpartum depression.11 Other studies have found that having any ACEs ( ≥ 1) vs no ACEs is a meaningful difference and is associated with a higher number of mental and behavioral health outcomes (eg, anxiety and depressive disorders, depressive symptoms, prenatal substance use) during early pregnancy.12 Moreover, pregnant women reporting 1–2 ACEs compared to none have 2.42 higher odds of depressive symptoms.13 Past research has also shown that maternal distress can impair parental behaviors, such as responsiveness and bonding,14 highlighting the importance of considering the intergenerational impact of ACEs. As such, maternal distress, including depressive and anxiety symptoms and perceived stress, are key targets for preventive and treatment interventions among perinatal individuals.15,16
Mothers and Babies (MB), an evidence-based intervention providing cognitive behavioral therapy (CBT) plus mindfulness enhancement and delivered during the prenatal and postpartum period9,17,18 was recommended as one of the effective interventions aimed at preventing perinatal depression in the 2019 US Preventive Services Task Force report.19 MB is effective in reducing depressive and anxiety symptoms and preventing new cases of prenatal and postpartum depression.9 Other studies have also demonstrated the benefits of CBT and mindfulness interventions during pregnancy on longer-term effects20 Of note, there is limited evidence on the effectiveness of these interventions on maternal regulation of stress and emotions for pregnant people who have had experiences of ACEs.21 The present study tested the effect of ACEs on intervention response to an adapted version of the MB intervention that was enhanced via personalized text messaging and responsive to real-time stress monitoring in the perinatal period (Mothers and Babies Personalized; MB-P).22
Purpose
The primary aim of this paper was to examine the theorized differential impact of the MB-P intervention on maternal mental health outcomes of distress (eg, depressive symptoms, stress, anxiety symptoms) and emotional regulation (eg, negative mood regulation and behavioral activation for depression) outcomes for individuals with ACEs presence ( ≥ 1 ACEs) vs absence (0 ACEs) from pregnancy through 3 months postpartum. The authors examined differential effects via longitudinal trajectories and contrasts at discrete time points via panel analysis (ie, cross-sectional and time series data), comparing those with and without ACEs. The authors hypothesized the MB-P intervention would show improvement for all participants. In these novel analyses, the authors also hypothesized that history of ACEs would be associated with differential intervention response. In particular, the hypothesis was that women without ACEs would benefit more from the intervention than those with ACEs exposure. The authors based this on evidence that there is a dose–response relationship between ACEs and adverse mental health outcomes, and those with ACEs exposure may have greater difficulty with regulation of distress and negative emotions.3,8 To the authors’ knowledge, this is the first direct exploration of whether women with a maternal history of ACEs relative to those without ACEs exposure have a differential response to a perinatal intervention.
Methods
This exploratory analysis was adjunctive to the Promoting Healthy Brain Project: Wellness for 2 Study, a randomized controlled trial, examining the feasibility of conducting the MB-P intervention augmented by personalized just-in-time text messaging and mindfulness content among pregnant and postpartum women. The institutional review board at Lurie Children’s Hospital approved all study procedures. Details on MB-P are published elsewhere.22
Recruitment
Between August 2019 and August 2021, pregnant individuals were recruited from 6 prenatal clinics affiliated with a large, urban Midwestern university-based hospital. Clinic-based recruiters and print advertisements were initially used to recruit potential participants. In-person recruitment was suspended during the COVID-19 pandemic. During this time, clinic recruitment staff shared information about potential participants using a secure institutional email system. Social media platforms were also used to advertise throughout the recruitment phase.
Participants
Pregnant individuals were eligible for study participation if they were at least 18 years old, under 22 weeks gestation, English speaking, had access to a smartphone and wireless network, were willing to wear a wireless adhesive biosensor to measure physiological stress for 12 weeks,23 and agreed to have their infant participate in neurodevelopmental assessments during the first 2 years of life. Exclusion criteria included having a known pregnancy complication or medical condition that may place their infant at risk for neurologic disorders, to exclude conditions that might contribute to neurodevelopmental atypicality outside of the stress pathway that the randomized controlled trial sought to experimentally change, or having a major maternal mental health disorder, which could interfere with study adherence.
To confirm eligibility after recruitment, an online screener and informed consent form were emailed to interested individuals via Research Electronic Data Capture (REDCap). After consenting and completing baseline surveys, participants (N = 100) were randomized into a personalized mobile health-enhanced cognitive behavioral intervention with mindfulness enhancement (ie, the MB-P intervention; n = 49) or stress monitoring plus usual prenatal care (n = 51). Stratified randomization was conducted to oversample for current stress levels via baseline perceived stress (PSS-10) sum scores, with a score of 16 as a comparison point ( > 16 = stressed, < 16 = not stressed) using the REDCap randomization function.
Following randomization, participants were immediately notified by research assistants whether they would participate in the MB-P intervention.
Intervention conditions
Trained facilitators delivered MB-P intervention sessions to participants individually in person, by phone, or via Zoom. Intervention participants received MB-P, a 12-session, manual-based intervention based on principles of CBT, attachment theory, and psychoeducation.18,24 This novel intervention was designed to support and encourage pregnant and postpartum individuals to engage in enjoyable activities, adopt healthy thought patterns, seek social support, and practice mindfulness, in addition to developing and strengthening the parental bond with the baby. The MB-P curriculum is divided into 3 sections: 1) engaging in pleasant activities, 2) identifying and reframing unhelpful thought patterns, and 3) establishing positive relationships with others. Throughout each module, participants receive skills training to manage stress and mood in addition to mindfulness as a strategy to help facilitate the practice of core CBT skills. The key principles of the MB-P intervention were reinforced through personalized just-in-time adaptive intervention (JITAI) text messaging via a smartphone. JITAI messages provided participants with supplementary mindfulness content and encouraged the practice of skills that were learned that week. JITAI messages were prompted by an algorithm that was based on a combination of an objective measure of physiological stress assessed continuously via a biosensor, which was worn daily, and monitored heart rate variability (ie, electrocardiogram signals) and motion (ie, accelerometer data) in addition to a subjective measure of self-reported perceived stress assessed throughout the day using ecological momentary assessment (EMA; up to 5 surveys per day). The control group received prenatal care services as usual, along with stress monitoring in which they wore biosensors and responded to EMAs but did not receive MB-P or JITAI messages.
Data collection
Participant data for the current investigation were collected via REDCap through a secure email link, in person during laboratory visits, or by phone at baseline, at postintervention, and at 3, 7–9, 12, and 24 months postpartum. This analysis focused on data collected from baseline to 3 months postnatal to reflect intervention effects from pregnancy through early postpartum. Maternal distress was assessed through 3 self-report survey measures indicating depressive symptoms, state anxiety symptoms, and perceived stress at 4 data collection time points: baseline, postintervention (M = 4.7 months from baseline, standard deviation [SD] = 3.2), 1 month postnatal, and 3 months postnatal. Emotion regulation was assessed through 2 self-report survey measures indicating negative mood regulation and behavioral activation at 3 data collection time points: baseline, postintervention, and 3 months postnatal.
ACEs Questionnaire
The ACEs questionnaire, assessing ACE exposures prior to 18 years of age, included 10 questions about abuse (emotional, physical, and sexual), neglect (physical and emotional), and household challenges (parental divorce or separation, substance use, mental illness, violent treatment of mother or stepmother, and incarceration of a household member).1 Three additional ACE items assessed bullying, community violence, and foster care.25 A composite ACE score for each participant was calculated from binary (yes or no) responses, with higher scores indicating greater ACE exposures. Due to the lack of variability of ACEs in this sample, the ACE score was dichotomized as ACEs presence ( ≥ 1) vs ACEs absence (0).
Patient-Reported Outcomes Measurement Information System Depression
The Patient-Reported Outcomes Measurement Information System (PROMIS) Depression Short Form 8b includes 8 questions related to depressed mood in the past 7 days.26 Responses were measured on a 5-point Likert scale ranging from 1 (never) to 5 (always). Items were automatically scored using item response theory and the REDCap PROMIS autoscoring feature, with higher T-scores indicating greater symptomology.27
State-Trait Anxiety Inventory
The 20 state-related item State–Trait Anxiety Inventory was administered to assess maternal anxiety.28 Only the state anxiety inventory items were used for this analysis. The State–Trait Anxiety Inventory-S evaluated feelings of apprehension, tension, nervousness, and worry that participants were experiencing at the time of survey administration. Participants responded on a 4-point scale from 1 (almost never) to 4 (almost always). Higher scores indicated higher levels of state anxiety.
Perceived Stress Scale
The 10-item Perceived Stress Scale (PSS-10) assessed the perception of an individual’s stress based on how unpredictable, uncontrollable, or overloaded they feel their life has been over the past month.29 Responses were measured on a 5-point Likert scale from 0 (never) to 4 (very often), with higher scores indicating greater perceived stress levels.
Negative Mood Regulation Scale
The 30-item Negative Mood Regulation (NMR) Scale assessed affective self-regulation ability and the degree to which participants believed that their behavior or cognition could alleviate negative moods.30 Responses were measured on a 5-point Likert scale, from 1 (strongly disagree) to 5 (strongly agree), with higher scores indicating a stronger belief in altering one’s negative mood.
Behavioral Activation for Depression Scale
A 9-item Behavioral Activation for Depression Short Form (BADS-SF) assessed behaviors hypothesized to underlie depression, specifically targeting any changes in mood over the past week.31 Responses are measured on a 7-point scale ranging from 0 (not at all) to 6 (completely), with higher scores indicating greater frequency of behavioral activation.
Analysis plan
The aim of this study was to examine whether women who had experienced ACEs responded differently to the MB-P intervention during pregnancy and up to 3 months postpartum compared to those without ACEs. The analysis involved 2 approaches: 1) examining longitudinal trajectories to identify differential effects, and 2) using panel analysis to compare individual time points between those with and without ACEs. All participants who were randomized and had at least one measurement were included in the analysis, and missing data were handled using a mixed-effects model.32 Demographic and key measures, including ACE status and maternal mental health indicators, were analyzed at baseline using descriptive statistics. The standardized mean difference (SMD) of the slopes between the intervention and control groups was calculated for each outcome to assess the progression of change over time. The Chow test was used to determine the differential effect in slopes for each outcome relative to ACEs absence vs presence.33 Panel analysis was used to graphically plot the adjusted marginal means, which were derived from three-way interactions (time point × intervention group × ACE status) via general linear mixed models, comparing those with and without ACEs. Adjusted mean differences were assessed to better understand the change in each outcome between time points for the ACEs absence and presence subgroups, respectively.
Covariate adjustment using propensity score weighting is commonly performed in subgroup randomized clinical trial analyses as a method for addressing covariate confounding.34 For this analysis, a pool of clinically relevant variables were selected, including maternal age, race/ethnicity, education, relationship status, and income-to-need ratio. Generalized Boosted Modeling, an automated algorithm for iteratively forming a collection of simple regression tree models, estimated the propensity scores by simultaneously considering multiple covariates.35,36 Once the propensity weights were determined, they were incorporated into the model to account for covariate imbalances and provide a more accurate assessment of treatment effects in the subgroup analyses. All analyses were performed using Stata version 17 (StataCorp LLC).37 Statistical significance was assessed at P < 0.05.
Results
Participant flow
Of the 344 individuals who were referred by clinic recruiters and contacted by the research team, 164 met the eligibility criteria and 121 individuals provided informed consent, of which 11 declined participation and 10 could not be reached. One participant withdrew consent and data, which resulted in a sample size of 99 participants, and 4 participants were excluded due to ≥ 1 ACE items being missing, resulting in an analytic sample of 95 (47 intervention, 48 control) participants.
Participant characteristics
Table 1 shows the participant demographics and baseline characteristics for this analysis by ACEs absence and presence. Within the entire analytic sample (N = 95), the majority of participants reported being non-Hispanic White (n = 83, 69.5%); married, engaged or living with a partner (n = 93, 97.9%); having a graduate or professional degree (n = 58, 61.1%); mean (M) and standard deviation (SD) of total household size of 2–3 people (M = 2.8, SD = 1.1); and a highly resourced income-to-need ratio (M = 5.6, SD = 3.3). Nearly three-quarters (n = 68, 71.6%) of the participants reported ≥ 1 ACEs with a mean and standard deviation of (M = 2.94, SD = 2.30) and median score of 2 (range = 0–11). The control group (n = 48, 57.4%) had a median score of 2 ACEs, whereas the intervention group (n = 47, 42.6%) had a median score of 1 ACE, with a trend toward significance in the difference between the study groups (P = 0.059).
Table 1:
Participant demographics and baseline characteristics by Adverse Childhood Experiences absence and presence (N = 95)
| Characteristic | ACEs absence (n = 27) | ACEs presence (n = 68) | P value |
|---|---|---|---|
| Study group, n (%) | |||
| Control | 9 (33.3%) | 39 (57.4%) | 0.059 |
| Intervention | 18 (66.7%) | 29 (42.6%) | |
| Maternal age | |||
| Mean (SD) | 32.7 (3.46) | 34.1 (5.00) | 0.109 |
| Median [min, max] | 31.9 [28.6, 40.8] | 33.7 [19.2, 45.3] | |
| Gestational age (weeks) | |||
| Mean (SD) | 16.3 (3.24) | 16.1 (3.64) | 0.727 |
| Median [min, max] | 17.1 [10.3, 22.3] | 16.4 [9.86, 22.7] | |
| Maternal race, n (%) | |||
| White | 21 (77.8%) | 45 (66.2%) | 0.037 |
| Asian | 6 (22.2%) | 4 (5.9%) | |
| Black | 0 (0%) | 11 (16.2%) | |
| More than 1 race | 0 (0%) | 3 (4.4%) | |
| Native American/Alaskan Native | 0 (0%) | 1 (1.5%) | |
| Other | 0 (0%) | 2 (2.9%) | |
| Missing, n (%) | 0 (0%) | 2 (2.9%) | |
| Maternal ethnicity, n (%) | |||
| Hispanic/Latina | 1 (3.7%) | 11 (16.2%) | 0.191 |
| Non-Hispanic | 26 (96.3%) | 57 (83.8%) | |
| Maternal education, n (%) | |||
| High school diploma/GED or less | 0 (0%) | 3 (4.4%) | 0.324 |
| College degree | 7 (25.9%) | 26 (38.2%) | |
| Graduate or professional degree | 20 (74.1%) | 38 (55.9%) | |
| Prefer not to answer | 0 (0%) | 1 (1.5%) | |
| Maternal relationship status, n (%) | |||
| Married, engaged and/or living with partner | 27 (100%) | 66 (97.1%) | 0.667 |
| No romantic partner/single | 0 (0%) | 1 (1.5%) | |
| Romantic partner, not living together | 0 (0%) | 1 (1.5%) | |
| Household size | |||
| Mean (SD) | 2.74 (1.10) | 2.76 (1.20) | 0.926 |
| Median [min, max] | 2.00 [2.00, 6.00] | 2.00 [1.00, 7.00] | |
| Income-to-need ratio | |||
| Mean (SD) | 5.55 (3.23) | 5.51 (3.44) | 0.956 |
| Median [min, max] | 4.73 [1.50, 17.4] | 4.73 [0.859, 17.7] | |
| Missing, n (%) | 0 (0%) | 3 (4.4%) | |
| ACEs, baseline | |||
| Mean (SD) | 0 (0) | 2.94 (2.30) | < 0.001 |
| Median [min, max] | 0 [0, 0] | 2.00 [1.00, 11.0] | |
| BADS, baseline | |||
| Mean (SD) | 36.7 (8.49) | 36.4 (8.31) | 0.878 |
| Median [min, max] | 36.0 [15.0, 51.0] | 36.0 [19.0, 53.0] | |
| NMR, baseline | |||
| Mean (SD) | 110 (12.7) | 110 (13.5) | 0.977 |
| Median [min, max] | 111 [85.0, 133] | 112 [74.0, 143] | |
| PROMIS Depression, baseline | |||
| Mean (SD) | 50.0 (7.57) | 47.7 (7.10) | 0.182 |
| Median [min, max] | 49.2 [37.1, 69.4] | 48.4 [37.1, 66.0] | |
| Missing, n (%) | 1 (3.7%) | 1 (1.5%) | |
| PSS-10, baseline | |||
| Mean (SD) | 14.0 (6.01) | 15.0 (6.78) | 0.485 |
| Median [min, max] | 13.0 [4.00, 32.0] | 15.5 [1.00, 27.0] | |
| Missing, n (%) | 1 (3.7%) | 0 (0%) | |
| SAI, baseline | |||
| Mean (SD) | 14.2 (9.29) | 15.4 (9.78) | 0.602 |
| Median [min, max] | 12.0 [1.00, 44.0] | 15.0 [0, 45.0] | |
| Missing, n (%) | 1 (3.7%) | 0 (0%) |
Note: Income-to-needs ratio was calculated based on income reported by enrolled participants at baseline and on household size (total size and number of children in home) reported at baseline, using federal poverty guidelines.
ACE, Adverse Childhood Experience; BADS, Behavioral Activation for Depression Scale;GED, General Education Development; max, maximum; min, minimum; NMR, Negative Mood Regulation;PROMIS, Patient-Reported Outcomes Measurement Information System; PSS, Perceived Stress Scale; SAI, State Anxiety Index; SD, standard deviation.
Effect size differences in slopes
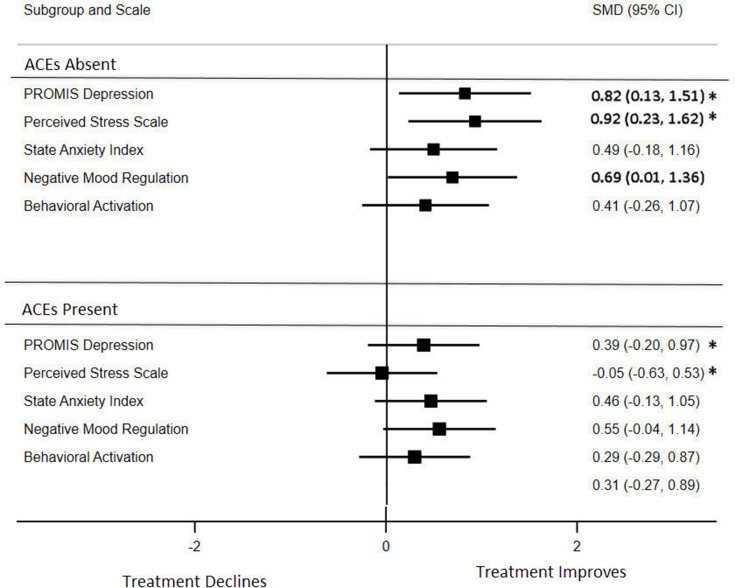
Figure 1 shows the effect size differences in linear slope trajectories between the intervention and control groups for those with an absence or presence of ACEs. These analyses are assessing progression over time (ie, slope) and testing whether intervention effects were different based on ACE status. Improvements in maternal distress are indicated as decreases in depression, perceived stress, and anxiety and increases in negative mood regulation and behavioral activation. The ACEs absence group demonstrated statistically significant between-group differences using the SMD of the slopes between the intervention and control groups in depressive symptoms, perceived stress, and negative mood regulation. Moderating effects for ACEs absence vs presence were statistically significant in depressive symptoms at P < 0.01 and for perceived stress at P < 0.001. Participants demonstrated differential rates of improvement in linear trajectories from baseline to 3 months postpartum for depressive symptoms ACEs absence (SMD = 0.82; 95% confidence interval [CI] = 0.13-1.51) vs ACEs presence (SMD = 0.39; 95% CI = −0.20 to 0.97) and perceived stress ACEs absence (SMD = 0.92; 95% CI = 0.23-1.62) vs ACEs presence (SMD = −0.05; 95% CI = −0.63 to 0.53). Participants without ACEs also had statistically significant increased negative mood regulation (SMD = 0.69; 95% CI = 0.01-1.36). Those without ACEs demonstrated larger effect size differences in the slope trajectories of all outcomes as compared to those with ACEs.
Figure 1:
Forest plot of the standardized mean difference of the effect size in the slopes between the intervention and control groups for participants with an absence or presence of ACEs. Effect size differences for depression, perceived stress, and anxiety were reversed from negative to positive to demonstrate improvement, ensuring that all of the scales were pointing in the same direction. The between-group statistical significance is indicated in bold, whereas the differential effect between ACEs absence vs presence for depressive symptoms and perceived stress is indicated with an asterisk. ACE = Adverse Childhood Experience; CI = confidence interval; PROMIS = Patient-Reported Outcomes Measurement Information System; SMD = standardized mean differences.
Contrasts between individual time points
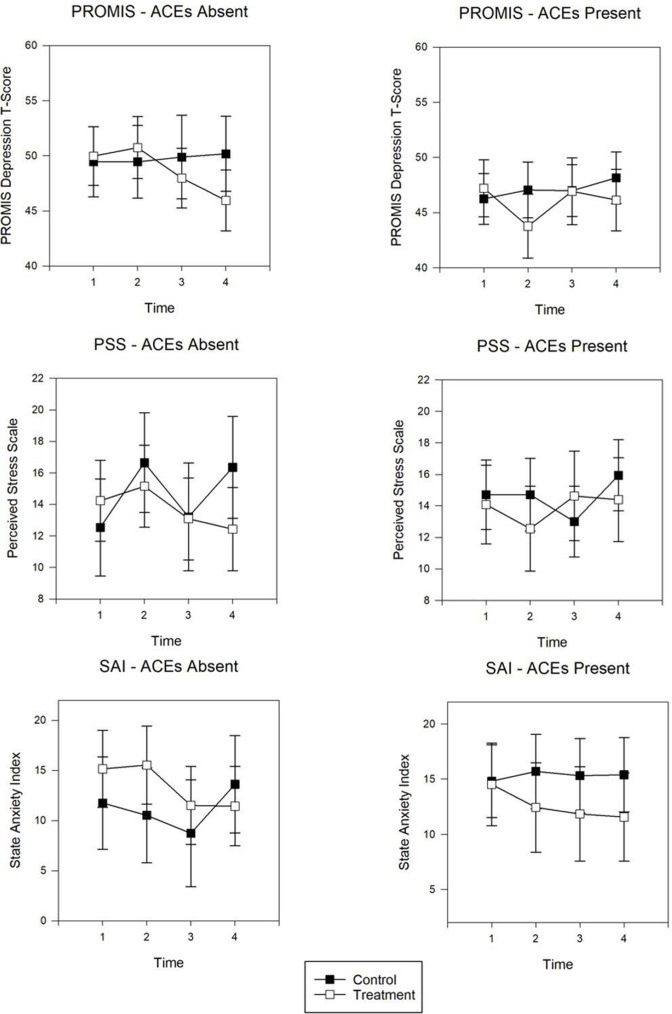
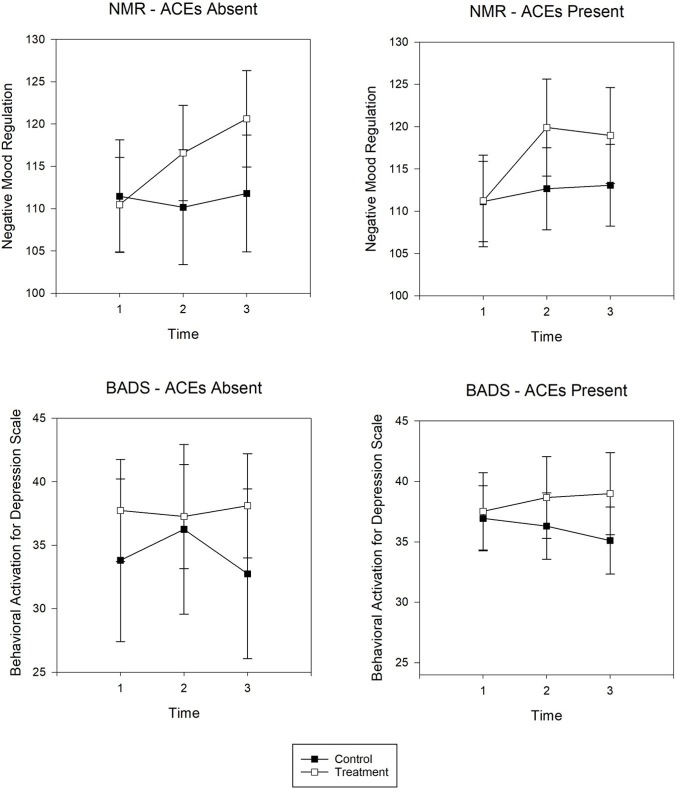
Cross-sectional panel analysis for maternal distress (Figure 2) and emotional regulation (Figure 3) display the adjusted marginal means for each outcome at discrete time points for those with and without ACEs. Whereas trajectory analyses examined patterns over time, the panel analyses examined group differences for each time point. Results are summarized below for the statistically significant adjusted mean differences (AdjMD) for contrasts between time points in the intervention group by ACEs absence and presence.
Figure 2:
Panel analysis of adjusted marginal means for PROMIS Depression, PSS, and SAI for individuals with ACEs absence or presence. Decreasing scores indicate improvement. Time points are designated as: 1 = baseline; 2 = postintervention; 3 = 1 month postnatal; 4 = 3 months postnatal. ACE = Adverse Childhood Experience; PROMIS = Patient-Reported Outcomes Measurement Information System; PSS = Perceived Stress Scale; SAI = State Anxiety Index.
Figure 3:
Panel analysis of adjusted marginal means for NMR Scale and BADS for those with ACEs absence and presence. Increasing scores indicate improvement. Time points are designated as: 1 = baseline; 2 = postintervention; 3 = 3 month postnatal. ACE = Adverse Childhood Experience; BADS = Behavioral Activation for Depression Scale; NMR = Negative Mood Regulation.
For individuals without ACEs, these data exhibited a decrease in depressive symptoms (AdjMD = –4.02, standard error [SE] = 1.49, P = 0.01) from baseline to 3 months postpartum and decreases in anxiety symptoms (AdjMD = –3.72, SE = 1.9, P = 0.05) and perceived stress (AdjMD = –2.73, SE = 1.26, P = 0.03) from postintervention to 3 months postpartum. Those without ACEs also demonstrated improvement in negative mood regulation (AdjMD = 10.15, SE = 2.17, P = 0.001) from baseline to 3 months postpartum.
For individuals with ACEs, depressive symptoms decreased (AdjMD = –3.44, SE = 1.53, P = 0.03) from baseline to postintervention yet was not sustained at 1 and 3 months postnatal. Negative mood regulation increased for those with ACEs (AdjMD = 7.75, SE = 2.17, P = 0.001) from baseline to 3 months postpartum. Behavioral activation did not show any notable changes over time for either those with or without ACEs.
Discussion
Given the consistent evidence that ACEs have negative long-term effects on both maternal and child outcomes,38–41 an exploratory analysis was performed to examine the extent of which the intervention addressed the psychological needs of individuals with a history of ACEs. Although this sample was relatively small and well resourced, the present analyses were an important first step in examining this question while taking advantage of real-time personalization of the intervention delivery based on maternal EMA and physiologic indicators. Consistent with the authors’ hypothesis, there were meaningful differences between participants with and without ACEs in the rate of growth in intervention impact on maternal outcomes that can generate further hypotheses for future trials testing stress reduction interventions in perinatal individuals over time. In contrast, there were no differences by ACE status at the timepoint level in the overall model.
The suggestion from the longitudinal analyses is that women with ACEs vs those with no ACEs exposure may benefit in a less comprehensive and sustained way from the perinatal intervention. This points to the need for replication and extension in larger, more representative trials that can generate and test the hypothesis that pregnant individuals with ACEs exposure may benefit from trauma-informed enhancement of evidence-based interventions. Rates of improvement for women’s ability to regulate mood were greater for those with no history of ACEs as compared to those with ACEs exposure. In addition, those with ACEs exhibited improvements in depressive symptoms at postintervention, which were not sustained in postpartum.
These exploratory results partially confirm the authors’ hypotheses. The study sample as a whole improved in well-being based on participation in the MB-P intervention. The authors’ hypothesis that those without ACEs would benefit more was partially supported by greater improvement in the intervention group over time. However, the hypothesis was not supported in analyses that examined these patterns cross-sectionally. This suggests the possibility that it is in the rate and sustainment of effects that differences may exist. Maternal distress outcomes may show less consistent improvement and be more challenging to maintain in the long term for individuals with a history of ACEs exposure who may already be experiencing difficulties in managing distress and regulating stress responses.
The current data demonstrating differential effects of the intervention on depressive symptoms and perceived stress between those with and without ACEs emphasize the possible importance of considering ACEs exposure when designing interventions and examining their effects on perinatal mental health outcomes. These findings must be understood as a first step in light of the limitations of the present study, including a relatively small sample size and a well-resourced sample. Extant research on the longstanding impact of childhood experiences on adulthood outcomes highlight the importance of understanding both current and historical experiences of pregnant people,11,42 which may impact individuals’ present-day experience and capacity for self-soothing and regulation and ability to benefit from supports.43 ACEs are inalterable life experiences, yet the psychosocial and behavioral consequences of ACEs are modifiable, and, in turn, improvement in these factors may lead to improved maternal health and well-being and better perinatal outcomes.44
One may theorize based on these preliminary findings that a trauma-informed intervention may enhance intervention responsiveness for individuals with a history of ACEs. Elucidating the mechanism by which ACEs may reduce intervention responsiveness would be important for any enhancements to be tested. Bolstering the intervention components conducive to emotional regulation in pregnancy is critical to reduce the intergenerational impact of ACEs and to increase resilience for the next generation.8 Although perinatal interventions are designed to target the pregnant individual, these interventions will likely have intergenerational impact because of their potential positive impact on parental capacity for bonding, attunement, and creation of a safe and stable environment for their child, as well as protecting against deleterious effects of perinatal exposure to stress on early self-regulation in offspring.45,46 As such, increased awareness of the prevalence and impact of ACEs on birthing populations is a first step in responding with two-generation strategies that can improve mental health and promote affect regulation to increase lifelong positive parent–child interactions.47
Study limitations
Several limitations are noted. The study sample was well educated and predominately White, raising caution about the generalizability of study findings. This was a post hoc analysis of a primary study that was not explicitly designed to examine the impact of the intervention among individuals experiencing ACEs. Multiple testing was not adjusted for in the model, and the overall sample was small to be examining three-way interactions, as was the number of individuals without ACEs. Further, we did not distinguish between different types of ACEs that may have impacted individuals differently. It is also possible that social desirability bias may have been present due to self-report assessments. Last, confounders and life circumstances may have been unaccounted for in this study’s measurement, particularly those related to traumatic birth events and postpartum recovery, and may have influenced the authors’ findings. These limitations highlight the need for more robust and focused studies specifically designed to elucidate mechanisms by which a history of ACEs may influence intervention response.
Future directions
The present analysis provides a glimpse of the role that a pregnant person’s history of ACEs may have on the influence of intervention responsiveness, yet the generalizability and mechanisms of these patterns must be established. Future large perinatal intervention studies with diverse populations and greater socioeconomic and ACEs exposure heterogeneity are needed to elucidate whether and how perinatal interventions should be tailored for those with ACEs. Ideally, these future studies would compare pathways across a range of evidence-based interventions to assess whether patterns are generalizable, as well as testing these pathways in individuals from historically marginalized communities who are likely to have experienced substantially more ACEs in addition to other trauma exposures. If these findings are replicated, perinatal populations with varying ACEs exposure may benefit from additional trauma-specific interventions. Certainly, the present findings point to the importance of assessing maternal ACEs within the context of perinatal stress reduction trials.
MB-P uniquely incorporates JITAI personalized text messaging that is individualized and responsive to objective and subjective reports of stress. This innovative aspect of the intervention could be further leveraged to mitigate against less benefit for individuals with ACEs by incorporating content that is specifically resilience- and trauma-informed, including self-regulation skills that are biologically based (eg, tracking, resourcing, grounding), and can help to restore the natural balance of the nervous system.48,49 It will also be important to design studies that test the impact of protective factors (eg, positive childhood experiences), as well as ACE consequences (eg, health-compromising coping behaviors and multiple interacting forms of disadvantage on intervention effectiveness).
Conclusion
Maternal perinatal stress reduction interventions are effective and may benefit from enhancements based on maternal characteristics and history. Findings from this study are an important step for informing the possibility of larger trials and can help us to understand how to use a person’s ACE history to support maternal mental health and well-being in routine prenatal care. Future studies that are adequately powered and designed to examine differences among individuals with varying levels and types of ACEs are critically needed to generate more robust evidence on whether and how ACEs shape the effects of MB-P and other interventions delivered to pregnant individuals and new parents with a history of ACEs exposure. In this way, we can tailor perinatal interventions that have outsized impact on intergenerational health and well-being to have maximal impact on individuals with varied lived experience.
Acknowledgments
The authors gratefully acknowledge contributions of the Promoting Healthy Brain Project investigative team, including Sheila Krogh-Jespersen, Elizabeth Norton, Nabil Alshurafa, Bill Grobman, Leena Mitthal, Erin Ward, Gina Giase, Amelie Petitclerc, Peter Cummings, Aditi Rangarajan, and John Rogers, as well as the collaboration and support of the Perinatal Origins of Disease collective, particularly Karen Mestan and Aaron Hamvas. The authors would also like to express sincere appreciation for the individuals who participated in this study.
Footnotes
Author Contributions: Ellen Goldstein, PhD, MFT, and Roger L Brown, PhD, had full access to the data in this study and take responsibility for the integrity of the data and accuracy of the data analysis. Concept and design: Goldstein, Brown, Jillian S Merrick, PhD, Renee C Edwards, PhD, Yudong Zhang, PhD, S Darius Tandon, PhD, and Lauren S Wakschlag, PhD. Acquisition, analysis, or interpretation of data: Goldstein, Brown, Merrick, Edwards, Zhang, Tandon, Wakschlag, Stephanie Krislov, BS, Julia Raven, MA, Judith T Moskowitz, PhD, MPH, and Brianna Sinche, MPH. Drafting of manuscript: Goldstein, Brown, Merrick, Tandon, Sinche, and Daniela Robledo, MS. Critical revision of manuscript for important intellectual content: All authors. Statistical analysis: Brown. Obtained funding: Wakschlag.
Conflict of Interest: None declared
Funding: This work was supported by a generous grant from Ann & Robert H Lurie Children’s Hospital and its Stanley Manne Children’s Research Institute (SP0055092) for its Perinatal Origins of Disease Strategic Research Initiative.
Data-Sharing Statement: Data are available upon request. Readers may contact the corresponding author to request underlying data.
References
- 1. Felitti VJ, Anda RF, Nordenberg D, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med. 1998;14(4):245–258. 10.1016/s0749-3797(98)00017-8 [DOI] [PubMed] [Google Scholar]
- 2.Brown H, Krogh-Jespersen S, Tandon D, Graham A, Mackiewicz Seghete K, Wakschlag L. Looking ahead: Pre- and perinatal interventions for maternal distress to prevent neurodevelopmental vulnerability. In: Wazana A, Székely E, Oberlander TF, eds. Prenatal Stress and Child Development. New York City, NY: Springer International Publishing; 2021:595–622. 10.1007/978-3-030-60159-1 [DOI] [Google Scholar]
- 3. Racine N, Devereaux C, Cooke JE, Eirich R, Zhu J, Madigan S. Adverse childhood experiences and maternal anxiety and depression: A meta-analysis. BMC Psychiatry. 2021;21(1):28. 10.1186/s12888-020-03017-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mersky JP, Lee CP. Adverse childhood experiences and poor birth outcomes in a diverse, low-income sample. BMC Pregnancy Childbirth. 2019;19(1):387. 10.1186/s12884-019-2560-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nguyen MW, Heberlein E, Covington-Kolb S, Gerstner AM, Gaspard A, Eichelberger KY. Assessing adverse childhood experiences during pregnancy: Evidence toward a best practice. AJP Rep. 2019;9(1):e54–e59. 10.1055/s-0039-1683407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tran N, Callaway L, Shen S, et al. Screening for adverse childhood experiences in antenatal care settings: A scoping review. Aust N Z J Obstet Gynaecol. 2022;62(5):626–634. 10.1111/ajo.13585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Flanagan T, Alabaster A, McCaw B, Stoller N, Watson C, Young-Wolff KC. Feasibility and acceptability of screening for adverse childhood experiences in prenatal care. J Womens Health (Larchmt). 2018;27(7):903–911. 10.1089/jwh.2017.6649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Penner F, Rutherford HJV. Emotion regulation during pregnancy: A call to action for increased research, screening, and intervention. Arch Womens Ment Health. 2022;25(2):527–531. 10.1007/s00737-022-01204-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tandon SD, Ward EA, Hamil JL, Jimenez C, Carter M. Perinatal depression prevention through home visitation: A cluster randomized trial of mothers and babies 1-on-1. J Behav Med. 2018;41(5):641–652. 10.1007/s10865-018-9934-7 [DOI] [PubMed] [Google Scholar]
- 10. Rudenstine S, Espinosa A, McGee AB, Routhier E. Adverse childhood events, adult distress, and the role of emotion regulation. Traumatology. 2019;25(2):124–132. 10.1037/trm0000176 [DOI] [Google Scholar]
- 11. Goldstein E, Brown RL. Influence of maternal adverse childhood experiences on birth outcomes in American Indian and non-Hispanic White women. MCN Am J Matern Child Nurs. 2023;48(5):258–265. 10.1097/NMC.0000000000000938 [DOI] [PubMed] [Google Scholar]
- 12. Foti TR, Watson C, Adams SR, et al. Associations between adverse childhood experiences (ACEs) and prenatal mental health and substance use. Int J Environ Res Public Health. 2023;20(13):6289. 10.3390/ijerph20136289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Young-Wolff KC, Alabaster A, McCaw B, et al. Adverse childhood experiences and mental and behavioral health conditions during pregnancy: The role of resilience. J Womens Health (Larchmt). 2019;28(4):452–461. 10.1089/jwh.2018.7108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Elhai JD, Dvorak RD, Levine JC, Hall BJ. Problematic smartphone use: A conceptual overview and systematic review of relations with anxiety and depression psychopathology. J Affect Disord. 2017;207:251–259. 10.1016/j.jad.2016.08.030 [DOI] [PubMed] [Google Scholar]
- 15. Madigan S, Wade M, Plamondon A, Maguire JL, Jenkins JM. Maternal adverse childhood experience and infant health: Biomedical and psychosocial risks as intermediary mechanisms. J Pediatr. 2017;187:282–289. 10.1016/j.jpeds.2017.04.052 [DOI] [PubMed] [Google Scholar]
- 16. Bethell C, Gombojav N, Solloway M, Wissow L. Adverse childhood experiences, resilience and mindfulness-based approaches: Common denominator issues for children with emotional, mental, or behavioral problems. Child Adolesc Psychiatr Clin N Am. 2016;25(2):139–156. 10.1016/j.chc.2015.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tandon SD, Perry DF, Mendelson T, Kemp K, Leis JA. Preventing perinatal depression in low-income home visiting clients: A randomized controlled trial. J Consult Clin Psychol. 2011;79(5):707–712. 10.1037/a0024895 [DOI] [PubMed] [Google Scholar]
- 18. Tandon SD, Leis JA, Mendelson T, Perry DF, Kemp K. Six-month outcomes from a randomized controlled trial to prevent perinatal depression in low-income home visiting clients. Matern Child Health J. 2014;18(4):873–881. 10.1007/s10995-013-1313-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Curry SJ, Krist AH, Owens DK, et al. Interventions to prevent perinatal depression: US Preventive Services Task Force recommendation statement. JAMA. 2019;321(6):580–587. 10.1001/jama.2019.0007 [DOI] [PubMed] [Google Scholar]
- 20. Roubinov DS, Epel ES, Coccia M, et al. Long-term effects of a prenatal mindfulness intervention on depressive symptoms in a diverse sample of women. J Consult Clin Psychol. 2022;90(12):942–949. 10.1037/ccp0000776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lorenc T, Lester S, Sutcliffe K, Stansfield C, Thomas J. Interventions to support people exposed to adverse childhood experiences: Systematic review of systematic reviews. BMC Public Health. 2020;20(1):657. 10.1186/s12889-020-08789-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wakschlag LS, Tandon D, Krogh-Jespersen S, et al. Moving the dial on prenatal stress mechanisms of neurodevelopmental vulnerability to mental health problems: A personalized prevention proof of concept. Dev Psychobiol. 2021;63(4):622–640. 10.1002/dev.22057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ng A, Wei B, Jain J, et al. Predicting the next-day perceived and physiological stress of pregnant women by using machine learning and explainability: Algorithm development and validation. JMIR Mhealth Uhealth. 2022;10(8):e33850 10.2196/33850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Muñoz RF, Le H-N, Ippen CG. Prevention of postpartum depression in low-income women: Development of the Mamás y Bebés/Mothers and Babies course (Muñoz et al.). Cognitive and Behavioral Practice. 2007;14(1):70–83. 10.1016/j.cbpra.2006.10.001 [DOI] [Google Scholar]
- 25. Cronholm PF, Forke CM, Wade R, et al. Adverse childhood experiences: Expanding the concept of adversity. Am J Prev Med. 2015;49(3):354–361. 10.1016/j.amepre.2015.02.001 [DOI] [PubMed] [Google Scholar]
- 26. Pilkonis PA, Choi SW, Reise SP, et al. Item banks for measuring emotional distress from the patient-reported outcomes measurement information system (PROMIS®): Depression, anxiety, and anger. Assessment. 2011;18(3):263–283. 10.1177/1073191111411667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rothrock NE, Amtmann D, Cook KF. Development and validation of an interpretive guide for PROMIS scores. J Patient Rep Outcomes. 2020;4(1):16. 10.1186/s41687-020-0181-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- 29.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd Edition. Hillsdale, NJ: Lawrence Erlbaum; 1988. [Google Scholar]
- 30. Catanzaro SJ, Mearns J. Measuring generalized expectancies for negative mood regulation: Initial scale development and implications. J Pers Assess. 1990;54(3–4):546–563. 10.1080/00223891.1990.9674019 [DOI] [PubMed] [Google Scholar]
- 31. Kanter JW, Rusch LC, Busch AM, Sedivy SK. Validation of the Behavioral Activation for Depression Scale (BADS) in a community sample with elevated depressive symptoms. J Psychopathol Behav Assess. 2009;31(1):36–42. 10.1007/s10862-008-9088-y [DOI] [Google Scholar]
- 32.Chakraborty H, Gu H. RTI Press Methods Report Series. A Mixed Model Approach for Intent-to-Treat Analysis in Longitudinal Clinical Trials with Missing Values. Research Triangle Park, NC: RTI Press; 2009. [PubMed] [Google Scholar]
- 33. Chow GC. Tests of equality between sets of coefficients in two linear regressions. Econometrica. 1960;28(3):591. 10.2307/1910133 [DOI] [Google Scholar]
- 34. Yang S, Li F, Thomas LE, Li F. Covariate adjustment in subgroup analyses of randomized clinical trials: A propensity score approach. Clin Trials. 2021;18(5):570–581. 10.1177/17407745211028588 [DOI] [PubMed] [Google Scholar]
- 35. McCaffrey DF, Griffin BA, Almirall D, Slaughter ME, Ramchand R, Burgette LF. A tutorial on propensity score estimation for multiple treatments using generalized boosted models. Stat Med. 2013;32(19):3388–3414. 10.1002/sim.5753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. McCaffrey DF, Ridgeway G, Morral AR. Propensity score estimation with boosted regression for evaluating causal effects in observational studies. Psychol Methods. 2004;9(4):403–425. 10.1037/1082-989X.9.4.403 [DOI] [PubMed] [Google Scholar]
- 37.StataCorp . Stata Statistical Software: Release 17. College Station, TX: StataCorp LLC; 2021. [Google Scholar]
- 38. Cooke JE, Racine N, Pador P, Madigan S. Maternal adverse childhood experiences and child behavior problems: A systematic review. Pediatrics. 2021;148(3):e2020044131. 10.1542/peds.2020-044131 [DOI] [PubMed] [Google Scholar]
- 39. Racine N, Ereyi-Osas W, Killam T, McDonald S, Madigan S. Maternal-child health outcomes from pre- to post-implementation of a trauma-informed care initiative in the prenatal care setting: A retrospective study. Children (Basel). 2021;8(11):1061. 10.3390/children8111061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Madigan S, Wade M, Plamondon A, Maguire JL, Jenkins JM. Maternal adverse childhood experience and infant health: Biomedical and psychosocial risks as intermediary mechanisms. J Pediatr. 2017;187:282–289. 10.1016/j.jpeds.2017.04.052 [DOI] [PubMed] [Google Scholar]
- 41. Racine NM, Madigan SL, Plamondon AR, McDonald SW, Tough SC. Differential associations of adverse childhood experience on maternal health. Am J Prev Med. 2018;54(3):368–375. 10.1016/j.amepre.2017.10.028 [DOI] [PubMed] [Google Scholar]
- 42. Kim HG, Kuendig J, Prasad K, Sexter A. Exposure to racism and other adverse childhood experiences among perinatal women with moderate to severe mental illness. Community Ment Health J. 2020;56(5):867–874. 10.1007/s10597-020-00550-6 [DOI] [PubMed] [Google Scholar]
- 43. Miu AC, Szentágotai-Tătar A, Balázsi R, Nechita D, Bunea I, Pollak SD. Emotion regulation as mediator between childhood adversity and psychopathology: A meta-analysis. Clin Psychol Rev. 2022;93:102141. 10.1016/j.cpr.2022.102141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Johnson S, Kasparian NA, Cullum AS, et al. Addressing adverse childhood and adult experiences during prenatal care. Obstet Gynecol. 2023;141(6):1072–1087. 10.1097/AOG.0000000000005199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Clark CAC, Espy KA, Wakschlag L. Developmental pathways from prenatal tobacco and stress exposure to behavioral disinhibition. Neurotoxicol Teratol. 2016;53:64–74. 10.1016/j.ntt.2015.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Talge NM, Neal C, Glover V, Early Stress, Translational Research and Prevention Science Network: Fetal and Neonatal Experience on Child and Adolescent Mental Health . Antenatal maternal stress and long-term effects on child neurodevelopment: How and why? J Child Psychol Psychiatry. 2007;48(3–4):245–261. 10.1111/j.1469-7610.2006.01714.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shonkoff JP, Fisher PA. Rethinking evidence-based practice and two-generation programs to create the future of early childhood policy. Dev Psychopathol. 2013;25(4 Pt 2):1635–1653. 10.1017/S0954579413000813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Leitch L, Miller-Karas E. A case for using biologically-based mental health intervention in post-earthquake China: Evaluation of training in the trauma resiliency model. Int J Emerg Ment Health. 2009;11(4):221–233. [PubMed] [Google Scholar]
- 49. Grabbe L, Miller-Karas E. The trauma resiliency model: A “bottom-up” intervention for trauma psychotherapy. J Am Psychiatr Nurses Assoc. 2018;24(1):76–84. 10.1177/1078390317745133 [DOI] [PubMed] [Google Scholar]