Abstract
Objectives
Insulin resistance is associated with elevations in plasma branched-chain amino acids (BCAAs). BCAAs compete with aromatic amino acids including tryptophan for uptake into β cells. To explore relationships between BCAAs and tryptophan metabolism, adiposity, and glucose tolerance, we compared urine metabolites in overweight/obese youth with type 2 diabetes (T2D) with those in nondiabetic overweight/obese and lean youth.
Methods
Metabolites were measured in 24-hour and first-morning urine samples of 56 nondiabetic adolescents with overweight/obesity, 42 adolescents with T2D, and 43 lean controls, aged 12 to 21 years. Group differences were assessed by Kruskal Wallis or ANOVA.
Results
Groups were comparable for age, pubertal status, and ethnicity. Youth with T2D were predominantly female and had highest percent body fat. BCAAs, branched-chain ketoacids (BCKAs), tryptophan, and kynurenine were higher in urine of subjects with T2D. There were no differences between lean controls and nondiabetic youth with overweight/obesity. T2D was associated with diversion of tryptophan from the serotonin to the kynurenine pathway, with higher urinary kynurenine/serotonin ratio and lower serotonin/tryptophan and 5-HIAA/kynurenine ratios. Urinary BCAAs, BCKAs, tryptophan, and ratios reflecting diversion to the kynurenine pathway correlated positively with metrics of body fat and hemoglobin A1c. Increases in these metabolites in the obese T2D group were more pronounced and statistically significant only in adolescent girls.
Conclusion
Increases in urinary BCAAs and BCKAs in adolescent females with T2D are accompanied by diversion of tryptophan metabolism from the serotonin to the kynurenine pathway. These adaptations associate with higher risks of T2D in obese adolescent females than adolescent males.
Keywords: BCAAs, BCKAs, tryptophan-kynurenine-serotonin pathway, youth-onset type 2 diabetes mellitus, obesity
Type 2 diabetes (T2D) has a more aggressive course in youth than in adults, with early and rapid deterioration of β-cell function, inadequate responses to oral hypoglycemic agents, rapid progression to insulin dependence, and higher prevalence and earlier presentation of diabetes complications (1). Obesity and insulin resistance (IR) correlate strongly with development of T2D in youth; however, only one-half of obese children are insulin resistant and a far lower percentage progress to T2D (2, 3). This suggests that factors in addition to obesity contribute to progression to overt glucose intolerance. It is essential to identify metabolic markers that can predict the development of IR and progression to T2D; this might enable us to reduce the incidence and prevent the complications of T2D in youth.
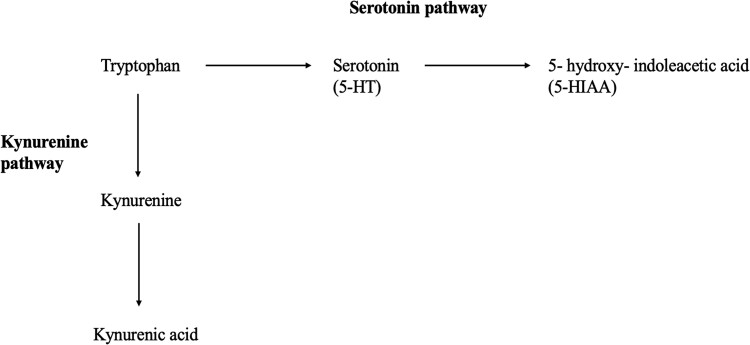
Emerging literature demonstrates that IR and T2D are associated with, and may be predicted by, derangements in amino acid metabolism (4-12). For example, we showed that IR in youth with obesity is associated with a sex-dependent metabolomic profile comprising increases in the fasting plasma concentrations of the branched-chain amino acids (BCAAs) and their catabolic by-products, including branched-chain keto acids (BCKAs), glutamate, and C3/C5 acylcarnitines (4-6). Plasma levels of these metabolites are higher in adolescent boys than girls of comparable body mass index (BMI) z score (4). Likewise, IR in adults is associated with increases in plasma BCAAs and related metabolites (13-20), as well as altered metabolism of tryptophan, an aromatic amino acid and the precursor of the neurotransmitter serotonin (21-24). Serotonin plays an important role in glucose homeostasis by increasing pancreatic β-cell replication, β-cell mass, and glucose-dependent insulin secretion (25-28). Serotonin production is regulated by the activity of tryptophan hydroxylase and limited by the availability of tryptophan (29). In peripheral tissues, tryptophan can be converted to kynurenine as well as serotonin (Fig. 1). In adults with obesity and IR, the metabolism of tryptophan is diverted preferentially to kynurenines, with a concomitant increase in the plasma kynurenine/tryptophan ratio (22-24). Thus, decreased serotonin production in obese, high-risk subjects might explain defects in β-cell function that lead to the development of overt hyperglycemia.
Figure 1.
Tryptophan metabolism via kynurenine and serotonin metabolic routes.
In this study, we analyzed 24-hour urine samples to identify factors associated with T2D among youth with overweight/obesity. In contrast to single point-in-time plasma analyses, urine metabolomic profiling integrates differences in metabolic status over a 24-hour period in a noninvasive manner (30). We hypothesized that: (1) urine excretion of BCAAs and BCKAs is higher in overweight/obese diabetic youth than in normal weight and overweight/obese, nondiabetic youth; (2) overweight/obesity and diabetes in youth are associated with decreases in the urinary ratio of 5-HIAA, the major metabolite of serotonin, to kynurenine; (3) the urinary concentrations of BCAAs and BCKAs and the ratio of 5-HIAA/kynurenine correlate with metrics of body fat and glycemic control; and (4) the urinary metabolites of BCAAs and tryptophan metabolism differ among adolescent boys and girls.
Methods
Participants and Anthropometric Measurements
To test these hypotheses, we recruited 56 adolescents with overweight/obesity without T2D (“obese”), 42 adolescents with overweight/obesity and T2D (“T2D”), and 43 normal weight controls (“lean”). The majority of the participants were recruited from Duke Children's primary care clinics, diabetes clinics at Duke's Lenox Baker Children's Hospital, and the Duke Children's Healthy Lifestyles program. The Healthy Lifestyles program is a comprehensive, interdisciplinary clinic for pediatric obesity treatment. The program uses motivational interviewing, as well as support from registered dieticians and pediatric physical therapists, to create individualized, evidence-based lifestyle modifications to help improve habits and reduce excess weight (5). Three participants with T2D were recruited from the Pediatric Endocrine and Diabetes Clinic at the University of North Carolina in Chapel Hill, NC. In addition, 2 participants with T2D were recruited from Pennington Biomedical Research Center in Baton Rouge, LA.
The participants were between ages 12 and 21 years. Weight was measured to the nearest .1 kg using the same calibrated scales. Normal weight was defined as a BMI between the 5th and 85th percentile, and overweight/obesity was defined as a BMI ≥ 85th percentile (18). Height was measured to the nearest .1 cm using wall-mounted stadiometers. Body composition was measured by a Tanita BC-418 segmental body composition analyzer, and pubertal status evaluated by the participants’ general practitioner including pediatricians, nurse practitioners who received training in pubertal staging, and pediatric endocrinologists per standard of care. Diabetes was defined as hemoglobin A1c (HbA1c) ≥ 6.5%, fasting plasma glucose ≥126 mg/dL, 2-hour glucose ≥200 mg/dL during an oral glucose tolerance test, and/or random plasma glucose ≥200 mg/dL with symptoms of hyperglycemia (31). For diabetes management, participants with T2D were on metformin and/or an insulin regimen (Supplemental Table 1) (32). Additionally, 3 participants with T2D were taking liraglutide, 3 were taking dulaglutide, and 1 was taking sitagliptin. The duration from onset of T2D to date of urine collection was 1.6 ± 1.5 years. No participants were ill or metabolically unstable at the time of urine collection.
Exclusion criteria included use of systemic corticosteroids, antipsychotics, medications for weight loss, topiramate, recent start of oral contraceptives (within the past 3 months), or medroxy-progesterone acetate either currently or within the month before urine collection, as well as use of over-the-counter medications such as acetaminophen or aspirin, either chronically or within the week before urine collection. In addition, those with a genetic syndrome causing obesity, Cushing syndrome, untreated hypothyroidism, persistent hyperprolactinemia, proteinuria, or chronic kidney dysfunction were also excluded.
At least 1 parent/guardian provided informed consent for all children aged younger than 18 years. Informed consent was obtained from the participant if older than age 18 years and assent was obtained for those younger than age 18 years. The protocol was approved by the Duke University Health System institutional review board (IRB), Pediatrics Clinical Research Unit. The other sites utilized IRB reliance on the Duke University Health System IRB for their approvals.
Because serotonin metabolism is highly affected by stress and depression, we extracted data from the Patient Health Questionnaire (PHQ)-2/PHQ-9 Modified for Teens from medical charts and the initial research visits (33). The PHQ-2/PHQ-9 Modified for Teens questionnaire is a reliable and valid method to detect depression and assess severity of depression; thus, all youth receive it as part of standard care for routine annual well-child checks at Duke's primary clinics. Youth who are obese/overweight or have diabetes also receive the same surveys as standard of care at their first visits and annually at Duke's diabetes clinics and the Healthy Lifestyles program.
Urine Collection and Metabolomic Profiling
All participants collected urine for 24 hours in a container, provided by the study team, at home under standardized conditions. The first specimen was discarded, and all subsequent urine was saved, stored, and kept cold, either on ice or in a refrigerator for the next 24 hours. A urine log was kept during collection to record the urine volume and voiding times. Subjects also provided a first morning urine in a urine cup at completion of the 24-hour period to reflect metabolic changes during fasting. The container and the urine cup were returned cold to the recruitment site in an US Food and Drug Administration-compliant thermal bag. The samples were aliquoted, frozen immediately, and stored at −80 °C until processing. All participants taking metformin were asked to stop the drug 24 hours before the start of collection of urine samples. Because of safety concerns, we did not stop insulin and/or liraglutide, dulaglutide, or sitagliptin.
BCAAs (5-1000 µmol/L, < 15%) were analyzed by tandem mass spectrometry (MS/MS) using a Waters TQD instrument, as described previously (34-38). BCKAs including the alpha-keto acids of leucine (α-keto-isocaproate [KIC]), isoleucine (α-keto-β-methylvalerate [KMV]), and valine (α-keto-valerate [KIV]) were analyzed by liquid chromatography MS/MS, as previously described (6, 39, 40). Urine concentrations of tryptophan, kynurenine, and kynurenic acid were measured by liquid chromatography MS/MS as previously described (41, 42). Briefly, 10 µL of urine was diluted 10-fold with water and spiked with the heavy isotope-labeled internal standards tryptophan-d5, kynurenine-d6 (Cambridge Isotope Laboratories) and kynurenic acid-d5 (CDN Isotopes). Metabolites were analyzed on a Waters Acquity UPLC system coupled to a Waters Xevo TQ-S triple quadrupole mass spectrometer (Milford, MA). The analytical column (Waters Acquity UPLC HSS T3 Column, 1.8 µm, 2.1 × 100 mm) equipped with a guard column (Agilent Rapid Resolution cartridge, ZORBAX SB-C8, 3.5 m, 2.1 30 mm) was maintained at 30 °C and the flow rate was set at .3 mL/min. The gradient began with 100% eluent A (.1% formic acid in water) and was then programmed as follows: 0 to 2 minutes 0% eluent B (95:5 acetonitrile-water, .1% formic acid); 2 to 10 minutes gradient to 40% eluent B; 10 to 11 minute gradient to 100% eluent B followed by a 2-minute wash and 2-minute equilibration. Mass transitions of m/z 205 > 146 for tryptophan, 210 > 150 for tryptophan-d5, m/z 209 > 146 for kynurenine, m/z 215 > 151 for kynurenine-d6, m/z 190 > 116 for kynurenic acid, and 195 > 121 for kynurenic acid-d5 were monitored in a positive ion electrospray ionization mode. Metabolite concentrations were computed using Waters TargetLynx Quantitative Analysis and an external calibration constructed from a serial dilution of the tryptophan, kynurenine, and kynurenic acid standards (Sigma, St. Louis, MO) in dialyzed fetal bovine serum.
Quantitative measurements of 5-HIAA were analyzed on a Thermo ARIA TX4 LC system coupled to a SCIEX API5000 mass spectrometer with HPLC/MS-MS. Serotonin in spot and 24-hour urine samples were measured using an immunoassay kit (DLD Diagnostika Cat# EA602/96, RRID:AB_3073757) on a Molecular Devices M2e plate reader (Mountain View, CA). To adjust for variation in dilution effects, sample volume, and rate of urine production, the metabolite levels were normalized to urinary creatinine; the rate of urinary creatinine excretion is fairly constant (43). Concentration values were normalized by creatinine, as measured using reagents from Beckman (Brea, CA) on a DxC 600 clinical analyzer.
HbA1c levels were measured in youth with overweight/obesity and/or T2D at each clinic visit and were also extracted from the participants’ medical charts.
Data Analysis
Chi-square test was used to assess differences in ethnicity, self-reported race, sex, and Tanner stage across 3 groups. Kruskal-Wallis test or ANOVA was used to assess differences across 3 groups for age, BMI%, BMI z-score, body fat percent (BF%), blood pressure, PHQ2/9 scores, HbA1c, metabolites related to BCAA catabolism (BCKAs) and tryptophan-kynurenine pathway. For the same comparisons between 2 groups, t tests or Wilcoxon rank sum tests were performed. We used a conservative P value threshold (P ≤ .01) when interpreting results. To address and explore the influences of sex as a biological variable (44, 45), we performed sex-stratified analyses of all data. Additionally, 2-sample t tests were performed to compare the anthropometric and demographic measurements between males and females within the T2D group. To determine if differences in urinary metabolites among the 3 groups were different between males and females, we calculated interaction P values from linear regression models with group, sex, and group by sex interaction as covariates. Interaction P < .05 was considered statistically significant. To evaluate the association between urinary metabolites of interest with glycemic control, Spearman Rho correlation coefficients were calculated. Metabolites related to BCAAs, BCKAs, and tryptophan-kynurenine-serotonin pathway were also assessed as dependent variables using multivariate linear regression models adjusting for Tanner stage, sex, BMI, age, and self-reported race. Among obese and T2D groups, we also performed analyses in which the models were additionally adjusted for glycemic control because HbA1c data were available. All analyses were performed in R version 4.1.2 (2021-11-01) and SAS v.9.4 (SAS Institute, Inc., Cary, NC, USA).
Results
Anthropometric and Demographic Characteristics
Anthropometric and demographic comparisons for the overall cohort are shown in Table 1, for males only in Table 2, and females only in Table 3 (17, 30). A total of 141 participants were studied, of which 43 (30%) had normal weight, 56 (40%) had overweight/obesity without T2D, and 42 (30%) had obesity with T2D. In the overall cohort, lean, obese, and T2D groups were comparable for age, ethnicity, and pubertal status. Obese youth with and without T2D were predominantly female and Black. Youth with T2D had higher BMI-z, BMI%, and BF% than lean and nondiabetic obese youth. Youth with T2D also had the highest systolic blood pressures (P = 5.49e−10), with comparable diastolic blood pressures across 3 groups. Compared with obese youth without T2D, youth with T2D had higher levels of HbA1c (T2D, 8.38 ± 2.70%; obese, 5.54 ± .37%; P = 2.70e−10). The PHQ-2 and PHQ-9 scores were comparable across groups.
Table 1.
Anthropometric and demographic comparisons across 3 groups in overall cohort
| Lean (n = 43) | Obese without T2D (n = 56) | Obese with T2D (n = 42) | P | |
|---|---|---|---|---|
| Age (y) | 14.93 ± 1.87 | 14.77 ± 1.93 | 15.68 ± 2.09 | .067 |
| Sex (females/males) | 17/26 | 33/23 | 28/14 | .030 |
| Race | AA = 21 (48.8%), Asian = 1 (2.3%), More than 1 = 5 (11.6%), NA = 1 (2.3%), White = 15 (34.9%) | AA = 37 (66.1%), Asian = 1 (1.8%), More than 1 = 2 (3.6%), NA = 8 (14.3%), White = 8 (14.3%) | AA = 31 (73.8%), Asian = 0, More than 1 = 2 (4.8%), NH = 1 (2.4%), NA = 3 (7.1%), White = 5 (11.9%) | .030 |
| Ethnicity | Hispanic/Latino = 2, Not Hispanic/Latino = 40, NA = 1 | Hispanic/Latino = 11, Not Hispanic/Latino = 43, NA = 2 | Hispanic/Latino = 6, Not Hispanic/Latino = 36, NA = 0 | .18 |
| Tanner staging (early/mid, late) |
5/35 (NA = 3) | 12/44 | 3/35 (NA = 4) | .171 |
| BMI-z | .06 ± .63 | 2 ± .49 | 2.36 ± .41 | 4.22e-45 |
| BMI% | 52.41 ± 21.92 | 96.37 ± 3.68 | 98.51 ± 1.91 | 5.89e-40 |
| BF% | 20.11 ± 6.28 | 37.25 ± 9.5 | 42.9 ± 9.87 | 2.58e-22 |
| Systolic blood pressure | 111.65 ± 9.13 | 115.36 ± 11.54 | 127.83 ± 12.08 | 5.49e-10 |
| Diastolic blood pressure | 67.91 ± 7.54 | 69.11 ± 8.98 | 71.51 ± 10.53 | .182 |
| PHQ2 score | .3 ± .62 | .54 ± 1.12 | 1.06 ± 1.56 | .053 |
| PHQ9 score | 1.09 ± 3.04 | 3.55 ± 5.12 | 4.53 ± 6.19 | .064 |
| HbA1c | — | 5.54 ± .37 | 8.38 ± 2.70 | <.0001 |
Abbreviations: AA, African American; BF%, percent body fat; BMI, body mass index; HbA1c, hemoglobin A1c; NA, not answered/not available; NH, Native Hawaiian/Pacific Islander; PHQ, Patient Health Questionnaire; T2D, type 2 diabetes.
Table 2.
Anthropometric and demographic comparisons across 3 groups in males
| Lean (n = 26) | Obese without T2D (n = 23) | Obese with T2D (n = 14) | P | |
|---|---|---|---|---|
| Age (y) | 15.44+/−1.79 | 14.53+/−1.55 | 15.68+/−1.61 | .076 |
| Race | Black = 13, Asian = 1, More than 1 = 1, NA = 0, White = 11 | Black = 17, Asian = 1, More than 1 = 1, NA = 1, White = 3 | Black = 11, Asian = 0, More than 1 = 0, NA = 1, White = 2 | .314 |
| Ethnicity | Hispanic/Latino = 0 Not Hispanic/Latino = 26 | Hispanic/Latino = 2 Not Hispanic/Latino = 21 | Hispanic/Latino = 0 Not Hispanic/Latino = 14 | .166 |
| Tanner staging (early/mid, late) |
Early/mid = 2 Late = 21 (NA = 3) | Early/mid = 7, Late = 16 (NA = 0) | Early = 3, Late = 10 (NA = 0) | .18 |
| BMI-z | .3 ± .67 | 1.98 ± .54 | 2.45 ± .51 | 1.33e-10 |
| BMI% | 51.51 ± 23.71 | 96.53 ± 3.46 | 98.32 ± 2.77 | 7.8e-16 |
| BF% | 16.04 ± 3.37 | 32.08 ± 8.22 | 36.11 ± 10.32 | 4.4e-12 |
| Systolic BP | 115.08 ± 7.9 | 115.65 ± 11.62 | 132.14 ± 9.86 | 2.8e-6 |
| Diastolic BP | 68.5 ± 8 | 67.91 ± 8.3 | 70.93 ± 8.07 | .53 |
| PHQ2 score | .17 ± .58 | .11 ± .32 | 1.17 ± 1.17 | .002 |
| PHQ9 score | .29 ± .73 | 1.28 ± 1.81 | 4.67 ± 7.37 | .022 |
| HbA1c | — | 5.69 ± .36 | 8.59 ± 3.76 | .050 |
Abbreviations: BF%, percent body fat; BMI, body mass index; BP, blood pressure; NA, not answered; NH, Native Hawaiian/Pacific Islander; PHQ, Patient Health Questionnaire: T2D, type 2 diabetes.
Table 3.
Anthropometric and demographic comparisons across 3 groups in females
| Lean (n = 17) | Obese without T2D (n = 33) |
Obese with T2D (n = 28) |
P | |
|---|---|---|---|---|
| Age, y | 14.16 ± 1.76 | 14.93 ± 2.16 | 15.67 ± 2.32 | .074 |
| Race | Black = 8, More than 1 = 4, NA = 1, White = 4 | Black = 20, More than 1 = 1, NA = 7, White = 5 | Black = 20, More than 1 = 2, NH = 1, NA = 2, White = 3 | .130 |
| Ethnicity | Hispanic/Latino = 2, not Hispanic/Latino = 14, NA = 1 | Hispanic/Latino = 9, not Hispanic/Latino = 22, NA = 2 | Hispanic/Latino = 6, not Hispanic/Latino = 22, NA = 0 | .494 |
| Tanner staging (early/mid, late) |
early/mid = 3, Late = 14 (NA = 0) | Early/mid = 5, Late = 28 (NA = 0) | Early/mid = 0, late =25 (NA = 3) | = 103 |
| BMI-z | .11+/−.57 | 2.05+/−.46 | 2.3+/−.35 | 2.83e-10 |
| BMI% | 53.73+/−19.62 | 96.26+/−3.88 | 98.61+/−1.27 | 2.83e-10 |
| BF% | 25.86+/−4.71 | 40.86+/−8.72 | 46.58+/−7.52 | 5e-12 |
| Systolic BP | 106.41+/−8.55 | 115.15+/−11.66 | 125.59+/−12.67 | 2.7e-6 |
| Diastolic BP | 67+/−6.91 | 69.94+/−9.46 | 71.81+/−11.73 | .3 |
| PHQ2 score | .5 +/− .65 | .9 +/− 1.41 | 1 +/− 1.79 | .59 |
| PHQ9 score | 2.33 +/− 4.66 | 5.12 +/− 6.05 | 4.45 +/− 5.84 | .47 |
| HbA1c | — | 5.45 +/− .35 | 8.28 +/− 2.06 | <.0001 |
Abbreviations: BF%, percent body fat; BMI, body mass index; BP, blood pressure; NA, not answered; NH, Native Hawaiian/Pacific Islander; PHQ, Patient Health Questionnaire: T2D, type 2 diabetes.
Urinary Metabolites of BCAA Metabolism and Tryptophan-kynurenine-serotonin Pathway
Metabolites related to BCAA catabolism and the tryptophan-kynurenine-serotonin pathways in the overall cohort are shown in Table 4 and Supplemental Table 2 (32).
Table 4.
Metabolites related to BCAA metabolism and tryptophan-kynurenine- serotonin- pathways in 24-hour urine samples across 3 groups in overall cohort, normalized for urine Cr (mmol/mol Cr)
| Lean (n = 43) | Obese without T2D (n = 56) | Obese with T2D (n = 42) | P, unadjusted | P, adjusteda | |
|---|---|---|---|---|---|
| BCAAs mmol/mol Cr | 9.82 ± 4.38 | 9.47 ± 3.83 | 14.07 ± 6.9 | .0005 | <.0001 |
| BCKAs mmol/mol Cr | 1.97 ± .94 | 1.92 ± 1.05 | 4.39 ± 4.05 | .0002054 | <.0001 |
| KIV mmol/mol Cr | .32 ± .15 | .35 ± .21 | .96 ± .95 | .000004572 | <.0001 |
| KMV mmol/mol Cr | 1.32 ± .69 | 1.22 ± .68 | 2.56 ± 2.15 | .0008751 | <.0001 |
| KIC mmol/mol Cr | .33 ± .13 | .35 ± .25 | .88 ± 1.08 | .00005518 | .0018 |
| Tryptophan mmol/mol Cr | 6.63 ± 3.36 | 6.53 ± 3.12 | 8.58 ± 3.48 | .004464 | .0037 |
| Kynurenine mmol/mol Cr | .32 ± .21 | .28 ± .17 | .52 ± .34 | .00008695 | <.0001 |
| Kynurenic acid mmol/mol Cr | 1.08 ± .44 | 1.13 ± .48 | 1.08 ± .46 | .794 | .9599 |
| Serotonin nmol/mmol Cr | 78.06 ± 30.63 | 81.61 ± 33.04 | 78.9 ± 25.26 | .8843 | .9714 |
| 5-HIAA mg/g Cr | 2.94 ± 1.45 | 2.75 ± 1.35 | 3.41 ± 3.55 | .5353 | .0244 |
| Kynurenine/tryptophan | .05 ± .02 | .04 ± .02 | .06 ± .03 | .0112 | .0139 |
| 5-HIAA/tryptophan | .51 ± .22 | .48 ± .19 | .44 ± .36 | .0272 | .7559 |
| 5-HIAA/kynurenine | 14.04 ± 12.84 | 13.03 ± 7.75 | 9.59 ± 9.24 | .0067 | .6504 |
| Kynurenine/serotonin | .0043 ± .0029 | .0035 ± .0018 | .0070 ± .0046 | <.0001 | .0001 |
| Serotonin/tryptophan | 13.13 ± 4.87 | 13.53 ± 4.71 | 10.84 ± 5.11 | .0036 | .0649 |
Abbreviations: BCAA, branched-chain amino acid; BCKA, branched-chain ketoacid; Cr, creatinine; KIC, α-keto-isocaproate; KIV, α-keto-valerate; KMV, α-keto-β-methylvalerate; T2D, type 2 diabetes.
a Model adjusted for Tanner stage, sex, body mass index, age, self-reported race.
BCAA and Their Catabolic Byproducts
In 24-hour urine samples, total BCAAs, BCKAs, KIV, KMV, and KIC were markedly increased in the T2D group (unadjusted 3-group comparison: P = .0005, P = .0002054, P = .000004572, P = .0008751, and P = .00005518, respectively) (Table 4). In pairwise group comparisons, the 24-hour urinary levels of BCAAs, BCKAs, KIV, KMV, and KIC were significantly higher in subjects with obesity and T2D than in subjects with obesity without T2D (P = .0004, P = .0002, P < .0001, P = .0004, and P < .0001, respectively) as well as lean subjects (P = .0009, P = .0008, P < .0001, P = .0039, and P = .0002, respectively). On the other hand, there were no significant differences between the obese and lean groups (P = .8495, P = .7781, P = .5916, P = .5394, and P = .5863, respectively; Supplemental Table 3) (32). The findings were similar in first morning urine samples: total BCAAs, BCKAs, KIV, and KIC were elevated in the T2D group (unadjusted 3-group comparisons P = .0045, P = .00151, P = .000000438, and P = .0000744, respectively) (Supplemental Table 2 and Supplemental Table 4)(32), whereas BCAA and BCKA levels were comparable between the lean group and obese without T2D group (Supplemental Table 4) (32).
When models were adjusted for multiple variables including Tanner stage, sex, BMI, age, and race, the levels of BCAAs (P < .0001), BCKAs (P < .0001), KIV (P < .0001), KMV (P < .0001), and KIC (P = .0018) in 24-hour urine samples were still significantly different across the 3 groups. Results from the adjusted models showed that the differences were driven by higher levels in the obese with T2D group compared with the obese without T2D group (least square mean differences between obese with and without T2D: BCAA 4.97, P < .0001; BCKA 2.47, P < .0001; KIV .58, P < .0001; KMV 1.37, P < .0001; KIC .52, P = .0004). Because HbA1c levels were available only among obese and T2D subjects, we also adjusted the models for HbA1c in addition to Tanner stage, sex, BMI, age, and race. After adjustment for these variables, the major determinant of BCKAs, KIV, KMV, and KIC was HbA1c (P = .0013, P = .0004, P = .0056, and P = .0009, respectively), with higher HbA1c levels associated with higher BCKAs, KIV, KMV, and KIC. The BCAA levels were significantly higher in subjects in the obese with T2D group compared with those in the obese without T2D group (P = .0056), whereas HbA1c approached nominal significance (P = .0684). After adjustment for HbA1c, levels of BCKA, KIV, KMV, and KIC were not significantly different between obese with and without T2D (P = .18, P = .23, P = .11, P = .54, respectively).
Tryptophan, Kynurenine, Serotonin, and 5-HIAA
In 24-hour urine samples, urinary concentrations of kynurenine increased (P = .00008695) and the ratio of kynurenine/serotonin was higher in the obese with T2D group than in the lean and obese without T2D groups (P < .0001). The ratio of kynurenine/tryptophan also trended higher in the T2D group (P = .0012). In contrast, the ratios of serotonin/tryptophan (P = .0036) and 5-HIAA/kynurenine (P = .0067) were lower in the obese with T2D group (Table 4). In pairwise group comparisons, the 24-hour urinary concentrations of tryptophan and kynurenine and the ratios of kynurenine/tryptophan and kynurenine/serotonin were significantly higher in subjects in the obese with T2D group than in subjects in the obesity without T2D group (P = .0027, P < .0001, and P = .0033, respectively). Likewise, the urinary levels of tryptophan and kynurenine and the ratio of kynurenine/serotonin were significantly higher in the obese with T2D group than the lean group (P = .0068, P = .0021, and P = .0021, respectively), but there were no differences between the obese and lean groups (P = .8560, P = .4110, P = .2713, and P = .3246, respectively) (Supplemental Table 3) (32). Analysis of first morning urine yielded similar results: the ratios of 5-HIAA/tryptophan (P = .001) and 5-HIAA/kynurenine (P = .0036) in spot morning urines were lowest in T2D (Supplemental Table 2 and Supplemental Table 4)(32). All tryptophan, kynurenine, and serotonin metabolites and calculated ratios were comparable between the lean group and obese without T2D group (Table 4 and Supplemental Table 2) (32).
When models were adjusted for multiple variables including Tanner stage, sex, BMI, age, and race, the levels of tryptophan (P = .0037), kynurenine (P < .0001), and kynurenine/serotonin ratio (P = .0001) in 24-hour urine samples remained significantly different across 3 groups. Again, these differences were driven by differences associated with T2D; the obese with T2D group had higher levels of kynurenine, tryptophan, and kynurenine/serotonin ratio than the obese group without T2D (least square mean differences between obese with and without T2D: kynurenine .25, P < .0001; tryptophan 2.34, P = .0011; kynurenine/serotonin ratio .0033, P < .0001). Because HbA1c levels were available only among obese and T2D subjects, we also adjusted the models for HbA1c in addition to Tanner stage, sex, BMI, age, and race. After adjustment for all those variables, the levels of kynurenine (P = .0008) and the ratios of kynurenine/tryptophan (P = .0100) and kynurenine/serotonin (P = .0093) remained higher among the T2D group compared with the obese group. Tryptophan levels also trended higher (P = .0222) in the T2D group. These findings were similar to those for the BCAAs but differed from those for the BCKAs in that HbA1c was not a primary driver of the differences in tryptophan metabolism.
Associations Between BCAA, BCAA Catabolic Byproducts, Tryptophan and its Byproducts, and Metrics of Body Fat and Glycemic Control
Bivariate Associations Between BCAA Metabolism, Tryptophan-Kynurenine-Serotonin Pathway, and Metrics of Body Fat
In 24-hour urine samples, the concentrations of BCAAs (r = .294, P = .0009), total BCKAs (r = .276, P = .0016), KIV (r = .315, P = .0003), KMV (r = .251, P = .0043), KIC (r = .259, P = .0031), tryptophan (r = .248, P = .0047), and kynurenine (r = .126, P = .0143) correlated positively with BF%. The ratios of 5-HIAA/tryptophan, 5-HIAA/kynurenine, and serotonin/tryptophan correlated negatively with BMI (r = −.321, P = .0002; r = −.245, P = .0044; and r = −.229, P = .0089, respectively) and BF% (r = −.272, P = .0019; r = −.188, P = .0340; and r = −.237, P = .0081, respectively) (Table 5).
Table 5.
Spearman correlations in 24-hour urine samples in the overall cohort
| Metabolite | HbA1c | BMI | BMI percent | BMI-z score | BF% |
|---|---|---|---|---|---|
| BCAAs mmol/mol Cr, 24 h | .389, P = .0006 | .179, P = .0418 | .192, P = .0296 | .19, P = .0308 | .294, P = .0009 |
| BCKAs mmol/mol Cr, 24 h | .401, P = .0002 | .136, P = .1163 | .128, P = .1438 | .128, P = .1444 | .276, P = .0016 |
| KIV mmol/mol Cr, 24 h | .457, P < .0001 | .222, P = .0096 | .208, P = .0173 | .208, P = .0174 | .315, P = .0003 |
| KMV mmol/mol Cr, 24 h | .387, P = .0004 | .114, P = .1871 | .108, P = .2174 | .108, P = .2178 | .251, P = .0043 |
| KIC mmol/mol Cr, 24 h | .439, P < .0001 | .124, P = .1504 | .103, P = .2424 | .102, P = .2466 | .259, P = .0031 |
| Tryptophan mmol/mol Cr, 24 h | .381, P = .0004 | .179, P = .0374 | .196, P = .0248 | .196, P = .0251 | .248, P = .0047 |
| Kynurenine mmol/mol Cr, 24 h | .377, P = .0005 | .202, P = .0191 | .215, P = .0138 | .214, P = .0140 | .216, P = .0143 |
| Kynurenic acid mmol/mol Cr, 24 h | −.259, P = .0195 | −.005, P = .9580 | .017, P = .8467 | .016, P = .8568 | .079, P = .3757 |
| 5 HIAA mg/g Cr, 24 h | .005, P = .9662 | −.161, P = .0635 | −.152, P = .0823 | −.152, P = .0828 | .032, P = .7177 |
| Serotonin nmol/mmol Cr, 24 h | −.014, P = .9036 | −.011, P = .9019 | .03, P = .7359 | .03, P = .7353 | .083, P = .3584 |
| Kynurenine/tryptophan, 24 h | .196, P = .0795 | .135, P = .1191 | .123, P = .1607 | .123, P = .1601 | .084, P = .3464 |
| 5-HIAA/tryptophan, 24 h | −.406, P = .0002 | −.321, P = .0002 | −.327, P = .0001 | −.327, P = .0001 | −.272, P = .0019 |
| 5-HIAA/kynurenine, 24 h | −.391, P = .0003 | −.245, P = .0044 | −.251, P = .0039 | −.251, P = .0039 | −.188, P = .0340 |
| Kynurenine/serotonin, 24 h | .443, P < .0001 | .212, P = .0157 | .199, P = .0240 | .199, P = .0243 | .193, P = .0319 |
| Serotonin/tryptophan, 24 h | −.423, P = .0001 | −.229, P = .0089 | −.206, P = .0198 | −.205, P = .0203 | −.237, P = .0081 |
Abbreviations: BCAA, branched-chain amino acid; BCKA, branched-chain ketoacid; BF%, percent body fat; BMI, body mass index; Cr, creatinine; HbA1c, hemoglobin A1c; KIC, α-keto-isocaproate; KIV, α-keto-valerate; KMV, α-keto-β-methylvalerate.
In first morning urine samples, the concentrations of BCAAs, total BCKAs, KIV, KMV, and KIC correlated positively with BMI-related metrics, with BF% being the strongest association. Similarly, tryptophan and kynurenine correlated positively with BMI-related metrics including BF%, whereas the ratios of 5-HIAA/tryptophan and 5-HIAA/kynurenine associated negatively with BMI-related metrics and BF% (Supplemental Table 5) (32). When data were stratified by sex, these correlations were driven primarily by females (Table 6, Supplemental Table 6, Table 7, and Supplemental Table7)(32).
Table 6.
Spearman correlations in 24-hour urine samples in males
| Metabolite | HbA1c | BMI | BMI percent | BMI-z score | BF% |
|---|---|---|---|---|---|
| BCAAs mmol/mol Cr, 24 h | .265, P = .1736 | .149, P = .2517 | .234, P = .0721 | .234, P = .0713 | .231, P = .0779 |
| BCKAs mmol/mol Cr, 24 h | .281, P = .1326 | .067, P = .6040 | .144, P = .2688 | .144, P = .2679 | .142, P = .2839 |
| KIV mmol/mol Cr, 24 h | .312, P = .0928 | .148, P = .2508 | .219, P = .0896 | .219, P = .0894 | .204, P = .1212 |
| KMV mmol/mol Cr, 24 h | .317, P = .0878 | .047, P = .7187 | .123, P = .3465 | .123, P = .3444 | .123, P = .3536 |
| KIC mmol/mol Cr, 24 h | .322, P = .0832 | .046, P = .7254 | .102, P = .4363 | .101, P = .4379 | .1, P = .4495 |
| Tryptophan mmol/mol Cr, 24 h | .37, P = .0440 | .176, P = .1707 | .242, P = .0606 | .243, P = .0588 | .285, P = .0289 |
| Kynurenine mmol/mol Cr, 24 h | .328, P = .0768 | .063, P = .6259 | .129, P = .3225 | .13, P = .3167 | .07, P = .5958 |
| Kynurenic acid mmol/mol Cr, 24 h | −.242, P = .1968 | −.064, P = .6196 | .013, P = .9238 | .011, P = .9313 | .021, P = .8766 |
| 5-HIAA mg/g Cr, 24 h | −.341, P = .0654 | −.248, P = .0523 | −.177, P = .1725 | −.177, P = .1731 | −.037, P = .7810 |
| Serotonin nmol/mmol Cr, 24 h | −.207, P = .2824 | −.002, P = .9906 | .066, P = .6212 | .066, P = .6198 | .063, P = .6407 |
| Kynurenine/tryptophan, 24 h | .236, P = .2089 | −.055, P = .6707 | −.029, P = .8233 | −.028, P = .8304 | −.136, P = .3046 |
| 5-HIAA/tryptophan, 24 h | −.567, P = .0011 | −.315, P = .0126 | −.333, P = .0087 | −.334, P = .0085 | −.285, P = .0284 |
| 5-HIAA/kynurenine, 24 h | −.484, P = .0067 | −.1, P = .4412 | −.133, P = .3053 | −.135, P = .3011 | −.034, P = .7980 |
| Kynurenine/serotonin, 24 h | .404, P = .0297 | .064, P = .6261 | .086, P = .5166 | .088, P = .5092 | .025, P = .8538 |
| Serotonin/tryptophan, 24 h | −.489, P = .0071 | −.197, P = .1304 | −.185, P = .1609 | −.186, P = .1584 | −.231, P = .0839 |
Abbreviations: BCAA, branched-chain amino acid; BCKA, branched-chain ketoacid; BF%, percent body fat; BMI, body mass index; Cr, creatinine; HbA1c, hemoglobin A1c; KIC, α-keto-isocaproate; KIV, α-keto-valerate; KMV, α-keto-β-methylvalerate.
Table 7.
Spearman correlations in 24-hour urine samples in females
| Metabolite | HbA1c | BMI | BMI percent | BMI-z score | BF% |
|---|---|---|---|---|---|
| BCAAs mmol/mol Cr, 24 h | .456, P = .0013 | .09, P = .4610 | .083, P = .4985 | .078, P = .5259 | .049, P = .6918 |
| BCKAs mmol/mol Cr, 24 h | .468, P = .0005 | .105, P = .3757 | .086, P = .4772 | .085, P = .4867 | .124, P = .3104 |
| KIV mmol/mol Cr, 24 h | .544, P < .0001 | .213, P = .0704 | .188, P = .1189 | .187, P = .1218 | .2, P = .1002 |
| KMV mmol/mol Cr, 24 h | .438, P = .0013 | .087, P = .4636 | .07, P = .5634 | .068, P = .5749 | .108, P = .3771 |
| KIC mmol/mol Cr, 24 h | .52, P < .0001 | .111, P = .3482 | .082, P = .5018 | .079, P = .5135 | .127, P = .2995 |
| Tryptophan mmol/mol Cr, 24 h | .418, P = .0023 | .077, P = .5184 | .108, P = .3737 | .105, P = .3876 | .035, P = .7737 |
| Kynurenine mmol/mol Cr, 24 h | .389, P = .0048 | .217, P = .0647 | .229, P = .0565 | .226, P = .0605 | .155, P = .2048 |
| Kynurenic acid mmol/mol Cr, 24 h | −.26, P = .0650 | −.057, P = .6328 | −.074, P = .5450 | −.078, P = .5236 | −.086, P = .4817 |
| 5-HIAA mg/g Cr, 24 h | .168, P = .2433 | −.24, P = .0426 | −.241, P = .0449 | −.242, P = .0437 | −.289, P = .0162 |
| Serotonin nmol/mmol Cr, 24 h | .139, P = .3518 | −.044, P = .7187 | −.006, P = .9605 | −.007, P = .9541 | −.043, P = .7311 |
| Kynurenine/tryptophan, 24 h | .166, P = .2453 | .248, P = .0344 | .236, P = .0497 | .234, P = .0509 | .193, P = .1117 |
| 5-HIAA/tryptophan, 24 h | −.336, P = .0172 | −.29, P = .0134 | −.302, P = .0110 | −.301, P = .0114 | −.264, P = .0281 |
| 5-HIAA/kynurenine, 24 h | −.363, P = .0096 | −.33, P = .0047 | −.345, P = .0034 | −.344, P = .0036 | −.293, P = .0145 |
| Kynurenine/serotonin, 24 h | .498, P = .0004 | .253, P = .0362 | .242, P = .0449 | .239, P = .0481 | .213, P = .0828 |
| Serotonin/tryptophan, 24 h | −.413, P = .0039 | −.189, P = .1199 | −.16, P = .1881 | −.156, P = .1995 | −.171, P = .1670 |
Abbreviations: BCAA, branched-chain amino acid; BCKA, branched-chain ketoacid; BF%, percent body fat; BMI, body mass index; Cr, creatinine; HbA1c, hemoglobin A1c; KIC, α-keto-isocaproate; KIV, α-keto-valerate; KMV, α-keto-β-methylvalerate.
Bivariate Associations Between BCAA Metabolism, Tryptophan-Kynurenine-Serotonin Pathway, and HbA1c
In the 24-hour urine samples of the overall cohort, the concentrations of BCAAs, total BCKAs, KIV, KMV, and KIC correlated positively with HbA1c (r = .389, P = .0006; r = .401, P = .0002; r = .457, P < .0001; r = .387, P = .0004; and r = .439, P < .0001, respectively; Table 5). Likewise, the concentrations of tryptophan and kynurenine correlated positively with HbA1c (r = .381, P = .0004; r = .377, P = .0005, respectively). The ratios of serotonin/tryptophan (r = −.423, P = .0001), 5-HIAA/tryptophan (r = −.406, P = .0002), and 5-HIAA/kynurenine (r = −.391, P = .0003) correlated negatively with hemoglobin A1C, whereas the ratio of kynurenine/serotonin (r = .443, P < .0001) correlated positively with HbA1c (Table 5).
Similarly, in the spot morning urine samples of the overall cohort, BCAAs (r = .462, P = .0001), total BCKAs (r = .458, P < .0001), KIV (r = .578, P < .0001), KMV (r = .369, P = .0021), KIC (r = .561, P < .0001), and tryptophan (r = .409, P = .0006) correlated positively with HbA1c, whereas the ratios of 5-HIAA/tryptophan and 5-HIAA/kynurenine correlated negatively with HbA1c (r = −.423, P = .0004; r = −.331, P = .0063, respectively) (Supplemental Table 5)(32). When data were stratified by sex, these correlations were driven primarily by females (Table 6, Supplemental Table 6, Table 6, and Supplemental Table 7)(32).
Correlations Between Urinary Metabolites of BCAAs and BCKAs and the Byproducts of Tryptophan Metabolism
In 24-hour urine samples, the concentrations of tryptophan, kynurenine, and kynurenic acid correlated positively with both BCAAs (r = .806, P < .0001; r = .649, P < .0001; r = .505, P < .0001, respectively) and BCKAs (r = .59, P < .0001; r = .596, P < .0001; r = .216, P = .01, respectively; Table 8). 5-HIAA and serotonin also correlated positively with BCAAs (r = .526, P < .0001 and r = .522, P < .0001) and BCKAs (r = .313, P = .0002 and r = .337, P < .0001). However, the ratios of 5-HIAA/tryptophan, 5-HIAA/kynurenine, and serotonin/tryptophan all correlated negatively with both BCAAs (r = −.426, P < .0001; r = −.361, P < .0001; and r = −.501, P < .0001) and BCKAs (r = −.338, P < .0001; r = −.408, P < .0001; r = −.438, P < .0001). Conversely, the ratio of kynurenine/serotonin correlated positively with BCAAs (.402, P < .0001) and BCKAs (r = .495, P < .0001) (Table 8).
Table 8.
Correlations between BCKAs, BCAAs, and tryptophan, kynurenine, kynurenic acid, 5-HIAA, serotonin, and the ratios in 24-hour urine samples
| Variable | BCKAs (mmol/mol Cr), 24 h | BCAAs (mmol/mol Cr), 24 h |
|---|---|---|
| Tryptophan mmol/mol Cr, 24 h | .59, P < .0001 | .806, P < .0001 |
| Kynurenine mmol/mol Cr, 24 h | .596, P < .0001 | .649, P < .0001 |
| Kynurenic acid mmol/mol Cr, 24 h | .216, P = .0119 | .505, P < .0001 |
| 5-HIAA mg/g Cr, 24 h | .313, P = .0002 | .526, P < .0001 |
| Serotonin nmol/mmol Cr, 24 h | .337, P < .0001 | .522, P < .0001 |
| Kynurenine/tryptophan, 24 h | .277, P = .0012 | .132, P = .1370 |
| 5-HIAA/tryptophan, 24 h | −.338, P < .0001 | −.426, P < .0001 |
| 5-HIAA/kynurenine, 24 h | −.408, P < .0001 | −.361, P < .0001 |
| Kynurenine/serotonin, 24 h | .495, P < .0001 | .402, P < .0001 |
| Serotonin/tryptophan, 24 h | −.438, P < .0001 | −.501, P < .0001 |
Abbreviations: BCAA, branched-chain amino acid; BCKA, branched-chain ketoacid; Cr, creatinine.
In first morning urine samples, tryptophan correlated positively with both BCAAs (r = .773, P < .0001) and BCKAs (r = .559, P < .0001). Kynurenine also correlated positively with BCAAs (r = .52, P < .0001) and BCKAs (r = .527, P < .0001). Kynurenic acid and 5-HIAA both correlated positively with BCAAs (r = .372, P < .0001 and r = .353, P = .0001, respectively). The kynurenine/serotonin ratio correlated positively with BCAAs (r = .343, P = .0002) and BCKAs (r = .443, P < .0001). In contrast, the ratios of 5-HIAA/tryptophan, 5-HIAA/kynurenine, and serotonin/tryptophan correlated negatively with BCAAs (r = −.442, P < .0001; r = −.337, P = .0003; and r = −.541, P < .0001, respectively) and BCKAs (r = −.352, P = .0001; r = −.337, P = .0002; and r = −.44, P < .0001) (Supplemental Table 8) (32).
Also of note, correlations of all metabolites in spot and 24-hour urine samples were positive and statistically different from zero (Table 9).
Table 9.
Correlations between spot and 24-hour urine metabolites
| Metabolite | Correlation | P |
|---|---|---|
| KIV mmol/mol Cr | .792 | <.0001 |
| KMV mmol/mol Cr | .733 | <.0001 |
| KIC mmol/mol Cr | .727 | <.0001 |
| BCKAs mmol/mol Cr | .735 | <.0001 |
| BCAAs mmol/mol Cr | .687 | <.0001 |
| Tryptophan mmol/mol Cr | .786 | <.0001 |
| Kynurenine mmol/mol Cr | .791 | <.0001 |
| Kynurenic acid mmol/mol Cr | .675 | <.0001 |
| 5-HIAA mg/g Cr | .575 | <.0001 |
| Serotonin nmol/mmol Cr | .597 | <.0001 |
Abbreviations: BCAA, branched-chain amino acid; BCKA, branched-chain ketoacid; Cr, creatinine; KIC, α-keto-isocaproate; KIV, α-keto-valerate; KMV, α-keto-β-methylvalerate.
Sex Differences in Urinary Metabolites of the BCAA Metabolism and Tryptophan-Kynurenine-Serotonin Pathway
We stratified the data to identify sex differences in the urinary BCAA and tryptophan metabolites. In males alone, there were no significant differences among groups in concentrations of BCAAs, BCKAs, or any of the metabolites related to the serotonin-tryptophan pathway (Table 10 and Supplemental Table 9) (32). Rather, the differences observed in the overall cohort were driven by females, as seen in Table 11 and Supplemental Table 10 (32). In 24-hour urine samples of females, the levels of BCAAs, BCKAs, KIV, KMV, KIC, tryptophan, and kynurenine were higher in the T2D group (3-group comparison: P = .0027, P = .00019, P = 6.4e-06, P = .00062, P = .00011, P = .011, and P = .00066). The ratio of kynurenine/serotonin (P < .0001) was higher in T2D, whereas the ratios of serotonin/tryptophan and 5-HIAA/kynurenine were lower (P = .013 and P = .0084, respectively), suggesting a diversion of tryptophan metabolism toward production of kynurenine rather than serotonin (Table 11). In pairwise group comparisons, the 24-hour levels of BCAAs, BCKAs, KIV, KMV, KIC, tryptophan, and kynurenine were significantly higher in the obese with T2D females than in females with obesity without T2D (P = .0008, P < .0001, P < .0001, P = .0002, P < .0001, P = .0020, and P = .0001 respectively; Supplemental Table 11)(32). The ratio of 24-hour kynurenine/serotonin was also significantly higher in the obese females with T2D (P < .0001, Supplemental Table 11)(32). Similarly, in spot morning urine samples of females, the concentrations of total BCKAs, KIV, and KIC were all significantly higher in obese females with T2D group compared with the lean and obese without T2D groups (3-group comparison P = .0086, P = 8.3e−06, and P = .00052, respectively). Conversely, 5-HIAA levels were lower (P = .0038) in obese females with T2D, with concomitant decreases in the ratios of 5-HIAA/kynurenine (P = .0003) and 5-HIAA/tryptophan (P = .0016) (Supplemental Table 10 and Supplemental Table 12 for pairwise group comparisons among females in spot urine samples)(32). Because 3-way comparisons were not different among males, we did not conduct pairwise group comparisons. In 24-hour urine samples, the differences among the 3 groups in total BCKAs, KIV, KMV, KIC, and kynurenine/tryptophan ratio were statistically different between males and females (Table 12, sex by group interaction P = .02, P = .012, P = .037, P = .028, and P = .028, respectively). In spot morning urine samples, only the differences among the 3 groups in kynurenine/tryptophan ratio were statistically different between males and females (Supplemental Table 13, sex by group interaction P = .031) (32).
Table 10.
Metabolites related to BCAA metabolism and tryptophan-kynurenine-serotonin pathways in 24-hour urine samples across 3 groups in males, normalized for urine Cr (mmol/mol Cr)
| Lean (n = 26) | Obese without T2D (n = 23) | Obese with T2D (n = 14) | P | |
|---|---|---|---|---|
| BCAAs mmol/mol Cr | 8.17 ± 2.5 | 9.16 ± 3.96 | 11.1 ± 6.24 | .2610 |
| BCKAs mmol/mol Cr | 1.72 ± .81 | 1.97 ± 1.30 | 2.59 ± 2.05 | .49 |
| KIV mmol/mol Cr | .29 ± .14 | .36 ± .26 | .53 ± .49 | .25 |
| KMV mmol/mol Cr | 1.13 ± .58 | 1.24 ± .80 | 1.63 ± 1.31 | .6 |
| KIC mmol/mol Cr | .30 ± .11 | .36 ± .34 | .44 ± .32 | .36 |
| Tryptophan mmol/mol Cr | 5.75 ± 2.49 | 6.64 ± 3.34 | 7.38 ± 4.12 | .34 |
| Kynurenine mmol/mol Cr | .31 ± .19 | .26 ± .16 | .41 ± .29 | .26 |
| Kynurenic acid mmol/mol Cr | .99 ± .33 | .96 ± .39 | 1.01 ± .43 | .91 |
| 5-HIAA mg/g Cr | 2.459 ± .757 | 2.705 ± 1.783 | 2.501 ± 1.279 | .73 |
| Serotonin nmol/mmol Cr | 73.20 ± 35.94 | 81.72 ± 30.03 | 68.25 ± 24.40 | .2197 |
| Kynurenine/tryptophan | .052 ± .025 | .042 ± .017 | .046 ± .019 | .47 |
| 5-HIAA/tryptophan | .49 ± .18 | .45 ± .20 | .42 ± .26 | .2884 |
| 5-HIAA/kynurenine | 11.29 ± 7.75 | 13.44 ± 7.68 | 11.47 ± 10.81 | .2656 |
| Kynurenine/serotonin | .0045 ± .0029 | .0035 ± .0022 | .0062 ± .0044 | .1818 |
| Serotonin/tryptophan | 13.17 ± 4.37 | 13.38 ± 5.41 | 11.05 ± 4.93 | .3490 |
Abbreviations: BCAA, branched-chain amino acid; BCKA, branched-chain ketoacid; Cr, creatinine; HbA1c, hemoglobin A1c; KIC, α-keto-isocaproate; KIV, α-keto-valerate; KMV, α-keto-β-methylvalerate; T2D, type 2 diabetes.
Table 11.
Metabolites related to BCAA metabolism and tryptophan-kynurenine-serotonin pathways in 24-hour urine samples across 3 groups in females, normalized for urine Cr (mmol/mol Cr)
| Lean (n = 17) | Obese without T2d (n = 33) | Obese with T2D (n = 28) | P | |
|---|---|---|---|---|
| BCAAs mmol/mol Cr | 12.34 ± 5.42 | 9.73 ± 3.77 | 15.55 ± 6.86 | .0027 |
| BCKAs mmol/mol Cr | 2.34 ± 1.02 | 1.89 ± .81 | 5.29 ± 4.51 | .00019 |
| KIV mmol/mol Cr | .36 ± .16 | .34 ± .15 | 1.17 ± 1.06 | 6.4e-06 |
| KMV mmol/mol Cr | 1.6 ± .76 | 1.21 ± .57 | 3.02 ± 2.35 | .00062 |
| KIC mmol/mol Cr | .38 ± .14 | .34 ± .14 | 1.1 ± 1.26 | .00011 |
| Tryptophan mmol/mol Cr | 7.93 ± 4.07 | 6.44 ± 2.99 | 9.17 ± 3.02 | .011 |
| Kynurenine mmol/mol Cr | .34 ± .25 | .29 ± .17 | .57 ± .36 | .00066 |
| Kynurenic acid mmol/mol Cr | 1.22 ± .55 | 1.27 ± .51 | 1.11 ± .47 | .47 |
| Serotonin nmol/mmol Cr | 84.94 ± 20.05 | 81.53 ± 35.77 | 85.11 ± 24.10 | .4726 |
| 5-HIAA mg/g Cr | 3.65 ± 1.89 | 2.78 ± .88 | 3.88 ± 4.23 | .15 |
| Kynurenine/tryptophan | .034 ± .012 | .047 ± .022 | .058 ± .036 | .043 |
| 5-HIAA/tryptophan | .54 ± .26 | .50 ± .19 | .46 ± .41 | .1131 |
| 5-HIAA/kynurenine | 18.10 ± 17.41 | 12.69 ± 7.93 | 8.61 ± 8.37 | .0084 |
| Kynurenine/serotonin | .0040 ± .0030 | .0036 ± .0015 | .0075 ± .0048 | <.0001 |
| Serotonin/tryptophan | 13.08 ± 5.65 | 13.66 ± 4.18 | 10.71 ± 5.31 | .0129 |
Abbreviations: BCAA, branched-chain amino acid; BCKA, branched-chain ketoacid; Cr, creatinine; HbA1c, hemoglobin A1c; KIC, α-keto-isocaproate; KIV, α-keto-valerate; KMV, α-keto-β-methylvalerate; T2D, type 2 diabetes.
Table 12.
Metabolites related to BCAA metabolism and tryptophan-kynurenine- serotonin pathways: P values from testing if differences among the 3 groups are different between males and females in 24-hour urine samples
| 24-h urine | |
|---|---|
| BCAAs mmol/mol Cr | .11 |
| BCKAs mmol/mol Cr | .020 |
| KIV mmol/mol Cr | .012 |
| KMV mmol/mol Cr | .037 |
| KIC mmol/mol Cr | .028 |
| Tryptophan mmol/mol Cr | .17 |
| Kynurenine mmol/mol Cr | .41 |
| Kynurenic acid mmol/mol Cr | .58 |
| Serotonin nmol/mmol Cr | .40 |
| 5-HIAA mg/g Cr | .33 |
| Kynurenine/tryptophan | .028 |
| 5-HIAA/tryptophan | .99 |
| 5-HIAA/kynurenine | .077 |
| Kynurenine/serotonin | .50 |
| Serotonin/tryptophan | .96 |
Abbreviations: BCAA, branched-chain amino acid; BCKA, branched-chain ketoacid; Cr, creatinine; KIC, α-keto-isocaproate; KIV, α-keto-valerate; KMV, α-keto-β-methylvalerate.
Discussion
As obesity and its complications become increasingly more common in youth, it is important to develop new tools to identify those who are at highest risk of developing T2D. Studies in adolescents and adults with obesity suggest that plasma BCAAs and BCKAs are associated with IR and T2D and predict future risk of metabolic syndrome and hypertriglyceridemia (4-12, 46-48). Recent studies in adults also highlight the altered metabolism of tryptophan in obesity, whereby diversion to the kynurenine pathway is associated with IR and T2D (22-24).
In this study, we analyzed 24-hour urine samples to identify factors associated with progression from IR to T2D among youth with obesity. In contrast to single point-in-time plasma analyses, urine metabolomic profiling integrates differences in metabolic status over a 24-hour period in a noninvasive manner (17). We hypothesized that: (1) urine excretion of BCAAs and BCKAs are higher in obese diabetic youth than in normal weight and obese, nondiabetic youth; (2) obesity and diabetes in youth are associated with decreases in the urinary ratio of 5-HIAA, the major metabolite of serotonin, to kynurenine; (3) the urinary concentrations of BCAAs and BCKA and the ratio of 5HIAA/kynurenine correlate with metrics of body fat and glycemic control; and (4) the urinary metabolites of BCAAs and tryptophan metabolism differ among adolescent boys and girls.
Our findings include 4 novel observations. First, urine excretion of BCAAs and BCKAs are higher in obese diabetic youth than in normal weight and obese, nondiabetic youth. Second, youth with T2D exhibit differences in urinary metabolites that are associated with diversion of tryptophan to the kynurenine pathway rather than the serotonin pathway; this is evident by higher urinary kynurenine concentrations, higher ratio of kynurenine/serotonin, lower ratios of serotonin/tryptophan, and 5-HIAA/kynurenine. Third, the urinary concentrations of BCAAs and BCKAs, and metabolites reflecting diversion to the kynurenine pathway were positively associated with metrics of body fat and HbA1c. In contrast, metabolites and ratios related to the serotonin pathway were negatively associated with metrics of body fat and HbA1c. Fourth, urinary BCAAs and BCKAs and byproducts of tryptophan metabolism differ strikingly among adolescent boys and girls.
These findings are of interest given that serotonin increases pancreatic β-cell replication, β-cell mass, and glucose-dependent insulin secretion (25-29). The higher urinary concentrations of kynurenine, higher ratios of kynurenine/tryptophan and kynurenine/serotonin, and lower ratios of serotonin/tryptophan and 5-HIAA/kynurenine in T2D may reflect a relative decrease in cellular serotonin availability. It is unclear why tryptophan metabolism is diverted toward production of kynurenine rather than serotonin; this may be related in part to inflammatory cytokine expression in obesity and T2D (25-28, 49). It should also be noted that elevations in BCAAs may inhibit cellular uptake of serotonin via their competition with tryptophan, the precursor of serotonin, for uptake into β cells and other tissues (42, 50, 51).
Similar observations have been described in prior investigations of tryptophan metabolism in states of obesity, impaired glucose tolerance, and T2D in adults. Studies by Oxenkrug et al (23) and others suggest that inflammation and/or stress-induced upregulation of tryptophan-kynurenine metabolism is a factor predisposing to IR (21, 22, 24). In a study of adults with obesity, the kynurenine/tryptophan ratio was elevated in plasma samples, similar to our findings in urine samples of youth with T2D. In addition, the levels of serotonin were decreased, consistent with a shift of tryptophan metabolism favoring kynurenine production over serotonin (49). In an integrative analysis of host genetics, diet, gut microbiome, and circulating metabolites, the levels of tryptophan, 4 kynurenine-pathway metabolites (kynurenine, kynurenate, xanthurenate, and quinolinate), and indolelactate were positively associated with T2D risk, whereas indolepropionate was inversely associated with T2D risk (52). Another study showed that the host and microbiota may play a significant dual role in either the production or regulation of kynurenine metabolites (53). In a recent investigation, the plasma levels of kynurenine, xanthurenic acid, and kynurenic acid were higher in patients with T2D (54). Similar findings were replicated in the PREDIMED Trial, with baseline tryptophan and 1-year increases in quinolinic acid positively associated with incident T2D (55). Finally, a recent study showed that bariatric surgery reduces serum levels of tryptophan and its downstream kynurenine metabolites; their reduction after surgery was associated with improvement in glycemic control (HbA1c) (56).
Despite these studies in adults, the literature in youth is limited and newly emerging. A study of targeted metabolomic profiling in plasma samples of youth with overweight and/or obesity found a strong positive association between Homeostatic Model Assessment for Insulin Resistance (HOMA-IR), BCAAs, and kynurenine levels (57). In a recent prospective cohort study of subjects aged 9 to 19 years with severe obesity (BMI ≥ 97th percentile), the plasma concentrations of kynurenine correlated positively with BMI z-score and body fat mass, whereas concentrations of serotonin correlated negatively with metrics of adiposity. Tryptophan and kynurenine, but not serotonin, also correlated with IR as assessed by HOMA-IR. In addition, plasma tryptophan was higher in children with prediabetes (58). The current investigation showed that the urinary concentrations of BCAAs, BCKAs, tryptophan, and kynurenine and the ratios reflecting diversion from the serotonin to the kynurenine pathway were positively associated with metrics of body fat and glycemic control assessed by HbA1c. In contrast, urinary metabolites and ratios related to the serotonin pathway were negatively associated with body fat and HbA1c.
Finally, the significant differences in urinary metabolites related to BCAA and tryptophan metabolism and their associations with body fat and glycemic control were largely driven by the female participants. Diversion of tryptophan metabolism to the kynurenine pathway has been characterized in other studies of adult women with obesity, IR, gestational diabetes, and T2D. For example, a recent study of adult women with polycystic ovary syndrome also found abnormal activation of the tryptophan-kynurenine pathway, as indicated by a decrease in plasma tryptophan/kynurenine and an increase in tryptophan/serotonin (59). The increased levels of kynurenine and tryptophan were positively associated with HOMA-IR. Similarly, a longitudinal metabolomics study of pregnant Chinese women found that tryptophan and purine metabolites were consistently upregulated in the urinary metabolome of patients diagnosed with gestational diabetes mellitus throughout pregnancy (60). Another study found that adult women with obesity and T2D had higher serum tryptophan and kynurenine levels than obese, normoglycemic women (61). In the aggregate, these findings suggest that glucose intolerance, rather than, or in addition to, obesity is a critical determinant of tryptophan, serotonin, and kynurenine metabolism.
Our models were adjusted for BMI-related metrics because subjects in the T2D group had higher BMI and BR% than subjects in the nondiabetic obese group. Even after adjustment for BMI-related metrics, Tanner stage, sex, age, and race, subjects in the T2D group had the highest urinary levels of BCKAs, BCAAs, tryptophan, and kynurenine. Because measurement of HbA1c was available only among obese and T2D subjects, we also adjusted the models for HbA1c in addition to Tanner stage, sex, BMI, age, and race. The major exploratory variable or correlate of BCKAs, KIV, KMV, and KIC was HbA1c. BCAAs, kynurenine, kynurenine/tryptophan, and kynurenine levels were significantly elevated among the obese with T2D group compared with the obese without T2D group. We speculate that the chronic hyperglycemia in the diabetic state may suppress the expression of the enzyme branch chain ketoacid dehydrogenase, which catalyzes the oxidative decarboxylation of BCKAs. Suppression of branch chain ketoacid dehydrogenase activity could explain in part the elevated levels of BCKAs as well as BCAAs in T2D. We cannot however, exclude the possibility that increases in BCAAs and/or BCKAs impair insulin action or secretion and thereby contribute to the development of T2D (62, 63).
Interestingly, our previous studies in youth found that plasma BCAAs and products of BCAA catabolism were higher in overweight and obese adolescent males than females (4-6). The previous studies did not include participants with T2D; it is possible that lower BCAA levels in nondiabetic females with obesity are overshadowed by more striking elevations in obese females with T2D.
Our study has a number of important strengths. The participants were well matched for age, pubertal status, and ethnicity. The T2D group comprised predominantly females and contained more Black participants, which is consistent with our clinical experience. Similarly, the T2D group also had higher body fat percentage and BMI, which is also consistent with general clinical practice.
There are certain limitations. Female participants were not studied at standard phases of the menstrual cycle, though none was actively menstruating during urine collection. We did not calculate measures of insulin sensitivity, insulin secretion, or insulin secretion adjusted for insulin sensitivity because our cohort included subjects with overt T2D, whose relative β-cell function is severely diminished. Moreover, participants with T2D were taking metformin, insulin, and/or other medications (liraglutide, dulaglutide, sitagliptin), all of which modify insulin secretion and action and make interpretation of fasting or postprandial insulin levels difficult or impossible. Insulin and GLP-1 analogs are known to stimulate BCAA catabolism and would therefore be expected to lower the BCAA and BCAA-related catabolic byproducts (64). Thus, use of these medications in selected patients cannot explain the higher urinary excretion of BCAAs and BCKAs in youth-onset T2D. The effects of the medications on tryptophan metabolism have not yet been characterized, but the very strong correlations between BCAA and kynurenine (r = .649, P < .001) in the cohort as a whole make it less likely that treatment of a minority of subjects had a major influence on the overall results. The cohort of diabetic subjects was too small to conduct a rigorous statistical analysis correlating metabolomic data with specific medication use. Finally, to minimize the potential for spurious conclusions resulting from multiple statistical comparisons, we used a threshold of P < .01 to indicate statistical significance. Nevertheless, the exploratory nature of our study requires that our findings be interpreted with caution.
The use of urine as a source for metabolic biomarkers comes with its own strengths and limitations. Currently in the literature, there has been little investigation of urine as a source for biomarkers in youth. Urine is useful because it is collected noninvasively and available in large quantities. Furthermore, 24-hour urine samples integrate differences in metabolic status over time and are not affected by level of protein intake, circadian rhythm, or elapsed time since the subject’s last meal (65). In our study, urine was preferable to plasma because of the challenges associated with measuring circulating serotonin levels. Serotonin circulates in very low concentrations, with the vast majority contained in platelets. Human free plasma serotonin in platelet-poor plasma has also proven difficult to measure, with a wide range of reference values reported (66).
However, urine can pose several analytical challenges because urine volume can vary widely depending on physiological status, hydration, and kidney function; sex, age, and BMI can also contribute to variations in the urine metabolome. As a biological waste material, it has broad chemical diversity that makes data analysis and interpretation challenging (67). We addressed these challenges by normalizing all measurements to urine creatinine. Our study is limited by lack of plasma samples to correlate with urine results; however, our findings in urine regarding the tryptophan/kynurenine pathway are consistent with findings in plasma in studies of adults and a handful of studies in children.
In summary, we showed that increased urinary excretion of BCAAs and BCKAs in youth-onset T2D is accompanied by diversion of tryptophan metabolism from the serotonin pathway to the kynurenine pathway. We hypothesize that these metabolic adaptations may associate with and/or contribute to β-cell dysfunction and glucose intolerance. Future studies investigating the cellular mechanisms by which BCAAs and serotonin interact in the control of glucose-stimulated insulin secretion could test this hypothesis. Urinary metabolites of BCAA catabolism are selectively higher in females with T2D than in lean and obese females without diabetes, and tryptophan metabolism is diverted from the serotonin to kynurenine pathway preferentially in females. These adaptations associate with, and may explain in part, the higher risks of T2D in obese adolescent females compared with males (68, 69).
Acknowledgments
We thank our patients and their families, as well as Duke Children's primary care clinics, Duke's Lenox Baker Children's Hospital Diabetes Clinics, Duke Children's Healthy Lifestyles Program, The Metabolomics Core Laboratory at the Stedman Center/Duke Molecular Physiology Institute, Duke Center for Childhood Obesity Research, Children's Clinical Research Unit, Duke Office of Clinical Research, Pennington Biomedical Research Center, and University of North Carolina's Pediatric Endocrine and Diabetes Clinic. We also thank Huaxia Cui for her technical assistance in running the Beckman DxC 600 clinical analyzer.
Abbreviations
- BCAA
branched-chain amino acid
- BCKA
branched-chain ketoacid
- BF%
percent body fat
- BMI
body mass index
- HbA1c
hemoglobin A1c
- HOMA-IR
Homeostatic Model Assessment for Insulin Resistance
- IR
insulin resistance
- IRB
institutional review board
- KIC
α-keto-isocaproate
- KIV
α-keto-valerate
- KMV
α-keto-β-methylvalerate
- MS/MS
tandem mass spectrometry
- PHQ
Patient Health Questionnaire
- T2D
type 2 diabetes
Contributor Information
Natalie Hernandez, Division of Pediatric Endocrinology and Diabetes, Duke University Medical Center, Durham, NC 27710, USA.
Yuliya Lokhnygina, Department of Biostatistics and Bioinformatics, Duke University School of Medicine, Durham, NC 27710, USA; Duke Clinical Research Institute, Duke University Medical Center, Durham, NC 27701, USA.
Megan Elizabeth Ramaker, Duke Molecular Physiology Institute (DMPI), Duke University Medical Center, Durham, NC 27701, USA.
Olga Ilkayeva, Duke Molecular Physiology Institute (DMPI), Duke University Medical Center, Durham, NC 27701, USA; Sarah W. Stedman Nutrition and Metabolism Center, Duke University Medical Center, Durham, NC 27705, USA; Division of Endocrinology, Metabolism, and Nutrition, Duke University Medical Center, Durham, NC 27710, USA.
Michael J Muehlbauer, Duke Molecular Physiology Institute (DMPI), Duke University Medical Center, Durham, NC 27701, USA; Sarah W. Stedman Nutrition and Metabolism Center, Duke University Medical Center, Durham, NC 27705, USA.
Matthew L Crawford, Department of Research and Development, LabCorp, Burlington, NC 27215, USA.
Russell P Grant, Department of Research and Development, LabCorp, Burlington, NC 27215, USA.
Daniel S Hsia, Clinical Trials Unit, Pennington Biomedical Research Center, Baton Rouge, LA 70808, USA.
Nina Jain, Division of Endocrinology, Department of Pediatrics, University of North Carolina, Chapel Hill, NC 27514, USA.
James R Bain, Duke Molecular Physiology Institute (DMPI), Duke University Medical Center, Durham, NC 27701, USA; Sarah W. Stedman Nutrition and Metabolism Center, Duke University Medical Center, Durham, NC 27705, USA; Division of Endocrinology, Metabolism, and Nutrition, Duke University Medical Center, Durham, NC 27710, USA.
Sarah Armstrong, Duke Clinical Research Institute, Duke University Medical Center, Durham, NC 27701, USA; Division of General Pediatrics and Adolescent Health, Duke University Medical Center, Durham, NC 27710, USA; Department of Family Medicine and Community Health, Duke University Medical Center, Durham, NC 27710, USA; Department of Population Health Sciences, Duke University Medical Center, Durham, NC 27710, USA.
Christopher B Newgard, Duke Molecular Physiology Institute (DMPI), Duke University Medical Center, Durham, NC 27701, USA; Sarah W. Stedman Nutrition and Metabolism Center, Duke University Medical Center, Durham, NC 27705, USA; Division of Endocrinology, Metabolism, and Nutrition, Duke University Medical Center, Durham, NC 27710, USA; Department of Pharmacology and Cancer Biology, Duke University Medical Center, Durham, NC 27710, USA.
Michael Freemark, Division of Pediatric Endocrinology and Diabetes, Duke University Medical Center, Durham, NC 27710, USA; Duke Molecular Physiology Institute (DMPI), Duke University Medical Center, Durham, NC 27701, USA; Sarah W. Stedman Nutrition and Metabolism Center, Duke University Medical Center, Durham, NC 27705, USA.
Pinar Gumus Balikcioglu, Division of Pediatric Endocrinology and Diabetes, Duke University Medical Center, Durham, NC 27710, USA; Duke Molecular Physiology Institute (DMPI), Duke University Medical Center, Durham, NC 27701, USA; Sarah W. Stedman Nutrition and Metabolism Center, Duke University Medical Center, Durham, NC 27705, USA.
Funding
P.G.B. was supported by National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under the award number DK117067, Children's Miracle Network Hospitals partnerships and programs benefiting Duke Children's, Derfner Foundation Research Grant, Duke University Pediatric Departmental Support, and Duke Strong Start Award Program. O.I. and J.R.B. and the metabolomics assays they performed were supported by National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under award number P30DK124723. C.B.N. received salary support from DK121710. J.R.B. received salary support from 5R01DK117491, 1U24DK129557, and 2P30AG027816. D.S.H. conducted the study in the Clinical Trials Unit, which is supported in part by U54 GM104940 from the National Institute of General Medical Sciences of the National Institutes of Health, which funds the Louisiana Clinical and Translational Science Center and in part by a NORC Center Grant #P30DK072476 entitled “Nutrition and Metabolic Health Through the Lifespan” sponsored by the National Institute of Diabetes and Digestive and Kidney Diseases. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author Contributions
N.H. was responsible for interpreting the data and writing the manuscript. Y.L. and M.E.R. were responsible for advanced statistical analysis. K.B. was responsible for interpretation of the data and critical review of the manuscript. M.L.C. and R.P.G. were responsible for mass spectrometry analysis of samples and critical review of the manuscript. J.R.B. and M.M. performed biochemical analysis and critical review of the manuscript. N.J., D.S.H., and S.A. contributed to data collection and critical review of the manuscript. C.B.N. and M.F. were responsible for development of the research question, conception and design of the research project, interpretation of the data, and critical review of the manuscript. P.G.B. was responsible for development of the research question, conception and design of the research project, obtaining funding, acquisition of data, statistical analysis and interpretation of the data, and writing the manuscript.
Disclosures
N.H., Y.L., M.E.R., O.I., M.J.M., D.S.H., N.J., S.A., C.B.N., and P.G.B. have no conflicts of interest to declare. M.F. is a co-investigator on a grant from the American Heart Association that deals with the pathogenesis and treatment of childhood obesity. M.F. is also the local principal investigator on a Rhythm-sponsored study of identification and treatment of children and adults with monogenic obesity and was previously a member of a Data Safety Monitoring Board for a separate Rhythm-sponsored study of treatment of patients with syndromic obesity. M.L.C. and R.P.G. own stock and are paid salary by Laboratory Corporation of America. J.R.B. has nonfinancial ties to Agilent Technologies, Inc.
Data Availability
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
Clinical Trial Information
ClinicalTrials.gov Identifier: 12326129.
References
- 1. Group TS, Zeitler P, Hirst K, et al. A clinical trial to maintain glycemic control in youth with type 2 diabetes. New Engl J Med. 2012;366(24):2247‐2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sinha R, Fisch G, Teague B, et al. Prevalence of impaired glucose tolerance among children and adolescents with marked obesity. New Engl J Med. 2002;346(11):802‐810. [DOI] [PubMed] [Google Scholar]
- 3. Weiss R, Taksali SE, Tamborlane WV, Burgert TS, Savoye M, Caprio S. Predictors of changes in glucose tolerance Status in obese youth. Diabetes Care. 2005;28(4):902‐909. [DOI] [PubMed] [Google Scholar]
- 4. Newbern D, Balikcioglu PG, Balikcioglu M, et al. Sex differences in biomarkers associated with insulin resistance in obese adolescents: metabolomic profiling and principal components analysis. J Clin Endocrinol Metab. 2014;99(12):4730‐4739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Trub CJ, Balikcioglu M, Freemark M, et al. Impact of lifestyle intervention on branched-chain amino acid catabolism and insulin sensitivity in adolescents with obesity. Endocrinol Diabetes Metab. 2021;4(3):e00250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Balikcioglu PG, Trub CJ, Balikcioglu M, et al. Branched-chain α-keto acids and glutamate/glutamine: biomarkers of insulin resistance in childhood obesity. Endocrinol Diabetes Metab. 2023;6(1):e388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Perng W, Gillman MW, Fleisch AF, et al. Metabolomic profiles and childhood obesity. Obesity. 2014;22(12):2570‐2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Butte NF, Liu Y, Zakeri IF, et al. Global metabolomic profiling targeting childhood obesity in the hispanic population. Am J Clin Nutr. 2015;102(2):256‐267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McCormack SE, Shaham O, McCarthy MA, et al. Circulating branched-chain amino acid concentrations are associated with obesity and future insulin resistance in children and adolescents. Pediatr Obes. 2013;8(1):52‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mihalik SJ, Michaliszyn SF, de las Heras J, et al. Metabolomic profiling of fatty acid and amino acid metabolism in youth with obesity and type 2 diabetes evidence for enhanced mitochondrial oxidation. Diabetes Care. 2012;35(3):605‐611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Michaliszyn SF, Sjaarda LA, Mihalik SJ, et al. Metabolomic profiling of amino acids and b-cell function relative to insulin sensitivity in youth. J Clin Endocrinol Metab. 2012;97(11):E2119‐E2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Frohnert BI, Rewers MJ. Metabolomics in childhood diabetes. Pediatr Diabetes. 2016;17(1):3‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Newgard CB, An J, Bain JR, et al. A branched-chain amino acid related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009;9(4):311‐326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang TJ, Larson MG, Vasan RS, et al. Metabolite profiles and the risk of developing diabetes. Nat Med. 2011;17(4):448‐453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Batch BC, Shah SH, Newgard CB, et al. Branched chain amino acids are novel biomarkers for discrimination of metabolic wellness. Metabolism. 2013;62(7):961‐969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shah SH, Bain JR, Muehlbauer MJ, et al. Association of a peripheral blood metabolic profile with coronary artery disease and risk of subsequent cardiovascular events. Circ Cardiovasc Genet. 2010;3(2):207‐214. [DOI] [PubMed] [Google Scholar]
- 17. Huffman KM, Shah SH, Stevens RD, et al. Relationships between circulating metabolic intermediates and insulin action in overweight to obese, inactive men and women. Diabetes Care. 2009;32(9):1678‐1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tai ES, Tan ML, Stevens RD, et al. Insulin resistance is associated with a metabolic profile of altered protein metabolism in Chinese and Asian-Indian men. Diabetologia. 2010;53(4):757‐767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Laferrère B, Reilly D, Arias S, et al. Differential metabolic impact of gastric bypass surgery versus dietary intervention in obese diabetic subjects despite identical weight loss. Science Transl. Med. 2011;3(80):80re2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gumus Balikcioglu P, Newgard C. Metabolomic signatures and metabolic complications in childhood obesity. In: Michael F, ed. Pediatric Obesity, Etiology, Pathogenesis, and Treatment. 2nd ed Springer Humana Press; 2018:343‐361. [Google Scholar]
- 21. Theofylaktopoulou D, Midttun Ø, Ulvik A, et al. A community-based study on determinants of circulating markers of cellular immune activation and kynurenines: the Hordaland health study. Clin Exp Immunol. 2013;173(1):121‐130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mangge H, Summers KL, Meinitzer A, et al. Obesity-related dysregulation of the tryptophan-kynurenine metabolism: role of age and parameters of the metabolic syndrome. Obesity (Silver Spring). 2014;22(1):195‐201. [DOI] [PubMed] [Google Scholar]
- 23. Oxenkrug G. Insulin resistance and dysregulation of tryptophan-kynurenine and kynurenine-nicotinamide adenine dinucleotide metabolic pathways. Mol Neurobiol. 2013;48(2):294‐301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Favennec M, Hennart B, Caiazzo R, et al. The kynurenine pathway is activated in human obesity and shifted toward kynurenine monooxygenase activation. Obesity. 2015;23(10):2066‐2074. [DOI] [PubMed] [Google Scholar]
- 25. Almaça J, Molina J, Menegaz D, et al. Human beta cells produce and release serotonin to inhibit glucagon secretion from alpha cells. Cell Rep. 2016;17(12):3281‐3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bennet H, Mollet IG, Balhuizen A, et al. Serotonin (5-HT) receptor 2b activation augments glucose-stimulated insulin secretion in human and mouse islets of Langerhans. Diabetologia. 2016;59(4):744‐754. [DOI] [PubMed] [Google Scholar]
- 27. Kim K, Oh CM, Ohara-Imaizumi M, et al. Functional role of serotonin in insulin secretion in a diet-induced insulin-resistant state. Endocrinology. 2015;156(2):444‐452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ohara-Imaizumi M, Kim H, Yoshida M, et al. Serotonin regulates glucose-stimulated insulin secretion from pancreatic β cells during pregnancy. Proc Natl Acad Sci U S A. 2013;110(48):19420‐19425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Namkung J, Kim H, Park S. Peripheral serotonin: a new player in systemic energy homeostasis. Mol Cells. 2015;38(12):1023‐1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Giessner S, Ramaker ME, Blew K, et al. Disrupted circadian rhythm of epinephrine in males with youth-onset type 2 diabetes. J Endocr Soc. 2022;7(2):bvac190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Committee ADAPP . 2. Classification and diagnosis of diabetes: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(Supplement_1):S17‐S38. [DOI] [PubMed] [Google Scholar]
- 32. Hernandez N, Lokhnygina Y, Ramaker ME, et al. Supplemental data for: Sex differences in branched-chain amino acid and tryptophan metabolism and pathogenesis of youth-onset type 2 diabetes [Data set]. Zenodo. 2023. 10.5281/zenodo.10050218 [DOI] [PMC free article] [PubMed]
- 33.Psychiatry AA of C& A. PHQ-9: Modified for Teens. Accessed January 24, 2023. http://www.aacap.org/App_Themes/AACAP/docs/member_resources/toolbox_for_clinical_practice_and_outcomes/symptoms/GLAD-PC_PHQ-9.pdf
- 34. An J, Muoio DM, Shiota M, et al. Hepatic expression of malonyl-CoA decarboxylase reverses muscle, liver and whole-animal insulin resistance. Nat Med. 2004;10(3):268‐274. [DOI] [PubMed] [Google Scholar]
- 35. Millington DS, Kodo N, Norwood DL, Roe CR. Tandem mass spectrometry: a new method for acylcarnitine profiling with potential for neonatal screening for inborn errors of metabolism. J Inherit Metab Dis. 1990;13(3):321‐324. [DOI] [PubMed] [Google Scholar]
- 36. Wu JY, Kao HJ, Li SC, et al. ENU mutagenesis identifies mice with mitochondrial branched-chain aminotransferase deficiency resembling human maple syrup urine disease. J Clin Invest. 2004;113(3):434‐440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chace DH, Hillman SL, Millington DS, Kahler SG, Roe CR, Naylor EW. Rapid diagnosis of maple syrup urine disease in blood spots from newborns by tandem mass spectrometry. Clin Chem. 1995;41(1):62‐68. [PubMed] [Google Scholar]
- 38. Ferrara CT, Wang P, Neto EC, et al. Genetic networks of liver metabolism revealed by integration of metabolic and transcriptional profiling. PLoS Genet. 2008;4(3):e1000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Olson KC, Chen G, Lynch CJ. Quantification of branched-chain keto acids in tissue by ultra fast liquid chromatography–mass spectrometry. Anal Biochem. 2013;439(2):116‐122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. White PJ, McGarrah RW, Grimsrud PA, et al. The BCKDH kinase and phosphatase integrate BCAA and lipid metabolism via regulation of ATP-citrate lyase. Cell Metab. 2018;27(6):1281‐1293.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Parker DC, Kraus WE, Whitson HE, et al. Tryptophan metabolism and neurodegeneration: longitudinal associations of kynurenine pathway metabolites with cognitive performance and plasma Alzheimer's disease and related dementias biomarkers in the duke physical performance across the LifeSpan study. J Alzheimer’s Dis. 2022;91(3):1141‐1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Coppola A, Wenner BR, Ilkayeva O, et al. Branched-chain amino acids alter neurobehavioral function in rats. Am J Physiol-Endocr. 2013;304(4):E405‐E413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Martin MD, Woods JS, Leroux BG, et al. Longitudinal urinary creatinine excretion values among preadolescents and adolescents. Transl Res. 2008;151(1):51‐56. [DOI] [PubMed] [Google Scholar]
- 44. Clayton JA. Applying the new SABV (sex as a biological variable) policy to research and clinical care. Physiol Behav. 2018;187:2‐5. [DOI] [PubMed] [Google Scholar]
- 45. Clayton JA. Studying both sexes: a guiding principle for biomedicine. FASEB J. 2016;30(2):519‐524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lee A, Jang HB, Ra M, et al. Prediction of future risk of insulin resistance and metabolic syndrome based on Korean boy's metabolite profiling. Obes Res Clin Pract. 2015;9(4):336‐345. [DOI] [PubMed] [Google Scholar]
- 47. Hellmuth C, Kirchberg FF, Lass N, et al. Tyrosine is associated with insulin resistance in longitudinal metabolomic profiling of obese children. J Diabetes Res. 2016;2016:2108909‐2108910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wiklund P, Zhang X, Tan X, Keinänen-KiukaanniemiS AM, Cheng S. Serum amino acid profiles in childhood predict triglyceride level in adulthood: a 7-year longitudinal study in girls. J Clin Endocrinol Metab. 2016;101(5):2047‐2055. [DOI] [PubMed] [Google Scholar]
- 49. Cussotto S, Delgado I, Anesi A, et al. Tryptophan metabolic pathways are altered in obesity and are associated with systemic inflammation. Front Immunol. 2020;11:557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Di Gialleonardo V, Signore A, Scheerstra EA, et al. 11C-Hydroxytryptophan uptake and metabolism in endocrine and exocrine pancreas. J Nucl Med. 2012;53(11):1755‐1763. [DOI] [PubMed] [Google Scholar]
- 51. Cheng Q, Beltran VD, Chan SM, Brown JR, Bevington A, Herbert TP. System-L amino acid transporters play a key role in pancreatic β-cell signalling and function. J Mol Endocrinol. 2016;56(3):175‐187. [DOI] [PubMed] [Google Scholar]
- 52. Qi Q, Li J, Yu B, et al. Host and gut microbial tryptophan metabolism and type 2 diabetes: an integrative analysis of host genetics, diet, gut microbiome and circulating metabolites in cohort studies. Gut. 2022;71(6):1095‐1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hyland NP, Cavanaugh CR, Hornby PJ. Emerging effects of tryptophan pathway metabolites and intestinal microbiota on metabolism and intestinal function. Amino Acids. 2022;54(1):57‐70. [DOI] [PubMed] [Google Scholar]
- 54. Oxenkrug GF. Increased plasma levels of Xanthurenic and kynurenic acids in type 2 diabetes. Mol Neurobiol. 2015;52(2):805‐810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yu E, Papandreou C, Ruiz-Canela M, et al. Association of tryptophan metabolites with incident type 2 diabetes in the PREDIMED trial: a case–cohort study. Clin Chem. 2018;64((8):1211‐1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yeung KTD, Penney N, Whiley L, et al. The impact of bariatric surgery on serum tryptophan–kynurenine pathway metabolites. Sci Rep. 2022;12(1):294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Perng W, Rifas-Shiman SL, Sordillo J, Hivert M, Oken E. Metabolomic profiles of overweight/obesity phenotypes during adolescence: a cross-sectional study in project Viva. Obesity. 2020;28(2):379‐387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lischka J, Schanzer A, Baumgartner M, de Gier C, Greber-Platzer S, Zeyda M. Tryptophan metabolism is associated with BMI and adipose tissue mass and linked to metabolic disease in pediatric obesity. Nutrients. 2022;14(2):286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wang S, Mu L, Zhang C, et al. Abnormal activation of tryptophan-kynurenine pathway in women with polycystic ovary syndrome. Front Endocrinol. 2022;13:877807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Law KP, Han TL, Mao X, Zhang H. Tryptophan and purine metabolites are consistently upregulated in the urinary metabolome of patients diagnosed with gestational diabetes mellitus throughout pregnancy: a longitudinal metabolomics study of Chinese pregnant women part 2. Clin Chim Acta. 2017;468:126‐139. [DOI] [PubMed] [Google Scholar]
- 61. Kubacka J, Staniszewska M, Sadok I, Sypniewska G, Stefanska A. The kynurenine pathway in obese middle-aged women with normoglycemia and type 2 diabetes. Metabolites. 2022;12(6):492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. White PJ, Newgard CB. Branched-chain amino acids in disease. Science. 2019;363(6427):582‐583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. White PJ, McGarrah RW, Herman MA, Bain JR, Shah SH, Newgard CB. Insulin action, type 2 diabetes, and branched-chain amino acids: a two-way street. Mol Metab. 2021;52:101261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Vanweert F, Schrauwen P, Phielix E. Role of branched-chain amino acid metabolism in the pathogenesis of obesity and type 2 diabetes-related metabolic disturbances BCAA metabolism in type 2 diabetes. Nutr Diabetes. 2022;12(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Slocum RH, Cummings JG. Chapter 8: amino acid analysis of physiological samples. In: Hommes FA, ed. Techniques in Diagostic Human Biochmical Genetics: A Laboratory Manual. Wiley; 1991:87‐126. [Google Scholar]
- 66. Brand T, Anderson GM. The measurement of platelet-poor plasma serotonin: a systematic review of prior reports and recommendations for improved analysis. Clin Chem. 2011;57(10):1376‐1386. [DOI] [PubMed] [Google Scholar]
- 67. Bouatra S, Aziat F, Mandal R, et al. The human urine metabolome. PLoS One. 2013;8(9):e73076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Copeland KC, Zeitler P, Geffner M, et al. Characteristics of adolescents and youth with recent-onset type 2 diabetes: the TODAY cohort at baseline. J Clin Endocrinol Metab. 2011;96(1):159‐167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. TODAY Study Group . A clinical trial to maintain glycemic control in youth with type 2 diabetes. N Engl J Med. 2012;366(24):2247‐2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Hernandez N, Lokhnygina Y, Ramaker ME, et al. Supplemental data for: Sex differences in branched-chain amino acid and tryptophan metabolism and pathogenesis of youth-onset type 2 diabetes [Data set]. Zenodo. 2023. 10.5281/zenodo.10050218 [DOI] [PMC free article] [PubMed]
Data Availability Statement
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.