Abstract
Gestational diabetes mellitus (GDM) is a known risk factor for gestational hypertension which further progress toward conditions like proteinuria, dyslipidemia, thrombocytopenia, pulmonary edema leading to Preeclampsia (PE). Pregnancy can be a challenging time for many women, especially those diagnosed with GDM and PE. Thus, the current prospective study investigates the association of OGTT glucose levels with systolic and diastolic blood pressure and lipid profile parameters in pregnant women diagnosed with GDM and PE. A total of 140 pregnant women were stratified into GDM (n = 50), PE (n = 40) and controls (n = 50). Two hour 75 g oral glucose tolerance test (OGTT) was performed for screening GDM. Biochemical parameters analysis of OGTT, total cholesterol (TC), triglyceride (Tg), high density lipoprotein-cholesterol (HDL-C), low density lipoprotein-cholesterol (LDL-C), urinary albumin and creatinine were tested to find urinary albumin creatinine ratio (uACR). Statistical analysis was performed using ANOVA followed by post hoc test and regression analysis. Among the studied groups, GDM and PE groups showed no significant difference in age and increased BMI. Increased 2 h OGTT & TC in GDM group; elevated uACR, systolic/diastolic blood pressure, Tg, HDL-C, LDL-C in PE group was observed and differ significantly (p < 0.0001) with other groups. A significant positive effect of 2 h OGTT was observed on blood pressure (R2: GDM = 0.85, PE = 0.71) and lipid profile determinants (R2: GDM = 0.85, PE = 0.33) at p < 0.0001. The current study concludes that glucose intolerance during the later weeks of pregnancy is associated with gestational hypertension and hyperlipidemia as a risk factor for PE. Further research is needed for a detailed assessment of maternal glucose metabolism at various pregnancy stages, including the use of more sensitive markers such as C-peptide and their relation to pregnancy-related hypertensive disorders.
Keywords: Gestational diabetes mellitus, Preeclampsia, Pregnancy, Hypertension, Dyslipidemia
Subject terms: Biochemistry, Biomarkers, Endocrinology
Background
Gestational diabetes mellitus (GDM) and preeclampsia (PE) are common pregnancy-related problems responsible for maternal and fetal mortality and morbidity1. GDM is characterized as the initial diagnosis of glucose intolerance during pregnancy2. The International Association of Diabetes and Pregnancy Study Groups (IADPSG) has formed the diagnostic criteria for GDM based on the findings of the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study3. The pathophysiological mechanisms in both GDM and PE entail oxidative stress, pro-inflammatory factor release, and vascular endothelial dysfunction, which conjointly raise the risk of future maternal diabetes and cardiovascular disease, suggesting a link between GDM and PE4,5.
According to the recent global report by the International Diabetes Federation (IDF), GDM complicates up to 80.3% of all pregnancies6. GDM is a major risk factor for gestational hypertension, which causes approximately 30,000 deaths per year among women7. Hyperglycemia induces a proinflammatory milieu and cytokine imbalance, resulting in placental vascular alterations, whilst insulin directly causes placental inflammation. One or more of the following conditions, such as proteinuria, dyslipidemia, thrombocytopenia, pulmonary edema, or persistent neurological symptoms, are also additional diagnostic indicators of gestational hypertension and PE8. The progressive PE manifests renal impairment, hepatic impairment, hemorrhagic stroke, ischemic acute respiratory distress syndrome, placental abruption, preterm labor, and delivery. A study conducted in Saudi Arabia by Subki et al. revealed that hypertensive disorders during pregnancy had a prevalence of 2.4%, with PE being the most common subtype at 54.9%9. It has been proposed that women with GDM exhibit a greater prevalence of dyslipidemia than their normoglycemic counterparts10. Additionally, a recent finding addresses the pathophysiology of PE, including dysregulations of fetal-maternal lipid metabolism11.
Nonetheless, an altered lipid profile causes vasoconstriction and endothelial dysfunction by suppressing endothelial prostacyclin and nitric oxide (NO), increasing oxidative stress. Although physiological hyperlipidemia is non-atherogenic until allied with associated severe hypertension12. The Fifth International Workshop-Conference on GDM recommended the treatment of GDM with lifestyle interventions like medical nutrition therapy, physical activity, weight management, and continuous glucose monitoring13. Pharmacological therapies like sulfonylureas and biguanides are contraindicated in GDM as they readily cross the placenta and are found to be associated with a higher rate of neonatal hypoglycemia, macrosomia, and increased neonatal abdominal circumference, hyperbilirubinemia14,15. Insulin is the recommended medication for treating hyperglycemia in GDM, and a low-dose aspirin at 12 to 16 weeks of gestation to lower the risk of preeclampsia13.
With the hypothesis that elevated maternal glucose concentrations during pregnancy can affect blood pressure and lipid profiles, consequently leading to PE, the present prospective study examined the association of OGTT glucose with systolic, diastolic blood pressure, and lipid profile parameters in pregnant women diagnosed with GDM and PE. This study is regionally important and has not been conducted before in Abha, the southern region of Saudi Arabia. However, a decade earlier, PE and eclampsia partake was observed on maternal and fetal mortality.
Materials and methods
Study setting
The study was conducted in the Basic Medical Sciences department of King Khalid University in collaboration with AlKhamis Maternity and Children Hospital, Khamis Mushayt. The study was approved by the ethical committee of King Khalid University, Abha, Saudi Arabia under approval number ECM#2021-601. An informed consent was obtained from the study subjects and methods were performed in accordance with the relevant guidelines and regulations of Helsinki.
Study participants inclusion and exclusion criteria
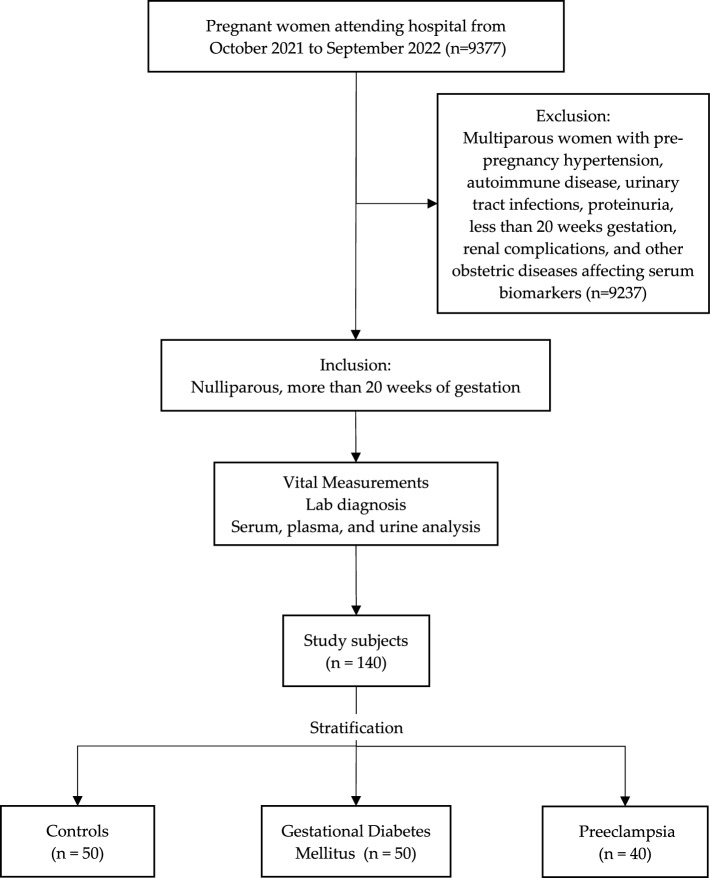
Nulliparous women arriving for antenatal service at AlKhamis Maternity and Children Hospital were consecutively screened and enrolled. The study excluded multiparous women with pre-pregnancy hypertension, autoimmune disease, urinary tract infections, proteinuria before 20 weeks of pregnancy, renal complications, and other obstetric diseases affecting serum biomarkers. A total of 140 pregnant women with not less than 20 weeks of pregnancy were included in the study from October 2021 to September 2022. Based on clinical and laboratory diagnosis, the total studied subjects were stratified into GDM (n = 50), PE (n = 40), and controls (n = 50) with no complications reported (Fig. 1). According to IADPSG recommendations3, a 2-h 75-g oral glucose tolerance test (OGTT) was performed to screen GDM in the study subjects. GDM is diagnosed when fasting plasma glucose = 92 mg/dl, 1-h OGTT = 180 mg/dl, 2-h OGTT ≥ 153 mg/dl3. According to the American Diabetes Association 16, PE is characterized by > 140/90 mmHg systolic and diastolic blood pressure on more than two occasions after 20 weeks of gestation with the addition of > 0.3gm of protein in 24 h of urine test. The recent readings of systolic and diastolic blood pressure were recorded at least 4 h apart. BMI was calculated using the metric formula weight in kilograms divided by height in meters squared.
Figure 1.
Flow chart representing study subjects recruitment and categorization.
Sample collection and analysis
Blood samples were collected by venipuncture in different color-coded labeled vacutainers. To estimate glucose levels, 3 mL of blood was collected in a purple-colored vacutainer coated with EDTA to isolate the plasma. For the biochemical analysis of TC, Tg, HDL-C, and LDL-C, a red-colored vacutainer was used to collect blood that was then centrifuged at 3000 rpm for 10 min in a tabletop centrifuge to separate the serum. The obtained serum was analyzed using an automatic analyzer, according to manufacturer instructions (Merck Ltd.). 24-h urine samples were received in wide-mouth, clean plastic universal containers for analysis. The proteinuria-positive cases identified using the dipstick method were further analyzed for urinary albumin and creatinine by Roche analyzer to estimate urinary albumin creatinine ratio (uACR). All laboratory results with vital signs were recorded.
Statistical analysis
Statistical analysis was performed using SPSS version 25. Data of studied biochemical parameters were presented as Mean ± SD (standard deviation) values. All the groups were compared using one-way ANOVA at 0.0001 & 0.05 significance level, followed by Post hoc Tukey’s honest test and indicated by superscript at 0.001 and 0.05 significance level. Multiple regression analysis was implemented to assess the association among study variables. Further, in Model 1, the association of 2 h OGTT with blood pressure (systolic and diastolic) and lipid profile parameters was done collectively. In Model 2, the associations were performed with individual variables excluding the irrelevant variables of Model 1. Both models were presented as coefficients, standard errors, and confidence intervals.
Results
The results of the present study include data analysis of 140 study subjects. According to physician diagnosis, subjects were grouped into controls (n = 50), GDM (n = 50) and PE (n = 40). Biochemical parameters were analyzed and presented as Mean ± SD values in Table 1.
Table 1.
Mean ± SD values of studied parameters and their comparison among different groups.
| CONTROL (n = 50) | GDM (n = 50) | PE (n = 40) | p value | |
|---|---|---|---|---|
| Maternal age (years) | 33 ± 4a | 38.74 ± 4.3b | 40.8 ± 3.12b | < 0.0001 |
| Gestational age (weeks) | 26 ± 1a | 26.88 ± 1.66b | 27.28 ± 1.18b | < 0.01 |
| Vitals | ||||
| Heart rate beats/min | 88.8 ± 6.27a | 104.4 ± 2.59b | 113.4 ± 1.08c | < 0.0001 |
| Respiratory rate breaths/min | 19.2 ± 1.5a | 20.7 ± 1.11b | 24.3 ± 1.03c | < 0.0001 |
| SpO2 | 96.6 ± 1.83a | 94.8 ± 0.6b | 92.5 ± 1.15c | < 0.0001 |
| Temperature ◦C | 34.9 ± 0.6a | 34.9 ± 0.8a | 37.4 ± 0.4b | < 0.0001 |
| BMI | 27.28 ± 1.13a | 32.32 ± 2.37b | 34.15 ± 2.73b | < 0.0001 |
| Systolic mm/Hg | 120.9 ± 3.3a | 137.6 ± 1.67b | 161 ± 3.2c | < 0.0001 |
| Diastolic mm/Hg | 81.2 ± 1.77a | 103.26 ± 6.15b | 106.6 ± 2.19c | < 0.0001 |
| Laboratory analysis | ||||
| FPG mg/dl | 89.32 ± 2.4a | 101.73 ± 5.51b | 131.74 ± 5.07c | < 0.0001 |
| 1 h OGTT mg/dl | 110.3 ± 7.7a | 190 ± 12.45b | 180.75 ± 14.92c | < 0.0001 |
| 2 h OGTT mg/dl | 120.6 ± 2.9a | 163.24 ± 4.66b | 152.7 ± 2.15c | < 0.0001 |
| Urinary albumin mg/dl | 2.15 ± 1.21a | 2.94 ± 2.22b | 334.25 ± 33.27c | < 0.0001 |
| Urinary creatinine mmol/l | 2.02 ± 0.32a | 1.72 ± 0.45b | 10.43 ± 0.81c | < 0.0001 |
| uACR mg/mmol | 1.08 ± 0.6a | 8.3 ± 3.2b | 32.13 ± 2.11c | < 0.0001 |
| TC mg/dl | 130.7 ± 2.9a | 143.2 ± 4.19b | 142.88 ± 2.33b | < 0.0001 |
| Tg mg/dl | 58.6 ± 3.09a | 73.34 ± 4.69b | 78.03 ± 4.83c | < 0.0001 |
| HDL-C mg/dl | 28.6 ± 3.2a | 20.14 ± 3.18b | 21.6 ± 1.41c | < 0.0001 |
| LDL-C mg/dl | 76.96 ± 3.23a | 90.82 ± 3.02b | 96.45 ± 2.58c | < 0.0001 |
| VLDL-C mg/dl | 11.73 ± 0.62a | 14.66 ± 0.94b | 15.61 ± 0.97c | < 0.0001 |
Mean ± SD values not sharing same superscripts differ significantly at 0.0001.
GDM Gestational diabetes mellitus, PE Preeclampsia, BMI Body mass index, FPG Fasting plasma glucose, OGTT Oral glucose tolerance test, uACR Urinary albumin creatinine ratio, TC Total cholesterol, Tg Triglycerides, HDL-C High density lipoprotein cholesterol, LDL-C Low-density lipoprotein cholesterol, VLDL-C Very low-density lipoprotein cholesterol.
Mean ± SD values of all the groups were compared using ANOVA and found to be significantly different from each other at p < 0.01 and p < 0.001. Further, the post hoc test allocates the difference among groups and is represented with alphabetical superscripts. Mean ± SD values not sharing the same superscript differ significantly at 0.001. Recorded vitals were significantly different among all groups except the body temperature of controls and GDM groups. Maternal age and gestational age showed no difference in GDM and PE groups. Increased BMI was observed in the GDM and PE groups with no difference. The systolic and diastolic blood pressure was observed to be elevated in the PE group and differed significantly from the controls and GDM group, p < 0.0001. The 2 h OGTT-glucose and uACR were relatively high in GDM and PE groups respectively, which differ from other groups. In the lipid index, the mean of TC was higher in GDM and PE with no difference, while Tg, LDL-C and VLDL-C markedly increased in PE group and HDL-C in controls compared to others.
This study is based on the hypothesis that maternal glucose levels are associated with alteration in blood pressure and lipid profile. To test this hypothesis, regression analysis was performed. Table 2 depicts 85% variance in blood pressure F(2,47 = 139.8, p < 0.001) and lipid indices F(5,45 = 87.22, p < 0.001) of the GDM group. In the PE group, 71% of variance on blood pressure F(2,37 = 48.8, p < 0.001) and 33% of the variance in lipid profile F(5,35 = 6.4, p < 0.001) can be accounted by GDM.
Table 2.
Adjusted R2 values of 2-h OGTT on overall blood pressure and lipid profile determinants among studied groups.
| CONTROL (n = 50) | GDM (n = 50) | PE (n = 40) | |
|---|---|---|---|
| Blood pressure (systolic & diastolic) | 0.02 | 0.85* | 0.71* |
| Lipid profile determinants (TC, Tg, HDL-C, LDL-C, VLDL-C) | − 0.10 | 0.85* | 0.33* |
GDM Gestational diabetes mellitus, PE Preeclampsia, OGTT Oral glucose tolerance test, TC Total cholesterol, Tg Triglycerides, HDL-C High density lipoprotein cholesterol, LDL-C Low-density lipoprotein cholesterol, VLDL-C Very low-density lipoprotein cholesterol.
*p value < 0.001.
Looking at Table 3 where glucose levels (2 h OGTT) reveal unique contribution on individual parameter, the result shows the regression coefficients of two models in control; GDM and PE group.
Table 3.
Multiple regression models of 2-h OGTT on blood pressure and lipid profile determinants among studied groups.
| CONTROL (n = 50) | GDM (n = 50) | PE (n = 40) | ||||
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | Model 1 | Model 2 | |
| Systolic mm/Hg |
− 0.03 (0.12) − 0.2 to 0.1 |
− 0.08 (0.12) − 0.3 to 0.1 |
2.39 (0.21)*** 1.9 to 2.8 |
2.57 (0.15)*** 2.2 to 2.8 |
0.55 (0.06)*** 0.4 to 0.6 |
0.57 (0.05)*** 0.4 to 0.6 |
| Diastolic mm/Hg |
0.37 (0.23) − 0.1 to 0.8 |
0.38 (0.22) − 0.07 to0.8 |
0.07 (0.05) − 0.04 to0.1 |
0.51 (0.07)*** 0.3 to 0.6 |
0.07 (0.08) − 0.1 to 0.2 |
0.31 (0.15)** 0.01 to 0.6 |
| TC mg/dl |
0.01 (0.14) − 0.2 to 0.3 |
0.009 (0.14) − 0.2 to 0.2 |
0.37 (0.11)*** 0.1 to 0.6 |
0.98 (0.07)*** 0.8 to 1.1 |
0.015 (0.13) − 0.2 to 0.2 |
0.25 (0.14) 0.0 to 0.5 |
| Tg mg/dl |
− 0.04 (0.14) − 0.3 to 0.2 |
− 0.05 (0.13) − 0.3 to 0.2 |
0.23 (0.12) − 0.01 to 0.4 |
0.88 (0.06)*** 0.7 to 1 |
0.19 (0.07)** 0.04 to 0.3 |
0.26 (0.05)*** 0.1 to 0.3 |
| HDL-C mg/dl |
0.03 (0.13) − 0.2 to 0.3 |
0.01 (0.12) − 0.2 to 0.2 |
− 0.25 (0.14) − 0.5 to 0.03 |
− 1.2 (0.11)*** − 1.4 to − 1 |
0.43 (0.21)* 0.0 to 0.8 |
0.73 (0.21)*** 0.2 to 1.1 |
| LDL-C mg/dl |
0.07 (0.13) − 0.1 to 0.3 |
0.07 (0.12) − 0.1 to 0.3 |
0.38 (0.18)* − 0.0 to 0.7 |
1.37 (0.09)*** 1.1 to 1.5 |
0.06 (0.12) − 0.1 to 0.3 |
0.31 (0.12)* 0.0 to 0.5 |
| VLDL-C mg/dl |
− 0.25 (0.67) − 1.6 to 1.1 |
4.4 (0.3)*** 3.7 to 5 |
1.31 (0.29)*** 0.7 to 1.9 |
|||
Numeric values are regression coefficients followed by robust standard errors in parenthesis and a 95% confidence interval (lower to upper range) in italics.
TC Total cholesterol, Tg Triglycerides, HDL-C High density lipoprotein cholesterol, LDL-C Low-density lipoprotein cholesterol, VLDL-C Very low-density lipoprotein cholesterol.
***p < 0.0001; **p < 0.01; *p < 0.05.
An insignificant effect of 2 h-OGTT-glucose was seen on the controls' blood pressure and lipid profile. In the GDM group, a significant positive effect of 2 h-OGTT was observed on systolic blood pressure (model 1: t value = 11.2; model 2: t value = 16.5), diastolic blood pressure (model 2: t value = 6.54) and lipid profile determinants (TC- model 1 & 2: t value = 3.25 & 13.4 respectively; Tg- model 2: t value = 13.7; HDL-C- model 2: t value = − 11.0; LDL-C- model 1 & 2 t-value = 2.0 &13.7 respectively; VLDL-C: t value = 13.7); except HDL-C showing a negative impact at p < 0.001. In the PE group, 2 h-OGTT-glucose portrays a significant effect on systolic blood pressure (model 1: t value = 9.13; model 2: t value = 9.89), diastolic blood pressure (model 2: t value = 2.12), Tg (model 1 & 2: t value = 2.6 & 4.4 respectively) , HDL-C (model 1 & 2: t value = 1.9 & 3.4 respectively), LDL-C (model 2: t value = 2.5), and VLDL-C (model 2: t value = 4.4), at p < 0.001, p < 0.01, and p < 0.05.
Discussion
GDM demonstrates imbalanced vascular, metabolic, and inflammatory processes by distinguishably accelerated concentrations of inflammatory molecules and placental genes encoding for inflammatory mediators17,18. GDM is a known risk factor for stillbirth, fetal macrosomia, fetal structural anomalies, premature delivery, and gestational hypertensive disorders19. GDM is often diagnosed in the second trimester of pregnancy. Over time, excessive glucose levels may be attributed to gestational hypertension disorders and related intricacies20.
In the present study, advanced maternal age is observed in the GDM and PE groups, consistent with the studies suggesting that older age can be a risk factor for the onset of GDM21,22. Other risk factors like multiple pregnancies and higher pre-pregnancy BMI are also disclosed in the pathogenesis of PE in women with early-onset GDM23. Alfadhli et al. reported the high prevalence of GDM in Saudi women due to older age, increased BMI, and hypertension24. A cohort study assessing the risk of PE in women diagnosed with GDM documented that the risk was eight times higher when GDM is detected during 20 weeks of pregnancy25. Our study participants in both GDM and PE groups have 26–28 weeks of pregnancy and elevated BMI with no statistical difference, suggesting that GDM in later weeks of pregnancy is likely to worsen the condition. Progressive maternal and gestation age altogether may be the reason for GDM and its exaggerated manifestation like PE. A study by Erkamp et al.20 reported the positive correlation of non-fasting glucose concentrations with blood pressure in early pregnancy, but not in later pregnancy.
Diabetes and hypertension frequently coexist and share risk factors and disease etiologies including genetics, obesity, insulin resistance, and inflammation26. The current study observed a significant impact of OGTT glucose on systolic and diastolic blood pressure in GDM and PE women. However, significant differences in OGTT glucose and systolic and diastolic blood pressure in GDM and PE groups suggest that the probable reason could be persistently elevated glucose to later trimester affecting the blood pressure. Various studies expressed that the risk of gestational hypertensive disorders and PE is significantly raised by GDM6,7. An earlier prospective research of healthy nulliparous women revealed that the probability of PE was positively linked with fasting blood glucose concentrations, even within the normal range27. On the contrary, a study described that normal glucose concentrations in early pregnancy are not associated with gestational hypertensive disorders20. It has been suggested that moderate exercising in pregnant women with GDM helps in controlling pregnancy weight and blood sugar levels, but it has little impact on the development of PE28,29. In a recent randomized trial, it was observed that gestational diabetics receiving proper immediate treatment before 20 weeks had a lower incidence of adverse effects on neonates30.
The presence of persistently increased glucose concentrations during pregnancy have a dramatic influence on lipid profile1. In the present line of work, GDM and PE groups present that hyperlipidemic profile is significantly affected by OGTT glucose (p < 0.001). Numerous effects like maternal risk of chronic hypertension, cardiovascular disease, diabetes, perinatal adverse events, and increased offspring BMI are noticed upon GDM complicated by PE31,32. Excessive lipid accumulation inside endothelial cells can lead to endothelial dysfunction and reduced prostacyclin release, which is a crucial factor in the development of PE33,34. However, the effect of GDM on dyslipidemia has been disregarded in routine screening, which might be the risk factor for PE. Dyslipidemia is a metabolic disorder defined by elevated levels of LDL-C, Tg, and low levels of HDL-C1. Our regression models in the GDM and PE groups align with other research reports which have documented an increase in TC, Tg, LDL-C, and a decrease in HDL-C concentrations associated with PE risk35–37. The National Health and Nutrition Examination Survey's research of parous women revealed no significant correlation between GDM and rising levels of LDL-C38. On the other hand, women who have had GDM exhibit a greater frequency of dyslipidemia than their normoglycemic counterparts39. Conflicting results have been reported while elucidating the relation between maternal lipid profile and PE40–43. Recent evidence reported deregulations of feto-maternal lipid metabolism are related to the pathogenesis of PE. Wojcik-Baszko et al.44 found dyslipidemia positively associated with PE11.
Nulliparous subjects diagnosed with gestational diabetes after 20 weeks of pregnancy strengthen the present study by depicting the need for monitoring blood glucose levels in early pregnancy to prevent the risk of developing maternal and fetal complications. It is important to note that this study represents its regional importance and has not been previously documented in Abha. However, the study is monocentric, and risk factors such as early pregnancy glucose concentrations and gestational weight gain were not assessed, which may affect the generalizability of the results. Examining these factors could provide valuable insights into the links between blood pressure, disrupted lipid levels, and other hypertensive disorders of pregnancy.
Conclusions
The current study indicates that glucose intolerance during the later weeks of pregnancy is associated with gestational hypertension and hyperlipidemia as a risk factor for PE. Further research is needed for a detailed assessment of maternal glucose metabolism at various pregnancy stages including the use of more sensitive markers such as C-peptide and their relation to pregnancy-related hypertensive disorders.
Acknowledgements
The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through Small group Research Project under Grant Number RGP1/189/44.
Abbreviations
- GDM
Gestational diabetes mellitus
- PE
Preeclampsia
- OGTT
Oral glucose tolerance test
- TC
Total cholesterol
- Tg
Triglyceride
- HDL-C
High density lipoprotein-cholesterol
- LDL-C
Low density lipoprotein-cholesterol
- uACR
Urinary albumin creatinine ratio
- IADPSG
International Association of Diabetes and Pregnancy Study Groups
- HAPO
Hyperglycemia and Adverse Pregnancy Outcome
Author contributions
F.A. Conceptualizing, planning, funding acquisition, methodology, and writing the manuscript; M.F.K. Statistical analysis, data curation, writing, review, editing; A.M. writing, review, editing. All authors read and approved the final manuscript.
Data availability
All data is included in this article.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Yang Y, Wu N. Gestational diabetes mellitus and preeclampsia: Correlation and influencing factors. Front. Cardiovasc. Med. 2022;9:831297. doi: 10.3389/fcvm.2022.831297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Metzger BE, Buchanan TA, Coustan DR, de Leiva A, Dunger DB, Hadden DR, et al. Summary and recommendations of the fifth international workshop-conference on gestational diabetes mellitus. Diabetes Care. 2007;30 Suppl 2:S251–S260. doi: 10.2337/dc07-s225. [DOI] [PubMed] [Google Scholar]
- 3.Metzger BE, et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33(3):676–682. doi: 10.2337/dc09-1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vounzoulaki E, Khunti K, Abner SC, Tan BK, Davies MJ, Gillies CL. Progression to type 2 diabetes in women with a known history of gestational diabetes: Systematic review and meta-analysis. BMJ. 2020;13:m1361. doi: 10.1136/bmj.m1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kramer CK, Campbell S, Retnakaran R. Gestational diabetes and the risk of cardiovascular disease in women: A systematic review and meta-analysis. Diabetologia. 2019;62(6):905–914. doi: 10.1007/s00125-019-4840-2. [DOI] [PubMed] [Google Scholar]
- 6.Boulton, P. A., Magliano, P. D. & Boyko, P. E. IDF Diabetes Atlas 2021—10th edition. J. Diabetes Res. (2021).
- 7.von Dadelszen P, Magee LA. Preventing deaths due to the hypertensive disorders of pregnancy. Best Pract. Res. Clin. Obstet. Gynaecol. 2016;36:83–102. doi: 10.1016/j.bpobgyn.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Magee LA, Smith GN, Bloch C, Côté AM, Jain V, Nerenberg K, et al. Guideline No. 426: Hypertensive disorders of pregnancy: Diagnosis, prediction, prevention, and management. J. Obstet. Gynaecol. Can. 2022;44(5):547–571. doi: 10.1016/j.jogc.2022.03.002. [DOI] [PubMed] [Google Scholar]
- 9.Subki AH, Algethami MR, Baabdullah WM, Alnefaie MN, Alzanbagi MA, Alsolami RM, et al. Prevalence, risk factors, and fetal and maternal outcomes of hypertensive disorders of pregnancy: A retrospective study in Western Saudi Arabia. Oman Med. J. 2018;33(5):409–415. doi: 10.5001/omj.2018.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chodick G, Tenne Y, Barer Y, Shalev V, Elchalal U. Gestational diabetes and long-term risk for dyslipidemia: A population-based historical cohort study. BMJ Open Diabetes Res Care. 2020;8(1):e000870. doi: 10.1136/bmjdrc-2019-000870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al-Maiahy T, Al-Gareeb A, Al-Kuraishy H. Role of dyslipidemia in the development of early-onset preeclampsia. J. Adv. Pharm. Technol. Res. 2021;12(1):73. doi: 10.4103/japtr.JAPTR_104_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patra S, Bhattacharya S, Bala A, Haldar PK. Antidiabetic effect of Drymaria cordata leaf against streptozotocin-nicotinamide-induced diabetic albino rats. J. Adv. Pharm. Technol. Res. 2020;11(1):44–52. doi: 10.4103/japtr.JAPTR_98_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elsayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, et al. 15. Management of diabetes in pregnancy: Standards of care in diabetes—2023. Diabetes Care. 2023;46:S254–S266. doi: 10.2337/dc23-S015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tarry-Adkins JL, Aiken CE, Ozanne SE. Comparative impact of pharmacological treatments for gestational diabetes on neonatal anthropometry independent of maternal glycaemic control: A systematic review and meta-analysis. PLoS Med. 2020;17(5):e1003126. doi: 10.1371/journal.pmed.1003126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sénat MV, Affres H, Letourneau A, Coustols-Valat M, Cazaubiel M, Legardeur H, et al. Effect of glyburide vs subcutaneous insulin on perinatal complications among women with gestational diabetes: A randomized clinical trial. JAMA. 2018;319(17):1773–1780. doi: 10.1001/jama.2018.4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.American Diabetes Association 2. Classification and diagnosis of diabetes. Diabetes Care. 2015;38(Supplement_1):S8–S16. doi: 10.2337/dc15-S005. [DOI] [PubMed] [Google Scholar]
- 17.Lowe LP, Metzger BE, Dyer AR, Lowe J, McCance DR, Lappin TRJ, et al. Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study: associations of maternal A1C and glucose with pregnancy outcomes. Diabetes Care. 2012;35(3):574–580. doi: 10.2337/dc11-1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hauguel-de Mouzon S, Guerre-Millo M. The placenta cytokine network and inflammatory signals. Placenta. 2006;27(8):794–798. doi: 10.1016/j.placenta.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 19.ACOG Practice Bulletin No. 190 Gestational diabetes mellitus. Obstet. Gynecol. 2018;131(2):e49–64. doi: 10.1097/AOG.0000000000002501. [DOI] [PubMed] [Google Scholar]
- 20.Erkamp JS, Geurtsen ML, Duijts L, Reiss IKM, Mulders AGMGJ, Steegers EAP, et al. Associations of maternal early-pregnancy glucose concentrations with placental hemodynamics, blood pressure, and gestational hypertensive disorders. Am. J. Hypertens. 2020;33(7):660–669. doi: 10.1093/ajh/hpaa070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Immanuel J, Simmons D. Screening and treatment for early-onset gestational diabetes mellitus: a systematic review and meta-analysis. Curr. Diab. Rep. 2017;17(11):115. doi: 10.1007/s11892-017-0943-7. [DOI] [PubMed] [Google Scholar]
- 22.Boriboonhirunsarn D, Sunsaneevithayakul P, Pannin C, Wamuk T. Prevalence of early-onset GDM and associated risk factors in a university hospital in Thailand. J. Obstet. Gynaecol. (Lahore). 2021;41(6):915–919. doi: 10.1080/01443615.2020.1820469. [DOI] [PubMed] [Google Scholar]
- 23.Mustafa M, Bogdanet D, Khattak A, Carmody LA, Kirwan B, Gaffney G, et al. Early gestational diabetes mellitus (GDM) is associated with worse pregnancy outcomes compared with GDM diagnosed at 24–28 weeks gestation despite early treatment. QJM Int. J. Med. 2021;114(1):17–24. doi: 10.1093/qjmed/hcaa167. [DOI] [PubMed] [Google Scholar]
- 24.Alfadhli EM, Osman EN, Basri TH, Mansuri NS, Youssef MH, Assaaedi SA, et al. Gestational diabetes among Saudi women: Prevalence, risk factors and pregnancy outcomes. Ann. Saudi Med. 2015;35(3):222–230. doi: 10.5144/0256-4947.2015.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Phaloprakarn C, Tangjitgamol S. Risk assessment for preeclampsia in women with gestational diabetes mellitus. JPME. 2009;37(6):617–621. doi: 10.1515/JPM.2009.108. [DOI] [PubMed] [Google Scholar]
- 26.Cheung BMY, Li C. Diabetes and hypertension: Is there a common metabolic pathway? Curr. Atheroscler. Rep. 2012;14(2):160–166. doi: 10.1007/s11883-012-0227-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Joffe GM, Esterlitz JR, Levine RJ, Clemens JD, Ewell MG, Sibai BM, et al. The relationship between abnormal glucose tolerance and hypertensive disorders of pregnancy in healthy nulliparous women. Calcium for Preeclampsia Prevention (CPEP) Study Group. Am. J. Obstet. Gynecol. 1998;179(4):1032–1037. doi: 10.1016/S0002-9378(98)70210-8. [DOI] [PubMed] [Google Scholar]
- 28.Yang X, Tian H, Zhang F, Zhang C, Li Y, Leng J, et al. A randomised translational trial of lifestyle intervention using a 3-tier shared care approach on pregnancy outcomes in Chinese women with gestational diabetes mellitus but without diabetes. J. Transl. Med. 2014;12(1):290. doi: 10.1186/s12967-014-0290-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown, J., Ceysens, G. & Boulvain, M. Exercise for pregnant women with pre-existing diabetes for improving maternal and fetal outcomes. Cochrane Database Syst. Rev. 2017(12) (2017). [DOI] [PMC free article] [PubMed]
- 30.Simmons D, Immanuel J, Hague WM, Teede H, Nolan CJ, Peek MJ, et al. Treatment of gestational diabetes mellitus diagnosed early in pregnancy. N. Engl. J. Med. 2023;388(23):2132–2144. doi: 10.1056/NEJMoa2214956. [DOI] [PubMed] [Google Scholar]
- 31.Kul Ş, Güvenç TS, Baycan ÖF, Çelik FB, Çalışkan Z, Çetin Güvenç R, et al. Combined past preeclampsia and gestational diabetes is associated with a very high frequency of coronary microvascular dysfunction. Microvasc. Res. 2021;134:104104. doi: 10.1016/j.mvr.2020.104104. [DOI] [PubMed] [Google Scholar]
- 32.Huang Y, Zhang W, Go K, Tsuchiya KJ, Hu J, Skupski DW, et al. Altered growth trajectory in children born to mothers with gestational diabetes mellitus and preeclampsia. Arch. Gynecol. Obstet. 2020;301(1):151–159. doi: 10.1007/s00404-020-05436-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ghio A, Bertolotto A, Resi V, Volpe L, Di Cianni G. Triglyceride metabolism in pregnancy. Adv. Clin. Chem. 2011;55:133–153. doi: 10.1016/B978-0-12-387042-1.00007-1. [DOI] [PubMed] [Google Scholar]
- 34.Zhang X, Sun D, Song JW, Zullo J, Lipphardt M, Coneh-Gould L, et al. Endothelial cell dysfunction and glycocalyx—A vicious circle. Matrix Biol. 2018;71–72:421–431. doi: 10.1016/j.matbio.2018.01.026. [DOI] [PubMed] [Google Scholar]
- 35.Bayhan G, Koçyigit Y, Atamer A, Atamer Y, Akkus Z. Potential atherogenic roles of lipids, lipoprotein(a) and lipid peroxidation in preeclampsia. Gynecol. Endocrinol. 2005;21(1):1–6. doi: 10.1080/09513590500097382. [DOI] [PubMed] [Google Scholar]
- 36.Sahu S, Abraham R, Vedavalli R, Daniel M. Study of lipid profile, lipid peroxidation and vitamin E in pregnancy induced hypertension. Indian J. Physiol. Pharmacol. 2009;53(4):365–369. [PubMed] [Google Scholar]
- 37.Uzun H, Benian A, Madazli R, Topçuoğlu MA, Aydin S, Albayrak M. Circulating oxidized low-density lipoprotein and paraoxonase activity in preeclampsia. Gynecol. Obstet. Investig. 2005;60(4):195–200. doi: 10.1159/000087205. [DOI] [PubMed] [Google Scholar]
- 38.Shostrom DCV, Sun Y, Oleson JJ, Snetselaar LG, Bao W. History of gestational diabetes mellitus in relation to cardiovascular disease and cardiovascular risk factors in US women. Front. Endocrinol. (Lausanne). 2017;8:144. doi: 10.3389/fendo.2017.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ajala O, Jensen LA, Ryan E, Chik C. Women with a history of gestational diabetes on long-term follow up have normal vascular function despite more dysglycemia, dyslipidemia and adiposity. Diabetes Res. Clin. Pract. 2015;110(3):309–314. doi: 10.1016/j.diabres.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 40.Thathagari V, Veerendra Kumar CM. Evaluation of serum lipids in preeclampsia: A comparative study. Int. J. Reprod. Contracept. Obstet. Gynecol. 2018;7(4):1372. doi: 10.18203/2320-1770.ijrcog20180998. [DOI] [Google Scholar]
- 41.Gallos ID, Sivakumar K, Kilby MD, Coomarasamy A, Thangaratinam S, Vatish M. Pre-eclampsia is associated with, and preceded by, hypertriglyceridaemia: A meta-analysis. BJOG. 2013;120(11):1321–1332. doi: 10.1111/1471-0528.12375. [DOI] [PubMed] [Google Scholar]
- 42.Agarwal V, Gupta BK, Vishnu A, Kiran J. Association of lipid profile and uric acid with pre-eclampsia of third trimester in nullipara women. J Clin Diagn Res. 2014;8(7):CC04–CC07. doi: 10.7860/JCDR/2014/7901.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baumfeld Y, Novack L, Wiznitzer A, Sheiner E, Henkin Y, Sherf M, et al. Pre-conception dyslipidemia is associated with development of preeclampsia and gestational diabetes mellitus. PLoS One. 2015;10(10):e0139164. doi: 10.1371/journal.pone.0139164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wojcik-Baszko D, Charkiewicz K, Laudanski P. Role of dyslipidemia in preeclampsia—A review of lipidomic analysis of blood, placenta, syncytiotrophoblast microvesicles and umbilical cord artery from women with preeclampsia. Prostaglandins Other Lipid Mediat. 2018;139:19–23. doi: 10.1016/j.prostaglandins.2018.09.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data is included in this article.