Abstract
Real-world data on coronary events (CE) in elderly patients with atrial fibrillation (AF) are lacking in the direct oral anticoagulant era. This prespecified sub-analysis of the ANAFIE Registry, a prospective observational study in > 30,000 Japanese patients aged ≥ 75 years with non-valvular AF (NVAF), investigated CE incidence and risk factors. The incidence and risk factors for new-onset CE (a composite of myocardial infarction [MI] and cardiac intervention for coronary heart diseases other than MI), MI, and cardiac intervention for coronary heart diseases other than MI during the 2-year follow-up were assessed. Bleeding events in CE patients were also examined. Among 32,275 patients, the incidence rate per 100 patient-years was 0.48 (95% confidence interval (CI): 0.42–0.53) for CE during the 2-year follow-up, 0.20 (0.16–0.23) for MI, and 0.29 (0.25–0.33) for cardiac intervention for coronary heart diseases other than MI; that of stroke/systemic embolism was 1.62 (1.52–1.73). Patients with CE (n = 287) likely had lower creatinine clearance (CrCL) and higher CHADS2 and HAS-BLED scores than patients without CE (n = 31,988). Significant risk factors associated with new-onset CE were male sex, systolic blood pressure of ≥ 130 mmHg, diabetes mellitus (glycated hemoglobin ≥ 6.0%), CE history, antiplatelet agent use, and CrCL < 50 mL/min. Major bleeding incidence was significantly higher in patients with new-onset CE vs without CE (odds ratio [95% CI], 3.35 [2.06–5.43]). In elderly patients with NVAF, CE incidence was lower than stroke/systemic embolism incidence. New-onset CE (vs no CE) was associated with a higher incidence of major bleeding.
Trial registration: UMIN000024006.
Graphical Abstract
Keywords: Coronary events, Elderly, Non-valvular atrial fibrillation, Coronary artery disease, Oral anticoagulant
Introduction
Atrial fibrillation (AF) is a major risk factor for stroke [1]. This is particularly relevant for elderly patients as the prevalence of AF increases with advancing age from 0.12–0.16% in people aged < 49 years to 1.7–4.0% among those aged 60–70 years; proportions may be as high as 13.5–17.8% among those aged > 80 years [2–4]. Both AF and coronary artery disease (CAD) are common cardiovascular conditions encountered in daily clinical practice in elderly patients. The diseases tend to coexist because of shared risk factors, such as hypertension, diabetes, advanced age, obesity, and smoking, and they have similar pathophysiological features, such as inflammation [5]. CAD is more common in patients with AF, ranging between 17% and 46.5% [6, 7]. Furthermore, several studies have reported that comorbid CAD and AF aggravate one another [8, 9]. AF is associated with a twofold increase in the risk of myocardial infarction (MI) [7]. According to the Framingham study, patients with AF and heart disease have a 2.2-times higher probability of developing new coronary events (CE) compared with patients with heart disease without AF [9]. CAD is the third leading cause of death worldwide, with approximately 18 million deaths annually attributed to CAD [10].
Patients with both CAD and AF pose additional challenges in terms of treatment—which includes rhythm management, anticoagulants, and antiplatelet agents (APA)—and may require more complex treatment strategies to mitigate possible increases in bleeding risk [5, 11]. The Japanese open-label AFIRE trial suggested that oral anticoagulant (OAC) monotherapy was superior for safety compared with OAC and APA combination therapy in patients with AF and CAD [12]. Additionally, a temporal association between major bleeding and subsequent cardiovascular events and death in patients with AF and stable CAD has been demonstrated [13].
Although clinical outcomes in AF patients based on the presence or absence of CAD (e.g., MI or percutaneous coronary intervention [PCI]) have been reported [10, 14–18], real-world data on the incidence and risk factors of CE in elderly patients—who are at the highest risk of events—are lacking, especially in the era of direct OACs (DOACs). The All Nippon Atrial Fibrillation In the Elderly (ANAFIE) Registry aimed to clarify the real-world clinical status and prognosis of elderly patients with non-valvular AF (NVAF) in Japan. Over 30,000 elderly (≥ 75 years of age) Japanese patients with NVAF were enrolled and followed up for 2 years to investigate anticoagulation therapy status and outcomes in routine clinical practice. The 2-year follow-up data [19] and several sub-analyses have been published [20–24].
The main objective of this prespecified sub-analysis of the ANAFIE Registry was to investigate the incidence and risk factors of CE in elderly Japanese patients with NVAF. The occurrence of bleeding events in CE patients was also examined.
Methods
Study design
The ANAFIE Registry was a multicenter, prospective, observational study conducted at 1273 sites across Japan between 2016 and 2020. Details of the study design and rationale have been published [25]. The trial was registered in the UMIN Clinical Trials Registry under the identifier UMIN000024006. The study was compliant with the Declaration of Helsinki and local requirements for registries. Ethics committees approved the study protocol. Written informed consent was obtained from patients or family members in case of communication disorders (i.e., aphasia) or cognitive impairment.
Patients
Enrolled outpatients were men and women ≥ 75 years of age, diagnosed with NVAF by electrocardiography, who were able to attend hospital visits. Patients were excluded from enrollment if they were participating/planning to participate in an interventional study; had a definite diagnosis of mitral stenosis or artificial heart valve replacement (either mechanical or tissue valve prostheses), or had experienced very recent cardiovascular events, including stroke, MI, cardiac intervention, heart failure requiring hospitalization, or any bleeding leading to hospitalization within 1 month prior to enrollment; life expectancy of < 1 year; or who were deemed inappropriate for participation by treating physicians.
Study endpoints
Specifically, in this pre-specified sub-analysis, we assessed the incidence for new-onset CE (defined as a composite of MI and cardiac intervention for coronary heart diseases other than MI), MI, cardiac intervention for coronary heart diseases other than MI, major bleeding, clinically relevant non-major bleeding (CRNMB), intracranial hemorrhage (ICH), and gastrointestinal (GI) bleeding during the 2-year follow-up period. Major bleeding was classified using the International Society on Thrombosis and Haemostasis definition.
Statistical analysis
The Kaplan–Meier method was used to estimate the probability of occurrence of CE and other clinical events. The incidences of CE and other clinical events were also estimated as incidence rates per 100 person-years with 95% confidence intervals (CIs). A multivariate analysis was performed to identify risk factors of CE calculated using the Cox proportional hazards model. This analysis was also performed by combining the history of CAD and APA use. For bleeding events, based on the presence or absence of CAD, odds ratios (ORs) were evaluated using a logistic regression model adjusted for prognostic factors. Statistical tests were two-sided, with a significance level of 5%. The statistical software used for these analyses was SAS version 9.4 (SAS Institute, Tokyo, Japan).
Results
Patient disposition and characteristics
Of the 32,275 patients analyzed in the ANAFIE Registry, 287 developed CE (MI, cardiac intervention for coronary heart diseases other than MI) (0.89%). Table 1 shows the characteristics of patients with new-onset CE and those without CE. Significantly more men than women had CE vs no CE. Creatinine clearance (CrCL) was significantly lower, and CHADS2 and HAS-BLED scores were significantly higher in patients with CE vs those without CE. Similarly, significantly higher proportions of patients with CE had diabetes mellitus, dyslipidemia, a history of CAD including prior MI and/or angina, a history of cerebrovascular diseases including lacunar infarction and peripheral arterial disease and falls within 1 year.
Table 1.
Background characteristics of patients at baseline by the presence of new-onset coronary events
| Total N = 32,275 | CE n = 287 | No CE n = 31,988 | P valuea | |
|---|---|---|---|---|
| Male | 18,482 (57.3) | 210 (73.2) | 18,272 (57.1) | < 0.001 |
| Age, years | 81.5 ± 4.8 | 81.2 ± 4.5 | 81.5 ± 4.8 | 0.438 |
| Body mass index, kg/m2 | 23.3 ± 3.6 | 23.4 ± 3.3 | 23.3 ± 3.6 | 0.875 |
| SBP, mmHg | 127.4 ± 17.0 | 128.5 ± 17.8 | 127.3 ± 17.0 | 0.267 |
| DBP, mmHg | 70.6 ± 11.6 | 69.3 ± 11.5 | 70.7 ± 11.6 | 0.056 |
| Creatinine clearance, mL/min | 48.4 ± 18.2 | 44.1 ± 16.6 | 48.4 ± 18.2 | < 0.001 |
| CHADS2 score | 2.9 ± 1.2 | 3.3 ± 1.3 | 2.9 ± 1.2 | < 0.001 |
| HAS-BLED score | 1.9 ± 0.9 | 2.2 ± 1.0 | 1.9 ± 0.9 | < 0.001 |
| History of major bleeding | 1439 (4.5) | 14 (4.9) | 1425 (4.5) | 0.729 |
| AF type | ||||
| Paroxysmal | 13,586 (42.1) | 129 (44.9) | 13,457 (42.1) | 0.397 |
| Persistent | 9701 (30.1) | 76 (26.5) | 9625 (30.1) | – |
| Permanent | 8988 (27.8) | 82 (28.6) | 8906 (27.8) | – |
| OACs | 29,830 (92.4) | 265 (92.3) | 29,565 (92.4) | 0.954 |
| Warfarin | 8233 (25.5) | 81 (28.2) | 8152 (25.5) | 0.278 |
| DOACs | 21,585 (66.9) | 184 (64.1) | 21,401 (66.9) | 0.285 |
| History of non-pharmacological therapy for AF | 5677 (17.6) | 56 (19.5) | 5621 (17.6) | 0.390 |
| Catheter ablation | 2970 (9.2) | 24 (8.4) | 2946 (9.2) | 0.621 |
| Comorbidities | ||||
| Hypertension | 24,312 (75.3) | 228 (79.4) | 24,084 (75.3) | 0.104 |
| Diabetes mellitus | 8733 (27.1) | 120 (41.8) | 8613 (26.9) | < 0.001 |
| Dyslipidemia | 13,728 (42.5) | 156 (54.4) | 13,572 (42.4) | < 0.001 |
| Chronic kidney disease | 6705 (20.8) | 66 (23.0) | 6639 (20.8) | 0.351 |
| Myocardial infarction | 1851 (5.7) | 52 (18.1) | 1799 (5.6) | < 0.001 |
| Angina | 5521 (17.1) | 117 (40.8) | 5404 (16.9) | < 0.001 |
| Heart failure, left ventricular systolic dysfunction | 12,116 (37.5) | 123 (42.9) | 12,154 (38.0) | 0.091 |
| Cerebrovascular disease | 7303 (22.6) | 85 (29.6) | 7218 (22.6) | 0.005 |
| Atherosclerotic infarction | 655 (2.0) | 8 (2.8) | 647 (2.0) | 0.360 |
| Cardiogenic infarction | 2377 (7.4) | 23 (8.0) | 2354 (7.4) | 0.672 |
| Lacunar infarction | 1436 (4.4) | 20 (7.0) | 1416 (4.4) | 0.038 |
| Peripheral arterial diseaseb | 1931 (6.0) | 41 (14.3) | 1890 (5.9) | < 0.001 |
| Fall within 1 year | 2347 (7.3) | 30 (10.5) | 2317 (7.2) | 0.026 |
| Antiplatelet agents | 5704 (17.7) | 121 (42.1) | 5583 (17.5) | < 0.001 |
Data are presented as n (%) or mean ± standard deviation
AF atrial fibrillation; CE coronary event; DBP diastolic blood pressure; DOAC direct oral anticoagulant; OAC oral anticoagulant; SBP systolic blood pressure
aComparison between CE and No CE groups
bAortic plaque, internal carotid artery stenosis, and arteriosclerosis obliterans
Incidence of events
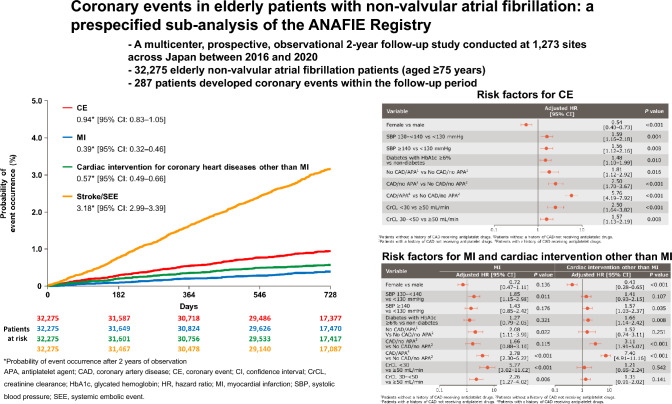
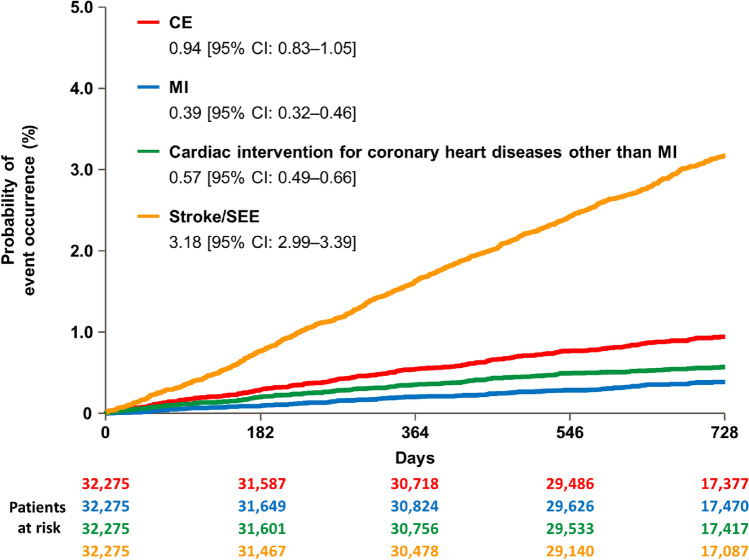
Figure 1 shows the probability of event occurrence for each clinical outcome. The probability of occurrence of CE after 2 years of observation was 0.94% [95% CI: 0.83–1.05]), which was lower than that of stroke/systemic embolic events (SEE) (3.18% [2.99–3.39]).
Fig. 1.
Kaplan–Meier curves for coronary events and stroke/SEE. Each occurrence and 95% CI show the data at 2 years. CE coronary event; MI myocardial infarction; SEE systemic embolic event
The incidence rate was 0.48 per 100 patient-years (95% CI: 0.42–0.53) for CE, 0.20 (0.16–0.23) for MI, and 0.29 (0.25–0.33) for that of cardiac intervention for coronary heart diseases other than MI (Table 2); these were lower than that of stroke/SEE, which was 1.62 (1.52–1.73) in the main analysis of the ANAFIE Registry [19].
Table 2.
Incidence rates of coronary events and stroke/SEE
| CE | MI | Cardiac intervention for coronary heart diseases other than MI | Stroke/SEEa | |
|---|---|---|---|---|
| Incidence, n (%) | 287 (0.89) | 118 (0.37) | 175 (0.54) | 970 (3.01) |
| Incidence rate (per 100 patient-years, [95% CI]) | 0.48 [0.42–0.53] | 0.20 [0.16–0.23] | 0.29 [0.25–0.33] | 1.62 [1.52–1.73] |
N = 32,275
CE coronary event; CI confidence interval; MI myocardial infarction; SEE systemic embolic event
aData from Yamashita et al. Eur Heart J Qual Care Clin Outcomes 2022;8:202–213 [19]
Risk factors of CE
The risk factors associated with new-onset CE were male sex, systolic blood pressure ≥ 130 mmHg, diabetes mellitus with glycated hemoglobin (HbA1c) ≥ 6.0%, CAD history with and without APA use, APA use without CAD history, and CrCL < 50 mL/min (Table 3). Of note, prior CAD and APA use each were significant risk factors, and their co-occurrence increased the risk even further.
Table 3.
Risk factors for new-onset CE, MI, and cardiac intervention for coronary heart diseases other than MI during follow-up, results of a multivariate analysis
| CE | MI | Cardiac intervention for coronary heart diseases other than MI | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Event (%) | HR (95% CI) | P value | N | Event (%) | HR (95% CI) | P value | N | Event (%) | HR (95% CI) | P value | |
| Overall | 32,275 | 287 (0. 9) | – | 32,275 | 118 (0.4) | – | 32,275 | 175 (0.5) | – | |||
| Sex | ||||||||||||
| Malea | 18,482 | 210 (1.1) | – | 18,482 | 75 (0.4) | – | 18,482 | 139 (0.8) | – | |||
| Female | 13,793 | 77 (0. 6) | 0.54 (0.40–0.73) | < 0.001 | 13,793 | 43 (0.3) | 0.72 (0.47–1.11) | 0.136 | 13,793 | 36 (0.3) | 0.43 (0.28–0.65) | < 0.001 |
| Age, years | ||||||||||||
| < 85a | 23,856 | 225 (0.9) | – | 23,856 | 84 (0.4) | – | 23,856 | 147 (0.6) | – | |||
| ≥ 85 | 8419 | 62 (0.7) | 0.80 (0.58–1.10) | 0.168 | 8419 | 34 (0.4) | 0.90 (0.57–1.43) | 0.664 | 8419 | 28 (0.3) | 0.67 (0.43–1.05) | 0.079 |
| Body mass index (kg/m2) | ||||||||||||
| < 18.5 | 2059 | 11 (0.5) | 0.49 (0.23–1.06) | 0.069 | 2059 | 4 (0.2) | 0.26 (0.06–1.06) | 0.060 | 2059 | 7 (0.3) | 0.72 (0.29–1.78) | 0.474 |
| > 18.5 and < 25.0a | 17,621 | 167 (0.9) | – | 17,621 | 70 (0.4) | – | 17,621 | 101 (0.6) | – | |||
| ≥ 25.0 | 8090 | 64 (0.8) | 0.89 (0.65–1.21) | 0.455 | 8090 | 27 (0.3) | 0.99 (0.62–1.60) | 0.977 | 8090 | 39 (0.5) | 0.83 (0.56–1.24) | 0.368 |
| Smoking habit | ||||||||||||
| Yes (current smoker) | 1243 | 12 (1.0) | 0.94 (0.53–1.69) | 0.843 | 1243 | 5 (0.4) | 1.07 (0.43–2.65) | 0.885 | 1243 | 8 (0.6) | 0.96 (0.47–1.96) | 0.908 |
| None (never smoked or stopped)a | 26,068 | 228 (0.9) | – | 26,068 | 97 (0.4) | – | 26,068 | 136 (0.5) | – | |||
| Blood pressure | ||||||||||||
| SBP < 130 mmHga | 16,218 | 125 (0.8) | – | 16,218 | 51 (0.3) | – | 16,218 | 77 (0.5) | – | |||
| SBP ≥ 130 mmHg to < 140 mmHg | 6937 | 72 (1.0) | 1.59 (1.16–2.18) | 0.004 | 6937 | 32 (0.5) | 1.85 (1.15–2.98) | 0.011 | 6937 | 42 (0.6) | 1.41 (0.93–2.15) | 0.107 |
| SBP ≥ 140 mmHg | 6528 | 65 (1.0) | 1.56 (1.12–2.16) | 0.008 | 6528 | 24 (0.4) | 1.43 (0.85–2.42) | 0.176 | 6528 | 41 (0.6) | 1.57 (1.03–2.37) | 0.035 |
| Diabetes mellitus | ||||||||||||
| Presence (HbA1c < 6.0%) | 1259 | 11 (0.9) | 0.91 (0.46–1.80) | 0.787 | 1259 | 5 (0.4) | 1.00 (0.36–2.76) | 0.993 | 1259 | 6 (0.5) | 0.82 (0.33–2.04) | 0.669 |
| Presence (HbA1c ≥ 6.0%) | 5824 | 87 (1.5) | 1.48 (1.10–1.99) | 0.010 | 5824 | 29 (0.5) | 1.27 (0.79–2.05) | 0.321 | 5824 | 61 (1.0) | 1.66 (1.14–2.42) | 0.008 |
| Nonea | 23,542 | 167 (0.7) | – | 23,542 | 75 (0.3) | – | 23,542 | 95 (0.4) | – | |||
| Heart failure, left ventricular systolic dysfunction | ||||||||||||
| Yes | 12,277 | 123 (1.00) | 1.15 (0.88–1.51) | 0.298 | 12277 | 50 (0.41) | 0.96 (0.63–1.46) | 0.855 | 12,277 | 77 (0.6) | 1.37 (0.97–1.93) | 0.070 |
| Nonea | 19,998 | 164 (0.8) | – | 19,998 | 68 (0.3) | – | 19,998 | 98 (0.5) | – | |||
| Previous CAD and/or taking APA | ||||||||||||
| No CAD/no APAa | 22,908 | 115 (0.5) | – | 22,908 | 55 (0.2) | – | 22,908 | 63 (0.3) | – | |||
| No CAD/with APA | 2653 | 23 (0.9) | 1.81 (1.12–2.92) | 0.016 | 2653 | 14 (0.5) | 2.08 (1.11–3.90) | 0.022 | 2653 | 10 (0.4) | 1.52 (0.74–3.11) | 0.251 |
| With CAD/no APA | 3663 | 51 (1.4) | 2.50 (1.70–3.67) | < 0.001 | 3663 | 16 (0.4) | 1.66 (0.88–3.10) | 0.115 | 3663 | 35 (1.0) | 3.11 (1.91–5.07) | < 0.001 |
| With CAD/with APA | 3051 | 98 (3.2) | 5.76 (4.19–7.92) | < 0.001 | 3051 | 33 (1.1) | 3.78 (2.30–6.22) | < 0.001 | 3051 | 67 (2.2) | 7.40 (4.91–11.16) | < 0.001 |
| Anticoagulants | ||||||||||||
| Warfarina | 8233 | 81 (1.0) | – | 8233 | 39 (0.5) | – | 8233 | 42 (0.5) | – | |||
| None | 2445 | 22 (0.9) | 0.83 (0.49–1.40) | 0.486 | 2445 | 11 (0.4) | 1.03 (0.51–2.06) | 0.937 | 2445 | 12 (0.5) | 0.74 (0.34–1.61) | 0.447 |
| DOAC | 21,585 | 184 (0.9) | 1.05 (0.78–1.41) | 0.747 | 21,585 | 68 (0.3) | 0.86 (0.56–1.34) | 0.512 | 21,585 | 121 (0.6) | 1.27 (0.86–1.88) | 0.234 |
| Dyslipidemia | ||||||||||||
| Yes | 13,728 | 156 (1.1) | 1.11 (0.85–1.46) | 0.432 | 13,728 | 62 (0.5) | 1.23 (0.82–1.86) | 0.317 | 13,728 | 98 (0.7) | 1.05 (0.74–1.50) | 0.780 |
| Nonea | 18,547 | 131 (0.7) | – | 18,547 | 56 (0.3) | – | 18,547 | 77 (0.4) | – | |||
| Creatinine clearance (mL/min) | ||||||||||||
| < 30 mL/min/severe renal dysfunction/dialysis | 4015 | 51 (1.3) | 2.50 (1.64–3.82) | < 0.001 | 4,015 | 32 (0.8) | 5.77 (3.02–11.02) | < 0.001 | 4015 | 19 (0.5) | 1.21 (0.65–2.24) | 0.542 |
| 30 mL/min to < 50 mL/min | 10,685 | 105 (1.0) | 1.57 (1.13–2.19) | 0.008 | 10,685 | 40 (0.4) | 2.26 (1.27–4.02) | 0.006 | 10,685 | 69 (0.6) | 1.35 (0.91–2.02) | 0.141 |
| ≥ 50 mL/mina | 11,561 | 77 (0.7) | – | 11,561 | 23 (0.2) | – | 11,561 | 56 (0.5) | – | |||
APA antiplatelet agent; CAD coronary artery disease; CE coronary event; CI confidence interval; DOAC direct oral anticoagulant; HbA1c glycated hemoglobin; HR hazard ratio; MI myocardial infarction; SBP systolic blood pressure
aReference group
Risk factors for MI were systolic blood pressure between 130 and 140 mmHg, no history of CAD with APA and history of CAD with APA vs no history of CAD without APA, and CrCL < 50 mL/min.
Risk factors for cardiac intervention for coronary heart diseases other than MI were male sex, systolic blood pressure ≥ 140 mmHg, diabetes mellitus with HbA1c ≥ 6.0%, history of CAD/no APA, and history of CAD/APA (Table 3). Of note, OAC use was not associated with the risk of CE.
Analysis of patient backgrounds based on the history of CAD and use of APA
Table 4 summarizes the comparison of background factors of patients with a history of CAD, with and without the use of APA. Patients using APA were significantly more likely to be male, slightly younger, and with higher BMI, lower CrCL, and higher HAS-BLED score. They were more likely to have paroxysmal AF, use OAC therapy, and had a higher proportion of comorbidities, diabetes mellitus, dyslipidemia, atherosclerotic diseases other than CAD (i.e., atherosclerotic infarction or peripheral artery disease), and chronic kidney disease.
Table 4.
Patient characteristics according to a history of coronary artery disease and the use of antiplatelet agents
| History of CAD | No history of CAD | P valued | ||||
|---|---|---|---|---|---|---|
| With APAa (n = 3051) | Without APA (n = 3663) | P valueb | With APAc (n = 2653) | Without APA (n = 22,908) | ||
| Male | 2215 (72.6) | 2141 (58.4) | < 0.001 | 1640 (61.8) | 12,486 (54.5) | < 0.001 |
| Age, years | 81.4 ± 4.7 | 81.9 ± 4.8 | < 0.001 | 81.7 ± 4.8 | 81.4 ± 4.8 | < 0.001 |
| Body mass index, kg/m2 | 23.7 ± 3.5 | 23.4 ± 3.5 | 0.004 | 23.5 ± 3.5 | 23.3 ± 3.6 | < 0.001 |
| SBP, mmHg | 126.0 ± 17.7 | 126.1 ± 16.6 | 0.753 | 127.7 ± 16.8 | 127.7 ± 17.0 | < 0.001 |
| DBP, mmHg | 68.7 ± 11.6 | 69.5 ± 11.4 | 0.010 | 70.4 ± 11.7 | 71.1 ± 11.6 | < 0.001 |
| Creatinine clearance, mL/min | 46.4 ± 16.9 | 46.9 ± 17.3 | 0.224 | 46.9 ± 17.6 | 49.0 ± 18.6 | < 0.001 |
| CHADS2 score | 3.2 ± 1.2 | 3.2 ± 1.2 | 0.460 | 3.3 ± 1.3 | 2.7 ± 1.1 | < 0.001 |
| HAS-BLED score | 2.6 ± 0.7 | 1.7 ± 0.8 | < 0.001 | 2.7 ± 0.7 | 1.7 ± 0.8 | < 0.001 |
| History of major bleeding | 141 (4.6) | 195 (5.3) | 0.189 | 128 (4.8) | 975 (4.3) | 0.022 |
| AF type | ||||||
| Paroxysmal | 1468 (48.1) | 1663 (45.4) | 0.004 | 1090 (41.1) | 9365 (40.9) | < 0.001 |
| Persistent | 744 (24.4) | 1025 (28.0) | – | 838 (31.6) | 7,094 (31.0) | – |
| Permanent | 839 (27.5) | 975 (26.6) | – | 725 (27.3) | 6,449 (28.2) | – |
| OACs | 2662 (87.3) | 3429 (93.6) | < 0.001 | 2202 (83.0) | 21,537 (94.0) | < 0.001 |
| Warfarin | 932 (30.5) | 945 (25.8) | < 0.001 | 766 (28.9) | 5590 (24.4) | < 0.001 |
| DOACs | 1729 (56.7) | 2484 (67.8) | < 0.001 | 1434 (54.1) | 15,938 (69.6) | < 0.001 |
| History of non-pharmacological therapy for AF | 516 (16.9) | 830 (22.7) | < 0.001 | 422 (15.9) | 3909 (17.1) | < 0.001 |
| Catheter ablation | 247 (8.1) | 379 (10.3) | 0.002 | 186 (7.0) | 2,158 (9.4) | < 0.001 |
| Comorbidities | ||||||
| Hypertension | 2520 (82.6) | 2979 (81.3) | 0.179 | 2105 (79.3) | 16,708 (72.9) | < 0.001 |
| Diabetes mellitus | 1342 (44.0) | 1422 (38.8) | < 0.001 | 805 (30.3) | 5164 (22.5) | < 0.001 |
| Dyslipidemia | 2150 (70.5) | 1993 (54.4) | < 0.001 | 1293 (48.7) | 8292 (36.2) | < 0.001 |
| Chronic kidney disease | 839 (27.5) | 719 (19.6) | < 0.001 | 602 (22.7) | 4545 (19.8) | < 0.001 |
| Myocardial infarction | 1158 (38.0) | 693 (18.9) | < 0.001 | 0 (0.0) | 0 (0.0) | < 0.001 |
| Angina | 2333 (76.5) | 3188 (87.0) | < 0.001 | 0 (0.0) | 0 (0.0) | < 0.001 |
| Heart failure, left ventricular systolic dysfunction | 1322 (43.3) | 1854 (50.6) | < 0.001 | 985 (37.1) | 8116 (35.4) | < 0.001 |
| Cerebrovascular disease | 797 (26.1) | 924 (25.2) | 0.402 | 1069 (40.3) | 4513 (19.7) | < 0.001 |
| Atherosclerotic infarction | 92 (3.0) | 64 (1.7) | < 0.001 | 178 (6.7) | 321 (1.4) | < 0.001 |
| Cardiogenic infarction | 163 (5.3) | 202 (5.5) | 0.757 | 268 (10.1) | 1744 (7.6) | < 0.001 |
| Lacunar infarction | 174 (5.7) | 183 (5.0) | 0.199 | 260 (9.8) | 819 (3.6) | < 0.001 |
| Peripheral arterial diseasee | 476 (15.6) | 398 (10.9) | < 0.001 | 327 (12.3) | 730 (3.2) | < 0.001 |
| Fall within 1 year | 237 (7.8) | 321 (8.8) | 0.054 | 208 (7.8) | 1,581 (6.9) | < 0.001 |
Data are presented as n (%)
APA antiplatelet agent; AF atrial fibrillation; CAD coronary artery disease; DBP diastolic blood pressure; DOAC direct oral anticoagulant; OAC oral anticoagulants; SBP systolic blood pressure
aIncluded 2,092 aspirin users, 849 P2Y12-inhibitor users, and 452 others (of whom 201 used dual antiplatelet therapy)
bComparison between patients with a history of CAD receiving APAs and those not receiving APAs
cIncluded 1,399 aspirin users, 529 P2Y12-inhibitor users, and 819 others (of whom 39 used dual antiplatelet therapy)
dComparison among the four groups
eAortic plaque, internal carotid artery stenosis, and arteriosclerosis obliterans
Bleeding events in patients with CE
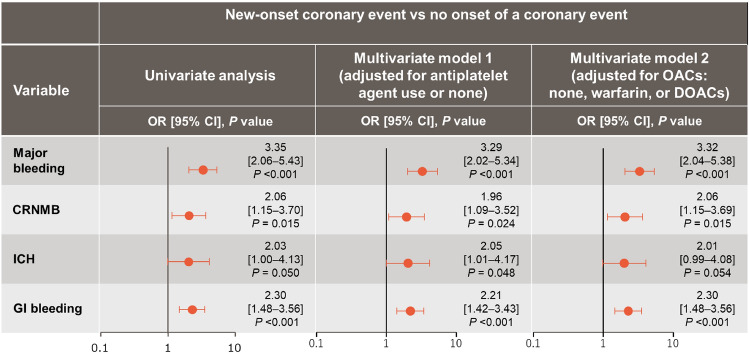
All bleeding events were observed in 2,557 cases (7.9%). Bleeding events in patients with or without new-onset CE are shown in Fig. 2. In the univariate analysis, new-onset CE was significantly associated with a higher incidence of major bleeding (OR: 3.35 [95% CI: 2.06–5.43]), CRNMB (2.06 [1.15–3.70]), ICH (2.03 [1.00–4.13]), and GI bleeding (2.30 [1.48–3.56]) compared with those without CE. In the multivariate analysis both when adjusting for the presence of APAs and when adjusting for the type of OACs, the incidence of major bleeding, CRNMB, ICH, and GI bleeding remained significantly higher for patients with new-onset CE compared with patients without CE. Of 287 patients who developed new-onset CE, the incidence of CE was 5.2% (n = 15) after all bleeding episodes, 2.4% (n = 7) after a major bleeding event, and 1.0% (n = 3) after a CRNMB event.
Fig. 2.
Risk of bleeding events with new-onset coronary event versus no onset of a coronary event. CI confidence interval; CRNMB clinically relevant non-major bleeding; DOAC direct oral anticoagulant; GI gastrointestinal; ICH intracranial hemorrhage; OAC oral anticoagulant; OR odds ratio
Discussion
The main findings of this sub-analysis were as follows. First, in the overall population, the incidence rates of CE, MI, and cardiac intervention for coronary heart diseases other than MI were 0.48, 0.20, and 0.29 per 100 patient-years, respectively, which were lower than that of stroke/SEE (1.62 per 100 patient-years) reported in the main analysis of the ANAFIE Registry [19]. Second, compared with patients without CE (n = 31,988), those with new-onset CE (n = 287) were more likely to have lower CrCL and higher CHADS2 and HAS-BLED scores. Third, risk factors significantly associated with the onset of CE were male sex, systolic blood pressure of ≥ 130 mmHg, diabetes mellitus with HbA1c ≥ 6.0%, history of CAD, APA use, and CrCL < 50 mL/min. Fourth, the incidence of major bleeding, CRNMB, ICH, and GI bleeding was significantly higher in patients with new-onset CE compared with those without CE.
The incidence of CE in the present study was consistent with a recent report on the trends of antithrombotic therapy status and outcomes in Japanese AF patients (mean age ± standard deviation, 73.6 ± 10.9 years), in which the incidence of MI was 0.2% and that of stroke/SEE, 2.2% per patient-year during a 5-year follow-up [26]. The trends observed for the onset of CE in patients with a history of CAD were similar to those reported in a previous study overseas in which cardiovascular outcomes of patients with a history of CAD (i.e., MI) were worse than those of patients without a history of CAD [11]. However, the incidence of MI during anticoagulant therapy was lower in Japanese than in Western patients—a finding that might be attributable to ethnic differences [27–29].
The risk factors for CE for elderly patients with NVAF in the present analysis were also generally consistent with previous reports [30]. An unexpected finding of our study was that APA use was a risk factor for CE. Based on the comparison of patient background data, advanced atherosclerotic diseases are likely the basis for APA use. Therefore, patient background factors, for instance a history of atherosclerotic disease such as peripheral arterial disease, may be associated with high CE risk in patients taking APA. In these patients, it may be difficult to reduce the risk of CE, even with APA. Conversely, OACs were not associated with CE risk.
Another explanation is that bleeding associated with antiplatelet administration may have increased CE. In the present study, new-onset CE was significantly associated with a higher incidence of bleeding compared with patients without CE. The incidence of major bleeding in patients with new-onset CE was significantly higher than that in patients without CE (OR: 3.35 [95% CI: 2.06–5.43]). The findings of the multivariate analysis suggest that APA use or type of OAC at baseline may not have contributed to the incidence of bleeding in patients with new-onset CE. On the other hand, it has also been noted that bleeding complications are followed by ischemic events. In the present study, the incidence of CE after the onset of all bleeding was 5.2%, which is consistent with a previous report [13]. This may be because of the cessation of antithrombotic therapy, blood transfusion, or other interventions. As noted, adjusting for antiplatelet or anticoagulant drug use did not significantly change the OR. Therefore, these drugs do not appear to contribute to increased bleeding events in new-onset CE patients, which may instead be attributable to risk factors identified in the high bleeding risk criteria for PCI patients [31], although this remains unclear.
Concerning risk factors specifically for MI, systolic blood pressure between 130 and 140 mmHg, no history of CAD/APA, history of CAD/APA, and CrCL < 50 mL/min were significant risk factors, which is generally consistent with the previously reported risk factors for MI [32, 33]. Nevertheless, it was surprising that dyslipidemia and diabetes mellitus were not among the relevant risk factors identified. It is possible that the small sample size in that subgroup precluded these factors from reaching a significant difference. Additionally, if the administration of statins was high in this population, it is possible that the risk was modified. Nevertheless, data on statin administration were not collected in this study. Furthermore, it is possible that traditional risk factors, such as hyperlipidemia and diabetes, have become relatively unimportant in the elderly [34, 35]. Treatment history of dyslipidemia was also not recorded in this study.
Regarding the clinical relevance and implications of these findings, it is important to characterize patients at higher risk of CE to prevent and reduce CE. Our results clarify the risk of developing CE in elderly NVAF patients. Although these patients were at a lower risk of CE than stroke/SEE, strict management is required to prevent CE in patients with risk factors for CE, especially those with a history of CAD and those who require APA therapy. Another important finding was that aspirin use was a negative predictor of the development of CE. Therefore, concomitant antiplatelet therapy for primary and secondary prevention of CE may not be recommended for elderly patients with AF. These findings are consistent with the AFIRE trial [12] and are a further advance on the ASPREE trial, in which the use of low-dose aspirin in elderly patients without atrial fibrillation resulted in a significantly increased risk of major bleeding without a decreased risk of cardiovascular disease [36], although the lack of data on treatment changes after each event in the present study means that this requires further verification.
Limitations
The main limitations of the ANAFIE Registry have been published previously [25, 37]. Information such as the withdrawal or change of APA and anticoagulants and use of statins or other treatments for dyslipidemia was not evaluated during the study. Because there was a low prevalence of a history of MI in the study population (5.7%), our findings cannot be extrapolated to populations with higher MI prevalence.
Conclusions
This sub-analysis of the ANAFIE Registry is the first large-scale study to report that CE incidence was lower than that of stroke/SEE in elderly patients with NVAF. Risk factors for CE in elderly Japanese patients with NVAF were male sex, systolic blood pressure of ≥ 130 mmHg, diabetes mellitus, CE history, antiplatelet agent use, and CrCL of < 50 mL/min. New-onset CE was associated with a higher incidence of major bleeding than no CE. Thus, the current findings may contribute to the understanding of the management of elderly NVAF patients.
Acknowledgements
We would like to take this opportunity to express our appreciation to approximately 2500 doctors and 1300 facilities from all over Japan for the support and guidance provided during the conduct of the ANAFIE Registry. We also wish to thank Keyra Martinez Dunn, MD, of Edanz (www.edanz.com) for providing medical writing support, which was supported by Daiichi Sankyo in accordance with Good Publication Practice (GPP) 2022 guidelines (https://www.ismpp.org/gpp-2022). In addition, the authors thank Daisuke Chiba, of Daiichi Sankyo Co., Ltd., for supporting preparation of the manuscript.
Author contributions
TY had full access to all the data in the study and takes responsibility for its integrity and the data analysis.
Funding
Daiichi Sankyo Co., Ltd supported this research.
Data availability
The study protocol will be made available. The deidentified participant data used in this study will be shared with researchers who participated in the study and provide a methodologically sound proposal for 36 months after article publication. The proposal may be reviewed by a committee led by Daiichi Sankyo. For any purpose, requests must be in writing and should be sent to yamt-tky@umin.ac.jp. To gain access, those requesting the data will need to sign a data access agreement.
Declarations
Conflict of interest
M.N. received remuneration from Daiichi Sankyo and Bayer, and received research funding from Daiichi Sankyo and Bayer. H.I. received consultancy fees from Daiichi Sankyo. T. Yamashita received research funding from Bristol-Myers Squibb, Bayer, and Daiichi Sankyo, manuscript fees from Daiichi Sankyo and Bristol-Myers Squibb, and remuneration from Daiichi Sankyo, Bayer, Pfizer Japan, and Bristol-Myers Squibb. M.A. received research funding from Bayer and Daiichi Sankyo, and remuneration from Bristol-Myers Squibb, Nippon Boehringer Ingelheim, Bayer, and Daiichi Sankyo. H.A. received remuneration from Daiichi Sankyo. T.I. received research funding from Daiichi Sankyo and Bayer, and remuneration from Daiichi Sankyo, Bayer, Nippon Boehringer Ingelheim, and Bristol-Myers Squibb. Y.K. received remuneration from Daiichi Sankyo, Bayer, and Nippon Boehringer Ingelheim. K.O. received remuneration from Nippon Boehringer Ingelheim, Daiichi Sankyo, Johnson & Johnson, and Medtronic. W.S. received research funding from Daiichi Sankyo and Nippon Boehringer Ingelheim, and remuneration from Daiichi Sankyo, Pfizer Japan, Bristol-Myers Squibb, Bayer, and Nippon Boehringer Ingelheim. S.S. received research funding from Daiichi Sankyo, and remuneration from Bristol-Myers Squibb and Daiichi Sankyo. H.T. received research funding from Daiichi Sankyo and Nippon Boehringer Ingelheim, remuneration from Daiichi Sankyo, Bayer, Nippon Boehringer Ingelheim, and Pfizer Japan, scholarship funding from Daiichi Sankyo, and consultancy fees from Pfizer Japan, Bayer, and Nippon Boehringer Ingelheim. K.T. received remuneration from Daiichi Sankyo, Otsuka, Novartis, Bayer, and Bristol-Myers Squibb. M.Y. received research funding from Nippon Boehringer Ingelheim, and remuneration from Nippon Boehringer Ingelheim, Daiichi Sankyo, Bayer, Bristol-Myers Squibb, and Pfizer Japan. T. Yamaguchi functioned as an advisory board member for Daiichi Sankyo and has received remuneration from Daiichi Sankyo and Bristol-Myers Squibb. S.T. received research funding from Nippon Boehringer Ingelheim and remuneration from Daiichi Sankyo, Sanofi, Takeda, Chugai Pharmaceutical, Solasia Pharma, Bayer, Sysmex, Nipro, NapaJen Pharma, Gunze, Kaneka, Kringle Pharma and Atworking. T.K., Y.M., and A.T. are employees of Daiichi Sankyo. A.H. participated in a course endowed by Boston Scientific Japan, has received research funding from Daiichi Sankyo and Bayer, and remuneration from Bayer, Daiichi Sankyo, Bristol-Myers Squibb, and Nippon Boehringer Ingelheim.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham study. Stroke. 1991;22:983–988. doi: 10.1161/01.STR.22.8.983. [DOI] [PubMed] [Google Scholar]
- 2.Kavousi M. Differences in epidemiology and risk factors for atrial fibrillation between women and men. Front Cardiovasc Med. 2020;7:3. doi: 10.3389/fcvm.2020.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heeringa J, van der Kuip DA, Hofman A, Kors JA, van Herpen G, Stricker BH, et al. Prevalence, incidence and lifetime risk of atrial fibrillation: the Rotterdam study. Eur Heart J. 2006;27:949–953. doi: 10.1093/eurheartj/ehi825. [DOI] [PubMed] [Google Scholar]
- 4.Zoni-Berisso M, Lercari F, Carazza T, Domenicucci S. Epidemiology of atrial fibrillation: European perspective. Clin Epidemiol. 2014;6:213–220. doi: 10.2147/CLEP.S47385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mekhael M, Marrouche N, Hajjar AHE, Donnellan E. The relationship between atrial fibrillation and coronary artery disease: understanding common denominators. Trends Cardiovasc Med. 2022 doi: 10.1016/j.tcm.2022.09.006. [DOI] [PubMed] [Google Scholar]
- 6.Michniewicz E, Mlodawska E, Lopatowska P, Tomaszuk-Kazberuk A, Malyszko J. Patients with atrial fibrillation and coronary artery disease - double trouble. Adv Med Sci. 2018;63:30–35. doi: 10.1016/j.advms.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 7.Soliman EZ, Safford MM, Muntner P, Khodneva Y, Dawood FZ, Zakai NA, et al. Atrial fibrillation and the risk of myocardial infarction. JAMA Intern Med. 2014;174:107–114. doi: 10.1001/jamainternmed.2013.11912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aronow WS, Ahn C, Mercando AD, Epstein S. Correlation of atrial fibrillation, paroxysmal supraventricular tachycardia, and sinus rhythm with incidences of new coronary events in 1359 patients, mean age 81 years, with heart disease. Am J Cardiol. 1995;75:182–184. doi: 10.1016/S0002-9149(00)80074-0. [DOI] [PubMed] [Google Scholar]
- 9.Liang F, Wang Y. Coronary heart disease and atrial fibrillation: a vicious cycle. Am J Physiol Heart Circ Physiol. 2021;320:H1–H12. doi: 10.1152/ajpheart.00702.2020. [DOI] [PubMed] [Google Scholar]
- 10.GBD 2017 Causes of Death Collaborators Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories 1980–2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2017;2018(392):1736–1788. doi: 10.1016/S0140-6736(18)32203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mahaffey KW, Stevens SR, White HD, Nessel CC, Goodman SG, Piccini JP, et al. Ischaemic cardiac outcomes in patients with atrial fibrillation treated with vitamin K antagonism or factor Xa inhibition: results from the ROCKET AF trial. Eur Heart J. 2014;35:233–241. doi: 10.1093/eurheartj/eht428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yasuda S, Kaikita K, Akao M, Ako J, Matoba T, Nakamura M, AFIRE Investigators et al. Antithrombotic therapy for atrial fibrillation with stable coronary disease. N Engl J Med. 2019;381:1103–1113. doi: 10.1056/NEJMoa1904143. [DOI] [PubMed] [Google Scholar]
- 13.Kaikita K, Yasuda S, Akao M, Ako J, Matoba T, Nakamura M, et al. Bleeding and subsequent cardiovascular events and death in atrial fibrillation with stable coronary artery disease: insights from the AFIRE trial. Circ Cardiovasc Interv. 2021;14:e010476. doi: 10.1161/CIRCINTERVENTIONS.120.010476. [DOI] [PubMed] [Google Scholar]
- 14.Zelniker TA, Ruff CT, Wiviott SD, Blanc JJ, Cappato R, Nordio F, et al. Edoxaban in atrial fibrillation patients with established coronary artery disease: insights from ENGAGE AF-TIMI 48. Eur Heart J Acute Cardiovasc Care. 2019;8:176–185. doi: 10.1177/2048872618790561. [DOI] [PubMed] [Google Scholar]
- 15.Hohnloser SH, Oldgren J, Yang S, Wallentin L, Ezekowitz M, Reilly P, et al. Myocardial ischemic events in patients with atrial fibrillation treated with dabigatran or warfarin in the RE-LY (randomized evaluation of long-term anticoagulation therapy) trial. Circulation. 2012;125:669–676. doi: 10.1161/CIRCULATIONAHA.111.055970. [DOI] [PubMed] [Google Scholar]
- 16.Bahit MC, Lopes RD, Wojdyla DM, Hohnloser SH, Alexander JH, Lewis BS, et al. Apixaban in patients with atrial fibrillation and prior coronary artery disease: Insights from the ARISTOTLE trial. Int J Cardiol. 2013;170:215–220. doi: 10.1016/j.ijcard.2013.10.062. [DOI] [PubMed] [Google Scholar]
- 17.Masunaga N, Ogawa H, Minami K, Ishigami K, Ikeda S, Doi K, et al. Association of concomitant coronary artery disease with cardiovascular events in patients with atrial fibrillation - the Fushimi AF registry. Circ J. 2022;86:1252–1262. doi: 10.1253/circj.CJ-22-0180. [DOI] [PubMed] [Google Scholar]
- 18.Abe M, Masunaga N, Ishii M, Doi K, Ishigami K, Ikeda S, et al. Current status of percutaneous coronary intervention in patients with atrial fibrillation: the Fushimi AF registry. J Cardiol. 2020;75:513–520. doi: 10.1016/j.jjcc.2019.09.014. [DOI] [PubMed] [Google Scholar]
- 19.Yamashita T, Suzuki S, Inoue H, Akao M, Atarashi H, Ikeda T, et al. Two-year outcomes of more than 30 000 elderly patients with atrial fibrillation: results from the all nippon AF in the elderly (ANAFIE) registry. Eur Heart J Qual Care Clin Outcomes. 2022;8:202–213. doi: 10.1093/ehjqcco/qcab025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamashita T, Akao M, Atarashi H, Ikeda T, Koretsune Y, Okumura K, et al. Effect of polypharmacy on clinical outcomes in elderly patients with non-valvular atrial fibrillation- a sub-analysis of the ANAFIE registry. Circ J. 2022;87:6–16. doi: 10.1253/circj.CJ-22-0170. [DOI] [PubMed] [Google Scholar]
- 21.Akishita M, Suzuki S, Inoue H, Akao M, Atarashi H, Ikeda T, et al. Frailty and outcomes in older adults with non-valvular atrial fibrillation from the ANAFIE registry. Arch Gerontol Geriatr. 2022;101:104661. doi: 10.1016/j.archger.2022.104661. [DOI] [PubMed] [Google Scholar]
- 22.Hiasa KI, Kaku H, Kawahara G, Inoue H, Yamashita T, Akao M, et al. Echocardiographic structure and function in elderly patients with atrial fibrillation in Japan- The ANAFIE echocardiographic substudy. Circ J. 2022;86:222–232. doi: 10.1253/circj.CJ-21-0180. [DOI] [PubMed] [Google Scholar]
- 23.Okumura K, Yamashita T, Akao M, Atarashi H, Ikeda T, Koretsune Y, et al. Oral anticoagulants in very elderly nonvalvular atrial fibrillation patients with high bleeding risks: ANAFIE registry. JACC Asia. 2022;2:720–733. doi: 10.1016/j.jacasi.2022.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kario K, Hasebe N, Okumura K, Yamashita T, Akao M, Atarashi H, et al. Home blood pressure can predict the risk for stroke/bleeding events in elderly patients with nonvalvular atrial fibrillation from the ANAFIE registry. Hypertension. 2022;79:2696–2705. doi: 10.1161/HYPERTENSIONAHA.122.19810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inoue H, Yamashita T, Akao M, Atarashi H, Ikeda T, Okumura K, et al. Prospective observational study in elderly patients with non-valvular atrial fibrillation: Rationale and design of the all nippon AF in the elderly (ANAFIE) registry. J Cardiol. 2018;72:300–306. doi: 10.1016/j.jjcc.2018.02.018. [DOI] [PubMed] [Google Scholar]
- 26.Akao M, Ogawa H, Masunaga N, Minami K, Ishigami K, Ikeda S, et al. 10-year trends of antithrombotic therapy status and outcomes in Japanese atrial fibrillation patients - the Fushimi AF registry. Circ J. 2022;86:726–736. doi: 10.1253/circj.CJ-22-0023. [DOI] [PubMed] [Google Scholar]
- 27.Cho H, Kang J, Kim HS, Park KW. Ethnic differences in oral antithrombotic therapy. Korean Circ J. 2020;50:645–657. doi: 10.4070/kcj.2020.0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Hoog VC, Lim SH, Bank IE, Gijsberts CM, Ibrahim IB, Kuan WS, et al. Ethnic differences in clinical outcome of patients presenting to the emergency department with chest pain. Eur Heart J Acute Cardiovasc Care. 2016;5:32–40. doi: 10.1177/2048872615623064. [DOI] [PubMed] [Google Scholar]
- 29.Saito Y, Kobayashi Y, Tanabe K, Ikari Y. Antithrombotic therapy after percutaneous coronary intervention from the Japanese perspective. Cardiovasc Interv Ther. 2020;35:19–29. doi: 10.1007/s12928-019-00633-6. [DOI] [PubMed] [Google Scholar]
- 30.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro heart survey on atrial fibrillation. Chest. 2010;137:263–272. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 31.Urban P, Mehran R, Colleran R, Angiolillo DJ, Byrne RA, Capodanno D, et al. Defining high bleeding risk in patients undergoing percutaneous coronary intervention: a consensus document from the academic research consortium for high bleeding risk. Eur Heart J. 2019;40:2632–2653. doi: 10.1093/eurheartj/ehz372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brown JC, Gerhardt TE, Kwon E. Risk Factors For Coronary Artery Disease. [Updated 2023 Jan 23]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023. Available from: https://www.ncbi.nlm.nih.gov/books/NBK554410/. [PubMed]
- 33.Sanchis-Gomar F, Perez-Quilis C, Leischik R, Lucia A. Epidemiology of coronary heart disease and acute coronary syndrome. Ann Transl Med. 2016;4:256. doi: 10.21037/atm.2016.06.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ahmadi SF, Streja E, Zahmatkesh G, Streja D, Kashyap M, Moradi H, et al. Reverse epidemiology of traditional cardiovascular risk factors in the geriatric population. J Am Med Dir Assoc. 2015;16:933–939. doi: 10.1016/j.jamda.2015.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dalton JE, Rothberg MB, Dawson NV, Krieger NI, Zidar DA, Perzynski AT. Failure of traditional risk factors to adequately predict cardiovascular events in older populations. J Am Geriatr Soc. 2020;68:754–761. doi: 10.1111/jgs.16329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McNeil JJ, Wolfe R, Woods RL, Tonkin AM, Donnan GA, Nelson MR, et al. Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. N Engl J Med. 2018;379:1509–1518. doi: 10.1056/NEJMoa1805819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koretsune Y, Yamashita T, Akao M, Atarashi H, Ikeda T, Okumura K, et al. Baseline demographics and clinical characteristics in the all nippon AF in the elderly (ANAFIE) registry. Circ J. 2019;83:1538–1545. doi: 10.1253/circj.CJ-19-0094. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The study protocol will be made available. The deidentified participant data used in this study will be shared with researchers who participated in the study and provide a methodologically sound proposal for 36 months after article publication. The proposal may be reviewed by a committee led by Daiichi Sankyo. For any purpose, requests must be in writing and should be sent to yamt-tky@umin.ac.jp. To gain access, those requesting the data will need to sign a data access agreement.