Abstract
Background
Colorectal cancer (CRC) significantly threatens human health with increasing incidence and mortality. A debate continues whether fruit consumption is associated with CRC, despite dietary habits having an impact on the disease. The study aims to examine the causal relationship between fruit consumption and CRC based on a two-sample Mendelian randomization method (MR).
Methods
Summary statistics for fruit consumption and CRC were obtained from the UK Biobank and the FinnGen Consortium, respectively. Analysis methods used in this study included the inverse-variance weighted (IVW), MR Egger, weighted median, simple mode, and weighted mode. Heterogeneity and horizontal pleiotropy were also assessed. Additionally, a leave-one-out analysis was performed to validate the robustness of the results.
Results
We found that fruit consumption was associated with a reduction in CRC risk by the IVW method (P = 0.021). This protective effect was predominantly observed in males (OR 0.374; 95% CI: 0.157-0.892; P = 0.027), while no protective effect was noted in females. However, causal correlations were not observed upon analyzing 16 individual types of fruits. Moreover, our results were unlikely to be influenced by horizontal pleiotropy and heterogeneity. Leave-one-out analysis confirmed the stability of the results.
Conclusion
Our findings suggest that a genetic predisposition for fruit consumption may be protective against CRC, underscoring the need for further research to elucidate the underlying mechanisms and dietary patterns involved.
Keywords: colorectal cancer, fruit consumption, Mendelian randomization, two-sample MR, single nucleotide polymorphisms
1. Introduction
In 2020, there were an estimated 1.9 million new colorectal cancer (CRC) cases globally, accounting for 10.0% of all new malignant tumor cases, only after breast and lung cancer (1). Approximately 916,000 people died from CRC, representing 9.4% of all malignant tumor-related deaths, second only to lung cancer (2). Additionally, most CRC patients are diagnosed at an advanced stage due to no early symptoms or subtle signs (3). Moreover, the 5-year survival rate for most stage IV CRC patients is below 10% (4). CRC poses a significant threat to human life and health, emphasizing the importance of early prevention. Based on migrant epidemiology and etiology research, CRC incidence differs between Eastern and Western populations primarily due to dietary and nutritional factors (5). A healthy diet can prevent about 30-50% of CRC cases (6). Fruits are rich in dietary fiber, vitamins, and other potential anti-tumor bioactive compounds, making them possible protective factors against CRC (7).
Inconsistent results have been found in previous studies on the correlation between fruit consumption and CRC (8–13). Some studies linked higher fruit and vegetable consumption with lower CRC mortality rates. A meta-analysis indicated a reduced risk of CRC associated with a high intake of citrus fruits, apples, watermelon, and kiwi (14). However, Aoyama et al. did not find a strong correlation between low or consistently low intake of vegetables and fruits and the risk of CRC (9). These conflicting research findings may be attributed to methodological differences, confounding factors, and variations in dietary habits. To mitigate potential confounding factors and enhance the robustness of empirical evidence, Mendelian randomization (MR) has been employed as a methodological approach. MR utilizes genetic variability as instrumental variables (IVs) for evaluating the causal association between an exposure and its corresponding outcomes (15, 16).
MR analysis assumes that genetic variations are randomly distributed in the population, similar to a randomized controlled trial. This method mitigates reverse causation and confounding biases in observational studies, providing a more precise representation of the exposure-outcome correlation (17). Moreover, many genetic variations are associated with the consumption of proteins, fats, and carbohydrates. These genetic variations not only affect the intake of individual nutrients but also the overall dietary preferences and patterns (18). As a result, this study investigates the risk of CRC associated with fruit consumption using MR. Genetic data were sourced from the Medical Research Council Integrative Epidemiology Unit (MRC-IEU) project.
2. Materials and methods
2.1. Study design and data resource
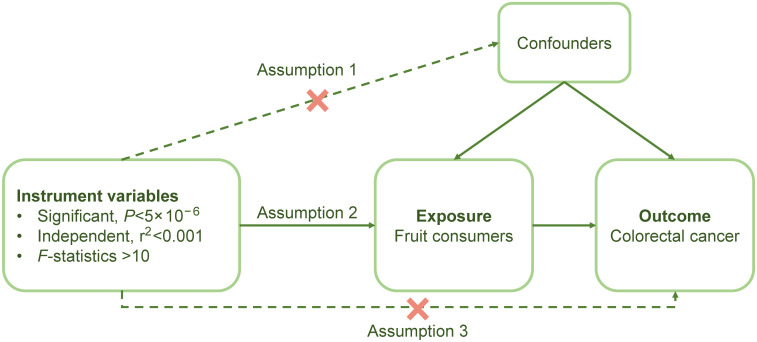
In this study, fruit consumption is considered the exposure variable, while CRC is regarded as the outcome. Single nucleotide polymorphisms (SNPs) have been selected as IVs for further analysis. The study adheres to the three fundamental assumptions of the two-sample MR design:
Assumption I: there is a significant association between genetic variants and exposure (P < 5 ×10-6, F-statistic > 10).
Assumption II: genetic variations are unrelated to any confounding factors within the exposure-outcome correlation.
Assumption III: genetic variants solely impact the outcome by virtue of their connection to the exposure (Figure 1).
Figure 1.
The process of MR analysis.
This MR investigation is based on publicly available GWAS datasets, thus obviating the need for additional ethical approval from institutional review boards.
The CRC GWAS summary data were acquired from the FinnGen consortium, comprising 3,022 cases and 174,006 controls, all of European ancestry who provided informed consent. In addition, summary data for fruit intake (females and males) was obtained from the IEU OPEN GWAS project (https://gwas.mrcieu.ac.uk/) with 64,949 individuals of European descent (including 11,960 controls) and encompassed 9,851,867 SNPs.
We obtained GWAS summary statistics for different types of fruit from GWAS meta-analysis, including: apple intake, banana intake, berry intake, cherry intake, grape intake, grapefruit intake, mango intake, melon intake, orange intake, peach/nectarine intake, pear intake, pineapple intake, plum intake, prune intake, satsuma intake, dried fruit intake.
2.2. Selection of instrumental variables
In order to perform MR analysis, it is imperative to adhere to three essential assumptions: relevance, independence, and exclusion restriction. Consequently, a rigorous selection process was applied to all IVs used for subsequent analysis. Initially, we identified SNPs closely linked to the exposure (P < 5 × 10-6) and excluded those with an F-statistic < 10, ensuring their significance and reducing the potential for weak IV bias.
The F-statistic utilized in this study is defined by the formula R2 × (N − K − 1)/(1 −R2) where R2 represents the variance in exposure explained by each IV. R2 = 2 × β2 × EAF × (1 − EAF)/(2 × β2 × EAF × (1 − EAF) + 2 × SE2 × N × EAF × (1 − EAF)), where β denotes the allele effect size, EAF signifies the effect allele frequency, K is the number of IVs, and N is the sample size of the GWAS study. Subsequently, to ensure the independence of IVs, SNPs with strong linkage disequilibrium were excluded (r2< 0.001, kb = 10,000). Thirdly, the chosen IVs were examined for associations with other phenotypes (http://www.phenoscanner.medschl.cam.ac.uk/) to further mitigate the potential impact of horizontal pleiotropy on the study. Finally, the datasets related to the exposure and outcome were harmonized to ensure that the effect alleles were consistently aligned.
2.3. Statistical analysis
In this study, all statistical analyses were conducted using R software (version 4.3.1) with the TwoSampleMR package. We assessed the causal correlation between fruit consumption and CRC using various methods, including the inverse-variance weighted (IVW) method (the primary approach), as well as MR Egger, weighted median, simple mode, and weighted mode methods. Significance for a causal effect between the exposure and outcome was defined as P < 0.05. The results from the MR analyses were reported as odds ratios (ORs) along with their corresponding 95% confidence intervals (CIs). To assess individual SNP heterogeneity, Cochrane’s Q statistic was employed. Heterogeneity was considered absent for P values exceeding 0.05. Additionally, we performed sensitivity analyses to identify possible horizontal pleiotropy and verify the consistency of associations. These analyses encompassed the weighted median method and tests for heterogeneity. The weighted median method yielded a consistent overall effect estimate when more than 50% of the IVs were effective. Furthermore, a leave-one-out test was conducted to confirm that the observed causality remained unaffected by individual SNPs.
Given that gender difference, we performed a gender-stratified MR analysis, aiming to avoid potential sexual bias. To minimize the potential interactions between different types of fruit and isolate the independent effect of each category, we conducted an MR analysis for each type of fruit and CRC.
3. Results
3.1. Instrumental variable selection
After the initial selection process, which involved identifying SNPs with strong relevance (P < 5 × 10-6, F-statistic > 10) and eliminating those exhibiting linkage disequilibrium (r2 < 0.001, kb = 10,000), we identified a set of 13 SNPs as preliminary IVs. The lowest F-statistic was 20.908, and a comprehensive overview of the F-statistics can be found in Table 1. It’s worth noting that despite conducting exclusion of SNPs associated with the outcome and potential confounding factors using Phenoscanner, no SNPs were excluded at this stage. Subsequently, after aligning the exposure and outcome data, we proceeded with these 13 SNPs as IVs for further analysis.
Table 1.
SNPs used as instrumental variables from fruit consumers and colorectal cancer GWASs.
| SNPs | Exposure | Outcome | Chr | Pos | F | R2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta | Se | P-value | Effect allele | Other allele | Beta | Se | P-value | Effect allele | Other allele | |||||
| rs11217077 | -0.012 | 0.002 | 3.00E-06 | C | T | 0.065 | 0.031 | 0.035 | C | T | 11 | 118746223 | 21.813 | 0.000335745 |
| rs118052164 | -0.040 | 0.009 | 2.80E-06 | A | T | 0.119 | 0.096 | 0.218 | A | T | 9 | 6978352 | 21.971 | 0.000338171 |
| rs17818946 | 0.017 | 0.004 | 1.70E-06 | G | A | -0.016 | 0.049 | 0.751 | G | A | 18 | 72390746 | 22.913 | 0.000352667 |
| rs36013074 | 0.027 | 0.006 | 4.00E-06 | A | G | 0.092 | 0.097 | 0.339 | A | G | 6 | 151026080 | 21.264 | 0.000327293 |
| rs4648553 | 0.012 | 0.002 | 5.50E-07 | A | G | -0.015 | 0.029 | 0.612 | A | G | 1 | 3648879 | 25.091 | 0.000386174 |
| rs4663869 | -0.016 | 0.004 | 4.80E-06 | G | A | -0.002 | 0.035 | 0.946 | G | A | 2 | 239175023 | 20.908 | 0.000321817 |
| rs4836713 | 0.019 | 0.004 | 1.40E-06 | G | A | -0.010 | 0.079 | 0.903 | G | A | 9 | 120998947 | 23.324 | 0.000359001 |
| rs4923532 | 0.011 | 0.002 | 7.60E-07 | G | A | -0.046 | 0.028 | 0.092 | G | A | 11 | 28401900 | 24.462 | 0.000376504 |
| rs55809544 | -0.014 | 0.003 | 4.40E-06 | G | A | 0.094 | 0.045 | 0.034 | G | A | 13 | 99119023 | 21.065 | 0.000324235 |
| rs72867233 | -0.048 | 0.010 | 8.20E-07 | G | A | 0.132 | 0.227 | 0.561 | G | A | 2 | 32616643 | 24.303 | 0.000374061 |
| rs75417443 | -0.016 | 0.004 | 3.50E-06 | A | G | -0.018 | 0.059 | 0.757 | A | G | 12 | 20027525 | 21.501 | 0.000330947 |
| rs76331651 | 0.024 | 0.005 | 1.80E-06 | G | A | 0.091 | 0.070 | 0.193 | G | A | 15 | 88441033 | 22.792 | 0.00035081 |
| rs7937987 | -0.012 | 0.003 | 3.40E-06 | T | C | 0.069 | 0.037 | 0.061 | T | C | 11 | 94690088 | 21.597 | 0.000332427 |
Se, standard error; SNP, single nucleotide polymorphism; Chr, chromosome; Pos, position.
3.2. Mendelian randomization analysis
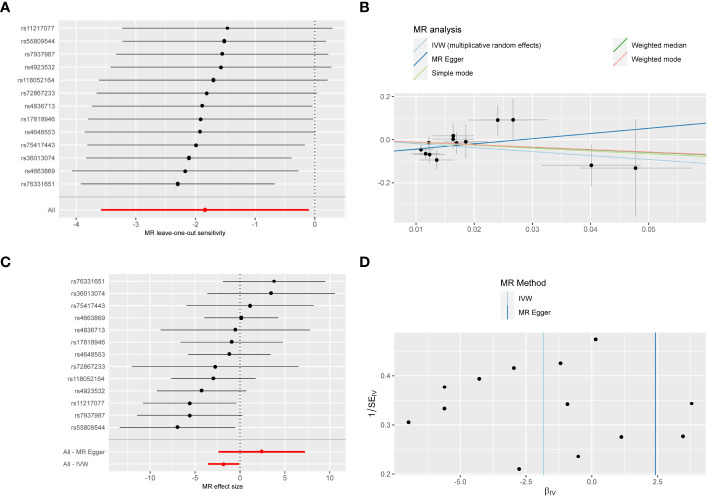
The genetic correlation between fruit intake and CRC was investigated using a random effects IVW approach. Table 2 presents MR results and the five methods used in our analysis. Our results indicate that fruit intake is negatively correlated with CRC (OR 0.159; 95% CI: 0.033-0.759; P = 0.021) (Figure 2). According to the Cochrane’s Q test, there was no evidence of heterogeneity between fruit intake and CRC (P = 0.247). The MR-Egger method detects horizontal pleiotropy through its intercept with the y-axis. The presence of horizontal pleiotropy is indicated when the intercept is non-zero. To satisfy the exclusion restriction assumption, horizontal pleiotropy must not be present. No horizontal pleiotropy was observed in our MR analysis results (P = 0.094) (Table 2). Our MR analysis results were not influenced by individual SNPs (Figure 2A) based on the leave-one-out test.
Table 2.
MR results of causal links between fruit consumers and colorectal cancer risk.
| Exposure | Outcome | Nsnp | Method | β | Se | P-value | OR (95%CI) | Heterogeneity | Horizontal pleiotropy | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cochran’s Q | P-value | Egger intercept | Se | P-value | ||||||||
| Fruit consumers | Colorectal cancer | 13 | MR Egger | 2.414 | 2.459 | 0.347 | 11.183 (0.090-1386.369) | 14.907 | 0.247 | -0.068 | 0.037 | 0.094 |
| Weighted median | -1.170 | 1.145 | 0.307 | 0.310 (0.033-2.925) | ||||||||
| IVW | -1.839 | 0.798 | 0.021 | 0.159 (0.033-0.759) | ||||||||
| Simple mode | -1.309 | 2.145 | 0.553 | 0.270 (0.004-18.097) | ||||||||
| Weighted mode | -1.139 | 1.795 | 0.538 | 0.320 (0.009-10.810) | ||||||||
MR, Mendelian randomization; Se, standard error; OR, odds ratio; CI, confidence intervals; IVW, inverse variance weighted.
Figure 2.
Sensitivity analysis (A), scatter plot (B), forest plot (C), and funnel plot (D) of the causal effect of fruit consumption on colorectal cancer risk.
Scatter plots demonstrated that the causal association estimates from IVW, MR-Egger, and weighted median methods were similar, as judged by the slope of the line (Figure 2B). A forest plot (Figure 2C) visualizes the causal correlation between each SNP and CRC. The funnel plot, representing each SNP as an IV, showed that the derived causal effects were symmetrically distributed, suggesting a low probability of potential bias and indicating that the results were stable and reliable (Figure 2D). Therefore, these findings were stable and robust.
3.3. Gender-stratified MR analysis
To further explore the causal-effect difference of genders on fruit intake, a gender-stratified MR analysis was performed. In total, 15 SNPs were identified as IVs for gender-stratified fruit consumers, among which 8 SNPs were identified for fruit consumers in females, and 7 SNPs for males. The F statistics of IVs ranged between 21.054 and 28.781, indicating no evidence of weak instrument bias. Detailed information on these IVs is listed in Supplementary Tables 3, 4.
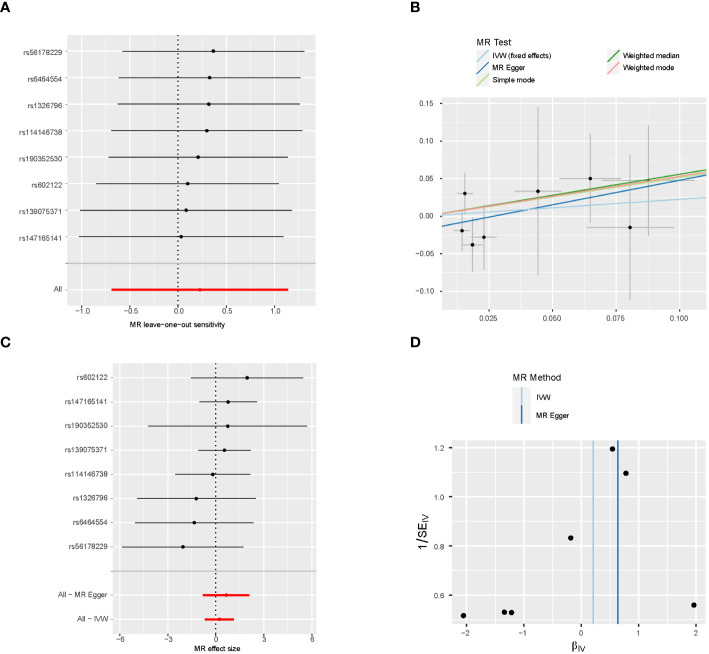
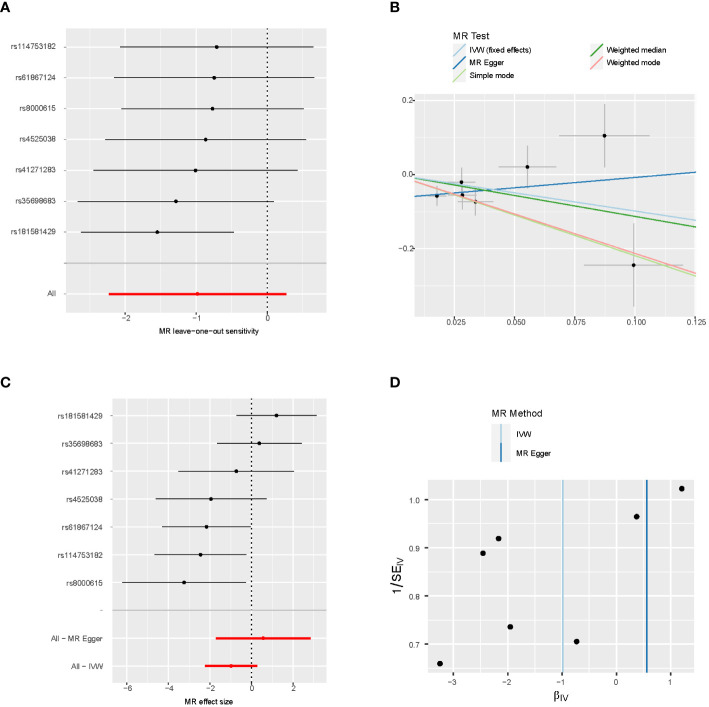
The MR results from gender-stratified fruit intake are listed in Tables 3, 4. Gender-stratified MR showed that genetically predicted fruit intake was associated with CRC in males (OR 0.374; 95% CI: 0.157-0.892; P = 0.027). The scatter plots of IVs are shown in Figures 3, 4. Moreover, the results from the Cochran’s Q and MR-Egger intercept tests are also shown in Tables 3, 4. There is no heterogeneity was observed between the genetic IVs for fruit intake in both females and males, for which the FE-IVW method was used. MR-Egger intercepts did not detect any pleiotropy, indicating no evidence of potential horizontal pleiotropy (both intercepts P > 0.05). The leave-one-out analysis showed that the significant results were not driven by any single SNP (Figures 3, 4).
Table 3.
MR analysis of the impact of fruit consumption on CRC in females.
| Exposure | Outcome | Nsnp | Method | β | Se | P-value | OR (95%CI) | Heterogeneity | Horizontal pleiotropy | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cochran’s Q | P-value | Egger intercept | Se | P-value | ||||||||
| Fruit consumers in females | Colorectal cancer | 8 | MR Egger | 0.655 | 0.736 | 0.408 | 1.925 (0.455-8.146) | 4.271 | 0.748 | -0.017 | 0.023 | 0.480 |
| Weighted median | 0.559 | 0.594 | 0.346 | 1.750 (0.546-5.602) | ||||||||
| IVW | 0.225 | 0.465 | 0.628 | 1.253 (0.503-3.120) | ||||||||
| Simple mode | 0.515 | 0.866 | 0.571 | 1.674 (0.306-9.143) | ||||||||
| Weighted mode | 0.532 | 0.607 | 0.409 | 1.703 (0.518-5.594) | ||||||||
MR, Mendelian randomization; Se, standard error; OR, odds ratio; CI, confidence intervals; IVW, inverse variance weighted.
Table 4.
MR analysis of the impact of fruit consumption on CRC in males.
| Exposure | Outcome | Nsnp | Method | β | Se | P-value | OR (95%CI) | Heterogeneity | Horizontal pleiotropy | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cochran’s Q | P-value | Egger intercept | Se | P-value | ||||||||
| Fruit consumers in males | Colorectal cancer | 7 | MR Egger | 0.557 | 1.161 | 0.651 | 1.746 (0.18-16.981) | 12.377 | 0.054 | -0.063 | 0.042 | 0.187 |
| Weighted median | -1.129 | 0.674 | 0.094 | 0.323 (0.086-1.212) | ||||||||
| IVW | -0.983 | 0.443 | 0.027 | 0.374 (0.157-0.892) | ||||||||
| Simple mode | -2.188 | 1.230 | 0.126 | 0.112 (0.010-1.250) | ||||||||
| Weighted mode | -2.127 | 1.458 | 0.195 | 0.119 (0.007-2.076) | ||||||||
MR, Mendelian randomization; Se, standard error; OR, odds ratio; CI, confidence intervals; IVW, inverse variance weighted.
Figure 3.
Sensitivity analysis of female (A), scatter plot (B), forest plot (C), and funnel plot (D) of the causal effect of fruit consumption on colorectal cancer risk.
Figure 4.
Sensitivity analysis of male (A), scatter plot (B), forest plot (C), and funnel plot (D) of the causal effect of fruit consumption on colorectal cancer risk.
3.4. MR analysis in different types of fruit
The genetic correlation between different types of fruit and CRC was investigated using a fixed effects IVW approach. Detailed information on these IVs is listed in Supplementary Tables.
4. Discussion
The causal correlation between fruit consumption and CRC was examined with a two-sample MR analysis. Our study suggests that fruit consumption may reduce the risk of CRC. This finding highlights the importance of fruit consumption in a healthy diet and the prevention of CRC.
Increasing research indicates that fruit consumption can reduce the risk of CRC. Jedrychowski et al. reported that individuals who ate one or more apples per day experienced approximately a 50% reduction in the risk of CRC (19). According to a meta-analysis of 24 studies involving 1,068,158 participants, citrus fruits, apples, watermelon, and kiwi were associated with 9%, 25%, 26%, and 13% lower risk of CRC, respectively (14). These studies illuminate the relation between fruit consumption and reduced CRC risk. Despite these correlations, our analysis across 16 types of fruit did not establish a causal correlation. The absence of a discernible causal link may be attributed to several factors. Due to the current limited extent of research on CRC, only the risk factors believed to be associated with CRC have been excluded. There may be other confounding factors, either undiscovered or lacking sufficient evidence, that have not been eliminated, thereby interfering with the relationship between fruit intake and CRC risk. Additionally, the inherently multifactorial etiology of diseases, where multiple contributors interplay, dilutes the measurable impact of individual factors. This complexity could be why the specific influence of certain fruits on CRC risk did not reach statistical significance. Therefore, to substantiate these preliminary observations, further high-quality, extensive research is essential.
The potential of fruits to reduce the risk of CRC necessitates attention to gender differences. Our study indicates that fruit consumption is protective in men, but no causal correlation was found in women. The divergent outcomes between men and women could be attributed to greater dietary heterogeneity among women, biological differences, increased measurement errors in women, different approaches to completing food frequency questionnaires between men and women, or other factors. Additionally, there are variations in health behavior characteristics between men and women within similar patterns, which might help explain why these patterns yield different outcomes (20).
Fruits are rich in various vitamins, minerals, dietary fiber, and antioxidants, such as vitamin C, vitamin A, vitamin K, potassium, magnesium, calcium, flavonoid, phenolic acid, carotenoid, and tannin, among others (21, 22). The potential biological mechanisms underlying the reduction in CRC risk associated with fruit consumption are as follows (1). Tumoricidal action: Vitamin C, polyphenol, and flavonoid exhibit toxicity towards tumor cells, inhibiting cell growth and proliferation (23, 24). Pires’ group found that pharmacological doses of vitamin C combined with certain conventional anticancer drugs can inhibit the growth of CRC cells (2, 25). Induction of apoptosis: Natural compounds such as flavonoids, terpenoids, and carotenoids have been evidenced to promote apoptosis in cancer cells (26–28). Quercetin is a flavonoid and exists in fruits like capers, apples, and berries (29). The quercetin derivative 8-C-(E-phenylmethyl) quercetin can trigger G2 phase arrest in CRC cells and inhibit proliferation. Furthermore, it induces autophagic cell death under extracellular signal-regulated kinase stimulation (3, 30). Inhibition of cell cycle progression: Flavonoids like quercetin (31) and carotenoids (32) can inhibit progression in the cancer cell cycle. Quercetin, for example, can regulate the expression of tumor suppressor genes, cycle- and apoptosis-related genes to inhibit the growth of CRC cells (4, 31). Enhanced immune system function: Components found in citrus fruits, pomegranates, and similar fruits (33), including vitamin C, polyphenolic compounds (34), and zinc (35), are believed to enhance immune system function. Additionally, researchers have found that vitamin C can increase the activity of natural killer cells (36) and T cells (37). These biological mechanisms may help explain the association between fruit consumption and a reduction of CRC risk.
A major strength of our study is that it uses large-scale GWAS to investigate causal correlations between fruit consumption and CRC. In comparison to traditional observational studies, the two-sample MR method offers a means to mitigate several common problems, including the presence of confounding factors, and the potential for biases. Then, all IVs were rigorously screened, with the lowest F-value of 20.908, indicating the accuracy of the results. Additionally, we tested for sensitivity, horizontal pleiotropy, and heterogeneity to confirm the robustness of the findings. Finally, this study utilized a CRC-related dataset, excluding patients with other types of cancer, making the conclusions more reliable.
However, the present study has some limitations. First, our findings may not apply to populations and regions beyond Europe. Studies on fruit consumption and CRC risk have varied significantly across populations and regions. A study of women in central Sweden reported an association between low fruit consumption and an increased risk of CRC (38), while a similar association was not observed in Japan (39). Second, this study did not delve into the intake frequencies and quantities. There is evidence to suggest that both low consumption and consistently low consumption of fruits may not have a significant association with CRC risk (9). Lastly, while MR analysis can reduce many confounding factors, our conclusions may be affected by environmental factors.
5. Conclusion
Fruit consumption may help to reduce the risk of CRC in men. Although our findings support existing public health guidelines that encourage including fruit as part of a healthy diet to lower the risk of CRC, further research is needed to validate our findings and explore the underlying mechanisms.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributions
LY: Conceptualization, Data curation, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding Statement
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by joint project of Chongqing Shapingba Health Commission and Science and Technology Burea (2022SQKWLH004).
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1362269/full#supplementary-material
MR Analysis of the association between consumption of 16 different fruits and colorectal cancer risk.
References
- 1. Morgan E, Arnold M, Gini A, Lorenzoni V, Cabasag CJ, Laversanne M, et al. Global burden of colorectal cancer in 2020 and 2040: incidence and mortality estimates from GLOBOCAN. Gut. (2023) 72:338–44. doi: 10.1136/gutjnl-2022-327736 [DOI] [PubMed] [Google Scholar]
- 2. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 3. Li X, Guo D, Chu L, Huang Y, Zhang F, Li W, et al. Potential diagnostic value of combining inflammatory cell ratios with carcinoembryonic antigen for colorectal cancer. Cancer Manage Res. (2019) 11:9631–40. doi: 10.2147/CMAR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wagner J, Kline CL, Zhou L, Khazak V, El-Deiry WS. Anti-tumor effects of ONC201 in combination with VEGF-inhibitors significantly impacts colorectal cancer growth and survival in vivo through complementary non-overlapping mechanisms. J Exp Clin Cancer research: CR. (2018) 37:11. doi: 10.1186/s13046-018-0671-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Reid WV, Mooney HA, Capistrano D, Carpenter SR, Chopra K, Cropper A, et al. Nature: the many benefits of ecosystem services. Nature. (2006) 443:749. doi: 10.1038/443749a [DOI] [PubMed] [Google Scholar]
- 6. Hu Y, McIntosh GH, Le Leu RK, Nyskohus LS, Woodman RJ, Young GP. Combination of selenium and green tea improves the efficacy of chemoprevention in a rat colorectal cancer model by modulating genetic and epigenetic biomarkers. PloS One. (2013) 8:e64362. doi: 10.1371/journal.pone.0064362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Holcombe RF, Martinez M, Planutis K, Planutiene M. Effects of a grape-supplemented diet on proliferation and Wnt signaling in the colonic mucosa are greatest for those over age 50 and with high arginine consumption. Nutr J. (2015) 14:62. doi: 10.1186/s12937-015-0050-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Michels KB, Edward G, Joshipura KJ, Rosner BA, Stampfer MJ, Fuchs CS, et al. Prospective study of fruit and vegetable consumption and incidence of colon and rectal cancers. J Natl Cancer Institute. (2000) 92:1740–52. doi: 10.1093/jnci/92.21.1740 [DOI] [PubMed] [Google Scholar]
- 9. Aoyama N, Kawado M, Yamada H, Hashimoto S, Suzuki K, Wakai K, et al. Low intake of vegetables and fruits and risk of colorectal cancer: the Japan Collaborative Cohort Study. J Epidemiol. (2014) 24:353–60. doi: 10.2188/jea.JE20130195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kunzmann AT, Coleman HG, Huang WY, Cantwell MM, Kitahara CM, Berndt SI. Fruit and vegetable intakes and risk of colorectal cancer and incident and recurrent adenomas in the PLCO cancer screening trial. Int J Cancer. (2016) 138:1851–61. doi: 10.1002/ijc.29922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lin J, Zhang SM, Cook NR, Rexrode KM, Liu S, Manson JE, et al. Dietary intakes of fruit, vegetables, and fiber, and risk of colorectal cancer in a prospective cohort of women (United States). Cancer Causes Control: CCC. (2005) 16:225–33. doi: 10.1007/s10552-004-4025-1 [DOI] [PubMed] [Google Scholar]
- 12. Park Y, Subar AF, Kipnis V, Thompson FE, Mouw T, Hollenbeck A, et al. Fruit and vegetable intakes and risk of colorectal cancer in the NIH-AARP diet and health study. Am J Epidemiol. (2007) 166:170–80. doi: 10.1093/aje/kwm067 [DOI] [PubMed] [Google Scholar]
- 13. Liang W, Binns CW. Fruit, vegetables, and colorectal cancer risk: the European Prospective Investigation into Cancer and Nutrition. Am J Clin Nutr. (2009) 90:1112. doi: 10.3945/ajcn.2009.28320 [DOI] [PubMed] [Google Scholar]
- 14. Wu ZY, Chen JL, Li H, Su K, Han YW. Different types of fruit intake and colorectal cancer risk: a meta-analysis of observational studies. World J Gastroenterol. (2023) 29:2679–700. doi: 10.3748/wjg.v29.i17.2679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Davey Smith G, Holmes MV, Davies NM, Ebrahim S. Mendel’s laws, Mendelian randomization and causal inference in observational data: substantive and nomenclatural issues. Eur J Epidemiol. (2020) 35:99–111. doi: 10.1007/s10654-020-00622-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Holmes MV, Ala-Korpela M, Smith GD. Mendelian randomization in cardiometabolic disease: challenges in evaluating causality. Nat Rev Cardiol. (2017) 14:577–90. doi: 10.1038/nrcardio.2017.78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mukamal KJ, Stampfer MJ, Rimm EB. Genetic instrumental variable analysis: time to call mendelian randomization what it is. The example of alcohol and cardiovascular disease. Eur J Epidemiol. (2020) 35:93–7. doi: 10.1007/s10654-019-00578-3 [DOI] [PubMed] [Google Scholar]
- 18. Guénard F, Bouchard-Mercier A, Rudkowska I, Lemieux S, Couture P, Vohl MC. Genome-wide association study of dietary pattern scores. Nutrients. (2017) 9(7):649. doi: 10.3390/nu9070649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jedrychowski W, Maugeri U, Popiela T, Kulig J, Sochacka-Tatara E, Pac A, et al. Case-control study on beneficial effect of regular consumption of apples on colorectal cancer risk in a population with relatively low intake of fruits and vegetables. Eur J Cancer Prev. (2010) 19:42–7. doi: 10.1097/CEJ.0b013e328333d0cc [DOI] [PubMed] [Google Scholar]
- 20. Reedy J, Wirfält E, Flood A, Mitrou PN, Krebs-Smith SM, Kipnis V, et al. Comparing 3 dietary pattern methods–cluster analysis, factor analysis, and index analysis–With colorectal cancer risk: The NIH-AARP Diet and Health Study. Am J Epidemiol. (2010) 171:479–87. doi: 10.1093/aje/kwp393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu RH. Health-promoting components of fruits and vegetables in the diet. Adv Nutr (Bethesda Md). (2013) 4:384s–92s. doi: 10.3945/an.112.003517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rampersaud GC, Valim MF. 100% citrus juice: Nutritional contribution, dietary benefits, and association with anthropometric measures. Crit Rev Food Sci Nutr. (2017) 57:129–40. doi: 10.1080/10408398.2013.862611 [DOI] [PubMed] [Google Scholar]
- 23. Seeram NP, Adams LS, Zhang Y, Lee R, Sand D, Scheuller HS, et al. Blackberry, black raspberry, blueberry, cranberry, red raspberry, and strawberry extracts inhibit growth and stimulate apoptosis of human cancer cells in vitro . J Agric Food Chem. (2006) 54:9329–39. doi: 10.1021/jf061750g [DOI] [PubMed] [Google Scholar]
- 24. Putchala MC, Ramani P, Sherlin HJ, Premkumar P, Natesan A. Ascorbic acid and its pro-oxidant activity as a therapy for tumours of oral cavity – a systematic review. Arch Oral Biol. (2013) 58:563–74. doi: 10.1016/j.archoralbio.2013.01.016 [DOI] [PubMed] [Google Scholar]
- 25. Pires AS, Marques CR, Encarnação JC, Abrantes AM, Marques IA, Laranjo M, et al. Ascorbic acid chemosensitizes colorectal cancer cells and synergistically inhibits tumor growth. Front Physiol. (2018) 9:911. doi: 10.3389/fphys.2018.00911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Echeverria C, Santibañez JF, Donoso-Tauda O, Escobar CA, Ramirez-Tagle R. Structural antitumoral activity relationships of synthetic chalcones. Int J Mol Sci. (2009) 10:221–31. doi: 10.3390/ijms10010221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yoo KH, Park JH, Lee DK, Fu YY, Baek NI, Chung IS. Pomolic acid induces apoptosis in SK-OV-3 human ovarian adenocarcinoma cells through the mitochondrial-mediated intrinsic and death receptor-induced extrinsic pathways. Oncol Lett. (2013) 5:386–90. doi: 10.3892/ol.2012.985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Scarano A, Olivieri F, Gerardi C, Liso M, Chiesa M, Chieppa M, et al. Selection of tomato landraces with high fruit yield and nutritional quality under elevated temperatures. J Sci Food Agricul. (2020) 100:2791–9. doi: 10.1002/jsfa.10312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rauf A, Imran M, Khan IA, Ur-Rehman M, Gilani SA, Mehmood Z, et al. Anticancer potential of quercetin: A comprehensive review. Phytother Res: PTR. (2018) 32:2109–30. doi: 10.1002/ptr.6155 [DOI] [PubMed] [Google Scholar]
- 30. Alzate-Yepes T, Pérez-Palacio L, Martínez E, Osorio M. Mechanisms of action of fruit and vegetable phytochemicals in colorectal cancer prevention. Molecules (Basel Switzerland). (2023) 28(11):4322. doi: 10.3390/molecules28114322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Seo HS, Ku JM, Choi HS, Choi YK, Woo JK, Kim M, et al. Quercetin induces caspase-dependent extrinsic apoptosis through inhibition of signal transducer and activator of transcription 3 signaling in HER2-overexpressing BT-474 breast cancer cells. Oncol Rep. (2016) 36:31–42. doi: 10.3892/or.2016.4786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ascenso A, Pedrosa T, Pinho S, Pinho F, de Oliveira JM, Cabral Marques H, et al. The effect of lycopene preexposure on UV-B-irradiated human keratinocytes. Oxid Med Cell Longev. (2016) 2016:8214631. doi: 10.1155/2016/8214631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jaganathan SK, Vellayappan MV, Narasimhan G, Supriyanto E. Role of pomegranate and citrus fruit juices in colon cancer prevention. World J Gastroenterol. (2014) 20:4618–25. doi: 10.3748/wjg.v20.i16.4618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. González-Gallego J, García-Mediavilla MV, Sánchez-Campos S, Tuñón MJ. Fruit polyphenols, immunity and inflammation. Br J Nutr. (2010) 104 Suppl 3:S15–27. doi: 10.1017/S0007114510003910 [DOI] [PubMed] [Google Scholar]
- 35. Prasad AS. Zinc in human health: effect of zinc on immune cells. Mol Med (Cambridge Mass). (2008) 14:353–7. doi: 10.2119/2008-00033.Prasad [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Toliopoulos IK, Simos YV, Daskalou TA, Verginadis II, Evangelou AM, Karkabounas SC. Inhibition of platelet aggregation and immunomodulation of NK lymphocytes by administration of ascorbic acid. Indian J Exp Biol. (2011) 49:904–8. [PubMed] [Google Scholar]
- 37. Grant MM, Mistry N, Lunec J, Griffiths HR. Dose-dependent modulation of the T cell proteome by ascorbic acid. Br J Nutr. (2007) 97:19–26. doi: 10.1017/S0007114507197592 [DOI] [PubMed] [Google Scholar]
- 38. Terry P, Giovannucci E, Michels KB, Bergkvist L, Hansen H, Holmberg L, et al. Fruit, vegetables, dietary fiber, and risk of colorectal cancer. J Natl Cancer Institute. (2001) 93:525–33. doi: 10.1093/jnci/93.7.525 [DOI] [PubMed] [Google Scholar]
- 39. Sato Y, Tsubono Y, Nakaya N, Ogawa K, Kurashima K, Kuriyama S, et al. Fruit and vegetable consumption and risk of colorectal cancer in Japan: The Miyagi Cohort Study. Public Health Nutr. (2005) 8:309–14. doi: 10.1079/PHN2004681 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
MR Analysis of the association between consumption of 16 different fruits and colorectal cancer risk.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.