Abstract
We previously reported that the region corresponding to amino acids 197 to 216 of the gp46 surface glycoprotein (gp46-197) served as a binding domain for the interaction between gp46 and trypsin-sensitive membrane components of the target cell, leading to syncytium formation induced by human T-cell lymphotropic virus type 1 (HTLV-1)-bearing cells. Our new evidence shows that the 71-kDa heat shock cognate protein (HSC70) acts as a cellular receptor for syncytium formation. Using affinity chromatography with the peptide gp46-197, followed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, we isolated three components (bands A, B, and C) from MOLT-4 cell lysate which exhibited specific interactions with gp46 and inhibitory activities for syncytium formation induced by HTLV-1-bearing cells. Band A and B components were identified as HSC70 and β-actin, respectively, through amino acid sequencing by tandem mass spectrometry and immunostaining with specific monoclonal antibodies. Band C is likely to be a nonprotein component, because full activity for syncytium formation was seen after extensive trypsin digestion. Anti-HSC70 monoclonal antibody clearly blocked syncytium formation in a coculture of HTLV-1-bearing cells and indicator cells, whereas no inhibition was seen with anti-β-actin monoclonal antibody. Furthermore, flow cytometric analysis indicated that anti-HSC70 antibody reacted with MOLT-4 cells. Thus, we propose that HSC70 expressed on the target cell surface acts as a cellular acceptor to gp46 exposed on the HTLV-1-infected cell for syncytium formation, thereby leading to cell-to-cell transmission of HTLV-1.
Human T-cell lymphotropic virus type 1 (HTLV-1) is the causative agent of adult T-cell leukemia/lymphoma (ATLL) (17, 32, 33, 47) and HTLV-1-associated myelopathy/tropical spastic paraparesis (2, 14, 22, 30). In addition, HTLV-1 has previously been implicated in causing other diseases, including polymyositis (21) and uveitis (28). Although HTLV-1 has previously been associated with human malignancies which exhibit the CD4+ T-lymphocyte cell surface phenotype (3, 15, 32), many human and mammalian cells, including sarcoma cell lines (5, 19, 29, 48) and epithelial and endothelial cells (18, 20), can be infected by a cocultivation technique. The infectivity of cell-free HTLV-1 is very low (4, 5, 29). These observations suggest that close cell-to-cell interaction between HTLV-1-bearing cells and target cells is important for transmission of the virus (46). Like many other retroviruses, the cell-to-cell interaction between HTLV-1-bearing cells and target cells also induces syncytium formation (19, 29). It is thought that these two phenomena are at least partly based on the same mechanism and that the viral envelope glycoproteins expressed on HTLV-1-bearing cells are primarily responsible for these phenomena (6, 8, 31).
The receptor for HTLV-1 has previously been mapped to the long arm of human chromosome 17 (37, 38), and the gene product of approximately 30 to 31 kDa was identified as a cell surface receptor for HTLV-1 (13). Several other candidate antigens have previously been suggested to be involved in HTLV-1 cell surface adhesion and syncytium formation; they include HLA A2 (7), interleukin-2 receptor (25), CD2 (9), membrane glycoprotein C33 (11), and an 80-kDa membrane glycoprotein (1). However, the identity of the HTLV-1 receptor has not been determined. Previously, we reported that the peptide, referred to as gp46-197, corresponding to amino acids 197 to 216 of the gp46 surface glycoprotein inhibited syncytium formation induced by HTLV-1-bearing cells (34) and that this region served as a binding domain for the interaction between gp46 and the trypsin digestion-sensitive components in the MOLT-4 cell membrane, leading to viral entry into the target cell (35). To identify cell surface molecules involved in HTLV-1-induced syncytium formation, we isolated three cellular components which are capable of inhibiting syncytium formation induced by HTLV-1 by using affinity chromatography with peptide gp46-197, followed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). In the present study, we show that the 71-kDa heat shock cognate (HSC) protein, HSC70, expressed on the target cell surface acts as a cellular receptor for syncytium formation induced by HTLV-1.
MATERIALS AND METHODS
Cells and compounds.
The HTLV-1-bearing T-cell line used was human T-cell line KT252, established from a patient with ATLL in Kyushu University Hospital. The HTLV-1-negative cell line used was the human T-cell line MOLT-4 (39). These cell lines were cultured in RPMI 1640 medium supplemented with 10% fetal calf serum. The mouse fibroblast cell line L-M(TK−) (Dainippon Pharmaceutical Co., Osaka, Japan) was maintained in the Dulbecco modification of Eagle’s medium supplemented with 10% fetal calf serum. Peptides were synthesized with a peptide synthesizer (model 431A; Applied Biosystems, Foster City, Calif.) by the tert-butoxycarbonyl method, as described previously (26). The purity of each peptide was examined by reverse-phase high-performance liquid chromatography (Waters 626 pump equipped with a 996 photodiode array detector; Millipore, Milford, Mass.) with an octadecyl (C18) silicated column. Peptides were isolated as single peaks. Mature gp46 and gp21 proteins (a generous gift from H. Miyakoshi, Diagnostics Research Laboratory, Fujirebio, Tokyo, Japan) were purified from culture fluids of HTLV-1-producing TCL-Kan cells by immunoaffinity chromatography and gel chromatography (27).
Syncytium formation assay.
The syncytium formation assay consisted of a coculture of 2.5 × 104 HTLV-1-bearing KT252 cells and 105 indicator MOLT-4 cells. MOLT-4 cells were suspended in RPMI 1640 medium supplemented 10% fetal calf serum and 0.5% normal human serum (hereafter referred to as RPMI medium) at 5 × 106 cells per ml. Aliquots (20 μl per well) were added to 155 μl of RPMI medium containing test samples or medium alone in each well of a U-bottom 96-well plate (cell wells 25850; Corning Glass Works, Corning, N.Y.). Then 25 μl of KT252 cell suspension (106 cells per ml of RPMI medium) was added to each well. After incubation at 37°C for 18 h in a 5% CO2 incubator, the coculture medium was gently mixed with a pipette and aliquots (40 μl of 200 μl of coculture medium) were transferred to 35-mm-diameter tissue culture dishes with a 2-mm grid (Nunc Inc., Naperville, Ill.). Syncytia containing more than five nuclei were counted under an inverted microscope. In this assay system, the HTLV-1-bearing cell line KT252 routinely formed 500 to 800 syncytia per 105 indicator MOLT-4 cells. All experiments were performed in triplicate wells.
Isolation of cellular components.
Sepharose 4B coupled with peptide was prepared by incubation of 3 mg of synthetic peptide and 0.6 g (dry weight) of cyanogen bromide-activated Sepharose 4B (Pharmacia AB, Uppsala, Sweden) according to the manufacturer’s instructions. MOLT-4 cells (5 × 108) were disrupted with 2 ml of 20 mM Tris-HCl buffer (pH 7.4) containing 2 μg (each) of protease inhibitors (leupeptin, pepstatin, and aprotinin; Wako Pure Chemicals, Osaka, Japan) per ml, 2 mM phenylmethylsulfonyl fluoride, 50 mM sucrose monocaprate (SM-1000; Dojin Kagaku, Kumamoto, Japan), 1 mM dithiothreitol, 1 mM EDTA, and 150 mM KCl (hereafter referred to as disruption buffer), and the supernatant was collected by centrifugation at 11,000 × g for 20 min. After insoluble materials had been removed through a Sepharose 4B column (3 ml), the run-through fractions were applied to an affinity column (3 ml) coupled with gp46-197 and were washed extensively in succession with cell disrupting buffer, 10 mM phosphate buffer (PB) (pH 7.4) containing 10 mM sucrose monocaprate, and PB containing 10 mg of the peptide (p19-100) corresponding to amino acids 100 to 130 of HTLV-1 p19 protein per ml as a peptide-matched negative control. Subsequently, the materials that bound the affinity column were eluted with PB containing 10 mg of peptide gp46-197 per ml (hereafter referred to as gp46-197 eluate).
SDS-PAGE analysis.
The gp46-197 eluate was mixed with 30 μl of 250 mM Tris-HCl buffer (pH 6.7) containing 4% SDS, 0.05% bromophenol blue, 50% glycerol, and 10% 2-mercaptoethanol and then incubated for 2 min at 100°C. The reaction mixture was applied to a 12.5% polyacrylamide gel. After being run, the gel was cut into 2-mm strips and homogenized in 400 μl of 10 mM phosphate-buffered saline (PBS) (pH 7.2). After overnight incubation at 4°C, the supernatant (2 μl/well) was used to determine the activity for syncytium formation. Molecular mass calibration curves were prepared with standard proteins, including myosin heavy chain (200 kDa), phosphorylase B (97 kDa), bovine serum albumin (BSA) (68 kDa), ovalbumin (43 kDa), carbonic anhydrase (29 kDa), and β-lactoglobulin (18 kDa).
Binding experiment with a plastic plate.
The wells of a 96-well microtiter plate (Nunc-Immuno Module Maxisorp; Nunc) were coated with 1 μg of protein or peptide dissolved in 10 mM NaHCO3 buffer (pH 9.55) and left overnight at 4°C. Unreacted sites on the solid phase were blocked with 3% BSA (fraction V; Sigma) in PBS for 3 h at room temperature. After six washings with PBS containing 0.05% Tween 20, samples (1 μl per well) were added to 100 μl of RPMI 1640 medium containing 20 mM HEPES (Sigma, St. Louis, Mo.) (pH 7.2) in each well and incubated for 3 h at 37°C. The supernatant was used to determine the activity for syncytium formation.
Tandem mass spectrometry.
Acetamidation and lysyl-endopeptidase digestion were carried out essentially as previously described (23). After electrophoresis of the gp46-197 eluate on a 12.5% polyacrylamide gel, the part of the gel corresponding to protein bands was washed with distilled water, homogenized in 400 μl of 20 mM Tris-HCl (pH 8.0) containing 1% SDS, and allowed to stand overnight at 4°C. The supernatant was added to a fivefold amount of ice-cold acetone, and the preparation was incubated at −80°C for 2 h. After centrifugation, the pellet was dissolved in medium (pH 7.7) containing 8 M urea, 0.4 M NH4HCO3, and 4 mM dithiothreitol, incubated at 50°C for 15 min, and then acetamidized with 12.5 mM iode acetamide for 15 min at room temperature. Acetamidated protein was digested overnight with 0.6 mU of lysyl-endopeptidase (Wako Pure Chemicals) at 37°C and stored at −20°C. The sample was applied to an LCQ mass spectrometer (Finnigan MAT Instruments Inc., San Jose, Calif.), equipped with a Monitor C18 column (2 by 150 mm; Column Bioengineering, Ontario, Calif.) and eluted with a linear gradient for 40 min at a flow rate of 0.2 ml/min with buffer A containing 0.1% acetic acid, 0.02% trifluoroacetic acid and buffer B containing 0.1% acetic acid, 0.02% trifluoroacetic acid, and 90% acetonitrile. The mass spectrum of peptide was assigned through database searches by using the peptide sequence tag.
Immunochemical analysis.
gp46-197 eluates were analyzed by SDS-PAGE (12.5% polyacrylamide gel). After blotting to a polyvinylidene difluoride membrane (Millipore, Bedford, Mass.), the sheet was incubated for 2 h in the presence of 5 μg of rat anti-HSC70 monoclonal antibody (immunoglobulin G [IgG] fraction) (SPA-815; StressGen, Victoria, Canada) or 100 μg of mouse anti-β-actin monoclonal antibody (ascites fluid) (clone AC-15; Sigma) per ml. In experiments for syncytium formation with monoclonal antibody, serial dilutions of anti-HSC70 antibody (IgG fraction) and anti-β-actin antibody (ascites fluid) were placed on 96-well microtiter plates before the addition of infected cells and target cells. For control experiments, we used monoclonal antibody BE11 (IgG fraction or ascites fluid), which recognizes VP2 of human parvovirus B19 (36).
Trypsin digestion.
Trypsin digestion was performed by incubation for 1 h at 37°C in serum-free RPMI 1640 medium containing 0.5 μg of trypsin (Sigma), and the reaction was stopped by the addition of 1 μg of trypsin inhibitor (Wako Pure Chemicals). The reaction mixture was used to determine the activity for syncytium formation.
Flow cytometric analysis.
Cells (106 per tube) were washed once with PBS containing 1% BSA, incubated with monoclonal antibody on ice for 1 h, and then incubated with fluorescein isothiocyanate-conjugated goat anti-rat IgG or anti-mouse IgG (MBL, Nagoya, Japan). After being washed, cells were suspended in 1% BSA–PBS and analyzed on a Cytoron Absolute (Ortho Diagnostic System, Tokyo, Japan). Rat monoclonal antibody LAT 27, which recognizes the region corresponding to amino acids 192 to 196 of gp46 (41), was used as a control antibody.
RESULTS
Cellular components for syncytium formation.
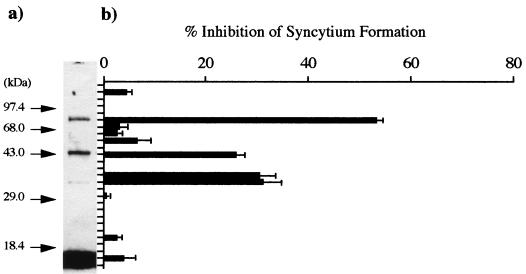
To isolate cellular components that interact with gp46 for syncytium formation induced by HTLV-1, we disrupted MOLT-4 cells (5 × 108), which were used as target cells in syncytium formation assays, with 2 ml of cell disruption buffer (pH 7.4). After centrifugation, the supernatant (2.5 ml) was applied to a Sepharose 4B column (3 ml) coupled with peptide gp46-197. For more specific elution of the materials that bound the affinity column, PB containing 10 mg of peptide gp46-197 was used. The affinity column was washed extensively in succession with cell disrupting buffer, PB containing 10 mM sucrose monocaprate, and PB containing 10 mg of peptide p19-100 per ml as a peptide-matched negative control. During these steps, we observed no inhibitory activity for the syncytium formation of eluates. Subsequently, the gp46-197 eluate was subjected to SDS-PAGE on a 12.5% polyacrylamide gel. As shown in Fig. 1, we observed three bands (bands A, B, and C) at positions corresponding to molecular masses of approximately 73, 43, and 34 kDa and inhibitory activities for syncytium formation in extracts from the gel corresponding to each band. These observations suggest that components in these bands are important for syncytium formation induced by HTLV-1.
FIG. 1.
SDS-PAGE analysis of the gp46-197 eluate. (a) Protein staining with 0.1% Coomassie brilliant blue. Arrows indicate the positions of molecular size marker proteins. (b) Inhibitory activity for syncytium formation in extracts from the gel. In the control experiment, the rate of formation was 806 syncytia per 105 indicator cells. Data are estimates in comparison with the syncytium count in a coculture without gel eluate and averages of triplicate determinations. Standard errors are also shown.
Characteristics of cellular components.
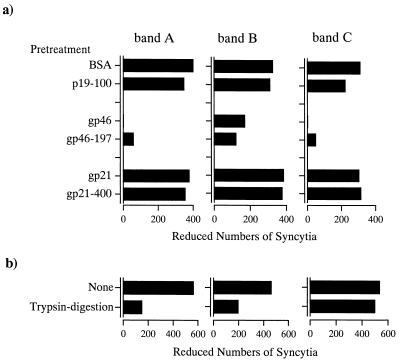
To clarify the binding specificity of each band, we performed absorption experiments on 96-well microtiter plates coated with mature gp46 and synthetic peptide gp46-197. In these experiments, extracts corresponding to each band on the SDS-PAGE gel were added to each well of the microtiter plate coated with the protein or synthetic peptide. After the incubation of plates, supernatants were used to determine the inhibitory activities for syncytium formation (Fig. 2a). The inhibitory activities of band A and C components were completely eliminated by preincubation with gp46, whereas no decrease was seen with gp21 or BSA as a negative control. Furthermore, the inhibitory activity of the band B component was clearly reduced by preincubation with gp46 for extracts with gp21 and BSA. These inhibitions were equivalent to the findings for components preincubated with peptide gp46-197, while no reduction was seen with p19-100 or gp21-400, which corresponds to the functional domain (amino acids 400 to 429) of the gp21 transmembrane protein (34) and served as a negative control. These results indicate that these three components interact specifically with the gp46 surface glycoprotein. We reported previously that the gp46 surface glycoprotein interacted with trypsin digestion-sensitive cellular component for syncytium formation (35). Therefore, these three components were treated with trypsin. The results of trypsin digestion on the inhibitory activity of each component for syncytium formation is shown in Fig. 2b. The inhibitory activities of band A and B components were remarkably reduced by trypsin digestion, whereas no reduction was seen for the band C component. These observations indicate that bands A and B carry protein components with inhibitory activities for syncytium formation.
FIG. 2.
Characteristics of the band A, B, and C components. All data are the means of triplicate experiments. (a) Specificity of binding to HTLV-1 proteins and peptides. The supernatant was used to determine the inhibitory activity for syncytium formation. In control experiments, the rate of formation was 822 syncytia per 105 indicator cells. (b) Effect of trypsin digestion on the inhibitory activity for syncytium formation. The reaction mixture was used to determine the activity for syncytium formation. In control experiments, the rate of formation was 1,075 syncytia per 105 indicator cells.
Identification of cellular components.
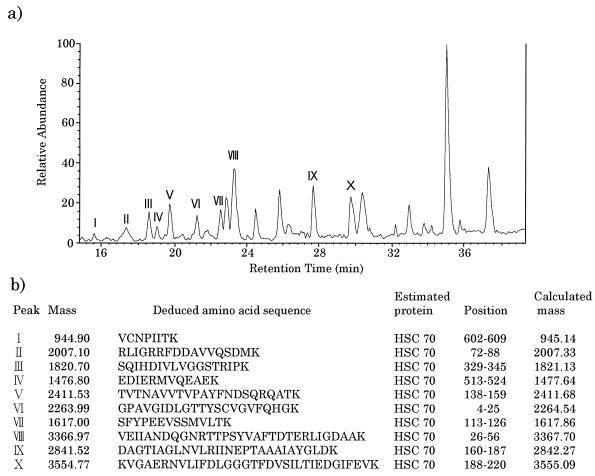
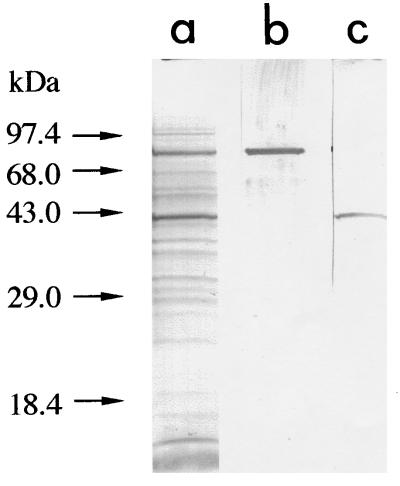
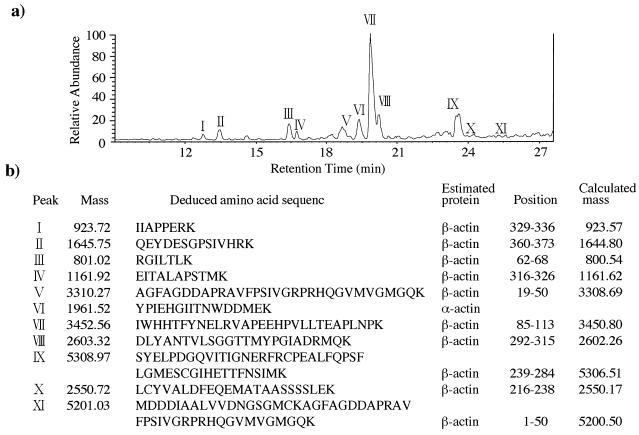
To identify the molecules in bands A and B, amino acid sequencing of the proteins was done by electron spray tandem mass spectrometry. The peptide extracts produced by lysyl-endopeptidase digestion of the extracts from bands A and B were applied to an LCQ mass spectrometer, equipped with a Monitor C18 column, and eluted with a linear gradient for 40 min at a flow rate of 0.2 ml/min with buffer A containing 0.1% acetic acid and 0.02% trifluoroacetic acid and buffer B containing 0.1% acetic acid and 0.02% trifluoroacetic acid, and 90% acetonitrile. As shown in Fig. 3, the peptide ion spectrum of the band A component showed multiple high-intensity peaks. The mass spectrum of all 10 peptide peaks derived from this component was assigned to a 71-kDa HSC protein (HSC70) through database searches by using the peptide sequence tag. Immunostaining with antibody against HSC70 supported this result. As shown in Fig. 4, the band A component in the gp46-197 eluate reacted with rat monoclonal antibody that recognizes HSC70 (IgG fraction) (SPA-815; StressGen). The mass spectrum of peptides derived from the band B component also showed multiple high-intensity peaks, of which all peaks but one were assigned to β-actin through database searches (Fig. 5). The component in band B reacted with mouse anti-β-actin monoclonal antibody (clone AC-15; Sigma) that recognizes the N-terminal part of β-actin (Fig. 4c). With respect to the component in band C, the ion signal could not be obtained by tandem mass spectrometry under our experimental conditions. This supported the previous result of trypsin digestion of the band C component.
FIG. 3.
Sequencing of a peptide produced by lysyl-endopeptidase digestion of the band A component. (a) Peptide ion spectrum. Peptide peaks are numbered according to their identifications by tandem mass spectrometry. (b) Deduced amino acid sequence of the peptide, as determined by the partial sequences obtained by tandem mass spectrometry.
FIG. 4.
Immunostaining of band A and B components with monoclonal antibodies to HSC70 and β-actin. (a) Protein staining with 0.1% Coomassie brilliant blue. (b) Rat anti-HSC70 monoclonal antibody (IgG fraction) (SPA-815; StressGen). (c) Mouse anti-β-actin monoclonal antibody (ascites fluid) (clone AC-15; Sigma). The gp46-197 eluates were analyzed by SDS-PAGE (12.5% polyacrylamide gel). After blotting to a polyvinylidene difluoride membrane, the sheet was incubated for 2 h in the presence of 5 μg of anti-HSC70 antibody or 100 μg of anti-β-actin antibody per ml.
FIG. 5.
Sequencing of a peptide produced by lysyl-endopeptidase digestion of the band B component. (a) Peptide ion spectrum. Peptide peaks are numbered according to their identifications by tandem mass spectrometry. (b) Deduced amino acid sequence of the peptide, as determined by the partial sequences obtained by tandem mass spectrometry.
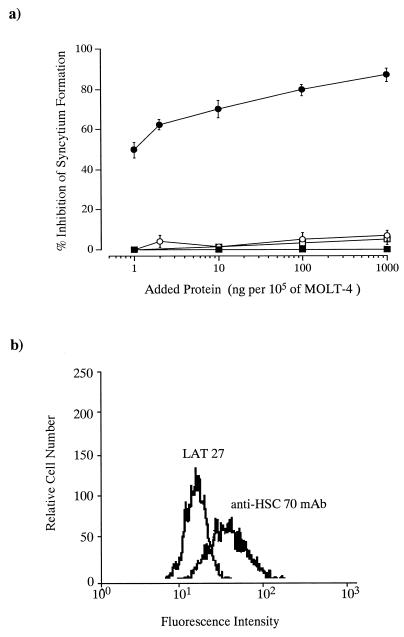
We obtained evidence that HSC70 and β-actin are trypsin digestion-sensitive components of the target cell, playing an important role for syncytium formation induced by HTLV-1-bearing cells. Our next concern was whether these components are expressed on the cell surface. Therefore, we examined the effect of monoclonal antibody to HSC70 or β-actin on syncytium formation. As shown in Fig. 6, syncytium formation was clearly blocked by the addition of monoclonal antibody to HSC70; 83% inhibition was seen at an antibody concentration of 1 μg per 105 MOLT-4 cells. In contrast, no blocking was seen with anti-β-actin or BE11 as a protein-matched negative control. Furthermore, flow cytometric analysis in the presence of monoclonal antibody at a concentration of 1 μg per 105 cells indicated that anti-HSC70 antibody reacted with MOLT-4 cells (Fig. 6b). Seventy-three percent of MOLT-4 cells scored positive for anti-HSC70 antibody binding. On the other hand, only 15% of L-M(TK−) cells, previously reported to be resistant for infection by HTLV-1 (37), scored positive. These data suggest that HSC70 expression on the surfaces of HTLV-1-permissive MOLT-4 cells is greater than that on nonpermissive or HTLV-1-resistant L-M(TK−) cells.
FIG. 6.
Effects of monoclonal antibodies to HSC70 and β-actin. (a) Effect on syncytium formation. Serial dilutions of test antibodies were placed on 96-well microtiter plates before the addition of infected cells and target cells. For control experiments, we used monoclonal antibody BE11 (IgG fraction or ascites fluid). In control experiments, the rate of formation was 857 syncytia per 105 indicator cells. Data are estimates in comparison with the syncytium count in a coculture without antibody and averages of triplicate determinations. Standard errors are also shown. •, anti-HSC70; ○, BE11 IgG fraction; ▪, anti-β-actin; □, BE11 ascites fluid. (b) Flow cytometric analysis. Flow cytometric analysis was performed in the presence of monoclonal antibody at a concentration of 1 μg per 105 cells. Rat monoclonal antibody LAT 27, which recognizes amino acids 192 to 196 of gp46 (41), was used as a control antibody.
DISCUSSION
This work shows that the 71-kDa HSC protein expressed on the target cell surface acts as a cellular receptor for syncytium formation induced by HTLV-1-bearing cells. The receptor for HTLV-1 has previously been mapped to the long arm of human chromosome 17 by infecting mouse-human somatic cell hybrids with pseudotyped vesicular stomatitis virus (HTLV-1) (13, 37, 38). However, recent work cells into question the previous assignment of the HTLV-1 receptor to human chromosome 17. By using an improved transient-transfection system, Sutton et al. (40) showed that L-M(TK−) cells, previously considered to be nonpermissive for HTLV-1 infection, were infected with pseudotyped human immunodeficiency virus (HTLV-1). This suggest that the receptor for HTLV-1 has a considerably wider cellular distribution than previously thought. The gene for HSC70 has previously been mapped to chromosome 11 (42), and its gene expression was relatively low in L-M(TK−) cells (24). Those findings support the results in our study.
Syncytium formation between retrovirus-infected cells and target cells is widely used to monitor virus-receptor interactions. The process consists of several steps, including correct binding to the cellular receptors of viral proteins, membrane fusion, and nuclei migration in the syncytial cytoplasm. Accordingly, many cellular components, including adhesion molecules, contribute to syncytium formation induced by HTLV-1; perturbation of any of these steps is likely to disturb syncytium formation. In the present study, we showed that β-actin itself inhibited syncytium formation induced by HTLV-1, but this inhibition did not occur at the cell surface. We cannot readily explain the role of β-actin in syncytium formation. It is possible that the inhibitory effect of β-actin or syncytium formation is due to the apparent binding of β-actin to the gp46 expressed on the surfaces of HTLV-1-bearing cells. Alternatively, the β-actin in target cells may act as an important cytoplasmic component for syncytium formation involvement in the migration or positioning of gp46. This is consistent with the involvement of actin-containing microfilaments in the migration and positioning of nuclei in syncytia induced by infection with parainfluenza virus (44). Further studies are needed for conclusive demonstrations of such cellular cofactors.
The nature of the band C component is unclear. Band C is likely to be a nonprotein component, because full activity for syncytium formation was seen after extensive trypsin digestion. Our experimental results showed that the band C component which allowed binding with gp46 had a molecular mass of approximately 34 kDa by SDS-PAGE. Recently, Gavalchin et al. (12) identified a monoclonal antibody which blocked HTLV-1 binding, syncytium formation, and HTLV-1 infection. This antibody reacted with antigens of approximately 30 to 31 kDa, which were found only in a hybrid cell line containing human chrosome 17q (13). We cannot readily explain the relationship between the band C component presented here and the antigens obtained with hybrid cell lines, because it is not clear whether the antigens are proteins. To further understand the molecular nature of the band C component, additional studies are under way to determine its fine structure.
HTLV-1 infection directly causes ATLL. However, infection with this virus indirectly contributes to many other disorders, including HTLV-1-associated myelopathy/tropical spastic paraparesis, polymyositis, and uveitis. Although the role of HTLV-1-infected cells in these disorders is unknown, it is likely that an autoimmune mechanism mediated by HTLV-1 infection has an important role (45). HSP70 family proteins have important intracellular functions, including protein trafficking, oligomer assembly, binding to damaged or aberrant proteins, and prevention of toxic aggregate formation (16). Furthermore, recent experimental results suggest the following possibilities for HSP and HSC protein functions in the development of autoimmune disease: (i) the HSP or HSC protein itself could act as a ligand to T-cell receptor γδ-type cells (10); (ii) the HSP or HSC protein itself could function as a presenting molecule complexed with an endogenously derived cellular peptide (43). This putative HSP or HSC protein function would be analogous to that of major histocompatibility complex class I molecules, and the expression of the HSP or HSC protein would be on the cell surface. Although the roles of the HSC protein itself or HSC protein-peptide complexes in a cellular immune response are unclear, it is highly likely that the direct interaction between HTLV-1 and putative cellular receptor HSC70 elicits numerous cellular responses. Thus, the characterization of HSC70 expressed on the cell surface will profoundly increase our understanding of the pathogenesis of HTLV-1-mediated diseases and provide certain insights into the host range and cellular tropism of HTLV-1 in vivo and in vitro.
ACKNOWLEDGMENTS
We thank M. Kanai, Finnigan MAT Instruments Inc., for technical assistance in amino acid sequencing with a tandem mass spectrometer and M. Ohara for helpful comments on the manuscript.
REFERENCES
- 1.Agadjanyan M G, Ugen K E, Wang B, Williams W V, Weiner D B. Identification of an 80-kilodalton membrane glycoprotein important for human T-cell leukemia virus type I and type II syncytium formation and infection. J Virol. 1994;68:485–493. doi: 10.1128/jvi.68.1.485-493.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhagavati S, Ehrlich G, Kula R, Kwok S, Sninsky J, Udani V, Poiesz B. Detection of human T-cell lymphoma/leukemia virus type I DNA and antigen in the spinal fluid and blood of patients with chronic progressive myelopathy. N Engl J Med. 1988;318:1141–1147. doi: 10.1056/NEJM198805053181801. [DOI] [PubMed] [Google Scholar]
- 3.Chen I S, McLaughlin J, Gasson J C, Clark S C, Golde D W. Molecular characterization of genome of a novel human T-cell leukemia virus. Nature. 1983;305:502–505. doi: 10.1038/305502a0. [DOI] [PubMed] [Google Scholar]
- 4.Chosa T, Yamamoto N, Tanaka Y, Koyanagi Y, Hinuma Y. Infectivity dissociated from transforming activity in a human retrovirus, adult T-cell leukemia virus. Gann. 1982;73:844–847. [PubMed] [Google Scholar]
- 5.Clapham P, Nagy K, Cheingsong-popov R, Exley M, Weiss R A. Productive infection and cell-free transmission of human T-cell leukemia virus in a nonlymphoid cell line. Science. 1983;222:1125–1127. doi: 10.1126/science.6316502. [DOI] [PubMed] [Google Scholar]
- 6.Clapham P, Nagy K, Weiss R A. Pseudotypes of human T-cell leukemia virus type 1 and 2: neutralization by patient’s sera. Proc Natl Acad Sci USA. 1984;81:2886–2889. doi: 10.1073/pnas.81.9.2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clarke M F, Gelmann E P, Reitz M S. Homology of human T-cell leukemia virus envelope gene with class I HLA gene. Nature. 1983;305:60–63. doi: 10.1038/305060a0. [DOI] [PubMed] [Google Scholar]
- 8.Delamarre L, Pique C, Pham D, Tursz T, Dokhelar M C. Identification of functional regions in the human T-cell leukemia virus type I SU glycoprotein. J Virol. 1994;68:3544–3549. doi: 10.1128/jvi.68.6.3544-3549.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duc Dodon M, Bernard A, Gazzolo L. Peripheral T-lymphocyte activation by human T-cell leukemia virus type I interferes with the CD2 but not with the CD3/TCR pathway. J Virol. 1989;63:5413–5419. doi: 10.1128/jvi.63.12.5413-5419.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fisch P, Malkovsky M, Kovats S, Sturm E, Braakman E, Klein B S, Voss S D, Morrissey L W, DeMars R, Welch W J, Bolhuis R L H, Sondel P M. Recognition by human Vg9/Vd2 T cells of a GroEL homolog on Daudi Burkitt’s lymphoma cells. Science. 1990;250:1269–1273. doi: 10.1126/science.1978758. [DOI] [PubMed] [Google Scholar]
- 11.Fukudome K, Furuse M, Imai T, Nishimura M, Takagi S, Hinuma Y, Yoshie O. Identification of membrane antigen C33 recognized by monoclonal antibodies inhibitory to human T-cell leukemia virus type 1 (HTLV-1)-induced syncytium formation: altered glycosylation of C33 antigen in HTLV-1 positive T-cells. J Virol. 1992;66:1394–1401. doi: 10.1128/jvi.66.3.1394-1401.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gavalchin J, Fan N, Lane M J, Papsidero L, Poiesz B J. Identification of a putative cellular receptor for HTLV-1 by a monoclonal antibody, Mab 34-23. Virology. 1993;194:1–9. doi: 10.1006/viro.1993.1228. [DOI] [PubMed] [Google Scholar]
- 13.Gavalchin J, Fan N, Waterbury P G, Corbett E, Faldasz B D, Peshick S M, Poiesz B J, Papsidero L, Lane M J. Regional localization of the putative cell surface receptor for HTLV-1 to human chromosome 17q23.2-17q25.3. Virology. 1995;212:196–203. doi: 10.1006/viro.1995.1468. [DOI] [PubMed] [Google Scholar]
- 14.Gessain A, Barin F, Vernent J C, Gout O, Maurs L, Calender A, de-The G. Antibodies to human T-lymphotropic virus type I in patients with tropical spastic paraparesis. Lancet. 1985;ii:407–410. doi: 10.1016/s0140-6736(85)92734-5. [DOI] [PubMed] [Google Scholar]
- 15.Hattori T, Uchiyama T, Toibana T, Takatsuki K, Uchino H. Surface phenotype of Japanese adult T-cell leukemia cells characterized by monoclonal antibodies. Blood. 1981;58:645–647. [PubMed] [Google Scholar]
- 16.Hightower L E. Heat shock, stress proteins, chaperones, and proteotoxicity. Cell. 1991;66:191–197. doi: 10.1016/0092-8674(91)90611-2. [DOI] [PubMed] [Google Scholar]
- 17.Hinuma Y, Nagata K, Hanaoka M, Matsumoti T, Kinoshita K, Shirakawa S, Miyoshi I. Adult T-cell leukemia: antigen in an ATL cell line and detection of antibodies to the antigen in human sera. Proc Natl Acad Sci USA. 1981;78:6476–6480. doi: 10.1073/pnas.78.10.6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ho D D, Rota T R, Hirsch M S. Infection of human endothelial cells by human T-lymphotropic virus type I. Proc Natl Acad Sci USA. 1984;81:7588–7590. doi: 10.1073/pnas.81.23.7588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoshino H, Shimoyama M, Miwa M, Sugimura T. Detection of lymphocytes producing a human retrovirus associated with adult T-cell leukemia by syncytia induction assay. Proc Natl Acad Sci USA. 1983;80:7337–7341. doi: 10.1073/pnas.80.23.7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoxie J A, Matthews D W, Cines D B. Infection of human endothelial cells by human T-cell leukemia virus type 1. Proc Natl Acad Sci USA. 1984;81:7591–7595. doi: 10.1073/pnas.81.23.7591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ishii K, Yamato K, Iwahara Y, Eguchi T, Takehara N, Ohtsuki Y, Taguchi H, Miyoshi I. Isolation of HTLV-I from muscle of a patient with polymyositis. Am J Med. 1991;90:267–269. [PubMed] [Google Scholar]
- 22.Jacobson S, Raine C S, Mingiolio E S, McFarlin D E. Isolation of an HTLV-I-like retrovirus from patients with tropical spastic paraparesis. Nature. 1988;331:540–543. doi: 10.1038/331540a0. [DOI] [PubMed] [Google Scholar]
- 23.Jekel P A, Weijer W, Beintema J. Use of endoproteinase Lys-C from lysobacter enzymogenes in protein sequence analysis. Anal Biochem. 1983;134:347–354. doi: 10.1016/0003-2697(83)90308-1. [DOI] [PubMed] [Google Scholar]
- 24.Kohl A, Alonso A, Hovemann B. Hsp 90, hsc73 and EF-1 alpha gene expression in nonheatshocked and heatshocked LMTK− cells. Nucleic Acids Res. 1987;15:6756. doi: 10.1093/nar/15.16.6756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kohtz D S, Altman A, Kohtz I D, Poszkin S. Immunological and structural homology between human T-cell leukemia virus type 1 envelope glycoprotein and a region of human interleukin-2 implicated in binding the β receptor. J Virol. 1988;62:659–662. doi: 10.1128/jvi.62.2.659-662.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuroda N, Washitani Y, Shiraki H, Kiyokawa H, Ohno M, Sato H, Maeda Y. Detection of antibodies to human T-lymphotropic virus type I using synthetic peptides. Int J Cancer. 1990;45:864–868. doi: 10.1002/ijc.2910450514. [DOI] [PubMed] [Google Scholar]
- 27.Miyakoshi H, Sugitani M, Igarashi H, Honda H, Fujino R, Mizukoshi M. Improvement of simultaneous detection of antibodies to Gag and envelope antigens of human T-lymphotropic virus type I by Western immunoblot assay. J Clin Microbiol. 1992;30:2555–2559. doi: 10.1128/jcm.30.10.2555-2559.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mochizuki M, Watanabe T, Yamaguchi K, Tajima K, Yoshikura I, Nakashima S, Shirao M, Araki S, Miyota N, Mori S, Takatsuki K. Uveitis associated with human T-lymphotropic virus type I: seroepidemiologic, clinical and virologic studies. J Infect Dis. 1992;166:943–944. doi: 10.1093/infdis/166.4.943. [DOI] [PubMed] [Google Scholar]
- 29.Nagy K, Clapham P, Cheingsong-Popov R, Weiss R. Human T-cell leukemia virus type I: induction of syncytia and inhibition by patients sera. Int J Cancer. 1983;32:321–328. doi: 10.1002/ijc.2910320310. [DOI] [PubMed] [Google Scholar]
- 30.Osame M, Usuku K, Izumo S, Ijichi N, Amitani H, Igata A, Matsumoto M, Tara M. HTLV-I associated myelopathy, a new clinical entity. Lancet. 1986;i:1031–1032. doi: 10.1016/s0140-6736(86)91298-5. [DOI] [PubMed] [Google Scholar]
- 31.Pique C, Pham D, Tursz T, Dokhelar M C. Human T-cell leukemia virus type I envelope protein maturation process: requirements for syncytium formation. J Virol. 1992;66:906–913. doi: 10.1128/jvi.66.2.906-913.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poiesz B J, Ruscetti F W, Gazdar A F, Bunn P A, Minna J D, Gallo R C. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci USA. 1980;77:7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Popovic M, Sarin P S, Robert-Guroff M, Kalyanaraman V S, Mann D, Minowada J, Gallo R C. Isolation and transmission of human retrovirus (human T-cell leukemia virus) Science. 1983;219:856–859. doi: 10.1126/science.6600519. [DOI] [PubMed] [Google Scholar]
- 34.Sagara Y, Inoue Y, Shiraki H, Jinno A, Hoshino H, Maeda Y. Identification and mapping of functional domains on human T-cell lymphotropic virus type 1 envelope proteins using synthetic peptides. J Virol. 1996;70:1564–1569. doi: 10.1128/jvi.70.3.1564-1569.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sagara Y, Ishida C, Inoue Y, Shiraki H, Maeda Y. Trypsin-sensitive and -resistant components in human T-cell membranes required for syncytium formation by human T-cell lymphotropic virus type 1-bearing cells. J Virol. 1997;71:601–607. doi: 10.1128/jvi.71.1.601-607.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sato H, Hirata J, Furukawa M, Kuroda N, Shiraki H, Maeda Y, Okochi K. Identification of the region including the epitope for a monoclonal antibody which can neutralize human parvovirus B19. J Virol. 1991;65:1667–1672. doi: 10.1128/jvi.65.4.1667-1672.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sommerfelt M A, Williams B P, Clapham P R, Solomon E, Goodfellow P N, Weiss R A. Human T-cell leukemia viruses use a receptor determined by human chromosome 17. Science. 1988;242:1557–1559. doi: 10.1126/science.3201246. [DOI] [PubMed] [Google Scholar]
- 38.Sommerfelt M A, Weiss R A. Receptor interference groups of 20 retroviruses plating on human cells. Virology. 1990;176:58–59. doi: 10.1016/0042-6822(90)90230-o. [DOI] [PubMed] [Google Scholar]
- 39.Srivastava B I, Minowada J. Terminal deoxynucleotidyl transferase activity in a cell line (Molt-4) derived from the peripheral blood of a patient with acute lymphoblastic leukemia. Biochem Biophys Res Commun. 1978;51:529–535. doi: 10.1016/0006-291x(73)91346-6. [DOI] [PubMed] [Google Scholar]
- 40.Sutton R E, Littman D R. Broad host range of human T-cell leukemia virus type 1 demonstrated with an improved pseudotyping system. J Virol. 1996;70:7322–7326. doi: 10.1128/jvi.70.10.7322-7326.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tanaka Y, Lee Z, Shiraki H, Shida H, Tozawa H. Identification of a neutralizing epitope on the envelope gp46 antigen of human T-cell leukemia virus type I and induction of neutralizing antibody by peptide immunization. J Immunol. 1991;146:354–360. [PubMed] [Google Scholar]
- 42.Tavaria M T, Gabriele R L, Anderson M E, Mirault E, Baker E, Sutherland G, Kola I. Localization of the gene encoding the human heat shock cognate protein, HSC73, to chromosome 11. Genomics. 1995;29:266–268. doi: 10.1006/geno.1995.1242. [DOI] [PubMed] [Google Scholar]
- 43.Udono H, Srivastava P K. Heat shock protein 70-associated peptides elicit specific cancer immunity. J Exp Med. 1993;178:1391–1396. doi: 10.1084/jem.178.4.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang E, Cross R K, Choppin P W. Involvement of microtubules and 10-nm filaments in the movement and positioning of nuclei in syncytia. J Cell Biol. 1979;83:320–337. doi: 10.1083/jcb.83.2.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamaguchi K. Human T-lymphotropic virus type 1 in Japan. Lancet. 1994;343:213–216. doi: 10.1016/s0140-6736(94)90994-6. [DOI] [PubMed] [Google Scholar]
- 46.Yamamoto N, Okada M, Koyamagi Y, Kannagi M, Hinuma Y. Transformation of human leukocytes by cocultivation with an adult T cell leukemia virus producer cell line. Science. 1982;217:737–739. doi: 10.1126/science.6980467. [DOI] [PubMed] [Google Scholar]
- 47.Yoshida M, Miyoshi I, Hinuma Y. Isolation and characterization of retrovirus from cell lines of human adult T-cell leukemia and its implication in the disease. Proc Natl Acad Sci USA. 1982;79:2031–2035. doi: 10.1073/pnas.79.6.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yoshikura H, Nishida J, Yoshida M, Kitamura Y, Takaku F, Ikeda S. Isolation of HTLV derived from Japanese adult T-cell leukemia patients in human diploid fibroblast strain IMR90 and the biological characters of the infected cells. Int J Cancer. 1984;33:745–749. doi: 10.1002/ijc.2910330606. [DOI] [PubMed] [Google Scholar]