Abstract
Physical exercise intervention can significantly improve the liver of patients with Non-alcoholic fatty liver disease (NAFLD), but it is unknown which exercise mode has the best effect on liver improvement in NAFLD patients. Therefore, we systematically evaluated the effect of exercise therapy on liver and blood index function of NAFLD patients through network meta-analysis (NMA). Through systematic retrieval of PubMed, Cochrane Library, Web of Science, EBSCO, and CNKI (National Knowledge Infrastructure), two reviewers independently screened the literature, extracted data, and assessed the risk of bias of the included studies by means of databases from inception to January 2023. The NMA was performed using the inconsistency model. A total of 43 studies, 2070 NAFLD patients were included: aerobic training (n = 779), resistance training (n = 159), high-intensity interval training (n = 160), aerobic training + resistance training (n = 96). The results indicate that aerobic training + resistance training could significantly improve serum total cholesterol (TC) (Surface under the cumulative ranking curve (SUCRA) = 71.7), triglyceride (TG) (SUCRA = 96.8), low-density lipoprotein cholesterol (LDL-C) (SUCRA = 86.1) in patients with NAFLD including triglycerides. Aerobic training is the best mode to improve ALT (SUCRA = 83.9) and high-density lipoprotein cholesterol (HDL-C) (SUCRA = 72.3). Resistance training is the best mode to improve aspartate transaminase (AST) (SUCRA = 81.7). Taking various benefits into account, we believe that the best modality of exercise for NAFLD patients is aerobic training + resistance training. In our current network meta-analysis, these exercise methods have different effects on the six indicators of NAFLD, which provides some reference for further formulating exercise prescription for NAFLD patients.
Subject terms: Health care, Health occupations
Introduction
Non-alcoholic fatty liver disease (NAFLD) was originally defined in 19801. It is defined as the presence of steatosis in more than 5% of hepatocytes, associated with metabolic risk factors (especially obesity and type 2 diabetes) and without excessive alcohol consumption (≥ 30 g/day in men and ≥ 20 g/day in women) or other chronic liver disease2. NAFLD is a major cause of cirrhosis and hepatocellular carcinoma. And it encompasses a spectrum of diseases ranging from steatosis with or without mild inflammation NAFLD to nonalcoholic steatohepatitis (NASH), which is characterized by necroinflammation and more rapid fibrosis progression than NAFLD3. Currently, NAFLD is considered one of the most common causes of chronic liver disease, causing 1.2 million deaths annually and rising to the eighth most common cause of death in the world4. In recent years, the prevalence of NAFLD has been increasing year by year in different regions of the world. According to a meta-analysis, the current global prevalence of NAFLD is an alarming 32.4%, which places a huge economic burden on society.5. In many parts of the world, NAFLD has become a more common chronic liver disease, and it is closely associated with obesity, type 2 diabetes mellitus (T2DM), dyslipidemia, and other patients6. For example, about 30.45% in South American states, Europe (23.71%), Korea (27.3%) and Japan (23–26%)7–9. As a result, complications of NAFLD place a significant health, economic, and patient experience burden on patients, their families, and society10. Because of the increasing prevalence of NAFLD, the search for an effective treatment has become an urgent issue.
NAFLD is a multi-system disease related to genetic, environmental and metabolic stress, including simple fatty liver disease, which progresses to NASH and cirrhosis (LC)11. The mechanism of NAFLD occurrence and development may be related to overproduction of reactive oxygen species (ROS) and oxygen species (OS). And it is also linked to DNA, lipid and protein oxidation and subsequent hepatocyte death12. Lipid metabolism disorder is also closely related to NAFLD, so Serum total cholesterol (TC), Triglyceride (TG), Low-Density Lipoprotein Cholesterol (LDL-C) and High-density Lipoprotein Cholesterol (HDL-C) in NAFLD patients show abnormalities13. In addition, Aspartate Transaminase (AST) and serum alanine aminotransferase (ALT) are good indicators for evaluating NAFLD patients14. However, NAFLD is the most prevalent liver disease worldwide and there is no approved pharmacotherapy. At present, pioglitazone and vitamin E are now recommended as effective drug therapy for NAFLD patients confirmed by biopsy15. Meanwhile diets high in caloric, high fat, and fructose-rich foods, along with a sedentary lifestyle, and obesity are significant risk factors implicated in the development and progression of NAFLD16. Therefore, making lifestyle changes may be a good choice. Exercise is a good way. It is an important factor affecting metabolism control by increasing physical activity of NAFLD patients. In some cross-sectional studies, it was found that NAFLD patients had low levels of physical activity17,18. Therefore, exercise intervention to improve the activity level of NAFLD patients is very important to cultivate a healthy lifestyle. The effect of exercise on NAFLD has been confirmed in previous studies19,20. Moderate intensity exercise of any degree is associated with a reduced risk of NAFLD and remission of NAFLD. The frequency of exercise plays a decisive role in reducing the incidence of NAFLD by 16% and improving the remission rate of NAFLD by 40%. Higher baseline exercise levels and increased weekly exercise over time were also associated with a reduced risk of NAFLD events and NAFLD resolution21. Exercise may be to reduce excessive ROS and OS production in NAFLD by regulating several mechanisms of action of exercise on NAFLD antioxidant enzymes and anti-inflammatory mediators. In addition, exercise on NAFLD patients is reflected in the effective improvement of TC, TG, LDL-C, HDL-C, AST and ALT levels22. This is very beneficial for the NAFLD patients.
Although previous systematic studies included different exercise interventions and indirect comparisons, it was not possible to determine the best exercise model23,24. Therefore, the effect of different exercise methods on NAFLD can be well determined through systematic review. In this study, we reviewed several randomized clinical trials (RCTs), discussed the efficacy of different exercise methods in NAFLD, and analyzed its characteristics using mesh meta-analysis. It aims to provide scientific and comprehensive reference for the formulation of exercise prescription for the treatment of NAFLD.
Methods
Network meta-analysis is performed according to the preferred reporting items in the System Review and Meta-analysis (PRISMA) guide25. (PROSPERO: CRD42023457428).
Literature search strategy
The literature search was performed for the related research studies, mainly from the following databases: PubMed, Cochrane Library, Web of Science, EBSCO, CNKI. The search keywords we used were (“Non-alcoholic fatty liver disease” OR “NAFLD” OR “non-alcoholic steatohepatitis” OR “NASH”) AND (“Randomized controlled trial” OR “Random” OR “RCT”) AND (“Exercise” OR “Training” OR “Aerobic training” OR Aerobic exercise OR “Resistance training” OR “Resistance exercise” OR “high-intensity interval training” OR “high-intensity interval exercise” OR “HIIT”). The meta-analysis is limited to January 2023, and the included study only includes randomized controlled trials.
Inclusion and exclusion criteria
Studies were included according to the following criteria: (1) RCTs with exercises as the intervention treating patients with NAFLD; (2) Subjects were diagnosed as NAFLD through pathological or imaging examination; (3) There was no significant difference in the basic indicators of patients included before intervention; (4) Results indicators included triglyceride (TG), total cholesterol (TC), low-density lipoprotein (LDL-C), high-density lipoprotein (HDL-C), Alanine aminotransferase (ALT), and Aspartate aminotransferase (AST); (5) The data before and after intervention were obtained. Studies were excluded based on the following criteria: (1) Unable to obtain exact data; (2) The detection indicators did not meet the inclusion criteria; (3) Studies such as animal experiments, abstracts, case reports, reviews, systematic reviews, and repeated publications; (4) The mode, duration, and period of the movement are unclear.
Data extraction
The two authors independently screened abstracts and full-text articles from these selected works, extracted and cross-checked the data. In case of disagreement, we consult a third party for mediation and reach consensus. In the literature screening process, the first thing is to read the title and abstract, and then the full text to determine the excluded literature. The following data were extracted from the selected works: research title; author name; publication time; sample size; interventions; type, intensity, frequency and duration of exercise; relevant patient outcomes (TC/TG/LDL-C/HDL-C/AST/ALT); and risks of literature bias.
Quality assessment
The quality of the included studies was evaluated. Then two authors assessed the quality of the included studies. Any disagreement was discussed with a third reviewer. The two authors/It is important to use the Cochrane Handbook for Systematic Interventions to assess the quality of studies. It includes the evaluation of randomization methods, concealment of distribution, blindness of patients and physicians, outcome evaluation, data integrity, selective reporting, and other biased sources26.
Statistical analysis
STATA (Version 17.0) command'mvmeta' was used to perform a multivariate network meta-analysis within a frequentist framework. The therapeutic effect of each study on NAFLD was calculated by standard mean deviation (SMD) and standard deviation (SD). For studies that provide only median and quartile ranges, we derive SMD and SD to overcome the heterogeneity between research interventions and results27,28. The I2 statistics are used to measure heterogeneity, which is considered high heterogeneity when it exceeds 50%. Consistency means that the treatment effect estimated by direct comparison is consistent with that estimated by indirect comparison. Statistical indicators include TG, TC, LDL-C, HDL-C, AST and ALT. Subsequently, interventions were ranked using the surface under the cumulative ranking curve (SUCRA). SUCRA is considered to be a more accurate estimate of the cumulative ranking probability. Simultaneously, SUCRA reported the overall probability based on the ranking of all interventions, that is, a given intervention is one of the best treatments29,30. When calculating the SUCRA impact of each exercise on NAFLD indicators, it is necessary to combine the corresponding indicators and observe the impact of exercise on the paired indicators.
Result
Literature selection
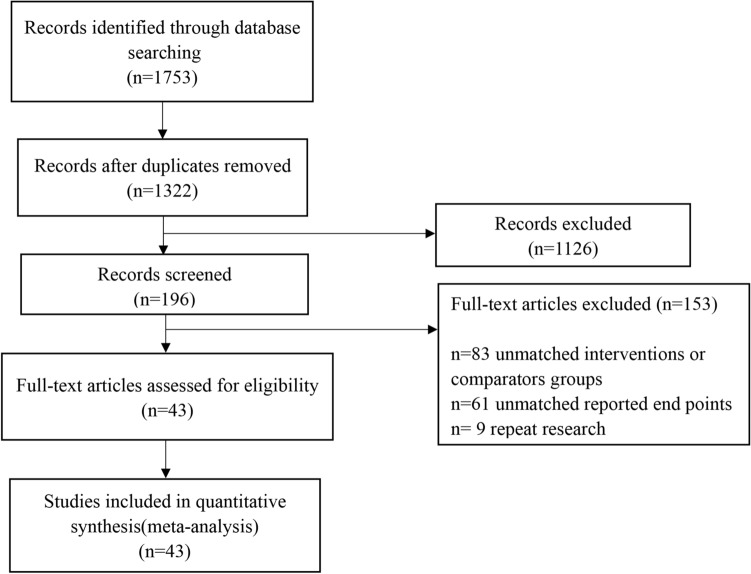
A total of 1753 studies were initially identified. After reviewing the title, summary and full text, 43 studies met the inclusion criteria. Among them, there are 21 Chinese studies31–51 and 22 English studies52–73 in this review (Fig. 1).
Figure 1.
Study flow diagram.
Characteristics of the included studies
The characteristics of these included studies are presented in Table1. A total of 2070 NAFLD patients were included in 42 studies, including 876 in the control group and 1194 in the intervention group. The exercise cycle varies from 8 to 24 weeks, and exercise modulations include aerobic exercise, resistance exercise, HIIT, aerobic exercise + resistance exercise. There is aerobic training (AT) (n = 779), resistance training (RT) (n = 159), high-intensity interval training (HIIT) (n = 160), aerobic training + resistance training (AT + RT) (n = 96) (Table 1).
Table 1.
Basic characteristics of the included studies.
| No. | Author | Sample size | Treatment | Interventiontime (weeks) | Outcome indicator | Other outcome | ||
|---|---|---|---|---|---|---|---|---|
| T | C | T | C | |||||
| 1 | Hallsworth et al.64 | 11 | 8 | RT | None | 8 | 1, 5, 6 | None |
| 2 | de Piano66 |
AT:15 RT:15 |
14 |
AT AT + RT |
None | 48 | 1, 2, 3, 4, 5, 6 | None |
| 3 | Sullivan53 | 12 | 6 | AT | None | 16 | 1, 2, 5 | None |
| 4 | Pugh et al.56 | 13 | 7 | AT | None | 16 | 1, 2, 3, 4, 5, 6 | Decreased liver fat content |
| 5 | O Al-Jiffri71 | 50 | 50 | AT | None | 12 | 4, 5 | None |
| 6 | Jakovljevic61 | 9 | 8 | RT | None | 8 | 1, 5, 6 | None |
| 7 | Hallsworth63 | 12 | 11 | HIIT | None | 12 | 1, 4, 5, 6 | None |
| 8 | Shamsoddini55 |
AT:10 RT:10 |
10 |
AT RT |
None | 8 | 4, 5 | None |
| 9 | Keating59 | 36 | 12 | AT | None | 8 | 1, 2, 3, 4, 5, 6 | None |
| 10 | Croci68 | 10 | 6 | AT | None | 24 | 2, 3, 6 | None |
| 11 | de Lira67 |
HIIT:26 AT:25 |
33 |
HIIT AT |
None | 12 | 1, 2, 3, 4, 5, 6 | None |
| 12 | Oh et al.57 |
AT:33 RT:19 |
AT RT |
12 | 4, 5, 6 | None | ||
| 13 | Winn et al.88 |
AT:5 HIIT:5 |
5 |
AT HIIT |
None | 4 | 1, 2, 3, 4, 5, 6 | None |
| 14 | Keating et al.60 | 7 | 5 | HIIT | None | 12 | 1, 2, 3, 4, 5, 6 | None |
| 15 | Stine et al.89 | 18 | 10 | AT | None | 20 | 1, 2, 3, 4, 5, 6 | None |
| 16 | Charatcharoenwitthaya et al.69 |
AT:18 RT:17 |
AT RT |
12 | 1, 2, 3, 4, 5, 6 | None | ||
| 17 | Whyte et al.52 |
HIIT:16 AT:15 |
16 |
HIIT AT |
None | 8 | 1, 2, 3, 5, 6 | None |
| 18 | Abdelbasset et al.73 | 15 | 12 | AT | None | 16 | 2 | None |
| 19 | Moradi58 | 12 | 11 | RT | None | 12 | 4, 5 | Structural changes in the liver |
| 20 | Ghamarchehreh65 |
AT:10 RT:10 |
8 |
AT RT |
None | 8 | 1, 2, 3, 6 | None |
| 21 | Banitalebi et al.70 |
AT:17 AT + RT:17 |
18 |
AT AT + RT |
None | 10 | 4, 5 | Fatty liver index decreased |
| 22 | Abdelbasset et al.72 | 16 | 16 | HIIT | None | 8 | 1, 2, 3, 5, 6 | None |
| 23 | Peng42 | 27 | 27 | AT | None | 12 | 1, 2, 3, 4, 5, 6 | Altered fatty deposits in the liver |
| 23 | Liu45 | 48 | 44 | HIIT | None | 12 | 1, 2, 3 | None |
| 25 | Luo44 | 30 | 30 | HIIT | None | 12 | 1, 2, 3, 4, 5, 6 | None |
| 26 | Zuo31 | 12 | 12 | AT | None | 24 | 1, 2, 3, 6 | None |
| 27 | Liu46 | 30 | 30 | AT | None | 16 | 1, 2, 3, 5, 6 | None |
| 28 | Fu48 |
AT:37 RT:37 |
36 |
AT RT |
None | 16 | 1, 2, 3, 6 | None |
| 29 | Yang37 |
AT:34 RT:34 |
35 |
AT RT |
None | 20 | 1, 2, 3, 5, 6 | None |
| 30 | Zhao32 | 17 | 14 | AT | None | 35 | 1, 3, 6 | |
| 31 | Yang36 | 48 | 48 | AT | None | 24 | 1, 2, 3, 6 | None |
| 32 | Yao35 | 15 | 15 | AT | None | 24 | 1, 2, 4, 5, 6 | None |
| 33 | Xu38 | 42 | 29 | AT | None | 12 | 1, 2, 3, 6 | None |
| 34 | Guo47 | 18 | 15 | AT | None | 24 | 1, 5, 6 | None |
| 35 | Chen50 | 36 | 51 | AT | None | 52 | 1, 6 | None |
| 36 | Mao43 | 30 | 30 | AT | None | 12 | 1, 2, 3, 6 | None |
| 37 | Fan49 | 11 | 10 | AT | None | 16 | 2, 3, 5, 6 | None |
| 38 | Zhang34 | 60 | 60 | AT | None | 16 | 1, 2, 3, 6 | None |
| 39 | Wu39 | 15 | 15 | AT | None | 16 | 2, 3, 5, 6 | None |
| 40 | Wu40 | 13 | 13 | AT | None | 16 | 2, 3, 6 | None |
| 41 | Tan41 | 18 | 19 | AT | None | 24 | 1, 2, 3, 6 | None |
| 42 | Zhang33 | 14 | 13 | AT | None | 24 | 1, 2, 3, 6 | None |
| 43 | Li ying51 | 64 | 64 | AT + RT | None | 12 | 1, 2, 3, 6 | None |
1 TC 2 HDL-C 3 LDL-C 4 AST 5 ALT 6 TG.
C Control group; T Training group; AT Aerobic training; RT Resistance training; HIIT: High-intensity-interval-training; AT + RT Aerobic training + Resistance training.
Result of assessment
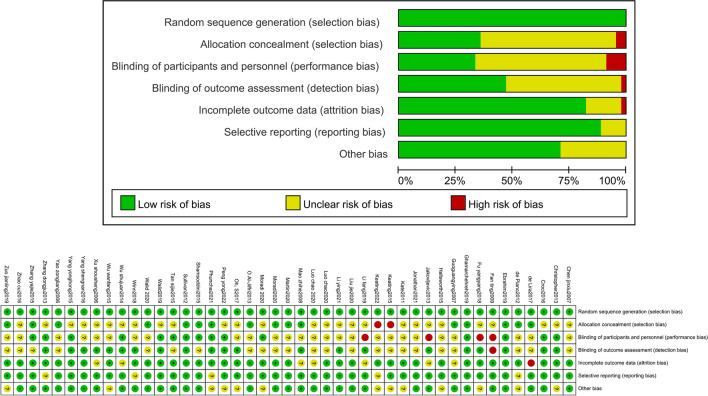
According to the Cochrane Intervention System Evaluation Manual, the quality of the study was assessed by the quality assessment method of the RCT. The Cochrane Bias Risk Assessment Chart shows the risks of different biases in 43 studies. In the included studies, the selection of new concepts to check whether blinding performance bias and outcome evaluation detection bias showed the highest risk. In addition, other biases such as attribution bias, reporting bias and random sequence generation show low risk (Fig. 2).
Figure 2.
Schematic of cochrane bias risk assessment.
Network meta-analysis
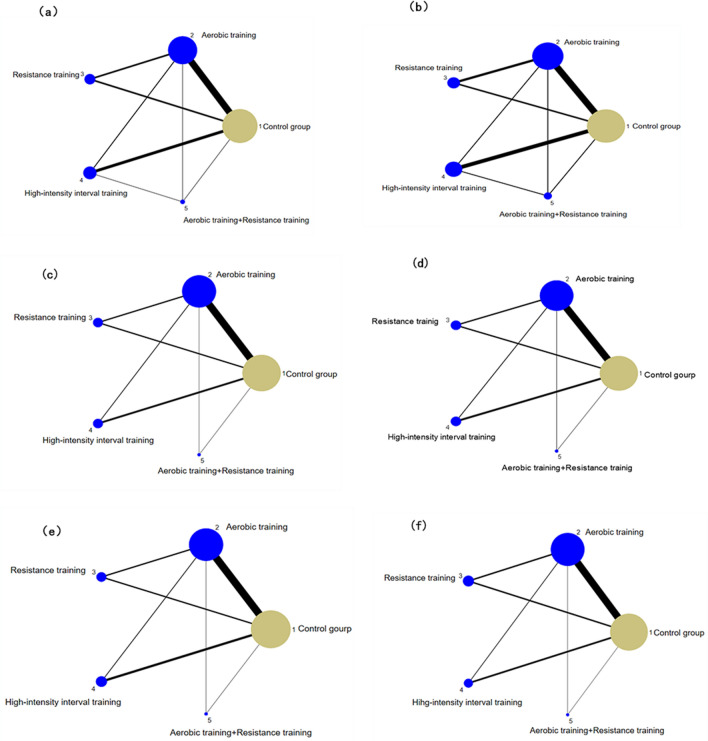
This study includes various types of exercise therapy, HIIT, RT, AT, and AT + RT. The effects of four different exercises on TC, TG, AST, ALT, HDL-C and LDL-C in NAFLD patients were analyzed. Figure 3 shows the network meta-analysis of the effects of different exercise interventions on efficacy. The size of the node is related to the number of participants in the exercise interventions, and the thickness of the lines between different nodes is related to the number of studies compared. Compared with the control group, AT (p < 0.01), RT, HITT, AT + RT and other exercise modes can reduce the TC level of NAFLD patients. Similarly, for TG, AT (p < 0.01), HIIT (p < 0.05) and AT + RT (p < 0.01) show significant differences. Different exercise modalities can reduce HDL-C and ALT levels in NAFLD patients, and AT (p < 0.01) shows a significant decrease. The high expression of LDL-C in vivo brings bad benefits, which can be effectively reduced by different exercise modalities. On the contrary, RT (0.16 ( − 0.30, 0.62)) did not show a good reduction effect. For AST, RT (0.26 ( − 2.62, 3.14)) and AT (0.09 ( − 2.75, 2.93)) have no effect on AST reduction. About ALT, a significant improvement effect is brought by AT (p < 0.01). However, AT + RT does not appear to offer any significant improvement benefits (Table 2). Not only the global inconsistency, but also the node-splitting method is used to continue the local inconsistency inspection. Then, the node splitting method confirmed the difference in the influence of different motion modes on NAFLD patients. It was found that the improvement effect of AT VS RT (p < 0.05) on NAFLD was significantly different between TC, TG and LDL-C (Table 3). Subsequently, we will calculate the effect of different exercise modes on NAFLD patients through SUCRA.
Figure 3.
Effects of different exercise modes on lipids, liver enzyme networking, and plasma cholesterol in patients with nonalcoholic fatty liver disease. (a) ALT (b) AST (c) HDL-C (d) LCL-C (e) TC (f) TG.
Table 2.
Global inconsistency detection.
| Non-conformance inspection | ||||
|---|---|---|---|---|
| (vs Control) | n | 95%CI | p | |
| TC | ||||
| AT | 25 | − 0.69 ( − 1.11, − 0.26) | 0.001* | |
| RT | 5 | − 0.41 ( − 1.45, 0.63) | 0.438 | |
| HIIT | 7 | − 0.61 ( − 1.86, 0.64) | 0.339 | |
| AT + RT | 2 | − 1.16 ( − 2.86, 0.533) | 0.179 | |
| TG | ||||
| AT | 28 | − 0.58 ( − 0.91, − 0.25) | 0.001* | |
| RT | 7 | − 0.23 ( − 1.08, 0.61) | 0.59 | |
| HIIT | 6 | − 1.10 ( − 2.16, − 0.37) | 0.043* | |
| AT + RT | 2 | − 1.98 ( − 3.37, − 0.59) | 0.005* | |
| HDL-C | ||||
| AT | 25 | 0.61 (0.09, 1.12) | 0.022* | |
| RT | 5 | 0.48 ( − 0.73, 1.69) | 0.434 | |
| HIIT | 6 | 0.61 ( − 0.87, 2.01) | 0.419 | |
| AT + RT | 2 | 1.22 ( − 0.81, 3.26) | 0.237 | |
| LDL-C | ||||
| AT | 23 | − 0.56 ( − 0.77, − 0.34) | 0.00* | |
| RT | 5 | 0.16 ( − 0.30, 0.62) | 0.497 | |
| HIIT | 6 | − 0.99 ( − 1.58, − 0.40) | 0.001* | |
| AT + RT | 2 | − 1.01 ( − 1.69, − 0.35) | 0.003* | |
| AST | ||||
| AT | 11 | − 0.66 ( − 1.83, 0.50) | 0.264 | |
| RT | 4 | 0.26 ( − 2.62, 3.14) | 0.860 | |
| HIIT | 5 | − 0.40 ( − 3.20, 2.39) | 0.777 | |
| AT + RT | 2 | 0.09 ( − 2.75, 2.93) | 0.950 | |
| ALT | ||||
| AT | 19 | − 0.85 ( − 1.26, − 0.43) | 0.00* | |
| RT | 6 | − 0.14 ( − 1.13, 0.84) | 0.773 | |
| HIIT | 7 | − 0.73 ( − 1.70, 0.24) | 0.140 | |
| AT + RT | 2 | 0.56 ( − 1.37, 1.49) | 0.938 | |
AT aerobic training; RT resistance training; HIIT high intensity interval training; AT + RT aerobic training + resistance training; *p < 0.05.
Table 3.
Node-splitting inconsistency detection.
| Group | Direct | Indirect | p |
|---|---|---|---|
| TC | |||
| AT VS RT | − 1.03 | 1.42 | 0.002* |
| AT VS HIIT | − 0.17 | 0.67 | 0.721 |
| AT VS AT + RT | − 0.32 | 0.96 | 0.772 |
| TG | |||
| AT VS RT | 0.23 | 1.45 | 0.048* |
| AT VS HIIT | − 0.17 | − 0.18 | 0.996 |
| AT VS AT + RT | − 0.44 | − 1.45 | 0.336 |
| HDL-C | |||
| AT VS RT | − 0.10 | − 0.08 | 0.986 |
| AT VS HIIT | 0.26 | − 0.77 | 0.207 |
| AT VS AT + RT | − 0.58 | 0.66 | 0.363 |
| LDL-C | |||
| AT VS RT | − 0.26 | 1.36 | 0.000* |
| AT VS HIIT | − 0.91 | − 0.40 | 0.510 |
| AT VS AT + RT | − 0.36 | − 0.58 | 0.450 |
| AST | |||
| AT VS RT | − 0.82 | 0.19 | 0.485 |
| AT VS HIIT | 0.31 | 0.27 | 0.975 |
| AT VS AT + RT | − 0.60 | 0.37 | 0.583 |
| HIIT VS AT + RT | − 0.22 | − 0.53 | 0.868 |
| ALT | |||
| AT VS RT | − 0.78 | 0.57 | 0.083 |
| AT VS HIIT | 0.07 | 0.09 | 0.971 |
| AT VS AT + RT | − 1.01 | 0.50 | 0.226 |
| HIIT VS AT + RT | 0.12 | − 0.64 | 0.569 |
AT aerobic training; RT resistance training; HIIT high intensity interval training; AT + RT aerobic training + resistance training; *p < 0.05.
The effect of different exercise modalities
TC and TG
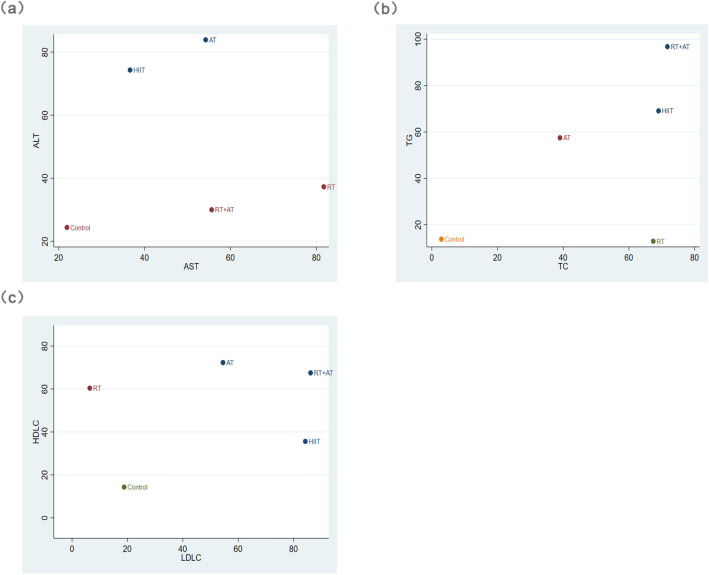
In the network analysis, SUCRA is considered to be a more accurate estimate of the cumulative ranking probability. It can use different ranking methods, maximum or minimum, according to the different benefits of sports. Among the effects of different exercise modes on TC in NAFLD patients, AT + RT (SUCRA = 71.7) has the best effect, HIIT (SUCRA = 69.0), RT (SUCRA = 67.4), AT (SUCRA = 39.0). For TG, RT + AT (SUCRA = 96.8) still provides the best intervention benefits. HIIT (SUCRA = 69.1), AT (SUCRA = 57.5), RT (SUCRA = 12.9) (Fig. 4). Subsequently, after fitting the effects of different exercise modes on TC and TG, it was found that AT + RT had the best overall effect on TC and TG reduction in NAFLD patients, followed by HIIT (Fig. 5a). HIIT also takes great improvement.
Figure 4.
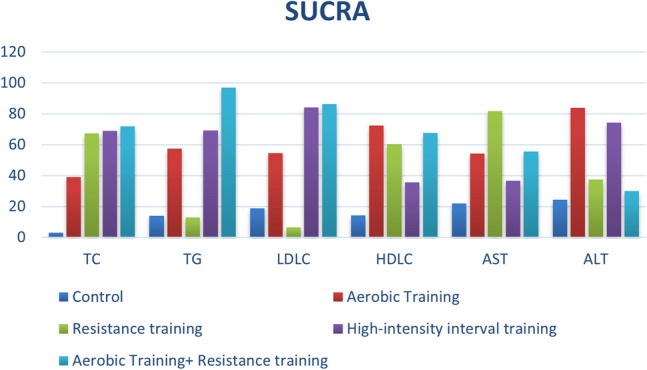
Ranking chart of different outcome indicators for each intervention.
Figure 5.
Efficacy of different exercise modalities. (a) ALT and AST (b) TG and TC (c) HDL-C and LDL-C.
ALT and AST
ALT and AST are two important indicators of liver function. By calculating the effect of different exercise modalities on ALT, AT (SUCRA = 83.9) had the best effect on ALT in NAFLD patients. HIIT (SUCRA = 74.3), RT (SUCRA = 37.3), RT + AT (SUCRA = 30). For AST, RT (SUCRA = 81.7), RT + AT (SUCRA = 55.6), AT (SUCRA = 54.2), HIIT (SUCRA = 36.6) (Fig. 4). Among them, AT has the best effect on reducing ALT and AST in patients with NAFLD (Fig. 5b).
LDL-C and HDL-C
LDL-C is a kind of bad cholesterol, which is usually too high in patients with NAFLD. By calculating the effect of different exercise modalities on LDL-C, RT + AT (SUCRA = 86.1) had the best effect on LDL-C in NAFLD patients. HIIT (SUCRA = 84.2), AT (SUCRA = 54.5), RT (SUCRA = 6.4). For HDL-C, AT (SUCRA = 72.3) is the best exercise modality in NAFLD patients, subsequently, RT + AT (SUCRA = 67.5), RT (SUCRA = 60.4), HIIT (SUCRA = 35.6) (Fig. 4). Through comprehensive effect verification, AT + RT is the best exercise modalities, which can improve HDL-C and reduce LDL-C in NAFLD patients. AT also brought great improvement (Fig. 5c).
Discussion
Exercise brings many benefits to NAFLD patients, such as promoting blood lipid metabolism, reducing liver fat, and improving quality of life, etc. Many studies have shown that exercise intervention is effective for NAFLD patients22,74,75. However, considering individual heterogeneity, it remains a challenge to develop appropriate exercise prescriptions for NAFLD patients. Therefore, it is of great significance to explore the best exercise mode for NAFLD patients to improve the exercise intervention and improve the symptoms of NAFLD patients. Our study found that AT + RT is the best for overall improvement of TC and TG in NAFLD patients. The results are consistent with previous studies76. For ALT and AST, we found that the best exercise modality to improve them is AT and RT, respectively. AT has the best effect on ALT levels in NAFLD patients, while RT has the best effect on AST levels in NAFLD patients. This is not consistent with the AT + RT obtained by ZHOU et al.76, which may be due to the fact that we included more RCTs than they did. In addition, we also added the effect of exercise on LDL-C and HDL-C, which are important for improving NAFLD patients. RT + AT and AT are the best exercise methods to improve LDL-C and HDL-C in NAFLD patients. In terms of overall effect, our conclusion is that AT + RT is the best exercise method to improve NAFLD patients.
AT + RT exercise mode is the best exercise mode we have found. It is a combination of AT and RT. The test indicators we selected include TG, TC, AST, ALT, LDL-C, HDL-C. Because TC is an independent factor in the development of cirrhosis, TG is an independent predictor of cardiovascular disease77,78. High levels of AST and ALT increase the risk of liver cell damage79. These two liver enzymes are also key parameters for determining NAFLD and hepatitis incidence. HDL-C is mainly synthesized in the liver and is an anti-atherosclerotic lipoprotein, which can transport cholesterol from extrahepatic tissues to the liver for metabolism and excrete it from bile. However, some studies have found that HDL-C levels are reduced in NAFLD patients80. LDL-C is the main lipoprotein in fasting plasma and the main vehicle for transporting cholesterol to extrahepatic tissues. Some studies have found that almost one in 10 children and adolescents with NAFLD have low LDL-C levels, which is about twice the expected level of the general population. Interestingly, patients with NAFLD and low LDL-C levels have similar liver disease severity as patients with normal or elevated LDL-C81. One characteristic of movement is that it consumes a lot of energy in the process. Long-term exercise significantly improves ALT and AST in Chinese patients with NAFLD82. Elevated transaminase levels are considered an independent predictor of advanced fibrosis, and have also been shown to correlate significantly with NASH83,84. And the ratio of AST to ALT is often used in medicine and is considered an independent indicator for predicting advanced liver fibrosis85. Improvements in this ratio from exercise can reflect the benefits that exercise brings to NAFLD patients. Exercise is beneficial for reducing visceral fat. A 4-week AT intervention experiment was conducted on 19 sedentary obese people. It has been found that AT can reduce visceral adipose tissue volume by 12% and liver fat content by 21% over a 4-week period. High intensity physical activity has been reported to effectively improve the pathological conditions of NAFLD, including fat accumulation, inflammation and fibrosis57. This strongly confirmed the benefits of physical exercise for improving NAFLD patients. However, in exploring which exercise can achieve the best effect, some studies have confirmed the benefits of combined exercise (AT + RT) through experiments86. Among obese adolescents diagnosed with metabolic syndrome, AT + RT is more effective than AT alone in improving the associated inflammatory process, including increasing adiponectin concentration and controlling cardiovascular risk factors87. One study found that the cure rate of AT + RT combined training for NAFLD patients was higher than that of AT alone after 60 patients received AT alone or AT + RT combined training for one year66. Regarding the exercise cycle, the research intervention duration we included is generally 12 weeks. Perhaps the effects of exercise need to be achieved over a long period of time. Combined training (AT + RT) was more effective than AT alone in promoting high amplitude changes in fat mass (kg), lean mass (kg, %), homeostasis model assessment-Insulin resistance (HOMA-IR), LDL-C, adiponectin, adiponectin/leptin ratio, and mean corpuscular hemoglobin (MCH)66. Most patients with NAFLD are middle-aged or older, and attention should be paid to the patient's physical condition when choosing exercise methods. Therefore, finding more scientific training methods is of great significance for the rehabilitation of NAFLD patients.
Although this NMA has many benefits, it must admit some limitations: (1) There is a large difference in the number of exercise modes included in each study; (2) Only the choice of motion mode is considered, and the choice of motion content, period and frequency is ignored; (3) The article only includes Chinese and English, and there are high-quality RCTs in other languages that are not included; (4) Only assessed changes in blood biomarkers, and lacked more intuitive indicators associated with NAFLD, including steatosis inside the liver, biopsy, ultrasound or elastography tests.
Conclusion
Through network meta-analysis, we found that for patients with NAFLD, AT + RT modalities is the best way to improve TC, TG and LDL-C. Next, AT is the best mode to improve ALT and HDL-C. RT is best mode to improve AST. For the overall effect, AT + RT has the best effect on improving TC, TG, LDL-C and HDL-C in NAFLD patients. AT has the best effect on improving AST and ALT in patients with NAFLD. Therefore, NAFLD patients are recommended to participate in AT OR AT + RT modes improving NAFLD. In addition, we also added the effect of exercise on LDL-C and HDL-C, which are important for improving NAFLD patients. Therefore, our results show that AT + RT at least three times a week, 50 min each time, for 10 weeks, has a better effect on liver improvement in NAFLD patients.
Acknowledgements
Thank each author for their hard work.
Abbreviations
- NAFLD
Nonalcoholic fatty liver disease
- NMA
Network meta-analysis
- T2DM
Type 2 diabetes mellitus
- ROS
Reactive oxygen species
- OS
Oxygen species
- TC
Triglycerides cholesterol
- TG
Triglyceride
- LDL-C
Low-density lipoprotein cholesterol
- HDL-C
High-density lipoprotein cholesterol
- AST
Aspartate transaminase
- ALT
Alanine aminotransferase
- RCTs
Randomized clinical trials
- PRISMA
Preferred reporting items in the system review and meta-analysis
- SMD
Standard mean deviation
- SD
Standard deviation
- SUCRA
Surface under the cumulative ranking curve
- C
Control group
- T
Training group
- AT
Aerobic training
- RT
Resistance training
- HIIT
High-intensity-interval-training
- AT + RT
Aerobic training + Resistance training
- HOMA-IR
Homeostasis model assessment-insulin resistance
- MCH
Mean corpuscular hemoglobin
Author contributions
Conceptualization, L.G., G.J.; methodology, L.G., Y.X.; software, L.G., Y.P.; validation, L.Z., Y.P. and Y.X.; data curation, Y.P., L.Z. and Y.X.; writing—original draft preparation, L.G., Y.P. and Y.X.; writing—review and editing, L.G., G.J., Y.X. and Y.B. All authors have read and agreed to the published version of the manuscript.
Data availability
All data generated or analysed during this study are included in this published article.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Yang Peng and Yaqi Xue.
References
- 1.Ludwig J, Viggiano TR, McGill DB, Oh BJ. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin. Proc. 1980;55(7):434–438. [PubMed] [Google Scholar]
- 2.European Association for the Study of the L, European Association for the Study of D, European Association for the Study of O: EASL–EASD–EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. Diabetologia 2016, 59(6):1121–1140. [DOI] [PubMed]
- 3.Powell EE, Wong VW, Rinella M. Non-alcoholic fatty liver disease. Lancet. 2021;397(10290):2212–2224. doi: 10.1016/S0140-6736(20)32511-3. [DOI] [PubMed] [Google Scholar]
- 4.DALYs GBD, Collaborators H, Murray CJ, Barber RM, Foreman KJ, Abbasoglu Ozgoren A, Abd-Allah F, Abera SF, Aboyans V, Abraham JP, et al. Global, regional, and national disability-adjusted life years (DALYs) for 306 diseases and injuries and healthy life expectancy (HALE) for 188 countries, 1990–2013: quantifying the epidemiological transition. Lancet. 2015;386(10009):2145–2191. doi: 10.1016/S0140-6736(15)61340-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Riazi K, Azhari H, Charette JH, Underwood FE, King JA, Afshar EE, Swain MG, Congly SE, Kaplan GG, Shaheen AA. The prevalence and incidence of NAFLD worldwide: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2022;7(9):851–861. doi: 10.1016/S2468-1253(22)00165-0. [DOI] [PubMed] [Google Scholar]
- 6.Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, Grundy SM, Hobbs HH. Prevalence of hepatic steatosis in an urban population in the United States: Impact of ethnicity. Hepatology. 2004;40(6):1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 7.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 8.Jeong EH, Jun DW, Cho YK, Choe YG, Ryu S, Lee SM, Jang EC. Regional prevalence of non-alcoholic fatty liver disease in Seoul and Gyeonggi-do, Korea. Clin. Mol. Hepatol. 2013;19(3):266–272. doi: 10.3350/cmh.2013.19.3.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamaguchi M, Kojima T, Takeda N, Nakagawa T, Taniguchi H, Fujii K, Omatsu T, Nakajima T, Sarui H, Shimazaki M, et al. The metabolic syndrome as a predictor of nonalcoholic fatty liver disease. Ann. Intern. Med. 2005;143(10):722–728. doi: 10.7326/0003-4819-143-10-200511150-00009. [DOI] [PubMed] [Google Scholar]
- 10.Younossi ZM. Non-alcoholic fatty liver disease: A global public health perspective. J. Hepatol. 2019;70(3):531–544. doi: 10.1016/j.jhep.2018.10.033. [DOI] [PubMed] [Google Scholar]
- 11.Rezende RE, Duarte SM, Stefano JT, Roschel H, Gualano B, de Sa Pinto AL, Vezozzo DC, Carrilho FJ, Oliveira CP. Randomized clinical trial: benefits of aerobic physical activity for 24 weeks in postmenopausal women with nonalcoholic fatty liver disease. Menopause. 2016;23(8):876–883. doi: 10.1097/GME.0000000000000647. [DOI] [PubMed] [Google Scholar]
- 12.Farzanegi P, Dana A, Ebrahimpoor Z, Asadi M, Azarbayjani MA. Mechanisms of beneficial effects of exercise training on non-alcoholic fatty liver disease (NAFLD): Roles of oxidative stress and inflammation. Eur. J. Sport Sci. 2019;19(7):994–1003. doi: 10.1080/17461391.2019.1571114. [DOI] [PubMed] [Google Scholar]
- 13.Deprince A, Haas JT, Staels B. Dysregulated lipid metabolism links NAFLD to cardiovascular disease. Mol. Metab. 2020;42:101092. doi: 10.1016/j.molmet.2020.101092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amernia B, Moosavy SH, Banookh F, Zoghi G. FIB-4, APRI, and AST/ALT ratio compared to FibroScan for the assessment of hepatic fibrosis in patients with non-alcoholic fatty liver disease in Bandar Abbas, Iran. BMC Gastroenterol. 2021;21(1):453. doi: 10.1186/s12876-021-02038-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sumida Y, Yoneda M. Current and future pharmacological therapies for NAFLD/NASH. J. Gastroenterol. 2018;53(3):362–376. doi: 10.1007/s00535-017-1415-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nseir W, Hellou E, Assy N. Role of diet and lifestyle changes in nonalcoholic fatty liver disease. World J. Gastroenterol. 2014;20(28):9338–9344. doi: 10.3748/wjg.v20.i28.9338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.George AS, Bauman A, Johnston A, Farrell G, Chey T, George J. Independent effects of physical activity in patients with nonalcoholic fatty liver disease. Hepatology. 2009;50(1):68–76. doi: 10.1002/hep.22940. [DOI] [PubMed] [Google Scholar]
- 18.Zelber-Sagi S, Nitzan-Kaluski D, Goldsmith R, Webb M, Zvibel I, Goldiner I, Blendis L, Halpern Z, Oren R. Role of leisure-time physical activity in nonalcoholic fatty liver disease: A population-based study. Hepatology. 2008;48(6):1791–1798. doi: 10.1002/hep.22525. [DOI] [PubMed] [Google Scholar]
- 19.Ghaffari M, Sadeghiyan S, Faramarzi M, Moghaddam M, Baghurst T. The effect of aerobic exercise on metabolic parameters of patients with non-alcoholic fatty liver disease: Systematic review and meta-analysis. J. Sports Med. Phys. Fitness. 2023;63(1):178–187. doi: 10.23736/S0022-4707.22.13801-6. [DOI] [PubMed] [Google Scholar]
- 20.Keating SE, Hackett DA, George J, Johnson NA. Exercise and non-alcoholic fatty liver disease: A systematic review and meta-analysis. J. Hepatol. 2012;57(1):157–166. doi: 10.1016/j.jhep.2012.02.023. [DOI] [PubMed] [Google Scholar]
- 21.Sung KC, Ryu S, Lee JY, Kim JY, Wild SH, Byrne CD. Effect of exercise on the development of new fatty liver and the resolution of existing fatty liver. J. Hepatol. 2016;65(4):791–797. doi: 10.1016/j.jhep.2016.05.026. [DOI] [PubMed] [Google Scholar]
- 22.Xiong Y, Peng Q, Cao C, Xu Z, Zhang B. Effect of different exercise methods on non-alcoholic fatty liver disease: A meta-analysis and meta-regression. Int. J. Environ. Res. Public Health. 2021;18(6):3242. doi: 10.3390/ijerph18063242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang ST, Zheng J, Peng HW, Cai XL, Pan XT, Li HQ, Hong QZ, Peng XE. Physical activity intervention for non-diabetic patients with non-alcoholic fatty liver disease: A meta-analysis of randomized controlled trials. BMC Gastroenterol. 2020;20(1):1–12. doi: 10.1186/s12876-020-01204-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Y, Zou J, Dan L, Zhang R, Feng Q. The efficacy of Qigong exercises for nonalcoholic fatty liver disease: A protocol for systematic review and meta-analysis. Medicine. 2020;99(44):e22753. doi: 10.1097/MD.0000000000022753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, Ioannidis JP, Straus S, Thorlund K, Jansen JP, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: Checklist and explanations. Ann. Intern. Med. 2015;162(11):777–784. doi: 10.7326/M14-2385. [DOI] [PubMed] [Google Scholar]
- 26.Cumpston M, Li TJ, Page MJ, Chandler J, Welch VA, Higgins JPT, Thomas J: Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst. Rev. 2019(10): ED000142. [DOI] [PMC free article] [PubMed]
- 27.Bennett MM, Crowe BJ, Price KL, Stamey JD, Seaman JW., Jr Comparison of Bayesian and frequentist meta-analytical approaches for analyzing time to event data. J. Biopharm. Stat. 2013;23(1):129–145. doi: 10.1080/10543406.2013.737210. [DOI] [PubMed] [Google Scholar]
- 28.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mbuagbaw L, Rochwerg B, Jaeschke R, Heels-Andsell D, Alhazzani W, Thabane L, Guyatt GH. Approaches to interpreting and choosing the best treatments in network meta-analyses. Syst. Rev. 2017;6(1):79. doi: 10.1186/s13643-017-0473-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shim S, Yoon BH, Shin IS, Bae JM. Network meta-analysis: application and practice using Stata. Epidemiol. Health. 2017;39:e2017047. doi: 10.4178/epih.e2017047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zuo J, Yan W, Wang Y, Wang W, Wang D, Wang C, Zhang F, Yan WG, Wang YB, Wang WX, et al. Effects of swimming and diet control on levels of SREBP-1c, RBP4 and FS in serum of female patients with non-alcoholic fatty liver disease. J. Hebei North Univ. Nat. Sci. Ed. 2019;35(4):22–25. [Google Scholar]
- 32.Zhao C. Effects of Nordic Walking Interventionon Abdominal Adiposity Hepatic Fat Contentand Serum Lipidof Postmenopausal Women with Pre-Diabetes and Non-Alcoholic Fatty Liver Disease. Shanghai University of Sport; 2016. [Google Scholar]
- 33.Zhang Y. Effects of Nordic Walking on Body Composition and Muscle Strength in Prediabetic Postmenopausal Women with Non-Alcoholic Fatty Liver Disease. Shanghai University of Sport; 2015. [Google Scholar]
- 34.Zhang D, Wang S, Wang S. Application of aerobic exercise training to patients with NAFLD. J. Qilu Nurs. 2013;19(03):25–26. [Google Scholar]
- 35.Yao Z. The Influence of Xuezhikang Associated Aerobic Movement in the Serum of TN-α and TGF-β of the Non-Alcoholic Fatty Liver Patients. Qingdao University; 2006. [Google Scholar]
- 36.Yang Y, Wang F, Mao J, Wang FL, Mao JJ. A comprehensive investigation of the effectiveness of aerobic exercise in patients with nonalcoholic fatty liver disease. Modern Pract. Med. 2015;27(08):1055–1057. [Google Scholar]
- 37.Yang S. Effect of Different Modalities of Exercise on Patients with Non-Alcoholic Fatty Liver. Nanjing University Of Chinese Medicine; 2016. [Google Scholar]
- 38.Xu S. Influences of walking on some blood biochemical index of NAFLD patients. J. Xi'an Phys. Educ. Univ. 2006;05:79–81. [Google Scholar]
- 39.Wu S, Gao L, Gao LP. Effect of diet control combined with aerobic exercise on TNF-a, SREB SP-1c levels in patients with non-alcoholic fatty liver. China Modern Doc. 2014;52(33):4–8. [Google Scholar]
- 40.Wu M, Lu A, Lu AM. Effects of aerobic exercise combined with controlled diet on the serum level of SREBP-1c and RBP4 in patients with non-alcoholic fatty liver disease. Chin. J. Rehabil. Med. 2015;30(02):132–137. [Google Scholar]
- 41.Tan S, Xu D, Cao L, Guo Z, Xu DQ, Cao LQ, Guo Z. Treatment of maximal fat oxidation intensity through exercise training on non-alcohol fatty liver disease in middle-aged women. J. Tianjin Univ. Sport. 2015;30(03):185–189. [Google Scholar]
- 42.Peng Y, Zhu H, Yang M, Zhou H, Liu X, Wang C, Zhu H, Yang M, Zhou HM, Liu XL, et al. The effects of 12-week FATmax intensity exercise on blood glucose, blood lipids and liver function in obese non-alcoholic fatty liver patients. Genomics Appl. Biol. 2022;41(03):648–658. [Google Scholar]
- 43.Mao Z. Effect of Oenothera erythrosepala Borb with aerobic exercise on serum lipid metabolism and liver histolomorph of non-alcoholic fatty liver patients. J. Beijing Sport Univ. 2008;08:1087–1089. [Google Scholar]
- 44.Luo C, Li H, Tian D, Song L, Yang Y, Wang M, Hu L, Cao Y, Lan Y, Song Q, et al. High intensityinterval exercise on NAFLD under exercise and medical integration: exercise method and evaluation. J. Beijing Normal Univ. (Nat. Sci.) 2020;56(01):132–140. [Google Scholar]
- 45.Liu J, Chang YN, Cao S, Chang YN, Cao SY. Effects of HIIT on visceral lipids, insulin resistance and health-related quality of life in obese patients with NAFLD and diabetes. Chin. Hepatol. 2020;25(04):426–428. [Google Scholar]
- 46.Liu F. Effect of aerobic exercise on liver function and blood lipid in patients with nonalcoholic fatty liver disease. Chin. Foreign Med. Res. 2019;17(10):147–148. [Google Scholar]
- 47.Guo G, Xing J, Lu G, Zhang Y, Xing J, Lu G, Zhang Y. Effect of antisecosis and exercise therapy on non-alcoholic fatty liver. Inner Mongolia Med. J. 2007;01:46–48. [Google Scholar]
- 48.Fu Y, Meng M, Rong N, Liu L, Zhang J, Chen S, Zhong Y, Meng MM, Rong N, Liu L, et al. Effect of aerobic exercise and resistance exercise on patients with nonalcoholic fatty liver disease. J. Nanjing Med. Univ. (Nat. Sci.) 2018;38(04):528–531. [Google Scholar]
- 49.Fan T. Effert of Aerobic Exercise and Diet Control on the Serum TNF-a, SREBP-1c of Non-Alcoholic Fatty Liver Disease Patients. Soochow University; 2009. [Google Scholar]
- 50.Chen J, He Y, Xiao X, Chen B, He Y, Xiao X, Chen B. Application of behavior interfering in the patients with non-alcohol fatty liver. J. Nurs. Sci. 2007;13:32–34. [Google Scholar]
- 51.Hm L. Effects of exercise prescription combined with dietary intervention on body shape and blood biochemical indicators in patients with nonalcoholic fatty liver disease. Chin. J. Prev. Control Chronic Dis. 2021;29(2):115–118. [Google Scholar]
- 52.Whyte MB, Shojaee-Moradie F, Sharaf SE, Cuthbertson DJ, Kemp GJ, Barrett M, Jackson NC, Herring RA, Wright J, Thomas EL, et al. HDL-apoA-I kinetics in response to 16 wk of exercise training in men with nonalcoholic fatty liver disease. Am. J. Physiol. Endocrinol. Metab. 2020;318(6):E839–E847. doi: 10.1152/ajpendo.00019.2020. [DOI] [PubMed] [Google Scholar]
- 53.Sullivan S, Kirk EP, Mittendorfer B, Patterson BW, Klein S. Randomized trial of exercise effect on intrahepatic triglyceride content and lipid kinetics in nonalcoholic fatty liver disease. Hepatology. 2012;55(6):1738–1745. doi: 10.1002/hep.25548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stine JG, Schreibman IR, Faust AJ, Dahmus J, Stern B, Soriano C, Rivas G, Hummer B, Kimball SR, Geyer NR, et al. NASHFit: A randomized controlled trial of an exercise training program to reduce clotting risk in patients with NASH. Hepatology. 2022;76(1):172–185. doi: 10.1002/hep.32274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shamsoddini A, Sobhani V, Ghamar CM, Alavian SM, Zaree A. Effect of aerobic and resistance exercise training on liver enzymes and hepatic fat in Iranian men with nonalcoholic fatty liver disease. Hepat. Mon. 2015;15(10):e31434. doi: 10.5812/hepatmon.31434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pugh CJ, Cuthbertson DJ, Sprung VS, Kemp GJ, Richardson P, Umpleby AM, Green DJ, Cable NT, Jones H. Exercise training improves cutaneous microvascular function in nonalcoholic fatty liver disease. Am. J. Physiol. Endocrinol. Metab. 2013;305(1):E50–58. doi: 10.1152/ajpendo.00055.2013. [DOI] [PubMed] [Google Scholar]
- 57.Oh S, So R, Shida T, Matsuo T, Kim B, Akiyama K, Isobe T, Okamoto Y, Tanaka K, Shoda J. High-intensity aerobic exercise improves both hepatic fat content and stiffness in sedentary obese men with nonalcoholic fatty liver disease. Sci. Rep. 2017;7:43029. doi: 10.1038/srep43029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moradi KB, Rahmati-Ahmadabad S, Farzanegi P, Helalizadeh M, Azarbayjani MA. Effects of non-linear resistance training and curcumin supplementation on the liver biochemical markers levels and structure in older women with non-alcoholic fatty liver disease. J. Bodyw. Mov. Ther. 2020;24(3):154–160. doi: 10.1016/j.jbmt.2020.02.021. [DOI] [PubMed] [Google Scholar]
- 59.Keating SE, Hackett DA, Parker HM, O'Connor HT, Gerofi JA, Sainsbury A, Baker MK, Chuter VH, Caterson ID, George J, et al. Effect of aerobic exercise training dose on liver fat and visceral adiposity. J. Hepatol. 2015;63(1):174–182. doi: 10.1016/j.jhep.2015.02.022. [DOI] [PubMed] [Google Scholar]
- 60.Keating SE, Croci I, Wallen MP, Cox ER, Thuzar M, Pham U, Mielke GI, Coombes JS, Macdonald GA, Hickman IJ. High-intensity interval training is safe, feasible and efficacious in nonalcoholic steatohepatitis: A randomized controlled trial. Dig. Dis. Sci. 2023;68(5):2123–2139. doi: 10.1007/s10620-022-07779-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jakovljevic DG, Hallsworth K, Zalewski P, Thoma C, Klawe JJ, Day CP, Newton J, Trenell MI. Resistance exercise improves autonomic regulation at rest and haemodynamic response to exercise in non-alcoholic fatty liver disease. Clin. Sci. 2013;125(3):143–149. doi: 10.1042/CS20120684. [DOI] [PubMed] [Google Scholar]
- 62.Houghton D, Thoma C, Hallsworth K, Cassidy S, Hardy T, Burt AD, Tiniakos D, Hollingsworth KG, Taylor R, Day CP, et al. Exercise reduces liver lipids and visceral adiposity in patients with nonalcoholic steatohepatitis in a randomized controlled trial. Clin. Gastroenterol. Hepatol. 2017;15(1):96–102.e103. doi: 10.1016/j.cgh.2016.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hallsworth K, Thoma C, Hollingsworth KG, Cassidy S, Anstee QM, Day CP, Trenell MI. Modified high-intensity interval training reduces liver fat and improves cardiac function in non-alcoholic fatty liver disease: A randomized controlled trial. Clin. Sci. 2015;129(12):1097–1105. doi: 10.1042/CS20150308. [DOI] [PubMed] [Google Scholar]
- 64.Hallsworth K, Fattakhova G, Hollingsworth KG, Thoma C, Moore S, Taylor R, Day CP, Trenell MI. Resistance exercise reduces liver fat and its mediators in non-alcoholic fatty liver disease independent of weight loss. Gut. 2011;60(9):1278–1283. doi: 10.1136/gut.2011.242073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ghamarchehreh ME, Shamsoddini A, Alavian SM. Investigating the impact of eight weeks of aerobic and resistance training on blood lipid profile in elderly with non-alcoholic fatty liver disease: A randomized clinical trial. Gastroenterol. Hepatol. Bed. Bench. 2019;12(3):190–196. [PMC free article] [PubMed] [Google Scholar]
- 66.de Piano A, de Mello MT, Sanches PL, Da SP, Campos RM, Carnier J, Corgosinho F, Foschini D, Masquio DL, Tock L, et al. Long-term effects of aerobic plus resistance training on the adipokines and neuropeptides in nonalcoholic fatty liver disease obese adolescents. Eur. J. Gastroenterol. Hepatol. 2012;24(11):1313–1324. doi: 10.1097/MEG.0b013e32835793ac. [DOI] [PubMed] [Google Scholar]
- 67.de Lira CT, Dos SM, Gomes PP, Fidelix YL, Dos SA, Tenório TR, Lofrano-Prado MC, Do PW. Aerobic training performed at ventilatory threshold improves liver enzymes and lipid profile related to non-alcoholic fatty liver disease in adolescents with obesity. Nutr. Health. 2017;23(4):281–288. doi: 10.1177/0260106017720350. [DOI] [PubMed] [Google Scholar]
- 68.Croci I, Byrne NM, Chachay VS, Hills AP, Clouston AD, O'Moore-Sullivan TM, Prins JB, Macdonald GA, Hickman IJ. Independent effects of diet and exercise training on fat oxidation in non-alcoholic fatty liver disease. World J. Hepatol. 2016;8(27):1137–1148. doi: 10.4254/wjh.v8.i27.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Charatcharoenwitthaya P, Kuljiratitikal K, Aksornchanya O, Chaiyasoot K, Bandidniyamanon W, Charatcharoenwitthaya N. Moderate-intensity aerobic vs resistance exercise and dietary modification in patients with nonalcoholic fatty liver disease: A randomized clinical trial. Clin. Transl. Gastroenterol. 2021;12(3):e00316. doi: 10.14309/ctg.0000000000000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Banitalebi E, Faramarzi M, Nasiri S, Mardaniyan M, Rabiee V. Effects of different exercise modalities on novel hepatic steatosis indices in overweight women with type 2 diabetes. Clin. Mol. Hepatol. 2019;25(3):294–304. doi: 10.3350/cmh.2018.0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Al-Jiffri O, Al-Sharif FM. Abd E-KS, Ashmawy EM: Weight reduction improves markers of hepatic function and insulin resistance in type-2 diabetic patients with non-alcoholic fatty liver. Afr. Health Sci. 2013;13(3):667–672. doi: 10.4314/ahs.v13i3.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Abdelbasset WK, Tantawy SA, Kamel DM, Alqahtani BA, Soliman GS. A randomized controlled trial on the effectiveness of 8-week high-intensity interval exercise on intrahepatic triglycerides, visceral lipids, and health-related quality of life in diabetic obese patients with nonalcoholic fatty liver disease. Medicine. 2019;98(12):e14918. doi: 10.1097/MD.0000000000014918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Abdelbasset WK, Tantawy SA, Kamel DM, Alqahtani BA, Elnegamy TE, Soliman GS, Ibrahim AA. Effects of high-intensity interval and moderate-intensity continuous aerobic exercise on diabetic obese patients with nonalcoholic fatty liver disease: A comparative randomized controlled trial. Medicine. 2020;99(10):e19471. doi: 10.1097/MD.0000000000019471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nam H, Yoo JJ, Cho Y, Kang SH, Ahn SB, Lee HW, Jun DW, Song DS, Choi M. Effect of exercise-based interventions in nonalcoholic fatty liver disease: A systematic review with meta-analysis. Dig. Liver Dis. 2023;55:1574–1575. doi: 10.1016/j.dld.2023.08.059. [DOI] [PubMed] [Google Scholar]
- 75.Orci LA, Gariani K, Oldani G, Delaune V, Morel P, Toso C. Exercise-based interventions for nonalcoholic fatty liver disease: A meta-analysis and meta-regression. Clin. Gastroenterol. Hepatol. 2016;14(10):1398–1411. doi: 10.1016/j.cgh.2016.04.036. [DOI] [PubMed] [Google Scholar]
- 76.Zhou BJ, Huang G, Wang W, Zhu LH, Deng YX, He YY, Ma FH. Intervention effects of four exercise modalities on nonalcoholic fatty liver disease: a systematic review and Bayesian network meta-analysis. Eur. Rev. Med. Pharmacol. 2021;25(24):7687–7697. doi: 10.26355/eurrev_202112_27615. [DOI] [PubMed] [Google Scholar]
- 77.Zelber-Sagi S, Godos J, Salomone F. Lifestyle changes for the treatment of nonalcoholic fatty liver disease: A review of observational studies and intervention trials. Therap. Adv. Gastroenterol. 2016;9(3):392–407. doi: 10.1177/1756283X16638830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Golabi P, Locklear CT, Austin P, Afdhal S, Byrns M, Gerber L, Younossi ZM. Effectiveness of exercise in hepatic fat mobilization in non-alcoholic fatty liver disease: Systematic review. World J. Gastroenterol. 2016;22(27):6318–6327. doi: 10.3748/wjg.v22.i27.6318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Johnson NA, Keating SE, George J. Exercise and the liver: Implications for therapy in fatty liver disorders. Semin. Liver Dis. 2012;32(1):65–79. doi: 10.1055/s-0032-1306427. [DOI] [PubMed] [Google Scholar]
- 80.Vos MB, Abrams SH, Barlow SE, Caprio S, Daniels SR, Kohli R, Mouzaki M, Sathya P, Schwimmer JB, Sundaram SS, et al. NASPGHAN Clinical Practice Guideline for the Diagnosis and Treatment of Nonalcoholic Fatty Liver Disease in Children: Recommendations from the Expert Committee on NAFLD (ECON) and the North American Society of Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN) J. Pediatr. Gastroenterol. Nutr. 2017;64(2):319–334. doi: 10.1097/MPG.0000000000001482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hartz J, Hegele RA, Wilson DP. Low LDL cholesterol—Friend or foe? J. Clin. Lipidol. 2019;13(3):367–373. doi: 10.1016/j.jacl.2019.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Smart NA, King N, McFarlane JR, Graham PL, Dieberg G. Effect of exercise training on liver function in adults who are overweight or exhibit fatty liver disease: A systematic review and meta-analysis. Br. J. Sports Med. 2018;52(13):834–843. doi: 10.1136/bjsports-2016-096197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hossain N, Afendy A, Stepanova M, Nader F, Srishord M, Rafiq N, Goodman Z, Younossi Z. Independent predictors of fibrosis in patients with nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatol. 2009;7(11):1224–1229. doi: 10.1016/j.cgh.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 84.de Alwis NMW, Day CP. Genetics of alcoholic liver disease and nonalcoholic fatty liver disease. Semin. Liver Dis. 2007;27(01):44–54. doi: 10.1055/s-2006-960170. [DOI] [PubMed] [Google Scholar]
- 85.Hadizadeh F, Faghihimani E, Adibi P. Nonalcoholic fatty liver disease: Diagnostic biomarkers. World J. Gastrointest. Pathophysiol. 2017;8(2):11–26. doi: 10.4291/wjgp.v8.i2.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Johnson NA, Sachinwalla T, Walton DW, Smith K, Armstrong A, Thompson MW, George J. Aerobic exercise training reduces hepatic and visceral lipids in obese individuals without weight loss. Hepatology. 2009;50(4):1105–1112. doi: 10.1002/hep.23129. [DOI] [PubMed] [Google Scholar]
- 87.de Mello MT, de Piano A, Carnier J, Sanches PD, Correa FA, Tock L, Ernandes RMY, Tufik S, Damaso AR. Long-term effects of aerobic plus resistance training on the metabolic syndrome and adiponectinemia in obese adolescents. J. Clin. Hypertens. 2011;13(5):343–350. doi: 10.1111/j.1751-7176.2010.00388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Winn NC, et al. Energy-matched moderate and high intensity exercise training improves nonalcoholic fatty liver disease risk independent of changes in body mass or abdominal adiposity - A randomized trial. Metabolism. 2018;78:128–140. doi: 10.1016/j.metabol.2017.08.012. [DOI] [PubMed] [Google Scholar]
- 89.Stine JG, et al. Nonalcoholic steatohepatitis Fitness Intervention in Thrombosis (NASHFit): Study protocol for a randomized controlled trial of a supervised aerobic exercise program to reduce elevated clotting risk in patients with NASH. Contemp Clin Trials Commun. 2020;18:100560. doi: 10.1016/j.conctc.2020.100560. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article.