Abstract
Social anxiety disorder (SAD) is a prevalent and disabling mental health condition, characterized by excessive fear and anxiety in social situations. Resting-state functional magnetic resonance imaging (fMRI) paradigms have been increasingly used to understand the neurobiological underpinnings of SAD in the absence of threat-related stimuli. Previous studies have primarily focused on the role of the amygdala in SAD. However, the amygdala consists of functionally and structurally distinct subregions, and recent studies have highlighted the importance of investigating the role of these subregions independently. Using multiband fMRI, we analyzed resting-state data from 135 participants (42 SAD, 93 healthy controls). By employing voxel-wise permutation testing, we examined group differences of fMRI connectivity and associations between fMRI connectivity and social anxiety symptoms to further investigate the classification of SAD as a categorical or dimensional construct. Seed-to-whole brain functional connectivity analysis using multiple ‘seeds’ including the amygdala and its subregions and the precuneus, revealed no statistically significant group differences. However, social anxiety severity was significantly negatively correlated with functional connectivity of the precuneus - perigenual anterior cingulate cortex and positively correlated with functional connectivity of the amygdala (specifically the superficial subregion) - parietal/cerebellar areas. Our findings demonstrate clear links between symptomatology and brain connectivity in the absence of diagnostic differences, with evidence of amygdala subregion-specific alterations. The observed brain-symptom associations did not include disturbances in the brain’s fear circuitry (i.e., disturbances in connectivity between amygdala - prefrontal regions) likely due to the absence of threat-related stimuli.
Subject terms: Neuroscience, Psychiatric disorders
Introduction
Social anxiety disorder (SAD) is a debilitating mental health condition characterized by a disproportionate level of fear or anxiety in social situations that causes significant distress or functional impairment with a global lifetime estimated prevalence of 4.0% [1, 2]. Accumulating evidence suggests that SAD may not exist as a discrete categorical entity (as defined by the Diagnostic and Statistical Manual of Mental Disorders, fifth edition; DSM-5) [1]. Instead, it is proposed that symptoms associated with SAD have a dimensional structure (e.g., as a range of severity of anxiety, fear, and avoidance) [3–6]. Given the high prevalence and subsequent impairments associated with SAD, there has been increased investigation to further understand the neurobiology of this disorder in the hope that it may improve its identification, classification, and treatment.
Advances in neuroimaging techniques have greatly assisted our understanding of the neurobiological mechanisms implicated in those with SAD [7, 8]. Most often, task-based functional magnetic resonance imaging (fMRI) approaches have elucidated the neural underpinnings of SAD under specific paradigms, such as in response to stimuli of facial expressions. In contrast, resting-state fMRI measures brain activity or connectivity in the absence of stimuli. In SAD, the use of resting-state fMRI allows for the identification of underlying neurobiological changes that are related to the characteristics of the disorder independent of any triggers from socially provoking situations (i.e., is the brain socially anxious outside the context of social threat?).
Findings from systematic reviews investigating task-based and resting-state fMRI in SAD have primarily identified the amygdala as a region of interest. That is, in response to socially relevant stimuli (i.e., threat-related facial expressions), those with SAD are commonly reported to have hyperactive amygdala responses compared to controls [9–11]. When examining only resting-state fMRI studies that used seed-based functional connectivity analyses, we recently found that the most frequently reported alterations in connectivity were between amygdala-frontal regions in those with SAD compared to controls [12]. However, there were mixed findings with regards to the direction of connectivity, with five studies reporting an increase [13–16] and four studies reporting a decrease [17–20].
There are several reasons to why these mixed findings may be occurring in the literature. Brain alterations at rest may depend on symptom severity, as supported by evidence of associations between social anxiety severity and resting-state functional connectivity of the amygdala-frontal regions in those with SAD [17, 21] and in a combined sample of participants with a diagnosis of SAD and those with no diagnosed psychiatric disorder [15]. Additionally, mixed evidence regarding the connectivity patterns of the amygdala with frontal regions in those with SAD may relate to the amygdala having functionally distinct subregions [22–24]. To date, there is little known about the amygdalostriatal subregion of the amygdala, with no studies having yet investigated this in SAD but animal studies demonstrating that it may be involved in the regulation of fear expression [25, 26]. Two studies have examined resting-state amygdala subregion connectivity of the centromedial, superficial and basolateral complexes in SAD [14, 16]. Both studies consistently found that those with SAD (compared to controls) had increased connectivity between each of the subregions and frontal regions (including the supplementary motor area, inferior frontal gyrus, superior frontal gyrus, dorsolateral prefrontal cortex, dorsomedial prefrontal cortex, and the anterior cingulate cortex). However, these findings and the majority of the previous studies showing that the amygdala and frontal regions are implicated in SAD have been limited in terms of interpretability for various reasons we discuss next.
Most studies to date have used relatively small sample sizes (average of n = 23 SAD) which have been shown to detect unreliable brain and brain-behavior findings that are unlikely to be reproduced [27]. Moreover, studies have used short resting-state scan lengths (ranging from 200 to 471 s) which have been associated with poor test-retest reliability of connectivity findings [28]. Then, there has been considerable heterogeneity in scanning acquisition and pre-processing procedures which hinders the identification of consistencies in findings across the literature [12].
In the current study, we aimed to further elucidate resting-state fMRI connectivity differences in people with SAD compared to controls. We attempted to address the aforementioned limitations by including a larger sample size, a longer scan duration using multiband imaging which reduces the signal-to-noise ratio, and a streamlined and reliable data processing (fMRIPrep) pipeline (to allow for easier replication). Our specific aims were three-fold: i) to examine whether each subregion of the amygdala had functionally distinct connectivity patterns (as a validation test); ii) to test whether resting-state functional connectivity from a range of ROIs or ‘seeds’ (including the amygdala and its subregions) displayed aberrant connectivity with other brain regions, in SAD compared to controls; and iii) to examine a dimensional approach to the study of SAD by exploring the association between resting-state fMRI connectivity and social anxiety severity across all participants (SAD and controls).
Methods
Participants
A total of 138 participants were included in this study, 43 of which had SAD and 95 of which were healthy controls. Participants were recruited using community-based advertising, and those with SAD were additionally recruited through online advertisements on the Anxiety Disorders Association of Victoria website and Facebook page.
Participants were included if they were aged between 18 to 55 years, fluent in English, and right-handed. They were excluded if they had a history of or current substance abuse (including smoking), taking psychotropic medication, head trauma (defined as being unconscious for ≥ 5 min), neurological condition, clinically significant medical illness (e.g., cardiovascular disease, diabetes), and MRI contraindications (e.g., metal objects that cannot be removed or unsafe for MRI). Additionally, the MINI 6.0.0 Screen (English version for the DSM-IV) was used to ensure that those in the control group had no prior or current psychiatric diagnosis. The MINI 6.0.0 English Version was used to determine whether those in the SAD group met the diagnostic criteria for SAD based on the DSM-IV or the DSM-5. The Liebowitz Social Anxiety Scale (LSAS) was used as an additional measure to determine whether participants in the clinical group met the diagnostic criteria for SAD, with a score ≥30 being required for inclusion [29, 30]. The social interaction anxiety scale (SIAS) has demonstrated good reliability and validity in measuring social anxiety severity across people with SAD and non-clinical samples [31]. It was administered to all participants as a measure of social anxiety symptom severity, with higher scores indicating greater levels of social anxiety. If comorbid mental health issues were reported by participants in the SAD group, they were only included if their primary diagnosis was SAD. That is, SAD had to be the condition for which the participant sought help, or which caused the most distress and impairment in functioning. Participants with a primary diagnosis of SAD who also reported current comorbid acute depressive disorder, bipolar disorder, and schizophrenia were excluded. Written informed consent was obtained from all participants. This study was approved by the Human Research Ethics Committee at Australian Catholic University.
Data acquisition
Data acquisition was performed on a Siemens MAGNETOM Tim Trio 3.0 Tesla scanner with a Siemens 12 channel head matrix coil (Erlangen, Germany) at Swinburne University of Technology, Australia. Padded foam cushions were used to minimize head movement throughout the scan. All participants were instructed to try to think about nothing in particular (i.e., a resting-state), remain awake, and fixate their gaze on a white crosshair displayed centrally on a black background.
A multiband echo-planar imaging sequence with an acceleration factor of 5 was used to acquire functional MRI data for 8 minutes 38 seconds, along the anterior commissure-posterior commissure (AC-PC) plane with A > P phase encode direction (voxel size = 2 × 2 x 2 mm; 65 slices; repetition time (TR) = 1020 ms; total volumes = 500, echo time (TE) = 30 ms; flip angle (FA) = 65°). A T1-weighted sagittal MPRAGE structural image (TR = 1900 ms, TE = 2.52 ms, FA = 9°, 176 slices; voxel size = 1 × 1 x 1 mm voxels) and T2-weighted image (TR = 3200 ms, TE = 402 ms, 176 slices; voxel size = 1 × 1 x 1 mm voxels) were also obtained for anatomical co-registration.
Data analysis
Pre-processing
T1- and T2-weighted MRI and resting-state fMRI images were converted to Brain Imaging Data Structure (BIDS) format [32]. Firstly, the data was pre-processed using fMRIPrep 20.1.1 (Esteban, Markiewicz, et al. (2018); Esteban, Blair, et al. (2018); RRID:SCR_016216), based on Nipype 1.5.0 (Gorgolewski et al. (2011); Gorgolewski et al. (2018); RRID:SCR_002502) using the Ozstar High-Performance Computer (see Supplementary materials for details).
Following this, FSL was used to regress eight parameters out of the fMRI time series (signal from white matter, cerebrospinal fluid in addition to transverse x, y, and z head motion, and rotation x, y, and z head motion). Three participants (SAD = 1, Controls = 2) with excessive head motion, defined as a mean framewise displacement (FD) greater than 0.5 mm, were excluded from the study. This resulted in a total of 42 participants with SAD and 93 control participants. The data were filtered between 0.01 and 0.08 Hz and smoothed to 8 mm full width at half maximum (FWHM). Only fMRI voxels residing within grey matter were used for final analysis.
Regions of Interest (ROIs)
Seed ROIs included the centromedial, basolateral, superficial, and amygdalostriatal subregions of the amygdala (see Fig. 1 and Table 1). These amygdala subregions were chosen as they are defined using cytoarchitectonic probability maps from the Anatomy Toolbox (Version 2.2b) in SPM12 [33, 34]. Additionally, regions that were frequently implicated in resting-state fMRI studies of SAD (as identified in the most recent systematic review on this topic [12]) were included to observe whether previous findings were replicable. These ROIs (i.e., the amygdala, subgenual anterior cingulate cortex (ACC), ventromedial prefrontal cortex (vmPFC), precuneus, and the temporoparietal junction (TPJ)) were identified using NeuroSynth (http://neurosynth.org), which is an online database that uses a meta-analytic approach to synthesize existing neuroimaging literature [35]. The relevant term was identified and the peak voxel of the region of interest was used as the MNI coordinate in this study (see Table 1). The whole amygdala was included as a ROI to examine whether findings differed between the region as a whole when compared to amygdala subregion findings.
Fig. 1.
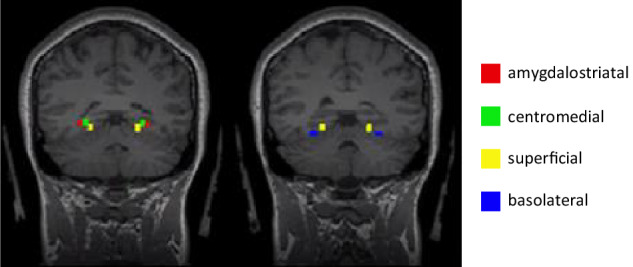
Amygdala subregion ROIs as identified in Anatomy Toolbox 2.2.
Table 1.
Coordinates and Size of Regions of Interests (ROIs).
| ROI | MNI coordinates | Size of radius sphere (mm) | How it was defined |
|---|---|---|---|
| Amygdala: amygdalostriatal | |||
| Right | 27, −11, −11 | 3 | Anatomy toolbox |
| Left | −27, −11, −11 | 3 | |
| Amygdala: basolateral | |||
| Right | 26, −5, −19 | 3 | Anatomy toolbox |
| Left | −26, −5, −19 | 3 | |
| Amygdala: centromedial | |||
| Right | 23, −9, −10 | 3 | Anatomy toolbox |
| Left | −23, −9, −10 | 3 | |
| Amygdala: superficial | |||
| Right | 19, −8, −14 | 3 | Anatomy toolbox |
| Left | −19, −8, −14 | 3 | |
| Amygdala: whole amygdala | |||
| Right | 24, −4, −18 | 5 | Neurosynth: searched term ‘emotional’ II |
| Left | −24, −4, −18 | 5 | |
| Precuneus | |||
| Right | 4, −58, 38 | 6 | Neurosynth: searched term ‘default mode’ I |
| Left | −4, −58, 38 | 6 | |
| ACC (subgenual) | |||
| Right | 4, 32, −6 | 6 | Neurosynth: searched term ‘emotional’ II |
| Left | −4, 32, −6 | 6 | |
| vmPFC | |||
| Right | 4, 48, −6 | 6 | Neurosynth: searched term ‘default mode’ I |
| Left | −4, 48, −6 | 6 | |
| TPJ | |||
| Right | 56, −50, 16 | 6 | Neurosynth: searched term ‘default mode’ I |
| Left | −56, −50, 16 | 6 | |
Ibased on 777 studies and 26256 activations; IIbased on 1708 studies and 58327 activations. ACC Anterior cingulate cortex, TPJ Temporoparietal junction, vmPFC Ventromedial prefrontal cortex.
fMRI connectivity analysis
To assess whether group differences existed between the defined ROIs and the whole-brain, seed-based functional connectivity analysis was completed using Data Processing Assistant for Resting-State fMRI (DPARSF) V5.1 [36] (http://rfmri.org/DPARSF) within the Data Processing and Analysis for Brain Imaging (DPABI) (http://rfmri.org/dpabi) [37] implemented in MATLAB R2017b. Pre-processed voxel-wise fMRI time-series were extracted from each seed, and Pearson’s correlation coefficients were calculated between the average seed time series, and the time series of all voxels in the brain (total number of grey matter voxels = 173,843). The correlation coefficient was transformed to a z-value using Fisher’s r-to-z transformation, and the resultant functional connectivity maps for each participant was entered into the two-sample t-test.
Statistical analysis
To examine differences in age and mean FD between groups, the non-parametric Mann-Whitney U test was used as the data was not normally distributed (as determined by Shapiro-Wilk test; p < 0.001). To examine differences in SIAS scores and sex between groups, an independent sample t-test and a chi-square analysis were used respectively.
To ensure the functional specificity of amygdala subregions being measured (aim i), average connectivity maps for each of the subregions were compared to one another. An independent sample t-test was used to analyze group differences in functional connectivity (SAD vs controls) for each ROI (aim ii). Two-tailed threshold-free cluster enhancement (TFCE) correction with the Permutation Analysis of Linear Models (PALM) software (p < 0.05, 5000 permutations, tail approximation acceleration method) was applied [38, 39]. Age, sex, and FD (average head movement) were included as covariates of no interest. Given the ongoing debate about the appropriateness of including covariates if they are not matched between groups [40], the t-tests were run a second time excluding FD as a covariate as head motion differed significantly between groups (control participants had greater head motion than SAD participants).
To examine associations between seeded fMRI connectivity maps and social anxiety severity (as measured by the SIAS) across all participants (aim iii), a voxel-wise Spearman’s partial correlation analysis was conducted (due to the non-normal distribution of social anxiety scores). Age, sex and FD were included as covariates. Since TFCE does not support correlation-based permutations, we employed a p-min permutation approach to perform multiple comparisons testing [41, 42]. In total, we randomized SIAS scores 5000 times (i.e., 5000 permutations) while keeping the fMRI connectivity data unchanged for all participants. For each permutation, we extract the minimal p-value across all 173,843 voxels, which in turn represents the null distribution (i.e., a ‘Bonferroni-like’ multiple comparison correction). This procedure asks the question: what is the strongest correlation any voxel can have ‘by chance’? The average of the 5000 random correlations obtained from the permutation testing was used to generate a statistical threshold. Any voxels with p-values less than this threshold were statistically significant. To avoid interpreting single voxels that may constitute a Type-1 error, we required 10 voxels to be interconnected to reach a statistical significance level.
Results
Demographics
The demographic and clinical characteristics of all participants (aside from 3 participants who were excluded from all further analyses due to excessive head motion) are included in Table 2. Five participants in the SAD group had comorbid secondary psychiatric disorders (generalized anxiety disorder (n = 3); post-traumatic stress disorder (n = 1); obsessive-compulsive disorder/attention deficit hyperactivity disorder (n = 1)). There were no significant differences in age and sex between groups. There was a significant difference in mean FD (greater in-scanner head motion in the control group) and SIAS (higher scores in the SAD group). Of note, there was an overlap of SIAS scores between people who were diagnosed with SAD and controls (see range in Table 2).
Table 2.
Demographic Information of Participants.
| SAD | Control | Statistic | p-value | |
|---|---|---|---|---|
| n | 42 | 93 | - | - |
| Sex (female/male) | 21/21 | 49/44 | χ2 = 0.084 | 0.772 |
| Age I |
27.57 (7.52) 19–54 |
26.06 (6.50) 18–49 |
U = 1718.000 | 0.264 |
| SIAS I |
51.62 (9.80) 28–72 |
18.29 (10.65) 1–48 |
t = 17.248 | < 0.001 |
| LSAS I |
81.24 (22.58) 41–139 II |
- | - | - |
| Mean FD I |
0.16 (0.07) 0.08–0.46 |
0.20 (0.08) 0.07–0.46 |
U = 1289.000 | 0.002 |
IMean (standard deviation) and range, IIscores were not reported for n = 5 due to missing data, FD Framewise displacement, LSAS Liebowitz Social Anxiety Scale, SIAS Social Interaction Anxiety Scale, t independent sample t-test, χ2 chi square analysis, U Mann-Whitney U test.
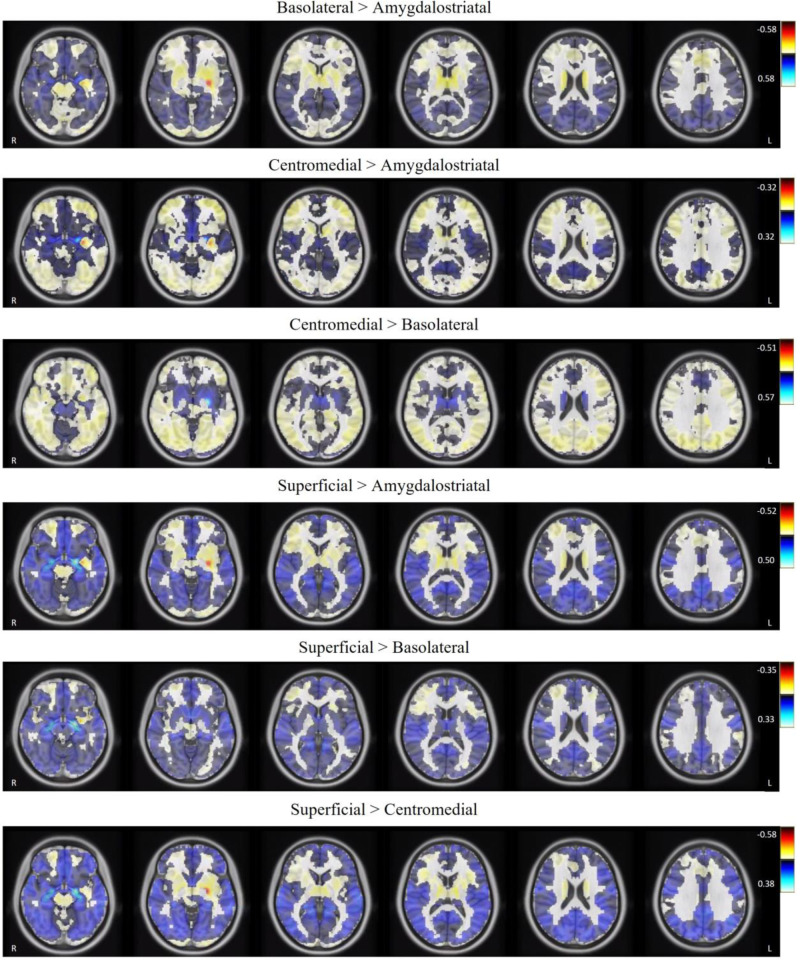
Amygdala subregions display divergent functional connectivity patterns
In the combined groups, differences in the average connectivity maps when comparing amygdala subregions were observed and presented in Fig. 2 (for illustration purposes we presented only the left hemispheric maps, but similar patterns were observed with right hemispheric subregions). These images show divergent connectivity patterns depending on the amygdala subregion ROI, providing visual evidence that the connectivity maps from different amygdala subregions in our study are functionally distinct. This has not been previously demonstrated in the existing literature examining resting-state functional connectivity of the amygdala subregions in those with SAD [14, 16].
Fig. 2.
Differences in the average functional connectivity maps of left hemispheric amygdala subregions.
No between-group differences in functional connectivity
Seed-based functional connectivity from 18 ROIs showed no statistically significant group differences between SAD and controls, based on 5000 permutations. This was observed when average FD was included and excluded as a covariate of no interest. It is worth noting that we observed moderate, but sub-threshold, effect sizes between groups (see Table 3 and Supplementary Figure 1).
Table 3.
Group Differences in Resting-State Functional Connectivity between those with SAD (n = 42) and Controls (n = 93).
| Seed # | Seed region | SAD vs. Controls | ||
|---|---|---|---|---|
| Regions showing peak altered connectivity | Peak MNI coordinate | Peak intensity | ||
| 1 | L. amygdalostriatal | R. calcarine gyrus | 20, −90, 4 | 3.892 |
| 2 | R. amygdalostriatal | R. superior frontal gyrus | 18, 56, 12 | 3.960 |
| 3 | L. basolateral | R. supramarginal gyrus (inferior parietal lobule) | 52, −38, 42 | 4.1655 |
| 4 | R. basolateral | L. cerebellum (IV-V) | −18 −28, −26 | 4.5144 |
| 5 | L. centromedial | R. medial temporal pole | 46, 16, −42 | 3.7061 |
| 6 | R. centromedial | R. superior frontal gyrus | 18, 56, 12 | 3.6414 |
| 7 | L. superficial | R. supramarginal gyrus (inferior parietal lobule) | 58, −38, 34 | 4.0305 |
| 8 | R. superficial | L. anterior agranular insula complex | −30, −62, 0 | 3.4051 |
| 9 | L. amygdala | R. supramarginal gyrus (inferior parietal lobule) | 52, −40, 42 | 4.1854 |
| 10 | R. amygdala | L. cerebellum (IV-V) | −20, −28, −26 | 3.7871 |
| 11 | L. precuneus | R. calcarine gyrus | 22, −90, 2 | 4.0954 |
| 12 | R. precuneus | R. frontal opercular area 2 | 20, −60, 42 | 3.9529 |
| 13 | L. ACC (subgenual) | R. cerebellum (IX) | 2, −50,−42 | −4.1908 |
| 14 | R. ACC (subgenual) | R. cerebellum (IX) | 4, −48, −42 | −3.7414 |
| 15 | L. vmPFC | L. cingulate gyrus, frontal opercular area 1 | −16, 8, 46 | −4.294 |
| 16 | R. vmPFC | L. cingulate gyrus, frontal opercular area 1 | −16, 8, 46 | −3.8349 |
| 17 | L. TPJ | R. anterior agranular insula complex | −42,−26, 0 | −4.3152 |
| 18 | R. TPJ | L. precuneus, frontal opercular area 3 | −18, −52, 56 | 3.8643 |
Region names were identified using the Automatic Anatomical Labelling Atlas and Glasser, Coalson [68] parcellation map. L left, R right, MNI Montreal Neurological Institute, ACC Anterior cingulate gyrus, PFC prefrontal cortex, TPJ Temperoparietal junction.
Significant associations between functional connectivity and social anxiety severity
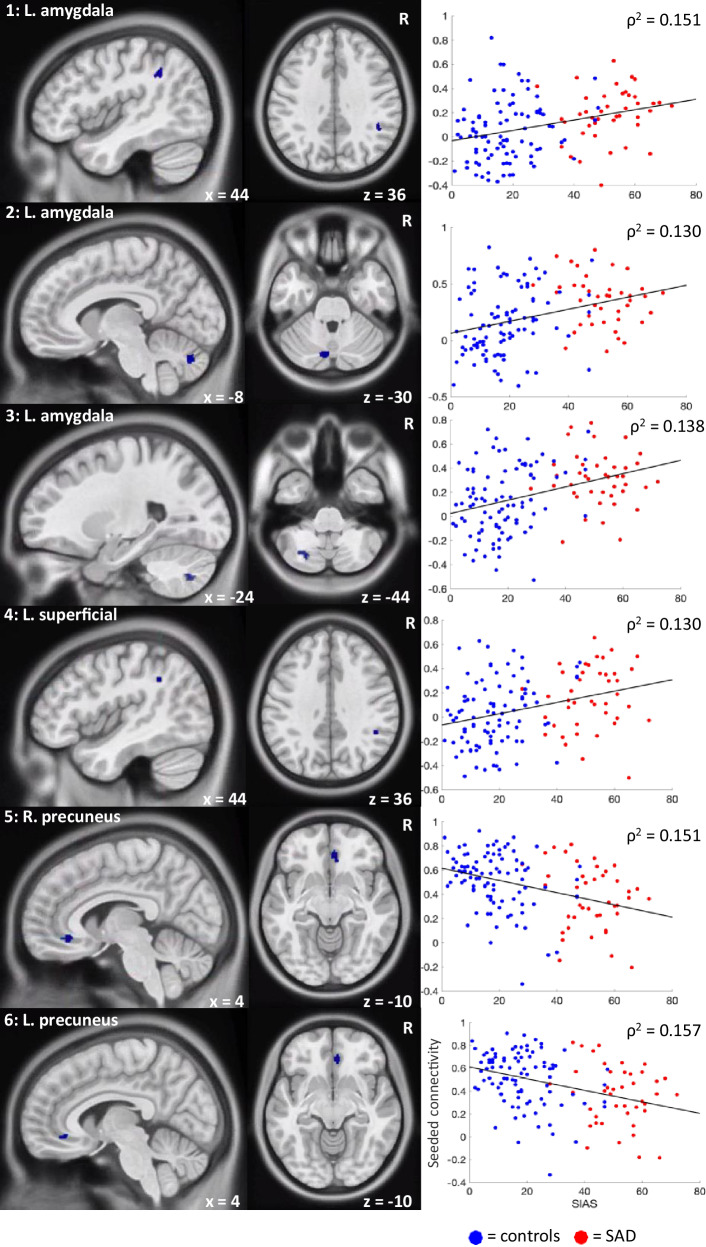
In the combined groups, significant associations (p < 0.001, corrected for multiple comparisons using the p-min method) were found between SIAS scores and seeded fMRI connectivity of several amygdala subregions and the precuneus (see Table 4 and Fig. 3).
Table 4.
Significant Associations between Resting-State Functional Connectivity and Social Anxiety Severity (SIAS Scores).
| Seed region | Regions showing altered connectivity | Peak MNI coordinate | Peak intensity (Spearman ρ-value) | p-value | Correlation |
|---|---|---|---|---|---|
| L. amygdala | L. cerebellum_crus2 | −8, −76, −30 | 0.360 | 0.00016 | Positive |
| R. supramarginal gyrus | 44, −44, 36 | 0.389 | 0.00016 | Positive | |
| L. cerebellum_7b | −24, −70, −44 | 0.372 | 0.00014 | Positive | |
| L. superficial subregion of the L. amygdala | R. supramarginal gyrus | 44, −46, 36 | 0.361 | 0.00013 | Positive |
| R. precuneus | R. peri-genu ACC (BA32) | 4, 38, −10 | −0.389 | 0.00013 | Negative |
| L. precuneus | R. peri-genu ACC (BA32) | 4, 38, −10 | −0.396 | 0.00001 | Negative |
N = 135, ACC Anterior cingulate cortex, BA Brodmann Area, L Left, MNI Montreal Neurological Institute, SIAS Social interaction anxiety scale, R Right.
Fig. 3. Connectivity maps and Spearman’s ρ correlations of the significant associations (all p < 0.001) between resting-state functional connectivity (y-axis) and social anxiety severity (SIAS scores; x-axis).
1 = L. amygdala – R. supramarginal gyrus; 2 = L. amygdala – L. cerebellum_crus2; 3 = L. amygdala – L. cerebellum_7b; 4 = L. superficial – R. supramarginal gyrus; 5 = R. precuneus – R. peri-genu ACC ; 6 = L. precuneus – R. peri-genu ACC.
Discussion
Using multiband fMRI, we found significant associations between social anxiety severity and resting-state functional connectivity across 135 participants (42 with SAD). Specifically, we found positive associations between the severity of social anxiety and functional connectivity between the left superficial amygdala – right supramarginal gyrus and the left amygdala – right supramarginal gyrus and left cerebellar regions. We also found negative associations between social anxiety severity and resting-state functional connectivity of the bilateral precuneus and the right peri-genu ACC. These associations were observed in the absence of statistically significant group differences in resting-state functional connectivity (control vs. SAD participants).
Amygdala subregion-specific associations with social anxiety
To date, very little is known about the functionality of the amygdala subregions in people with SAD given that no previous resting-state fMRI studies have examined these four commonly classified subregions within this population. Here, we showed that the positive association between social anxiety severity and functional connectivity between the left amygdala and the supramarginal gyrus is driven specifically by the superficial subregion of the amygdala. Increased connectivity between the superficial amygdala and the supramarginal gyrus at rest may indicate enhanced emotional surveillance of socially anxious self-relevant information and an increased tendency for socially anxious people to have negative social-evaluative cognitions, which is thought to maintain the disorder (i.e., negative thoughts/feelings they have about themselves are put onto others thus increasing feelings of fear/anxiety) [19, 43]. This is because the superficial subregion of the amygdala has been implicated in the processing of socially relevant information [44]. Additionally, the supramarginal gyrus is thought to play a role in downregulating egocentricity bias (i.e., the tendency to project one’s mental state onto others) [45], with evidence from a recent meta-analysis finding that those with SAD (compared to controls) had significantly decreased activation in this region when viewing disorder-related scenes (e.g., being in a conference room, harsh faces) compared to neutral scenes [46]. The positive association between social anxiety severity and functional connectivity between the left amygdala and left cerebellum regions was not observed in any of the amygdala subregions. This suggests that this result may be driven by connectivity patterns across amygdala subregions, and highlights the importance of examining the functionally and structurally distinct amygdala subregions.
There were no significant associations between social anxiety severity and the amygdalostriatal, basolateral and centromedial subregions of the amygdala. This suggests that these subregions may play a role in socio-emotion processing (as measured by task-based fMRI studies) rather than in the absence of any stimuli (i.e., at rest). The centromedial subregion, which is known to be the major output area of the amygdala, sends signals to other neural areas to generate emotional, behavioral, autonomic, and motor responses [44]. Therefore, aberrant patterns of connectivity in this subregion may only occur in task-based paradigms that require a response (and therefore an output signal). This is supported by findings of greater activation in the centromedial amygdala in response to negatively valenced stimuli compared to positively valenced stimuli in people genetically enriched for SAD [47] and significantly increased activation in the centromedial subregion of the amygdala in those with SAD (compared to controls) when viewing disorder-related scenes compared to neutral scenes [48]. Similarly, the basolateral subregion is involved in the integration of sensory information from the environment [49]. Less is known about the amygdalostriatal subregion, but it is thought to have shared pathways with the basolateral subregion [50]. The absence of significant findings in these three subregions may reflect the lack of necessity to integrate information in the absence of stimuli during resting-state fMRI.
Precuneus to ACC associations with social anxiety
In addition to the positive associations, we also observed negative associations between the connectivity of the precuneus to the peri-genu ACC and social anxiety severity. This finding is in line with Brühl, Delsignore [7] neurobiological model of SAD which posited a decrease in connectivity between the precuneus and ACC in those with SAD compared to controls. The precuneus plays a role in organizing attentional processes such as assessing the context of stimuli [51] and is a prominent component of the default mode network which is involved in self-referential processing [52]. The peri-genu ACC is involved in emotion regulation and, in a recent meta-analysis, has been identified as having altered functional connectivity to other neural regions across anxiety and affective disorders [53]. Disrupted connectivity between the peri-genu ACC and the precuneus is thought to be related to a decreased ability in being able to regulate negative self-relevant emotions which contributes to the maintenance of SAD, and has similarly been found in people with major depressive disorder [54].
Theoretical implications
It is of note that, despite observing moderate effect sizes for group differences in functional connectivity, none of these findings remained significant after statistical thresholding (p < 0.05, 5000 permutations, TFCE corrected). Voxel-wise permutation testing, which was used in this study, has become an increasingly popular choice to deal with multiple comparisons that may occur in fMRI analyses. This is due to its high sensitivity and its recognition that voxels are not activated independently of their neighboring voxels [55, 56]. However, it has also been found that using such a stringent thresholding approach has its limitations. In addition to fewer degree-of-freedom in TFCE between-group analysis compared to permutation-based correlation analysis, Noble, Scheinost and Constable [57] found that the TFCE approach was not able to detect any medium-sized effects in large sample sizes ranging from 480 to 493 healthy participants using the fMRI data in the Human Connectome Project. They concluded that numerous true effects may have been missed due to the prioritization of controlling family-wise error rates. The link between false-positive errors (which we can control) and false-negative errors (which we cannot control) is a non-trivial problem in contemporary science, but it remains imperative to minimize the former error type. Therefore, we believe that the differences reported in functional connectivity between groups in this study (but which did not survive thresholding) may be a relevant finding of interest and could be tested in a more hypothesis-driven way in future studies by pre-selecting voxels-of-interest or larger ROI based anxiety-specific a priori hypotheses which will result in fewer multiple comparisons.
Significant associations between functional connectivity and social anxiety severity (in the absence of significant group differences) further contribute to evidence of a dimensional or spectrum conceptualization of SAD, this time from a neurobiological perspective. This is consistent with the National Institute of Mental Health’s Research Domain Criteria (RDoC) framework which is advocating for a dimensional approach for the investigation of neurobiological markers of psychiatric disorders [58]. Other studies that have used resting-state fMRI in a range of psychiatric and neurodevelopmental disorders (such as attention-deficit hyperactivity disorder, autism spectrum disorder, and major depressive disorder) have similarly found evidence to support the conceptualization of these disorders as being dimensional, with associations between functional connectivity and symptom severity [59–61]. Findings from taxometric analyses [5] and previous fMRI studies in those with SAD also support this approach, with significant positive associations (but no significant group differences when comparing SAD to controls) between social anxiety severity and brain activity (e.g. in the dorsal ACC and right anterior insular cortex) in response to threat stimuli [62], and between emotion regulation and amygdala functional connectivity at rest [63].
Our findings of significant associations in the absence of statistically significant group differences (control vs. SAD participants) is also consistent with the most recently proposed integrated etiological and maintenance (IAM) model of SAD [43] in which contributing factors (both neurobiological and cognitive) to the etiology and maintenance of the disorder are identified as being dimensional. However, the most recently proposed neurobiological model of SAD [7] uses a categorical approach to conceptualize changes in neural activity and connectivity occurring in those with SAD compared to controls. Our findings show a similar pattern to the neurobiological model, including decreased connectivity between the precuneus and ACC in those with SAD compared to controls. However, our finding of increased connectivity between the amygdala (including the superficial subregion) and the supramarginal gyrus being associated with increased social anxiety severity is contrary to the model which indicates decreased connectivity between the amygdala and parietal regions in those with SAD compared to controls. Therefore, we provide further insights to this model by highlighting the importance of conceptualizing symptoms associated with SAD dimensionally (by examining associations and not only group comparisons) and the necessity to examine amygdala subregion specific effects that are linked to social anxiety severity. It is therefore critical that both these points are considered in future proposed neurobiological models of SAD.
It is well-known that broader disturbances between the amygdala and frontal regions are strongly implicated in fear processing, and altered connectivity between the amygdala and frontal regions in those with SAD compared to controls has been the most consistently reported across resting-state fMRI studies (reported by 9 of 18 fMRI studies in a systematic review) [12]. However, we found no alterations in connectivity between amygdala-frontal regions between groups and no associations between amygdala-frontal connectivity and social anxiety severity. This suggests that people with social anxiety do not have disturbances in fear processing in the absence of explicit social stimuli (i.e., at rest), perhaps due to a lesser need to be hypervigilant to threat and a reduction in negative cognitions related to being evaluated by others (both factors contributing to the maintenance of social anxiety) [43].
Conclusion
In conclusion, our study found significant associations between resting-state functional connectivity (with evidence of subregion-specific amygdala effects) and social anxiety severity scores in the absence of significant group differences. Limitations of this study was the lack of visual inspection and collection of physiological measures (e.g., heart rate) during the resting-state scan. To minimize confounds in results, future studies should use visual inspection to ensure that participants remain awake and to track their gaze and denoise physiological noise based on external recordings of physiological measures [64]. Additionally, while the connectivity patterns of amygdala subregions differed (see Fig. 2), it is possible that there is some degree of overlap between amygdala subregions due to the spatial resolution of fMRI and our smoothing parameter of 8 mm FWHM. Future studies should explore how using different smoothing kernels (e.g., 4 mm or 6 mm FWHM) impacts the signal-to-noise of BOLD signals of the amygdala subregions. Finally, although a cluster correction threshold of 10 voxels has been used in the literature to balance the probability of type I and II errors [65], future studies examining correlations between connectivity and behavioral/symptom measures may consider using programs (e.g., AFNI’s 3dClustSim) to determine cluster extent thresholds. Relative to previous resting-state fMRI studies examining SAD, the strengths of this study were the use of a larger sample of participants (n = 135) and longer scan length time (518 s; known to improve test-retest reliability) [66]. Additionally, our use of multiband fMRI imaging (improving spatial and temporal resolution)[67], stringent fMRI thresholding, and use of fMRIprep for preprocessing provides a strong basis for future studies to continue studying and/or replicate these patterns. Based on the current findings, the IAM model of SAD [43], and the current RDoC framework, we believe that future studies would benefit from examining changes in brain activity and connectivity in relation to dimensional symptoms (e.g., social anxiety severity) rather than the presence or absence of a diagnosis of SAD (i.e., a categorical approach). This will lead to a more nuanced understanding of the neurobiological mechanisms underlying social anxiety at rest and may contribute to a future dimensional neurobiological model of SAD.
Supplementary information
Acknowledgements
This work was supported by funding from the Australian Catholic University Research Fund Program Grant (ACURF2013000557), Australian Catholic University Early Career Research Fund (14HS4027IL), and by the Australian Government Research Training Program Scholarship (SM). S.L.R. holds a Senior National Health and Medical Research Council (NHMRC) Fellowship (GNT1154651). This article was published as a preprint on medRxiv: 10.1101/2022.02.27.22271587.
Author contributions
SM assisted with recruitment and gathering data, conducted analyses, interpreted results, and wrote up the manuscript. MP assisted with analyses, interpretation and editing of the manuscript. SR, PR, GT, and MH provided feedback on the manuscript. IL was the chief investigator on this project. She obtained funding, designed the study, and provided feedback and consultation at each step of this paper.
Data availability
Deidentified data for this study and codes used for analyses are available upon request to the corresponding author.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Simone Mizzi, Email: simone.mizzi2@rmit.edu.au.
Izelle Labuschagne, Email: izelle.labuschagne@acu.edu.au.
Supplementary information
The online version contains supplementary material available at 10.1038/s41398-024-02844-9.
References
- 1.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; (2013).
- 2.Stein DJ, Lim CCW, Roest AM, de Jonge P, Aguilar-Gaxiola S, Al-Hamzawi A, et al. The cross-national epidemiology of social anxiety disorder: Data from the World Mental Health Survey Initiative. BMC Med. 2017;15:143. doi: 10.1186/s12916-017-0889-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boyers GB, Broman-Fulks JJ, Valentiner DP, McCraw K, Curtin L, Michael KD. The latent structure of social anxiety disorder and the performance only specifier: A taxometric analysis. Cogn Behav Ther. 2017;46:507–21. doi: 10.1080/16506073.2017.1338310. [DOI] [PubMed] [Google Scholar]
- 4.Hyett MP, McEvoy PM. Social anxiety disorder: Looking back and moving forward. Psychol Med. 2018;48:1937–44. doi: 10.1017/S0033291717003816. [DOI] [PubMed] [Google Scholar]
- 5.Ruscio AM. The latent structure of social anxiety disorder: Consequences of shifting to a dimensional diagnosis. J Abnorm Psychol. 2010;119:662–71. doi: 10.1037/a0019341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Skocic S, Jackson H, Hulbert C. Beyond DSM-5: An alternative approach to assessing social anxiety disorder. J Anxiety Disord. 2015;30:8–15. doi: 10.1016/j.janxdis.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 7.Brühl AB, Delsignore A, Komossa K, Weidt S. Neuroimaging in social anxiety disorder—A meta-analytic review resulting in a new neurofunctional model. Neurosci Biobehav Rev. 2014;47:260–80. doi: 10.1016/j.neubiorev.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 8.Bas-Hoogendam JM, Westenberg PM. Imaging the socially-anxious brain: recent advances and future prospects. F1000Res. 2020;9:230. doi: 10.12688/f1000research.21214.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hattingh CJ, Ipser J, Tromp SA, Syal S, Lochner C, Brooks SJ, et al. Functional magnetic resonance imaging during emotion recognition in social anxiety disorder: An activation likelihood meta-analysis. Front Human Neurosci. 2013;6:347. doi: 10.3389/fnhum.2012.00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Binelli C, Subirà S, Batalla A, Muñiz A, Sugranyés G, Crippa JA, et al. Common and distinct neural correlates of facial emotion processing in social anxiety disorder and Williams syndrome: A systematic review and voxel-based meta-analysis of functional resonance imaging studies. Neuropsychologia. 2014;64:205–17. doi: 10.1016/j.neuropsychologia.2014.08.027. [DOI] [PubMed] [Google Scholar]
- 11.Kraus J, Frick A, Fischer H, Howner K, Fredrikson M, Furmark T. Amygdala reactivity and connectivity during social and non-social aversive stimulation in social anxiety disorder. Psychiatry Res: Neuroimag. 2018;280:56–61. doi: 10.1016/j.pscychresns.2018.08.012. [DOI] [PubMed] [Google Scholar]
- 12.Mizzi S, Pedersen M, Lorenzetti V, Heinrichs M, Labuschagne I. Resting-state neuroimaging in social anxiety disorder: a systematic review. Mol Psychiatry. 2021;27:164–179. doi: 10.1038/s41380-021-01154-6. [DOI] [PubMed] [Google Scholar]
- 13.Yuan ML, Zhu HR, Qiu CJ, Meng YJ, Zhang Y, Shang J, et al. Group cognitive behavioral therapy modulates the resting-state functional connectivity of amygdala-related network in patients with generalized social anxiety disorder. BMC Psychiatry. 2016;16:198. doi: 10.1186/s12888-016-0904-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anteraper SA, Triantafyllou C, Sawyer AT, Hofmann SG, Gabrieli JD, Whitfield-Gabrieli S. Hyper-connectivity of subcortical resting-state networks in social anxiety disorder. Brain Connect. 2014;4:81–90. doi: 10.1089/brain.2013.0180. [DOI] [PubMed] [Google Scholar]
- 15.Geiger MJ, Domschke K, Ipser J, Hattingh C, Baldwin DS, Lochner C, et al. Altered executive control network resting-state connectivity in social anxiety disorder. World J Biol Psychiatry. 2016;17:47–57. doi: 10.3109/15622975.2015.1083613. [DOI] [PubMed] [Google Scholar]
- 16.Yoon H-J, Kim JS, Shin Y-B, Choi S-H, Lee S-K, Kim J-J. Neural activity during self-referential working memory and the underlying role of the amygdala in social anxiety disorder. Neurosci Lett. 2016;627:139–47. doi: 10.1016/j.neulet.2016.05.068. [DOI] [PubMed] [Google Scholar]
- 17.Dodhia S, Hosanagar A, Fitzgerald DA, Labuschagne I, Wood AG, Nathan PJ, et al. Modulation of resting-state amygdala-frontal functional connectivity by oxytocin in generalized social anxiety disorder. Neuropsychopharmacology. 2014;39:2061–9. doi: 10.1038/npp.2014.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prater KE, Hosanagar A, Klumpp H, Angstadt M, Phan KL. Aberrant amygdala–frontal cortex connectivity during perception of fearful faces and at rest in generalized social anxiety disorder. Depression Anxiety. 2013;30:234–41. doi: 10.1002/da.22014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jung YH, Shin JE, Lee YI, Jang JH, Jo HJ, Choi SH. Altered Amygdala resting-state functional connectivity and hemispheric asymmetry in patients with social anxiety disorder. Front Psychiatry. 2018;9:164. doi: 10.3389/fpsyt.2018.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hahn A, Stein P, Windischberger C, Weissenbacher A, Spindelegger C, Moser E, et al. Reduced resting-state functional connectivity between amygdala and orbitofrontal cortex in social anxiety disorder. NeuroImage. 2011;56:881–9. doi: 10.1016/j.neuroimage.2011.02.064. [DOI] [PubMed] [Google Scholar]
- 21.Liao W, Qiu C, Gentili C, Walter M, Pan Z, Ding J, et al. Altered effective connectivity network of the amygdala in social anxiety disorder: a resting-state FMRI study. Plos One. 2010;5:e15238. doi: 10.1371/journal.pone.0015238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Balderston NL, Schultz DH, Hopkins L, Helmstetter FJ. Functionally distinct amygdala subregions identified using DTI and high-resolution fMRI. Soc Cogn Affect Neurosci. 2015;10:1615–22. doi: 10.1093/scan/nsv055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qiao J, Tao S, Wang X, Shi J, Chen Y, Tian S, et al. Brain functional abnormalities in the amygdala subregions is associated with anxious depression. J Affect Disord. 2020;276:653–9. doi: 10.1016/j.jad.2020.06.077. [DOI] [PubMed] [Google Scholar]
- 24.Wang C, Wang Y, Lau WKW, Wei X, Feng X, Zhang C, et al. Anomalous static and dynamic functional connectivity of amygdala subregions in individuals with high trait anxiety. Depression Anxiety. 2021;38:860–73. doi: 10.1002/da.23195. [DOI] [PubMed] [Google Scholar]
- 25.Leitermann RJ, Rostkowski AB, Urban JH. Neuropeptide Y input to the rat basolateral amygdala complex and modulation by conditioned fear. J Comp Neurol. 2016;524:2418–39. doi: 10.1002/cne.23960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shammah-Lagnado SJ, Alheid GF, Heimer L. Afferent connections of the interstitial nucleus of the posterior limb of the anterior commissure and adjacent amygdalostriatal transition area in the rat. Neuroscience. 1999;94:1097–123. doi: 10.1016/S0306-4522(99)90280-4. [DOI] [PubMed] [Google Scholar]
- 27.Grady CL, Rieck JR, Nichol D, Rodrigue KM, Kennedy KM. Influence of sample size and analytic approach on stability and interpretation of brain-behavior correlations in task-related fMRI data. Hum Brain Mapp. 2021;42:204–19. doi: 10.1002/hbm.25217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Noble S, Spann MN, Tokoglu F, Shen X, Constable RT, Scheinost D. Influences on the Test–Retest Reliability of Functional Connectivity MRI and its Relationship with Behavioral Utility. Cereb Cortex. 2017;27:5415–29. doi: 10.1093/cercor/bhx230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liebowitz MR. Social phobia. In: Klein DF, editor. Anxiety. 22. New York, NY: Karger Publishers; 1987. p. 141–73.
- 30.Mennin DS, Fresco DM, Heimberg RG, Schneier FR, Davies SO, Liebowitz MR. Screening for social anxiety disorder in the clinical setting: using the Liebowitz Social Anxiety Scale. J Anxiety Disord. 2002;16:661–73. doi: 10.1016/S0887-6185(02)00134-2. [DOI] [PubMed] [Google Scholar]
- 31.Mattick RP, Clarke JC. Development and validation of measures of social phobia scrutiny fear and social interaction anxiety. Behav Res Ther. 1998;36:455–70. doi: 10.1016/S0005-7967(97)10031-6. [DOI] [PubMed] [Google Scholar]
- 32.Gorgolewski KJ, Auer T, Calhoun VD, Craddock RC, Das S, Duff EP, et al. The brain imaging data structure, a format for organizing and describing outputs of neuroimaging experiments. Sci Data. 2016;3:160044. doi: 10.1038/sdata.2016.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, et al. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25:1325–35. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- 34.Amunts K, Kedo O, Kindler M, Pieperhoff P, Mohlberg H, Shah NJ, et al. Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps. Anat Embryol (Berl) 2005;210:343–52. doi: 10.1007/s00429-005-0025-5. [DOI] [PubMed] [Google Scholar]
- 35.Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, Wager TD. Large-scale automated synthesis of human functional neuroimaging data. Nat Methods. 2011;8:665–70. doi: 10.1038/nmeth.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chao-Gan Y, Yu-Feng ZD. a MATLAB toolbox for “pipeline” data analysis of resting-state fMRI. Front Syst Neurosci. 2010; 4:13. Epub 2010/06/26. 10.3389/fnsys. 2010.00013 PMID: 20577591; 2010. [DOI] [PMC free article] [PubMed]
- 37.Yan C-G, Wang X-D, Zuo X-N, Zang Y-F. DPABI: Data Processing & Analysis for (Resting-State) Brain Imaging. Neuroinformatics. 2016;14:339–51. doi: 10.1007/s12021-016-9299-4. [DOI] [PubMed] [Google Scholar]
- 38.Winkler AM, Ridgway GR, Douaud G, Nichols TE, Smith SM. Faster permutation inference in brain imaging. NeuroImage. 2016;141:502–16. doi: 10.1016/j.neuroimage.2016.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen X, Lu B, Yan C-G. Reproducibility of R-fMRI metrics on the impact of different strategies for multiple comparison correction and sample sizes. Hum Brain Mapp. 2018;39:300–18. doi: 10.1002/hbm.23843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miller GA, Chapman JP. Misunderstanding analysis of covariance. J Abnorm Psychol. 2001;110:40–8. doi: 10.1037/0021-843X.110.1.40. [DOI] [PubMed] [Google Scholar]
- 41.Westfall PH, Young SS Resampling-Based Multiple Testing: Examples and Methods for p-Value Adjustment. US: John Wiley & Sons Inc; 1993.
- 42.Rempala GA, Yang Y. On Permutation Procedures for Strong Control in Multiple Testing with Gene Expression Data. Stat Interface. 2013;6: 10.4310/SII.2013.v6.n1.a8. [DOI] [PMC free article] [PubMed]
- 43.Wong QJJ, Rapee RM. The aetiology and maintenance of social anxiety disorder: A synthesis of complimentary theoretical models and formulation of a new integrated model. J Affect Disord. 2016;203:84–100. doi: 10.1016/j.jad.2016.05.069. [DOI] [PubMed] [Google Scholar]
- 44.Roy AK, Shehzad Z, Margulies DS, Kelly AMC, Uddin LQ, Gotimer K, et al. Functional connectivity of the human amygdala using resting state fMRI. NeuroImage. 2009;45:614–26. doi: 10.1016/j.neuroimage.2008.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Silani G, Lamm C, Ruff CC, Singer T. Right Supramarginal Gyrus Is Crucial to Overcome Emotional Egocentricity Bias in Social Judgments. J Neurosci. 2013;33:15466–76. doi: 10.1523/JNEUROSCI.1488-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yu X, Ruan Y, Zhang Y, Wang J, Liu Y, Zhang J, et al. Cognitive Neural Mechanism of Social Anxiety Disorder: A Meta-Analysis Based on fMRI Studies. Int J Environ Res Public Health. 2021;18:5556. doi: 10.3390/ijerph18115556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bas-Hoogendam JM, van Steenbergen H, van der Wee NJA, Westenberg PM. Amygdala hyperreactivity to faces conditioned with a social-evaluative meaning– a multiplex, multigenerational fMRI study on social anxiety endophenotypes. NeuroImage: Clin. 2020;26:102247. doi: 10.1016/j.nicl.2020.102247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Heitmann CY, Feldker K, Neumeister P, Zepp BM, Peterburs J, Zwitserlood P, et al. Abnormal brain activation and connectivity to standardized disorder‐related visual scenes in social anxiety disorder. Hum Brain Mapp. 2016;37:1559–72. doi: 10.1002/hbm.23120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Klumpp H, Fitzgerald JM. Neuroimaging Predictors and Mechanisms of Treatment Response in Social Anxiety Disorder: an Overview of the Amygdala. Curr Psychiatry Rep. 2018;20:89. doi: 10.1007/s11920-018-0948-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang C, Kang-Park M-H, Wilson WA, Moore SD. Properties of the Pathways From the Lateral Amygdal Nucleus to Basolateral Nucleus and Amygdalostriatal Transition Area. J Neurophysiol. 2002;87:2593–601. doi: 10.1152/jn.2002.87.5.2593. [DOI] [PubMed] [Google Scholar]
- 51.Utevsky AV, Smith DV, Huettel SA. Precuneus Is a Functional Core of the Default-Mode Network. J Neurosci. 2014;34:932–40. doi: 10.1523/JNEUROSCI.4227-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Raichle ME. The Brain’s Default Mode Network. Annu Rev Neurosci. 2015;38:433–47. doi: 10.1146/annurev-neuro-071013-014030. [DOI] [PubMed] [Google Scholar]
- 53.Marusak HA, Thomason ME, Peters C, Zundel C, Elrahal F, Rabinak CA. You say ‘prefrontal cortex’ and I say ‘anterior cingulate’: meta-analysis of spatial overlap in amygdala-to-prefrontal connectivity and internalizing symptomology. Transl Psychiatry. 2016;6:e944–e. doi: 10.1038/tp.2016.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Peng X, Wu X, Gong R, Yang R, Wang X, Zhu W, et al. Sub-regional anterior cingulate cortex functional connectivity revealed default network subsystem dysfunction in patients with major depressive disorder. Psychol Med. 2021;51:1687–95. doi: 10.1017/S0033291720000434. [DOI] [PubMed] [Google Scholar]
- 55.Smith SM, Nichols TE. Threshold-free cluster enhancement: Addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage. 2009;44:83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- 56.Heller R, Stanley D, Yekutieli D, Rubin N, Benjamini Y. Cluster-based analysis of FMRI data. NeuroImage. 2006;33:599–608. doi: 10.1016/j.neuroimage.2006.04.233. [DOI] [PubMed] [Google Scholar]
- 57.Noble S, Scheinost D, Constable RT. Cluster failure or power failure? Evaluating sensitivity in cluster-level inference. NeuroImage. 2020;209:116468. doi: 10.1016/j.neuroimage.2019.116468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Insel TR. The NIMH Research Domain Criteria (RDoC) Project: Precision Medicine for Psychiatry. Am J Psychiatry. 2014;171:395–7. doi: 10.1176/appi.ajp.2014.14020138. [DOI] [PubMed] [Google Scholar]
- 59.Chabernaud C, Mennes M, Kelly C, Nooner K, Di Martino A, Castellanos FX, et al. Dimensional Brain-Behavior Relationships in Children with Attention-Deficit/Hyperactivity Disorder. Biol Psychiatry. 2012;71:434–42. doi: 10.1016/j.biopsych.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Elton A, Di Martino A, Hazlett HC, Gao W. Neural Connectivity Evidence for a Categorical-Dimensional Hybrid Model of Autism Spectrum Disorder. Biol Psychiatry. 2016;80:120–8. doi: 10.1016/j.biopsych.2015.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Saris IMJ, Penninx BWJH, Dinga R, van Tol M-J, Veltman DJ, van der Wee NJA, et al. Default Mode Network Connectivity and Social Dysfunction in Major Depressive Disorder. Sci Rep. 2020;10:194. doi: 10.1038/s41598-019-57033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Savage HS, Davey CG, Fullana MA, Harrison BJ. Threat and safety reversal learning in social anxiety disorder – an fMRI study. J Anxiety Disord. 2020;76:102321. doi: 10.1016/j.janxdis.2020.102321. [DOI] [PubMed] [Google Scholar]
- 63.Rabany L, Diefenbach GJ, Bragdon LB, Pittman BP, Zertuche L, Tolin DF, et al. Resting-State Functional Connectivity in Generalized Anxiety Disorder and Social Anxiety Disorder: Evidence for a Dimensional Approach. Brain Connect. 2017;7:289–98. doi: 10.1089/brain.2017.0497. [DOI] [PubMed] [Google Scholar]
- 64.Caballero-Gaudes C, Reynolds RC. Methods for cleaning the BOLD fMRI signal. NeuroImage. 2017;154:128–49. doi: 10.1016/j.neuroimage.2016.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lieberman MD, Cunningham WA. Type I and Type II error concerns in fMRI research: re-balancing the scale. Soc Cogn Affect Neurosci. 2009;4:423–8. doi: 10.1093/scan/nsp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Birn RM, Molloy EK, Patriat R, Parker T, Meier TB, Kirk GR, et al. The effect of scan length on the reliability of resting-state fMRI connectivity estimates. Neuroimage. 2013;83:550–8. doi: 10.1016/j.neuroimage.2013.05.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bhandari R, Kirilina E, Caan M, Suttrup J, De Sanctis T, De Angelis L, et al. Does higher sampling rate (multiband + SENSE) improve group statistics - An example from social neuroscience block design at 3T. NeuroImage. 2020;213:116731. doi: 10.1016/j.neuroimage.2020.116731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Glasser MF, Coalson TS, Robinson EC, Hacker CD, Harwell J, Yacoub E, et al. A multi-modal parcellation of human cerebral cortex. Nature. 2016;536:171–8. doi: 10.1038/nature18933. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Deidentified data for this study and codes used for analyses are available upon request to the corresponding author.