Abstract
Nanovaccines have gathered significant attention for their potential to elicit tumor-specific immunological responses. Despite notable progress in tumor immunotherapy, nanovaccines still encounter considerable challenges such as low delivery efficiency, limited targeting ability, and suboptimal efficacy. With an aim of addressing these issues, engineering customized nanovaccines through modification or functionalization has emerged as a promising approach. These tailored nanovaccines not only enhance antigen presentation, but also effectively modulate immunosuppression within the tumor microenvironment. Specifically, they are distinguished by their diverse sizes, shapes, charges, structures, and unique physicochemical properties, along with targeting ligands. These features of nanovaccines facilitate lymph node accumulation and activation/regulation of immune cells. This overview of bespoke nanovaccines underscores their potential in both prophylactic and therapeutic applications, offering insights into their future development and role in cancer immunotherapy.
Keywords: Nanovaccines, Customized structure, Tailored-ligand, Enhanced cancer immunotherapy, Prophylactic and therapeutic applications
Graphical abstract

Highlights
-
•
Customized structured nanovaccines are classified and elucidated.
-
•
Tailored-ligand-based of nanovaccines are engineered to augment efficacy of cancer immunotherapy.
-
•
Custom nanovaccines unveil superior capabilities in targeting lymph nodes, enhancing antigen presentation, and modulating immunosuppression. Custom nanovaccines enable targeting lymph nodes, enhancing antigen presentation, and modulating immunosuppression.
-
•
Progress in both prophylactic and therapeutic nanovaccines is detailed and explored in-depth.
1. Introduction
Since bacterial toxins were first proposed as an immunotherapy strategy to treat soft-tissue and bone sarcomas in 1891, cancer immunotherapy has become a burgeoning field revolutionizing oncology [1,2]. Differing from conventional tumor treatments, cancer immunotherapy focuses on stimulating immune responses, modulating tumor microenvironment (TME), and providing unique therapeutic effects by integrating tumor-specific antibodies with cellular immune effectors [3]. Primary cancer immunotherapy strategies include vaccines-based (active), antigens-based (passive), adaptive cells, cytokines, and immune checkpoint blockade (ICB) immunotherapy [[4], [5], [6]]. For vaccines-based immunotherapy, cancer vaccines are primarily composed of tumor-associated antigens (TAA), immunotherapeutic agents (e.g., adjuvants), and/or nanocarriers, which are elaborately-designed to activate of immune cells and provoke effective antitumor immunity [7]. For example, Sipuleucel-T, as a well-known vaccine formulation, that has already been approved by the Food and Drug Administration (FDA) and is widely used in clinical settings, which owned an ability of initiating immune responses from prostatic acid phosphatase and then eliciting the patient's own immune system to proficiently identify and engage in combatting cancer [8]. Nonetheless, a growing body of evidence underscores that efficacy of the cancer vaccines is compromised by intrinsic tumor cell resistance, potential off-target risks, and induction of local or systemic immunosuppressive mechanisms [9]. These impediments present significant challenges in both advancement and broader clinical adoption of tumor-associated vaccines [10].
Nanovaccine is a subset of cancer vaccines with unique structures and physicochemical properties, which is accumulated in lymph nodes (LN) and taken up by antigen presenting cells (APC) in order to trigger immune responses [11]. A prominent feature of the nanovaccines is tunable nanosizes, which determines entrance of active components into the LN and subsequent passive delivery of them in the TME [12]. In particular, nanovaccines with diameters of 10–100 nm easily access draining LN through peripheral lymphatic vessels (LV), whereas nanovaccines of larger sizes (>100 nm) are generally internalized by peripheral APC and migrated in LN after an accumulation at injection sites [13]. Dimensions of nanovaccines have also been shown to influence effectiveness of cross-presentation, with fine nanoparticles showing greater efficiency [14,15]. Tunable physicochemical properties (e.g., balanced surface charges, suitable shape, trade-off elasticity, targeting ligands, etc.) of the nanovaccines also play a crucial role in enhancing cancer immunotherapy [16]. For instance, customized nanovaccines with positive charges were attracted to negatively-charged membranes via the electrostatic interaction, which effectively enhanced cellular internalization and antigen presentation [[17], [18], [19]]. Shape of engineered nanoparticles also influences antibody and cytokine secretions. A study comparing spherical, rod, and cubic gold nanovaccines in stimulating antibody production against West Nile Virus showed that the nanorods induced secretions of inflammasome-dependent cytokines (interleukin(IL)-1β, IL-18), while the sphere- and cube-vaccines induced secretions of pro-inflammatory cytokines (tumor necrosis factor (TNF)-α, IL-6, IL-12, etc.) [20]. Furthermore, nanovaccines with high permeability, biocompatibility, and stability also affect pharmacological properties of immune reagents and protect biological drugs from premature release or degradation [21].
Inorganic (hollow, core-shell, and nanosheet), polymeric (micelle and nanogel), and biomimetic (vesicle-like, nanodisc, and virus-like) nanovaccines with diverse morphologies have been systematically-explored, where proficiencies and efficiencies of these nanovaccines in delivering active components to tumor sites have been summarized [22]. For example, polymer-based nanovaccines are identified with properties of exceptional stability, biocompatibility, adjuvanticity, and abilities of high endocytosis and trafficking within TME [23]. Inorganic carriers like manganese ion-based nanovaccines enhance antigen presentation and amplify synergistic effects towards photothermal and photodynamic therapies [24]. Moreover, inorganic carriers such as ferric ions mitigate risks associated with off-targeting effects, instability, and rapid clearance in vivo also facilitate timely unloading of active components after being quickly arrived at LN [25]. Notably, incorporation of the targeting ligands involving artificial APC (aAPC), chemokines, agonists, inhibitors, cytokines, adjuvants, receptors, or tumor-specific peptides endows nanovaccines with the high specificity to combat tumors [26]. This specificity allows for precise migration to LN and activation/regulation of immune cells [27]. For instance, Wang et al. reported that α-peptides-based nanovaccines M2-like tumor-associated macrophages (TAM) dual-targeting nanoparticle (M2NP) specifically depleted M2-like TAM and blocked M2-like TAM signaling pathways by loading anti-colony stimulating RNA [28]. Hence, customized nanovaccines elaborately-designed from nanocarriers and active components on the basis of a specific clinical scenario, will effectively accumulate TME to induce activity of body's own immunological effectors, suppress intrinsic resistance of tumor cells, and reduce immunosuppressive effects [29].
After delivery of major active vaccine components towards the TME, personalized nanovaccines unearth capacities to amplify LN accumulation, bolster antigen presentation, and finely regulate immunosuppressive mechanisms at the single-cell level [30]. Process of enhanced antigen presentation primarily involves inducing dendritic cell (DC) maturation, activating cytotoxic T lymphocytes (CTL), and targeting both B cells and natural killer (NK) cells [31]. As principal APC, employing appropriate active or passive targeting strategies for DC (e.g., modifications involving mannose, C-type Lectin, etc.) can promote effective nanovaccine phagocytosis [32]. Moreover, stimulating activation and proliferation of CTL is of paramount importance for achieving efficacious immunotherapy [6]. Concurrently, leveraging both B and NK cells as effective anchors can enhance nanovaccine accumulation and retention within tumors [33]. Furthermore, meticulous modulation of the immunosuppressive microenvironment at the single-cell level primarily entails a targeted approach involving immune cells, including TAM, tumor-associated neutrophils (TAN), myeloid-derived suppressor cells (MDSC), natural killer T (NKT) cells, and regulatory T (Treg) Cells. Customized nanovaccines, furnished with functional ligands such as M2 macrophage-targeting peptides (M2pep), folate (FA), and stimulatory cytokines, have been employed with success in directing regulation of TAM [34]. In a parallel manner, nanovaccines with a plethora of functional ligands, such as α-galactosylceramide (GalCer), have feasibility in modulating NKT cell functions. Additionally, TAN regulation has been achieved by administration of the captopril-based vaccines, while MDSC can be anchored via tadalafil (TAD)-based vaccines.
This review presents a comprehensive overview of the most recent progress in design and applicability of nanovaccines, especially for the bespoke nanovaccines with tweaked antigens and/or adjuvants. These nanovaccines exhibit versatilities by effectively targeting LN, activating and/or regulating diverse immune cells, as depicted in Scheme 1. Firstly, it is imperative to categorize diverse nanovaccines in a comprehensive manner, delineating their characteristics based on distinct typologies, including inorganic (hollow, core-shell, and nanosheet), polymeric (micelle and nanogel), and biomimetic (vesicle-like, nanodisc, and virus-like) vaccines. Secondly, it is worth noting that tailored nanovaccines function versatility at level of immune cells, which can be stratified into distinct stages. These functions encompass following as: 1) escalation of LN accumulation, 2) enhancement of antigen presentation at the single-cell level, and 3) modulation of immunosuppression at the single-cell level. Ultimate goal of such painstakingly-customized nanovaccines is a convergence of tumor eradication, tumor recurrence inhibition, and TME modulation. Finally, objective evaluations of advantages, disadvantages, and challenges of these customized nanovaccines applied in clinical trials are also presented. This review provides an exhaustive and in-depth exposition of the meticulously-tailored nanovaccines and their pivotal role in advancing cancer immunotherapy.
Scheme 1.
Customized nanovaccines for enhanced cancer immunotherapy.
2. Engineering customized nanovaccines
Valid cancer vaccines including customized nanovaccines should meet a requirement of effectively co-delivering anti-tumor antigens as well as immunologic adjuvants to lymphoid tissues and APC (e.g., macrophages and DC) for successful activation of immune system [35]. Considering principal mechanism of the nanovaccines, various types of antigens and adjuvants have been developed and applied in nanoscale vaccines. The antigens, as crucial components, which constitute a pivotal component in cancer vaccines, often classified into pre-defined and unidentified antigens [36]. Predefined antigens predominantly encompass TAA or tumor-specific antigens (TSA), such as neoantigens arising from aberrant gene expression or stochastic somatic mutations within cancer cells. Besides, biomimetic carriers, derived from biological entities like cell membranes (CM) or liposomes, skillfully integrate a diverse range of antigens. This integration yields personalized nanovaccines by embraced multi-targeting and multi-epitope capabilities. Up to now, nanovaccines harboring blends of unidentified antigens such as whole cancer cells or cellular lysates, have emerged as a promising avenue in cancer immunotherapy. As another essential component, the universal adjuvant mainly includes stimulator of interferon genes (STING) agonists (e.g. 2′,3′-cGAMP, 3′,3′-cGAMP, c-di-GMP, etc.) [37,38], cytokines [39], costimulatory ligands (e.g., 4-1 BBL (CD137L)) [40], toll-like receptor (TLR) agonists (e.g., poly(I:C) [41], cytosine-phosphate-guanosine oligodeoxynucleotide (CpG-ODN) 1826 [42], R837 [43], resiquimod (R848) [44], etc.) and monophosphoryl lipid A (MPLA) [45]. Specific inorganic ions, like manganese ions, and polymers, such as polyethylene glycol (PEG), are notable for their dual functionality in nanovaccine development, simultaneously acting as carriers and exhibiting adjuvant or adjuvant-like properties. This multifaceted role of nanoparticles enhances design flexibility of bespoke nanovaccines and amplifies benefits of their inherent characteristics (shape, size, charge, etc.). These dual capabilities are also instrumental in fine-tuning the balance between adjuvants and antigens, optimizing these nanovaccines for specific therapeutic targets. Their distinct structural composition serves dual purposes: functioning as efficient vehicles and promoting antigen presentation, thereby activating immune responses and eliminating cancers.
Challenges faced by engineering nanoscale vaccines are how to effectively immobilize and precisely deliver various immune reagents (antigens and adjuvants) to ensure prompt and efficient immune responses [46]. After bearing substantial active components, nanomaterials, functioning as vehicles and/or adjuvants, are precisely delivered to TME via various routes such as tissues (skin or mucosa), blood vessels, and nasal passages. Meanwhile, these components are released with or without endogenous or exogenous responses, activating specific immune responses against cancer through immune signal pathways [47]. In this chapter, custom designs of nanovaccines comprising inorganic- (hollow structure, core-shell structure, and nanosheet), polymeric- (micelle and nanogel), and biomimetic-nanovaccines (vesicle-like, nanodisc, and virus-like) are elucidated, along with their intrinsic mechanisms and behavior profiles in cancer immunotherapy. Such precise engineering profoundly influences active components’ loading, delivery, internalization, and release dynamics within TME [48]. Compared with conventional vaccines, unique physical properties and physiological/biological advantages of customized nanovaccines boost immune responses, and detailed information about meticulous engineering of the nanovaccine is outlined in Table 1 [49].
Table 1.
Engineering customized nanovaccines.
| Customized morphologies | Antigens | Adjuvants | Nano-carriers | Nanovaccine types | Refs |
|---|---|---|---|---|---|
| Hollow structure-based | miR-145 | Amino-modified oligonucleotide CpG |
Manganese dioxide (H–MnO2) | Therapeutic vaccine | [50] |
| Tumor fragment (TF) | Hollow silica with spike-like aluminum hydroxide nanoparticle (SiAl NP) | SiAl NP | Therapeutic vaccine | [51] | |
| OVA | Ammonium bicarbonate (ABC) | Mesoporous silica nanoparticle (MSN) | Prophylactic and therapeutic vaccines | [52] | |
| OVA | Metal-phenolic network (MPN) | Polyethylenimine (PEI)-based MSN | Prophylactic and therapeutic vaccines | [27] | |
| Ovalbumin (OVA) | CpG | Europium-doped GdPO4 hollow sphere | Prophylactic and therapeutic vaccines | [53] | |
| Core-shell structure-based | OVA | PEI/CaCO3 and CpG | PEI/CaCO3 | Prophylactic and therapeutic vaccines | [54] |
| OVA | CaO2 | CaO2 | Therapeutic vaccine | [55] | |
| OVA | R848 | MSN | Therapeutic vaccine | [56] | |
| Tumor cell lysate | CpG-ODN | Aluminum hydroxyphosphate (Alum) nanoparticle (NP) | Prophylactic and therapeutic vaccines | [25] | |
| Nanosheets | Endogenous TAA | γ-MnO2 nanodot | Ti3C2Tx | Therapeutic vaccine | [57] |
| Tumor autoantigen (TA) | R848 | Boron bulk | Prophylactic and therapeutic vaccines | [58] | |
| OVA | CpG | Layered double hydroxide (LDH) | Therapeutic vaccine | [59] | |
| Phenylalanine-lysine-phenylalanine tripeptide-modified antigen epitope (FKF-OVAp) | Black phosphorus (BP) nanosheet | BP nanosheet | Prophylactic vaccine | [60] | |
| Micelles | OVA | CL264 | Carboxylated-NP | Therapeutic vaccine | [61] |
| Palmitoylated polypeptide | MPLA | PEG-phosphatidylethanolamine (PE) | Therapeutic vaccine | [62] | |
| OVA | PEG-b-poly(l-lysine)-b-poly(l-leucine) (PEG-PLL-PLLeu) | PEG-PLL-PLLeu | Therapeutic vaccine | [63] | |
| Endogenous TAA | R848 | PEG-ss-polycaprolactone (PCL) (PsP) | Therapeutic vaccine | [64] | |
| Nanogels | Tumor-associated antigen | R848 | Hyaluronan (HA) dynamic hydrogel messenger RNA/R848/lipid nanoparticle (HA-mRLNP) | Therapeutic vaccine | [65] |
| OVA encoding mRNA | R848 | Graphene oxide-low molecular weight PEI hydrogel | Therapeutic vaccine | [66] | |
| OVA | Bioreducible alginate-PEI nanogel | Bioreducible alginate-poly(ethylenimine) nanogel (AP-SS) | Prophylactic and therapeutic vaccines | [67] | |
| OVA | Imidazo-quinolinetype TLR7/8 agonist | pH-responsive nanogel | Therapeutic vaccine | [68] | |
| Vesicle-like | OVA | HA-decorated cationic lipid-poly(lactide-co-glycolide) acid (PLGA) hybrid NP (HA-DOTAP-PLGA NP) | HA-DOTAP-PLGA NP | Prophylactic and therapeutic vaccines | [69] |
| Protein antigen | CpG | PH9-Aln-8020 | Therapeutic vaccine | [70] | |
| Melanoma antigen peptide TRP2 | MPLA | Liposomes-coated gold nanocage | Therapeutic vaccine | [71] | |
| Whole CM antigen | CpG and TLR9 | Manganese porphyrin-based metal-organic framework (Mn-MOF) | Therapeutic vaccine | [72] | |
| Antigen from tumor cell death | MnO2 NP | Hollow biomimetic nanoplatform (CMM-DiR) | Prophylactic and therapeutic vaccines | [73] | |
| Antigenic motif from tumor cell membrane (TM) | Bacterial cytoplasmic membrane adjuvant | Hybrid membrane (HM) vesicle | Therapeutic vaccine | [74] | |
| Nanodisc | Antigen from tumor cell death | CpG and docetaxel (DTX) | Synthetic high-density lipoprotein mimicking nanodisc (sHDL) | Prophylactic and therapeutic vaccines | [75] |
| Antigen peptide | Cholesterol-modified CpG | sHDL | Therapeutic vaccine | [76] | |
| Aldehyde dehydrogenase (ALDH) antigen peptide | CpG | ALDH/CpG-based nanodisc | Therapeutic vaccine | [77] | |
| Virus-like | E75369-377 peptide | Bacteriophage λF7 | Bacterio-phage λF7 | Therapeutic vaccine | [78] |
| Insulin-like growth factor-1 receptor | Human parvovirus B19 | Human parvovirus B19 | Prophylactic vaccine | [79] | |
| M and M2 proteins | P22 virus-like particle (VLP) | P22 VLP | Prophylactic vaccine | [80] |
2.1. Inorganic nanovaccines
Inorganic nanomaterials possess significant value-addition potential for their numerous advantages essential to nanovaccine design, such as unique size-dependency, extremely high surface area-to-volume ratio, and strong affinity for antigen binding [81]. For instance, these materials resist chemical degradation, directly influencing their lifespan in vivo. Gaussian size distribution of synthesized or extracted inorganic materials, with their variable number of surface atoms, facilitates formation of multilayer structures, like peripheral biocompatible polymer coatings. Additionally, tunable net charges of functional groups on inorganic materials determine endocytic efficiency of custom-designed nanovaccines. These features collectively play a strategic role formulation of bespoke nanovaccines, enhancing both cellular absorption and interactions with other biomolecules, thereby optimizing their efficacy and bioactivity.
2.1.1. Hollow structures
A vast hollow cavity is beneficial for encapsulation of components, where sufficient amounts of antigens are utilized to activate immune responses at tumor sites [82]. These sizable cavities facilitate efficient component loading and reduce risks of clearance during transportations of adjuvants and/or antigens. Functional genes-covered biodegradable H–MnO2 as an example, offers a sizable hole to be filled and/or conjugated with a significant quantity of immunological reagents (Fig. 1A−D) [50]. Ce6 and DOX are encapsulated internally in this system, whereas amino-modified oligonucleotide CpG, S6-aptamers, and miR-145 are conjugated through amide reactions (Fig. 1E). Hollow MnO2 reacts with hydrogen peroxide in a slightly acidic environment and decomposes to produce manganese ion (Mn2+) and oxygen, which releases loaded drugs and alleviates tumor's hypoxic environment. A hollow SiAl NP, featuring aluminum hydroxide spikes on its surface, exhibits high loading efficiencies, as demonstrated by its capacity to accommodate DOX (17%), TF (37.0%), and OVA (29.7%) (Fig. 1F−H) [51]. This structural design significantly enhances potential for diverse payload integration, facilitating efficient drug and antigen delivery (Fig. 1I, J). Hollow porous structures like MSN also serve as a versatile framework adaptable for designs of personalized nanovaccines based on variations in their loaded active components. A MSNs-ABC@polydopamine-OVA (MSNs-ABC@PDA-OVA) nanovaccine as an example, employed polydopamine (PDA)-modified MSNs as a porous backbone, encapsulating ABC and conjugating thiolate ovalbumin via Michael addition reaction [52]. Similarly, a pH-sensitive MSN-based nanovaccine is constructed by electrostatically adsorbing OVA and encapsulating MPN with numerous reducible disulfide bonds [27]. The PMSN@OVA-MPN nanovaccine embraces a high loading efficiency for OVA, and the PEI shell with “proton sponge effect” promotes antigen lysosome escape. Meanwhile, disulfide bonds in external MPN induce OVA release in presence of the reduced glutathione. Furthermore, reports on large cavity structures include lanthanide-doped GdPO4 hollow spheres (GdPO4:Eu), which efficiently co-deliver protein antigens like OVA and CpG-ODN to TME. These hollow sphere-based vaccines also serve as dual-modal imaging agents for tracking immune processes within the TME [53].
Fig. 1.
Fabrication processes of hollow structure-based nanovaccines for cancer immunotherapy. Transmission electron microscopy (TEM) images depicting (A) initial H–MnO2, (B) modification of H–MnO2 with an external coating of acrylic acid and the polyelectrolyte poly(allylamine hydrochloride), accompanied by internal loading of doxorubicin (DOX) and Chlorin e6 (Ce6) (H-M-pp/C&D), and (C) subsequent conjugation of modified structure with amino-modified oligonucleotides including CpG, S6-aptamers, and miR-145 (H-M-pp/C&D + 3). (D) Ultraviolet–visible (UV–vis)−near-infrared (NIR) spectra of free Ce6, DOX, H–MnO2, and H-M-pp/C&D. (E) Schematic of synthesis process and various functions of finally prepared nanovaccine. Reproduced with permission [50]. Copyright 2022, American Chemical Society. (F) Synthesis of SiAl NP by successive reverse microemulsion and hydrothermal processes. (G) TEM image and (H) Scanning TEM image of SiAl NP. (I) TF loading efficiencies in SiAl and its alongside incorporation of DOX. (J) OVA release profiles of SiAl@OVA in phosphate-buffered saline solution (PBS) with different pH values. Reproduced with permission [51]. Copyright 2022, Wiley.
2.1.2. Core-shell structures
Oxide-based nanocarriers with adjuvants property have been employed for developing nanovaccines. Active components are strategically modified on exterior of core-shell structure of nanocarriers to make nanovaccines with cancer immunotherapy abilities [83]. The core-shell nanovaccines are created by utilizing PEI-coated calcium carbonate (CaCO3) nanocarriers (PEI/CaCO3), which efficiently absorb both OVA and CpG [54]. Additionally, PEI/CaCO3 works as an underlying adjuvant to activate bone marrow-derived dendritic cell (BMDC) dose-dependently, leading to an increase in CD86 expression. Together with the assistant nanoparticles PEG/PEI/pSpam1 (pSpam1@NPs), the nanovaccines activate T cells and gene-mediated extracellular matrix scavengers for tumor elimination. Another biomineralized nanovaccine is also developed by assembling OVA onto an in-situ growth of calcium peroxide [55]. The nanovaccine has a superhigh density of OVA antigen with a necessary calcium peroxide adjuvant (8.9%). Concerning acidic TME, the nanovaccine achieves lysosome escape and antigen cross-presentation within the cytoplasm. Furthermore, in the formulation of nanovaccines, a biopolymer-coated copper oxide core functioned as an adjuvant, eliciting an immunoglobulin (Ig) G response [84]. This adjuvant effectively inhibits proliferation of breast cancer (MCF-7) and cervical cancer (HeLa) cells, following activations of type 1 helper T (Th1) cells and type 2 helper T (Th2) cells.
Core-shell structure of nanoparticles excels in delivering and strategically releasing active components at multiple levels. This is demonstrated through two concise examples. The first involves synthesis of the core-shell mesoporous silica nanovaccines (MSN-R848-OVAp) utilizing a co-condensation method that incorporates cetyltrimethylammonium chloride and tetraethyl orthosilicate [56]. These nanoparticles feature phenyl-functionalized cores to accommodate hydrophobic R848, coupling with a pH-responsive acetal linker and a biotin-avidin complex/OVA on the surface. This configuration facilitates antigen delivery and induces T cell responses. The second is the Alum-CpG@Fe-Shikonin nanovaccine, which is composed of an Alum NP core infused with CpG-ODN and enveloped in Fe-Shikonin networks [25]. Upon disassembly, they release iron ion (II) and Shikonin, triggering immunogenic cell death (ICD) in tumor cells via ferroptosis and necroptosis. Subsequently, these components are absorbed by Alum NP and co-delivered with CpG-ODN to antigen-presenting cells, initiating a targeted, multistep antitumor immune response. Collectively, these examples underscore core-shell structure's adaptability and efficiency in targeted delivery and release of complex therapeutic agents within cancer immunotherapy.
2.1.3. Nanosheets
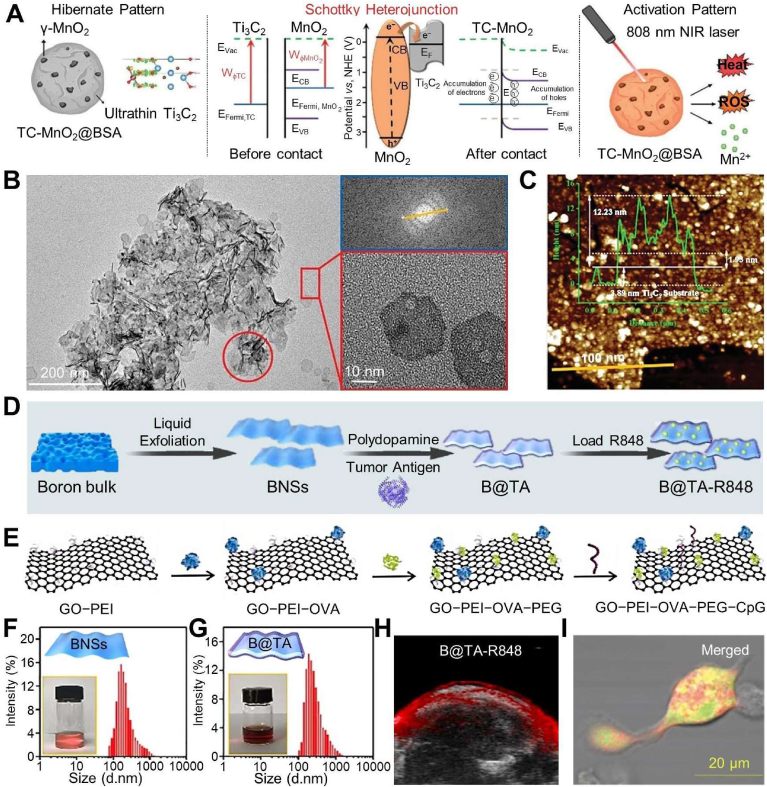
Active components are also immobilized onto two-dimensional nanomaterials, capitalizing on their distinctive attributes, including exceptional electrochemical and mechanical properties. These advantages confer upon two-dimensional nanomaterials elevated thermal conductivity and outstanding photothermal conversion efficiency. Notably, intrinsic metallic-based nanosheets (Ti3C2Tx, graphene oxide (GO), and two-dimensional MoSe2 nanosheets) have become predictable reactive oxygen species (ROS) manufacturers, triggering ICD to release TAA and damage-associated molecular patterns [[85], [86], [87]]. In this context, a Schottky heterojunction is engineered in the TC-MnO2@BSA nanovaccine by immobilizing ultrasmall γ-MnO2 nanodots onto intrinsic metallic Ti3C2Tx nanoflakes, followed by bull serum albumin (BSA) decoration (Fig. 2A) [57]. This Schottky heterojunction endows the nanovaccine with exceptional photoelectric performance and capabilities towards ROS generation and photothermal conversion. TEM and atomic force microscopy (AFM) images reveal a tightly integrated heterojunction, with Ti3C2 maintaining its monolayer morphology and exhibiting distinctive wrinkles (Fig. 2B, C). Experimental findings also indicate that the nanovaccines induce ROS production after 24 h. Simultaneously, they also lead to expression of CD44 on CD8+ T cells and a reduced proportion of Treg cells within TME. In addition, ultrathin thickness and high surface area-to-mass ratio of two-dimensional nanomaterials enhance drug release profiles alongside optimization of delivery and efficacy of therapeutics. As an illustration, boron nanosheet-based nanovaccine (B@TA-R848) is constructed with nanosheet from exfoliated boron bulk, coated PDA, and then modified with TA from triple-negative breast cancer (TNBC) as well as adjuvant of R848 (Fig. 2D, F, 2G) [58]. Such B@TA-R848 increases DC internalization by intracellular endocytosis, thus promoting antigen release and presentation based on an ultrathin of β-rhombohedral hexahedral boron structure. As expected, the B@TA-R848 also bespeaks high enrichment at tumor sites and desired photoacoustic imaging and synergetic photothermal therapy (PTT) in TNBC model (Fig. 2H). Similarly, a GO/polymer-famulated nanovaccine enhances antigen cross-presentation ability and amplifies cytokine productions of immune cells (Fig. 2E, I) [87].
Fig. 2.
Fabrication processes of layered structure-based nanovaccines for cancer immunotherapy. (A) Schematic diagram of NIR activated Schottky nanovaccine TC-MnO2@BSA and its mechanisms towards immune effects. (B) TEM images and (C) AFM image and height of TC-MnO2@BSA. Reproduced with permission [57]. Copyright 2023, Wiley. (D) Preparations of personalized nanovaccine of B@TA-R848. Size, dispersity, and photo images of (F) boron nanosheet and (G) its loading TA. (H) Photoacoustic images of tumor site in B@TA-R848 groups at 12 h post injection. Reproduced with permission [58]. Copyright 2021, Walter de Gruyter. (E) Formation illustration of GO/polymer-famulated nanovaccine. (I) Confocal microscope image of BMDCs incubated with GO/polymer-famulated nanovaccine and fluorescein isothiocyanate (FITC). Reproduced with permission [87]. Copyright 2022, Elsevier.
Moreover, well-defined layered structures of these nanovaccines augment blood flow, concurrently modifying interstitial pressure and increasing accumulation in tumor sites [88]. For instance, LDH is utilized as nanoadjuvants with an average size from 77 to 285 nm, and prompts accumulation in spleen as the most prominent secondary lymphoid organ owns highest density of APC and B/T cells. The LDH-based CO-LDH nanovaccine is also built with LDH, CpG, and OVA [59]. Customized LDH structure endows this nanovaccine with a high spleen enrichment at 24 h post i.v. injection. Taking in situ nanovaccine (LDHs-cGAMP) formed by LDH carrying cGAMP and adsorbed TAAs as another paradigm, it is demonstrated that this vaccine effectively accumulates in TME, inducing a robust response of type I interferon (IFN–I) [89]. Furthermore, BP-based nanovaccine possesses biocompatibility, biodegradability, and multiple immunostimulatory properties. For instance, phenylalanine-lysine-phenylalanine (FKF) tripeptide-modified antigen was coated onto BP to generate a minimalized nanovaccine of FKF-OVAp@BP [60]. The FKF-OVAp@BP nanovaccine harnessed immunomodulatory properties of BP and displayed significant compatibility with ICB, resulting in robust therapeutic efficacy in a melanoma mouse model. Besides, adjuvant effect mediated by BP is evidenced by efficient delivery of the mRNA nanovaccines. Yang et al. introduced the mRNA nanovaccines highlighting BP-mediated adjuvant effect being contributed efficient protein expression and enhanced immune activation [90]. Compared to nanoerythrosomes (NER) without BP, the BP-NER combination efficiently delivered mRNA for coronavirus receptor-binding domain, significantly increasing antibody titers and pseudovirus neutralization effect.
In short, inorganic materials, characterized by innate attributes such as photophysical properties, hold promise for developing customized nanovaccines. However, these “hard NP materials” (referred to as inorganic materials) often emanate limited inherent solubility, necessitating surface modifications to hasten their dissolution or maintain stability in aqueous environments [81]. Such modifications in tandem with intrinsic characteristics of inorganic materials significantly determine versatility of inorganic-based vaccines, thereby influencing chemical properties recognized by biological organisms and dictating nature of their interactions with immune cells. Consequently, when employing these materials as carriers or adjuvants, there is a critical need to strike an optimal balance between surface functionalization with soluble polymers and efficient loading of antigens.
2.2. Polymeric nanovaccines
Polymers are particularly-important in the personalized design of nanovaccines due to their high biocompatibility, adjustable side chain length, stimuli-responsive surface as a point of anchored chain, and structural flexibility, enabling their practical use as carriers and/or adjuvants. In this field, polymer modifications are indispensable in nanovaccines based on inorganic or biomimetic materials. They enhance solubility of inorganic nanovaccines and facilitate co-delivery of antigens and adjuvants in biomimetic formulations. As a pivotal component of nanovaccines, their discussion primarily focuses on nanomicelles with a two-dimensional arrangement and nanogels forming three-dimensional polymeric networks. A detailed examination of the polymer structures reveals their distinct properties and functions, providing critical insights into their mechanisms and instrumental role in advancement of nanovaccine technologies. As for other secondary or bridging components, they are succinctly described in other sections dedicated to inorganic and biomimetic-based nanovaccines.
2.2.1. Micelles
Amphiphilic block copolymers typically engage in self-assembly to form micelles, and these micelle-centered nanovaccines possess several advantageous characteristics including targeting capabilities, enhanced cellular uptake, and specific stimulus responsiveness [91,92]. Owing to their functional embedded designs, micelles inherit endocytic receptor targeting properties, presenting an alternative avenue for enhancing vaccine internalization, as exemplified by Pluronics [[93], [94], [95]]. Amphiphilic diblock copolymer of poly(2-ethyl-2-oxazoline)-poly(d, l-lactide) (PEOz-PLA) and carboxylterminated-pluronic F127 are utilized to yield micelles (denoted as carboxylated-NPs) as an example [61]. These micelles demonstrate aptitudes to infiltrate LV and precisely reach LN due to strategic incorporation of carboxylic groups. This design confers targeting capability to the micelles, allowing them to utilize a scavenger receptor-mediated endocytosis pathway for precise delivery.
Polymeric micelles, including polypeptides, exhibit efficient cytosolic delivery and enhanced adjuvanticity [96,97]. As depicted, the self-assembled micelles are consisted of PEG-PE, palmitoylated polypeptide, and MPLA, where palmitoylated polypeptide and MPLA are inserted into hydrophobic core of micelles (Fig. 3A) [62]. Designed PEG-PE micelles not only serve as chaperons for TLR signaling and perform adjuvant effects towards DC, but also transform soluble peptides into α-helix and enhance cytosolic delivery efficiency (Fig. 3C). These self-assembled cationic micelles, derived from PEG-PLL-PLLeu, exhibited particle stability during OVA transportation [63]. In vivo results showed that PEG-PLL-PLLeu-based nanovaccine significantly enhanced adjuvanticity, vaccine-induced germinal center formation, and antibody production. Efficient delivery of contents is also evidenced by mannose-modified stearic acid-grafted chitosan (M–CS–SA) micelles; upon being taken up by DC via mannose receptors, these micelles escape lysosomes and disperse in the cytoplasm [98]. The dispersed micelles trigger mitochondrial ROS generation, activating cytosolic DNA/cyclic GMP-AMP synthase (cGAS)-STING signaling pathway and stimulating IFN-I secretion. Moreover, Hubbell et al. reported two different PEG-conjugated-poly(propylene sulfide) (PEG-PPS)-based micelles [99,100]. These micelles were capable of conjugating antigens and adjuvants comprising CpG [99] or MPLA [100], thereby enhancing antigen presentations. Also, such micellar vaccine formulation markedly enhanced cellular and humoral responses while eliminating the need for dual adjuvants, simplifying platform preparation, and reducing costs.
Fig. 3.
Fabrications and applications of micelles in cancer immunotherapy. (A) Schematic diagram of self-assembly micelle, comprising PEG-PE, palmitoylated polypeptide, and MPLA, where hydrophobic components, palmitic acid, and MPLA are integrated into micelle's hydrophobic core. (C) Accumulation of different FITC formulations (FITC; FITC-labeled micelle, F-M; FITC-labeled liposome, F-L) in draining LN (DLN) at indicated time points (axillary LNs are referred as “aLNs” and inguinal LNs are referred as “iLNs”). Reproduced with permission [62]. Copyright 2017, Springer. (B) Schematic illustration of Man-VIPER delivery systems, consisting of disulfide-conjugated OVA antigens and membranolytic melittin (either releasable Man-VIPER-R or non-releasable Man-VIPER-NR). System self-assembles into micelles is endocytosed via mannosylated segments, leading to disassembly in endosomes and subsequent MHC I or MHC II epitope presentation to respective T-cell subsets. (D) Size distributions of non-membranolytic D-melittin-free analogues (Man-AP), Man-VIPER-R and Man-VIPER-NR characterized by dynamic light scattering (DLS; dmean = 27.6 nm, 51.6 nm, 40 nm, respectively). (E) pH-dependent micellization of Man-AP, Man-VIPER-R and Man-VIPER-NR. Reproduced with permission [102]. Copyright 2023, Elsevier.
The personalized micelles are meticulously engineered to incorporate sensitivity groups, such as pH-responsive functionalization and disulfide bonds, enabling them to effectively deliver and release their contents upon deconstruction. pH-sensitive micelles have been formulated when peptide antigens are conjugated to pH-sensitive hydrophobic blocks [[101], [102], [103]]. A notable example is a mannosylated Virus-Inspired Polymers for Endosomal Release (Man-VIPER) nanosystem comprising formulations with disulfide-conjugated OVA antigens. These formulations include either releasable disulfide-conjugated membranolytic melittin (Man-VIPER-R) or non-releasable pentafluorobenzyl-conjugated melittin (Man-VIPER-NR) (Fig. 3B) [102]. These formulations undergo self-assembly to form well-defined micelles, characterized by their preferential endocytosis mediated by mannosylated hydrophilic segments. The micelles disintegrate upon endosomal maturation, leading to either endosomal disruption and cross-presentation of major histocompatibility complex (MHC) I epitopes or lysosomal maturation and presentation of MHC II epitopes, targeting specific T cell subsets (Fig. 3D, E). In a similar vein, amphiphilic diblock of PCL-based arginine-glycine-aspartic acid (RGD)-PEG-ss-PCL (RPsP) is introduced where PCL is grafted with PEI via disulfide bonds and modifies with RGD [64]. These nano-sized micelles are disintegrated by a high concentration of glutathione in tumor cells, leading to on-demand release of DOX and R848 and inducing activation of DC.
2.2.2. Nanogels
Nanogels constitute nanoscale three-dimensional polymeric networks with the inherent capacity to encapsulate a diverse arrange of immune reagents. This encapsulation process is accomplished through widespread utilization of cross-linking agents, harnessing electrostatic interactions and oxidative self-polymerization mechanisms, culminating in sustained immune potency [104,105]. In cancer vaccine delivery, nanogels have demonstrated their overwhelming utility in facilitating controlled and prolonged release of encapsulated therapeutic agents [34,106]. For instance, HA-mRLNPs were developed to co-load mRNA-based lipid nanoparticles (LNP) and R838 into a hyaluronic acid (HA) dynamic hydrogel with a high molecular weight, resulting in long-lasting cancer immunotherapy [65]. HA-based hydrogels controlled release of the mRLNPs after undergoing state transitions in a physiological environment. Further, the nanovaccines in the LNPs-hydrogel system retained their functions after 14 days of storage at room temperature. Similarly, an injectable hydrogel based on graphene oxide and low molecular weight PEI is electrostatically combined and employed to encapsulate mRNA (OVA and antigen) and adjuvants (R848) [66]. After an injection into subcutaneous layers, these transformable nanogels release mRNA for at least thirty days.
Apart from their outstanding biocompatibility, abundant raw material resources, and substantial antigen-loading capacities, nanogels also possess abilities to facilitate controlled release and degradation of antigens [107]. Based on these, AP-SS nanogel is fabricated by disulfide cross-linking before electrostatic interaction of negatively-charged alginate sodium with branched PEI2k [67]. These nanogels exhibit minimal cytotoxicity, great antigen-loading capacity, and enhanced vaccine-elicited humoral and cellular immune responses. In addition to promoting antigen uptake by BMDC, reducible AP-SS nanogels also promote antigen release and cytosolic degradation. Except for this, a pH-degradable nanogel containing block copolymer micelles covalently functionalized with TLR7/8 agonist 1-(4-(aminomethyl)benzyl)-2-butyl-1H-imidazo[4,5-c]quinolin-4-amine (IMDQ) and Texas Red cadaverine is proposed [68]. Precise calculation of hydrodynamic radii deduced from resultant autocorrelation functions, substantiates not only conjugation of dibenzyl cyclooctyne-modified OVA but also degradation of IMDQ-loaded nanogels upon acidification. Such a nanogel carrier system is pre-defined in morphology, and guarantees control over co-delivery of each vaccine component. This nanogel emphasizes importance of incorporations of immune adjuvant and antigen into the same carrier system. Also, the self-adjuvant nanovaccine that is assembled by amphiphilic pH-sensitive galactosyl dextran-retinal and OVA, facilitates cytosolic antigen release and improves cancer vaccine efficacy after triggering a lysosomal rupture [108].
In brief, utilizing polymeric micelles and nanogels provides outstanding functionality and adaptability in engineering bespoke nanovaccine. Micelles, synthesized from amphiphilic block copolymers, exhibit their distinctive structural and compositional attributes, which is crucial for significant cellular uptake and specific stimulus responsiveness. These engineered micelles enable structure transformation, precise targeting and potentiate effective immune activation. Meanwhile, meticulously-engineered polymeric network structures within the nanogels ensure stable encapsulation and prolonged release of the vaccine constituents. Such a configuration of nanogels is pivotal in preserving biocompatibility while facilitating effective encapsulation and incremental release of diverse immunogenic elements. This strategic approach significantly augments therapeutic efficacy and longevity of the personalized nanovaccines. However, the development of polymeric nanovaccines underscores the crucial balance between innovation and practicality. While the structural design of micelles and nanogels provides promising pathways for targeted delivery and sustained release, the associated complexities in clinical translation highlight the need for continued research to optimize their therapeutic efficacy and safety in varied treatment contexts. Challenges such as potential toxicity from degradation products, non-specific immune reactions including allergic responses, and barriers to stable antigen release are notable considerations.
2.3. Biomimetic nanovaccines
Biomimetic syntheses entail synthesis and presentation of protein cargos on cell surfaces, offering a promising approach for efficient development of the ligand-targeted nanovaccines. Such biosynthesis process has been upheld native conformations, structures, and activities of functional proteins/lipids of interest. Depending on different preparation sources or approaches, biomimetic nanovaccines can be broadly categorized into vesicle-like, nanodisc, virus-like-oriented nanovaccines.
2.3.1. Vesicle-like
Large amphiphilic molecules form vesicles as nanoscale containers extending liposome skeletons. Their self-assembly into vesicles protects biomolecules, facilitating endocytosis and subsequent release in early endosomes before lysosomal fusion. This design is leveraged in lipid-based antigen delivery systems, inducing membrane fusion or endosomal destabilization for effective cytoplasmic antigen delivery. Nanocarriers with natural lipid-embedded vesicular structures are amenable to functionalization to form personalization nanovaccines, which achieve antigen-specific immune responses via multiple signaling pathways [109]. An example of HA-DOTAP-PLGA NPs has been investigated as vaccine delivery vehicles, exhibiting enhanced antigen presentation via both MHC I/II pathways after HA-CD44 receptor-mediated endocytosis [69]. Self-assembled liposomes-based nanovaccines, that were composed of monophosphatidyl (A) and CpG-ODN, had desirable antitumor immunities, especially Th1 cell response through activations of nuclear factor-κB (NF-κB) and protein kinase (MAPK) signaling pathways [110]. Liposomal vesicles based on pH-responsive systems represent one of the most common design strategies towards nanovaccines [111]. Yuba and colleagues have documented a series of highly pH-sensitive liposomes that have been shown promise in augmenting cancer immunotherapy [[112], [113], [114], [115]]. By incorporating pH-sensitive 3-methylglutarylated residues (MGlu-Dex) as the antigen delivery system, the mGlu-Dex-modified liposome is internalized by DCs through endocytosis and subsequently entrapped within endosomal compartments [112]. A mildly acidic microenvironment destabilizes liposomes, thereby prompting release of antigenic molecules confined within the endosome. Besides solid lipids [116], phospholipids [117], lipids [118], and cationic/helper polymers [70] -centered nanovesicles have also been explored for personalized treatment of cancer.
Exosomes represent a category of diminutive extracellular vesicles, characterized by approximate diameters spanning from 30 to 100 nm [119]. They encompass considerable therapeutic potential by virtue of their multifaceted cargo, containing proteins, lipids, nucleic acids, microRNAs, and long non-coding RNAs [120]. From diverse cellular sources, the exosome-based vaccines manifest a broad spectrum of immunomodulatory activities, endowing them with remarkable versatility as antigen-delivery vehicles [121]. For instance, exosomes derived from DCs possess capacity to activate CD4+ and CD8+ T cells via antigenic complexes adorning their surface [122]. Similarly, exosomes derived from B cell lymphoma cells can induce both clonal expansion of T cells and secretion of cytokines including IL-6 and TNF-α [123]. Given their interactions with immune cells and ability to instigate downstream signaling cascades, these exosome-based vaccines function as indispensable antigen carriers and immunomodulatory adjuvants within cancer immunotherapy [124]. Furthermore, exosomes have been identified as effective biomarkers for gauging adaptive immune activation, playing a crucial role in the diagnosis and treatment of cancers [121].
Besides, artificial cytomembranes-based nanovesicles are also derived from various sources, including DC [125], cancer cells [126], bacteria [127], red CM [128], TM [85], etc. The cytomembrane-base vesicle widened nanovaccine applications for multiple tumor types [71]. For instance, a personalized cancer vaccine based on recombinant adenovirus-infected DC membranes was genetically engineered, inheriting complete surface functional proteins of mature DC and enabling direct antigen presentation to naive T cells [125]. Immune reagents coated on CM [129], such as TMs, allow acquisitions of a comprehensive array of CM protein antigens, thereby eliciting a specific immune response against corresponding tumors (Fig. 4A) [130]. For instance, the cMnMOF@CM nanovaccine is established from TM derived from OVA-overexpressing melanoma B16 cells after encapsulations of CpG and TLR9 within Mn-MOF (Fig. 4B) [72]. cMn-MOF@CM nanovaccines obtain a comprehensive CM antigen array exposed to calreticulin and enhance DC endocytosis. Similarly, an advanced biomimetic nanovaccine, CMM-DiR, encapsulates STING agonists (MnO2 nanoparticles) and photothermal agent DiR within TM (Fig. 4D) [73]. This formulation promotes a rapid, explosive release of Mn2+ and enhances tumor cell uptake via homotypic adhesion properties (Fig. 4C, E). Notably, Mn2+ activates intracellular STING pathway and is a robust activator of cGAS within innate immune system's response against malignancies [131,132]. Mechanistically, Mn2+ enhances binding affinity between cGAMP and STING while simultaneously increasing sensitivity of cGAS to double-stranded DNA, amplifying STING signaling and eliciting downstream immunostimulatory responses (Fig. 4F−H). Moreover, membrane fusion technology facilitates formations of HM vesicles, combining Escherichia coli cytoplasmic membrane with TM derived from resected autologous tumor tissue [74]. These autologous HM vesicles extend antigen-specific tolerance, while pathogen-associated molecular patterns (PAMP) in bacterial membranes trigger innate immune responses by engaging pattern recognition receptors on epithelial and immune cells. Fusion of tumor neoantigens and bacterial PAMP is also an effective cancer vaccine strategy, potentially eliciting robust antitumor immunity.
Fig. 4.
Fabrications and applications of vesicles or nanodisc in cancer immunotherapy. (A) Schematic illustration of personalized nanovaccine by coating adjuvant R837-loaded PLGA NP with calcinetin-expressed Luc-4T1 cell membrane antigens. Reproduced with permission [130]. Copyright 2021, American Chemical Society. (B) Diagram of cMn-MOF@CM nanovaccine. Reproduced with permission [72]. Copyright 2021, Elsevier. (C) Detail mechanism of in situ STING-activating vaccination strategy. (D) Composition of CMM-DiR and functions of each component. (E) Mn2+ release profiles in pH 7.4 and pH 6.8 solutions (H2O2). (F) Expression of proteins on different groups. (G) Western blot for activation of cGAS-STING pathway in DC 2.4 cells with different treatments: Complete reaction liquid (CMM-DiR1), supernatant (CMM-DiRsup), and precipitation (CMM-DiRpre) from co-incubated CMM-DiR and H2O2 solution (pH 6.8). (H) Real-time quantitative polymerase chain reaction analysis for relative expression of cGAS-STING axis in tumor sites of mice with different treatment (n = 5). Reproduced with permission [73]. Copyright 2021, Elsevier. (I) Whole cancer cell derived of TM, followed by incubation with MPLA and styrene-maleic acid to form MPLA-loaded cancer cell membrane nanodisc (CCND/MPLA). (J) Size of cancer cell membrane-based nanodisc (CCND) and CCND/MPLA (n = 3). (K) TEM images of CCND (left) and CCND/MPLA (right) negatively stained with uranyl acetate. (L) Protein profiles of MC38 cell membrane (1), CCND (2), and CCND/MPLA (3) after gel electrophoresis. (M) Western blot probing for tumor antigens in MC38 cell membrane, CCND, and CCND/MPLA. Reproduced with permission [135]. Copyright 2023, American Chemical Society.
2.3.2. Nanodiscs
Among the systems above, high-density lipoprotein (HDL) represents a naturally occurring nanodisc characterized by its prolonged circulation in plasma (with a half-life of approximately 3∼4 days), distinguishing it from many synthetically engineered nanovesicles [75]. Integration of small molecule drugs into HDLs has been shown to augment therapeutic efficacy, principally by enhancing the small molecules’ solubility, circulation half-life, and distribution profile. Pre-formed sHDL nanodiscs offer a streamlined approach, wherein they can be readily combined with cholesteryl-CpG and tumor antigen peptides, including neoantigens identified through tumor DNA sequencing [76]. This process yields homogeneous, stable, and ultra-small nanodiscs at ambient temperature in less than 2 h. Employing sHDL as a matrix for encapsulating various bioactive compounds has proven effective in promoting immunotherapeutic interventions targeting MC38 tumors, glioblastoma multiforme, and cancer immunotherapy directed against cancer stem cells [77,133]. Furthermore, alternative formulations, such as CM fortified with styrene-maleic acid as a scaffold, have been employed in construction of nanodiscs, in which designs function as potent modalities for anti-tumor vaccination strategies (Fig. 4I) [134,135]. Following characterization, the CCND/MPLA nanodiscs, measuring 16 nm in diameter and displaying circular morphology, exhibit protein profiles akin to the MC38 plasma membrane (Fig. 4J−L). Notably, these nanodiscs have been confirmed to contain tumor-promoting markers such as CD44, programmed death ligand 1 (PD-L1), and the tumor-associated antigen EphA247 (Fig. 4M). This nanodisc-based formulation thus holds potential for applicability across a broad spectrum of cancer types.
2.3.3. Virus-like
Biomimetic nanovaccines, originating from viral sources, employ self-assembled viral capsid proteins devoid of genetic material and have found widespread applications in vaccine design, especially in the context of cancer vaccines [78,79,136]. These VLPs, functioning as biomaterial scaffolds characterized by pathogen-like polyvalent structures and versatile chimeric multiepitope, hold the potential to serve as nanocarriers for antigen delivery and the induction of immune responses [137]. Cervarix, the FDA-approved human papillomavirus (HPV) vaccine, consisting of HPV16 and HPV18 L1-protein-based VLP has been produced using the virus-like nanoformulation. Another instance involved engineered Salmonella typhimurium bacteriophage P22-based VLP, which were co-encapsulated with two proteins of respiratory syncytial virus (M and M2) [80]. In vivo results showed that the P22-M/M2-treated mice exhibited significantly decreased lung viral titers, indicating potential of the VLP-based strategies in designing nanovaccines generating immune responses towards multiple subunit antigens. Except for that, those of prokaryotic or eukaryotic origin, which are called protein cages and vaults, are generally for immune molecular transportation and induce robust immune responses. Cages like the 13 nm cage of human heavy chain ferritin (an iron storage protein) [138], 25 nm E2 cage derived from the pyruvate dehydrogenase complex of Bacillus stearothermophilus [139], and 24 nm encapsulins of bacteria and archaea, have all been employed as nanocarriers for delivering antigens and eliciting immune responses post-immunization [140]. In addition, eukaryotic ribonucleoproteins assembly of a cage-like barrel-shaped structure is also provided to design protein-based nanovaccines, increasing antigen-specific CD4+ T cell responses with multiple cytokine secretion patterns [140,141].
Biomimetic nanovaccines, comprising vesicles, nanodiscs, and VLP, advance cancer immunotherapy by optimizing antigen delivery and immune response specificity. These nanoscale entities emulate biological processes for targeted antigen presentation and activation of immune cells. However, their fabrication, being more complex than nanovaccines derived from inorganic materials and polymers, poses significant challenges in selecting and processing diverse nanomaterials for development of tailored vaccines, necessitating a sophisticated approach to their production methodology.
3. Nanovaccines-based strategies for enhanced cancer immunotherapy
Based on the above description, engineering, and characterization of nanocarriers from a diverse array of sources, including inorganic, polymeric, and biomimetic materials, alongside their adjuvant attributes, are of paramount importance. Efficacy of these nanovaccines in the context of enhanced cancer immunotherapy depends on various factors: vaccination methodologies, antigen specificity, active constituents anchored to different immune cells, and dynamic profiles of TME specific to diseases.
In strategies aimed at augmenting cancer immunotherapy, nanovaccines necessitate a focused approach on LN targeting for antigen delivery, efficacious antigen presentation, initiation of immune response signaling pathways, and synchronized modulation of TME under distinct disease profiles. Integration of different active components, encompassing adjuvants, receptors, agonists, cytokines, and chemokines, into formation of custom nanovaccines is advantageous for targeting immune cells and mitigating immunosuppressive milieu (Table 2). Specifically, efficacy of LN targeting and subsequent accumulation is intricately-linked to the vaccination strategy employed, including in situ and tumor-specific modalities. For activation of the immune system, stimulation and proliferation of T and B cells are fundamental. Addressing immunosuppressive TME, which encompasses TAM, and MDSC, among others, necessitates a strategic focus on personalized targeting ligands or antigens and modulation of associated signaling pathways. Subsequent sections will extensively examine these critical aspects in context of enhancing therapeutic potential of custom nanovaccines towards enhanced cancer immunotherapy.
Table 2.
Customized nanovaccines targeting LN and immune cells.
| Targeting sites | Nanovaccine names | Essential components | Features | Tumor types | Refs |
|---|---|---|---|---|---|
| LN | AlO(OH)-polymer NP | OVA and CpG | Nanosized vaccine (90 nm) for LN | B16-OVA and B16–F10 melanomas | [142] |
| Melittin-lipid based nanovaccine | Whole-cell tumor antigen | Nanosized vaccine (10−20 nm) for LN | B16–F10 melanoma | [143] | |
| Amphiphiles-based molecular vaccine | Peptide antigen | “Albumin hitchhiking” strategy | TC-1 tumor and B16–F10 melanoma | [144] | |
| Tu-NPFN(+)/Ln-NPR848 | Ferrimagnetic nanocube (FN) and R848 | Nanosized vaccine (∼100 nm) for LN | CT26 colon tumor | [145] | |
| OVA/CpG | OVA and CpG | Small size, narrow distribution, negative charge, and good stability for LN targeting | E.G7-OVA tumor | [146] | |
| Gold nanoparticle (GNP) | OVA | Nanosized vaccine (10−20 nm) for LN | E.G7-OVA tumor | [147] | |
| Pluronic-stabilized poly(propylene) sulfide NP | CpG and paclitaxel | Nanosized vaccine (30 nm) for LN | B16–F10 melanoma | [148] | |
| OVA@CpG (mNV) | OVA and CpG-SH | Extended “antigen depot” effect | B16-OVA melanoma | [149] | |
| T Cell | Poly-lactide-co-glycolide (PLG) scaffold | Granulocyte-macrophage colony-stimulating factor (GM-CSF) and CpG-ODN | Release cytokine to recruit and house host DC | B16–F10 melanoma | [150] |
| Multifunctional bacterial membrane-coated nanoparticle (BNP) | PC7A/CpG and Neoantigen | Capturing cancer neoantigens resulting in enhancement of DC uptake and cross presentation | B78 melanoma and NXS2 neuroblastoma | [151] | |
| MSN-TY | Screened peptide (TY), OVA, and CpG | DC targeting peptide | B16-OVA melanoma | [152] | |
| NP-supported cytomembranes (NP@FM) | Whole tumor antigen complexes and immunological co-stimulatory molecules | Mimicking both APC and cancer cells | 4T1 breast cancer | [153] | |
| PLA-PEI NP | TLR9, CpG, and protein antigen | Antigen cross-presentation through “proton sponge effect” | B16-OVA, MC38, and E0771 tumors | [154] | |
| iDR-NCs/neoantigen complex | CpG, Stat3 shRNA, and PPT-g-PEG | Neoantigen-specific T cell response | MC38 tumor | [155] | |
| DNA nanodevice-based vaccine | Tumor antigen peptide, dsRNA, CpG loop, locking strands | Amenable size and pH-responsive manner in respond to acidic endosomal environment | B16-OVA melanoma | [156] | |
| mRNA/LNP | LNP, mRNA encoding CMV glycoproteins gB, and pentameric complex | Higher affinity between antigen and T cell | – | [157] | |
| B Cell | DCDX modified liposome (DCDX-sLip) | Brain-targeted D-peptide ligand (DCDX) and OVA | IgM-Fc receptor (FcμR) pathway to targeting B cell | – | [158] |
| NK Cell | Cyclic diguanylate monophosphate (cdGMP)/MPLA-encapsulated immuno-NP | cdGMP and MPLA | Cytokine gradient drive of NK cell upregulation | TNBC | [159] |
| Fe3O4/SiO2 core/shell NP | Cyanine5.5 (Cy5.5) and NK-92MI cell | Modulation NK cell | Human B cell lymphoma | [160] | |
| Fe3O4@PDA NP | Labeled NK cell | NK cell recruitment and its infiltration into tumor site | A549 cancer | [161] | |
| TAM | Hybrid PEG micelle | BLZ-945, OVA, and NLG919 | BLZ-945 and NLG919 caused effective M2-like TAM depletion | E.G7-OVA tumor | [162] |
| PEG-FA-based liposome (PEG-FA-Lip) | CpG, FA, and DOX | FA-receptor mediate endocytosis | 4T1 breast cancer | [163] | |
| Dual-inhibitor loaded supramolecular NP | MCSF 1 receptor (CSF1R) and MAPK inhibitor | Inhibitions of CSF1R and MAPK signaling pathway to enhance repolarization of M2-TAM | 4T1 breast cancer | [164] | |
| M2NP | α-peptide | M2-TAM binding peptide | B16 melanoma | [165] | |
| Peptide hydrogel | TLR7/8a | TLR7/8 for driving polarization of macrophage towards M1-TAM | 4T1 and B16 tumors | [166] | |
| Fe-MOF | Diclofenac and peptide | Decrease efflux by hepcidin/ferroportin signaling pathway | H22 tumor | [167] | |
| TAN | Mesoporous silica | Fe(III)-captopril | Repolarized N2 phenotype | H22 tumor | [168] |
| MDSC | TAD-based (FIT) NP | TAD and indocyanine green | Inhibit MDSC function by TAD | Colon tumor | [169] |
| Lipid nanocapsule | Gemcitabine-C12 | Gemcitabine-C12 targeting monocytic MDSC subset | Lymphoma and melanoma tumors | [170] | |
| Glyceryl-monooleate-based liquid-crystal core NP | CCL2, OVA, and RNAi sequence | Chemokine targeting MDSC | MCA-203 fibrosarcoma | [171] | |
| NKT Cell | PEGylated lipid-PLGA NP | CpG-ODN and MPLA | Presentation of GalCer via MHC I-like molecule (CD1) to NKT cell | B16–F10 melanoma | [172] |
| Treg Cell | Layer-by-layer hybrid NP | IR 780, Imatinib, and glucocorticoid-induced TNF receptor | Reductions of transcription factors of STAT3 and STAT5 in Treg cell | B16 and BL6 tumors | [173] |
| CpG/cGAMP-hybrid liposome-Man (C/G-HL-Man) | Fenofibrate, cGAMP, CpG, and TM | Fenofibrate, activated peroxisome proliferator-activated receptor (PPAR)-α pathway, and downstream genes related to FA metabolism | B16–F10 melanoma | [174] |
3.1. Escalation of lymph node accumulation
In addition to their conventional surgical removal, increasing attention has been paid to modulation of the LN as a cancer treatment [175]. LN are secondary-lymphoid tissues regarded as crucial hubs linking immune cells and regulating adaptive immune responses [176]. They are distributed throughout the body and recruited leukocytes from the circulation screen antigen-laden lymph draining to different tissues [177]. Efficient delivery of vaccines to LN is crucial in achieving cancer immunotherapy, with administration modes significantly influencing antigen accumulation and enrichment within LN. Nanovaccine delivery to LN commonly employs intratumoral and interstitial methods, resulting in a “depot effect” that prolongs antigen exposure within TME, thus inducing a potent immune response.
Intratumoral injection is the direct and effective way for delivering in situ vaccines directly towards LN. This process involves engineered delivery of exogenous antigens directly into LN within tumors, eliciting immune responses [142]. For instance, a high-density lipoprotein-mimicking peptide-phospholipid scaffold (termed α-peptide-NP) was developed after lading melittin, forming an ultra-small (10−20 nm) melittin-lipid nanoparticle (named α-melittin-NP) [143]. Compared with melittin, which had a molecular weight of 2840 Da, as-fabricated α-melittin-NP, within an ultra-small size range of 10–20 nm, was favorable for LN uptake, and induced release of whole tumor antigen, retaining multiple immunogenic epitopes to trigger a robust immune response. Exogenous antigens are structurally encoded for LN delivery [178]. Another innovative approach is the “albumin hitchhiking” strategy, designed for specific LN targeting by molecular vaccines [144]. This strategy exploits albumin's role as a fatty acid transporter, whereby antigens or adjuvants, modified with a lipophilic albumin-binding domain, accumulate in lymphoid organs post-injection through in situ complexation and transportation with endogenous albumin. In situ vaccine programming within tumor sites provides another targeting-LN delivery method. For example, intratumoral injection of a composite containing adjuvant-loaded Ln-NP and antigen-inducing Tu-NP yields nanovaccines approximately 100 nm in size with neutral/negative charge (Fig. 5A) [145]. Under an alternating magnetic field, Tu-NPFN (100 nm) generates prolifically antigens, while Ln-NPR848 (35 nm) captures a portion of the produced antigens, inducing in situ nanovaccines targeting LN after traversing interstitial space and LV (Fig. 5B, C). After intratumoral injection, the in situ programming nanovaccines convert primary tumors into whole-cell antigens for targeted LN delivery. Leveraging a hydrogel-based system, these in situ nanovaccines enable ROS-responsive DOX and nanoadjuvant (CpG-P-ss-M) releases, accumulating abundant tumor antigens within tumors [15]. The CpG-P-ss-M's charge reversal facilitates adsorption and conversion into small, negatively charged tumor vaccines, optimizing LN priming.
Fig. 5.
Nanovaccines for LN-targeted delivery and their enhanced cancer immunotherapy. (A) In situ programming of vaccines via two synergetic nanomedicines, Tu-NPFN and Ln-NPR848. (B) Sodium dodecyl sulfate-polyacrylamide gel electrophoresis of total proteins generated by Tu-NPFN under an alternating magnetic field and proteins captured by Ln-NPR848. Proteins were stained with Coomassie Brilliant Blue. (C) Relative abundances of tumor antigens captured by Ln-NPR848 as determined by liquid chromatography/tandem mass spectrometry. Reproduced with permission [145]. Copyright 2022, American Chemical Society. (D) Schematic illustration of mNV-triggered antitumor immune responses. (G) Representative fluorescence images of DLN for groups of mNV that FAM-labeled CpG (mNVF) at the indicated time points. Reproduced with permission [149]. Copyright 2018, American Chemical Society. (E) Personalized nanoDC that efficiently accumulates in LN after subcutaneous injection, directly stimulating TAA-specific T cells to kill tumor cells. (F) Ex vivo fluorescence image of FITC, FITC-labeled immature DCs membrane-based vaccines (nanoiDCs) or nanoDCs treated LN. Reproduced with permission [185]. Copyright 2022, Wiley.
Intratumoral injection, while direct and efficient, often requires surgical, ultrasonic, or tracer dye guidance, adding complexity to the vaccination process [179]. An alternative approach is to utilize interstitial administration methods such as subcutaneous, intramuscular, and intradermal injections to enable nanovaccines to enter capillary LV and reach LN. Efficiency of this transfer depends on the nanovaccines’ characteristics like size, charge, and hydrophilic modifications, etc. These characteristics influence their movement through lymphatic pathways, interstitial structures (compositions of tangled collagen fibers and negatively charged HA), and blood flow rates [146]. Size of antigen-bearing carriers reconciles diameter of LN vascular channels, approximately 100 nm; larger nanoparticles fail to reach capillary LVs and are intercepted by tissue-resident DC, while particles smaller than 10 nm are typically cleared through blood capillaries from intestine due to blood capillary flow rates being 100–500 times faster than LV [180]. GNP-based nanovaccines of various sizes have been synthesized to evaluate their capacity for delivering payloads to the LN [147]. Specifically, GNPs with 7, 14, and 28 nm diameters are functionalized with OVA, resulting in corresponding hydrodynamic diameters of 10, 22, and 33 nm, respectively. Significantly, OVA-GNPs with dimensions of 22 and 33 nm exhibit superior delivery efficiency in targeting the draining LN compared to those with a diameter of 10 nm. An adjuvant-targeted strategy also achieves passive accumulation within tumor-draining lymph nodes (TDLN), modulating effector responses and counteracting tumor-induced immunosuppression [181]. Polymeric nanoparticles with a size of 30 nm are prone to TDLN, mainly when administered in limbs on the same side as the tumor [148].
The “minimalist” nanovaccine design, which effectively captures nearly all cancer-related antigens [149], exemplifies how extended antigen “depot” enhances LN-targeting potential of the nanovaccines. The nanovaccine (mNV), with a diameter of 50 nm, is formulated using OVA and thiol-modified CpG (CpG-SH), resulting in a composition of 500 antigen molecules per nanoparticle [149]. This substantial antigen-loading capacity of the nanovaccine facilitates efficient drainage into the LV and subsequent high retention in the LN, thereby initiating immune responses (Fig. 5D, G). Furthermore, an efficient accumulation of cargo within LN is achieved through application of the external forces, such as magnetic guiding mechanisms [182]. Notably, functionalization of magnetic responsive components such as superparamagnetic oxides are harnessed as frameworks or scaffolds to guide homing of LN, with classical examples including ferric oxide as a magnetic nanocarrier. These intricately-crafted nanovaccines showcase a remarkable propensity for accumulation within profound regions of LN. Another example of superparamagnetic iron oxide coated with zinc oxide efficiently expedited LN homing through synergistic interactions between external forces and LN-homing leukocytes [183]. Reports also indicate that DC-based vaccines exhibit enhanced LN targeting efficiency by incorporating key protein CC-chemokine receptor 7 from DC membrane vesicles, facilitating lymphatic homing [184]. By maintaining co-stimulatory markers, MHC I antigen complexes, and lymphocyte homing receptors, a personalized DC-mimicking nanovaccine (nanoDC) also efficiently migrates to LN and elicits potent antigen-specific T-cell responses (Fig. 5E, F) [185].
In short, administration of the vaccination generally employs intratumoral and interstitial methods, efficiently facilitating antigen accumulation within LN and consequently triggering robust immune responses. Nanovaccine delivery strategies, encompassing intratumoral and interstitial routes, are meticulously designed to align with specific characteristics and structural complexities of LN. These passive targeting approaches, whether implemented via subcutaneous, intramuscular, or direct introduction into LV, are adept at ensuring targeted antigen accumulation within LNs in various tumor models. Furthermore, incorporation of active targeting mechanisms, driven by external forces, enhances precision of nanovaccine localization in LN. This holistic and versatile vaccine delivery approach significantly advances customized nanovaccines development in cancer immunotherapy. While these strategies hold promise, they also present challenges such as potential systemic toxicity, the necessity for precise targeting, and the complex interplay within TME that may impede effective vaccine delivery and action. The “albumin hitchhiking” strategy and in situ vaccine programming within tumor sites further illustrate the innovative approaches being explored to improve cancer immunotherapy. Addressing these challenges requires a nuanced understanding of the TME and a development of sophisticated nanovaccine systems capable of precise navigation and targeted delivery. As such, future research should not only aim to optimize these delivery methods but also to explore new targeting ligands and nanocarrier designs to enhance specificity, efficacy, and safety of the custom nanovaccines in cancer treatment.
3.2. Enhancing antigen presentation in single-cell level
3.2.1. Enhancement of antigen presentation
DC plays a central role in antigen presentation within context of cancer immunotherapy, which initiates and sustains effective immune responses through their efficient antigen presentation mechanisms [186]. To ensure executing sufficient immune responses, customized nanovaccines are initially engulfed by DC. These tailored nanovaccines are internalized via receptor-mediated pathways in DCs, guaranteeing an effective immune response [187,188]. Specifically, the antigen presentation mediated by receptors from the C-type lectin family, such as DC-SIGN receptor, is one strategy for targeting DCs with custom nanovaccines [189]. Additionally, nanovaccines engineered with mannose fragments, targeting overexpressed mannose receptors on APC surfaces, enhance cross-presentation of antigens [190]. Moreover, appropriate delivery of regulatory cytokines, such as GM-CSF, creates a simulated infection microenvironment, enabling precise DC recruitments and proliferations [150,191,192]. Omar et al. have underscored a critical role of GM-CSF released from PLG scaffolds in enhancing DC recruitment and proliferation in a dose-dependent manner [150]. Experimental results demonstrated that implanting GM–CSF–based PLG matrices into mice significantly increased proportion of DC and regulated their maturation and dispersion. Furthermore, BNPs, abundant in PAMP such as TLR agonists, are recognized for their capacity to stimulate innate immunity and activate DC [151]. Peptides are specifically binding to BMDCs and splenic DCs, identified through phage display technology, have also been incorporated into nanovaccine to targeting DCs effectively [152,193]. In brief, engineering inherent or specifically designed targets is a key strategy for activating and recruiting DCs with nanovaccines [32].
T cells, being integral to cell-mediated immunity and expressed by T-cell receptor (TCR), are pivotal in advanced cancer immunotherapy strategies [194]. Central to these strategies is activation and proliferation of cytotoxic T-cells, notably CD8+ T cells. Low immunogenicity and stability of antigens necessitate efficient antigen-specific delivery systems, with an emphasis on nanovaccines that stimulate CD8+ T cell-mediated immunity [195]. An ideal process should proficiently transport antigens to the cytoplasm of APC for MHC I-antigen complex formation through cross-presentation, thereby activating T cells for immune defense and pathogen clearance [196]. Customized nano-based vaccination strategies, aimed at enhanced cross-presentation and T-cell responses can be categorized into several methods: aAPC based on membrane fusion, endosomal escape (e.g., endosomal swelling rupture), photochemical internalization, and nucleic acid vaccines capable of sustained expression in APC [197]. For example, a cytomembrane nanovaccine, engineered to mimic tumor cells and function as an APC, has demonstrated significant therapeutic effects (Fig. 6A) [153]. Fusions of two types of cells result in high expressions of immunological co-stimulatory molecules and whole tumor antigen complexes on FM, allowing nanoparticle-supported FM (NP@FM) to behave as APC for T cell activation. Lipid bilayer, emulating natural APC membrane system, incites expansion of antigen-specific cytotoxic T-cell subpopulations via T-cell receptor stimulation [198]. Similarly, nanosized artificial APC (naAPC)-based formulation induces antigen cross-presentation to T cells via size-transformation and peptide-MHC (pMHC) I complex/TCR signaling (Fig. 6C−G) [199].
Fig. 6.
Enhancement of antigen presentation with customized nanovaccines in cancer immunotherapy. (A) Preparation of MOF@cytomembrane. (B) Confocal laser scanning microscopy observation of fusion of DC (green fluorescence from anti-MHC II-FITC antibody) and 4T1 cell (red fluorescence from anti-CD44-APC antibody). Reproduced with permission [153]. Copyright 2019, Springer. (C) Schematic illustration of mannan-decorated pathogen-like polymeric nanoparticle system (MPVax) and mechanism in eliciting antitumor immune responses. MPVax-CpG/Antigen is constructed with PLA-PEI core, loaded with CpG and antigens and shielded with oxidized mannan. MPVax-CpG/Antigen efficiently accumulates in LN by passive drainage or pDC and mannose-binding lectin mediated active transportation. After arriving at CD8+ DC, antigens are presented to CD8+ T cells and results in antigen-specific tumor eradication. Reproduced with permission [154]. Copyright 2022, Elsevier. (D) Formation of size-transformable naAPC. Achieved through self-assembly of copolymer biotin-PEG-b-poly(N-2-hydroxypropyl methacrylamide-g-thiol)-b-poly[2-(dimethylamino) ethyl methacrylate], naAPC encapsulates IL-2 within their aqueous core and features a surface adorned with pMHC monomer and αCD28. (E) Size and structure characterization of naAPC tested by DLS and TEM, respectively. In vitro CD3+CD8+ T cell proliferation by (F) co-incubation of naïve T cells with mature DC 2.4 cells, and co-incubation of mature DC 2.4 cells and dying EG7-OVA cells with (G) T cells; and (H) preactivated T cells by photodynamic therapy (PDT) after treatment with different groups including (1) PBS, (2) naAPC, (3) OVA, (4) NP-OVA, (5) OVA-naAPC, and (6) NP-OVA/naAPC. Mixed cells had PBS or naAPC alone serve as control. (n = 3; **P < 0.01, ***P < 0.001; ns, not significant; one-way ANOVA with multiple comparisons). Reproduced with permission [199]. Copyright 2020, American Association for the Advancement of Science. (I) Schematic illustrations of responsive release and process for triggering anti-tumor immune response of cyclodextrin/PEI-based nanovaccine. Reproduced with permission [201]. Copyright 2023, Wiley.
Polymers like PEI or PEG are increasingly used in nanovaccine development to enhance antigen cross-presentation through the “proton sponge effect’’. This effect involves rupturing endosomal membranes and releasing antigens into cytosol, thereby facilitating increased antigen cross-presentation and T-cell priming (Fig. 6B) [154]. Nanovaccines, such as those with PEG-grafted polypeptide (PPT-g-PEG) copolymers, leverage acid-labile nature of the PEG for optimal solubility and to amplify the “proton sponge effect” [155]. This effect enhances intracellular delivery following PEG detachment in an acidic endolysosomal environment, along with exposure to cationic polypeptide for improved cellular uptake and antigen presentation. PEG-based nanovaccines with pyridyl disulfide groups facilitate antigen conjugation through thiol-disulfide exchange, destabilizing acidic endosomes and releasing vaccine contents into cytosol for MHC I presentation and differentiation-associated protein 5 signaling pathway activation [200]. Moreover, a streamlined and scalable platform, incorporating beta-cyclodextrin and PEI, provides a distinctive cavity structure, high charge density, and reactive disulfide bonds, facilitating endosomal escape of the neoantigens without inducing damage (Fig. 6H) [201]. This unique composition not only enhances cross-presentation significantly but also mediates activation and expansion of naïve T lymphocytes, thus effectively strengthening antitumor immunity.
Nucleic acid-based vaccines like DNA and mRNA are emerging as effective alternatives to traditional protein or peptide-based subunit vaccines, capable of inducing robust T-cell responses through host cellular mechanisms [202]. Specifically, DNA illustrates this by transferring gene sequences to APC, which releases payloads in acidic endosomal environments, thereby eliciting strong antigen-specific T-cell responses [203]. This mechanism is further exemplified by DNA nanodevices that also release payloads in response to acidic endosomal environments [156]. These nanovaccines are particularly adept at stimulating CD8+ T cell-mediated cellular immunity owing to their mode of intracellular expression [204]. In comparison, mRNA vaccines present lower genetic risks and operate without need for nuclear entry, degrading after activation. This characteristic renders them promising for clinical applications, particularly in LNP-based systems [205,206]. These LNPs, including liposome-entrapped mRNA vaccines, have been successful in stimulating virus-specific CTLs [207]. Furthermore, a versatile platform of the mRNA/LNP nanovaccine is demonstrated in its application for heterologous prime/boost T-cell responses [157]. The recent remarkable success and demonstrated safety of mRNA lipid nanoparticle technology have been pivotal in production of the vaccines for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [208].
Photochemical internalization offers a powerful approach for endosomal escape via light-induced rupture [209]. This technique involves integrating photosensitizers into nanovaccines which triggers ROS generation and endosome rupture upon exposure to specific light. This strategy enhances antigen internalization and cross-presentation, effectively stimulating antigen-specific CD8+ T cell activation [210]. The HA-OVA-AuNPs nanovaccine, based on gold NP adorned with HA and OVA, demonstrates this by generating a prompt antigen-specific CD8+ T cell response when exposed to NIR irradiation [211]. This response is attributed to increased proteasome activity and MHC I antigen presentation, driven by heat-mediated disruption of endo/lysosomes. Besides, a protein-based nanovaccine has also shown potential in photochemical internalization, causing CD8+ T cell infiltration and caspase-3-dependent death, both of which inhibit tumor progression [212].
Simply put, activation and expansion of the cytotoxic T cells, facilitated by advanced nanovaccine strategies, represent a cornerstone in evolution of the cancer immunotherapy. The utilization of aAPC based on membrane fusion, endosomal escape mechanisms, photochemical internalization, and nucleic acid vaccines delineate a multifaceted approach towards optimizing antigen presentation and T-cell activation. These strategies, while promising, underscore the complexities of immune modulation at the cellular level, highlighting the delicate balance required to elicit a potent controlled immune response. The challenge lies not only in achieving efficient antigen delivery to APCs but also in navigating the myriad of immune regulatory mechanisms to ensure a sustained and targeted antitumor response. Furthermore, the integration of nucleic acid-based vaccines introduces a novel dimension to traditional immunotherapy paradigms, offering a dynamic and adaptable platform for inducing robust T-cell responses. However, success of these innovative approaches necessitates a comprehensive understanding of underlying biological interactions and development of sophisticated delivery systems capable of precise targeting and controlled release. As such, future research should endeavor to refine these strategies, exploring the potential of novel nanocarrier designs and targeting ligands to enhance specificity and efficacy of the bespoke nanovaccines in mediating cancer immunotherapy.
3.2.2. Activating B cells
B cells play a pivotal role in orchestrating adaptive immune responses through communication mediated by B-cell receptor (BCR) [[213], [214], [215]]. Signaling pathway of BCR entails participation of a membrane-anchored surface Ig, the distal portion of which is characterized by variable regions with antigen-binding sites [[216], [217], [218]]. IgM is recombined Ig variable region genes and expressed on immature or mature B cells [219]. Based on this finding, IgM-based nanovaccines (DCDX-sLip/OVA) are formulated using DCDX-modified liposomes, OVA, and IgM via electrostatic interactions, demonstrating a significantly-targeting ability to splenic B cells [158]. IgM, alongside decorations of FcμR and CD21/35, plays a crucial role in enhancing antigen presentation and uptake by B cells residing in the splenic marginal zone. This phenomenon is evidenced by a robust biosafety profile observed in response to DCDX-sLip/OVA, characterized by sustained higher antibody titers (IgG1), which is facilitated through both IgM-complement and IgM-FcμR pathways. Complement-mediated B-cell targeting approach is also demonstrated by lectin-coated surfaces of dextran coated ferrous-based nanovaccines where surface-deposited active complement factor 3 (C3) results in predominant targeting to CR1/2-expressing B cells [220]. BCR signaling is additionally believed to contribute to how malignant B cells proliferate and survive in individuals with lymphomas or leukemias [219]. Development of the therapeutic medications that emphasize BCR signaling is gaining momentum. Examples of these medications are bruton tyrosine kinase inhibitors [221] and phosphoinositide 3-kinase isoform-specific inhibitors [222], which sparks further enthusiasm for development of the customized nanovaccines targeting B cells-related therapeutic pathways.
3.2.3. Activating natural killer cells
Given their capacity for cytokine production and participation in immunosurveillance, NK cells have emerged as compelling targets for cancer immunotherapeutic approaches [223]. An effective approach to enhance infiltration of NK cells into tumors involves inductions of stimulatory cytokines associated with NK cell activation [224]. Utilizing functionalized ligands as an active targeting strategy, precisely-tailored nanovaccines can be designed to stimulate cytokine production and facilitate the trafficking of NK cells to tumor sites. Immune-NPs, which are engineered by cdGMP (a STING agonist), MPLA, and a TLR4 agonist, have shown promise in catalyzing production of type I IFN-β and increasing accumulation of NK cells in TME [159]. These nanovaccines are demonstrated to have significant therapeutic effects in a murine model of metastatic TNBC. In addition, the passive strategy to mediate NK cell activation is to sense chemokine attraction by an external stimulator. Magnetic-mediation nanovaccines enhance infiltration of NK cells. For example, Fe3O4/SiO2 core/shell NPs conjugated with Cy5.5 exhibited a satisfactory cancer immunotherapy effect and significantly-increased infiltration of NK-92MI cells (17-fold greater than that detected without NP treatment) under an external magnetic field [160]. Magnetic nanoparticles alongside PDA layer are also confirmed to improve accumulation and retention of NK in TME [161]. By focusing on the intricacies of cytokine modulation, it underscores potential of customized nanovaccines to activate NK cells, enhancing efficacy of cancer immunotherapies.
3.3. Modulating immunosuppression in single-cell level
3.3.1. Modulating tumor-associated macrophage strategies
Macrophages are integral components of the mononuclear phagocytic system and play pivotal roles in tumor progression [225]. These versatile cells exhibit two distinct phenotypes: M1 macrophages with pro-inflammatory and anti-tumorigenic properties, and M2 macrophages with anti-inflammatory and pro-tumorigenic properties [226]. Their inherent plasticity allows macrophages to adapt their functions and phenotypes based on the specific tissue microenvironment, making them amenable to reprogramming for targeted immunotherapies [227]. TAM is the predominant cell type in TME, and clinical research has revealed a strong correlation between increased TAM infiltration in tumors, poor prognosis, and heightened metastasis risk. Therefore, targeting TAM involves activating M1 phenotype while inhibiting M2 phenotype [228]. In this context, strategies for nanovaccines targeting TAM emphasize two aspects: inhibiting recruitment and viability of M2-type TAM and reprogramming TAM phenotypes [227].
Nanovaccines incorporated functionalized ligands, including small molecule CSF1R kinase inhibitors (pexidartinib, PLX7486, RG7155, and BLZ-945) and FA receptor-targeting agents, effectively redound conversion of M2-type to M1-type TAM [162,163,229]. For example, a newly-developed bioactive nanovaccine generated a “depot effect” for NLG-919 and BLZ-945 into tumors, inducing M1-like TAM and depleting M2-like TAM. This approach also led to inhibition of indoleamine 2,3-dioxygenase activity and effective depletion of M2-like TAM [28,230]. In addition, targeting overexpressed FA receptors in various solid malignancies, FA-mediated endocytosis-driven strategies have been applied. The matrix metalloprotease 2 responsive liposome (PEG-FA-Lip), focuses on M2-TAM and tumor cells, inhibiting Treg cell activation, reversing M2-TAM-mediated immunosuppression, and suppressing tumor growth and metastasis [231]. Additionally, supramolecular nanoparticles loaded with CSF1R and MAPK inhibitors advance macrophage-targeting immunotherapy by blocking relevant signaling pathways [164].
Engineered nanovaccines are also effectively reprogramming TAM by incorporating anchored ligands like M2pep and TLR agonists [232,233]. These nanovaccines, such as M2NPs, integrate α-peptides with targeting abilities to specifically deplete M2-like TAM and block their signaling pathways [165]. Moreover, TLR agonist-based nanovaccines are repolarized M2-type macrophages towards an anti-tumor M1 phenotype, as demonstrated by a TLR7/8a conjugated hydrogel that aids in reprogramming TAM and enhances efficacy of PD1-blockade (Fig. 7A−C) [166]. The DNA-polymer hybrid nanocomplex containing co-assembly of TLR9 agonist (CpG) and TLR7 agonist (loxoribine) also converts of suppressive M2 macrophage to active M1 macrophage (Fig. 7D−F) [234]. Furthermore, iron ions are being explored for their role in repolarizing M2-like TAM, with innovations like an iron-based nanovaccine (Dic@M2pep-Fe-MOF) amplifying this repolarization and bolstering efficacy of cancer immunotherapy. These advancements underscore potential of customized nanovaccines in modulating immunosuppressive TME and improving efficacy of cancer immunotherapy [167].
Fig. 7.
Designs of targeting TAM-specific nanovaccines in cancer immunotherapy. (A) Schematic illustration of macrophage repolarization regulated by peptide hydrogel (Smac-TLR7/8 hydrogel) for overcoming radioresistance. Smac-TLR7/8 peptide self-assembles into nanofibrous hydrogel. Then, (i) after peritumoral injection, Smac-TLR7/8 hydrogel effectively reprograms TAM from M2 type toward M1 type, secreting inflammatory factors and activating anti-tumor functions. (ii) Immunosuppressive TME is rebuilt through tumor infiltrating lymphocytes recruitment, and downregulating Treg cells. (iii) Radioresistance is overcame which further improves anti-tumor efficacy combined with immunotherapy. (B) Percentage of M2-related (F4/80+CD206+) macrophages (n = 5). (C) Protein expression level of NF-κB, TNF-α, inducible isoform of nitric oxide synthase (iNOS), IL-10, arginase 1 in macrophages determined by western blotting. RT: Radiotherapy. Reproduced with permission [166]. Copyright 2022, KeAi Communications Co. Expression level of CD40 (D) and CD206 (E) on bone-marrow-derived macrophage cells that treated with different groups. All the data represented as mean ± SD, n = 3. (F) Application of CpG-Lox-DPNFs vaccines composed of CpG, loxoribine, and co-loaded DNA-polymer hybrid nanocomplex in synergistic reprogramming tumor immune microenvironment for efficient cancer immunotherapy. Reproduced with permission [234]. Copyright 2024, Elsevier.
Furthermore, surface markers on macrophages, particularly on TAM, are being targeted for phenotype reprogramming in cancer immunotherapy. For instance, overexpression of placental growth factor (PIGF) and vascular endothelial growth factor (VEGF) on M2-TAM and breast cancer cells led to their synergistic down-regulation, repolarization of M2-TAM as well as inhibiting breast cancer cell proliferation [235,236]. Similarly, a specialized nanoplatform referred to as PEG = MT/PC NPs (PEI, and mannose doubly modified trimethyl chitosan (PEG = MT) along with citraconic anhydride grafted poly (allylamine hydrochloride) (PC)) is engineered to facilitate deliveries of VEGF siRNA (siVEGF) and PIGF siRNA (siPIGF) to M2-TAM, thereby reprogramming of TAM [237]. Vaccination with miR155-loaded of detachable PEG-PLL/galactose-PLL-poly(l-cysteine) (sPEG/GLC/155) nanovaccines, as an example, not only enhanced expression of miR155 and M1-TAM markers like IL-2 and MHC II but also suppressed expressions of M2-TAM markers such as arginase 1 and macrophage scavenger receptor 2 [238]. Furthermore, an innovative “two-in-one” nanovaccine formulation, incorporating a legumain-derived antigenic sequence (a TAM biomarker) and trichosanthin, effectively stimulates cytokine secretion and initiates antitumor immunity by specifically targeting TAM and orchestrating a transformation in TME [239,240].
Essentially, the focused modulation of TAM within TME is crucial for enhancing efficacy of the nanovaccine-based cancer immunotherapies. This section has highlighted the significance of selectively inhibiting M2 type TAMs while promoting activation of M1 type TAMs, a strategy that directly addresses immunosuppressive nature of TME. The successful application of this approach relies on the precise engineering of nanovaccines to target specific TAM receptors and signaling pathways, thereby reprogramming TAM phenotypes to support anti-tumor immunity. However, challenges remain in achieving selective targeting without affecting systemic immune response and in understanding dynamic interactions within TME. Future research must focus on overcoming these hurdles to fully realize the potential of TAM-modulating nanovaccines in cancer immunotherapy.
3.3.2. Modulating tumor-associated neutrophil strategies
Neutrophils, the most abundant circulating leukocytes in humans, are a type of myeloid cells derived from the bone marrow, characterized by their short half-life and multilobed nuclear morphology [[241], [242], [243]]. They are crucial players in tumor progression, especially within TME, and like TAM, TAN shows significant plasticity [244,245]. This variability is influenced by tissue types and specific molecular cues of their environment [246,247]. In the context of cancer immunotherapy, manipulating TAN polarization, which means transitioning the pro-tumor N2 phenotype to the anti-tumor N1 phenotype, is a key therapeutic target [248]. Custom-designed nanovaccines have been developed to induce this polarization [249]. For instance, Fe(III)-captopril-based nanovaccines had successfully repolarized TAN in a hepatocellular carcinoma model [168]. Experimental results revealed that captopril was activated within the acidic TME, facilitating conversion of protumoral TAN into an antitumor phenotype. This transformation led to significant immune effects, resulting in complete tumor regression (83%) in H22 tumor-bearing mice. Additionally, Bacillus Calmette-Guérin has also shown potential benefits in modulating TAN function [250]. Although TAN are promising targets in cancer immunotherapy, further research is needed to understand molecular mechanisms for effectively targeting TAN with the customized nanovaccines.
3.3.3. Modulating myeloid-derived suppressor cell strategies
MDSC constitute another pivotal class of immunosuppressive immune cells. Numerous high-quality studies, encompassing both preclinical and clinical research, have elucidated the strong correlation between abundant infiltration of MDSC within TME and unfavorable patient prognosis [181]. Recent years have witnessed significant advancements in development of the inhibitory agent-based nanovaccine targeting MDSC at tumor sites. These advancements are grounded in various mechanisms, such as deactivation or depletion of MDSC, inhibition of MDSC development, and induction of MDSC differentiation into mature cells, among others [251]. Notably, FDA's recent approval of the phosphodiesterase 5 inhibitor TAD has shown promise in enhancing antitumor immunity by inhibiting MDSC function and revitalizing T-cell responses [252]. In this context, a supramolecular TAD nanovaccine has been developed to mitigate MDSC-induced suppression and enhance immunogenicity (Fig. 8) [169]. These TAD-based vaccines are formulated by assembling indocyanine green and TAD in presence of Fe3+ to form FIT NPs (Fig. 8A). The FIT NPs trigger ICD and relieve immunosuppressive activity of MDSC by releasing TAD at tumor sites, a process validated by observed apoptosis of MDSC (Fig. 8B−D). Besides, other strategies like manipulating chemokine like CCL2 via CCAAT/enhancer-binding protein beta pathways or employing low-dose gemcitabine-loaded lipid nanocapsules, have been effective in targeting MDSC, thus enhancing cancer immunotherapy by modulating TME [170,171]. Despite these advancements, further exploration in MDSC-targeting vaccines is needed to develop more effective nanovaccines to reshape TME and enhance antitumor efficacy [181].
Fig. 8.
Deductive procedure of FIT nanovaccine for cancer immunotherapy. (A) Preparation process of FIT nanoparticle, mechanism of MDSC regulation, ICD induction process, and dual-imaging medicated enhanced cancer immunotherapy. In vitro cellular experiment with FIT NP, (B) confocal laser scanning microscopy images of CT26 multicellular spheroids treated with different samples for 6 h and stained with FDA (green) and propidium iodide (red). (C) Flow cytometry and (D) qualitative analysis of MDSC in tumors (n = 3; *P < 0.05, **P < 0.01). Reproduced with permission [169]. Copyright 2021, Wiley.
3.3.4. Modulating invariant natural killer T cell strategies
NKT cells divide into different types (I and II) as follows: Type II includes variant NKT cells identified by sulfatide/CD1d-tetramers [[253], [254], [255]], whereas type I includes as invariant NKT cells that communicate with a semi-invariant TCR (Vα14Jα18 gene segment paired with Vβ2, Vβ7, and Vβ8.2 in mice and Vα24Jα18 paired with Vβ11 in humans) [256,257]. These NKT cells function as a bridge between adaptive and innate immune responses, influencing DC maturation, and activating downstream immune effectors such as B cells, NK cells, CD4+ T cells, and CD8+ T cells [258]. Since they are recognized as both self and foreign by DC via the MHC I-like molecule CD1 (CD1-9 TCR ligand), type I NKT cells have attracted significant attention, and are activated by glycosphingolipid such as GalCer [172,258,259]. GalCer-based nanovaccines modified with melanoma peptides and TLR ligands suppress melanoma [172]. These nanovaccines enhance infiltrations of NKT and NK cells, and promote high-level secretions of INF-γ and IL-4. It is anticipated that development of more tailored nanovaccines targeting NK cells will be a focus of future research endeavors in this field.
3.3.5. Depletion of regulatory T cells
Treg cells, known for their immunosuppressive role, are prevalent in TME, where they impede antitumor immunity, contributing to tumor progression [260]. These cells are chemotactically attracted to the TME via specific chemokine gradients like CCR4-CCL17/22 and CCR8-CCL1, among others [261]. Their high infiltration correlates with poor prognosis in various cancers. Treg cells suppress immune responses through mechanisms such as inhibition of co-stimulatory signals, interleukin-2 consumption, and metabolic modulation [262]. Consequently, cancer immunotherapy focuses on depleting Treg cells or modulating their functions to enhance antitumor responses. Targeted therapies include antibodies or small molecules against Treg-specific markers like immune checkpoint molecules, chemokine receptors, metabolites, etc., with aim of strengthening antitumor effects while avoiding systemic autoimmunity. Combinational cancer immunotherapy involving imatinib (IMT)-loaded glucocorticoid-induced TNF receptor family-related protein/PLGA (GITR-PLGA) with IR780 dye has shown promise [173]. IMT triggers signal transducer and activator of transcription- (STAT)3 and STAT5 activation, thereby inhibiting Foxp3 expression and rendering Treg dysfunctionality. Additionally, genetically modified megakaryocytes stably expressing murine PD-1 have been developed to produce PD-1-presenting platelets, capable of carrying cyclophosphamide [263]. This approach not only targets PD-L1 but also depletes Tregs in TME, enhancing antitumor effects of CD8+ T lymphocytes. Furthermore, a combination of PPAR-α agonist fenofibrate, PD-1 antibody, and custom nanovaccines has shown potential in modulating T-cell metabolism and enhancing CTL activity through PD-1 blockade [174]. However, studies indicate that PD-1-blocked Treg cells exhibit reduced immunosuppressive activity, necessitating further research to fully understand the role of PD-1 in effector T cells and Treg cells in cancer contexts [264].
4. Nanovaccines applied in cancer immunotherapies
As discussed previously, nanovaccines have demonstrated capabilities to elicit a robust immune response and activate immunogenicity, owing to their adaptable nano-size ranges and customizable structures [265]. These inherent advantages equip nanovaccines with diverse functionalities, allowing them to effectively-target immune cells and find applications in immunotherapies. These vaccines under consideration can be categorized as prophylactic, intended to avert future infections, or therapeutic, to treat existing diseases, including cancer and/or bacterial diseases. Prophylactic nanovaccines are designed to bolster immunogenic memory and trigger both antibody-mediated and cell-mediated immune responses. For instance, nanovaccines had been demonstrated a significant potential for preventing infectious diseases like acquired immunodeficiency syndrome, malaria, tuberculosis, influenza, and cancer [202]. On the therapeutic front, custom-designed nanovaccines induce accumulation and expression of exogenous or neoantigens at tumor sites, curtailing tumor growth. Studies suggest that nanovaccines adeptly modulate the immune system, offering targeted preventive or therapeutic interventions for various pathological conditions. While prophylactic nanovaccines primarily focus on infection prevention, therapeutic nanovaccines are increasingly utilized in cancer immunotherapy.
4.1. Prophylactic functionalities
Despite progress in vaccine development, creation of effective vaccines for viruses like HIV and influenza is still a work in progress [266]. Coronavirus disease 2019 (COVID-19) pandemic has accelerated development of viral vaccines, although there's a risk of these vaccines reverting to a pathogenic form [267]. Prophylactic vaccines are given prior to onset of a disease to establish long-lasting immunogenic memory against infections [126]. In response to these challenges, bespoke nanovaccines, have shown promise, enhancing both antibody-mediated and cell-mediated immune responses. There's growing recognition of efficacy of CD8+ T cell-mediated cellular immunity in combating viruses and providing extended protection beyond traditional focused on neutralizing antibodies [268]. This form of immunity targets eradicating viral infections within the body, while humoral immunity works by neutralizing the viruses in bodily fluids by producing soluble antibodies.
The COVID-19 pandemic, caused by SARS-CoV-2, has rapidly spread globally, leading to significant mortality [269]. Vaccination remains the most effective defense against COVID-19, with multiple innovative approaches being developed. One example was the SARS-CoV-2 DLNP vaccine, which was shown robust immunities in various animal models and offered single-shot protection against lethal challenges [270]. Another prophylactic nanovaccines involved formulations of the virus's S1 subunit and dual adjuvants, successfully eliciting robust humoral immune responses and potent IgA antibodies in mice [271]. Dual-adjuvant systems have been shown to enhance systemic and lung immunities by combining MnOx and CpG with a dimeric antigen version of the virus's receptor-binding domain [272]. Additionally, several nanoadjuvants like selenium [273], manganese [274], and antigen-caged-adjuvants [275], have also been employed in developing subunit vaccines for SARS-CoV-2. Innovative inhalable nanovaccines, such as biomimetic coronavirus structure-based [276] and chitosan-based, have also been reported [277]. Interestingly, the Bacillus Calmette-Guerin (BCG) vaccine has shown effectiveness against COVID-19, being attributed to its nonspecific protective effects against respiratory infections [278]. Research indicated that BCG induced a robust biphasic innate immune response and antigen-specific Th1 cell response in lungs [279]. In mouse models, BCG also protected against weight loss following infection with SARS-CoV-2 BA.5, SARS-CoV, and SHC014 coronaviruses. Overall, nanovaccines demonstrate significant potential in eliciting durable immune responses against pathogen infections or re-infections, with biosafety being a crucial consideration, especially for clinical preventive applications.
4.2. Therapeutic functionalities
Therapeutic nanovaccines have shown promising potential in cancer treatment, particularly in development of highly potent foreign non-self-antigens (TSA), which are overexpressed at specific tumor sites but minimally presented in normal tissues [265]. For instance, an intertwining DNA-RNA nanocapsule (referred to as iDR-NCs) has been demonstrated favorable prospects in cancer immunotherapy [155]. The iDR-NCs nanovaccines efficiently delivered APC in draining LN, inducing durable neoantigen-specific T cell responses and significantly inhibiting progression of neoantigens-specific colorectal tumors. These neoantigen-based nanovaccines exhibited robust cancer immunotherapy, as evidenced by the syngeneic C57BL/6 mice bearing MC38 syngeneic colon adenocarcinoma tumor models. Similarly, a dual adjuvant-based nanoformulation (R848 and CpG) for colorectal cancer, combining neoantigens and adjuvants, has enhanced immunogenicity of neoantigens and reduced acute systemic toxicity [280]. Personalized neoantigens nanovaccines have also shown promise in treatment of advanced pancreatic cancer [281].
Although cancer therapy faces numerous limitations, therapeutic nanovaccines offer effective alternatives or complements to conventional treatments, especially in combination with other modalities such as ICB therapy, PTT, PDT, chemotherapy, etc. [[282], [283], [284], [285], [286]] PD-1 checkpoint blockade immunotherapy is the primary method and synergistic with nanovaccines for cancer immunotherapy [287]. Su et al. reported on nanovaccines engineered to enhance efficacies of ICB, aiming to broaden repertoire of antitumor cells and increasing immune checkpoint expression levels to sensitize ICB [35]. These vaccines, by co-delivering bi-adjuvants (R848 and CpG) along with TSA to LN, successfully induced enduring antigen-specific T cell responses and immune memory. When combined with ICB, these nanovaccines demonstrate significant therapeutic effects in different mouse tumor models [288,289]. Besides, tandem nanovaccines with PD-1 checkpoint blockade immunotherapy have also shown remarkable therapeutic effects in diverse cancer models, particularly when integrations of RT [89], chemo-immunotherapy [290], ultrasound [291], PTT [292], and PDT [293]. Although therapeutic nanovaccines have demonstrated immense potential through formulation of effective antigens and their synergistic interactions with existing therapies, therapeutic efficacies of nanovaccines across various models and practical considerations of treatment strategies in clinical applications necessitate further assessment.
5. Conclusions and perspectives
The last decade has witnessed a shift from a focus on cytotoxic anti-cancer therapies towards approaches that increase anti-tumor immunity, as emerging immunotherapy shows promise in extending survival of patients [294]. As a subtype of cancer immunotherapy, nano-based cancer vaccines have gained significant momentum owing to their advantage of size and proximity to pathogens as well as their controllable physicochemical and biophysical properties. A remarkable characteristic of nanovaccines lies in their exceptional diversity and tunability, which is engineered with various morphologies such as hollow, core-shell, nanosheet, micelle, nanogel, vesicle-like, nanodisc, and virus-like [295]. These inherent characteristics bestow nanovaccines with notable targeting capabilities, prolonged bio-persistence, highly efficient transport capabilities, safeguarding of antigens against degradation, and potential for long-term therapeutic efficacy. Individualized nanovaccines, when laden with antigens and/or adjuvants, tend to accumulate within LN via LV. This distinctive accumulation pattern serves as a mitigation strategy against risks linked to leaky tumor vasculature, impaired lymphatic drainage, and potential off-target effects [296]. Furthermore, tailored targeting nanovaccines are encapsulated or conjugated with antigens and/or adjuvants that exhibit targeting recognition capabilities for immune cells. As a result, a diverse range of nanovaccines has been meticulously-designed and deployed to passively or actively target different immune cell populations [297]. This strategic targeting aims to enhance antigen presentation and modulate immunosuppression upon accumulation within LN. Consequently, these nanovaccines contribute to eradication of tumor cells, augmentation of the immune response, mitigation of TME resistance development, and inhibition of cancer recurrence [298]. Considering complexity of the TME during tumor development, prophylactic and/or therapeutic nanovaccines are always combined with radiotherapy, ICB, PDT, PTT, and other therapeutic modalities to further magnify immune responses and generate sufficient long-lasting immune memory [266,292,299,300]. Overall, nanovaccines with design flexibility have numerous inherent advantages as they are tailored to target various immune cells. These attributes not only position nanovaccines as a viable substitute for conventional cancer treatments but also establish them as valuable clinical tools for screening vaccines tailored to different types of cancer.
While nanovaccines represent a promising frontier in the field of personalized vaccines, there remain several formidable challenges that need to be addressed before their successful translation into clinical practice [288]. In nanovaccine formulation, it requires a precise architecture for effective immune cell engagement and stability, ensuring antigen to release and targeting circulating immune cells [16]. An integrated nanovaccine system, incorporating all vital components, is key to achieving varied therapeutic and diagnostic objectives in cancer treatment. In terms of nanovaccine fabrication, meticulous consideration and assessment of streamlined synthesis processes for purification of nanovaccines, coupled with minimizing use of organic solvents, are imperative. Synergy effects between immunotherapeutic agents and targeting carriers, in aspects of dosage and classification, are critical in securing efficacious therapeutic results. Second, toxicity assessment of the nanovaccines is currently in an incipient stage, and the long-term safety profile of these engineered nanovaccines demands further evaluation. Special attention should be directed towards potential emergence of the autoimmune responses. It is imperative to recognize that physicochemical properties of the nanoparticles may undergo alterations as they interact with various biological entities within human bodies; hence, an exhaustive examination of the final metabolites is warranted. Furthermore, there is a major hurdle to immunogenicity of the nanomaterial-based vaccines [301]. Nanovaccines exhibit antigenic characteristics but often face swift clearance prior to manifestation of their anti-tumor therapeutic efficacy. Not only that, a vigorous immune response may inadvertently precipitate severe complications, such as hemolysis and thrombogenesis [302]. Finally, limited knowledge of immune networks during tumorigenesis hinders widespread applications of the nanovaccines in cancer immunotherapy. Innate and adaptive immune systems are complex networks, and the influence of depletion or inhibition of one component on the entire network remains controversial [303].
Taken together, research progress in customized targeting nanovaccines lays a theoretical foundation for further development of novel cancer therapy options, and many excellent studies are exploring their potential in targeting and regulating/training immune cells. In the future, refinement of the nanovaccine design is likely to necessitate a closer alignment with clinical translation and the establishment of collaborative immune responses. This endeavor aims to enhance patient compliance by integrating diverse components (such as biepitope antigens) within the same nanosystem [304]. This multifaceted approach seeks to achieve precise targeting of distinct elements, controlled release mechanisms, and tailored treatments at various stages of the disease. Nanovaccine development is also set to be shaped by key advancements in precise immunomodulation, focusing on targeted interventions within TME to mitigate systemic toxicity. Exploration of the therapeutic synergies, combining nanovaccines with existing treatments like radiation therapy and checkpoint inhibitors, promises to amplify immune responses and enhance efficacy. Technological innovations, such as mRNA-based and STING-activating nanovaccines, are pivotal for improving vaccine performance and patient compliance. Critical to this progress is the clinical translation of nanovaccines, necessitating rigorous safety evaluations and the development of scalable, stable formulations. Moreover, addressing economic and accessibility barriers will broaden patient access to these advanced therapies. Collectively, vaccine nanotechnology has been demonstrated with significant promise in experimental investigations. Synergistic integration of expertise from nanomaterial science, immunology, virology, oncology, and pharmaceutical sector is pivotal for advancing clinical translation and implementation of nanovaccines. This collaborative approach is essential for effectively addressing and managing a spectrum of formidable infectious diseases and malignancies.
Ethical statement
Hereby, I Prof. Jin Zhang, consciously assure that for the manuscript entitled “Engineering customized nanovaccines for enhanced cancer immunotherapy” the following is fulfilled:
-
1)
This material is the authors' own original work, which has not been previously published elsewhere.
-
2)
The paper is not currently being considered for publication elsewhere.
-
3)
The paper reflects the authors' own research and analysis in a truthful and complete manner.
-
4)
The paper properly credits the meaningful contributions of coauthors and co-researchers.
-
5)
The results are appropriately placed in the context of prior and existing research.
-
6)
All sources used are properly disclosed (correct citation). Literally copying of text must be indicated as such by using quotation marks and giving proper reference.
-
7)
All authors have been personally and actively involved in substantial work leading to the paper, and will take public responsibility for its content.
CRediT authorship contribution statement
Jinyu Guo: Writing – review & editing, Writing – original draft, Supervision, Investigation, Formal analysis, Conceptualization. Changhua Liu: Writing – review & editing, Methodology. Zhaoyang Qi: Validation. Ting Qiu: Supervision. Jin Zhang: Supervision, Funding acquisition, Conceptualization. Huanghao Yang: Supervision.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgments
This work was financially supported by the National Key R&D Program of China (Grant No. 2022YFB3808000/2022YFB3808001; Grant No. 2021YFC2400600/2021 YFC2400604), the Medical Innovation Project of Science and Technology Program of Fujian Provincial Health Commission (Grant No. 2021CXA006), the Project for High-Level Talent Innovation and Entrepreneurship of Quanzhou (Grant No. 2022C016R), and the Key Program of Qingyuan Innovation Laboratory (Grant No. 00221002). The authors also gratefully acknowledged the kind financial support provided by the Top Young Talents of Foal Eagle Program of Fujian Province and the Youth Promotion Talent Project of Fujian Province to Jin Zhang.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Contributor Information
Jin Zhang, Email: J_Zhang929@fzu.edu.cn.
Huanghao Yang, Email: hhyang@fzu.edu.cn.
References
- 1.McCarthy E.F. The toxins of William B. Coley and the treatment of bone and soft-tissue sarcomas. Lowa Orthop. J. 2006;26:154. [PMC free article] [PubMed] [Google Scholar]
- 2.Nam J., Son S., Park K.S., et al. Cancer nanomedicine for combination cancer immunotherapy. Nat. Rev. Mater. 2019;(4):398–414. [Google Scholar]
- 3.Ribas A., Haining W.N., Schumacher T.N.M. When cancer cells become the enablers of an antitumor immune response. Cancer Discov. 2022;(12):2244–2248. doi: 10.1158/2159-8290.CD-22-0706. [DOI] [PubMed] [Google Scholar]
- 4.Schuster M., Nechansky A., Kircheis R. Cancer immunotherapy. Biotechnol. J.: Healthcare, Nutrition, Technology. 2006;(1):138–147. doi: 10.1002/biot.200500044. [DOI] [PubMed] [Google Scholar]
- 5.Saxena M., van der Burg S.H., Melief C.J.M., et al. Therapeutic cancer vaccines. Nat. Rev. Cancer. 2021;(21):360–378. doi: 10.1038/s41568-021-00346-0. [DOI] [PubMed] [Google Scholar]
- 6.Want M.Y., Bashir Z., Najar R.A. T cell based immunotherapy for cancer: approaches and strategies. Vaccines. 2023;(11):835. doi: 10.3390/vaccines11040835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Y. Nanotechnology for next-generation cancer immunotherapy: state of the art and future perspectives. J. Contr. Release. 2023;356:14–25. doi: 10.1016/j.jconrel.2023.02.016. [DOI] [PubMed] [Google Scholar]
- 8.Cheever M.A., Higano C.S. PROVENGE (Sipuleucel-T) in prostate cancer: the first FDA-approved therapeutic cancer vaccine therapeutic prostate cancer vaccine. Clin. Cancer Res. 2011;17:3520–3526. doi: 10.1158/1078-0432.CCR-10-3126. [DOI] [PubMed] [Google Scholar]
- 9.Immunoengineering M.S. Goldberg. How nanotechnology can enhance cancer immunotherapy. Cell. 2015;161:201–204. doi: 10.1016/j.cell.2015.03.037. [DOI] [PubMed] [Google Scholar]
- 10.Ding D., Jiang X. Advances in immunogenic cell death for cancer immunotherapy. Small Methods. 2023;(7) doi: 10.1002/smtd.202300354. [DOI] [PubMed] [Google Scholar]
- 11.Wang Q.T., Liu Y.X., Wang J., et al. Advances in cancer nanovaccines: harnessing nanotechnology for broadening cancer immune response. ChemMedChem. 2023;18 doi: 10.1002/cmdc.202200673. [DOI] [PubMed] [Google Scholar]
- 12.Nakamura T., Harashima H. Dawn of lipid nanoparticles in lymph node targeting: potential in cancer immunotherapy. Adv. Drug Deliv. Rev. 2020;167:78–88. doi: 10.1016/j.addr.2020.06.003. [DOI] [PubMed] [Google Scholar]
- 13.Li Y., Li S., Jiang Z., et al. Targeting lymph node delivery with nanovaccines for cancer immunotherapy: recent advances and future directions. J. Nanobiotechnol. 2023;21:212. doi: 10.1186/s12951-023-01977-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Q., Wang Z., Sun X.X., et al. Lymph node-targeting nanovaccines for cancer immunotherapy. J. Contr. Release. 2022;351:102–122. doi: 10.1016/j.jconrel.2022.09.015. [DOI] [PubMed] [Google Scholar]
- 15.Zhang H., Zhang Y., Hu H., et al. In situ tumor vaccine for lymph nodes delivery and cancer therapy based on small size nanoadjuvant. Small. 2023;19 doi: 10.1002/smll.202301041. [DOI] [PubMed] [Google Scholar]
- 16.Liu J., Zhang R., Xu Z.P. Nanoparticle-based nanomedicines to promote cancer immunotherapy: recent advances and future directions. Small. 2019;15 doi: 10.1002/smll.201900262. [DOI] [PubMed] [Google Scholar]
- 17.Mant A., Chinnery F., Elliott T., et al. The pathway of cross-presentation is influenced by the particle size of phagocytosed antigen. Immunology. 2012;136:163–175. doi: 10.1111/j.1365-2567.2012.03558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carmona-Ribeiro A.M., Pérez-Betancourt Y. Cationic nanostructures for vaccines design. Biomimetics. 2020;(5):32. doi: 10.3390/biomimetics5030032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou J.R., Kroll A.V., Holay M., et al. Biomimetic nanotechnology toward personalized vaccines. Adv. Mater. 2019;32 doi: 10.1002/adma.201901255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Niikura K., Matsunaga T., Suzuki T., et al. Gold nanoparticles as a vaccine platform: influence of size and shape on immunological responses in vitro and in vivo. ACS Nano. 2013;7:3926–3938. doi: 10.1021/nn3057005. [DOI] [PubMed] [Google Scholar]
- 21.Ochyl L.J., Bazzill J.D., Park C., et al. PEGylated tumor cell membrane vesicles as a new vaccine platform for cancer immunotherapy. Biomaterials. 2018;(182):157–166. doi: 10.1016/j.biomaterials.2018.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson L., Duschl A., Himly M. Nanotechnology-based vaccines for allergen-specific immunotherapy: potentials and challenges of conventional and novel adjuvants under research. Vaccines. 2020;(8):237. doi: 10.3390/vaccines8020237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xekouki K., Lagopati N., Demetzos C., et al. A mini review for lipid-based nanovaccines: from their design to their applications. J. Liposome Res. 2023;33:214–233. doi: 10.1080/08982104.2023.2170408. [DOI] [PubMed] [Google Scholar]
- 24.Fan H., Guo Z. Tumor microenvironment-responsive manganese-based nanomaterials for cancer treatment. Coord. Chem. Rev. 2023;480 [Google Scholar]
- 25.Shi W., Feng W., Li S., et al. Ferroptosis and necroptosis produced autologous tumor cell lysates co-delivering with combined immnoadjuvants as personalized in situ nanovaccines for antitumor immunity. ACS Nano. 2023;17:14475–14493. doi: 10.1021/acsnano.3c00901. [DOI] [PubMed] [Google Scholar]
- 26.Su Y., Xu W., Wei Q., et al. Chiral polypeptide nanoparticles as nanoadjuvants of nanovaccines for efficient cancer prevention and therapy. Sci. Bull. 2023;68:284–294. doi: 10.1016/j.scib.2023.01.024. [DOI] [PubMed] [Google Scholar]
- 27.Zhou X., Su Q.H., Zhao H.W., et al. Metal-phenolic network-encapsulated nanovaccine with pH and reduction dual responsiveness for enhanced cancer immunotherapy. Mol. Pharm. 2020;17:4603–4615. doi: 10.1021/acs.molpharmaceut.0c00802. [DOI] [PubMed] [Google Scholar]
- 28.Wang J.X., Shen S., Li J., et al. Precise depletion of tumor seed and growing soil with shrinkable nanocarrier for potentiated cancer chemoimmunotherapy. ACS Nano. 2021;15:4636–4646. doi: 10.1021/acsnano.0c08996. [DOI] [PubMed] [Google Scholar]
- 29.Xie C., You X., Zhang H., et al. A nanovaccine based on adjuvant peptide FK-13 and L-phenylalanine poly(ester amide) enhances CD8+ T cell-mediated antitumor immunity. Adv. Sci. 2023;10 doi: 10.1002/advs.202300418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li D., Li W., Zheng P., et al. A “trained immunity” inducer-adjuvanted nanovaccine reverses the growth of established tumors in mice. J. Nanobiotechnol. 2023;21:74. doi: 10.1186/s12951-023-01832-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Su R., Gu J., Sun J., et al. CaCO3 powder-mediated biomineralization of antigen nanosponges synergize with PD-1 blockade to potentiate anti-tumor immunity. J. Nanobiotechnol. 2023;21:120. doi: 10.1186/s12951-023-01870-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.El-Sayed N., Korotchenko E., Scheiblhofer S., et al. Functionalized multifunctional nanovaccine for targeting dendritic cells and modulation of immune response. Int. J. Pharm. 2021;593 doi: 10.1016/j.ijpharm.2020.120123. [DOI] [PubMed] [Google Scholar]
- 33.Qiao N., Wang H., Xu Y., et al. A MnAl double adjuvant nanovaccine to induce strong humoral and cellular immune responses. J. Contr. Release. 2023;358:190–203. doi: 10.1016/j.jconrel.2023.04.036. [DOI] [PubMed] [Google Scholar]
- 34.Cheng F., Su T., Zhou S., et al. Single-dose injectable nanovaccine-in-hydrogel for robust immunotherapy of large tumors with abscopal effect. Sci. Adv. 2023;9 doi: 10.1126/sciadv.ade6257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Su T., Liu X., Lin S., et al. Ionizable polymeric nanocarriers for the codelivery of bi-adjuvant and neoantigens in combination tumor immunotherapy. Bioact. Mater. 2023;(26):169–180. doi: 10.1016/j.bioactmat.2023.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Diao L., Liu M. Rethinking antigen source: cancer vaccines based on whole tumor cell/tissue lysate or whole tumor cell. Adv. Sci. 2023;10 doi: 10.1002/advs.202300121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van Herck S., Feng B., Tang L. Delivery of STING agonists for adjuvanting subunit vaccines. Adv. Drug Deliv. Rev. 2021;179 doi: 10.1016/j.addr.2021.114020. [DOI] [PubMed] [Google Scholar]
- 38.Hopfner K.P., Hornung V. Molecular mechanisms and cellular functions of cGAS-STING signalling. Nat. Rev. Mol. Cell Biol. 2020;21:501–521. doi: 10.1038/s41580-020-0244-x. [DOI] [PubMed] [Google Scholar]
- 39.Boyaka P.N., McGhee J.R. Cytokines as adjuvants for the induction of mucosal immunity. Adv. Drug Deliv. Rev. 2001;51:71–79. doi: 10.1016/s0169-409x(01)00170-3. [DOI] [PubMed] [Google Scholar]
- 40.Bukczynski J., Wen T., Ellefsen K., et al. Costimulatory ligand 4-1BBL (CD137L) as an efficient adjuvant for human antiviral cytotoxic T cell responses. Proc. Natl. Acad. Sci. USA. 2004;101:1291–1296. doi: 10.1073/pnas.0306567101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Uppu D.S., Turvey M.E., Sharif A.R.M., et al. Temporal release of a three-component protein subunit vaccine from polymer multilayers. J. Contr. Release. 2020;317:130–141. doi: 10.1016/j.jconrel.2019.11.022. [DOI] [PubMed] [Google Scholar]
- 42.Ding Y., Yang J., Wei H., et al. Construction of pH-sensitive nanovaccines encapsulating tumor cell lysates and immune adjuvants for breast cancer therapy. Small. 2023;19 doi: 10.1002/smll.202301420. [DOI] [PubMed] [Google Scholar]
- 43.Huang J., Leng X., Jiang T., et al. Oxygen-carrying nanoplatform to reprogram tumor immunosuppressive microenvironment and enhance photothermal-immunotherapy. Mater. Today Bio. 2023;19 doi: 10.1016/j.mtbio.2023.100555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Banete A., Gee K., Basta S. Sustained IL-4 priming of macrophages enhances the inflammatory response to TLR7/8 ligand R848. J. Leukoc. Biol. 2022;111:401–413. doi: 10.1002/JLB.3A0520-293RR. [DOI] [PubMed] [Google Scholar]
- 45.Pires I.S., Ni K., Melo M.B., et al. Controlled lipid self-assembly for scalable manufacturing of next-generation immune stimulating complexes. Chem. Eng. J. 2023;464 doi: 10.1016/j.cej.2023.142664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jung M., Lee S., Park S., et al. A therapeutic nanovaccine that generates anti-amyloid antibodies and amyloid-specific regulatory T cells for alzheimer's disease. Adv. Mater. 2023;35 doi: 10.1002/adma.202207719. [DOI] [PubMed] [Google Scholar]
- 47.Carreno B.M., Collins M. The B7 family of ligands and its receptors: new pathways of costimulation and inhibition of immune responses. Annu. Rev. Immunol. 2002;20:29. doi: 10.1146/annurev.immunol.20.091101.091806. [DOI] [PubMed] [Google Scholar]
- 48.Shao K., Singha S., Casares X.C., et al. Nanoparticle-based immunotherapy for cancer. ACS Nano. 2015;9:16–30. doi: 10.1021/nn5062029. [DOI] [PubMed] [Google Scholar]
- 49.Yang M., Li J., Gu P., et al. The application of nanoparticles in cancer immunotherapy: targeting tumor microenvironment. Bioact. Mater. 2021;(6):1973–1987. doi: 10.1016/j.bioactmat.2020.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang W., Chen X., Li J., et al. Hollow MnO2 nanoparticles loaded with functional genes as nanovaccines for synergistic cancer therapy. ACS Appl. Nano Mater. 2022;(5):10537–10547. [Google Scholar]
- 51.Tan J., Ding B., Zheng P., et al. Hollow aluminum hydroxide modified silica nanoadjuvants with amplified immunotherapy effects through immunogenic cell death induction and antigen release. Small. 2022;18 doi: 10.1002/smll.202202462. [DOI] [PubMed] [Google Scholar]
- 52.Huang C.L., Zhang L., Guo Q., et al. Robust nanovaccine based on polydopamine-coated mesoporous silica nanoparticles for effective photothermal-immunotherapy against melanoma. Adv. Funct. Mater. 2021;31 [Google Scholar]
- 53.Li Z., Liu Z., Yin M., et al. Combination delivery of antigens and CpG by lanthanides-based core-shell nanoparticles for enhanced immune response and dual-mode imaging. Adv. Healthcare Mater. 2013;(2):1309–1313. doi: 10.1002/adhm.201200364. [DOI] [PubMed] [Google Scholar]
- 54.Hu Y., Lin L., Chen J., et al. Synergistic tumor immunological strategy by combining tumor nanovaccine with gene-mediated extracellular matrix scavenger. Biomaterials. 2020;(252) doi: 10.1016/j.biomaterials.2020.120114. [DOI] [PubMed] [Google Scholar]
- 55.Su R., Chong G., Dong H., et al. Nanovaccine biomineralization for cancer immunotherapy: a NADPH oxidase-inspired strategy for improving antigen cross-presentation via lipid peroxidation. Biomaterials. 2021;(277) doi: 10.1016/j.biomaterials.2021.121089. [DOI] [PubMed] [Google Scholar]
- 56.Wagner J., Gossl D., Ustyanovska N., et al. Mesoporous silica nanoparticles as pH-responsive carrier for the immune-activating drug resiquimod enhance the local immune response in mice. ACS Nano. 2021;15:4450–4466. doi: 10.1021/acsnano.0c08384. [DOI] [PubMed] [Google Scholar]
- 57.Song X.R., Zhou X.Y., Pan Y.F., et al. Schottky heterojunction realizes in situ vaccine-like antitumor efficacy and microenvironment remodeling upon near-infrared laser response in cold tumors. Adv. Funct. Mater. 2023;33 [Google Scholar]
- 58.Sun Z., Fan T.J., Liu Q., et al. Autologous tumor antigens and boron nanosheet-based nanovaccines for enhanced photo-immunotherapy against immune desert tumors. Nanophotonics. 2021;10:2519–2535. [Google Scholar]
- 59.Zhang L.X., Jia Y.B., Huang Y.R., et al. Efficient delivery of clay-based nanovaccines to the mouse spleen promotes potent anti-tumor immunity for both prevention and treatment of lymphoma. Nano Res. 2021;14:1326–1334. [Google Scholar]
- 60.Li W.H., Wu J.J., Wu L., et al. Black phosphorous nanosheet: a novel immune-potentiating nanoadjuvant for near-infrared-improved immunotherapy. Biomaterials. 2021;273 doi: 10.1016/j.biomaterials.2021.120788. [DOI] [PubMed] [Google Scholar]
- 61.Li C.X., Zhang X.X., Chen Q., et al. Synthetic polymeric mixed micelles targeting lymph nodes trigger enhanced cellular and humoral immune responses. ACS Appl. Mater. Interfaces. 2018;(10):2874–2889. doi: 10.1021/acsami.7b14004. [DOI] [PubMed] [Google Scholar]
- 62.Liu Z.D., Zhou C., Qin Y., et al. Coordinating antigen cytosolic delivery and danger signaling to program potent cross-priming by micelle-based nanovaccine. Cell Discov. 2017;(3) doi: 10.1038/celldisc.2017.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Luo Z., Li P., Deng J., et al. Cationic polypeptide micelle-based antigen delivery system: a simple and robust adjuvant to improve vaccine efficacy. J. Contr. Release. 2013;170:259–267. doi: 10.1016/j.jconrel.2013.05.027. [DOI] [PubMed] [Google Scholar]
- 64.Yan P., Luo Y., Li X.Y., et al. A redox-responsive nanovaccine combined with A2A receptor antagonist for cancer immunotherapy. Adv. Healthcare Mater. 2021;10 doi: 10.1002/adhm.202101222. [DOI] [PubMed] [Google Scholar]
- 65.Jia F., Huang W., Yin Y., et al. Stabilizing RNA nanovaccines with transformable hyaluronan dynamic hydrogel for durable cancer immunotherapy. Adv. Funct. Mater. 2023;33 [Google Scholar]
- 66.Yin Y., Li X., Ma H., et al. In situ transforming RNA nanovaccines from polyethylenimine functionalized graphene oxide hydrogel for durable cancer immunotherapy. Nano Lett. 2021;21:2224–2231. doi: 10.1021/acs.nanolett.0c05039. [DOI] [PubMed] [Google Scholar]
- 67.Li P., Luo Z., Liu P., et al. Bioreducible alginate-poly(ethylenimine) nanogels as an antigen-delivery system robustly enhance vaccine-elicited humoral and cellular immune responses. J. Contr. Release. 2013;168:271–279. doi: 10.1016/j.jconrel.2013.03.025. [DOI] [PubMed] [Google Scholar]
- 68.Stickdorn J., Stein L., Arnold Schild D., et al. Systemically administered TLR7/8 agonist and antigen-conjugated nanogels govern immune responses against tumors. ACS Nano. 2022;16:4426–4443. doi: 10.1021/acsnano.1c10709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu L., Cao F., Liu X., et al. Hyaluronic acid-modified cationic lipid-PLGA hybrid nanoparticles as a nanovaccine induce robust humoral and cellular immune responses. ACS Appl. Mater. Interfaces. 2016;(8):11969–11979. doi: 10.1021/acsami.6b01135. [DOI] [PubMed] [Google Scholar]
- 70.Gu W., An J., Li Y., et al. Tuning the organ tropism of polymersome for spleen-selective nanovaccine delivery to boost cancer immunotherapy. Adv. Mater. 2023 doi: 10.1002/adma.202301686. [DOI] [PubMed] [Google Scholar]
- 71.Liang R., Xie J., Li J., et al. Liposomes-coated gold nanocages with antigens and adjuvants targeted delivery to dendritic cells for enhancing antitumor immune response. Biomaterials. 2017;149:41–50. doi: 10.1016/j.biomaterials.2017.09.029. [DOI] [PubMed] [Google Scholar]
- 72.Zhan G.T., Xu Q.B., Zhang Z.L., et al. Biomimetic sonodynamic therapy-nanovaccine integration platform potentiates anti-PD-1 therapy in hypoxic tumors. Nano Today. 2021;38 [Google Scholar]
- 73.Yang X., Yang Y., Bian J., et al. Converting primary tumor towards an in situ STING-activating vaccine via a biomimetic nanoplatform against recurrent and metastatic tumors. Nano Today. 2021;38 [Google Scholar]
- 74.Chen L., Qin H., Zhao R.F., et al. Bacterial cytoplasmic membranes synergistically enhance the antitumor activity of autologous cancer vaccines. Sci. Transl. Med. 2021;13 doi: 10.1126/scitranslmed.abc2816. [DOI] [PubMed] [Google Scholar]
- 75.Kadiyala P., Li D., Nunez F.M., et al. High-density lipoprotein-mimicking nanodiscs for chemo-immunotherapy against glioblastoma multiforme. ACS Nano. 2019;13:1365–1384. doi: 10.1021/acsnano.8b06842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kuai R., Ochyl L.J., Bahjat K.S., et al. Designer vaccine nanodiscs for personalized cancer immunotherapy. Nat. Mater. 2017;16:489–496. doi: 10.1038/nmat4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hassani Najafabadi A., Zhang J., Aikins M.E., et al. Cancer immunotherapy via targeting cancer stem cells using vaccine nanodiscs. Nano Lett. 2020;20:7783–7792. doi: 10.1021/acs.nanolett.0c03414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Arab A., Nicastro J., Slavcev R., et al. Lambda phage nanoparticles displaying HER2-derived E75 peptide induce effective E75-CD8+ T response. Immunol. Res. 2018;66:200–206. doi: 10.1007/s12026-017-8969-0. [DOI] [PubMed] [Google Scholar]
- 79.Salazar Gonzalez J.A., Ruiz-Cruz A.A., Bustos-Jaimes I., et al. Expression of breast cancer-related epitopes targeting the IGF-1 receptor in chimeric human parvovirus B19 virus-like particles. Mol. Biotechnol. 2019;61:742–753. doi: 10.1007/s12033-019-00198-y. [DOI] [PubMed] [Google Scholar]
- 80.Schwarz B., Morabito K.M., Ruckwardt T.J., et al. Virus-like particles encapsidating respiratory syncytial virus M and M2 proteins induce robust T cell responses. ACS Biomater. Sci. Eng. 2016;(2):2324–2332. doi: 10.1021/acsbiomaterials.6b00532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hess K.L., Medintz I.L., Jewell C.M. Designing inorganic nanomaterials for vaccines and immunotherapies. Nano Today. 2019;27:73–98. doi: 10.1016/j.nantod.2019.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Praetorius N.P., Mandal T.K. Engineered nanoparticles in cancer therapy. Recent Pat. Drug Delivery Formulation. 2007;1:37–51. doi: 10.2174/187221107779814104. [DOI] [PubMed] [Google Scholar]
- 83.Vinardell M.P., Mitjans M. Antitumor activities of metal oxide nanoparticles. Nanomaterials. 2015;(5):1004–1021. doi: 10.3390/nano5021004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dey A., Manna S., Kumar S., et al. Immunostimulatory effect of chitosan conjugated green copper oxide nanoparticles in tumor immunotherapy. Cytokine. 2020;127 doi: 10.1016/j.cyto.2019.154958. [DOI] [PubMed] [Google Scholar]
- 85.He L.Z., Nie T.Q., Xia X.J., et al. Designing bioinspired 2D MoSe2 nanosheet for efficient photothermal-triggered cancer immunotherapy with reprogramming tumor-associated macrophages. Adv. Funct. Mater. 2019;29 [Google Scholar]
- 86.Huang J., Yang B., Peng Y., et al. Nanomedicine-boosting tumor immunogenicity for enhanced immunotherapy. Adv. Funct. Mater. 2021;31 [Google Scholar]
- 87.Zhang L., Xu L., Wang Y., et al. A novel therapeutic vaccine based on graphene oxide nanocomposite for tumor immunotherapy. Chin. Chem. Lett. 2022;33:4089–4095. [Google Scholar]
- 88.Liu Q., Fan T., Zheng Y., et al. Immunogenic exosome-encapsulated black phosphorus nanoparticles as an effective anticancer photo-nanovaccine. Nanoscale. 2020;12:19939–19952. doi: 10.1039/d0nr05953f. [DOI] [PubMed] [Google Scholar]
- 89.Tian Z., Hu Q., Sun Z., et al. A booster for radiofrequency ablation: advanced adjuvant therapy via in situ nanovaccine synergized with anti-programmed death ligand 1 immunotherapy for systemically constraining hepatocellular carcinoma. ACS Nano. 2023;17:19441–19458. doi: 10.1021/acsnano.3c08064. [DOI] [PubMed] [Google Scholar]
- 90.Yang L., Wang T., Zhang D., et al. Black phosphorus nanosheets assist nanoerythrosomes for efficient mrna vaccine delivery and immune activation. Adv. Healthcare Mater. 2023;(12) doi: 10.1002/adhm.202300935. [DOI] [PubMed] [Google Scholar]
- 91.Blanco E., Kessinger C.W., Sumer B.D., et al. Multifunctional micellar nanomedicine for cancer therapy. Exp. Biol. Med. 2009;234:123–131. doi: 10.3181/0808-MR-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ghosh B., Biswas S. Polymeric micelles in cancer therapy: state of the art. J. Contr. Release. 2021;332:127–147. doi: 10.1016/j.jconrel.2021.02.016. [DOI] [PubMed] [Google Scholar]
- 93.Van Der Vlies A.J., O'Neil C.P., Hasegawa U., et al. Synthesis of pyridyl disulfide-functionalized nanoparticles for conjugating thiol-containing small molecules, peptides, and proteins. Bioconjugate Chem. 2010;21:653–662. doi: 10.1021/bc9004443. [DOI] [PubMed] [Google Scholar]
- 94.Hubbell J.A. Enhancing drug function. Science. 2003;300:595–596. doi: 10.1126/science.1083625. [DOI] [PubMed] [Google Scholar]
- 95.O'Neil C.P., van der Vlies A.J., Velluto D., et al. Extracellular matrix binding mixed micelles for drug delivery applications. J. Contr. Release. 2009;137:146–151. doi: 10.1016/j.jconrel.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 96.Zhou Q., Wang T., Li K., et al. Biofilm microenvironment-responsive polymeric CO releasing micelles for enhanced amikacin efficacy. J. Contr. Release. 2023;357:561–571. doi: 10.1016/j.jconrel.2023.04.025. [DOI] [PubMed] [Google Scholar]
- 97.Chen T., Qin Y., Li Y., et al. Chiral polymer micelles alleviate adriamycin cardiotoxicity via iron chelation and ferroptosis inhibition. Adv. Funct. Mater. 2023;33 [Google Scholar]
- 98.Zhang S., Zeng Y., Wang K., et al. Chitosan-based nano-micelles for potential anti-tumor immunotherapy: synergistic effect of cGAS-STING signaling pathway activation and tumor antigen absorption. Carbohydr. Polym. 2023;321 doi: 10.1016/j.carbpol.2023.121346. [DOI] [PubMed] [Google Scholar]
- 99.Eby J.K., Dane K.Y., O'Neil C.P., et al. Polymer micelles with pyridyl disulfide-coupled antigen travel through lymphatics and show enhanced cellular responses following immunization. Acta Biomater. 2012;(8):3210–3217. doi: 10.1016/j.actbio.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 100.Brubaker C.E., Panagiotou V., Demurtas D., et al. A cationic micelle complex improves CD8+ T cell responses in vaccination against unmodified protein antigen. ACS Biomater. Sci. Eng. 2016;(2):231–240. doi: 10.1021/acsbiomaterials.5b00456. [DOI] [PubMed] [Google Scholar]
- 101.Peeler D.J., Yen A., Luera N., et al. Lytic polyplex vaccines enhance antigen‐specific cytotoxic T cell response through induction of local cell death. Adv. Ther. 2021;4 [Google Scholar]
- 102.Song K., Nguyen D.C., Luu T., et al. A mannosylated polymer with endosomal release properties for peptide antigen delivery. J. Contr. Release. 2023;356:232–241. doi: 10.1016/j.jconrel.2023.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lv S., Song K., Yen A., et al. Well-defined mannosylated polymer for peptide vaccine delivery with enhanced antitumor immunity. Adv. Healthcare Mater. 2022;11 doi: 10.1002/adhm.202101651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ferreira S.A., Gama F.M., Vilanova M. Polymeric nanogels as vaccine delivery systems. Nanomed-Nanotechnol. 2013;(9):159–173. doi: 10.1016/j.nano.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 105.Muraoka D., Harada N., Shiku H., et al. Self-assembled polysaccharide nanogel delivery system for overcoming tumor immune resistance. J. Contr. Release. 2022;347:175–182. doi: 10.1016/j.jconrel.2022.05.004. [DOI] [PubMed] [Google Scholar]
- 106.Yuki Y., Harada N., Sawada S.I., et al. Biodistribution assessment of cationic pullulan nanogel, a nasal vaccine delivery system, in mice and non-human primates. Vaccine. 2023;(41):4941–4949. doi: 10.1016/j.vaccine.2023.06.065. [DOI] [PubMed] [Google Scholar]
- 107.Molofsky A.B., Locksley R.M. The ins and outs of innate and adaptive type 2 immunity. Immunity. 2023;(56):704–722. doi: 10.1016/j.immuni.2023.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang C., Li P., Liu L.L., et al. Self-adjuvanted nanovaccine for cancer immunotherapy: role of lysosomal rupture-induced ROS in MHC class I antigen presentation. Biomaterials. 2016;79:88–100. doi: 10.1016/j.biomaterials.2015.11.040. [DOI] [PubMed] [Google Scholar]
- 109.Wang N., Chen M., Wang T. Liposomes used as a vaccine adjuvant-delivery system: from basics to clinical immunization. J. Contr. Release. 2019;303:130–150. doi: 10.1016/j.jconrel.2019.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Liu D., Deng B., Liu Z., et al. Enhanced antitumor immune responses via a self-assembled carrier-free nanovaccine. Nano Lett. 2021;21:3965–3973. doi: 10.1021/acs.nanolett.1c00648. [DOI] [PubMed] [Google Scholar]
- 111.Tretiakova D.S., Vodovozova E.L. Liposomes as adjuvants and vaccine delivery systems. Biochem. (Mosc) Suppl. Ser. A Membr. Cell Biol. 2022;(16):1–20. doi: 10.1134/S1990747822020076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yuba E., Tajima N., Yoshizaki Y., et al. Dextran derivative-based pH-sensitive liposomes for cancer immunotherapy. Biomaterials. 2014;35:3091–3101. doi: 10.1016/j.biomaterials.2013.12.024. [DOI] [PubMed] [Google Scholar]
- 113.Yoshizaki Y., Yuba E., Sakaguchi N., et al. Potentiation of pH-sensitive polymer-modified liposomes with cationic lipid inclusion as antigen delivery carriers for cancer immunotherapy. Biomaterials. 2014;35:8186–8196. doi: 10.1016/j.biomaterials.2014.05.077. [DOI] [PubMed] [Google Scholar]
- 114.Yoshizaki Y., Yuba E., Sakaguchi N., et al. pH-sensitive polymer-modified liposome-based immunity-inducing system: effects of inclusion of cationic lipid and CpG-DNA. Biomaterials. 2017;141:272–283. doi: 10.1016/j.biomaterials.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 115.Yuba E., Harada A., Sakanishi Y., et al. A liposome-based antigen delivery system using pH-sensitive fusogenic polymers for cancer immunotherapy. Biomaterials. 2013;34:3042–3052. doi: 10.1016/j.biomaterials.2012.12.031. [DOI] [PubMed] [Google Scholar]
- 116.Prasanna P., Kumar P., Kumar S., et al. Current status of nanoscale drug delivery and the future of nano-vaccine development for leishmaniasis-A review. Biomed. Pharmacother. 2021;141 doi: 10.1016/j.biopha.2021.111920. [DOI] [PubMed] [Google Scholar]
- 117.Kang T., Huang Y.K., Zhu Q.Q., et al. Necroptotic cancer cells-mimicry nanovaccine boosts anti-tumor immunity with tailored immune-stimulatory modality. Biomaterials. 2018;164:80–97. doi: 10.1016/j.biomaterials.2018.02.033. [DOI] [PubMed] [Google Scholar]
- 118.Hu X.M., Wu T.T., Qin X.Y., et al. Tumor lysate-loaded lipid hybrid nanovaccine collaborated with an immune checkpoint antagonist for combination immunotherapy. Adv. Healthcare Mater. 2019;(8) doi: 10.1002/adhm.201800837. [DOI] [PubMed] [Google Scholar]
- 119.Thakur A., Parra D.C., Motallebnejad P., et al. Exosomes: small vesicles with big roles in cancer, vaccine development, and therapeutics. Bioact. Mater. 2022;10:281–294. doi: 10.1016/j.bioactmat.2021.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Huda M.N., Nurunnabi M. Potential application of exosomes in vaccine development and delivery. Pharm. Res. (N. Y.) 2022;39:2635–2671. doi: 10.1007/s11095-021-03143-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Xu Z., Zeng S., Gong Z., et al. Exosome-based immunotherapy: a promising approach for cancer treatment. Mol. Cancer. 2020;19:160. doi: 10.1186/s12943-020-01278-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hao S., Liu Y., Yuan J., et al. Novel exosome-targeted CD4+ T cell vaccine counteracting CD4+25+ regulatory T cell-mediated immune suppression and stimulating efficient central memory CD8+ CTL responses. J. Immunol. 2007;179:2731–2740. doi: 10.4049/jimmunol.179.5.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chen Z., You L., Wang L., et al. Dual effect of DLBCL-derived EXOs in lymphoma to improve DC vaccine efficacy in vitro while favor tumorgenesis in vivo. J. Exp. Clin. Cancer Res. 2018;37:190. doi: 10.1186/s13046-018-0863-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Naseri M., Bozorgmehr M., Zoller M., et al. Tumor-derived exosomes: the next generation of promising cell-free vaccines in cancer immunotherapy. OncoImmunology. 2020;9 doi: 10.1080/2162402X.2020.1779991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Liu C., Liu X., Xiang X., et al. A nanovaccine for antigen self-presentation and immunosuppression reversal as a personalized cancer immunotherapy strategy. Nat. Nanotechnol. 2022;17:531–540. doi: 10.1038/s41565-022-01098-0. [DOI] [PubMed] [Google Scholar]
- 126.Sun W., Ji P., Zhou T., et al. Ultrasound responsive nanovaccine armed with engineered cancer cell membrane and RNA to prevent foreseeable metastasis. Adv. Sci. 2023;10 doi: 10.1002/advs.202301107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Long Q., Zheng P., Zheng X., et al. Engineered bacterial membrane vesicles are promising carriers for vaccine design and tumor immunotherapy. Adv. Drug Deliv. Rev. 2022;186 doi: 10.1016/j.addr.2022.114321. [DOI] [PubMed] [Google Scholar]
- 128.Guo Y.Y., Wang D., Song Q.L., et al. Erythrocyte membrane-enveloped polymeric nanoparticles as nanovaccine for induction of antitumor immunity against melanoma. ACS Nano. 2015;9:6918–6933. doi: 10.1021/acsnano.5b01042. [DOI] [PubMed] [Google Scholar]
- 129.Li W., Ma T., He T., et al. Cancer cell membrane-encapsulated biomimetic nanoparticles for tumor immuno-photothermal therapy. Chem. Eng. J. 2023;463 [Google Scholar]
- 130.Xiong X., Zhao J.Y., Pan J.M., et al. Personalized nanovaccine coated with calcinetin-expressed cancer cell membrane antigen for cancer immunotherapy. Nano Lett. 2021;21:8418–8425. doi: 10.1021/acs.nanolett.1c03004. [DOI] [PubMed] [Google Scholar]
- 131.Wang X., Liu Y., Xue C., et al. A protein-based cGAS-STING nanoagonist enhances T cell-mediated anti-tumor immune responses. Nat. Commun. 2022;13:5685. doi: 10.1038/s41467-022-33301-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lv M., Chen M., Zhang R., et al. Manganese is critical for antitumor immune responses via cGAS-STING and improves the efficacy of clinical immunotherapy. Cell Res. 2020;30:966–979. doi: 10.1038/s41422-020-00395-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kuai R., Yuan W.M., Son S.J., et al. Elimination of established tumors with nanodisc-based combination chemoimmunotherapy. Sci. Adv. 2018;(4) doi: 10.1126/sciadv.aao1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Noh I., Guo Z., Zhou J., et al. Cellular nanodiscs made from bacterial outer membrane as a platform for antibacterial vaccination. ACS Nano. 2022;17:1120–1127. doi: 10.1021/acsnano.2c08360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Guo Z., Noh I., Zhu A.T., et al. Cancer cell membrane nanodiscs for antitumor vaccination. Nano Lett. 2023;23:7941–7949. doi: 10.1021/acs.nanolett.3c01775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Asadi K., Gholami A. Virosome-based nanovaccines: a promising bioinspiration and biomimetic approach for preventing viral diseases: a review. Int. J. Biol. Macromol. 2021;182:648–658. doi: 10.1016/j.ijbiomac.2021.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tsoras A.N., Champion J.A. Protein and peptide biomaterials for engineered subunit vaccines and immunotherapeutic applications. Annu. Rev. Chem. Biomol. Eng. 2019;10:337–359. doi: 10.1146/annurev-chembioeng-060718-030347. [DOI] [PubMed] [Google Scholar]
- 138.Qi M., Zhang X.E., Sun X., et al. Intranasal nanovaccine confers homo-and hetero-subtypic influenza protection. Small. 2018;14 doi: 10.1002/smll.201703207. [DOI] [PubMed] [Google Scholar]
- 139.Molino N.M., Neek M., Tucker J.A., et al. Viral-mimicking protein nanoparticle vaccine for eliciting anti-tumor responses. Biomaterials. 2016;(86):83–91. doi: 10.1016/j.biomaterials.2016.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Encapsulins T.W. Giessen. Microbial nanocompartments with applications in biomedicine, nanobiotechnology and materials science. Curr. Opin. Chem. Biol. 2016;34:1–10. doi: 10.1016/j.cbpa.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 141.Jiang J., Liu G., Kickhoefer V.A., et al. A protective vaccine against chlamydia genital infection using vault nanoparticles without an added adjuvant. Vaccines. 2017;(5):3. doi: 10.3390/vaccines5010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Jiang H., Wang Q., Li L., et al. Turning the old adjuvant from gel to nanoparticles to amplify CD8+ T cell responses. Adv. Sci. 2018;(5) doi: 10.1002/advs.201700426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yu X., Dai Y.F., Zhao Y.F., et al. Melittin-lipid nanoparticles target to lymph nodes and elicit a systemic anti-tumor immune response. Nat. Commun. 2020;11:1110. doi: 10.1038/s41467-020-14906-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Liu H., Moynihan K.D., Zheng Y., et al. Structure-based programming of lymph-node targeting in molecular vaccines. Nature. 2014;507:519–522. doi: 10.1038/nature12978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Jin L., Yang D., Song Y., et al. In situ programming of nanovaccines for lymph node-targeted delivery and cancer immunotherapy. ACS Nano. 2022;16:15226–15236. doi: 10.1021/acsnano.2c06560. [DOI] [PubMed] [Google Scholar]
- 146.Xi X.B., Zhang L.J., Lu G.H., et al. Lymph node-targeting nanovaccine through antigen-CpG self-assembly potentiates cytotoxic T Cell activation. J. Immunol. Res. 2018 doi: 10.1155/2018/3714960. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kang S., Ahn S., Lee J., et al. Effects of gold nanoparticle-based vaccine size on lymph node delivery and cytotoxic T-lymphocyte responses. J. Contr. Release. 2017;256:56–67. doi: 10.1016/j.jconrel.2017.04.024. [DOI] [PubMed] [Google Scholar]
- 148.Thomas S.N., Vokali E., Lund A.W., et al. Targeting the tumor-draining lymph node with adjuvanted nanoparticles reshapes the anti-tumor immune response. Biomaterials. 2014;(35):814–824. doi: 10.1016/j.biomaterials.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 149.Wang K., Wen S.M., He L.H., et al. “Minimalist” nanovaccine constituted from near whole antigen for cancer immunotherapy. ACS Nano. 2018;12:6398–6409. doi: 10.1021/acsnano.8b00558. [DOI] [PubMed] [Google Scholar]
- 150.Ali O.A., Huebsch N., Cao L., et al. Infection-mimicking materials to program dendritic cells in situ. Nat. Mater. 2009;(8):151–158. doi: 10.1038/nmat2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Patel R.B., Ye M., Carlson P.M., et al. Development of an in situ cancer vaccine via combinational radiation and bacterial-membrane-coated nanoparticles. Adv. Mater. 2019;31 doi: 10.1002/adma.201902626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Liu Y.T., Yao L.T., Cao W.P., et al. Dendritic cell targeting peptide-based nanovaccines for enhanced cancer immunotherapy. ACS Appl. Bio Mater. 2019;(2):1241–1254. doi: 10.1021/acsabm.8b00811. [DOI] [PubMed] [Google Scholar]
- 153.Liu W.L., Zou M.Z., Liu T., et al. Cytomembrane nanovaccines show therapeutic effects by mimicking tumor cells and antigen presenting cells. Nat. Commun. 2019;10:3199. doi: 10.1038/s41467-019-11157-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Xu Y.D., Ma S., Zhao J.Y., et al. Mannan-decorated pathogen-like polymeric nanoparticles as nanovaccine carriers for eliciting superior anticancer immunity. Biomaterials. 2022;(284) doi: 10.1016/j.biomaterials.2022.121489. [DOI] [PubMed] [Google Scholar]
- 155.Zhu G., Mei L., Vishwasrao H.D., et al. Intertwining DNA-RNA nanocapsules loaded with tumor neoantigens as synergistic nanovaccines for cancer immunotherapy. Nat. Commun. 2017;(8):1482. doi: 10.1038/s41467-017-01386-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Liu S., Jiang Q., Zhao X., et al. A DNA nanodevice-based vaccine for cancer immunotherapy. Nat. Mater. 2021;20:421–430. doi: 10.1038/s41563-020-0793-6. [DOI] [PubMed] [Google Scholar]
- 157.John S., Yuzhakov O., Woods A., et al. Multi-antigenic human cytomegalovirus mRNA vaccines that elicit potent humoral and cell-mediated immunity. Vaccine. 2018;36:1689–1699. doi: 10.1016/j.vaccine.2018.01.029. [DOI] [PubMed] [Google Scholar]
- 158.Jiang Z.X., Liu J.C., Guan J., et al. Self-adjuvant effect by manipulating the bionano interface of liposome-based nanovaccines. Nano Lett. 2021;21:4744–4752. doi: 10.1021/acs.nanolett.1c01133. [DOI] [PubMed] [Google Scholar]
- 159.Atukorale P.U., Raghunathan S.P., Raguveer V., et al. Nanoparticle encapsulation of synergistic immune agonists enables systemic codelivery to tumor sites and IFNβ-driven antitumor immunity. Cancer Res. 2019;79:5394–5406. doi: 10.1158/0008-5472.CAN-19-0381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Jang E.S., Shin J.H., Ren G., et al. The manipulation of natural killer cells to target tumor sites using magnetic nanoparticles. Biomaterials. 2012;(33):5584–5592. doi: 10.1016/j.biomaterials.2012.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wu L.Y., Zhang F.Q., Wei Z.H., et al. Magnetic delivery of Fe3O4@polydopamine nanoparticle-loaded natural killer cells suggest a promising anticancer treatment. Biomater. Sci. 2018;(6):2714–2725. doi: 10.1039/c8bm00588e. [DOI] [PubMed] [Google Scholar]
- 162.Xie X.X., Feng Y., Zhang H.X., et al. Remodeling tumor immunosuppressive microenvironment via a novel bioactive nanovaccines potentiates the efficacy of cancer immunotherapy. Bioact. Mater. 2022;(16):107–119. doi: 10.1016/j.bioactmat.2022.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Deng C.F., Zhang Q., Jia M.D., et al. Tumors and their microenvironment dual-targeting chemotherapy with local immune adjuvant therapy for effective antitumor immunity against breast cancer. Adv. Sci. 2019;6 doi: 10.1002/advs.201801868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ramesh A., Brouillard A., Kumar S., et al. Dual inhibition of CSF1R and MAPK pathways using supramolecular nanoparticles enhances macrophage immunotherapy. Biomaterials. 2020;227 doi: 10.1016/j.biomaterials.2019.119559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Qian Y., Qiao S., Dai Y.F., et al. Molecular-targeted immunotherapeutic strategy for melanoma via dual-targeting nanoparticles delivering small interfering RNA to tumor-associated macrophages. ACS Nano. 2017;11:9536–9549. doi: 10.1021/acsnano.7b05465. [DOI] [PubMed] [Google Scholar]
- 166.Zhang Y.M., Feng Z.J., Liu J.J., et al. Polarization of tumor-associated macrophages by TLR7/8 conjugated radiosensitive peptide hydrogel for overcoming tumor radioresistance. Bioact. Mater. 2022;(16):359–371. doi: 10.1016/j.bioactmat.2021.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Wei Z.H., Zhang X.Q., Zhang Z.L., et al. Engineered iron-based nanoplatform amplifies repolarization of M2-like tumor-associated macrophages for enhanced cancer immunotherapy. Chem. Eng. J. 2022;433 [Google Scholar]
- 168.Wang Y.H., Zhao Q.F., Zhao B.Y., et al. Remodeling tumor-associated neutrophils to enhance dendritic cell-based HCC neoantigen nano-vaccine efficiency. Adv. Sci. 2022;9 doi: 10.1002/advs.202105631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zhang T., Xiong H.G., Ma X.B., et al. Supramolecular tadalafil nanovaccine for cancer immunotherapy by alleviating myeloid-derived suppressor cells and heightening immunogenicity. Small Methods. 2021;5 doi: 10.1002/smtd.202100115. [DOI] [PubMed] [Google Scholar]
- 170.Sasso M.S., Lollo G., Pitorre M., et al. Low dose gemcitabine-loaded lipid nanocapsules target monocytic myeloid-derived suppressor cells and potentiate cancer immunotherapy. Biomaterials. 2016;(96):47–62. doi: 10.1016/j.biomaterials.2016.04.010. [DOI] [PubMed] [Google Scholar]
- 171.Ledo A.M., Sasso M.S., Bronte V., et al. Co-delivery of RNAi and chemokine by polyarginine nanocapsules enables the modulation of myeloid-derived suppressor cells. J. Contr. Release. 2019;295:60–73. doi: 10.1016/j.jconrel.2018.12.041. [DOI] [PubMed] [Google Scholar]
- 172.Sainz V., Moura L.I.F., Peres C., et al. α-galactosylceramide and peptide-based nano-vaccine synergistically induced a strong tumor suppressive effect in melanoma. Acta Biomater. 2018;76:193–207. doi: 10.1016/j.actbio.2018.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ou W., Jiang L., Thapa R.K., et al. Combination of NIR therapy and regulatory T cell modulation using layer-by-layer hybrid nanoparticles for effective cancer photoimmunotherapy. Theranostics. 2018;8:4574–4590. doi: 10.7150/thno.26758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Chang L., Fu S., Gao T., et al. Regulating T-cell metabolic reprogramming and blocking PD-1 co-promote personalized postoperative autologous nanovaccines. Biomaterials. 2023;297 doi: 10.1016/j.biomaterials.2023.122104. [DOI] [PubMed] [Google Scholar]
- 175.Qian Y., Jin H.L., Qiao S., et al. Targeting dendritic cells in lymph node with an antigen peptide-based nanovaccine for cancer immunotherapy. Biomaterials. 2016;98:171–183. doi: 10.1016/j.biomaterials.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 176.Bois H.D., Heim T.A., Lund A.W. Tumor-draining lymph nodes: at the crossroads of metastasis and immunity. Sci. Immunol. 2021;(6) doi: 10.1126/sciimmunol.abg3551. eabg3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Wen Z., Liu H., Qiao D., et al. Nanovaccines fostering tertiary lymphoid structure to attack mimicry nasopharyngeal carcinoma. ACS Nano. 2023;17:7194–7206. doi: 10.1021/acsnano.2c09619. [DOI] [PubMed] [Google Scholar]
- 178.Jiang H., Wang Q., Sun X. Lymph node targeting strategies to improve vaccination efficacy. J. Contr. Release. 2017;267:47–56. doi: 10.1016/j.jconrel.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 179.Trevaskis N.L., Kaminskas L.M., Porter C.J.H. From sewer to saviour-targeting the lymphatic system to promote drug exposure and activity. Nat. Rev. Drug Discov. 2015;14:781–803. doi: 10.1038/nrd4608. [DOI] [PubMed] [Google Scholar]
- 180.Yang Z., Tian R., Wu J.J., et al. Impact of semiconducting perylene diimide nanoparticle size on lymph node mapping and cancer imaging. ACS Nano. 2017;11:4247–4255. doi: 10.1021/acsnano.7b01261. [DOI] [PubMed] [Google Scholar]
- 181.Wang H., Mooney D.J. Biomaterial-assisted targeted modulation of immune cells in cancer treatment. Nat. Mater. 2018;17:761–772. doi: 10.1038/s41563-018-0147-9. [DOI] [PubMed] [Google Scholar]
- 182.Kim J., Archer P.A., Thomas S.N. Innovations in lymph node targeting nanocarriers. Semin. Immunol. 2021;56 doi: 10.1016/j.smim.2021.101534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Li J.X., Ren H., Sun Y.P., et al. Magnetic metal micelles for enhanced delivery of self-immolating CD8+ T Cell epitopes for cancer immunotherapy. Chem. Mater. 2021;33:9780–9794. [Google Scholar]
- 184.Xu J., Liu H., Wang T., et al. CCR7 mediated mimetic dendritic cell vaccine homing in lymph node for head and neck squamous cell carcinoma therapy. Adv. Sci. 2023;10 doi: 10.1002/advs.202207017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Zhang J., Fan B., Cao G., et al. Direct presentation of tumor-associated antigens to induce adaptive immunity by personalized dendritic cell-mimicking nanovaccines. Adv. Mater. 2022;34 doi: 10.1002/adma.202205950. [DOI] [PubMed] [Google Scholar]
- 186.Iwasaki A., Medzhitov R. Regulation of adaptive immunity by the innate immune system. Science. 2010;327:291–295. doi: 10.1126/science.1183021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Tacken P.J., de Vries I.J.M., Torensma R., et al. Dendritic-cell immunotherapy: from ex vivo loading to in vivo targeting. Nat. Rev. Immunol. 2007;(7):790–802. doi: 10.1038/nri2173. [DOI] [PubMed] [Google Scholar]
- 188.Shi G.N., Zhang C.N., Xu R., et al. Enhanced antitumor immunity by targeting dendritic cells with tumor cell lysate-loaded chitosan nanoparticles vaccine. Biomaterials. 2017;113:191–202. doi: 10.1016/j.biomaterials.2016.10.047. [DOI] [PubMed] [Google Scholar]
- 189.Cruz L.J., Tacken P.J., Fokkink R., et al. Targeted PLGA nano-but not microparticles specifically deliver antigen to human dendritic cells via DC-SIGN in vitro. J. Contr. Release. 2010;144:118–126. doi: 10.1016/j.jconrel.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 190.Chen J., Fang H.P., Hu Y.Y., et al. Combining mannose receptor mediated nanovaccines and gene regulated PD-L1 blockade for boosting cancer immunotherapy. Bioact. Mater. 2022;(7):167–180. doi: 10.1016/j.bioactmat.2021.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Ali O.A., Tayalia P., Shvartsman D., et al. Inflammatory cytokines presented from polymer matrices differentially generate and activate DCs in situ. Adv. Funct. Mater. 2013;23:4621–4628. doi: 10.1002/adfm.201203859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Bhattacharya P., Budnick I., Singh M., et al. Dual role of GM-CSF as a pro-inflammatory and a regulatory cytokine: implications for immune therapy. J. Interferon Cytokine Res. 2015;(35):585–599. doi: 10.1089/jir.2014.0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Lu Y., Liu Z.H., Li Y.X., et al. Targeted delivery of nanovaccine to dendritic cells via DC-binding peptides induces potent antiviral immunity in vivo. Int. J. Nanomed. 2022;17:1593–1608. doi: 10.2147/IJN.S357462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Du J., Zhang Y.S., Hobson D., et al. Nanoparticles for immune system targeting. Drug Discov. Today. 2017;22:1295–1301. doi: 10.1016/j.drudis.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 195.Qiu L., Valente M., Dolen Y., et al. Endolysosomal-escape nanovaccines through adjuvant-induced tumor antigen assembly for enhanced effector CD8+ T cell activation. Small. 2018;14 doi: 10.1002/smll.201703539. [DOI] [PubMed] [Google Scholar]
- 196.Wang M., Yin B., Wang H.Y., et al. Current advances in T-cell-based cancer immunotherapy. Immunotherapy. 2014;(6):1265–1278. doi: 10.2217/imt.14.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Kim C.G., Kye Y.C., Yun C.H. The role of nanovaccine in cross-presentation of antigen-presenting cells for the activation of CD8+ T cell responses. Pharmaceutics. 2019;11:612. doi: 10.3390/pharmaceutics11110612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Cheung A.S., Zhang D.K.Y., Koshy S.T., et al. Scaffolds that mimic antigen-presenting cells enable ex vivo expansion of primary T cells. Nat. Biotechnol. 2018;36:160–169. doi: 10.1038/nbt.4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Yang W.J., Deng H.Z., Zhu S.J., et al. Size-transformable antigen-presenting cell-mimicking nanovesicles potentiate effective cancer immunotherapy. Sci. Adv. 2020;(6) doi: 10.1126/sciadv.abd1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Carson C.S., Becker K.W., Garland K.M., et al. A nanovaccine for enhancing cellular immunity via cytosolic co-delivery of antigen and polyIC RNA. J. Contr. Release. 2022;345:354–370. doi: 10.1016/j.jconrel.2022.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Mao J., Jin Z., Rui X., et al. A universal cyclodextrin-based nanovaccine platform delivers epitope peptides for enhanced antitumor immunity. Adv. Healthcare Mater. 2023;12 doi: 10.1002/adhm.202301099. [DOI] [PubMed] [Google Scholar]
- 202.Liu G., Zhu M., Zhao X., et al. Nanotechnology-empowered vaccine delivery for enhancing CD8+ T cells-mediated cellular immunity. Adv. Drug Deliv. Rev. 2021;176 doi: 10.1016/j.addr.2021.113889. [DOI] [PubMed] [Google Scholar]
- 203.Liu M.A. DNA vaccines: an historical perspective and view to the future. Immunol. Rev. 2011;239:62–84. doi: 10.1111/j.1600-065X.2010.00980.x. [DOI] [PubMed] [Google Scholar]
- 204.Pardi N., Hogan M.J., Porter F.W., et al. mRNA vaccines-A new era in vaccinology. Nat. Rev. Drug Discov. 2018;17:261–279. doi: 10.1038/nrd.2017.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Mandl C.W., Aberle J.H., Aberle S.W., et al. In vitro-synthesized infectious RNA as an attenuated live vaccine in a flavivirus model. Nat. Med. 1998;(4):1438–1440. doi: 10.1038/4031. [DOI] [PubMed] [Google Scholar]
- 206.Kose N., Fox J.M., Sapparapu G., et al. A lipid-encapsulated mRNA encoding a potently neutralizing human monoclonal antibody protects against chikungunya infection. Sci. Immunol. 2019;(4) doi: 10.1126/sciimmunol.aaw6647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Jirikowski G.F., Sanna P.P., Maciejewski-Lenoir D., et al. Reversal of diabetes insipidus in Brattleboro rats: intrahypothalamic injection of vasopressin mRNA. Science. 1992;255:996–998. doi: 10.1126/science.1546298. [DOI] [PubMed] [Google Scholar]
- 208.Baden L.R., El Sahly H.M., Essink B., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Selbo P.K., Weyergang A., Høgset A., et al. Photochemical internalization provides time-and space-controlled endolysosomal escape of therapeutic molecules. J. Contr. Release. 2010;148:2–12. doi: 10.1016/j.jconrel.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 210.Pinillos Bayona A.M.D., Moore C.M., Loizidou M., et al. Enhancing the efficacy of cytotoxic agents for cancer therapy using photochemical internalisation. Int. J. Cancer. 2016;138:1049–1057. doi: 10.1002/ijc.29510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Cao F., Yan M., Liu Y., et al. Photothermally controlled MHC class I restricted CD8+ T-cell responses elicited by hyaluronic acid decorated gold nanoparticles as a vaccine for cancer immunotherapy. Adv. Healthcare Mater. 2018;(7) doi: 10.1002/adhm.201701439. [DOI] [PubMed] [Google Scholar]
- 212.Hakerud M., Selbo P.K., Waeckerle M.Y., et al. Photosensitisation facilitates cross-priming of adjuvant-free protein vaccines and stimulation of tumour-suppressing CD8 T cells. J. Contr. Release. 2015;198:10–17. doi: 10.1016/j.jconrel.2014.11.032. [DOI] [PubMed] [Google Scholar]
- 213.Suchanek O., Ferdinand J.R., Tuong Z.K., et al. Tissue-resident B cells orchestrate macrophage polarisation and function. Nat. Commun. 2023;14:7081. doi: 10.1038/s41467-023-42625-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Li W.H., Su J.Y., Li Y.M. Rational design of T-cell- and B-cell-based therapeutic cancer vaccines. Acc. Chem. Res. 2022;55:2660–2671. doi: 10.1021/acs.accounts.2c00360. [DOI] [PubMed] [Google Scholar]
- 215.Chen J.J., Pompano R.R., Santiago F.W., et al. The use of self-adjuvanting nanofiber vaccines to elicit high-affinity B cell responses to peptide antigens without inflammation. Biomaterials. 2013;34:8776–8785. doi: 10.1016/j.biomaterials.2013.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Argos B.V., Chiodin G., Forconi F., et al. Lymphoma-specific subversion of B-cell receptor signaling by macrophage lectins. Blood. 2018;132:2865. [Google Scholar]
- 217.Jia H.X., Shang Y.N., Cao H.M., et al. A minimalist supramolecular nanovaccine forcefully propels the Tfh cell and GC B cell responses. Chem. Eng. J. 2022;435 [Google Scholar]
- 218.Li F.S., Feng X.Y., Huang J.X., et al. Periodic mesoporous organosilica as a nanoadjuvant for subunit vaccines elicits potent antigen-specific germinal center responses by activating naive B cells. ACS Nano. 2023;17:15424–15440. doi: 10.1021/acsnano.3c00991. [DOI] [PubMed] [Google Scholar]
- 219.Burger J.A., Wiestner A. Targeting B cell receptor signalling in cancer: preclinical and clinical advances. Nat. Rev. Cancer. 2018;(18):148–167. doi: 10.1038/nrc.2017.121. [DOI] [PubMed] [Google Scholar]
- 220.Shen L.M., Tenzer S., Storck W., et al. Protein corona-mediated targeting of nanocarriers to B cells allows redirection of allergic immune responses. J. Allergy Clin. Immunol. 2018;142:1558–1570. doi: 10.1016/j.jaci.2017.08.049. [DOI] [PubMed] [Google Scholar]
- 221.Pal Singh S., Dammeijer F., Hendriks R.W. Role of Bruton's tyrosine kinase in B cells and malignancies. Mol. Cancer. 2018;17:57. doi: 10.1186/s12943-018-0779-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Chen S.S., Barrientos J.C., Ferrer G., et al. Duvelisib eliminates CLL B Cells, impairs CLL-supporting cells, and overcomes ibrutinib resistance in a Xenograft model. Clin. Cancer Res. 2023;29:1984–1995. doi: 10.1158/1078-0432.CCR-22-2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Raza A., Rossi G.R., Janjua T.I., et al. Nanobiomaterials to modulate natural killer cell responses for effective cancer immunotherapy. Trends Biotechnol. 2023;(41):77–92. doi: 10.1016/j.tibtech.2022.06.011. [DOI] [PubMed] [Google Scholar]
- 224.Phung C.D., Tran T.H., Kim J.O. Engineered nanoparticles to enhance natural killer cell activity towards onco-immunotherapy: a review. Arch Pharm. Res. (Seoul) 2020;43:32–45. doi: 10.1007/s12272-020-01218-1. [DOI] [PubMed] [Google Scholar]
- 225.Dunkelberger J.R., Song W.C. Complement and its role in innate and adaptive immune responses. Cell Res. 2010;20:34–50. doi: 10.1038/cr.2009.139. [DOI] [PubMed] [Google Scholar]
- 226.Cheng Y.X., Song S.Y., Wu P.Y., et al. Tumor associated macrophages and TAMs-based anti-tumor nanomedicines. Adv. Healthcare Mater. 2021;10 doi: 10.1002/adhm.202100590. [DOI] [PubMed] [Google Scholar]
- 227.Yang Q.Y., Guo N.N., Zhou Y., et al. The role of tumor-associated macrophages (TAMs) in tumor progression and relevant advance in targeted therapy. Acta Pharm. Sin. B. 2020;10:2156–2170. doi: 10.1016/j.apsb.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Ruffell B., Coussens L.M. Macrophages and therapeutic resistance in cancer. Cancer Cell. 2015;27:462–472. doi: 10.1016/j.ccell.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Gomez-Roca C.A., Italiano A., Le Tourneau C., et al. Phase I study of emactuzumab single agent or in combination with paclitaxel in patients with advanced/metastatic solid tumors reveals depletion of immunosuppressive M2-like macrophages. Ann. Oncol. 2019;30:1381–1392. doi: 10.1093/annonc/mdz163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Shen S., Li H.J., Chen K.G., et al. Spatial targeting of tumor-associated macrophages and tumor cells with a pH-sensitive cluster nanocarrier for cancer chemoimmunotherapy. Nano Lett. 2017;17:3822–3829. doi: 10.1021/acs.nanolett.7b01193. [DOI] [PubMed] [Google Scholar]
- 231.Wang X., Li J., Wang Y.Q., et al. HFT-T, a targeting nanoparticle, enhances specific delivery of paclitaxel to folate receptor-positive tumors. ACS Nano. 2009;3:3165–3174. doi: 10.1021/nn900649v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Rong L., Zhang Y., Li W.S., et al. Iron chelated melanin-like nanoparticles for tumor-associated macrophage repolarization and cancer therapy. Biomaterials. 2019;(225) doi: 10.1016/j.biomaterials.2019.119515. [DOI] [PubMed] [Google Scholar]
- 233.Wang M., Hu Q.D., Huang J.M., et al. Engineered a dual-targeting biomimetic nanomedicine for pancreatic cancer chemoimmunotherapy. J. Nanobiotechnol. 2022;20:85. doi: 10.1186/s12951-022-01282-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Li F., Ding X., Lv Z., et al. A DNA-polymer hybrid nanocomplex based bi-adjuvant vaccine for tumor immunotherapy. Nano Today. 2024;54 [Google Scholar]
- 235.Costa da Silva M., Breckwoldt M.O., Vinchi F., et al. Iron induces anti-tumor activity in tumor-associated macrophages. Front. Immunol. 2017;8:1479. doi: 10.3389/fimmu.2017.01479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Moradi C.M., Shojaei S., Mohammad Y.S., et al. Transfer of miRNA in tumor-derived exosomes suppresses breast tumor cell invasion and migration by inducing M1 polarization in macrophages. Life Sci. 2021;282 doi: 10.1016/j.lfs.2021.119800. [DOI] [PubMed] [Google Scholar]
- 237.Song Y.D., Tang C., Yin C.H. Combination antitumor immunotherapy with VEGF and PIGF siRNA via systemic delivery of multi-functionalized nanoparticles to tumor-associated macrophages and breast cancer cells. Biomaterials. 2018;185:117–132. doi: 10.1016/j.biomaterials.2018.09.017. [DOI] [PubMed] [Google Scholar]
- 238.Liu L.L., Yi H.Q., He H.M., et al. Tumor associated macrophage-targeted microRNA delivery with dual-responsive polypeptide nanovectors for anti-cancer therapy. Biomaterials. 2017;134:166–179. doi: 10.1016/j.biomaterials.2017.04.043. [DOI] [PubMed] [Google Scholar]
- 239.Tang Y.S., Fan W.F., Chen G.H., et al. Recombinant cancer nanovaccine for targeting tumor-associated macrophage and remodeling tumor microenvironment. Nano Today. 2021;40 [Google Scholar]
- 240.Chen G.H., Xiong W., Gu Z.Y., et al. Mannosylated engineered trichosanthin-legumain protein vaccine hydrogel for breast cancer immunotherapy. Int. J. Biol. Macromol. 2022;223:1485–1494. doi: 10.1016/j.ijbiomac.2022.11.045. [DOI] [PubMed] [Google Scholar]
- 241.Zhang Y., Diao N., Lee C.K., et al. Neutrophils deficient in innate suppressor IRAK-M enhances anti-tumor immune responses. Mol. Ther. 2020;(28):89–99. doi: 10.1016/j.ymthe.2019.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Sheng S.P., Jin L.M., Zhang Y., et al. A twindrive precise delivery system of platelet-neutrophil hybrid membrane regulates macrophage combined with CD47 blocking for postoperative immunotherapy. ACS Nano. 2024;18:4981–4992. doi: 10.1021/acsnano.3c10862. [DOI] [PubMed] [Google Scholar]
- 243.Yan M., Zheng M.Y., Niu R., et al. Roles of tumor-associated neutrophils in tumor metastasis and its clinical applications. Front. Cell Dev. Biol. 2022;10:1–16. doi: 10.3389/fcell.2022.938289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Yin H.Y., Lu H.D., Xiong Y.K., et al. Tumor-associated neutrophil extracellular traps regulating nanocarrier-enhanced inhibition of malignant tumor growth and distant metastasis. ACS Appl. Mater. Interfaces. 2021;(13):59683–59694. doi: 10.1021/acsami.1c18660. [DOI] [PubMed] [Google Scholar]
- 245.Fan D.P., Wang S.Q., Huang R., et al. Light-assisted “nano-neutrophils” with high drug loading for targeted cancer therapy. Int. J. Nanomed. 2023;18:6487–6502. doi: 10.2147/IJN.S432854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Rogers T., DeBerardinis R.J. Metabolic plasticity of neutrophils: relevance to pathogen responses and Cancer. Trends Cancer. 2021;(7):700–713. doi: 10.1016/j.trecan.2021.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Hao M.X., Zhu L.L., Hou S.Y., et al. Sensitizing tumors to immune checkpoint blockage via STING agonists delivered by tumor-penetrating neutrophil cytopharmaceuticals. ACS Nano. 2023;17:1663–1680. doi: 10.1021/acsnano.2c11764. [DOI] [PubMed] [Google Scholar]
- 248.Pylaeva E., Harati M.D., Spyra I., et al. NAMPT signaling is critical for the proangiogenic activity of tumor-associated neutrophils. Int. J. Cancer. 2019;144:136–149. doi: 10.1002/ijc.31808. [DOI] [PubMed] [Google Scholar]
- 249.Fridlender Z.G., Sun J., Kim S., et al. Polarization of tumor-associated neutrophil phenotype by TGF-β: “N1” versus “N2” TAN. Cancer Cell. 2009;16:183–194. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Moorlag S., Rodriguez-Rosales Y.A., Gillard J., et al. BCG vaccination induces long-term functional reprogramming of human neutrophils. Cell Rep. 2020;33 doi: 10.1016/j.celrep.2020.108387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Wesolowski R., Markowitz J., Carson W.E. Myeloid derived suppressor cells-a new therapeutic target in the treatment of cancer. J. Immunother. Cancer. 2013;(1):10. doi: 10.1186/2051-1426-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Hassel J.C., Jiang H., Bender C., et al. Tadalafil has biologic activity in human melanoma, results of a pilot trial with Tadalafil in patients with metastatic Melanoma (TaMe) OncoImmunology. 2017;6 doi: 10.1080/2162402X.2017.1326440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Gebremeskel S., Slauenwhite D., Johnston B. Reconstitution models to evaluate natural killer T cell function in tumor control. Immunol. Cell Biol. 2016;94:90–100. doi: 10.1038/icb.2015.67. [DOI] [PubMed] [Google Scholar]
- 254.Arrenberg P., Halder R., Kumar V. Cross-regulation between distinct natural killer T cell subsets influences immune response to self and foreign antigens. J. Cell. Physiol. 2009;218:246–250. doi: 10.1002/jcp.21597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Jahng A., Maricic I., Aguilera C., et al. Prevention of autoimmunity by targeting a distinct, noninvariant CD1d-reactive T cell population reactive to sulfatide. J. Exp. Med. 2004;199:947–957. doi: 10.1084/jem.20031389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Iyoda T., Yamasaki S., Ueda S., et al. Natural killer T and natural killer cell-based immunotherapy strategies targeting cancer. Biomolecules. 2023;13:348. doi: 10.3390/biom13020348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Ghinnagow R., Cruz L.J., Macho-Fernandez E., et al. Enhancement of adjuvant functions of natural killer T cells using nanovector delivery systems: application in anticancer immune therapy. Front. Immunol. 2017;8:879. doi: 10.3389/fimmu.2017.00879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Faveeuw C., Trottein F. Optimization of natural killer T cell-mediated immunotherapy in cancer using cell-based and nanovector vaccines. Cancer Res. 2014;74:1632–1638. doi: 10.1158/0008-5472.CAN-13-3504. [DOI] [PubMed] [Google Scholar]
- 259.Verbeke R., Lentacker I., Breckpot K., et al. Broadening the message: a nanovaccine co-loaded with messenger RNA and α-GalCer induces antitumor immunity through conventional and natural killer T cells. ACS Nano. 2019;13:1655–1669. doi: 10.1021/acsnano.8b07660. [DOI] [PubMed] [Google Scholar]
- 260.Yang W., Zhou Z., Lau J., et al. Functional T cell activation by smart nanosystems for effective cancer immunotherapy. Nano Today. 2019;27:28–47. [Google Scholar]
- 261.Tanaka A., Sakaguchi S. Regulatory T cells in cancer immunotherapy. Cell Res. 2017;27:109–118. doi: 10.1038/cr.2016.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Ohue Y., Nishikawa H. Regulatory T (Treg) cells in cancer: can Treg cells be a new therapeutic target? Cancer Sci. 2019;110:2080–2089. doi: 10.1111/cas.14069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Zhang X., Wang J., Chen Z., et al. Engineering PD-1-presenting platelets for cancer immunotherapy. Nano Lett. 2018;18:5716–5725. doi: 10.1021/acs.nanolett.8b02321. [DOI] [PubMed] [Google Scholar]
- 264.Gianchecchi E., Fierabracci A. Inhibitory receptors and pathways of lymphocytes: the role of PD-1 in Treg development and their involvement in autoimmunity onset and cancer progression. Front. Immunol. 2018;9:2374. doi: 10.3389/fimmu.2018.02374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Bhardwaj P., Bhatia E., Sharma S., et al. Advancements in prophylactic and therapeutic nanovaccines. Acta Biomater. 2020;108:1–21. doi: 10.1016/j.actbio.2020.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Zhu M., Wang R., Nie G. Applications of nanomaterials as vaccine adjuvants. Hum. Vaccines Immunother. 2014;(10):2761–2774. doi: 10.4161/hv.29589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Kim J.H., Marks F., Clemens J.D. Looking beyond COVID-19 vaccine phase 3 trials. Nat. Med. 2021;(27):205–211. doi: 10.1038/s41591-021-01230-y. [DOI] [PubMed] [Google Scholar]
- 268.Janssens Y., Joye J., Waerlop G., et al. The role of cell-mediated immunity against influenza and its implications for vaccine evaluation. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.959379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Lee J., Khang D. Mucosal delivery of nanovaccine strategy against COVID-19 and its variants. Acta Pharm. Sin. B. 2022;(13):2897–2925. doi: 10.1016/j.apsb.2022.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Konrath K.M., Liaw K., Wu Y., et al. Nucleic acid delivery of immune-focused SARS-CoV-2 nanoparticles drives rapid and potent immunogenicity capable of single-dose protection. Cell Rep. 2022;38 doi: 10.1016/j.celrep.2022.110318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Liu L.X., Liu Z.J., Chen H.L., et al. Subunit nanovaccine with potent cellular and mucosal immunity for COVID-19. ACS Appl. Bio Mater. 2020;(3):5633–5638. doi: 10.1021/acsabm.0c00668. [DOI] [PubMed] [Google Scholar]
- 272.Li Y., Chen Z., Lu X., et al. STING and TLR9 agonists synergistically enhance the immunogenicity of SARS-CoV-2 subunit vaccine. Nano Res. 2023;(16):13322–13334. [Google Scholar]
- 273.Lai H., Xu L., Liu C., et al. Universal selenium nanoadjuvant with immunopotentiating and redox-shaping activities inducing high-quality immunity for SARS-CoV-2 vaccine. Signal Transduct. Targeted Ther. 2023;(8):88. doi: 10.1038/s41392-023-01371-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Guo M., Cao M., Sun J., et al. Durable and enhanced immunity against SARS-CoV-2 elicited by manganese nanoadjuvant formulated subunit vaccine. Signal Transduct. Targeted Ther. 2023;(8):462. doi: 10.1038/s41392-023-01718-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Chen K., Zhang Z., Zhang J., et al. Antigen-caged-adjuvant nanovaccines elicit potent humoral and cellular immune responses. Nano Today. 2023;53 [Google Scholar]
- 276.Zheng B., Peng W., Guo M., et al. Inhalable nanovaccine with biomimetic coronavirus structure to trigger mucosal immunity of respiratory tract against COVID-19. Chem. Eng. J. 2021;418 doi: 10.1016/j.cej.2021.129392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Zhuo S.H., Wu J.J., Zhao L., et al. A chitosan-mediated inhalable nanovaccine against SARS-CoV-2. Nano Res. 2022;(15):4191–4200. doi: 10.1007/s12274-021-4012-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Yitbarek K., Abraham G., Girma T., et al. The effect of Bacillus Calmette-Guerin (BCG) vaccination in preventing severe infectious respiratory diseases other than TB: implications for the COVID-19 pandemic. Vaccine. 2020;(38):6374–6380. doi: 10.1016/j.vaccine.2020.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Lee A., Floyd K., Wu S., et al. BCG vaccination stimulates integrated organ immunity by feedback of the adaptive immune response to imprint prolonged innate antiviral resistance. Nat. Immunol. 2024;(25):41–53. doi: 10.1038/s41590-023-01700-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Ni Q.Q., Zhang F.W., Liu Y.J., et al. A bi-adjuvant nanovaccine that potentiates immunogenicity of neoantigen for combination immunotherapy of colorectal cancer. Sci. Adv. 2020;(6) doi: 10.1126/sciadv.aaw6071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Shao J., Liu Q., Shen J., et al. Advanced pancreatic cancer patient benefit from personalized neoantigen nanovaccine based immunotherapy: a case report. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.799026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Zhang Y., Lin S., Wang X.Y., et al. Nanovaccines for cancer immunotherapy. Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol. 2019;11:e1559. doi: 10.1002/wnan.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Liu H., Chen H., Liu Z., et al. Therapeutic nanovaccines sensitize EBV-associated tumors to checkpoint blockade therapy. Biomaterials. 2020;(255) doi: 10.1016/j.biomaterials.2020.120158. [DOI] [PubMed] [Google Scholar]
- 284.Liu X., Su Q., Song H., et al. PolyTLR7/8a-conjugated, antigen-trapping gold nanorods elicit anticancer immunity against abscopal tumors by photothermal therapy-induced in situ vaccination. Biomaterials. 2021;(275) doi: 10.1016/j.biomaterials.2021.120921. [DOI] [PubMed] [Google Scholar]
- 285.Zhang L., Wang K., Huang Y., et al. Photosensitizer-induced HPV16 E7 nanovaccines for cervical cancer immunotherapy. Biomaterials. 2022;(282) doi: 10.1016/j.biomaterials.2022.121411. [DOI] [PubMed] [Google Scholar]
- 286.Liu X., Feng Z., Wang C., et al. Co-localized delivery of nanomedicine and nanovaccine augments the postoperative cancer immunotherapy by amplifying T-cell responses. Biomaterials. 2020;230 doi: 10.1016/j.biomaterials.2019.119649. [DOI] [PubMed] [Google Scholar]
- 287.Zhang Y., Ma S., Liu X.M., et al. Supramolecular assembled programmable nanomedicine as in situ cancer vaccine for cancer immunotherapy. Adv. Mater. 2021;33 doi: 10.1002/adma.202007293. [DOI] [PubMed] [Google Scholar]
- 288.Kim Y.J., Kang S., Shin H., et al. Sequential and timely combination of a cancer nanovaccine with immune checkpoint blockade effectively inhibits tumor growth and relapse. Angew. Chem. 2020;132:14736–14746. doi: 10.1002/anie.202006117. [DOI] [PubMed] [Google Scholar]
- 289.Xu J., Wang H., Xu L., et al. Nanovaccine based on a protein-delivering dendrimer for effective antigen cross-presentation and cancer immunotherapy. Biomaterials. 2019;207:1–9. doi: 10.1016/j.biomaterials.2019.03.037. [DOI] [PubMed] [Google Scholar]
- 290.Chen G., Li X., Li R., et al. Chemotherapy-induced neoantigen nanovaccines enhance checkpoint blockade cancer immunotherapy. ACS Nano. 2023;17:18818–18831. doi: 10.1021/acsnano.3c03274. [DOI] [PubMed] [Google Scholar]
- 291.Meng Z., Zhang Y., She J., et al. Ultrasound-mediated remotely controlled nanovaccine delivery for tumor vaccination and individualized cancer immunotherapy. Nano Lett. 2021;21:1228–1237. doi: 10.1021/acs.nanolett.0c03646. [DOI] [PubMed] [Google Scholar]
- 292.Li T., Chen G.J., Xiao Z.C., et al. Surgical tumor-derived photothermal nanovaccine for personalized cancer therapy and prevention. Nano Lett. 2022;(22):3095–3103. doi: 10.1021/acs.nanolett.2c00500. [DOI] [PubMed] [Google Scholar]
- 293.Zhang J., Li W., Qi Y., et al. PD-L1 aptamer-functionalized metal-organic framework nanoparticles for robust photo-immunotherapy against cancer with enhanced safety. Angew. Chem., Int. Ed. 2023;(62) doi: 10.1002/anie.202214750. [DOI] [PubMed] [Google Scholar]
- 294.Gun S.Y., Lee S.W.L., Sieow J.L., et al. Targeting immune cells for cancer therapy. Redox Biol. 2019;25 doi: 10.1016/j.redox.2019.101174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Luo M., Wang H., Wang Z., et al. A STING-activating nanovaccine for cancer immunotherapy. Nat. Nanotechnol. 2017;(12):648–654. doi: 10.1038/nnano.2017.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Azharuddin M., Zhu G.H., Sengupta A., et al. Nano toolbox in immune modulation and nanovaccines. Trends Biotechnol. 2022;(40):1195–1212. doi: 10.1016/j.tibtech.2022.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Shi Y., Pan C., Wang K., et al. Construction of orthogonal modular proteinaceous nanovaccine delivery vectors based on mSA-biotin binding. Nanomaterials. 2022;12:734. doi: 10.3390/nano12050734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Han X., Wang R., Xu J., et al. In situ thermal ablation of tumors in combination with nano-adjuvant and immune checkpoint blockade to inhibit cancer metastasis and recurrence. Biomaterials. 2019;(224) doi: 10.1016/j.biomaterials.2019.119490. [DOI] [PubMed] [Google Scholar]
- 299.Luo M., Liu Z., Fu Y. Combining nanovaccine and radiotherapy to revert the immunosuppressive environment in tumor. Nano Biomed. Eng. 2018;10:310. [Google Scholar]
- 300.Gou S.S., Liu W.W., Wang S.A., et al. Engineered nanovaccine targeting clec9a+ dendritic cells remarkably enhances the cancer immunotherapy effects of STING agonist. Nano Lett. 2021;21:9939–9950. doi: 10.1021/acs.nanolett.1c03243. [DOI] [PubMed] [Google Scholar]
- 301.Gebre M.S., Brito L.A., Tostanoski L.H., et al. Novel approaches for vaccine development. Cell. 2021;184:1589–1603. doi: 10.1016/j.cell.2021.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Li Y., Ayala O.C., Rauta P.R., et al. The application of nanotechnology in enhancing immunotherapy for cancer treatment: current effects and perspective. Nanoscale. 2019;11:17157–17178. doi: 10.1039/c9nr05371a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Roy P., Orecchioni M., Ley K. How the immune system shapes atherosclerosis: roles of innate and adaptive immunity. Nat. Rev. Immunol. 2022;(22):251–265. doi: 10.1038/s41577-021-00584-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Chen S., Xu X., Zhang Y., et al. Nanovaccine based on a biepitope antigen to potentiate the immunogenicity of a neoantigen. ACS Macro Lett. 2023;(12):281–287. doi: 10.1021/acsmacrolett.2c00742. [DOI] [PubMed] [Google Scholar]