Abstract
Natural killer (NK) cells are a subset of cytotoxic lymphocytes within the innate immune system. While they are naturally cytotoxic, genetic modifications can enhance their tumor-targeting capability, cytotoxicity, persistence, tumor infiltration, and prevent exhaustion. These improvements hold the potential to make NK-cell-based immunotherapies more effective in clinical applications. Currently, several viral and non-viral technologies are used to genetically modify NK cells. For nucleic acid delivery, non-viral methods such as electroporation, lipid nanoparticles, lipofection, and DNA transposons have gained popularity in recent years. On the other hand, viral methods including lentivirus, gamma retrovirus, and adeno-associated virus, remain widely used for gene delivery. Furthermore, gene editing techniques such as clustered regularly interspaced short-palindromic repeats-based, zinc finger nucleases, and transcription activator-like effector nucleases are the pivotal methodologies in this field. This review aims to provide a comprehensive overview of chimeric antigen receptor (CAR) arming strategies and discuss key gene editing techniques. These approaches collectively aim to enhance NK cell/NK cell CAR-based immunotherapies for clinical translation.
Keywords: immunotherapy, natural killer cells, cytokine-induced memory-like NK cells, CAR-NK cells, gene editing, gene delivery, CRISPR
Graphical Abstract
Graphical Abstract.
Significance Statement.
There is an urgent need to develop novel and more efficient adoptive cell therapies, especially for solid tumors. NK cell-based approaches are attractive for immunotherapy because of their excellent safety profile, and “their ability to kill” function without prior priming. Nevertheless, the cytotoxicity, anti-tumor targeting, and therapeutic efficiency of NK cells can be improved by arming them with chimeric antigen receptors (CARs).The focus of this review is to give a broad overview of the various CAR arming approaches and discuss key gene editing techniques employed to enhance further NK cell/NK cell CAR-based immunotherapies for clinical translation.
Background
Natural killer (NK) cells are a type of innate lymphocytes characterized by the expression of CD56 and the absence of CD3 and CD19 cell surface markers. NK cells represent around 5%-15% of circulating lymphocytes in healthy donors and they exhibit a strong cytotoxic and cytokine response against virus-infected and malignant cells without requiring prior antigen exposure, unlike T cells.1 NK-cell function is regulated by a balance of activating and inhibitory receptors; when activating signals overcome the inhibitory, NK cells become activated and initiate a cytotoxic response by releasing perforin and granzyme molecules.2 NK cells also mediate cytotoxicity via antibody-dependent cellular cytotoxicity (ADCC) as well as by engaging death cell receptors expressed by malignant/virus-infected cells through Fas ligand (FasL) and tumor necrosis factor-related apoptosis-inducing ligand (TRAIL).3,4 Upon activation, NK cells also secrete several modulatory proteins including IFNγ, TNFα, GM-CSF, and MIP1α.5 Although NK cells have traditionally been considered a part of the innate immune system with no recall, recent studies suggest that they exhibit memory-like features after viral infection, hapten exposure, or cytokine stimulation.6,7 Brief stimulation (12-16 hours) of NK cells with IL-12, IL-15, and IL-18 cytokines is sufficient to reprogram them into what are called cytokine-induced memory-like (CIML) NK cells with an enhanced cytotoxic response, IFNγ secretion, proliferative capacity, and in vivo persistence.8,9
NK cell-based approaches are attractive for immunotherapy because of their excellent safety profile (limited severe cytokine release syndrome, graft versus host disease, and neurotoxicity), their intrinsic propensity to target HLA class low/negative tumor cells, and “their ability to kill” function without needing prior priming.10 However, their relatively short half-life, the low yield from peripheral blood aphaeresis, and the low in vivo persistence and expansion are some of the major limitations of using conventional NK cells for clinical studies.11 Therapeutically, poor tumor infiltration, lack of tumor antigen recognition and/or escape, and functional exhaustion in the tumor microenvironment (TME) are other major hurdles in advancing adoptive cell therapies including NK-cell-based approaches. Many of these shortcomings can be overcome by genetically engineered NK cells to add various advantageous properties. Recently several key advances have been made to genetically modify NK cells allowing arming them with novel chimeric antigen receptor (CAR) constructs for enhanced tumor targeting.12 Furthermore, the use of advanced gene editing approaches enhances their in vivo persistence, improves metabolic fitness, and allows the secretion of molecules that modulate the tumor microenvironment (TME).13-15 In this review, we summarize current techniques used for genetic manipulation/engineering of the NK cells and major gene editing approaches including clustered regularly interspaced short palindromic repeats (CRISPR)-Cas9 based, zinc finger nucleases (ZFNs), transcription activator-like effector nucleases (TALENs), to further enhance their function.
Source of NK cells
The source of NK-cell products may impact the choice and or efficiency of genetic manipulation methods used and have their respective advantages and disadvantages, as summarized in Table 1. Peripheral blood mononuclear cells (PBMCs) in a healthy donor typically contain approximately 5%-10% of NK cells.16 PBMC-derived NK cells offer several advantages, including easy accessibility, a mature phenotype, and high cytotoxic function. However, they also have some drawbacks, such as donor variability and low cell numbers often necessitating ex vivo expansion in the context of using multiple infusions.10 Donor variability is also reflected in the differences in the efficiency of genetic engineering.15,17-19 In cord blood (CB), NK cells constitute approximately 20%-30% of the nuclear cell population.20,21 Wide availability in CB banks and the high proliferative potential of CB-derived NK cells make this source an attractive choice.10 Nonetheless, major challenges for this cell source include the necessity for ex vivo expansion, and donor variability.10 In recent years induced pluripotent stem cells (iPSCs) have also been successfully used to generate NK cells. iPSC-derived NK cells have the benefit of high proliferative potential during differentiation and the homogenous cell populations.22-24 However, iPSC-derived NK cells often exhibit an immature phenotype and require extended in vitro culture conditions leading to prolonged manufacturing times.22-24 Additionally, NK cells can be differentiated from hematopoietic stem cells (HSCs), which are also available in CB banks.25 However, this approach requires extended ex vivo expansion, there is often high variability between donors, and CB-derived NK cells typically lack significant expression of CD16 and thus display low antibody-dependent cell cytotoxicity (ADCC).10,22 Understanding the key limitations of these various NK-cell sources is critical for devising advancing NK-cell engineering therapies, including CAR-NK cells.
Table 1.
NK-cell sources and respective advantages and disadvantages.
| NK-cell source | Advantages | Disadvantages | Ref |
|---|---|---|---|
| PBMCs | Easy accessibility; mature phenotype; high cytotoxic function. | Donor variability; low cell numbers; require ex vivo expansion. | 10 |
| Cord blood | Available in cord blood banks; high proliferative potential. | Donor variability; require ex vivo expansion. | 10 |
| iPSCs | High proliferative potential; homogeneous cell population | Immature phenotype; extensive cell culture procedure. | 22-24 |
| HSCs | Available in cord blood banks. | Extensive cell culture procedure; high variability between donors; NK cells display low ADCC. | 22,25 |
Genetic Engineering of NK Cells
Genetic manipulation has enabled the improvement of NK-cell persistence, cytotoxicity, and tumor targeting capacities, thereby rendering a more promising approach for improving the efficacy of immunotherapy. Nevertheless, the engineering of NK cells still faces major technical challenges, including the difficulty of gene delivery into NK cells, their subsequent sensitivity to apoptosis, and low levels of gene expression.26,27 Recently, several engineering methods have been developed and adapted for NK-cell manipulation, including non-viral methods such as electroporation, lipid nanoparticles, lipofection, and DNA transposons and viral methods such as lentivirus, gamma retrovirus, and adeno-associated virus. The following sections provide a summary of the most relevant methodologies that are currently used for the genetic manipulation of NK cells, including both chimeric antigen receptor (CAR)-based and non-CAR-based strategies, and are summarized in Fig. 1 and Table 2.
Figure 1.
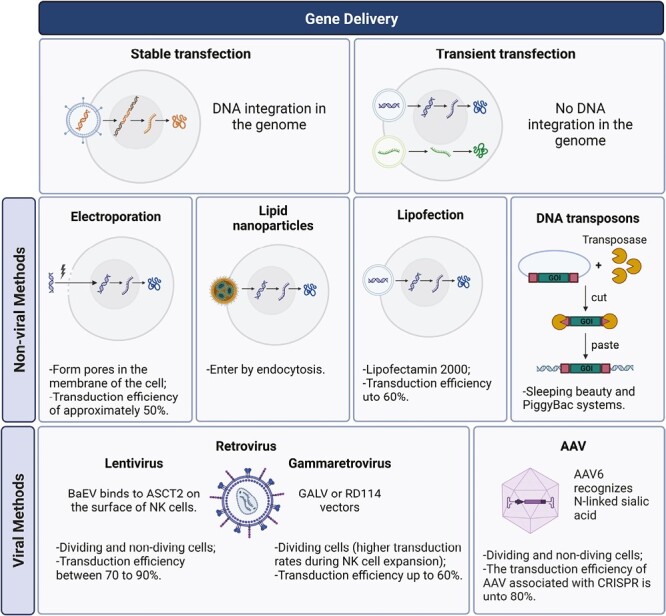
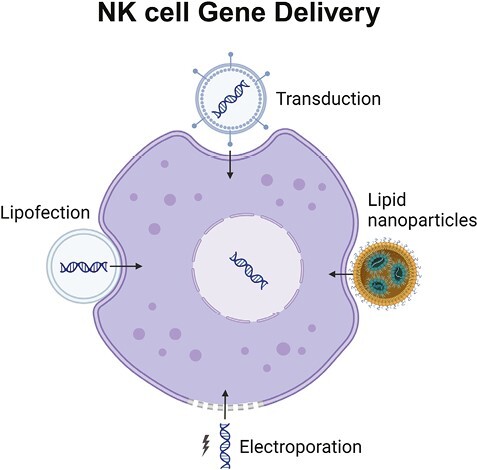
Major gene delivery methods used for genetic manipulation of NK cells. Cells can be genetically modified transiently when DNA is not integrated into the genome or in a stable manner when DNA is integrated into the genome. Currently, there are different methods for gene delivery which can be viral dependent using gamma retrovirus, lentivirus, adeno-associated virus, or non-viral-dependent methodologies such as lipofection, electroporation, DNA transposons, and lipid nanoparticles.
Table 2.
Characteristics of non-viral and viral gene delivery methods used in NK-cell engineering.
| Methodology | Efficiency | Advantages | Disadvantages | Applications | Ref | |
|---|---|---|---|---|---|---|
| Non-viral delivery methods | Electroporation | Approx. 50 %. | High transfection efficacy; versatility; In vivo and in vitro applications; safety. | High cell damage; only applicable on dividing cells. | CXCR4-NK cells; BCMA-NK cells; CCR7-CD19-NK cells; NKG2D-NK cells; CXCR1-NK cells; etc. | 46-49 |
| Lipid nanoparticles | Not reported. | Efficient gene delivery; stable transfection; safety; in vivo and in vitro applications. | Cost; limited understanding. | TRAIL-engineered NK cells. | 59 | |
| Lipofection | Approx. 60 %. | Cost effect; applicable for DNA, RNA, or plasmids. | Low transfection rate; High levels of toxicity. | HER2-CAR NK cells. | 59 | |
| DNA transposons | Not reported. | Efficient integration; single hit mutations. | Possible transient expression; possible off-target effects. | Meso-NK cells; CD73-NK92 cells; SLAMF7-CD19-CAR T cells. | 67,68,71 | |
| Viral delivery methods | Lentivirus | Between 70 – 90 %. | High transduction efficiency; applicable for non-dividing cells; stable integration. | Limited cargo capacity; safety; limited in vivo applications. | CD19-NK cells; NPM1-CIML-NK cells; etc. | 41,78,83 |
| Gammaretrovirus | Approx. 60 %. | High transduction efficiency. | Inability to transduce non-dividing cells; safety; limited in vivo applications. | CD19-NK cells; IL15-Cas9-CD19-NK cells; etc. | 85 | |
| Adeno-associated virus | Approx. 80 %. | High transduction efficiency; applicable for non-dividing cells; stable integration. | Limited cargo capacity; possible integration into the host genome. | CD33-CAR NK cells; PD1KO and ADAM17KO NK cells. | 89,131 |
CAR and Non-CAR Genetic Engineering of the NK Cells
Recent advances in CAR technology have revolutionized the adoptive cell therapy (ACT) field.28-30 CAR arming of immune cells allows specific recognition of an antigen in a major histocompatibility (MHC) independent fashion and provides an activation signal to trigger cytotoxicity and/or cytokine secretion.31 The essential components of a CAR include the single chain variable fragment (scFv) which binds to a tumor-specific antigen, a transmembrane domain, and one or more intracellular domains that typically include immunoreceptor tyrosine-based activation motifs (ITAM) which mediate activation of the CAR-engineered cell.32 The first-generation CAR design comprised only one intracellular domain, namely CD3ζ, while the second and third generations also added additional co-stimulatory domains, such as 2B4, 4-1BB, DAP10, DAP12, and OX40.33-35 The fourth-generation CARs incorporate additional edits allowing them to secrete molecules such as cytokines to improve cell proliferation and modulate the tumor microenvironment (TME).36 Recently, NK-cell CARs have been generated against a wide variety of tumor targets including CD19, CD20, BCMA, CD38, CD5, CD70, CD123, CD33, EGFR, HER2, mesothelin, and NKG2D ligands, among others.33,37-40 Our group has recently generated CIML-NK -cell CARs taking advantage of the enhanced anti-tumor function and proliferative capacity of CIML-NK cells compared to the conventional NK cells.41 CIML-NK cells armed with a neoepitope-specific CAR (TCR-mimetic CAR) showed enhanced antitumor response against nucleophosmin-1 (NPM1)-mutated AML cells while avoiding off-target toxicity.41 In another study, a CD19 CIML-NK-cell CAR demonstrated potent responses to NK-resistant lymphomas.42
Major non-CAR genetic manipulations have mostly focused on knocking out genes coding for proteins that are involved with dampening of the NK-cell-based immune responses. These genes include CIS, which codes for cytokine-inducible SH2-containing protein (CISH) protein, CD38, and TGFBR1/2, which codes for the TGF-β receptor.15,17-19 For these genetic modifications, CRISPR/Cas-9 approaches have been used and are further discussed in the section “Gene editing approaches” later in this review.
Non-Viral Delivery Methods
Non-viral methods are typically preferred for gene delivery due to their enhanced safety compared to viral methods and their broader applicability in vivo, making non-viral methods more versatile.43 Non-viral methods can be divided into electroporation in which no carrier is involved, and carrier-based systems including lipid nanoparticles, lipofection, and DNA transposons.
Electroporation
Electroporation is based on the use of electric pulses that induce small and temporary pores in the cell membrane allowing entry of genetic material into the cell.44 Electroporation can be used with most cell types, and it can generate stable and transient genetic modifications, based on the genetic material used.
In transient transfection, genetic material is not passed from generation to generation since it is not integrated into the genome of the cell and only stays for a limited period, usually for a few days as the genetic material used (mRNA) is degraded from the cells.45 Recently, 2 mRNAs encoding a CAR targeting NKG2D ligands as well as chemokine receptor CXCR1 were introduced by electroporation into PBMC-derived NK cells with high transfection efficiency and the expression lasted for 72 hours. These CXCR1-modified NK cells displayed increased migration toward tumors supernatant in vitro and augmented infiltration into human tumors in vivo in subcutaneous and intraperitoneal xenograft models.46 Furthermore, local treatment of metastatic colorectal cancer by intraperitoneal infusion and intratumoral injection of the CAR NK cells was tested in a clinical trial where PBMC-derived NK cells were transfected by RNA electroporation to generate CAR NK cells with transiently enhanced activity against NKG2D-ligand-expressing cancer cells.47 PBMC-derived NK cells were also electroporated with mRNA encoding the chemokine receptor CXCR4 to improve their migration, and, in addition, CXCR4-modified NK cells were electroporated with mRNA encoding a CAR targeting B-cell maturation antigen (BCMA), resulting in higher tumor recognition.48 Furthermore, Ingegnere et al generated CCR7-CD19-CAR NK cells through mRNA electroporation in PBMC-derived NK cells, reaching approximately 50% transfection efficiency.49 This novel CAR allowed the recognition of CD19 and the CCR7 receptor in the tumor cells.49 Importantly, when delivering mRNA or plasmid DNA, it leads to transient expression, requiring the exploration of alternative methods to achieve stable expression. One widely used approach involves the use of DNA transposons, a topic that will be discussed in the following sections.
The use of electroporation for gene insertions offers several advantages, including its suitability for all cell types, the capacity to handle a wide range of gene sizes, the ability to perform both transient and stable gene insertions, in vitro and in vivo applications, and safety benefits.46-49 However, this methodology also has some major limitations including significant cell membrane damage following electroporation leading to cell apoptosis, and its efficiency limited to dividing cells.46-49 Furthermore, to achieve high transfection efficiency, NK cells require prior stimulation with cytokines or feeder cells, pre-warmed material and media, and optimized electroporation parameters.43,50 For clinical applications, electroporated T cells expressing CARs mediating tumor regression after multiple doses have been reported, and this strategy may also be relevant to engineered NK cells.51
Charge-Altering Releasable Transporters (CARTs)
Recently, a new method has been described to introduce the CAR mRNA into non-dividing cells, named charge-altering releasable transporters (CARTs).52 CARTs are synthetic nanoparticles that change their own surface charge when encountering a specific trigger, such as receptors on the surface of the cells. This change in the charge enables them to bind to the target cell and CARTs are internalized by endocytosis. In response to the acidic environment inside the endosome, CARTs release their cargo.52 Compared to the electroporation, CARTs transfected NK cells more effectively, better-preserved cell viability, and caused minimal reconfiguration of NK-cell phenotype and function.53 However, this technique is only applicable to small nucleic acids, may require big optimizations, and presents limited in vivo delivery.53
Lipid Nanoparticles
Lipid nanoparticles (LNPs) encapsulate the mRNA with a lipid layer to avoid its degradation. LNPs are often called liposomes due to their similarities however, while liposomes have one or more rings of a lipid bilayer of phospholipids, LNPs usually have a single phospholipid outer layer combined with cholesterol molecules.54 LNPs enter the cells via endocytosis, and then they release the cargo into the cytosol after endosome acidification.55 Recently, CAR-T cells were produced in vivo to treat cardiac injury using LNPs containing mRNA encoding a CAR designed to bind to T cells allowing the LNP fusion with T cell.56 Chandrasekaran et al reported the development of what they call “super NK cells,” due to the addition of TRAIL by liposomes into mouse lymph-node derived-NK cells. TRAIL-engineered NK cells showed enhanced anti-tumor activity by inducing apoptosis in tumor-draining lymph nodes in vivo.57 LNPs present efficient gene delivery, induce stable transfection, and are suitable for in vitro and in vivo applications however, fine-tuning nanoparticles for specific immune cell targeting can be costly, and there is still limited understanding of this technology.58
Lipofection
Lipofection involves encapsulating the nucleic acids in cationic liposomes that then fuse with the target cell membrane releasing their cargo into the cell cytosol.43 Lipofectamine 2000 has been used to generate CAR NK cells and, for example, in one study HER2-CAR NK cells were developed from PBMC-derived NK cells using this system with approximately 60% transduction efficiency. These HER2-CAR NK cells demonstrated anti-tumor capacities in vivo.59 This methodology presents low cost, and it is applicable for DNA, RNA, and plasmids. Nevertheless, this technique presents some limitations, including toxicity due to the charged lipids used and it still presents relatively low transfection rates in most procedures.60
DNA Transposons (PiggyBac and Sleeping Beauty)
DNA transposons or transposable elements are fragments of DNA that move from one site to another, also known as “jumping genes.”61 DNA transposons can be delivered into the cytosol by several methods such as electroporation and lipid-based nanoparticles.62,63 DNA transposons consist of a transposon vector housing the sequences to be transferred by terminal inverted repeats (TIR), along with a transposase enzyme responsible for recognizing TIR sequences and facilitating the excision and reintegration of the transposon.64 There are several different DNA transposons, the more commonly used for gene editing are the sleeping beauty (SB) transposon system and the piggyBac (PB) system, both inducing a stable DNA integration.43,65,66 In one study, the NK-92 cell line was used to screen mesothelin-specific CAR constructs using SB and PB systems and showed an improved anti-tumor response in mesothelin-positive tumors.67 In addition, CAR NK-92 cells have been developed using the PB system, recognizing the CD73 marker, with improved killing abilities against CD73-positive lung cancer in vivo model.68 Both SB and PB have been extensively used for T-cell editing, suggesting their great potential for gene editing of primary human immune cells.69,70 A CD19-CAR and a signaling lymphocyte activation molecule family member 7 (SLAMF7)-CAR introduced into PBMC-derived T cells have already entered phase I/II clinical trials for the treatment of leukemia, lymphoma, or multiple myeloma (NCT04499339).71 DNA transposons exhibit certain advantages over alternative methodologies, including their efficient integration and the induction of single-hit mutations. Nevertheless, they are constrained by limitations, including the potential for off-target effects and the possibility of transient expression.67,68,71
Viral Methods for Gene Delivery in NK Cells
Retroviruses (lentivirus, gammaretrovirus, alpharetrovirus), and adeno-associated viruses (AAV) are the most commonly used viral vectors for gene delivery allowing stable (retroviruses) and transient (AAV) gene expression in NK cells (Fig. 1).72 The retrovirus family members consist of lipid-enveloped virion particles that englobe 2 copies of a single-stranded RNA and an RNA-dependent DNA polymerase called reverse transcriptase in addition to other proteins. AAV belongs to the Parvoviridae family, lacks a lipid envelope (naked virus), has a single copy of single-stranded DNA, and depends on co-infection with other viruses to replicate (replication-defective).73
Lentiviral Delivery System
Lentivirus is a genus of retroviruses that includes the human immunodeficiency virus (HIV) and its use in gene delivery presents several advantages compared to other retroviruses including their ability to transduce non-dividing cells, stably incorporate their genome into the host cell, and lack of immunogenic viral proteins.74
Vesicular stomatitis virus G protein (VSV-G) is widely used as an envelope protein to pseudo-type the lentiviral particles, binding to the low-density lipid receptors (LDLR) on the surface of human lymphocytes to gain entrance into the cell.75 Contrary to T-cells, VSV-G does not effectively transduce NK cells since only a small fraction of NK cells express LDLR.76 The addition of statins, specifically rosuvastatin, can improve VSV-G transfection efficiency by inducing higher LDLR expression in NK cells.77 Gong et al reported that the combination of rosuvastatin with geranylgeranyl-pyrophosphate (GGPP) improved viral transduction without affecting the cytotoxic properties of the NK cells.77 On the other hand, the use of Baboon envelope pseudo-typed lentiviral vectors (BaEV) allows favorable transduction efficiency in NK cells, around 20-fold higher compared to VSV-G, this is since BaEV receptors, mainly ASCT-1 and ASCT-2, are expressed on the NK-cell surface at much higher levels compared to the LDLR.78,79 Indeed, in one study activated PBMC-derived NK cells transduced with BaEV showed a transduction rate of 84%, which is much higher than other lentiviruses.79 In another study, using the BaEV method, CD19-CAR was successfully expressed in around 70% of the primary PBMC-derived NK cells from multiple donors.78 Furthermore, the same high efficiency was obtained when generating NPM1-CAR CIML-NK cells (isolated from PBMCs) against AML using BaEV by our group.41 We found that CIML differentiation was associated with a significant increase in the expression of ASCT-2 leading to enhanced transduction efficiency using the BaEV system.41 While lentiviruses are extensively used in gene manipulation and offer several advantages as previously described, their principal limitations are their limited cargo capacity and restricted in vivo applicability.80
Gammaretrovirus delivery system
Gammaretrovirus vectors like Gibbon ape leukemia virus (GALV) and RD114 were some of the first vectors to be used for transduction in gene therapy, although initially with low transduction efficiency.81 Recently, Guven et al demonstrated that performing 2 rounds of gammaretroviral transduction (GALV) in NK cells can increase efficiency by 75% after 21 days of NK-cell expansion.82 The retroviruses were produced using a PG13 retrovirus packaging cell line derived from TK-NIH/3T3 (mouse fibroblast) cells.82 Several studies have used RD114 pseudo-typed retrovirus to successfully transduce NK cells.83 RD114 pseudo-type vector fused to the cytoplasmic tail (TR) of the amphitropic murine leukemia virus (MLV-A), named RD114-RT, showed augmented transduction of human primary blood lymphocytes and CD34+cells.84 In one study, cord blood derived CD19-CAR NK cells were generated with this system achieving approximately 67% transduction efficiency.85 In addition, the CD19-CAR construct also included a secreted version of IL-15 and an inducible caspase-9.85 In the clinical setting, 73% of patients responded with 7 out of 8 patients reaching complete remission.85 Similarly, Mϋller et al generated CD19-CAR-NK cells with RD114-TR pseudotyped retroviral vectors and reported almost 3 times higher NK-cell transduction on day 3 in combination with vectofusin-1 when compared to lentiviral vectors.83 Despite these advances, this system presents some major limitations including the inability to transduce non-dividing cells at high rates.11
Adeno-Associated Viral (AAV) Delivery System
Recombinant AAVs are protein-based nanoparticles engineered to access the cell membrane by recognizing attachment factors and then delivering the DNA cargo.86 Depending on the serotype, the entry process is mediated by different receptors, for example, the AAV6 attaches to the N-linked sialic acid on the target cell membranes.87 While AAV can deliver DNA to dividing and non-dividing cells, it is still difficult to produce the vectors, and the cargo capacity is limited to ~5kb which is one of the major limitations. Lately, this methodology has been used as a gene delivery system in generating CAR NK cells. When combined with CRISPR/Cas9, AAV showed highly efficient and stable CAR expression in the PBMC-derived NK cells.88 In this study, Kararoudi et al generated CD33-CAR NK cells that demonstrated efficacy against AML. They reported around 78% transducing efficiency 7 days after electroporation and transduction.88 Furthermore, a highly efficient knockout of A Disintegrin and Metalloproteinase-17 (ADAM17) and PD-1 in PBMC-derived NK cells was performed using CRISPR combined with AAV6, with approximately 100% efficiency, improved NK-cell activity, cytotoxicity, and cytokine production.89
Several auxiliary reagents are often used to enhance transduction efficiency in NK cells. Polybrene (cationic polymer), vectofusin-1 (synthetic cationic peptide), and rectronectin (recombinant fibronectin fragment) promote adhesion and fusion between the viral particles and cell membranes.90 In one study, retronectin enhanced lentiviral/VSV-G gene delivery to NK cells while vectofusin-1 enhanced the transduction efficiency of RD114-TR pseudotyped retroviral vectors.83 Furthermore, spinfection (centrifugation at a low speed for a prolonged time) is often used to improve transduction efficacy compared with static transduction.91
Overall, viral vectors are the most used methods for gene therapy, but there are still several limitations, including the high cost of production, batch-to-batch variability, GMP grade-related restrictions, and safety concerns.92 Non-viral delivery methods have been developed to overcome some limitations in recent years.
Gene Editing Approaches
The first gene editing tool described was the use of restriction enzymes, which cleave specific regions of the DNA, providing the possibility to remove or introduce new genetic sequences.93 Recent advancements in precise gene editing methodologies have enabled the alteration of the cell’s genome at specific loci, which has been exploited in immunotherapy.94 The gene editing approaches used for NK-cell engineering are summarized in Table 3 and Fig. 2.
Table 3.
Gene editing approaches used in NK-cell engineering.
| Gene editing approaches | |||||
|---|---|---|---|---|---|
| Methodology | NK-cell sources | Advantages | Disadvantages | Applications | Ref |
| CRISPR | All NK-cell sources. | Versatility; in vivo and in vitro applications; cost-effective. | DSBs; chromosomal translocations; off-target effects. | CD33-CAR NK cells; CISHKO NK cells; CD38KO NK cells; BCMA-CD16-IL5 NK cells; etc. | 107,108 |
| Zinc finger nucleases | iPSC-derived NK cells | Safety; DNA binding specificity. | DSBs; off-target effects. | EpCAM-CAR NK cells. | 94,116 |
| TALEN | iPSC-derived NK cells | Specificity; flexibility. | DSBs; off-target effects. | TGFβR2KO NK cells. | 118,119 |
Figure 2.
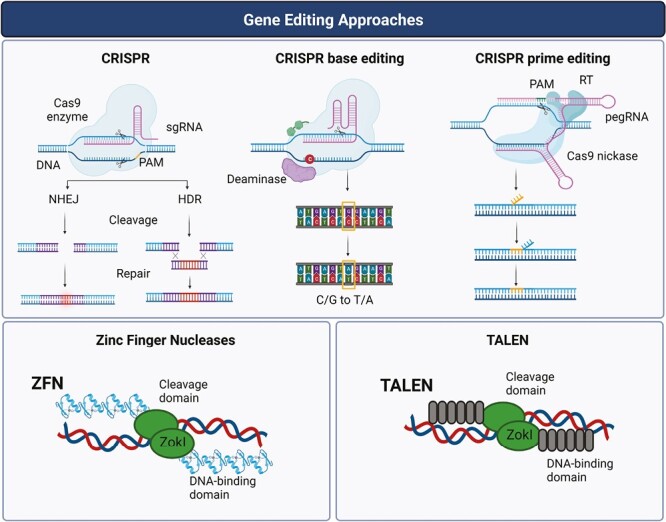
Gene editing strategies used for NK-cell editing. The precise genetic alteration of NK cells is allowed by the development of key gene editing methodologies such as zinc finger nucleases (ZFN), transcription activator-like effector nucleases (TALEN), clustered regularly interspaced short palindromic repeats (CRISPR)/Cas9 technology, and its derivatives like base editing and prime sediting methodologies.
CRISPR Gene Editing
Clustered regularly interspaced short palindromic repeats (CRISPR) represent a system used by bacteria to overcome viral infections by editing foreign DNA and its discovery has revolutionized the gene editing field.95,96 CRISPR is a class of repeated DNA sequences that act in coordination with CRISPR-associated (Cas) proteins, which are endonucleases that uses a single guide RNA and protospacer adjacent motif (PAM) to target and cleave DNA sequences, generating double-strand breaks (DSBs).96 These DSBs are repaired by a non-homologous end-joining (NHEJ) pathway, which enables gene insertions or deletions, or by a homology-directed repair (HDR) that precisely repairs the DSBs.97 Indeed, the DNA encoding of a CAR can be delivered as an HDR template by AAV vectors following electroporation of Cas9/RNP as described by Kararoudi et al.98 In this study, CD33-targeting CAR NK cells were developed, isolated from PBMCs, with improved efficacy against AML targets generated by electroporating Cas9/RNP and using AAV6 gene delivery.98
In the past years, different approaches using CRISPR technologies have been used to enhance the anti-tumor capacity of NK cells. For example, several groups have used CRISPR/Cas9 approaches to abrogate the expression of CISH in NK and T cells.99,100 The CIS gene encodes the CISH protein, which is an important checkpoint in NK cells.15 Daher et al used CRISPR/Cas9 to abrogate the expression of CISH protein which enhanced the metabolic fitness and anti-tumor activity of armored IL-15-secreting CB-derived CAR NK cells.15 Moreover, iPSC-NK cells with CISHKO exhibited improved expansion and cytotoxicity and increased IL-15-mediated-STAT signaling activity.101 In addition, when Bernard et al deleted CISH using CRISPR in human PBMC-derived NK cells, they also observed an enhancement of the IL-15 signaling pathway, antitumor functions as well as the signaling of natural cytotoxic receptors, such as NKp46.102 Recently, the anti-tumor functions of PBMC-derived NK cells against glioblastoma were enhanced by direct blockade of the αv integrin or TGF-β or by electroporation coupled to CRISPR gene editing of TGFBR2 gene, which codes for TGF-β receptor 2, on allogeneic NK cells.103 Kararoudi et al developed CRISPR-modified CD38KO NK cells to overcome the Daratumumab (DARA)-induced fratricide.18 DARA is a human monoclonal antibody targeting CD38 and it is used in the treatment of multiple myeloma and, consequently, DARA also induces the depletion of CD38high NK cells. The CD38 depletion of PBMC-derived NK cells eliminated DARA-induced fratricide and boosted their effector activity, in MM and AML.17,18 Furthermore, quadruple gene-engineered iPSC-derived NK cells (iNK) were generated by CRISPR followed by lentiviral transduction. They added BCMA CAR, a high affinity, non-cleavable CD16 to augment ADCC, membrane-bound IL-15 to enhance function and persistence coupled with knock out of CD38 to prevent antibody-mediated fratricide and enhance NK-cell metabolic fitness.104 However, this tool still presents some limitations due to the formation of DSBs that can cause large DNA deletions and chromosomal translocations. In addition, HDR presents low efficacy in non-dividing cells105,106 While CRISPR-Cas9 gene editing has advanced our efforts to mediate genetic modification of immune cells, including NK cells, however, the introduction of unintended mutations in off-target genes remains a concern.107 Recent research has revealed that CRISPR-Cas9 can induce large structural variants (SVs) not only at the intended target sites but also at off-target locations in vivo. This unintended disruption of non-targeted genes can have serious consequences, affecting their function or regulation.107,108 These findings highlight the importance of carefully monitoring CRISPR-edited cells for off-target effects long term.
Several variations of the CRISPR/Cas9 technology have emerged recently, including alternative CRISPR-associated nucleases like Cas12, also known as Cpf1. Like Cas9, Cas12 utilizes a guided sgRNA to induce DSBs at specific DNA sequences. However, a key difference is that while Cas9 targets PAMs of NGG, Cas12 can recognize T-rich PAMs, which can impact its specificity and efficiency.109 Additionally, Cas12 is of smaller size compared to Cas9, potentially leading to more efficient delivery into cells. Its application in human NK-cell engineering has been explored to optimize the nuclear localization signal (NLS) composition.110 In addition to Cas12, an RNA-specific nuclease, known as Cas13, has been reported and unlike Cas9 and Cas12, Cas13 does not require PAMs and instead, it contains 2 higher eukaryotes and prokaryotes nucleotide-binding (HEPN) domains that specifically cleave RNA.111 However, Cas13 may have potential drawbacks, including off-target effects.112 These advancements in CRISPR/Cas9 technology, along with the emergence of Cas12 and Cas13 variants provide exciting opportunities for gene editing modifications in immune cell engineering.
Zinc Finger Nucleases
Zinc finger nucleases (ZFN) are composed of 2 components: a cleavage domain which is an engineered nuclease (Fok I) fused to a DNA-binding domain composed of zinc finger proteins. When zinc finger proteins bind to specific sequences of DNA, they recruit the FokI cleavage domain to the target site, allowing it to cut the DNA by a double-stranded break.113 ZFNs can be engineered to target longer DNA regions increasing their specificity compared to conventional restriction enzymes.114,115 Recently, human iPSCs were genetically modified with ZFN technology to generate EpCAM-CAR iNK cells.116 These EpCAM-CAR iNK cells showed lytic activity against NK-cell resistant, EpCAM-positive cancer cells, but not to EpCAM-positive normal cells.116 While ZFNs are considered safe and present DNA binding specificity, this methodology also entails certain limitations, including the induction of DSBs and off-target effects and have overall fallen out of favor.94
Transcription Activator-Like Effector Nucleases (TALENs)
The transcription activator-like effector nucleases (TALENs) are like the ZFNs, both contain a DNA-binding domain and a cleavage domain inducing double-stranded breaks in the DNA. TALENs differ from ZFNs mainly in their DNA binding domain, while ZFNs use zinc finger proteins, TALENs use transcription activator-like effector (TALE) repeat proteins as their DNA-binding domain, which consists of tandem arrays of 33-35 amino acid repeats increasing specificity. The first-in-human use of TALEN gene-edited CD52 depleted T cells was described in 2 infants with refractory relapsed B cell acute lymphoblastic leukemia (B-ALL).117 Recently, the use of TALEN technology to alter NK cells was reported by Chen et al where they developed an iNK-cell platform with Cellectis TALEN gene editing technology, which differentiates iPSC into iNK cells with upregulation of IL-15 and downregulation of TGFβ receptor and these iNK cells showed enhanced antitumor activity.118 Both ZFNs and TALENs can induce non-specific modifications causing off-target effects which makes them somewhat less attractive than more precise CRISPR-based gene editing.119
Challenges and Future Perspectives
The gene editing approaches are advancing rapidly; however, the following key challenges need to be overcome to further accelerate the development of innovative and novel cell therapies:
Off-target effects: The use of the current gene editing tools is associated with off-target effects by producing unintentional alterations in the DNA with the potential to cause deleterious effects.108,120 Recent versions of Cas9 with higher fidelity have successfully been developed to reduce off-target activity.121,122
Gene delivery: While viral gene delivery methods are more commonly used than non-viral methods, they still present some limitations such as high cost of production, batch variability, and safety.123 Because of these concerns, more recently non-viral methods like electroporation have been explored. However, transient expression (when using RNA-based methods), lower expression efficiency, and some poor cell viability after electroporation remain some of the major areas that still need further optimization.
Tumor heterogeneity: Tumors, particularly solid tumors are composed of malignant cells with significant intra-tumor and interpatient gene expression and mutation which negatively impacts the efficiency of the cell therapy-based treatments.124 Furthermore, the tumor microenvironment is complex and often mediates the suppression and exhaustion of the adoptively transferred immune cells and thus further hampering the efficacy of these approaches.
Long-term effects: considering that the gene editing field is very recent, there are limited long-term safety data from patients having received genetically modified immune cells. Some of the approaches that have been explored recently include the integration of caspase-controlled suicide vectors in genetically modified immune cells potentially allowing the elimination of these cells in a controlled way however long-term safety remained to be evaluated.85
Access and affordability: The current gene editing-based therapies are complex requiring highly skilled personnel and facilities to develop and deliver them and thus limiting them to very advanced centers.125 Furthermore, these therapies are very expensive which further restricts their broader application.
While major advances have been made in recent years in developing novel gene editing and delivery technologies, further research is crucial to address some of the key challenges in the field. Recently, alternative CRISPR-based gene editing tools have been developed, such as base and prime editing, which represent important steps in overcoming the limitations of established gene editing methodologies.126-128
Base editing systems, pioneered by Liu’s lab, enable the irreversible conversion of one target DNA base into another without requiring DSBs or a donor template, for example, cytosine base editors target and convert CG to TA.126 This approach involves fusing a “catalytically dead” Cas9 to DNA deaminases, allowing the alteration of a single nucleotide.126 Base editing has been applied in therapeutic gene editing approaches to correct point mutations that cause diseases.127
Another gene editing approach developed by Liu’s lab is known as prime editing.127 While base editing tools can edit SNPs, they cannot mediate targeted insertions, deletions, or perform all single-nucleotide conversions. Prime editing employs a reverse transcriptase Cas9 nickase, guided by an engineered prime editing guide RNA (pegRNA) to induce single-stranded breaks in the DNA. This technique has the potential to allow various substitutions, insertions, and/or deletions over dozens of base pairs.128,129
More recently, a newer CRISPR-based tool known as Programmable Addition via Site-specific Targeting Elements (PASTE) permits targeted insertion of large DNA sequences avoiding DSBs.130 It builds upon prime editing and integrates a serine integrase that recognizes an attachment site placed near the target sequence. The development of these CRIPSR-based tools enables the substitution of different lengths of DNA without creating DSBs, thereby increasing the safety of gene editing methodologies.130
Significant research efforts continue to focus on developing more efficient, safer gene delivery, and gene editing methods with minimal off-target toxicity. These advancements hold the promise of relatively rapid translation to clinical applications in the near future.
Contributor Information
Andreia Maia, Champalimaud Centre for the Unknown, Champalimaud Foundation, Lisbon, Portugal; NOVA Medical School of NOVA University of Lisbon, Lisbon, Portugal; Division of Cellular Therapy and Stem Cell Transplant, Dana-Farber Cancer Institute, Harvard Medical School, MA, USA.
Mubin Tarannum, Division of Cellular Therapy and Stem Cell Transplant, Dana-Farber Cancer Institute, Harvard Medical School, MA, USA.
Rizwan Romee, Division of Cellular Therapy and Stem Cell Transplant, Dana-Farber Cancer Institute, Harvard Medical School, MA, USA.
Conflict of Interest
The author indicated no financial relationships.
Author Contributions
A.M., M.T., R.R.: conception and design, manuscript writing, final approval of manuscript.
Data Availability
No new data were generated or analyzed in support of this research.
References
- 1. Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S.. Functions of natural killer cells. Nat Immunol. 2008;9(5):503-510. 10.1038/ni1582 [DOI] [PubMed] [Google Scholar]
- 2. Prager I, Watzl C.. Mechanisms of natural killer cell-mediated cellular cytotoxicity. J Leukoc Biol. 2019;105(6):1319-1329. 10.1002/JLB.MR0718-269R [DOI] [PubMed] [Google Scholar]
- 3. lo Nigro C, Macagno M, Sangiolo D, et al. NK-mediated antibody-dependent cell-mediated cytotoxicity in solid tumors: biological evidence and clinical perspectives. Ann Transl Med. 2019;7(5):105. 10.21037/atm.2019.01.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Barnes, SA, Trew, I, de Jong E, Foley, B.. Making a killer: selecting the optimal natural killer cells for improved immunotherapies. Front Immunol. 2021;12:1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Paul S, Lal G.. The molecular mechanism of natural killer cells function and its importance in cancer immunotherapy. Front Immunol. Preprint at 2017;8:1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sheppard S, Sun JC.. Virus-specific NK cell memory. replaced the correct journal title. 2021;218(4):e20201731. 10.1084/jem.20201731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brillantes M, Beaulieu AM.. Memory and memory-like NK cell responses to microbial pathogens. Front Cell Infect Microbiol. Preprint at 2020;10:102. 10.3389/fcimb.2020.00102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cooper MA, Elliott JM, Keyel PA, et al. Cytokine-induced memory-like natural killer cells. Proc Natl Acad Sci USA. 2009;106(6):1915-1919. 10.1073/pnas.0813192106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Romee R, Schneider SE, Leong JW, et al. Cytokine activation induces human memory-like NK cells. Blood. 2012;120(24):4751-4760. 10.1182/blood-2012-04-419283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Laskowski TJ, Biederstädt A, Rezvani K.. Natural killer cells in antitumour adoptive cell immunotherapy. Nat Rev Cancer. 2022;22(10):557-575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Heipertz EL, Zynda ER, Stav-Noraas TE, et al. Current perspectives on “off-the-shelf” allogeneic NK and CAR-NK cell therapies. Front Immunol. 2021;12:732135. 10.3389/fimmu.2021.732135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang L, Meng Y, Feng X, Han Z.. CAR-NK cells for cancer immunotherapy: from bench to bedside. Biomark Res. 2022;10(12):1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liu E, Marin D, Banerjee P, Rezvani K.. Use of CAR-transduced NK cells in CD19-positive lymphoid tumors. N Engl J Med. 2020;382:545-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Choi C, Finlay DK.. Optimising NK cell metabolism to increase the efficacy of cancer immunotherapy. Stem Cell Res Ther. Preprint at 2021;12(1):1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Daher M, Basar R, Gokdemir E, et al. Targeting a cytokine checkpoint enhances the fitness of armored cord blood CAR-NK cells. Blood. 2021;137(5):624-636. 10.1182/blood.2020007748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zotto GD, Antonini F, Pesce S, et al.. Comprehensive phenotyping of human PB NK cells by flow cytometry. J Quant Cell Sci. 2020: 97(9):1-9. 10.1002/cyto.a.24001 [DOI] [PubMed] [Google Scholar]
- 17. Gurney M, Stikvoort A, Nolan E, et al. CD38 knockout natural killer cells expressing an affinity optimized CD38 chimeric antigen receptor successfully target acute myeloid leukemia with reduced effector cell fratricide. Haematologica. 2022;107(2):437-445. 10.3324/haematol.2020.271908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kararoudi M, et al. CD38 deletion of human primary NK cells eliminates daratumumab-induced fratricide and boosts their effector activity. Blood. 2020;136(21):2416-2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gerew A, Sexton S, Wasko KM, et al. Deletion of CISH and TGFβR2 in iPSC-derived NK cells promotes high cytotoxicity and enhances in vivo tumor killing. Blood. 2021;138(Supplement 1):2780. [Google Scholar]
- 20. Sarvaria A, Jawdat D, Madrigal JA, Saudemont A.. Umbilical cord blood natural killer cells, their characteristics, and potential clinical applications. Front Immunol. 2017;8:329. 10.3389/fimmu.2017.00329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shpall EJ, Rezvani K.. Cord blood expansion has arrived. Blood. Preprint at 2021;138(16):1381-1382. 10.1182/blood.2021012725 [DOI] [PubMed] [Google Scholar]
- 22. Zhu H, Blum RH, Bjordahl R, et al. Pluripotent stem cell-derived NK cells with high-affinity noncleavable CD16a mediate improved antitumor activity. Blood. 2020;135(6):399-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Goldenson BH, Hor P, Kaufman DS.. iPSC-derived natural killer cell therapies—expansion and targeting. Front Immunol. 2022;13:841107. 10.3389/fimmu.2022.841107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li Y, Hermanson DL, Moriarity BS, Kaufman DS.. Human iPSC-derived natural killer cells engineered with chimeric antigen receptors enhance anti-tumor activity. Cell Stem Cell. 2018;23(2):181-192.e5. 10.1016/j.stem.2018.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Luevano M, Madrigal A, Saudemont A.. Generation of natural killer cells from hematopoietic stem cells in vitro for immunotherapy. Cell Mol Immunol. Preprint at 2012;9(4):310-320. 10.1038/cmi.2012.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wu X, Matosevic S.. Gene-edited and CAR-NK cells: opportunities and challenges with engineering of NK cells for immunotherapy. Mol Ther Oncolytics. 2022;27:224-238. 10.1016/j.omto.2022.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Carlsten M, Childs RW.. Genetic manipulation of NK cells for cancer immunotherapy: techniques and clinical implications. Front Immunol. 2015;6:266. 10.3389/fimmu.2015.00266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lee DW, Kochenderfer JN, Stetler-Stevenson M, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet (London, England). 2015;385(9967):517-528. 10.1016/S0140-6736(14)61403-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Albinger N, Hartmann J, Ullrich E.. Current status and perspective of CAR-T and CAR-NK cell therapy trials in Germany. Gene Ther. 2021;28(9):513-527. 10.1038/s41434-021-00246-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sterner RC, Sterner RM.. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J. 2021;11(4):69. 10.1038/s41408-021-00459-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Benmebarek MR, Karches CH, Cadilha BL, et al. Killing mechanisms of chimeric antigen receptor (CAR) T cells. Int J Mol Sci. Preprint at 2019;20(6):1283. 10.3390/ijms20061283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rafei H, Daher M, Katayoun R.. Chimeric antigen receptor (CAR) natural killer (NK)-cell therapy: leveraging the power of innate immunity. Br J Haematol. 2020;193(2):216-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Xu Y, Liu Q, Zhong M, et al. 2B4 costimulatory domain enhancing cytotoxic ability of anti-CD5 chimeric antigen receptor engineered natural killer cells against T cell malignancies. J Hematol Oncol. 2019;12(1):1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pfe A, Huntington ND.. You have got a fast CAR: chimeric antigen receptor NK cells in cancer therapy. Cancers (Basel). 2020;12(3):1-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kawalekar OU, O'Connor RS, Fraietta JA, et al. Distinct signaling of coreceptors regulates specific metabolism pathways and impacts memory development in CAR T cells. Immunity. 2016;44(2):380-390. 10.1016/j.immuni.2016.01.021 [DOI] [PubMed] [Google Scholar]
- 36. Daher M, Rezvani K.. Next generation natural killer cells for cancer immunotherapy: the promise of genetic engineering. Curr Opin Immunol. 2018;51:146-153. 10.1016/j.coi.2018.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Leivas A, Valeri A, Córdoba L, et al. NKG2D-CAR-transduced natural killer cells efficiently target multiple myeloma. Blood Cancer J. 2021;11(8):1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chu Y, Hochberg J, Yahr A, et al. Targeting CD20+ aggressive B-cell non-hodgkin lymphoma by anti-CD20 CAR mRNA-modified expanded natural killer cells in vitro and in NSG Mice. Cancer Immunol Res. 2015;3(4):333-344. 10.1158/2326-6066.CIR-14-0114 [DOI] [PubMed] [Google Scholar]
- 39. Genßler S, Burger MC, Zhang C, et al. Dual targeting of glioblastoma with chimeric antigen receptor-engineered natural killer cells overcomes heterogeneity of target antigen expression and enhances antitumor activity and survival. Oncoimmunology. 2016;5(4):e1119354. 10.1080/2162402X.2015.1119354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Romanski A, Uherek C, Bug G, et al. CD19-CAR engineered NK-92 cells are sufficient to overcome NK cell resistance in B-cell malignancies. J Cell Mol Med. 2016;20(7):1287-1294. 10.1111/jcmm.12810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dong H, Ham JD, Hu G, et al. Memory-like NK cells armed with a neoepitope-specific CAR exhibit potent activity against NPM1 mutated acute myeloid leukemia. PNAS. 2022;119(25):1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gang M, Wong P, Berrien-Elliott MM, Fehniger TA.. Memory-like natural killer cells for cancer immunotherapy. Semin Hematol. 2020;57(4):185-193. 10.1053/j.seminhematol.2020.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Robbins GM, Wang M, Pomeroy EJ, Moriarity BS.. Nonviral genome engineering of natural killer cells. Stem Cell Res Ther. 2021;12(1):350. 10.1186/s13287-021-02406-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Deipolyi AR, Golberg A, Yarmush ML, Arellano RS, Oklu R.. Irreversible electroporation: evolution of a laboratory technique in interventional oncology. Diagn Interv Radiol (Ankara, Turkey). 2014;20(2):147-154. 10.5152/dir.2013.13304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fus-Kujawa A, Prus P, Bajdak-Rusinek K, et al. An overview of methods and tools for transfection of eukaryotic cells in vitro. Front Bioeng Biotechnol. 2021;9:701031. 10.3389/fbioe.2021.701031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ng YY, Tay JCK, Wang S.. CXCR1 expression to improve anti-cancer efficacy of intravenously injected CAR-NK cells in mice with peritoneal xenografts. Mol Ther Oncolytics. 2020;16:75-85. 10.1016/j.omto.2019.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Xiao L, Cen D, Gan H, et al. Adoptive transfer of NKG2D CAR mRNA-engineered natural killer cells in colorectal cancer patients. Mol Ther. 2019;27(6):1114-1125. 10.1016/j.ymthe.2019.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ng YY, Du Z, Zhang X, Chng WJ, Wang S.. CXCR4 and anti-BCMA CAR co-modified natural killer cells suppress multiple myeloma progression in a xenograft mouse model. Cancer Gene Ther. 2022;29(5):475-483. 10.1038/s41417-021-00365-x [DOI] [PubMed] [Google Scholar]
- 49. Ingegnere T, Mariotti FR, Pelosi A, et al. Human CAR NK cells: a new non-viral method allowing high efficient transfection and strong tumor cell killing. Front Immunol. 2019;10:1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jordan ET, Collins M, Terefe J, Ugozzoli L, Rubio T.. Optimizing electroporation conditions in primary and other difficult-to-transfect cells. J Biomol Tech. 2008;19(5):328-334. [PMC free article] [PubMed] [Google Scholar]
- 51. Zhao Y, Moon E, Carpenito C, et al. Multiple injections of electroporated autologous T cells expressing a chimeric antigen receptor mediate regression of human disseminated tumor. Cancer Res. 2010;70(22):9053-9061. 10.1158/0008-5472.CAN-10-2880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. McKinlay CJ, Vargas JR, Blake TR, et al. Charge-altering releasable transporters (CARTs) for the delivery and release of mRNA in living animals. Proc Natl Acad Sci USA. 2017;114(4):E448-E456. 10.1073/pnas.1614193114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wilk AJ, Weidenbacher NL, Vergara R, et al. Charge-altering releasable transporters enable phenotypic manipulation of natural killer cells for cancer immunotherapy. Blood Adv. 2020;4(17):4244-4255. 10.1182/bloodadvances.2020002355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Li M, Li Y, Li S, et al. The nano delivery systems and applications of mRNA. Eur J Med Chem. Preprint at 2022;227:1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gilleron J, Querbes W, Zeigerer A, et al. Image-based analysis of lipid nanoparticle-mediated siRNA delivery, intracellular trafficking and endosomal escape. Nat Biotechnol. 2013;31(7):638-646. 10.1038/nbt.2612 [DOI] [PubMed] [Google Scholar]
- 56. Rurik JG, Tombácz I, Yadegari A, et al. CAR T cells produced in vivo to treat cardiac injury. Science (New York, N.Y.). 2022;375(6576):91-96. 10.1126/science.abm0594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Chandrasekaran S, Chan MF, Li J, King MR.. Super natural killer cells that target metastases in the tumor draining lymph nodes. Biomaterials. 2016;77:66-76. 10.1016/j.biomaterials.2015.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kiaie SH, Majidi Zolbanin N, Ahmadi A, et al. Recent advances in mRNA-LNP therapeutics: immunological and pharmacological aspects. J Nanobiotechnol. 2022;20(1):276. 10.1186/s12951-022-01478-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kruschinski A, Moosmann A, Poschke I, et al. Engineering antigen-specific primary human NK cells against HER-2 positive carcinomas. Proc Natl Acad Sci USA. 2008;105(45):17481-17486. 10.1073/pnas.0804788105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lv H, Zhang S, Wang B, Cui S, Yan J.. Toxicity of cationic lipids and cationic polymers in gene delivery. J Control Release. 2006;114(1):100-109. 10.1016/j.jconrel.2006.04.014 [DOI] [PubMed] [Google Scholar]
- 61. Slotkin RK, Martienssen R.. Transposable elements and the epigenetic regulation of the genome. Nat Rev Genet. 2007;8(4):272-285. 10.1038/nrg2072 [DOI] [PubMed] [Google Scholar]
- 62. Huang X, Wilber A C, Bao L, et al. Stable gene transfer and expression in human primary T cells by the Sleeping Beauty transposon system. Blood. 2006;107(2):483-491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ma K, Fu D, Yu D, et al. Targeted delivery of in situ PCR-amplified sleeping beauty transposon genes to cancer cells with lipid-based nanoparticle-like protocells. 2016;121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Tipanee J, VandenDriessche T, Chuah MK.. Transposons: moving forward from preclinical studies to clinical trials. Hum Gene Ther. 2017;28(11):1087-1104. 10.1089/hum.2017.128 [DOI] [PubMed] [Google Scholar]
- 65. Izsvák Z, Ivics Z.. Sleeping Beauty transposition: biology and applications for molecular therapy. Mol Ther. Preprint at 2004;9(2):147-156. 10.1016/j.ymthe.2003.11.009 [DOI] [PubMed] [Google Scholar]
- 66. Woodard LE, Wilson MH.. PiggyBac-ing models and new therapeutic strategies. Trends Biotechnol. Preprint at 2015;33(9):525-533. 10.1016/j.tibtech.2015.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Cao B, Liu M, Huang J, et al. Development of mesothelin-specific car nk-92 cells for the treatment of gastric cancer. Int J Biol Sci. 2021;17(14):3850-3861. 10.7150/ijbs.64630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wang J, Lupo KB, Chambers AM, Matosevic S.. Purinergic targeting enhances immunotherapy of CD73+ solid tumors with piggyBac-engineered chimeric antigen receptor natural killer cells. J ImmunoTher Cancer. 2018;6(1):136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Tsai HC, Pietrobon V, Peng M, et al. Current strategies employed in the manipulation of gene expression for clinical purposes. J Transl Med. Preprint at 2022;20(1):535. 10.1186/s12967-022-03747-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Huang X, Guo H, Tammana S, et al. Gene transfer efficiency and genome-wide integration profiling of sleeping beauty, Tol2, and PiggyBac transposons in human primary T cells. Mol Ther. 2010;18(10):1803-1813. 10.1038/mt.2010.141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kebriaei P, Singh H, Huls MH, et al. Phase I trials using sleeping beauty to generate CD19-specific CAR T cells. J Clin Investig. 2016;126(9):3363-3376. 10.1172/jci86721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Wang X, Yang X, Yuan X, Wang W, Wang Y.. Chimeric antigen receptor-engineered NK cells: new weapons of cancer immunotherapy with great potential. Exp Hematol Oncol. 2022;11(1):85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Issa SS, Shaimardanova AA, Solovyeva VV, Rizvanov AA.. Various AAV serotypes and their applications in gene therapy: an overview. Cells. Preprint at 2023;12(5):785. 10.3390/cells12050785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Dufait I, Liechtenstein T, Lanna A, et al. Retroviral and lentiviral vectors for the induction of immunological tolerance. Scientifica. 2012;2012:1-14. 10.6064/2012/694137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Nikolic J, Belot L, Raux H, et al. Structural basis for the recognition of LDL-receptor family members by VSV glycoprotein. Nat Commun. 2018;9(1):1029. 10.1038/s41467-018-03432-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. de Sanctis JB, Blanca I, Radzioch D, Bianco NE.. Expression and function of low-density lipoprotein receptors in CD3− CD16+ CD56+ cells: effect of interleukin 2. Cell Immunol. 1996;167(1):18-29. 10.1006/cimm.1996.0003 [DOI] [PubMed] [Google Scholar]
- 77. Gong Y, Klein Wolterink RGJ, Janssen I, et al. Rosuvastatin enhances VSV-G lentiviral transduction of NK cells via upregulation of the low-density lipoprotein receptor. Mol Ther Methods Clin Dev. 2020;17:634-646. 10.1016/j.omtm.2020.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Bari R, Granzin M, Tsang KS, et al. A distinct subset of highly proliferative and lentiviral vector (LV)-transducible NK cells define a readily engineered subset for adoptive cellular therapy. Front Immunol. 2019;10:2784. 10.3389/fimmu.2019.02784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Colamartino ABL, Lemieux W, Bifsha P, et al. Efficient and robust NK-cell transduction with baboon envelope pseudotyped lentivector. Front Immunol. 2019;10:2873. 10.3389/fimmu.2019.02873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Milone MC, O’Doherty U.. Clinical use of lentiviral vectors. Leukemia. Preprint at 2018;32(7):1529-1541. 10.1038/s41375-018-0106-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Nagashima S, Mailliard R, Kashii Y, et al. Stable transduction of the interleukin-2 gene into human natural killer cell lines and their phenotypic and functional characterization in vitro and in vivo. Blood. 1998;91(10):3850-3861. [PubMed] [Google Scholar]
- 82. Guven H, Konstantinidis KV, Alici E, et al. Efficient gene transfer into primary human natural killer cells by retroviral transduction. Exp Hematol. 2005;33(11):1320-1328. 10.1016/j.exphem.2005.07.006 [DOI] [PubMed] [Google Scholar]
- 83. Müller S, Bexte T, Gebel V, et al. High cytotoxic efficiency of lentivirally and alpharetrovirally engineered CD19-specific chimeric antigen receptor natural killer cells against acute lymphoblastic leukemia. Front Immunol. 2020;10:3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Sandrin V, Boson B, Salmon P, et al. Lentiviral vectors pseudotyped with a modified RD114 envelope glycoprotein show increased stability in sera and augmented transduction of primary lymphocytes and CD34+ cells derived from human and nonhuman primates. Blood. 2002;100(3):823-832. 10.1182/blood-2001-11-0042 [DOI] [PubMed] [Google Scholar]
- 85. Liu E, Tong Y, Dotti G, et al. Cord blood NK cells engineered to express IL-15 and a CD19-targeted CAR show long-term persistence and potent anti-tumor activity. Catalysis A Z. 2020;32(2):520-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Meyer NL, Chapman MS.. Adeno-associated virus (AAV) cell entry: structural insights. Trends Microbiol. 2022;30(5):432-451. 10.1016/j.tim.2021.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Wu Z, Miller E, Agbandje-McKenna M, Samulski RJ.. α2,3 and α2,6 N-linked sialic acids facilitate efficient binding and transduction by adeno-associated virus types 1 and 6. J Virol. 2006;80(18):9093-9103. 10.1128/JVI.00895-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Naeimi Kararoudi M, Likhite S, Elmas E, et al. Optimization and validation of CAR transduction into human primary NK cells using CRISPR and AAV. Cell Rep Methods. 2022;2(6):100236. 10.1016/j.crmeth.2022.100236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Pomeroy EJ, Hunzeker JT, Kluesner MG, et al. A genetically engineered primary human natural killer cell platform for cancer immunotherapy. Mol Ther. 2020;28(1):52-63. 10.1016/j.ymthe.2019.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Fenard D, Ingrao D, Seye A, et al. Vectofusin-1, a new viral entry enhancer, strongly promotes lentiviral transduction of human hematopoietic stem cells. Mol Ther Nucleic Acids. 2013;2(5):e90. 10.1038/mtna.2013.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Boissel L, Betancur M, Lu W, et al. Comparison of mRNA and lentiviral based transfection of natural killer cells with chimeric antigen receptors recognizing lymphoid antigens. Leuk Lymphoma. 2012;53(5):958-965. 10.3109/10428194.2011.634048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Sayed N, Allawadhi P, Khurana A, et al. Gene therapy: comprehensive overview and therapeutic applications. Life Sci. 2022;294:120375. 10.1016/j.lfs.2022.120375 [DOI] [PubMed] [Google Scholar]
- 93. Cohen SN. DNA cloning: a personal view after 40 years. Proc Natl Acad Sci USA. 2013;110(39):15521-15529. 10.1073/pnas.1313397110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Li H, Yang Y, Hong W, et al. Applications of genome editing technology in the targeted therapy of human diseases: mechanisms, advances and prospects. Signal Transduct Target Ther. 2020;5(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Ran FA, Hsu PD, Wright J, et al. Genome engineering using the CRISPR-Cas9 system. Nat Protoc. 2013;8(11):2281-2308. 10.1038/nprot.2013.143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Doudna JA, Charpentier E.. The new frontier of genome engineering with CRISPR-Cas9. Science (1979). 2014;346(6213). [DOI] [PubMed] [Google Scholar]
- 97. San Filippo J, Sung P, Klein H.. Mechanism of eukaryotic homologous recombination. Annu Rev Biochem. 2008;77:229-257. 10.1146/annurev.biochem.77.061306.125255 [DOI] [PubMed] [Google Scholar]
- 98. Naeimi Kararoudi M, Likhite S, Elmas Eet al. CRISPR-targeted CAR gene insertion using Cas9/RNP and AAV6 1 enhances anti-AML activity of primary NK cells. bioRxiv 2021.03.17.435886. 10.1101/2021.03.17.435886 [DOI]
- 99. Palmer DC, Guittard GC, Franco Z, et al. Cish actively silences TCR signaling in CD8+ T cells to maintain tumor tolerance. J Exp Med. 2015;212(12):2095-2113. 10.1084/jem.20150304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Lv J, Qin Le, Zhao R, et al. Disruption of CISH promotes the antitumor activity of human T cells and decreases PD-1 expression levels. Mol Ther Oncolytics. 2023;28:46-58. 10.1016/j.omto.2022.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Zhu H, Blum RH, Bernareggi D, et al. Metabolic reprograming via deletion of CISH in human iPSC-derived NK cells promotes in vivo persistence and enhances anti-tumor activity. Cell Stem Cell. 2020;27(2):224-237.e6. 10.1016/j.stem.2020.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Bernard PL, Delconte R, Pastor S, et al. Targeting CISH enhances natural cytotoxicity receptor signaling and reduces NK cell exhaustion to improve solid tumor immunity. J ImmunoTher Cancer. 2022;10(5):e004244. 10.1136/jitc-2021-004244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Shaim H, Shanley M, Basar R, et al. Targeting the αv integrin/TGF-β axis improves natural killer cell function against glioblastoma stem cells. J Clin Investig. 2021;131(14):e142116. 10.1172/JCI142116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Cichocki F, Bjordahl R, Goodridge JP, et al. Quadruple gene-engineered natural killer cells enable multi-antigen targeting for durable antitumor activity against multiple myeloma. Nat Commun. 2022;13(1):7341. 10.1038/s41467-022-35127-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Song Y, Liu Z, Zhang Y, et al. Large-fragment deletions induced by Cas9 cleavage while not in the BEs system. Mol Ther Nucleic Acids. 2020;21:523-526. 10.1016/j.omtn.2020.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Benjamin D, Cox T, Platt RJ, Zhang F.. Therapeutic genome editing: prospects and challenges. Nat Med. 2015;21(2):121-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Höijer I, Emmanouilidou A, Östlund R, et al. CRISPR-Cas9 induces large structural variants at on-target and off-target sites in vivo that segregate across generations. Nat Commun. 2022;13(1):627. 10.1038/s41467-022-28244-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Fu Y, Foden JA, Khayter C, et al. High-frequency off-target mutagenesis induced by CRISPR-Cas nucleases in human cells. Nat Biotechnol. 2013;31(9):822-826. 10.1038/nbt.2623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. He Y, Yan W, Long L, et al. The CRISPR/Cas system: a customizable toolbox for molecular detection. Genes. 2023;14(4):850. 10.3390/genes14040850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Luk, K., Liu P, Zeng J, et al. Optimization of NLS composition improves CRISPR-Cas12a editing rates in human primary cells. Gen Biotechnology 2022;1(2). 10.1089/genbio.2022.0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Liu Z, Shi M, Ren Y, et al. Recent advances and applications of CRISPR-Cas9 in cancer immunotherapy. Mol Cancer. Preprint at 2023;22(1):35. 10.1186/s12943-023-01738-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Ai Y, Liang D, Wilusz JE.. CRISPR/Cas13 effectors have differing extents of off-target effects that limit their utility in eukaryotic cells. Nucleic Acids Res. 2022;50(11):e65-E65. 10.1093/nar/gkac159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Urnov FD, Rebar EJ, Holmes MC, Zhang HS, Gregory PD.. Genome editing with engineered zinc finger nucleases. Nat Rev Genet. 2010;11(9):636-646. 10.1038/nrg2842 [DOI] [PubMed] [Google Scholar]
- 114. Palpant NJ, Dudzinski D.. Zinc finger nucleases: looking toward translation. Gene Ther. 2013;20(2):121-127. 10.1038/gt.2012.2 [DOI] [PubMed] [Google Scholar]
- 115. Urnov FD, Miller JC, Lee Y-L, et al. Highly efficient endogenous human gene correction using designed zinc-finger nucleases. Nature. 2005;435(7042):646-651. 10.1038/nature03556 [DOI] [PubMed] [Google Scholar]
- 116. Tang SY, Zha S, Du Z, et al. Targeted integration of EpCAM-specific CAR in human induced pluripotent stem cells and their differentiation into NK cells. Stem Cell Res Ther. 2021;12(1):580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Qasim W, Zhan H, Samarasinghe S, et al. Molecular remission of infant B-ALL after infusion of universal TALEN gene-edited CAR T cells. Sci Transl Med. 2017;9(374):eaaj2013. 10.1126/scitranslmed.aaj2013 [DOI] [PubMed] [Google Scholar]
- 118. Chen A-P, Gao P, Ashok Pet al. TALEN-based gene edited iPSC-derived NK (iNK) cells demonstrate enhanced antitumor activity. J ImmunoTher Cancer. 2022;10:A339. 10.1136/jitc-2022-SITC2022.0323 [DOI] [Google Scholar]
- 119. Gupta RM, Musunuru K.. Expanding the genetic editing tool kit: ZFNs, TALENs, and CRISPR-Cas9. J Clin Invest. 2014;124(10):4154-4161. 10.1172/JCI72992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Lin Y, Cradick TJ, Brown MT, et al. CRISPR/Cas9 systems have off-target activity with insertions or deletions between target DNA and guide RNA sequences. Nucleic Acids Res. 2014;42(11):7473-7485. 10.1093/nar/gku402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Blattner G, Cavazza A, Thrasher AJ, Turchiano G.. Gene editing and genotoxicity: targeting the off-targets. Front Genome Ed. 2020;2:613252. 10.3389/fgeed.2020.613252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Vakulskas CA, Dever DP, Rettig GR, et al. A high-fidelity Cas9 mutant delivered as a ribonucleoprotein complex enables efficient gene editing in human hematopoietic stem and progenitor cells. Nat Med. 2018;24(8):1216-1224. 10.1038/s41591-018-0137-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Ghosh S, Brown AM, Jenkins C, Campbell K.. Viral vector systems for gene therapy: a comprehensive literature review of progress and biosafety challenges. Appl Biosaf. Preprint at 2020;25(1):7-18. 10.1177/1535676019899502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Marusyk A, Janiszewska M, Polyak K.. Intratumor heterogeneity: the Rosetta stone of therapy resistance. Cancer Cell. 2020;37(4):471-484. 10.1016/j.ccell.2020.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Muigai AWT. Expanding global access to genetic therapies. Nat Biotechnol. 2022;40(1):20-21. 10.1038/s41587-021-01191-0 [DOI] [PubMed] [Google Scholar]
- 126. Komor AC, Kim YB, Packer MS, Zuris JA, Liu DR.. Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature. 2016;533(7603):420-424. 10.1038/nature17946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Newby GA, Liu DR.. In vivo somatic cell base editing and prime editing. Mol Ther. 2021;29(11):3107-3124. 10.1016/j.ymthe.2021.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Anzalone AV, Randolph PB, Davis JR, et al. Search-and-replace genome editing without double-strand breaks or donor DNA. Nature. 2019;576(7785):149-157. 10.1038/s41586-019-1711-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Chen PJ, Hussmann JA, Yan J, et al. Enhanced prime editing systems by manipulating cellular determinants of editing outcomes. Cell. 2021;184(22):5635-5652.e29. 10.1016/j.cell.2021.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Yarnall MTN, Ioannidi EI, Schmitt-Ulms C, et al. Drag-and-drop genome insertion of large sequences without double-strand DNA cleavage using CRISPR-directed integrases. Nat Biotechnol. 2022;41(4):500-512. 10.1038/s41587-022-01527-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Naeimi Kararoudi M, Likhite S, Elmas E, et al. CD33 targeting primary CAR-NK cells generated by CRISPR mediated gene insertion show enhanced anti-AML activity. Blood. 2020;136(Supplement 1):3.32614960 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated or analyzed in support of this research.


