Abstract
Background
Antihyperglycemic drug utilization studies are conducted frequently and describe the uptake of new drug therapies across may jurisdictions. An increasingly important, yet often absent, aspect of these studies is the impact of rurality on drug utilization.
Objective(s)
The objective of this study was to explore the association between place of residence (rural, urban, metropolitan) and the use of dipeptidyl peptidase 4 inhibitors (DPP-4i) for first treatment intensification of type 2 diabetes.
Methods
A retrospective cohort study was conducted from April 1, 2008 to March 31, 2019 of new metformin users. A multivariable logistic regression analysis was performed to determine the association between place of residence (using postal codes) and likelihood of DPP-4i dispensing.
Results
After adjusting for confounders, analysis revealed that rural-dwellers are less likely to have a DPP-4i dispensed, compared with metropolitan-dwellers (aOR:0.64; 95%CI:0.61–0.67) and over-time, the uptake in rural areas was slower.
Conclusions
This study demonstrates that rurality can have an impact on drug therapy decisions at first treatment intensification, with respect to the utilization of new therapies.
Keywords: Type 2 diabetes, Rural-urban continuum, Residence, Dipeptidyl peptidase 4 inhibitor, Treatment intensification
1. Introduction
Many have described trends in the dispensing of antihyperglycemic therapies globally, however, few consider the impact of rurality.1, 2, 3 Some of the earliest literature in this area suggests that rurality is associated with low achievement of glycated hemoglobin A1C, blood pressure, and cholesterol targets, underuse of aspirin therapy, and lower use of combination drug therapy to treat type 2 diabetes and hypertension when targets are not being met.4, 5, 6 Others have identified rural and remote dwellers as 2 to 3 times more likely to experience a hospitalization for hypo or hyperglycemia as an acute complication of diabetes.7 More recently, it has been reported that individuals living with type 2 diabetes and in rural areas are more likely to be dispensed a sulfonylurea at first treatment intensification (FTI), compared to their metropolitan-dwelling counterparts.8 Considering the increased use of sulfonylureas in rural areas and lower use of combination therapies, the question remains whether residence impacts the uptake of newer drug therapies such as dipeptidyl peptidase 4 inhibitors (DPP-4i).
The first DPP-4i was approved for use by Health Canada in 2008 and was recommended at treatment intensification for being weight neutral and having a low risk of hypoglycemia beginning in the 2008 Canadian Diabetes Association Clinical Practice Guidelines.9., 10, 11 In the Canadian province of Alberta, (population: 4 million people) DPP-4i are eligible for publicly funded provincial drug insurance coverage if an individual previously trialed metformin, a sulfonylurea, and where insulin is not an option.12,13
Taken together, the reports of differential diabetes management according to residence, despite having access to the same provincial drug availability, publicly funded provincial drug insurance programs, and national clinical practice guidelines, is concerning. The objective of this study was to further this line of research by examining the uptake of DPP-4i along the rural-urban continuum and since its approval for use. The primary hypothesis of this research was that uptake will be slower in rural areas and lag behind urban and metropolitan utilization.
2. Methods
This retrospective cohort study analyzed administrative health records of adult new metformin users in Alberta between April 1, 2008 and March 31, 2019. A new metformin user was defined as an individual age 18 years or older with no history of antihyperglycemic drug therapy in the past 12 months, and their first instance of antihyperglycemic drug therapy being metformin.14 Additionally, individuals were excluded if they were diagnosed with gestational diabetes (International Classification of Diseases-10 code O24.xx) in the 9 months before FTI or at all during follow up.15 Individuals were followed until they experienced the outcome of interest, a pharmacy dispensing record for FTI with a DPP-4i either alone or in combination with other therapies. At the time an individual received FTI, their postal code was used to categorize their place of residence as rural, urban, or metropolitan, based on Alberta Health's geographic boundaries.16,17
Multivariable logistic regression was used to determine whether there was an association between place of residence and FTI with a DPP-4i. Several baseline characteristics were adjusted for in the model to control for confounding including age, sex, time since first metformin was dispensed, healthcare utilization, and diabetes complications based on the available data and other literature (Table 1).18, 19, 20, 21 Additionally, a count of the unique number of prescription drug therapies dispensed in the baseline year prior to first metformin was included in the model to control for possible confounding related to the burden of comorbid conditions and polypharmacy. Knowing that laboratory data should guide clinical decision making, a subgroup analysis was performed for individuals with laboratory data available (estimated glomerular filtration rate or creatinine clearance and glycated hemoglobin HbA1c). As this research is an extension of previously reported findings, these methods have been further detailed elsewhere.8
Table 1.
Baseline demographics across the rural-urban continuum.
| Metropolitan (n = 41,646) | Urban (n = 6800) | Rural (n = 17,638) | Standardized Difference⁎ | |
|---|---|---|---|---|
| Fiscal Year, n (%)a | 0.03 (M-U) | |||
| 2009/2010 | 3129 (7.5) | 390 (5.7) | 1159 (6.6) | |
| 2010/2011 | 3413 (8.2) | 511 (7.5) | 1475 (8.4) | |
| 2011/2012 | 3647 (8.8) | 594 (8.7) | 1520 (8.6) | |
| 2012/2013 | 3694 (8.9) | 731 (10.8) | 1636 (9.3) | |
| 2013/2014 | 4169 (10.0) | 648 (9.5) | 1811 (10.3) | |
| 2014/2015 | 4810 (11.5) | 809 (11.9) | 2065 (11.7) | |
| 2015/2016 | 5770 (13.9) | 974 (14.3) | 2454 (13.9) | |
| 2016/2017 | 6252 (15.0) | 1103 (16.2) | 2687 (15.2) | |
| 2017/2018 | 6762 (16.2) | 1040 (15.3) | 2831 (16.0) | |
| Age (years), mean (SD) | 55.3 (12.5) | 53.7 (12.6) | 56.0 (13.0) | 0.18 (U-R) |
| Male, n (%) | 25,593 (61.5) | 4360 (64.1) | 10,812 (61.3) | 0.06 (U-R) |
| Time since first metformin (years), mean (SD) | 1.5 (1.9) | 1.5 (1.9) | 1.6 (1.9) | 0.05 (U-R) |
| Number of physician visitsb, n (%) | 0.18 (M-R) | |||
| 0–6 | 8627 (20.7) | 1785 (26.3) | 4850 (27.5) | |
| 7–12 | 10,781 (25.9) | 1850 (27.2) | 4704 (26.7) | |
| 13–24 | 11,721 (28.1) | 1736 (25.5) | 4479 (25.4) | |
| ≥ 25 | 10,517 (25.3) | 1429 (21.0) | 3605 (20.4) | |
| Hospitalizationb, n (%) | 5227 (12.6) | 1032 (15.2) | 3350 (19.0) | 0.18 (M-R) |
| Number of unique prescriptionsb, n (%) | 0.17 (M-R) | |||
| 0–2 | 13,243 (31.8) | 1926 (28.3) | 4533 (25.7) | |
| 3–5 | 11,248 (27.0) | 1826 (26.9) | 4527 (25.7) | |
| 6–8 | 7714 (18.5) | 1328 (19.5) | 3458 (19.6) | |
| ≥ 9 | 9441 (22.7) | 1720 (25.3) | 5120 (29.0) | |
| Diabetes Complications, n (%) | ||||
| Retinopathy | 9524 (22.9) | 1604 (23.6) | 2983 (16.9) | 0.17 (U-R) |
| Nephropathy | 2027 (4.9) | 329 (4.8) | 950 (5.4) | 0.02 (U-R) |
| Neuropathy | 4233 (10.2) | 789 (11.6) | 2196 (12.5) | 0.07 (M-R) |
| Ischemic Heart Disease | 10,743 (25.8) | 1569 (23.1) | 4710 (26.7) | 0.08 (U-R) |
| Prior Stroke | 1731 (4.2) | 197 (2.9) | 704 (4.0) | 0.07 (M-U) |
| Peripheral Vascular Disease | 1724 (4.1) | 327 (4.8) | 1102 (6.2) | 0.10 (M-R) |
| Hyperlipidemia | 18,632 (44.7) | 2643 (38.9) | 7275 (41.2) | 0.12 (M-U) |
| Diabetic Foot Infection | 2748 (6.6) | 545 (8.0) | 2155 (12.2) | 0.19 (M-R) |
| Prior Amputation | 237 (0.6) | 41 (0.6) | 120 (0.7) | 0.01 (M-R) |
| Dental Complications | 3659 (8.8) | 733 (10.8) | 2436 (13.8) | 0.16 (M-R) |
| Hypoglycemia | 724 (1.7) | 179 (2.6) | 484 (2.7) | 0.07 (M-R) |
| Number of other chronic conditionsc, n (%) | 0.14 (M-R) | |||
| 0–1 | 6644 (16.0) | 1040 (15.3) | 2337 (13.2) | |
| 2 | 11,618 (27.9) | 1711 (25.2) | 4285 (24.3) | |
| 3–4 | 15,221 (36.5) | 2563 (37.7) | 6815 (38.6) | |
| ≥ 5 | 8163 (19.6) | 1486 (21.9) | 4201 (23.8) |
Standardized difference is both the absolute value and maximum among the 3 pairwise comparisons.
Percentage by fiscal year (Alberta Health fiscal year runs April to March), M-U = metropolitan-urban comparison, SD = standard deviation, U-R = urban-rural comparison, M-R = metropolitan-rural comparison.
In the year prior to first metformin.
As listed in Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27.
All analyses were performed in STATA version 16.1. The University of Alberta Research Ethics Board approved this study (Pro00066037).
3. Results
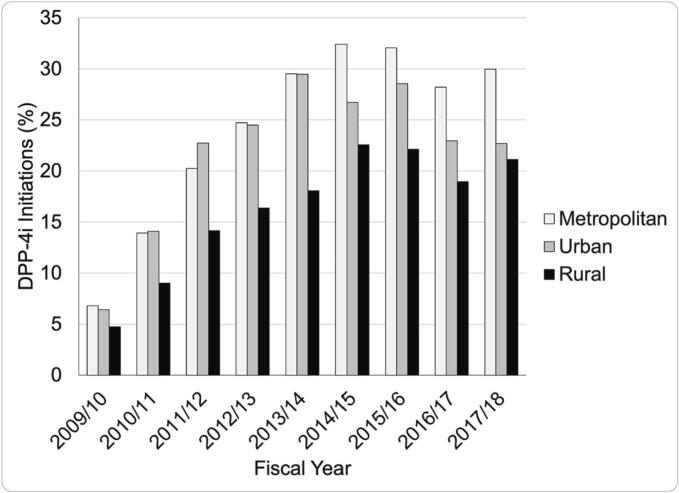
Of 171,759 adult new metformin users, 66,064 received treatment intensification and 15,467 (23%) were intensified with a DPP-4i. Baseline demographics are described in Table 1. At the beginning of the observation period, the proportion of DPP-4i dispensed according to place of residence was similar (7% metropolitan, 6% urban, 5% rural) -see Fig. 1. However, year over year, the gap widened between rural and metropolitan/urban to a maximum 10% difference in 2014/15 (32% metropolitan, 27% urban, 22% rural). Thereafter, DPP-4i dispensing dipped slightly in all locations (after the 2014/15 fiscal year) which corresponds with the market approval of SGLT2i, and then level-off.9
Fig. 1.
Dipeptidyl peptidase 4 inhibitor (DPP-4i) dispensations over time across the rural-urban continuum.
White bars = metropolitan; grey bars = urban; black bars = rural.
After adjusting for potential confounders, individuals living in rural areas were 36% less likely to receive a DPP-4i at FTI, compared with individuals living in metropolitan areas (aOR: 0.64; 95% CI: 0.61–0.67). This remained unchanged in the subgroup of those with laboratory data available (aOR: 0.64; 95% CI: 0.59–0.69). Of note, no interaction between sex and place of residence was found in these models.
4. Discussion
Key findings demonstrate that not only are individuals living with type 2 diabetes in rural areas less likely to have a DPP-4i dispensed at FTI, but also year over year the uptake of this drug class in rural areas substantially lags behind urban and metropolitan locations. Healthcare disparities among rural-dwellers in Alberta has been described in the literature for decades and these results demonstrate that over time, not much has changed.4, 5, 6,8,22 Despite programs to attract healthcare professionals to rural areas to improve healthcare access, different management practices still exist across the rural-urban continuum, as evidenced by this research.23,24 Of particular concern however, is the delayed incorporation of new drug therapies into practice, as seen with DPP-4is.
While it is likely that the limited use of DPP-4is in rural areas is a result of the sustained use of sulfonylureas in these locations, as previously reported, justification for these clinical decisions remains unknown and is beyond the scope of this study.8 Current literature is also sparse in identifying patient and clinician factors underpinning differential processes of care and management strategies. Some speculate a difference in patient expectations of the healthcare system based on where they live, or differences in the use or methods of engagement with clinical practice guidelines and continuing education initiatives based on where a clinician practices.25, 26, 27, 28, 29 Considering that individuals with publicly funded provincial drug insurance require a trial of metformin and a sulfonylurea before a DPP-4i will be covered, this may partly explain these findings however, as previously described, the largest caseloads of individuals with these drug insurance plans reside in metropolitan locations.8 In light of this, these findings are likely a result of multifaceted patient and clinician factors which require further investigation.
Acknowledging limitations of this work include possible residual confounding from unmeasured factors which may impact drug therapy utilization such as drug insurance coverage, income level, education level, and medication taking beliefs. However, these data are not routinely collected by Alberta Health or made available in their administrative datasets.30 Despite this, the study is strengthened by the large sample size, which represents all Albertans with type 2 diabetes when FTI is required. Additionally, the lengthy observation window enables not only trends over time, but also analysis beginning when DPP-4is were first available for use in Canada.
5. Conclusion
This study provides further evidence of differential type 2 diabetes care received among those living in rural locations, with a particular focus on the uptake of new drug therapies. A necessary next step in this line of research, which is currently underway, is to determine whether the differences in the management of type 2 diabetes along the rural-urban continuum results in jeopardized health outcomes, namely risk of microvascular and macrovascular complications.
CRediT authorship contribution statement
Danielle K. Nagy: Writing – original draft, Methodology, Formal analysis, Conceptualization. Lauren C. Bresee: Writing – review & editing, Methodology, Conceptualization. Dean T. Eurich: Writing – review & editing, Methodology, Data curation, Conceptualization. Scot H. Simpson: Writing – review & editing, Project administration, Methodology, Data curation, Conceptualization.
Declaration of competing interest
No potential conflicts of interest relevant to this article were reported. S.H.S. is supported as the Chair in Patient Health Management, jointly held by the Faculty of Pharmacy and Pharmaceutical Sciences and Faculty of Medicine and Dentistry, University of Alberta. This study is based in part on data provided by Alberta Health. The interpretation and conclusions contained herein are those of the researchers and do not necessarily represent the views of the Government of Alberta. Neither the Government of Alberta nor Alberta Health express any opinion in relation to this study.
Acknowledgements
This study received funding support from the Chair in Patient Health Management, University of Alberta.
References
- 1.Secrest M.H., Azoulay L., Dahl M., et al. A population-based analysis of antidiabetic medications in four Canadian provinces: secular trends and prescribing patterns. Pharmacoepidemiol Drug Saf. 2020;29(suppl 1):86–92. doi: 10.1002/pds.4878. [published Online First: 2019/08/30] [DOI] [PubMed] [Google Scholar]
- 2.Curtis H.J., Dennis J.M., Shields B.M., et al. Time trends and geographical variation in prescribing of drugs for diabetes in England from 1998 to 2017. Diabetes Obes Metab. 2018;20(9):2159–2168. doi: 10.1111/dom.13346. [published Online First: 2018/05/08] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strain W.D., Tsang C., Hurst M., et al. What next after metformin in type 2 diabetes? Selecting the right drug for the right patient. Diabetes Ther. 2020;11(6):1381–1395. doi: 10.1007/s13300-020-00834-w. [published Online First: 2020/05/20] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klinke J.A., Johnson J.A., Guirguis L.M., et al. Underuse of aspirin in type 2 diabetes mellitus: prevalence and correlates of therapy in rural Canada. Clin Ther. 2004;26(3):439–446. doi: 10.1016/s0149-2918(04)90040-9. [DOI] [PubMed] [Google Scholar]
- 5.Supina A.L., Guirguis L.M., Majumdar S.R., et al. Treatment gaps for hypertension management in rural Canadian patients with type 2 diabetes mellitus. Clin Ther. 2004;26(4):598–606. doi: 10.1016/s0149-2918(04)90062-8. [DOI] [PubMed] [Google Scholar]
- 6.Toth E.L., Majumdar S.R., Guirguis L.M., et al. Compliance with clinical practice guidelines for type 2 diabetes in rural patients: treatment gaps and opportunities for improvement. Pharmacotherapy. 2003;23(5):659–665. doi: 10.1592/phco.23.5.659.32203. [DOI] [PubMed] [Google Scholar]
- 7.Booth G.L., Hux J.E., Fang J., et al. Time trends and geographic disparities in acute complications of diabetes in Ontario, Canada. Diabetes Care. 2005;28(5):1045–1050. doi: 10.2337/diacare.28.5.1045. [DOI] [PubMed] [Google Scholar]
- 8.Nagy D.K., Bresee L.C., Eurich D.T., et al. Rural residence is associated with a delayed trend away from sulfonylurea use for treatment intensification of type 2 diabetes. Diabetes Care. 2023;46(3):613–619. doi: 10.2337/dc22-1223. [DOI] [PubMed] [Google Scholar]
- 9.Health Canada Drug Product Database: Government of Canada; Version 4.0.1. https://health-products.canada.ca/dpd-bdpp/search#results Available from. accessed February 26, 2024.
- 10.Canadian Diabetes Association Clinical Practice Guidelines Expert Committee Canadian Diabetes Association 2008 clinical practice guidelines for the prevention and Management of Diabetes in Canada. Can J Diabetes. 2008;31(suppl 1):S1–S201. doi: 10.1016/j.jcjd.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 11.Canadian Diabetes Association Clinical Practice Guidelines Expert Committee Canadian Diabetes Association 2013 clinical practice guidelines for the prevention and Management of Diabetes in Canada. Can J Diabetes. 2013;37(suppl 1):S1–S212. doi: 10.1016/j.jcjd.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 12.Province of Alberta . Alberta Health Care Insurance Regulation Alberta Regulation 76/2006. Alberta King’s Printer; Edmonton, Alberta: 2022. Alberta Health care insurance act. [Google Scholar]
- 13.Alberta Blue Cross Interactive Drug Benefit List: Government of Alberta. 2024. https://idbl.ab.bluecross.ca/idbl/load.do?reset=true&_cid=c5dd9d2d-5ae3-4fb4-90de-cc7700327057 Available from: accessed February 26, 2024.
- 14.Ray W.A. Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol. 2003;158(9):915–920. doi: 10.1093/aje/kwg231. [published Online First: 2003/10/31] [DOI] [PubMed] [Google Scholar]
- 15.Shah B.R., Booth G.L., Feig D.S., et al. Validation of algorithms to identify gestational diabetes from population-level Health-care administrative data. Can J Diabetes. 2023;47(1):25–30. doi: 10.1016/j.jcjd.2022.06.010. [published Online First: 2022/08/26] [DOI] [PubMed] [Google Scholar]
- 16.Alberta Health Services and Alberta Health Official Standard Geographic Areas. 2018. https://open.alberta.ca/publications/official-standard-geographic-areas Available from: (accessed February 26, 2024)
- 17.Alberta Health . September 2021. Postal Code Translator File (PCTF) [Google Scholar]
- 18.Quan H., Sundararajan V., Halfon P., et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [published Online First: 2005/10/15] [DOI] [PubMed] [Google Scholar]
- 19.Quan H., Li B., Saunders L.D., et al. Assessing validity of ICD-9-CM and ICD-10 administrative data in recording clinical conditions in a unique dually coded database. Health Serv Res. 2008;43(4):1424–1441. doi: 10.1111/j.1475-6773.2007.00822.x. [published Online First: 2008/08/30] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tonelli M., Wiebe N., Fortin M., et al. Methods for identifying 30 chronic conditions: application to administrative data. BMC Med Inform Decis Mak. 2015;15:31. doi: 10.1186/s12911-015-0155-5. [published Online First: 2015/04/18] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elixhauser A., Steiner C., Harris D.R., et al. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27. doi: 10.1097/00005650-199801000-00004. [published Online First: 1998/02/07] [DOI] [PubMed] [Google Scholar]
- 22.Liu X., Shahid R., Patel A.B., et al. Geospatial patterns of comorbidity prevalence among people with osteoarthritis in Alberta Canada. BMC Public Health. 2020;20(1):1551. doi: 10.1186/s12889-020-09599-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rural Health Services Review Committee . Government of Alberta; 2015. Rural Health Services Review Final Report: Understanding the Concerns and Challenges of Albertans Who Live in Rural and Remote Communities. [Google Scholar]
- 24.Alberta's Rural Health Professions Action Plan The Rural Health Professions Action Plan. Strategic Plan. 2020–2024. https://rhpap.ca/resources/ Available from:
- 25.Sibley L.M., Weiner J.P. An evaluation of access to health care services along the rural-urban continuum in Canada. BMC Health Serv Res. 2011;11:20. doi: 10.1186/1472-6963-11-20. [published Online First: 2011/02/02] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fischer F., Lange K., Klose K., et al. Barriers and strategies in guideline implementation-a scoping review. Healthcare (Basel) 2016;4(3) doi: 10.3390/healthcare4030036. [published Online First: 2016/07/16] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Registered Nurses’’ Association of Ontario . 2nd ed. 2012. Toolkit: Implementation of best practice guidelines. [Google Scholar]
- 28.Hunt S., Hunt E. Barriers to practice of rural and remote nursing in Canada. Eur Sci J. 2016;12(36) 56/69. [Google Scholar]
- 29.Bulmer T., Volders D., Kamal N. Analysis of thrombolysis process for acute ischemic stroke in urban and rural hospitals in Nova Scotia Canada. Front Neurol. 2021;12 doi: 10.3389/fneur.2021.645228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Government of Alberta Health research data access Edmonton, AB. 2023. https://www.alberta.ca/health-research.aspx Available from: [accessed February 26, 2024]