Abstract
Acyl-CoA thioesterase 4 (ACOT4) has been reported to be related to acetyl-CoA carboxylase activity regulation; However, its exact functions in liver lipid and glucose metabolism are still unclear. Here, we discovered explored the regulatory roles of ACOT4 in hepatic lipid and glucose metabolism in vitro. We found that the expression level of ACOT4 was significantly increased in the hepatic of db/db and ob/ob mice as well as obese mice fed a high fat diet. Adenovirus-mediated overexpression of ACOT4 promoted gluconeogenesis and high-glucose/high-insulin-induced lipid accumulation and impaired insulin sensitivity in primary mouse hepatocytes, whereas ACOT4 knockdown notably suppressed gluconeogenesis and decreased the triglycerides accumulation in hepatocytes. Furthermore, ACOT4 knockdown increased insulin-induced phosphorylation of AKT and GSK-3β in primary mouse hepatocytes. Mechanistically, we found that upregulation of ACOT4 expression inhibited AMP-activated protein kinase (AMPK) activity, and its knockdown had the opposite effect. However, activator A769662 and inhibitor compound C of AMPK suppressed the impact of the change in ACOT4 expression on AMPK activity. Our data indicated that ACOT4 is related to hepatic glucose and lipid metabolism, primarily via the regulation of AMPK activity. In conclusion, ACOT4 is a potential target for the therapy of non-alcoholic fatty liver (NAFLD) and type 2 diabetes.
Keywords: NAFLD, Type 2 diabetes, ACOT4, AMPK, Gluconeogenesis, Lipogenesis
1. Introduction
The abnormal liver fat accumulation (>5%) without excessive alcohol intake or other reasons for liver steatosis is a feature of non-alcoholic fatty liver disease (NAFLD), the most common chronic liver disease globally [1,2]. Increased prevalence of NAFLD corresponds to the worldwide increase in metabolic syndrome, obesity, and type 2 diabetes mellitus (T2DM) [[3], [4], [5]]. Despite the progress made in the elucidation of the pathophysiology, identification of therapeutic targets, and drug development for NAFLD, significant unresolved challenges remain, with no approved drugs for the treatment of this disease [3]. Therefore, it is important to elucidate the underlying mechanism and detect potential targets for NAFLD.
AMP-activated protein kinase (AMPK) is connected to lipid and glucose metabolism regulation via distinct mechanisms [6,7]. AMPK is a key metabolic mechanisms regulator and may be therapeutically beneficial in the treatment of insulin resistance, T2D, obesity, NAFLD, and cardiovascular disease (CVD) [8]. In order to restore cellular energy homeostasis, active AMPK suppresses anabolic pathways to decrease ATP consumption and activates a catalytic mechanism to create ATP [6,9]. For example, AMPK regulates cellular lipid metabolism by directly enhancing phosphorylating acetyl-CoA carboxylase (ACC)-1 and ACC2, promoting fatty acid oxidation and inhibiting fatty acid synthesis [10,11]. Recent studies have demonstrated that pharmacological ACC suppressors that mimic the AMPK phosphorylation impacts can reduce lipogenesis by diminishing ACC dimer formation, while increasing fatty acid oxidation in isolated primary mouse hepatocytes, thereby alleviating NAFLD symptoms [12]. Moreover, continuous activation of the hepatic AMPK α2 subunit has been reported to decrease blood glucose levels and hepatic gluconeogenesis-related gene expression in diabetic and wild-type mice [13,14].
Acyl-CoA thioesterase 4 (ACOT4) is a member of the ACOT family that hydrolyzes and deactivates fatty acyl-CoA into non-esterified fatty acids (NEFAs) and CoA [15]. ACOTs are divided into two distinct types according to their structure: types I and II [16]. Type I ACOTs (ACOT1–6) are linked to hepatic lipid metabolism modulation by regulating hepatic fatty acid oxidation and adaptive thermogenesis [17]. According to prior research, ACOT1 balances the oxidation flux and capacity to regulate fatty acid metabolism in the fasting liver [18]. In addition, in hepatic mitochondria, ACOT2 has been revealed to promote fatty acid oxidation [19]. ACOT4 is a peroxisomal thioesterase that has a lesser impact on mitochondrial fatty acid oxidation than ACOT2 and ACOT1 have [16]. A recent investigation reported that microRNA (miR)-23b overexpression in db/db mice livers can substantially decrease ACOT4 expression, thereby increasing ACC phosphorylation and improving fatty liver symptoms in mice [20]. However, the ACOT4 function in metabolic homeostasis is not completely understood. In this study, we confirmed a rise in the ACOT4 expression levels in the livers of db/db and high-fat diet (HFD)-induced obese mice. Fasting also increased the hepatic ACOT4 expression. Our results showed that ACOT4 is related to hepatic glucose and lipid metabolism regulation by modulating the AMPK signaling pathway.
2. Materials and methods
2.1. Animals
Male db/db (leptin receptor-deficient mice; rodent model for obesity and T2DM), C57BL/6J mice, ob/ob, and db/m (corresponding control db/db mice) aged 6–8 weeks were purchased from Gempharmatech Co., Ltd. All animals were kept in a pathogen-free environment at a temperature of 22–24 °C with a 12-h light/dark photoperiod and unlimited access to drink and food. For building diet-induced obesity and control models, C57BL/6J mice were nourished for 16 weeks either a standard chow food (9% fat; Lab Diet) or HFD (45% fat; Research Diets). The fasting mouse model was established by removing food and providing free water to C57BL/6J mice for 48 h. All animal studies were performed following the ethical guidelines of the Animal Center of Anhui Medical University, and all experimental protocols treated with animals were permitted by the Laboratory Animal Ethics Committee of Anhui Medical University.
2.2. Reagents and antibodies
Roswell Park Memorial Institute (RPMI)-1640 medium were purchased from Gibco. Fetal bovine serum (FBS) was acquired from Lonsera. Insulin was purchased from Novo Nordisk. Adenyl cyclase activators, forskolin (Fsk) and dexamethasone (Dex), were obtained from Beyotime Biotech Inc. The AMPK inhibitor and activator (Compound C) and A-769662 were purchased from Med Chem Express. Primary antibodies against AKT, phospho-Ser473 AKT, glycogen synthase kinase (GSK)-3β, phospho-GSK-3β, AMPK, phospho-Thr172 AMPK, phospho-ACC, and α-tubulin were obtained from Cell Signaling Technology. Thermo Fisher Scientific was used to obtain a rabbit polyclonal antibody against ACOT4. Other primary antibodies against phosphoenolpyruvate carboxylase (PEPCK) and peroxisome proliferator-activated receptor γ coactivator-1α (PGC-1α) were obtained from Proteintech Group, Inc. Glucose-6-phosphatase (G6pase) antibody was acquired from Abclonal. Anti-FLAG tag antibody was acquired from Sigma-Aldrich.
2.3. RNA extraction and real-time quantitative PCR (qPCR)
TRIzol reagent (Life Technologies) was employed to extract total RNA from the cells or fresh liver tissues. First Strand cDNA Synthesis Kit (Thermo Fisher Scientific) was utilized to reverse-transcribe one total RNA microgram into cDNA. Real-time qPCR was conducted using the SYBR Green I qPCR kit (Promega, Madison, WI, USA) on a Light Cycler 96 (Roche) method to quantify the target genes' mRNA levels. All PCR data are presented relative to the 36B4 mRNA levels. All primer pairs used for qPCR are listed in Table S1.
2.4. Protein extraction and western blotting analysis
Radioimmunoprecipitation assay lysis buffer treated with 1 × protease cocktail suppressor and 1 × phosphatase suppressor was employed to obtain total proteins from cultured cells or pulverized liver tissues. At 4 °C and for 15 min, protein lysates were centrifuged at 12000 rpm before being introduced to 1 × sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) loading buffer and heated for 10 min at 100 °C in a water bath. Protein extracts were loaded and isolated on SDS-PAGE gels of 8 or 10% and electrotransferred to polyvinylidene difluoride membranes (Millipore). The blotted membranes were individually incubated with primary antibodies overnight at 4 °C after blocking at room temperature for 1 h in 0.1% Tris-buffered saline with Tween-20 (TBST) with 5% skim milk. After three 10-min washes with TBST, membranes were incubated at room temperature for 1 h with secondary antibodies at a dilution of 1:5000 or 1:10000. Membranes were then rinsed 3 times with TBST. The blots were subjected to the enhanced chemiluminescence (ECL) reagent for 1 min in the dark in order to observe the proteins, which were then viewed utilizing chemiluminescence system. The protein samples were controlled using α-tubulin.
2.5. Adenovirus expressing ACOT4 and ACOT4 short hairpin RNA (shRNA) preparation
Recombinant adenovirus expressing Ad-ACOT4 (Ad-GFP as the control) was generated by GenePharma, and recombinant adenovirus Ad-shACOT4 (Ad-shNC as the control) was produced by OBiO Technology (Shanghai, China). For the purification of adenoviruses, cesium chloride density gradient centrifugation was conducted, as explained before [21].
2.6. Primary mouse hepatocyte isolation and management
A technique of two-step collagenase retrograde perfusion via the inferior vena cava was utilized to obtain primary mouse hepatocytes from male C57BL/6J mice (age, 6–8 weeks), as reported [22]. After adenovirus infection with Ad-ACOT4, Ad-GFP, Ad-shNC, or Ad-shACOT4 for 24–48 h, primary mouse hepatocytes were replaced with serum-free medium and supplemented with Fsk (10 μM) and Dex (1 μM) for 6 h for further analysis. For insulin treatment, after infection with the indicated adenoviruses for 24–48 h, primary mouse hepatocytes were starved for 6 h in a serum-free medium before being treated for 5 min with 100 nM insulin and then harvested for further analysis.
2.7. Glucose output assay
In RPMI 1640 medium with 10% FBS, primary mouse hepatocytes were implanted in a 6-well plate. Hepatocytes were subjected to the specified adenovirus for 2 days following the 4–6 h of attachment. Following three ice-cold PBS washes, the cells were grown in a glucose production medium made up of DMEM that was phenol red-free and glucose-free and treated for 3 h with 20 mM sodium lactate and 2 mM sodium pyruvate. The glucose production medium was collected, and the glucose released into the medium was measured using a glucose assay kit (Applygen Technologies Inc.). Hepatocytes were lysed, and the amount of protein in each lysate was determined. The glucose production was expressed as g/mg protein after being standardized to the protein levels.
2.8. Hepatic triglyceride (TG) production assay in vitro
TG production in primary mouse hepatocytes was measured, as previously defined [23]. Briefly, after infection with the indicated adenovirus, the supernatant used to culture the cells was obtained, and the amount of cell lysates protein was measured. The TG content in the supernatant was detected utilizing a TG assay kit (Applygen Technologies Inc.) and expressed as mg/g protein.
2.9. Oil red O staining
Primary mouse hepatocytes were double washed with PBS, fixed in 4% polyformaldehyde at room temperature for 60 min, and again double washed with distilled water. The specimens were then stained with fresh Oil Red O working solution for 10 min after being immersed in 60% isopropanol for 3–5 min. Lastly, Oil Red O staining was used to assess intracellular lipid droplet deposition. Images were shown and photographed under a microscope.
2.10. Statistical analysis
Statistical results were analyzed and processed using GraphPad Prism9 and ImageJ software, and the data represent the mean ± standard deviation of the results of three independent experiments. The t-test was employed for comparisons between groups, multiple comparisons between two groups via 2-way ANOVA; *p < 0.05, **p < 0.01, and ***p < 0.001 were regarded as statistically significant.
3. Results
3.1. Hepatic ACOT4 expression levels are increased in diet-induced obese and diabetic mice
Although prior investigations have reported that ACOT4 is related to non-alcoholic steatohepatitis (NASH) regulation [20], its role in hepatic lipid and glucose metabolism is unclear. In this study, we found that the mRNA and protein levels of ACOT4 were significantly higher in the livers of ob/ob and db/db mice than those in control mice (Fig. 1A–D). Similarly, we discovered that ACOT4 expression levels in the HFD-fed obese mice livers were significantly greater than those in mice nourished with a standard chow diet (Fig. 1E and F). We further investigated whether the expression of ACOT4 was regulated by the nutritional status of the body. Our results confirmed that hepatic ACOT4 expression levels were significantly increased after starvation in normal C57BL/6J mice (Fig. 1G and H). ACOT4 expression was consistent with that of significant gluconeogenic genes, such as PEPCK and G6pase (Fig. 1G and H). Furthermore, our data showed that co-treatment with Dex and the cAMP agonist Fsk for 48 h significantly elevated the ACOT4 expression levels in primary mouse hepatocytes (Figs. S1A and B). Taken together, these results revealed that ACOT4 may play a pivotal function in hepatic glucose and lipid homeostasis.
Fig. 1.
Hepatic acyl-CoA thioesterase 4 (ACOT4) expression levels are increased in diabetic and diet-induced obese mice.
(A–B) mRNA and protein levels of ACOT4 in the livers of db/m and db/db mice were determined via quantitative polymerase chain reaction (qPCR) and western blotting.(C–D) qPCR (C) and western blotting (D) analyses revealed the mRNA and protein levels of ACOT4, respectively, in the livers of C57BL/6J and ob/ob mice.(E–F) qPCR (E) and western blotting (F) analyses revealed the mRNA and protein levels of ACOT4, respectively, in the livers of C57BL/6J mice fed a normal diet (Chow) or high-fat diet (HFD) for 12 weeks. (G–H) qPCR (G) and western blotting (H) analyses revealed ACOT4, peroxisome proliferator-activated receptor γ coactivator-1α (PGC-1α), phosphoenolpyruvate carboxylase (PEPCK), and glucose-6-phosphatase (G6pase) mRNA and protein expression levels, respectively, in the livers of 8-week-old male C57BL/6J mice after fasting for 48 h. The uncropped versions of figures are shown in Supplementary of Original Images for Blots and Gels. Data represent the mean ± standard error of the mean (SEM) of at least three independent experiments. *p < 0.05, **p < 0.01, and ***p < 0.001 via Student’s t-test.
3.2. Upregulation of ACOT4 expression impairs gluconeogenesis in mouse primary hepatocytes
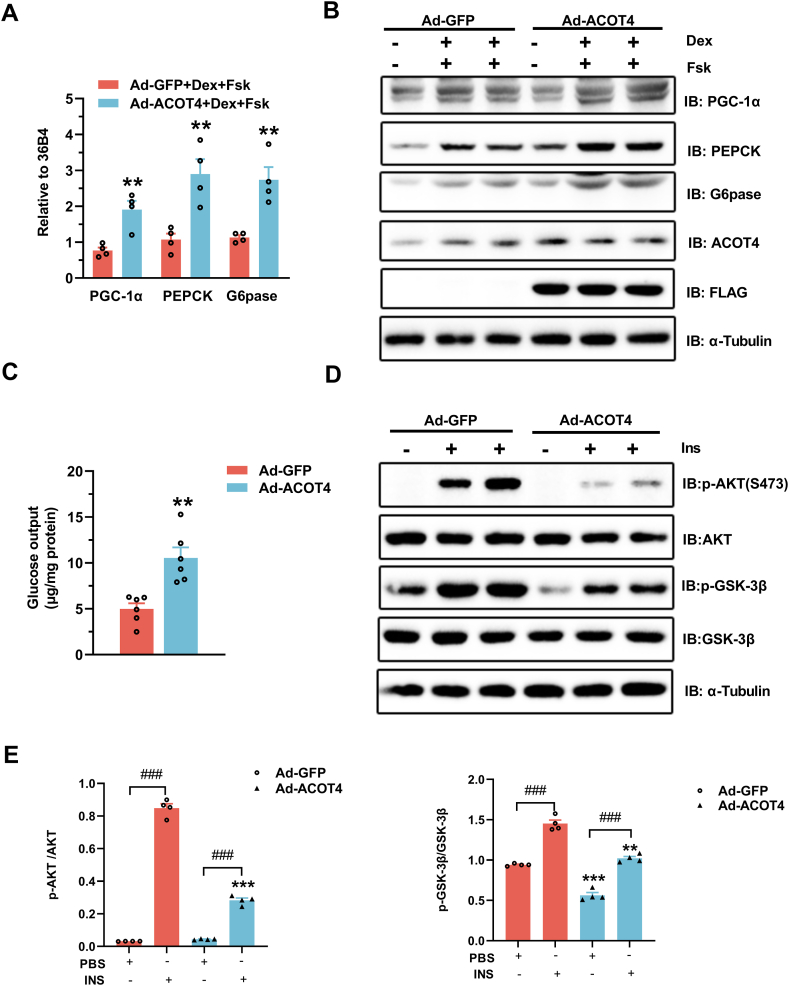
In primary mouse hepatocytes, to define whether ACOT4 can regulate liver gluconeogenesis in a cell-autonomous manner, we employed adenoviral Ad-ACOT4 to upregulate its expression. Western blotting assay confirmed that Ad-ACOT4 effectively increased ACOT4 protein levels compared to Ad-GFP (Fig. 2B). Our data showed that upregulation of ACOT4 expression increased the PEPCK and G6pase mRNA expression levels in the presence of Dex and Fsk, the key genes involved in liver gluconeogenesis (Fig. 2A). Western blotting results further confirmed that forced expression of ACOT4 increased the PEPCK and G6pase protein contents (Fig. 2B). Moreover, the PGC-1α mRNA and protein levels (Fig. 2A and B), a key regulator of increased hepatocyte gluconeogenic gene transcription and glucose production [24], were also elevated. Accordingly, significantly elevated hepatocyte glucose levels were detected in the Ad-ACOT4 group (Fig. 2C). In primary mouse hepatocytes, ACOT4 overexpression notably impaired the insulin-mediated AKT and glycogen synthase kinase 3β (GSK-3β) phosphorylation (Fig. 2D and E). In summary, our data indicate that the upregulation of ACOT4 expression increases hepatic gluconeogenesis and suppresses insulin signaling in vitro.
Fig. 2.
ACOT4 overexpression promotes gluconeogenesis and impairs insulin sensitivity in mouse primary hepatocytes.
(A–B) qPCR (A) and western blotting (B) analyses revealed the mRNA and protein levels, respectively, of the gluconeogenetic genes, PGC-1α, PEPCK, and G6pase, in mouse primary hepatocytes infected with either Ad-GFP or Ad-ACOT4 adenovirus for 24 h and then treated with 1 μM dexamethasone (Dex) and 10 μM forskolin (Fsk) for 6 h. (C) Measurement of cellular glucose production in primary hepatocytes infected with either Ad-GFP or Ad-ACOT4 adenovirus for 24 h. (D) Western blotting revealed the phosphorylation levels of AKT and glycogen synthase kinase (GSK)-3β in mouse primary hepatocytes infected with either Ad-GFP or Ad-ACOT4 adenovirus for 24 h and then treated with 100 nM insulin (Ins) for 5 min. (E) Quantitation of the western blotting data shown in (D). The uncropped versions of figures are shown in Supplementary of Original Images for Blots and Gels. Data represent the means ± SEM of at least three independent experiments. Student’s t-test was used for comparison between the two groups, and 2-way ANOVA was used for comparison between the two groups of multiple factors. *p < 0.05, **p < 0.01, ***p < 0.001, compare to the same treatment group with Ad-GFP; #p < 0.05, ##p < 0.01, ###p < 0.001, INS group compare with PBS group.
3.3. Overexpression of ACOT4 promotes TG accumulation in mouse primary hepatocytes induced by high-glucose/high-insulin (HGlu/HIns)
A recent study reported that ACOT4 can regulate the phosphorylation of ACC [20], indicating its involvement in the regulation of lipogenesis. Here, we explored whether ACOT4 affected lipogenesis in hepatocytes. Oil red O staining indicated that ACOT4 expression upregulation enhanced the number and size of cellular fat droplets induced by HGlu (30 mM)/HIns (100 nM) in primary mouse hepatocytes in comparison with the Ad-GFP control (Fig. 3A). According to quantitative analysis, overexpression of ACOT4 enhanced the TG content in primary mouse hepatocytes following treatment with HGlu/HIns compared to the Ad-GFP group (Fig. 3B), which is matched with the Oil red O staining findings. However, overexpression of ACOT4 exhibited no significant alteration in lipid accumulation in the absence of HGlu/HIns in comparison with that in the control group (Fig. S2A). Next, it was determined whether ACOT4 overexpression could increase the key lipogenic gene expression levels in vitro. Our results showed that ACOT4 overexpression upregulated the mRNA and protein levels of the genes associated with lipogenesis regulation, including ACC, fatty acid synthase (FAS), and stearyl-coenzyme A desaturase 1 (SCD1), in the HGlu/HIns cell model (Fig. 3C and D). However, overexpression of ACOT4 had no impact on sterol regulatory element-binding protein-1c (SREBP1c) expression, the main enzyme transcriptional modulator related to de novo lipogenesis (Fig. S2B). Moreover, forced ACOT4 expression did not affect the genes expression levels related to lipid uptakes or fatty acid oxidation, such as fatty acid translocase CD36 (CD36) and carnitine palmitoyl transferase I α (CPT1α) (Figs. S2B and C). Notably, ACOT4 overexpression reduced the AMPK and its substrate ACC phosphorylation levels. However, the AMPK-specific activator, A-769662, suppressed the ACOT4 inhibitory impacts on AMPK and ACC, as well as the influence of TG content (Fig. 3E and F). Collectively, these data indicate that ACOT4 may be connected to hepatic lipid metabolism modulation by regulating AMPK activity.
Fig. 3.
Overexpression of ACOT4 increases triglyceride (TG) accumulation in mouse primary hepatocytes.
(A) Oil red O staining revealed the number of lipid droplets in primary mouse hepatocytes infected with either Ad-GFP or Ad-ACOT4 adenovirus for 24 h and then treated with 100 nM insulin, 30 mM glucose, and 0.25% bovine serum albumin in a serum-free medium for 24 h. Scale bar, 50 μm. (B) Intracellular TG content in primary mouse hepatocytes infected with either Ad-GFP or Ad-ACOT4 adenovirus for 24 h and then treated with 100 nM insulin and 30 mM glucose for 24 h. (C–D) qPCR(C) and western blotting (D) analyses revealed the mRNA and protein levels of genes involved in lipogenesis in mouse primary hepatocytes infected with either Ad-GFP or Ad-ACOT4 adenovirus for 24 h and then treated with 100 nM insulin and 30 mM glucose for 24 h. (E) Primary mouse hepatocytes were infected with either Ad-GFP or Ad-ACOT4 for 24 h and then treated with AMP-activated protein kinase (AMPK)-specific agonist, A-769662 (10 μM), for 3 h. Phosphorylation levels of AMPK and acetyl-CoA carboxylase (ACC) were determined via western blotting. (F) Intracellular TG content in primary mouse hepatocytes infected with either Ad-GFP or Ad-ACOT4 adenovirus for 24 h and then treated with A-769662 for 3 h. The uncropped versions of figures are shown in Supplementary of Original Images for Blots and Gels. Data represent the means ± SEM of at least three independent experiments. Student’s t-test was used for comparison between the two groups, and 2-way ANOVA was used for comparison between the two groups of multiple factors. *p < 0.05, **p < 0.01, ***p < 0.001, compare to the same treatment group with Ad-GFP; #p < 0.05, ##p < 0.01, ###p < 0.001, A-769662 group compare with DMSO group.
3.4. Knockdown of ACOT4 reduces hepatic gluconeogenesis in primary mouse hepatocytes
We discovered whether the knockdown of ACOT4 was sufficient to suppress gluconeogenesis in primary mouse hepatocytes. Ad-shACOT4 effectively reduced ACOT4 expression (Fig. 4A and B). Our results confirmed that the downregulation of ACOT4 expression in primary mouse hepatocytes decreased the mRNA and protein levels of PEPCK, G6pase, and PGC-1α (Fig. 4C and D). Furthermore, knockdown of ACOT4 markedly reduced glucose synthesis in primary mouse hepatocytes supplemented with Dex and Fsk compared to the Ad-shNC control (Fig. 4E). In addition, knockdown of ACOT4 increased the AKT and GSK-3β phosphorylation in cultured mouse primary liver cells after insulin treatment (Fig. 4F and G). These results suggest that ACOT4 knockdown decreases cellular glucose production and improves insulin sensitivity.
Fig. 4.
Knockdown of ACOT4 inhibits gluconeogenic gene expression, reduces glucose production, and improves insulin sensitivity.
(A–B) qPCR (A) and western blotting (B) analyses revealed the mRNA and protein levels of ACOT4 in mouse primary hepatocytes infected with either Ad-shNC or Ad-shACOT4 adenovirus for 48 h. (C–D) qPCR(C) and western blotting (D) analyses revealed the mRNA and protein levels, respectively, of the gluconeogenetic genes, PGC-1α, PEPCK, and G6pase, in mouse primary hepatocytes infected with either Ad-shNC or Ad-shACOT4 adenovirus for 48 h and then treated with 1 μM Dex and 10 μM Fsk for 6 h. (E) Measurement of cellular glucose production in primary hepatocytes infected with either Ad-shNC or Ad-shACOT4 adenovirus for 48 h. (F) Western blotting revealed the phosphorylation levels of AKT and GSK-3β in mouse primary hepatocytes infected with either Ad-shNC or Ad-shACOT4 adenovirus for 48 h and then treated with 100 nM insulin for 5 min. (G) Quantitation of the western blotting data shown in (F). The uncropped versions of figures are shown in Supplementary of Original Images for Blots and Gels. Data represent the means ± SEM of at least three independent experiments. Student’s t-test was used for comparison between the two groups, and 2-way ANOVA was used for comparison between the two groups of multiple factors. *p < 0.05, **p < 0.01, ***p < 0.001, compare to the same treatment group with Ad-shNC; #p < 0.05, ##p < 0.01, ###p < 0.001, INS group compare with PBS group.
3.5. Knockdown of ACOT4 reduces TG content in primary mouse hepatocytes
We also examined how overnutrition-induced TG accumulation in hepatocytes was impacted by ACOT4 knockdown. Oil red O staining demonstrated that the number and size of lipid droplets induced by HGlu/HIns in primary mouse hepatocytes were decreased when ACOT4 expression was knocked down (Fig. 5A). However, the knockdown of ACOT4 had no significant effect on the lipid droplet accumulation in primary hepatocytes at the basal level (Fig. S3A). According to the quantitative analysis of TGs, ACOT4 knockdown decreased TG accumulation in mouse primary hepatocytes exposed to HGlu/HIns (Fig. 5B). Our results showed that the mRNA and protein levels of FAS, ACC, and SCD1 were elevated in primary hepatocytes exposed to HGlu/HIns, which were decreased after treatment with Ad-shACOT4, in comparison with those in Ad-shNC-treated hepatocytes (Fig. 5C and D). Similarly, we found that knockdown of ACOT4 did not significantly change the expression of SREBP1c and the gene related to fatty acid transport and oxidation, such as CD36 and CPT1α (Figs. S3B and C). In addition, knockdown of ACOT4 enhanced the AMPK and its substrate ACC phosphorylation levels, but co-treatment with the AMPK suppressor, compound C, attenuated these effects (Fig. 5E). Furthermore, compound C can reverse the effect of knocking down ACOT4 on TG content (Fig. 5F). These outcomes further indicate that ACOT4 may be linked to hepatocyte lipid metabolism modulation via the AMPK activity regulation.
Fig. 5.
Knockdown of ACOT4 reduces TG content in primary mouse hepatocytes.
(A) Oil red O staining revealed the number of lipid droplets in mouse primary hepatocytes infected with either Ad-shNC or Ad-shACOT4 adenovirus for 36 h and then treated with 100 nM insulin, 30 mM glucose, and 0.25% bovine serum albumin in a serum-free medium for 24 h. Scale bar, 50 μm. (B) Intracellular TG content in primary mouse hepatocytes infected with either Ad-shNC or Ad-shACOT4 adenovirus for 36 h and then treated with 100 nM insulin and 30 mM glucose for 24 h. (C–D) qPCR(C) and western blotting (D) analyses revealed the mRNA and protein levels, respectively, of genes involved in lipogenesis in mouse primary hepatocytes infected with either Ad-shNC or Ad-shACOT4 adenovirus for 36 h and then treated with 100 nM insulin and 30 mM glucose for 24 h. (E) Western blotting revealed the AMPK and ACC phosphorylation levels in primary mouse hepatocytes infected with either Ad-shNC or Ad-shACOT4 adenovirus for 36 h and then treated with the AMPK-specific inhibitor, Compound C (10 μM), for 12 h. (F) Intracellular TG content in primary mouse hepatocytes infected with either Ad-shNC or Ad-shACOT4 adenovirus for 36 h and then treated with Compound C for 12 h. The uncropped versions of figures are shown in Supplementary of Original Images for Blots and Gels. Data represent the means ± SEM of at least three independent experiments. Student’s t-test was used for comparison between the two groups, and 2-way ANOVA was used for comparison between the two groups of multiple factors. *p < 0.05, **p < 0.01, ***p < 0.001, compare to the same treatment group with Ad-shNC; #p < 0.05, ##p < 0.01, ###p < 0.001, Compound C group compare with DMSO group.
4. Discussion
The main ACOT family members' function is to catalyze the hydrolysis of acyl-CoA thioesters to NEFA and CoA. Based on its tissue expression, subcellular distribution, and substrate selectivity, ACOT regulates fatty acid metabolism [16]. In the current investigation, we demonstrated that ACOT4 has a critical function in the AMPK signaling pathway in hepatic lipid and glucose metabolism in vitro. The finding provides a new target for the avoidance and intervention of T2DM and fatty liver disease.
Our findings confirmed the significant upregulation in ACOT4 expression in the db/db and HFD-induced obese mice livers as well as normal C57BL/6J mice livers after 24 h of fasting. These outcomes revealed that ACOT4 is connected to liver glucose and lipid metabolism modulation. A recent study also reported significantly increased ACOT4 expression levels in the HFD-induced obese mice livers [20]. Although ACOT4 has a vital function in modulating fatty acid oxidation and ACC activity [20], its action pathway is not fully understood. Our outcomes confirmed that, in primary mouse hepatocytes, upregulation of ACOT4 expression significantly promoted gluconeogenesis in the existence of Dex and Fsk, and elevated the key hepatic gluconeogenic genes mRNA and protein expression levels, like PEPCK and G6pase. Glucose output assay also revealed that the overexpression of ACOT4 promoted glucose synthesis in primary mouse hepatocytes. In contrast, ACOT4 knockdown decreased glucose synthesis in the hepatocytes. In line with these results, down-regulation of ACOT4 decreased the gluconeogenic enzymes gene mRNA and protein expression levels, G6pase and PEPCK, in primary mouse hepatocytes. Previous studies have confirmed that dysregulation of glucose production in the liver is a key event in diabetes [25]. Gluconeogenesis is essential for maintaining body functions, especially the central nervous system, during prolonged fasting. However, in T2DM, excessive gluconeogenesis leads to chronic hyperglycemia with serious consequences, such as blindness, kidney failure, and cardiovascular events [26]. Therefore, we believe that inhibition of hepatic ACOT4 expression can appropriately control leptin resistance and HFD-induced diabetes. Insulin represses the main gluconeogenic gene expression both in isolated hepatocytes and in vivo [27]. Indeed, our data revealed that ACOT4 overexpression reduced the AKT and GSK3β phosphorylation levels induced by insulin. Conversely, knockdown of ACOT4 promoted the AKT and GSK3β phosphorylation levels. Insulin pathway mediates gluconeogenesis suppression in the liver [28]. This data reveals the cell-autonomous impact of ACOT4 on insulin signaling.
A previous study showed that overexpression of miR-23b can regulate ACC activity by acting on ACOT4 [20], and ACC activity depends on its phosphorylation level. AMPK phosphorylates ACC1, and ACC2 blocks ACC dimerization, resulting in decreased ACC activity, which lowers malonyl-CoA levels and causes fatty acid synthesis suppression and promotes mitochondrial fatty acid oxidation [10,29]. Indeed, our data showed that ACOT4 overexpression significantly reduced ACC and AMPK phosphorylation levels, and its knockdown diminished ACC activities. Several activators, such as AMP, A769662, and AICAR, allosterically activated AMPK [30,31]. Our outcomes confirmed that A769662 reversed the inhibitory effect of ACOT4 on AMPK activity and led to a decrease in ACC activity. We also found that the AMPK inhibitor, compound C, blocked ACOT4 knockdown by promoting AMPK activity and reducing ACC phosphorylation levels. These results reveal that ACOT4 regulates ACC activity in an AMPK-dependent manner. As an important kinase, AMPK is related to the modulation of multiple metabolic pathways, like obesity and insulin resistance pathways, in T2DM, NAFLD, and CVD [8]. AICAR, an AMPK activator, regulates the AMPK activity and inhibits the crucial gluconeogenic gene expression, such as G6pase and PEPCK [32,33]. Additionally, CRTC2 (cyclic AMP-regulated transcriptional coactivator 2) and class II HDACs histone deacetylases were phosphorylated and excluded from the nucleus by AMPK, which suppressed the transcriptional activation of gluconeogenic genes (HDACs) [34,35]. Our findings revealed that ACOT4 may be connected to the modulation of liver gluconeogenesis by regulating AMPK activity. Previous studies have reported that metformin inhibits mouse liver gluconeogenesis independently of the LKB1/AMPK pathway by reducing liver energy status rather than transcriptional dependent processes [36]. We speculate that ACOT4 may regulate gluconeogenesis through this mechanism. We also found that upregulated ACOT4 expression elevated the FAS, ACC, and SCD1 expression levels, induced by HGlu/HIns, thereby promoting hepatocyte lipogenesis. As opposed to that, ACOT4 knockdown suppressed these genes' expression and reduced hepatocyte lipid accumulation. In addition, knockdown of ACOT4 inhibited the expression of SREBP1 induced by HGlu/HIns stimulation in primary hepatocytes, but its overexpression had no effect on SREBP1 expression (data not presented). ACOT4 is suggested to have a vital function in the β-oxidation of peroxisomes in adipose tissue, but has a minor function in the β-oxidation of fatty acids in mitochondria [16]. Although ACOT1 and ACOT2 have been shown to be involved in mitochondrial fat oxidation [18,19], our data exhibited that intervention of ACOT4 expression in vitro did not have any significant effect on key fatty acid oxidation genes, like CPT1α. Therefore, we believe that ACOT4 is involved in liver lipid synthesis modulation, mainly by regulating AMPK activity; however, the underlying mechanism requires further investigation. In future studies, we aim to investigate its roles in liver glucose and lipid metabolism by constructing liver-specific ACOT4 knockout mice.
5. Conclusions
In conclusion, our results revealed that increased ACOT4 expression in the liver may be one of the factors related to T2DM and NAFLD development. The increase in ACOT4 expression level can to some extent inhibit AMPK activity, thereby increasing lipid synthesis and gluconeogenesis in hepatocytes. Therefore, ACOT4 may be used as a potential target for T2DM and NAFLD therapy.
Funding
This work was supported by collective grants from the Key Research and Development Program of Anhui Province (Grants No. 2022i01020023), Anhui Science Fund for Distinguished Young Scholars (Grants No. 2208085J45), National Natural Science Foundation of China (Grants No. 81870402) and the Project Funded by Basic and Clinical Cooperative Research Promotion Program of Anhui Medical University (Grants No. 2022xkjT026).
Data availability statement
All available data are presented within the article.
CRediT authorship contribution statement
Qianqian Yuan: Writing – original draft. Xiaomin Zhang: Data curation. Xiaonan Yang: Data curation. Qing Zhang: Investigation. Xiang Wei: Formal analysis. Zhimin Ding: Formal analysis. Jiajie Chen: Software. Hongting Hua: Software. Dake Huang: Methodology. Yongxia Xu: Investigation. Xiuyun Wang: Supervision. Chaobing Gao: Visualization, Supervision. Shengxiu Liu: Visualization, Supervision. Huabing Zhang: Writing – review & editing, Visualization, Supervision, Project administration.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e27618.
Contributor Information
Chaobing Gao, Email: gcb110011@163.com.
Shengxiu Liu, Email: liushengxiu@ahmu.edu.cn.
Huabing Zhang, Email: slzhang1977@163.com, huabingzhang@ahmu.edu.cn.
Appendix A. Supplementary data
The following is/are the supplementary data to this article:
References
- 1.Loomba R., Sanyal A.J. The global nafld epidemic. Nat. Rev. Gastroenterol. Hepatol. 2013;10(11):686–690. doi: 10.1038/nrgastro.2013.171. [DOI] [PubMed] [Google Scholar]
- 2.Younossi Z., Anstee Q.M., Marietti M., et al. Global burden of nafld and nash: trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2018;15(1):11–20. doi: 10.1038/nrgastro.2017.109. [DOI] [PubMed] [Google Scholar]
- 3.Friedman S.L., Neuschwander-Tetri B.A., Rinella M., Sanyal A.J. Mechanisms of nafld development and therapeutic strategies. Nat. Med. 2018;24(7):908–922. doi: 10.1038/s41591-018-0104-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith B.K., Marcinko K., Desjardins E.M., Lally J.S., Ford R.J., Steinberg G.R. Treatment of nonalcoholic fatty liver disease: role of ampk. Am. J. Physiol. Endocrinol. Metab. 2016;311(4):E730–E740. doi: 10.1152/ajpendo.00225.2016. [DOI] [PubMed] [Google Scholar]
- 5.Cusi K. Role of obesity and lipotoxicity in the development of nonalcoholic steatohepatitis: pathophysiology and clinical implications. Gastroenterology. 2012;142(4):711–725. doi: 10.1053/j.gastro.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 6.Hardie D.G., Ross F.A., Hawley S.A. Ampk: a nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Biol. 2012;13(4):251–262. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruderman N.B., Carling D., Prentki M., Cacicedo J.M. Ampk, insulin resistance, and the metabolic syndrome. J. Clin. Invest. 2013;123(7):2764–2772. doi: 10.1172/JCI67227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Day E.A., Ford R.J., Steinberg G.R. Ampk as a therapeutic target for treating metabolic diseases. Trends Endocrinol. Metabol. 2017;28(8):545–560. doi: 10.1016/j.tem.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 9.Carling D., Thornton C., Woods A., Sanders M.J. Amp-activated protein kinase: new regulation, new roles? Biochem. J. 2012;445(1):11–27. doi: 10.1042/BJ20120546. [DOI] [PubMed] [Google Scholar]
- 10.Garcia D., Shaw R.J. Ampk: mechanisms of cellular energy sensing and restoration of metabolic balance. Mol. Cell. 2017;66(6):789–800. doi: 10.1016/j.molcel.2017.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fullerton M.D., Galic S., Marcinko K., et al. Single phosphorylation sites in acc1 and acc2 regulate lipid homeostasis and the insulin-sensitizing effects of metformin. Nat. Med. 2013;19(12):1649–1654. doi: 10.1038/nm.3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harriman G., Greenwood J., Bhat S., et al. Acetyl-coa carboxylase inhibition by nd-630 reduces hepatic steatosis, improves insulin sensitivity, and modulates dyslipidemia in rats. Proc. Natl. Acad. Sci. U.S.A. 2016;113(13):E1796–E1805. doi: 10.1073/pnas.1520686113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foretz M., Ancellin N., Andreelli F., et al. Short-term overexpression of a constitutively active form of amp-activated protein kinase in the liver leads to mild hypoglycemia and fatty liver. Diabetes. 2005;54(5):1331–1339. doi: 10.2337/diabetes.54.5.1331. [DOI] [PubMed] [Google Scholar]
- 14.Garcia D., Hellberg K., Chaix A., et al. Genetic liver-specific ampk activation protects against diet-induced obesity and nafld. Cell Rep. 2019;26(1):192–208. doi: 10.1016/j.celrep.2018.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cohen D.E. New players on the metabolic stage: how do you like them acots? Adipocyte. 2013;2(1):3–6. doi: 10.4161/adip.21853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steensels S., Ersoy B.A. Fatty acid activation in thermogenic adipose tissue. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2019;1864(1):79–90. doi: 10.1016/j.bbalip.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 17.Dongol B., Shah Y., Kim I., Gonzalez F.J., Hunt M.C. The acyl-coa thioesterase i is regulated by pparalpha and hnf4alpha via a distal response element in the promoter. J. Lipid Res. 2007;48(8):1781–1791. doi: 10.1194/jlr.M700119-JLR200. [DOI] [PubMed] [Google Scholar]
- 18.Franklin M.P., Sathyanarayan A., Mashek D.G. Acyl-coa thioesterase 1 (acot1) regulates pparalpha to couple fatty acid flux with oxidative capacity during fasting. Diabetes. 2017;66(8):2112–2123. doi: 10.2337/db16-1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moffat C., Bhatia L., Nguyen T., et al. Acyl-coa thioesterase-2 facilitates mitochondrial fatty acid oxidation in the liver. J. Lipid Res. 2014;55(12):2458–2470. doi: 10.1194/jlr.M046961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li H., Li X., Yu S., et al. Mir-23b ameliorates nonalcoholic steatohepatitis by targeting acyl-coa thioesterases 4, Exp. Cell Res. 2021;407(1) doi: 10.1016/j.yexcr.2021.112787. [DOI] [PubMed] [Google Scholar]
- 21.Luo J., Deng Z.L., Luo X., et al. A protocol for rapid generation of recombinant adenoviruses using the adeasy system. Nat. Protoc. 2007;2(5):1236–1247. doi: 10.1038/nprot.2007.135. [DOI] [PubMed] [Google Scholar]
- 22.Wang L., Tong X., Gu F., et al. The klf14 transcription factor regulates hepatic gluconeogenesis in mice. J. Biol. Chem. 2017;292(52):21631–21642. doi: 10.1074/jbc.RA117.000184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang H., Chen Q., Yang M., et al. Mouse klf11 regulates hepatic lipid metabolism. J. Hepatol. 2013;58(4):763–770. doi: 10.1016/j.jhep.2012.11.024. [DOI] [PubMed] [Google Scholar]
- 24.Puigserver P., Rhee J., Donovan J., et al. Insulin-regulated hepatic gluconeogenesis through foxo1-pgc-1alpha interaction. Nature. 2003;423(6939):550–555. doi: 10.1038/nature01667. [DOI] [PubMed] [Google Scholar]
- 25.Cao H., Sekiya M., Ertunc M.E., et al. Adipocyte lipid chaperone ap2 is a secreted adipokine regulating hepatic glucose production. Cell Metabol. 2013;17(5):768–778. doi: 10.1016/j.cmet.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Emambokus N., Granger A., Messmer-Blust A. Exercise metabolism. Cell Metabol. 2015;22(1):1. doi: 10.1016/j.cmet.2015.06.020. [DOI] [PubMed] [Google Scholar]
- 27.Rines A.K., Sharabi K., Tavares C.D., Puigserver P. Targeting hepatic glucose metabolism in the treatment of type 2 diabetes. Nat. Rev. Drug Discov. 2016;15(11):786–804. doi: 10.1038/nrd.2016.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cross D.A., Alessi D.R., Cohen P., Andjelkovich M., Hemmings B.A. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase b. Nature. 1995;378(6559):785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 29.Zhang H.A., Yang X.Y., Xiao Y.F. Ampkalpha1 overexpression alleviates the hepatocyte model of nonalcoholic fatty liver disease via inactivating p38mapk pathway. Biochem. Biophys. Res. Commun. 2016;474(2):364–370. doi: 10.1016/j.bbrc.2016.04.111. [DOI] [PubMed] [Google Scholar]
- 30.Goransson O., Mcbride A., Hawley S.A., et al. Mechanism of action of a-769662, a valuable tool for activation of amp-activated protein kinase. J. Biol. Chem. 2007;282(45):32549–32560. doi: 10.1074/jbc.M706536200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Namgaladze D., Kemmerer M., von Knethen A., Brune B. Aicar inhibits ppargamma during monocyte differentiation to attenuate inflammatory responses to atherogenic lipids. Cardiovasc. Res. 2013;98(3):479–487. doi: 10.1093/cvr/cvt073. [DOI] [PubMed] [Google Scholar]
- 32.Bergeron R., Previs S.F., Cline G.W., et al. Effect of 5-aminoimidazole-4-carboxamide-1-beta-d-ribofuranoside infusion on in vivo glucose and lipid metabolism in lean and obese zucker rats. Diabetes. 2001;50(5):1076–1082. doi: 10.2337/diabetes.50.5.1076. [DOI] [PubMed] [Google Scholar]
- 33.Lochhead P.A., Salt I.P., Walker K.S., Hardie D.G., Sutherland C. 5-aminoimidazole-4-carboxamide riboside mimics the effects of insulin on the expression of the 2 key gluconeogenic genes pepck and glucose-6-phosphatase. Diabetes. 2000;49(6):896–903. doi: 10.2337/diabetes.49.6.896. [DOI] [PubMed] [Google Scholar]
- 34.Koo S.H., Flechner L., Qi L., et al. The creb coactivator torc2 is a key regulator of fasting glucose metabolism. Nature. 2005;437(7062):1109–1111. doi: 10.1038/nature03967. [DOI] [PubMed] [Google Scholar]
- 35.Mcbride A., Ghilagaber S., Nikolaev A., Hardie D.G. The glycogen-binding domain on the ampk beta subunit allows the kinase to act as a glycogen sensor. Cell Metabol. 2009;9(1):23–34. doi: 10.1016/j.cmet.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Foretz M., Hebrard S., Leclerc J., et al. Metformin inhibits hepatic gluconeogenesis in mice independently of the lkb1/ampk pathway via a decrease in hepatic energy state. J. Clin. Invest. 2010;120(7):2355–2369. doi: 10.1172/JCI40671. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All available data are presented within the article.