Summary
Background
Women with glucose intolerance after gestational diabetes mellitus (GDM) are at high risk to develop type 2 diabetes. Traditional lifestyle interventions in early postpartum have limited impact. We investigated the efficacy of a blended mobile-based lifestyle intervention in women with glucose intolerance after a recent history of GDM.
Methods
Prospective, double-arm, non-masked, multicentre randomised controlled trial (RCT) in which women with glucose intolerance, diagnosed 6–16 weeks after a GDM-complicated pregnancy, were assigned 1:1 to a one-year blended-care, telephone- and mobile-based lifestyle program (intervention) or usual care (control). Primary endpoint was the proportion of women able to achieve their weight goal (≥5% weight loss if prepregnancy BMI ≥ 25 kg/m2 or return to prepregnancy weight if prepregnancy BMI < 25 kg/m2) in the intention-to-treat sample. Key secondary outcomes were frequency of glucose intolerance, diabetes and metabolic syndrome, and lifestyle-related outcomes assessed with self-administered questionnaires. The study was registered in ClinicalTrials.gov (NCT03559621).
Findings
Between April 10th 2019 and May 13th 2022, 240 participants were assigned to the intervention (n = 121) or control group (n = 119), of which 167 (n = 82 in intervention and n = 85 in control group) completed the study. Primary outcome was achieved by 46.3% (56) of intervention participants compared to 43.3% (52) in the control group [odds ratio (OR) 1.13, 95% confidence interval (CI) 0.63–2.03, p = 0.680; risk ratio 1.07, 95% CI (0.78–1.48)]. Women in the intervention group developed significantly less often metabolic syndrome compared to the control group [7.3% (6) vs. 16.5% (14), OR 0.40, CI (0.22–0.72), p = 0.002], reported less sedentary behaviour and higher motivation for continuing healthy behaviours. In the intervention group, 84.1% (69) attended at least eight telephone sessions and 70.7% (58) used the app at least once weekly.
Interpretation
A blended, mobile-based lifestyle intervention was not effective in achieving weight goals, but reduced the risk to develop metabolic syndrome.
Funding
Research fund of University Hospitals Leuven, Novo Nordisk, Sanofi, AstraZeneca, Boehringer-Ingelheim, Lilly.
Keywords: Gestational diabetes mellitus, Prediabetes, Lifestyle intervention, Mobile-based, Prevention, Weight retention
Research in context.
Evidence before this study
We searched PubMed for English literature published up to 8 September 2023, using search terms including but not limited to “gestational diabetes mellitus”, “postpartum”, “lifestyle”, “diet”, “physical activity”, “weight”, “IGT”, “impaired glucose tolerance”, “IFG”, “impaired fasting glycaemia”, “prediabetes”, “glucose intolerance” and “type 2 diabetes”. Besides the Diabetes Prevention Program, we found only two other studies that investigated the use of a lifestyle intervention (offered face-to-face) in women with glucose intolerance after a more recent history of GDM. To date, it is still not clear whether a blended-care, mobile-based lifestyle intervention can help women with glucose intolerance achieve weight goals after a recent history of GDM to prevent progression to T2DM.
Added value of this study
The MELINDA (Mobile-based lifestyle intervention in women with glucose intolerance after gestational diabetes) study is a prospective, multicentre RCT to compare a unique blended-care, mobile-based one-year lifestyle intervention with usual care to reach weight goals in women with glucose intolerance after a recent history of GDM. Findings of our study revealed that the intervention was not effective in achieving weight goals. Women in the intervention group developed significantly less often the metabolic syndrome compared to women receiving usual care, but no difference in the frequency of glucose intolerance or diabetes was observed between both groups.
Implications of all the available evidence
To our knowledge, this trial is the first to investigate the use of a unique blended-care, mobile-based lifestyle intervention in women with glucose intolerance shortly after GDM. The intervention was not effective in achieving weight goals, but reduced the risk to develop a metabolic syndrome and decreased sedentary behaviour compared to usual care. The adherence rates to the lifestyle program confirm that this approach is feasible to offer to a group of women at high risk for type 2 diabetes in early postpartum.
Introduction
Gestational diabetes mellitus (GDM) is a frequent medical complication during pregnancy and is defined as diabetes diagnosed during pregnancy, provided that overt diabetes has been excluded in early pregnancy.1 Although glucose values generally normalise shortly after delivery, the underlying β-cell dysfunction often persists postpartum and increases the risk of developing type 2 diabetes (T2DM) in the long term.2 Women with glucose intolerance in early postpartum are a particularly high-risk group, with up to 50% who will develop T2DM within five years after delivery.3 These data reflect the importance of recognizing the early postpartum period as a window of opportunity to prevent progression towards T2DM. Lifestyle modifications have been shown to be effective in the prevention or delay of T2DM in high-risk, middle-aged populations.4 Subgroup analyses of the Diabetes Prevention Program (DPP) trial, focusing on women with previous GDM that have followed an intensive lifestyle intervention over a 3-year period, demonstrated a 53% reduction in T2DM incidence at the end of the trial and a 35% reduction after 10 years.5,6 However, this trial took place on average 12 years after the index pregnancy, thereby excluding women with earlier postpartum conversion to diabetes. In addition, the DPP trial included only adults with impaired glucose tolerance (IGT). A recent meta-analysis found that lifestyle interventions could reduce diabetes incidence in individuals with IGT [with or without impaired fasting glycaemia (IFG)] but not in those with isolated IFG.7 Moreover, a recent meta-analysis on lifestyle interventions for the prevention of T2DM in women with prior GDM found a 24% reduction in T2DM incidence with lifestyle modification compared with standard of care.8 However, this finding was associated with a considerable degree of publication bias, which may limit confidence in translating the effectiveness of lifestyle interventions into clinical practice. Effectively implementing lifestyle interventions in early postpartum is challenging due to low compliance rates and lack of sustained behavioural change, because of barriers such as resumption of work, need for childcare, and limited family support. Lifestyle interventions in women with a recent history of GDM need to be adjusted to address these specific barriers to behaviour change. Digital health interventions have shown potential for improved metabolic outcomes in other high-risk populations, such as people with overweight and obesity.9 Recent studies investigating the use of a smartphone application (alone or in a blended approach) in women with prior GDM differ in timing and duration of the intervention and in their selection of outcomes, and none could demonstrate a significant difference in the primary outcome (weight, diet and/or physical activity).10, 11, 12 These studies included all women with prior GDM (also with normal glucose tolerance postpartum), while women with GDM and glucose intolerance shortly after pregnancy might benefit more from a health promotion intervention, given their high risk to develop T2DM. Since there is a lack of studies evaluating blended-care, mobile-based lifestyle interventions in women with glucose intolerance after a recent history of GDM, we designed the MELINDA (Mobile-based lifestyle intervention in women with glucose intolerance after gestational diabetes) study. This is a multicentre, non-masked randomised controlled trial (RCT) to investigate the efficacy and feasibility of a blended-care, telephone- and mobile-based lifestyle intervention to reach weight goals as a proxy for reduced diabetes risk in women with glucose intolerance (IFG, IGT or both) after a recent history of GDM.
Methods
Study design and participants
The MELINDA study was a one-year, double-arm, parallel-group, non-masked, multicentre RCT to test the efficacy of a telephone- and mobile-based lifestyle intervention to reach weight goals in women with glucose intolerance after a previous pregnancy with GDM. The study was registered in ClinicalTrials.gov (identifier: NCT03559621) and the protocol was published previously.13 Participants were recruited in the diabetes clinics of six regional and two university medical centres in Belgium at the routine postpartum 75 g oral glucose tolerance test (OGTT) 6–16 weeks after delivery. Due to COVID-19 measures, the window-of-visit period was temporarily extended to 6–25 weeks postpartum from May 2020 until March 2021 and from September 2021 until December 2021. Sociodemographic, clinical and biochemical characteristics were collected at the postpartum OGTT from 1201 women with a recent history of GDM based on the 2013 World Health Organization (WHO) criteria (baseline visit).14 GDM screening was done according to the Flemish guidelines from 2019 (with an universal one-step approach with 75 g OGTT in high risk women and with a two-step screening strategy with a 50 g glucose challenge test in women at lower risk).15 Results of the baseline study were published earlier.16 Dutch, English or French speaking women aged 18 years or older with a diagnosis of glucose intolerance at the postpartum OGTT were eligible for inclusion in the RCT. Glucose intolerance was defined as impaired fasting glycaemia (IFG) [fasting plasma glucose (FPG) 5.6–6.9 mmol/l] and/or impaired glucose intolerance (IGT) (2-h glucose value on the OGTT between 7.8 and 11.1 mmol/l) as described by the American Diabetes Association (ADA).17 Exclusion criteria for the RCT were current use of medication that can affect glycaemia (such as glucocorticoids) or receiving treatment for glucose intolerance (such as metformin), health limitations or treatments (any psychiatric conditions or chronic diseases that based on the assessment by the local investigator would not allow for a normal adherence to the intervention and follow-up in the study) which would restrict participation in the RCT, and not possessing a suitable smartphone.
Ethics
The study was approved by the independent Ethics Committee of University Hospitals Leuven and the local ethics committees of all participating centres (reference number B322201837047), and was performed in accordance with the Declaration of Helsinki. Participants gave written informed consent prior to any trial-related activity.
Randomisation and masking
Eligible participants were randomised 1:1, stratified by centre and prepregnancy Body Mass Index (BMI) (cut-off 25 kg/m2) to the lifestyle intervention or control group. A password-protected, computer-generated, permuted block randomisation was used. Varying block sizes of two and four were used to prevent disclosure of the allocation sequence to recruiters. Given the nature of the intervention, this was an open-label study.
Procedures
Baseline characteristics were collected at the routine 75 g postpartum OGTT through blood collection, clinical examination and self-administered questionnaires. Women in the control group were referred to primary care for follow-up in line with normal routine (with the recommendation of diabetes screening once yearly with FPG measurement).18 Women in the intervention group received a blended-care, mobile-based lifestyle intervention for one year to promote healthy lifestyle behaviours. The lifestyle program for the MELINDA study has been developed by several research teams of KU Leuven and was built on interventions from previous studies by our research group, and adapted to meet particular needs of women with a recent history of GDM.9,19,20 The program included one individual face-to-face session at the clinic, a smartphone application with tailored advice and skills training on diet and physical activity, and monthly telephone coaching sessions. Within one month after the postpartum OGTT, participants received the individual face-to-face coaching session of 1.5 h, given by a health care provider (HCP) trained in motivational interviewing. During this session, participants received information on the long-term risks associated with GDM, the importance of a healthy lifestyle, and the use of the MELINDA smartphone application. The app consisted of a data entry module, a coaching module and a library. The data entry module requested monthly input of weight and motivational status, and three-monthly input of abdominal circumference. The coaching module provided a 12-week dietary coaching trajectory interspersed with a 12-week physical activity coaching trajectory with individualized tips to adhere to a healthy lifestyle. If goals were achieved within 6 months, the app helped to maintain and support the current healthy lifestyle. If goals were not achieved, based on the telephone contact and the monitored data, new goals and/or recommendations were formulated in consultation with the participant and new tailored modules were offered to optimize lifestyle.13 A set of limited questions on food literacy was completed to produce tailored goals and tips, focusing on processes such as food planning, selecting the right foods, food preparation, eating habits and evaluating information about food.21 Physical activity was automatically monitored through a pedometer (Mi Band 2 and Mi Band 5, Xiaomi) connected to the app (step counts) and manually entered in the physical activity coaching module (duration and intensity of physical exercise) in order to tailor the physical activity advice and skills training. The library included educational texts and videos on nutrition, physical activity, breastfeeding and the consequences of GDM. In addition, a variety of healthy recipes and workout videos adapted to the postpartum period were available. The lifestyle coach had access to a dashboard, which provided an overview of the evolution of weight, abdominal circumference, physical activity and motivational status of the participant, as well as information on app use. During the monthly telephone coaching sessions, the lifestyle coach consulted the dashboard and used this information to personalise the advice. All coaches received training by an experienced psychologist in motivational interviewing and a standardized procedure was established to perform the telephone coaching sessions. However, the competency of the lifestyle coaches was not measured. A more detailed description of the MELINDA program was published previously.13 One year after the 6–16 weeks postpartum OGTT (baseline visit), all participants received another 75 g OGTT with the same examinations as during the baseline visit (one-year visit).
Outcomes
The predefined primary outcome was the proportion of women able to achieve the weight goal of ≥5% weight loss if prepregnancy BMI was ≥25 kg/m2 or returning to prepregnancy weight if prepregnancy BMI was <25 kg/m2 (weight target calculated based on measured body weight at the one-year visit and prepregnancy weight or, if not available, weight in early pregnancy). Secondary outcomes, evaluated at one year, were frequency of glucose intolerance and diabetes based on the ADA criteria,17 frequency of the metabolic syndrome based on the WHO criteria,22 frequency of overweight, obesity and postpartum weight retention (PPWR), measures of insulin resistance (Matsuda Index, 1/HOMA-IR) and beta-cell function (HOMA-B, the insulinogenic index divided by HOMA-IR, and the insulin secretion sensitivity index (ISSI-2)), mean weight loss, and duration of breastfeeding.13 Secondary lifestyle-related outcomes were dietary quality (assessed with a Food Frequency Questionnaire (FFQ)), measures of physical activity level (assessed with the International Physical Activity Questionnaire—Long Form (IPAQ-LF)), symptoms of depression and anxiety (assessed with the Centre for Epidemiologic Studies-Depression (CES-D) and Spielberger State-Trait Anxiety Inventory (STAI-6) questionnaire), quality of life (assessed with the 36-Item Short Form Health Survey (SF-36)), motivation for behaviour change (assessed with the Treatment Self-Regulation Questionnaire (TSRQ)), perceived risk to develop diabetes (assessed with the Risk Perception Survey For Developing Diabetes (RPS-DD)), and comprehensibility, manageability, and meaningfulness of one’s life (assessed with the Sense of Coherence (SOC) questionnaire).13,16 Adherence to the intervention was assessed by monitoring the use of the MELINDA app and the number of telephone coaching sessions attended.
Statistical analysis
Based on the results of previous studies of our research group with a blended mobile-based lifestyle intervention, we assumed that 20% of women in the control group would reach the weight goal compared to 40% in the intervention group.9 The sample size was calculated to show a difference in the proportion reaching the weight goal after one year with 80% power and 5% significance level, based on a two-sided Chi-square test. Assuming a dropout rate of 30%, a total sample size of 236 for the RCT was planned. Descriptive statistics were presented as frequencies and percentages for categorical variables, and means with standard deviations, or medians with interquartile range for continuous variables. Since there was no evidence for imbalance between the study groups with respect to the dropout rate, inferential analyses were performed by complete case analysis. For the primary outcome, comparison of study groups was based on logistic regression analyses with target weight achievement as binary response variable and study group as explanatory variable. The principle of intention-to-treat (ITT) was adopted, hence, patients were analysed according to the intervention group to which they were randomised, regardless if the intervention was actually received. However, due to the design of the study, data were only collected at baseline and 1 year after the start of the inclusion in the study (at the time of final study visit with 75 g OGTT). The aim of the study was to evaluate the feasibility and effectiveness of a low intensity lifestyle intervention (which was mostly delivered remotely). There was therefore no interim data collection. Once participants stopped with the study, no further data collection occurred. These women did not receive a one-year (final) study visit. Therefore, due to the design of the study, the intention-to-treat analysis is mostly a per protocol analysis (as no data collection could be done of participants who dropped-out before the final study visit). To account for possible bias in the results of complete case analysis due to specific drop-out patterns, multiple imputation was applied in a sensitivity analysis. Fifty imputation data sets were constructed by fully conditional specification (FCS). Analysis variables treatment arm and centre were included in the imputation model as well as pre-pregnancy weight as an auxiliary variable related to missingness. Analyses were performed on each completed data set and combined into unique summary statistics accounting for uncertainty due to the imputations. The primary outcome was therefore analysed as ITT with multiple imputation and as ITT without multiple imputation.
For the secondary outcomes, comparison of the study groups was based on logistic regression analyses for binary outcomes, proportional odds models for ordinal data, and linear models for continuous outcomes. All analysis accounted for clustering by centre by means of generalized estimating equations with independent working correlation structure for binary or ordinal outcomes, or a random intercept for continuous outcomes. All analyses were performed as two-sided tests at 5% significance level using SAS software (version 9.4 for Windows) by statistician Annouschka Laenen (KU Leuven).
Role of the funding source
Funding for this investigator-initiated study was provided by the research fund of University Hospitals Leuven and by an unrestricted grant from Novo Nordisk. The following companies provided limited research grants: Sanofi, AstraZeneca, Boehringer-Ingelheim and Lilly. Funders had no role in the design of the study, nor in data collection, dataanalysis, interpretation, writing of the manuscript, or the decision to submit for publication.
Results
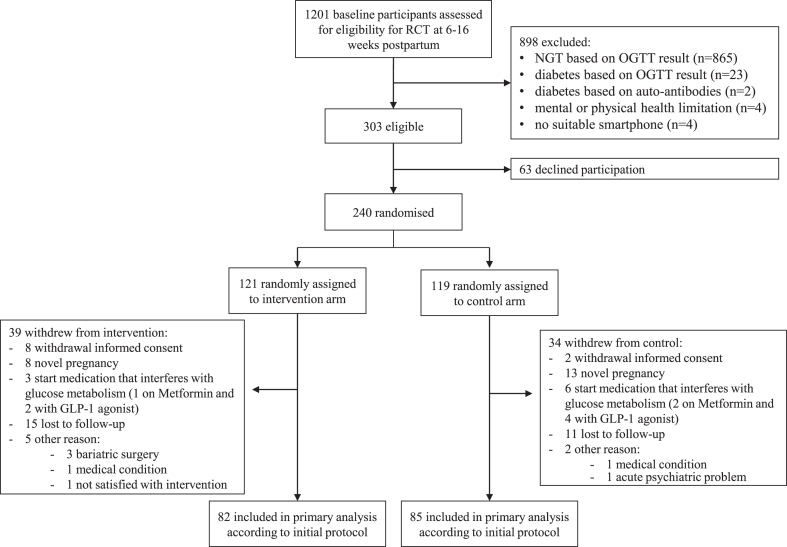
Between April 10th 2019 and May 13th 2022, 1201 women were assessed for eligibility, of which 303 were eligible. Of all eligible women, 79.2% (240) were willing to participate and randomly assigned to the intervention (121) or control group (119). Eligible women who declined participation were more often from an ethnic minority, lower educated and unemployed compared to eligible women who consented to participate (Supplementary Table S1, appendix p. 1). Women who declined participation also had lower scores for role physical and vitality on the SF-36 questionnaire, worse perception of benefits and barriers to behaviour change, and a lower score for autonomous regulation at the baseline visit (Supplementary Table S1, appendix p. 1). Of all randomised participants, 167 participants completed the study and were included in the final analysis (82 in intervention group and 85 in control group). A total of 73 participants (39 in intervention group and 34 in control group) withdrew from the study, corresponding with a dropout rate of 30.4%. Reasons for withdrawal are presented in Fig. 1. Participants who withdrew from the study had a higher body weight at the start of their pregnancy and at the baseline visit in early postpartum. They had a higher abdominal circumference, had more often hypertension, a higher FPG, higher HOMA-IR, and they breastfed less often at baseline (Supplementary Table S2, appendix p. 4).
Fig. 1.
Study flowchart. Eligible participants were randomly assigned in a 1:1 ratio to either a one-year blended-care mobile-based lifestyle program (intervention) or usual care (control). Assessments at baseline and after one year included a 75 g oral glucose tolerance test, clinical examination and self-administered questionnaires. Abbreviations: RCT: randomized controlled trial; NGT: normal glucose tolerant; OGTT: oral glucose tolerance test; GLP-1: glucagon-like-peptide-1.
At a median of 12 weeks postpartum, baseline characteristics of the participants in the intervention and control group did not differ significantly (Table 1). The average age of participants at baseline was 32.1 years (SD 4.1). Of all participants, 82.8% (198) were White and 74.5% (178) were highly educated. Mean BMI at baseline was 27.7 kg/m2 (SD 5.4), 32.9% (79) was overweight and 32.1% (77) obese. Participants underestimated their risk of developing diabetes, with 33.6% (80) of all participants estimating having only a small risk and 2.5% (6) estimating having almost no risk. An extensive overview of all baseline characteristics is presented in Supplementary Table S3 in the appendix (p. 7).
Table 1.
Baseline characteristics.
| Control group N = 119 | Intervention group N = 121 | |
|---|---|---|
| Age at baseline (years) | 32.5 ± 4.1 | 31.8 ± 4.1 |
| % (n) Ethnic minority | 20.3 (24) | 14.0 (17) |
| Ethnic minorities: | ||
| % North-African | 6.8 (8) | 4.1 (5) |
| % Black-African | 2.5 (3) | 0.8 (1) |
| % Latin-American | 1.7 (2) | 0.0 (0) |
| % Middle-East | 1.7 (2) | 0.8 (1) |
| % Asian | 6.8 (8) | 8.3 (10) |
| % Other | 0.8 (1) | 0.0 (0) |
| % (n) Smoking at baseline | 9.3 (11) | 3.3 (4) |
| Highest degree: | ||
| % (n) None/primary school | 0.8 (1) | 0.0 (0) |
| % (n) Secondary school | 20.3 (24) | 29.8 (36) |
| % (n) Higher education | 78.8 (93) | 70.2 (85) |
| % (n) Paid job | 85.6 (101) | 88.4 (107) |
| % (n) Multiparous | 53.8 (64) | 50.4 (61) |
| % (n) First degree family history of T2DM | 36.4 (40) | 29.1 (34) |
| Prepregnancy weight (kg) | 74.9 ± 15.5 | 74.2 ± 15.6 |
| Prepregnancy BMI (kg/m2) | 27.4 ± 5.6 | 27.2 ± 5.4 |
| % (n) Insulin treatment during pregnancy | 35.3 (42) | 29.7 (36) |
| % (n) Cesarean section | 31.9 (38) | 22.3 (27) |
| Time since delivery (weeks) | 12.6 ± 2.9 | 12.2 ± 3.1 |
| Weight (kg) | 76.4 ± 15.1 | 74.8 ± 15.6 |
| BMI (kg/m2) | 28.0 ± 5.4 | 27.4 ± 5.5 |
| % (n) Overweight | 32.8 (39) | 33.1 (40) |
| % (n) Obese | 35.3 (42) | 28.9 (35) |
| Waist circumference (cm) | 93.8 ± 13.5 | 91.6 ± 13.0 |
| % metabolic syndrome | 13.4 (16) | 9.1 (11) |
| PPWR: | ||
| % (n) ≤ 0 kg | 42.9 (51) | 45.5 (55) |
| % (n) > 0 and ≤ 5 kg | 33.6 (40) | 43.0 (52) |
| % (n) > 5 kg | 23.5 (28) | 11.6 (14) |
| HbA1c (%) | 5.3 (5.1–5.6) | 5.4 (5.2–5.5) |
| HbA1c (mmol/mol) | 34.4 (32.2–37.7) | 35.5 (33.3–36.6) |
| Fasting total cholesterol (mmol/l) | 4.7 (4.1–5.5) | 4.7 (4.2–5.4) |
| Fasting HDL-cholesterol (mmol/l) | 1.4 (1.2–1.7) | 1.5 (1.3–1.8) |
| Fasting LDL-cholesterol (mmol/l) | 2.8 (2.3–3.5) | 2.7 (2.3–3.5) |
| Fasting Triglycerides (mmol/l) | 1.0 (0.7–1.6) | 1.0 (0.7–1.4) |
| % (n) IFG | 47.1 (56) | 43.0 (52) |
| % (n) IGT | 67.2 (80) | 68.6 (83) |
| % (n) IFG + IGT | 14.3 (17) | 11.6 (14) |
| Matsuda insulin sensitivity | 3.4 (2.4–4.7) | 3.1 (2.1–4.6) |
| HOMA-IR | 2.4 (1.3–3.3) | 2.2 (1.4–3.1) |
| HOMA-B | 113.7 (77.5–147.7) | 109.2 (78.8–158.2) |
| ISSI-2 | 1.4 (1.1–1.6) | 1.4 (1.2–1.6) |
| Insulinogenic index/HOMA-IR | 0.1 (0.1–0.2) | 0.2 (0.1–0.2) |
| % (n) Breastfeeding | 49.1 (57) | 45.4 (54) |
| % (n) Use of contraceptive | 63.6 (75) | 67.8 (82) |
| Dietary Quality Index (%) | 77.4 (71.9–82.8) | 77.1 (69.5–82.7) |
| IPAQ-LF category at time of OGTT: | ||
| % (n) Low | 11.0 (12) | 10.9 (13) |
| % (n) Moderate | 44.0 (48) | 43.7 (52) |
| % (n) High | 44.9 (49) | 45.4 (54) |
| Average sitting time (minutes/day) | 257.1 (180.0–394.3) | 282.9 (162.9–402.9) |
| % (n) Depression (≥16 on CES-D) | 22.9 (27) | 22.3 (27) |
Categorical variables are presented as frequencies %(n); continuous variables are presented as mean ± SD if normally distributed and as median ± IQR if not normally distributed. Overweight = BMI 25–29.9 kg/m2, obesity = BMI ≥ 30 kg/m2.
T2DM: type 2 diabetes mellitus; BMI: Body Mass Index; PPWR: postpartum weight retention; HbA1c: haemoglobin A1c; HDL: high-density lipoprotein; LDL: low-density-lipoprotein; IFG: impaired fasting glycaemia; IGT: impaired glucose tolerance; HOMA-IR: Homeostatic Model Assessment for Insulin Resistance; HOMA-B: Homeostatic Model Assessment for β-cell function; ISSI-2: insulin secretion-sensitivity index-2; IPAQ-LF: International Physical Activity Questionnaire—Long Form; CES-D: Centre for Epidemiologic Studies—Depression.
The primary outcome, reaching target weight after one year, analysed as ITT with multiple imputation for missing data, was achieved by 46.3% (56) of participants in the intervention group compared with 43.3% (52) in the control group [odds ratio (OR) 1.13, 95% confidence interval (CI) (0.63–2.03) and risk ratio 1.07, 95% CI (0.78–1.48)]. The primary outcome, analysed as ITT without imputation, was achieved by 48.8% (40) of participants in the intervention group compared with 43.5% (37) in the control group [OR 1.24, 95% confidence interval (CI) 0.89–1.71, p = 0.202] (Table 2). No significant differences were observed between the groups for secondary metabolic outcomes such as the prevalence of glucose intolerance and diabetes, overweight and obesity, weight loss, and measures of insulin sensitivity and beta-cell function (Table 2). Women in the intervention group developed significantly less often the metabolic syndrome compared to women in the control group [7.3% (6) vs. 16.5% (14), OR 0.40, CI (0.22–0.72), p = 0.002]. Lifestyle-related outcomes were similar in both groups, except for less mean sedentary time in the intervention group [ 282.7 ± 144.3 vs. 338.4 ± 166.2 min per day, mean difference (MD) −55.68 (−106.60 to 4.73), p = 0.032]. There was a trend toward better diet quality in the intervention group, reflected by a higher mean Dietary Quality Index (DQI), calculated from the FFQ, but the difference did not reach statistical significance [78.3 ± 8.0 vs. 75.6 ± 10. %, MD 2.71 (−0.11 to 5.52), p = 0.059]. Data analysed from the SF-36, CES-D, STAI-6 and SOC questionnaires revealed no statistical significant differences between both groups with regard to quality of life, depression, anxiety, and sense of coherence after one year. Women in the intervention group had a significantly higher degree of both autonomous and controlled motivation for continuing healthy behaviours than women in the control group, reflected by higher average scores on the subscales for autonomous and controlled regulation of the TSRQ. The perceived risk of developing diabetes, assessed by the RPS-DD, did not differ significantly between the intervention and control group (Table 2). Overall, the risk of diabetes was still underestimated after one year, as 35.8% (59) of all participants indicated that they perceived their risk to develop T2DM to be small and 5.9% (9) of participants indicated that they perceived to have no risk to develop T2DM. An extensive overview of all characteristics at the one-year visit is presented in Supplementary Table S4 in the appendix (p. 13).
Table 2.
Primary and secondary outcomes.
| Control group N = 85 | Intervention group N = 82 | Odds ratio (95% CI) | Mean difference (95% CI) | p-value | |
|---|---|---|---|---|---|
| Primary outcome analysis | |||||
| % (n) target weight achieved (ITT analyses with multiple imputation)a | 43.3 (52) | 46.3 (56) | 1.13 (0.63–2.03) | – | 0.680 |
| % (n) target weight achieved (ITT analyses without imputation) | 43.5 (37) | 48.8 (40) | 1.24 (0.89–1.71) | – | 0.202 |
| BMI < 25 kg/m2 | 72.4 (21/29) | 77.8 (28/36) | |||
| BMI ≥ 25 kg/m2 | 28.6 (16/56) | 26.1 (12/46) | |||
| Secondary outcome analysis | |||||
| Metabolic outcomes | |||||
| % (n) diabetes | 5.9 (5) | 3.7 (3) | 0.61 (0.16–2.33) | – | 0.468 |
| % (n) glucose intolerance | 57.6 (49) | 52.4 (43) | 0.81 (0.57–1.18) | – | 0.274 |
| % (n) IFG | 31.8 (14) | 25.0 (10) | |||
| % (n) IGT | 47.7 (21) | 52.5 (21) | |||
| % (n) IFG + IGT | 20.5 (9) | 22.5 (9) | |||
| % (n) metabolic syndrome | 16.5 (14) | 7.3 (6) | 0.40 (0.22–0.72) | – | 0.002 |
| Matsuda index | 3.9 ± 2.4 | 4.0 ± 2.0 | – | 0.09 (−0.60 to 0.798) | 0.801 |
| HOMA-IR | 3.1 ± 3.0 | 2.6 ± 1.9 | – | −0.52 (−1.26 to 0.23) | 0.172 |
| HOMA-B | 121.9 ± 63.0 | 121.3 ± 56.7 | – | −0.17 (−18.12 to 17.77) | 0.985 |
| ISSI-2 | 1.6 ± 0.8 | 1.6 ± 0.6 | – | −0.01 (−0.22 to 0.22) | 0.992 |
| Insulinogenic index/HOMA-IR | 0.3 ± 0.3 | 0.2 ± 0.1 | – | −0.04 (−0.11; 0.04) | 0.355 |
| Weight difference between baseline visit and one-year visit | 0.1 ± 5.8 | 1.6 ± 5.3 | – | 1.44 (−0.20 to 3.09) | 0.085 |
| % (n) PPWR: | |||||
| ≤0 kg | 50.6 (43) | 59.8 (49) | 0.91 (0.43–2.00) | – | 0.817 |
| >0 and ≤ 5 kg | 27.1 (23) | 32.9 (27) | |||
| >5 kg | 31.8 (27) | 20.7 (17) | |||
| % (n) BMI category: | |||||
| Normal | 41.2 (35) | 50.0 (41) | 0.84 (0.48–1.47) | – | 0.532 |
| Overweight | 27.1 (23) | 29.3 (24) | |||
| Obese | 31.8 (27) | 20.7 (17) | |||
| Lifestyle-related outcomes from self-administered questionnaires | |||||
| Dietary Quality Index (%) | 75.6 ± 10.8 | 78.3 ± 8.0 | – | 2.71 (−0.11 to 5.52) | 0.059 |
| IPAQ-LF: | |||||
| Total walking (MET-minutes/week) | 1173.3 ± 1741.7 | 1203.0 ± 1330.3 | – | 29.70 (−477.50 to 536.93) | 0.908 |
| Total moderate (MET-minutes/week) | 2463.0 ± 2800.4 | 2461.6 ± 3751.2 | – | −1.40 (−1085.00 to 1082.00) | 0.998 |
| Total vigorous (MET-minutes/week) | 1018.1 ± 2759.1 | 920.5 ± 2360.0 | – | −97.53 (−937.80 to 742.77) | 0.819 |
| Total overall (MET-minutes/week) | 4654.4 ± 5418.2 | 4585.1 ± 4732.2 | – | −69.24 (−1734.00 to 1595.70) | 0.935 |
| Average sitting time (minutes/day) | 338.4 ± 166.2 | 282.7 ± 144.3 | – | −55.68 (−106.60 to 4.73) | 0.032 |
| % (n) IPAQ-LF category: | 0.92 (0.62–1.37) | – | 0.677 | ||
| Low | 13.7 (10) | 2.7 (2) | |||
| Moderate | 37.0 (27) | 41.1 (30) | |||
| High | 49.3 (36) | 56.2 (41) | |||
| % (n) months of breastfeeding: | 1.22 (0.74–2.01) | – | 0.427 | ||
| 0–1 months | 13.4 (9) | 7.2 (4) | |||
| 1–2 months | 7.5 (5) | 8.9 (5) | |||
| 2–3 months | 9.0 (6) | 16.1 (9) | |||
| 3–6 months | 20.9 (14) | 12.5 (7) | |||
| >6 months | 49.2 (33) | 55.4 (31) | |||
| Psychosocial outcomes from self-administered questionnaires | |||||
| SF-36: | |||||
| Physical functioning | 88.1 ± 17.371.3 ± 19.7 | 90.4 ± 11.3 | – | 2.27 (−2.22 to 6.76) | 0.319 |
| Role physical | 71.8 ± 20.7 | – | 0.52 (−5.67 to 6.71) | 0.869 | |
| Bodily pain | 71.7 ± 18.6 | 74.4 ± 17.2 | – | 2.70 (−2.80 to 8.20) | 0.333 |
| General health | 52.3 ± 14.4 | 52.3 ± 16.2 | – | −0.06 (−4.75 to 4.62) | 0.978 |
| Vitality | 77.5 ± 17.2 | 76.5 ± 16.5 | – | −0.93 (−6.10 to 4.23) | 0.722 |
| Social functioning | 56.4 ± 21.9 | 56.1 ± 21.4 | – | −0.30 (−6.95 to 6.35) | 0.929 |
| Role emotional | 79.0 ± 22.7 | 77.3 ± 23.5 | – | −1.63 (−8.72 to 5.45) | 0.650 |
| Mental health | 68.3 ± 15.5 | 68.2 ± 16.6 | – | −0.10 (−5.02 to 4.82) | 0.967 |
| Total score on CES-D | 10.8 ± 8.6 | 11.2 ± 10.4 | – | 0.41 (−2.51 to 3.33) | 0.782 |
| Total score on STAI-6 | 12.7 ± 3.6 | 12.4 ± 3.7 | – | −0.23 (−1.35 to 0.89) | 0.688 |
| TSRQ autonomous regulation average | 4.6 ± 1.4 | 5.3 ± 1.3 | – | 0.72 (0.31–1.13) | <0.001 |
| TSRQ controlled regulation average | 2.1 ± 1.0 | 2.7 ± 1.0 | – | 0.60 (0.28–0.91) | <0.001 |
| % (n) perceived risk of getting diabetes: | 0.89 (0.53–1.50) | – | 0.669 | ||
| Almost no chance | 5.9 (5) | 4.9 (4) | |||
| Slight chance | 33.3 (28) | 38.3 (31) | |||
| Moderate chance | 47.6 (40) | 44.4 (36) | |||
| High chance | 13.1 (11) | 12.4 (10) | |||
| % (n) perceived risk of getting diabetes without lifestyle changes: | 0.88 (0.59–1.29) | – | 0.515 | ||
| Almost no chance | 3.6 (3) | 3.7 (3) | |||
| Slight chance | 28.6 (24) | 35.8 (29) | |||
| Moderate chance | 50.0 (42) | 39.5 (32) | |||
| High chance | 17.9 (15) | 21.0 (17) | |||
| % (n) Recent lifestyle changes | 53.6 (45) | 49.4 (40) | 0.85 (0.59–1.22) | – | 0.369 |
| % (n) Planning lifestyle changes | 73.8 (62) | 81.5 (66) | 1.56 (0.64–3.81) | – | 0.328 |
| Knowledge of diabetes risk factors (sum) | 5.8 ± 2.0 | 6.2 ± 1.4 | – | 0.41 (−0.11 to 0.94) | 0.124 |
| Personal control (average) | 3.0 ± 0.4 | 3.1 ± 0.5 | – | 0.042 (−0.10 to 0.18) | 0.549 |
| Optimistic bias (average) | 2.0 ± 0.6 | 1.9 ± 0.5 | – | −0.10 (−0.28 to 0.07) | 0.247 |
| Perception of benefits and barriers of preventive behaviours (average) | 3.6 ± 0.5 | 3.8 ± 0.5 | – | 0.20 (0.04–0.35) | 0.013 |
| Average score on SOC | 4.8 ± 0.9 | 4.7 ± 0.9 | – | −0.12 (−0.40 to 0.16) | 0.397 |
Normal weight (BMI < 25 kg/m2), overweight (BMI 25–29.9 kg/m2), obesity (BMI ≥ 30 kg/m2). Categorical variables are presented as frequencies %(n); continuous variables are presented as mean ± SD. Group comparisons were performed using the Mann–Whitney U test for continuous or ordinal outcomes, or, Fisher exact test for categorical outcomes. Results were presented with as odds ratios with 95% confidence intervals for categorical variables and as mean difference with 95% confidence intervals for continuous variables. OR>(<)1: higher (lower)probability of event or levels in intervention group. Mean difference>(<)0: higher (lower) outcome value in intervention group. Differences are considered significant at p-value < 0.05 (in bold).
HOMA-B: Homeostatic Model Assessment for β-cell function; IFG: impaired fasting glycaemia; IGT: impaired glucose tolerance; ITT: intention-to-treat analyses; ISSI-2: insulin secretion-sensitivity index-2; PPWR: postpartum weight retention; NMI: Body Mass Index; IPAQ-LF: International Physical Activity Questionnaire—Long Form; SF-36: 36-Item Short Form Health Survey; CES-D: Centre for Epidemiologic Studies—Depression; STAI: State-Trait Anxiety Inventory; TSRQ: Treatment Self-Regulation Questionnaire; SOC: Sense of coherence.
To account for possible bias in the results of complete case analysis due to specific drop-out patterns, multiple imputation was applied in a sensitivity analysis.
Among women randomised to the intervention that completed the study (82), 48.8% (40) attended all ten telephone coaching sessions, 84.1% (69) attended at least eight telephone coaching sessions, and 96.3% (79) attended at least five telephone coaching sessions. Women who completed all ten telephone coaching sessions were older and more often highly educated compared to those who did not complete all telephone coaching sessions. In addition, women who completed all sessions had a lower degree of PPWR, higher HDL cholesterol, less often IFG, and reported less often that they had made recent lifestyle changes at the baseline visit in early postpartum. By the end of the study, they achieved more often their target weight, had a higher DQI, and they reported more often that they had made recent changes in their lifestyle behaviours (Supplementary Table S5, appendix p.16). Regarding the use of the MELINDA app, 70.7% (58) of intervention participants used the app at least once every week, 93.9% (77) used it at least once every two weeks, and 95.1% (78) used it at least once every month. During the study, no adverse events were causally linked to the intervention.
Discussion
To our knowledge, this is the first RCT investigating the use of a unique blended-care, mobile-based lifestyle intervention in women with glucose intolerance shortly after a pregnancy with GDM. Two previous RCTs have investigated the effectiveness of lifestyle interventions in women with glucose intolerance after a more recent history of GDM than the DPP trial, but did not find a significant impact on metabolic outcomes.23,24 Both trials delivered their lifestyle intervention in a conventional face-to-face approach, which often has been found to be unfeasible in a real-life setting due to barriers associated with the early postpartum period, such as resumption of work, need for child care, and limited family support. The lifestyle program in the present study has been developed evidence-based, consisting of a unique combination of one individual face-to-face session at the clinic with a HCP, the use of a smartphone application with tailored advice and skills training on diet and physical activity, and monthly telephone coaching sessions. It was specifically adapted to the early postpartum period in order to overcome barriers identified in previous research and optimise adherence with the lifestyle program.
Our findings indicate that a blended, mobile-based lifestyle intervention was not effective in achieving weight goals compared to the control group [46.3% (56) vs. 43.3% (52), OR 1.13, 95% CI 0.63–2.0]. Weight goal achievement in the intervention arm was in line with the estimation for the power calculation but was higher than anticipated in the control arm. The power calculation was based on a previous study of our research group with a blended mobile-based lifestyle intervention in adults with obesity,9 indicating that 20% of women in the control group reached the weight goal compared to 40% in the intervention group. The sample size calculation overestimated therefore the predicted effect size. A meta-analysis on the impact of digital and telemedicine interventions (with or without in-person contact) on weight and BMI following GDM also identified a statistically non-significant reduction in BMI and weight, but none of the included trials reported T2DM incidence or cardiovascular risk.25 The largest trial to date of a lifestyle intervention for preventing T2DM in women with prior GDM was published in 2022 by Tandon et al. in South Asia.26 In this RCT, 1612 women with recent GDM were allocated 3–18 months postpartum to a 12-month resource- and context-appropriate lifestyle intervention program or to usual care. In line with our findings, the intervention did not prevent glycaemic deterioration, including development of T2DM, or improve any secondary outcome such as body weight. Although this trial combined face-to-face sessions with remote engagement, the use of a smartphone application was not integrated in the lifestyle intervention. A few other recent RCTs investigated the use of a smartphone application (alone or in a blended approach) in women with prior GDM.10, 11, 12 The interventions in these trials differed in timing (immediately postpartum up to 5 years) and duration (4–12 months), and none of these studies could demonstrate a significant difference in their primary outcome. However, several benefits of the lifestyle intervention were observed in these trials, such as an overall healthier lifestyle with reduced self-reported caloric intake and improved health-directed behaviours in the SPAROW trial and significant improvements in self-efficacy-for-exercise scores, anxiety levels and quality of life in the Baby Steps RCT. A small pilot RCT evaluating the immediate effect of text messages to promote a healthy lifestyle in conjunction with an activity monitor, showed that this low intensity intervention was feasible and was associated with an increase of step count in the hours following supportive text messages.27,28 Direct comparison between trials remains difficult due to the high degree of variability in the selected study population, outcome measures, and the offered intervention in terms of mode of delivery, intensity, timing, and duration of follow-up. Recently, an initiative was set up to develop a core outcome set for behaviour change interventions to prevent diabetes after pregnancy, with the aim to enhance opportunities for comparison of future studies.29,30
In the present study, women who received the lifestyle intervention developed significantly less often the metabolic syndrome (7.3%) compared to those receiving usual care (16.5%) [OR 0.40, CI (0.22–0.72)]. To our knowledge, no other blended lifestyle intervention RCTs in women with previous GDM have included the metabolic syndrome as an outcome measure. It is therefore important to also consider the development of metabolic syndrome in future studies in women with a history of GDM, as this is associated with an increased cardiovascular risk in the long term. We could not detect a significant difference in the prevalence of glucose intolerance and diabetes between both groups in our cohort. To demonstrate such a difference, RCTs with a larger sample size and longer follow-up are needed.
Most previous trials have reported on metabolic and anthropometric outcomes, but not on psychosocial and lifestyle changes. The prevalence of symptoms of depression was high in our cohort (22%). A recent meta-analysis has shown that the frequency of postnatal depression is highly variable (between 5 and 47% of all women with a history of GDM), depending on the questionnaire used to screen for depression and on the timing postpartum.31 However, the intervention in our study did not include mental health support. This highlights the importance for future diabetes prevention programs to include mental health support in women with a history of GDM, as women with GDM and depression are less likely to adhere to a healthy lifestyle in pregnancy and postpartum.32 In our study, significantly less sedentary time per day and a non-significant trend towards better diet quality were observed in the intervention group. We found no differences between the intervention and control group in terms of quality of life, feelings of depression or anxiety, sense of coherence and perceived risk of developing diabetes. Overall, a considerable group of women (about 40%) underestimated their risk of diabetes at the end of the intervention, even though education on the increased risk of T2DM was provided at various times during and after pregnancy. In line with our findings, the PAIGE trial found no significant differences in psychosocial factors, quality of life, or lifestyle, with the exception of significantly reduced bodily pain after six months in the intervention group.33 Women in the intervention group had significantly higher levels of both autonomous and controlled motivation for continuing healthy behaviours than women in the control group. In addition, high attendance rates for the telephone coaching sessions and frequent app use were observed, highlighting the feasibility of the lifestyle program. Moreover, women who attended all telephone coaching sessions were also more likely to achieve their weight goal. These findings suggest that insufficient penetration of the lifestyle intervention is probably not the reason for the limited impact on key outcomes in our trial. A recent meta-analysis stipulated that lifestyle interventions alone may not be sufficient for effectively preventing diabetes in women with prior GDM, and raised the question whether additional pharmacological interventions should be considered in this population.8 The recurrent negative or weak effects shown by most RCTs investigating lifestyle interventions after a recent history of GDM, could partially be related to the psychological distress (with high perceived medicalisation of eating behaviours) associated with the diagnosis of GDM.34 This might also explain the (paradoxically) avoidance and high rates of women in our study that underestimated their future risk to develop T2DM.
Our study has several strengths, including its randomised design and long intervention period of 12 months. Moreover, nearly 80% of all eligible women were included in the RCT and the actual dropout rate (30.4%) was in line with our power calculation. The design of our intervention was built on previous successful interventions of our research group, specifically adapted to our target population, and delivered in a unique blended-care format. The adherence to the lifestyle program was high, highlighting the feasibility for young mothers to adhere to such a blended-care lifestyle intervention. Moreover, lifestyle coaches received structured training and the content of the intervention was offered in three languages (Dutch, French and English) to include a population as diverse as possible and thus minimize recruitment bias. However, we also acknowledge some limitations of our study. Due to the nature of the intervention, both participants and research personnel were non-masked. Despite our effort to include a diverse population, our cohort was overall highly educated and primarily White, leading to possible socioeconomic recruitment bias. Eligible non-participants were more often from an ethnic minority, lower educated and unemployed. Participants who withdrew from the study had a higher prepregnancy body weight, a worse metabolic profile, and breastfed less often in early postpartum. Future interventions should be designed to maximally avoid socioeconomic recruitment bias and minimise dropout rates so that prevention strategies are able to reach all layers of the population. Due to the COVID-19 pandemic, the window-of-visit period for the baseline OGTT was temporarily extended to 25 weeks postpartum. As the intervention was mostly delivered remotely, there was no significant impact of the COVID-19 pandemic on the lifestyle intervention. However, we cannot exclude that due to the general constraints (and stress) associated with the pandemic, it might have been more difficult to adhere to a healthy lifestyle during this period. In addition, as the aim of the study was to evaluate a low intensity lifestyle intervention (which was mostly delivered remotely), no interim data collection was performed. We have therefore no data after the baseline visit of participants who prematurely stopped with the study. In addition, the competency of the lifestyle coaches was not measured. We can therefore not exclude that the limited efficacy on the primary outcome was due to the way the coaches delivered the intervention.
Despite the fact that the present study could not demonstrate a significant effect of a blended-care, mobile-based lifestyle intervention on weight goals in women with glucose intolerance shortly after a pregnancy with GDM, it is important to add the findings of this study to the research field on prevention strategies for T2DM in mothers with prior GDM. This study provides useful guidance for developing future health promotion interventions for women with a recent history of GDM. Future trials should consider the incorporation of mental health support with psychosocial outcomes, and the involvement of the family system in their intervention. The Face-it study, for example, is an ongoing health promotion intervention for women with prior GDM and their partners, addressing the individual, family and healthcare system levels through a combination of home visits by a health visitor and digital coaching with a smartphone application.35
In conclusion, this study found that a blended-care, mobile-based lifestyle intervention was not effective in achieving weight goals among women with glucose intolerance shortly after a pregnancy with GDM, but observed significant impact on some secondary outcomes, including less development of metabolic syndrome, less sedentary behaviour and a higher degree of both autonomous and controlled motivation for continuing healthy behaviours. Moreover, adherence to the lifestyle program was high. Our findings indicate that additional preventive strategies for T2DM should be explored in this high-risk population, including family-based health promotion and pharmacological interventions.
Contributors
K.B. and Car.M. conceived the project. Car.M. prepared the data and A.L. performed the statistical analysis. Car.M. and K.B. wrote the first draft of the manuscript. Car.M contributed to figure and table creating. All authors contributed to the study design, including data collection, data interpretation, and manuscript revision. Car.M. and K.B. are the guarantors of this work and, as such, had full access to the underlying data in the study and take responsibility for the integrity of the data and accuracy of the data analysis. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication. All authors read and approved the final version of the manuscript.
Data sharing statement
Anonymous data are shared with centres participating in the MELINDA trial, based on research questions mentioned in the MELINDA study protocol. Selected anonymous data collected in the study and additional documents can be made available to others not involved in the MELINDA trial upon reasonable request.
Declaration of interests
NM reports consulting fees from AstraZeneca and Boehringer Ingelheim, and serves on the speaker bureau for Novo Nordisk, AstraZeneca, Boehringer Ingelheim, Merck Sharp & Dohme. CBD reports consulting fees and honoraria for speaking for Abbott, AstraZeneca, Boehringer-Ingelheim, A. Menarini Diagnostics, Eli Lilly, Insulet, Medtronic, and Novo Nordisk. WV reports serving on the Merck Sharp & Dohme Advisory board on diabetes. LL has served on the speaker bureau for Novo Nordisk and Sanofi, and received support for attending a conference from Novo Nordisk. ChaM reports consulting fees from Novo Nordisk, Sanofi, Merck Sharp & Dohme, Eli Lilly, AstraZeneca, Boehringer Ingelheim, Roche, Medtronic, ActoBio Therapeutics, Pfizer, Insulet, and Zealand Pharma; and serves or has served on the speaker bureau for Novo Nordisk, Sanofi, Eli Lilly, Boehringer Ingelheim, AstraZeneca, and Novartis. Financial compensation for these activities has been received by KU Leuven. ChaM is president of EASD. All external support of EASD is to be found on www.easd.org. ChrM’s research is funded through internal KU Leuven funding, Fonds voor Wetenschappelijk Onderzoek (FWO), Vlaams Fonds voor Innovatie en Ondernemen (VLAIO), Horizon Europe and VLIR-UOS. He is chair of the KU Leuven Fund ‘Nutrition’, a donation-based fund to stimulate research on nutrition. He is a nonfunded member of the advisory board of Belgian Health and Nutrition Conference, non-funded board member of the Belgian Nutrition Society and the Flemish Society of Clinical Nutrition and Metabolism, non-funded associate editor of Frontiers in Nutrition and Scientific Reports. He is recipient of travel/accommodation expenses and small participation fee (<100€/meeting) as member of working groups of the Belgian Superior Health Council and Belgian Federal Agency of the Safety of the Food Chain. He is recipient of travel/accommodation expenses as member of the scientific advisory body of the Joint Programme Initiative Healthy Diet, Healthy Life, as co-chair ILSI Europe Task Force Dietary Intake and Exposure, as member ILSI Europe Task Force Nutrient Intake Optimisation. He is recipient of honoraria (<500€/year) as active member of the advisory board of NutriNews (Belgian Nutrition Information Centre). He is recipient of honoraria as jury-member of nutrition-related awards and reviewing EU-grants. He is recipient of royalties form a textbook (Handboek Voeding, ACCO). Honoraria are used to support research of PhD Students. KB reports research funding and receipt of study devices from Medtronic for the investigator-initiated CRISTAL study, receipt of study devices from Dexcom for the investigator-initiated GLORIA-study, receipt of study medication of Novo Nordisk for the investigator-initiated SERENA study, consulting fees from AstraZeneca and Lilly, and served on the speaker bureau for Novo Nordisk, AstraZeneca and Mundipharma. CaM received a doctoral grant for strategic basic research from Fonds Wetenschappelijk Onderzoek Flanders. KB received a fundamental clinical investigatorship from Fonds Wetenschappelijk Onderzoek Flanders (1800220 N). All disclosures are unrelated to the present work. All other authors declare no competing interests.
Acknowledgements
University Hospitals Leuven, Leuven, Belgium (sponsor of the MELINDA study) acted as sponsor and received a research grant from the research fund of University Hospitals Leuven and an unrestricted grant of Novo Nordisk. Sanofi, AstraZeneca, Boehringer-Ingelheim and Lilly provided limited research grants, but had no role in the design of the study, collection and analysis of data, writing of the manuscript, or the decision to submit for publication. The authors wish to thank the local investigators, study nurses and their teams for recruiting and following up the participants, completing the case reporting forms, and collecting data. The authors would like to especially thank all women who participated in the trial.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2024.102523.
Appendix ASupplementary data
References
- 1.American Diabetes Association Standards of medical care in diabetes-2021. Diabetes Care. 2021;44:S1–S232. [Google Scholar]
- 2.Buchanan T.A. Pancreatic B-cell defects in gestational diabetes: implications for the pathogenesis and prevention of type 2 diabetes. J Clin Endocrinol Metab. 2001;86:989–993. doi: 10.1210/jcem.86.3.7339. [DOI] [PubMed] [Google Scholar]
- 3.Gerstein H.C., Santaguida P., Raina P., et al. Annual incidence and relative risk of diabetes in people with various categories of dysglycemia: a systematic overview and meta-analysis of prospective studies. Diabetes Res Clin Pract. 2007;78:305–312. doi: 10.1016/j.diabres.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 4.Knowler W.C., Barrett-Connor E., Fowler S.E., et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ratner R.E., Christophi C.A., Metzger B.E., et al. Prevention of diabetes in women with a history of gestational diabetes: effects of metformin and lifestyle interventions. J Clin Endocrinol Metab. 2008;93:4774–4779. doi: 10.1210/jc.2008-0772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aroda V.R., Christophi C.A., Edelstein S.L., et al. The effect of lifestyle intervention and metformin on preventing or delaying diabetes among women with and without gestational diabetes: the diabetes prevention program outcomes study 10-year follow-up. J Clin Endocrinol Metab. 2015;100:1646–1653. doi: 10.1210/jc.2014-3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sathish T., Khunti K., Narayan K.M.V., et al. Effect of conventional lifestyle interventions on type 2 diabetes incidence by glucose-defined prediabetes phenotype: an individual participant data meta-analysis of randomized controlled trials. Diabetes Care. 2023;46:1903–1907. doi: 10.2337/dc23-0696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Retnakaran M., Viana L.V., Kramer C.K. Lifestyle intervention for the prevention of type 2 diabetes in women with prior gestational diabetes: a systematic review and meta-analysis. Diabetes Obes Metab. 2023;25:1196–1202. doi: 10.1111/dom.14966. [DOI] [PubMed] [Google Scholar]
- 9.Hurkmans E., Matthys C., Bogaerts A., Scheys L., Devloo K., Seghers J. Face-to-Face versus mobile versus blended weight loss program: randomized clinical trial. JMIR Mhealth Uhealth. 2018;6:e14. doi: 10.2196/mhealth.7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lim K., Chan S.Y., Lim S.L., et al. A smartphone app to restore optimal weight (SPAROW) in women with recent gestational diabetes mellitus: randomized controlled trial. JMIR Mhealth Uhealth. 2021;9 doi: 10.2196/22147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Potzel A.L., Gar C., Banning F., et al. A novel smartphone app to change risk behaviors of women after gestational diabetes: a randomized controlled trial. PLoS One. 2022;17 doi: 10.1371/JOURNAL.PONE.0267258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khunti K., Sukumar N., Waheed G., et al. Structured group education programme and accompanying mHealth intervention to promote physical activity in women with a history of gestational diabetes: a randomised controlled trial. Diabet Med. 2023;40 doi: 10.1111/DME.15118. [DOI] [PubMed] [Google Scholar]
- 13.Minschart C., Maes T., De Block C., et al. Mobile-based lifestyle intervention in women with glucose intolerance after gestational diabetes mellitus (MELINDA), A multicenter randomized controlled trial: methodology and design. J Clin Med. 2020;2635:2635. doi: 10.3390/jcm9082635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization (WHO) 2013. Diagnostic criteria and classification of hyperglycaemia first detected in pregnancy. [PubMed] [Google Scholar]
- 15.Benhalima K., Minschart C., Van Crombrugge P., et al. The 2019 Flemish consensus on screening for overt diabetes in early pregnancy and screening for gestational diabetes mellitus. Acta Clin Belg. 2020;75:340–347. doi: 10.1080/17843286.2019.1637389. [DOI] [PubMed] [Google Scholar]
- 16.Minschart C., Myngheer N., Maes T., et al. Weight retention and glucose intolerance in early postpartum after gestational diabetes. Eur J Endocrinol. 2023;188:438–447. doi: 10.1093/ejendo/lvad053. [DOI] [PubMed] [Google Scholar]
- 17.American Diabetes Association Standards of medical care in diabetes - 2017. Diabetes Care. 2017;40:S11–S24. [Google Scholar]
- 18.Benhalima K., Verstraete S., Muylle F., et al. Implementing a reminder system in the northern part of Belgium to stimulate postpartum screening for glucose intolerance in women with gestational diabetes: the “Sweet Pregnancy” Project. Int J Endocrinol. 2017;2017 doi: 10.1155/2017/3971914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boedt T., Dancet E., Lie Fong S., et al. Effectiveness of a mobile preconception lifestyle programme in couples undergoing in vitro fertilisation (IVF): the protocol for the PreLiFe randomised controlled trial (PreLiFe-RCT) BMJ Open. 2019;9 doi: 10.1136/bmjopen-2019-029665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bogaerts A., Bijlholt M., Mertens L., et al. Development and field evaluation of the INTER-ACT app, a pregnancy and interpregnancy coaching app to reduce maternal overweight and obesity: mixed methods design. JMIR Form Res. 2020;4 doi: 10.2196/16090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boedt T., Steenackers N., Verbeke J., et al. A mixed-method approach to develop and validate an integrated food literacy tool for personalized food literacy guidance. Front Nutr. 2022;21 doi: 10.3389/fnut.2021.760493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grundy S.M., Brewer H.B., Cleeman J.I., Smith S.C., Lenfant C. Definition of metabolic syndrome: report of the national heart, lung, and blood institute/American heart association conference on scientific issues related to definition. Circulation. 2004;109:433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 23.Shek N.W.M., Ngai C.S.W., Lee C.P., Chan J.Y.C., Lao T.T.H. Lifestyle modifications in the development of diabetes mellitus and metabolic syndrome in Chinese women who had gestational diabetes mellitus: a randomized interventional trial. Arch Gynecol Obstet. 2014;289:319–327. doi: 10.1007/s00404-013-2971-0. [DOI] [PubMed] [Google Scholar]
- 24.O’Dea A., Tierney M., McGuire B.E., et al. Can the onset of type 2 diabetes be delayed by a group-based lifestyle intervention in women with prediabetes following gestational diabetes mellitus (GDM)? Findings from a randomized control mixed methods trial. J Diabetes Res. 2015;2015 doi: 10.1155/2015/798460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Halligan J., Whelan M.E., Roberts N., Farmer A.J. Reducing weight and BMI following gestational diabetes: a systematic review and meta-analysis of digital and telemedicine interventions. BMJ Open Diabetes Res Care. 2021;9 doi: 10.1136/BMJDRC-2020-002077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tandon N., Gupta Y., Kapoor D., et al. Effects of a lifestyle intervention to prevent deterioration in glycemic status among South asian women with recent gestational diabetes: a randomized clinical trial. JAMA Netw Open. 2022;5 doi: 10.1001/JAMANETWORKOPEN.2022.0773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheung N.W., Blumenthal C., Smith B.J., et al. A pilot randomised controlled trial of a text messaging intervention with customisation using linked data from wireless wearable activity monitors to improve risk factors following gestational diabetes. Nutrients. 2019;11:590. doi: 10.3390/nu11030590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheung N.W., Thiagalingam A., Smith B.J., et al. Text messages promoting healthy lifestyle and linked with activity monitors stimulate an immediate increase in physical activity among women after gestational diabetes. Diabetes Res Clin Pract. 2022;190 doi: 10.1016/j.diabres.2022.109991. [DOI] [PubMed] [Google Scholar]
- 29.Nielsen K.K., O’Reilly S., Wu N., Dasgupta K., Maindal H.T. Development of a core outcome set for diabetes after pregnancy prevention interventions (COS-DAP): a study protocol. Trials. 2018;19:708. doi: 10.1186/s13063-018-3072-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu N., O’Reilly S., Nielsen K.K., Maindal H.T., Dasgupta K. Core outcome set for diabetes after pregnancy prevention across the life span: international Delphi study. BMJ Open Diabetes Res Care. 2020;8 doi: 10.1136/bmjdrc-2020-001594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilson C.A., Newham J., Rankin J., et al. Is there an increased risk of perinatal mental disorder in women with gestational diabetes? A systematic review and meta-analysis. Diabet Med. 2020;37:602–622. doi: 10.1111/dme.14170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Minschart C., De Weerdt K., Elegeert A., et al. Antenatal depression and risk of gestational diabetes, adverse pregnancy outcomes, and postpartum quality of life. J Clin Endocrinol Metab. 2021;106:e3110–e3124. doi: 10.1210/clinem/dgab156. [DOI] [PubMed] [Google Scholar]
- 33.Holmes V.A., Draffin C.R., Patterson C.C., et al. Postnatal lifestyle intervention for overweight women with previous gestational diabetes: a randomized controlled trial. J Clin Endocrinol Metab. 2018;103:2478–2487. doi: 10.1210/jc.2017-02654. [DOI] [PubMed] [Google Scholar]
- 34.Benton M., Silverio S.A., Ismail K. “It feels like medically promoted disordered eating”: the psychosocial impact of gestational diabetes mellitus in the perinatal period. PLoS One. 2023;18 doi: 10.1371/journal.pone.0288395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maindal H.T., Timm A., Dahl-Petersen I.K., et al. Systematically developing a family-based health promotion intervention for women with prior gestational diabetes based on evidence, theory and co-production: the Face-it study. BMC Publ Health. 2021;21:1–14. doi: 10.1186/s12889-021-11655-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.