Abstract
Chronic wounds are among the most devastating and difficult to treat consequences of diabetes. Dysregulation of the skin renin-angiotensin system is implicated in abnormal wound healing in diabetic and older adults. Given this, we sought to determine the effects of topical reformulations of the angiotensin type 1 receptor blockers losartan and valsartan and the angiotensin-converting enzyme inhibitor captopril on wound healing in diabetic and aged mice with further validation in older diabetic pigs. The application of 1% valsartan gel compared with other tested formulations and placebo facilitated and significantly accelerated closure time and increased tensile strength in mice, and was validated in the porcine model. One percent of valsartan gel-treated wounds also exhibited higher mitochondrial content, collagen deposition, phosphorylated mothers against decapentaplegic homologs 2 and 3 and common mothers against decapentaplegic homolog 4, alpha-smooth muscle actin, CD31, phospho-vascular endothelial growth factor receptor 2, and p42/44 mitogen-activated protein kinase. Knockout of the angiotensin subtype 2 receptors abolished the beneficial effects of angiotensin type 1 receptor blockers, suggesting a role for angiotensin subtype 2 receptors in chronic wound healing.
INTRODUCTION
Chronic wounds are among the most common, painful, and debilitating consequences of diabetes (Abbott et al., 2002; Boulton et al., 2005; Singh and Newman, 2011). The biology of normal wound healing includes sequential yet overlapping inflammatory, proliferative, and remodeling phases that involve complex biological signaling. Dysregulation of this signaling is thought to underlie skin breakdown, poor healing, and the development of chronic wounds (Stadelmann et al., 1998a). The renin-angiotensin system (RAS) is involved in the inflammatory response, collagen deposition, and transforming growth factor-beta (TGFβ) signaling necessary for wound healing (Cooper, 2004; Hao et al., 2011; Rodgers et al., 2011; Steckelings et al., 2004). The RAS is known to be dysregulated in both aging and diabetes, with increased angiotensin II type 1 receptor (AT1R) and decreased angiotensin II type 2 receptor (AT2R) expression in diabetic wound healing and aging (Hao et al., 2011), which may play a role in the skin vulnerability associated with aging and diabetes (Abadir et al., 2011; Cooper, 2004; Hao et al., 2011). Indeed, the altered dermal AT1R and AT2R ratio is associated with thinning of the epidermis, degeneration of collagen, fracture of the dermal layer, and atrophy of subcutaneous fat in diabetic rats (Hao et al., 2011). To date, one study suggests that the use of oral angiotensin type 1 receptor blockers (ARBs) in animal models impaired fibroblast migration and delayed wound healing (Yahata et al., 2006). The AT2R is less studied than AT1R in the context of wound healing, but its anti-inflammatory, antiapoptotic, and cell differentiation effects likely oppose the effects of AT1R (Vajapey et al., 2014). The overarching hypothesis of this study is that the topical and phase-targeted use of angiotensin receptor blockers would accelerate wound healing and collagen deposition and enhance the quality of healing skin via the TGFβ signaling pathway.
RESULTS
To ascertain the impact of ARBs on wound healing, downstream effectors, and healed skin quality, we reformulated oral RAS blockers into topical treatments (gels). Topical ARBs were selected to avoid the systemic impacts of ARBs and to focus on the effects of the local skin RAS. We studied the effects of these treatments on wounds in young diabetic (Leprdb/J) and aged nondiabetic (C57BL/6) mice, as well as aged diabetic pigs.
Delayed application of topical losartan accelerated wound healing in diabetic mice
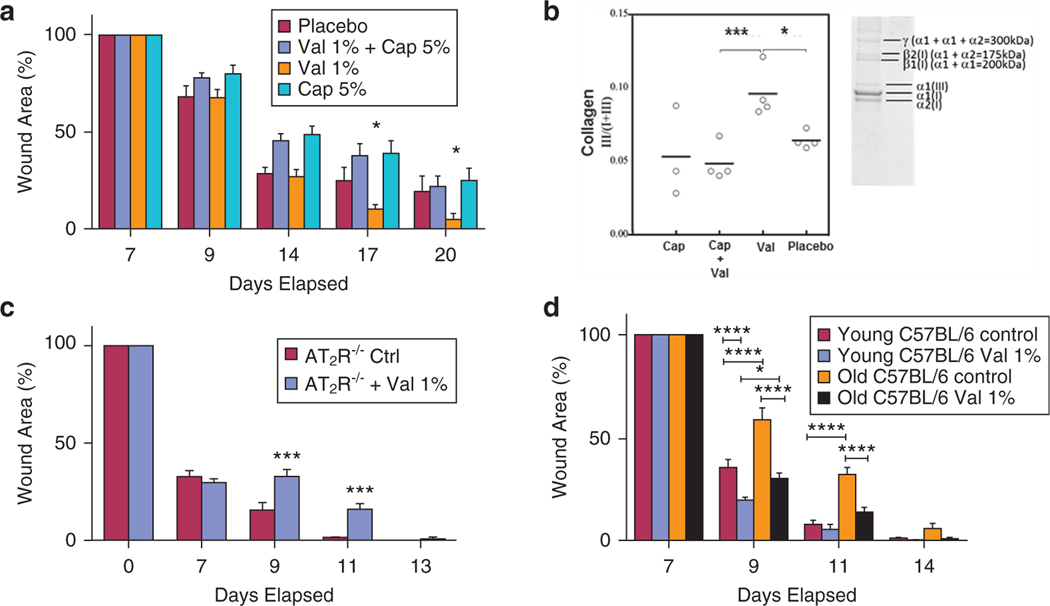
Previous research supports the notion that the activation of the RAS in skin plays a crucial role in wound healing (Cooper, 2004; Hao et al., 2011; Yevdokimova and Podpryatov, 2007). It has also been suggested that systemic AT1R blockers impair fibroblast migration and delay wound healing (Yahata et al., 2006) and that the impact of AT1R and AT2R on wound healing may be phase dependent (Faghih et al., 2015). To test the effects of topical ARBs on different phases of wound healing, we utilized standardized wounding techniques to generate 8-mm dorsal wounds on the backs of 8-week-old BKS.Cg-m+/+ Leprdb/Jdb/db mice. We applied 5% topical losartan (dose extrapolated from skeletal muscle findings (Burks et al., 2011, 2015) to three different groups (Supplementary Figure S1 online) that correspond to wound phases (group 1, treatment up to day 3 after wounding to target the inflammatory phase; group 2, treatment starting on day 7 after wounding to target the proliferative/remodeling phase; group 3, treatment from the first day until closure to target all phases; group 4, vehicle treatment as the control). As shown in Figure 1a, the results suggest that treatment with 5% losartan starting on day 7 after wounding significantly accelerated the time to closure compared with other treatment groups and the control (P < 0.05; Figure 1a). We next sought to determine if there was a difference in the healed skin’s physical characteristics (peak force, total work, and compliance). Our results show that the accelerated healing rate observed in the delayed losartan group was associated with stronger healed skin that required more force to break (P<0.05; Figure 1b). Topical treatment with losartan increased the blood flow to the woundbed (P<0.05; Figure 1c), which is consistent with the vasodilation properties of ARBs.
Figure 1. Wound closure measurements in mice treated with different angiotensin system blockers at different time points of wound healing.
Planimetric assessment of (a) the wound closure rate and biomechanical assessment, n = 6 mice per group, and of (b) healed skin in diabetic (Leprdb/J) mice treated with 5% topical losartan, n = 3–5 mice per group. (c) Laser Doppler perfusion imaging of wound area blood flow, n = 5 mice per group. (d) Planimetric assessment of the wound closure rate in Leprdb/J mice treated with different doses of valsartan and losartan gels applied 7 days after wounding, n = 7–8 mice per group. (e) Altered mRNA expression of wound angiotensin receptors and TGFβ isoforms in Leprdb/J mice treated with losartan 1% and valsartan 1%, n = 3–5 mice per group. (f) Kaplan-Meier analysis of complete wound closure in Leprdb/J mice treated with 1% valsartan n = 7–8 mice per group. *P < 0.05, **P < 0.01, ***P < 0.001, +++P < 0.001 los 1% versus val 1% early stage, +P < 0.05 los 1% versus val 1% late stage of wound healing. AT1R, angiotensin II type 1 receptor; TGFβ, transforming growth factor-beta.
Topical losartan versus valsartan.
Despite the similarity in the specificity of different ARBs toward AT1R, each ARB has unique properties, affinity to AT1R, and impacts on cellular functions (Gring and Francis, 2004). Losartan is a selective AT1R blocker, but it also has partial PPARƔ agonist properties. Furthermore, 14% of losartan is metabolized by cytochrome P450 2C9 enzyme to its metabolite EXP 3174 (Aulakh et al., 2007; Jackson, 2006). Valsartan was selected as a comparative ARB based on its AT1R specificity and because it is an active drug that does not require metabolism. We therefore compared the efficacy of 1%, 5%, and 10% losartan gel with that of 0.5%, 1%, and 5% valsartan gel applied during the proliferation/remodeling phase of wound healing in diabetic mice. The results (Figure 1d) demonstrated that valsartan was more efficacious in accelerating wound healing compared with losartan. Even though each valsartan dose significantly accelerated healing compared with placebo (val 1%; P = 0.006, val 0.5%; P = 0.02, val 5%; P = 0.01), there was no significant difference in healing time between any of the valsartan doses. However, val 1% had a greater overall impact on total closure compared with other agents. In contrast, the application of 10% losartan was associated with worse wound healing (P < 0.05), suggesting possible toxicity related to that higher dose. The smallest wound area over time compared with placebo was seen with losartan 1%. The rate of wound closure was significantly faster with valsartan 1% compared with losartan 1% (P < 0.001 during the early stage and P < 0.05 during the later stage of wound healing). To determine the mechanism associated with the differential effects of the best dose of valsartan 1% compared with the best dose of losartan 1%, we examined the changes in mRNA expression of the angiotensin receptors (AT1R and AT2R) and the three isoforms of TGFβ (1, 2, and 3) in wounds treated with valsartan 1% and losartan 1%. Wounds treated with 1% valsartan had a lower AT1R mRNA quantity (0.7-fold, P < 0.05) than those treated with losartan 1%. The valsartan treatments also caused a nonsignificant increase in the AT2R mRNA and a decrease in TGFγ3 mRNA (0.8-fold, P < 0.05) (Figure 1e). Additionally, the Kaplan-Meier analysis (Figure 1f) revealed that when 50% of the animals in the valsartan 1% cohort achieved complete wound healing, only 10% of the placebo-treated mice achieved complete wound healing (P < 0.001). These findings provided a rationale for the choice of 1% valsartan applied starting at day 7 after wounding as the dose for the experiments described below.
Topical valsartan versus captopril.
Clinically, both ARBs and angiotensin-converting enzyme (ACE) inhibitors have yielded comparable results in terms of blood pressure control and cardiovascular protection (Li et al., 2014). Pharmacologically, ARBs and ACE inhibitors differ in their mechanism of action and the level at which they block the RAS. Although ARBs block the RAS distally at the AT1R level, ACE inhibitors block the conversion of angiotensin I to angiotensin II and thereby decrease the amount of available angiotensin II to bind to either AT1Rs or AT2Rs. Topical treatment with captopril 5% significantly delayed the wound closure rate compared with valsartan 1% (P < 0.05). Interestingly, the addition of valsartan 1% to captopril 5% did not alleviate the negative effects of captopril (Figure 2a). Our data also suggest that topical treatment with 1% valsartan increased the ratio of type III collagen to total collagen compared with placebo (P < 0.05; Figure 2b). Given the mechanistic difference between the agents described above, the contrast between valsartan and captopril may suggest a role for AT2Rs in mediating the effects of topical valsartan (captopril deactivates both AT1R and AT2R, whereas valsartan deactivates only AT1R and augments AT2R). To further clarify the possibility of a role for AT2R in wound healing, we applied valsartan 1% gel to AT2R−/− mice. Our data (Figure 2c) show that valsartan 1% paradoxically delayed wound healing in AT2R−/− mice (P < 0.001 on day 9 and day 11). Together, these results demonstrate that the application of 1% valsartan gel starting on day 7 after wounding accelerated the time to wound closure and that AT2R plays a role in mediating the effects of topical valsartan. Changes in wound tissue inflammatory markers (Supplementary Figure S2 online) suggest that the pharmacological blockade of the angiotensin type 1 receptor with topical valsartan during wound healing may alter inflammatory response but also clearly demonstrate that this interaction is complex and changes with duration or onset of administration of the topical ARB.
Figure 2. Impact of topical ARB and ACEi on wounds of aged, diabetic and AT2R−/− mice.
(a) Comparison between 1% valsartan gel and 5% captopril gel applied starting on seventh day after wounding, demonstrating delayed healing with captopril, n = 7–9 mice per group. (b) Collagen type III/I+III ratio quantification and representative image, n= 3–4miceper group in wounds of Leprdb/J mice treated with valsartan 1% and/or captopril 5%. The highest ratio was found in the wounds treated with valsartan. (c) One percent valsartan gel failed to accelerate wound closure in AT2R−/− mice, n = 5–6 mice per group. (d) Planimetric assessment of the wound closure rate in young (6 weeks old) and aged (104 weeks old) C57BL/6 male mice treated with 1% valsartan gel applied starting on seventh day after wounding, n = 7–10 mice per group. The data are presented as the mean ± standard error of the mean. *P < 0.05, ***P < 0.001. ****P < 0.0001. AT2R−/−, angiotensin II type 2 receptor knockout.
Finally, to determine if the effects of valsartan are specific to diabetic wound healing or if it applies as well to wounds in aging models, we studied the effects of 1% valsartan treatment on young and aged C57BL/6J mice. We compared control and valsartan treated 6- to 8-week-old male C57BL/6J mice with 104-week-old male C57BL/6J mice. Our data (Figure 2d) demonstrate that aged C57BL/6J mice have slower wound healing rate as compared with the young controls. Our data also demonstrate that 1% valsartan gel accelerated wound healing in young and aged C57BL/6J mice, but the impact was more pronounced in the aged animals.
Testing valsartan 1% in the diabetic porcine model of chronic wound healing.
Pig skin has been shown to have similar physiohistological properties to human skin and is suggested as a good model for human wounds (FDA, 2006). Driven by the promising effects of 1% valsartan gel on accelerating wound healing in mice, we sought to determine the effects of this agent on an aged and diabetic porcine model of wound healing. Diabetic (36 months) Yucatan miniature swine were studied. Diabetes was alloxan induced at 7 months of age. Animals were 3 years old at the time of wounding and had blood sugars ranging from 200 to 400 mg/dl to approximate older diabetic humans with poor glucose control. Wounds treated with valsartan 1% exhibited superior healing compared with those treated with placebo gel (Supplementary Video S1 online and Figure 3a and b). Consistent with these photographic results, wounds receiving valsartan showed faster wound closure rates than corresponding placebo gel-treated wounds over a period of 57 days (Figure 3c; P < 0.0001). Ultraperformance liquid chromatography was utilized to determine whether valsartan was systemically absorbed from the pig wounds treated with topical valsartan. The results revealed valsartan plasma concentrations ranging from 1 nM to a peak of 50 nM early in the course of treatment. Valsartan was undetectable in the blood later in the treatment course (for comparison, an expected blood level of valsartan in a human after oral valsartan is 4,000–5,000 nM). All wounds treated with 1% valsartan gel were closed on day 50, compared with none of the placebo-treated wounds. Using an automated digital analysis of daily wound images (Supplementary Figure S3a online) to monitor changes in different wound compartments, lower rates of accumulation of slough at the wound base (P < 0.0001; Supplementary Figure S3b, top) and higher rates of epithelialization (P < 0.0001; Supplementary Figure S3b, bottom) were demonstrated in valsartan-treated wounds.
Figure 3. Wound closure measurements in diabetic pigs treated with daily 1% valsartan gel applied starting on seventh day after wounding.
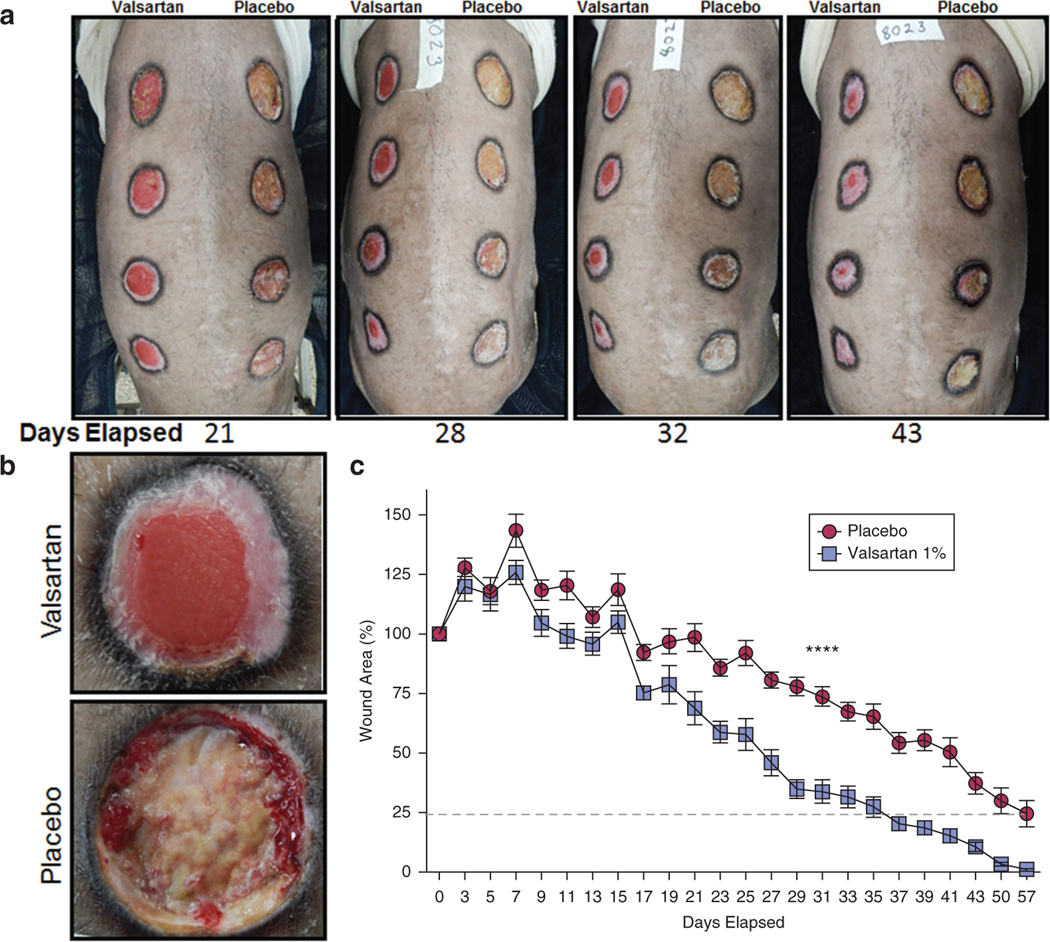
(a) Representative images of pig wounds as days elapsed showing marked difference between valsartan- and placebo-treated wounds. (b) A close-up image to provide more details of healing differences on day 22 in valsartan-(top) and placebo-treated (bottom) wounds. (c) Planimetric assessment of the changes in wound size in aged diabetic pigs treated with 1% valsartan gel. Total n = 12 wounds per treatment group. The data are presented as the mean ± standard error of the mean. ****P < 0.0001.
Selective activation of the SMAD signaling pathway.
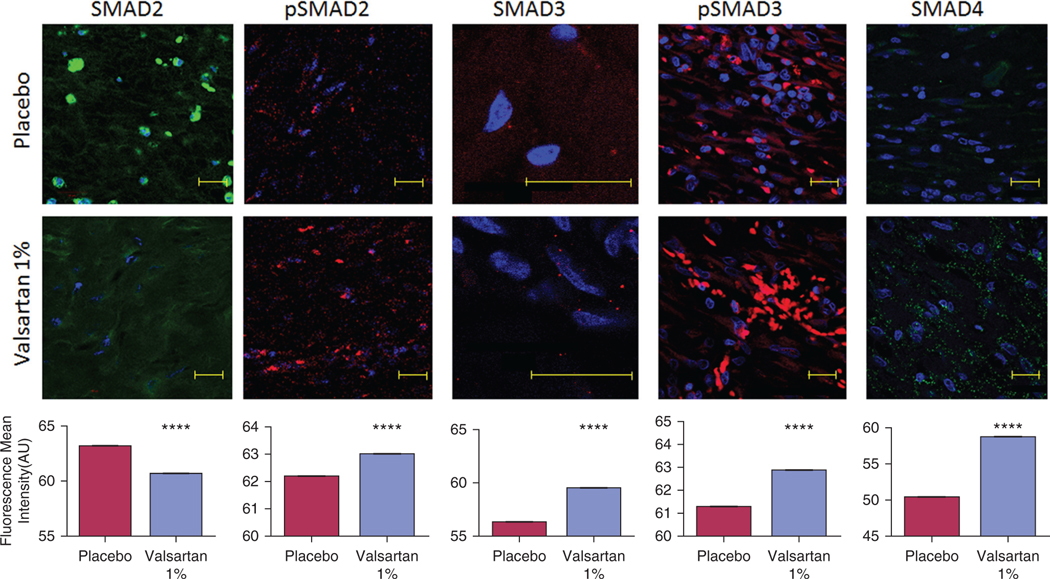
Although not completely characterized, wound healing is greatly influenced by subtle changes in the TGFβ superfamily, which is strongly influenced by the RAS (Crowe et al., 2000; Finnson et al., 2013; Roberts et al., 1988; Steckelings et al., 2004; Yevdokimova and Podpryatov, 2007). The TGFβ superfamily includes TGFβ, bone morphogenetic proteins, and activins. TGFβ signaling mediates the phosphorylation of mothers against decapentaplegic (SMAD) family proteins (Zandifar et al., 2012). Phosphorylated SMADs then translocate to the nucleus with the common mediator (co-SMAD) SMAD4 (Bergstrom et al., 2010; Moustakas and Heldin, 2009). Additionally, SMAD activity is regulated by phosphorylation through nonreceptor kinases such as p42/44 mitogen-activating protein kinase (MAPK) and p38 MAPK (Matsuura et al., 2005; Valluru et al., 2011). In chronic wounds, failure of TGFβ SMAD2 and 3 phosphorylation and a reduction in p42/44 MAPK activity were associated with a slower proliferative rate and impaired healing (Kim et al., 2003). The RAS has been tightly linked to TGFβ activity, but the specific effects of AT1R on the different SMADs during wound healing are not known. To determine the molecular mechanisms by which valsartan may have influenced the wound healing process, we quantified the changes in SMADs (1, 2, 3, 4, 5, and 9) using immunohistochemistry. We included members of the bone morphogenetic protein family (SMAD 1, 5, and 9) to determine the specificity of valsartan impact on the TGFβ superfamily and to serve as an internal validation. Our results show that topical valsartan inhibited SMADs 1 and 2 but activated SMAD3 (Figure 4 and Supplementary Figure S4 online) in the wounds of diabetic aged pigs. To examine the impact of the changes in SMADs 2 and 3, we quantified the changes in phosphorylated SMADs and co-SMAD4 and demonstrated an increase in phosphorylated SMAD2 and phosphorylated SMAD3 and an increase in co-SMAD4 (Figure 4). Treatment with valsartan also decreased the phosphorylation of SMAD1, 5, and 9 (Supplementary Figure S4).
Figure 4. Changes in the transforming growth factor-beta superfamily downstream signaling proteins in wounds of aged diabetic pigs.
Valsartan-treated wounds express higher levels of SMAD3, phosphorylated SMAD2 and phosphorylated SMAD3, and the common mediator SMAD4 in healed skin compared with placebo. A decrease in the expression of SMAD2 was also observed in the valsartan-treated wounds. The photomicrographs present red or green fluorescent staining with a blue DAPI nuclear counter stain at ×63 magnification. Quantification of the levels of SMADs in porcine wounds is shown. Total n = 12 wounds per treatment group. Scale bar, 20 μm. The data are presented as the mean fluorescence intensity ± standard error of the mean. ****P < 0.0001. SMAD, mothers against decapentaplegic.
Enriched mitochondrial, proliferation, and angiogenesis markers in aged diabetic pig wounds treated with valsartan.
Because topical valsartan treatment in aged diabetic pig wounds increased phosphorylation of SMAD2 and SMAD3 and increased the rate of granulation tissue formation and re-epithelialization, we examined the effect of valsartan on factors linked to SMAD3 and involved in wound healing. Previous research suggested that in chronic wounds, the decrease in SMAD3 was associated with a parallel decrease in MAPK and that exogenous SMAD3 administration enhanced alpha-smooth muscle actin (α-SMA) and vascular endothelial growth factor (Sumiyoshi etal.,2004). In diabetic aged pig wounds, valsartan enhanced the phosphorylation of p42/44 MAPK (Figure 5). We also observed an increase in α-SMA and phosphorylated vascular endothelial growth factor receptor 2 (Figure 5) as well as CD31(Supplementary Figure S5 online). Finally, we previously reported the presence of a functional RAS within the mitochondria that played a role in bioenergetics regulation (Abadiretal., 2011). Other groups reported that the knockout of the AT1R receptor leads to significant increases in mitochondria (Benigni et al., 2009). The evaluation of mitochondrial content in healing wounds treated with valsartan revealed a significant increase in the number of mitochondria (Figure 5).
Figure 5. Enhanced mitochondrial, proliferation, and angiogenesis markers in aged diabetic pig wounds treated with valsartan.
Higher mitochondrial Cox IV expression was found in wounds treated with daily valsartan gel. Treated wounds also exhibited higher levels of alpha-smooth muscle actin (α-SMA) and increased phosphorylation of p42/44 mitogen-activating protein kinase (MAPK) and vascular endothelial growth factor (VEGF) receptor 2. The photomicrographs present red or green fluorescent staining with a blue DAPI nuclear counter stain at ×63 magnification. Quantification of the levels in porcine wounds is shown. Total n = 12 wounds per treatment group. Scale bar, 10 μm (COX IV) or 20 μm (all others). The data are presented as the mean fluorescence intensity ± standard error of the mean. ****P < 0.0001.
Valsartan increases skin biomechanical tensile strength.
Because faster wound closure and healthy closure may not be synonymous (Faghih et al., 2015), the impact of valsartan 1% on the quality of wound repair was also assessed. Using Masson’s trichrome (Figure 6a–h) and hematoxylin and eosin stains (Figure 6i), we examined the collagen content and other histological changes in healing skin. Tensiometry was employed to quantify the differences in tensile strength between placebo- and valsartan-treated wounds. Consistent with our mouse data (Figure 2b), the accelerated wound healing rate observed with valsartan treatment in aged diabetic pigs was associated with a significantly thicker epidermal layer (192 ± 11 μm vs. 110 ± 22 μm; valsartan vs. placebo, P < 0.001; Figure 6c, d, and g) and dermal collagen layer (6 ± 0.2 mm vs. 3.9 ± 0.1 mm; valsartan vs. placebo, P < 0.001; Figure 6a, b, and h). Ultrastructural analysis revealed a more organized collagen fiber arrangement in valsartan-treated wounds (Figure 6e) than the coarser and irregular fiber outlines, consistent with scar tissue in placebo-treated wounds (Figure 6f). Biomechanically, valsartan treatment yielded significantly stronger healing skin with a higher tensile strength (Supplementary Video S1 and Figure 6j and k), suggesting more resilience against wound dehiscence and recurrence, a highly relevant concern in diabetic patients.
Figure 6. Impact of topical valsartan 1% on the quality of wound repair.
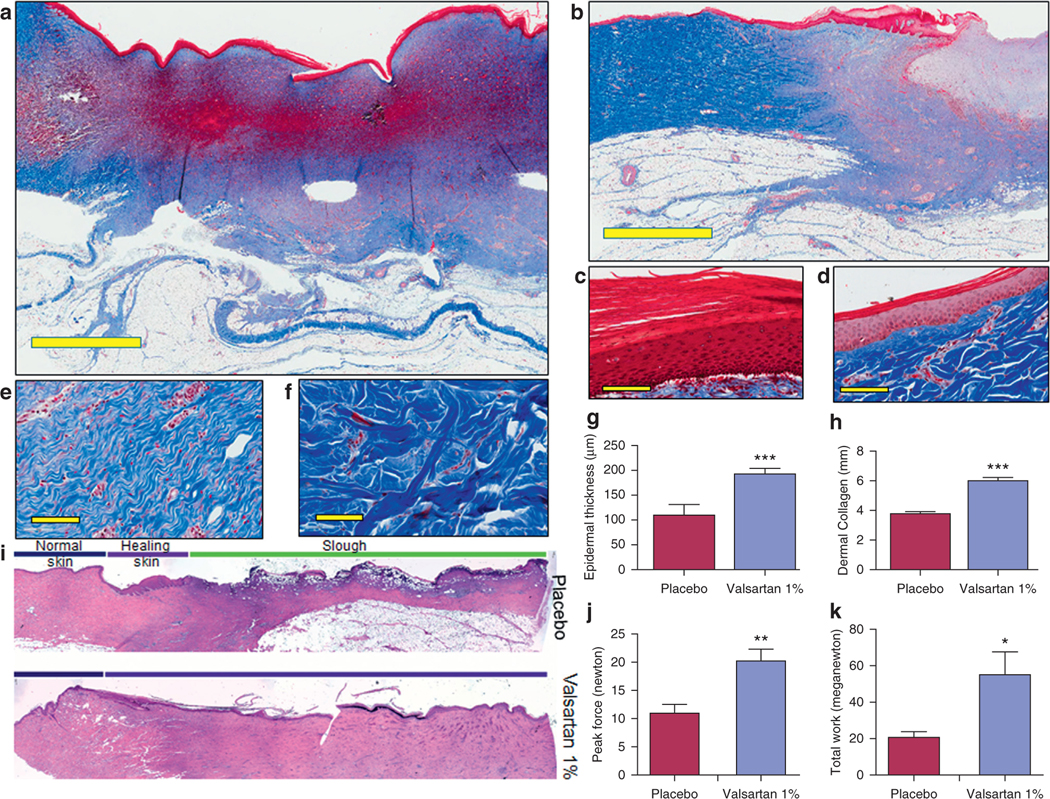
Masson’s trichrome staining of skin sections from aged diabetic pigs of (a) valsartan- and (b) placebo-treated wounds (scale bar, 4 mm). Representative image of epidermis in (c) valsartan- and (d) placebo-treated skin (scale bar, 50 μm). Representative image showing collagen arrangement in (e) valsartan- and (f) placebo-treated skin (scale bar, 50 μm). (g, h) Quantification of thickness of epidermis and dermal collagen layers in porcine wounds. (i) Hematoxylin and eosin image. Biomechanical assessment of healed skin in pig cohorts showing comparison of the (j) average tension at the break point and (k) average work at the break point in healed wounds. Total n = 12 wounds per treatment group. *P < 0.05, **P < 0.01, ***P < 0.001.
DISCUSSION
Together, our results demonstrate that 1% valsartan enhances chronic wound healing in diabetic mice and aging diabetic pigs. The accelerated healing rate was associated with increased wound blood flow, collagen deposition, and re-epithelialization and led to an increased tensile strength of healing skin. The improved skin parameters were associated with selective activation of SMAD2, SMAD3, and co-SMAD4 along with increased MAPK, α-SMA, CD31, and vascular endothelial growth factor receptor 2 expression and a higher mitochondrial content in tissues obtained from the wound bed. A schematic representation of the observed biologic changes related to valsartan treatment compared with placebo is shown in Figure 7. Our results are consistent with prior reports on the effects of the RAS on skeletal muscle repair and demonstrate the efficacy of topical ARBs in chronic wound healing. Furthermore, our results suggest that the beneficial effects of RAS blockade seen with ARBs do not extend to ACE inhibitor.
Figure 7. Schematic representation of the biologic changes observed in chronic wounds treated with valsartan compared with those treated with placebo.
α-SMA, alpha-smooth muscle actin; ARB, angiotensin II type 1 receptors blocker; iNOS, inducible nitric oxide synthase; MAPK, mitogen-activating protein kinase; SMAD, mothers against decapentaplegic; VEGF, vascular endothelial growth factor.
AT1R amplifies inflammatory signaling, a necessary activating function that leads to the proliferation phase but a function with potential negative consequences on wound healing in aging and diabetes as the inflammatory phase does not sufficiently resolve to allow proliferation and remodeling in granulation tissue. The blockade of the AT1R during the early stages of wound healing was associated with a slower closure rate, perhaps resulting from the disruption of the inflammatory phase and impairment of the transition to the proliferative and remodeling phases (Falanga, 2005; Pradhan et al., 2009; Scimeca et al., 2010; Stadelmann et al., 1998a; Van de Kerkhof et al., 1994). In previous reports, wounds in AT1R−/− mice had a delayed healing pattern when compared with controls (Yahata et al., 2006). This result is also supported by our previous results showing a significantly slower healing rate as well as a reduction in both proliferating cell nuclear antigen and phospho-histone H3 in the healing skin of AT1R−/− mice (Faghih et al., 2015). The AT1R−/− wound healing results correspond to the application of topical ARBs throughout all phases of wound healing.
In contrast, starting the selective blockade of AT1R with ARBs, and most specifically with 1% valsartan, in diabetic and aged mice as the healing wounds were transitioning to the proliferative phase caused a significant increase in wound blood flow and collagen deposition along with an accelerated rate of healing. In a recent study by Kamber et al. (2015), oral treatment with losartan in diabetic mice immediately after wounding was associated with accelerated wound healing. Differences in drug metabolism, tissue distribution, and systemic effects (effects on blood pressure, heart rate, and immune system) may explain this apparent discrepancy.
The pattern of healing (increased buildup of slough and plateau of the healing rate) seen in the wounds of placebo-treated aged diabetic pigs resembles the impaired healing seen in older humans with chronic wounds (Stadelmann et al., 1998b). A key characteristic of chronic wounds is the failure to progress through wound phases and to get “stuck” in the inflammatory phase (Stadelmann et al., 1998b). Cells from patients with chronic wounds also reveal a failure of phosphorylation of the SMAD pathway (Kim et al., 2003). SMAD proteins are required for signaling in the TGFβ superfamily. Our results demonstrate selective phosphorylation of SMAD2 and SMAD3 and inhibition of SMAD1, 2, 5, and 9 with valsartan treatment. The implication of higher SMAD2 phosphorylation despite lower SMAD2 levels is unclear. The impact of RAS blockers on the continuously dynamic equilibrium and the nucleocytoplasmic shuttling and degradation of SMADs (Lin et al., 2006; Schmierer and Hill, 2005) remains an uncharted territory. The association between the activation of valsartan-induced SMAD2 phosphorylation, SMAD3 phosphorylation, upregulated co-SMAD4, and accelerated rate of healing aligns with prior reports demonstrating augmented wound healing (increased granulation tissue area, number of capillaries, and re-epithelialization rate) with the administration of exogenous SMAD3 (Sumiyoshi et al., 2004) and TGFβ (Roberts, 1995; Sporn et al., 1983) in the wounds. Furthermore, it has also been shown that SMAD3 phosphorylation is associated with increased collagen gene transcription and promotion of collagen production (Inagaki et al., 2001; Rozen-Zvi et al., 2013; Wang et al., 2007), which is consistent with the results from our mouse and pig models, which demonstrated increased collagen deposition with topical valsartan treatment. This increased collagen deposition and improved collagen arrangement provides an important scaffold for healing cells and explains the increased tensile strength of treated skin. The effects of valsartan on wound collagen deposition and arrangement may open a new avenue for the use of topical ARBs in skin wrinkling and maxilla-facial reconstructive surgery.
MATERIALS AND METHODS
The following sections contain a brief overview of the methods used for results presented in this article. For further details of these methodologies, see Supplementary Materials and Methods online.
Mouse experiments
These experiments were approved by the Johns Hopkins Animal Care and Use Committee. To ascertain the influence of angiotensin receptors on wound healing, downstream effectors, and healed skin quality, we compared control and treated 6- to 8-week-old female BKS.Cg-Dock7m+/+ Leprdb/J (Jackson Laboratories, Bar Harbor, ME), AT1R−/− (Jackson Laboratories), and AT2R−/− knockout (AT2R ) mice (supplied by Dr Tedashi Inagami, Vanderbilt University, Nashville, TN) (Ichiki et al., 1995; Tanaka et al., 1999). Blood glucose was measured in all BKS.Cg-Dock7-m+/+ Leprdb/J, and animals studied were confirmed to be diabetic at the time of experiments.
For the aging experiments, we compared control and treated 6- to 8-week-old male C57BL/6J wild-type mice (National Institute of Aging Aged Rodent Colonies, Germantown, MD) with 100-week-old male C57BL/6J mice. A full thickness 8-mm wound was created by punch biopsy as previously described (Faghih et al., 2015).
Pig experiments
All pig experiments were performed in Sinclair Research Farm (Columbia, MO) under protocols approved by the Animal Care and Use Committee at the research farm. Diabetic (36 months) Yucatan miniature swine were studied. Diabetes was alloxan induced at 7 months of age. Three diabetic Yucatan miniature swine underwent surgical creation of eight circular 5-cm-diameter (approximately 20 cm2) full thickness excisional skin wounds on the paraspinous areas (four per side) under general anesthesia.
Reformulation of RAS inhibitors
The Johns Hopkins Research Pharmacy compounded placebo, valsartan, losartan, and captopril gels. Placebo wounds always received a placebo gel containing the antiseptic.
Longitudinal tissue composition analysis of porcine wounds
To analyze the tissue composition of the wounds over the time of the study, an automated analytical tool that combines machine learning and computer vision was utilized (Tissue Analytics, Baltimore, MD, https://www.tissue-analytics.com).
Laser Doppler perfusion imaging
Blood flow in the wound areas was measured using a 633-nm, He-Ne scanning laser Doppler imaging device (Moor Instruments, Devon, UK), as previously described (Zhang et al., 2010).
Physical measurements of tissue strength
Peak force, work to rupture, and flexibility of healed skin were calculated at day 21 using an FGV-10XY tensiometer (Checkline by Electromatic, Cedarhurst, NY) as previously described (Douetal.,2014).
Collagen biochemical analysis and quantification
Collagen quantification in mice skin was performed as previously described (Kligman et al., 1989).
Measurement of blood valsartan level
Untreated pig plasma collected at baseline and days 8, 18, and 56 of treatment were separated on an Agilent 1290 Ultra Performance Liquid Chromatography system. The limit of quantitation was 1 nM in porcine plasma.
Histology/immunofluorescence
Healing skin tissues at the end the termination of each study were stained with Masson’s trichrome (Polysciences, Warrington, PA) or using immunofluorescence techniques as previously described (Faghih et al., 2015).
Quantitative real-time reverse transcription PCR
Real-time PCR was performed using Brilliant II SYBR Green QPCR Master Mix (Agilent Technologies, Santa Clara, CA) and Mx3000P QPCR System (Agilent Technologies).
Statistical analysis
The data are presented as the mean ± standard error of the mean. Differences in mean values between groups were analyzed for significance using a two-way analysis of variance, followed by the Holm-Sidak post hoc analysis when appropriate. A probability value of <0.05 was considered statistically significant.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by the Johns Hopkins Older Americans Independence Center National Institute on Aging (grants P30 AG021334, R21AG043284, R01AG046441, and K23 AG035005), Nathan Shock in Aging Scholarship Award and the Wound Healing Society Foundation 3M Scholarship (PA), Maryland Technology Development Grant phase 1&2 (JW and PA), and NIH Grant HL58205 (TI).
Abbreviations:
- α-SMA
alpha-smooth muscle actin
- ACE
angiotensin converting enzyme
- ARB
angiotensin II type 1 receptors blocker
- AT1R
angiotensin II type 1 receptors
- AT2R−/−
angiotensin II type 2 receptor knockout
- MAPK
mitogen-activating protein kinase
- RAS
renin-angiotensin system
- SMAD
mothers against decapentaplegic
- TGFβ
transforming growth factor-beta
Footnotes
CONFLICT OF INTEREST
PA, NF, and JW have filed an international patent application involving wound healing, topical RAS blocker treatment. The rest of the authors state no conflict of interest.
SUPPLEMENTARY MATERIAL
Supplementary material is linked to the online version of the paper at www.jidonline.org, and at https://doi.org/10.1016/j.jid.2017.09.030.
REFERENCES
- Abadir PM, Foster DB, Crow M, Cooke CA, Rucker JJ, Jain A, et al. Identification and characterization of a functional mitochondrial angiotensin system. Proc Natl Acad Sci USA 2011;108:14849–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott CA, Carrington AL, Ashe H, Bath S, Every LC, Griffiths J, et al. The North-West Diabetes Foot Care Study: incidence of, and risk factors for, new diabetic foot ulceration in a community-based patient cohort. Diabet Med 2002;19:377–84. [DOI] [PubMed] [Google Scholar]
- Aulakh GK, Sodhi RK, Singh M. An update on non-peptide angiotensin receptor antagonists and related RAAS modulators. Life Sci 2007;81:615–39. [DOI] [PubMed] [Google Scholar]
- Benigni A, Corna D, Zoja C, Sonzogni A, Latini R, Salio M, et al. Disruption of the Ang II type 1 receptor promotes longevity in mice. J Clin Invest 2009;119:524–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergstrom R, Savary K, Moren A, Guibert S, Heldin CH, Ohlsson R, et al. Transforming growth factor beta promotes complexes between Smad proteins and the CCCTC-binding factor on the H19 imprinting control region chromatin. J Biol Chem 2010;285:19727–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulton AJ, Vileikyte L, Ragnarson-Tennvall G, Apelqvist J. The global burden of diabetic foot disease. Lancet 2005;366:1719–24. [DOI] [PubMed] [Google Scholar]
- Burks TN, Andres-Mateos E, Marx R, Mejias R, Van EC, Simmers JL, et al. Losartan restores skeletal muscle remodeling and protects against disuse atrophy in sarcopenia. Sci Transl Med 2011;3:82ra37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burks TN, Marx R, Powell L, Rucker J, Bedja D, Heacock E, et al. Combined effects of aging and inflammation on renin-angiotensin system mediate mitochondrial dysfunction and phenotypic changes in cardiomyopathies. Oncotarget 2015;6:11979–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper ME. The role of the renin-angiotensin-aldosterone system in diabetes and its vascular complications. Am J Hypertens 2004;17:16S–20S. [DOI] [PubMed] [Google Scholar]
- Crowe MJ, Doetschman T, Greenhalgh DG. Delayed wound healing in immunodeficient TGF-beta 1 knockout mice. J Invest Dermatol 2000;115:3–11. [DOI] [PubMed] [Google Scholar]
- Dou C, Lay F, Ansari AM, Rees DJ, Ahmed AK, Kovbasnjuk O, et al. Strengthening the skin with topical delivery of keratinocyte growth factor-1 using a novel DNA plasmid. Mol Ther 2014;22:752–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faghih M, Hosseini SM, Smith BA, Ansari AM, Lay F, Ahmed AK, et al. Knockout of Angiotensin AT2 receptors accelerates healing but impairs quality. Aging 2015;7:185–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falanga V. Wound healing and its impairment in the diabetic foot. Lancet 2005;366:1736–43. [DOI] [PubMed] [Google Scholar]
- FDA. Guidance for industry: chronic cutaneous ulcer and burn wounds-developing products for treatment; 2006. https://www.fda.gov/downloads/drugs/guidances/ucm071324.pdf (accessed 1 November 2017). [DOI] [PubMed]
- Finnson KW, McLean S, Di Guglielmo GM, Philip A. Dynamics of transforming growth factor beta signaling in wound healing and scarring. Adv Wound Care (New Rochelle) 2013;2:195e214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gring CN, Francis GS. A hard look at angiotensin receptor blockers in heart failure. J Am Coll Cardiol 2004;44:1841e6. [DOI] [PubMed] [Google Scholar]
- Hao SY, Ren M, Yang C, Lin DZ, Chen LH, Zhu P, et al. Activation of skin renin-angiotensin system in diabetic rats. Endocrine 2011;39:242–50. [DOI] [PubMed] [Google Scholar]
- Ichiki T, Labosky PA, Shiota C, Okuyama S, Imagawa Y, Fogo A, et al. Effects on blood pressure and exploratory behaviour of mice lacking angiotensin II type-2 receptor. Nature 1995;377:748–50. [DOI] [PubMed] [Google Scholar]
- Inagaki Y, Mamura M, Kanamaru Y, Greenwel P, Nemoto T, Takehara K, et al. Constitutive phosphorylation and nuclear localization of Smad3 are correlated with increased collagen gene transcription in activated hepatic stellate cells. J Cell Physiol 2001;187:117–23. [DOI] [PubMed] [Google Scholar]
- Jackson EK. Renin and angiotensin. In: Brunton LL, Lazo JS, Parker KL, editors. Goodman & Gilman’s the pharmacological basis of therapeutics. 11th edn. New York: McGraw Hill; 2006. p. 789–821. [Google Scholar]
- Kamber M, Papalazarou V, Rouni G, Papageorgopoulou E, Papalois A, Kostourou V. Angiotensin II inhibitor facilitates epidermal wound regeneration in diabetic mice. Front Physiol 2015;6:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BC, Kim HT, Park SH, Cha JS, Yufit T, Kim SJ, et al. Fibroblasts from chronic wounds show altered TGF-beta-signaling and decreased TGF-beta type II receptor expression. J Cell Physiol 2003;195:331e6. [DOI] [PubMed] [Google Scholar]
- Kligman LH, Gebre M, Alper R, Kefalides NA. Collagen metabolism in ultraviolet irradiated hairless mouse skin and its correlation to histochemical observations. J Invest Dermatol 1989;93:210e4. [DOI] [PubMed] [Google Scholar]
- Li EC, Heran BS, Wright JM. Angiotensin converting enzyme (ACE) inhibitors versus angiotensin receptor blockers for primary hypertension. Cochrane Database Syst Rev 2014;8:CD009096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X, Duan X, Liang YY, Su Y, Wrighton KH, Long J, et al. PPM1A functions as a Smad phosphatase to terminate TGFβeta signaling. Cell 2006;125: 915–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura I, Wang G, He D, Liu F. Identification and characterization of ERK MAP kinase phosphorylation sites in Smad3. Biochemistry 2005;44: 12546–53. [DOI] [PubMed] [Google Scholar]
- Moustakas A, Heldin CH. The regulation of TGFβeta signal transduction. Development 2009;136:3699–714. [DOI] [PubMed] [Google Scholar]
- Pradhan L, Nabzdyk C, Andersen ND, LoGerfo FW, Veves A. Inflammation and neuropeptides: the connection in diabetic wound healing. Expert Rev Mol Med 2009;11:e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AB. Transforming growth factor-beta: activity and efficacy in animal models of wound healing. Wound Repair Regen 1995;3: 408–18. [DOI] [PubMed] [Google Scholar]
- Roberts AB, Flanders KC, Kondaiah P, Thompson NL, Van Obberghen-Schilling E, Wakefield L, et al. Transforming growth factor beta: biochemistry and roles in embryogenesis, tissue repair and remodeling, and carcinogenesis. Recent Prog Horm Res 1988;44:157e97. [DOI] [PubMed] [Google Scholar]
- Rodgers K, Verco S, Bolton L, diZerega G. Accelerated healing of diabetic wounds by NorLeu(3)-angiotensin (1–7). Expert Opin Investig Drugs 2011;20:1575–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozen-Zvi B, Hayashida T, Hubchak SC, Hanna C, Platanias LC, Schnaper HW. TGF-beta/Smad3 activates mammalian target of rapamycin complex-1 to promote collagen production by increasing HIF-1alpha expression. Am J Physiol Renal Physiol 2013;305: F485–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmierer B, Hill CS. Kinetic analysis of Smad nucleocytoplasmic shuttling reveals a mechanism for transforming growth factor beta-dependent nuclear accumulation of Smads. Mol Cell Biol 2005;25: 9845–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scimeca CL, Bharara M, Fisher TK, Kimbriel H, Mills JL, Armstrong DG. An update on pharmacological interventions for diabetic foot ulcers. Foot Ankle Spec 2010;3:285–302. [DOI] [PubMed] [Google Scholar]
- Singh T, Newman AB. Inflammatory markers in population studies of aging. Ageing Res Rev 2011;10:319–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporn MB, Roberts AB, Shull JH, Smith JM, Ward JM, Sodek J. Polypeptide transforming growth factors isolated from bovine sources and used for wound healing in vivo. Science 1983;219:1329–31. [DOI] [PubMed] [Google Scholar]
- Stadelmann WK, Digenis AG, Tobin GR. Physiology and healing dynamics of chronic cutaneous wounds. Am J Surg 1998a;176:26S–38S. [DOI] [PubMed] [Google Scholar]
- Stadelmann WK, Digenis AG, Tobin GR. Physiology and healing dynamics of chronic cutaneous wounds. Am J Surg 1998b;176:26S–38S. [DOI] [PubMed] [Google Scholar]
- Steckelings UM, Wollschlager T, Peters J, Henz BM, Hermes B, Artuc M. Human skin: source of and target organ for angiotensin II. Exp Dermatol 2004;13:148–54. [DOI] [PubMed] [Google Scholar]
- Sumiyoshi K, Nakao A, Setoguchi Y, Okumura K, Ogawa H. Exogenous Smad3 accelerates wound healing in a rabbit dermal ulcer model. J Invest Dermatol 2004;123:229–36. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Tsuchida S, Imai T, Fujii N, Miyazaki H, Ichiki T, et al. Vascular response to angiotensin II is exaggerated through an upregulation of AT1 receptor in AT2 knockout mice. Biochem Biophys Res Commun 1999;258: 194–8. [DOI] [PubMed] [Google Scholar]
- Vajapey R, Rini D, Walston J, Abadir P. The impact of age-related dysregulation of the angiotensin system on mitochondrial redox balance. Front Physiol 2014;5:439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valluru M, Staton CA, Reed MW, Brown NJ. Transforming growth factor-beta and endoglin signaling orchestrate wound healing. Front Physiol 2011;2:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Kerkhof PC, Van BB, Spruijt K, Kuiper JP. Age-related changes in wound healing. Clin Exp Dermatol 1994;19:369–74. [DOI] [PubMed] [Google Scholar]
- Wang Z, Gao Z, Shi Y, Sun Y, Lin Z, Jiang H, et al. Inhibition of Smad3 expression decreases collagen synthesis in keloid disease fibroblasts. J Plast Reconstr Aesthet Surg 2007;60:1193–9. [DOI] [PubMed] [Google Scholar]
- Yahata Y, Shirakata Y, Tokumaru S, Yang L, Dai X, Tohyama M, et al. A novel function of angiotensin II in skin wound healing. Induction of fibroblast and keratinocyte migration by angiotensin II via heparin-binding epidermal growth factor (EGF)-like growth factor-mediated EGF receptor transactivation. J Biol Chem 2006;281:13209–16. [DOI] [PubMed] [Google Scholar]
- Yevdokimova N, Podpryatov S. The up-regulation of angiotensin II receptor type 1 and connective tissue growth factor are involved in high-glucose-induced fibronectin production by cultured human dermal fibroblasts. J Dermatol Sci 2007;47:127–39. [DOI] [PubMed] [Google Scholar]
- Zandifar E, Sohrabi BS, Zandifar A, Haghjooy JS. The effect of captopril on impaired wound healing in experimental diabetes. Int J Endocrinol 2012;2012:785247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Wei X, Liu L, Marti GP, Ghanamah MS, Arshad MJ, et al. Association of increasing burn severity in mice with delayed mobilization of circulating angiogenic cells. Arch Surg 2010;145:259–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.