Abstract
Objective
To investigate the association between opioid replacement therapy (ORT) and benzodiazepine (BZD) coprescription and all-cause mortality compared with the prescription of ORT alone.
Design
Population-based cohort study.
Setting
Scotland, UK.
Participants
Participants were people prescribed ORT between January 2010 and end of December 2020 aged 18 years or above.
Main outcome measures
All-cause mortality, drug-related deaths and non-drug related deaths.
Secondary outcome
ORT continuous treatment duration.
Analysis
Cox regression with time-varying covariates.
Results
During follow-up, 5776 of 46 899 participants died: 1398 while on coprescription and 4378 while on ORT only. The mortality per 100 person years was 3.11 during coprescription and 2.34 on ORT only. The adjusted HR for all-cause mortality was 1.17 (1.10 to 1.24). The adjusted HR for drug-related death was 1.14 (95% CI, 1.04 to 1.24) and the hazard for death not classified as drug-related was 1.19 (95% CI, 1.09 to 1.30).
Conclusion
Coprescription of BZDs in ORT was associated with an increased risk of all-cause mortality, although with a small effect size than the international literature. Coprescribing was also associated with longer retention in treatment. Risk from BZD coprescription needs to be balanced against the risk from illicit BZDs and unplanned treatment discontinuation. A randomised controlled trial is urgently needed to provide a clear clinical direction.
Trial registration number
Keywords: Substance misuse, EPIDEMIOLOGY, CLINICAL PHARMACOLOGY
Strengths and limitations of this study.
A strength of this analysis is the population-based analysis that included the whole opioid replacement therapy treatment population in Scotland.
A strength of this analysis is that follow-up took place over 10 years.
A weakness of this study is that the analysis has not considered dose of opioid replacement therapy, or benzodiazepine (BZD), which will be variable within individuals over time.
A weakness of the study is that there is potential residual unmeasured confounding that means that the relationship between BZD coprescription and mortality cannot be assumed to be causal.
Introduction
We have an ongoing challenge in the UK and abroad on how to address the risks associated with illicit drug use. Opioid replacement treatment (ORT) is a well-evidenced treatment which has provided a safe and effective treatment to reduce the risks of illicit opiate use.1 Despite this, in recent years, there have been remarkably high numbers of deaths reported in Scotland, with increasing numbers recorded in England and Wales and Northern Ireland. The opioid crisis of north America is also well documented.2 A strong feature associated with increasing deaths in the UK is that of concurrent use of benzodiazepines (BZDs) alongside opiate drugs.3 This does not occur in isolation and may be compounded by use of alcohol, cocaine and gabapentinoids.3 4
Nowhere is the issue more apparent than in Scotland where the rise of the use of non-prescription BZDs is clear. In 2008, BZDs were implicated in 26% (n=149) of drug-related deaths (DRDs) and were mainly drugs licensed for prescription such as diazepam. By 2018, BZDs and BZD-type drugs were implicated in 67% (792) of DRDs, reducing slightly to 57% in 2022.3 BZDs identified are predominately substances not licensed for prescription in the UK such as etizolam (a thenodiazepine), but there is an ongoing trend of novel BZDs emerging.5
People who use non-prescription BZDs, of unknown constituents and potency, can consume ‘megadoses’ of BZDs many times in excess of safe therapeutic doses, often with alcohol and other drugs, which combine to increase the risk of harm and death.5 6 People presenting to addiction services for initial assessment frequently report illicit BZD use in the month prior to assessment, an average of 2561 (29%) per year in a 5 year period.7 The prevalence of illicit BZD use is known to be higher among people with other substance use disorders, especially problematic opiate and/or alcohol dependence.8 9 A systematic review identified a high prevalence (typically>40%) of illicit BZD use among people on opiate replacement therapy (ORT).10 In Scotland, the Drug Deaths Taskforce, as a pragmatic approach, developed interim guidance for clinicians to support the management of problematic ‘street’ BZD use alongside opiate use.11 While some addiction services are now exploring maintenance prescribing to reduce the risks associated with illicit BZD use among ORT patients, there is considerable and understandable reluctance given the potential risk and lack of evidence of risk and benefit. The available clinical guidance only supports maintenance prescribing in exceptional cases.11 12
Evidence of patient safety and other outcomes is developing internationally with recent studies added to the evidence base. A recent systematic review of these studies found that of six identified studies that looked at all-cause mortality (ACM), four recorded coprescription to be associated with an increased risk.13–16 However, of the seven studies that looked at retention in treatment, there were favourable findings in three studies with those coprescribed a BZD with ORT remaining in treatment longer.14 16 17 There was no difference in two studies and variable findings depending on time for one study.18–20 One study that analysed the impact of prescribed versus street BZD use among ORT patients receiving methadone found prescribed BZD improved treatment retention, whereas non-prescribed BZD (ie, street drug use) was predictive of treatment drop out.21 Thus, there are opposing risks and benefits associated with BZD prescribing for those receiving ORT. Much of the existing evidence is based on large epidemiological studies of administrative prescribing and outcome data sets. However, the follow-up time for many of these studies is limited and it is important to understand the longer-term implications of coprescription. Given the particular problems highlighted in Scotland, this study sought to further understand patterns of, and outcomes from BZD prescribing among ORT patients over a 10 year period to inform safe and effective clinical practice.
Methods
This was an observational, retrospective cohort study using routinely collected administrative data in Scotland. Participants were followed from their first ORT prescription after 1 January 2010 until the time they were known to have died, or until 31 December 2020.
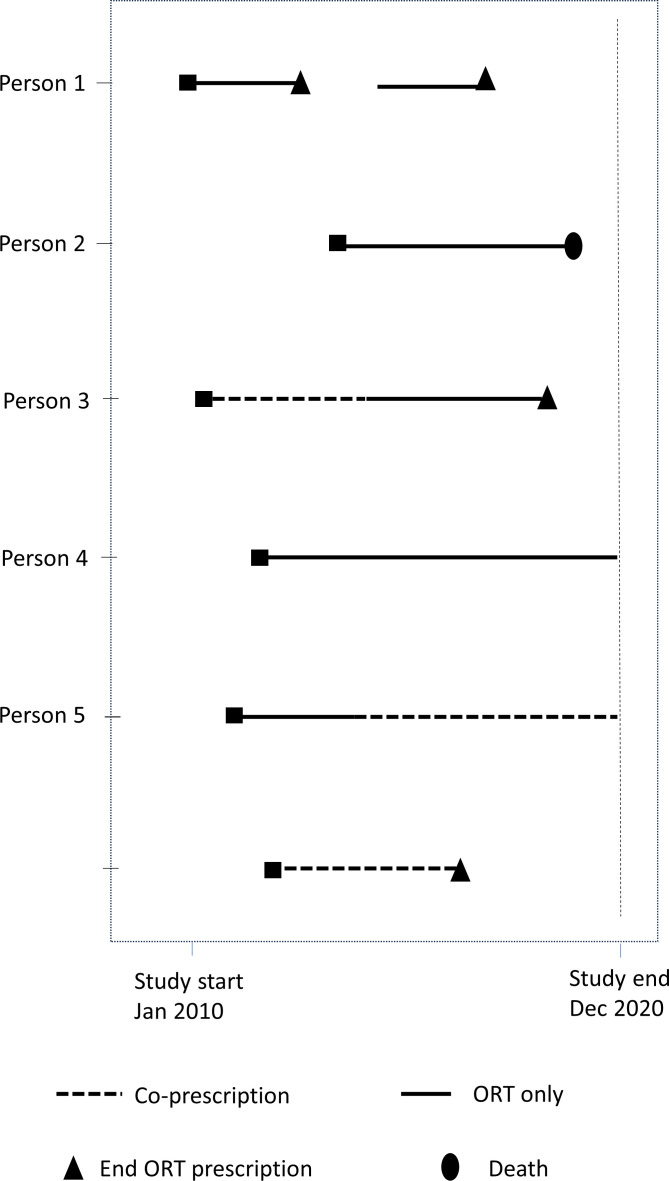
Figure 1 time to event analysis: lines denote time in study for each participant.
Figure 1.
Time to event analysis. ORT, opioid replacement therapy.
Cohort identification
The study population were people dispensed ORT where prescribing was coded using British National Formulary codes for ‘drugs used in substance dependence’.22 The inclusion criteria were all individuals prescribed ORT between 1 January 2010 and 31 December 2020 and who were aged 18 years or above.
This study used data from Public Health Scotland which included the Prescribing Information System (PIS) and the National Records of Scotland (NRS) Vital Events.23 The PIS contains information on all medicines and their costs that are prescribed and dispensed in the community in Scotland. The information is supplied by Practitioner and Counter Fraud Services Division who are responsible for the processing and pricing of all prescriptions dispensed in Scotland. General practitioners write the majority of these prescriptions, with the remainder written by other authorised prescribers such as nurses, psychiatrists, pharmacists and dentists. Also included in the data set are prescriptions written in hospitals that are dispensed in the community. Prescriptions dispensed within hospitals are not included. Linkage of data from diverse sources was conducted by electronic Data Research and Innovation Service (eDRIS) which is part of Public Health Scotland. Data sets were joined by deterministic linkage based on each patients’ unique Community Health Index number.24 Data were held in the national Safe Haven and all analyses were undertaken in the Safe Haven by approved researchers.25
‘On treatment’ definition
To determine the ‘on treatment’ definition, we examined the time interval between repeat prescription of ORT in the data set. Prescription intervals of 90 days were found to be the most common. Individuals were defined as being ‘on ORT treatment’ if the time was less than 101 days from the dispensed date of their previous ORT prescription as this allows some leeway for holidays and illness around the most common prescription interval of 90 days. Thus, the time period used in the ‘on treatment’ definition was defined empirically from the distribution of observed dispense date intervals for ORT prescription. Individuals were included in the analyses while they were ‘on treatment’.
ORT and BZDs included
ORT drugs included methadone and buprenorphine. BZDs included alprazolam, chlordiazepoxide, clobazam, clonazepam, diazepam, flurazepam, loprazolam, lorazepam, lormetazepam, nitrazepam, oxazepam, and temazepam.
Time varying exposure definition
The exposure was defined as an individual being within 40 days of the dispensing of their most recent prescription of a BZD. The time of 40 days was defined empirically as the time window that captured the majority of interprescription time periods for repeat BZD prescriptions in this cohort.
Continuous treatment episode definition
A treatment episode is defined here as a continuous time period where an individual was receiving ORT prescriptions at intervals of 100 days or less.
Demographic variables
Socioeconomic deprivation was assessed by the Scottish Index of Multiple Deprivation (SIMD) based on post code of residence. Scotland is divided into around 7000 small areas which are ranked in terms of deprivation across the domains of: income, employment, education, health access to services, crime and housing to create the SIMD.26 Area of residence was also categorised using the Scottish Government’s Urban Rural Classification which is based on population and accessibility.27 Age in years and sex were also available for the cohort.
Analysis
Descriptive statistics were used to characterise the demographics (age, gender, urban/rural classification, area-level socioeconomic deprivation) of exposed and unexposed groups.
The primary analysis was a time-to-event analysis by Cox Regression. Figure 1 is an illustration of data for the time to event analysis. The Cox proportional hazards model allowed us to compare the instantaneous hazard for mortality during time periods where there was coprescription of ORT and a BZD compared with the hazard where ORT was prescribed alone. The exposure was included as a time varying covariate. All models presented are adjusted for age at first ORT prescription dispensed, age at first dispense squared and age at first dispense cubed, sex, Scottish Index of Multiple Deprivation and Scottish urban rural classification, ever prescription of z-drugs and ever prescription of opioid analgesics.
Three outcomes were examined in separate regression models: ACM, DRDs and non-DRDs. Effect sizes are presented as HRs and their 95% CIs.
In secondary analyses, we examined a different definition of the exposure: any prescription of BZD during the study period. That is, we examined whether any prescription of a BZD during the study period was associated with increased mortality. This was done by including BZD prescription as a time-invariant covariate. Then, we tested whether the observed effects differed by the type of ORT prescription. That is, we examined the effects of methadone and buprenorphine separately.
In further analyses, we examined the average continuous treatment episode duration for episodes where ORT was prescribed alone compared with episodes of coprescription with a BZD. Differences in duration were tested by regression analysis adjusted for age and sex.
As the definition of ‘on treatment’ for ORT was determined from prescription intervals observed in this data set, we performed sensitivity analyses varying the time window for defining being on ORT treatment and for the exposure that is, BZD coprescription. If the effect of coprescription on mortality outcomes was only observed under one particular definition of ‘on treatment’ or exposure then this would indicate that the association may be a chance observation. However, if the effect is robust under a number of definitions, then this is support for the association.
All analyses were conducted in Stata V.17.28
Patient and public involvement
The research questions were informed by consulting people with personal experience of substance use and/or addiction care and/or non-fatal overdose and/or affected by another person’s DRD. Members of two voluntary sector recovery communities were consulted in 2019: Aberdeen in Recovery and Forth Valley Recovery Community. Nineteen people were consulted and received a £10 supermarket voucher stipend for their time and contributions. All those consulted supported the study concept and research questions. All supported analysis of pseudonymised patient data on the condition that individuals could not be identified by academic researchers or in project outputs. All appreciated the plan to develop a public-facing, accessible, plain language summary of results for dissemination to people who use drugs.
The research team and the project Advisory Group both include at least two people with lived experience of problematic substance use and addiction service use.
Results
Description of sample
The total number of prescriptions dispensed for the cohort was approximately 17 million of which 5 494 857 prescriptions were for ORT or BZD. The cohort was made up of 48 588 individuals and was approximately two-thirds male. The cohort was disproportionately from areas characterised by high levels of deprivation relative to the general population reflecting the fact those in low SIMD deciles (high deprivation) are more likely to be receiving ORT and/or BZD as shown in table 1. There was also higher ORT prescribing in urban areas. Of the full cohort, 55.9% received a BZD prescription at some time between January 2010 and December 2020. Sociodemographics are presented in table 1 according to whether the participant had BZD exposure at any time during the study period (irrespective of length or number of prescriptions). Slightly more women had ever received a BZD compared with men (62.8% of females, 52.6% of males on BZD) which was statistically significant (χ2=447, df=1, p<0.001). There was no association between exposure and either social deprivation or urbanicity.
Table 1.
Sociodemographic characteristics of cohort
| Any BZD (exposed) | No BZD (unexposed) | Full sample | P value | |
| N | 27 184 | 21 404 | 48 588 | |
| Sex | ||||
| Male | 17 155 (63.11%) | 15 451 (72.19%) | 32 606 (67.11%) | <0.001 |
| Female | 10 029 (36.89%) | 5953 (27.81%) | 15 982 (32.89%) | |
| SIMD decile | ||||
| 1 (highest deprivation) | 8811 (32.41%) | 7055 (32.96%) | 15 866 (32.65%) | 0.704 |
| 2 | 5477 (20.15%) | 4367 (20.40%) | 9844 (20.26%) | |
| 3 | 3925 (14.44%) | 2933 (13.70%) | 6858 (14.11%) | |
| 4 | 2757 (10.14%) | 2091 (9.77%) | 4848 (9.98%) | |
| 5 | 1880 (6.92%) | 1417 (6.62%) | 3297 (6.79%) | |
| 6 | 1388 (5.11%) | 1026 (4.79%) | 2414 (4.97%) | |
| 7 | 1005 (3.70%) | 723 (3.38%) | 1728 (3.56%) | |
| 8 | 723 (2.66%) | 598 (2.79%) | 1321 (2.72%) | |
| 9 | 525 (1.93%) | 400 (1.87%) | 925 (1.90%) | |
| 10 (lowest deprivation) | 367 (1.35%) | 357 (1.67%) | 724 (1.49%) | |
| Missing | 326 (1.20%) | 437 (2.04%) | 763 (1.57%) | |
| Urban–rural classification 2016 | ||||
| Large urban areas | 13 359 (49.14%) | 10 249 (47.88%) | 23 608 (48.59%) | 0.689 |
| Other urban areas | 9516 (35.01%) | 7746 (36.19%) | 17 262 (35.53%) | |
| Accessible small towns | 1508 (5.55%) | 1183 (5.53 %) | 2691 (5.54%) | |
| Remote small towns | 824 (3.03%) | 587 (2.74 %) | 1411 (2.90%) | |
| Accessible rural areas | 1164 (4.28%) | 869 (4.06%) | 2033 (4.18%) | |
| Remote rural areas | 476 (1.75%) | 325 (1.52%) | 801 (1.65%) | |
| Missing | 337 (1.24%) | 445 (2.08%) | 782 (1.61%) |
BZD, benzodiazepine; SIMD, Scottish Index of Multiple Deprivation.
All-cause mortality, drug-related deaths and non-drug-related deaths
During follow-up, 5776 participants died: 1398 while on coprescription of a BZD and ORT and 4378 while on ORT only. The total time spent in the study for all participants was 2 32 282 years. The total time in the study while on BZD prescription and ORT was 45 046 years (mean per participant 2.21 years, median per participant 1.09 years) and the total time on ORT was only 1 87 236 years (mean per participant 4.09, median per participant 3.36 years). The mortality per 100 person years was 3.11 during coprescription and 2.34 on ORT only.
This section outlines the results of three Cox regressions examining the effect of coprescription of BZDs on the outcomes: ACM, DRDs and deaths not classified as drug related.
The total number of participants included in the Cox regression analysis was 46 899. There were 5776 deaths from any cause during the time period. Of these, 2938 were DRDs and 2838 were not classified as DRDs.
Table 2 shows the HR and 95% CI for the effect of BZD coprescription versus ORT only on the three mortality outcomes.
Table 2.
Effect of coprescription of a BZD on outcomes in people receiving opioid replacement therapy
| Outcome | HR* | P value | 95% CI |
| All-cause mortality | 1.17 | <0.001 | 1.10 to 1.24 |
| Drug-related death | 1.14 | 0.003 | 1.04 to 1.24 |
| Not drug-related death | 1.19 | <0.001 | 1.09 to 1.30 |
*Adjusted for age at first ORT prescription dispensed, age at first dispense squared and age at first dispense cubed, sex, Scottish Index of Multiple Deprivation, Scottish urban-rural classification, ever prescription of z-drugs and ever prescription of opioid analgesics.
BZD, benzodiazepine; ORT, opioid replacement therapy.
After adjustment, the effect of exposure (coprescription of BZDs in the last 40 days) increased the hazard for ACM relative to ORT alone, by 17% (HR 1.17; 95% CI, 1.10 to 1.24) it increased the hazard for DRD by 14% (HR 1.14; 95% CI, 1.04 to 1.24) and it increased the hazard for death not classified as drug-related by 19% (HR 1.19; 95% CI, 1.09 to 1.30).
All-cause mortality by ORT drug
ACM was analysed by type of ORT (methadone and buprenorphine). Being ‘on ORT treatment’ was defined as being within 100 days of the last methadone prescription. Then, we repeated the analysis with the definition that on treatment was being within 100 days of the last buprenorphine prescription.
Table 3 shows that methadone with a coprescribed BZD was associated with an increase hazard of ACM compared with methadone alone, whereas buprenorphine plus coprescribed BZDs was not associated with an increased hazard for ACM.
Table 3.
Comparison of results with methadone versus buprenorphine opioid prescription
| ORT definition | HR | P value | 95% CI |
| Methadone | 1.41 | <0.001 | 1.32 to 1.50 |
| Buprenorphine | 1.16 | 0.189 | 0.93 to 1.44 |
ORT, opioid replacement therapy.
Retention in treatment
Table 4 shows the descriptive statistics for treatment episodes broken down by whether the treatment episodes was for ORT only or ORT and a BZD.
Table 4.
Treatment episode length for ORT episodes with and without a BZD coprescription
| All ORT episodes | ORT with any BZD coprescription | ORT with no BZD prescriptions | |
| N episodes | 121 435 | 37 022 | 84 413 |
| Median (days) | 375 | 678 | 312 |
| IQR (days) | 153–1005 | 222–1645 | 131–749 |
| Mean (days) | 766.27 | 1110.29 | 615.38 |
| SD (days) | 920.06 | 1118.86 | 770.75 |
BZD, benzodiazepine; ORT, opioid replacement therapy.
A comparison of episode duration between ORT episodes with no BZD coprescription and episodes of ORT with BZD coprescription by linear regression with adjustment for age and sex found the coefficient for a BZD episode was 540.58 days (95% CI, 528.56 to 552.61 days). This indicates that treatment episodes are around 541 days longer (ie, retention in treatment better), when there is BZD coprescription than episodes with ORT alone after adjustment for age and sex of the person receiving treatment.
Model refinement and sensitivity analysis
We considered the possibility that there may be some dilution of the model due to inclusion of non-ORT opiates for example, for pain or the use chlordiazepoxide for alcohol detoxification. Frequencies of prescriptions for these drugs are as follows:
Chlordiazepoxide: 0.2% of all prescriptions, and 0.841% of all BZD prescriptions, 6.5% of patients were ever prescribed this.
Temgesic: 0.03% of prescriptions, and 0.30% analgesic opioid prescriptions, 0.56% of patients were ever prescribed this.
Buprenorphine patches: 0.10% of all prescriptions, and 0.92% of all analgesic opioid prescriptions, were for buprenorphine patches; 0.6% of patients were ever prescribed buprenorphine patches.
We concluded that Temgesic and buprenorphine patches were present in very small percentage of opioid prescriptions and patients and are therefore unlikely to affect the model. Chlordiazepoxide represented less than 1% of BZD prescriptions, however it was present in 6.5% of patients.
Further sensitivity analyses were conducted to test the effects of varying the time periods used to define continuous treatment episodes for ORT (100 days in main analysis but varied to 365 days here) and continuous treatment episodes for BZD (40 days for main and analysis but varied to 60 and 28 days here). The results shown in table 5 are from cox regression analyses and are adjusted for the same covariates as the main analyses. The analysis found the association between BZD coprescription and increased hazard for mortality was robust to variations in the time frame used to define continuous treatment episodes. When either the time frame for BZD or ORT continuous treatment was extended, then the HR between groups was larger. When both were extended at the same time, this was not the case.
Table 5.
Sensitivity analyses
| All cause mortality | ||||
| ORT duration | BZD duration | HR | P value | 95% CI |
| 100 | 60 | 1.41 | <0.001 | 1.33 to 1.49 |
| 100 | 28 | 1.17 | <0.001 | 1.10 to 1.25 |
| 365 | 60 | 1.17 | <0.001 | 1.10 to 1.24 |
| 365 | 28 | 1.58 | <0.001 | 1.49 to 1.69 |
| Drug-related deaths | ||||
| 100 | 60 | 1.39 | <0.001 | 1.27 to 1.51 |
| 100 | 28 | 1.17 | <0.001 | 1.08 to 1.26 |
| 365 | 60 | 1.13 | <0.001 | 1.04 to 1.22 |
| 365 | 28 | 1.55 | <0.001 | 1.42 to 1.69 |
| Non-drug-related deaths | ||||
| 100 | 60 | 1.42 | <0.001 | 1.30 to 1.54 |
| 100 | 28 | 1.17 | <0.001 | 1.08 to 1.26 |
| 365 | 60 | 1.20 | <0.001 | 1.11 to 1.30 |
| 365 | 28 | 1.60 | <0.001 | 1.46 to 1.74 |
BZD, benzodiazepine; ORT, opioid replacement therapy.
Discussion
Summary of main findings
Findings indicated an increased risk of ACM, DRD and non-DRD in our cohort when comparing those coprescribed a BZD compared with ORT with no prescribed BZD exposure. However, when analysed by ORT drug, methadone with a coprescribed BZD increased hazard of ACM, whereas buprenorphine plus a coprescribed BZD did not. Retention in treatment was increased when coprescribed a BZD alongside ORT compared with ORT alone.
The increased risk of coprescribing opiates and BZDs are well documented in a range of clinical groups covering opiates for analgesia29 and in veterans.30 These studies highlight the significant increased risk of overdose29 and overdose death.30 31 Our study focused on those with a history of using illicit substances who are at increased risk of premature mortality without treatment.1 Given the increasing literature specifically covering the ORT population who also use BZDs, it has been possible to compare findings against the international literature.
The ACM HR for combined ORT (methadone and buprenorphine) concurs with the international literature although the risk in this study appears to be lower (17% increase of ACM, 14% for DRD and 19% for non-drug death) than in other studies of equivalent size and methodological approach (range 70%–90% for ACM).13 15 17 There was a higher level of non-DRD than DRD within ACM which is indicative of other risks being posed by BZD use. This group of drugs, indeed sedatives in general, has long been known to increase risk of accidents and falls so this finding could reflect this general risk associated with this drug group. It is possible that the association we have found is due to residual confounding however, a number of other studies have found larger effect sizes for the association between BZD coprescribing and ACM after adjusting for a greater range of potential confounders. For example Abrahamsson and colleagues13 controlled for sex, age, previous non-fatal overdose, previous psychiatric in-patient treatment, previous suicide attempt and ORT status and found a HR of 1.75 (1.28–2.39).
This study was able to compare ACM by ORT drug. Analysis did not find evidence of increased risk of ACM among patients prescribed buprenorphine. One study in the literature looked specifically at buprenorphine ACM and while there was an increased risk (HR 1.9),17 this is lower than for the studies that combined ORT drugs or looked at methadone alone. Taken together, these findings suggest that buprenorphine poses less risk in combination with a BZD. This may be because buprenorphine causes less respiratory depression than methadone.32 There may, however, be bias in treatment allocation to methadone or buprenorphine as people who are prescribed methadone could have particular characteristics which predispose them to increased risk of harm. Methadone is associated with more sedation than buprenorphine,33 which is welcomed by some compared with the ‘clear headedness’ that buprenorphine provides.34 Buprenorphine is a partial agonist in relation to respiratory depression in humans. A detailed pharmacological review concluded that there is a favourable safety profile with less sedation, respiratory depression and potentially less immunosuppression than other opioids and is not impacted by renal disease.35 In addition, it is possible that the smaller number of participants in buprenorphine-only sample, reduced the statistical power to detect effects of coprescription in this group.
Retention in treatment was significantly longer for those with a BZD coprescription than those on ORT alone. This finding concurs with the literature.36 Evidence strongly implies that treatment is protective of overdose,1 therefore keeping people in contact with treatment services, and avoiding unplanned discharge is generally considered protective. The sensitivity analysis found extending the period of ORT (365 day compared with 100 days) and BZD prescribing (60 days compared with 40 days) reduced the relative effect of BZD prescription on ACM. This could be because increasing the time window for both BZD and ORT prescription means we include more people who have disengaged from treatment within the analysis meaning the baseline risk increases and therefore there is less of an effect of coprescription. This interpretation requires further research to confirm or refute.
Overall, the effect size was lower than other studies in the literature. To explore this, we undertook further sensitivity analysis. First, we considered if there had been a dilution effect for example, due to opiate drugs for non ORT purposes, specifically Temgesic and buprenorphine patches, prescribed for pain. In addition, we considered the potential inclusion of chlordiazepoxide for alcohol detoxification. However, these formed a very small percentage (<1%) of all prescriptions and cases so were not considered to have affected the findings. Therefore, we can conclude that while there is a raised ACM overall, Scotland appears to have a lower HR ACM compared with other countries. This may well be a factor related to the characteristics of the Scottish treatment population. For example, we have high levels of mental and physical comorbidity in the Scottish drug using population.37 Brands et al also noted the different clinical profile in people who use BZD, highlighting that there are more women and more psychiatric conditions. In other words, this is evidence that BZD and opiate users have more comorbid risk.38 This was not specifically tested in our analysis but would be an important plausible explanation given the known high levels of co-occurring mental health problems.39
There is also a high level of other drugs (as well as BZDs) implicated in DRD in Scotland, which has increased over time, specifically gabapentinoids, cocaine and alcohol are all relatively frequently implicated. This is indicative of a higher risk pattern of drug use in this population. It may be that the many in the ORT group were also using street BZDs so were already exposed to increased risk.
Methodological considerations
A strength of this analysis is the large and inclusive population approach that included the whole ORT treatment population over 10 years. Compared with the existing literature, this study is one of the larger studies conducted. The analysis has not considered dose of ORT, or BZD, which will be variable within individuals over time.
There are some important caveats to this analysis that must be taken into consideration in any further reporting or referencing of this work. This is a treatment population and does not compare ACM for those prescribed a BZD and ORT with those not receiving a prescribed BZD and a prescribed ORT, that is, those still using street drugs. The risk of ACM for people who are using non-medical opioids, from a recent meta-analysis is a standardised mortality ratio of 10 (95% CI, 7.6 to 13.2).39 This does not account for BZD prescribing.
Clinical implications
Clinicians need to asses the risks to patients of being exposed to the street market of illicit drugs and the impact of a controlled prescribed alternative, recognising that street BZDs will still be available. Overall improved retention in treatment is an important clinical consideration. ORT reduces the spread of blood borne virus and injecting injuries (as well as criminal activity)11 and engaging people in ORT longer will reduce overall harm. Retaining people also using BZDs alongside ORT in treatment for longer provides opportunities to address comorbidities and other factors that may contribute to street BZD use. However, it is acknowledged that maintenance prescribing of BZDs is ‘off-label’ in the UK. Clinical decision making should consider other substances an individual may also take alongside their mental and physical health.
Conclusion
In the absence of a randomised controlled trial for definitive evidence of risk versus benefit, treatment planning should consider risk on an individual basis. Risk of BZD coprescription needs to be balanced against the risk from illicit BZDs and unplanned treatment discontinuation. A randomised controlled trial is urgently needed.
Supplementary Material
Footnotes
Twitter: @cathbest, @catrionaMath, @trinaritchie
Contributors: CM and JS (leads) JR, TR, FC, JD, CD, KK and CW conceptualised the study, CSB (lead) managed data and conducted analysis, drafted results and methods sections. CM drafted introduction and discussion. All authors revised drafts to refine interpretation and for intellectual content. CM and JS managed the project. CSB is the guarantor.
Funding: This project was funded by the Scottish Government. Funder reference DDTFRF15. The funder had no role in the conduct of the research and the conclusions presented are those the research team and do not necessarily represent those of the funder.
Competing interests: All authors have completed the ICMJE uniform disclosure form at http://www.icmje.org/disclosure-of-interest/ and declare: no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work. TR received honoraria from Camurus for educational inputs in the last 3 years.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data may be obtained from a third party and are not publicly available. Data may be obtained from Public Health Scotland.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Access for data to conduct this study was approved (ref. 2021–0154) by the National Health Service (NHS) Scotland Public Benefit and Privacy Panel for Health and Social Care which is a governance structure of NHS Scotland.
References
- 1.Wakeman SE, Larochelle MR, Ameli O, et al. Comparative effectiveness of different treatment pathways for opioid use disorder. JAMA Netw Open 2020;3:e1920622. 10.1001/jamanetworkopen.2019.20622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The Lancet . Managing the opioid crisis in North America and beyond. Lancet 2022;399. 10.1016/S0140-6736(22)00200-8 [DOI] [PubMed] [Google Scholar]
- 3.National Records of Scotland . Vital events Edinburgh: national records of Scotland. 2023. Available: https://www.nrscotland.gov.uk/statistics-and-data/statistics/statistics-by-theme/vital-events
- 4.Office for National Statistics (ONS) . Statistical Bulletin, deaths related to drug poisoning in England and Wales: 2022 registrations. 2023. Available: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/bulletins/deathsrelatedtodrugpoisoninginenglandandwales/2022registrations
- 5.McAuley A, Matheson C, Robertson JR. From the clinic to the street: the changing role of benzodiazepines in the Scottish overdose epidemic. Int J Drug Policy 2022;100:103512. 10.1016/j.drugpo.2021.103512 [DOI] [PubMed] [Google Scholar]
- 6.Johnson C, Barnsdale L, McAuley A. Investigating the role of benzodiazepines in drug-related mortality: a systematic review undertaken on behalf of the Scottish national forum on drug-related deaths; 2016.
- 7.EMCDDA . The misuse of benzodiazepines among high-risk opioid users in Europe. Portugal: EMCDDA, 2018. [Google Scholar]
- 8.Information Services Division . Scottish drug misuse database: overview of initial assessments for specialist drug treatment 2018/19. Edinburgh: NHS Scotland; 2020. [Google Scholar]
- 9.Chen KW, Berger CC, Forde DP, et al. Benzodiazepine use and misuse among patients in a methadone program. BMC Psychiatry 2011;11:90. 10.1186/1471-244X-11-90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Votaw VR, Geyer R, Rieselbach MM, et al. The epidemiology of benzodiazepine misuse: a systematic review. Drug Alcohol Depend 2019;200:95–114. 10.1016/j.drugalcdep.2019.02.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clinical Guidelines on Drug Misuse and Dependence Independent Expert Working Group . Drug misuse and dependence: UK guidelines on clinical management. London: Department of Health, 2017. [Google Scholar]
- 12.Public Health Scotland . MAT standards informed response for benzodiazepine harm reduction. 2021. Available: https://drugdeathstaskforce.scot/media/1229/mat-standards-informed-response-for-benozdiazepine-harm-reduction_interim-guidance_june21.pdf
- 13.Abrahamsson T, Berge J, Öjehagen A, et al. Benzodiazepine, Z-drug and pregabalin prescriptions and mortality among patients in opioid maintenance treatment-A nation-wide register-based open cohort study. Drug Alcohol Depend 2017;174:58–64. 10.1016/j.drugalcdep.2017.01.013 [DOI] [PubMed] [Google Scholar]
- 14.Macleod J, Steer C, Tilling K, et al. Prescription of benzodiazepines, Z-drugs, and Gabapentinoids and mortality risk in people receiving opioid agonist treatment: observational study based on the UK clinical practice research datalink and office for National Statistics death records [no pagination]. PLoS Med 2019;16:e1002965. 10.1371/journal.pmed.1002965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharma V, Simpson SH, Samanani S, et al. Concurrent use of opioids and benzodiazepines/Z-drugs in Alberta, Canada and the risk of hospitalisation and death: a case cross-over study. BMJ Open 2020;10:e038692. 10.1136/bmjopen-2020-038692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bakker A, Streel E. Benzodiazepine maintenance in opiate substitution treatment: good or bad? A retrospective primary care case-NOTE review. J Psychopharmacol 2017;31:62–6. 10.1177/0269881116675508 [DOI] [PubMed] [Google Scholar]
- 17.Park TW, Larochelle MR, Saitz R, et al. Associations between prescribed benzodiazepines, overdose death and buprenorphine discontinuation among people receiving buprenorphine. Addiction 2020;115:924–32. 10.1111/add.14886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Durand L, Boland F, O’Driscoll D, et al. Factors associated with early and later dropout from methadone maintenance treatment in specialist addiction clinics: a six-year cohort study using proportional hazards frailty models for recurrent treatment episodes. Drug Alcohol Depend 2021;219:108466. 10.1016/j.drugalcdep.2020.108466 [DOI] [PubMed] [Google Scholar]
- 19.Maremmani AGI, Bacciardi S, Rugani F, et al. Outcomes of clonazepam maintained benzodiazepine-heroin addicted patients during methadone maintenance: a descriptive case series. Heroin Addict Relat Clin Probl 2014;16:55–64. [Google Scholar]
- 20.Eibl JK, Wilton AS, Franklyn AM, et al. Evaluating the impact of prescribed versus nonprescribed benzodiazepine use in methadone maintenance therapy: results from a population-based retrospective cohort study. J Addict Med 2019;13:182–7. 10.1097/ADM.0000000000000476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schuman-Olivier Z, Hoeppner BB, Weiss RD, et al. Benzodiazepine use during buprenorphine treatment for opioid dependence: clinical and safety outcomes. Drug Alcohol Depend 2013;132:580–6. 10.1016/j.drugalcdep.2013.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Institute for Health and Care Excellence . Substance dependence: National Institute for health and care excellence. 2023. Available: https://bnf.nice.org.uk/treatment-summaries/substance-dependence
- 23.NHS Scotland . Prescribing information system. Edinburgh: NHS Scotland; 2022. Available: https://www.ndc.scot.nhs.uk/National-Datasets/data.asp?SubID=9 [Google Scholar]
- 24.NHS Scotland . CHI number. Edinburgh: NHS National Services Scotland; 2021. Available: https://www.ndc.scot.nhs.uk/Dictionary-A-Z/Definitions/index.asp?Search=C&ID=128&Title=CHI%20Number [Google Scholar]
- 25.NHS Scotland . Use of the national safe haven. Edinburgh: NHS Scotland; 2023. Available: https://www.isdscotland.org/products-and-services/edris/use-of-the-national-safe-haven [Google Scholar]
- 26.Scottish Government . Scottish index of multiple deprivation 2020. 2020. Available: https://www.gov.scot/collections/scottish-index-of-multiple-deprivation-2020 [Accessed 17 Nov 2023].
- 27.Scottish Government . Overview - Scottish government urban rural classification. 2016. Available: www.gov.scot [Accessed 17 Nov 2023].
- 28.StataCorp . Stata statistical software, 17 ed. College Station, TX, USA: StataCorp LLC; 2021. [Google Scholar]
- 29.Cho J, Spence MM, Niu F, et al. Risk of overdose with exposure to prescription opioids, benzodiazepines, and nonbenzodiazepine sedative-hypnotics in adults: a retrospective cohort study. J Gen Intern Med 2020;35:696–703. 10.1007/s11606-019-05545-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park TW, Saitz R, Ganoczy D, et al. Benzodiazepine prescribing patterns and deaths from drug overdose among US veterans receiving opioid analgesics: case-cohort study. BMJ 2015;350:h2698. 10.1136/bmj.h2698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dasgupta N, Funk MJ, Proescholdbell S, et al. Cohort study of the impact of high-dose opioid analgesics on overdose mortality. Pain Med 2016;17:85–98. 10.1111/pme.12907 [DOI] [PubMed] [Google Scholar]
- 32.Whelan PJ, Remski K. Buprenorphine vs methadone treatment: a review of evidence in both developed and developing worlds. J Neurosci Rural Pract 2012;3:45–50. 10.4103/0976-3147.91934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Macleod J, Whittaker A, Robertson JR. Changes in opiate treatment during attendance at a community drug service--findings from a clinical audit. Drug Alcohol Rev 1998;17:19–25. 10.1080/09595239800187561 [DOI] [PubMed] [Google Scholar]
- 34.Matheson C, Foster R, Schofield J, et al. Long-acting depot buprenorphine in people who are homeless: views and experiences. J Subst Abuse Treat 2022;139:108781. 10.1016/j.jsat.2022.108781 [DOI] [PubMed] [Google Scholar]
- 35.Pergolizzi J, Aloisi AM, Dahan A, et al. Current knowledge of buprenorphine and its unique pharmacological profile. Pain Pract 2010;10:428–50. 10.1111/j.1533-2500.2010.00378.x [DOI] [PubMed] [Google Scholar]
- 36.Matheson C, Best C, Cowden F. BENZORT final report. University of Stirling; [Google Scholar]
- 37.Scottish Drugs Forum . Older people with drug problems in Scotland: addressing the needs of an ageing population. Glasgow: Scottish Drugs Forum; 2017. [Google Scholar]
- 38.Brands B, Blake J, Marsh DC, et al. The impact of benzodiazepine use on methadone maintenance treatment outcomes. J Addict Dis 2008;27:37–48. 10.1080/10550880802122620 [DOI] [PubMed] [Google Scholar]
- 39.Larney S, Tran LT, Leung J, et al. All-cause and cause-specific mortality among people using extramedical opioids: a systematic review and meta-analysis. JAMA Psychiatry 2020;77:493. 10.1001/jamapsychiatry.2019.4170 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data may be obtained from a third party and are not publicly available. Data may be obtained from Public Health Scotland.