Abstract
Objective
To assess the risk of serious infection associated with different targeted therapies for psoriatic arthritis (PsA) in real-world settings.
Methods
This nationwide cohort study used the administrative healthcare database of the French health insurance scheme linked to the hospital discharge database to identify all adults with PsA who were new users of targeted therapies (adalimumab, etanercept, golimumab, certolizumab pegol, infliximab, secukinumab, ixekizumab, ustekinumab, and tofacitinib) from 1 January 2015 to 30 June 2021. The primary outcome was a serious infection (ie, requiring hospitalisation), in a time-to-event analysis using propensity score-weighted Cox models, with adalimumab as the comparator, estimating weighted HRs (wHRs) and their 95% CIs.
Results
A total of 12 071 patients were included (mean age 48.7±12.7 years; 6965 (57.7%) women). We identified 367 serious infections (3.0% of patients), with a crude incidence rate of 17.0 per 1000 person-years (95% CI, 15.2 to 18.7). After inverse propensity score weighting and adjustment for time-dependent covariates and calendar year, risk of serious infection was significantly lower for new users of etanercept (wHR 0.72; 95% CI, 0.53 to 0.97) or ustekinumab (wHR, 0.57; 95% CI, 0.35 to 0.93) than adalimumab new users. This risk was not statistically modified with the other targeted therapies.
Conclusions
The incidence of serious infection was low for PsA patients who were new users of targeted therapies in real-world settings. Relative to adalimumab new users, this risk was lower among new users of etanercept and ustekinumab and unmodified for the other molecules.
Keywords: Arthritis, Psoriatic; Biological Therapy; Epidemiology
WHAT IS ALREADY KNOWN ON THIS TOPIC
Targeted therapies have been suspected to increase the risk of serious infection among patients with inflammatory diseases.
For patients with psoriasis or rheumatoid arthritis, a different infectious risk according to each targeted therapy has been reported. Some risk is known for psoriatic arthritis (PsA).
WHAT THIS STUDY ADDS
In this cohort of PsA patients, the risk of serious infection was significantly lower among new users of etanercept and ustekinumab than adalimumab new users.
Risk of serious infection was not modified in new users of the other targeted therapies as compared with adalimumab new users.
Risk of serious infection was statistically increased with the use of concomitant systemic corticosteroids.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
These findings could help physicians choose a targeted therapy for each PsA patient, considering their risk of infection.
Introduction
Psoriatic arthritis (PsA) is a chronic inflammatory disease with heterogeneous clinical presentation including articular, periarticular and extramusculoskeletal manifestations. In general, it occurs in patients with psoriasis.1 PsA can also be associated with other inflammatory diseases such as inflammatory bowel disease (IBD) and uveitis as well as several comorbidities.2 Current treatment guidelines recommend targeted therapies when standard treatments (including non-steroidal anti-inflammatory drugs (NSAIDs) and conventional synthetic disease-modifying antirheumatic drugs (csDMARDs)) fail to control the disease or are not tolerated. The recent updating of the European Alliance of Associations for Rheumatology (EULAR)3 for PsA treatment aims to help clinicians choose, for a given patient, the best treatment among the different available drugs: tumour necrosis factor inhibitors (TNFi), interleukin 17A and A/F inhibitors (IL-17i), interleukin 12/23 inhibitor (IL-12/23i), interleukin 23 inhibitors (IL-23i), Janus kinase inhibitors (JAKi) and phosphodiesterase 4 inhibitor (PDE4i). The efficacy of these treatments has been clearly proven, but some concerns remain regarding their potential infectious risk.4–7
Randomised clinical trials comparing some targeted treatments in PsA are of limited interest in terms of safety in real life: sample sizes are rather small, the inclusion criteria are highly selective and the comparative follow-up is short.8–12 Several observational studies have assessed the risk of serious infection among new users of targeted therapies as compared with users of standard treatments; however, interpreting their results is difficult because of risk of immortal time bias induced by comparing prevalent users of non-targeted treatments and incident users of targeted treatments.13 Some cohort studies have shown differences in the risk of serious infection in new users of targeted therapies depending on the drug used.14–16 However, these studies mostly involved patients with various rheumatic diseases or isolated plaque psoriasis and thus are difficult to extrapolate to patients with PsA. Moreover, only limited data are available on the most recent treatments (IL-17i, IL-12/23i, IL-23i, and JAKi).17–19 Therefore, with the emergence of increasingly targeted treatments, there is a growing need to know whether some of those drugs have a better profile than others in terms of infectious risk.
The main objective of this study was to assess and compare the risk of serious infection with different targeted therapies used to treat PsA in a large and exhaustive French population.
Methods
Data source and study design
This study was based on data from the French national health insurance database (Système National des Données de Santé (SNDS)).20 The SNDS contains data from the health insurance system linked to the national hospital discharge base (Programme de Médicalisation des Systèmes d’Information (PMSI)). The French health insurance system provides equal access to healthcare services for all residents of France. Thus, the database covers approximately 99% of the French population, corresponding to more than 67 million individuals, each identified by a unique anonymous number. This database provides information on outpatients: sociodemographic data, including year of birth, sex, area of residence, degree of social deprivation in the geographic area, and vital status;21 eligibility for complementary universal health insurance, which provides free access to healthcare for low-income people; data on all pharmacy-dispensed medications, including number of units and date of reimbursed drug dispensation; date and nature of medical and paramedical interventions; and information on patient eligibility for fully reimbursed care related to severe and costly chronic diseases (long-term diseases), such as moderate to severe PsA. This information can be linked to detailed medical data from the PMSI concerning all admissions to French public-sector and private-sector hospitals: dates of hospital admission and discharge; diagnosis codes according to the International Classification of Diseases, 10th Revision (ICD-10) for the main, related, or accompanying diagnosis, and medical procedures; and costly drugs, such as targeted therapies, administered in the hospital. This national database has been used for several pharmacoepidemiological studies.22–24
Specific approval was obtained to conduct this study from the French data protection agency (Commission Nationale de l’Informatique et des Libertés: SLN/CBO/AR197671). This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines.
Study population and exposure definition
All adults (≥18 years old) registered in the SNDS with PsA (ie, identified by a specific ICD-10 code (M07 except M07.4 and M07.5) according to an algorithm previously published25 26) from 1 January 2015 to 30 June 2021 were eligible for inclusion. We identified patients with at least one prescription for a targeted therapy. Among these individuals, we selected new users of a targeted therapy, defined as those who had not filled a prescription for one of the targeted therapies studied for at least 1 year. Finally, we excluded patients with HIV infection, history of cancer, transplantation, or serious infection (defined by a hospital discharge diagnosis of an infection) during the 2 years before the index date. The targeted therapies studied were adalimumab, certolizumab pegol, etanercept, golimumab and infliximab (TNFi); ixekizumab and secukinumab (IL-17i); ustekinumab (IL-12/23i); and tofacitinib (JAKi). We did not assess abatacept, brodalumab, guselkumab, risankizumab, tildrakizumab, and upadacitinib because they did not receive marketing authorisation for PsA and/or reimbursement in France until June 2021.27 We did not study apremilast, a targeted synthetic DMARD indicated for milder PsA, in particular PsA without structural aggression.28 Targeted therapies were identified in outpatient and hospital discharge databases by Anatomical Therapeutic Chemical codes (online supplemental table 1). Time of coverage by a molecule was defined as the delay from drug initiation to discontinuation. Initiation was defined as the date of the first reimbursement for a targeted therapy during the study period and discontinuation by a term of at least 60 days without filling a prescription for the same targeted treatment after the period covered by the previous reimbursement (ie, 28 days for all treatments except infliximab (56 days) and ustekinumab (84 days)). The end of the 60-day period was defined as the discontinuation date. We only considered the first therapeutic sequence in the analysis, regardless of the dosage of the molecule prescribed. The index date was the date of initiation of a targeted therapy during the study period. Patients were followed until 31 December 2021.
rmdopen-2023-003865supp001.pdf (295KB, pdf)
Outcome
The main outcome was the occurrence of a serious infection during follow-up, defined by any hospitalisation with a discharge main diagnosis of an infection (ICD-10 codes are in the online supplemental table 2). This algorithm, specifically developed to identify serious infections in the French health insurance database, has shown a very good positive predictive value (97%, 95% CI 93 to 100).29In addition, it has been previously used in another study published using data from the SNDS.15 All cases of serious infection were assessed according to the affected organ: pulmonary, gastrointestinal, cutaneous, genitourinary tract, musculoskeletal, ear-nose-throat, nervous system, and other infections. Only the occurrence of a first serious infection (ie, the first hospital stay for a serious infection, which could involve several organs) was studied; the risk of recurrent infections was not assessed in this study.
Covariates
Data were collected for all patients: basic demographics (age, sex, complementary universal health coverage and French deprivation index21 (geographical indicator of social disadvantage specifically adapted to health studies of the French population)), inflammatory diseases associated with PsA (active skin psoriasis defined by at least four deliveries of topical vitamin D derivatives or topical corticosteroids within a 2 year period, IBD, uveitis), and comorbidities (diabetes, chronic heart failure, coronary heart disease, chronic respiratory disease, chronic liver disease, chronic renal failure, sequelae of stroke, obesity (proxy), tobacco use (proxy), alcohol intake (proxy) in the 2 years before the index date). Data linked to care consumption in the 2 years before the index date were also collected (hospitalisation for PsA flare exceeding 1 day, consultations with a rheumatologist, corticosteroids injections, weak and strong opioids analgesics use, and work stoppage). These covariates are defined in online supplemental table 3. We studied the use of treatments of interest other than targeted therapies (csDMARDs (methotrexate, leflunomide, and sulfasalazine), NSAIDs, or prednisone) in the 2 years before the index date. The combination of add-on therapy (csDMARDs, NSAIDs, or prednisone) and targeted therapy at baseline was defined as a 30-day period between reimbursements for the two treatments.
Vital status and exposure to add-on therapies were also compiled during follow-up.
Statistical analyses
Categorical covariates are reported as numbers and percentages. Quantitative covariates are reported as median with IQR or mean±SD. Patient characteristics at baseline are described overall and for each molecule. Incidence rates of serious infection, expressed per 1000 person-years with their 95% CIs, are estimated for the entire study population, for each molecule, and for each infection, according to the organ affected.
Patients were followed until the occurrence of the main outcome (a serious infection) or censorship, that is, death from any cause, end of exposure to a targeted therapy (discontinuation or switch to another targeted therapy), lost to follow-up (defined as no reimbursement for 12 consecutive months), or the study end date (31 December 2021), whichever came first.
The occurrence of serious infections over time was estimated for each molecule by using a Kaplan-Meier method.
To identify factors associated with a serious infection, we used multivariate Cox proportional-hazards regression models to estimate HRs and their CIs. Assumption of linearity and Cox proportional-hazards regression assumption were tested formally by using martingale and Schoenfeld residuals, respectively.
To control for potential confounders, a propensity score was estimated with a multinomial logistic regression model that included all covariates known in the literature to be associated with the occurrence of serious infections and those identified by a complete univariate analysis. These covariates were age, sex, complementary universal health coverage, active skin psoriasis, diabetes, chronic heart failure, coronary heart disease, chronic respiratory disease, chronic liver disease, sequelae of stroke, obesity (proxy), tobacco use (proxy), alcohol intake (proxy), consumption of csDMARDs, NSAIDs, or prednisone in the 2 years before the index date and at the index date, and weak and strong opioids analgesics use in the 2 years before the index date. To reduce potential bias from treatment allocation and to account for the variance estimates that can be inflated by estimated weights, we used the inverse probability of treatment weighting (IPTW) method and the sandwich estimator.30 In addition, stabilised weights were calculated to preserve the sample size of the original data and produce an appropriate estimation of the main effect variance.31 Standardised differences were used to examine the balance of covariates distribution between the several treatment groups. The final model was adjusted for other treatments of interest used as add-on therapies during follow-up (time-dependent covariates) and calendar year. Adalimumab, the most widely prescribed molecule for PsA in France, was the reference molecule.23 32
A prespecified subgroup analysis of patients without active skin psoriasis was performed.
To assess the sensitivity of the estimated weighted HRs (wHRs) with respect to several possible models, we performed the following additional analyses: (1) computing IPTW sub-HRs in a Fine and Gray analysis to account for the competing risk between all-cause out-of-hospital death and hospitalisation for serious infections; (2) using a conventional multivariate Cox proportional hazards regression model estimating adjusted HRs (aHRs); (3) using a 1–1 propensity score-matched analysis; (4) using propensity score weighting and trimming; (5) defining treatment discontinuation as >90 days without filling a prescription for the same treatment after the period covered by the previous prescription; (6) defining new users of a targeted therapy as those who had not filled a prescription for one of the targeted therapies studied for 5 years; and (7) excluding the COVID pandemic period by modifying the end date to 31 December 2019.
All tests were two-tailed, and the threshold for statistical significance was set at two-sided p<0.05. We did not correct for multiple comparisons. All analyses were performed with SAS Enterprise Guide, V.7.1 (SAS Institute).
Patient and public involvement
Patients and/or the public were not involved in the design, conduct, reporting, or dissemination plans of this research.
Results
Characteristics of the study population
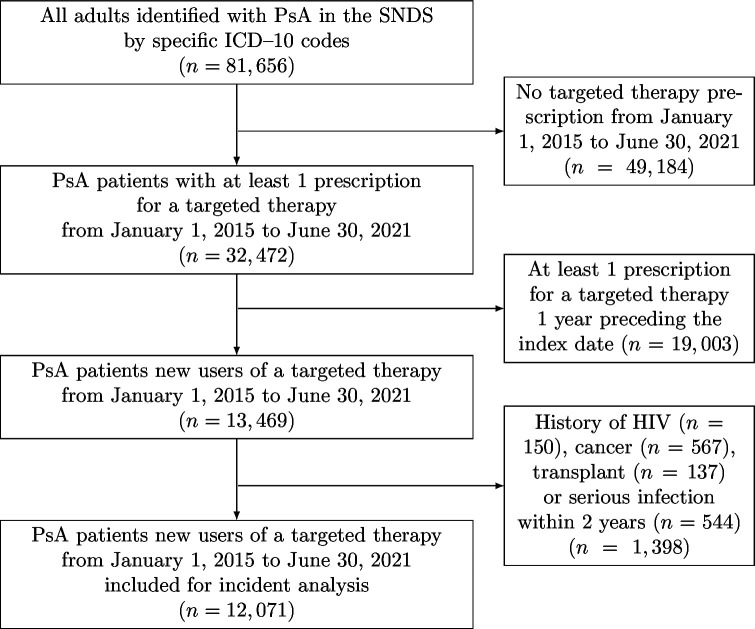
After applying our exclusion criteria, we included 12 071 adults with PsA who were new users of a targeted therapy from 1 January 2015 to 30 June 2021 (mean age 48.7±12.7 years; 6965 (57.7%) women; median follow-up 13.2 (IQR 6.4–31.4) months) (table 1). The flowchart of the study is in figure 1. Most patients included (8946 (74.1%)) were new users of TNFi, mainly (51.6%) adalimumab (see table 1 for the other molecules). There were many similarities between the treatment groups, although a few differences can be highlighted. The sex ratio in the whole cohort was 0.7 man for one woman, with an even higher proportion of women in the certolizumab pegol and tofacitinib groups. Active skin psoriasis was more frequent in new users of IL-17i and IL-12/23i, whereas IBD was uncommon in new users of etanercept and IL-17i. A csDMARD (mainly methotrexate) was prescribed for 43% of patients at the index date. All characteristics are summarised in table 1.
Table 1.
Characteristics of targeted therapies new users for psoriatic arthritis from 2015 to 2021
| Characteristics, n (%) | TNFi | ||||
| Adalimumab | Etanercept | Golimumab | Certolizumab | Infliximab | |
| (n=4616) | (n=2623) | (n=954) | (n=683) | (n=70) | |
| Follow-up, median (IQR), mo.* | 12.6 (6.2–29.5) | 13.4 (5.6–36.5) | 14.0 (6.5–36.2) | 11.2 (5.5–27.9) | 10.7 (6.1–32.7) |
| Deaths | 8 (0.2) | 9 (0.3) | 3 (0.3) | 2 (0.3) | 0 |
| Sociodemographic characteristics | |||||
| Age, mean (SD), year | 48.0 (12.5) | 50.5 (13.2) | 47.7 (12.3) | 44.3 (12.7) | 50.1 (14.8) |
| Female sex | 2538 (55.0) | 1554 (59.3) | 557 (58.4) | 513 (75.1) | 41 (58.6) |
| Deprivation index† | |||||
| First quantile | 295 (6.5) | 251 (9.7) | 79 (8.4) | 44 (6.5) | 4 (5.9) |
| Second quantile | 979 (21.6) | 628 (24.2) | 189 (20.1) | 147 (21.8) | 24 (35.3) |
| Third quantile | 1504 (33.1) | 725 (28.0) | 274 (29.2) | 247 (36.5) | 17 (25.0) |
| Fourth quantile | 1556 (34.3) | 866 (33.4) | 356 (37.9) | 198 (29.3) | 19 (27.9) |
| Fifth quantile | 206 (4.5) | 124 (4.8) | 41 (4.4) | 40 (5.9) | 4 (5.9) |
| CUH coverage | 653 (14.2) | 356 (13.6) | 124 (13.0) | 108 (15.8) | 10 (14.3) |
| Associated inflammatory diseases | |||||
| Active psoriasis | 1382 (29.9) | 645 (24.6) | 163 (17.1) | 139 (20.4) | 12 (17.1) |
| IBD | 378 (8.2) | 14 (0.5) | 46 (4.8) | 21 (3.1) | 21 (30.0) |
| Uveitis | 23 (0.5) | 2 (0.08) | 1 (0.1) | 2 (0.3) | 0 |
| Comorbidities | |||||
| Diabetes | 384 (8.3) | 243 (9.3) | 58 (6.1) | 40 (5.9) | 7 (10.0) |
| Chronic heart failure | 28 (0.6) | 23 (0.9) | 2 (0.2) | 2 (0.3) | 1 (1.4) |
| Coronary heart disease | 145 (3.1) | 91 (3.5) | 30 (3.1) | 30 (4.4) | 7 (10.0) |
| Chronic respiratory disease | 361 (7.8) | 242 (9.2) | 71 (7.4) | 47 (6.9) | 6 (8.6) |
| Chronic liver disease | 99 (2.1) | 74 (2.8) | 11 (1.2) | 8 (1.2) | 4 (5.7) |
| Chronic renal failure | 11 (0.2) | 7 (0.3) | 0 | 2 (0.3) | 0 |
| Sequelae of stroke | 41 (0.9) | 32 (1.2) | 11 (1.2) | 3 (0.4) | 1 (1.4) |
| Obesity (proxy) | 424 (9.2) | 244 (9.3) | 85 (8.9) | 82 (12.0) | 6 (8.6) |
| Tobacco use (proxy) | 299 (6.5) | 149 (5.7) | 66 (6.9) | 39 (5.7) | 3 (4.3) |
| Alcohol intake (proxy) | 57 (1.2) | 42 (1.6) | 9 (0.9) | 4 (0.6) | 1 (1.4) |
| Other treatments of interest within 2 years | |||||
| csDMARDs | 2837 (61.5) | 1718 (65.5) | 598 (62.7) | 420 (61.5) | 30 (42.9) |
| Methotrexate | 2553 (55.3) | 1510 (57.6) | 516 (54.1) | 365 (53.4) | 27 (38.6) |
| Leflunomide | 280 (6.1) | 249 (9.5) | 90 (9.4) | 45 (6.6) | 1 (1.4) |
| Sulfasalazine | 191 (4.1) | 126 (4.8) | 49 (5.1) | 44 (6.4) | 2 (2.9) |
| NSAIDs | 2950 (63.9) | 1820 (69.4) | 682 (71.5) | 494 (72.3) | 27 (38.6) |
| Prednisone | 883 (19.1) | 668 (25.5) | 231 (24.2) | 178 (26.1) | 10 (14.3) |
| Care consumption within 2 years | |||||
| Hospitalisation for PsA‡ | 401 (8.7) | 296 (11.3) | 108 (11.3) | 90 (13.2) | 3 (4.3) |
| Specialist consultation (≥2) | 3402 (73.7) | 2133 (81.3) | 566 (82.9) | 771 (80.8) | 36 (51.4) |
| Specialist consultation (≥4) | 1755 (38.0) | 1362 (51.9) | 469 (49.2) | 341 (49.9) | 14 (20.0) |
| Corticosteroids injections (≥4) | 508 (11.0) | 360 (13.7) | 117 (12.3) | 105 (15.4) | 6 (8.6) |
| Opioids use | 3186 (69.0) | 1869 (71.3) | 677 (71.0) | 528 (77.3) | 52 (74.3) |
| Work stoppage (≥1) | 1379 (29.9) | 681 (26.0) | 283 (29.7) | 220 (32.2) | 19 (27.1) |
| Add-on therapies§ at index date | |||||
| csDMARDs | 2021 (43.8) | 1282 (48.9) | 478 (50.1) | 306 (44.8) | 23 (32.9) |
| Methotrexate | 1806 (39.1) | 1129 (43.0) | 405 (42.5) | 264 (38.7) | 23 (32.9) |
| Leflunomide | 150 (3.3) | 123 (4.7) | 52 (5.5) | 23 (3.4) | 0 |
| Sulfasalazine | 85 (1.8) | 38 (1.5) | 25 (2.6) | 23 (3.4) | 0 |
| NSAIDs | 1644 (35.6) | 1035 (39.5) | 376 (39.4) | 289 (42.3) | 12 (17.1) |
| Prednisone | 712 (15.4) | 498 (19.0) | 180 (18.9) | 126 (18.5) | 8 (11.4) |
| Add-on therapies¶ during follow-up | |||||
| csDMARDs | 1895 (41.1) | 1169 (44.6) | 442 (46.3) | 279 (40.9) | 23 (32.9) |
| Methotrexate | 1708 (37.0) | 1063 (40.5) | 383 (40.2) | 245 (35.9) | 23 (32.9) |
| Leflunomide | 136 (3.0) | 94 (3.6) | 45 (4.7) | 22 (3.2) | 0 |
| Sulfasalazine | 64 (1.4) | 24 (0.9) | 17 (1.8) | 17 (2.5) | 0 |
| NSAIDs | 1673 (36.2) | 1090 (41.6) | 432 (45.3) | 297 (43.5) | 19 (27.1) |
| Prednisone | 605 (13.1) | 437 (16.7) | 145 (15.2) | 115 (16.8) | 8 (11.4) |
| Characteristics, n (%) | IL-17i | IL-12/23i | JAKi | ||
| Secukinumab | Ixekizumab | Ustekinumab | Tofacitinib | Total | |
| (n=1452) | (n=344) | (n=1177) | (n=152) | (n=12 071) | |
| Follow-up, median (IQR), mo.* | 15.0 (7.3–30.4) | 13.2 (7.1–21.7) | 16.8 (8.6–36.5) | 8.5 (4.7–14.3) | 13.2 (6.4–31.4) |
| Deaths | 0 | 0 | 3 (0.3) | 0 | 25 (0.2) |
| Sociodemographic characteristics | |||||
| Age, mean (SD), year | 49.3 (12.2) | 49.4 (11.7) | 49.7 (13.0) | 52.7 (13.0) | 48.7 (12.7) |
| Female sex | 818 (56.3) | 181 (52.6) | 649 (55.1) | 114 (75.0) | 6965 (57.7) |
| Deprivation index† | |||||
| First quantile | 95 (6.6) | 22 (6.4) | 83 (7.2) | 10 (6.7) | 883 (7.4) |
| Second quantile | 283 (19.8) | 90 (26.2) | 249 (21.7) | 40 (26.7) | 2629 (22.1) |
| Third quantile | 520 (36.4) | 92 (26.8) | 404 (35.2) | 61 (40.7) | 3844 (32.3) |
| Fourth quantile | 461 (32.2) | 122 (35.6) | 369 (32.2) | 35 (23.3) | 3982 (33.5) |
| Fifth quantile | 71 (5.0) | 17 (5.0) | 42 (3.7) | 4 (2.7) | 549 (4.6) |
| CUH coverage | 248 (17.1) | 59 (17.2) | 174 (14.8) | 19 (12.5) | 1751 (14.5) |
| Associated inflammatory diseases | |||||
| Active psoriasis | 556 (38.3) | 174 (50.6) | 601 (51.1) | 28 (18.4) | 3700 (30.7) |
| IBD | 11 (0.8) | 2 (0.6) | 69 (5.9) | 7 (4.6) | 569 (4.7) |
| Uveitis | 3 (0.2) | 0 | 0 | 0 | 31 (0.3) |
| Comorbidities | |||||
| Diabetes | 154 (10.6) | 34 (9.9) | 127 (10.8) | 12 (7.9) | 1059 (8.8) |
| Chronic heart failure | 11 (0.8) | 5 (1.5) | 17 (1.4) | 5 (3.3) | 94 (0.8) |
| Coronary heart disease | 57 (3.9) | 16 (4.7) | 41 (3.5) | 5 (3.3) | 422 (3.5) |
| Chronic respiratory disease | 135 (9.3) | 36 (10.5) | 106 (9.0) | 20 (13.2) | 1024 (8.5) |
| Chronic liver disease | 31 (2.1) | 7 (2.0) | 52 (4.4) | 3 (2.0) | 289 (2.4) |
| Chronic renal failure | 2 (0.1) | 0 | 6 (0.5) | 1 (0.7) | 29 (0.2) |
| Sequelae of stroke | 28 (1.9) | 6 (1.7) | 11 (0.9) | 1 (0.7) | 134 (1.1) |
| Obesity (proxy) | 174 (12.0) | 28 (8.1) | 163 (13.9) | 10 (6.6) | 1216 (10.1) |
| Tobacco use (proxy) | 126 (8.7) | 37 (10.8) | 99 (8.4) | 12 (7.9) | 830 (6.9) |
| Alcohol intake (proxy) | 24 (1.7) | 11 (3.2) | 24 (2.0) | 1 (0.7) | 173 (1.4) |
| Other treatments of interest within 2 years | |||||
| csDMARDs | 842 (58.0) | 197 (57.3) | 662 (56.2) | 104 (68.4) | 7408 (61.4) |
| Methotrexate | 748 (51.5) | 179 (52.0) | 600 (51.0) | 87 (57.2) | 6585 (54.6) |
| Leflunomide | 92 (6.3) | 20 (5.8) | 62 (5.3) | 19 (12.5) | 858 (7.1) |
| Sulfasalazine | 46 (3.2) | 10 (2.9) | 33 (2.8) | 7 (4.6) | 508 (4.2) |
| NSAIDs | 849 (58.5) | 167 (48.6) | 555 (47.2) | 80 (52.6) | 7624 (63.2) |
| Prednisone | 238 (16.4) | 40 (11.6) | 162 (13.8) | 56 (36.8) | 2466 (20.4) |
| Care consumption within 2 years | |||||
| Hospitalisation for PsA‡ | 106 (7.3) | 25 (7.3) | 93 (7.9) | 11 (7.2) | 1133 (9.4) |
| Specialist consultation (≥2) | 962 (66.3) | 190 (55.2) | 650 (55.2) | 115 (75.7) | 8825 (73.1) |
| Specialist consultation (≥4) | 456 (31.4) | 53 (15.4) | 361 (30.7) | 61 (40.1) | 4872 (40.4) |
| Corticosteroids injections (≥4) | 144 (9.9) | 24 (7.0) | 116 (9.9) | 15 (9.9) | 1395 (11.6) |
| Opioids use | 1010 (69.6) | 209 (60.8) | 738 (62.7) | 102 (67.1) | 8371 (69.4) |
| Work stoppage (≥1) | 362 (24.9) | 93 (27.0) | 263 (22.3) | 35 (23.0) | 3335 (27.6) |
| Add-on therapies§ at index date | |||||
| csDMARDs | 520 (35.8) | 124 (36.1) | 373 (31.7) | 80 (52.6) | 5207 (43.1) |
| Methotrexate | 448 (30.9) | 111 (32.3) | 325 (27.6) | 69 (45.4) | 4580 (37.9) |
| Leflunomide | 56 (3.9) | 11 (3.2) | 39 (3.3) | 9 (5.9) | 463 (3.8) |
| Sulfasalazine | 22 (1.5) | 3 (0.9) | 11 (0.9) | 2 (1.3) | 209 (1.7) |
| NSAIDs | 504 (34.7) | 82 (23.8) | 322 (27.4) | 33 (21.7) | 4297 (35.6) |
| Prednisone | 204 (14.1) | 41 (11.9) | 136 (11.6) | 44 (29.0) | 1949 (16.2) |
| Add-on therapies¶ during follow-up | |||||
| csDMARDs | 455 (31.3) | 97 (28.2) | 335 (28.5) | 65 (42.8) | 4760 (39.4) |
| Methotrexate | 408 (28.1) | 85 (24.7) | 285 (24.2) | 56 (36.8) | 4256 (35.3) |
| Leflunomide | 40 (2.8) | 11 (3.2) | 42 (3.6) | 8 (5.3) | 398 (3.3) |
| Sulfasalazine | 13 (0.9) | 1 (0.3) | 10 (0.9) | 2 (1.3) | 148 (1.2) |
| NSAIDs | 555 (38.2) | 95 (27.6) | 441 (37.5) | 36 (23.7) | 4638 (38.4) |
| Prednisone | 193 (13.3) | 27 (7.9) | 147 (12.5) | 38 (25.0) | 1715 (14.2) |
*Two patients were lost to follow-up in the adalimumab group.
†Data were missing for 184 patients; individuals in first quantile are least disadvantaged.
‡PsA code as main diagnosis for a hospitalisation>1 day.
§Defined as a period of <30 days between reimbursements of a csDMARD, NSAIDs, or prednisone and the targeted therapy.
csDMARDs, conventional synthetic disease-modifying antirheumatic drugs; CUH, complementary universal health; IBD, inflammatory bowel disease; IL-17i, interleukin 17 inhibitors; IL-12/23i, interleukin 12 and 23 inhibitor; JAKi, Janus kinase inhibitors; NSAIDs, non-steroidal anti-inflammatory drugs; PsA, psoriatic arthritis; TNFi, tumour necrosis factor inhibitors.
Figure 1.
Flow of participants in the study. ICD-10, International Classification of Diseases 10th revision; PsA, Psoriatic Arthritis; SNDS, Système National des Données de Santé.
Description of serious infections
During follow-up, 367 serious infections were identified. With a total of 21 649 person-years, the overall crude incidence rate was 17.0 per 1000 person-years (95% CI, 15.2 to 18.7). The median time to event was 9.5 months (IQR 3.2–23.4). The most frequent serious infections were pulmonary infections (95 (25.9%)), gastrointestinal infections (90 (24.5%)), and cutaneous infections (73 (19.9%)). Among all serious infections, we noted 14 (3.8%) opportunistic infections (four cases of legionella, three cases of tuberculosis, two cases of Candida esophagitis, one case of Aspergillus infection, one case of cryptosporidiosis infection, one case of Herpes Zoster Virus meningitis, one case of Pneumocystis pneumonia, and one case of Toxoplasma gondii infection). We found 29 (7.9%) cases of serious infection requiring admission to an intensive care unit. Serious infection details are summarised in online supplemental table 4. Relative to adalimumab, the crude incidence rate of serious infection was higher with secukinumab and lower with etanercept or ustekinumab. Details per therapeutic drug are available in table 2.
Table 2.
Serious infection cases among targeted therapies new users for psoriatic arthritis from 2015 to 2021
| Total (n=12 071) | N (%) of serious infection cases | Incidence rate of serious infection cases per 1000 PY (95% CI) | Time before serious infection, median (IQR), mo. |
| Serious infection cases | 367 (3.0) | 17.0 (15.2 to 18.7) | 9.5 (3.2–23.4) |
| Details per organ | |||
| Pulmonary | 95 (0.8) | 4.4 (3.5 to 5.3) | 11.5 (3.7–30.4) |
| Gastrointestinal | 90 (0.8) | 4.2 (3.2 to 5.0) | 9.6 (3.7–22.8) |
| Cutaneous | 73 (0.6) | 3.4 (2.6 to 4.1) | 9.4 (3.0–22.7) |
| Genitourinary tract | 28 (0.2) | 1.3 (0.8 to 1.8) | 6.8 (1.9–17.4) |
| ENT | 24 (0.2) | 1.1 (0.7 to 1.6) | 6.9 (4.4–12.2) |
| Musculoskeletal | 11 (0.09) | 0.5 (0.2 to 0.8) | 7.4 (1.4–18.0) |
| Nervous system | 6 (0.05) | 0.3 (0.06 to 0.5) | 14.9 (11.9–34.6) |
| Other | 40 (0.3) | 1.9 (1.3 to 2.4) | 11.1 (3.2–24.9) |
| Details per molecules | |||
| TNFi | |||
| Adalimumab (n=4616) | |||
| Serious infection cases | 150 (3.3) | 18.9 (15.9 to 21.9) | 7.6 (2.5–23.3) |
| Etanercept (n=2623) | |||
| Serious infection cases | 73 (2.8) | 14.2 (11.0 to 17.5) | 10.0 (3.9–22.8) |
| Golimumab (n=954) | |||
| Serious infection cases | 24 (2.5) | 12.9 (7.7 to 18.1) | 10.6 (4.2–33.9) |
| Certolizumab (n=683) | |||
| Serious infection cases | 17 (2.5) | 15.2 (8.0 to 22.4) | 15.2 (7.6–25.3) |
| Infliximab (n=70) | |||
| Serious infection cases | 5 (7.1) | 41.1 (5.1 to 77.1) | 19.4 (2.1–19.8) |
| IL-17i | |||
| Secukinumab (n=1452) | |||
| Serious infection cases | 55 (3.8) | 22.0 (16.2 to 27.9) | 11.9 (3.7–26.0) |
| Ixekizumab (n=344) | |||
| Serious infection cases | 10 (2.9) | 22.1 (8.4 to 35.9) | 7.9 (4.7–12.5) |
| IL-12/23i | |||
| Ustekinumab (n=1177) | |||
| Serious infection cases | 28 (2.4) | 11.8 (7.4 to 16.2) | 11.0 (6.5–28.1) |
| JAKi | |||
| Tofacitinib (n=152) | |||
| Serious infection cases | 5 (3.3) | 34.8 (4.3 to 65.2) | 10.4 (8.1–11.8) |
ENT, ear-nose-throat; IL-17i, interleukin 17 inhibitors; IL-12/23i, interleukin 12 and 23 inhibitor; JAKi, Janus kinase inhibitors; PY, person-years; TNFi, tumour necrosis factor inhibitors.
Risk of serious infection among new users of targeted therapies
The linearity assumption for the continuous covariate (age at index date) and the proportional-hazard assumption were met. After stabilised weights allocation, the distribution of all characteristics was balanced, with standardised differences less than │0.1│ between the treatment classes (online supplemental figure 1). The results of the main analysis are presented in table 3. After IPTW propensity score application and adjustment for time-dependent covariates and calendar year, risk of serious infection was significantly reduced with etanercept (wHR 0.72; 95% CI, 0.53 to 0.97) and ustekinumab (wHR 0.57; 95% CI, 0.35 to 0.93) relative to adalimumab. The risk was not statistically different from adalimumab for certolizumab pegol, golimumab, infliximab, ixekizumab, secukinumab, and tofacitinib. Comparisons with one molecule relative to another and by targeted therapies class with TNFi as the comparator are available in online supplemental tables 5 and 6. Risk of serious infection was significantly increased with concomitant use of systemic corticosteroids (prednisone) (wHR 1.90; 95% CI, 1.47 to 2.46) and was not statistically different with concomitant use of csDMARDs or NSAIDs.
Table 3.
Risk of serious infection among new users of targeted therapies for psoriatic arthritis from 2015 to 2021
| Unadjusted stabilised IPTW* Cox model† | Adjusted stabilised IPTW* Cox model†‡ | |||||
| cHR | 95% CI | P value | wHR | 95% CI | P value | |
| Treatments (reference: adalimumab) | ||||||
| Etanercept | 0.72 | 0.54 to 0.96 | 0.0261 | 0.72 | 0.53 to 0.97 | 0.0305 |
| Golimumab | 0.79 | 0.39 to 1.60 | 0.5148 | 0.79 | 0.40 to 1.58 | 0.506 |
| Certolizumab | 0.86 | 0.48 to 1.54 | 0.6156 | 0.84 | 0.47 to 1.50 | 0.5488 |
| Infliximab | 2.01 | 0.67 to 6.02 | 0.2127 | 1.75 | 0.54 to 5.63 | 0.3486 |
| Secukinumab | 1.09 | 0.79 to 1.51 | 0.6015 | 1.07 | 0.77 to 1.50 | 0.6821 |
| Ixekizumab | 0.81 | 0.41 to 1.60 | 0.5383 | 0.77 | 0.38 to 1.57 | 0.4767 |
| Ustekinumab | 0.58 | 0.35 to 0.94 | 0.0258 | 0.57 | 0.35 to 0.93 | 0.0256 |
| Tofacitinib | 1.16 | 0.37 to 3.66 | 0.801 | 1.07 | 0.33 to 3.44 | 0.9089 |
| Time-dependent covariates | ||||||
| csDMARDs | NA | NA | NA | 0.9 | 0.71 to 1.15 | 0.4035 |
| NSAIDs | NA | NA | NA | 1.04 | 0.80 to 1.35 | 0.7668 |
| Prednisone | NA | NA | NA | 1.9 | 1.47 to 2.46 | <0.0001 |
Values in bold correspond to significant results.
*Estimated with a multinomial logistic regression including age, sex, complementary universal health coverage, active skin psoriasis, diabetes, chronic heart failure, coronary heart disease, chronic respiratory disease, chronic liver disease, sequelae of stroke, obesity (proxy), tobacco use (proxy), alcohol intake, (proxy) consumption of csDMARDS, NSAIDs, or prednisone in the 2 years before inclusion and at inclusion, and weak and strong opioids analgesics use in the 2 years before inclusion.
†Using the Sandwich estimator, with adalimumab as the comparator.
‡Adjusted for time-dependent covariates (csDMARDs, NSAIDs, and prednisone) and calendar year.
cHR, crude HR; csDMARDs, conventional synthetic disease-modifying antirheumatic drugs; IPTW, inverse probability of treatment weighting; NA, not applicable; NSAIDs, non-steroidal anti-inflammatory drugs; wHR, weighted HR.
Subgroup analysis
After excluding patients with active skin psoriasis, the results did not differ from the main analysis with a significant lower risk of serious infection in new users of etanercept and ustekinumab relative to new users of adalimumab. The result of the subgroup analysis is available in online supplemental table 7.
Sensitivity analysis
The results of the sensitivity analysis were consistent with our principal analysis, with decreased risk of serious infection in new users of etanercept and ustekinumab as compared with adalimumab.
We performed a sensitivity analysis using a conventional multivariate Cox proportional hazards regression model (online supplemental table 9), the results of which were consistent with our main analysis. In this analysis, other factors associated with the occurrence of a serious infection were increasing age, precarity (ie, Complementary Universal Health coverage), chronic respiratory disease at baseline, opioid use 2 years prior to inclusion, and prednisone use during follow-up.
During the pandemic, both the initiation and the median persistence of targeted therapies appeared to be similar to those of previous years (online supplemental table 14). The sensitivity analysis performed excluding the COVID pandemic period found similar results to the main analysis (online supplemental table 15).
Some variations were found, notably in the analysis using propensity score weighting and trimming in which the risk of serious infection was significantly lower for golimumab new users than adalimumab new users (wHR 0.54; 95% CI, 0.34 to 0.85) and in the analysis defining new users of targeted therapies as patients who had not filled a prescription for one of the targeted therapies studied for 5 years, in which the risk of serious infection was also significantly lower for golimumab new users than adalimumab new users (wHR 0.43; 95% CI, 0.25 to 0.74).
Results of the sensitivity analyses are available in online supplemental tables 8-13 and 15.
Discussion
In this French cohort study including 12 071 new users of targeted therapies for PsA, the overall risk of serious infection was low, which confirms the good safety of the studied treatments. After accounting for potential confounding factors, this risk was significantly lower for new users of etanercept and ustekinumab than adalimumab new users and remained unchanged with the other molecules. In addition, the risk of infection was increased with the use of systemic corticosteroids as add-on therapy but did not differ with csDMARDs or NSAIDs.
To the best of our knowledge, our study is the first to specifically assess the risk of serious infection associated with targeted therapy use for PsA in real-world settings. The crude incidence rate of serious infection (17.0 per 1000 person-years (95% CI, 15.2 to 18.7)) was similar to those found in two studies of patients with psoriasis or PsA based on US medicoadministrative databases.17 18 Furthermore, similar rates have been observed in other databases of patients with psoriasis only, which supports the hypothesis of a similar infectious risk whatever the underlying disease.14 15
The results of this study are important because the risk of serious infection associated with recent targeted therapies, including secukinumab, ixekizumab, ustekinumab, and tofacitinib, has been assessed under real-world conditions. The two previously published cohort studies examining the risk of serious infection in psoriatic patients (psoriasis alone or PsA) receiving targeted therapies were based on Optum and/or MarketScan databases, US commercial health data sets whose results may be difficult to generalise to real-world patients, as demonstrated in a recent study.33 The first study found no significant difference in infection risk in the PsA cohort, but only therapeutic classes (ie, TNFi, IL-17i, and IL-12/23i) and not molecules were investigated.18 In our study, we also found no significant differences among new users of IL-17i, IL-12/23i, or JAKi as compared with TNFi new users (online supplemental table 6), but the risk of serious infection seemed significantly lower for new users of etanercept than adalimumab new users, which reinforces the need to compare molecules separately because of differences within the same therapeutic class. This finding agrees with previously published studies, mainly in psoriasis,14 15 17 34 and could be explained by the particular structure of etanercept, which differs from other TNFi. As shown by a recent study,35 the structural features of the five available TNFi confer a different mechanism of action towards TNF, notably with regard to antibody-dependent cellular cytotoxicity, complement-dependent cytotoxicity, downregulation of cytokine expression via reverse signalling pathways, and cell apoptosis. Therefore, it could lead to different clinical outcomes in terms of risk of serious infection. In particular, TNFi vary in their ability to interact with transmembrane TNF (tmTNF), a precursor of the soluble form of TNF that plays an important role in host defence against infections (action against T cells, monocytes/macrophages, and natural killer cells).36 As several studies have shown,37 38 etanercept seems to bind to tmTNF with less avidity and stability than monoclonal antibodies, which could explain in part the lower risk of infection with this molecule. In addition, etanercept, which acts as a type 2 TNF decoy receptor, seems to have a faster dissociation rate between the ligand and type 2 TNF receptors than type 1 TNF receptors. This dissociation rate could result in a more transient impact in terms of biological effects of TNF, which may explain its greater safety against serious infections.
Consistent with our results, the other cohort study of psoriatic patients highlighted a significantly higher risk of serious infection with each studied molecule (ie, adalimumab, apremilast, certolizumab pegol, etanercept, golimumab, infliximab, ixekizumab, and secukinumab) than ustekinumab new users.17 Of note, the safety profile of ustekinumab had previously been reported in psoriasis patients14 15 and has been recently described in a large observational study comparing ustekinumab and TNFi in PsA patients over 3 years.39 These findings could be attributed to structural and pharmacokinetic considerations. Ustekinumab binds to the common p40 subunit of IL-12 and IL-23 which prevents them from binding to the receptor chain of IL-12 and the IL-23 receptor complexes on the surfaces of natural killer and T cells.40 As a result, it neutralises IFNγ, IL-17A, IL-17F, and IL-22 production,41 but does not contribute to antibody-dependent cellular cytotoxicity or complement-dependent cytotoxicity, unlike most TNFi. An in vitro study showed that in contrast to adalimumab, etanercept and infliximab, ustekinumab did not significantly reduce leukocyte recruitment when the endothelium was activated,42 which may explain the difference observed in our study.
To take into account the COVID pandemic period and the risk of behaviour change, we performed a sensitivity analysis excluding this period and found similar results.
This study has several limitations. The definition of PsA population was based on either ICD-10 diagnostic codes for PsA (M07 except M07.4 and M07.5, which correspond to arthropathy in Crohn’s disease and ulcerative colitis, respectively) applied to in-patients or out-patients with fully reimbursed PsA-related care procedures.25 26 We did not exclude patients with ICD-10 code M07.6. However, out of the total population included, we found only eight (<0.1%) patients identified only via an ICD-10 code M07.6. Treatment exposure was based on fulfilment of prescription data, which is not necessarily equivalent to days of use and could therefore lead to classification bias. However, the study of repeat dispensing allowed for limiting this bias, and in published reports, adherence rates for targeted therapies have been generally higher than those for other treatment categories in inflammatory diseases.43 Although we applied a propensity score and adjusted on time-dependent covariates,44 45 the presence of unmeasured residual confounders cannot be ruled out. Another limitation is data availability for some covariates, such as disease severity, tobacco use, alcohol intake, and obesity. Nevertheless, the covariates were assessed with a proxy previously used in other studies.23 46 In addition, defining treatment discontinuation as >60 days without filling a prescription for the same treatment after the period covered by the previous prescription may be an issue. However, this definition has been used in other studies,23 46 and a sensitivity analysis performed by modifying this definition confirmed the results of our main analysis. The dosage of each molecule was not taken into account in our study. Further analysis is required to determine whether the dosage has an impact on the risk of serious infection in these patients. Another limitation is the lack of correction for multiple tests because this was an exploratory study. Nevertheless, the comparison between the different molecules was predefined (comparison provided for in the initial protocol), and a sandwich estimator was used to adequately calculate the variance of the treatment effect estimates.47 Furthermore, when considering the low number of events in some treatment groups, notably with certolizumab pegol, golimumab, infliximab, ixekizumab, and tofacitinib, these results need to be confirmed. Finally, abatacept (not reimbursed for the treatment of PsA in France) and the most recently marketed targeted therapies (ie, brodalumab, guselkumab, risankizumab, tildrakizumab, and upadacitinib) were not included in our analysis, and further studies will be necessary in the coming years.
This study has several strengths. This cohort encompassed a large number of patients from a comprehensive national database with a data quality and consistency plan ensuring homogeneous data processing, which allowed for mitigating selection bias.20 Our definition of PsA was based on either ICD-10 diagnostic codes for PsA applied to in-patients or out-patients with fully reimbursed PsA-related care procedures. We are confident that our definition is specific: most of the patients had at least one encounter with a rheumatologist 2 years before their inclusion, patients received targeted therapies that are reimbursed in the context of PsA, and we have previously shown that our population has much the same characteristics as described populations of patients with moderate to severe PsA.25 26 The definition of new users of targeted therapies allowed us to obtain an initial incident population that was generally at the same stage of the disease, which limited the risk of attributing the occurrence of a serious infection to past exposure to a targeted therapy. Moreover, the sensitivity analysis performed with a modified definition of new users (ie, defining new users of a targeted therapy as those who had not filled a prescription for one of the targeted therapies studied for 5 years) gave similar results. Additionally, the risk of serious infection was assessed among targeted therapy users rather than non-targeted therapy users (ie, csDMARDs or NSAIDs users or without treatment) to avoid the risk of immortal bias and to approach ‘real-life’ clinical situations for which the indications for initiating targeted therapies or non-targeted therapies are different. Finally, results of all sensitivity analyses supported the integrity of our main results.
Conclusion
This study found an overall low risk of serious infection among new users of targeted therapies for moderate to severe PsA. Risk of serious infection was significantly reduced with etanercept and ustekinumab as compared with adalimumab and was not modified for new users of the other studied targeted therapies. Risk of serious infection was increased with concomitant use of systemic corticosteroids during targeted treatment exposure. Considering the number of current treatment options for PsA, further studies, especially of other large-scale cohorts in real-world settings, are needed to confirm these results and evaluate the most recent treatments to help physicians optimise their therapeutic choice for each patient.
rmdopen-2023-003865supp002.pdf (47.9KB, pdf)
Acknowledgments
L. Bastard received a Master 2 grant from the French Society of Rheumatology (Bourse Master 2ème année 2022). We thank Laura Smales for English editing.
Footnotes
Twitter: @Lea_Bstard, @LauraPnVg
Contributors: L. Bastard, L. Pina Vegas, E. Sbidian, P. Claudepierre conceived the study idea and concept. L. Bastard, L. Penso, L. Pina Vegas, E. Sbidian, P. Claudepierre contributed to data collection. L. Bastard, L. Pina Vegas and E. Sbidian conducted the data anlysis. L. Bastard and L. Pina Vegas drafted the article and act as guarantors of the study. L. Bastard, L. Penso, L. Pina Vegas, E. Sbidian, P. Claudepierre interpreted the results, critically revised the manuscript and approved the final version.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: L. Bastard, L. Penso and E. Sbidian have no conflicts of interest to declare.P. Claudepierre has received consulting fees from Abbvie, Amgen, Pfizer, Roche-Chugai, BMS, MSD, UCB, Novartis, Janssen, Lilly, Galapagos, and Celgene (less than $10,000 each) and has been an investigator for Roche Chugai, Sanofi Aventis, Celgene, Pfizer, MSD, Novartis and BMS.L. Pina Vegas received support from Novartis to attend a meeting.
Patient and public involvement statement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Consent obtained from parent(s)/guardian(s).
Ethics approval
The French public instutition which conducted this study has permanent access to the SNDS database in application of the provisions of Articles R. 1461-12 et seq. of the French Public Health Code and the French data protection authority decision CNIL-2016-316. No informed consent was therefore required.
References
- 1.Ritchlin CT, Colbert RA, Gladman DD. Psoriatic arthritis. N Engl J Med 2017;376:957–70. 10.1056/NEJMra1505557 [DOI] [PubMed] [Google Scholar]
- 2.Perez-Chada LM, Merola JF. Comorbidities associated with psoriatic arthritis: review and update. Clin Immunol 2020;214:108397. 10.1016/j.clim.2020.108397 [DOI] [PubMed] [Google Scholar]
- 3.Aletaha D, Askling J, Bae SC, et al. Annals of the rheumatic diseases publishes original work on all aspects of rheumatology and disorders of connective tissue. laboratory and clinical studies are equally welcome. 2023.
- 4.Christensen IE, Lillegraven S, Mielnik P, et al. Serious infections in patients with rheumatoid arthritis and psoriatic arthritis treated with tumour necrosis factor inhibitors: data from register linkage of the NOR-DMARD study. Ann Rheum Dis 2022;81:398–401. 10.1136/annrheumdis-2021-221007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Minozzi S, Bonovas S, Lytras T, et al. Risk of infections using anti-TNF agents in rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis: a systematic review and meta-analysis. Expert Opin Drug Saf 2016;15:11–34. 10.1080/14740338.2016.1240783 [DOI] [PubMed] [Google Scholar]
- 6.Lortholary O, Fernandez-Ruiz M, Baddley JW, et al. Infectious complications of rheumatoid arthritis and psoriatic arthritis during targeted and biological therapies: a viewpoint in 2020. Ann Rheum Dis 2020;79:1532–43. 10.1136/annrheumdis-2020-217092 [DOI] [PubMed] [Google Scholar]
- 7.Winthrop KL, Novosad SA, Baddley JW, et al. Opportunistic infections and biologic therapies in immune-mediated inflammatory diseases: consensus recommendations for infection reporting during clinical trials and postmarketing surveillance. Ann Rheum Dis 2015;74:2107–16. 10.1136/annrheumdis-2015-207841 [DOI] [PubMed] [Google Scholar]
- 8.McInnes IB, Anderson JK, Magrey M, et al. Trial of upadacitinib and adalimumab for psoriatic arthritis. N Engl J Med 2021;384:1227–39. 10.1056/NEJMoa2022516 [DOI] [PubMed] [Google Scholar]
- 9.McInnes IB, Behrens F, Mease PJ, et al. Secukinumab versus adalimumab for treatment of active psoriatic arthritis (EXCEED): a double-blind, parallel-group, randomised, active-controlled, phase 3B trial. Lancet 2020;395:1496–505. 10.1016/S0140-6736(20)30564-X [DOI] [PubMed] [Google Scholar]
- 10.Kristensen L-E, Okada M, Tillett W, et al. Ixekizumab demonstrates consistent efficacy versus adalimumab in biologic disease-modifying anti-rheumatic drug-Naïve psoriatic arthritis patients regardless of psoriasis severity: 52-week post hoc results from SPIRIT-H2H. Rheumatol Ther 2022;9:109–25. 10.1007/s40744-021-00388-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mease P, Hall S, FitzGerald O, et al. Tofacitinib or adalimumab versus placebo for psoriatic arthritis. N Engl J Med 2017;377:1537–50. 10.1056/NEJMoa1615975 [DOI] [PubMed] [Google Scholar]
- 12.Mease PJ, Lertratanakul A, Anderson JK, et al. Upadacitinib for psoriatic arthritis refractory to Biologics: SELECT-PSA 2. Ann Rheum Dis 2021;80:312–20. 10.1136/annrheumdis-2020-218870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chiu YM, Chen DY. Infection risk in patients undergoing treatment for inflammatory arthritis: non-biologics versus biologics. Expert Rev Clin Immunol 2020;16:207–28. 10.1080/1744666X.2019.1705785 [DOI] [PubMed] [Google Scholar]
- 14.Kalb RE, Fiorentino DF, Lebwohl MG, et al. Risk of serious infection with biologic and systemic treatment of psoriasis: results from the psoriasis longitudinal assessment and Registry (PSOLAR). JAMA Dermatol 2015;151:961–9. 10.1001/jamadermatol.2015.0718 [DOI] [PubMed] [Google Scholar]
- 15.Penso L, Dray-Spira R, Weill A, et al. Association between biologics use and risk of serious infection in patients with psoriasis. JAMA Dermatol 2021;157:1056–65. 10.1001/jamadermatol.2021.2599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haddad A, Li S, Thavaneswaran A, et al. The incidence and predictors of infection in psoriasis and psoriatic arthritis: results from longitudinal observational cohorts. J Rheumatol 2016;43:362–6. 10.3899/jrheum.140067 [DOI] [PubMed] [Google Scholar]
- 17.Jin Y, Lee H, Lee MP, et al. Risk of hospitalized serious infection after initiating ustekinumab or other biologics for psoriasis or psoriatic arthritis. Arthritis Care Res (Hoboken) 2022;74:1792–805. 10.1002/acr.24630 [DOI] [PubMed] [Google Scholar]
- 18.Li X, Andersen KM, Chang H-Y, et al. Comparative risk of serious infections among real-world users of biologics for psoriasis or Psoriatic arthritis. Ann Rheum Dis 2020;79:285–91. 10.1136/annrheumdis-2019-216102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Balanescu A-R, Citera G, Pascual-Ramos V, et al. Infections in patients with rheumatoid arthritis receiving tofacitinib versus tumour necrosis factor inhibitors: results from the open-label, randomised controlled ORAL surveillance trial. Ann Rheum Dis 2022;81:1491–503. 10.1136/ard-2022-222405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tuppin P, Rudant J, Constantinou P, et al. Value of a national administrative database to guide public decisions: from the Système national D’Information Interrégimes de L’Assurance Maladie (SNIIRAM) to the Système national des Données de Santé (SNDS) in France. Rev Epidemiol Sante Publique 2017;65 Suppl 4:S149–67. 10.1016/j.respe.2017.05.004 [DOI] [PubMed] [Google Scholar]
- 21.Temam S, Varraso R, Pornet C, et al. Ability of ecological deprivation indices to measure social inequalities in a French cohort. BMC Public Health 2017;17:956. 10.1186/s12889-017-4967-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pol S, Fouad F, Lemaitre M, et al. Impact of extending direct antiviral agents (DAA) availability in France: an observational cohort study (2015-2019) of data from French administrative Healthcare databases (SNDS). Lancet Reg Health Eur 2022;13:100281. 10.1016/j.lanepe.2021.100281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pina Vegas L, Penso L, Claudepierre P, et al. Long-term persistence of first-line biologics for patients with psoriasis and psoriatic arthritis in the French health insurance database. JAMA Dermatol 2022;158:513–22. 10.1001/jamadermatol.2022.0364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoisnard L, Pina Vegas L, Dray-Spira R, et al. Risk of major adverse cardiovascular and venous thromboembolism events in patients with rheumatoid arthritis exposed to JAK inhibitors versus adalimumab: a nationwide cohort study. Ann Rheum Dis 2023;82:182–8. 10.1136/ard-2022-222824 [DOI] [PubMed] [Google Scholar]
- 25.Pina Vegas L, Sbidian E, Penso L, et al. Epidemiologic study of patients with psoriatic arthritis in a real-world analysis: a cohort study of the French health insurance database. Rheumatology (Oxford) 2021;60:1243–51. 10.1093/rheumatology/keaa448 [DOI] [PubMed] [Google Scholar]
- 26.Castagné B, Viprey M, Caillet-Pascal P, et al. Algorithms to identify chronic inflammatory rheumatism and psoriasis in medico-administrative databases: a review of the literature. Rev Epidemiol Sante Publique 2021;69:225–33. 10.1016/j.respe.2021.02.002 [DOI] [PubMed] [Google Scholar]
- 27.Wendling D, Hecquet S, Fogel O, et al. French society for rheumatology (SFR) recommendations on the everyday management of patients with spondyloarthritis, including psoriatic arthritis. Joint Bone Spine 2022;89:105344. 10.1016/j.jbspin.2022.105344 [DOI] [PubMed] [Google Scholar]
- 28.Gossec L, Baraliakos X, Kerschbaumer A, et al. EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2019 update. Ann Rheum Dis 2020;79:700. 10.1136/annrheumdis-2020-217159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sahli L, Lapeyre-Mestre M, Derumeaux H, et al. Positive predictive values of selected hospital discharge diagnoses to identify infections responsible for hospitalization in the French national hospital database. Pharmacoepidemiol Drug Saf 2016;25:785–9. 10.1002/pds.4006 [DOI] [PubMed] [Google Scholar]
- 30.Hardin JW. The sandwich estimate of variance. In: Advances in econometrics. Bingley: Emerald (MCB UP), 2003: 45–73. Available: https://www.emerald.com/insight/content/doi/10.1016/S0731-9053(03)17003-X/full/html [Google Scholar]
- 31.Xu S, Ross C, Raebel MA, et al. Use of stabilized inverse propensity scores as weights to directly estimate relative risk and its confidence intervals. Value Health 2010;13:273–7. 10.1111/j.1524-4733.2009.00671.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jourdain H, Hoisnard L, Sbidian E, et al. TNF-alpha inhibitors biosimilar use in France: a nationwide population-based study using the French national health data system. Sci Rep 2022;12:19569. 10.1038/s41598-022-24050-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alberto IRI, Alberto NRI, Ghosh AK, et al. The impact of commercial health datasets on medical research and health-care algorithms. Lancet Digit Health 2023;5:e288–94. 10.1016/S2589-7500(23)00025-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Desai RJ, Thaler KJ, Mahlknecht P, et al. Comparative risk of harm associated with the use of targeted immunomodulators: a systematic review. Arthritis Care Res (Hoboken) 2016;68:1078–88. 10.1002/acr.22815 [DOI] [PubMed] [Google Scholar]
- 35.Fernández-Ruiz M, Aguado JM. Risk of infection associated with anti-TNF-Α therapy. Expert Rev Anti Infect Ther 2018;16:939–56. 10.1080/14787210.2018.1544490 [DOI] [PubMed] [Google Scholar]
- 36.Mitoma H, Horiuchi T, Tsukamoto H, et al. Mechanisms for cytotoxic effects of anti-tumor necrosis factor agents on transmembrane tumor necrosis factor alpha-expressing cells: comparison among Infliximab, etanercept, and adalimumab. Arthritis Rheum 2008;58:1248–57. 10.1002/art.23447 [DOI] [PubMed] [Google Scholar]
- 37.Marotte H, Cimaz R. Etanercept – TNF receptor and Igg1 FC fusion protein: is it different from other TNF blockers? Expert Opin Biol Ther 2014;14:569–72. 10.1517/14712598.2014.896334 [DOI] [PubMed] [Google Scholar]
- 38.Scallon B, Cai A, Solowski N, et al. Binding and functional comparisons of two types of tumor necrosis factor antagonists. J Pharmacol Exp Ther 2002;301:418–26. 10.1124/jpet.301.2.418 [DOI] [PubMed] [Google Scholar]
- 39.Gossec L, Siebert S, Bergmans P, et al. Long-term effectiveness and persistence of ustekinumab and TNF inhibitors in patients with psoriatic arthritis: final 3-year results from the Psabio real-world study. Ann Rheum Dis 2023;82:496–506. 10.1136/ard-2022-222879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.López-Ferrer A, Laiz A, Puig L. The safety of ustekinumab for the treatment of psoriatic arthritis. Expert Opin Drug Saf 2017;16:733–42. 10.1080/14740338.2017.1323864 [DOI] [PubMed] [Google Scholar]
- 41.Benson JM, Peritt D, Scallon BJ, et al. Discovery and mechanism of ustekinumab: a human monoclonal antibody targeting Interleukin-12 and Interleukin-23 for treatment of immune-mediated disorders. MAbs 2011;3:535–45. 10.4161/mabs.3.6.17815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ríos-Navarro C, de Pablo C, Collado-Diaz V, et al. Differential effects of anti-TNF-Α and anti-IL-12/23 agents on human Leukocyte-endothelial cell interactions. Eur J Pharmacol 2015;765:355–65. 10.1016/j.ejphar.2015.08.054 [DOI] [PubMed] [Google Scholar]
- 43.Hsu DY, Gniadecki R. Patient adherence to biologic agents in psoriasis. Dermatology 2016;232:326–33. 10.1159/000444581 [DOI] [PubMed] [Google Scholar]
- 44.Austin PC. The performance of different propensity score methods for estimating marginal hazard ratios. Stat Med 2013;32:2837–49. 10.1002/sim.5705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Austin PC. The performance of different propensity-score methods for estimating differences in proportions (risk differences or absolute risk reductions) in observational studies. Statist Med 2010;29:2137–48. 10.1002/sim.3854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pina Vegas L, Hoisnard L, Bastard L, et al. Long-term persistence of second-line biologics in psoriatic arthritis patients with prior TNF inhibitor exposure: a nationwide cohort study from the French health insurance database (SNDS). RMD Open 2022;8:e002681. 10.1136/rmdopen-2022-002681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Desai RJ, Franklin JM. Alternative approaches for confounding adjustment in observational studies using weighting based on the propensity score: a primer for practitioners. BMJ 2019;367:l5657. 10.1136/bmj.l5657 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2023-003865supp001.pdf (295KB, pdf)
rmdopen-2023-003865supp002.pdf (47.9KB, pdf)
Data Availability Statement
Data are available upon reasonable request. All data relevant to the study are included in the article or uploaded as supplementary information.