Abstract
Objective
To assess the long-term efficacy and safety of oral saffron, a natural antioxidant, in treating mild/moderate age-related macular degeneration (AMD).
Methods and analysis
Open-label, extension trial of 93 adults (>50 years) with mild/moderate AMD and vision >20/70 Snellen equivalent in at least 1 eye. Exclusion criteria included confounding visual lesions or significant gastrointestinal disease impairing absorption.
Participants were given oral saffron supplementation (20 mg/day) for 12 months. Those already consuming Age-Related Eye Diseases Study (AREDS) supplements or equivalent maintained these.
Primary outcomes included changes in multifocal electroretinogram (mfERG) response density and latency, and changes in best-corrected visual acuity (BCVA). Secondary outcomes included safety outcomes, changes in mfERG and BCVA among participants on AREDS supplements and changes in microperimetry.
Results
At 12 months, mean mfERG response density was significantly higher in rings 1, 2 and overall (p<0.001 for all) but not in rings 3–6, and there was no difference in response between those taking AREDS supplements and those not (p>0.05). Mean mfERG latency was not significantly different in any of rings 1–6 or overall (p>0.05 for all), again with no difference between those taking AREDS supplements or not (p>0.05). Mean BCVA was 1.6 letters worse (p<0.05) with no difference between those on AREDS supplements or not, and this may have been related to cataract progression. No saffron-related serious adverse events were detected.
Conclusion
Saffron supplementation modestly improved mfERG responses in participants with AMD, including those using AREDS supplements. Given the chronic nature of AMD, longer-term supplementation may produce greater benefits.
Keywords: Clinical Trial, Macula, Degeneration
WHAT IS ALREADY KNOWN ON THIS TOPIC
Saffron has previously been shown to have short-term efficacy in slowing the progression of mild/moderate age-related macular degeneration (AMD).
WHAT THIS STUDY ADDS
Saffron is associated with preservation of multifocal electroretinogram responses in patients with mild/moderate AMD of a prolonged period of therapy, with minimal side effects.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
This study guides further research into dietary supplements and dietary modifications to reduce vision loss and progression of AMD, and the design of future research studies.
Background
Despite age-related macular degeneration (AMD) remaining a leading cause of vision loss,1 therapies for the more common mild/moderate stages of AMD remain few.2 In these stages of the disease, vision loss is relatively limited, and many patients retained good visual function, including meeting driving criteria, whereas advanced disease is associated with significant visual loss and associated loss of function.3 Given that it is the earlier stages of AMD where intervention may be most successful in preventing further progression and vision loss, there is a growing recognition of the need for therapies for mild/moderate AMD.
Of the range of interventions trialled for the early and intermediate stages of AMD, successful therapies are still essentially limited to the use of Age-Related Eye Disease Study 2 (AREDS2) supplements, which contain a mix of vitamins, nutrients and carotenoids, namely lutein and zeaxanthin.4 Previous studies of other supplements for mild/moderate AMD, such as omega-3 fatty acids, did not show these to be of benefit,4 and currently, the AREDS2 supplements remain the only well-recognised treatment for the early/intermediate stages of AMD, although the evidence for their benefit in intermediate AMD derives from post hoc analysis of the AREDS trial. Although other therapies exist for the late stages of AMD, including intravitreal therapy of differing agents for both neovascular AMD (nAMD) and geographical atrophy, these therapies generally aim to limit already established damage and vision loss.5 6 Additionally, they are burdensome on patients and healthcare systems alike, are more invasive than oral nutritional supplementation and have specific risks associated with their use.7
It has previously been shown that supplementation with the spice saffron (crocus sativus) may preserve retinal function in the early/intermediate stages of AMD, potentially due to the high concentration of carotenoids found within saffron.8 9 Although a potentially promising therapy for AMD, little long-term data exist on the efficacy of saffron as a therapeutic in this role. As the pathogenesis of AMD remains under investigation, the exact effect of saffron in AMD is still unclear. However, proposed disease mechanisms include autoimmune damage to the retina through mechanisms such as complement dysfunction and oxidative stress.10 These can result in damage to the retinal pigment epithelium as well as the choriocapillaris, causing further strain on the photoreceptors eventually cell loss.10 As saffron is a powerful antioxidant, it is possible that it acts to reduce oxidative damage and preserve retinal function through this action, and it is additionally thought to have some effect in downregulating inflammatory cytokines, potentially also reducing autoimmune-mediated damage.11 12
Given the need for additional treatments for mild/moderate AMD, and the potential benefits of saffron in this setting, we investigated the role of longer-term saffron supplementation as a therapy for mild/moderate AMD.
Materials and Methods
A prospective, randomised, placebo-controlled, double-blind cross-over trial (Registered on Australian and New Zealand Clinical Trials Registry, 9 July 2012, ACTRN12612000729820) was conducted on 100 participants attending a single tertiary retinal clinic between January 2013 and March 2015. All participants who successfully completed this earlier cross-over trial were then invited to join this 1-year, open-label, single-arm extension trial following completion of that study, which concluded in May 2016. Of the original cohort, 93 participants enrolled in the extension trial.
Inclusion/exclusion criteria
All participants underwent baseline dilated ophthalmic examination and general medical review to confirm the presence of AMD and to assess eligibility under the exclusion/inclusion criteria listed below. Inclusion criteria were as follows: (a) age greater than 50 years, (b) moderate severity AMD (defined as AREDS grade 2 or 3) in at least one eye,13 (c) best-corrected visual acuity (BCVA) greater than 55 Early Treatment of Diabetic Retinopathy Study (ETDRS) letters (approximately 20/70 Snellen equivalent) in the eye(s) meeting criteria (a) and (b), and (d) ability to provide written consent.5 Exclusion criteria were as follows: (a) the presence of any ocular lesion in the study eye(s) that might confound results, including nAMD, proliferative diabetic retinopathy, macular hole or epiretinal membrane, prior macula-off retinal detachment, uncontrolled glaucoma, significant corneal or lenticular opacities or active uveitis, (b) prior macular laser therapy for AMD or other retinal disorders, (c) prior or current intravitreal pharmacotherapy and (d) gastric or hepatic disorders altering either absorption or metabolism of orally administered saffron, such as prior intestinal resection, inflammatory bowel disease or liver cirrhosis.8 In participants in whom both eyes met eligibility criteria, both eyes were included in the analysis, with 153 eyes meeting inclusion/exclusion criteria.
Age-related macular degeneration
The diagnosis of AMD was confirmed by both dilated retinal examination by a retinal specialist (AAC) and dilated retinal fundus photography (Zeiss Visucam NM/FA, Zeiss Industries, Dublin). Macular centred fundus photos (45°) were graded according to the AREDS trial criteria.13 All participants also underwent baseline spectral domain optical coherence tomography (SD-OCT) and fundus autofluorescence (FAF). Where necessary, additional investigations, including fundus fluorescein angiography and indocyanine green angiography, were undertaken to evaluate potential exclusion criteria such as nAMD.
Study protocol
The initial cross-over study consisted of 100 participants with mild/moderate AMD who underwent a 6-month, double-blinded, placebo-controlled cross-over study of 20 mg saffron versus placebo (3 months of either saffron or placebo followed by cross-over to the other arm of the study). All participants met the inclusion criteria detailed above, and baseline investigations for that study included a dilated fundus examination with lens grading, SD-OCT, FAF, intraocular pressure (IOP) and BCVA measurement, multifocal electroretinogram (mfERG), microperimetry (MP) and a single once off full field electroretinogram to exclude occult retinal diseases.8 14
All participants were offered saffron supplementation. Participants were provided oral capsules containing 20 mg saffron and instructed to consume one capsule daily for the duration of the study. This dosage was chosen based on prior small pilot studies that had suggested that this dose was sufficient to have a neuroprotective effect on the retina.9 Compliance was evaluated via self-reporting at interview at scheduled regular assessments, which may have impacted the accuracy of the reported compliance. In the case of missed doses, these were instructed not to be retaken or ‘double dosed’, but to instead be recorded as missed or absent and regular daily dosing continued from the next day. No participant reported <80% compliance with dosing throughout the study.
All participants underwent 3-monthly assessment for a period of 12 months. At each visit, complete ophthalmic examination was undertaken, including (a) IOP monitoring via Goldmann applanation tonometry, (b) adverse event monitoring, (c) standardised BCVA assessment in ETDRS letters and (d) colour fundus photography. As mentioned above, at each visit, compliance with supplementation use was assessed by interview.
Additionally, at the baseline and 12 months visits, participants also underwent (MP, SD-OCT, FAF, lens grading (cataract grading) using AREDS lens assessment criteria15 and mfERG assessment. Electroretinography and perimetric examinations were performed prior to any investigations that may have affected photoreceptor response, such as fundus examination, colour photography, OCT, FAF or fundus photography.
Autofluorescence and OCT
SD-OCT was conducted using a 19-line, 1024 A-scans per line scan via a Heidelberg Spectalis system (Heidelberg Industries, Heidelberg, Germany), and inbuilt Heidelberg licensed software with eye tracking and image recognition (Tru-Track and AutoRescan respectively) was employed to ensure continuity of the scan location. All scans were reviewed, recentred and resegmented as necessary by two independent graders, with any disputes adjudicated by a third, independent grader. Central macular thickness was measured via SD-OCT and was defined as the distance between the Internal Limiting Membrane and Bruch’s Membrane within the central 1 mm of the ETDRS subfield.
FAF was conducted using a Heidelberg Spectralis FAF acquisition module, and hypoautofluorescence area (a measure of retinal pigment epithelial atrophy) was measured using FAF images by two independent graders, with any disputes >15% in area being adjudicated by a third, independent grader.
Microperimetry
MP was undertaken with a Macular Assessment Integrity Analyser (MAIA, CenterVue, Padova, Italy). The MAIA uses a scanning light ophthalmoscope to perform fundus tracking, using the whole fundus as a reference. Participants were tested using an automated macular assessment protocol. Fixation was ensured via the use of a red circle target of 1° diameter, and stimuli were presented in a 4–2 strategy across an array of 37 points at 0°, 1°, 3° and 5° from central fixation. Throughout the test, Goldmann III stimuli are displayed across a dynamic range of 36 dB, with a background luminescence of 1.27 cd×cm2 .
All participants were dilated/redilated after earlier mfERG with 1% tropicamide/2.5% phenylephrine prior to testing, and all received a standardised set of instructions regarding test performance prior to test commencement. Tests were conducted in a standardised, non-illuminated room, prior to any tests that may have affected photoreceptor response (such as fundus photos). Participants with false positive responses of >25% were retested, and if these responses persisted, were excluded from analysis.
Results were grouped into concentric rings at 1°, 3° and 5° from central fixation (rings 1–3, respectively) and analysed as the average sensitivity of each of these rings, as well as the overall average macular sensitivity.
Multifocal electroretinography
mfERG is an objective test of retinal function that measures macular function and was acquired using a VERIS Science (Veris) device following International Society for Electroretinogaphy in Vision (ISCEV) guidelines.16 All participants’ pupils were maximally dilated to at least 7 mm diameter using 0.5% tropicamide and/or 2.5% phenylephrine, with the cornea anaesthetised with 0.4% oxybupivicaine hydrochloride. The mfERG data were acquired using a gold foil electrode. Test stimuli consisted of 103 scaled hexagons presented in a pseudorandom fashion at a rate of 75 Hz, using a luminescence of 200 cd for the white hexagons and 1.0 cd for black hexagons. Fixation was ensured using a fixation device, and recordings that contained blinks or other artefacts were not saved and were rerecorded. Signals obtained were band pass filtered from 10 to 100 Hz and amplified 100 000 times. Noise-contaminated segments were rejected and repeated.
The mfERG responses for the hexagons across the retina were separated into six concentric rings (rings 1–6) for data analysis. The latencies and average response densities of the six concentric rings were measured (figure 1), with greater response density and lower latency indicative of better retinal function. The rings of 1–6 correspond to the foveola at 1°, 4°, 8°, 12°, 17° and 22°, respectively, according to the eccentricity, with the fixation target at the central 0.75°. The response amplitudes in each ring were measured between the first negative trough (N1) and the first positive peak (P1), yielding the N1P1 response densities (amplitudes per unit retinal area in nV/deg2). The P1 peak latencies (ms) of the positive waveform were also measured.
Figure 1.
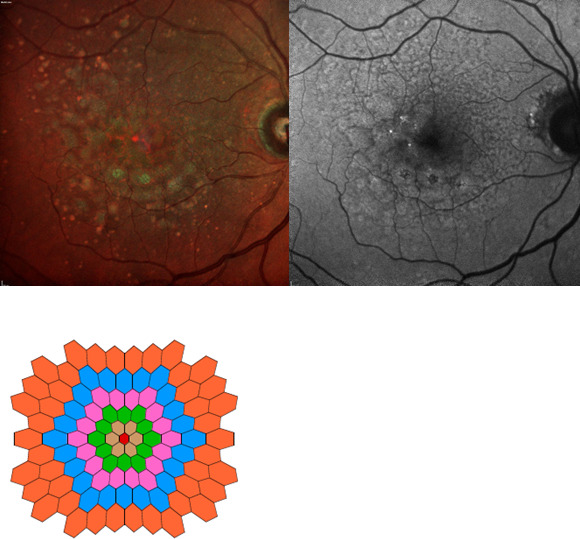
Imaging and functional output from a trial participant. Clockwise from top left: pseudocolour fundus imaging, autofluorescence fundus imaging, multifocal electroretinogram output.
All participants had previously undergone a full field ERG under ISCEV conditions14 to exclude the presence of potential confounding retinal degenerative diseases that may have mimicked AMD.
Statistical analysis
The primary outcomes were mean change in mfERG N1P1 response density, mean change in BCVA and mean change in mfERG latency. Secondary outcomes included change in individual ring mfERG N1P1 response density and latency, mean change in MP ring response, and safety of saffron longer-term. Incidence of serious adverse events (SAEs) was recorded.
Additional exploratory analyses were also conducted to explore the effect of saffron on those participants already consuming other supplementation therapy, and the effect of baseline atrophy on response observed. Participants on AREDS supplementation (current best-practice treatment at trial commencement) were analysed to assess the efficacy of saffron in this subgroup.
Of the 93 participants enrolled, 85 completed the full year of the trial (figure 2). Two participants passed away during the trial, one withdrew soon after enrolment due to travel difficulties, and five failed to attend for final follow-up despite repeated reminders and efforts at communication. All participants enrolled in the study were included in the safety data. Efficacy analysis was conducted on a modified intention to treat basis, however, participants who developed confounding visual pathologies (nAMD) during the trial had the eye(s) thus affected excluded from visual outcome analysis. There were 5 cases of nAMD development in their only eligible eye during the 12-month period, leaving 80 participants for with complete visual outcome data.
Figure 2.
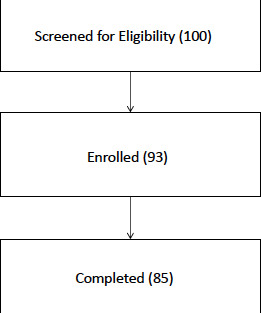
Flow diagram of participants through the trial.
Given the hierarchical nature of data (two eyes for some patients, multiple time points and six rings for mfERG results) a linear mixed effects model was used to account for within patient, eye and ring correlations using the lme and lmer functions in R packages nlme and lme4, respectively. To combine mfERG results over all rings, a linear mixed effects model was fitted on the mfERG logarithm with a quadratic term for reduction of log(mfERG) by ring. The choice for taking the logarithm of mfERG results, and for using a quadratic term for reduction by ring, was made to ensure assumptions on residual values were not violated. The fixed effects were time point only for BCVA and time point, ring and ring squared for log (mfERG) and for latency. Random effects were chosen to be consistent with the parent study and were intercept only for BCVA and individual mfERG ring analysis; and intercept, ring and ring squared terms for mfERG. The effects of AREDS supplementation were assessed with additional fixed effects.
All analyses were conducted using the software R: A Language and Environment for Statistical Computing V.3.6.3 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Baseline characteristics of trial participants are shown in table 1. Briefly, 37% of participants were male, with a mean age of 74.6 years at enrolment, 75% of participants were consuming AREDS supplements and 66% of eyes were phakic.
Table 1.
Baseline characteristics of trial participants
| Characteristic | No |
| Male (%) | 34 (37) |
| Age (years) | 74.6 |
| Age range (years) | 51.0–91.2 |
| BCVA (ETDRS letters and Snellen equivalent) | 82.7 (20/25+) |
| Smoking status (non-smoker: ex-smoker: current smoker%) | 54:44:2 |
| Lens status (%phakic:%pseudophakic) | 66:34 |
| AREDS supplement use (%) | 75 |
| Not using AREDS supplements (%) | 25 |
| Lutein supplement use (%) | 41 |
| Not using lutein supplements (%) Central macular thickness (µm) Hypoautofluorescent area (mm2) |
59 290.5 0.21 |
AREDS, Age-Related Eye Disease Study; BCVA, best-corrected visual acuity; ETDRS, Early Treatment of Diabetic Retinopathy Study.
Vision
Mean BCVA declined by 1.59 letters at 12 months (p<0.001; 95% CI 0.89 to 2.30). There was no difference in change in BCVA between those consuming AREDS supplements and those not (p>0.05). Progression of cataract may have affected the visual acuity outcomes observed.
Multifocal electroretinography
There was a significant improvement in central rings (rings 1 and 2) mean response density measured in nanovolts/degree squared at 12 months (p<0.01 for both), however, no significant change was noted in rings 3–6 (p>0.05 for each ring of rings 3–6; table 2). Pooled analysis of all rings showed a significant increase in response density at 12 months (8.7% increase; p<0.001). There was no significant difference in response between those consuming AREDS supplements and those not (p>0.05).
Table 2.
Multifocal electroretinogram (mfERG) results seen with saffron supplementation
| mfERG response | 12-month change | P value |
| Ring 1 | ||
| Response density* | 6.05 | <0.001 |
| Latency† | 0.15 | 0.75 |
| Ring 2 | ||
| Response density* | 2.38 | 0.0017 |
| Latency† | 0.03 | 0.88 |
| Ring 3 | ||
| Response density* | 0.89 | 0.066 |
| Latency† | −0.15 | 0.53 |
| Ring 4 | ||
| Response density* | 0.51 | 0.18 |
| Latency† | 0.05 | 0.77 |
| Ring 5 | ||
| Response density* | −0.13 | 0.77 |
| Latency† | 0.11 | 0.56 |
| Ring 6 | ||
| Response density* | 0.51 | 0.19 |
| Latency† | 0.08 | 0.69 |
| Pooled‡ | ||
| Response density* | 8.7% | <0.001 |
| Latency† | 0.038 | 0.66 |
*Nanovolts/degree squared.
†Milliseconds.
‡Proportional Increase in mfERG response compared with baseline.
No significant change in mean latency measured in milliseconds in any ring (p>0.05 for all rings, table 2), or for pooled analysis of all rings (p>0.05) was detected at 12 months. There was also no evidence for a difference in pooled change in latency for those consuming AREDS supplements and those not (p>0.05).
Microperimetry
Mean MP overall threshold declined by 0.43 dB across the duration of the study (p=0.001), and a decline was observed in all three rings (p<0.001 for all).
Hypoautofluorscence area
There was a non-statistically significant increase in mean hypoautofluorscence area by 0.04 mm2 on average across the 12 months (p>0.05).
Adverse events
There were a total of 14 SAEs during the trial, including 2 deaths (table 3). None of the SAEs were thought to be linked to the use of saffron supplementation. IOP did not significantly change across the course of the trial (p>0.05). Cataract surgery occurred in two cases within 3 months of trial completion, and there was a non-statistically significant trend towards worsening cataract across the trial duration (p>0.05 for worsening of all cataract grades combined).
Table 3.
Serious adverse events in Saffron supplementation for mild/moderate age-related macular degeneration
| Adverse event | No of events during trial |
| Death (nalignancy) | 1 |
| Death (pneumonia) | 1 |
| Aortic valve replacement | 1 |
| Fall | 1 |
| Neovascular AMD | 7 |
| SCC requiring excision | 1 |
| Idiopathic pancreatitis | 1 |
| Bowel cancer | 1 |
AMD, age-related macular degeneration; SCC, squamous cell carcinoma.
Discussion
Saffron supplementation is associated with the preservation of mfERG responses in patients with early stages of AMD, and this effect is maintained over a longer period of treatment compared with the 3 months of supplementation received in the initial cross-over trial. It is interesting to note that there was a more marked effect on response density, which is thought to represent photoreceptor survival, than on latency, which is hypothesised to be a marker of photoreceptor stress. This is of interest given that the initial 3-month cross-over trial showed more marked changes in latency than response density.8 It has been suggested that in AMD there is a pool of ‘at-risk’ photoreceptors that are diseased but not yet dead. Potentially in the short term, use of saffron stabilises these, leading to survival of these photoreceptors over a longer period of time, potentially explaining these findings, although further research is needed to investigate this hypothesis. The association between saffron usage and macular mfERG function was observed both in the participants receiving AREDS supplementation and those who did not, with no difference in response observed between those two groups. This suggests that these effects are independent of other supplement use and may offer alternate means and pathways of preserving vision in intermediate AMD then those observed with current therapies.
Previous studies have shown that mfERG response density decreases over time in mild-moderate AMD, with the changes seen earliest in the central ring (ring 1).17 Additionally, loss of response density in ring 1 has also been associated with visual decline in mild-moderate AMD,18 potentially as a result of this representing the area closest to the fovea and hence most responsible for central clear vision. This, therefore, offers the possibility of preservation of function in this region in the early/intermediate stages of AMD. Although the exact mechanisms by which saffron may be associated with mfERG function are not known, saffron and its constituents have previously been shown to have both antioxidant and anti-inflammatory properties, including downregulation of autoimmune cytokines.11 12 Given that current theories of AMD pathogenesis heavily implicate autoimmune-mediated inflammation, including oxidative damage, as a probable cause of AMD,10 it seems possible that association between saffron usage and mfERG responses seen in this study may relate to a reduction in auto-immune related injury and oxidative stress.
The reasons for the overall decline in visual acuity despite the mfERG suggesting preservation of visual function are not entirely clear, although there are a few potential explanations. Cataract progression was significant enough to warrant surgery in two eyes, and it is possible that cataract contributed somewhat to visual decline, although the overall rate of stepwise progression was not statistically significant. However, given that cataract is known to progress slowly and approximately two-thirds of the cohort were phakic, even a relatively minor, non-clinically significant progression in cataract may account for a relatively small visual decline of 1–2 letters, and it is, therefore, possible that cataract progression may have affected the observed visual acuity outcomes. Additionally, previous long-term follow-up of the AREDS cohort and other studies have shown a rate of approximately 0–1 ETDRS letters decline in vision in the first year for participants who did not develop advanced disease, which is similar to the findings in our cohort.18 19 It is, therefore, possible that despite the preserved mfERG responses, a degree of AMD progression was responsible for this visual change, although the magnitude was below the thresholds generally considered to be a clinically significant decline (ie, more than five ETDRS letters loss). The combination of mild cataract progression and mild AMD progression, both of which would be expected with time, may have also had a cumulative effect to produce a visual decline in the order of the 1–2 letters seen, without either being individually statistically significant. Similarly, it is not obvious why there was a difference in the response seen between the mfERG and MP, with one showing an improvement in results and the other a decline. Previous studies have suggested that mfERG and MP results may not correlate, particularly in intermediate AMD, and this may also explain the differing results seen here.20
There are some limitations of this study. The single-arm nature of this trial means that it is not fully possible to assess the effect of saffron compared with other therapies, such as AREDS or AREDS2 supplements alone (although the benefit of these supplements in intermediate AMD was seen in post hoc analysis of the AREDS trial rather than as a primary outcome), and additionally means it is not possible to include a placebo-control arm either participants were of limited demographic diversity, being recruited from a single city in Australia, and this may limit the generalisability of these findings to wider populations. The improvement noted in mfERG retinal function is of uncertain clinical significance, and without a control arm it is not possible to fully determine if there would have been a greater decline in BCVA had participants not taken saffron. Additionally, even longer follow-up would allow for better understanding of the longer-term effects of saffron in this setting, particularly given the slow, chronic nature of mild/moderate AMD. Patients who developed nAMD were excluded due to the potential confounding effect of treatment for this condition, however, this may have affected the visual outcomes seen.
Conclusions
Longer-term saffron supplementation was associated with preserved mfERG responses in patients with mild/moderate AMD, without significant safety concerns. Although not able to reverse existing damage, saffron was associated with preserved mfERG function across a prolonged period of supplementation. Combined with the findings of earlier cross-over placebo controlled trials, these results suggest that saffron supplementation may be useful in preserving retinal function in those with mild/moderate AMD, and further trials to evaluate the efficacy of prolonged saffron supplementation in these patients are warranted. These results were somewhat limited by the lack of a control group and the duration of follow-up, with a longer-follow up potentially offering great insights into to role of saffron in mild/moderate AMD given the slowly progressive nature of the disease. Future studies, particularly controlled, larger and longer-term follow-up studies, would be useful to further investigate the long-term efficacy of saffron for the treatment of mild/moderate AMD.
Footnotes
Contributors: GKB contributed to data collection, data analysis and manuscript preparation, and acted as guarantor. JG contributed to study design, data interpretation and manuscript preparation. PJM contributed to study design and manuscript preparation. TH contributed to data collection and manuscript preparation. TES contributed to data analysis and manuscript preparation. EC contributed to data collection and manuscript preparation. AAC contributed to study design, data collection and manuscript preparation. All authors read and approved the final manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: AAC has previously acted as a consultant for Novartis, Roche, Zeiss, Bayer and Alcon. No other authors have any interests to declare.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available on reasonable request. Deidentified data used or analysed are available from the corresponding author on reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
All participants provided written consent prior to their enrolment for both participation in the trial and for future publication of results, and Institutional Ethical Review was obtained via the prior to study commencement (HREC/12/RPAH/20), and all procedures and investigations were performed in accordance with relevant guidelines and regulations. This research adhered to the tenets of the Declaration of Helsinki.
References
- 1.Klein R, Lee KE, Gangnon RE, et al. Incidence of visual impairment over a 20-year period: the beaver dam eye study. Ophthalmology 2013;120:1210–9. 10.1016/j.ophtha.2012.11.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller JW. Developing therapies for age-related macular degeneration: the art and science of problem-solving: the 2018 Charles L. Ophthalmol Retina 2019;3:900–9. 10.1016/j.oret.2019.07.015 [DOI] [PubMed] [Google Scholar]
- 3.Brown JC, Goldstein JE, Chan TL, et al. Characterizing functional complaints in patients seeking outpatient low-vision services in the United States. Ophthalmology 2014;121:1655–62.S0161-6420(14)00198-5. 10.1016/j.ophtha.2014.02.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Age-Related Eye Disease Study 2 Research Group . Lutein + Zeaxanthin and Omega-3 fatty acids for age-related macular degeneration: the age-related eye disease study 2 (Areds2) randomized clinical trial. JAMA 2013;309:2005–15. 10.1001/jama.2013.4997 [DOI] [PubMed] [Google Scholar]
- 5.Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for Neovascular age-related macular degeneration. N Engl J Med 2006;355:1419–31. 10.1056/NEJMoa054481 [DOI] [PubMed] [Google Scholar]
- 6.Heir JS, Lad EM, et al. n.d. Pegcetoacoplan for the treatment of geographic atrophy secondary to age-related macular degeneration (OAKS and DERBY): two Multicentre, randomised, double-masked, sham-controlled, phase 3 trials. Lancet;2023:1434–48. [DOI] [PubMed] [Google Scholar]
- 7.Reitan G, Kjellevold Haugen IB, Andersen K, et al. Through the eyes of patients: understanding treatment burden of intravitreal anti-VEGF injections for nAMD patients in Norway. Clin Ophthalmol 2023;17:1465–74. 10.2147/OPTH.S409103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Broadhead GK, Grigg JR, McCluskey P, et al. Saffron therapy for the treatment of mild/moderate age-related macular degeneration: a randomised clinical trial. Graefes Arch Clin Exp Ophthalmol 2019;257:31–40. 10.1007/s00417-018-4163-x [DOI] [PubMed] [Google Scholar]
- 9.Falsini B, Piccardi M, Minnella A, et al. Influence of Saffron supplementation on retinal flicker sensitivity in early age-related macular degeneration. Invest Ophthalmol Vis Sci 2010;51:6118–24. 10.1167/iovs.09-4995 [DOI] [PubMed] [Google Scholar]
- 10.Fernandes AR, Zielińska A, Sanchez-Lopez E, et al. Exudative versus Nonexudative age-related macular degeneration: pathophysiology and treatment options. Int J Mol Sci 2022;23:2592. 10.3390/ijms23052592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Broadhead GK, Chang A, Grigg J, et al. Efficacy and safety of Saffron supplementation: Current clinical findings. Crit Rev Food Sci Nutr 2016;56:2767–76. 10.1080/10408398.2013.879467 [DOI] [PubMed] [Google Scholar]
- 12.Butnariu M, Quispe C, Herrera-Bravo J, et al. The pharmacological activities of Crocus sativus L.:A review on the mechanisms and therapeutic Opportunties of its Phytoconstituents. Oxid Med Cell Longev 2022;2022:8214821. 10.1155/2022/8214821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Age-Related Eye Disease Study Research Group . A simplified severity scale for age-related macular degeneration: AREDS report number 18. Arch Ophthalmol 2005;123:570–4. 10.1001/archopht.123.11.1484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCulloch DL, Marmor MF, Brigell MG, et al. ISCEV standard for full-field clinical electroretinography. Doc Ophthalmol 2015;130:1–12. 10.1007/s10633-014-9473-7 [DOI] [PubMed] [Google Scholar]
- 15.Age-Related Eye Disease Study Research Group . The age-related eye disease study (AREDS) system for classifying cataracts from photographs: AREDS report No.4. Am J Ophthalmol 2001;131:167–75. 10.1016/s0002-9394(00)00732-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hood DC, Bach M, Brigell M, et al. ISCEV standard for clinical multifocal electroretinography (mfERG). Doc Ophthalmol 2012;124:1–13. 10.1007/s10633-011-9296-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.González-García E, Vilela C, Navea A, et al. Electrophysiological and clinical tests in dry age-related macular degeneration follow-up: differences between mfERG and OCT. Doc Ophthalmol 2016;133:31–9. 10.1007/s10633-016-9545-y [DOI] [PubMed] [Google Scholar]
- 18.Ambrosio L, Ambrosio G, Nicoletti G, et al. The value of multifocal electroretinography to predict progressive visual loss in early AMD. Doc Ophthalmol 2015;131:125–35. 10.1007/s10633-015-9507-9 [DOI] [PubMed] [Google Scholar]
- 19.Chew EY, Clemons TE, Agrón E, et al. Ten-year follow-up of age-related macular degeneration in the age-related eye disease study [AREDS report no.36]. JAMA Ophthalmol 2014;132:272. 10.1001/jamaophthalmol.2013.6636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu Z, Ayton LN, Guymer RH, et al. Comparison between multifocal electroretinography and Microperimetry in age-related macular degeneration. Invest Ophthalmol Vis Sci 2014;55:6431–9. 10.1167/iovs.14-14407 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available on reasonable request. Deidentified data used or analysed are available from the corresponding author on reasonable request.


