Abstract
Background
The rate of pulmonary tuberculosis (TB) recurrence is substantial. Identifying risk factors can support the development of prevention strategies.
Methods
We retrieved studies published between 1 January 1980 and 31 December 2022 that assessed factors associated with undifferentiated TB recurrence, relapse or reinfection. For factors reported in at least four studies, we performed random-effects meta-analysis to estimate a pooled relative risk (RR). We assessed heterogeneity, risk of publication bias and certainty of evidence.
Results
We included 85 studies in the review; 81 documented risk factors for undifferentiated recurrence, 17 for relapse and 10 for reinfection. The scope for meta-analyses was limited given the wide variety of factors studied, inconsistency in control for confounding and the fact that only few studies employed molecular genotyping. Factors that significantly contributed to moderately or strongly increased pooled risk and scored at least moderate certainty of evidence were: for undifferentiated recurrence, multidrug resistance (MDR) (RR 3.49; 95% CI 1.86 to 6.53) and fixed-dose combination TB drugs (RR 2.29; 95% CI 1.10 to 4.75) in the previous episode; for relapse, none; and for reinfection, HIV infection (RR 4.65; 95% CI 1.71 to 12.65). Low adherence to treatment increased the pooled risk of recurrence 3.3-fold (95% CI 2.37 to 4.62), but the certainty of evidence was weak.
Conclusion
This review emphasises the need for standardising methods for TB recurrence research. Actively pursuing MDR prevention, facilitating retention in treatment and providing integrated care for patients with HIV could curb recurrence rates. The use of fixed-dose combinations of TB drugs under field conditions merits further attention.
PROSPERO registration number
CRD42018077867.
Keywords: Tuberculosis, Respiratory Infection, Clinical Epidemiology
WHAT IS ALREADY KNOWN ON THIS TOPIC
Recurrence of pulmonary tuberculosis (TB) has been linked to sociodemographic and behavioural patient factors, clinical and treatment characteristics of the previous episode, and presence of comorbidities. Existing reviews on individual-level risk factors for recurrence do not provide pooled estimates of their effects, or do not distinguish between relapse and reinfection, or look at only one specific factor.
WHAT THIS STUDY ADDS
Our review shows that methodological divergence of original research studies limits the scope for meta-analyses. We found, with at least moderate certainty of evidence, that the following factors are associated with a moderate or strong increase in risk: for undifferentiated recurrence, multidrug resistance and fixed-dose combination TB drugs in the previous episode; for relapse, none; and for reinfection, HIV comorbidity. We also observed, but with weak evidence, that low adherence to treatment is a moderate risk factor for recurrence.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Identification of risk factors should provide a basis for designing preventive strategies that will reduce the recurrence rate of TB. In addition, it seems advisable to carefully reassess the use of fixed-dose TB drug combinations.
Introduction
In 2020, an estimated 155 million people had survived tuberculosis (TB).1 Life after TB may encompass chronic complications such as bronchiectasis and obstructive pulmonary disease, socioeconomic hardship due to catastrophic health expenditure2 and psychosocial problems potentially aggravated by stigma.3–6 Mortality in people previously treated for TB is three times higher than in the general population, which is partially due to recurrent TB, a new TB episode after a successfully treated one.7
Recurrence can result from an exogenous new infection or from relapse through endogenous reactivation of the first infection.8 9 In high TB incidence settings, the recurrence rate has been estimated to be 4.10 (95% CI 2.67 to 6.28) per 100 person-years among patients followed-up for on average 2.3 years after cure.10 The probability of recurrent TB has been linked to suboptimal treatment (irregular medication intake, use of regimens with low bactericidal potency, inadequate treatment duration or poor dosing and failure to adapt treatment to pre-existing resistance),11 12 to individual vulnerability resulting from immune status (such as in HIV infection)13 and to the probability of exposure to the agent (for reinfections), which depends on the local TB burden, transmission patterns, contact networks, and living and working conditions.14
Identifying patients with TB at high risk for recurrent TB supports the design of prevention strategies such as selective and close follow-up to ensure early detection and treatment. Few studies have systematically reviewed risk factors for recurrent TB and their mechanisms. Lambert et al8 found that the frequency of reinfection varied widely (ranging from 0% to 100% of the cases of recurrence) but only found one study that identify HIV infection as a significant risk factor for reinfection. Panjabi et al15 identified associations of smoking, poor treatment adherence, residual lung cavitation, large area of affected lung tissue, positive sputum culture at 2 months of treatment and HIV infection with undifferentiated TB recurrences, but they did not provide pooled estimates of their strength.
Systematic reviews from the last decade have looked into the role of specific factors: HIV in Africa,16 diabetes mellitus17 18 and duration and type of treatment.19 Research on risk factors for recurrences differentiated by genotyping has arisen lately. A review20 that included studies which used genotyping methods extracted a restricted set of a priori defined factors and analysed the likelihood for relapse versus reinfection given their presence, but not the relative risks (RRs) for these adverse outcomes associated with the presence versus absence of the factors. An individual patient meta-analysis of three trials21 found that cavitary disease and smear positivity at 2 months of treatment were the best markers for relapse in patients treated with a standard 6-month regimen.
We follow up here on our previous review10 that looked, at an ecological level, into environmental and study factors that were determinants of the incidence of recurrent TB and the proportion of reinfections and relapses. The present systematic review and meta-analysis aims to summaries the literature on patient-level factors associated with recurrence, relapse and reinfection.
Methods
Search strategy and selection process
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses checklist22 (online supplemental appendix 1) and registered the review protocol via the PROSPERO platform (CRD42018077867). We searched MEDLINE/PubMed, Cochrane, LILACS and SciELO for observational studies and clinical trials assessing risk factors for TB recurrences, reinfections or relapses, published between 1 January 1980 and 31 December 2022 in English, French or Spanish. The full search strategy is detailed in online supplemental appendix 2. In summary, for all databases, key search terms included variations on the concept “tuberculosis AND (recurrence OR reinfection OR relapse OR reactivation)”and for Medline/PubMed we used, additionally, related medical subject headings (MeSH) terms. We also reviewed references of included articles.
bmjresp-2023-002281supp001.pdf (1.5MB, pdf)
We checked for duplicates using Covidence systematic review software, Veritas Health Innovation (available at www.covidence.org) and manually. We included studies reporting, or providing data that allowed us to derive, estimates of the strength of association between candidate risk factors (exposure) and recurrent TB, relapses or reinfections (outcome). We defined recurrent TB as a new TB diagnosis following a successfully treated pulmonary TB episode, according to WHO reporting framework definition23; relapses and reinfections were recurrent episodes differentiated by means of genotyping techniques comparing the initial and recurrent TB strain in the primary studies. Three authors (VV, JC-S and SR) independently screened titles and abstracts, reviewed full texts and extracted data. Uncertainties on study inclusion and data extraction were solved by consensus, or by discussion with a fourth author (LO).
Data collection
We extracted data on general study characteristics, methods of ascertainment of exposure factors and of recurrent episodes, and type and length of follow-up. For studies differentiating between reinfection and relapse, we extracted genotyping methods used and the percentage of DNA sample availability to conduct genotyping for both episodes. We extracted unadjusted and adjusted estimates of strength of association (rate, risk, ORs and HRs) with CIs as reported by the authors. In case a multivariable analysis was done, we also extracted the set of variables included in the final model. If no association estimates were reported for a factor we extracted, when provided, data to calculate the corresponding association estimate. We calculated the estimates based on the raw data reported in the studies using the epiR package in Rstudio V.1.4.1106. In case of zero events, we added 0.5 to all cells in the 2×2 table. For factors related to treatment regimen characteristics, we extracted data from clinical trials that included TB recurrence as an outcome.
Study risk of bias assessment
We appraised the quality of the studies using the Quality In Prognostic Factor Studies (QUIPS) tool24 which includes the domains: study characteristics, study attrition, factor measurement, outcome measurement, adjustment for other factors, and statistical analysis and reporting. Full criteria are described in online supplemental appendix 3. The assessment was performed independently by two reviewers (VV, SR and JC-S in pairs) and discrepancies were solved by discussion with a fourth author (LO). We also obtained an overall judgement. Studies were classified as good quality if they had low risk of bias for all domains, poor quality if they had high risk of bias in at least one domain and fair quality otherwise.
Data analysis
We grouped the extracted factors in (1) sociodemographic characteristics, (2) behavioural characteristics of the patient, (3) clinical characteristics of the previous TB episode, (4) treatment characteristics of the previous TB episode and (5) comorbidities. To be included in the quantitative pooled estimate synthesis, a factor had to be reported in at least four studies.
We performed meta-analyses of association estimates of factors related with recurrent TB, relapse and reinfection. We obtained the pooled estimates using random-effects approaches (DerSimonian and Laird method) available in the meta package via the command ‘metagen’ in Rstudio V.1.4.1106. In view of the low incidence of recurrent TB and the limited average risk period considered, we assumed HR and OR to adequately approximate RR.
Assessment of heterogeneity
We assessed heterogeneity by inspection of forest plots for all factors included in the quantitative synthesis. In addition, we also considered the I2 statistic and the corresponding χ2 statistical test. We explored causes of heterogeneity and reported it in the assessment of certainty of evidence.
Assessment of reporting bias
For exposure factors evaluated in 10 or more studies, we generated funnel plots and used Egger’s test to check their symmetry.
Assessment of certainty of evidence
We evaluated the certainty of evidence using the Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) approach25 for all factors included in the quantitative analyses.
Patient and public involvement
None.
Results
Study selection and study characteristics
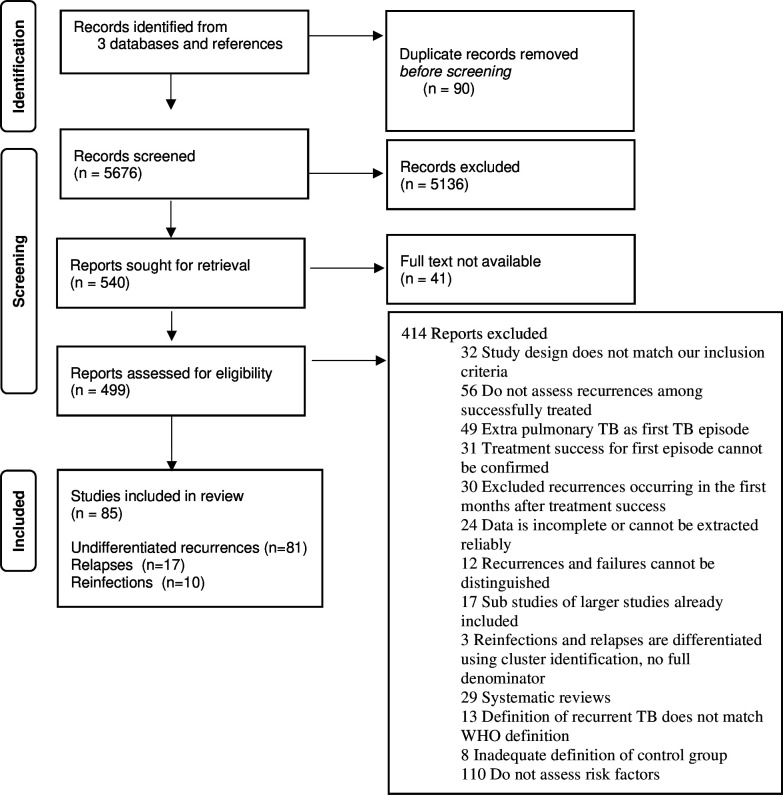
Our search yielded 5676 unique records (figure 1). Through screening of titles and abstracts, we identified 540 records for full-text review. Forty-one full-text records were not available, thus we reviewed 499 records. Eighty-five records assessed factors associated with either recurrent TB, reinfection or relapse. Thirty-one were trials, 27 prospective cohort studies, 17 retrospective cohort studies, 8 case–control studies, 1 cross-sectional study and 1 individual patient data meta-analysis that provided more information than the primary studies. Among them, 81 studies reported factors for undifferentiated recurrence, 17 for relapses and 10 for reinfections. Enough data for meta-analysis was available for 56 studies for undifferentiated recurrence, 15 for relapses and 8 for reinfections. Online supplemental appendix 6 lists the included articles and online supplemental appendix 7 the excluded articles, with a reason for exclusion. The characteristics of included studies are presented in online supplemental appendix 4, online supplemental table S1. Twenty-nine studies included a multivariable analysis; details on variable selection and model building are presented in online supplemental table S2.
Figure 1.
Flowchart of study selection
Study risk of bias assessment
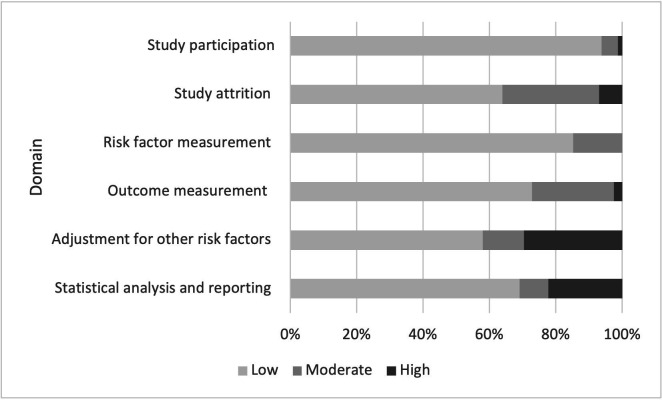
Figures 2 and 3 show the risk of bias assessment using the QUIPS Tool. The complete results of quality appraisal per record and per domain are available in online supplemental tables S3 and S4. Overall evaluation of studies on undifferentiated recurrence resulted in 26 studies with good, 26 with fair and 29 with poor overall quality. The main reasons for low-quality rating were that the observed effects of the factor were very likely to be distorted by confounding, and that the reported results were very likely to be spurious or biased for methodological reasons. Other causes were inadequate reporting on patient follow-up, on methods to measure risk factors and on non-microbiological diagnostic criteria for recurrent TB.
Figure 2.
Risk of bias assessment with the QUIPS Tool for studies of undifferentiated recurrence. QUIPS, Quality In Prognostic Factor Studies.
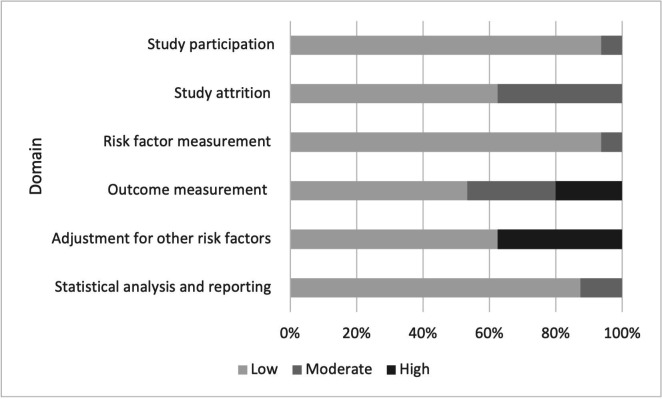
Figure 3.
Risk of bias assessment with the QUIPS Tool for studies of relapse and/or reinfection. QUIPS, Quality In Prognostic Factor Studies.
For studies addressing factors related to reinfection or relapse, there were eight with good, two with fair and seven with poor overall quality. In addition to the main reasons mentioned above, low availability of DNA samples from both episodes for genotyping compromised the quality of several studies.
Overview of factors analysed
We included 20 factors in our quantitative analysis: Sociodemographic characteristics: male sex, illiteracy, rural resident versus urban; Behavioural characteristics of the patient: smoking and alcohol consumption; Clinical characteristics of the previous TB episode: cavitary disease, smear positivity at diagnosis and smear or culture positivity at month 2 after treatment initiation, previous TB episodes, multidrug resistance (MDR), any drug resistance patterns and advanced radiographical extent of TB disease; Treatment characteristics of the previous TB episode: rifampicin less than 6 months vs 6 months, rifampicin more than 6 months vs 6 months, use of fixed-dose combinations of TB drugs and low TB treatment adherence; Comorbidities: HIV infection, body mass index <18.5 kg/m2, chronic lung disease and diabetes mellitus. The individual study estimates for these factors are detailed in online supplemental table S5 and S6.
Data synthesis
Pooled unadjusted RR estimates for undifferentiated recurrences and for reinfections and relapses for factors reported by at least four studies are presented in tables 1 and 2, respectively. Forest plots are provided in online supplemental appendix 5, figure S1–S24. For undifferentiated recurrences, the risk increase is below twofold for all factors except for low TB treatment adherence (pooled RR 3.31) and MDR (pooled RR 3.49) in the previous episode. For relapses, none of the factors showed a significant association, and for reinfections, HIV infection, the only factor that could be pooled, showed a strong significant association (pooled RR 4.65).
Table 1.
Pooled estimates of the crude effects of risk factors for recurrent tuberculosis reported by at least four studies*
| Risk factor | N of studies | RR | 95% CI | P value χ2 for heterogeneity | I2* | Certainty of evidence† | |
| Sociodemographic characteristics | |||||||
| Male sex (yes) | 23 | 1.40 | 1.25 to 1.57 | 0.0175 | 42% | Low | |
| Illiteracy (yes) | 5 | 1.51 | 1.09 to 2.09 | 0.1979 | -- | Very low | |
| Rural resident (vs urban) | 4 | 0.94 | 0.63 to 1.41 | <0.0001 | -- | Very low | |
| Behavioural characteristics of the patient | |||||||
| Smoking (yes) | 10 | 1.68 | 1.45 to 1.95 | 0.1860 | -- | Low | |
| Alcohol consumption (yes) | 7 | 1.74 | 1.40 to 217 | 0.3549 | -- | Low | |
| Clinical characteristics of the previous TB episode | |||||||
| Cavitary disease (yes) | 14 | 1.47 | 1.34 to 1.62 | 0.6424 | 0.00% | Moderate | |
| Smear positivity at diagnosis (yes) | 6 | 1.30 | 1.11 to 1.53 | 0.3839 | -- | Low | |
| Smear or culture positivity at 2 months after treatment initiation (yes) | 9 | 1.63 | 1.28 to 2.08 | 0.7368 | -- | Very low | |
| Previous TB episode (yes) | 7 | 1.98 | 1.31 to 2.97 | <0.0001 | -- | Moderate | |
| Multidrug resistance (vs no resistance) | 6 | 3.49 | 1.86 to 6.53 | <0.0001 | -- | Moderate | |
| Any drug resistance (yes) | 4 | 1.59 | 0.73 to 3.43 | 0.0110 | -- | Very low | |
| Advanced radiographical extent of tuberculosis disease (yes) | 4 | 1.83 | 1.28 to 2.61 | 0.4263 | -- | Low | |
| Treatment characteristics of the previous TB episode | |||||||
| Rifampicin less than 6 months vs 6 months | 9 | 1.94 | 1.51 to 2.49 | 0.0572 | -- | Low | |
| Rifampicin more than 6 months vs 6 months | 4 | 0.37 | 0.13 to 1.11 | 0.0129 | -- | Very low | |
| Fixed-dose combination tuberculosis drug (yes) | 6 | 2.29 | 1.10 to 4.75 | 0.1645 | -- | High | |
| Low TB treatment adherence (yes) | 4 | 3.31 | 2.37 to 4.62 | 0.7751 | -- | Low | |
| Comorbidities | |||||||
| HIV infection (yes) | 23 | 1.78 | 1.38 to 2.31 | 0.0022 | 51.80% | Low | |
| Body mass index <18.5 (yes) | 8 | 1.52 | 1.17 to 1.96 | 0.1719 | -- | Very low | |
| Chronic lung disease (yes) | 4 | 1.42 | 1.08 to 1.87 | 0.1757 | -- | Low | |
| Diabetes mellitus (yes) | 11 | 1.69 | 1.08 to 2.64 | 0.0001 | -- | Very low | |
*I2 was considered for factors that were evaluated in 10 or more studies.
†Full details regarding certainty of evidence in online supplemental table S9.
.RR, relative risk; TB, tuberculosis.
Table 2.
Pooled estimates of the crude effects of risk factors for reinfection and relapse reported by at least four studies
| Relapses | Reinfection | |||||||||||
| Risk factor | N of studies | RR | 95% CI | P value χ2 for heterogeneity | I2 | Certainty of evidence | N of studies | RR | 95% CI | P value χ2 for heterogeneity | I2 | Certainty of evidence |
| Sociodemographic characteristics | ||||||||||||
| Male sex (yes) | 5 | 1.44 | 0.83 to 2.48 | 0.0265 | -- | Low | 0 | -- | -- | -- | -- | -- |
| Rifampicin less than 6 months vs 6 months | 4 | 1.64 | 0.46 to 5.85 | 0.0001 | -- | Very low | 0 | -- | -- | -- | -- | -- |
| Comorbidities | ||||||||||||
| HIV infection (yes) | 9 | 1.10 | 0.72 to 1.56 | 0.0795 | -- | Low | 8 | 4.65 | 1.71 to 12.65 | 0.0220 | -- | Moderate |
*Full details regarding certainty of evidence in online supplemental table S9.
.RR, relative risk.
Online supplemental table S8 summarises the results of studies that report multivariable analyses for the above factors—controlling for vastly varying sets of confounding factors—and specifies the number of studies, the number that found a significant association, and the minimum, median and maximum of the adjusted estimates.
Assessment of heterogeneity
For studies on undifferentiated TB recurrences, heterogeneity was categorised as high for rural resident (vs urban), previous TB episode, MDR, any drug resistance, rifampicin more than 6 months vs rifampicin for 6 months and diabetes mellitus; as moderate for male sex, literacy, HIV, chronic lung disease and rifampicin less than 6 months vs rifampicin for 6 months during the previous episode; and as low for, smoking and alcohol consumption, smear or culture positivity at 2 months treatment, cavitary disease, smear positivity at diagnosis, advanced radiographical extent of previous TB disease, low treatment adherence and body mass index <18.5 kg/m2.
For relapses, male sex and HIV infection have moderate heterogeneity, and rifampicin less than 6 months has high heterogeneity. For reinfections, HIV shows moderate heterogeneity.
Assessment of reporting bias
No manifest publication bias was identified on inspection of the funnel plots (online supplemental figure S25–S29) and with the Egger’s test for the association between the factors gender, HIV, cavitary disease and smoking and diabetes mellitus.
Assessment of certainty of evidence
The GRADE evidence assessment is shown in online supplemental table S9. For this review, we did not downgrade quality of evidence based on study design as observational studies are adequate to assess risk factors. Regarding undifferentiated recurrences, we found moderate certainty of evidence for clinical characteristics of the previous TB episode: cavitary disease, MDR and smear positivity at diagnosis and high certainty of evidence for treatment characteristics of the previous episode: fixed-dose drug combination. We found moderate certainty of evidence for the association between HIV infection and TB reinfection. For all other risk factors, be it for recurrence, relapse or reinfection, we found certainty to be low or very low.
Discussion
We reviewed studies on individual risk factors for TB recurrence, relapse or reinfection and document a wide array of sociodemographic and behavioural factors, clinical and treatment characteristics of the previous episode, and comorbidities that have been investigated over the past four decades. Our meta-analysis identified only a few factors that significantly contribute to at least a moderately or strongly increased risk and at the same time score at least moderate certainty of evidence: MDR and fixed-dose combination TB drugs in the previous episode for recurrence; none for relapse; and HIV infection for reinfection.
We aimed to address risk factors for relapses and reinfections independently, but the small number of studies employing molecular genotyping severely hampered separate meta-analyses and compelled us to also analyse risk factors for undifferentiated recurrences. While the underlying mechanisms of relapses and reinfections are fundamentally different, overlap of risk factors cannot not be excluded, but when studying undifferentiated recurrence, they become intertwined. Furthermore, a fully comprehensive approach should have considered alongside individual factors environmental characteristics, background TB incidence and control programme attributes to start with, as well as characteristics of the included studies such as design and length of patient follow-up. This could in theory be done using multilevel meta-analysis methods, but the available data did not allow it. The above is further compounded by widely different factors being included in the reviewed studies and different definitions being used for the same factor and substantial lack of consistency across studies in the sets of variables used to control for confounding which limited us to rely on unadjusted estimates for our main meta-analyses. All this taken together might in part explain the meagre catch of our meta-analyses. Noteworthy, the aforementioned characteristics constitute sources of methodological heterogeneity that add to the clinical heterogeneity that arises from differences in patient characteristics such as age, sex and comorbidities.
Sociodemographic and behavioural characteristics of patients, although generally statistically significant, only showed weak pooled associations with undifferentiated recurrence and low to very low certainty of evidence. Although the studies included in our meta-analysis pointed at smoking and alcohol consumption as risk factors for recurrence, inadequate analysis and indirectness was a major issue for the certainty of the evidence. Of note, the adjusted effect of smoking tended to be of moderate strength in the studies that somehow controlled for confounding. The absence of data prevented a separate analysis of its effect on relapse and reinfection. Still, smoking and alcohol consumption are known to be associated to the risk of TB as such26 27 and could be addressed by TB programme interventions.
Regarding the characteristics of the previous TB episode, MDR showed a strong positive pooled association with undifferentiated recurrence, with moderate certainty of evidence. MDR TB treatment is less effective than treatment of susceptible TB28 29 and causes more side effects,30 which hampers adherence. For cavitary disease, we found, with moderate certainty of evidence, a significant but small effect on recurrence. It is known that poor vascularisation in cavities limits delivery of TB drugs, possibly resulting in incomplete sterilisation increasing the risk of relapse.31 We also document a weak pooled risk for smear positivity at month 2 of treatment, with low evidence due to high risk of bias and indirectness in the primary studies. Previous meta-analyses21 32 have concluded that cavitary TB and smear positivity at month 2 were potentially useful prognostic factors, but our review casts doubts on that. On the other hand, more than one previous TB episode moderately increased the pooled risk for undifferentiated recurrence, but major heterogeneity among studies compromised the certainty of evidence. We hypothesise that this finding reflects that higher background TB incidence increases the occurrence of reinfection, as found previously.10 33 Finally, although our review did not provide evidence that clinical sequelae of the previous episode increase the risk of reinfection, this is attributable to lack of data and signals that pathological structural changes such as bronchiectasis, fibrosis or residual cavitation, which lead to post-TB lung disease,34 require further research.
Our meta-analysis singled out low adherence as a treatment characteristic of the previous TB episode that increases the risk of recurrence over threefold. However, the certainty is low, and no included study reported an estimate of this effect separately for relapse. In line with the results of previous reviews on drug treatment,19 35 our meta-analyses for both relapse and recurrence pointed to a moderately increased risk for regimens including less than 6 months of rifampicin, be it with low certainty due to indirectness and heterogeneity among studies. For treatment of the previous episode with fixed-dose TB drug formulations, we found a moderate, significantly positive pooled effect on recurrence, with high certainty. Low bioavailability, particularly of rifampicin, has been described for such formulations,36 37 which could hamper elimination of bacilli. On the other hand, these formulations have been found to increase adherence. As can somewhat be expected, no TB treatment-related factors were studied separately in relation to reinfection.
Comorbidities’ effects on recurrence of TB are related to immune depression increasing the risk of TB progression once reinfection occurrs and potentially due to possible continuous exposure at high-risk places such as health facilities.38 39 We found only a weak pooled association of HIV infection with undifferentiated recurrence, with low certainty of evidence. However, while there was no indication of an association between HIV and relapse, our separate meta-analysis for reinfection indicated an RR close to 5 with moderate certainty of evidence. Overall, this is consistent with older reviews from the 2000s15 33 that looked into undifferentiated recurrence. Unfortunately, very few studies assess the effect of HIV stage. For diabetes mellitus, we found a significant but weak pooled association with recurrence. No study looked separately into relapse and reinfection. A systematic review specifically on the subject of diabetes mellitus18 similarly found an RR below 2 and considerable heterogeneity. Adequate glycaemic control reduces TB recurrence.40 It was never adequately adjusted for in the studies we included, which could have contributed to the observed heterogeneity.
From a methodological point of view, our review calls attention to the conceptual complexity of studying risk factors for recurrent TB, reinfection and relapse, as well as to the need for standardising methods and definitions, more systematically assessing confounding, and meticulously using DNA fingerprinting in research studies. We recommend to adopt strict terminology and to reserve the term recurrence for any new episode after treatment and use reinfection or relapse only if genotyping was performed. We also encourage more transparency and completeness in describing the methods used. This will allow better insight into methodological as opposed to contextual heterogeneity, the latter being due to differences in study setting characteristics. We propose to collect information on and adjust for age, sex, HIV status and treatment adherence as a minimum set of factors to address confounding at the individual level. Improving the quality and the scope of routine data collection and systematic exploitation of the information contained in health information systems could further contribute to obtaining more robust evidence on the risk factors for recurrence. In addition, studies on mechanisms that address the intermediary pathogenetic process are required.
As for the subject matter, the characteristics of the environment and TB control programme implementation play an important role in the frequency of recurrence and the relative proportion of reinfection and relapse, as previously demonstrated.10 With the present systematic review, we identified major risk factors at the individual level that are personal characteristics or related to TB programme strategies for treatment delivery and care. Behavioural factors could be addressed, among others, by implementing existing guidelines, for example, on smoking cessation interventions. TB programmes should also more actively pursue retention in treatment and provide integrated care for patients with HIV or diabetes. Secondary prophylaxis with isoniazid after TB treatment completion was found to be protective in HIV-infected people41 42 and could be systematically considered. Finally, successfully treated individuals must be made aware of post-TB health and the risk of recurrence and be encouraged to immediately seek care if symptoms arise.
Footnotes
Contributors: Conceptualisation and methodology: LO, CS, PVdS and VV. Data collection and risk of bias assessment: SR, VV and JC-S. Data analysis and interpretation: PVdS, LO, TV and VV. All authors critically revised, provided important conceptual input and approved the final version of the manuscript. Guarantor: VV.
Funding: The study received financial support from Institutional Collaboration Framework Agreement IV Institute of Tropical Medicine Antwerp, Belgium—Instituto de Medicina Tropical Alexander von Humboldt, Universidad Peruana Cayetano Heredia. LO is supported by an Emerging Global Leader Award from the Fogarty International Center at the National Institutes of Health (K43TW011137).
Disclaimer: The funders had no role in the design and conduction of this study.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as online supplemental information.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Dodd PJ, Yuen CM, Jayasooriya SM, et al. Quantifying the global number of tuberculosis survivors: a Modelling study. Lancet Infect Dis 2021;21:984–92. 10.1016/S1473-3099(20)30919-1 Available: http://www.thelancet.com/article/S1473309920309191/fulltext [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization . Global tuberculosis report 2021; 2021. Available: Licence: CC BY-NC-SA 3.0 IGO
- 3.Allwood B, van der Zalm M, Makanda G, et al. The long shadow post-tuberculosis. Lancet Infect Dis 2019;19:1170–1. 10.1016/S1473-3099(19)30564-X Available: https://linkinghub.elsevier.com/retrieve/pii/S147330991930564X [DOI] [PubMed] [Google Scholar]
- 4.van Kampen SC, Wanner A, Edwards M, et al. International research and guidelines on post-tuberculosis chronic lung disorders: A systematic Scoping review. BMJ Glob Health 2018;3:e000745. 10.1136/bmjgh-2018-000745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Basham CA, Romanowski K, Johnston JC. Life after tuberculosis: planning for health. Lancet Respir Med 2019;7:1004–6. 10.1016/S2213-2600(19)30371-6 Available: https://linkinghub.elsevier.com/retrieve/pii/S2213260019303716 [DOI] [PubMed] [Google Scholar]
- 6.Harries AD, Dlodlo RA, Brigden G, et al. 'Should we consider a “fourth 90” for tuberculosis'. Int J Tuberc Lung Dis 2019;23:1253–6. 10.5588/ijtld.19.0471 Available: https://www.ingentaconnect.com/content/10.5588/ijtld.19.0471 [DOI] [PubMed] [Google Scholar]
- 7.Romanowski K, Baumann B, Basham CA, et al. Long-term all-cause mortality in people treated for tuberculosis: a systematic review and meta-analysis. Lancet Infect Dis 2019;19:1129–37. 10.1016/S1473-3099(19)30309-3 [DOI] [PubMed] [Google Scholar]
- 8.Lambert M-L, Hasker E, Van Deun A, et al. Recurrence in tuberculosis: relapse or reinfection Lancet Infect Dis 2003;3:282–7. 10.1016/s1473-3099(03)00607-8 [DOI] [PubMed] [Google Scholar]
- 9.Rieder HL, Chiang CY, Rusen ID, et al. A method to determine the utility of the third diagnostic and the second follow-up sputum smear examinations to diagnose tuberculosis cases and failures. Int J Tuberc Lung Dis 2005;9:384–91. [PubMed] [Google Scholar]
- 10.Vega V, Rodríguez S, Van der Stuyft P, et al. Recurrent TB: a systematic review and meta-analysis of the incidence rates and the proportions of relapses and reinfections. Thorax 2021;76:494–502. 10.1136/thoraxjnl-2020-215449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moreno-Martínez R, Rodríguez-Ábrego G, Martínez-Montañez OG. Incidencia de Recaída Y Factores de Riesgo Asociados en Pacientes con tuberculosis Pulmonar. Rev Med Inst Mex Seguro Soc 2007;45:335–42. [PubMed] [Google Scholar]
- 12.Perriëns JH, St Louis ME, Mukadi YB, et al. Pulmonary tuberculosis in HIV-infected patients in Zaire. A controlled trial of treatment for either 6 or 12 months. N Engl J Med 1995;332:779–84. 10.1056/NEJM199503233321204 Available: http://www.nejm.org/doi/abs/10.1056/NEJM199503233321204 [DOI] [PubMed] [Google Scholar]
- 13.Sonnenberg P, Murray J, Glynn JR, et al. HIV-1 and recurrence, relapse, and Reinfection of tuberculosis after cure: a cohort study in South African Mineworkers. Lancet 2001;358:1687–93. 10.1016/S0140-6736(01)06712-5 Available: https://linkinghub.elsevier.com/retrieve/pii/S0140673601067125 [DOI] [PubMed] [Google Scholar]
- 14.Trinh QM, Nguyen HL, Nguyen VN, et al. Tuberculosis and HIV Co-infection—focus on the Asia-Pacific region. Int J Infect Dis 2015;32:170–8. 10.1016/j.ijid.2014.11.023 Available: https://linkinghub.elsevier.com/retrieve/pii/S1201971214017123 [DOI] [PubMed] [Google Scholar]
- 15.Panjabi R, Comstock GW, Golub JE. Recurrent tuberculosis and its risk factors: adequately treated patients are still at high risk. Int J Tuberc Lung Dis 2007;11:828–37. [PubMed] [Google Scholar]
- 16.Moodley Y, Govender K. A systematic review of published literature describing factors associated with tuberculosis recurrence in people living with HIV in Africa. Afr Health Sci 2015;15:1239–46. 10.4314/ahs.v15i4.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baker MA, Harries AD, Jeon CY, et al. The impact of diabetes on tuberculosis treatment outcomes: A systematic review. BMC Med 2011;9:81. 10.1186/1741-7015-9-81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huangfu P, Ugarte-Gil C, Golub J, et al. The effects of diabetes on tuberculosis treatment outcomes: an updated systematic review and meta-analysis. Int J Tuberc Lung Dis 2019;23:783–96. 10.5588/ijtld.18.0433 [DOI] [PubMed] [Google Scholar]
- 19.Menzies D, Benedetti A, Paydar A, et al. Effect of duration and Intermittency of rifampin on tuberculosis treatment outcomes: A systematic review and meta-analysis. PLoS Med 2009;6:e1000146. 10.1371/journal.pmed.1000146 Available: https://dx.plos.org/10.1371/journal.pmed.1000146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qiu B, Wu Z, Tao B, et al. Risk factors for types of recurrent tuberculosis (reactivation versus Reinfection): A global systematic review and meta-analysis. Int J Infect Dis 2022;116:14–20. 10.1016/j.ijid.2021.12.344 Available: https://linkinghub.elsevier.com/retrieve/pii/S120197122101242X [DOI] [PubMed] [Google Scholar]
- 21.Romanowski K, Balshaw RF, Benedetti A, et al. Predicting tuberculosis relapse in patients treated with the standard 6-month regimen: an individual patient data meta-analysis. Thorax 2019;74:291–7. 10.1136/thoraxjnl-2017-211120 Available: https://thorax.bmj.com/lookup/doi/10.1136/thoraxjnl-2017-211120 [DOI] [PubMed] [Google Scholar]
- 22.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:71.:n71. 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.World Health Organization . Definitions and reporting framework for tuberculosis – 2013 revision: updated December 2014 and January 2020. World Health Organization; 2013. [Google Scholar]
- 24.Riley RD, Moons KGM, Snell KIE, et al. A guide to systematic review and meta-analysis of Prognostic factor studies. BMJ 2019;364:k4597. 10.1136/bmj.k4597 [DOI] [PubMed] [Google Scholar]
- 25.Schunemann H, Brozek J, Guyatt G, et al. GRADE Handbook for grading quality of evidence and strength of recommendations. updated October 2013. The GRADE Working Group 2013. Available: guidelinedevelopment.org/handbook [Google Scholar]
- 26.Obore N, Kawuki J, Guan J, et al. Association between indoor air pollution, tobacco smoke and tuberculosis: an updated systematic review and meta-analysis. Public Health 2020;187:24–35. 10.1016/j.puhe.2020.07.031 Available: https://linkinghub.elsevier.com/retrieve/pii/S0033350620303267 [DOI] [PubMed] [Google Scholar]
- 27.Imtiaz S, Shield KD, Roerecke M, et al. Alcohol consumption as a risk factor for tuberculosis: meta-analyses and burden of disease. Eur Respir J 2017;50:1700216. 10.1183/13993003.00216-2017 Available: http://erj.ersjournals.com/lookup/doi/10.1183/13993003.00216-2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nunn AJ, Phillips PPJ, Meredith SK, et al. A trial of a shorter regimen for rifampin-resistant tuberculosis. N Engl J Med 2019;380:1201–13. 10.1056/NEJMoa1811867 [DOI] [PubMed] [Google Scholar]
- 29.Van Deun A, Maug AKJ, Salim MAH, et al. Short, highly effective, and inexpensive standardized treatment of multidrug-resistant tuberculosis. Am J Respir Crit Care Med 2010;182:684–92. 10.1164/rccm.201001-0077OC Available: https://www.atsjournals.org/doi/10.1164/rccm.201001-0077OC [DOI] [PubMed] [Google Scholar]
- 30.World Health Organization . WHO Consolidated guidelines on tuberculosis: Module 4: treatment - drug-resistant tuberculosis treatment. Geneva; 2020. [PubMed] [Google Scholar]
- 31.Urbanowski ME, Ordonez AA, Ruiz-Bedoya CA, et al. Cavitary tuberculosis: the Gateway of disease transmission 20 Internet, the lancet infectious diseases. Lancet Publishing Group July 6, 2020. 10.1016/S1473-3099(20)30148-1 Available: http://www.thelancet.com/article/S1473309920301481/fulltext [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Horne DJ, Royce SE, Gooze L, et al. Sputum monitoring during tuberculosis treatment for predicting outcome: systematic review and meta-analysis. Lancet Infect Dis 2010;10:387–94. 10.1016/S1473-3099(10)70071-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Korenromp EL, Scano F, Williams BG, et al. Effects of human immunodeficiency virus infection on recurrence of tuberculosis after rifampin-based treatment: an Analytical review. Clin Infect Dis 2003;37:101–12. 10.1086/375220 [DOI] [PubMed] [Google Scholar]
- 34.Allwood BW, Byrne A, Meghji J, et al. Post-tuberculosis lung disease: clinical review of an under-recognised global challenge. Respiration 2021;100:751–63. 10.1159/000512531 Available: https://www.karger.com/DOI/10.1159/000512531 [DOI] [PubMed] [Google Scholar]
- 35.Grace AG, Mittal A, Jain S, et al. Shortened treatment regimens versus the standard regimen for drug-sensitive pulmonary tuberculosis. Cochrane Database Syst Rev 2019;12:CD012918. 10.1002/14651858.CD012918.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hao L-H, Guo S-C, Liu C-C, et al. Comparative Bioavailability of Rifampicin and isoniazid in fixed-dose combinations and single-drug formulations. Int J Tuberc Lung Dis 2014;18:1505–12. 10.5588/ijtld.13.0647 Available: http://openurl.ingenta.com/content/xref?genre=article&issn=1027-3719&volume=18&issue=12&spage=1505 [DOI] [PubMed] [Google Scholar]
- 37.Gallardo CR, Rigau Comas D, Valderrama Rodríguez A, et al. Fixed-dose combinations of drugs versus single-drug formulations for treating pulmonary tuberculosis. Cochrane Database Syst Rev 2016:CD009913. 10.1002/14651858.CD009913.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crampin AC, Mwaungulu JN, Mwaungulu FD, et al. Recurrent TB: relapse or Reinfection? the effect of HIV in a general population cohort in Malawi. AIDS 2010;24:417–26. 10.1097/QAD.0b013e32832f51cf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marais BJ, Lönnroth K, Lawn SD, et al. Tuberculosis Comorbidity with communicable and non-communicable diseases: integrating health services and control efforts. Lancet Infect Dis 2013;13:436–48. 10.1016/S1473-3099(13)70015-X [DOI] [PubMed] [Google Scholar]
- 40.Ma Y, Pang Y, Shu W, et al. Metformin reduces the relapse rate of tuberculosis patients with diabetes mellitus: experiences from 3-year follow-up. Eur J Clin Microbiol Infect Dis 2018;37:1259–63. 10.1007/s10096-018-3242-6 [DOI] [PubMed] [Google Scholar]
- 41.Ayles H, Muyoyeta M. Isoniazid to prevent first and recurrent episodes of TB. Trop Doct 2006;36:83–6. 10.1258/004947506776593521 Available: http://journals.sagepub.com/doi/10.1258/004947506776593521 [DOI] [PubMed] [Google Scholar]
- 42.Fitzgerald DW, Desvarieux M, Severe P, et al. Effect of post-treatment isoniazid on prevention of recurrent tuberculosis in HIV-1-infected individuals: a randomised trial. Lancet 2000;356:1470–4. 10.1016/S0140-6736(00)02870-1 Available: https://linkinghub.elsevier.com/retrieve/pii/S0140673600028701 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjresp-2023-002281supp001.pdf (1.5MB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as online supplemental information.