Abstract
Cooling sensations arise inside the mouth during ingestive and homeostasis behaviors. Oral presence of cooling temperature engages the cold and menthol receptor TRPM8 (transient receptor potential melastatin 8) on trigeminal afferents. Yet, how TRPM8 influences brain and behavioral responses to oral temperature is undefined. Here we used in vivo neurophysiology to record action potentials stimulated by cooling and warming of oral tissues from trigeminal nucleus caudalis neurons in female and male wild-type and TRPM8 gene deficient mice. Using these lines, we also measured orobehavioral licking responses to cool and warm water in a novel, temperature-controlled fluid choice test. Capture of antidromic electrophysiological responses to thalamic stimulation identified that wild-type central trigeminal neurons showed diverse responses to oral cooling. Some neurons displayed relatively strong excitation to cold <10°C (COLD neurons) while others responded to only a segment of mild cool temperatures below 30°C (COOL neurons). Notably, TRPM8 deficient mice retained COLD-type but lacked COOL cells. This deficit impaired population responses to mild cooling temperatures below 30°C and allowed warmth-like (≥35°C) neural activity to pervade the normally innocuous cool temperature range, predicting TRPM8 deficient mice would show anomalously similar orobehavioral responses to warm and cool temperatures. Accordingly, TRPM8 deficient mice avoided both warm (35°C) and mild cool (≤30°C) water and sought colder temperatures in fluid licking tests, whereas control mice avoided warm but were indifferent to mild cool and colder water. Results imply TRPM8 input separates cool from warm temperature sensing and suggest other thermoreceptors also participate in oral cooling sensation.
Keywords: behavior, cold, temperature, trigeminal, TRPM8, warm
Significance Statement
TRPM8 contributes to the detection of cutaneous cooling. Yet how TRPM8 influences neural representations for temperatures in somatosensory circuits in the brain is not fully understood. Here we show that in central trigeminal neurons, TRPM8 input drives a neural information breakpoint between cool and warm temperature sensing inside the mouth. Mice gene deficient for TRPM8 showed reduced, but not abolished, neural responses to oral cooling that were impaired to distinguish mild cool temperatures from warmth. This neural deficit predicted a behavioral generalization between mild cooling and warmth that appeared in licking responses to temperature-controlled fluids in TRPM8 deficient mice. These data reveal a novel role for TRPM8 in thermosensory coding and have implications for the multiplicity of oral cooling receptors.
Introduction
We receive pleasure and protective information from thermal sensations. Cutaneous temperature sensing is mediated by diverse thermosensory neurons in somatosensory pathways. Such diversity is apparent among cooling-responsive neurons excited by temperatures below ∼35°C. These cells are variably sensitive to moderately cool and colder temperatures and can sometimes activate to mechanical and noxious stimuli (e.g., Landgren, 1960; Craig and Dostrovsky, 2001; Craig et al., 2001). Humans show greater perceived responsiveness to cooling temperatures applied to oral tissues, like the tongue, compared to bodily extremities, such as the fingertip (Green, 1984). Strong sensitivity to oral cooling may stem from the high resting temperature of the tongue surface near 36°C (Green, 1984, 1986), which promotes cooling sensations in mammals during mouth openings for heat exchange, communication, and feeding.
Cooling of oral epithelia excites trigeminal ganglion neurons, which supply craniofacial somatosensation. Orolingual trigeminal afferents express temperature sensitive transient receptor potential (TRP) ion channels including TRP melastatin 8 (TRPM8) (Abe et al., 2005; Dhaka et al., 2008; Yarmolinsky et al., 2016), which is excited by cool temperatures near and below 30°C and cooling mimetics like menthol (McKemy et al., 2002; Bautista et al., 2007). Primary trigeminal fibers that respond to oral cooling compose heterogeneous types distinguished by their sensitivities to cool and cold temperatures. For instance, in vivo calcium imaging found that different subpopulations of mouse trigeminal ganglion cells preferentially respond when temperatures applied inside the mouth decrease from mild cooling below 30°C to cold <10°C (Yarmolinsky et al., 2016; Leijon et al., 2019). These results associate with in vitro functional data that show mouse trigeminal ganglion neurons appear as low- or high-threshold based on sensitivity to, respectively, small or comparably larger temperature drops, with low-threshold cells showing greater expression of TRPM8 and failing to develop in TRPM8 gene knock-out mice (Bautista et al., 2007; Madrid et al., 2009). Thus, variability in cooling sensitivity across trigeminal afferents associates with TRPM8 function.
On entering the brain, the axons of trigeminal ganglion neurons partly terminate in the medullary trigeminal nucleus caudalis (Vc) (Capra and Dessem, 1992). Vc neurons are implicated for orolingual nociceptive and thermosensory processing (Carstens et al., 1995, 1998) and project to rostral brain stem and forebrain structures (Cechetto et al., 1985; Jasmin et al., 1997; Craig and Dostrovsky, 2001; Saito et al., 2017). Presently, there are only scant functional data on how oral cooling information is represented by Vc neural activity. Both innocuous and more extreme cooling temperature stimulation of oral tissues can excite rat Vc neurons (Carstens et al., 1998; Zanotto et al., 2007), with cool-driven Vc cells in mice composing subgroups responsive to select ranges of mild cool or intense cold oral temperatures (Lemon et al., 2016). The latter trend follows the cooling response heterogeneity found among peripheral trigeminal neurons (Poulos and Lende, 1970a; Bautista et al., 2007; Madrid et al., 2009; Yarmolinsky et al., 2016; Leijon et al., 2019) and could suggest some features of neural codes for oral cooling information in the periphery are recapitulated in the brain. Further delineation of this requires identification of how TRPM8 input influences diverse cooling-active Vc neurons and the central projection targets of these cells, which remain unresolved.
Here, we used in vivo neurophysiology to characterize oral cooling responses in mouse Vc neurons that project to the thalamus. We also recorded Vc neurons in TRPM8 gene deficient mice to gauge how silencing TRPM8 impacted CNS oral thermal activity. We found trigeminothalamic neurons responsive to cooling formed heterogeneous types tuned to mild cool or colder temperatures. Vc neurons responsive to mild cooling were absent in TRPM8 deficient mice, revealing their dependence on TRPM8 function. Furthermore, neural distinctions between mild cooling and warmth were impaired in TRPM8 deficient mice and predicted similarities in their responses to these temperatures in an orobehavioral task. TRPM8 deficient mice also showed residual orobehavioral responses to cool and cold temperatures, implying non-TRPM8 receptors importantly participate in oral cooling sensation.
Materials and Methods
Three experiments were performed. First, neural recordings were made to characterize how mouse Vc neurons that project to the thalamus respond to oral delivery of cool and warm temperature fluids. We then compared Vc cellular responses between mice gene deficient for TRPM8 and controls to quantify how the neural representation of oral temperature in the trigeminal brain stem changes in the absence of TRPM8. Finally, using a novel behavioral testing apparatus and approach, we examined orosensory-guided licking responses to temperature-controlled fluids in behaving TRPM8 deficient and control mice to test predictions about TRPM8 function that arose from our neural data.
Mice
Naive adult female and male C57BL/6J (B6; approximate wild type) mice (n = 40; body weight 20.1–32.1 g; stock #000664, The Jackson Laboratory) and TRPM8 homozygous gene deficient (knock-out; labeled TRPM8-KO in figures) mice (n = 21; body weight 18.6–35.6 g; stock #008198, The Jackson Laboratory) were used in neurophysiological studies. Additional squads of naive adult B6 (n = 10, 5 per sex; body weight 18.9–30.8 g) and TRPM8 deficient (n = 10, 5 per sex; body weight 18.0–30.6 g) mice entered noninvasive behavioral experiments. All mice were housed in a vivarium that maintained an ambient temperature of ∼23°C and a 12:12 hour light/dark cycle. Food and water were available ad libitum, except during water restriction conditions during behavioral studies. All neurophysiological and behavioral testing procedures were approved by the Institutional Animal Care and Use Committee of the University of Oklahoma and performed in accordance with standards set by the National Institutes of Health Guide for Care and Use of Laboratory Animals.
Neurophysiology
The present in vivo electrophysiological studies recorded extracellular action potentials (APs) from single Vc neurons in anesthetized mice that were stimulated with oral delivery of temperature-controlled fluids and chemesthetic stimuli. Many, but not all, of the recorded neurons were also tested for antidromic APs in response to weak electrical stimulation of the orosensory thalamus to characterize their axonal projection status. Antidromic response testing was largely performed on Vc cells recorded from B6 mice, as below.
Surgery and preparation
Mice that entered in vivo neurophysiological studies were inducted by an intraperitoneal (i.p.) injection of ketamine/xylazine (ketamine,10 mg/ml; xylazine, 1 mg/ml; dosage, 10 ml/kg). Atropine (24 µg/kg, i.p.) was also administered to reduce bronchial secretions. Once anesthetized, a tracheal tube was inserted to facilitate breathing during stimulation of the oral cavity with temperature-controlled liquids and maintenance of isoflurane gas anesthesia. Mice were secured in a stereotaxic instrument with ear bars (model 930; David Kopf Instruments). The distal end of the tracheal tube was then positioned inside the tip of concentric pressure/vacuum tubing that allowed mice to freely inhale vaporizer-controlled isoflurane (1.2–1.5%, in 100% oxygen gas) and scavenged waste respiratory gas, as described (Li et al., 2020).
Under anesthesia, a rostrocaudal mid-line incision was made on the scalp. Lambda and bregma were exposed and brought into the same dorsoventral plane by adjusting the head position in the stereotaxic device. A small, lateral portion of the occipital bone was then removed to expose the dorsal surface of the medulla and allow recording electrode access to the dorsal Vc. For studies where antidromic testing was performed, a small craniotomy was also made at the coordinate-determined area above the caudal medial region of the contralateral ventral posteromedial nucleus of the thalamus (VPM), as below.
Next, a small silk thread was passed behind the lower incisors and drawn lightly taut to deflect the mandible downward. The tongue was then extended from the mouth by a small rostroventral suture clasped with a small, lightweight bulldog clamp. Using miniature rongeurs, the lower incisors were trimmed so that they did not deflect or damage the protruding tongue. Heart rate and blood oxygen level were monitored by a pulse oximeter (MouseSTAT Jr., Kent Scientific). Body temperature was maintained at 36.5–37°C with a feedback-controlled heating pad.
Electrophysiology
To test Vc neurons for antidromic spikes, the tip of a concentric bipolar stimulating electrode (200 µm O.D.; Microprobes) was positioned unilaterally in the VPM by stereotaxic coordinates and electrophysiological guidance. To do this, we first targeted a smaller-diameter tungsten recording microelectrode to the orosensory VPM using stereotaxic measurements (0.5–1.9 mm caudal of bregma, 1.0–1.2 mm lateral of the midline, and 3.5–4.4 mm ventral to the brain surface) (Franklin and Paxinos, 2008) and an electronic micro-positioner (model 2660; David Kopf Instruments). We recorded the exact coordinates where neurons were found to respond to cooling fluids applied to the warmth-adapted mouth, as below, or touching/brushing the tongue with a cotton swab. These coordinates were then used to direct the concentric bipolar stimulating electrode to the orosensory VPM, after the recording microelectrode was withdrawn. The bipolar stimulating electrode was slowly lowered to 200 µm dorsal of the VPM area. The bipolar electrode was placed into the brain only once to mitigate potential tissue damage.
Extracellular APs were recorded from single Vc neurons using a tungsten microelectrode (3–10 MΩ; FHC, Bowdoin, ME) positioned in the dorsal, caudal trigeminal nucleus. Because of decussation, we recorded neurons in Vc sites contralateral (Lipshetz and Giesler, 2016; Saito et al., 2017) to the VPM stimulating electrode in preparations where this was installed. When isolating Vc cells, the recording electrode was slowly lowered into the trigeminal nucleus using a hydraulic micro-positioner (Model MHW-4, Narishige) attached to a stereotaxic micromanipulator (Model SM-11, Narishige). The electrode was advanced in ∼2 µm increments while monitoring for neural activity to cool fluid stimulation of the oral cavity, as below. Vc neural signals were AC amplified (Grass P511, with high-impedance probe), bandpass filtered (0.3–10 kHz), and monitored on an oscilloscope and loudspeaker. Single Vc neurons were identified based on waveform consistency. APs were digitally sampled at 25 kHz (1401 interface and Spike2 software; CED) and time-stamped to the nearest 0.1 msec.
Vc neurons were examined for antidromic thalamic responses by delivering weak cathodal electrical pulses to the VPM-positioned bipolar electrode. The bipolar electrode was gradually advanced ventrally by ∼100 µm during initial tests. If VPM-directed pulses (100 µA, 0.2 msec) could trigger APs in an online Vc neuron, the VPM stimulation electrode was moved in 10 µm steps in its dorsoventral tract while pulse current was adjusted to identify stimulation threshold (i.e., the smallest electrical current amplitude applied to the VPM that could trigger APs in the Vc cell; thresholds ranged from approximately 60–70 µA across recordings). Threshold current was used for antidromic circuit testing. Vc neurons were considered to show antidromic APs to VPM shocks if they displayed the following: (1) a stable evoked AP response latency, (2) an ability to generate APs that followed high-frequency (≥200 Hz) VPM pulse trains, and (3) evidence of collision between orthodromic and antidromic APs (Lipski, 1981). Collision, which arises when one AP encounters the refractory period of another travelling in the opposite direction on the same axon, was evidenced by the absence of VPM-backfired spikes triggered at short, but not long, delays after orthodromic APs. Vc neurons that were successfully antidromically invaded were considered to project to the VPM (projection neurons).
Oral sensory stimuli
Temperature-controlled purified water was continually applied to the oral cavity at a baseline adaptation temperature of 35°C. Flow switched to brief periods of stimulation with cool and warm temperature water respectively below and above this baseline to create oral temperature changes that could excite Vc thermosensory cells. The 35°C baseline intended to mimic closed-mouth physiological temperature for mammals (Green, 1986), with similar adaptation temperatures used in other studies of oral thermosensory trigeminal neurons (Lemon et al., 2016; Yarmolinsky et al., 2016; Leijon et al., 2019).
Oral delivery of thermal fluids was accomplished using a custom apparatus, as described for mice (Wilson and Lemon, 2013; Li and Lemon, 2015). In brief, this system allowed for timed presentations of different temperature-controlled stimulus and rinse solutions to oral tissues, and rapid switching between these solutions during thermal stimulation trials. Flow rates observed at the output of this system, which is a small diameter PE elbow/tube that is positioned inside the mouth, were ∼1.4 ml/sec. Fluid delivered in this manner broadly bathed oral epithelia, including rostral and caudal regions of the tongue and oral cavity (Wilson and Lemon, 2013).
Vc neurons were stimulated with oral delivery of different thermal, and also chemesthetic, stimuli presented individually on discrete trials. Each trial was composed of sequential pre-stimulus, stimulus, and after-stimulus periods that differed in length for thermal and chemesthetic stimuli, as defined below. During the pre-stimulus period, the 35°C water adaptation rinse bathed the oral cavity. Flow switched to the stimulus solution to begin the stimulus period and then back to the 35°C adaptation rinse to begin the after-stimulus period and finish the trial. Once a trial completed, the fluid passages of the delivery system were rinsed with purified water to neutralize system temperature and remove potential remnants of the previous stimulus. In between trials, the mouth was continuously adapted to oral delivery of 35°C purified water.
The temperature of the adaptation rinse/stimulus fluid stream delivered to the oral cavity was continuously monitored by an IT-1E miniature thermocouple probe (time constant = 0.005 s, Physitemp Instruments). This probe was embedded in the distal tip of the intraoral fluid delivery tubing, positioned inside the mouth, and coupled to a digital thermometer with analog output (BAT-12, Physitemp Instruments). Thus, fluid temperatures reported here reflect oral °C – the temperature of a fluid as it entered the mouth. Temperature data were sampled at 1 kHz by the data acquisition system (Li and Lemon, 2019), which facilitated real-time measurements of oral temperature change alongside Vc cellular AP response trains. Each stated stimulus temperature step is the mean oral °C across trials that tested fluids targeted to this temperature, calculated during the last 4 s of the stimulus period.
Vc neurons were tested to respond to oral delivery of purified water at each of seven temperature steps (7°, 13°, 21°, 28°, 35°, 46°, and 54°C), randomly ordered across cells. For these trials, the pre-stimulus, stimulus, and after-stimulus periods were each 5 s long. Cooling stimulus temperatures below 35°C included mild cooling to 28°C, which is near the temperature drop threshold for cooling excitation of TRPM8 (McKemy, 2007; Tajino et al., 2011), and colder temperatures like 13° and 7°C, which span the cold response range of TRPM8 (McKemy et al., 2002). Heat included 46° and 54°C, which are associated, in part, with excitation of the polymodal nocisensor TRP vanilloid 1 (TRPV1) and sensory neurons that express this ion channel (Caterina et al., 1997, 2000). Vc neurons were also tested with oral delivery of water warmed to 35°C, which was neutral/isothermal with the adaptation rinse. Finally, a subset of Vc neurons recorded from B6 mice and all cells sampled from TRPM8 deficient mice were tested with an eighth temperature: oral delivery of water at 40°C. This aimed to gauge sensitivity to innocuous warming.
Vc neurons were also tested to respond to oral delivery of solutions of chemesthetic stimuli including 35°C 1.28 mM L-menthol (referred to herein as menthol), 35°C 0.1 mM allyl isothiocyanate (AITC; mustard oil), 35°C 1.0 mM AITC, and room temperature 1.0 mM capsaicin. The cooling agent menthol can activate TRPM8 (McKemy et al., 2002) and at the selected concentration is detected by mice in orobehavioral tests (Lemon et al., 2019). AITC engages the nocisensors TRP ankyrin 1 (TRPA1) and, at high concentrations, TRPV1 (Jordt et al., 2004; Everaerts et al., 2011). Sensitivity to capsaicin is a hallmark of excitation of TRPV1 and nociceptive neurons (Caterina et al., 1997, 2000).
Menthol and AITC were dissolved in purified water and presented to the oral cavity using our stimulus delivery system. For these trials, the pre-stimulus period was 5 s, the stimulus period was 20 s, followed by a 95 s after-stimulus period to ensure capture of potential delayed or lasting responses typical of chemesthetic agents. The order of menthol and AITC trials randomly varied across cells.
Capsaicin was dissolved in a vehicle of 1.5% ethanol and 1.5% Tween 80 in purified water (Ellingson et al., 2009; Li and Lemon, 2019; Li et al., 2022). Unlike menthol and AITC, capsaicin was uniquely brushed onto the rostral tongue at room temperature, as described (Li et al., 2022). Capsaicin trials were structured to include a 5 s pre-stimulus period, 20 s stimulus period, and a 95 s after-stimulus period. On a separate control trial, the vehicle solution used to dissolve capsaicin was also tested by brushing the vehicle-only solution onto the tongue, in the same way capsaicin was applied. Capsaicin tests were always performed last due to the lingering neural effects of lingual capsaicin (Carstens et al., 1998; Li et al., 2022).
Some neurons that remained isolated and showed baseline-level firing approximately 20 min following completion of the above stimulus tests entered additional tests with a different set of controlled water temperatures, orally presented. These included 6°, 13°, 18°, 26°, 30°, 33°, 38°, 46°, and 54°C, tested using a lower oral adaptation rinse temperature of 30°C. This aimed to study if mild warming from a lower baseline temperature (e.g., from 30° to 33°C) could excite Vc cells. These tests constituted a second attempt at characterizing neural excitability to warmth, which is reportedly rare/absent in trigeminal neurons (Landgren, 1960; Poulos and Lende, 1970a,b; Mosso and Kruger, 1973; Craig and Dostrovsky, 1991, 2001; Yarmolinsky et al., 2016; Leijon et al., 2019). The order of the different temperatures was randomized, as the above.
Identification of VPM stimulation sites
At the conclusion of Vc recording sessions, mice were euthanized by an overdose of sodium pentobarbital (≥130 mg/kg, i.p.) combined with 2% isoflurane. Mice then underwent transcardial perfusion with 0.9% NaCl followed by 4% paraformaldehyde and 3% sucrose dissolved in 0.1 M phosphate buffer. Brains were removed and stored in 4% paraformaldehyde and 20% sucrose dissolved in 0.1 M phosphate buffer refrigerated to ∼4°C. A sliding microtome (SM2010R, Leica) was used to cut coronal sections (40 µm) of forebrain tissue that included the thalamus to verify stimulating electrode sites. Sections were mounted onto gelatin-coated slides. The slides were Nissl stained, with electrode tracts compared to anatomical landmarks identified using a mouse brain atlas (Franklin and Paxinos, 2008).
Behavioral testing
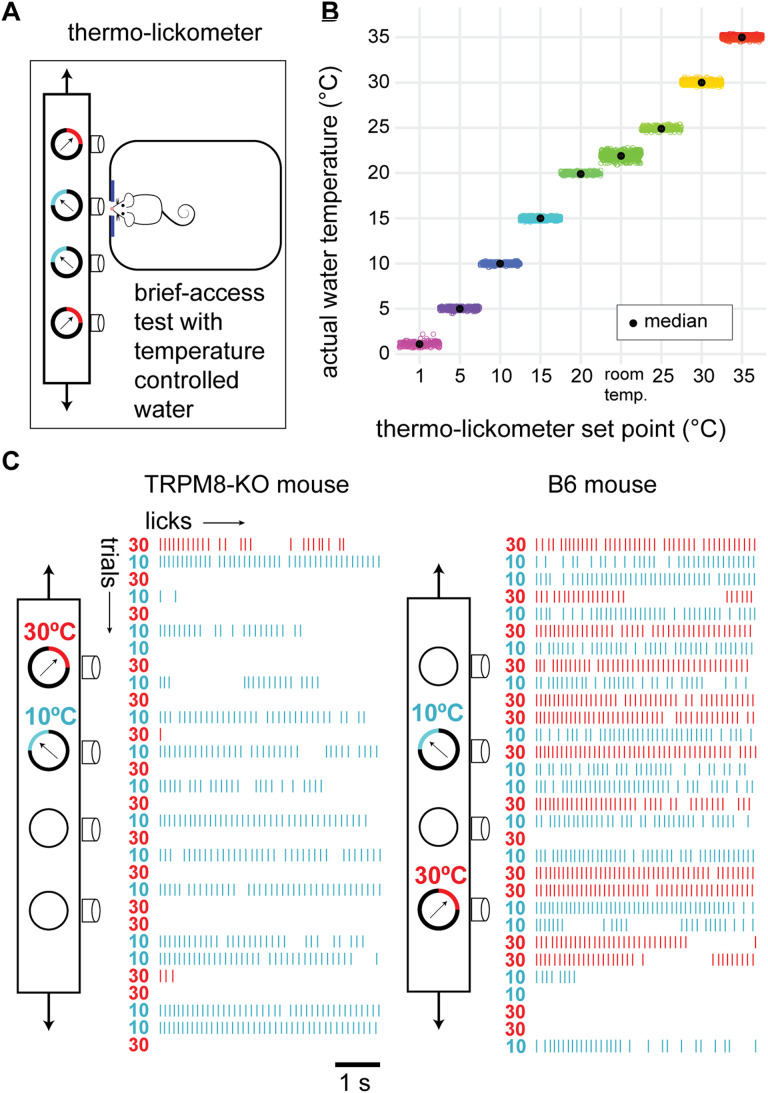
We measured licking responses to cooled and warmed water in behaving TRPM8 deficient and B6 mice to test predictions about TRPM8 orosensory function that arose from the neural data. These studies were carried out using a novel, custom-built thermo-lickometer that could independently chill (e.g., 1°C) and warm (e.g., 35°C) different fluids sampled by mice during brief-access lickometry tests. Brief-access tests measure immediate licking responses to small volumes of fluid to capture tongue/orosensory control of rodent licking behavior (Smith, 2001; Boughter et al., 2002).
The thermo-lickometer was based on a commercial Davis MS-160 contact lickometer (DiLog Instruments). Briefly, the MS-160 can present, in random order, several different sipper tubes to a mouse, and record their licking response on each tube, over a sequence of brief-access (i.e., seconds-long) fluid exposure trials. Licking response data measured by the MS-160 include inter-lick intervals (1 msec resolution). To perform thermo-lickometry, we modified this device to fit the metal tips of four of its standard fluid sipper tubes through precision machined holes in metal lick blocks. Attached to each block was a miniature thermo-electric/heat exchange apparatus that warmed or cooled the block and, in turn, the fluid in the sipper tube tip. The temperature of each block was independently regulated by a thermostat and calibrated to achieve a set point fluid temperature. For the thermo-lickometer, tube positioning relative to a mouse and measurements of licking performance were achieved like a stock MS-160 lickometer, with our modification adding independent and precise control of each stimulus fluid temperature.
Blinding
Before training, mice were singly housed in shoebox-style cages. A lab confederate then matched a random nondescript alphanumeric code to each mouse, which was used to label their cage in lieu of all other identifying information. This blinded the experimenter, who collected all data for these studies, to mouse line. Both TRPM8 deficient and B6 mice have black coats and indistinguishable outward appearances, facilitating blinding. The experimenter was un-blinded to mouse genotype only after all data were collected.
Training
To begin training, mice were placed on an overnight water-restriction schedule and initial body weights recorded. Food was freely available during all study phases. Each mouse was individually trained to receive fluid in the thermo-lickometer over 4 d. On training days 1 and 2, mice were allowed free access to a sipper tube filled with room temperature water for 30 min to habituate them to the apparatus. Days 3 and 4 familiarized mice with the brief-access procedure, offering them sipper tubes of room temperature water over 30, 5 s access trials. Once a tube was presented, mice were allowed 30 s to make a lick, which started the trial. Zero licks were recorded if no licks were made after 30 s. Inter-trial intervals were 10 s. During all study phases, mice were given 1-hr access to water in their home cage if their daily body weight fell below ∼80% of baseline.
Testing
After an approximate 2 d break with ad lib access to water, mice again entered overnight water restriction conditions and began “two-fluid” thermal lick rate tests in the thermo-lickometer. This assay was partly inspired by prior two choice temperature preference tests that, by measuring binary thermal place preference in TRPM8 mutant mice, found roles for TRPM8 in temperature recognition presumably contributed by bodily (spinal) thermosensation (Bautista et al., 2007; Dhaka et al., 2007; Knowlton et al., 2010, 2013). The present method gauged TRPM8 involvement in trigeminal-guided thermosensory behavior driven by focused thermal stimulation of the tongue.
Daily two-fluid test sessions lasted <20 min. During tests, each mouse was proffered water at two temperatures over a series 5 s brief-access trials arranged in random interleaved order, with each temperature offered on 15 trials (30 trials total per session). Trials were structured as for brief-access training. Testing lasted several days, with one of the water temperatures used as a reference temperature that was held constant across test days. The other was a comparison (test) temperature that was randomly selected, without replacement, for each test day from an eight-temperature series: 35°, 30°, 25°, 20°, 15°, 10°, 5°, and 1°C. Tests of each reference/comparison temperature pair were completed over eight consecutive days, with mice tested with these pairings for three different reference temperatures (35°, 30°, and 15°C) over sequential 8 d blocks. Mice were given at least a 2 d break with ad lib access to water in between blocks.
During daily tests, clean sipper tubes and fresh water were used for each mouse. Sipper tubes were allowed to rest in the thermo-lickometer for a few minutes before testing started to ensure temperature acclimation. Actual fluid temperatures were recorded before and after each test session using a small thermocouple probe positioned in fluid just inside each sipper tube tip. Across all cold and warm set points, variation in actual fluid temperature was low, within 0.2°C standard deviations.
Test sessions always began in the morning, typically between 08:30 and 09:30. Because mice were serially tested, the order that mice entered their thermo-lickometer session was randomized each day to mitigate potential time-of-day confounds. Furthermore, the order of the three reference/comparison temperature blocks was randomized between two squads of TRPM8 deficient and B6 mice, with four or six mice per genotype in each squad, that were sequentially tested. This controlled potential block ordering effects and divided the experiment to accommodate the time it took to clean the thermo-lickometer and temperature-equilibrate test fluids between mice, which precluded testing all mice examined in each daily session. Mice were returned to ad lib water access after all data were collected. After testing blocks were completed, there were instances where some mice were retested with select reference and comparison temperature pairs due to lack of responding or equipment issues that arose during their initial tests.
Temperatures tested in behavioral studies spanned a deeper cold range than those tested in the present neural recordings due to differences in fluid temperature control/delivery in these settings. For example, each temperature channel of the thermo-lickometer held only a small fluid volume that eased heat exchange and fluid cooling to ∼0°C. On the other hand, the oral stimulation system used with neural recordings worked with a larger, moving fluid volume that limited cold to ∼7°C. Nevertheless, comparable trends in responses emerged across temperatures in the present neural and behavioral data.
Experimental design and statistical analysis
Neural sample
Overall, 77 Vc neurons were recorded from B6 mice, with 35 cells sampled from females and 42 from males. Thirty-nine Vc neurons were recorded from TRPM8 deficient mice, with 21 neurons from females and 18 from males. For each line, there was no significant sex × stimulus interaction (two-way ANOVAs, B6 neurons, p = 0.9; TRPM8 deficient neurons, p = 0.5) or main effect of sex (B6 neurons: p = 0.9; TRPM8 deficient neurons: p = 0.7) on Vc cellular thermal responses (in Hz; measured as below). Sex was not considered in further analyses. In several cases, multiple Vc neurons were sequentially recorded from the same mouse, but at different coordinates. Thus, they were analyzed as independent units.
Measurements of neural activity
Neural activity to temperature was quantified in multiple ways. Foremost, a time-averaged response was calculated for each temperature stimulation trial to gauge cellular responsiveness across temperatures. Time-averaged activity to thermal stimuli was the difference in mean firing rate between the stimulus and pre-stimulus trial periods, expressed in APs per second (Hz). Time-averaged responses were also calculated for chemesthetic stimuli, taken as the average firing rate across the stimulus and after-stimulus periods minus the firing rate to isothermal water/vehicle; this accommodated long and variable latency responses that can be elicited by these stimuli. These calculations yielded baseline-corrected measurements of sensory response magnitude for Vc neurons. For analysis, stimulus onset time for each trial was considered to start 400 msec after computer-actuated stimulus onset to accommodate a small dead space in the delivery apparatus that could create a brief cooling artifact at the start of warming trials (e.g., Fig. 1A). Finally, after-stimulus, or “off”, responses to each temperature were calculated for neurons as the difference in mean firing rate (in Hz) between the after-stimulus and pre-stimulus trial periods.
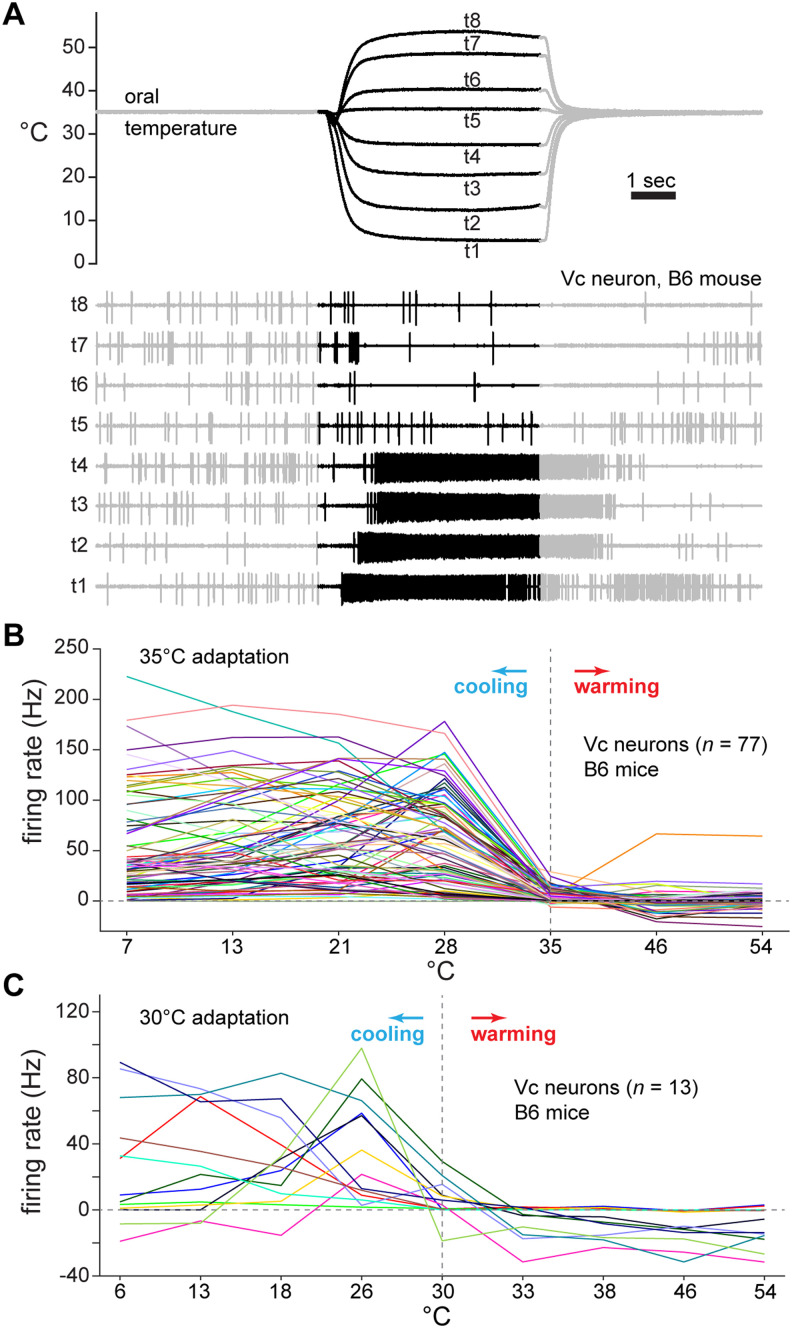
Figure 1.
Oral cooling, but not warming, frequently excites mouse Vc neurons. A, Simultaneous monitoring of change in oral temperature (upper traces) and evoked electrophysiological activity in a Vc neuron (lower traces) in a B6 mouse. Thermal traces reflect water temperature at its point of entry inside the mouth (oral °C) on each of eight different trials (t1–t8). The blackened regions of the traces mark the stimulus period, which was 5 s. This cell fired strongly, and selectively, to cooling <35°C (t1–4). Note that a brief period of inhibition (i.e., decrease in spikes compared to baseline) appears to precede the onset of AP discharge to cooling (t1–4). This feature was observed in only a handful of neurons and was rare across the cellular sample. B, Time-averaged thermal response profiles for 77 Vc neurons (colored lines) recorded from B6 mice. Neurons were tested with oral delivery of water at seven different temperatures (abscissa) following oral adaptation to 35°C. Nearly all neurons showed substantial increases in responding to cool (<35°C) but not warm (>35°C) temperatures. C, Same as (B), except data are from a subset of B6 Vc neurons tested with an expanded set of oral temperatures presented following adaptation to 30°C. Temperatures (abscissa) included mild warming (33°C, 38°C), albeit neurons (colored lines) were excited by only cooling <30°C.
Time-averaged, during-stimulus responses were used to classify the tuning orientation of individual Vc neurons to cooling temperatures. To do this, a Spearman correlation coefficient was computed between each cell's responses to the four cooling temperatures (7°, 13°, 21°, and 28°C) and the integer sequence 1 to 4. The sign (±) of this coefficient was used to sort neurons into one of two thermal tuning groups. Negative correlation coefficients emerged for cells that showed relatively strong responses to the coldest temperature tested (7°C); these cells were classified as COLD neurons. On the other hand, positive coefficients were found for neurons that generally increased their response rate as temperature stepped upward from the cold limit (7°C) to mild cooling (21°–28°C). These latter neurons, which were tuned to a segment of mild cool temperatures and gave low responses to cold, were classified as COOL cells. Notably, this simple delineation of COLD and COOL neurons produced cell groups comparable to those identified by cluster analysis in our prior studies of Vc cooling neurons with unidentified axonal projections (Lemon et al., 2016).
We quantified the frequencies of neurons that showed significant excitation or inhibition to each temperature/stimulus, and their latencies to significantly fire, using a Poisson-based algorithm (Chase and Young, 2007; Wilson and Lemon, 2014). To test for excitation, this method estimated the probability that firing rates iteratively computed over sequential APs during the stimulus period of a given trial were the same as the baseline firing rate during the pre-stimulus period. If this probability fell lower than 10−6, the null hypothesis (i.e., no excitation) was rejected and the time of the AP when this drop occurred was taken to mark the latency to significant response. On the other hand, we retained the null and considered the neuron insensitive to the input if this criterion was not met for spikes emerging within 4 s after stimulus onset. To test for inhibition, the procedure was the same except that the algorithm estimated the probability that firing rates iteratively computed over sequential APs during the pre-stimulus period were the same as the mean firing rate during the stimulus period. In this case, rejecting the null reflected pre-stimulus firing that was greater than activity during the stimulus period (i.e., the stimulus caused a significant inhibition in responding). For these tests, the stimulus period was considered to start at 400 msec following stimulus onset, as above.
The cooling threshold for each neuron was determined by identifying the temperature drop required to stimulate significant excitatory firing during the cold ramp of the 7°C trial. This was accomplished using the Poisson method above to identify response latency and comparing this to the simultaneous real-time temperature monitoring data. Thresholds were expressed relative to the average adaptation temperature measured across cells during the pre-stimulus period.
Each neuron's response to the cold limit of 7°C was also used to compute their phasic response index. This index estimated the proportion of a cell's overall response to cold that was due to the phasic increase in AP discharge associated with the cold ramp falling through innocuous cool temperatures to reach cold. This index has implications for delineating the thermal specificity of Vc neurons. By measuring Vc neural firing alongside real-time fluid temperature change inside the mouth, we were able to calculate each cell's response (in Hz) during the initial mild cooling transition, defined as the epoch where oral temperature fell from 32°C to 15°C, and the later steady cold (<10°C) period of the cold ramp to compose the phasic response index. This index ranged from 0 to 1 and was given by as follows:
where a value near 1 meant that most of a cell's activity to cold was attributed to a phasic increase in firing during the initial mild cooling transition. In contrast, an index approaching zero reflected most APs were generated during the latter and sustained cold period that followed the initial ramp phase.
We examined how phasic response indices associated with the temporal response features of neurons across temperatures and their thermal tuning. To do this, we quantified the response time course on each temperature stimulation trial as AP counts in consecutive, nonoverlapping 200 msec bins, beginning from stimulus onset to trial end. For analysis calculations, stimulus onset was delayed by 400 msec, as above. Response time course data were input to cluster analysis to identify the different patterns of thermal response kinetics displayed across Vc cells. For cluster analysis, a data vector that concatenated time-coursed firing to 7°, 13°, 21°, 28°, 35°, 46°, and 54°C was constructed for each neuron and standardized by dividing the AP count in each bin by the standard deviation of the cell's AP counts across all bins for the seven temperature stimuli. This standardization aimed to reduce magnitude differences between neurons and facilitate clustering based on the shapes of their time-evolved responses (Lemon et al., 2016). A pairwise Euclidean distance matrix was computed for all neurons using their standardized temporal responses. Hierarchical clustering under Ward's amalgamation method was then applied to this matrix. Neuronal groups were identified in the clustering solution by finding the amalgamation step at which distance drops between groups were minimized, as in our prior work (e.g., Li et al., 2022). Cluster analysis was performed using the linkage function in MATLAB (release 2021a; MathWorks).
Comparisons of neural activity between TRPM8 deficient and B6 mice
Forty-nine Vc neurons recorded from B6 mice were tested with the same eight-temperature series (7°, 13°, 21°, 28°, 35°, 40°, 46°, and 54°C) as the 39 Vc cells sampled from TRPM8 deficient mice. This subgroup of B6 cells was used as a wild-type (approximate) group to determine how silencing TRPM8 impacted Vc cellular responses. Not all Vc neurons recorded from TRPM8 deficient mice were tested for antidromic responses to thalamic stimulation; thalamic projection status was not assessed in analyses of TRPM8 mutant cells. Some recordings from B6 and TRPM8 deficient neurons were interspersed for time-of-study control.
Thermal responses were compared between TRPM8 deficient and B6 neurons using parametric/nonparametric methods, as below. Standardized Euclidean distances were calculated for Vc neural population responses to temperatures and compared between mouse lines to explore how silencing TRPM8 influenced population-level thermal codes. Distance standardization accommodated the different cell numbers in each mouse line. Distances were computed by the MATLAB function pdist and visualized using colormap matrices. Inspection of these matrices arrived at interpretations of associations between Vc population responses to temperatures that were also borne out by multivariate analyses, such as principal components, albeit these are not presented here for simplicity.
Analysis of behavioral data
Orobehavioral responses to water presented at three different reference temperatures and eight comparison (test) temperatures in two-fluid tests were analyzed across 10 B6 mice and 9 to 10 TRPM8 deficient mice. While 10 initially entered these studies, one TRPM8 deficient mouse began to show poor licking performance and did not complete all test sessions; this mouse was not included in analyses when missing data. Sex was not analyzed in behavioral studies due to low group numbers (4 and 5) for this factor and the lack of a neural effect, as above.
We calculated the number of licks mice emitted on each thermal fluid trial by adding 1 to the number of inter-lick intervals that were >50 msec. This criterion filtered any erroneously recorded phantom/noise “licks” (Ellingson et al., 2009; Lemon et al., 2019). For each mouse, their lick rate to each daily tested reference and test water temperature was calculated by applying a 20% trimmed mean to lick counts recorded across the 15 trials that offered each temperature, ignoring trials where no sampling occurred (i.e., zero licks). These data (licks per trial) served as the basis for analyses and plotting. The trimmed mean accommodated outliers by computing average licks after dropping the lower 20% and upper 20% of lick counts recorded across sampled trials.
General
Inferential analyses of neural responses were carried out using factorial ANOVA (SPSS, version 28, IBM Corporation). Mauchly's test evaluated the sphericity assumption for repeated measures, albeit no interpretative differences emerged if the ANOVA df were corrected by the Greenhouse–Geisser method to accommodate this assumption. For simplicity, we report statistical outcomes based on only the standard df. Effect size was gauged using partial eta squared , which ranged from 0 to 1 in proportion to the variance contributed by the effect in context of the error variance. Nonparametric methods, including the rank sum test, were used when compared neural data distributions showed marked differences in shape and spread. McNemar's test evaluated paired nominal data. Nonparametric analyses were also applied to the behavioral data, which were of lower sample sizes. Within mouse line, Wilcoxon signed rank tests for matched samples compared licking responses between reference and test water temperatures by test day and collapsed across days. Statistical outcomes were evaluated using α = 0.05. When applicable, p-levels were corrected for multiple comparisons using the Benjamini and Hochberg (1995) method to control false discovery rate (FDR) to <0.05. For plotting, confidence intervals for measures of location (e.g., mean) were bootstrapped using the MATLAB function bootci with 1,000 resamples. All custom analyses were coded and performed in MATLAB.
For neural data, all interactions and major effects observed in factorial ANOVAs were also analyzed using the robust statistics package WRS2 (version 1.1–4; Mair and Wilcox, 2020) in R (version 4.2.2; R Core Team (2022)). Robust statistics accommodated data that, for some comparisons, were not normally distributed or displayed group differences in variance or skew. Robust methods offer alternatives to classical inferential statistics when data challenge test assumptions (Mair and Wilcox, 2020). Robust ANOVAs were performed using 20% trimmed means. Notably, robust analyses applied to the present neural data led to the same general interpretations and results offered by standard parametric two-way ANOVAs. While affording cross-validation including in cases where standard test assumptions may have been violated, robust statistics are comparatively new and uncommon in thermosensory literature. For simplicity, we report only the outcome of conventional inferential methods in the results.
Results
Trigeminothalamic neurons respond to mild oral cooling or cold
We measured extracellular APs in vivo from 77 Vc thermosensory neurons in B6 mice. These cells were stimulated with brief, seconds-long changes in fluid temperatures delivered to the oral cavity that were fast-onset (e.g., decreased by an average of 42°C/sec during a cold ramp) and followed oral adaptation to 35°C (Fig. 1A). Nearly all Vc neurons we sampled substantially increased their AP discharge to oral cooling below 35°C (Fig. 1B). Tests for sensitivity to oral delivery of menthol in 69 neurons revealed most cells (n = 60, 87%) also showed significant excitation to this cooling mimetic (data not shown). In contrast, the recorded neurons infrequently showed excitatory responses to oral warming, including heating to 46°C and above (Fig. 1B). Furthermore, we tested some neurons with innocuous warming to 33° and 38°C following adaptation to a reduced temperature of 30°C to gauge if moderate temperature increases from a lower baseline might reveal excitatory sensitivity to warmth. However, all neurons examined showed null or inhibitory (i.e., below baseline) responses (Fig. 1C). These observations agree with prior data that show warmth-excited neurons are rare, or absent, in trigeminal pathways (Poulos and Lende, 1970a,b; Mosso and Kruger, 1973; Craig and Dostrovsky, 1991; Lemon et al., 2016; Yarmolinsky et al., 2016; Leijon et al., 2019). None of the sampled Vc cells gave significant excitation to oral stimulation with the nociceptive agents AITC or capsaicin; these trials were not further analyzed.
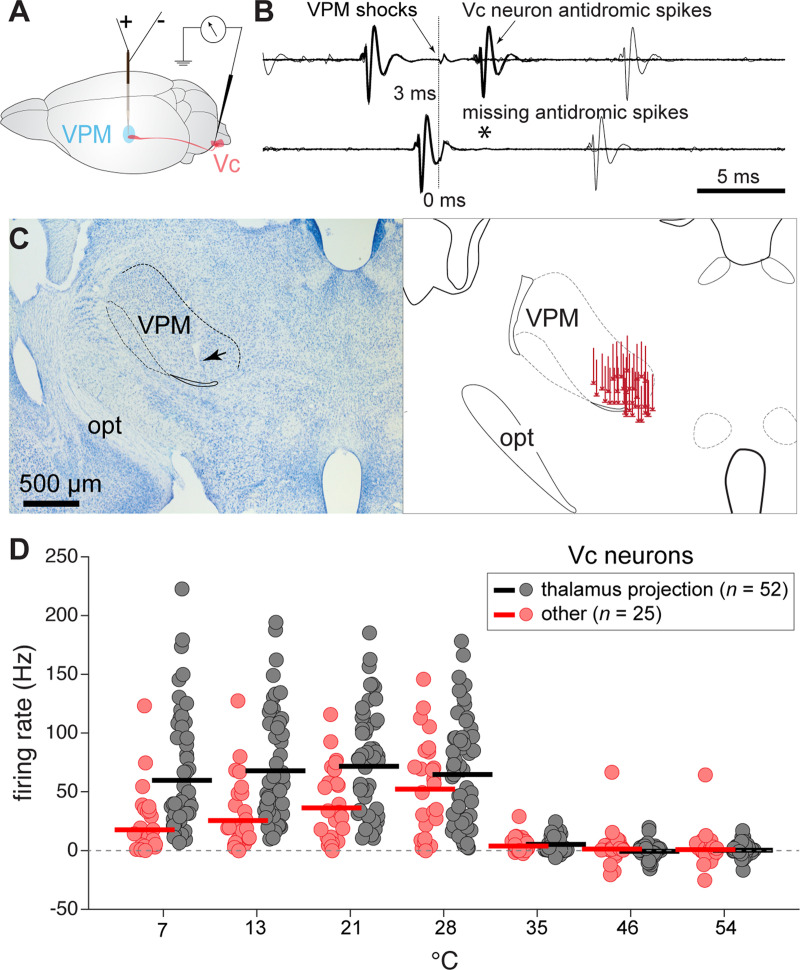
By capturing antidromic responses to electrical pulse stimulation of the VPM (Fig. 2A–C), we identified 52 Vc neurons in our sample that projected to the thalamus (projection neurons) and separated them from the remaining 25 cells that could not be antidromically invaded from the VPM. Identification of projection neurons confirmed recordings from central cells with soma in the spinal trigeminal nucleus and axons that tracked to thalamic circuits. Projection status did not influence baseline neural firing rates measured during the 35°C adaptation rinse (mean = 4.8 Hz; n.s. main effect of group, p = 0.8). However, projection neurons showed greater responsiveness to change in oral temperature (neural group × temperature interaction, F(6,450) = 10.1, p < 0.001, = 0.12), as evident in their larger time-averaged responses to cooling below 35°C (Fig. 2D). Projection neurons also showed broad, equivalent mean responses to cooling temperatures <35°C (n.s. simple effect of temperature, p = 0.4) whereas the remainder of the sample (“other” neurons) displayed greater excitation to moderate cool (e.g., 28°C) over colder temperatures (simple effect of temperature, F(3,225) = 6.6, p < 0.001, = 0.08; Fig. 2D).
Figure 2.
Response features of Vc thermosensory neurons that project to the thalamus in B6 mice. A, Experimental schematic showing circuit implemented to capture antidromic responses in Vc neurons. A bipolar stimulation electrode was placed in the orosensory thalamus (VPM) while a recording electrode monitored extracellular APs in the contralateral Vc. B, Collision test in one Vc neuron showing antidromic APs. Antidromic spikes appeared when weak electrical shocks of the VPM were triggered at comparably long (upper) but not short (*, lower) delays after random orthodromic spikes, indicative of successful spike collision and axonal projection to the VPM. Multiple sweeps are superimposed. C, Reconstruction of VPM electrode stimulation sites. Micrograph (left) shows an example coronal brain section depicting the electrode tract and tip placement (arrow) in the VPM in one mouse. Drawing (right) shows stimulating electrode tract/tip locations in the VPM for 40 mice. Rostrocaudal dimension is conflated for simplification. opt, optic tract. D, Time-averaged responses (ordinate) by thalamus projection (n = 52) and other (n = 25) Vc neurons (circles) to oral delivery of cool (<35°C) and warm (>35°C) temperature water (abscissa). Bars represent 10% trimmed means. On average, projection neurons showed broad, equal (p > 0.05) responses across cooling temperatures. Other neurons, which could not be antidromically invaded from the VPM, displayed greater excitation to mild cooling (e.g., 28°C) than colder temperatures (p < 0.05).
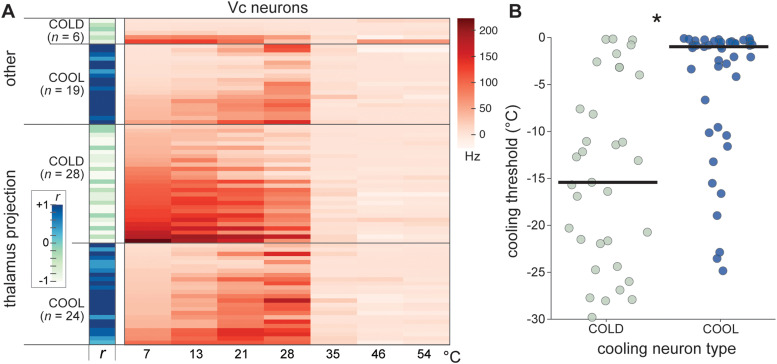
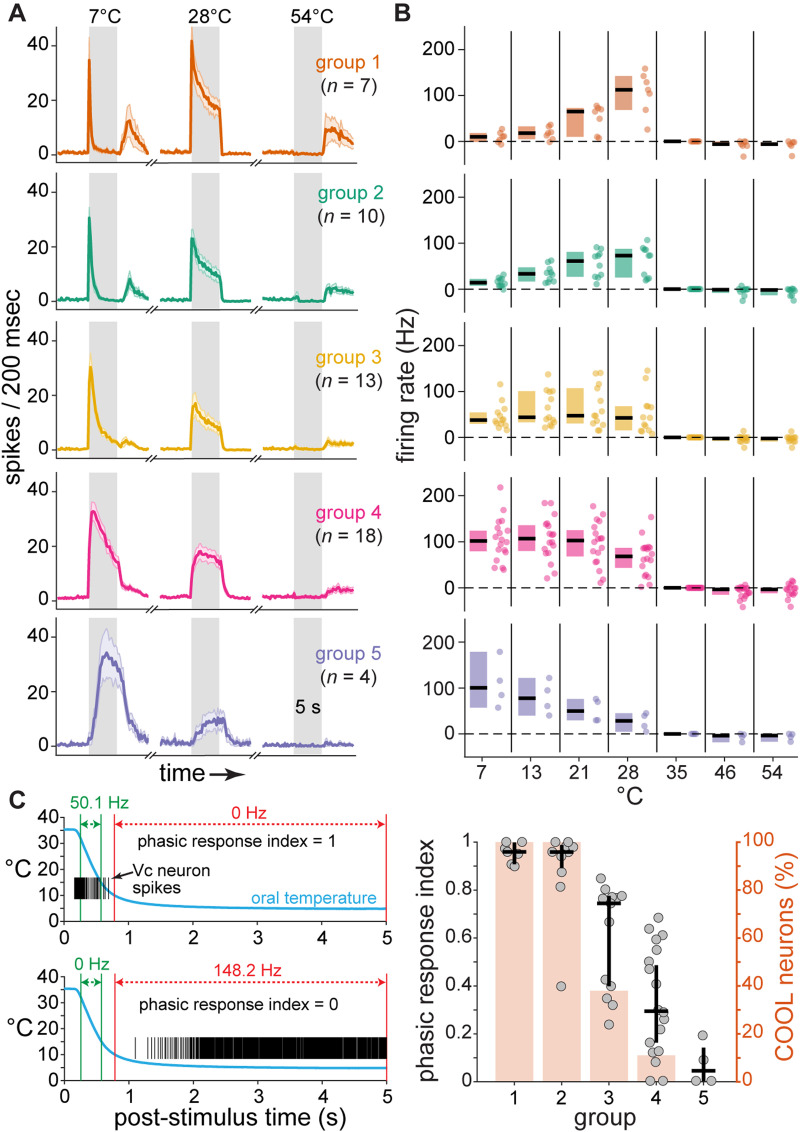
The broad mean responsiveness of projection neurons across cool temperatures was attributable to averaging across cells with diverse, and opposing, tuning to moderate oral cooling and cold. Based on time-averaged responses, about half (n = 28, 54%) of the projection neurons were classified as COLD cells that showed relatively strong AP discharge rates to oral delivery of water at the coldest temperatures tested, including 7°C (Fig. 3A). The remainder (n = 24, 46%) were COOL neurons that, in contrast, were primarily tuned towards only a segment of moderate temperatures. COOL-type projection neurons frequently gave their highest AP discharge to mild cooling drops to 28° and 21°C, with comparably low responsiveness to colder temperatures (Fig. 3A). Analysis of the time course of neural firing revealed that cold temperatures like 7°C predominantly evoked only a transient onset response in COOL-type cells (Fig. 4A,B), with most COOL neurons displaying phasic response indices near or equal to 1 (Fig. 4C). Thus, cold excitation of COOL neurons was associated with their sensitivity to the initial mild cooling phase of the cold ramp but not steady cold. This implies COOL cells demonstrate veridical tuning towards only a limited range of moderate temperatures. Accordingly, COOL neurons showed low cooling response thresholds and could impressively begin to respond when temperature fell by <1–2°C below adapted warmth (Fig. 3B). In contrast, COLD cells typically required much deeper temperature drops to begin to fire (test of the null hypothesis that COLD and COOL neurons showed equal median cooling thresholds, Wilcoxon rank sum test, p < 0.001).
Figure 3.
Vc cooling neuron types in B6 mice. A, Heatmap shows time-averaged responses (color legend) by 77 Vc neurons (rows) to oral delivery of cool (<35°C) and warm (>35°C) temperature water (abscissa). Vc neurons that were antidromically invaded from the orosensory thalamus were classified as thalamus projection cells; otherwise, cells were labeled as “other”. Within these groups, cells are subdivided by their tuning orientation to cooling temperatures, where the sign (±) of the correlation coefficient (r) computed between all cooling responses and an integer sequence classified neurons as COOL or COLD. COLD neurons showed relatively strong responses to the coldest temperatures tested whereas COOL cells were maximally excited by mild cooling and gave low responses to colder temperatures. B, Cooling temperature thresholds (ordinate) for COLD and COOL neurons (circles) from panel A. COLD neurons showed higher cooling excitation thresholds (i.e., required deeper cooling temperature drops to fire) compared to COOL cells (*, p < 0.05), which frequently showed significant excitation to temperature decreases of <1° to 2°C below 35°C. Bars are medians.
Figure 4.
COOL neurons show phasic responses to cold temperatures. A, Time-dependent responses to oral delivery of cold (7°C), mild cool (28°C), and hot (54°C) water observed across five groups of thalamus-projection Vc neurons (n = 52, sampled from B6 mice) recovered by hierarchical cluster analysis of temporal firing. For each plot, solid line tracks mean spikes per 200 msec over the pre-stimulus, stimulus (grayed area, 5 s), and after-stimulus periods. Shaded region surrounding mean line denotes standard deviation. B, Time-averaged responses (ordinate) to temperatures (abscissa) by individual cells (points) within each corresponding neural cluster (row aligned) in panel A. The median (horizontal bar) and its 95% confidence interval (vertical shaded area) are shown to the left of each response distribution. Comparing panels reveals projection neurons that gave a transient response to cold onset (groups 1 and 2, 33% of sample; panel A) were oriented to mild cooling temperatures (28° and 21°C, panel B). C, Analysis of phasic response indices for thalamus-projection Vc neurons in B6 mice. The left column shows data for calculations of phasic response indices for two different example cells (upper and lower plots). This index ranged from 0 to 1 and gauged the proportion of each cell's response to cold (spikes raster plot) contributed by the initial mild cooling transition phase of the cold ramp (blue trace). At one extreme, an index equal to 1 (upper plot) resulted for neurons sensitive to only the mild cooling transition (denoted by green vertical bars). An index of 0 (lower plot) was found for neurons that responded only during the later steady cold period (denoted by red vertical bars). The right column of panel C plots phasic response indices (left ordinate) for projection neurons (circles) in the temporal response groups (abscissa) defined in panel A. For each group, the index median (horizontal bar) and its 95% confidence interval (vertical bar) are shown. A shaded rectangle gives the percent of neurons in each group that were classified as COOL cells (right ordinate), which were oriented to mild cooling by time-averaged firing. All neurons in groups 1 and 2 were COOL-type and showed phasic response indices that were near and equal to 1. This implied their activation to cold was due to phasic excitation during the initial mild cooling transition of the cold ramp but not cold temperature. Phasic response indices were larger in groups 1 and 2 compared to the other groups (Kruskal–Wallis test, χ2 = 37.1, df = 4, p < 0.001), which included comparably few or no COOL neurons.
Central trigeminal neurons responsive to mild cooling require TRPM8
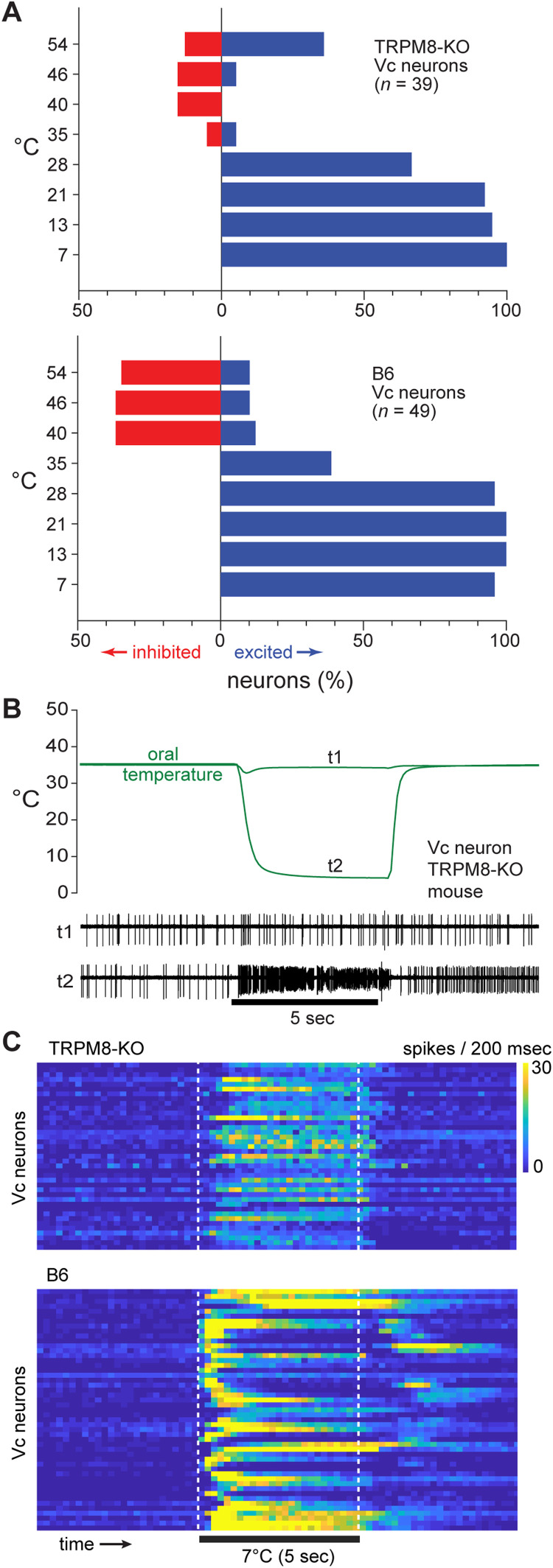
We next examined the dependence of COLD and COOL central trigeminal neurons on the presence of active TRPM8 channels. To do this, Vc cellular responses to oral temperature were compared between TRPM8 knock-out (39 cells) and wild-type B6 (49 cells) mice. We observed that in vivo neural sensitivity to cold temperatures showed robust persistence in TRPM8 deficient mice. In this line, more than half of the sampled Vc neurons were significantly excited at each cooling temperature step <35°C (Fig. 5A), with several TRPM8 deficient cells showing notably strong responses to the coldest temperature tested (Fig. 5B,C). Thus, non-TRPM8 mechanisms can participate in responses to oral cooling in CNS trigeminal pathways.
Figure 5.
Vc neurons in TRPM8 deficient mice maintain sensitivity to oral cooling. A, Bar plots give the percentage of Vc neurons that were significantly inhibited or excited (abscissa) by cool (<35°C) and warm (>35°C) temperatures (ordinate) in TRPM8 deficient (n = 39 cells, top) and B6 control (n = 49 cells, bottom) mice. TRPM8 deficient neurons showed frequent excitation to cooling temperatures <35°C but displayed a lower (FDR-corrected Fisher's exact tests, p < 0.05) incidence of inhibition to warm temperatures compared to B6 cells. Excitation to 35°C is attributable to subtle differences between the adaptation rinse and stimulus fluid temperature (e.g., trace t1 in panel B). B, Electrophysiological activity (lower traces) measured from an example Vc neuron in a TRPM8 deficient mouse during oral stimulation with neutral (t1) and cold (t2) temperatures (upper traces). Cold strongly excited this cell in the absence of TRPM8. C, Heatmaps showing time-dependent responses (color scale) by Vc neurons (rows) to the cold limit tested (7°C) in TRPM8 deficient (top) and B6 (bottom) mice. Many TRPM8 deficient neurons displayed robust responses to cold.
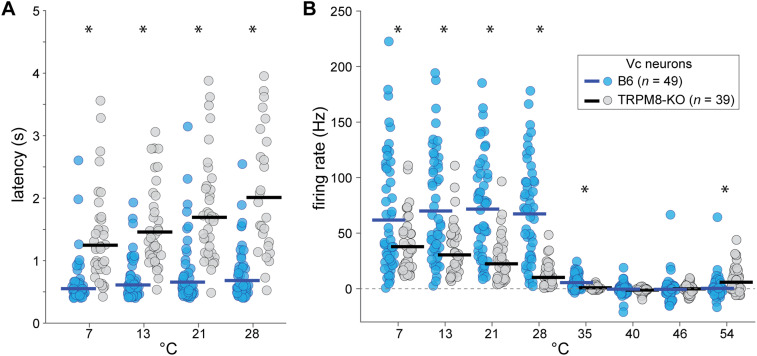
Nevertheless, Vc thermosensory neurons showed several differences between TRPM8 deficient and B6 mice. For instance, B6 neurons more frequently displayed inhibition than excitation to warm temperatures >35°C (McNemar's statistic corrected for multiple tests, p < 0.05). However, this same trend was not significant for TRPM8 deficient cells (McNemar's statistic corrected for multiple tests, p > 0.05; Fig. 5A), agreeing with a role for TRPM8 in warm-evoked inhibition of cooling neurons (Paricio-Montesinos et al., 2020). Furthermore, TRPM8 deficient neurons showed longer excitatory response latencies (line × temperature interaction, F(3,204) = 26.5, p < 0.001, = 0.28, Fig. 6A) and lower response magnitudes (line × temperature interaction, F(7,602) = 23.7, p < 0.001, = 0.22, Fig. 6B) to all cooling steps <35°C. Considering mean firing, B6 neurons displayed equivalent responses across the cooling steps (n.s. simple effect of cool temperatures <35°C on firing rate, p = 0.47) whereas TRPM8 deficient units showed only a monotonically increasing graded response to cold (simple effect of cool temperatures <35°C on firing rate, F(3,258) = 10.4, p < 0.001, = 0.11, Fig. 6B).
Figure 6.
Vc cellular responses to oral cooling are slower and lower in TRPM8 deficient mice. A, Latency (ordinate) to respond to oral delivery of cooling temperatures below 35°C (abscissa) for Vc neurons (circles) in TRPM8 deficient (n = 39 cells) and B6 control (n = 49 cells) mice (see legend in B). Only latencies for significant responses are shown. An interaction (p < 0.05) revealed latencies were longer in TRPM8 deficient cells (*, p < 0.001, tests for a simple effect of mouse line at each cool temperature). B, Time-averaged responses to cool (<35°C) and warm (>35°C) temperatures by Vc neurons in TRPM8 deficient and B6 mice. Post hoc analyses that followed a significant interaction (p < 0.05) revealed that Vc responses to all cooling steps were lower in TRPM8 deficient mice (*, p < 0.02, tests for a simple effect of mouse line at each temperature). Nonetheless, TRPM8 deficient cells continued to respond to cooling with graded increases in firing to increasing cold (p < 0.05; simple effect of cool temperatures on firing rate). Horizontal bars (both panels) are 10% trimmed means.
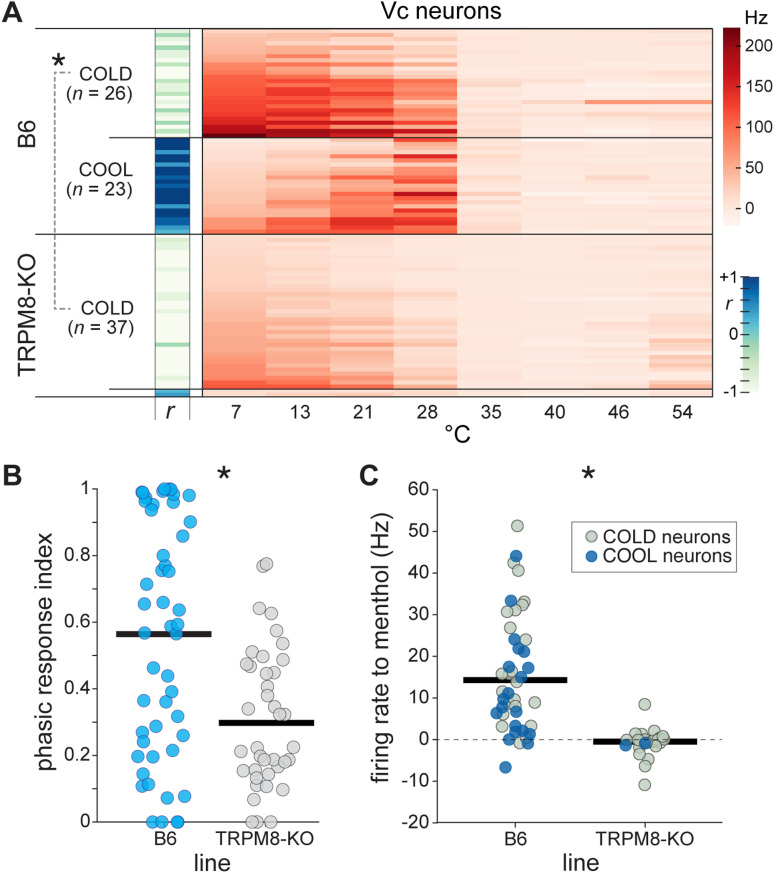
Accordingly, among the 39 cooling-active Vc neurons recorded from TRPM8 deficient mice, nearly all of them, n = 37 (95%), were COLD-type cells that showed relatively low responsiveness to mild cooling and stronger AP discharge to the colder temperatures tested (Fig. 7A). The two TRPM8 deficient neurons that did not fall into this group gave reduced firing overall, leaving Vc neurons with robust excitation to mild cooling absent from the TRPM8-null line. Thus, there was a persistence of COLD but an absence of COOL neurons in TRPM8 deficient mice. In marked contrast, the comparator sample of Vc neurons recorded from B6 mice with intact TRPM8 function retained nearly equal numbers of COLD (n = 26, 53%) and COOL (n = 23, 47%) cells (Fig. 7A).
Figure 7.
TRPM8 deficient mice show cell type-specific deficits in cooling responses. A, Heatmap shows time-averaged responses (Hz, color legend) to oral delivery of cool (<35°C) and warm (>35°C) temperature water (abscissa) by 88 Vc neurons across TRPM8 deficient (n = 39 cells) and B6 control (n = 49 cells) mice. Neurons are divided by mouse line and then by thermal tuning, which was gauged by the sign (±) of the correlation coefficient (r) computed between their responses to cooling temperatures and an integer sequence (–, COLD; +, COOL). COOL and COLD neurons were found at similar frequencies in B6 mice. In contrast, robust COOL neurons did not appear in TRPM8 deficient mice, where COLD neurons were ubiquitously encountered. The COLD neurons that persisted in TRPM8 mutants gave lower (*, p < 0.05) responses to cooling temperatures <35°C compared to B6 COLD cells. B, Phasic response indices were lower (*, p < 0.05) for Vc neurons (circles) in TRPM8 deficient mice, reflecting the predominance of cold-tuned neurons in this line. C, Oral delivery of menthol produced larger responses in Vc neurons from B6 than TRPM8 deficient mice (*, p < 0.05), where menthol was largely ineffective. Horizontal bars in panels B and C are 10% trimmed means.
These data reveal that embryonic silencing of TRPM8 channels reduced, but did not abolish, neural responses to oral cooling and did so in a cell type-specific way. COOL neurons tuned to mild cooling temperatures, which are normally involved with the trigeminothalamic pathway (Fig. 3), do not develop in central trigeminal circuits in TRPM8 deficient mice. Instead, COLD-type neurons predominate without TRPM8 – a trend also supported by reduced phasic response indices, indicative of activation to colder temperatures, for cells in TRPM8 knock-out mice (Wilcoxon rank sum test against data from B6 mice, p = 0.002; Fig. 7B). Nonetheless, Vc COLD neurons showed lower responses across cooling temperatures <30°C in TRPM8 deficient compared to B6 mice (comparison of COLD neurons between lines, main effect of line, F(1,60) = 39.7, p < 0.001, = 0.40, Fig. 7A), suggesting COLD neurons do show partial reliance on active TRPM8 channels. Furthermore, oral delivery of menthol produced larger Vc cellular responses in B6 compared to TRPM8 deficient mice (Wilcoxon rank sum test, p < 0.001), where menthol was largely ineffective (Fig. 7C). Thus, menthol-insensitive thermoception mechanisms drive Vc neural responses to oral cold in TRPM8-null mice.
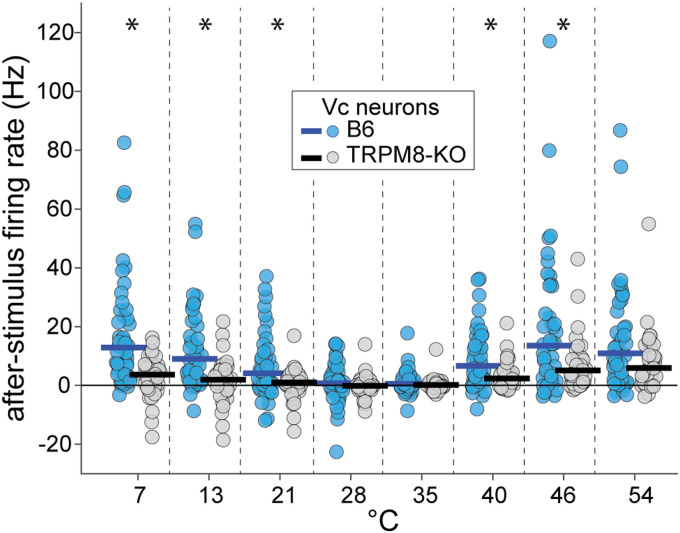
Finally, we found that after-stimulus, or “off”, responses to temperatures, which appeared as rebound excitatory firing that followed cessation of the thermal stimulus in some cells (e.g., group 1, Fig. 4A), were reduced in TRPM8 deficient compared to wild-type mice (line × temperature interaction, F(7,595) = 4.0, p < 0.001, = 0.04, Fig. 8). This result suggests TRPM8 is involved with generating temperature-off activity in trigeminal neurons under select conditions.
Figure 8.
“Off” neural responses to temperatures are suppressed in TRPM8 deficient mice. Plot shows after-stimulus discharge rates (ordinate) to oral delivery of cool (<35°C) and warm (>35°C) temperature water (abscissa) by Vc neurons (circles) in TRPM8 deficient (n = 39 cells) and B6 (n = 49 cells) mice. After-stimulus activity in Vc neurons followed, and was evoked by, cessation of the thermal stimulus (i.e., stimulus-off responses). The time course of after-stimulus firing is visible in the temporal response data for B6 neurons in Fig. 4. Post hoc analyses that followed a significant interaction (p < 0.05) revealed that off responses to cool/cold (21°, 13°, and 7°C) and warm/hot (40° and 46°C) temperature steps were lower in TRPM8-null mice (*, p < 0.01, tests for a simple effect of mouse line at each temperature). Horizontal bars are 10% trimmed means.
Neural effects of TRPM8 associate with orosensory behaviors to temperatures
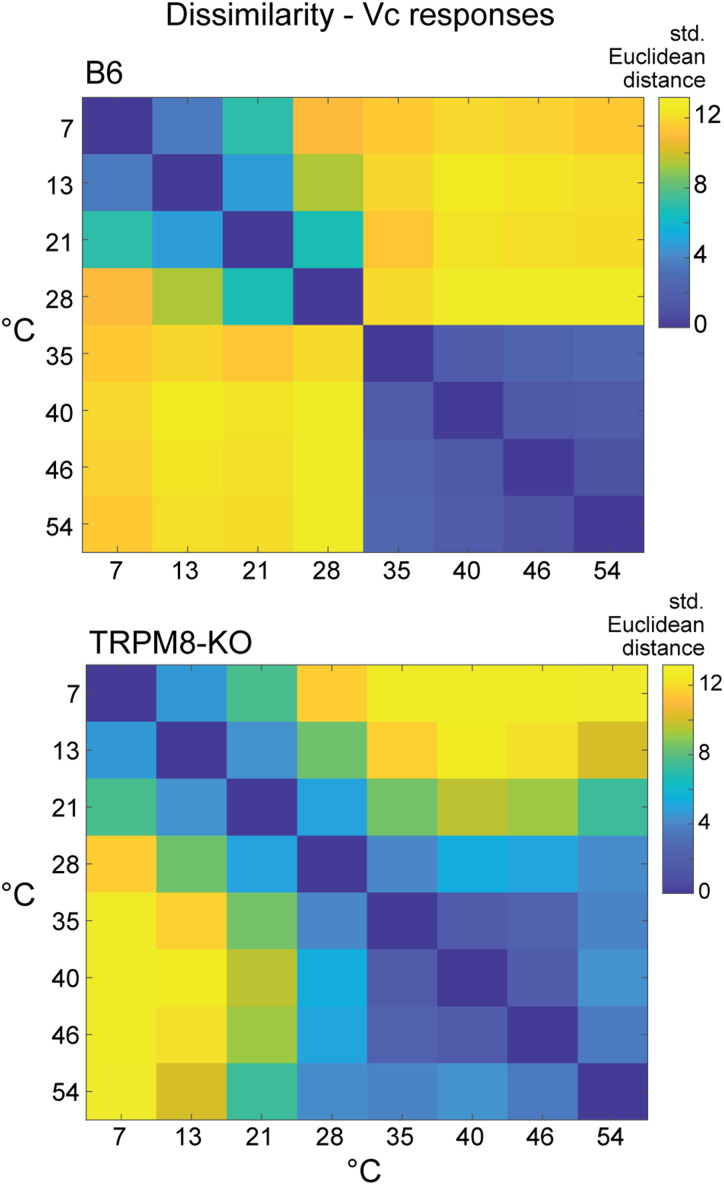
Calculation of multivariate distances between Vc neural population responses to thermal stimuli revealed that TRPM8 deficient mice lacked a sharp neural contrast and distinction between warming (≥35°C) and mild cooling (<30°C) that was present in wild-type B6 mice (Fig. 9). Instead of dissimilarity, neural population responses in TRPM8 mutants showed similarity to, and diverged only gradually from, warmth with drops to lower temperatures. Notably, mild cooling (e.g., 28°C) evoked warmth-like (≥35°C) activity and cold unique responses appeared at substantially lower temperatures (e.g., 7°C) compared to wild-type (Figs. 7A, 9). These data suggest that TRPM8 input normally establishes a neural information boundary in trigeminal circuits that separates excitatory cooling signals from warm temperatures. The loss of this boundary when TRPM8 was silenced extended the reduced neural activity pattern for warmth to adjacent cool temperatures. Assuming the sampled neurons can influence thermal coding, the appearance of warmth-like activity at lower temperatures predicts TRPM8 deficient mice would show orobehavioral responses to moderate cooling that reflect sensory generalization to warmth.
Figure 9.
Silencing TRPM8 decreases neural contrast between mild cool and warm temperatures. Matrices plot standardized Euclidean distances (legend) between Vc neural population responses to temperatures in B6 control (n = 49 cells, top) and TRPM8 deficient (n = 39 cells, bottom) mice. When compared to distances shown for B6 mice, responses to mild oral cooling (28°C and 21°C) in TRPM8-null mice show unusually increased similarity (i.e., reduced distance) to warm temperatures ≥35°C.
This prediction was tested by measuring orosensory hedonic responses to temperature-controlled fluids in behaving TRPM8 deficient (n = 9 to 10) and wild-type B6 (n = 10) mice performing in our thermo-lickometer. This novel device supported automated and random presentation of multiple temperature-controlled fluids to a mouse (Fig. 10A,B) over a series of brief-access trials while monitoring licking responses to each fluid (Fig. 10C). Using two of the fluid channels of the thermo-lickometer, we first examined “two-fluid” lick rates to water held at a warm (35°C) reference and each of a series of cooler test temperatures that straddled and fell below TRPM8 cooling excitation threshold, which is around 28°C (McKemy, 2007; Tajino et al., 2011). This aimed to initially characterize how control and TRPM8 mutant mice would respond to warming in an orosensory setting when also offered a choice of lower temperatures.
Figure 10.
The thermo-lickometer. A, Summary schematic of this device, which monitored mouse licking responses to water proffered at different controlled temperatures over a series of brief-access trials. A computer-controlled movable table (arrows) shuttled between fluid sipper tubes/temperatures to randomize their order of presentation across trials. B, Points show actual water temperatures (ordinate) measured before and after all fluid test sessions presently performed in the thermo-lickometer. Water temperatures follow thermo-lickometer set point temperatures (abscissa) and show high stability across animals/trials. Room temperature data represent temperature-uncontrolled water presented during brief-access training and show more thermal variance than controlled fluid temperatures. C, Example raw data collected from a TRPM8 deficient (left) and a wild-type B6 (right) mouse during two-fluid tests. Stacked raster plots show for each animal their licks to water randomly presented at 30°C (reference temperature) and 10°C (test) over 30 consecutive 5 s trials (10 s inter-trial interval). Schematic depicts use of different thermo-lickometer tubes for temperature control, which was randomized across sessions. Based on these plots, the TRPM8 deficient mouse clearly preferred to lick 10°C water, whereas the B6 mouse appeared indifferent to both temperatures.
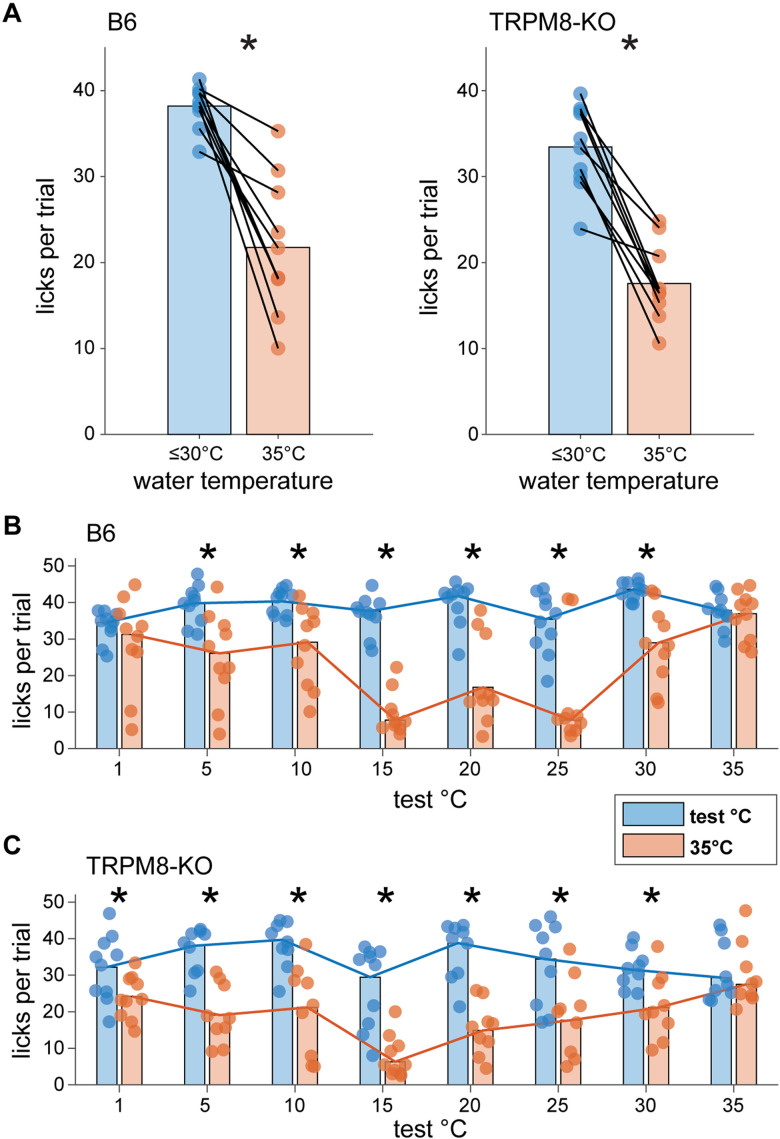
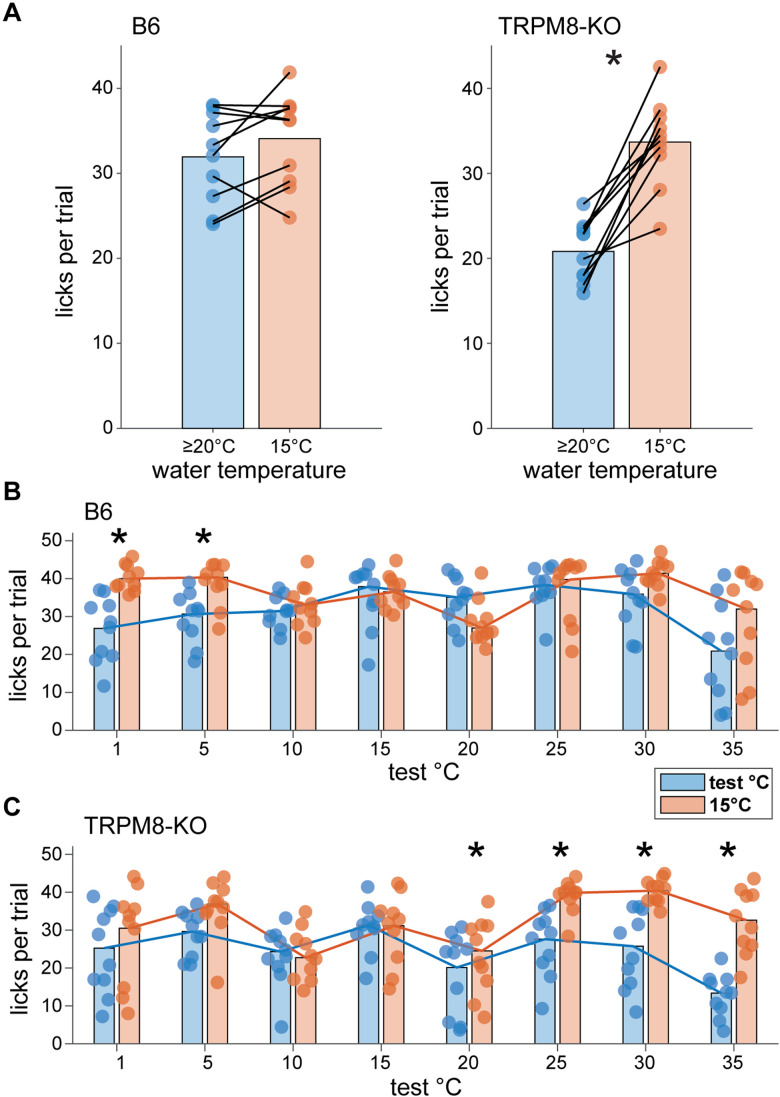
Results showed that TRPM8 deficient and wild-type mice both made more licks to water cooled to test temperatures <35°C than to water warmed to a 35°C reference temperature (data collapsed across days with ≤30°C test temperatures; B6 mice: p = 0.002; TRPM8 deficient mice: p = 0.002, Wilcoxon signed rank tests; Fig. 11A). Specifically, wild-type mice emitted more licks to water proffered at cool temperatures from 30°C to 5°C compared to warmth at 35°C (p < 0.04, FDR-corrected Wilcoxon signed rank tests; Fig. 11B). TRPM8 deficient mice showed higher lick rates across 30°C to 1°C water than to water warmed to 35°C (p < 0.009, FDR-corrected Wilcoxon signed rank tests; Fig. 11C). Notably, cool test temperatures ≤30°C stimulated mean lick rates that approached the maximum number of licks that B6 mice could make in a 5 s fluid exposure trial with room temperature water (about 40 licks), as estimated from their established peak inter-lick interval (∼124 msec; Boughter et al., 2007). This observation and the reduced licks to warm water displayed by both mouse lines implies TRPM8 deficient and B6 mice actively avoid oral warmth in favor of cool temperatures in an orosensory setting.
Figure 11.
Mice avoid licking warm (35°C) water when given a choice of cooler fluids in two-fluid tests. A, Mean licks made by B6 (n = 10, left) and TRPM8 deficient (n = 9 to 10, right) mice (circles) to water proffered at a reference temperature of 35°C and test temperatures ≤30°C during brief-access trials in two-fluid tests. Data for each water temperature condition are averaged over the 7 testing days where test temperatures were below 35°C. Lines connect mean test and reference temperature licking responses made by individual mice. Bar plots give mean licks per trial to each water temperature condition, collapsed across mice. On average, both B6 and TRPM8 deficient mice made more licks to water at cooler temperatures below 35°C (*, p < 0.05). Panels B, C display licks per trial made by individual mice (circles) to water presented at each test and 35°C reference temperature pair in two-fluid tests. Bar plots give average (20% trimmed mean) licks for each pair collapsed across mice, with trend lines connecting means to enhance visualization. These data represent specific effects that composed the averages in panel A. As shown here, B6 mice reduced their licks to warm 35°C water when also offered water at cooler temperatures from 30°C to 5°C in two-fluid tests (*, p < 0.05; panel B). Similarly, TRPM8 deficient mice showed reduced licks to warm 35°C water when offered 30°C to 1°C water (*, p < 0.05; panel C). Notably, cooler water temperatures generally elicited near maximal lick rates in mice (see Results), implying their reduced licking to 35°C water reflects active avoidance of warmth and a preference for the cooler fluid.
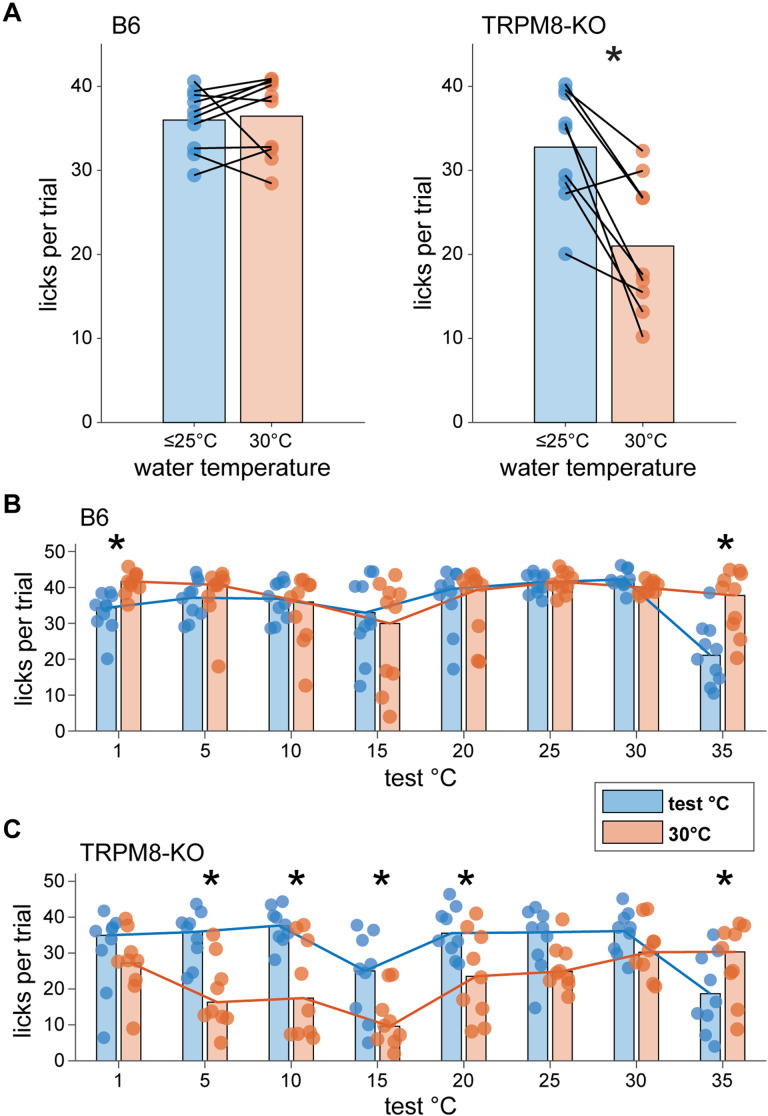
The same mice were subjected to additional two-fluid tests using water moderately cooled to 30°C as the reference. This temperature bordered on the cooling excitation threshold for TRPM8. Results revealed that, on average, wild-type B6 mice showed equal licks across trials that presented water at 30°C and test temperatures <30°C (data collapsed across days with ≤25°C test temperatures; p = 0.43, Wilcoxon signed rank test; Fig. 12A). B6 mice specifically appeared indifferent to 30°C and fluid temperatures from 25 to 5°C, licking water proffered at these cool to cold test temperatures and the 30°C reference at the same rate (p > 0.3, FDR-corrected Wilcoxon signed rank tests; Fig. 12B). In contrast, TRPM8 deficient mice emitted more licks to water proffered at test temperatures <30°C compared to the 30°C reference (data collapsed across days with ≤25°C test temperatures; p = 0.008, Wilcoxon signed rank test; Fig. 12A). This effect specifically emerged as test temperatures sequentially stepped down to 20°C (p = 0.04), 15°C (p = 0.02), 10°C (p = 0.02), and then to 5°C (p = 0.02, FDR-corrected Wilcoxon signed rank tests; Fig. 12C). This unique preference for cooler water temperatures in TRPM8 deficient mice was largely driven by reductions in licking to the 30°C reference. These reductions paralleled the licking suppression observed towards warm 35°C water when it was used as the reference (Fig. 11C).
Figure 12.
TRPM8 deficient mice uniquely avoid licking mild cool (30°C) water for colder water in two-fluid tests. A, Mean licks per trial made by B6 (n = 10, left) and TRPM8 deficient (n = 9, right) mice (circles) to water proffered at a reference temperature of 30°C and test temperatures ≤25°C in two-fluid tests. Data represent averaged responses collapsed across the 6 testing days where test temperatures were below 30°C. Lines connect test and reference temperature licking responses made by individual mice. Bar plots give mean licks to each water temperature condition collapsed across mice. On average, B6 mice showed equal lick rates to 30°C and lower water temperatures (p > 0.05). In contrast, TRPM8 deficient mice made more licks to water at cooler temperatures below 30°C (*, p < 0.05). Panels B, C show licking responses by individual mice (circles) to water at each specific test and 30°C reference temperature pair offered in two-fluid tests. Bar plots give average licks (20% trimmed means) made to each pair of temperatures collapsed across mice, with trend lines connecting means. While making more licks to 30°C than 1°C cold water (*, p < 0.05), B6 mice showed indifference and emitted equal licks to water across a broad range of cooling temperatures from 30°C to 5°C (p > 0.05; panel B). In contrast, TRPM8 deficient mice reduced their licks to 30°C water and showed greater licking to cooler water temperatures specifically from 20°C to 5°C in two-fluid tests (*, p < 0.05; panel C). This unique avoidance of mild cooling displayed by TRPM8-null mice is similar to mouse licking avoidance of warm water (Fig. 11).
Collectively, these data demonstrate that while mice will avoid warmth and favor cold water when given a choice in an orosensory task, temperature avoidance in this setting anomalously extends to lower, moderate cool temperatures in mice that lack TRPM8. This was evidenced by similar reductions in licking to both warm (35°C) and normally innocuous cool (30°C) water, and preference for lower water temperatures, in TRPM8 deficient mice performing in two-fluid tests. This common behavioral response to warm and cool temperatures was remarkably predicted by the unique similarity, and “blurring”, between avoided warming (≥35°C) and mild oral cooling (∼30°C) that appeared in central trigeminal neural responses in TRPM8 deficient animals (Fig. 9).
Notably, TRPM8 deficient mice showed an ability to recognize and respond to the lower of two temperatures over a broad cooling range in two-fluid tests. With the 30°C reference, TRPM8 mutants systematically increased their focus and licking towards cooler water as comparison temperatures stepped below this reference (Fig. 12). Furthermore, we ran the squads of mice in additional two-fluid tests that used 15°C water as the reference. Results showed that TRPM8 deficient mice emitted more licks to 15°C cold water than to water proffered at less cool (20°C, p = 0.04; 25°C, p = 0.02; 30°C, p = 0.008) and warm (35°C, p = 0.008) temperatures (FDR-corrected Wilcoxon signed rank tests, Fig. 13C; data collapsed across days with >15°C test temperatures: p = 0.002, Wilcoxon sign rank test, Fig. 13A). In contrast, B6 mice were indifferent to lick 15°C and less cool/warmer fluids (p > 0.1, FDR-corrected Wilcoxon signed rank tests, Fig. 13B; data collapsed across days with >15°C test temperatures: p = 0.23, Wilcoxon sign rank test, Fig. 13A). These data further suggest that TRPM8 deficient mice uniquely prefer colder over moderate cool temperatures in orosensory settings and reveal they are not “blind” to react to the lower of two cool or cold temperatures sensed by the mouth.
Figure 13.
TRPM8 deficient mice uniquely make more licks to cold (15°C) than to less cool water in two-fluid tests. A, Mean licks emitted by B6 (n = 10, left) and TRPM8 deficient (n = 10, right) mice (circles) to water presented on brief-access trials at a reference temperature of 15°C and test temperatures ≥20°C in two-fluid tests. Data represent averaged responses collapsed across the 4 testing days where test water temperatures were above 15°C. Lines connect responses made by individual mice, with bar plots displaying mean licks for each temperature condition on the abscissae. When proffered water at 15°C cold and less cool/warmer temperatures ≥20°C, B6 mice showed indifference (p > 0.05) whereas TRPM8 deficient mice made more licks to 15°C cold water (*, p < 0.05). Panels B, C show licking responses by individual mice (circles) for each test and 15°C reference water temperature pair. Bar plots give average licks (20% trimmed means) made to each pair collapsed across mice. Trend lines connect means. TRPM8 deficient mice emitted more licks to water presented at a cold temperature of 15°C than less cool temperatures of 20°, 25°, and 30°C (*, p < 0.05; panel C), which, on the other hand, all evoked licking responses equal to 15°C in B6 mice (p > 0.05, panel B). These data further suggest that TRPM8 deficient mice uniquely prefer colder over moderate cool temperatures in orosensory settings and reveal they are not impaired to react to the lower of two cool or cold temperatures sensed by the mouth.
Finally, TRPM8 also appeared to influence orosensory reactions that involved the coldest temperatures tested in two-fluid tests. For instance, wild-type B6 mice made more licks to water at 15°C (p = 0.008) or 30°C (p = 0.02) compared to 1°C, whereas TRPM8 deficient mice were indifferent (p > 0.2) to these temperature pairings (Figs. 12, 13; FDR-corrected Wilcoxon signed rank tests). This reduced orobehavioral response to cold temperature in TRPM8 deficient mice associates with the reduced firing of COLD-type Vc neurons, which excite to low temperatures, in this line (Fig. 7A).
Discussion
Silencing TRPM8 impaired, but did not abolish, brain and behavioral responses to oral cooling, which implies this ion channel is only one of multiple cold temperature receptors in orosensory pathways. Furthermore, the pattern of these impairments suggested a novel role for TRPM8 in thermosensory coding for cool and warm.
The COOL- and COLD-type neurons presently found to populate the trigeminothalamic tract demonstrated different dependencies on TRPM8, which is expressed by presynaptic trigeminal ganglion cells. Furthermore, these CNS cell types, which showed segmented tuning to mild cooling (COOL) or relatively strong responding to cold temperatures (COLD), displayed response characteristics observed in peripheral thermosensory neurons. Neurons differentially excited by small (mild) and deeper cooling temperature decreases were described in functional studies of mouse trigeminal ganglia in vitro (Bautista et al., 2007; Madrid et al., 2009) and in vivo (Yarmolinsky et al., 2016; Leijon et al., 2019). In the latter work, some cool neurons in trigeminal ganglia gave only transient responses to the onset of lower temperature stimulation of oral tissues – a response feature of COOL-type neurons observed here in Vc circuits. Moreover, while COLD-like neurons persisted, COOL neurons presently did not appear in Vc recordings from TRPM8-null mice, implying active TRPM8 channels are required for the development of COOL response profiles in Vc cells. Relatedly, the appearance of neurons particularly sensitive to small temperature decreases in cultured trigeminal ganglia also critically requires TRPM8 signaling (Bautista et al., 2007; Madrid et al., 2009). Altogether, these data imply that functional and TRPM8-dependent properties of peripheral cooling cells are recapitulated in central trigeminal neurons that transmit to the thalamus, suggesting these properties are critical for thermosensory coding.
TRPM8 thermal input was presently found to influence the balance between cool and warm temperature signaling in the brain. The loss of COOL, and suppressed firing of COLD, neurons in TRPM8-null mice impaired Vc responses to mild cooling temperatures near and below 30°C. This allowed reduced, warmth-like (≥35°C) neural activity to extend into the innocuous cool temperature range, with neural divergence from warming anomalously observed at cold temperatures (<15°C) in TRPM8 deficient mice. In contrast, a clear distinction between warm and all cooling temperatures emerged in Vc neural responses in wild-type B6 mice.
The appearance of warm neural activity at cool temperatures in TRPM8-null mice associated with their behavior in the present two-fluid thermal licking tests. When offered a choice between warm (35°C) and cooler (≤30°C) temperatures, thirsty wild-type and TRPM8 deficient mice both avoided warm and favored licking cooler water. However, when offered mildly cool (e.g., 30°C) and colder water, wild-type mice showed indifference whereas TRPM8 deficient mice displayed warmth-like avoidance of mild cooling, still preferring to lick colder water. Thus, while both mouse lines avoided oral warmth, orobehavioral avoidance uniquely extended to lower, normally innocuous cool temperatures in TRPM8 deficient mice.
Collectively, these results imply that TRPM8 thermal input defines a neural information breakpoint in trigeminal pathways that separates cool from warm temperature coding. Without TRPM8, this breakpoint does not develop, and warmth-like neural and behavioral responses encroach on and arise in the mild cool temperature range. Our data suggest that TRPM8 deficient mice recognize innocuous cool temperatures inside the mouth as less preferred, warmth-like sensations.
There are caveats to this interpretation, including the focus of our two-fluid tests on hedonic, but not sensory-discriminative, behavior. Relatedly, water-restricted mice avoided warmth, and mild cooling in the case of TRPM8 mutants, only if given the option to sample cooler fluid in the present two-fluid tests. Otherwise, they licked warm and warmth-like fluids likely to satisfy thirst drive, which is a component of the measured behavior in addition to temperature preference.
Silencing TRPM8 suppressed Vc neural population responses to cooling and increased their similarity to warmth, which evoked low or null responses across the present Vc neurons. The latter agrees with prior neurophysiological studies that described a paucity, and absence, of trigeminal neurons excited by orofacial warmth (Poulos and Lende, 1970a, b; Craig and Dostrovsky, 1991; Lemon et al., 2016). However, a rare presence of neurons excited by oral warming was reported in in vivo imaging studies of mouse trigeminal ganglia (Yarmolinsky et al., 2016; Leijon et al., 2019). While we observed “off” responses to warmth in Vc neurons, other experimental conditions could be needed to capture warmth-on activity, even if rare. Nevertheless, an absence, and suppression, in responding to warmth in cooling-active cells was discussed to be a component of the neural message for warmth (Poulos and Benjamin, 1968; Poulos and Lende, 1970a; Pogorzala et al., 2013; Paricio-Montesinos et al., 2020). In this context, a suppression of population-level activity could bring neural information for cool temperatures closer to a warmth-like signal, as implied by the present neural and behavioral responses of TRPM8-null mice.
Notably, the current orosensory data show mice avoid licking 35°C, near-physiological warm water in favor of cold fluids, which is opposite of their normal preference for ∼35°C warmth and avoidance of cold in body-sensed thermal preference assays (Dhaka et al., 2007; Pogorzala et al., 2013). This may relate to differences in thermoregulatory sensory drive between contexts (Lemon, 2021). Furthermore, in the latter studies, which used binary thermal place preference and thermal gradient assays, TRPM8 deficient mice shifted their preferred body-sensed environmental temperatures to include cooler values (∼25°C) and displayed some avoidance of warm temperatures (∼35°C) optimally preferred by wild-type mice (Dhaka et al., 2007; Pogorzala et al., 2013). This shift in preference to cooler temperatures in TRPM8 mutants could arise if they recognize lower body-sensed temperatures as warmth, agreeing the present neural and behavioral results implying warm-like orosensory responses appear at lower temperatures without TRPM8. Relatedly, ablation of TRPM8-expressing dorsal root afferents reduces the number of mild cooling (29° to 26°C) sensitive spinal neurons and appears to increase similarity between cutaneous (skin) cooling population activity and warmth (Ran et al., 2016).
TRPM8 deficient mice clearly responded to mild cool temperatures in the present two-fluid tests. When 30°C was the reference, TRPM8 deficient mice made more licks to water at moderately lower temperatures beginning around 25°C. TRPM8 mutants also uniquely preferred to lick water at the 15°C cold reference when pitted against less cool/warmer temperatures (20°, 25°, and 30°C). Although displaying a warmth-shifted hedonic reaction to moderate cooling, TRPM8 null mice appeared to continually recognize the lower of two moderate cool temperatures and did not exhibit a thermal blind spot in the present orosensory tests. This is a major departure from the deficits in recognition of moderate cool temperatures that TRPM8 deficient mice show in body-sensed thermal preference assays. For instance, in binary thermal place settings, wild-type mice can prefer 30°C over a slightly lower temperature (e.g., 26°C) surface, whereas TRPM8 deficient mice show impaired preferences, and indifference, for 30°C against surfaces cooled down to 20° or ∼15°C (Bautista et al., 2007; Dhaka et al., 2007; Knowlton et al., 2010, 2013; Pogorzala et al., 2013). These data have implied TRPM8 deficient mice are challenged to distinguish between mild cool temperatures ≤30°C (see also Isaacson and Hoon, 2021).
This discrepancy suggests TRPM8 function partly differs between the body and mouth and implies receptors and neurons independent of the canonical TRPM8 cold receptor also sense oral cooling. While the menthol-insensitive COLD-type Vc neurons that remained in TRPM8-null mice could be involved, there are extra-trigeminal afferents that also respond to oral temperature change. Cooling the tongue excites gustatory- and thermo-active fibers of the facial (CN VII) nerve (e.g., Ogawa et al., 1968; Yokota and Bradley, 2016) in parallel with trigeminal fibers. Facial afferents supply taste buds, which do not contain TRPM8 fibers (Abe et al., 2005; Dhaka et al., 2008). The significance of thermal activity in CN VII remains unknown, although recent data suggest neurons outside of traditional somatosensory pathways can have roles in signaling cutaneous temperature (Vestergaard et al., 2023). Finally, our two-fluid tests measured behavior driven by thermal stimulation of a localized, small region of (orofacial) epithelia, which may influence differences with prior studies.
The present, and our prior (Lemon et al., 2016), data found mouse Vc cooling neurons are insensitive to oral delivery of nociceptive stimuli, including capsaicin or noxious heat. This contrasts electrophysiological data concerning rat Vc cooling cells, which can respond to oral presence of capsaicin and heat (Carstens et al., 1998; Zanotto et al., 2007). It is difficult to assert if this discrepancy represents a species effect when considering methodological differences between studies, including some differences in stimulus presentation and oral adaptation temperature control, and present verification of neuronal projection targets. Notably, our prior data show oral capsaicin and heat delivered as presently can excite mouse parabrachial neurons (Li and Lemon, 2019; Li et al., 2022), showing stimulus effectiveness.
Mapping thermoreceptors to brain and behavioral responses is critical for establishing their function. The present data define a novel role for TRPM8 in cool and warm temperature processing by trigeminal neurons and stimulate questions about how additional receptors and afferent systems participate in orofacial thermosensation. Future studies in this vein may further establish general principles and specializations of thermosensory coding.
References
- Abe J, Hosokawa H, Okazawa M, Kandachi M, Sawada Y, Yamanaka K, Matsumura K, Kobayashi S (2005) TRPM8 protein localization in trigeminal ganglion and taste papillae. Brain Res Mol Brain Res 136:91–98. 10.1016/j.molbrainres.2005.01.013 [DOI] [PubMed] [Google Scholar]
- Bautista DM, Siemens J, Glazer JM, Tsuruda PR, Basbaum AI, Stucky CL, Jordt SE, Julius D (2007) The menthol receptor TRPM8 is the principal detector of environmental cold. Nature 448:204–208. 10.1038/nature05910 [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate – a practical and powerful approach to multiple testing. J R Stat Soc B 57:289–300. 10.1111/j.2517-6161.1995.tb02031.x [DOI] [Google Scholar]
- Boughter JD Jr, Baird JP, Bryant J, St John SJ, Heck D (2007) C57BL/6J and DBA/2J mice vary in lick rate and ingestive microstructure. Genes Brain Behav 6:619–627. 10.1111/j.1601-183X.2006.00293.x [DOI] [PubMed] [Google Scholar]
- Boughter JD Jr, St John SJ, Noel DT, Ndubuizu O, Smith DV (2002) A brief-access test for bitter taste in mice. Chem Senses 27:133–142. 10.1093/chemse/27.2.133 [DOI] [PubMed] [Google Scholar]
- Capra NF, Dessem D (1992) Central connections of trigeminal primary afferent neurons: topographical and functional considerations. Crit Rev Oral Biol Med 4:1–52. 10.1177/10454411920040010101 [DOI] [PubMed] [Google Scholar]
- Carstens E, Kuenzler N, Handwerker HO (1998) Activation of neurons in rat trigeminal subnucleus caudalis by different irritant chemicals applied to oral or ocular mucosa. J Neurophysiol 80:465–492. 10.1152/jn.1998.80.2.465 [DOI] [PubMed] [Google Scholar]
- Carstens E, Saxe I, Ralph R (1995) Brainstem neurons expressing c-Fos immunoreactivity following irritant chemical stimulation of the rat's tongue. Neuroscience 69:939–953. 10.1016/0306-4522(95)00297-V [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D (2000) Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 288:306–313. 10.1126/science.288.5464.306 [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D (1997) The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389:816–824. 10.1038/39807 [DOI] [PubMed] [Google Scholar]
- Cechetto DF, Standaert DG, Saper CB (1985) Spinal and trigeminal dorsal horn projections to the parabrachial nucleus in the rat. J Comp Neurol 240:153–160. 10.1002/cne.902400205 [DOI] [PubMed] [Google Scholar]
- Chase SM, Young ED (2007) First-spike latency information in single neurons increases when referenced to population onset. Proc Natl Acad Sci U S A 104:5175–5180. 10.1073/pnas.0610368104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD, Dostrovsky JO (1991) Thermoreceptive lamina I trigeminothalamic neurons project to the nucleus submedius in the cat. Exp Brain Res 85:470–474. 10.1007/BF00229424 [DOI] [PubMed] [Google Scholar]
- Craig AD, Dostrovsky JO (2001) Differential projections of thermoreceptive and nociceptive lamina I trigeminothalamic and spinothalamic neurons in the cat. J Neurophysiol 86:856–870. 10.1152/jn.2001.86.2.856 [DOI] [PubMed] [Google Scholar]
- Craig AD, Krout K, Andrew D (2001) Quantitative response characteristics of thermoreceptive and nociceptive lamina I spinothalamic neurons in the cat. J Neurophysiol 86:1459–1480. 10.1152/jn.2001.86.3.1459 [DOI] [PubMed] [Google Scholar]
- Dhaka A, Earley TJ, Watson J, Patapoutian A (2008) Visualizing cold spots: TRPM8-expressing sensory neurons and their projections. J Neurosci 28:566–575. 10.1523/JNEUROSCI.3976-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhaka A, Murray AN, Mathur J, Earley TJ, Petrus MJ, Patapoutian A (2007) TRPM8 is required for cold sensation in mice. Neuron 54:371–378. 10.1016/j.neuron.2007.02.024 [DOI] [PubMed] [Google Scholar]
- Ellingson JM, Silbaugh BC, Brasser SM (2009) Reduced oral ethanol avoidance in mice lacking transient receptor potential channel vanilloid receptor 1. Behav Genet 39:62–72. 10.1007/s10519-008-9232-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everaerts W, et al. (2011) The capsaicin receptor TRPV1 is a crucial mediator of the noxious effects of mustard oil. Curr Biol 21:316–321. 10.1016/j.cub.2011.01.031 [DOI] [PubMed] [Google Scholar]
- Franklin K, Paxinos G (2008) The mouse brain in stereotaxic coordinates, Ed 3rd. San Diego: Academic Press. [Google Scholar]
- Green BG (1984) Thermal perception on lingual and labial skin. Percept Psychophys 36:209–220. 10.3758/BF03206361 [DOI] [PubMed] [Google Scholar]
- Green BG (1986) Oral perception of the temperature of liquids. Percept Psychophys 39:19–24. 10.3758/BF03207579 [DOI] [PubMed] [Google Scholar]
- Isaacson M, Hoon MA (2021) An operant temperature sensory assay provides a means to assess thermal discrimination. Mol Pain 17:17448069211013633. 10.1177/17448069211013633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasmin L, Burkey AR, Card JP, Basbaum AI (1997) Transneuronal labeling of a nociceptive pathway, the spino-(trigemino-)parabrachio-amygdaloid, in the rat. J Neurosci 17:3751–3765. 10.1523/JNEUROSCI.17-10-03751.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordt SE, Bautista DM, Chuang HH, McKemy DD, Zygmunt PM, Hogestatt ED, Meng ID, Julius D (2004) Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature 427:260–265. 10.1038/nature02282 [DOI] [PubMed] [Google Scholar]
- Knowlton WM, Bifolck-Fisher A, Bautista DM, McKemy DD (2010) TRPM8, but not TRPA1, is required for neural and behavioral responses to acute noxious cold temperatures and cold-mimetics in vivo. Pain 150:340–350. 10.1016/j.pain.2010.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowlton WM, Palkar R, Lippoldt EK, McCoy DD, Baluch F, Chen J, McKemy DD (2013) A sensory-labeled line for cold: TRPM8-expressing sensory neurons define the cellular basis for cold, cold pain, and cooling-mediated analgesia. J Neurosci 33:2837–2848. 10.1523/JNEUROSCI.1943-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgren S (1960) Thalamic neurones responding to cooling of the cat’s tongue. Acta Physiol Scand 48:255–267. 10.1111/j.1748-1716.1960.tb01860.x [DOI] [PubMed] [Google Scholar]
- Leijon SCM, Neves AF, Breza JM, Simon SA, Chaudhari N, Roper SD (2019) Oral thermosensing by murine trigeminal neurons: modulation by capsaicin, menthol and mustard oil. J Physiol 597:2045–2061. 10.1113/JP277385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemon CH (2021) Tasting temperature: neural and behavioral responses to thermal stimulation of oral mucosa. Curr Opin Physiol 20:16–22. 10.1016/j.cophys.2020.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemon CH, Kang Y, Li J (2016) Separate functions for responses to oral temperature in thermo-gustatory and trigeminal neurons. Chem Senses 41:457–471. 10.1093/chemse/bjw022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemon CH, Norris JE, Heldmann BA (2019) The TRPA1 ion channel contributes to sensory-guided avoidance of menthol in mice. eNeuro 6. 10.1523/ENEURO.0304-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Ali MSS, Lemon CH (2020) A simple method for acute gas anesthesia in mice via tracheostomy tube. Kopf Carrier 98:1–5. https://osf.io/74qm5 [Google Scholar]
- Li J, Ali MSS, Lemon CH (2022) TRPV1-Lineage somatosensory fibers communicate with taste neurons in the mouse parabrachial nucleus. J Neurosci 42:1719–1737. 10.1523/JNEUROSCI.0927-21.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Lemon CH (2015) Influence of stimulus and oral adaptation temperature on gustatory responses in central taste-sensitive neurons. J Neurophysiol 113:2700–2712. 10.1152/jn.00736.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Lemon CH (2019) Mouse parabrachial neurons signal a relationship between bitter taste and nociceptive stimuli. J Neurosci 39:1631–1648. 10.1523/JNEUROSCI.2000-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipshetz B, Giesler GJ Jr. (2016) Effects of scratching and other counterstimuli on responses of trigeminothalamic tract neurons to itch-inducing stimuli in rats. J Neurophysiol 115:520–529. 10.1152/jn.00326.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipski J (1981) Antidromic activation of neurones as an analytic tool in the study of the central nervous system. J Neurosci Methods 4:1–32. 10.1016/0165-0270(81)90015-7 [DOI] [PubMed] [Google Scholar]
- Madrid R, de la Pena E, Donovan-Rodriguez T, Belmonte C, Viana F (2009) Variable threshold of trigeminal cold-thermosensitive neurons is determined by a balance between TRPM8 and Kv1 potassium channels. J Neurosci 29:3120–3131. 10.1523/JNEUROSCI.4778-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mair P, Wilcox R (2020) Robust statistical methods in R using the WRS2 package. Behav Res Methods 52:464–488. 10.3758/s13428-019-01246-w [DOI] [PubMed] [Google Scholar]
- McKemy DD (2007) TRPM8: The Cold and Menthol Receptor. In: TRP Ion channel function in sensory transduction and cellular signaling cascades (Liedtke WB, Heller S, eds). Boca Raton, FL: CRC Press/Taylor & Francis. [PubMed] [Google Scholar]
- McKemy DD, Neuhausser WM, Julius D (2002) Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 416:52–58. 10.1038/nature719 [DOI] [PubMed] [Google Scholar]
- Mosso JA, Kruger L (1973) Receptor categories represented in spinal trigeminal nucleus caudalis. J Neurophysiol 36:472–488. 10.1152/jn.1973.36.3.472 [DOI] [PubMed] [Google Scholar]
- Ogawa H, Sato M, Yamashita S (1968) Multiple sensitivity of chorda tympani fibres of the rat and hamster to gustatory and thermal stimuli. J Physiol 199:223–240. 10.1113/jphysiol.1968.sp008650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paricio-Montesinos R, Schwaller F, Udhayachandran A, Rau F, Walcher J, Evangelista R, Vriens J, Voets T, Poulet JFA, Lewin GR (2020) The sensory coding of warm perception. Neuron 106:830–841 e833. 10.1016/j.neuron.2020.02.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogorzala LA, Mishra SK, Hoon MA (2013) The cellular code for mammalian thermosensation. J Neurosci 33:5533–5541. 10.1523/JNEUROSCI.5788-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulos DA, Benjamin RM (1968) Response of thalamic neurons to thermal stimulation of the tongue. J Neurophysiol 31:28–43. 10.1152/jn.1968.31.1.28 [DOI] [PubMed] [Google Scholar]
- Poulos DA, Lende RA (1970a) Response of trigeminal ganglion neurons to thermal stimulation of oral-facial regions. II. Temperature change response. J Neurophysiol 33:518–526. 10.1152/jn.1970.33.4.518 [DOI] [PubMed] [Google Scholar]
- Poulos DA, Lende RA (1970b) Response of trigeminal ganglion neurons to thermal stimulation of oral-facial regions. I. Steady-state response. J Neurophysiol 33:508–517. 10.1152/jn.1970.33.4.508 [DOI] [PubMed] [Google Scholar]
- Ran C, Hoon MA, Chen X (2016) The coding of cutaneous temperature in the spinal cord. Nat Neurosci 19:1201–1209. 10.1038/nn.4350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team (2022) R: a language and environment for statistical computing. R Foundation for Statistical Computing. [Google Scholar]
- Saito H, et al. (2017) Ascending projections of nociceptive neurons from trigeminal subnucleus caudalis: a population approach. Exp Neurol 293:124–136. 10.1016/j.expneurol.2017.03.024 [DOI] [PubMed] [Google Scholar]
- Smith JC (2001) The history of the “Davis Rig”. Appetite 36:93–98. 10.1006/appe.2000.0372 [DOI] [PubMed] [Google Scholar]
- Tajino K, Hosokawa H, Maegawa S, Matsumura K, Dhaka A, Kobayashi S (2011) Cooling-sensitive TRPM8 is thermostat of skin temperature against cooling. PLoS One 6:e17504. 10.1371/journal.pone.0017504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestergaard M, Carta M, Guney G, Poulet JFA (2023) The cellular coding of temperature in the mammalian cortex. Nature 614:725–731. 10.1038/s41586-023-05705-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DM, Lemon CH (2013) Modulation of central gustatory coding by temperature. J Neurophysiol 110:1117–1129. 10.1152/jn.00974.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DM, Lemon CH (2014) Temperature systematically modifies neural activity for sweet taste. J Neurophysiol 112:1667–1677. 10.1152/jn.00368.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarmolinsky DA, Peng Y, Pogorzala LA, Rutlin M, Hoon MA, Zuker CS (2016) Coding and plasticity in the mammalian thermosensory system. Neuron 92:1079–1092. 10.1016/j.neuron.2016.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota Y, Bradley RM (2016) Receptive field size, chemical and thermal responses, and fiber conduction velocity of rat chorda tympani geniculate ganglion neurons. J Neurophysiol 115:3062–3072. 10.1152/jn.00045.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanotto KL, Merrill AW, Carstens MI, Carstens E (2007) Neurons in superficial trigeminal subnucleus caudalis responsive to oral cooling, menthol, and other irritant stimuli. J Neurophysiol 97:966–978. 10.1152/jn.00996.2006 [DOI] [PubMed] [Google Scholar]