Abstract
Powder quality in additive manufacturing (AM) electron beam melt (EBM) of Ti-6Al-4V components is crucial in determining the critical material properties of the end item. In this study, we report on the effect of powder oxidation on the Charpy impact energy of Ti-6Al-4V parts manufactured using EBM. In addition to oxidation, the effects on impact energy due to hot isostatic pressing (HIP), specimen orientation, and EBM process defects were also investigated. This research has shown that excessive powder oxidation (oxygen mass fraction above 0.25 % and up to 0.46 %) dramatically decreases the impact energy. It was determined that the room temperature impact energy of the parts after excessive oxidation was reduced by about seven times. We also report that HIP post-processing significantly increases the impact toughness, especially for specimens with lower or normal oxygen content. The specimen orientation effect was found to be more significant for low oxidation levels.
Keywords: Additive Manufacturing, Ti-6Al-4V Powder, EBM, Oxidation, Charpy impact toughness, X-ray computed tomography
1 Introduction
Among other additive manufacturing (AM) technologies, metallic powder bed fusion electron beam melt (EBM) has recently gained considerable attention in the medical, automotive, and aerospace communities for the fabrication of production components [1–4] directly from 3D CAD files bringing enhanced design freedom, minimal material waste, and little post-processing [5]. The EBM process involves spreading a layer of pre-alloyed metallic powder in the evacuated build space, selectively melting regions in the layer with an electron beam, spreading another layer of powder and repeating the process until a three-dimensional solid metal part is contained within the powder ‘cake’. An in-depth description of the Arcam AB1 EBM process can be found in [6, 7]. The process can be used on many materials including stainless steel, nickel-based super alloys, titanium, and aluminum. The Ti-6Al-4V alloy is arguably the most widely studied [4, 8–12] owing to potential cost benefits of EBM over traditional titanium manufacturing methods and the high tensile strength-to-weight ratio of this alloy making adoption of AM enticing on intricate, highly-engineered components [13].
Many studies have shown static material properties of EBM Ti-6Al-4V to be comparable to typical annealed wrought products [14–17]. The fatigue properties of the material can approach those of wrought or cast product with post-processing—where hot isostatic pressing (HIP) is an important post-processing step, and machining (or other surface finishing techniques) is significant owing to the rough as-built finish of EBM components [1, 14, 18–20]. It is important to note that these promising properties can be achieved when chemistry is kept within traditional limits for wrought and cast product (e.g. see ASTM F2924), when processing parameters are optimal and maintained in-control, and with some or considerable post-processing (including machining in many cases).
A limited number of studies have investigated the effects of powder quality and chemistry on critical properties of EBM Ti-6Al-4V [11, 21]. In a recent study on EBM Ti-6Al-4V powder recycling [21], the authors systematically measured powder shape and size, powder oxygen content, and resulting tensile properties as a function of the number of powder reuses. Their study showed that oxygen content increased with powder reuse, and the resulting strength increased with little influence on ductility (%-elongation) [21]. Tang et al. found that powder recycling could be continued up to 21 times with acceptable static tensile properties [21], as long as oxygen content stays below the ASTM F2924 mass fraction limit of 0.20 %. To the current authors’ knowledge, no studies have systematically investigated the effects of increased oxygen content (above 0.20 % mass fraction) on EBM Ti-6Al-4V, nor have any looked at the effects of oxygen on other related measures of ductility and/or toughness, such as Charpy impact energy.
It has been shown that for Ti-6Al-4V powder consolidated using ambient pressure sintering (not EBM), tensile ductility (%-elongation) drops dramatically with oxygen mass fractions above about 0.33 % [22]. Similarly, for Ti-6Al-4V powder consolidated using a HIP-process, Kim et al. [23] showed that specimens made from powder with high oxygen mass fractions (0.28 %) fail in a brittle manner, with elongation of only 1 % [23]. It should be noted that the raw feedstock for these processes may be noticeably different than for the EBM process, and the resultant microstructures can also be substantially different [22, 23]. Nonetheless, it is expected that the ductility (and toughness) of EBM Ti-6Al-4V will be reduced with increasing oxygen content. The question is at what point does oxygen content become unacceptable, and should the traditional limit of 0.20 % mass fraction necessarily hold true for EBM?
2. Approach
In order to investigate the effects of oxygen content on the impact toughness of EBM Ti-6Al-4V, an entirely new approach for powder oxidation was devised in this research. The goal was to investigate oxygen content significantly higher than 0.2 % mass fraction using EBM instead of powder metallurgy [23], by precisely controlling the amount of oxygen as a function of time and temperature instead of the number of reuses or runs [21]. To accomplish the goal and to expedite the process without using large quantities of powder, small amounts of powder were first artificially oxidized in air at elevated temperature and then mixed in with low oxygen content powder. By measuring oxygen contents of normal and artificially oxidized powder, a simple rule of mixtures approach was used to create mixtures of powder with targeted overall/average oxygen contents. These mixtures of powder were then used to create Charpy specimen blanks with the EBM process. Specimens were fabricated in three distinct orientations, as it was expected the EBM process would exhibit non-isotropic behavior. Further, half of the blanks were post-processed using a standard hot-isostatic pressing (HIP) procedure common for Ti-6Al-4V castings and EBM produced components. Specimen blanks were then machined into Charpy specimens and tested at room temperature. The absorbed energies and fracture appearances were compared for all test conditions.
3. Methodology
3.1 Powder Batch Preparation
Four batches of plasma-atomized Ti-6Al-4V powders supplied by Arcam AB (Mölndal, Sweden) were used in this study with 5th and 95th percentile diameters of approximately 45 µm and 120 µm, respectively. Four batches of powders were used: A) virgin powder; B) five-times reused powder; C) marginally oxidized powder, made up of a mixture of five-times reused powder mixed with artificially oxidized powder; and D) highly oxidized powder, similar to ‘C’ but with a larger mass fraction of artificially oxidized powder (Table 1). The artificially oxidized powder was processed by heating five-times reused powder to 650 °C for 4 h in air. The oxygen mass fraction of the five-times recycled powder was approximately 0.16 %, and it increased to 4.1 % after artificial oxidation. For the ’C’ and ‘D’ batches, the five-times reused and artificially oxidized powders were combined and mixed together. The targeted average oxygen mass fractions of the ‘C’ and ‘D’ batches of powder were 0.3 % and 0.5 %, respectively. Prior to building specimen blanks, each powder batch was mixed using the Arcam powder recovery system and subsequently sifted through a 100 µm screen.
Table 1.
Powder lot descriptions and powder oxygen contents. Uncertainties in oxygen readings are indicated value ± 2.5 %.
| Powder Oxygen Content (% mass fraction) |
|||
|---|---|---|---|
| Identifier | Powder Mixture Description | Before build | After build |
| A | 100 % virgin powder | 0.11 | 0.123 |
|
| |||
| B | 100 % 5× reused powder | 0.142 | 0.157 |
|
| |||
| C | Mixture of 96.5 % 5× reused and 3.5 % aged at 650 °C for 4 h | 0.340 | 0.292 |
|
| |||
| D | Mixture of 88.8 % 5× reused and 11.2 % aged at 650 °C for 4 h | 0.525 | 0.455 |
Other artificial oxidizing conditions were investigated in air at 200 °C for 2 h and 24 h, as well as 400 °C, 450 °C, 500 °C, 550 °C, and 600 °C for 4 h to determine the effect of time and temperature on the morphology of the oxidized powders. In general, an increase in temperature and time produced more powder oxidation. The most severe condition (4 h at 650 °C) was chosen because the EBM process starts with each layer of powder pre-heated by the electron beam to approximately 650 °C to 700 °C. However, the selected approach may not be the optimal procedure for the delivery of oxygen into the EBM Ti-6Al-4V systems; more research may be required to fully optimize the process.
3.2 Powder Characterization
3.2.1 X-ray Computed Tomography of Powders
Samples of the virgin, five-times reused, and highly oxidized (~4 % mass fraction) Ti-6Al-4V powder were imaged using a Skyscan X-ray CT system at a pixel size of 4.11 µm for the virgin and five-times recycled powders, and 3.76 µm for the highly oxidized powder. The samples were prepared by dispersing powder in epoxy and using a vacuum pump to draw the mixture into an approximately 3 mm outer diameter straw. The resulting images were segmented into white particles and black background, stacked into 3D data structures, analyzed with custom code that identified each valid particle and multi-particle, and fit to a spherical harmonic series. This analysis procedure is described in detail elsewhere [24, 25]. The code was used to compute many quantities describing the particle shapes, including the length L, which is the longest straight-line distance between any two points of the particle; the width W, which is the longest distance between any two points of the particle with the constraint that the line going through those points must be perpendicular to the line defining L; and the thickness T, which is the longest distance between any two points of the particle while being perpendicular to both L and W.
3.2.2 Scanning Electron Microscopy
Samples of virgin, five-times reused, and highly oxidized (~4 % mass fraction) powders were investigated using a JEOL model JSM 5800 LV scanning electron microscope (SEM) to evaluate their surface morphologies. Additionally, cross-sections of the powders were examined by first mounting in epoxy then grinding with SiC paper and polishing with 6 µm and 1 µm diamond paste.
3.3 Charpy Specimen Preparation
All Charpy specimens were built on separate Arcam model A2 and A2X systems. The machine settings used were those recommended by the manufacturer for the material system. The standard EBM coordinate system convention (ref. ASTM F2971) was followed to define the specimen orientation, where the X and Y axes are parallel to the build layer and the Z-axis is in the build direction. The blanks were oversized by approximately 4.0 mm in each dimension. Hot isostatic pressing (HIP) was performed on half of the as-built blanks prior to final machining. The HIP process involved treating under an inert atmosphere at 100 MPa and 954 °C for 3 h, and then cooling under the inert atmosphere to below 425 °C. The blanks were machined into standard Charpy V-notch specimens (ASTM E23, Type A), with the notch in one of three distinct orientations with respect to the build chamber coordinate system: X-Y, X-Z, and Z-X, where the first letter indicates the direction normal to the fracture plane and the second letter indicates the direction of intended crack propagation.
3.4 Chemical Analysis
Samples of virgin and oxidized powders were taken from the mixture before and after each build and were analyzed for oxygen content using the inert gas fusion process per ASTM E1409. Additionally, samples were taken from the as-built specimen blanks and analyzed for oxygen, nitrogen, carbon, hydrogen, aluminum, vanadium, iron, and silicon content. Carbon content was determined using combustion infrared detection per ASTM E1941; oxygen and nitrogen using inert gas fusion per ASTM E1409; hydrogen using inert gas fusion per ASTM E1447; and all others using direct current plasma emission spectroscopy per ASTM E2371.
3.5 Charpy Testing and SEM Analysis of Fracture Surfaces after Impact
Instrumented impact tests on the Charpy specimens were performed at room temperature (21 °C ± 1 °C), in accordance with the ASTM Test Method E23. Tests were performed on an impact machine with potential energy (machine capacity) of 954 J and impact speed of approximately 5.5 m/s, equipped with an instrumented striker with 8 mm radius of the striking edge. The strain-gages on the machine striker allowed force/deflection curves to be acquired for every test performed, and comparisons between typical force/deflection test records were used to gain additional insight on the differences observed between the material conditions. For every test, absorbed energy at fracture (KV) was measured with the impact machine digital encoder, while lateral expansion (LE) was measured as the increase in specimen thickness due to fracture by means of a digital caliper. Three specimens were tested for each condition including oxygen content, orientation and HIP and no-HIP. Fracture surfaces of the Charpy specimens were analyzed using the JEOL model JSM 5800 LV SEM. Care was taken to image fracture surfaces in the same orientation and location with respect to the specimen notch.
3.6 Hardness Measurements
Rockwell C hardness was measured on the sides of the broken Charpy specimens using a Wilson® model 574 Rockwell Hardness Tester. Measurements were made using a 150 kg load with a 120° diamond cone indenter. Six hardness readings were averaged for each oxygen level and orientation tested.
3.7 X-ray Diffraction Analysis
X-ray diffraction patterns were generated from specimens built with the virgin and high oxygen content powder batches using Cu Kα radiation in the Bragg-Brentano geometry. The diffracting surface for both specimens was perpendicular to the build direction and was from a location close to the final build layer. The patterns were taken with a thin slurry of fine LaB6 powder (NIST Standard Reference Material 660 [26]) painted on each of the surfaces as an internal standard.
4. Results and Preliminary Observations
The general powder shape and appearance as seen in the SEM were compared between the virgin, five-times reused, and artificially oxidized powders, as seen in Fig. 1 for virgin and artificially oxidized powder. No discernable difference was observed between the virgin and five-times reused powders. The artificially oxidized powder, however, had an obvious oxide layer (Fig. 2a) with a thickness of about 3 µm after 4 h at 650 °C. The oxide layer is also observable in the cross-section of Fig. 1d. Notably, the microstructure seen in the virgin powder cross-section (Fig. 1b) was α’ martensitic, while that of the five-times reused and artificially oxidized powders appeared acicular α+β, with an oxygen induced alpha case in the artificially oxidized powder that was not seen in the five-times reused powder.
Figure 1.
Example SEM images of (a, b) virgin and (c, d) highly oxidized (650 °C for 4 h in air) powder. (b, d) Example cross-sections of the same powders.
Figure 2.
(a) Example SEM image showing the oxide outer-layer of the highly oxidized (650 °C for 4 h in air) powder used to create the powder mixtures. (b) Same cross section as Fig. 1d, but with a line showing the diameter used for (c) an EDS line scan of Ti and O.
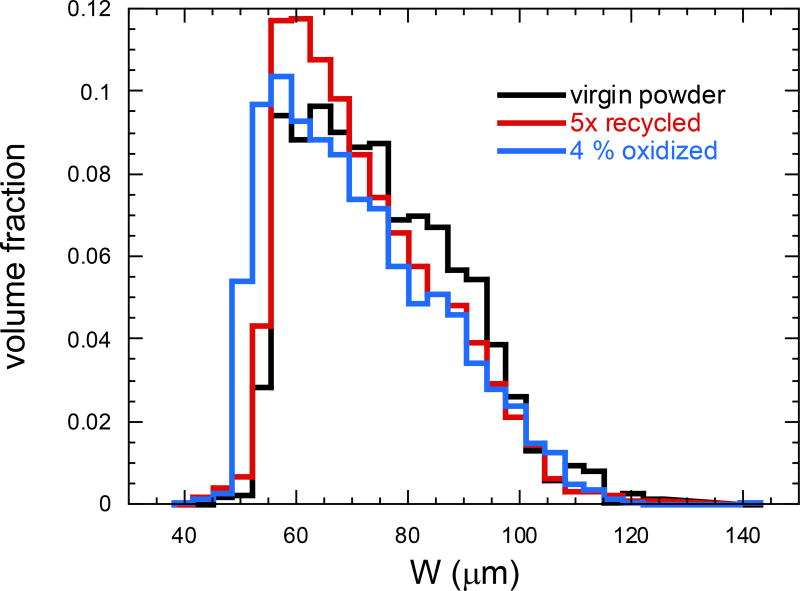
Higher concentrations of oxygen were detected with energy dispersive spectroscopy (EDS) on the surface of the artificially oxidized powders (Fig. 2b & c). The W-distributions, which is a measure of the particle “size” distributions, from the powder X-ray CT scan and analysis are shown in Fig. 3 for virgin, five-times reused, and artificially oxidized powders. The uncertainty in W for each particle corresponds to about 2 voxels in the original particle X-ray CT images, or about 8 µm. The size distribution appears to change somewhat between the virgin and five-times reused powders, but it can be seen that the five-times reused and artificially oxidized powders are rather similar, showing that the artificial oxidation process does not drastically alter the powder shape and size. Further, the five-times reused and artificially oxidized powder size distributions are slightly depleted with respect to the virgin powder in the range of 80 µm < W < 100 µm.
Figure 3.
Width W distribution for virgin, five-times reused, and highly oxidized (650 °C for 4 h in air) powder determined using X-ray computed tomography and spherical harmonic analysis.
The powder oxygen content results are summarized in Table 1, which shows the four lots of powder used to manufacture Charpy specimens. The virgin and five-times reused powder batches picked up a small amount of oxygen after the specimen build cycles. The marginally oxidized and highly oxidized powder mixtures, on the other hand, showed a slight reduction in oxygen content after the specimen build cycles. In addition, the total chemistry of the consolidated material (i.e. EBM Ti-6Al-4V product) is shown in Table 2. Note that two batches of specimens were built from five-times reused and marginally oxidized powder lots. With the exception of oxygen, the chemical contents were within the acceptable ASTM F2924 ranges in all cases. The oxygen contents of the consolidated material were similar to those of the powders when measured after the build.
Table 2.
Percent mass fraction of elements of consolidated product. Note, two batches each were built from the five-times reused powder and marginally oxidized powder mixture.
| Batch ID | Al | V | Fe | O | C | N | H |
|---|---|---|---|---|---|---|---|
| A | 6.01 | 4.09 | 0.20 | 0.129 | 0.012 | 0.043 | 0.0007 |
| B1 | 5.82 | 4.31 | 0.21 | 0.137 | 0.026 | 0.019 | 0.0007 |
| B2 | 5.66 | 4.05 | 0.19 | 0.134 | 0.019 | 0.019 | 0.0006 |
| C1 | 5.80 | 4.04 | 0.19 | 0.272 | 0.023 | 0.029 | 0.0009 |
| C2 | 5.73 | 4.07 | 0.19 | 0.288 | 0.015 | 0.025 | 0.0007 |
| D | 5.75 | 4.09 | 0.20 | 0.458 | 0.014 | 0.028 | 0.0007 |
| ASTM F2924 | 5.50–6.75 | 3.50–4.50 | 0.30 max. | 0.20 max. | 0.08 max. | 0.05 max. | 0.015 max. |
SEM backscatter electron images of the consolidated material made from virgin powder are shown in Fig. 4 in an X-Z plane and an X-Y plane. Similar images were produced for the material made from the five-times reused powder (not shown here). The microstructure of the consolidated material made from virgin and five-times reused powder was typical for EBM Ti-6Al-4V; it consisted of fine acicular α-Ti grains surrounded by β-phase interfacial regions, where prior-β grains were delineated by grain boundary α, and the prior-β grains were highly columnar in the Z-direction. The microstructure of the consolidated material made from the marginally and highly oxidized powder mixtures (Fig. 5) had shorter laths, less regular α lath spacing and orientation, and the prior-β grain boundaries were less discernable.
Figure 4.
SEM backscatter electron images of the consolidated material made from virgin powder on (a) an X-Z plane and (b) an X-Y plane. The build direction Z is indicated by the arrow/arrow point. Scale bars are 10 µm.
Figure 5.
SEM backscatter electron images of the consolidated material made from 0.46 % mass fraction oxidized powder mixture on (a) an X-Z plane and (b) an X-Y plane. The build direction Z is indicated by the arrow/arrow point. Scale bars are 10 µm.
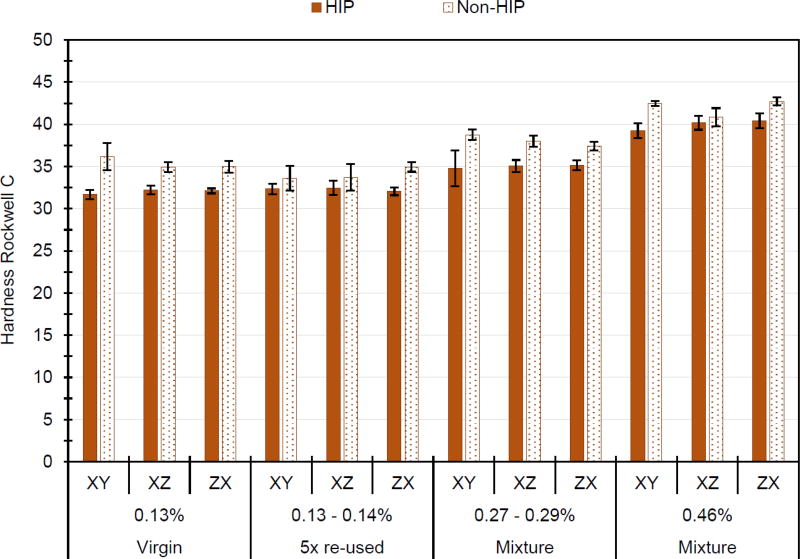
The Rockwell C hardness values presented in Fig. 6 show the non-HIP specimens always had noticeably higher values than their HIP-processed counterparts. The data also show that hardness increased slightly, from about Rockwell C 32 to about 40, as oxygen content increased. No discernable orientation effect was evident in the hardness readings.
Figure 6.
Hardness Rockwell C values from the ends of the Charpy specimens made from the four powder mixtures, including the effects of the three specimen orientations and non-HIP versus HIP post-processing. Consolidated material mass fraction of oxygen is listed for each batch. Error bars represent ± 1 standard deviation.
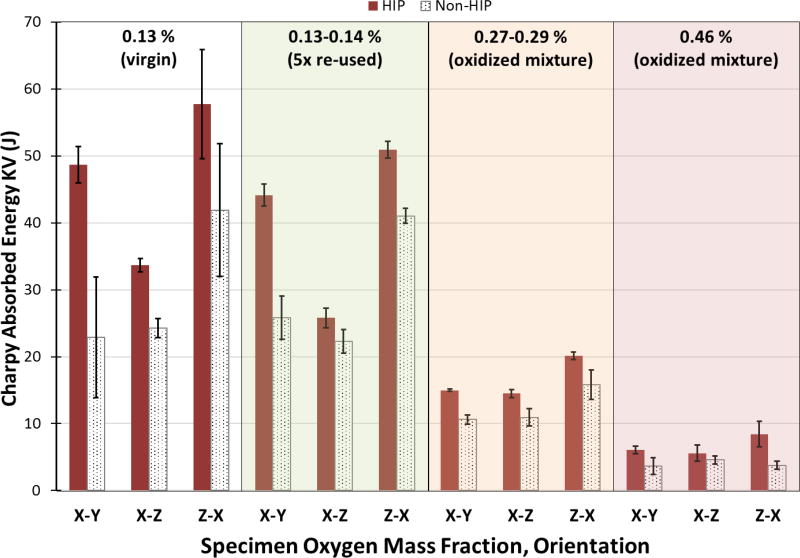
The Charpy absorbed impact energy of HIP versus non-HIP post-processing, X-Y versus X-Z versus Z-X orientations, and the four powder oxygen levels is summarized in Fig. 7. It can be seen that HIP post-processing always increased the absorbed energy levels. The Z-X orientation was found to be generally the toughest. Most importantly, the Charpy absorbed impact energies decreased dramatically with increasing oxygen content, with the HIP and orientation effects much less discernable for the highly oxidized specimens. The same trends were observed in the lateral expansion (LE) results, where higher oxygen contents resulted in lower LE values, HIP post-processing increased LE values, and a slight orientation effect on LE was observed. Additionally, the instrumented striker force-time histories indicated relative differences in ductility between the specimens, where the most ductile behavior was exhibited by the specimens with the lowest oxygen contents, the most brittle behavior for the highly oxidized specimens, and intermediate behavior for the moderately oxidized specimens. The HIP effect could also be discerned by comparing the force-time histories.
Figure 7.
Charpy absorbed impact energies (average of three tests per condition) for the four oxygen levels tested, including the effects of the three specimen orientations and non-HIP versus HIP post-processing. Consolidated material mass fraction of oxygen is listed for each batch. Error bars represent ± 1 standard deviation.
Figs. 8 and 9 show SEM images of the fracture surfaces taken from the Charpy samples after impact. The surface characteristics for the samples manufactured from the virgin powder (Fig. 8) exhibited typical features associated with a ductile type of fracture: highly irregular surfaces with numerous dimples, ridges, and depressions. In the highly oxidized samples, fracture surfaces appeared to be much flatter in comparison with the least oxidized samples, and more typical for very brittle fracture surfaces. For a given oxygen content, other than orientations of directional features, no differences were observed in the overall roughness and appearance between the samples tested in different orientations. For the five-times reused and marginally oxidized samples, the fracture surface features had characteristics of both the virgin and highly oxidized samples, with more brittle-type features in the artificially oxidized samples and more ductile-type features in the five-times reused samples. Importantly, voids were always detected on the fracture surfaces of the non-HIP specimens, irrespective of their oxidation levels and orientations. Voids were not observed on the HIP surfaces. This was also true for the CT scan results of HIP and non-HIP specimens: small voids were readily observable for non-HIP specimens only.
Figure 8.
SEM images of Charpy specimen fracture surfaces made from virgin powder in the (a, d) X-Y, (b, e) X-Z, and (c, f) Z-X orientations with (a, b, c) HIP or (d, e, f) non-HIP post-processing. The V-notch is above each figure. The build direction Z is indicated by arrow/arrow point. Scale bars are 200 µm.
Figure 9.
SEM images of Charpy specimen fracture surfaces made from 0.46 % mass fraction oxidized powder mixture in the (a, d) X-Y, (b, e) X-Z, and (c, f) Z-X orientations with (a, b, c) HIP or (d, e, f) non-HIP post-processing. The V-notch is above each figure. The build direction Z is indicated by arrow/arrow point. Scale bars are 200 µm.
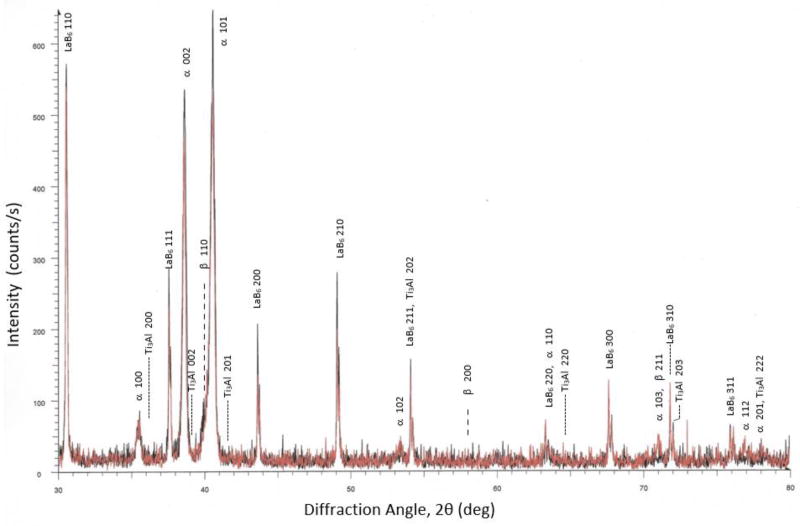
Fig. 10 shows X-ray diffraction patterns of the virgin (black) and highly oxidized (red) specimens, taken with Cu Kα radiation. The diffraction patterns of the two samples essentially overlap each other. The peaks for α-Ti and β-Ti are clearly visible. The patterns show no evidence of peaks for Ti-oxides or Ti3Al (α2). The expected peak positions of the Ti3Al (α2) phase are indicated. The patterns include peaks from NIST Standard Reference Material 660 (LaB6) [26] used as an internal standard.
Figure 10.
X-ray diffraction patterns of specimens built from the virgin powder (black) and 0.46 % mass fraction oxidized powder mixture (red), taken with Cu Kα radiation. Expected and observed peaks for α-Ti, β-Ti, α2 (Ti3Al), and LaB6 are annotated.
5. Additional Observations
Of important note, a major manufacturing flaw was observed in this study on some of the Z-X specimens manufactured using the five-times reused and less oxidized powders. This defect was attributable to a machine component malfunction rather than normal variation in the process. The defect was composed of several incomplete melt layers / lack of fusion, which appeared to be present across the entire Arcam A2 build volume. Upon microscopic examination of the fracture surfaces of the defect, it was observed that the adjacent layer was fully melted, meaning that the defect was abrupt and likely present on very few layers of the build process (Fig. 11). Examination of the fracture surfaces showed that some small regions did melt within the incomplete melt layer. The defective Charpy specimens were able to absorb some level of impact energy prior to failure because many of the powder particles were sintered to the adjacent layers. However, the specimens did not fail at the machined V-notch as intended, but rather failed in the defective layer, which in some cases was approximately 10 mm away from the notch. The fact that this defect affected the specimens’ impact energies more than excessive oxygen content (up to 0.5 % mass fraction), further sheds light on the importance of process control and quality control inspections for production of EBM components.
Figure 11.
Example SEM image of Z-X oriented Charpy specimen fracture surface with major laminar defect (incomplete melt/unfused layer), which failed away from the V-notch. The build direction Z is indicated by arrow point. Scale bar is 200 µm.
A 45 kg batch of powder was uniformly oxidized in air at 500 °C for 4 h in order to achieve approximately 0.3 % mass fraction oxygen. The batch of uniformly oxidized powder was created as a control for comparison with the marginally oxidized powder mixture with 0.3 % mass fraction oxygen (powder lot ‘C’ in Table 1). The EBM process was found to fail repeatedly--the machine faulted out due to shorting in the electron beam column--when attempting to build specimens using this uniformly oxidized batch of powder with the standard EBM Ti-6Al-4V processing settings, even after cleaning the system between build attempts.
6. Discussion
6.1 Oxidation of Ti-6Al-4V Powder
The artificially oxidized powders used in this study exhibited clearly noticeable surface oxide layers. It is known that the mass change of pure titanium and Ti-6Al-4V subject to surface oxidation progresses in a parabolic manner [27, 28], where the oxygen dissolves and occupies octahedral sites of α-Ti [29], as well as forms surface oxides. After the surface material reaches a critical oxygen composition of approximately TiO0.35, heavy oxide formation occurs [27]. After this point, further oxygen dissolution into the Ti matrix is substantially slowed, and the mass change is dominated by further surface oxide (mainly TiO2 Rutile) nucleation and growth [26]. Importantly, the presence of water accelerates the oxidation rate [30, 31].
The exact composition of the oxide layers in the oxidized particles of this study was not determined. Takahashi et al. has shown that Ti-Al and Ti-V alloys form oxide layers made primarily of TiO2 Rutile and some Al2O3 [32]. It should be noted that TiO2 rutile melts at 1843 °C and Al2O3 melts at 2072 °C. Additionally, the Ti-O phase diagram [33] shows for atomic percentages of oxygen above 10 % and below 30 %, various oxides form (Ti3O, Ti2O, Ti3O2), which transform to α-Ti at elevated temperatures but below the melting point of titanium. For very high atomic percentages (i.e. above 66.6 %), TiO2 Rutile is the dominant oxide and is stable up to its melting temperature of 1843 °C.
Since Ti-6Al-4V melts at 1660 °C, it is reasonable to assume that a significant portion of the oxygen in the powder surface oxides were absorbed into the metal as interstitials during the EBM process. However, there is no reason to assume that all of the TiO2 Rutile or Al2O3 would become fully dissolved during the EBM process. Nonetheless, no appreciable peaks for TiO2 or Al2O3 were observed during XRD on the consolidated material from virgin powder nor for the highly oxidized mixture (see Fig. 10). Oxides were also not detected in the microstructures of the samples shown in Figs. 4 and 5 nor on the fracture surfaces in Figs. 8 and 9. Therefore, it can be concluded that the surface Ti-oxides and any Al2O3 present on the artificially oxidized powder particles did in fact dissolve during the EBM process or were in too small a quantity to be detected by XRD. This means that all or most of the oxygen in the oxides went to interstitial sites in α-Ti of the consolidated material, without resulting in any undesirable micro- or nano-inclusions of various oxides.
The oxygen delivery process presented in the current work can be precisely controlled to achieve specific levels of oxygen content without compromising other chemical components during the EBM process (see Tables 1 and 2). This is a useful method to relate mechanical properties (Charpy impact toughness shown here) as a function of oxygen content, as an alternative to the typical approach of relating properties to “number of runs”, e.g. Tang [21], which can be strongly dependent on manufacturing conditions. The oxygen delivery process developed in this study could still be optimized through further research. The ratio of the virgin powder to the artificially oxidized powder is perhaps not ideal and could be altered.
6.2 Effect of Oxygen on Impact Energy
It has been shown in this research that the impact energy (and by extension, toughness) of EBM Ti-6Al-4V parts is greatly affected by excessive oxygen content. A similar conclusion was reported in the recent study on sintered Ti-6Al-4V components by Yan et al. [22], which showed that tensile %-elongation was greatly reduced from approximately 14 % to 3.5 % for samples with oxygen mass fractions of approximately 0.16 % and 0.49 %, respectively [22]. Yan et al. [22] also claimed that the maximum mass fraction of oxygen allowed in Ti-6Al-4V parts (made by sintering) should not exceed about 0.33 %. They showed that their Ti-6Al-4V specimens were significantly affected by oxygen, with the appearance of unique nano-scale microstructural features as well as evidence of fine α2 (Ti3Al) precipitates for oxygen mass fractions at and above 0.33 %. Conversely, in their study on powder reuse for EBM Ti-6Al-4V, Tang et al. [21] did not show any detrimental effects of oxygen on tensile properties, but the oxygen levels studied were relatively low (less than 0.19 % mass fraction) [21]. Most importantly, no studies are available illustrating the effect of oxygen on mechanical properties of EBM Ti-6Al-4V material systems while including the effects of orientation and HIP post processing. This was shown for the first time in Fig. 7.
The data in Fig. 7 clearly demonstrate that the impact energy of EBM Ti-6Al-4V parts can be reduced by a factor of almost seven for oxygen mass fractions approaching 0.5 %. As compared to the virgin powder samples, the impact energy of the five-times reused samples was only slightly reduced, but the scatter in the five-times reused data was noticeably less than that of the virgin powder data. If all the data in Fig. 7 are considered for both the HIP and non-HIP Charpy specimens and the three different orientations (X-Y, X-Z and Z-X), the effect of oxygen on the impact energies of EBM Ti-6Al-4V parts can be predicted (see Fig. 12). The data curves in Fig. 12 are relatively smooth for all conditions with a limited amount of scatter. These curves (and similar ones for other properties) can be of great practical utility. The Charpy impact test is a readily available and standardized test to compare relative levels of toughness/brittleness; however, other mechanical properties can easily be generated for the same set of conditions studied in this research. Curves for tensile strength and fracture toughness, for example, similar to those in Fig. 12 can be valuable to design engineers.
Figure 12.
Charpy absorbed impact energy as a function of consolidated material oxygen content, including the effects of the three specimen orientations and non-HIP versus HIP post-processing.
6.3 Effect of Orientation on Impact Energy
Fig. 7 shows a strong specimen orientation effect, with the Z-X orientation being the toughest in all cases. The typical microstructure of EBM-produced Ti-6Al-4V components has been characterized by others [14, 15, 34–36] to be fine acicular α-Ti grains surrounded by β-phase interfacial regimes. Fine α’ martensite microstructures produced by EBM have also been observed for relatively thin components or regions near the top of the build chamber (i.e. last few build layers) [35–38]. The microstructures observed in EBM Ti-6Al-4V exhibit strong texture in the build direction (Z-axis; “up” in build chamber), where the <001> β direction preferentially aligns with the direction of highest temperature gradient [8]. The textural alignment with the Z-direction is pronounced for thicker sections, but as sections become thinner the surrounding powder bed influences the texture to be more in-plane with the build layer [8]. Additionally, the prior-β grains are highly columnar in the Z-direction, and they can span many tens of layers of the build process [8, 14]. The Z-axis textural alignment and Z-oriented columnar grain structure has been shown to express a slight dependence of properties on specimen orientation. However, fully separating microstructural effects from the detrimental effects of voids and defects is difficult [39].
Compared to the above mentioned literature, very similar microstructural features were observed in this research (Figs. 8 and 9), and, most importantly, the effect of specimen orientation on toughness was quantified (Figs. 7 and 12). Owing to the known microstructural anisotropy, and the appearance of elongated Z-oriented directional features especially on the fracture surfaces of the lower oxygen content specimens (Fig. 8), it is postulated that the fracture path must progress in an intergranular fashion favoring the grain boundary α “coating” prior-β grains. For the Z-X orientation, the long dimension of the highly columnar prior-β grains is perpendicular to the intended crack plane, meaning the crack front must pass through the prior-β grain boundary α more often than for the other two orientations. In the same regard, the crack path for the X-Y orientation has to meander or weave between prior-β grains, making the X-Y orientation typically the second “toughest.” Further, for the X-Z orientation, once the crack front has been established amongst the prior-β columns, it continues to propagate along the same grain boundaries only shifting slightly between adjacent columns. This orientation effect, however, is reduced as oxygen content is greatly increased and fracture becomes more brittle and trans-granular (Fig. 9).
6.4 Effect of HIP Post-Processing on Impact Energy
HIP post-processing always improved the impact energy compared to as-built samples. It can also be noticed in Fig. 7 that the orientation and HIP post-processing effects were greatly reduced in the most severely oxidized samples, with the impact energies all dropping to around 3 J to 5 J. Many studies have investigated the effects of EBM processing parameters on material properties, with the aim of identifying the optimal parameters for near-fully-dense parts [10, 12, 40, 41]. The EBM process has many parameters that can be altered by the user, but recommended settings for Ti-6Al-4V are provided by the EBM manufacturer. It has been shown that HIP post-processing removes the typical micro-voids encountered, and it also improves the ductility of EBM Ti-6Al-4V while maintaining the strength at acceptable levels [14, 42]. The HIP process is typically performed at α+β annealing temperatures, and thus results in a coarsened microstructure compared to the as-built condition.
In this research, HIP post-processing increased the impact energy of the samples containing oxygen mass fractions up to about 0.3 %. It was noticed that the void content on the HIP post-processed Charpy specimen fracture surfaces was significantly reduced (ref. Figs 8 and 9). HIP post-processing increased the impact energies of the samples not only by closing voids and porosities, but also by annealing. This effect is clearly demonstrated in the hardness data shown in Fig. 6, combined with the impact energy data in Fig. 7, where all HIP post-processed samples exhibited noticeably lower Rockwell hardness, indicating lower yield strength and presumably higher ductility, leading to improved dynamic fracture resistance.
6.5 Reasons for Oxygen Embrittlement
For traditional Ti-6Al-4V products (e.g. plate, bar), it is well known that increasing the content of oxygen, and other interstitial elements, increases the yield and ultimate tensile strength while decreasing ductility and toughness [29, 43, 44]. Interstitial oxygen has been shown to strengthen α-Ti through the resistance to the motion of screw dislocations [43]. In general, lower levels of oxygen, nitrogen, and aluminum will result in increased ductility and improved damage tolerance [44]. The Ti-6Al-4V alloy is available in extra-low interstitial grades with high damage tolerance properties where the oxygen, nitrogen, and iron contents are reduced from the standard grade [44]. An empirical relation termed the “Rosenberg criterion” [45] states that there is a practical limit to the amount of α-Ti stabilizing elements that can be added to Ti alloys. In the case of Ti-6Al-4V, the Rosenberg criterion indicates embrittlement will occur above combined oxygen-carbon-nitrogen contents of 0.3 % mass fraction, and that above these levels the formation of the Ti3Al (α2) phase occurs [45]. In our case, the criterion was satisfied for an oxygen content of about 0.3 %. However, no practical limit can be noticed in Fig. 12 since the toughness data were continuously decreasing with oxygen for all tested specimens.
In the previously discussed study on sintered Ti-6Al-4V components by Yan et al. [22], the researchers observed marked changes in the microstructure and the appearance of Ti3Al (α2) precipitates for samples with oxygen mass fraction of 0.33 % and higher. They attributed the drastic reduction in ductility to these microstructural changes and the α2 phase. In the current study, the XRD patterns for low and very high oxygen content shown in Fig. 10 are very similar, indicating that phase content, crystallite size and preferred orientation in the build direction were all very similar (to the level of resolution for this technique). The peaks from the α and β phases could not be separated due to their close proximity, and extensive peak broadening occurred due to fine crystallite size and strain. There was no evidence of peaks from the Ti3Al (α2) phase, indicating that this phase, if present, was less than one or two percent of the total. Therefore, it can be assumed that most, if not all, oxygen in the consolidated samples went to interstitial sites, which as shown by Yu et al. [43] results in screw dislocation pinning and bulk material strengthening, while at the same time reducing resistance to dynamic fracture.
7. Conclusions
A newly developed method of mixing highly oxidized Ti-6Al-4V powder in small percentages with low oxygen content powders was applied to evaluate the effect on impact energy of powder oxygen mass fraction up to 0.5 %, without any detrimental effects such as residual oxide based particles. Charpy impact energy of Ti-6Al-4V decreased in a smooth but rapid fashion from approximately 20 J to 50 J (depending on orientation and post-processing) to 3 J to 5 J as the powder oxygen mass fraction increased from 0.11 % to 0.53 %. A strong orientation effect on toughness was detected, with fracture perpendicular to the build direction (Z-X orientation) exhibiting the highest impact energies. HIP post-processing increased toughness of the alloy in all directions, especially for low and medium oxygen content. Both the HIP post-processing and orientation effects on toughness largely disappeared at the 0.5 % mass fraction level.
Using the data presented in this work, impact energies can be related to oxygen concentrations for three orientations and HIP versus non-HIP post-processing. This work has contributed to the ongoing discussions regarding the effect of oxygen on the mechanical properties of Ti-based alloys. It is claimed that the reduction in toughness (similarly, in tensile %-elongation) and associated increase in hardness (similarly, in yield strength) of the alloy is most likely caused by the blockage of screw dislocation motion by interstitial oxygen as shown by Yu et al. [43], however further study of the dynamic mechanisms of deformation and fracture would be required to determine if this is the fundamental cause.
Supplementary Material
Acknowledgments
The authors would like to thank Nik Hrabe of NIST and Joe Hoffman of University of Denver for several candid discussions on the results of this study.
This work was predominantly supported by the National Science Foundation Industry/University Cooperative Research Center for Novel High Voltage/Temperature Materials and Structures under grant #IIP 1362135 and by the National Institute of Standards and Technology. Additional funds were provided independently by the Lockheed Martin Corporation.
Footnotes
Partial contribution of NIST – not subject to US copyright
Certain commercial equipment and/or materials are identified in this report in order to adequately specify the experimental procedure. In no case does such identification imply recommendation or endorsement by the National Institute of Standards and Technology, nor does it imply that the equipment and/or materials used are necessarily the best available for the purpose.
References
- 1.Frazier WE. Metal Additive Manufacturing: A Review. J. Mater. Eng. Perform. 2014;23:1917–1928. [Google Scholar]
- 2.Rawal S, Brantley J, Karabudak N. Additive manufacturing of Ti-6Al-4V alloy components for spacecraft applications; Recent Advances in Space Technologies 6th International Conference; 2013. pp. 5–11. [Google Scholar]
- 3.Ford SL. Additive manufacturing technology: Potential implications for US manufacturing competitiveness. J Int. Commer. Econ. 2014:1–35. [Google Scholar]
- 4.Gong X, Anderson T, Chou K. Review on powder-based electron beam additive manufacturing technology; ASME/ISCIE 2012 international symposium on flexible automation; 2012. pp. 507–515. [Google Scholar]
- 5.Gibson I, Rosen D, Stucker B. Additive manufacturing technologies: 3D printing, rapid prototyping, and direct digital manufacturing. Springer; New York: 2014. [Google Scholar]
- 6.Murr LE, Gaytan SM, Ramirez DA, Martinez E, Hernandez J, Amato KN, Shindo PW, Medina FR, Wicker RB. Metal fabrication by additive manufacturing using laser and electron beam melting technologies. J. Mater. Sci. Technol. 2012;28:1–14. [Google Scholar]
- 7.Murr LE, Martinez E, Amato KN, Gaytan SM, Hernandez J, Ramirez DA, Shindo PW, Medina F, Wicker RB. Fabrication of metal and alloy components by additive manufacturing: Examples of 3D materials science. J. Mater Res. Technol. 2012;1:42–54. [Google Scholar]
- 8.Antonysamy AA, Meyer J, Prangnell PB. Effect of build geometry on the β-grain structure and texture in additive manufacture of Ti6Al4V by selective electron beam melting. Mater. Charact. 2013;84:153–168. [Google Scholar]
- 9.Simonelli M, Tse YY, Tuck C. Effect of the build orientation on the mechanical properties and fracture modes of SLM Ti-6Al-4V. Mater. Sci. Eng.: A. 2104;616:1–11. [Google Scholar]
- 10.Juechter V, Scharowsky T, Singer RF, Körner C. Processing window and evaporation phenomena for Ti–6Al–4V produced by selective electron beam melting. Acta Mater. 2014;76:252–258. [Google Scholar]
- 11.Karlsson J, Snis A, Engqvist H, Lausmaa J. Characterization and comparison of materials produced by electron beam melting (EBM) of two different Ti-6Al-4V powder fractions. J. Mater. Process. Technol. 2013;213:2109–2119. [Google Scholar]
- 12.Gong H, Rafi K, Gu H, Starr T, Stucker B. Analysis of Defect Generation in Ti-6Al-4V Parts Made using Powder Bed Fusion Additive Manufacturing Processes. Addit. Manuf. 2014;1–4:87–98. [Google Scholar]
- 13.O’Brien MJ, Davis D. Mission Assurance for Additive Manufacturing of Metal Parts. 29th Aerospace Testing Seminar. 2015:138–155. [Google Scholar]
- 14.Al-Bermani SS, Blackmore ML, Zhang W, Todd I. The origin of microstructural diversity, texture, and mechanical properties in electron beam melted Ti-6Al-4V. Metall. Mater. Trans. A. 2010;41:3422–3434. [Google Scholar]
- 15.Hrabe N, Quinn T. Effects of processing on microstructure and mechanical properties of a titanium alloy (Ti-6Al-4V) fabricated using electron beam melting (EBM), part 1: Distance from build plate and part size. Mater. Sci. Eng.: A. 2013;573:264–270. [Google Scholar]
- 16.Hrabe N, Quinn T. Effects of processing on microstructure and mechanical properties of a titanium alloy (Ti-6Al-4V) fabricated using electron beam melting (EBM), part 2: Energy input, orientation, and location. Mater. Sci. Eng.: A. 2013;573:271–277. [Google Scholar]
- 17.Draper S, Lerch B, Rogers R, Martin R, Locci I, Garg A. Materials Characterization of Electron Beam Melted Ti-6Al-4V; In Proceedings of the 13th World Conference on Titanium; 2016. pp. 1433–1440. [Google Scholar]
- 18.Li P, Warner DH, Fatemi A, Phan N. Critical assessment of the fatigue performance of additively manufactured Ti–6Al–4V and perspective for future research. Inter. J. Fatigue. 2016;85:130–143. [Google Scholar]
- 19.Edwards P, O'Conner A, Ramulu M. Electron beam additive manufacturing of titanium components: Properties and performance. J. Manuf. Sci. Eng. 2013;135:1–7. [Google Scholar]
- 20.Chan KS, Koike M, Mason RL, Okabe T. Fatigue life of titanium alloys fabricated by additive layer manufacturing techniques for dental implants. Metall. Mater. Trans. A. 2013;44A:1010–1022. [Google Scholar]
- 21.Tang HP, Qian M, Liu N, Zhang XZ, Yang GY, Wang J. Effect of powder reuse times on additive manufacturing of Ti-6Al-4V by selective electron beam melting. JOM. 2015;67:555–563. [Google Scholar]
- 22.Yan M, Dargusch MS, Ebel T, Qian M. A transmission electron microscopy and three-dimensional atom probe study of the oxygen-induced fine microstructural features in as-sintered Ti–6Al–4V and their impacts on ductility. Acta Mater. 2014;68:196–206. [Google Scholar]
- 23.Kim Y, Kim EP, Song YB, Lee SH, Kwon YS. Microstructure and mechanical properties of hot isostatically pressed Ti–6Al–4V alloy. J. Alloys Compd. 2014;603:207–212. [Google Scholar]
- 24.Garboczi EJ. Three-dimensional mathematical analysis of particle shape using x-ray tomography and spherical harmonics: Application to aggregates used in concrete. Cem. Conc. Res. 2002;32:1621–1638. [Google Scholar]
- 25.Garboczi EJ, Bullard JW. 3D analytical mathematical models of random star-shape particles via a combination of X-ray computed microtomography and spherical harmonic analysis. Adv. Powder Technol. 2016. in press. [DOI]
- 26.National Institute of Standards and Technology. U.S. Department of Commerce; Gaithersburg, MD: Jun 1, 1989. SRM 660; Instrument Line Position and Profile Shape Standard for X-Ray Powder Diffraction. [Google Scholar]
- 27.Kofstad P, Anderson PB, Krudtaa OJ. Oxidation of titanium in the temperature range 800–1200 C. J. Less Common Met. 1961;2:89–97. [Google Scholar]
- 28.Poquillon D, Armand C, Huez J. Oxidation and Oxygen Diffusion in Ti–6Al–4V Alloy: Improving Measurements During Sims Analysis by Rotating the Sample. Oxid. Met. 2013;79:249–259. [Google Scholar]
- 29.Conrad H. Effect of interstitial solutes on the strength and ductility of titanium. Prog. Mater. Sci. 1981;26:123–403. [Google Scholar]
- 30.Wouters Y, Galerie A, Petit JP. Thermal oxidation of titanium by water vapour. Solid State Ionics. 1997;104:89–96. [Google Scholar]
- 31.Karlsson J, Norell M, Ackelid U, Engqvist H, Lausmaa J. Surface oxidation behavior of Ti–6Al–4V manufactured by Electron Beam Melting (EBM®) J. Manuf. Process. 2015;17:120–126. (2015) [Google Scholar]
- 32.Takahashi T, Minamino Y, Hirasawa H, Ouchi T. High-Temperature Oxidation and Its Kinetics Study of Ti–Al and Ti–V Alloys in Air. Mater. Trans. 2014;55:290–297. [Google Scholar]
- 33.Murray JL, Wriedt HA. The O− Ti (oxygen-titanium) system. J. Ph. Equilib. 1987;8:148–165. (1987) [Google Scholar]
- 34.Safdar A, Wei L, Snis A, Lai Z. Evaluation of microstructural development in electron beam melted Ti-6Al-4V. Mater. Charact. 2012;65:8–15. [Google Scholar]
- 35.Murr LE, Gaytan SM, Medina F, Lopez MI, Martinez E, Wicker RB. Additive Layered Manufacturing of Reticulated Ti-6Al-4V Biomedical Mesh Structures by Electron Beam Melting; 25th Southern Biomedical Engineering Conference; 2009. pp. 23–28. [Google Scholar]
- 36.Murr LE, Amato KN, Li SJ, Tian YX, Cheng XY, Gaytan SM, Martinez E, Shindo PW, Medina F, Wicker RB. Microstructure and mechanical properties of open-cellular biomaterials prototypes for total knee replacement implants fabricated by electron beam melting. J. Mech. Behav. Biomed. Mater. 2011;7:1396–1411. doi: 10.1016/j.jmbbm.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 37.Li SJ, Murr LE, Cheng XY, Zhang ZB, Hao YL, Yang R, Medina F, Wicker RB. Compression fatigue behavior of Ti–6Al–4V mesh arrays fabricated by electron beam melting. Acta Mater. 2012;60:793–802. doi: 10.1016/j.jmbbm.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 38.Hrabe NW, Heinl P, Flinn B, Körner C, Bordia RK. Compression-compression fatigue of selective electron beam melted cellular titanium (Ti-6Al-4V) J. Biomed. Mater. Res. Part B. 2011;99:313–320. doi: 10.1002/jbm.b.31901. [DOI] [PubMed] [Google Scholar]
- 39.Galarraga H, Lados DA, Dehoff RR, Kirka MM, Nandwana P. Effects of the microstructure and porosity on properties of Ti-6Al-4V ELI alloy fabricated by electron beam melting (EBM) Addit. Manuf. 2016;10:47–57. [Google Scholar]
- 40.Tammas-Williams S, Zhao H, Léonard F, Derguti F, Todd I, Prangnell PB. XCT analysis of the influence of melt strategies on defect population in Ti–6Al–4V components manufactured by selective electron beam melting. Mater. Charact. 2015;102:47–61. [Google Scholar]
- 41.Guo C, Ge W, Lin F. Effects of scanning parameters on material deposition during Electron Beam Selective Melting of Ti-6Al-4V powder. J. Mater. Process. Technol. 2015;217:148–157. [Google Scholar]
- 42.Tammas-Williams S, Withers PJ, Todd I, Prangnell PB. The Effectiveness of Hot Isostatic Pressing for Closing Porosity in Titanium Parts Manufactured by Selective Electron Beam Melting. Metall. Mater. Trans. A. 2016;47:1939–1946. [Google Scholar]
- 43.Yu Q, Qi L, Tsuru T, Traylor R, Rugg D, Morris JW, Minor AM. Origin of dramatic oxygen solute strengthening effect in titanium. Sci. 2015;347:635–639. doi: 10.1126/science.1260485. (2015) [DOI] [PubMed] [Google Scholar]
- 44.Welsch G, Boyer R, Collings EW. Materials Properties Handbook: Titanium Alloys. ASM International; Materials Park (OH): 1994. [Google Scholar]
- 45.Vydehi J. Titanium Alloys, an Atlas of Structures and Fractures Features. 1. Taylor &Francis; FL, USA: 2006. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.