Within a large population-based birth cohort, we examined the relationship between AD assessed at multiple time points during childhood and adolescence, and four measures of intellectual ability (IQ) between 1.5 and 15.5 years of age. We found no meaningful associations between AD activity or severity and general cognition; the results remained similar in analyses stratified by comorbid psychiatric or learning disorders and in an exploratory longitudinal analysis across all four outcome assessments. Future studies should incorporate objective measures of AD severity and investigate outcomes beyond IQ.
Abstract
Background
Atopic dermatitis (AD) may affect cognitive function, but studies are limited and inconsistent. The effect of AD severity on cognition remains underexplored and few previous studies have examined clinically validated or repeated measures of cognition throughout childhood.
Objectives
To evaluate the relationship of AD activity and severity with validated measures of general cognition in a longitudinal birth cohort.
Methods
We conducted cross-sectional analyses using data from the Avon Longitudinal Study of Parents and Children (ALSPAC), a UK cohort of 14 975 individuals followed prospectively since their birth in 1991–92. AD was assessed 11 times between the age of 6 and 166 months. Mothers were asked if their child had an ‘itchy, dry skin rash in the joints and creases’, and AD status was time-updated accordingly as ‘never’, ‘maybe’, ‘inactive’, ‘active/mild’ or ‘active/moderate–severe’. General cognition [i.e. intelligence quotient (IQ)] was measured at 18, 49, 103 and 186 months of age using the Griffiths Mental Development Scales (GMDS), Wechsler Preschool and Primary Scale of Intelligence (WPPSI), Wechsler Intelligence Scale for Children (WISC) and Wechsler Abbreviated Scale of Intelligence (WASI), respectively. Multivariable linear regression was used to compare IQ with respect to nearest time-updated AD status. Secondary analyses were stratified by the presence or absence of psychiatric or learning disorders. An exploratory longitudinal analysis of IQ across all four outcome assessments was conducted using generalized estimating equations.
Results
No significant associations between AD status and full-scale IQ scores on the GMDS, WPPSI, WISC and WASI were observed after adjustment for sociodemographic factors, atopic comorbidities and sleep characteristics. However, at 8 years of age, WISC Performance IQ was slightly, although statistically significantly, lower among children with active/moderate–severe AD [β coefficient –2.16, 95% confidence interval (CI) –4.12 to –0.19] and Verbal IQ was slightly, but statistically significantly, higher among those with inactive AD (β coefficient 1.31, 95% CI 0.28–2.34) compared with those without AD. Analyses stratified by psychiatric or learning disorders, and exploratory longitudinal analyses of cognition revealed similar findings.
Conclusions
We did not find any clinically meaningful associations between AD activity and severity and general cognitive function during early childhood and adolescence. Future studies should incorporate objective measures of AD severity and investigate outcomes beyond IQ.
Plain language summary available online
What is already known about this topic?
Paediatric atopic dermatitis (AD) has been associated with affective disorders, sleep disturbances and learning problems.
Recent epidemiological data suggest that paediatric AD may affect cognitive outcomes, but studies are limited and inconsistent.
The impact of AD severity on this relationship is underexplored and few previous studies have employed clinically validated or repeated measures of cognition throughout childhood.
What does this study add?
Within a large population-based birth cohort, we examined the relationship between AD, which was assessed at multiple time points during childhood, and four measures of intellectual ability between 1.5 and 15.5 years of age, finding no meaningful associations between AD activity or severity and general cognition.
The results remained similar in analyses stratified by comorbid psychiatric or learning disorders and in an exploratory longitudinal analysis across all four outcome assessments.
Atopic dermatitis (AD) affects up to 20% of children and has been associated with attention deficit/hyperactivity disorder (ADHD), affective issues, learning disorders and sleep disturbances.1–7 Considering these associations, some studies have investigated the relationship between AD and cognitive function, but their conclusions have been inconsistent.8–13 While some studies found lower intelligence quotient (IQ) scores in children with AD vs. those without,8,12 other studies found no associations between AD and IQ.9,12,13 Moreover, previous studies have been limited by the lack of robust definitions for AD, detailed AD severity data and longitudinal assessments of both AD and cognition. Therefore, we sought to examine the relationship between AD activity and severity with general cognitive function at multiple timepoints in childhood within a large population-based cohort.
Patients and methods
Study design and participants
We conducted a series of cross-sectional analyses on prospectively collected data from the Avon Longitudinal Study of Parents and Children (ALSPAC), a UK birth cohort designed to examine the health and development of children for which details are available elsewhere.14–17 Briefly, pregnant women residing in Avon, UK, with estimated delivery dates between 1 April 1991 and 31 December 1992 met the inclusion criteria. Overall, 14 833 unique mothers had 15 447 pregnancies that resulted in 14 975 live births. Child participants were longitudinally followed from birth via questionnaires and clinic visits.18 In the present study, three analysable subsamples were derived from the ALSPAC cohort using the number of participants who attended the clinics at which each cognitive assessment was administered. In the first subsample, 1432 children attended at least 1 of 10 early-childhood clinics, during which the Griffiths Mental Development Scales, Extended 0–8 years (GMDS) was administered at 18 months of age and the Wechsler Preschool and Primary Scale of Intelligence, UK Revised, Third Edition (WPPSI) at 49 months. In the second subsample, 7488 children attended the clinic at 8 years of age, when the Wechsler Intelligence Scale for Children, Third Edition (WISC) was administered. In the third subsample, 5530 children attended the teen clinic at 15.5 years of age, when the Wechsler Abbreviated Scale of Intelligence, Second Edition, Two-Subtest (WASI) was administered. Participants provided informed consent at the time of ALSPAC enrolment. The reporting of the study follows STROBE guidelines.19
Exposure, outcomes and covariates
Child AD status was assessed 11 times via questionnaires from 6 to 166 months of age (Table S1; see Supporting Information).15 Mothers were asked if their child had an ‘itchy, dry skin rash in the joints and creases (e.g. behind the knees, elbows, under the arms)’ over the past year and, if so, asked to rate its severity as ‘no problem’, ‘mild’, ‘quite bad’ or ‘very bad’. Reflecting the distribution of AD in young children, questionnaires at 6 and 18 months of age additionally assessed convexity dermatitis (‘itchy, dry, oozing or crusted rash on the face, forearms or shins’), which we integrated into our AD definition. Each time these period prevalence questions were asked, AD status was time-updated via categorization as ‘never’ (never answered yes), ‘maybe’ (ever yes once only), ‘inactive’ (ever yes ≥ 2 times but currently no), ‘active – mild’ (ever yes ≥ 2 times and currently yes with severity of no problem or mild) or ‘active – moderate/severe’ (ever yes ≥ 2 times, currently yes with severity quite bad or very bad). Such approaches to defining AD have been used in previous studies of AD in the ALSPAC cohort.1,20–23 Although we could not validate our AD definition against physician-confirmed diagnoses, the AD question used in ALSPAC reflects the International Study of Allergies and Asthma in Children and United Kingdom Working Party diagnostic criteria for AD,24,25 which are validated assessments used in epidemiological studies.
Our outcome was general cognitive function measured at 18, 49, 102 and 186 months of age using the GMDS, WPPSI, WISC and WASI, respectively (Table S1). These are well-validated assessments of development (GMDS) and intelligence (WPPSI, WISC, WASI) that provide age-adjusted, population-standardized scores [mean (SD) 100 (15)].26–30 The primary outcome for each test was its total score, namely the developmental quotient (DQ) for the GMDS and the Full-Scale Intelligence Quotient (FSIQ) for the WPPSI, WISC and WASI. Secondary outcomes were subscale scores for verbal IQ [i.e. hearing/speech score in GMDS, verbal IQ (VIQ) in WPPSI and WISC, and Vocabulary T-score (V-Tsc) in WASI] and nonverbal IQ [i.e. performance score in GMDS, performance IQ (PIQ) in WPPSI and WISC, and Matrix Reasoning T-score (P-Tsc) in WASI]. Although a developmental assessment scale, the GMDS was used as an early proxy for cognitive functioning. All cognitive tests were administered during in-person study visits by trained ALSPAC personnel in the psychology team and supervised by trained psychometricians.15
Covariates included data collected prenatally such as parental history of atopy (eczema, asthma or hay fever), parental education, maternal financial difficulties score (FDS; ability to afford lodging, heating, food, clothes and items for the child, where higher scores indicate greater financial need)31 and crowding index (number of people living in the home occupied by the mother and child divided by the number of rooms – excluding bathrooms – where higher numbers indicate greater crowding)32 (Table S1). At birth, child sex, ethnicity/race, gestational age and maternal age were recorded.
Similar to previous studies of AD using ALSPAC,1,22 we considered both sleep quantity (total daytime and night-time hours slept calculated from bed and wake times) and sleep quality (at least one night-time awakening per night, regular difficulty going to sleep, regular nightmares and/or regular early-morning awakenings) in our analyses. Sleep data described experiences over the past year and were collected at 18, 42, 81 and 115 months of age (Table S1). Atopic comorbidities were measured at several timepoints and included food allergies (54, 65, 81, 103 and 157 months), asthma and hay fever (both at 81, 91, 103, 128, 157 and 166 months).
For learning disorders, questionnaires administered at 115 months of age asked whether the mother had ever been told that her child had a ‘diagnosis’ of dyslexia, dyspraxia, dysgraphia, dysorthographia or dyscalculia (Table S1).15 For psychiatric disorders, participants’ parents and teachers completed clinically validated Development and Well-Being Assessment (DAWBA) measures at 91 months of age,33 which were reviewed by paediatric psychologists to ascribe Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) diagnoses of any depressive disorder [major depressive, depression not otherwise specified (NOS)], anxiety disorder (separation anxiety, specific phobia, social phobia, post-traumatic stress, obsessive–compulsive, general anxiety, anxiety NOS), oppositional/conduct disorder (oppositional defiant, conduct) or ADHD (inattentive, hyperactive–impulsive, combined) to the children.34
Missing data
As expected with longitudinal cohorts, missing data were encountered in ALSPAC. Across the three GMDS/WPPSI, WISC and WASI subsamples, the greatest proportions of missing data for any variable were 31.8%, 34.5% and 33.4%, respectively, all corresponding to paternal atopic history. For the GMDS, WPPSI, WISC and WASI analyses, the within-subsample missingness of AD exposure was 10.5%, 18.0%, 15.1% and 7.6%, respectively, and that of cognitive outcome data was 20.4%, 29.3%, 2.0% and 4.7%, respectively. Missingness was assumed to be at random, and missing data on AD, covariates and cognitive outcomes were multiply imputed 50 times for each of the three subsamples using multiple imputation by chained equations.35–37
Statistical analysis
GMDS, WPPSI, WISC and WASI scores were compared with respect to AD status using multivariable linear regression, adjusted for potential confounders defined a priori, including sex, gestational age at birth, maternal age at birth, parental education, crowding index, maternal FDS, atopic comorbidities, parental atopic history and sleep characteristics. Race/ethnicity was not directly adjusted for because it is a social construct without biological basis for inclusion and > 97% of ALSPAC participants are White. In each cross-sectional analysis, repeated variables (i.e. AD status and atopic comorbidities) were time-updated to the closest timepoint to when the outcome was assessed (Tables S1–S7; see Supporting Information). Similarly, sleep data used in each analysis were those nearest in time to the cognitive assessment. In secondary analyses, we examined WISC and WASI outcomes stratified by the presence or absence of learning or psychiatric disorders. As these disorders were diagnosed after the GMDS and WPPSI were administered, stratified analyses were not conducted for GMDS and WPPSI. Finally, in an exploratory longitudinal analysis of children with AD data at 18 months of age and at least two of the four cognitive outcomes, we used generalized estimating equations to examine total IQ scores across time with respect to AD status. All analyses were conducted using STATA 17 (StataCorp, College Station, TX, USA).
Results
Participant characteristics
Of 14 975 live-born children, AD data were available for 11 063 (73.9%) participants at 18 months, 9952 (66.5%) participants at 42 months, 8123 (54.2%) participants at 103 months and 6998 (46.7%) participants at 166 months (Table 1; Table S2). Among those with data available at 166 months of age, 12.7% had active AD, 32.0% had inactive AD, 20.8% had indeterminate AD and 34.5% never had AD (Table 1). Across all AD groups, participants were mostly White and born at full term. The more severe AD groups had greater proportions of females, higher parental educational attainment and less household crowding. Atopic comorbidities in the child and parents were more common with more active and severe AD (Table 1; Table S2). Rates of anxiety diagnoses increased as AD status worsened, but rates of other psychiatric or learning disorders did not significantly differ across AD groups (Table 1). The number of hours slept nightly by the child was minimally greater in the inactive and active AD groups vs. the never/maybe AD groups at 166 months (Table 1), but it did not significantly differ across AD groups at earlier timepoints (Table S2). Across all timepoints, the proportion of children with at least one night-time wakening per night increased with more active and severe AD (Table 1; Table S2).
Table 1.
Avon Longitudinal Study of Parents and Children (ALSPAC) study population characteristics by atopic dermatitis (AD) status at 166 months of age (n = 6998)
| Characteristica | AD status | P-valueb | ||||
|---|---|---|---|---|---|---|
| Never (n = 2413; 34.5%) | Maybe (n = 1452; 20.7%) | Inactive (n = 2241; 32.0%) | Active/mild (n = 656; 9.4%) | Active/moderate–severe (n = 236; 3.4%) | ||
| Sex | < 0.001 | |||||
| Male | 1291 (53.5) | 732 (50.4) | 1090 (48.6) | 276 (42.1) | 115 (48.7) | |
| Female | 1122 (46.5) | 720 (49.6) | 1151 (51.4) | 380 (57.9) | 121 (51.3) | |
| Race/ethnicity | 0.82 | |||||
| White | 2097 (98.4) | 1341 (98.2) | 2137 (98.4) | 606 (97.7) | 217 (98.2) | |
| Other group | 35 (1.6) | 25 (1.8) | 34 (1.6) | 14 (2.3) | < 5 (< 2) | |
| Missing | 281 (11.6) | 86 (5.9) | 70 (3.1) | 36 (5.5) | 15 (6.4) | |
| Gestational age (weeks), median (IQR) | 40 (39–41) | 40 (39–41) | 40 (39–41) | 40 (39–41) | 40 (39–41) | 0.21 |
| Missing | 196 (8.1) | 47 (3.2) | 23 (1.0) | 26 (4.0) | 8 (3.4) | |
| Maternal age at birth (years), median (IQR) | 29 (26–32) | 29 (26–32) | 29 (26–32) | 27 (26–32) | 28 (26–32) | 0.24 |
| Missing | 196 (8.1) | 47 (3.2) | 23 (1.0) | 26 (4.0) | 8 (3.4) | |
| Maternal FDS,c median (IQR) | 1 (0–4) | 1 (0–4) | 1 (0–4) | 1 (0–3.5) | 1 (0–4) | 0.34 |
| Missing | 319 (13.2) | 114 (7.9) | 131 (5.8) | 48 (7.3) | 19 (8.1) | |
| Highest parental education attainmentd | 0.001 | |||||
| CSE/none | 191 (8.9) | 107 (7.8) | 144 (6.6) | 39 (6.3) | 16 (7.2) | |
| Vocational | 111 (5.2) | 64 (4.7) | 90 (4.1) | 37 (5.9) | 15 (6.8) | |
| O-level/equivalent | 585 (27.3) | 356 (25.9) | 526 (24.2) | 131 (21.1) | 47 (21.3) | |
| A-level/equivalent | 735 (34.3) | 458 (33.4) | 775 (35.6) | 230 (37.0) | 78 (35.3) | |
| University degree | 523 (24.4) | 387 (28.2) | 640 (29.4) | 185 (29.7) | 65 (29.4) | |
| Missing | 268 (11.1) | 80 (5.5) | 66 (2.9) | 34 (5.2) | 15 (6.4) | |
| Household crowding indexe | 0.02 | |||||
| ≤ 0.5 | 1000 (47.5) | 694 (51.4) | 1113 (51.7) | 303 (48.9) | 118 (53.6) | |
| 0.5–0.75 | 671 (31.9) | 418 (30.9) | 640 (29.7) | 224 (36.2) | 61 (27.7) | |
| 0.75–1.0 | 346 (16.4) | 190 (14.1) | 329 (15.3) | 74 (12.0) | 33 (15.0) | |
| > 1.0 | 89 (4.2) | 49 (3.6) | 72 (3.3) | 18 (2.9) | 8 (3.6) | |
| Missing | 307 (12.7) | 101 (7.0) | 87 (3.9) | 37 (5.6) | 16 (6.8) | |
| History of atopic comorbiditiesf | ||||||
| Food allergies | 269 (11.4) | 215 (15.1) | 472 (21.2) | 195 (30.0) | 81 (34.5) | < 0.001 |
| Missing | 57 (2.4) | 24 (1.7) | 11 (0.5) | 5 (0.8) | < 5 (< 1) | |
| Asthma | 328 (13.6) | 236 (16.3) | 569 (25.4) | 208 (31.7) | 95 (40.4) | < 0.001 |
| Missing | < 5 (< 1) | < 5 (< 1) | < 5 (< 1) | < 5 (< 1) | < 5 (< 1) | |
| Hay fever | 495 (20.5) | 323 (22.3) | 737 (32.9) | 306 (46.7) | 126 (53.4) | < 0.001 |
| Missing | < 5 (< 1) | < 5 (< 1) | < 5 (< 1) | < 5 (< 1) | < 5 (< 1) | |
| Parental atopy (eczema, asthma, hay fever) | ||||||
| Maternal | 854 (41.0) | 624 (46.8) | 1088 (51.2) | 323 (53.3) | 133 (60.5) | < 0.001 |
| Missing | 328 (13.6) | 119 (8.2) | 118 (5.3) | 50 (7.6) | 16 (6.8) | |
| Paternal | 552 (36.1) | 375 (39.4) | 726 (45.4) | 240 (51.5) | 76 (48.4) | < 0.001 |
| Missing | 883 (36.6) | 500 (34.4) | 642 (28.6) | 190 (29.0) | 79 (33.5) | |
| Sleep characteristicsg | ||||||
| No. of night-time hours, median (IQR) | 8.2 (7.5–8.8) | 8.0 (7.4–8.7) | 8.3 (7.5–8.8) | 8.3 (7.6–8.8) | 8.3 (7.7–8.8) | 0.04 |
| Missing | 1038 (43.0) | 601 (41.4) | 862 (38.5) | 232 (35.4) | 99 (41.9) | |
| ≥ 1 night-time awakening per night | 542 (42.9) | 320 (41.2) | 526 (41.6) | 187 (46.9) | 64 (50.8) | 0.11 |
| Missing | 1150 (47.7) | 676 (46.6) | 977 (43.6) | 257 (39.2) | 110 (46.6) | |
| History of psychiatric diagnosish | ||||||
| ADHD | 31 (1.6) | 28 (2.3) | 35 (1.7) | 10 (1.7) | < 5 (< 2) | 0.75 |
| Oppositional/conduct disorder | 39 (2.0) | 37 (3.0) | 52 (2.6) | 22 (3.8) | 8 (3.8) | 0.11 |
| Anxiety | 32 (1.7) | 35 (2.8) | 58 (2.9) | 23 (4.0) | 8 (3.8) | 0.01 |
| Depression | < 5 (< 1) | 7 (0.6) | 9 (0.4) | < 5 (< 1) | < 5 (< 1) | 0.13 |
| Missing | 489 (20.3) | 210 (14.5) | 215 (9.6) | 78 (11.9) | 28 (11.9) | |
| History of learning disorderh | 97 (4.7) | 74 (5.9) | 103 (5.0) | 28 (4.8) | 10 (4.7) | 0.65 |
| Missing | 366 (15.2) | 200 (13.8) | 196 (8.7) | 72 (11.0) | 24 (10.2) | |
Data are presented as n (%) unless otherwise stated. ADHD, attention deficit/hyperactivity disorder; CSE, Certificate of Secondary Education; FDS, financial difficulties score; IQR, interquartile range. aPercentages refer to that variable’s nonmissing total in each AD status category and thus sum to 100. Missing percentages refer to the total count in each AD status category. Per ALSPAC protocol, all cell counts < 5 (including 0) are denoted as such instead of the actual number. bFisher’s exact or χ2 tests used for categorical variables; Kruskal–Wallis test used for continuous variables. cMother’s financial ability to afford lodging, food, heating, clothing and items for child, recorded during gestational period. dRecorded during the gestational period. eCalculated by dividing the number of people living in the home inhabited by the mother and child by the number of rooms (excluding bathrooms), recorded during gestational period. fHistory of food allergies by 157 months of age, history of asthma or hay fever by 166 months of age. gSleep variables asked at 186 months of age in reference to the past year. hPsychiatric disorders indicated by diagnosis of any depressive disorder, anxiety disorder, behavioural disorder or ADHD levied by child psychologists using writeups provided by parents and teachers on the Development and Well-Being Assessment (DAWBA) at 91 months of age. Learning disorders defined by maternal report at 115 months old of having been told that child has any of the following conditions: dyslexia, dysgraphia, dysorthographia, dyscalculia or dyspraxia.
Intelligence quotient scores
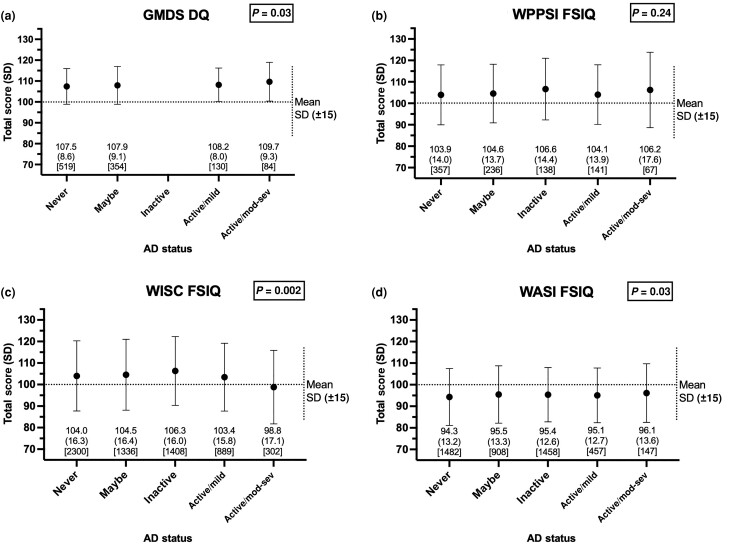
Average GMDS DQ, WPPSI FSIQ and WISC FSIQ scores across the five AD groups were generally higher than population means, while average WASI FSIQ scores were slightly lower than population means (Figure 1). GMDS DQ, WISC FSIQ and WASI FSIQ scores were statistically significantly different with respect to AD status but overlapped greatly with small absolute differences in scores (Figure 1). A similar trend was seen across verbal IQ scores for GMDS, WISC and WASI (Table S3). WPPSI scores and nonverbal IQ scores did not significantly differ with respect to AD status (Figure 1; Table S3).
Figure 1.
Total intelligence quotient (IQ) scores by atopic dermatitis (AD) status. Total scores for (a) Griffiths Mental Development Scales, Extended 0–8 years (GMDS) Development Quotient (DQ) at 18 months of age; (b) Wechsler Preschool and Primary Scale of Intelligence (WPPSI) Full-Scale Intelligence Quotient (FSIQ) at 49 months of age; (c) Wechsler Intelligence Scale for Children (WISC) FSIQ at 102 months of age; and (d) Wechsler Abbreviated Scale of Intelligence, Second Edition, Two-Subset (WASI) FSIQ at 186 months of age. AD status was defined by that recorded nearest to each outcome measure, i.e. within (a) the same month for GMDS, (b) 7 months of WPPSI, (c) 1 month of WISC and (d) 20 months of WASI. The mean (SD) [count] score is noted below the corresponding AD status category. One-way Anova used to compare mean scores by AD status. Dotted horizontal and vertical lines show the standardized population mean (SD) 100 (15) for all tests. mod-sev, moderate–severe.
Cross-sectional analyses of cognition by atopic dermatitis status
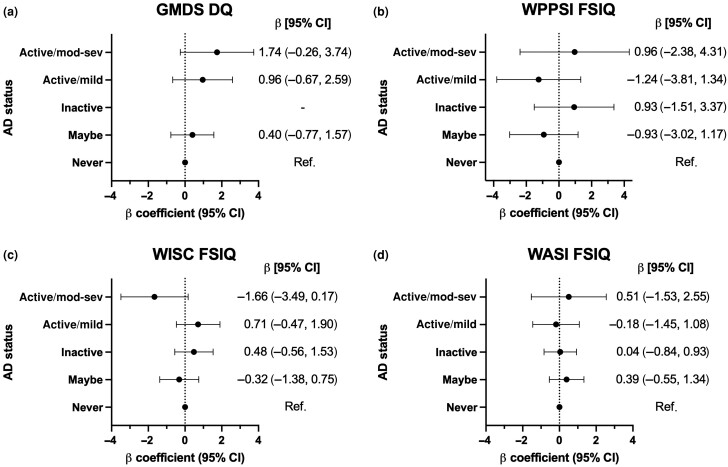
In adjusted multivariable analyses, GMDS DQ and subscale scores at 18 months did not differ significantly with respect to AD status (Figure 2; Table S4). However, scores for DQ and hearing/speech increased in parallel with more active/severe AD, with the highest mean scores in the active/moderate–severe AD group [β coefficient 1.74, 95% confidence interval (CI) –0.26 to 3.74; and β coefficient 3.43, 95% CI –0.07 to 6.93, respectively], but these were not statistically significant. At 8.5 years old, WISC FSIQ did not differ by AD status but was slightly lower in the active/moderate–severe group (β coefficient –1.66, 95% CI –3.49 to 0.17) vs. the never AD; VIQ was slightly higher in the inactive AD group (β coefficient 1.32, 95% CI 0.28–2.36) and PIQ was slightly lower in the active/moderate–severe group (β coefficient –2.21, 95% CI –4.19 to –0.23) relative to participants without AD (Figure 2; Table S6). WPPSI and WASI scores at 49 months and 15.5 years old, respectively, did not differ by AD status in adjusted models (Figure 2; Tables S5, S7). Among the adjusted covariates, female sex was associated with higher scores on early-childhood assessments and higher parental education was associated with higher scores at all timepoints, while fewer hours of sleep and the presence of night-time awakenings were associated with lower scores (Tables S4–S7).
Figure 2.
Adjusted multivariable linear regression models of total intelligence quotient (IQ) scores by atopic dermatitis (AD) status, relative to no history of AD. β coefficients for AD status with respect to (a) Griffiths Mental Development Scales, Extended 0–8 years (GMDS) Development Quotient (DQ) at 18 months of age; (b) Wechsler Preschool and Primary Scale of Intelligence (WPPSI) Full-Scale Intelligence Quotient (FSIQ) at 49 months of age; (c) Wechsler Intelligence Scale for Children (WISC) FSIQ at 102 months of age; and (d) Wechsler Abbreviated Scale of Intelligence, Second Edition, Two-Subset (WASI) FSIQ at 186 months of age, adjusted for sex, child gestational age at birth, maternal age at birth, parental education, crowding index, maternal financial difficulties score, parental atopy, comorbid child atopy and sleep quantity and quality. AD status defined by that recorded nearest to each outcome measure, i.e. within (a) the same month for GMDS, (b) 7 months of WPPSI, (c) 1 month of WISC and (d) 20 months of WASI. CI, confidence interval; mod-sev, moderate–severe.
Analyses stratified by psychiatric comorbidities
In analyses stratified by comorbid learning or psychiatric disorders, no association was observed between AD and WISC or WASI FSIQ in either subgroup (Table S8; see Supporting Information). Although the inverse association between active/moderate–severe AD and WISC PIQ was no longer statistically significant in the stratified analyses, similar estimates remained in the subgroup without learning or psychiatric disorders (β coefficient –2.04, 95% CI –4.17 to 0.09). The association between inactive AD and WISC VIQ remained statistically significant only in the subgroup without learning or psychiatric disorders (β coefficient 1.21, 95% CI 0.14–2.28). However, these effect sizes were small and there was no consistent evidence for a gradient by AD severity.
Longitudinal analysis of intelligence quotient by atopic dermatitis status
In total, 3086 participants had AD data available at 18 months old (17.6% with definite AD, 33.2% with maybe AD and 49.3% without any AD) and at least two cognitive outcomes measured. In a longitudinal analysis of these participants, we found no significant association between AD and total IQ scores over time after adjustment for sociodemographic characteristics and sleep (Table 2).
Table 2.
Longitudinal analysis of total intelligence quotient scores between 18 months and 15.5 years of age by atopic dermatitis (AD) status (n = 3086)
| Variable | β coefficient (95% CI) |
|---|---|
| AD status (ref.: never AD) | |
| Maybe | –0.33 (–1.24, 0.57) |
| Definitea | –0.09 (–1.22, 1.03) |
| Sex (ref.: male) | |
| Female | –0.11 (–0.92, 0.71) |
| Gestational age, per 1 week | 0.08 (–0.17, 0.32) |
| Maternal age at birth, per 1 year | 0.17 (0.07–0.28) |
| Highest parental education attainment (ref.: CSE/none)b | |
| Vocational | –1.68 (–4.47, 1.10) |
| O-level/equivalent | 2.97 (0.74–5.20) |
| A-level/equivalent | 6.41 (4.20–8.61) |
| University degree | 13.48 (11.20–15.76) |
| Crowding index (ref.: ≤ 0.5)c | |
| 0.5–0.75 | –1.06 (–1.99, –0.14) |
| 0.75–1 | –3.10 (–4.42, –1.78) |
| > 1 | –1.75 (–4.41, 0.91) |
| Maternal FDSd | –0.27 (–0.42, –0.12) |
| Parental history of atopy (ref.: none)b | |
| Maternal | 1.16 (0.36–1.97) |
| Paternal | 0.44 (–0.37, 1.26) |
| Sleep characteristicse | |
| No. of hours in a 24-h period, per 1 h | –0.11 (–0.46, 0.24) |
| ≥ 1 night-time awakening per night (ref.: < 1 awakening) | 0.03 (–0.81, 0.88) |
Multivariable generalized estimating equation model with general cognition as the dependent variable [measured by Griffiths Mental Development Scales, Extended 0–8 years (GMDS) Development Quotient (DQ) at 18 months of age; Wechsler Preschool and Primary Scale of Intelligence, UK Revised, Third Edition (WPPSI) Full-Scale Intelligence Quotient (FSIQ) at 49 months of age; Wechsler Intelligence Scale for Children, Third Edition (WISC) FSIQ at 102 months of age; and Wechsler Abbreviated Scale of Intelligence, Second Edition, Two-Subtest (WASI) FSIQ at 186 months of age]. AD status was defined as that at 18 months of age. Estimates with a P-value < 0.05 are indicated in bold. CI, confidence interval; CSE, Certificate of Secondary Education; FDS, financial difficulties score. aIncludes the following AD status categories: active/mild, active/moderate–severe. bRecorded during gestation. cCalculated by dividing the number of people living in the home inhabited by the mother and child by the number of rooms (excluding bathrooms), recorded during gestation. dMother’s financial ability to afford lodging, food, heating, clothing and items for the child, recorded during gestation. eSleep variables asked at 18 months of age in reference to the past year.
Discussion
Overall, we found no major association between AD and general cognitive function in childhood. While we observed statistically significant differences in nonverbal and verbal IQ by AD status at the age of 8 years, these small differences are unlikely to be clinically meaningful considering the WISC’s standard error of measurement of ±3 points.28
Several previous studies have also found no association between AD and general cognition, but many had limitations. Unlike our study’s serial assessment of cognitive function using clinically validated instruments,26–30 cognitive outcomes in previous studies were often a single measure and/or inadequately standardized.9,10 Moreover, AD definitions varied greatly, with one study finding an AD prevalence rate of < 1%, which was significantly lower than expected,10,38 and another that included individuals with atopic conditions other than AD.9 Our study was strengthened by its robust assessment of AD activity and severity over multiple timepoints,24,25 allowing us to account for the chronic, waxing-and-waning nature of AD.39,40 Unlike several past studies, which examined mostly young adult males,10,12,41 our study evaluated a broader population of children across their first 16 years of life.
In contrast to our findings, previous studies in different populations or using different outcome measures found worse cognitive function in select patients with AD. For example, one study found WISC FSIQ scores to be 17 points lower in children with AD than in those without.8 However, the study only included 41 children and almost 90% of those with AD had moderate-to-severe disease. In another study of adults with AD, patient-reported cognitive function declined with worsening AD severity, but the study did not include a non-AD comparator group.42 Nevertheless, given previous findings that children with more severe AD also have greater rates of learning problems than peers with milder AD,3,7 it remains possible that AD impacts cognition only when the skin disease is severe. As our cohort was enriched for children with milder AD, future studies focused on moderate-to-severe AD would be informative.
As AD has been associated with learning problems and psychiatric disorders, including depression, anxiety and ADHD,2–5,7 we considered whether such comorbidities might moderate the relationship between AD and cognition. It is possible that children with such disorders have greater difficulty managing the itch or sleep disruptions caused by AD and thus have worse cognitive performance than their peers without AD.8 However, our stratified analyses revealed no association between AD and cognition, regardless of the presence or absence of learning or psychiatric disorders. Although we adjusted for parental educational attainment, it is also possible that higher parental education, which is more prevalent among children with AD and is also associated with higher child IQ, could counteract any negative effects of AD on cognition; however, analyses stratified by parental education did not suggest such an effect modification (data not shown).
While direct comparisons across different IQ measures are not typically recommended,43 we explored whether there could be changes in cognition over time across different AD groups, as few longitudinal studies exist and our dataset was unique in having four repeated assessments of cognition.11,13 Similar to the cross-sectional findings, the exploratory longitudinal analysis did not show any association between AD and cognitive function over time. Nevertheless, future studies with repeated measures of the same cognitive instrument are needed to confirm our exploratory findings.
Our study had potential limitations. Firstly, as AD was parentally reported, misclassification is possible. However, similar approaches have been employed in other epidemiological studies of AD;1,4,22,40,44,45 the ALSPAC questions for AD reflect validated diagnostic criteria;24,25 and age-specific AD prevalence in our study aligns with estimates from other cohorts.39,44,46,47 Secondly, as AD was assessed via mailed questionnaires, while cognitive assessments were conducted in person, our exposure and outcome variables were not necessarily concurrent (e.g. a child with recently active/moderate–severe AD may not have had this exact degree of AD on the day of cognitive testing). However, AD status reflected annual period prevalence, which likely provides a reasonable approximation, and the timing of AD and cognition measures generally did not differ by more than 6 months. Thirdly, we could not account for all potential confounders or mediators of IQ test performance in our analysis. For example, data on school attendance, grades achieved and academic performance were limited in ALSPAC, precluding us from assessing their potential effects on IQ outcomes. Fourthly, our findings may not be generalizable beyond the UK population as many region-specific factors related to healthcare and education may have influenced our findings. Also, the paediatric data in ALSPAC were collected 15–30 years ago and are fairly dated now. The UK population has since become more racially/ethnically diverse,48 and AD epidemiology has also changed, including its rising prevalence over the last 30 years.49 Such changes may further limit the generalizability of our findings. Nevertheless, ALSPAC is one of few prospective cohorts to have collected detailed information on both AD and cognition across childhood and thus remains a unique resource for examining our study question. Finally, bias may have been introduced by the presence of missing data and cohort attrition over time. We imputed missing data using chained equations for the primary analysis and repeated our analyses using nonimputed data, which led to similar findings (not shown).35–37,50
In summary, our study within a UK-based birth cohort suggested no independent association between AD and general cognitive function in childhood. However, further characterization of cognitive function in select subpopulations, such as children with moderate-to-severe AD, is warranted. Moreover, although the cognitive instruments in this study are validated and widely used in practice, IQ is not the only or definitive measure of cognitive ability, and examinations of cognitive measures beyond IQ are needed.51,52 Finally, prospective studies with repeated measures of both objective AD severity and a diverse array of cognitive outcomes will further clarify the longitudinal relationship of AD with cognition.
Supplementary Material
Acknowledgements
We are extremely grateful to all the families who took part in this study, the midwives for their help in recruiting them and the whole Avon Longitudinal Study of Parents and Children (ALSPAC) team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists and nurses.
Contributor Information
Patrick G Sockler, Departments of Dermatology; Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA, USA.
Stephen R Hooper, Department of Health Sciences, School of Medicine, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA.
Katrina Abuabara, Department of Dermatology, University of California-San Francisco School of Medicine, San Francisco, CA, USA.
Emily Z Ma, University of Maryland School of Medicine, Baltimore, MD, USA.
Sarah Radtke, Child and Adolescent Psychiatry, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Aaron Bao, Departments of Dermatology.
Elle Kim, Departments of Dermatology; Departments of Biostatistics.
Rashelle J Musci, Mental Health.
Karin Kartawira, Departments of Dermatology.
Joy Wan, Departments of Dermatology; Epidemiology, Johns Hopkins University Bloomberg School of Public Health, Baltimore, MD, USA.
Funding sources
This study was supported by a grant from the National Institutes of Health [K23AR077629 (J.W.)]. The sponsors of this work played no role in the design of the study, the collection, analysis and interpretation of data and the writing of the manuscript. The Medical Research Council and Wellcome (grant ref.: 217065/Z/19/Z) and the University of Bristol provide core support for ALSPAC. This publication is the work of the authors, who will all serve as guarantors for the contents of this paper. A comprehensive list of grants funding is available on the ALSPAC website (http://www.bristol.ac.uk/alspac/external/documents/grant-acknowledgements.pdf). This research was specifically funded by the Wellcome Trust and Medical Research Council (Core) (grant ref.: 076467/Z/05/Z).
Conflicts of interest
J.W. has received research and fellowship funding (paid to her institution) from Pfizer and has served as an advisor to Janssen and Sun Pharmaceuticals (DMC), receiving honoraria. K.A. has received consulting fees from TARGET RWE and grants (paid to her institution) from Pfizer and Cosmetique Internacional SNC. The other authors declare no conflicts of interest.
Data availability
The ALSPAC website contains details of all the data that are available through a fully searchable data dictionary and variable search tool (http://www.bristol.ac.uk/alspac/researchers/our-data/).
Ethics statement
Ethical approval for the study was obtained from the Avon Longitudinal Study of Parents and Children (ALSPAC) Ethics and Law Committee and the Local Research Ethics Committees. Informed consent for the use of data collected via questionnaires and clinics was obtained from participants following the recommendations of the ALSPAC Ethics and Law Committee at the time. This current study was approved as exempt by the Johns Hopkins University Institutional Review Board.
Supporting Information
Additional Supporting Information may be found in the online version of this article at the publisher’s website.
References
- 1. Ramirez FD, Chen S, Langan SM. et al. Association of atopic dermatitis with sleep quality in children. JAMA Pediatr 2019; 173:e190025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Strom MA, Fishbein AB, Paller AS. et al. Association between atopic dermatitis and attention deficit hyperactivity disorder in U.S. children and adults. Br J Dermatol 2016; 175:920–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wan J, Mitra N, Hooper SR. et al. Association of atopic dermatitis severity with learning disability in children. JAMA Dermatol 2021; 157:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wan J, Takeshita J, Shin DB. et al. Mental health impairment among children with atopic dermatitis: a United States population-based cross-sectional study of the 2013–2017National Health Interview Survey. J Am Acad Dermatol 2020; 82: 1368–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liao T-C, Lien Y-T, Wang S. et al. Comorbidity of atopic disorders with autism spectrum disorder and attention deficit/hyperactivity disorder. J Pediatr 2016; 171:248–55. [DOI] [PubMed] [Google Scholar]
- 6. Meltzer LJ, Booster GD. Sleep disturbance in caregivers of children with respiratory and atopic disease. J Pediatr Psychol 2016; 41:643–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wan J, Shin DB, Gelfand JM. Association between atopic dermatitis and learning disability in children. J Allergy Clin Immunol Pract 2020; 8:2808–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Camfferman D, Kennedy JD, Gold M. et al. Sleep and neurocognitive functioning in children with eczema. Int J Psychophysiol 2013; 89:265–72. [DOI] [PubMed] [Google Scholar]
- 9. Daramola OO, Ayoola OO, Ogunbiyi AO. The comparison of intelligence quotients of atopic and nonatopic children in Ibadan, Nigeria. Indian J Dermatol 2010; 55:221–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Smirnova J, von Kobyletzki LB, Lindberg M. et al. Atopic dermatitis, educational attainment and psychological functioning: a national cohort study. Br J Dermatol 2019; 180:559–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Abuabara K. Atopic dermatitis and academic achievement – more to learn. JAMA Dermatol 2021; 157:637–8. [DOI] [PubMed] [Google Scholar]
- 12. Vittrup I, Andersen YMF, Skov L. et al. The association between atopic dermatitis, cognitive function and school performance in children and young adults. Br J Dermatol 2022; 188:341–9. [DOI] [PubMed] [Google Scholar]
- 13. Sockler PG, Hooper SR, Kartawira K. et al. Cognitive function and academic achievement in children with early childhood atopic dermatitis. J Invest Dermatol 2023; 10.1016/j.jid.2023.07.015 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Golding J, Pembrey M, Jones R; ALSPAC Study Team . ALSPAC – the Avon Longitudinal Study of Parents and Children. Paediatr Perinatal Epidemiol 2001; 15:74–87. [DOI] [PubMed] [Google Scholar]
- 15. Boyd A, Golding J, Macleod J. et al. Cohort profile: the ‘children of the 90s’ – the index offspring of the Avon Longitudinal Study of Parents and Children. Int J Epidemiol 2013; 42:111–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fraser A, Macdonald-Wallis C, Tilling K. et al. Cohort profile: the Avon Longitudinal study of parents and children: ALSPAC mothers cohort. Int J Epidemiol 2013; 42:97–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Northstone K, Ben Shlomo Y, Teyhan A. et al. The Avon Longitudinal Study of Parents and Children ALSPAC G0 Partners: A cohort profile [version 2; peer review: 1 approved with reservations]. Wellcome Open Research. Available at: https://wellcomeopenresearch.org/articles/8-37 (last accessed 12 December 2023). [Google Scholar]
- 18. University of Bristol . Avon Longitudinal Study of Parents and Children. Available at: https://www.bristol.ac.uk/alspac/(last accessed 12 December 2023).
- 19. von Elm E, Altman DG, Egger M. et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med 2007; 147:573–7. [DOI] [PubMed] [Google Scholar]
- 20. Perkin MR, Strachan DP, Williams HC. et al. Natural history of atopic dermatitis and its relationship to serum total immunoglobulin E in a population-based birth cohort study. Pediatr Allergy Immunol 2004; 15:221–9. [DOI] [PubMed] [Google Scholar]
- 21. Wadonda-Kabondo N, Sterne JAC, Golding J. et al. Association of parental eczema, hayfever, and asthma with atopic dermatitis in infancy: birth cohort study. Arch Dis Child 2004; 89:917–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kern C, Wan J, LeWinn KZ. et al. Association of atopic dermatitis and mental health outcomes across childhood: a longitudinal cohort study. JAMA Dermatol 2021; 157:1200–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Henderson J, Northstone K, Lee SP. et al. The burden of disease associated with filaggrin mutations: a population-based, longitudinal birth cohort study. J Allergy Clin Immunol 2008; 121:872–7. [DOI] [PubMed] [Google Scholar]
- 24. Asher M, Keil U, Anderson H. et al. International Study of Asthma and Allergies in Childhood (ISAAC): rationale and methods. Eur Respir J 1995; 8:483–91. [DOI] [PubMed] [Google Scholar]
- 25. Williams HC, Burney PG, Pembroke AC. et al. The U.K. Working Party's Diagnostic Criteria for Atopic Dermatitis. III. Independent hospital validation. Br J Dermatol 1994; 131:406–16. [DOI] [PubMed] [Google Scholar]
- 26. Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI). Pearson; , 1999. [Google Scholar]
- 27. Wechsler D. Wechsler Preschool and Primary Scale of Intelligenc – Third Edition (WPPSI-III). APA PsycTests; , 2002. [Google Scholar]
- 28. Wechsler D. Wechsler Intelligence Scale for Children – Third Edition (WISC-III). San Antonio, TX: The Psychological Corp; ., 1991. [Google Scholar]
- 29. Griffiths R. The Abilities of Young Children: A Comprehensive System of Mental Measurement for the First Eight Years of Life. London: Child Development Research Centre; , 1970. [Google Scholar]
- 30. Griffiths R, Huntley M. Griffiths Mental Development Scales – Revised: Birth to 2 Years (GMDS 0-2), 2nd edn. APA PsycTests; , 1996. [Google Scholar]
- 31. Russell AE, Ford T, Russell G. The relationship between financial difficulty and childhood symptoms of attention deficit/hyperactivity disorder: a UK longitudinal cohort study. Soc Psychiatry Psychiatr Epidemiol 2018; 53:33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Melki IS, Beydoun HA, Khogali M. et al. Household crowding index: a correlate of socioeconomic status and inter-pregnancy spacing in an urban setting. J Epidemiol Community Health 2004; 58:476–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Goodman R, Ford T, Richards H. et al. The Development and Well-Being Assessment: description and initial validation of an integrated assessment of child and adolescent psychopathology. J Child Psychol Psychiatry 2000; 41:645–55. [PubMed] [Google Scholar]
- 34. APA . Diagnostic and Statistical Manual of Mental Disorders, 4th edn. American Psychological Association; , 1994. [Google Scholar]
- 35. Azur MJ, Stuart EA, Frangakis C. et al. Multiple imputation by chained equations: what is it and how does it work? Int J Methods Psychiatr Res 2011; 20:40–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Resche-Rigon M, White IR. Multiple imputation by chained equations for systematically and sporadically missing multilevel data. Stat Methods Med Res 2018; 27:1634–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Little TD, Rhemtulla M. Planned missing data designs for developmental researchers. Child Dev Perspect 2013; 7:199–204. [Google Scholar]
- 38. Johansson EK, Bergström A, Kull I. et al. Prevalence and characteristics of atopic dermatitis among young adult females and males-report from the Swedish population-based study BAMSE. J Eur Acad Dermatol Venereol 2022; 36:698–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Odhiambo JA, Williams HC, Clayton TO. et al. Global variations in prevalence of eczema symptoms in children from ISAAC Phase Three. J Allergy Clin Immunol 2009; 124:1251–8. [DOI] [PubMed] [Google Scholar]
- 40. Silverberg JI, Patel N, Immaneni S. et al. Assessment of atopic dermatitis using self-report and caregiver report: a multicentre validation study. Br J Dermatol 2015; 173:1400–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Teasdale TW. The Danish draft board's intelligence test, Børge Priens Prøve: psychometric properties and research applications through 50 years. Scand J Psychol 2009; 50:633–8. [DOI] [PubMed] [Google Scholar]
- 42. Silverberg JI, Lei D, Yousaf M. et al. Association of atopic dermatitis severity with cognitive function in adults. J Am Acad Dermatol 2020; 83:1349–59. [DOI] [PubMed] [Google Scholar]
- 43. Axelrod BN. Validity of the Wechsler Abbreviated Scale of Intelligence and Other very short forms of estimating intellectual functioning. Assessment 2002; 9:17–23. [DOI] [PubMed] [Google Scholar]
- 44. Shaw TE, Currie GP, Koudelka CW. et al. Eczema prevalence in the United States: data from the 2003 National Survey of Children's Health. J Invest Dermatol 2011; 131:67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yaghmaie P, Koudelka CW, Simpson EL. Mental health comorbidity in patients with atopic dermatitis. J Allergy Clin Immunol 2013; 131:428–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Silverberg JI, Simpson EL. Associations of childhood eczema severity: a US population-based study. Dermatitis 2014; 25:107–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Taylor-Robinson DC, Williams H, Pearce A. et al. Do early-life exposures explain why more advantaged children get eczema? Findings from the U.K. Millennium Cohort Study. Br J Dermatol 2016; 174:569–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Office for National Statistics . Ethnic group, England and Wales: Census 2021. Available at: https://www.ons.gov.uk/peoplepopulationandcommunity/culturalidentity/ethnicity/bulletins/ethnicgroupenglandandwales/census2021 (last accessed 29 November 2022).
- 49. Odhiambo JA, Williams HC, Clayton TO. et al. Global variations in prevalence of eczema symptoms in children from ISAAC Phase Three. J Allergy Clin Immunol 2009; 124:1251–8. [DOI] [PubMed] [Google Scholar]
- 50. White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med 2011; 30:377–99. [DOI] [PubMed] [Google Scholar]
- 51. von Stumm S, Plomin R. Socioeconomic status and the growth of intelligence from infancy through adolescence. Intelligence 2015; 48:30–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Nisbett RE, Aronson J, Blair C. et al. Intelligence: new findings and theoretical developments. Am Psychol 2012; 67:130–59. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The ALSPAC website contains details of all the data that are available through a fully searchable data dictionary and variable search tool (http://www.bristol.ac.uk/alspac/researchers/our-data/).