Introduction
Major depression is a highly prevalent condition, affecting approximately 10% of the population.1 It is also a growing global problem,2 and has been consistently associated with increased risk of coronary heart disease (CHD).3 It is therefore not surprising that depression is highly comorbid with CHD, being two to three times more common among patients with CHD than in the general population. The prevalence of depression is 15–30% in patients with CHD,4 and is approximately twice as high in women than men, especially affecting young women in the aftermath of acute myocardial infarction (MI).5
Depression as a risk factor for CHD has been characterized from mild depressive symptoms to a clinical diagnosis of major depression. As defined by the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5), clinical depression, or major depression, is characterized by depressed mood or anhedonia (loss of interest or pleasure) for at least 2 weeks accompanied by significant functional impairment and additional somatic or cognitive symptoms.6 Most epidemiological studies of depression and incidence of CHD have used depressive symptom scales, and have frequently demonstrated a dose–response pattern, with higher levels of depressive symptoms being associated with higher risk.3
The exact mechanisms linking depression to increased CHD risk are complex and multifactorial, and still incompletely understood.7 Although adverse lifestyle behaviours and traditional CHD risk factors, such as smoking and sedentary lifestyle, largely contribute to the risk, they do not explain it entirely. In CHD patients, depression is also associated with severity of functional impairment, lower adherence to therapy and lower participation in cardiac rehabilitation. Whether and to what extent these factors explain the relationship between depression and CHD deserves future study. The present paper summarizes key aspects in our current knowledge linking depression and CHD within the intersecting fields of neuroscience, cardiovascular physiology, and behavioural medicine, with the objective of bringing attention to this area and stimulating interdisciplinary research, clinical awareness, and improved care.
Epidemiological aspects
Depression and coronary heart disease
Many studies have shown a relationship between major depression, or depressive symptoms, and CHD.3,8,9 This literature has been summarized by a number of meta-analyses,8–10 all providing evidence for an association between clinical depression (or depressive symptoms) and CHD. This link is seen in individuals initially free of CHD and in a variety of CHD patient populations, including patients with acute coronary syndromes (ACS), heart failure, stable CHD, and post-coronary bypass surgery. However, individual studies have produced heterogeneous risk estimates and have varied in their ability to adjust for other factors such as smoking, physical inactivity, other risk factors, and severity of CHD. Indeed, depression is associated with several CHD risk factors and health behaviours as described above. In statistical models that adjust for these risk factors, depression usually remains an independent risk factor for CHD, suggesting a biological relationship between these two disease states that remains in part unexplained by an increase in traditional risk factors or lifestyle behaviours.
In one of the relatively recent meta-analyses, which included 30 prospective cohort studies of individuals initially free of CHD, depression was associated with a 30% increased risk of future coronary events.9 The association remained significant in the group of studies that adjusted for socio-demographic factors and lifestyle behaviours.8 In community samples and in general practice clinics, the rate of depression is about, 10%11 but it goes up to about 15–30% in patients with CHD.11,12
Studies have also suggested that specific subtypes of depression may be more strongly associated with CHD risk than others. For instance, patients with a new-onset of depression after ACS, with treatment resistant depression, or with somatic depressive symptoms as opposed to cognitive symptoms, are all at increased risk of developing adverse CHD outcomes. However, there is no clear consensus on whether these different phenotypes carry variations in risk.13
Gender differences
Among women, depression is approximately twice as prevalent as in men and has shown some of the most robust associations with CHD.14 Depression in women is also on average more severe than in men and has an earlier age of onset. Women with CHD similarly have twice the rates of depression as men with CHD.15–17 The condition is especially common in young women who have survived a MI15,16,18; about half of women younger than 60 years with a previous MI have a history of major depression.16–18 Of note, young women are more likely to die MI than men.19 Depression is linked to early life adversities and psychological trauma, which tend to be more common in girls than boys and may result in chronic dysregulation of neurohormonal stress systems. This may begin at an early age, setting the stage for an increase in cardiovascular risk in women many years before CHD becomes manifest.5
Among women, depression increases their risk for CHD between 30% and two-fold depending on depression measures and CHD endpoints.20,21 Two follow-up studies of young community samples (<40 years old) found that the impact of depression on CHD risk was higher among women than men.22,23 In the Third National Health and Nutrition Examination Survey (NHANES III), a history of major depression or suicide attempt was associated with almost 15-fold increased risk of ischaemic heart disease among women, and 3.5 in men.22 In the prospective Community Mental Health Epidemiology Study of Washington County, MD, women younger than 40 years with depression had a six-fold increased risk of CHD compared with women of the same age without depression, while depression was not associated with CHD in men or older individuals.23 Even among patients referred for coronary angiography, depression is more predictive of adverse cardiovascular outcomes in young women than in other groups.24 After an acute MI, however, depression seems to affect prognosis to a similar extent in women and men.25 Overall, the evidence suggests that depression is more closely associated with CHD for women than for men, with the strongest effects for younger women.
Clinical and prognostic considerations
Depression as a prognostic factor in acute coronary syndromes
Despite some heterogeneity of findings, the bulk of the evidence supports the notion that depression after ACS is a risk factor for all-cause and cardiac mortality, as well as for composite outcomes including mortality or non-fatal cardiac events.4 Among patients hospitalized for ACS, the increased risk occurs regardless of whether depression pre-dated the ACS event or developed subsequently,4,26,27 although some evidence suggests that that depressive episodes that develop soon after an ACS may carry a higher risk than episodes that begin before the event.28–30 Depression is also a major determinant of unplanned rehospitalizations within 30 days after a hospital discharge for MI.31
Some studies have found that the somatic symptoms of depression may carry a high risk than cognitive symptoms.32–34 Depressive episodes that do not respond to standard treatments have also been identified as high-risk subtypes.35 However, evidence suggests that recognition and treatment of depression improves prognosis. In a previous study, patients with depression that was recognized or treated during an MI hospitalization or at discharge had similar 1-year mortality than those without depression, while a higher mortality was confined to patients with untreated depression.36 These data are important since depression in ACS patients is frequently under-recognized and untreated.33,37,38
Patients with comorbid depression and CHD have lower adherence to treatments and lifestyle changes; for example, they are significantly less likely to adhere to medication regimens39,40 and to follow lifestyle recommendations (e.g. smoking cessation, exercise) and practice self-management (e.g. weight monitoring in heart failure).41 They are also less likely to participate in cardiac rehabilitation programmes, and more likely to drop out of these programmes.39,42,43 Improvement in depression is associated with better self-reported adherence to medications and secondary prevention lifestyle.44,45
During the first year post-MI the presence of depression is associated with about 40% higher healthcare costs, including outpatient care and hospital readmissions.46 In addition, the presence of major depression in the past 12 months can affect societal costs indirectly through work absence.47 For all the above reasons, major depression has been proposed as a risk factor for adverse medical outcomes in patients with ACS.4 The application of collaborative care interventions for depression in CHD populations has emerged as a promising healthcare model to reduce the societal impact of this common comorbidity.48,49
Depression and quality of life
In the setting of CHD, depression is the strongest predictor of quality of life (QoL).50 Depression has a greater impact on QoL than symptoms related to the severity of cardiac disease, such as functional impairment or dyspnoea in patients with heart failure, and angina or exercise capacity in patients with stable CHD.26,44,51,52 After a MI, depressive symptoms are associated with more physical limitations and worse QoL.26,53 In patients with systolic dysfunction, depression is a major determinant of QoL, whereas cardiac indicators of severity of the disease (i.e. NT-proBNP and left ventricular ejection fraction) are not related to QoL.51 A change in depressive symptoms is the strongest predictor of 1-year health-related QoL in this population, even after accounting for functional status and clinical variables.54
Depression and chest pain
Epidemiological evidence suggests a close relationship between depression and angina, with these two clinical entities frequently co-existing. Presence of depression is associated with increased reporting of shortness of breath and/or chest pain symptoms in patients with established CHD.55 Not only is depression associated with everyday life angina independently of CHD severity, but it is a stronger predictor of angina than severity of coronary artery disease or other traditional risk indicators.56 Depression post-MI predicts new angina during follow-up,26 and improvement in depression leads to improvement in angina symptoms. A cause and effect relationship between depression and angina, however, is difficult prove. Over-reporting of chest pain in depressed patients could be related to alterations in pain perception. Furthermore, patients with chronic pain, including angina, may develop depressive symptoms as a consequence of their symptom burden or disability.57 Evidence also suggests that chest pain and depression share common neurohormonal pathways57 and a common genetic background,58 which could explain their co-existence. The links between depression and chest pain are summarized in Figure 1.
Figure 1.
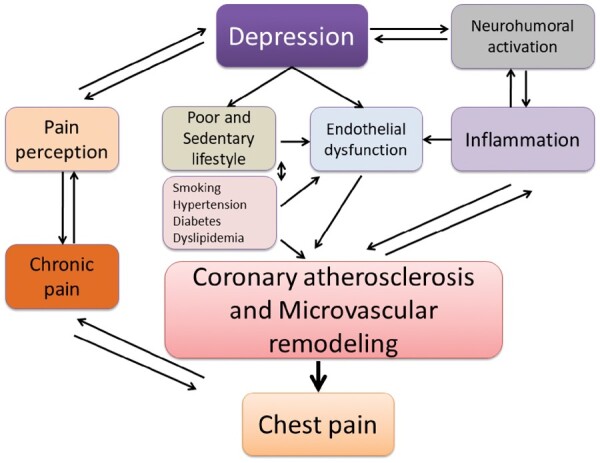
Common links between depression and chest pain in coronary heart disease.
Depression, atrial fibrillation, and ventricular arrhythmias
Atrial fibrillation (AF) can profoundly affect patients’ QoL and long-term outcome, and depression, which has been associated with AF, may worsen the symptoms and clinical course of this condition.59–63 Depression is associated with increased inflammatory and adrenergic activity and reduced heart rate variability (HRV), which is the normal beat-to-beat variability of heart rate. These factors can shorten atrial refractory periods, trigger AF, and foster a substrate that perpetuates AF, suggesting a mechanism for the observed association of depression with AF.59,63,64
Depression has been associated with an almost three-fold increase in the odds of the reoccurrence of AF after successful electric cardioversion,65 and negative emotions have been shown to trigger AF episodes in persons with paroxysmal AF.66 Furthermore, negative life events like the death of a partner have been associated with transiently increased risk of AF.67 The opposite pathway may also be true, however, as AF can have substantial impact on the risk or worsening of depression.62,68 Thus, AF can cause depression and anxiety in patients, and depression and anxiety, in turn, may create an environment that is conducive for the initiation and perpetuation of AF.68
Individuals with depression, as well as those exposed to various forms of chronic and acute psychological distress, have also an increased risk of developing ventricular arrhythmias and sudden cardiac arrest, a finding reported both in initially CHD-free populations and in patients with CHD.69–72 Yet, whether treating depression would affect cardiac arrhythmias still remains an open question.
Mechanisms linking depression to coronary heart disease
Neurobiological aspects of relevance to coronary heart disease
The well-documented association between depression and CHD has prompted a search for underlying mechanisms. One possibility is that changes in neurobiology in depressed patients alter cardiovascular function and structure.73–75 Additionally, because of the known link between stressful exposures and depression,76 dysregulation of stress-response pathways may contribute to CHD in vulnerable individuals. Thus, neurobiological mechanisms associated with stress and depression may be relevant for CHD risk. These mechanisms include changes in sympathetic nervous system and neurohormonal function as well as alterations in central brain function.77,78
Neuroendocrine pathways
Acute and chronic stress exposure can lead to altered neurochemical function, such as disruptions in the synthesis or activity of norepinephrine, dopamine, or serotonin,79 which, in turn, may influence mood and cardiovascular risk.80,81 Endocrine changes associated with depression include alterations in corticotropin-releasing factor (CRF),82 dysregulated adrenocorticotropic hormone (ACTH) responses to CRF,83 enhanced adrenal responses to ACTH,83 and elevated circulating cortisol levels.84 Several of these changes may affect the immune system leading to excessive secretion of cytokines such as interleukin (IL)-1, IL-6, and tumour necrosis factor (TNF)-α. Enhanced inflammation is common in mood disorders and cardiovascular disease and thus might play a role in the association of these conditions.
Brain systems and cardiovascular physiology
Brain areas that likely play a role in cardiovascular regulation based on imaging studies include those involved in stress and memory that have also been shown to be altered in patients with major depression. These include the amygdala, hippocampus, medial prefrontal cortex, and anterior cingulate (part of the prefrontal cortex).85–87 Structural and functional magnetic resonance imaging (MRI) studies have shown changes in hippocampal structure and function in depression.88–108 Moreover, MRI data have demonstrated that acute psychological stressors may reduce baroreflex sensitivity by increasing the functional connectivity of a discrete area of the anterior insula with both the cingulate cortex and the amygdala.109 Asymmetric sympathetic inputs from these brain areas to the heart may increase the risk of ventricular arrhythmias.110 Studies have shown that asymmetric brain responses to stress result in pro-arrhythmic sympathetic inputs to the heart.111 In a recent study,112 amygdalar activity measured by 18F-fluorodexoyglucose positron emission tomography independently predicted cardiovascular disease events, providing further evidence of brain mechanisms through which emotional stress can lead to cardiovascular disease.
Subjects who exhibit a larger cardiovascular reactivity and acute mental stress are at risk for hypertension and other cardiovascular risk indicators.113 There is an association between increased blood pressure and heart rate during mental stress and activation in the right insula, cerebellum, and anterior cingulate.114 Furthermore, myocardial ischaemia provoked by acute psychological stress in CHD patients has been associated with increased activation of the anterior cingualate.115 Studies have also implicated the insula and the somatosensory cortex in peripheral autonomic function.116 These studies, as a whole, suggest that brain regulatory systems are implicated in CHD pathophysiology, and imply that disruption of these systems may contribute to the observed associations of stress and depression with CHD risk.
Depression and mental stress
Brain areas involved in stress may modulate peripheral vascular and autonomic function,115,117 which may mediate the effects of stress acting through the brain to cause myocardial ischaemia in patients with CHD. Mental stress, which can be studied in the laboratory, can induce myocardial ischaemia in susceptible patients with CHD,73,118,119 and this phenomenon has been linked to depression.120,121 Such observations suggest that some individuals with CHD, especially those with depression, may experience stress-induced myocardial ischaemia on a daily basis, even in the absence of symptoms,74,122–124 possibly through a mechanism of increased coronary or peripheral vasoconstriction due to sympathetic nervous system stimulation during emotional stress.125,126 This phenomenon may be especially pronounced among women.5,127,128
Depression and autonomic dysfunction
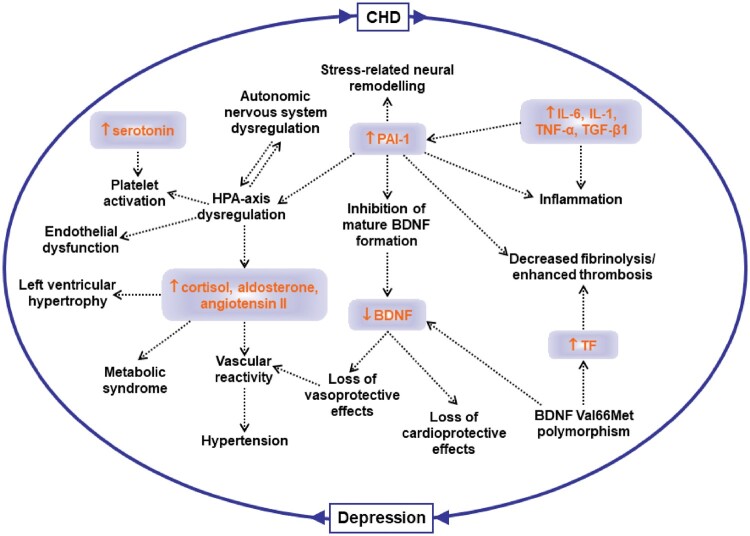
In part as a consequence of neurobiological alterations described above, chronic dysregulation of autonomic function (Figure 2), characterized by an imbalance between the sympathetic and parasympathetic systems, is thought to be a key mechanism linking depression to CHD risk and adverse cardiovascular outcomes.7 Sympathetic hyperactivity and parasympathetic withdrawal may lower the threshold for myocardial ischaemia and ventricular arrhythmias, and potentially pre-dispose to sudden cardiac death. Data from animal models suggest that depression is associated with cardiovascular and autonomic imbalance, characterized by elevated heart rate, reduced HRV,129 and elevated cardiac sympathetic tone.130,131
Figure 2.
Links between depression, autonomic dysregulation, inflammation, endothelial dysfunction, and thrombosis. BDNF, brain-derived neurotrophic factor; CHD, coronary heart disease; HPA, hypothalamic–pituitary–adrenal axis; IL, interleukin; PAI, plasminogen activator inhibitor; TGF, transforming growth factor; TNF, tumour necrosis factor.
Most studies of patients with CHD have found lower HRV and higher heart rate in patients with depression compared with those without depression, together with other indicators of cardiac autonomic dysregulation including decreased baroreceptor sensitivity, increased QT interval variability (reflecting abnormal ventricular repolarization) and increased heart rate turbulence.3,7 Heart rate variability is probably the most widely used method to assess cardiac autonomic function in humans. Lower HRV, reflecting cardiac autonomic imbalance, predicts mortality after MI,132,133 and morbidity and mortality in the general population and among patients with stable CHD.134,135 However, the association between depression and reduced HRV (or other measures of autonomic dysregulation) is not entirely consistent across all studies.136–138 Part of the effect may be driven by antidepressant medications.136,137,139,140 Furthermore, the link between depression and HRV is likely bidirectional,140 and depression and HRV may also share a genetic substrate, suggesting shared neurobiological alterations pre-disposing to both depression and autonomic dysfunction.141
Depression and inflammation
Another hypothesized mechanism for the increased risk of CHD associated with depression is chronic inflammation (Figure 2), which is a known risk factor for development of atherosclerosis and CHD.142 Depression has been associated with a sustained state of inflammation and increased concentrations of inflammatory molecules, including C-reactive protein and various cytokines, such as TNF-α, IL-1β, and IL-6143,144 with known adverse effects on the heart and circulation.145,146 Depression has been also associated with elevated markers of oxidative stress,147,148 which is involved in the initiation, progression, and complications of atherosclerosis. Nevertheless, the direction of the association between depression and inflammation and/or oxidative stress remains unclear. Some studies suggested that depression drives the inflammatory state rather than the reverse,149,150 while others supported the opposite pathway of inflammation predicting depression.151–153 In fact, inflammation has been considered a potential aetiological factor and treatment target for clinically depressed patients.144 It also remains unclear to what degree inflammation is the result of depression-related co-morbidities and risk factors, such as smoking, obesity, diabetes, and physical inactivity.142,143,150,154–157 Finally, as for autonomic function, depression and inflammation could share a pathophysiological pathway, such as common genetic precursors or shared behavioural or environmental risk factors.158,159
Depression and endothelial dysfunction
Various studies support an inverse correlation between depressed mood and endothelial function, as measured by flow-mediated dilation (FMD).160–162 The relationship between depression and endothelial dysfunction (Figure 2) is likely due to reduced endothelium-derived nitric oxide (NO),163 as shown by lower FMD of brachial arteries and reduced NO bioavailability in subcutaneous micro-vessels in patients with depressed mood.161,164,165 Animal models of human depression are useful to gain deeper understanding of underlying mechanisms. One such model is the so-called ‘unpredictable chronic mild stress (UCMS)’ rat model.166 Due to activation of neuroendocrine and immune systems the level of TNF-α is elevated in the UCMS rats. The endothelium dependent vasomotor response to carbachol is substantially reduced, whereas the direct NO donor, sodium nitroprusside, or the non-NO dependent agonist, papaverine, elicit similar dilation in vessels of UCMS and control rats.166 The reduced NO-dependent response is likely due to lower expression of endothelial NO synthase (eNOS). Higher levels of cortisol and increased inflammation in depression can down-regulate eNOS expression and NO production.167,168 In addition to the NO system, other endothelial mechanisms may play a role, for example, the arachidonic acid pathway with production of constrictor prostanoids.169 There is also evidence from experimental models that reduction in endothelium-dependent hyperpolarization is an important mechanism underlying the reduced endothelial function in the microcirculation. Endothelin is a powerful vasoconstrictor, and its levels are higher in patients with depression.170,171 The metabolic syndrome is associated with depression172 and can contribute to the development of vascular endothelial dysfunction.156 It is also likely that the effects of depression on the endothelium are due to upstream alterations of autonomic system circuits related to stress. Psychological stress evokes autonomic, hemodynamic, and metabolic changes that may contribute to endothelial dysfunction. Indeed, acute mental stress in the laboratory induces transient endothelial dysfunction, as measured by FMD, which lasts up to 4 h.173 This effect may be mediated through sympathetic activation,174 and may have implications for patients with increased sympathetic outflow including those with depression.
Platelet activation and thrombosis
Increased platelet activation and thrombosis represent another pathological mechanism for the association between depression and CHD. Several studies have shown increased platelet activity in major depression.175–179 Of note, plasminogen activator inhibitor (PAI)-1, an anti-fibrinolytic factor, may also play a pivotal role,155 as increased PAI-1 levels have been reported in major depression.180,181 PAI-1 may also affect hypothalamic–pituitary–adrenal (HPA) axis function and cardiovascular risk factors such as metabolic syndrome and hypertension (Figure 2). Furthermore, PAI-1 inhibits the formation of mature brain-derived neurotrophic factor (BDNF), and decreased BDNF levels have been described as a potential link between thrombosis and depression.182 These data extend a growing body of evidence linking increased PAI-1 concentration with major depression. Furthermore, they provide support for the vascular hypothesis of depression which has been implicated in the two-way association between CHD and depression.183,184 This hypothesis postulates that deficits in perfusion caused by small-vessel disease (which could be a result of hypercoagulability) induce structural and functional changes in the white matter, which, in turn, may affect brain function and mood.185
Health behaviours and cardiometabolic risk factors
Although positive behaviour changes for CHD primary and secondary prevention is recommended,186 a sizeable proportion of patients do not make any changes.187 One factor that may shape individuals’ responses to a health behaviour change is their emotional state, such as the presence of depression. Prior studies have extensively documented the association of depression with adverse health behaviours, including smoking,188 excessive drinking,189 physical inactivity, and overeating.190–192 For example, depression is associated with an increased risk of becoming a smoker, with an increased rate of daily smoking, and with a lower probability of quitting smoking.193–196 Depression is also associated with overweight and obesity, and with approximately 40% higher risk of developing Type 2 diabetes.197–204 Some of these associations appear bidirectional.205,206 Obesity and other cardiometabolic risk factors have been linked to increased oxidative stress, inflammation, and microvascular dysfunction,156,207,208 which lend further support for a central role of inflammation and microvascular disease as possible links between cardiometabolic disturbances, depression and CHD (Figure 2).209–212
Depression, CHD, and genetic vulnerability
Genetic studies can be instructive in clarifying the association between depression and CHD. The heritability of depression, or the proportion of the variance due to genetic factors, is estimated to be 37%,213 and individuals with a first-degree relative with depression have an almost three-fold higher risk for depression themselves, compared to others from the general population.213 This has led to genome-wide association studies aimed at the identification of common genetic variants that contribute to the risk of depression. Despite comprehensive efforts, no consistent genetic variation has yet been identified.214 These surprising results may be due to the heterogeneity of the depression phenotype, and the co-existence of depression and CHD may contribute to this heterogeneity.215
It is also possible that there is a core biological pathway that leads to both depression and CHD, as suggested by twin studies showing a common genetic vulnerability between these two phenotypes.212,216 Additional work has suggested shared, genetically influenced biological pathways underlying the association between depression and CHD that involve autonomic function,217 inflammation,218,219 and the serotoninergic system.158 Patients with depression also show distinct patterns of DNA methylation that are also associated with an increase in inflammatory markers, suggesting epigenetics as another pathway by which core biological changes may lead to both disorders.220
All these observations point to common genetic pathways involving neuroendocrine, immune and inflammatory systems that, when disrupted, may simultaneously increase the risk for both depression and CHD. Thus, genetically predisposed individuals could be at risk for both depression and CHD.
Telomere length and depression
Telomeres, the caps at the end of DNA strands, shorten with each cell division and have been proposed to reflect biological age. Studies have shown a relationship between shortened telomere length and risk for CHD.221–223 Results from studies examining the association between telomere length and depression have been conflicting. Some studies have reported an association,224,225 whereas other studies have shown no association.226,227 A study227 on a sample of more than 67 000 individuals from the Danish general population found that those who attended the hospital for depression treatment had shorter telomere length compared with those who did not attend hospital for depression or use antidepressant medication. However, a large part of this association was explained by confounders such as age, gender, lifestyle factors, and chronic disease. Furthermore, shorter telomere length was not prospectively associated with increased risk of depression and a Mendelian randomization approach showed no causal relationship with depression. This suggests that shortening of telomere length per se does not increase risk of depression.
Evaluation of depression in coronary heart disease patients
Recognition and screening
Recognition of depression is an important part of the management of patients with CHD. Depressive symptoms are highly prevalent in this population and can affect patients’ well-being and QoL. They can also influence treatment adherence, including fidelity to taking medications as prescribed, cooperating with follow-up care, and making risk factor and lifestyle changes needed to enhance recovery. In spite of this, depression is often unrecognized and untreated in CHD patients. Barriers to recognition of depression include lack of mental health expertise and training in cardiology practices, and the perception that this is not part of the treatment mission. Additionally, many symptoms of psychological distress are easily confused with physical disease, for example, fatigue, weight loss, poor appetite, or trouble sleeping.
There is no consensus on whether screening for and treatment of emotional problems, such as depression, should become a routine part of the cardiology practice. This is related to the fact that there is little evidence one way or another whether screening for and treating these problems will translate into better QoL or improved prognosis.4 The few studies of interventions for psychiatric disorders in patients with CHD that have been performed have shown only modest improvements in psychological status and no clear evidence of an improvement in cardiac outcomes.3,228 Nevertheless, psychological interventions such stress management, individual, or group counselling, and support for self-care and pharmacotherapy, are recommended for patients with CHD and comorbid depression. This is because these interventions can help promote modifications in standard risk factors, encourage lifestyle changes, and mitigate distress when added to standard cardiac rehabilitation or as part of a coordinated care management approach.48
Current clinical guidelines in the USA only mention depression as a psychosocial factor that is reasonable for the non-mental health clinician to recognize if patients have access to adequate care support systems (class of recommendation IIa, level of evidence B). These guidelines further state that treatment of depression may be reasonable for its clinical benefits other than improving CHD outcomes (class IIb, level of evidence C).229 In contrast, the European guidelines, while noting limitations for depression screening, recognize the importance of a comprehensive approach for the detection of psychosocial risk factors, using at least a preliminary assessment with a short series of yes/no questions and recommend a multimodal behavioural intervention approach integrating health education, physical activity, and psychological therapy (class Ia, level of evidence A).186 In the case of clinically significant symptoms of depression or other psychosocial factors, the European guidelines recommend consideration of interventions such as psychotherapy, medication, or collaborative care (class IIa, level of evidence A). These treatments are reviewed in more detail in the following sections of this paper.
Assessment of depression
Several reliable and valid instruments have been developed for the assessment of depression. The standard for research in the field at least in the USA is the Structured Clinical Interview for the DSM-5 (SCID) interview.230 This interview requires training and must be administered by someone with clinical experience or with close supervision by a mental health professional. It permits diagnosis based on DSM-5 criteria of major depression and related disorders, including dysthymia and bipolar disorder. Assessment of severity of depressive symptoms can be performed with the Hamilton Depression Scale, a reliable and valid measure of depressive symptoms based on a clinician interview.231 A score of greater than 9 is indicative of moderate to severe depression.232 Both of these instruments, however, rely on a mental health clinician to administer, which is not usually practical in busy cardiology clinics. An alternative that can be self-administered by patients with CHD is the Beck Depression Inventory. This is a reliable and valid assessment that can be used to screen for the presence of depression, although it does not provide a diagnosis.233 If suicidal ideation is a concern, the Sheehan Suicidality Tracking Index is another self-report instrument that can be employed. A score greater than 0 indicates the need for further timely follow-up by a mental health clinician.234
Management of depression in patients with coronary heart disease
A number of interventions can be useful for CHD patients with depression. Psychotherapy helps people with depression understand the behaviours, emotions, and ideas that contribute to depression, regain a sense of control and pleasure in life, and learn coping skills.235 Psychodynamic therapy is based on the assumption that a person is depressed because of unresolved, generally unconscious conflicts, often stemming from childhood. Interpersonal therapy focuses on patient’s behaviours and interactions with family and friends. The primary goal of this therapy is to improve communication skills and increase self-esteem during a short period of time. Cognitive behavioural therapy (CBT) involves examining thought patterns that can be negative and self-defeating, and going over the basis of such thoughts and how they contribute to negative emotions. Other therapies useful for depression include stress management and stress reduction techniques such as deep breathing, progressive muscle relaxation, yoga, meditation, and mindfulness-based stress reduction. These interventions can be provided in group format or individually by trained personnel. Psychotherapy has been shown to be equally effective for depression as medications, and some people, especially with early life stress issues, may not respond to medication without psychotherapy.
The Enhanced Recovery in Coronary Heart Disease Patients (ENRICHD) trial could not demonstrate a benefit for CBT, with medication intervention for severe depression, for the improvement of cardiac outcomes in depressed or socially isolated patients with CHD.236 The effects of the intervention on depression, however, were modest, and patients who responded to treatment did have a better outcome than those who did not respond.13 Unanswered questions, therefore, remain on whether treatment of depression may improve CHD outcomes.
Antidepressant medications
Antidepressant medications (Table 1) are a useful tool for the treatment of depression in patients with CHD, especially those with moderate-to-severe depression.237,238 Antidepressants act on the serotonin, dopamine, and norepinephrine systems and other neurotransmitter circuits in the brain.
Table 1.
Pharmacological management of depression in patients with coronary heart disease
| Drug classification/ generic name | Indication | Cardiovascular adverse effects | Other adverse effects |
|---|---|---|---|
| Selective serotonin reuptake inhibitors | |||
| Fluoxetine Sertraline Paroxetine Fluvoxamine Citalopram Escitalopram |
|
|
Nausea, diarrhoea, headache, insomnia, agitation, loss of libido, delayed ejaculation, and erectile dysfunction |
| Tricyclic antidepressants | |||
| Imipramine Doxepine Amoxapine Nortriptyline Amitriptyline |
|
|
Anticholinergic effects: dry mouth, constipation, memory problems, confusion, blurred vision, sexual dysfunction, and decreased urination, and memory impairment especially in the elderly |
| Serotonin-norepinephrine reuptake inhibitors | |||
| Desvenlafaxine Duloxetine Levomilnacipran Milnacipran Venlafaxine |
|
|
Dizziness, constipation, dry mouth, headache, changes in sleep, or more rarely a serotonin syndrome, with restlessness, shivering, and sweating |
| Antidepressants with novel mechanisms of action | |||
| Buproprion |
|
|
Weight loss, restlessness, high doses can rarely cause seizures |
| Mirtazapine |
|
|
Sweating and shivering, tiredness, strange dreams, dyslipidemia, weight gain, anxiety, and agitation |
| Trazodone |
|
|
Rarely, it can cause priapism |
CHD, coronary heart disease; MI, myocardial infarction; QTc: corrected QT interval.
Tricyclic antidepressants
Tricyclics represent the first class of medications found to work for the treatment of depression. Tricyclics increase norepinephrine and serotonin levels in the synapse. These medications have been associated with a lengthening of the PR interval, QRS duration, and QT interval, and a flattening of the T wave on the electrocardiogram (Table 1). Likely because of these effects, tricyclics have been linked to malignant ventricular arrhythmias and sudden cardiac death. For patients who suffer a cardiac event while being treated with a tricyclic or who develop a lengthening of the QT interval, abrupt withdrawal from the tricyclic medication can be associated with an increased risk of arrhythmias. Therefore, these medications should be tapered slowly over a period of time. For all of these reasons’ tricyclics should be avoided in patients with CHD, especially those with pre-existing cardiac conduction defects, congestive heart failure, or recent MI, and elderly patients.
Selective serotonin reuptake inhibitors
The selective serotonin reuptake inhibitors (SSRIs) block the transporter that brings the serotonin back from the synapse into the neuron (Table 1). Because of their different mechanism of action, they have fewer to no anticholinergic and cardiac effects. Therefore, they are first line of treatment for CHD patients.
The SSRIs have only modest efficacy over placebo,239,240 and about 80% of the improvement is due to placebo response. They show their greatest effect on patients with severe depression.238 Antidepressants without sexual dysfunction side effects can be given instead of an SSRI in case this is an issue, for example, bupropion. The SSRIs stopped suddenly can result in a potent withdrawal syndrome, including agitation, nervousness, and sometimes suicidal thoughts. Patients on aspirin or other antiplatelet/anticoagulation treatment can have an increase in bleeding risk with SSRIs.
Studies of SSRIs have found them to be safe and effective for patients with CHD, although their effects on improving cardiac outcomes are unclear. Some data suggest that patients whose depression improves with SSRIs, typically those with severe depression, may have better cardiac outcomes.13 However, some data suggest that SSRIs, like tricyclics, when used long-term may increase the risk of cardiac events and death.21,241 These events are rare, however, and a proper risk-benefit evaluation should be performed case by case.
Serotonin and norepinephrine dual reuptake inhibitors
The latest group of antidepressants has dual reuptake inhibition for serotonin and norepinephrine (Table 1). These drugs are moderately more effective than the SSRIs for the treatment of depression, although they can have more side effects. Venlafaxine has been associated with a dose-dependent increase in blood pressure, which is of particular concern for CHD patients, especially those with pre-existing hypertension. In addition, venlafaxine seems to carry the greatest risk of suicidality amongst all of the antidepressants, with a three-fold increased risk of attempted or completed suicides.
Antidepressants with novel mechanisms of action
Some drugs act on various neurotransmitter systems or have poorly understood mechanisms of action (Table 1). Buproprion primarily acts on dopamine systems and is used for both depression and smoking cessation. Mirtazapine is a quatrocyclic antidepressant that has actions on a number of different receptor systems. It blocks pre-synaptic noradrenergic alpha-2 receptors with associated enhancement of norepinephrine release. Mirtazapine also increases serotonin release. It can be associated with mild orthostatic hypotension and anticholinergic side effects. Trazodone is a safe and effective antidepressant that can also be an effective non-addicting sleep aid.
Electroconvulsive therapy
Electroconvulsive therapy (ECT) is used as a last resort for the treatment of depression in patients who have had multiple failed trials of psychotherapy and medication. Electroconvulsive therapy has an 80% overall response rate, and contrary to popular belief, is a safe procedure. Although ECT causes profound hemodynamic changes, including bradycardia (up to frank asystole which may last for a few seconds), tachycardia and hypertension, these effects are transient and typically resolve within 20 min. Possible complications include persistent hypertension, arrhythmias, asystole lasting more than 5 s, ischaemia, and heart failure. Older age and pre-existing cardiovascular diseases, including hypertension, CHD, congestive heart failure, aortic stenosis, implanted cardiac devices, and AF, have been associated with increased complication rates. However, most complications remain minor and transient, and the vast majority of patients can safely complete treatment. The procedure should be delayed in patients who are haemodynamically unstable or have new-onset or uncontrolled hypertension. In patients with stable CHD and controlled hypertension, medications can be continued through the morning of the procedure. Electroconvulsive therapy appears safe in patients with an implantable cardioverter defibrillator with detection mode turned off during ECT and continuous electrocardiographic monitoring and life resuscitation equipment on hand. Pacemakers should be tested before and after ECT and the magnet should be placed at the patient bedside.
Exercise
Exercise has been consistently found to be efficacious for the treatment of depression, at least equivalent to the effects of SSRIs or psychotherapy.242,243 Aerobic exercise seems to work best; therefore, aerobic exercise at a dose consistent with public health recommendations for CHD prevention is an effective treatment for mild-to-moderate depression. Exercise may also complement the effects of antidepressant medications in depressed patients who do not have a complete response to medications. Finally, in patients with CHD, cardiac rehabilitation is highly effective in improving mental health, including depression, as well as physical health outcomes including subsequent CHD events and mortality.244 Cardiac rehab enhanced by stress management training has been shown to be effective in reducing stress and improving medical outcomes compared with standard cardiac rehabilitation.245
Summary of management considerations
There are several treatments options for the CHD patient with depression, from medications to various forms of psychotherapy, to exercise and stress management approaches. Although treatment of depression has not been shown to improve cardiovascular outcomes in CHD patients, depression should still be addressed if severe enough, in order to promote patient wellness and QoL. Tricyclics should be avoided in this patient population.
Concluding remarks
Converging evidence from both experimental and epidemiological studies indicates that there is a bidirectional association between depression and CHD. Depression is very common in patients with CHD and is an independent risk factor for poorer CHD outcomes. The underlying mechanisms linking depression and worse CHD outcomes are complex and potentially multifactorial. Further research is necessary to elucidate them. Nonetheless, there is growing consensus for considering depression as a modifiable prognostic factor for CHD, and for the need of improved efforts towards better recognition and management of this problem in the clinical practice of cardiology.3,4 Whether effective and safe treatment of depression may improve CHD outcomes, and whether specific patient subgroups may benefit more from such treatments, require further evaluation.
Recommendations
Clinicians should be aware of the high prevalence of depression in CHD patients. Screening for depression is recommended if patients have access to adequate care support systems
Patients with positive screening results should be referred to a qualified health care provider in the management of depression
Non-pharmacologic interventions such as exercise and psychotherapy should be considered as additional treatment options for CHD patients
Harmonization of care between healthcare providers is essential in patients with combined CHD and depression
Author notes
ESC Committee for Practice Guidelines: Stephan Windecker (Chairperson), Switzerland; Victor Aboyans, France; Stefan Agewall, Norway; Emanuele Barbato, Belgium; Hector Bueno, Spain; Antonio Coca, Spain; Jean-Philippe Collet, France; Ioan Mircea Coman, Romania; Victoria Delgado, Netherlands; Donna Fitzsimons, UK; Oliver Gaemperli, Switzerland; Gerhard Hindricks, Germany; Bernard Iung, France; Peter Juni, Canada; Hugo Katus, Germany; Jujani Knuuti, Finland; Patrizio Lancellotti, Belgium; Christophe Leclerq, France; Theresa McDonagh, UK; Massimo Francesco Piepoli, FESC, Italy; Piotr Ponikowski, Poland; Dimitrios Richter, Greece; Marco Roffi, Switzerland; Evgeny Shlyakhto, Russia; Iain Simpson, UK; Michel Sousa Uva, Portugal; Jose Luis Zamorano (past CPG Chairperson), Spain; Veronica Dean (Head of Practice Guidelines Department) ESC, France.
Conflict of interest: none declared.
Contributor Information
Viola Vaccarino, Department of Epidemiology, Rollins School of Public Health, Emory University, 1518 Clifton Road Northeast, Atlanta, GA, 30322, USA; Department of Medicine (Cardiology), Emory University School of Medicine, 1518 Clifton Road Northeast, Atlanta, GA, 30322, USA.
Lina Badimon, Cardiovascular Program (ICCC), IR-Hospital de la Santa Creu i Sant Pau. CiberCV-Institute Carlos III. Autonomous University of Barcelona, C/ Sant Antoni Maria Claret, 167, 08025, Barcelona, Spain.
J Douglas Bremner, Department of Psychiatry and Behavioral Sciences, Emory University School of Medicine, 12 Executive Park Drive Northeast, Atlanta, GA, 30329, USA; Department of Radiology, Emory University School of Medicine, 1364 Clifton Road Northeast, Atlanta, GA, 30322, USA; Atlanta Veterans Administration Medical Center, 670 Clairmont Road, Decatur, GA, 30033, USA.
Edina Cenko, Department of Experimental, Diagnostic and Specialty Medicine, University of Bologna, Via Giuseppe Massarenti 9, 40138, Bologna, Italy.
Judit Cubedo, Cardiovascular Program (ICCC), IR-Hospital de la Santa Creu i Sant Pau. CiberCV-Institute Carlos III. Autonomous University of Barcelona, C/ Sant Antoni Maria Claret, 167, 08025, Barcelona, Spain.
Maria Dorobantu, Cardiology Department, University of Medicine and Pharmacy ‘Carol Davila’ of Bucharest, Emergency Clinical Hospital of Bucharest, Calea Floreasca 8, Sector 1, Bucuresti, 014461, Romania.
Dirk J Duncker, Division of Experimental Cardiology, Department of Cardiology, Thoraxcenter, Cardiovascular Research Institute COEUR, Erasmus MC, University Medical Center, Rotterdam, Dr. Molewaterplein 40, 3015 GD, Rotterdam, The Netherlands.
Akos Koller, Institute of Natural Sciences, University of Physical Education, Alkotas street, 44, 1123, Budapest, Hungary; Department of Physiology, New York Medical College, Valhalla, NY, 10595, USA.
Olivia Manfrini, Department of Experimental, Diagnostic and Specialty Medicine, University of Bologna, Via Giuseppe Massarenti 9, 40138, Bologna, Italy.
Davor Milicic, Department for Cardiovascular Diseases, University Hospital Center Zagreb, University of Zagreb, Kispaticeva 12, HR-10000, Zagreb, Croatia.
Teresa Padro, Cardiovascular Program (ICCC), IR-Hospital de la Santa Creu i Sant Pau. CiberCV-Institute Carlos III. Autonomous University of Barcelona, C/ Sant Antoni Maria Claret, 167, 08025, Barcelona, Spain.
Axel R Pries, Department of Physiology, Charitè-University Medicine, Thielallee 71, D-14195, Berlin, Germany.
Arshed A Quyyumi, Department of Medicine (Cardiology), Emory University School of Medicine, 1518 Clifton Road Northeast, Atlanta, GA, 30322, USA.
Dimitris Tousoulis, First Department of Cardiology, Hippokration Hospital, University of Athens Medical School, Vasilissis Sofias 114, TK 115 28, Athens, Greece.
Danijela Trifunovic, Department of Cardiology, University Clinical Center of Serbia, Pasterova 2, 11000, Belgrade, Serbia; School of Medicine, University of Belgrade, Dr Subotica 8, 11000, Belgrade, Serbia.
Zorana Vasiljevic, School of Medicine, University of Belgrade, Dr Subotica 8, 11000, Belgrade, Serbia.
Cor de Wit, Institut für Physiologie, Universität zu Lübeck and Deutsches Zentrumfür Herz-Kreislauf-Forschung (DZHK), Ratzeburger Allee 160, 23538, Lübeck, Germany.
Raffaele Bugiardini, Department of Experimental, Diagnostic and Specialty Medicine, University of Bologna, Via Giuseppe Massarenti 9, 40138, Bologna, Italy.
ESC Scientific Document Group Reviewers:
References
- 1. Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication (NCS-R). Arch Gen Psychiatry 2005;62:617–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Whiteford HA, Degenhardt L, Rehm J, Baxter AJ, Ferrari AJ, Erskine HE, Charlson FJ, Norman RE, Flaxman AD, Johns N, Burstein R, Murray CJ, Vos T. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet 2013;382:1575–1586. [DOI] [PubMed] [Google Scholar]
- 3. Carney RM, Freedland KE. Depression and coronary heart disease. Nat Rev Cardiol 2017;14:145–155. [DOI] [PubMed] [Google Scholar]
- 4. Lichtman JH, Froelicher ES, Blumenthal JA, Carney RM, Doering LV, Frasure-Smith N, Freedland KE, Jaffe AS, Leifheit-Limson EC, Sheps DS, Vaccarino V, Wulsin L.; American Heart Association Statistics Committee of the Council on Epidemiology and Prevention and the Council on Cardiovascular and Stroke Nursing. Depression as a risk factor for poor prognosis among patients with acute coronary syndrome: systematic review and recommendations: a scientific statement from the American Heart Association. Circulation 2014;129:1350–1369. [DOI] [PubMed] [Google Scholar]
- 5. Vaccarino V, Bremner JD. Behavioral, emotional and neurobiological determinants of coronary heart disease risk in women. Neurosci Biobehav Rev 2017;74:297–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed.Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- 7. Penninx BW. Depression and cardiovascular disease: epidemiological evidence on their linking mechanisms. Neurosci Biobehav Rev 2017;74:277–286. [DOI] [PubMed] [Google Scholar]
- 8. Meijer A, Conradi HJ, Bos EH, Thombs BD, van Melle JP, de Jonge P. Prognostic association of depression following myocardial infarction with mortality and cardiovascular events: a meta-analysis of 25 years of research. Gen Hosp Psychiatry 2011;33:203–216. [DOI] [PubMed] [Google Scholar]
- 9. Gan Y, Gong Y, Tong X, Sun H, Cong Y, Dong X, Wang Y, Xu X, Yin X, Deng J, Li L, Cao S, Lu Z. Depression and the risk of coronary heart disease: a meta-analysis of prospective cohort studies. BMC Psychiatry 2014;14:371.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nicholson A, Kuper H, Hemingway H. Depression as an aetiologic and prognostic factor in coronary heart disease: a meta-analysis of 6362 events among 146 538 participants in 54 observational studies. Eur Heart J 2006;27:2763–2774. [DOI] [PubMed] [Google Scholar]
- 11. Cassano P, Fava M. Depression and public health: an overview. J Psychosom Res 2002;53:849–857. [DOI] [PubMed] [Google Scholar]
- 12. Ziegelstein RC. Depression in patients recovering from a myocardial infarction. JAMA 2001;286:1621–1627. [DOI] [PubMed] [Google Scholar]
- 13. Freedland KE, Carney RM. Depression as a risk factor for adverse outcomes in coronary heart disease. BMC Med 2013;11:131.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vaccarino V. Psychosocial risk factors in women: Special reference to depression and posttraumatic stress disorder. In: Orth-Gomer K, Vaccarino V, Schneiderman N, Deter HC, eds. Psychosocial Stress and Cardiovascular Disease in Women: Concepts, Findings and Future Perspectives. Switzerland: Springer International Publishing; 2015, pp. 63–86. [Google Scholar]
- 15. Mallik S, Spertus JA, Reid KJ, Krumholz HM, Rumsfeld JS, Weintraub WS, Agarwal P, Santra M, Bidyasar S, Lichtman JH, Wenger NK, Vaccarino V; PREMIER Registry Investigators. Depressive symptoms after acute myocardial infarction: evidence for highest rates in younger women. Arch Intern Med 2006;166:876–883. [DOI] [PubMed] [Google Scholar]
- 16. Vaccarino V, Shah AJ, Rooks C, Ibeanu I, Nye JA, Pimple P, Salerno A, D’Marco L, Karohl C, Bremner JD, Raggi P. Sex differences in mental stress-induced myocardial ischemia in young survivors of an acute myocardial infarction. Psychosom Med 2014;76:171–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Smolderen KG, Strait KM, Dreyer RP, D'Onofrio G, Zhou S, Lichtman JH, Geda M, Bueno H, Beltrame J, Safdar B, Krumholz HM, Spertus JA. Depressive symptoms in younger women and men with acute myocardial infarction: insights from the VIRGO study. J Am Heart Assoc 2015;4:e001424.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vaccarino V, Sullivan S, Hammadah M, Wilmot K, Al Mheid I, Ramadan R, Elon L, Pimple PM, Garcia EV, Nye J, Shah AJ, Alkhoder A, Levantsevych O, Gay H, Obideen M, Huang M, Lewis TT, Bremner JD, Quyyumi AA, Raggi P. Mental stress-induced-myocardial ischemia in young patients with recent myocardial infarction: sex differences and mechanisms. Circulation 2018;137:794–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cenko E, Yoon J, Kedev S, Stankovic G, Vasiljevic Z, Krljanac G, Kalpak O, Ricci B, Milicic D, Manfrini O, van der Schaar M, Badimon L, Bugiardini R. Sex differences in outcomes after STEMI: effect modification by treatment strategy and age. JAMA Intern Med 2018;178:632–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wassertheil-Smoller S, Shumaker S, Ockene J, Talavera GA, Greenland P, Cochrane B, Robbins J, Aragaki A, Dunbar-Jacob J. Depression and cardiovascular sequelae in postmenopausal women. The Women's Health Initiative (WHI). Arch Intern Med 2004;164:289–298. [DOI] [PubMed] [Google Scholar]
- 21. Whang W, Kubzansky LD, Kawachi I, Rexrode KM, Kroenke CH, Glynn RJ, Garan H, Albert CM. Depression and risk of sudden cardiac death and coronary heart disease in women: results from the Nurses’ Health Study. J Am Coll Cardiol 2009;53:950–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shah AJ, Veledar E, Hong Y, Bremner JD, Vaccarino V. Depression and history of attempted suicide as risk factors for heart disease mortality in young individuals. Arch Gen Psychiatry 2011;68:1135–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wyman L, Crum RM, Celentano D. Depressed mood and cause-specific mortality: a 40-year general community assessment. Ann Epidemiol 2012;22:638–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shah AJ, Ghasemzadeh N, Zaragoza-Macias E, Patel R, Eapen DJ, Neeland IJ, Pimple PM, Zafari AM, Quyyumi AA, Vaccarino V. Sex and age differences in the association of depression with obstructive coronary artery disease and adverse cardiovascular events. J Am Heart Assoc 2014;3:e000741.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Parashar S, Rumsfeld JS, Reid KJ, Buchanan D, Dawood N, Khizer S, Lichtman J, Vaccarino V; PREMIER Registry Investigators. Impact of depression on sex differences in outcome after myocardial infarction. Circ Cardiovasc Qual Outcomes 2009;2:33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Parashar S, Rumsfeld JS, Spertus JA, Reid KJ, Wenger NK, Krumholz HM, Amin A, Weintraub WS, Lichtman J, Dawood N, Vaccarino V. Time course of depression and outcome of myocardial infarction. Arch Intern Med 2006;166:2035–2043. [DOI] [PubMed] [Google Scholar]
- 27. Leung YW, Flora DB, Gravely S, Irvine J, Carney RM, Grace SL. The impact of premorbid and postmorbid depression onset on mortality and cardiac morbidity among patients with coronary heart disease: meta-analysis. Psychosom Med 2012;74:786–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Spijkerman T, de Jonge P, van den Brink RH, Jansen JH, May JF, Crijns HJ, Ormel J. Depression following myocardial infarction: first-ever versus ongoing and recurrent episodes. Gen Hosp Psychiatry 2005;27:411–417. [DOI] [PubMed] [Google Scholar]
- 29. Carney RM, Freedland KE, Steinmeyer B, Blumenthal JA, de Jonge P, Davidson KW, Czajkowski SM, Jaffe AS. History of depression and survival after acute myocardial infarction. Psychosom Med 2009;71:253–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. de Jonge P, van den Brink RH, Spijkerman TA, Ormel J. Only incident depressive episodes after myocardial infarction are associated with new cardiovascular events. J Am Coll Cardiol 2006;48:2204–2208. [DOI] [PubMed] [Google Scholar]
- 31. Hess CN, Wang TY, McCoy LA, Messenger JC, Effron MB, Zettler ME, Henry TD, Peterson ED, Fonarow GC. Unplanned inpatient and observation rehospitalizations after acute myocardial infarction: insights from the treatment with adenosine diphosphate receptor inhibitors: longitudinal assessment of treatment patterns and events after acute coronary syndrome (TRANSLATE-ACS) study. Circulation 2016;133:493–501. [DOI] [PubMed] [Google Scholar]
- 32. Martens EJ, Hoen PW, Mittelhaeuser M, de Jonge P, Denollet J. Symptom dimensions of post-myocardial infarction depression, disease severity and cardiac prognosis. Psychol Med 2010;40:807–814. [DOI] [PubMed] [Google Scholar]
- 33. Smolderen KG, Spertus JA, Reid KJ, Buchanan DM, Krumholz HM, Denollet J, Vaccarino V, Chan PS. The association of cognitive and somatic depressive symptoms with depression recognition and outcomes after myocardial infarction. Circ Cardiovasc Qual Outcomes 2009;2:328–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. de Jonge P, Ormel J, van den Brink RH, van Melle JP, Spijkerman TA, Kuijper A, van Veldhuisen DJ, van den Berg MP, Honig A, Crijns HJ, Schene AH. Symptom dimensions of depression following myocardial infarction and their relationship with somatic health status and cardiovascular prognosis. Am J Psychiatry 2006;163:138–144. [DOI] [PubMed] [Google Scholar]
- 35. Carney RM, Freedland KE. Treatment-resistant depression and mortality after acute coronary syndrome. Am J Psychiatry 2009;166:410–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Smolderen KG, Buchanan DM, Gosch K, Whooley M, Chan PS, Vaccarino V, Parashar S, Shah AJ, Ho PM, Spertus JA. Depression treatment and 1-year mortality after acute myocardial infarction: insights from the TRIUMPH registry (Translational Research Investigating Underlying Disparities in Acute Myocardial Infarction Patients' Health Status). Circulation 2017;135:1681–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Huffman JC, Smith FA, Blais MA, Beiser ME, Januzzi JL, Fricchione GL. Recognition and treatment of depression and anxiety in patients with acute myocardial infarction. Am J Cardiol 2006;98:319–324. [DOI] [PubMed] [Google Scholar]
- 38. Cepoiu M, McCusker J, Cole MG, Sewitch M, Belzile E, Ciampi A. Recognition of depression by non-psychiatric physicians—a systematic literature review and meta-analysis. J Gen Intern Med 2008;23:25–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gehi A, Haas D, Pipkin S, Whooley MA. Depression and medication adherence in outpatients with coronary heart disease: findings from the Heart and Soul Study. Arch Intern Med 2005;165:2508–2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Benner JS, Glynn RJ, Mogun H, Neumann PJ, Weinstein MC, Avorn J. Long-term persistence in use of statin therapy in elderly patients. JAMA 2002;288:455–461. [DOI] [PubMed] [Google Scholar]
- 41. Rumsfeld JS, Ho PM. Depression and cardiovascular disease: a call for recognition. Circulation 2005;111:250–253. [DOI] [PubMed] [Google Scholar]
- 42. Swardfager W, Herrmann N, Marzolini S, Saleem M, Farber SB, Kiss A, Oh PI, Lanctôt KL. Major depressive disorder predicts completion, adherence, and outcomes in cardiac rehabilitation: a prospective cohort study of 195 patients with coronary artery disease. J Clin Psychiatry 2011;72:1181–1188. [DOI] [PubMed] [Google Scholar]
- 43. Blumenthal JA, Williams RS, Wallace AG, Williams RB, Needles TL. Physiological and psychological variables predict compliance to prescribed exercise therapy in patients recovering from myocardial infarction. Psychosom Med 1982;44:519–527. [DOI] [PubMed] [Google Scholar]
- 44. Hare DL, Toukhsati SR, Johansson P, Jaarsma T. Depression and cardiovascular disease: a clinical review. Eur Heart J 2014;35:1365–1372. [DOI] [PubMed] [Google Scholar]
- 45. Bauer LK, Caro MA, Beach SR, Mastromauro CA, Lenihan E, Januzzi JL, Huffman JC. Effects of depression and anxiety improvement on adherence to medication and health behaviors in recently hospitalized cardiac patients. Am J Cardiol 2012;109:1266–1271. [DOI] [PubMed] [Google Scholar]
- 46. Frasure-Smith N, Lespérance F, Gravel G, Masson A, Juneau M, Talajic M, Bourassa MG. Depression and health-care costs during the first year following myocardial infarction. J Psychosom Res 2000;48:471–478. [DOI] [PubMed] [Google Scholar]
- 47. Stein MB, Cox BJ, Afifi TO, Belik SL, Sareen J. Does co-morbid depressive illness magnify the impact of chronic physical illness? A population-based perspective. Psychol Med 2006;36:587–596. [DOI] [PubMed] [Google Scholar]
- 48. Katon WJ, Lin EH, Von Korff M, Ciechanowski P, Ludman EJ, Young B, Peterson D, Rutter CM, McGregor M, McCulloch D. Collaborative care for patients with depression and chronic illnesses. N Engl J Med 2010;363:2611–2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tully PJ, Baumeister H. Collaborative care for the treatment of comorbid depression and coronary heart disease: a systematic review and meta-analysis protocol. Syst Rev 2014;3:127.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lane D, Carroll D, Ring C, Beevers DG, Lip GY. Effects of depression and anxiety on mortality and quality-of-life 4 months after myocardial infarction. J Psychosom Res 2000;49:229–238. [DOI] [PubMed] [Google Scholar]
- 51. Müller-Tasch T, Peters-Klimm F, Schellberg D, Holzapfel N, Barth A, Jünger J, Szecsenyi J, Herzog W. Depression is a major determinant of quality of life in patients with chronic systolic heart failure in general practice. J Card Fail 2007;13:818–824. [DOI] [PubMed] [Google Scholar]
- 52. Ruo B, Rumsfeld JS, Hlatky MA, Liu H, Browner WS, Whooley MA. Depressive symptoms and health-related quality of life: the Heart and Soul Study. JAMA 2003;290:215–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Dickens CM, McGowan L, Percival C, Tomenson B, Cotter L, Heagerty A, Creed FH. Contribution of depression and anxiety to impaired health-related quality of life following first myocardial infarction. Br J Psychiatry 2006;189:367–372. [DOI] [PubMed] [Google Scholar]
- 54. Dekker RL, Lennie TA, Albert NM, Rayens MK, Chung ML, Wu JR, Song EK, Moser DK. Depressive symptom trajectory predicts 1-year health-related quality of life in patients with heart failure. J Card Fail 2011;17:755–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Barnett LA, Prior JA, Kadam UT, Jordan KP. Chest pain and shortness of breath in cardiovascular disease: a prospective cohort study in UK primary care. BMJ Open 2017;7:e015857.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hayek SS, Ko YA, Awad M, Del Mar Soto A, Ahmed H, Patel K, Yuan M, Maddox S, Gray B, Hajjari J, Sperling L, Shah A, Vaccarino V, Quyyumi AA. Depression and chest pain in patients with coronary artery disease. Int J Cardiol 2017;230:420–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Frasure-Smith N, Lespérance F, Irwin MR, Talajic M, Pollock BG. The relationships among heart rate variability, inflammatory markers and depression in coronary heart disease patients. Brain Behav Immun 2009;23:1140–1147. [DOI] [PubMed] [Google Scholar]
- 58. van Hecke O, Hocking LJ, Torrance N, Campbell A, Padmanabhan S, Porteous DJ, McIntosh AM, Burri AV, Tanaka H, Williams FM, Smith BH. Chronic pain, depression and cardiovascular disease linked through a shared genetic predisposition: analysis of a family-based cohort and twin study. PLoS One 2017;12:e0170653.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. McCabe PJ. Psychological distress in patients diagnosed with atrial fibrillation. The state of the science. J Cardiovasc Nurs 2010;25:40–51. [DOI] [PubMed] [Google Scholar]
- 60. Andersson T, Magnuson A, Bryngelsson IL, Frøbert O, Henriksson KM, Edvardsson N, Poçi D. All-cause mortality in 272, 186 patients hospitalized with incident atrial fibrillation 1995-2008: a Swedish nationwide long-term case-control study. Eur Heart J 2013;34:1061–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. von Eisenhart Rothe A, Hutt F, Baumert J, Breithardt G, Goette A, Kirchhof P, Ladwig KH. Depressed mood amplifies heart-related symptoms in persistent and paroxysmal atrial fibrillation patients: a longitudinal analysis–data from the German Competence Network on Atrial Fibrillation. Europace 2015;17:1354–1362. [DOI] [PubMed] [Google Scholar]
- 62. Akintade BF, Chapa D, Friedmann E, Thomas SA. The influence of depression and anxiety symptoms on health-related quality of life in patients with atrial fibrillation and atrial flutter. J Cardiovasc Nurs 2015;30:66–73. [DOI] [PubMed] [Google Scholar]
- 63. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener H-C, Heidbuchel H, Hendriks J, Hindricks G, Manolis AS, Oldgren J, Popescu BA, Schotten U, Van Putte B, Vardas P; ESC Scientific Document Group. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J 2016;37:2893–2962. [DOI] [PubMed] [Google Scholar]
- 64. Phillips KP. Role of inflammation in initiation and perpetuation of atrial fibrillation: a systematic review of the published data. J Atr Fibrillation 2013;6:935.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lange HW, Herrmann-Lingen C. Depressive symptoms predict recurrence of atrial fibrillation after cardioversion. J Psychosom Res 2007;63:509–513. [DOI] [PubMed] [Google Scholar]
- 66. Lampert R, Jamner L, Burg M, Dziura J, Brandt C, Liu H, Li F, Donovan T, Soufer R. Triggering of symptomatic atrial fibrillation by negative emotion. J Am Coll Cardiol 2014;64:1533–1534. [DOI] [PubMed] [Google Scholar]
- 67. Graff S, Fenger-Grøn M, Christensen B, Pedersen HS, Christensen J, Li J, Vestergaard M. Long-term risk of atrial fibrillation after the death of a partner. Open Heart 2016;3:e000367.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Patel D, Mc Conkey ND, Sohaney R, Mc Neil A, Jedrzejczyk A, Armaganijan L. A systematic review of depression and anxiety in patients with atrial fibrillation: the mind-heart link. Cardiovasc Psychiatry Neurol 2013;2013:1.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Dimsdale JE. Psychological stress and cardiovascular disease. J Am Coll Cardiol 2008;51:1237–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Lampert R. Mental stress and ventricular arrhythmias. Curr Cardiol Rep 2016;18:118.. [DOI] [PubMed] [Google Scholar]
- 71. Peacock J, Whang W. Psychological distress and arrhythmia: risk prediction and potential modifiers. Prog Cardiovasc Dis 2013;55:582–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Liang JJ, Huang CX, Yang B, Huang H, Wan J, Tang YH, Zhao QY. Depressive symptoms and risk factors in Chinese patients with premature ventricular contractions without structural heart disease. Clin Cardiol 2009;32:E11–E17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Burg MM, Soufer R. Psychological stress and induced ischemic syndromes. Curr Cardiovasc Risk Rep 2014;8:377.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Soufer R. Neurocardiac interaction during stress-induced myocardial ischemia: how does the brain cope?. Circulation 2004;110:1710–1713. [DOI] [PubMed] [Google Scholar]
- 75. Vaccarino V, Bremner JD. Traumatic stress is heartbreaking. Biol Psychiatry 2013;74:790–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Kendler KS, Thornton LM, Gardner CO. Stressful life events and previous episodes in the etiology of major depression in women: an evaluation of the “kindling” hypothesis. Am J Psychiatry 2000;157:1243–1251. [DOI] [PubMed] [Google Scholar]
- 77. Veith RC, Lewis N, Linares OA, Barnes RF, Raskind MA, Villacres EC, Murburg MM, Ashleigh EA, Castillo S, Peskind ER. Sympathetic nervous system activity in major depression. Basal and desipramine-induced alterations in plasma norepinephrine kinetics. Arch Gen Psychiatry 1994;51:411–422. [DOI] [PubMed] [Google Scholar]
- 78. Carney RM, Freedland KE, Veith RC, Cryer PE, Skala JA, Lynch T, Jaffe AS. Major depression, heart rate, and plasma norepinephrine in patients with coronary heart disease. Biol Psychiatry 1999;45:458–463. [DOI] [PubMed] [Google Scholar]
- 79. Adell A, Trullas R, Gelpi E. Time course of changes in serotonin and noradrenaline in rat brain after predictable or unpredictable shock. Brain Res 1988;459:54–59. [DOI] [PubMed] [Google Scholar]
- 80. Knardahl S, Sanders BJ, Johnson AK. Effects of adrenal demedullation on stress-induced hypertension and cardiovascular responses to acute stress. Acta Physiol Scand 1988;133:477–483. [DOI] [PubMed] [Google Scholar]
- 81. Corbalan R, Verrier R, Lown B. Psychological stress and ventricular arrhythmias during myocardial infarction in the conscious dog. Am J Cardiol 1974;34:692–696. [DOI] [PubMed] [Google Scholar]
- 82. van Praag HM. The cognitive paradox in posttraumatic stress disorder: a hypothesis. Prog Neuropsychopharmacol Biol Psychiatry 2004;28:923–935. [DOI] [PubMed] [Google Scholar]
- 83. Froger N, Palazzo E, Boni C, Hanoun N, Saurini F, Joubert C, Dutriez CI, Enache M, Maccari S, Barden N, Cohen-Salmon C, Hamon M, Lanfumey L. Neurochemical and behavioral alterations in glucocorticoid receptor-impaired transgenic mice after chronic mild stress. J Neurosci 2004;24:2787–2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Weber B, Lewicka S, Deuschle M, Colla M, Vecsei P, Heuser I. Increased diurnal plasma concentrations of cortisone in depressed patients. J Clin Endocrinol Metab 2000;85:1133–1136. [DOI] [PubMed] [Google Scholar]
- 85. Smith GS, Eyler LT. Structural neuroimaging in geriatric psychiatry. Am J Geriatr Psychiatry 2006;14:809–811. [DOI] [PubMed] [Google Scholar]
- 86. Bremner JD. Changes in brain volume in major depression. Depression: Mind and Body 2005;2:38–46. [Google Scholar]
- 87. Kumar A, Gupta RC, Albert Thomas M, Alger J, Wyckoff N, Hwang S. Biophysical changes in normal-appearing white matter and subcortical nuclei in late-life major depression detected using magnetization transfer. Psychiatry Res 2004;130:131–140. [DOI] [PubMed] [Google Scholar]
- 88. Bremner JD, Narayan M, Anderson ER, Staib LH, Miller HL, Charney DS. Hippocampal volume reduction in major depression. Am J Psychiatry 2000;157:115–118. [DOI] [PubMed] [Google Scholar]
- 89. Vythilingam M, Heim C, Newport J, Miller AH, Anderson E, Bronen R, Brummer M, Staib L, Vermetten E, Charney DS, Nemeroff CB, Bremner JD. Childhood trauma associated with smaller hippocampal volume in women with major depression. Am J Psychiatry 2002;159:2072–2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Mervaala E, Föhr J, Könönen M, Valkonen-Korhonen M, Vainio P, Partanen K, Partanen J, Tiihonen J, Viinamäki H, Karjalainen AK, Lehtonen J. Quantitative MRI of the hippocampus and amygdala in severe depression. Psychol Med 2000;30:117–125. [DOI] [PubMed] [Google Scholar]
- 91. Bremner JD, Vythilingam M, Vermetten E, Vaccarino V, Charney DS. Deficits in hippocampal and anterior cingulate functioning during verbal declarative memory encoding in midlife major depression. Am J Psychiatry 2004;161:637–645. [DOI] [PubMed] [Google Scholar]
- 92. Bremner JD, Vythilingam M, Vermetten E, Nazeer A, Adil J, Khan S, Staib LH, Charney DS. Reduced volume of orbitofrontal cortex in major depression. Biol Psychiatry 2002;51:273–279. [DOI] [PubMed] [Google Scholar]
- 93. Kumar A, Bilker W, Lavretsky H, Gottlieb G. Volumetric asymmetries in late-onset mood disorders: an attenuation of frontal asymmetry with depression severity. Psychiatry Res 2000;100:41–47. [DOI] [PubMed] [Google Scholar]
- 94. Ballmaier M, Toga AW, Blanton RE, Sowell ER, Lavretsky H, Peterson J, Pham D, Kumar A. Anterior cingulate, gyrus rectus, and orbitofrontal abnormalities in elderly depressed patients: an MRI-based parcellation of the prefrontal cortex. Am J Psychiatry 2004;161:99–108. [DOI] [PubMed] [Google Scholar]
- 95. Lacerda AL, Keshavan MS, Hardan AY, Yorbik O, Brambilla P, Sassi RB, Nicoletti M, Mallinger AG, Frank E, Kupfer DJ, Soares JC. Anatomic evaluation of the orbitofrontal cortex in major depressive disorder. Biol Psychiatry 2004;55:353–358. [DOI] [PubMed] [Google Scholar]
- 96. Greenwald BS, Kramer-Ginsberg E, Krishnan KR, Ashtari M, Auerbach C, Patel M. Neuroanatomic localization of magnetic resonance imaging signal hyperintensities in geriatric depression. Stroke 1998;29:613–617. [DOI] [PubMed] [Google Scholar]
- 97. Hickie I, Scott E, Wilhelm K, Brodaty H. Subcortical hyperintensities on magnetic resonance imaging in patients with severe depression–a longitudinal evaluation. Biol Psychiatry 1997.;42:367–374. [DOI] [PubMed] [Google Scholar]
- 98. Lenze E, Cross D, McKeel D, Neuman RJ, Sheline YI. White matter hyperintensities and gray matter lesions in physically healthy depressed subjects. Am J Psychiatry 1999;156:1602–1607. [DOI] [PubMed] [Google Scholar]
- 99. Austin MP, Dougall N, Ross M, Murray C, O’Carroll RE, Moffoot A, Ebmeier KP, Goodwin GM. Single photon emission tomography with 99mTc-exametazime in major depression and the pattern of brain activity underlying the psychotic/neurotic continuum. J Affect Disord 1992;26:31–43. [DOI] [PubMed] [Google Scholar]
- 100. Biver F, Goldman S, Delvenne V, Luxen A, De Maertelaer V, Hubain P, Mendlewicz J, Lotstra F. Frontal and parietal metabolic disturbances in unipolar depression. Biol Psychiatry 1994;36:381–388. [DOI] [PubMed] [Google Scholar]
- 101. Mayberg HS, Brannan SK, Mahurin RK, Jerabek PA, Brickman JS, Tekell JL, Silva JA, McGinnis S, Glass TG, Martin CC, Fox PT. Cingulate function in depression: a potential predictor of treatment response. Neuroreport 1997;8:1057–1061. [DOI] [PubMed] [Google Scholar]
- 102. Drevets WC, Price JL, Simpson JR, Todd RD, Reich T, Vannier M, Raichle ME. Subgenual prefrontal cortex abnormalities in mood disorders. Nature 1997;386:824–827. [DOI] [PubMed] [Google Scholar]
- 103. George MS, Ketter TA, Parekh PI, Rosinsky N, Ring HA, Pazzaglia PJ, Marangell LB, Callahan AM, Post RM. Blunted left cingulate activation in mood disorder subjects during a response interference task (the Stroop). J Neuropsychiatry Clin Neurosci 1997;9:55–63. [DOI] [PubMed] [Google Scholar]
- 104. Bremner JD, Innis RB, Salomon RM, Staib LH, Ng CK, Miller HL, Bronen RA, Krystal JH, Duncan J, Rich D, Price LH, Malison R, Dey H, Soufer R, Charney DS. Positron emission tomography measurement of cerebral metabolic correlates of tryptophan depletion-induced depressive relapse. Arch Gen Psychiatry 1997;54:364–374. [DOI] [PubMed] [Google Scholar]
- 105. Bremner JD, Vythilingam M, Ng CK, Vermetten E, Nazeer A, Oren DA, Berman RM, Charney DS. Regional brain metabolic correlates of alpha-methylparatyrosine-induced depressive symptoms: implications for the neural circuitry of depression. JAMA 2003;289:3125–3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Smith GS, Reynolds CF, Houck PR, Dew MA, Ma Y, Mulsant BH, Pollock BG. Glucose metabolic response to total sleep deprivation, recovery sleep, and acute antidepressant treatment as functional neuroanatomic correlates of treatment outcome in geriatric depression. Am J Geriatr Psychiatry 2002;10:561–567. [PubMed] [Google Scholar]
- 107. Mayberg HS, Brannan SK, Tekell JL, Silva JA, Mahurin RK, McGinnis S, Jerabek PA. Regional metabolic effects of fluoxetine in major depression: serial changes and relationship to clinical response. Biol Psychiatry 2000;48:830–843. [DOI] [PubMed] [Google Scholar]
- 108. Goodwin GM, Austin M-P, Dougall N, Ross M, Murray C, O’Caroll RE, Moffoot A, Prentice N, Ebmeier KP. State changes in brain activity shown by the uptake of 99mTc-exametazime with single photon emission tomography in major depression before and after treatment. J Affect Disord 1993;29:243–253. [DOI] [PubMed] [Google Scholar]
- 109. Gianaros PJ, Onyewuenyi IC, Sheu LK, Christie IC, Critchley HD. Brain systems for baroreflex suppression during stress in humans. Hum Brain Mapp 2012;33:1700–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Lane RD, Jennings JR. Hemispheric asymmetry, autonomic asymmetry and the problem of sudden cardiac death. In: Davidson RJ, Hugdahl K, eds. Brain Asymmetry. Cambridge, MA: MIT Press; 1995, pp. 271–304. [Google Scholar]
- 111. Critchley HD, Taggart P, Sutton PM, Holdright DR, Batchvarov V, Hnatkova K, Malik M, Dolan RJ. Mental stress and sudden cardiac death: asymmetric midbrain activity as a linking mechanism. Brain 2004;128:75–85. [DOI] [PubMed] [Google Scholar]
- 112. Tawakol A, Ishai A, Takx RA, Figueroa AL, Ali A, Kaiser Y, Truong QA, Solomon CJ, Calcagno C, Mani V, Tang CY, Mulder WJ, Murrough JW, Hoffmann U, Nahrendorf M, Shin LM, Fayad ZA, Pitman RK. Relation between resting amygdalar activity and cardiovascular events: a longitudinal and cohort study. Lancet 2017;389:834–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Chida Y, Steptoe A. Greater cardiovascular responses to laboratory mental stress are associated with poor subsequent cardiovascular risk status: a meta-analysis of prospective evidence. Hypertension 2010;55:1026–1032. [DOI] [PubMed] [Google Scholar]
- 114. Critchley HD, Corfield DR, Chandler MP, Mathias CJ, Dolan RJ. Cerebral correlates of autonomic cardiovascular arousal: a functional neuroimaging investigation in humans. J Physiol 2000;523 Pt 1:259–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Bremner JD, Campanella C, Khan Z, Shah M, Hammadah M, Wilmot K, Al Mheid I, Lima BB, Garcia EV, Nye J, Ward L, Kutner MH, Raggi P, Pearce BD, Shah AJ, Quyyumi AA, Vaccarino V. Brain correlates of mental stress-induced myocardial ischemia. Psychosom Med 2018;80:515–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Critchley HD, Mathias CJ, Dolan RJ. Neuroanatomical basis for first- and second-order representations of bodily states. Nat Neurosci 2001;4:207–212. [DOI] [PubMed] [Google Scholar]
- 117. Thayer JF, Hansen AL, Saus-Rose E, Johnsen BH. Heart rate variability, prefrontal neural function, and cognitive performance: the neurovisceral integration perspective on self-regulation, adaptation, and health. Ann Behav Med 2009;37:141–153. [DOI] [PubMed] [Google Scholar]
- 118. Wei J, Rooks C, Ramadan R, Shah AJ, Bremner JD, Quyyumi AA, Kutner M, Vaccarino V. Meta-analysis of mental stress-induced myocardial ischemia and subsequent cardiac events in patients with coronary artery disease. Am J Cardiol 2014;114:187–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Arri SS, Ryan M, Redwood SR, Marber MS. Mental stress-induced myocardial ischaemia. Heart 2016;102:472–480. [DOI] [PubMed] [Google Scholar]
- 120. Wei J, Pimple P, Shah AJ, Rooks C, Bremner JD, Nye JA, Ibeanu I, Murrah N, Shallenberger L, Raggi P, Vaccarino V. Depressive symptoms are associated with mental stress-induced myocardial ischemia after acute myocardial infarction. PLoS One 2014;9:e102986.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Burg MM, Meadows J, Shimbo D, Davidson KW, Schwartz JE, Soufer R. Confluence of depression and acute psychological stress among patients with stable coronary heart disease: effects on myocardial perfusion. J Am Heart Assoc 2014;3:e000898.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Arrighi JA, Burg M, Cohen IS, Soufer R. Simultaneous assessment of myocardial perfusion and function during mental stress in patients with chronic coronary artery disease. J Nucl Cardiol 2003;10:267–274. [DOI] [PubMed] [Google Scholar]
- 123. Stone PH, Krantz DS, McMahon RP, Goldberg AD, Becker LC, Chaitman BR, Taylor HA, Cohen JD, Freedland KE, Bertolet BD, Coughlan C, Pepine CJ, Kaufmann PG, Sheps DS. Relationship among mental stress-induced ischemia and ischemia during daily life and during exercise: the Psychophysiologic Investigations of Myocardial Ischemia (PIMI) study. J Am Coll Cardiol 1999;33:1476–1484. [DOI] [PubMed] [Google Scholar]
- 124. Ramachandruni S, Fillingim RB, McGorray SP, Schmalfuss CM, Cooper GR, Schofield RS, Sheps DS. Mental stress provokes ischemia in coronary artery disease subjects without exercise- or adenosine-induced ischemia. J Am Coll Cardiol 2006;47:987–991. [DOI] [PubMed] [Google Scholar]
- 125. Ramadan R, Sheps D, Esteves F, Zafari AM, Bremner JD, Vaccarino V, Quyyumi AA. Myocardial ischemia during mental stress: role of coronary artery disease burden and vasomotion. J Am Heart Assoc 2013;2:e000321.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Hammadah M, Alkhoder A, Al Mheid I, Wilmot K, Isakadze N, Abdulhadi N, Chou D, Obideen M, O’Neal WT, Sullivan S, Tahhan AS, Kelli HM, Ramadan R, Pimple P, Sandesara P, Shah AJ, Ward L, Ko YA, Sun Y, Uphoff I, Pearce B, Garcia EV, Kutner M, Bremner JD, Esteves F, Sheps DS, Raggi P, Vaccarino V, Quyyumi AA. Hemodynamic, catecholamine, vasomotor and vascular responses: determinants of myocardial ischemia during mental stress. Int J Cardiol 2017;243:47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Sullivan S, Hammadah M, Al Mheid I, Wilmot K, Ramadan R, Alkhoder A, Isakadze N, Shah A, Levantsevych O, Pimple PM, Kutner M, Ward L, Garcia EV, Nye J, Mehta PK, Lewis TT, Bremner JD, Raggi P, Quyyumi AA, Vaccarino V. Sex differences in hemodynamic and microvascular mechanisms of myocardial ischemia induced by mental stress. Arterioscler Thromb Vasc Biol 2018;38:473–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Pimple P, Hammadah M, Wilmot K, Ramadan R, Al Mheid I, Levantsevych O, Sullivan S, Garcia EV, Nye J, Shah AJ, Ward L, Mehta P, Raggi P, Bremner JD, Quyyumi AA, Vaccarino V. Chest pain and mental stress-induced myocardial ischemia: sex differences. Am J Med 2018;131:540–547.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Jangpangi D, Mondal S, Bandhu R, Kataria D, Gandhi A. Alteration of heart rate variability in patients of depression. J Clin Diagn Res 2016;10:CM04–CM06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Grippo AJ, Beltz TG, Johnson AK. Behavioral and cardiovascular changes in the chronic mild stress model of depression. Physiol Behav 2003;78:703–710. [DOI] [PubMed] [Google Scholar]
- 131. Shi S, Liang J, Liu T, Yuan X, Ruan B, Sun L, Tang Y, Yang B, Hu D, Huang C. Depression increases sympathetic activity and exacerbates myocardial remodeling after myocardial infarction: evidence from an animal experiment. PLoS One 2014;9:e101734.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Xhyheri B, Manfrini O, Mazzolini M, Pizzi C, Bugiardini R. Heart rate variability today. Prog Cardiovasc Dis 2012;55:321–331. [DOI] [PubMed] [Google Scholar]
- 133. Manfrini O, Pizzi C, Trerè D, Fontana F, Bugiardini R. Parasympathetic failure and risk of subsequent coronary events in unstable angina and non-ST-segment elevation myocardial infarction. Eur Heart J 2003;24:1560–1566. [DOI] [PubMed] [Google Scholar]
- 134. Dekker JM, Crow RS, Folsom AR, Hannan PJ, Liao D, Swenne CA, Schouten EG. Low heart rate variability in a 2-minute rhythm strip predicts risk of coronary heart disease and mortality from several causes: the ARIC Study. Atherosclerosis Risk In Communities. Circulation 2000;102:1239–1244. [DOI] [PubMed] [Google Scholar]
- 135. Manfrini O, Pizzi C, Viecca M, Bugiardini R. Abnormalities of cardiac autonomic nervous activity correlate with expansive coronary artery remodeling. Atherosclerosis 2008;197:183–189. [DOI] [PubMed] [Google Scholar]
- 136. Kemp AH, Brunoni AR, Santos IS, Nunes MA, Dantas EM, Carvalho de Figueiredo R, Pereira AC, Ribeiro AL, Mill JG, Andreão RV, Thayer JF, Benseñor IM, Lotufo PA. Effects of depression, anxiety, comorbidity, and antidepressants on resting-state heart rate and its variability: an ELSA-Brasil cohort baseline study. Am J Psychiatry 2014;171:1328–1334. [DOI] [PubMed] [Google Scholar]
- 137. Licht CM, Naarding P, Penninx BW, van der Mast RC, de Geus EJ, Comijs H. The association between depressive disorder and cardiac autonomic control in adults 60 years and older. Psychosom Med 2015;77:279–291. [DOI] [PubMed] [Google Scholar]
- 138. Gehi A, Mangano D, Pipkin S, Browner WS, Whooley MA. Depression and heart rate variability in patients with stable coronary heart disease: findings from the Heart and Soul Study. Arch Gen Psychiatry 2005;62:661.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Licht CM, de Geus EJ, van Dyck R, Penninx BW. Longitudinal evidence for unfavorable effects of antidepressants on heart rate variability. Biol Psychiatry 2010;68:861–868. [DOI] [PubMed] [Google Scholar]
- 140. Huang M, Shah A, Su S, Goldberg J, Lampert RJ, Levantsevych OM, Shallenberger L, Pimple P, Bremner JD, Vaccarino V. Association of depressive symptoms and heart rate variability in vietnam war-era twins: a longitudinal twin difference study. JAMA Psychiatry 2018;75:705–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Vaccarino V, Lampert R, Bremner JD, Lee F, Su S, Maisano C, Murrah NV, Jones L, Jawed F, Afzal N, Ashraf A, Goldberg J. Depressive symptoms and heart rate variability: evidence for a shared genetic substrate in a study of twins. Psychosom Med 2008;70:628–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Mason JC, Libby P. Cardiovascular disease in patients with chronic inflammation: mechanisms underlying premature cardiovascular events in rheumatologic conditions. Eur Heart J 2015;36:482–9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Raedler TJ. Inflammatory mechanisms in major depressive disorder. Curr Opin Psychiatry 2011;24:519–525. [DOI] [PubMed] [Google Scholar]
- 144. Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol 2016;16:22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Das UN. Free radicals, cytokines and nitric oxide in cardiac failure and myocardial infarction. Mol Cell Biochem 2000;215:145–152. [DOI] [PubMed] [Google Scholar]
- 146. Francis J, Chu Y, Johnson AK, Weiss RM, Felder RB. Acute myocardial infarction induces hypothalamic cytokine synthesis. Am J Physiol Heart Circ Physiol 2004;286:H2264–H2271. [DOI] [PubMed] [Google Scholar]
- 147. Black CN, Bot M, Scheffer PG, Cuijpers P, Penninx BW. Is depression associated with increased oxidative stress? A systematic review and meta-analysis. Psychoneuroendocrinology 2015;51:164–175. [DOI] [PubMed] [Google Scholar]
- 148. Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, Lanctôt KL. A meta-analysis of cytokines in major depression. Biol Psychiatry 2010;67:446–457. [DOI] [PubMed] [Google Scholar]
- 149. Miller GE, Freedland KE, Carney RM, Stetler CA, Banks WA. Pathways linking depression, adiposity, and inflammatory markers in healthy young adults. Brain Behav Immun 2003;17:276–285. [DOI] [PubMed] [Google Scholar]
- 150. Copeland WE, Shanahan L, Worthman C, Angold A, Costello EJ. Cumulative depression episodes predict later C-reactive protein levels: a prospective analysis. Biol Psychiatry 2012;71:15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Khandaker GM, Pearson RM, Zammit S, Lewis G, Jones PB. Association of serum interleukin 6 and C-reactive protein in childhood with depression and psychosis in young adult life: a population-based longitudinal study. JAMA Psychiatry 2014;71:1121–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Valkanova V, Ebmeier KP, Allan CL. CRP, IL-6 and depression: a systematic review and meta-analysis of longitudinal studies. J Affect Disord 2013;150:736–744. [DOI] [PubMed] [Google Scholar]
- 153. Zalli A, Jovanova O, Hoogendijk WJ, Tiemeier H, Carvalho LA. Low-grade inflammation predicts persistence of depressive symptoms. Psychopharmacology (Berl) 2016;233:1669–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Nemeroff CB, Goldschmidt-Clermont PJ. Heartache and heartbreak–the link between depression and cardiovascular disease. Nat Rev Cardiol 2012;9:526–539. [DOI] [PubMed] [Google Scholar]
- 155. Savoy C, Van Lieshout RJ, Steiner M. Is plasminogen activator inhibitor-1 a physiological bottleneck bridging major depressive disorder and cardiovascular disease?. Acta Physiol (Oxf) 2017;219:715–727. [DOI] [PubMed] [Google Scholar]
- 156. Badimon L, Bugiardini R, Cenko E, Cubedo J, Dorobantu M, Duncker DJ, Estruch R, Milicic D, Tousoulis D, Vasiljevic Z, Vilahur G, De Wit C, Koller A. Position paper of the European Society of Cardiology-working group of coronary pathophysiology and microcirculation: obesity and heart disease. Eur Heart J 2017;38:1951–1958. [DOI] [PubMed] [Google Scholar]
- 157. Libby P. Inflammation in atherosclerosis. Arterioscler Thromb Vasc Bio 2012;32:2045–2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Su S, Zhao J, Bremner JD, Miller AH, Tang W, Bouzyk M, Snieder H, Novik O, Afzal N, Goldberg J, Vaccarino V. Serotonin transporter gene, depressive symptoms, and interleukin-6. Circ Cardiovasc Genet 2009;2:614–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Bufalino C, Hepgul N, Aguglia E, Pariante CM. The role of immune genes in the association between depression and inflammation: a review of recent clinical studies. Brain Behav Immun 2013;31:31–47. [DOI] [PubMed] [Google Scholar]
- 160. Broadley AJ, Korszun A, Jones CJ, Frenneaux MP. Arterial endothelial function is impaired in treated depression. Heart 2002;88:521–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Rajagopalan S, Brook R, Rubenfire M, Pitt E, Young E, Pitt B. Abnormal brachial artery flow-mediated vasodilation in young adults with major depression. Am J Cardiol 2001;88:196–198, A7. [DOI] [PubMed] [Google Scholar]
- 162. Sherwood A, Hinderliter AL, Watkins LL, Waugh RA, Blumenthal JA. Impaired endothelial function in coronary heart disease patients with depressive symptomatology. J Am Coll Cardiol 2005;46:656–659. [DOI] [PubMed] [Google Scholar]
- 163. Pries AR, Badimon L, Bugiardini R, Camici PG, Dorobantu M, Duncker DJ, Escaned J, Koller A, Piek JJ, de Wit C. Coronary vascular regulation, remodelling, and collateralization: mechanisms and clinical implications on behalf of the working group on coronary pathophysiology and microcirculation. Eur Heart J 2015;36:3134–3146. [DOI] [PubMed] [Google Scholar]
- 164. Cooper DC, Tomfohr LM, Milic MS, Natarajan L, Bardwell WA, Ziegler MG, Dimsdale JE. Depressed mood and flow-mediated dilation: a systematic review and meta-analysis. Psychosom Med 2011;73:360–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Greenstein AS, Paranthaman R, Burns A, Jackson A, Malik RA, Baldwin RC, Heagerty AM. Cerebrovascular damage in late-life depression is associated with structural and functional abnormalities of subcutaneous small arteries. Hypertension 2010;56:734–740. [DOI] [PubMed] [Google Scholar]
- 166. Demirtaş T, Utkan T, Karson A, Yazır Y, Bayramgürler D, Gacar N. The link between unpredictable chronic mild stress model for depression and vascular inflammation? Inflammation 2014;37:1432–1438. [DOI] [PubMed] [Google Scholar]
- 167. Wallerath T, Witte K, Schäfer SC, Schwarz PM, Prellwitz W, Wohlfart P, Kleinert H, Lehr HA, Lemmer B, Förstermann U. Down-regulation of the expression of endothelial NO synthase is likely to contribute to glucocorticoid-mediated hypertension. Proc Natl Acad Sci USA 1999;96:13357–13362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Le Mellédo JM, Mahil N, Baker GB. Nitric oxide: a key player in the relation between cardiovascular disease and major depressive disorder? J Psychiatry Neurosci 2004;29:414–416. [PMC free article] [PubMed] [Google Scholar]
- 169. Yui K, Imataka G, Nakamura H, Ohara N, Naito Y. Eicosanoids derived from arachidonic acid and their family prostaglandins and cyclooxygenase in psychiatric disorders. Curr Neuropharmacol 2015;13:776–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Bouzinova EV, Wiborg O, Aalkjaer C, Matchkov VV. Role of peripheral vascular resistance for the association between major depression and cardiovascular disease. J Cardiovasc Pharmacol 2015;65:299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Burg MM, Martens EJ, Collins D, Soufer R. Depression predicts elevated endothelin-1 in patients with coronary artery disease. Psychosom Med 2011;73:2–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Huffman JC, Celano CM, Beach SR, Motiwala SR, Januzzi JL. Depression and cardiac disease: epidemiology, mechanisms, and diagnosis. Cardiovasc Psychiatry Neurol 2013;2013:1.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Ghiadoni L, Donald AE, Cropley M, Mullen MJ, Oakley G, Taylor M, O’Connor G, Betteridge J, Klein N, Steptoe A, Deanfield JE. Mental stress induces transient endothelial dysfunction in humans. Circulation 2000;102:2473–2478. [DOI] [PubMed] [Google Scholar]
- 174. Hijmering ML, Stroes ES, Olijhoek J, Hutten BA, Blankestijn PJ, Rabelink TJ. Sympathetic activation markedly reduces endothelium-dependent, flow-mediated vasodilation. J Am Coll Cardiol 2002;39:683–688. [DOI] [PubMed] [Google Scholar]
- 175. Laghrissi-Thode F, Wagner WR, Pollock BG, Johnson PC, Finkel MS. Elevated platelet factor 4 and beta-thromboglobulin plasma levels in depressed patients with ischemic heart disease. Biol Psychiatry 1997;42:290–295. [DOI] [PubMed] [Google Scholar]
- 176. Sanner JE, Frazier L. The role of serotonin in depression and clotting in the coronary artery disease population. J Cardiovasc Nurs 2011;26:423–429. [DOI] [PubMed] [Google Scholar]
- 177. Zafar MU, Paz-Yepes M, Shimbo D, Vilahur G, Burg MM, Chaplin W, Fuster V, Davidson KW, Badimon JJ. Anxiety is a better predictor of platelet reactivity in coronary artery disease patients than depression. Eur Heart J 2010;31:1573–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Serebruany VL, Glassman AH, Malinin AI, Nemeroff CB, Musselman DL, van Zyl LT, Finkel MS, Krishnan KRR, Gaffney M, Harrison W, Califf RM, O’Connor CM; Group SAHARTS. Platelet/endothelial biomarkers in depressed patients treated with the selective serotonin reuptake inhibitor sertraline after acute coronary events: the sertraline anti-depressant heart attack randomized trial (SADHART) platelet substudy. Circulation 2003;108:939–944. [DOI] [PubMed] [Google Scholar]
- 179. Pollock BG, Laghrissi-Thode F, Wagner WR. Evaluation of platelet activation in depressed patients with ischemic heart disease after paroxetine or nortriptyline treatment. J Clin Psychopharmacol 2000;20:137–140. [DOI] [PubMed] [Google Scholar]
- 180. Lahlou-Laforet K, Alhenc-Gelas M, Pornin M, Bydlowski S, Seigneur E, Benetos A, Kierzin JM, Scarabin PY, Ducimetiere P, Aiach M, Guize L, Consoli SM. Relation of depressive mood to plasminogen activator inhibitor, tissue plasminogen activator, and fibrinogen levels in patients with versus without coronary heart disease. Am J Cardiol 2006;97:1287–1291. [DOI] [PubMed] [Google Scholar]
- 181. Geiser F, Meier C, Wegener I, Imbierowicz K, Conrad R, Liedtke R, Oldenburg J, Harbrecht U. Association between anxiety and factors of coagulation and fibrinolysis. Psychother Psychosom 2008;77:377–383. [DOI] [PubMed] [Google Scholar]
- 182. Amadio P, Colombo GI, Tarantino E, Gianellini S, Ieraci A, Brioschi M, Banfi C, Werba JP, Parolari A, Lee FS, Tremoli E, Barbieri SS. BDNFVal66met polymorphism: a potential bridge between depression and thrombosis. Eur Heart J 2017;38:1426–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Alexopoulos GS. The vascular depression hypothesis: 10 years later. Biol Psychiatry 2006;60:1304–1305. [DOI] [PubMed] [Google Scholar]
- 184. Newberg AR, Davydow DS, Lee HB. Cerebrovascular disease basis of depression: post-stroke depression and vascular depression. Int Rev Psychiatry 2006;18:433–441. [DOI] [PubMed] [Google Scholar]
- 185. Taylor WD, Aizenstein HJ, Alexopoulos GS. The vascular depression hypothesis: mechanisms linking vascular disease with depression. Mol Psychiatry 2013;18:963–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, Cooney MT, Corrà U, Cosyns B, Deaton C, Graham I, Hall MS, Hobbs FDR, Løchen ML, Löllgen H, Marques-Vidal P, Perk J, Prescott E, Redon J, Richter DJ, Sattar N, Smulders Y, Tiberi M, van der Worp HB, van Dis I, Verschuren WMM, Binno S; ESC Scientific Document Group. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: the Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J 2016;37:2315–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. McBride CM, Puleo E, Pollak KI, Clipp EC, Woolford S, Emmons KM. Understanding the role of cancer worry in creating a “teachable moment” for multiple risk factor reduction. Soc Sci Med 2008;66:790–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Mykletun A, Overland S, Aarø LE, Liabø HM, Stewart R. Smoking in relation to anxiety and depression: evidence from a large population survey: the HUNT study. Eur Psychiatry 2008;23:77–84. [DOI] [PubMed] [Google Scholar]
- 189. Levola J, Holopainen A, Aalto M. Depression and heavy drinking occasions: a cross-sectional general population study. Addict Behav 2011;36:375–380. [DOI] [PubMed] [Google Scholar]
- 190. Ferrer-Garcia M, Pla-Sanjuanelo J, Dakanalis A, Vilalta-Abella F, Riva G, Fernandez-Aranda F, Sánchez I, Ribas-Sabaté J, Andreu-Gracia A, Escandón-Nagel N, Gomez-Tricio O, Tena V, Gutiérrez-Maldonado J. Eating behavior style predicts craving and anxiety experienced in food-related virtual environments by patients with eating disorders and healthy controls. Appetite 2017;117:284–293. [DOI] [PubMed] [Google Scholar]
- 191. Bruch H. Psychological aspects of overeating and obesity. Psychosomatics 1964;5:269–274. [DOI] [PubMed] [Google Scholar]
- 192. van Strien T, Herman CP, Verheijden MW. Eating style, overeating and weight gain. A prospective 2-year follow-up study in a representative Dutch sample. Appetite 2012;59:782–789. [DOI] [PubMed] [Google Scholar]
- 193. Breslau N, Peterson EL, Schultz LR, Chilcoat HD, Andreski P. Major depression and stages of smoking. A longitudinal investigation. Arch Gen Psychiatry 1998;55:161–166. [DOI] [PubMed] [Google Scholar]
- 194. Fergusson DM, Goodwin RD, Horwood LJ. Major depression and cigarette smoking: results of a 21-year longitudinal study. Psychol Med 2003;33:1357–1367. [DOI] [PubMed] [Google Scholar]
- 195. Anda RF, Williamson DF, Escobedo LG, Mast EE, Giovino GA, Remington PL. Depression and the dynamics of smoking. A national perspective. JAMA 1990;264:1541–1545. [PubMed] [Google Scholar]
- 196. Hall SM, Muñoz RF, Reus VI, Sees KL. Nicotine, negative affect, and depression. J Consult Clin Psychol 1993;61:761–767. [DOI] [PubMed] [Google Scholar]
- 197. Petri E, Bacci O, Barbuti M, Pacchiarotti I, Azorin JM, Angst J, Bowden CL, Mosolov S, Vieta E, Young AH, Perugi G; BRIDGE-II-Mix Study Group. Obesity in patients with major depression is related to bipolarity and mixed features: evidence from the BRIDGE-II-Mix study. Bipolar Disord 2017;19:458–464. [DOI] [PubMed] [Google Scholar]
- 198. Mason TB, Lewis RJ. Profiles of binge eating: the interaction of depressive symptoms, eating styles, and body mass index. Eat Disord 2014;22:450–460. [DOI] [PubMed] [Google Scholar]
- 199. Lee JH, Park SK, Ryoo JH, Oh CM, Mansur RB, Alfonsi JE, Cha DS, Lee Y, McIntyre RS, Jung JY. The association between insulin resistance and depression in the Korean general population. J Affect Disord 2017;208:553–559. [DOI] [PubMed] [Google Scholar]
- 200. Pine DS, Goldstein RB, Wolk S, Weissman MM. The association between childhood depression and adulthood body mass index. Pediatrics 2001;107:1049–1056. [DOI] [PubMed] [Google Scholar]
- 201. Goodman E, Whitaker RC. A prospective study of the role of depression in the development and persistence of adolescent obesity. Pediatrics 2002;110:497–504. [DOI] [PubMed] [Google Scholar]
- 202. Kawakami N, Takatsuka N, Shimizu H, Ishibashi H. Depressive symptoms and occurrence of type 2 diabetes among Japanese men. Diabetes Care 1999;22:1071–1076. [DOI] [PubMed] [Google Scholar]
- 203. Golden SH, Williams JE, Ford DE, Yeh HC, Paton Sanford C, Nieto FJ, Brancati FL; Atherosclerosis Risk in Communities study. Depressive symptoms and the risk of type 2 diabetes: the Atherosclerosis Risk in Communities study. Diabetes Care 2004;27:429–435. [DOI] [PubMed] [Google Scholar]
- 204. Arroyo C, Hu FB, Ryan LM, Kawachi I, Colditz GA, Speizer FE, Manson J. Depressive symptoms and risk of type 2 diabetes in women. Diabetes Care 2004;27:129–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Pan A, Lucas M, Sun Q, van Dam RM, Franco OH, Manson JE, Willett WC, Ascherio A, Hu FB. Bidirectional association between depression and type 2 diabetes mellitus in women. Arch Intern Med 2010;170:1884–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Pan A, Sun Q, Czernichow S, Kivimaki M, Okereke OI, Lucas M, Manson JE, Ascherio A, Hu FB. Bidirectional association between depression and obesity in middle-aged and older women. Int J Obes (Lond) 2012;36:595–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Ngo DT, Farb MG, Kikuchi R, Karki S, Tiwari S, Bigornia SJ, Bates DO, LaValley MP, Hamburg NM, Vita JA, Hess DT, Walsh K, Gokce N. Antiangiogenic actions of vascular endothelial growth factor-A165b, an inhibitory isoform of vascular endothelial growth factor-A, in human obesity. Circulation 2014;130:1072–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Sorop O, Olver TD, van de Wouw J, Heinonen I, van Duin RW, Duncker DJ, Merkus D. The microcirculation: a key player in obesity-associated cardiovascular disease. Cardiovasc Res 2017;113:1035–1045. [DOI] [PubMed] [Google Scholar]
- 209. van Agtmaal MJM, Houben AJHM, Pouwer F, Stehouwer CDA, Schram MT. Association of microvascular dysfunction with late-life depression: a systematic review and meta-analysis. JAMA Psychiatry 2017;74:729–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Capuron L, Su S, Miller AH, Bremner JD, Goldberg J, Vogt GJ, Maisano C, Jones L, Murrah NV, Vaccarino V. Depressive symptoms and metabolic syndrome: is inflammation the underlying link? Biol Psychiatry 2008;64:896–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Vaccarino V, McClure C, Johnson BD, Sheps DS, Bittner V, Rutledge T, Shaw LJ, Sopko G, Olson MB, Krantz DS, Parashar S, Marroquin OC, Merz CN. Depression, the metabolic syndrome and cardiovascular risk. Psychosom Med 2008;70:40–48. [DOI] [PubMed] [Google Scholar]
- 212. Vaccarino V, Votaw J, Faber T, Veledar E, Murrah NV, Jones LR, Zhao J, Su S, Goldberg J, Raggi JP, Quyyumi AA, Sheps DS, Bremner JD. Major depression and coronary flow reserve detected by positron emission tomography. Arch Intern Med 2009;169:1668–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Sullivan PF, Neale MC, Kendler KS. Genetic epidemiology of major depression: review and meta-analysis. Am J Psychiatry 2000;157:1552–1562. [DOI] [PubMed] [Google Scholar]
- 214.Major Depressive Disorder Working Group of the Psychiatric GWAS Consortium; Ripke S, Wray NR, Lewis CM, Hamilton SP, Weissman MM, Breen G, Byrne EM, Blackwood DH, Boomsma DI, Cichon S, Heath AC, Holsboer F, Lucae S, Madden PA, Martin NG, McGuffin P, Muglia P, Noethen MM, Penninx BP, Pergadia ML, Potash JB, Rietschel M, Lin D, Müller-Myhsok B, Shi J, Steinberg S, Grabe HJ, Lichtenstein P, Magnusson P, Perlis RH, Preisig M, Smoller JW, Stefansson K, Uher R, Kutalik Z, Tansey KE, Teumer A, Viktorin A, Barnes MR, Bettecken T, Binder EB, Breuer R, Castro VM, Churchill SE, Coryell WH, Craddock N, Craig IW, Czamara D, De Geus EJ, Degenhardt F, Farmer AE, Fava M, Frank J, Gainer VS, Gallagher PJ, Gordon SD, Goryachev S, Gross M, Guipponi M, Henders AK, Herms S, Hickie IB, Hoefels S, Hoogendijk W, Hottenga JJ, Iosifescu DV, Ising M, Jones I, Jones L, Jung-Ying T, Knowles JA, Kohane IS, Kohli MA, Korszun A, Landen M, Lawson WB, Lewis G, Macintyre D, Maier W, Mattheisen M, McGrath PJ, McIntosh A, McLean A, Middeldorp CM, Middleton L, Montgomery GM, Murphy SN, Nauck M, Nolen WA, Nyholt DR, O'Donovan M, Oskarsson H, Pedersen N, Scheftner WA, Schulz A, Schulze TG, Shyn SI, Sigurdsson E, Slager SL, Smit JH, Stefansson H, Steffens M, Thorgeirsson T, Tozzi F, Treutlein J, Uhr M, van den Oord EJ, Van Grootheest G, Völzke H, Weilburg JB, Willemsen G, Zitman FG, Neale B, Daly M, Levinson DF, Sullivan PF.. A mega-analysis of genome-wide association studies for major depressive disorder. Mol Psychiatry 2013;18:497–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Mulle JG, Vaccarino V. Cardiovascular disease, psychosocial factors, and genetics: the case of depression. Prog Cardiovasc Dis 2013;55:557–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Scherrer JF, Xian H, Bucholz KK, Eisen SA, Lyons MJ, Goldberg J, Tsuang M, True WR. A twin study of depression symptoms, hypertension, and heart disease in middle-aged men. Psychosom Med 2003;65:548–557. [DOI] [PubMed] [Google Scholar]
- 217. Su S, Lampert R, Lee F, Bremner JD, Snieder H, Jones L, Murrah NV, Goldberg J, Vaccarino V. Common genes contribute to depressive symptoms and heart rate variability: the Twins Heart Study. Twin Res Hum Genet 2010;13:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Vaccarino V, Brennan ML, Miller AH, Bremner JD, Ritchie JC, Lindau F, Veledar E, Su S, Murrah NV, Jones L, Jawed F, Dai J, Goldberg J, Hazen SL. Association of major depressive disorder with serum myeloperoxidase and other markers of inflammation: a twin study. Biol Psychiatry 2008;64:476–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Su S, Miller AH, Snieder H, Bremner JD, Ritchie J, Maisano C, Jones L, Murrah NV, Goldberg J, Vaccarino V. Common genetic contributions to depressive symptoms and inflammatory markers in middle-aged men: the Twins Heart Study. Psychosom Med 2009;71:152–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Crawford B, Craig Z, Mansell G, White I, Smith A, Spaull S, Imm J, Hannon E, Wood A, Yaghootkar H, Ji Y, Mullins N, Lewis CM, Mill J, Murphy TM; Major Depressive Disorder Working Group of the Psychiatric Genomics Consortium. DNA methylation and inflammation marker profiles associated with a history of depression. Hum Mol Genet 2018;27:2840–2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Brouilette SW, Moore JS, McMahon AD, Thompson JR, Ford I, Shepherd J, Packard CJ, Samani NJ; West of Scotland Coronary Prevention Study Group. Telomere length, risk of coronary heart disease, and statin treatment in the West of Scotland Primary Prevention Study: a nested case-control study. Lancet 2007;369:107–114. [DOI] [PubMed] [Google Scholar]
- 222. Codd V, Nelson CP, Albrecht E, Mangino M, Deelen J, Buxton JL, Hottenga JJ, Fischer K, Esko T, Surakka I, Broer L, Nyholt DR, Mateo Leach I, Salo P, Hägg S, Matthews MK, Palmen J, Norata GD, O'Reilly PF, Saleheen D, Amin N, Balmforth AJ, Beekman M, de Boer RA, Böhringer S, Braund PS, Burton PR, de Craen AJ, Denniff M, Dong Y, Douroudis K, Dubinina E, Eriksson JG, Garlaschelli K, Guo D, Hartikainen AL, Henders AK, Houwing-Duistermaat JJ, Kananen L, Karssen LC, Kettunen J, Klopp N, Lagou V, van Leeuwen EM, Madden PA, Mägi R, Magnusson PK, Männistö S, McCarthy MI, Medland SE, Mihailov E, Montgomery GW, Oostra BA, Palotie A, Peters A, Pollard H, Pouta A, Prokopenko I, Ripatti S, Salomaa V, Suchiman HE, Valdes AM, Verweij N, Viñuela A, Wang X, Wichmann HE, Widen E, Willemsen G, Wright MJ, Xia K, Xiao X, van Veldhuisen DJ, Catapano AL, Tobin MD, Hall AS, Blakemore AI, van Gilst WH, Zhu H, Erdmann J, Reilly MP, Kathiresan S, Schunkert H, Talmud PJ, Pedersen NL, Perola M, Ouwehand W, Kaprio J, Martin NG, van Duijn CM, Hovatta I, Gieger C, Metspalu A, Boomsma DI, Jarvelin MR, Slagboom PE, Thompson JR, Spector TD, van der Harst P, Samani NJ; CARDIoGRAM consortium. Identification of seven loci affecting mean telomere length and their association with disease. Nat Genet 2013;45:422–427, 427e1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Said MA, Eppinga RN, Hagemeijer Y, Verweij N, van der Harst P. Telomere length and risk of cardiovascular disease and cancer. J Am Coll Cardiol 2017;70:506–507. [DOI] [PubMed] [Google Scholar]
- 224. Wikgren M, Maripuu M, Karlsson T, Nordfjäll K, Bergdahl J, Hultdin J, Del-Favero J, Roos G, Nilsson LG, Adolfsson R, Norrback KF. Short telomeres in depression and the general population are associated with a hypocortisolemic state. Biol Psychiatry 2012;71:294–300. [DOI] [PubMed] [Google Scholar]
- 225. Karabatsiakis A, Kolassa IT, Kolassa S, Rudolph KL, Dietrich DE. Telomere shortening in leukocyte subpopulations in depression. BMC Psychiatry 2014;14:192.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Shaffer JA, Epel E, Kang MS, Ye S, Schwartz JE, Davidson KW, Kirkland S, Honig LS, Shimbo D. Depressive symptoms are not associated with leukocyte telomere length: findings from the Nova Scotia Health Survey (NSHS95), a population-based study. PLoS One 2012;7:e48318.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Wium-Andersen MK, Ørsted DD, Rode L, Bojesen SE, Nordestgaard BG. Telomere length and depression: prospective cohort study and Mendelian randomisation study in 67 306 individuals. Br J Psychiatry 2017;210:31–38. [DOI] [PubMed] [Google Scholar]
- 228. Whalley B, Rees K, Davies P, Bennett P, Ebrahim S, Liu Z, West R, Moxham T, Thompson DR, Taylor RS. Psychological interventions for coronary heart disease. Cochrane Database Syst Rev 2011;CD002902. [DOI] [PubMed] [Google Scholar]
- 229. Smith SC, Benjamin EJ, Bonow RO, Braun LT, Creager MA, Franklin BA, Gibbons RJ, Grundy SM, Hiratzka LF, Jones DW, Lloyd-Jones DM, Minissian M, Mosca L, Peterson ED, Sacco RL, Spertus J, Stein JH, Taubert KA; World Heart Federation and the Preventive Cardiovascular Nurses Association. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation. Circulation 2011;124:2458–2473. [DOI] [PubMed] [Google Scholar]
- 230. First MB, Williams JBW, Gibbon M. Structured Clinical Interview for DSMIV-Patient Edition (SCID-P). Washington, DC: American Psychiatric Press; 1995. [Google Scholar]
- 231. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry 1960;23:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Carneiro AM, Fernandes F, Moreno RA. Hamilton depression rating scale and Montgomery-Asberg Depression Rating Scale in depressed and bipolar I patients: psychometric properties in a Brazilian sample. Health Qual Life Outcomes 2015;13:42.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- 234. Coric V, Stock EG, Pultz J, Marcus R, Sheehan DV. Sheehan Suicidality Tracking Scale (Sheehan-STS): preliminary results from a multicenter clinical trial in generalized anxiety disorder. Psychiatry (Edgmont) 2009;6:26–31. [PMC free article] [PubMed] [Google Scholar]
- 235. Hirschfeld RM. The epidemiology of depression and the evolution of treatment. J Clin Psychiatry 2012;73 Suppl 1:5–9. [DOI] [PubMed] [Google Scholar]
- 236. Berkman LF, Blumenthal J, Burg M, Carney RM, Catellier D, Cowan MJ, Czajkowski SM, DeBusk R, Hosking J, Jaffe A, Kaufmann PG, Mitchell P, Norman J, Powell LH, Raczynski JM, Schneiderman N; Enhancing Recovery in Coronary Heart Disease Patients Investigators (ENRICHD). Effects of treating depression and low perceived social support on clinical events after myocardial infarction: the Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) Randomized Trial. JAMA 2003;289:3106–3116. [DOI] [PubMed] [Google Scholar]
- 237. Davis L, Hamner M, Bremner JD. Pharmacotherapy for PTSD: effects on PTSD symptoms and the brain. In: Bremner JD, ed. Posttraumatic Stress Disorder: From Neurobiology to Treatment. Hoboken, NJ: Wiley Blackwell; 2016. p389–412. [Google Scholar]
- 238. Fournier JC, DeRubeis RJ, Hollon SD, Dimidjian S, Amsterdam JD, Shelton RC, Fawcett J. Antidepressant drug effects and depression severity: a patient-level meta-analysis. JAMA 2010;303:47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Jakobsen JC, Katakam KK, Schou A, Hellmuth SG, Stallknecht SE, Leth-Møller K, Iversen M, Banke MB, Petersen IJ, Klingenberg SL, Krogh J, Ebert SE, Timm A, Lindschou J, Gluud C. Selective serotonin reuptake inhibitors versus placebo in patients with major depressive disorder. A systematic review with meta-analysis and Trial Sequential Analysis. BMC Psychiatry 2017;17:58.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Cipriani A, Furukawa TA, Salanti G, Chaimani A, Atkinson LZ, Ogawa Y, Leucht S, Ruhe HG, Turner EH, Higgins JPT, Egger M, Takeshima N, Hayasaka Y, Imai H, Shinohara K, Tajika A, Ioannidis JPA, Geddes JR. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet 2018;391:1357–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Weeke P, Jensen A, Folke F, Gislason GH, Olesen JB, Andersson C, Fosbøl EL, Larsen JK, Lippert FK, Nielsen SL, Gerds T, Andersen PK, Kanters JK, Poulsen HE, Pehrson S, Køber L, Torp-Pedersen C. Antidepressant use and risk of out-of-hospital cardiac arrest: a nationwide case-time-control study. Clin Pharmacol Ther 2012;92:72–79. [DOI] [PubMed] [Google Scholar]
- 242. Kvam S, Kleppe CL, Nordhus IH, Hovland A. Exercise as a treatment for depression: a meta-analysis. J Affect Disord 2016;202:67–86. [DOI] [PubMed] [Google Scholar]
- 243. Blumenthal JA, Babyak MA, Moore KA, Craighead WE, Herman S, Khatri P, Waugh R, Napolitano MA, Forman LM, Appelbaum M, Doraiswamy PM, Krishnan KR. Effects of exercise training on older patients with major depression. Arch Intern Med 1999;159:2349–2356. [DOI] [PubMed] [Google Scholar]
- 244. Rutledge T, Redwine LS, Linke SE, Mills PJ. A meta-analysis of mental health treatments and cardiac rehabilitation for improving clinical outcomes and depression among patients with coronary heart disease. Psychosom Med 2013;75:335–349. [DOI] [PubMed] [Google Scholar]
- 245. Blumenthal JA, Sherwood A, Smith PJ, Watkins L, Mabe S, Kraus WE, Ingle K, Miller P, Hinderliter A. Enhancing cardiac rehabilitation with stress management training: a randomized, clinical efficacy trial. Circulation 2016;133:1341–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]