Abstract
Background:
The surprise question is widely used to identify patients nearing the last phase of life. Potential differences in accuracy between timeframe, patient subgroups and type of healthcare professionals answering the surprise question have been suggested. Recent studies might give new insights.
Aim:
To determine the accuracy of the surprise question in predicting death, differentiating by timeframe, patient subgroup and by type of healthcare professional.
Design:
Systematic review and meta-analysis.
Data sources:
Electronic databases PubMed, Embase, Cochrane Library, Scopus, Web of Science and CINAHL were searched from inception till 22nd January 2021. Studies were eligible if they used the surprise question prospectively and assessed mortality. Sensitivity, specificity, negative predictive value, positive predictive value and c-statistic were calculated.
Results:
Fifty-nine studies met the inclusion criteria, including 88.268 assessments. The meta-analysis resulted in an estimated sensitivity of 71.4% (95% CI [66.3–76.4]) and specificity of 74.0% (95% CI [69.3–78.6]). The negative predictive value varied from 98.0% (95% CI [97.7–98.3]) to 88.6% (95% CI [87.1–90.0]) with a mortality rate of 5% and 25% respectively. The positive predictive value varied from 12.6% (95% CI [11.0–14.2]) with a mortality rate of 5% to 47.8% (95% CI [44.2–51.3]) with a mortality rate of 25%. Seven studies provided detailed information on different healthcare professionals answering the surprise question.
Conclusion:
We found overall reasonable test characteristics for the surprise question. Additionally, this study showed notable differences in performance within patient subgroups. However, we did not find an indication of notable differences between timeframe and healthcare professionals.
Keywords: Surprise question, advance care planning, palliative care, systematic review, meta-analysis
What is already known about the topic?
The surprise question (‘Would I be surprised if this patient were to die in the next 12 months?’) is widely used to identify patients nearing the last phase of life. Earlier meta-analyses showed a sensitivity of 67.0% and a specificity of 80.2% and a pooled accuracy of 74.8%.
The surprise question seems to perform better in cancer patients compared to other patient subgroups.
It is suggested that doctors appear to be more accurate than nurses in recognising people in the last year of life.
What this paper adds?
This study is based on 88.268 surprise question assessments and shows that the surprise question has an estimated sensitivity of 71.4% (95% CI [66.3–76.4]) and specificity of 74.0% (95% CI [69.3–78.6]). The negative predictive value of the surprise question remains high with varying mortality rates.
Analysis of timeframe subgroups showed similar sensitivity for 6- and 12-month timeframe: 74.5% (95% CI [67.6–81.4]) and 73.4% (95% CI [68.2–78.6]) respectively. Specificity was lower for a 6-month timeframe 64.3% (95% CI [56.8–71.8]) compared to a 12-month timeframe 72.9% (95% CI [67.6–78.1]).
A sensitivity of 83.8% (95% CI [75.6–92.0]) was observed for patients with cancer and 82.5% (95% CI [60.1–100.0]) for patients with pulmonary disease, whereas the sensitivity for the emergency department was 49.1 (95% CI [35.7–62.5]). Specificity showed less variation with values between 67.3% (95% CI [53.2–81.3]) for cancer patients and 80.0% (95% CI [60.0–99.9]) for primary care patients.
Seven studies provided detailed information on different healthcare professionals answering the surprise question. Based on these studies we did not find an indication of notable differences between the accuracy of healthcare professionals answering the surprise question.
Implications for practice, theory or policy
The surprise question has a reasonable accuracy and is therefore an appropriate screening tool to identify patients that could benefit from advance care planning.
The surprise question should not solely be seen as an indicator of prognostication of death but rather as an opportunity for renewed attention for quality of care and shared decision making by timely initiating advance care planning.
Introduction
Palliative care aims to improve quality of life and end of life care of patients with life-threatening illnesses and to support their families. Improving end of life care is challenging due to the unpredictable course of chronic diseases. In order to benefit from palliative care, the definition of palliative care by the World Health Organisation emphasises timely identification of patients. 1 The surprise question was proposed by Lynn et al. 2 as a screening method to identify patients who might benefit from palliative care. It requires the healthcare professional to answer the question: ‘Would I be surprised if this patient were to die in the next 12 months?’ 2 (or a different timeframe other than 12 months).
Two earlier meta-analyses have been performed to study the accuracy of the surprise question.3,4 Results from Downar et al. 3 showed a sensitivity of 67.0% and specificity of 80.2%. White et al. 4 showed a pooled accuracy of 74.8%. Both meta-analyses included studies with different timeframes, patient subgroups and healthcare professionals. Downar et al. included studies with a 6, 12 and 18 months timeframe but did not differentiate between timeframes in their results. White et al. included studies with timeframes of 7 days, 30 days, 6 months, 6–12 months and 12 months and stated that an increase in timeframe did not impact the diagnostic accuracy. Both meta-analyses concluded that the surprise question performs better in cancer patients compared to other subgroups. White et al. suggested that doctors appear to be more accurate than nurses in recognising people in their last year of life. 4 However, the accuracy of the surprise question by type of healthcare professional is based on one study and more research is needed.
Many studies on the surprise question have been published in recent years, potentially giving new insights, not only into the overall accuracy of the surprise question, but also into potential differences between timeframes, patient subgroups and healthcare professionals answering the surprise question. Therefore, the aim of this systematic review and meta-analysis is to determine the accuracy of the surprise question in predicting death, investigating potential differences by timeframe, patient subgroup and type of healthcare professional answering the surprise question by answering the following questions: 1. How accurate is the surprise question in identifying patients in the last year of life? 2. Are there differences in accuracy of the surprise question between various timeframes? 3. Are there differences between patient subgroups to identify patients in the last year of life when using the surprise question? 4. Are there differences between healthcare professionals in identifying patients in the last year of life when using the surprise question?
Methods
Study design
This study entails a systematic review and meta-analysis of articles studying the accuracy of the surprise question. This study followed the reporting guideline of the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA).5,6
Data sources and search strategy
A systematic search was performed in six databases from inception till January 22nd 2021: PubMed, Embase, Cochrane Library, Scopus, Web of Science and Cumulative Index to Nursing and Allied Health Literature (CINAHL). The search terms ‘surprise question’, ‘Gold Standards Framework’ and ‘NECPAL’ were combined using the Boolean operator OR. The latter two are more elaborate tools to predict the need for end of life care that also use the surprise question7,8 and were added after an initial pilot search. No filters or limits were applied in the search. Details of the search strategy can be found in Appendix 1. Cross-referencing of included studies was performed.
Eligibility criteria
Inclusion criteria
Studies were included if they met the following criteria:
Prospective studies of any design, including non-peer reviewed publications.
Using the surprise question as a prognostic indicator.
Death as outcome.
Exclusion criteria
Studies were excluded if they met the following criteria:
Text was not in English.
Design was retrospective.
Reversed surprise question (‘Would I be surprised if this patient were still alive in 12 months?’) was used.
The results were not obtainable from the text or after contact with the corresponding authors.
Timeframe of surprise question and follow-up did not match (e.g. a surprise question timeframe of 6 months and follow-up ‘this admission’).
Study selection
Two reviewers (EvL and LI) independently screened all studies by title and abstract to identify potentially relevant studies. Subsequently full texts of the remaining studies were assessed by the same two reviewers. Screening of the studies was performed using Rayyan. 9 Disagreements were resolved by discussion until consensus was reached. In case of doubt a third reviewer was consulted (JvD). In case of non-peer reviewed publications, databases were searched for full text versions and requested by contacting the corresponding author. In case of incomplete data or if interpretation of data was unclear, the corresponding author of (potentially) relevant studies was contacted to obtain additional data or information.
Quality of studies assessment
The Quality in Prognosis Studies (QUIPS) tool 10 was used for risk of bias assessment. Studies were considered of high quality if (1) the size of the eligible population and baseline characteristics were available (2) loss to follow-up was less than mortality rate and reasons for loss to follow-up were described (3) the setting and person asking the surprise question was described (4) outcome measurement was described and (5) if the risk of confounding was considered low. Studies were considered to have high confounding if decisions on limiting treatment, potentially leading to death, took place in the study setting (e.g. at the Intensive Care or dialysis unit) or when an intervention (consultation of palliative care team or advance care planning conversation) was planned based on surprise question outcome. Articles were critically appraised by two reviewers (EvL and LI). Disagreements were discussed until consensus was reached. Quality assessment did not affect the inclusion of studies.
Data extraction and statistical analysis
Two reviewers (EvL and LI) independently extracted the following data: study population, type of healthcare professional answering the surprise question, study setting, total subjects, total surprise question assessments, surprise question timeframe, mean age, gender and mortality. A ‘no’ answer to the surprise question will be referred to as a positive answer to the surprise question, whereas a ‘yes’ answer will be referred to as negative answer to the surprise question. In studies where multiple healthcare professionals answered the surprise question, the study’s definition was used to determine whether the answer was positive (this could require consensus in case of a multidisciplinary team or require at least one healthcare professional answering ‘no’). If multiple healthcare professionals answered the surprise question and the study provided data separately, the physician’s response was used for the meta-analysis when possible. In studies where a third option for answering the surprise question besides ‘yes’ and ‘no’ was possible (e.g. ‘unsure’) data extraction was performed conform the study’s definition of a positive surprise question answer (e.g. ‘unsure’ was regarded as ‘No, I would not be surprised’).
Studies were divided in subgroups based on timeframe and patient group (cancer, cardiac disease, emergency department, kidney disease, primary care and pulmonary disease). The patient groups consisting of too few studies for analysis were combined as various. If a study cohort could potentially be classified into two groups (e.g. cardiac and emergency department), the cohort was classified into the underlying organ specific disease (e.g. cardiac disease). A ‘6 to 12’ month timeframe was considered equivalent to a ‘12-month’ timeframe. In case a study contained a derivation and a validation cohort, these were counted as separate cohorts. When a study investigated two different timeframes of the surprise question, both timeframes were included in the analysis.
The accuracy of the surprise question was analysed by constructing 2 × 2 tables of the surprise question response and mortality for each study. A true positive was considered as ‘No, I would not be surprised’ and deceased within the predetermined timeframe and a true negative was considered as ‘Yes, I would be surprised’ and alive. Sensitivity, specificity, negative predictive value (NPV), positive predictive value (PPV) and confidence intervals (CI’s) were calculated for each study. CI’s were calculated with Wilson’s method. 11 We considered for sensitivity a correct outcome corresponding to a positive surprise question answer (‘No, I would not be surprised’) patients that died during the specified timeframe, and for specificity a correct outcome corresponding to patients with a negative surprise question answer (‘Yes, I would be surprised’) that did not die during the specified timeframe. NPV represents the percentage of patients surviving when the healthcare professionals predicted survival and PPV represents the percentage of patients dying when healthcare professionals predicted death within the specified timeframe. A bivariate random effects logistic regression model was used to pool sensitivity and specificity. 12 This model analyses the combination of sensitivity and specificity, estimates heterogeneity of sensitivity and specificity between studies and the correlation between these measures. Results from the analyses are presented as pooled sensitivity and specificity. PPV and NPV depend on prevalence of disease or mortality rate. Hence, pooled sensitivity and specificity were used to estimate pooled PPV and NPV with 95% CI for various mortality rates: 5%, 10% and 25%. From the results from this analysis, the summary c-statistic (area under the summary receiver operating characteristic curve) was estimated with formulas described by Walter. 13 The corresponding standard error (SE) was estimated with the Delta method. 14 The heterogeneity measure (τ 2 ), differences between studies beyond the uncertainty captured by confidence intervals, was used to estimate the I2 statistic. 15
In a second step, we assessed the impact of timeframe, patient group and peer reviewed versus non-peer reviewed studies by including these characteristics in the model. Reporting the results from the analysis with timeframe was limited to 6 and 12 months, as these were considered most relevant. We performed a likelihood ratio test to assess the influence of non-peer reviewed publications. For each subgroup we estimated pooled sensitivity, specificity, NPV, PPV and the c-statistic with CI’s. For the subgroups cardiac, emergency department and pulmonary disease, the analysis showed convergence difficulties, as the correlation between sensitivity and specificity over studies was estimated close to zero. For these analyses, we removed the correlation to obtain reliable results. Statistical analysis was performed with SAS version 9.4. 16 Forest plots were made using Microsoft Excel version 2016. 17
According to Dutch law, ethics approval was not required for this study.
Results
Study selection
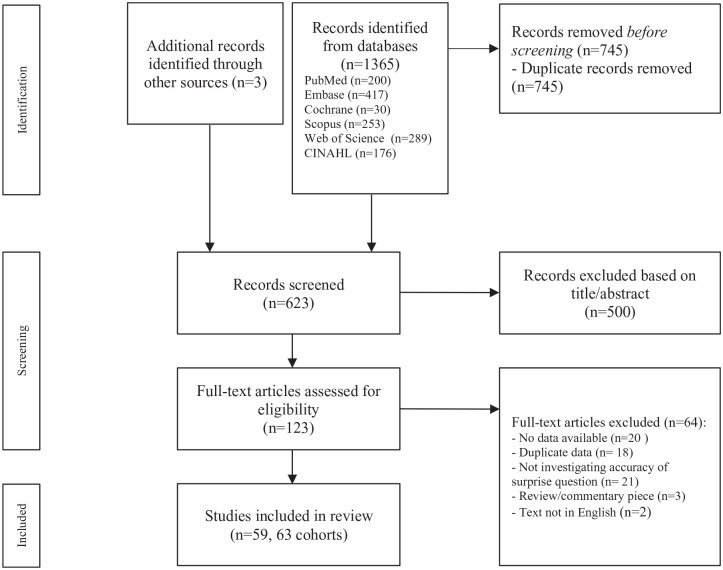
The systematic search identified 1365 studies, of which 745 were duplicates. Cross-referencing resulted in the inclusion of three extra studies.18 –20 Of the remaining 623 studies, 500 articles were excluded based on title/abstract screening. Full texts were assessed of 123 studies. Based on full text, 64 articles were excluded. In total 59 studies were included in the meta-analysis.18,21 –78 The flowchart of the included studies can be found in Figure 1. Four studies consisted of multiple cohorts: three studies consisted of a derivation and a validation cohort22,38,52 and one study consisted of two different patient subgroups. 70 In total 63 cohorts were included in our analysis. Four studies used two variants of the surprise question with varying timeframes.31,44,64,74
Figure 1.
PRISMA flow diagram of screening process. 6
Corresponding authors of 35 potentially relevant studies were contacted in order to obtain full text or additional data in order to construct the 2 × 2 table. 18 studies were included after the authors provided additional data.22,23,36,38,39,42,43,46,47,52,53,55,59–61,70,72,75 Of the remaining 17 articles, two studies were excluded since they did not use the surprise question to predict death.79,80 Eight studies were excluded since the author was not able to not provide extra data.81–88 Seven studies were excluded since the corresponding author did not respond after various attempts.89–95 For eight other potential relevant studies (all non-peer reviewed), no contact details were available nor could these be obtained after extensive searching.96–103
Study characteristics
Characteristics of included studies can be found in Appendix 2. Studies were heterogeneous in timeframe, population, setting and healthcare professional answering the surprise question (e.g. nurse v medical specialist). Most studies originated in the United States (20 studies), United Kingdom (9 studies) and The Netherlands (six studies). Forty-five studies took place in the hospital. Of these, 12 studies were performed at haemodialysis units and eight in outpatient clinics. Of the remaining 14 studies, eight took place in general practice/primary care, three in hospice care settings, one in a nursing home and one in a neurorehabilitation centre. One study took place at multiple settings (three primary care centres, one general hospital, one intermediate care centre and four nursing homes). 37 Most studies investigated a 12-month timeframe of the surprise question (48 cohorts). Other timeframes were 3 days, 67 1 week, 31 1 month,31,36,43,51,56,74 3 months, 44 6 months22,38,42,46,47,53,64 and 24 months. 57 Four studies used two variants of the surprise question with varying timeframes.31,44,64,74 In general, patients included were adults (>18 years), except for one study performed in children. 44 Eighteen studies included patients with kidney disease, 12 patients with cancer, seven with cardiac disease, seven included a diverse group of patients in general practice/primary care, six studies included patients with pulmonary disease and five studies included patients from the emergency department. In seven studies the surprise question was answered by various healthcare professionals.26,45,50,57,60,71,75 In two studies answering the surprise question was based on consensus of a multidisciplinary team.30,44 Mortality rate of all studies was on average 11.85% and varied between studies from 0.99% (primary care) 76 to 78.78% (advanced cancer patients at the emergency department). 63
In total five of the included studies added a third option for answering the surprise question besides ‘yes’ and ‘no’, including ‘don’t know this patient well enough’, 26 ‘don’t know’, 66 ‘unsure’, 48 ‘uncertain’ 49 and ‘defer’. 71 In total these answers represent 61 of 88.268 surprise question assessments, varying from 6% 48 to 9% 71 per study. In two studies this percentage could not be retrieved.26,49
Quality assessment: Risk of bias
A detailed overview of the risk of bias assessment is presented in Appendix 3. Three studies had a high risk of bias (two non-peer reviewed), 13 studies (eight non-peer reviewed) had a moderate risk of bias and 43 studies (six non-peer reviewed) had a low risk of bias. Most methodological issues were in study population (domain 1: eight high and 30 intermediate risk of bias) and study confounding (domain 5: two high and 17 intermediate risk of bias). A risk of selection bias was in many studies caused by not specifying the eligible population. An intermediate or high-risk assessment in study confounding was in most studies due to the setting and patient population (e.g. haemodialysis patients) or caused by planning an intervention based on the outcome of the surprise question.
Meta-analysis
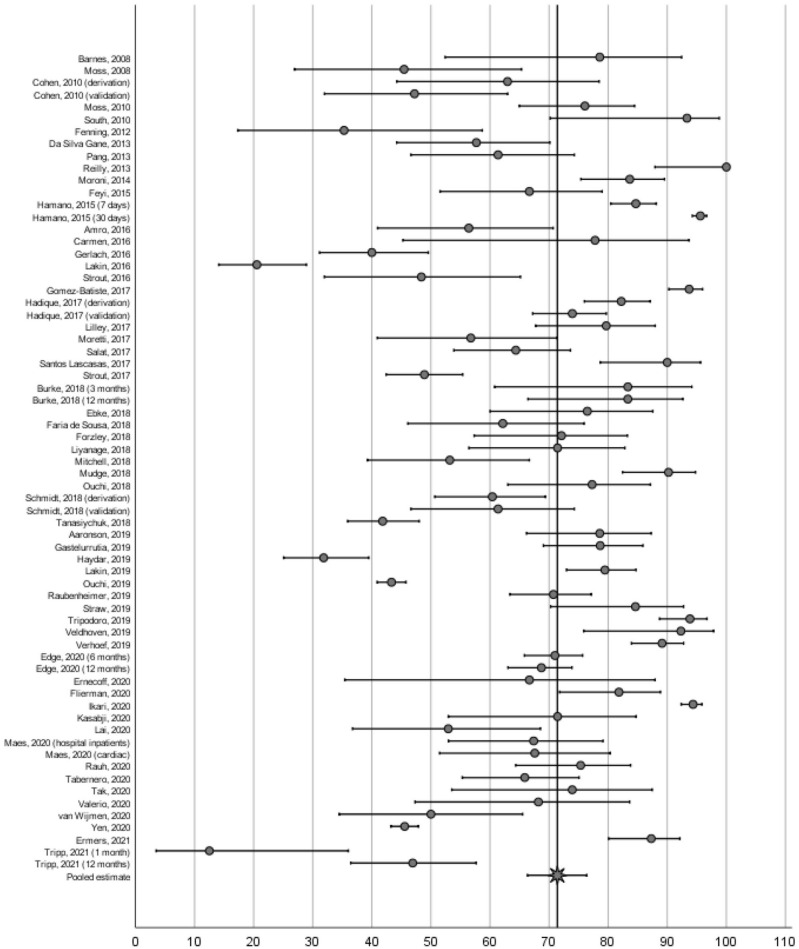
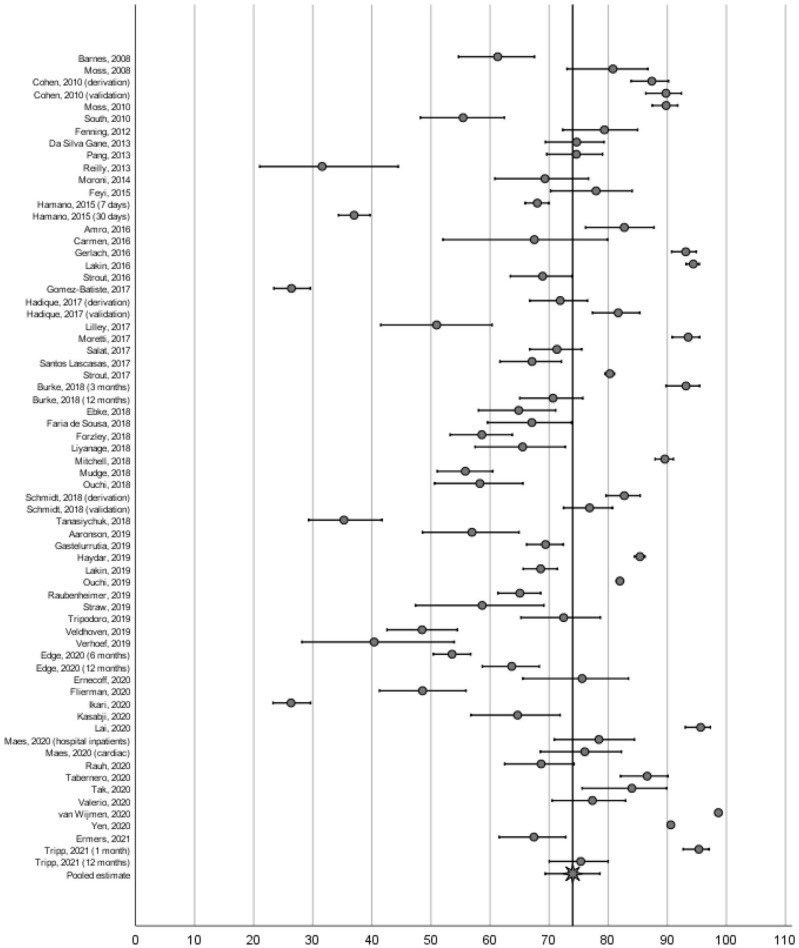
In total 88.268 assessments were included from 59 different studies and 63 different cohorts. Sensitivity between individual studies varied from 12.5% 74 to 100%, 28 specificity varied from 26.3% 67 to 98.6%, 76 NPV from 35.1% 53 to 100% 28 and PPV from 5.4%43,56 to 84.7%. 63 Individual study results and forest plots of the sensitivity and specificity can be found in Appendices 4–6. A likelihood ratio test showed that inclusion of non-peer reviewed publications did not significantly change the results (p value 0.84). Non-peer reviewed publications were therefore retained in all analyses.
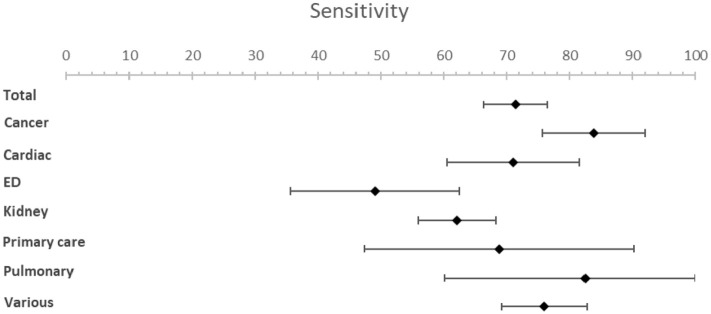
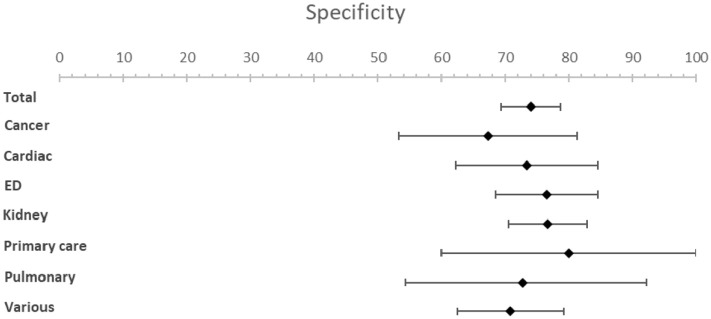
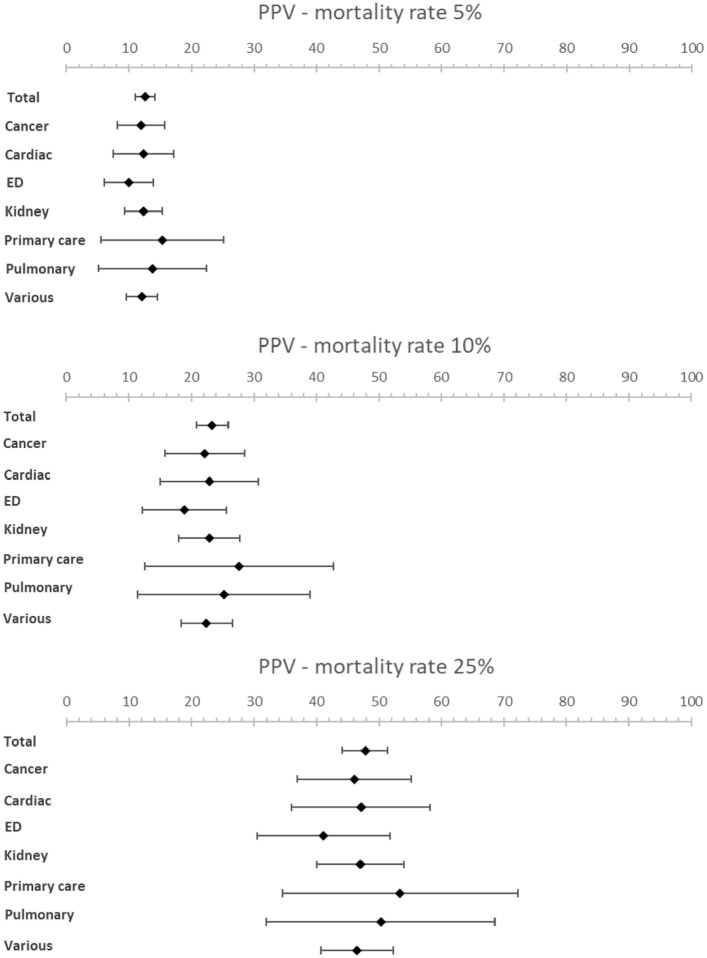
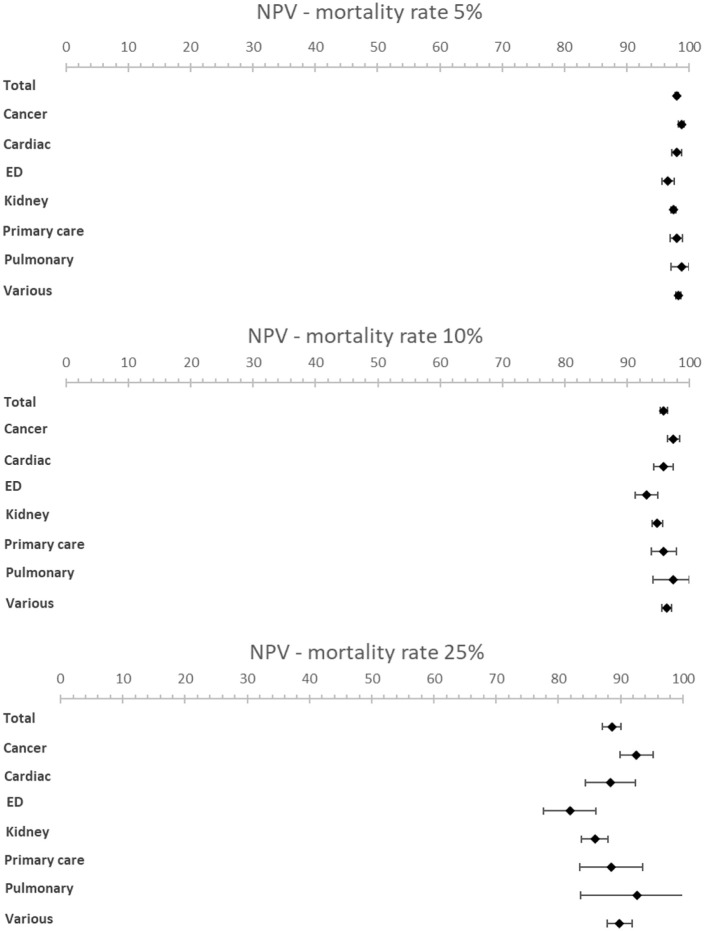
The meta-analysis resulted in an estimated sensitivity of 71.4% (95% CI [66.3–76.4]), an estimated specificity of 74.0% (95% CI [69.3–78.6]) (Table 1, Figures 2 and 3). The estimated NPV varied from 98.0% (95% CI [97.7–98.3]) to 88.6% (95% CI [87.1–90.0]) with a mortality rate of 5% and 25% respectively (Table 1, Figures 4 and 5). The estimated PPV varied from 12.6% (95% CI [11.0–14.2]) with a mortality rate of 5%–47.8% (95% CI [44.2–51.3]) with a mortality rate of 25%. The c-statistic value was 0.79 (95% CI [0.77–0.81]) in the overall analysis. Heterogeneity (I2) in the overall analysis was 98.2% and 98.4% for sensitivity and specificity respectively.
Table 1.
Diagnostic accuracy of the surprise question.
| Patient subgroup | No. of cohorts | Sensitivity [95% CI] | I2, % | Specificity [95% CI] | I2, % | AUC [95% CI] | PPV – mortality rate 5% [95% CI] | NPV – mortality rate 5% [95% CI] | PPV – mortality rate 10% [95% CI] | NPV – mortality rate 10% [95% CI] | PPV – mortality rate 25% [95% CI] | NPV – mortality rate 25% [95% CI] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | 63 | 71.4 [66.3–76.4] | 98.2 | 74.0 [69.3–78.6] | 98.4 | 0.79 [0.77–0.81] | 12.6 [11.0–14.2] | 98.0 [97.7–98.3] | 23.4 [20.8–25.9] | 95.9 [95.3–96.4] | 47.8 [44.2–51.3] | 88.6 [87.1–90.0] |
| Timeframe | ||||||||||||
| 6 months | 7 | 74.5 [67.6–81.4] | 96.4 | 64.3 [56.8–71.8] | 97.4 | 0.75 [0.71–0.80] | 9.9 [8.3–11.6] | 98.0 [97.5–98.4] | 18.8 [16.0–21.7] | 95.8 [94.8–96.7] | 41.0 [36.5–45.5] | 88.3 [85.9–90.8] |
| 12 months | 48 | 73.4 [68.2–78.6] | 98.0 | 72.9 [67.6–78.1] | 98.3 | 0.80 [0.77–0.82] | 12.5 [10.8–14.2] | 98.1 [97.8–98.4] | 23.1 [20.4–25.9] | 96.1 [95.5–96.7] | 47.5 [43.6–51.3] | 89.2 [87.7–90.7] |
| Subgroups | ||||||||||||
| Cancer a | 12 a | 83.8 [75.6–92.0] | 90.4 | 67.3 [53.2–81.3] | 90.7 | 0.83 [0.79–0.88] | 11.9 [8.0–15.7] | 98.8 [98.3–99.2] | 22.2 [15.8–28.5] | 97.4 [96.4–98.4] | 46.1 [37.0–55.1] | 92.6 [89.9–95.2] |
| Cardiac | 7 | 71.0 [60.5–81.5] | 82.4 | 73.4 [62.2–84.6] | 86.9 | 0.78 [0.69–0.87] | 12.3 [7.5–17.1] | 98.0 [97.2–98.8] | 22.9 [15.0–30.8] | 95.8 [94.2–97.4] | 47.1 [36.0–58.2] | 88.4 [84.3–92.4] |
| ED | 5 | 49.1 [35.7–62.5] | 80.9 | 76.5 [68.5–84.6] | 82.8 | 0.68 [0.58–0.79] | 9.9 [6.0–13.8] | 96.6 [95.7–97.6] | 18.9 [12.2–25.6] | 93.1 [91.3–94.9] | 41.1 [30.5–51.7] | 81.9 [77.7–86.1] |
| Kidney | 18 | 62.1 [55.9–68.3] | 91.4 | 76.7 [70.5–83.0] | 94.4 | 0.76 [0.70–0.81] | 12.3 [9.3–15.3] | 97.5 [97.0–97.9] | 22.9 [18.0–27.8] | 94.8 [93.9–95.7] | 47.1 [40.1–54.0] | 85.9 [83.7–88.0] |
| Primary care | 7 | 68.8 [47.3–90.3] | 86.8 | 80.0 [60.0–99.9] | 87.5 | 0.81 [0.74–0.89] | 15.3 [5.5–25.1] | 98.0 [97.0–99.0] | 27.6 [12.5–42.7] | 95.8 [93.9–97.8] | 53.4 [34.5–72.2] | 88.5 [83.4–93.6] |
| Pulmonary b | 6 b | 82.5 [60.1–100.0] | 79.1 | 72.8 [54.3–91.3] | 82.8 | 0.85 [0.68–1.00] | 13.8 [5.1–22.5] | 98.8 [97.1–100.0] | 25.2 [11.4–39.0] | 97.4 [94.1–100.0] | 50.3 [32.0–68.6] | 92.6 [83.6–100.0] |
| Various | 12 c | 75.9 [69.2–82.7] | 89.9 | 70.8 [62.5–79.2] | 91.3 | 0.80 [0.76–0.83] | 12.1 [9.6–14.5] | 98.2 [97.9–98.6] | 22.4 [18.4–26.5] | 96.4 [95.6–97.1] | 46.5 [40.7–52.2] | 89.8 [87.8–91.8] |
| Type of publication | ||||||||||||
| Peer reviewed | 47 d | 72.0 [66.4–77.6] | 97.7 | 73.9 [68.6–79.3] | 97.9 | 0.79 [0.77–0.82] | 12.7 [10.9–14.5] | 98.1 [97.7–98.4] | 23.5 [20.5–26.4] | 96.0 [95.3–96.6] | 47.9 [43.8–52.1] | 88.8 [87.2–90.4] |
| Non-peer reviewed | 16 e | 69.1 [58.4–79.8] | 93.0 | 74.1 [64.9–83.3] | 93.9 | 0.78 [0.73–0.83] | 12.3 [9.2–15.4] | 97.9 [97.3–98.5] | 22.9 [17.9–27.9] | 95.6 [94.4–96.8] | 47.1 [40.0–54.2] | 87.8 [84.8–90.8] |
ED: emergency department; AUC: area under the curve; I2: Heterogeneity; CI: confidence interval; PPV: positive predictive value; NPV: negative predictive value.
2/12 cohorts were analysed with two separate timeframes.
1/6 cohort was analysed with two separate timeframes.
1/12 cohort was analysed with two separate timeframes.
3/47 cohorts were analysed with two separate timeframes.
1/16 cohort was analysed with two separate timeframes.
Figure 2.
Forest plots for sensitivity.
Figure 3.
Forest plots for specificity.
Figure 4.
Forest plots showing PPV for various mortality rates (5%, 10% and 25%).
Figure 5.
Forest plots showing NPV for various mortality rates (5%, 10% and 25%).
Results from the subgroup analysis including timeframe subgroups (6- and 12-months), patient subgroups and peer reviewed versus non-peer reviewed subgroups can be found in Table 1 and Figures 2 to 5. Analysis of timeframe subgroups showed similar sensitivity for 6- and 12-month timeframe: 74.5% (95% CI [67.6–81.4]) and 73.4% (95% CI [68.2–78.6]) respectively. Specificity was lower for a 6-month timeframe 64.3% (95% CI [56.8–71.8]) compared to a 12-month timeframe 72.9% (95% CI [67.6–78.1]).
Subgroup analysis of patient subgroups showed a lower sensitivity for the surprise question at the emergency department (49.1%; 95% CI [35.7–62.5]) compared to higher sensitivities for cancer patients (83.8%; 95% CI [75.6–92.0]) and patients with pulmonary disease (82.5%; 95% CI [60.1–100]). Specificity varied from 67.3% (95% CI [53.2−81.3]) in cancer patients to 80.0% (95% CI [60.0–99.9]) in primary care patients. NPV was the lowest in the emergency department with a NPV of 96.6% (95% CI [95.8–97.6]) and the highest in pulmonary patients with a NPV of 98.8% (95% CI [97.1–100.0]) at a mortality rate of 5%. NPV varied from 81.9% (95% CI [77.7–86.1]) in patients at the emergency department to 92.6% (95% CI [83.6–100]) in patients with pulmonary disease and 92.6% (95% CI [89.9–95.2]) in patients with cancer at a mortality rate of 25%.
In seven studies multiple healthcare professionals answered the surprise question. Due to the heterogeneity of the results (different patient subgroups, different healthcare professionals answering the surprise question with different seniority and different intensity in care provision to the patient) we could not perform a meta-analysis on this subgroup. An overview of the accuracy of the surprise question by different healthcare professionals can be found in Table 2. The study by Da Silva Gane et al. 26 investigated the variability between nephrologists and nurses of different levels of seniority (referred to as ‘bands’). They conclude that nephrologists perform better compared to nurses based on a higher sensitivity and similar specificity. The study of Lakin et al. 57 also show that primary care physicians have a higher sensitivity compared to nurse care coordinators. On the contrary, the results of Valerio and Farinha 75 show that nurses have a higher sensitivity and lower specificity compared to nephrologists and the results of Straw et al. 60 show that heart failure nurses have a higher sensitivity compared to cardiologists, trainee-grade doctors and non-specialist nurses. Similar performances between healthcare professionals are seen in the study by Mudge et al. 50 when comparing doctors and senior nurses and by Rauh et al. 71 when comparing doctors, nurses and advanced practice providers. Ebke et al. 45 compare the accuracy of answering the surprise question by neurorehabilitation physicians and palliative care physicians, with palliative care physicians having a higher sensitivity and lower specificity. In five other studies multiple healthcare professionals answered the surprise question, however, no separate data was reported.40,52,55,68,74
Table 2.
Accuracy of the surprise question by type of healthcare professional.
| Study | Type of healthcare professional | Sensitivity, % [95% CI]** | Specificity, % [95% CI]** | PPV, % [95% CI]** | NPV, % [95% CI]** |
|---|---|---|---|---|---|
| Da Silva Gane et al. 26 | Nephrologists | 73.7 [64.6–82.8] | 73.9 [66.3–81.5] | 33.5 [27.3–39.7] | 94.3 [90.9–97.7] |
| Nurse band 5* | 35.6 [21.3–49.9] | 85.4 [77.8–93.0] | 32.4 [21.7–43.1 | 88.3 [86.5–90.1] | |
| Nurse band 6* | 51.1 [31.5–70.7] | 78.5 [69.1–87.9] | 31.1 [24.4–37.8] | 90.1 [87.3–92.9] | |
| Nurse band 7/8* | 51.4 [18.1–33.1] | 79.1 [72.4–85.8] | 30.0 [20.7–39.3] | 90.3 [87.8–92.8] | |
| Ebke et al. 45 | Neurorehabilitation physicians | 50.0 [32–67] | 86.1 [81–91] | 37.8 [27–50] | 91.1 [88–94] |
| Palliative care physicians | 67.7 [50–83] | 70.3 [64–77] | 27.7 [22–34] | 92.8 [89–96] | |
| At least one clinician | 76.5 | 64.9 | 26.8 | 94.2 | |
| Mudge et al. 50 | Doctors | 81 | 70 | 38 | 94 |
| Senior nurses | 80 | 68 | 36 | 93 | |
| Either discipline | 90 | 56 | 31 | 96 | |
| Straw et al. 60 | Cardiologists | 85 | 59 | 52 | 88 |
| Trainee-grade doctor | 75 | 62 | 51 | 83 | |
| Heart failure nurse | 90 | 44 | 45 | 90 | |
| Non-specialist nurse | 66 | 73 | 58 | 79 | |
| Lakin et al. 57 | Primary care physician | 79.4 | 68.6 | 31.6 | 94.8 |
| Nurse care coordinators | 52.6 | 80.6 | 31.8 | 90.8 | |
| Either healthcare professional says ‘no’ | 82.6 | 62.7 | 28.1 | 95.3 | |
| Both healthcare professionals say ‘no’ | 50.3 | 86.7 | 40 | 90.8 | |
| Valerio and Farinha 75 | Nephrologists | 68.2 | 77.3 | 27.8 | 95 |
| Nurses | 81.8 | 64.5 | 22.8 | 96.5 | |
| Rauh et al. 71 | Medical doctor | 75 | 69 | 43 | 90 |
| Nurses | 71 | 61 | 42 | 84 | |
| Advanced practice providers | 83 | 67 | 44 | 93 | |
| Combined | 75 | 66 | 43 | 89 |
PPV: positive predictive value; NPV: negative predictive value.
Band 5 nurses are less senior nurses. Band 6 nurses are of intermediate seniority and band 7/8 are senior nurses.
CI’s are only provided when presented in the original study.
Discussion
Main findings
This meta-analysis evaluated the accuracy of the surprise question in predicting death, differentiating by timeframe, patient subgroup and by type of healthcare professional answering the surprise question. In total, 59 studies encompassing 63 cohorts were identified including 88.268 surprise question assessments. The pooled sensitivity was 71.4% (95% CI [66.3–76.4]) and the pooled specificity 74.0% (95% CI [69.3–78.6]). The c-statistic value was 0.79 (95% CI [0.77–0.81]) in the overall analysis. Analysis of timeframe subgroups showed similar sensitivity for 6- and 12-month timeframe (74.5% (95% CI [67.6–81.4]) and 73.4% (95% CI [68.2–78.6]) respectively) and lower specificity for 6-month timeframe compared to a 12-month timeframe (64.3% (95% CI [56.8–71.8]) and 72.9% (95% CI [67.6–78.1]) respectively). Pooled estimates showed variation between patient groups. A sensitivity of 83.8% (95% CI [75.6–92.0]) was observed for patients with cancer and 82.5% (95% CI [60.1–100]) for patients with pulmonary disease, whereas the sensitivity for the emergency department was 49.1 (95% CI [35.7–62.5]). Specificity showed less variation with values between 67.3% (95% CI [53.2 and 81.3]) for cancer patients and 80.0% (95% CI [60.0–99.9]) for primary care patients. The estimated NPV varied from 98.0% (95% CI [97.7–98.3]) to 88.6% (95% CI [87.1–90.0]) with a mortality rate of 5% and 25% respectively. The estimated PPV varied from 12.6% (95% CI [11.0 to 14.2]) with a mortality rate of 5% to 47.8% (95% CI [44.2–51.3]) with a mortality rate of 25%. The NPV remains high with increasing mortality rate in all subgroups. Seven studies provided detailed information on different healthcare professionals answering the surprise question. Based on these studies we did not find clear evidence for a difference between the accuracy of healthcare professionals answering the surprise question.
Strengths and limitations
This study has a number of strengths. First of all, each part of the review process was independently undertaken by two reviewers. Furthermore, a high number of studies have been included. This can be explained by (1) the increased attention for palliative care and the surprise question, resulting in a high amount of recently published studies (2) the effort made to obtain additional data by contacting authors and (3) including non-peer reviewed studies: 16 of the 59 included studies were non-peer reviewed studies, mostly conference abstracts. We also included the non-peer reviewed studies in an effort to avoid publication bias of favourable outcomes. 104 A limitation of including non-peer reviewed studies is that they did not provide sufficient information for a comprehensive quality assessment, which could have led to a relatively negative quality assessment. Furthermore, we observed a high degree of heterogeneity, with an overall I2 of 98.2% and 98.4% for sensitivity and specificity respectively. The analysis with subgroups (i.e. timeframe, patient subgroups and type of publication) still showed a high degree of heterogeneity. This can be explained by the enormous diversity in included studies, reflecting the different real-life circumstances in which the surprise question is used, and its versatile nature. Furthermore, the accuracy of the surprise question may be overestimated due to a possible self-fulling prophecy: a positive answer to the surprise question (‘No, I would not be surprised’) could lead to, consciously or subconsciously, discussing goals of care, thereby potentially influencing outcome. Finally, c-statistics were estimated with an easy to apply formula, which may result in a slight over-estimation. 13
Comparison to other literature
As described earlier, two meta-analyses were performed on the accuracy of the surprise question by Downar et al. 3 and White et al. 4 Despite this, the subjectiveness and accuracy of using the surprise question are still debated.105,106 The previous meta-analyses included 17 and 22 cohorts, with 11.621 and 25.718 surprise question assessments respectively, compared to 63 cohorts and 88.268 SQ assessments in this study. Moreover, Downar et al. did not include ‘Gold Standards Framework’ in the search, therefore missing studies that did not mention the surprise question in title or abstract. Furthermore, both meta-analyses report a substantial risk of bias in their included studies. Indeed, in our assessment, most pre-2017 studies have an increased risk of bias whereas more recent studies seem to be of better methodological quality. Hence, our results may be more reliable due to the increase of surprise question assessments included and improved methodological quality of included studies.
This study shows similar results in overall accuracy in predicting death compared to the previous meta-analyses. Downar et al. reported a sensitivity of 67.0% and a specificity of 80.2% compared to 71.4% and 74.0% respectively in our study. The c-statistic (area under the curve) of Downar et al. 3 was 0.81 [0.78–0.84] compared to 0.79 [0.77–0.81] in our meta-analysis. De Bock et al. 107 studied the accuracy of the Supportive and Palliative Care Indicators Tool (SPICT) in a geriatric population and report a higher sensitivity of 84.1% and a lower specificity of 57.9% compared to our results of the surprise question.
White et al. stated that an increase in timeframe did not impact the diagnostic accuracy. Our study showed similar sensitivity for 6- and 12-month timeframe. However we found a lower specificity for 6-month timeframe compared to a 12-month timeframe. Our study confirms the previous conclusions that the surprise question performs better in cancer patients compared to other subgroups. We did not find clear evidence for a difference between the accuracy of healthcare professionals answering the surprise question, in contrast to an earlier suggestion by White et al. 4 that doctors seem to be more accurate than nurses in recognising people in the last year of life.
Implications for practice
A systematic review by Cardona-Morrell et al. 108 indicated that on average 33%–38% of patients nearing their end of life receive non-beneficial treatments in the last 6 months of their life. Advance care planning can have a positive effect on end of life care, decrease life-sustaining treatment, increase use of hospice and palliative care, prevent hospital admissions and improve goal-concordant care. 109 Timely identification of patients who could potentially benefit from advance care planning is important. 110 The importance of advance care planning increases when nearing the end of life. Hence, prognostication of mortality can be used as a proxy for initiating advance care planning. The surprise question is an easy to use tool 2 and does not require large amounts of clinical data compared to other available screening tools. 111 These characteristics and the reasonable accuracy in predicting death with fairly high NPV with various mortality rates make the surprise question an appropriate screening tool for initiating advance care planning. Additionally, patients with a positive answer to the surprise question (‘No, I would not be surprised’) are likely to be vulnerable and may therefore benefit from advance care planning regardless of whether they die exactly within the specified timeframe. Furthermore, initiating advance care planning ‘too early’ does not seem to cause damage. 109 The results of this systematic review and meta-analysis encourage the use of the surprise question as screening tool by various healthcare professionals, not exclusively by doctors. We think the surprise question should not solely be seen as an indicator of prognostication of death but rather as an opportunity for renewed attention for quality of care and shared decision making by timely initiating advance care planning.
Conclusion
We found overall reasonable test characteristics for the surprise question. Additionally, this study showed notable differences in performance within patient subgroups. However, we did not find an indication of notable differences between timeframe and healthcare professionals. We submit that the surprise question is an appropriate tool for initiating advance care planning.
Acknowledgments
We would like to thank all of the corresponding authors of the studies listed in this meta-analysis for providing us with additional data and/or information.
Appendix
Appendix 1.
Search strategy.
| Source | Date of search | Search strategy |
|---|---|---|
| PubMed | 22-01-2021 | ((surprise question*[Title/Abstract]) OR “gold standards framework”[Title/Abstract]) OR NECPAL[Title/Abstract] |
| Embase | 22-01-2021 | ‘surprise question*’:ti,ab,kw OR ‘gold standards framework’:ti,ab,kw OR necpal:ti,ab,kw |
| Cochrane | 22-01-2021 | (‘Gold Standards Framework’):ti,ab,kw OR (‘surprise question’):ti,ab,kw OR (NECPAL):ti,ab,kw |
| Scopus | 22-01-2021 | TITLE-ABS-KEY (‘surprise question*’) OR TITLE-ABS-KEY ( ‘gold standards framework’ ) OR TITLE-ABS-KEY |
| Web of Science | 22-01-2021 | TOPIC:(‘surprise question*’) OR TOPIC:(‘gold standards framework’) OR TOPIC: (NECPAL) |
| CINAHL | 22-01-2021 | TI ‘surprise question*’ OR AB ‘surprise question*’ OR MH ‘surprise question*’ OR TI ‘gold standards framework’ OR AB ‘gold standards framework*’ OR MH ‘gold standards framework*’ OR TI ‘necpal*’ OR AB ‘necpal*’ OR MH ‘necpal*’ |
No filters/limits were applied in the searches.
Appendix 2.
Characteristics of included studies.
| Author | Country | Population | Healthcare professional answering the surprise question | Setting | Total subjects (n) | Total surprise question assessments (n) | Surprise question timeframe | Subject age mean (SD) | Gender M:F |
|---|---|---|---|---|---|---|---|---|---|
| Barnes et al. 18 | United Kingdom | Heart failure, 60 years or older | General practitioners | General practice | 542 | 231 | 12 months | 77 (71–82)** | 121:110**** |
| Moss et al. 21 | United States | Haemodialysis | Nurse practitioners | 3 haemodialysis units | 147 | 147 | 12 months | 66.4 (14.8) | 81:66 |
| Cohen et al. 22 | United States | Haemodialysis | Nephrologists | Haemodialysis unit | 512 (d) | 447 (d) | 6 months | 60 (17) (d) | 254:195 (d) |
| 514 (v) | 427 (v) | 63 (16) (v) | 245:182 (v) | ||||||
| Moss et al. 23 | United States | Breast, lung or colon cancer | Oncologists | Outpatient clinic | 853 | 826 | 12 months | 60 (13) | 126:727 |
| South et al. 24 (a) | United Kingdom | Acute exacerbation COPD | NR | COPD unit | 199 | 199 | 6–12 months | 70 (NR) | 92:107 |
| Fenning et al. 25 | United Kingdom | Acute coronary artery disease | Medical staff | Cardiology ward | 172 | 172 | 6–12 months | 66 (14) | 105:67 |
| Da Silva Gane et al. 26 | United Kingdom | Haemodialysis | Nephrologists and nurses | 3 haemodialysis units | 344 | 344 | 12 months | 71.9 (11.0) (died) 62.2 (15.8) (alive) | 221:123 |
| Pang et al. 27 | Hong Kong | Peritoneal dialysis patients | Nephrologists | Dialysis centre | 367 | 367 | 12 months | 60.2 (12.3) | 205:162 |
| Reilly et al. 28 (a) | United Kingdom | Non-cancer respiratory patients | Pulmonologists | Ward of general hospital | 85 | 85 | 12 months | 68 (NR) | 36:49 |
| Moroni et al. 29 | Italy | Stage IV cancer | General practitioners | General practice | 231 | 231 | 12 months | 70.2 (SE 0.9) | 117:114 |
| Feyi et al. 30 | United Kingdom | End-stage renal disease | Multidisciplinary | 1 dialysis unit | 178 | 178 | 12 months | NR | NR |
| Hamano et al. 31 | Japan | Advanced cancer | Palliative care physicians | 58 palliative care services | 2361 | 2361 | 7 and 30 days | 69.1 (12.8) | 1358:1003 |
| Amro et al. 32 | United States | Haemodialysis | Nephrologists | Dialysis centre | 201 | 201 | 12 months | 71 (65–77)** | 105:96 |
| Maria Carmen et al. 33 (a) | Spain | Haemodialysis | Medical staff | Haemodialysis unit | 49 | 49 | 12 months | NR | NR |
| Gerlach et al. 34 (a) | Germany | Cancer (haemato-oncology) | Physicians | Hemato-oncology outpatient clinic | 828 | 672 | 12 months | NR | NR |
| Stem cell transplantation unit | |||||||||
| Lakin et al. 35 (a) | United States | High risk patients | General practitioners | Academic primary care centre | 1737 | 1737 | 12 months | 65 (NR) | 696:1041 |
| Strout et al. 36 (a) | United States | Emergency department patients with sepsis | Emergency physicians | Emergency department | 330 | 330 | 30 days | 71 (0–97)*** | 182:148 |
| Gómez-Batiste et al. 37 | Spain | Advanced chronic disease | Healthcare professional | Three primary care centres, one general hospital, one intermediate care centre, four nursing homes | 1064 | 1059 | 12 months | 81.3 (11.8) | 378:686 |
| Hadique et al. 38 | United States | Medical ICU patients | ICU physicians | Medical ICU | 500 (d) | 500 (d) | 6 months | 61.1 (17.7) (d) | 257:243 |
| 549 (v) | 543 (v) | 61.1 (16.2) (v) | 296:253 | ||||||
| Lilley et al. 39 | United States | Acute surgical conditions, 65 years or older | Surgical residents and attendings | Emergency general surgery service | 119 | 163* | 12 months | 79.3 (7.9) (SQ+) | 78:85 |
| 73.5 (7.0) (SQ−) | |||||||||
| Moretti et al. 40 | United Kingdom; Italy | Acute coronary artery disease | Medical and nursing staff | Cardiology ward | 629 | 470 | 6–12 months | 67.5 (12.7) | 432:197 |
| Salat et al. 41 | United States | Advanced chronic kidney disease (no dialysis) >60 years | Nephrologists | Outpatient clinic | 488 | 488 | 12 months | 71 (65–77)* | 239:249 |
| Santos Lascasas et al. 42 (a) | Portugal | Dialysis patients, 65 years or older | NR | Dialysis unit | 360 | 360 | 6 months | 76 (NR) | 169:191 |
| Strout et al. 43 (a) | United States | Admitted adult emergency department patients | Emergency physician and inpatient physician | Emergency department | 10,334 | 9923 | 30 days | 67 (18–103)*** | 50.3% female |
| Hospital wards | |||||||||
| Burke et al. 44 | United Kingdom | Children (0–21 years) with life-threatening disease | Multidisciplinary team | Children’s hospice | 327 | 325 (3 m) 306 (12 m) | 3 months and 12 months | 7.7 (5.3) | 185:142 |
| Ebke et al. 45 | Germany | Neurorehabilitation | Neurologists and palliative care physicians | Neurorehabilitation centre | 279 | 236 | 12 months | 63 (14) | 161:118 |
| Faria de Sousa et al. 46 (a) | Portugal | Advanced stages of: cancer, heart failure, kidney disease, COPD | General practitioners | General practice | 209 | 201 | 6 months | 72.6 (12.6) | 113:96 |
| Forzley et al. 47 | Canada | Haemodialysis | Nephrologists | Haemodialysis unit | 374 | 374 | 6 months | 68 (15) | 221:153 |
| Liyanage et al. 48 | Australia | Nursing home residents | Director of Nursings | Nursing home | 187 | 187 | 12 months | 82.4 (9.09) | 83:104 |
| Mitchell et al. 49 | Australia | Patients, 70 years or older | General practitioners | 19 general practices | 4375 | 1525 | 12 months | 79.1 (6.9)** | 1013:512**** |
| Mudge et al. 50 | Australia | Hospital inpatients | Senior nurses and medical staff | Teaching hospital | 524 | 513 | 12 months | 60.2 (18.9) | 276:237**** |
| Ouchi et al. 51 | United States | Emergency department patients, 65 years or older | Emergency physicians | Emergency department | 207 | 207 | 12 months | 75 (7.5) | NR |
| Schmidt et al. 52 | United States | Advanced chronic kidney disease (no dialysis) | Clinicians and nurse practitioners | Outpatient clinics | 749 (d) | 749 (d) 437 (v) | 12 months | 69.3 (14.6) (d) 70.0 (13.3) (v) | 381:368 (d)166:271 (v) |
| 437 (v) | |||||||||
| Tanasiychuk et al. 53 | Israel | Acute Kidney Injury with unscheduled dialysis | Nephrologists | Hospital | 475 | 475 | 6 months | 72.8 (12.2) | 238:192 |
| Aaronson et al. 54 | United States | Heart failure | Emergency physicians | Emergency department | 193 | 193 | 12 months | 74.5 (12.6) | NR |
| Gastelurrutia et al. 55 (a) | Spain | Heart failure | Physicians and nurses | 3 outpatient heart failure clinics | 922 | 922 | 12 months | 69.3 (12.2) | 648:274 |
| Haydar et al. 56 | United States | Emergency department patients, 18 years or older | Emergency physicians | Emergency department | 6122 | 6089 | 30 days | 66 (51–79)** | 3168:2954 |
| Lakin et al. 57 | United States | Chronically ill primary care patients | Primary care physician and nurse care coordinators | Primary care | 1163 | 1163* | 24 months | 70.1 (14.8) | 500:663 |
| Ouchi et al. 58 | United States | Admitted Emergency department patients, 65 years or older | Attending physicians | Emergency department | 10,737 | 16,223* | 30 days | 75.9 (8.8) | 5205:5532 |
| Raubenheimer et al. 59 | South Africa | Admitted to acute medical services | Two trained clinicians | Acute medical services | 822 | 822 | 12 months | 52 (37–67)** | 378:444 |
| Straw et al. 60 | United Kingdom | Decompensated heart failure | Cardiologists. Trainee-grade doctors and heart failure nurses | Cardiology ward | 129 | 114 | 12 months | 71 (14) | 73:41 |
| Tripodoro et al. 61 | Argentina | Cancer | Treating physicians | All patients in 1 centre | 317 | 313 | 12 months | 77 (21–99)*** | 106:211 |
| Veldhoven et al. 62 | The Netherlands | Primary care patients, 75 years or older | Two general practitioners | General practice | 292 | 292 | 12 months | 84 (5.46) | 117:175 |
| Verhoef et al. 63 | The Netherlands | Advanced cancer at emergency department | Attending physicians | Emergency department | 245 | 245 | 12 months | 62 (45–79)** | 118:127 |
| Edge et al. 64 (a) | United States | Metastatic cancer | Oncologists | Outpatient clinic | 1276 (6 m) 655 (12 m) | 1276 (6 m) 655 (12 m) | 6 and 12 months | NR | NR |
| Ernecoff et al. 65 (a) | United States | Advance chronic kidney patients, 60 years or older | Physicians | Outpatient nephrology clinic | 510 | 95 | 12 months | NR | NR |
| Flierman et al. 66 | The Netherlands | Hospital inpatients, 70 years or older | Nurses | Ward of general hospital | 252 | 252 | 12 months | 81.2 (6.56) | 122:130 |
| Ikari et al. 67 | Japan | Metastatic cancer | Physicians | Palliative care unit | 1411 | 1411 | 3 days | 72.6 (12.2) | 716:659 |
| Estifan Kasabji et al. 68 (a) | Spain | Haemodialysis | Nephrologists and nurses | Haemodialysis unit | 180 | 178 | 6–12 months | 69 (14.1) | 124:56 |
| Lai et al. 69 | Taiwan | Peritoneal dialysis patients | Nurses | Haemodialysis unit | 401 | 401 | 12 months | 56.2 (14) | 214:213 |
| Maes et al. 70 | Belgium | Hospital inpatients, 75 years or older: first cohort acute geriatric ward; second cohort cardiac patients | Physicians | Geriatric ward and cardiology ward | 458 | 368 | 6–12 months | NR | 184:195 |
| Rauh et al. 71 | United States | Gynaecological oncology | Attending physicians, ‘physician assistants, nurses’ | Two tertiary centres | 358 | 341 | 12 months | NR | 0:358 |
| Tabernero Huguet et al. 72 (a) | Spain | Patients on respiratory ward | NR | Respiratory ward of hospital | 363 | 361 | 12 months | NR | NR |
| Tak et al. 73 (a) | The Netherlands | Idiopathic pulmonary fibrosis | Health care providers | Outpatient clinic | 123 | 123 | 12 months | 74 (63) | 108:15 |
| Valerio and Farinha 75 (a) | Portugal | Haemodialysis | Nephrologists and nurses | Haemodialysis unit | 194 | 194 | 12 months | 69.9 | NR |
| van Wijmen et al. 76 | The Netherlands | General population | General practitioners | General practice | 3640 | 3640 | 12 months | NR | NR |
| Yen et al. 77 | Taiwan | Hospital inpatients | Nurses | Hospital | 23,444 | 21,098 | 6–12 months | 62.8 | 11,230:9879 |
| Ermers et al. 78 | The Netherlands | Cancer | Oncologists | Outpatient clinic | 382 | 379 | 12 months | 59.4 | 211:168 |
| Tripp et al. 74 | United States | COPD patients | Physicians and advanced practice providers | Emergency department (30 days) and general ward of hospital (12 months) | 428 | 381 (30 days) 365 (12 months) | 30 days and 12 months | NR | 210:218 |
(a): abstract or other non-peer reviewed publication; NR: not reported; (d): derivation cohort; (v): validation cohort; SQ+: surprise question answered with ‘no’; SQ−: surprise question answered with ‘yes’.
Multiple assessments per patient possible. **Median (IQR). ***Median (range). ****SQ group only.
Appendix 3.
Critical appraisal.
| Article | 1. Study participation | 2. Study attrition | 3. Prognostic factor measurement | 4. Outcome measurement | 5. Study confounding | 6. Statistical analysis and reporting | Total |
|---|---|---|---|---|---|---|---|
| Barnes et al. 18 | High | Low | Low | Low | Low | Low | Low |
| Moss et al. 21 | Low | Low | Low | Low | Moderate | Low | Low |
| Cohen et al. 22 | Moderate | Low | Low | Low | Moderate | Low | Low |
| Moss et al. 23 | Low | Low | Low | Low | Low | Low | Low |
| South et al. 24 (a) | High | Low | Moderate | High | Moderate | Low | High |
| Fenning et al. 25 | Moderate | Low | Low | Low | Low | Low | Low |
| Da Silva Gane et al. 26 | Low | Low | Low | Low | Moderate | Low | Low |
| Pang et al. 27 | Low | Low | Low | Low | Low | Low | Low |
| Reilly et al. 28 (a) | High | Low | Moderate | Moderate | Moderate | Low | Moderate |
| Moroni et al. 29 | Moderate | Low | Low | Low | Low | Low | Low |
| Feyi et al. 30 | High | Low | High | High | High | Low | High |
| Hamano et al. 31 | Moderate | Moderate | Low | Low | Low | Low | Low |
| Amro et al. 32 | Low | Low | Low | Low | High | Low | Moderate |
| Maria Carmen et al. 33 (a) | Moderate | Low | High | Low | Moderate | Low | Moderate |
| Gerlach et al. 34 (a) | Moderate | Low | Moderate | Moderate | Low | Low | Moderate |
| Lakin et al. 35 (a) | High | Low | Moderate | Low | Low | Low | Low |
| Strout et al. 36 (a) | Moderate | Low | Low | Moderate | Low | Low | Moderate |
| Gómez-Batiste et al. 37 | Low | Low | Low | Low | Low | Low | Low |
| Hadique et al. 38 | Low | Low | Low | Low | Low | Low | Low |
| Lilley et al. 39 | Moderate | Low | Low | Low | Low | Low | Low |
| Moretti et al. 40 | Moderate | Low | Low | Moderate | Low | Low | Low |
| Salat et al. 41 | Moderate | Low | Low | Low | Low | Low | Low |
| Santos Lascasas et al. 42 (a) | Moderate | Low | Low | Low | Moderate | Low | Low |
| Strout et al. 43 (a) | Moderate | High | Low | Moderate | Low | Low | High |
| Burke et al. 44 | Moderate | Low | Low | Low | Low | Low | Low |
| Ebke et al. 45 | Low | High | Low | Low | Low | Low | Moderate |
| Faria de Sousa et al. 46 (a) | Moderate | Low | Low | Low | Low | Low | Low |
| Forzley et al. 47 | Low | Low | Low | Low | Moderate | Low | Low |
| Liyanage et al. 48 | Low | Low | Low | Low | Low | Low | Low |
| Mitchell et al. 49 | Moderate | High | Moderate | Low | Low | Low | Moderate |
| Mudge et al. 50 | Low | Low | Low | Low | Low | Low | Low |
| Ouchi et al. 51 | Moderate | Low | Low | Low | Low | Low | Low |
| Schmidt et al. 52 | Moderate | Low | Low | Low | Moderate | Low | Low |
| Tanasiychuk et al. 53 | Moderate | Low | Low | Low | Moderate | Low | Moderate |
| Aaronson et al. 54 | Moderate | Low | Low | Low | Low | Low | Low |
| Gastelurrutia et al. 55 (a) | Low | Low | Low | Low | Low | Low | Low |
| Haydar et al. 56 | Low | Low | Low | Low | Low | Low | Low |
| Lakin et al. 57 | Moderate | Low | Low | Low | Low | Low | Low |
| Ouchi et al. 58 | Low | Low | Low | Low | Low | Low | Low |
| Raubenheimer et al. 59 | Moderate | Low | Low | Low | Low | Low | Low |
| Straw et al. 60 | Moderate | Low | Low | Low | Low | Low | Low |
| Tripodoro et al. 61 | Low | Low | Low | Low | Low | Low | Low |
| Veldhoven et al. 62 | Low | Low | Low | Low | Low | Low | Low |
| Verhoef et al. 63 | Moderate | Low | Low | Low | Low | Low | Low |
| Edge et al. 64 (a) | Moderate | Low | Low | Low | Low | Low | Low |
| Ernecoff et al. 65 (a) | High | Low | Low | Low | Low | Low | Moderate |
| Flierman et al. 66 | Moderate | Low | Low | Low | Low | Low | Low |
| Ikari et al. 67 | Moderate | Low | Low | Low | Low | Low | Low |
| Estifan Kasabji et al. 68 (a) | High | Low | Moderate | Low | Moderate | Low | Moderate |
| Lai et al. 69 | Low | Low | Low | Low | Moderate | Low | Low |
| Maes et al. 70 | High | Moderate | Low | Moderate | Low | Low | Moderate |
| Rauh et al. 71 | Moderate | Low | Low | Low | Low | Low | Low |
| Tabernero Huguet et al. 72 (a) | Moderate | Low | Low | Low | Moderate | Low | Moderate |
| Tak et al. 73 (a) | Moderate | Low | Low | Low | Low | Low | Low |
| Valerio and Farinha 75 (a) | Moderate | Low | Low | Low | Moderate | Low | Moderate |
| Van Wijmen etal. 76 | Low | Low | Low | Low | Low | Low | Low |
| Yen et al. 77 | Low | Low | Low | Low | Moderate | Low | Low |
| Ermers et al. 78 | Low | Low | Low | Low | Moderate | Low | Low |
| Tripp et al. 74 | Low | Low | Low | Low | Moderate | Low | Low |
Note: (a): abstract or other non-peer reviewed publication.
Appendix 4.
Individual study results.
| Study (authors) | Patient group | Timeframe (months) | Abstract (Yes/No) | Total SQ responses (n) | Mortality rate (%) | Sensitivity (95% CI)* | Specificity (95% CI)* |
|---|---|---|---|---|---|---|---|
| Barnes et al. 18 | Cardiac disease | 12 | No | 231 | 6.1 | 78.6 [52.4–92.4] | 61.3 [54.7–67.5] |
| Moss et al. 21 | Kidney disease | 12 | No | 147 | 15.0 | 45.5 [26.9–65.3] | 80.8 [73.0–86.7] |
| Cohen et al. 22 (derivation cohort) | Kidney disease | 6 | No | 447 | 6.0 | 63.0 [44.2–78.5] | 87.4 [83.9–90.2] |
| Cohen et al. 22 (validation cohort) | Kidney disease | 6 | No | 427 | 8.4 | 47.2 [32.0–63.0] | 89.8 [86.4–92.4] |
| Moss et al. 23 | Cancer | 12 | No | 826 | 8.6 | 76.1 [65.0–84.5] | 89.8 [87.4–91.8] |
| South et al. 24 | Pulmonary disease | 12 | Yes | 199 | 7.5 | 93.3 [70.2–98.8] | 55.4 [48.2–62.4] |
| Fenning et al. 25 | Cardiac disease | 12 | No | 172 | 9.9 | 35.3 [17.3–58.7] | 79.4 [72.3–85.0] |
| Da Silva Gane et al. 26 | Kidney disease | 12 | No | 344 | 15.1 | 57.7 [44.2–70.1] | 74.7 [69.4–79.3] |
| Pang et al. 27 | Kidney disease | 12 | No | 367 | 12.0 | 61.4 [46.6–74.3] | 74.6 [69.6–79.1] |
| Reilly et al. 28 | Pulmonary disease | 12 | Yes | 85 | 32.9 | 100 [87.9–100] | 31.6 [21.0–44.5] |
| Moroni et al. 29 | Cancer | 12 | No | 231 | 45.0 | 83.7 [75.4–89.5] | 69.3 [60.8–76.6] |
| Feyi et al. 30 | Kidney disease | 12 | No | 178 | 23.6 | 66.7 [51.6–79.0] | 77.9 [70.3–84.1] |
| Hamano et al. 31 – 7 day timeframe | Cancer | 0.25 | No | 2361 | 14.1 | 84.7 [80.4–88.2] | 68.0 [65.9–70.0] |
| Hamano et al. 31 – 1 month timeframe | Cancer | 1 | No | 2361 | 47.2 | 95.6 [94.2–96.7] | 37.0 [34.4–39.7] |
| Amro et al. 32 | Kidney disease | 12 | No | 201 | 19.4 | 56.4 [41–70.7] | 82.7 [76.2–87.8] |
| Maria Carmen et al. 33 | Kidney disease | 12 | Yes | 49 | 18.4 | 77.8 [45.3–93.7] | 67.5 [52.0–79.9] |
| Gerlach et al. 34 | Cancer | 12 | Yes | 672 | 15.6 | 40.0 [31.1–49.6] | 93.1 [90.7–94.9] |
| Lakin et al. 35 | Primary care | 12 | Yes | 1737 | 6.4 | 20.5 [14.1–28.9] | 94.4 [93.2–95.4] |
| Strout et al. 36 | Emergency department | 1 | Yes | 330 | 9.4 | 48.4 [32.0–65.2] | 68.9 [63.4–73.9] |
| Gomez-Batiste, 2017 37 | Primary care | 12 | No | 1059 | 27.0 | 93.7 [90.3–96.0] | 26.4 [23.4–29.6] |
| Hadique et al. 38 (derivation cohort) | Intensive care | 6 | No | 500 | 36.0 | 82.2 [76.0–87.1] | 71.9 [66.7–76.5] |
| Hadique et al. 38 (validation cohort) | Intensive care | 6 | No | 543 | 34.6 | 73.9 [67.2–79.7] | 81.7 [77.3–85.4] |
| Lilley et al. 39 | Acute surgical conditions | 12 | No | 163 | 36.2 | 79.7 [67.7–88.0] | 51.0 [41.5–60.4] |
| Moretti et al. 40 | Cardiac disease | 12 | No | 470 | 7.9 | 56.8 [40.9–71.3] | 93.5 [90.8–95.5] |
| Salat et al. 41 | Kidney disease | 12 | No | 488 | 17.8 | 64.4 [53.9–73.6] | 71.3 [66.7–75.5] |
| Santos Lascasas et al. 42 | Kidney disease | 6 | Yes | 360 | 13.9 | 90.0 [78.6–95.7] | 67.1 [61.7–72.1] |
| Strout et al. 43 | Emergency department | 1 | Yes | 9923 | 2.3 | 48.9 [42.4–55.4] | 80.3 [79.5–81.1] |
| Burke et al. 44 – 3 month timeframe | Children | 3 | No | 325 | 5.5 | 83.3 [60.8–94.2] | 93.2 [89.8–95.5] |
| Burke et al. 44 – 12 month timeframe | Children | 12 | No | 306 | 9.8 | 83.3 [66.4–92.7] | 70.7 [65.0–75.7] |
| Ebke et al. 45 | Neurorehabilitation | 12 | No | 236 | 14.4 | 76.5 [60.0–87.6] | 64.9 [58.0–71.1] |
| Faria de Sousa et al. 46 | Primary care | 6 | Yes | 201 | 18.4 | 62.2 [46.1–75.9] | 67.1 [59.6–73.8] |
| Forzley et al. 47 | Kidney disease | 6 | No | 374 | 11.5 | 72.1 [57.3–83.3] | 58.6 [53.2–63.8] |
| Liyanage et al. 48 | Nursing home | 12 | No | 187 | 22.5 | 71.4 [56.4–82.8] | 65.5 [57.5–72.8] |
| Mitchell et al. 49 | Primary care | 12 | No | 1525 | 3.1 | 53.2 [39.2–66.7] | 89.6 [87.9–91.0] |
| Mudge et al. 50 | Hospital inpatients | 12 | No | 513 | 17.9 | 90.2 [82.4–94.8] | 55.8 [51.0–60.5] |
| Ouchi et al. 51 | Emergency department | 12 | No | 207 | 21.3 | 77.3 [63.0–87.2] | 58.3 [50.6–65.6] |
| Schmidt et al. 52 (derivation cohort) | Kidney disease | 12 | No | 749 | 13.5 | 60.4 [50.6–69.4] | 82.7 [79.6–85.4] |
| Schmidt et al. 52 (validation cohort) | Kidney disease | 12 | No | 437 | 10.1 | 61.4 [46.6–74.3] | 76.8 [72.4–80.7] |
| Tanasiychuk et al. 53 | Kidney disease | 6 | No | 475 | 52.8 | 41.8 [35.9–48.0] | 35.3 [29.3–41.7] |
| Aaronson et al. 54 | Cardiac disease & emergency department | 12 | No | 193 | 29.0 | 78.6 [66.2–87.3] | 56.9 [48.6–64.9] |
| Gastelurrutia et al. 55 | Cardiac disease | 12 | Yes | 922 | 9.7 | 78.7 [69.0–85.9] | 69.4 [66.2–72.4] |
| Haydar et al. 56 | Emergency department | 1 | No | 6089 | 2.6 | 31.8 [25.1–39.5] | 85.4 [84.4–86.2] |
| Lakin et al. 57 | Primary care | 24 | No | 1163 | 15.5 | 79.4 [73.0–84.7] | 68.6 [65.6–71.4] |
| Ouchi et al. 58 | Emergency department | 1 | No | 16,223 | 9.7 | 43.3 [40.9–45.8] | 82.0 [81.3–82.6] |
| Raubenheimer et al. 59 | Acute medical services | 12 | No | 822 | 20.0 | 70.7 [63.4–77.2] | 65.0 [61.3–68.6] |
| Straw et al. 60 | Cardiac disease | 12 | No | 114 | 34.2 | 84.6 [70.3–92.8] | 58.7 [47.4–69.1] |
| Tripodoro et al. 61 | Cancer | 12 | No | 313 | 46.6 | 93.8 [88.7–96.7] | 72.5 [65.2–78.7] |
| Veldhoven et al. 62 | Primary care | 12 | No | 292 | 8.9 | 92.3 [75.9–97.9] | 48.5 [42.6–54.5] |
| Verhoef et al. 63 | Cancer and ED | 12 | No | 245 | 78.8 | 89.1 [83.9–92.8] | 40.4 [28.2–53.9] |
| Edge et al. 64 – 6 month timeframe | Cancer | 6 | Yes | 1276 | 25.4 | 71.0 [65.8–75.7] | 53.6 [50.4–56.7] |
| Edge et al. 64 – 12 month timeframe | Cancer | 12 | Yes | 655 | 42.4 | 68.7 [63.0–73.9] | 63.7 [58.7–68.4] |
| Ernecoff et al. 65 | Kidney disease | 12 | Yes | 95 | 9.5 | 66.7 [35.4–87.9] | 75.6 [65.5–83.4] |
| Flierman et al. 66 | Hospital inpatients | 12 | No | 252 | 30.6 | 81.8 [71.8–88.8] | 48.6 [41.3–55.9] |
| Ikari et al. 67 | Cancer | 0.1 | No | 1411 | 47.8 | 94.4 [92.4–95.9] | 26.3 [23.3–29.6] |
| Estifan Kasabji et al. 68 | Kidney disease | 12 | Yes | 178 | 15.7 | 71.4 [52.9–84.7] | 64.7 [56.7–71.9] |
| Lai et al. 69 | Kidney disease | 12 | No | 401 | 8.5 | 52.9 [36.7–68.5] | 95.6 [93.0–97.3] |
| Maes et al. 70 – subgroup 1 | Hospital inpatients | 12 | No | 185 | 24.9 | 67.4 [53.0–79.1] | 78.4 [70.9–84.4] |
| Maes et al. 70 – subgroup 2 | Cardiac disease | 12 | No | 183 | 20.2 | 67.6 [51.5–80.4] | 76.0 [68.5–82.2] |
| Rauh et al. 71 | Cancer | 12 | No | 309 | 23.6 | 75.3 [64.4–83.8] | 68.6 [62.5–74.2] |
| Tabernero Huguet et al. 72 | Pulmonary disease | 12 | Yes | 361 | 23.5 | 65.9 [55.3–75.1] | 86.6 [82.1–90.1] |
| Tak et al. 73 | Pulmonary disease | 12 | Yes | 123 | 18.7 | 73.9 [53.5–87.5] | 84.0 [75.6–89.9] |
| Valerio and Farinha 75 | Kidney disease | 12 | Yes | 194 | 11.3 | 68.2 [47.3–83.6] | 77.3 [70.5–82.9] |
| van Wijmen et al. 76 | Primary care | 12 | No | 3640 | 1.0 | 50.0 [34.5–65.5] | 98.6 [98.2–99.0] |
| Yen et al. 77 | Hospital inpatients | 12 | No | 21,098 | 8.3 | 45.6 [43.2–47.9] | 90.6 [90.2–91.0] |
| Ermers et al. 78 | Cancer | 12 | No | 379 | 31.1 | 87.3 [80.1–92.1] | 67.4 [61.5–72.8] |
| Tripp et al. 74 – 1 month timeframe | Pulmonary disease | 1 | No | 381 | 4.2 | 12.5 [3.5–36.0] | 95.3 [92.7–97.1] |
| Tripp et al. 74 – 12 month timeframe | Pulmonary disease | 12 | No | 365 | 22.2 | 46.9 [36.4–57.7] | 75.4 [70.0–80.0] |
Confidence intervals (CI) were calculated using Wilson’s method and can differ slightly from the CI’s presented by the original studies.
Appendix 5.
Forest plot of the sensitivity of individual studies.
Appendix 6.
Forest plot of the specificity of individual studies.
Footnotes
Authorship: EvL, LI and JvD conceived and designed the study. EvL and LI collected the data, critically appraised the articles and drafted the manuscript. NZ performed the statistical analyses. All authors critically revised the manuscript and approved the final version to be published.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Eline VTJ van Lummel  https://orcid.org/0000-0003-3063-7937
https://orcid.org/0000-0003-3063-7937
References
- 1. WHO. Palliative care, https://www.who.int/health-topics/palliative-care (accessed 18 May 2021).
- 2. Lynn J, Schall MW, Milne C, et al. Quality improvements in end of life care: insights from two collaboratives. Jt Comm J Qual Improv 2000; 26: 254–267. [DOI] [PubMed] [Google Scholar]
- 3. Downar J, Goldman R, Pinto R, et al. The ‘surprise question’ for predicting death in seriously ill patients: a systematic review and meta-analysis. CMAJ 2017; 189: E484–E493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. White N, Kupeli N, Vickerstaff V, et al. How accurate is the ‘Surprise Question’ at identifying patients at the end of life? A systematic review and meta-analysis. BMC Med 2017; 15(1): 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6(7): e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Internet J Surg 2021; 88: 105906. [DOI] [PubMed] [Google Scholar]
- 7. Thomas K, Noble B. Improving the delivery of palliative care in general practice: an evaluation of the first phase of the Gold Standards Framework. Palliat Med 2007; 21: 49–53. [DOI] [PubMed] [Google Scholar]
- 8. Gómez-Batiste X, Martínez-Muñoz M, Blay C, et al. Identifying patients with chronic conditions in need of palliative care in the general population: development of the NECPAL tool and preliminary prevalence rates in Catalonia. BMJ Support Palliat Care 2013; 3(3): 300–308. [DOI] [PubMed] [Google Scholar]
- 9. Ouzzani M, Hammady H, Fedorowicz Z, et al. Rayyan-a web and mobile app for systematic reviews. Syst Rev 2016; 5: 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hayden JA, Côté P, Bombardier C. Evaluation of the quality of prognosis studies in systematic reviews. Ann Intern Med 2006; 144: 427–437. [DOI] [PubMed] [Google Scholar]
- 11. Wilson EB. Probable inference, the law of succession, and statistical inference. J Am Stat Assoc 1927; 22: 209–212. [Google Scholar]
- 12. Reitsma JB, Glas AS, Rutjes AW, et al. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol 2005; 58: 982–990. [DOI] [PubMed] [Google Scholar]
- 13. Walter SD. Properties of the summary receiver operating characteristic (SROC) curve for diagnostic test data. Stat Med 2002; 21: 1237–1256. [DOI] [PubMed] [Google Scholar]
- 14. Greene WH. Econometric analysis. 7th ed. Boston, MA: Prentice Hall, 2012. [Google Scholar]
- 15. Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002; 21: 1539–1558. [DOI] [PubMed] [Google Scholar]
- 16. SAS ® Reference. The statistical analysis was performed with SAS statistical software version 9.4 (SAS Institute, North-Carolina, USA). [Google Scholar]
- 17. Microsoft Corporation. Microsoft Excel [Internet], https://office.microsoft.com/excel (2018).
- 18. Barnes S, Gott M, Payne S, et al. Predicting mortality among a general practice-based sample of older people with heart failure. Chronic Illn 2008; 4: 5–12. [DOI] [PubMed] [Google Scholar]
- 19. Faria de Sousa P, Julião M, Rodrigues A, et al. Accuracy of the Surprise Question on Patients with Advanced Chronic Disease in the Primary Care Setting 2019. [Google Scholar]
- 20. Gibbins J, Bloor S, Reid C, et al. The use of a modified ‘surprise’ question to identify and recruit dying patients into a research project in an acute hospital setting. BMJ Support Palliat Care 2012; 2: A8. [Google Scholar]
- 21. Moss AH, Ganjoo J, Sharma S, et al. Utility of the “Surprise” question to identify dialysis patients with high mortality. Clin J Am Soc Nephrol 2008; 3: 1379–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cohen LM, Ruthazer R, Moss AH, et al. Predicting six-month mortality for patients who are on maintenance hemodialysis. Clin J Am Soc Nephrol 2010; 5: 72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Moss AH, Lunney JR, Culp S, et al. Prognostic significance of the “Surprise” question in cancer patients. J Palliat Med 2010; 13: 837–840. [DOI] [PubMed] [Google Scholar]
- 24. South G, Reddington O, Hatfield L, et al. End of life in COPD: there may be no surprises! Eur Respir J 2011; 38: 1241.22045799 [Google Scholar]
- 25. Fenning S, Woolcock R, Haga K, et al. Identifying acute coronary syndrome patients approaching end-of-life. PLoS One 2012; 7: e35536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Da Silva Gane M, Braun A, Stott D, et al. How robust is the ‘surprise question’ in predicting short-term mortality risk in haemodialysis patients. Nephron Clin Pract 2013; 123: 185–193. [DOI] [PubMed] [Google Scholar]
- 27. Pang W-F, Kwan BC, Chow K-M, et al. Predicting 12-month mortality for peritoneal dialysis patients using the ‘surprise’ question. Perit Dial Int 2013; 33: 60–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Reilly L, Reilly K, McCloskey M, et al. Prognostic significance of the ‘surprise question’ in an respiratory inpatient population in a DGH. Ir J Med Sci 2013; 182: S484. [Google Scholar]
- 29. Moroni M, Zocchi D, Bolognesi D, et al. The ‘surprise’ question in advanced cancer patients: a prospective study among general practitioners. Palliat Med 2014; 28: 959–964. [DOI] [PubMed] [Google Scholar]
- 30. Feyi K, Klinger S, Pharro G, et al. Predicting palliative care needs and mortality in end stage renal disease: use of an at-risk register. BMJ Support Palliat Care 2015; 5: 19–25. [DOI] [PubMed] [Google Scholar]
- 31. Hamano J, Morita T, Inoue S, et al. Surprise questions for survival prediction in patients with advanced cancer: a multicenter prospective cohort study. Oncologist 2015; 20: 839–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Amro OW, Ramasamy M, Strom JA, et al. Nephrologist-facilitated advance care planning for hemodialysis patients: a quality improvement project. Am J Kidney Dis 2016; 68: 103–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Maria Carmen J, Santiago P, Elena D, et al. Frailty, surprise question and mortality in a hemodilaysis cohort question and mortality in a hemodialysis cohort. Nephrol Dial Transplant 2016; 31: i553. [Google Scholar]
- 34. Gerlach C, Halbe L, Goebel S, et al. The role of the “Surprise”-Question in hematooncology: “Would I be surprised if this patient died in the next 12 months?” Quantitative and qualitative analyses of a pilot project in the care of outpatients of an academic hospital in Germany. Palliat Med 2016; 30: S12. [Google Scholar]
- 35. Lakin JR, Robinson MG, Bernacki RE, et al. Estimating 1-year mortality for high-risk primary care patients using the ‘Surprise’ question. JAMA Intern Med 2016; 176: 1863–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Strout TD, Haydar SA, Eager E, et al. Identifying unmet palliative care needs in the ED: use of the ‘surprise question’ in patients with sepsis. Acad Emerg Med 2016; 23: S196. [Google Scholar]
- 37. Gómez-Batiste X, Martínez-Muñoz M, Blay C, et al. Utility of the NECPAL CCOMS-ICO© tool and the surprise question as screening tools for early palliative care and to predict mortality in patients with advanced chronic conditions: a cohort study. Palliat Med 2017; 31: 754–763. [DOI] [PubMed] [Google Scholar]
- 38. Hadique S, Culp S, Sangani RG, et al. Derivation and validation of a prognostic model to predict 6-month mortality in an intensive care unit population. Ann Am Thorac Soc 2017; 14: 1556–1561. [DOI] [PubMed] [Google Scholar]
- 39. Lilley EJ, Gemunden SA, Kristo G, et al. Utility of the ‘Surprise’ question in predicting survival among older patients with acute surgical conditions. J Palliat Med 2017; 20: 420–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Moretti C, Iqbal J, Murray S, et al. Prospective assessment of a palliative care tool to predict one-year mortality in patients with acute coronary syndrome. Eur Heart J Acute Cardiovasc Care 2017; 6: 272–279. [DOI] [PubMed] [Google Scholar]
- 41. Salat H, Javier A, Siew ED, et al. Nephrology provider prognostic perceptions and care delivered to older adults with advanced kidney disease. Clin J Am Soc Nephrol 2017; 12: 1762–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Santos Lascasas J, Fonseca I, Malheiro J, et al. Predicting six month mortality in elderly dialysis patients: a simple prognostic score. Nephrol Dial Transplant 2017; 32: iii346. [Google Scholar]
- 43. Strout TD, Haydar SA, Vogt A. Identifying unmet palliative care needs: use and utility of the “surprise question” in emergency and inpatient settings. Acad Emerg Med 2017; 24: S149. [DOI] [PubMed] [Google Scholar]
- 44. Burke K, Coombes LH, Menezes A, et al. The ‘surprise’ question in paediatric palliative care: a prospective cohort study. Palliat Med 2018; 32: 535–542. [DOI] [PubMed] [Google Scholar]
- 45. Ebke M, Koch A, Dillen K, et al. The ‘Surprise Question’ in neurorehabilitation—prognosis estimation by neurologist and palliative care physician; a longitudinal, prospective, observational study. Front Neurol 2018; 9: 792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Faria de Sousa P, Julião M, Rodrigues AP, et al. Accuracy of the Surprise Question on Patients with Advanced Chronic Disease in the Primary Care Setting: Preliminary Results. J Palliat Med 2018; 21: 410–411. [Google Scholar]
- 47. Forzley B, Er L, Chiu HHL, et al. External validation and clinical utility of a prediction model for 6-month mortality in patients undergoing hemodialysis for end-stage kidney disease. Palliat Med 2018; 32: 395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Liyanage T, Mitchell G, Senior H. Identifying palliative care needs in residential care. Aust J Prim Health 2018; 24: 524–529. [DOI] [PubMed] [Google Scholar]
- 49. Mitchell GK, Senior HE, Rhee JJ, et al. Using intuition or a formal palliative care needs assessment screening process in general practice to predict death within 12 months: a randomised controlled trial. Palliat Med 2018; 32: 384–394. [DOI] [PubMed] [Google Scholar]
- 50. Mudge AM, Douglas C, Sansome X, et al. Risk of 12-month mortality among hospital inpatients using the surprise question and SPICT criteria: a prospective study. BMJ Support Palliat Care 2018; 8: 213–220. [DOI] [PubMed] [Google Scholar]
- 51. Ouchi K, Jambaulikar G, George NR, et al. The ‘Surprise Question’ asked of emergency physicians may predict 12-month mortality among older emergency department patients. J Palliat Med 2018; 21: 236–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Schmidt RJ, Landry DL, Cohen L, et al. Derivation and validation of a prognostic model to predict mortality in patients with advanced chronic kidney disease. Nephrol Dial Transplant 2019; 34: 1517–1525. [DOI] [PubMed] [Google Scholar]
- 53. Tanasiychuk T, Antebi A, Kushnir D, et al. Prognostic tool in dialysis treated AKI. J Am Soc Nephrol 2018; 29: 876–877. [Google Scholar]
- 54. Aaronson EL, George N, Ouchi K, et al. The surprise question can be used to identify heart failure patients in the emergency department who would benefit from palliative care. J Pain Symptom Manag 2019; 57: 944–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gastelurrutia P, Zamora E, Domingo M, et al. Palliative care needs in heart failure. A multicenter study using the NECPAL Questionnaire. Rev Esp Cardiol 2019; 72: 870–872. [DOI] [PubMed] [Google Scholar]
- 56. Haydar SA, Strout TD, Bond AG, et al. Prognostic value of a modified surprise question designed for use in the emergency department setting. Clin Exp Emerg Med 2019; 6: 70–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lakin JR, Robinson MG, Obermeyer Z, et al. Prioritizing primary care patients for a communication intervention using the ‘Surprise Question’: a prospective cohort study. J Gen Intern Med 2019; 34: 1467–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ouchi K, Strout T, Haydar S, et al. Association of emergency clinicians’ assessment of mortality risk with actual 1-month mortality among older adults admitted to the hospital. JAMA Netw Open 2019; 2: e1911139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Raubenheimer PJ, Day C, Abdullah F, et al. The utility of a shortened palliative care screening tool to predict death within 12 months - a prospective observational study in two South African hospitals with a high HIV burden. BMC Palliat Care 2019; 18: 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Straw S, Byrom R, Gierula J, et al. Predicting one-year mortality in heart failure using the ‘Surprise Question’: a prospective pilot study. Eur J Heart Fail 2019; 21: 227–234. [DOI] [PubMed] [Google Scholar]
- 61. Tripodoro VA, Llanos V, De Lellis S, et al. Prognostic factors in cancer patients with palliative needs identified by the NECPAL CCOMS-ICO© tool. Medicina 2019; 79: 95–103. [PubMed] [Google Scholar]
- 62. Veldhoven CMM, Nutma N, De Graaf W, et al. Screening with the double surprise question to predict deterioration and death: an explorative study. BMC Palliat Care 2019; 18(1): 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Verhoef M-J, de Nijs EJM, Fiocco M, et al. Surprise question and performance status indicate urgency of palliative care needs in patients with advanced cancer at the emergency department: an observational cohort study. J Palliat Med 2020; 23: 801–808. [DOI] [PubMed] [Google Scholar]
- 64. Edge SB, Liu L, Case AA, et al. Value of oncologist generated “surprise question” in predicting survival in metastatic cancer. J Clin Oncol 2020; 38: 12082. [Google Scholar]
- 65. Ernecoff NC, Abdel-Kader K, Cai M, et al. Implementation of surprise question assessments using the electronic health record in older adults with advanced CKD. J Am Soc Nephrol 2020; 31: 224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Flierman I, van Rijn M, Willems DL, et al. Usability of the surprise question by nurses to identify 12-month mortality in hospitalized older patients: a prospective cohort study. Int J Nurs Stud 2020; 109: 103609. [DOI] [PubMed] [Google Scholar]
- 67. Ikari T, Hiratsuka Y, Yamaguchi T, et al. ‘3-Day Surprise Question’ to predict prognosis of advanced cancer patients with impending death: multicenter prospective observational study. Cancer Med 2021; 10: 1018–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Estifan Kasabji J, Lucas C, Sastre A, et al. Is the surprise question useful as a predictor of mortality in hemodialysis patients? Nephrol Dial Transplant 2020; 35: iii1452. [Google Scholar]
- 69. Lai C-F, Cheng C-I, Chang C-H, et al. Integrating the surprise question, palliative care screening tool, and clinical risk models to identify peritoneal dialysis patients with high one-year mortality. J Pain Symptom Manag 2020; 60(3): 613–621.e6. [DOI] [PubMed] [Google Scholar]
- 70. Maes H, Van Den Noortgate N, De Brauwer I, et al. Prognostic value of the surprise question for one-year mortality in older patients: a prospective multicenter study in acute geriatric and cardiology units. Acta Clin Belg 2022; 77: 286–294. [DOI] [PubMed] [Google Scholar]
- 71. Rauh LA, Sullivan MW, Camacho F, et al. Validation of the surprise question in gynecologic oncology: a one-question screen to promote palliative care integration and advance care planning. Gynecol Oncol 2020; 157: 754–758. [DOI] [PubMed] [Google Scholar]
- 72. Tabernero Huguet E, Ortiz de Urbina Antia B, González Quero B, et al. Prevalence and mortality of patients with palliative needs in an acute respiratory setting. Arch Bronconeumol 2021; 57: 729. [DOI] [PubMed] [Google Scholar]
- 73. Tak N, Moor C, Owusuaa C, et al. The value of the surprise question to predict mortality in idiopathic pulmonary fibrosis. Eur Respir J 2020; 56: 1800. [Google Scholar]
- 74. Tripp D, Janis J, Jarrett B, et al. How well does the surprise question predict 1-year mortality for patients admitted with COPD? J Gen Intern Med 2021; 36: 2656–2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Valerio P, Farinha A. Surprise question: a mortality predictor in hemodialysis patients? J Am Soc Nephrol 2020; 31: 407. [Google Scholar]
- 76. van Wijmen MPS, Schweitzer BPM, Pasman HR, et al. Identifying patients who could benefit from palliative care by making use of the general practice information system: the surprise question versus the SPICT. Fam Pract 2020; 37: 641–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Yen Y-F, Lee Y-L, Hu HY, et al. Early palliative care: the surprise question and the palliative care screening tool—better together. BMJ Support Palliat Care. Epub ahead of print 25 May 2020. DOI: 10.1136/bmjspcare-2019-002116. [DOI] [PubMed] [Google Scholar]
- 78. Ermers DJ, Kuip EJ, Veldhoven C, et al. Timely identification of patients in need of palliative care using the double surprise question: a prospective study on outpatients with cancer. Palliat Med 2021; 35: 592–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Milnes S, Orford NR, Berkeley L, et al. A prospective observational study of prevalence and outcomes of patients with gold standard framework criteria in a tertiary regional Australian Hospital. BMJ Support Palliat Care 2019; 9: 92–99. [DOI] [PubMed] [Google Scholar]
- 80. Ramsenthaler C, Haberland B, Schneider S, et al. Identifying patients with cancer appropriate for early referral to palliative care using the integrated palliative care outcome scale (IPOS)-a cross-sectional study of acceptability and deriving valid cut-points for screening. Palliat Med 2018; 32: 98. [Google Scholar]
- 81. Haga K, Murray S, Reid J, et al. Identifying community based chronic heart failure patients in the last year of life: a comparison of the Gold Standards Framework Prognostic Indicator Guide and the Seattle Heart Failure Model. Heart 2012; 98: 579–583. [DOI] [PubMed] [Google Scholar]
- 82. O’Callaghan A, Laking G, Frey R, et al. Can we predict which hospitalised patients are in their last year of life? A prospective cross-sectional study of the Gold Standards Framework Prognostic Indicator Guidance as a screening tool in the acute hospital setting. Palliat Med 2014; 28: 1046–1052. [DOI] [PubMed] [Google Scholar]
- 83. Vick JB, Pertsch N, Hutchings M, et al. The utility of the surprise question in identifying patients most at risk of death. J Clin Oncol 2015; 33: 8. [Google Scholar]
- 84. Zertuche-Maldonado T, Tellez-Villarreal R, Pascual A, et al. Palliative care needs in an acute internal medicine ward in Mexico. J Palliat Med 2018; 21: 163–168. [DOI] [PubMed] [Google Scholar]
- 85. Kittanamongkolchai W, Suarez MLG, Gregoire JR. Dialysis: palliative and end-of-life care external validation of a short-term prognostic model for patients who are on maintenance hemodialysis. J Am Soc Nephrol 2017; 28: 638. [Google Scholar]
- 86. Gillespie S, Lane ND, Echevarria C, et al. The ‘surprise question’ in patients surviving severe COPD exacerbation. Eur Respir J 2020; 56: 109. [Google Scholar]
- 87. Sangani R, Mokaya E, Mujahid H, et al. Early high-risk patient identification and palliative care intervention does not lead to self-fulfilling prophecy in the ICU. Chest 2020; 158: A1849. [Google Scholar]
- 88. Sangani R, Mokaya E, Mujahid H, et al. Early palliative care intervention reduces ICU readmissions in high-risk patients. Chest 2020; 158: A1841. [Google Scholar]
- 89. Duenk RG, Verhagen C, Bronkhorst EM, et al. Development of the ProPal-COPD tool to identify patients with COPD for proactive palliative care. Int J Chron Obstruct Pulmon Dis 2017; 12: 2121–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Rice J, Hunter L, Hsu AT, et al. Using the ‘Surprise Question’ in nursing homes: a prospective mixed-methods study. J Palliat Care 2018; 33: 9–18. [DOI] [PubMed] [Google Scholar]
- 91. Carvalho JR, Vasconcelos M, Marques da, Costa P, et al. Identifying palliative care needs in a Portuguese liver unit. Liver Int 2018; 38: 1982–1987. [DOI] [PubMed] [Google Scholar]
- 92. Gaffney L, Judge C, Morrison L, et al. Use of the ‘Surprise Question’ in predicting adverse outcomes among frail older patients after hospital admission. Age Ageing 2018; 47: v1–v12. [Google Scholar]
- 93. Glick J, Marin BG, Chelluri J, et al. Utility of the” surprise question” in critically ill emergency department patients. Ann Emerg Med 2018; 72: S68. [Google Scholar]
- 94. Singh S, Graham Z, Rodriguez A, et al. Accuracy of the surprise question on an inpatient oncology service: a multidisciplinary perspective. J Hosp Palliat Nurs 2019; 21: 300–304. [DOI] [PubMed] [Google Scholar]
- 95. Li DY, Prigmore HL, Stewart TG, et al. Development and internal validation of a mortality risk prediction model in older adults with advanced non-dialysis-dependent (NDD) CKD. J Am Soc Nephrol 2020; 31: 213. [Google Scholar]
- 96. Thiagarajan R, Morris J, Harkins KJ. Can simple intuitive questions identify patients in the last year of their life?-a pragmatic study comparing the “paired surprise questions” with the “single surprise question”. Age Ageing 2012; 41: i61. [Google Scholar]
- 97. Lledo MD, Ahumada M, Puche AM, et al. Palliative care in cardiological patients, a forgotten problem. Eur Heart J 2014; 35: 835–836. [Google Scholar]
- 98. Lefkowits C, Chandler C, Sukumvanich P, et al. Validation of the “surprise question” in gynecologic oncology: comparing physicians, advanced practice providers and nurses. Gynecol Oncol 2016; 141: 128.26867989 [Google Scholar]
- 99. Gopinathan J, Aboobacker I, Hafeeq B, et al. Predicting death on maintenance hemodialysis-a complex task in prevalent elders. Nephrol Dial Transplant 2016; 31: i550. [Google Scholar]
- 100. Wong J, Gott M, Frey R, et al. Palliative care presentations to emergency departments in a secondary and a sub-acute hospital: a one year incidence study. Prog Palliat Care 2017; 25: 235–241. [Google Scholar]
- 101. Mastandrea M, Rojas L, Costa D, et al. Frailty and functional status in elderly patients with acute coronary syndrome: prospective cohort study to assess mortality risk. Eur Heart J 2018; 39: 704. [Google Scholar]
- 102. Rubinfeld G, Boodram P, Ho Cho M, et al. The prognostic accuracy of the ‘surprise question’ in geriatric patients at a large New York City hospital. J Am Geriatr Soc 2019; 67: S290. [Google Scholar]
- 103. De La Puente MÍNM, Del Rosario Evangelista Cabrera L, Mendoza SD, et al. Advanced chronic disease and palliative needs in an acute geriatric unit. Eur Geriatr Med 2019; 10: S49. [Google Scholar]
- 104. Ahmed I, Sutton AJ, Riley RD. Assessment of publication bias, selection bias, and unavailable data in meta-analyses using individual participant data: a database survey. BMJ 2012; 344: d7762. [DOI] [PubMed] [Google Scholar]
- 105. Elliott M, Nicholson C. A qualitative study exploring use of the surprise question in the care of older people: perceptions of general practitioners and challenges for practice. BMJ Support Palliat Care 2017; 7: 32–38. [DOI] [PubMed] [Google Scholar]
- 106. Haydar SA, Almeder L, Michalakes L, et al. Using the surprise question to identify those with unmet palliative care needs in emergency and inpatient settings: what do clinicians think? J Palliat Med 2017; 20: 729–735. [DOI] [PubMed] [Google Scholar]
- 107. De Bock R, Van Den Noortgate N, Piers R. Validation of the supportive and palliative care indicators tool in a geriatric population. J Palliat Med 2018; 21(2): 220–224. [DOI] [PubMed] [Google Scholar]
- 108. Cardona-Morrell M, Kim J, Turner RM, et al. Non-beneficial treatments in hospital at the end of life: a systematic review on extent of the problem. Int J Qual Health Care 2016; 28: 456–469. [DOI] [PubMed] [Google Scholar]
- 109. Brinkman-Stoppelenburg A, Rietjens JA, van der Heide A. The effects of advance care planning on end-of-life care: a systematic review. Palliat Med 2014; 28: 1000–1025. [DOI] [PubMed] [Google Scholar]
- 110. Billings JA, Bernacki R. Strategic targeting of advance care planning interventions: the Goldilocks phenomenon. JAMA Intern Med 2014; 174: 620–624. [DOI] [PubMed] [Google Scholar]
- 111. Walsh RI, Mitchell G, Francis L, et al. What diagnostic tools exist for the early identification of palliative care patients in general practice? A systematic review. J Palliat Care 2015; 31: 118–123. [DOI] [PubMed] [Google Scholar]