Abstract
The human T-cell leukemia/lymphotropic virus type 1 (HTLV-1) induces a malignant lymphocytic disease. The HTLV-1 transactivator protein, Tax, is believed to be crucial for the development of the disease since it is transforming in vitro and induces tumors in transgenic animals. Although the transcriptional modulation of viral and cellular gene expression by Tax has been analyzed thoroughly, it has remained unclear how the Tax functions act on the cell cycle of primary T cells. To investigate the mechanism of Tax-mediated T-cell stimulation, we transduced primary human cord blood T cells with a conditional, tetracycline repressor-based tax expression system. Permanent Tax expression results in an abnormal proliferation of T cells which closely resemble HTLV-1-infected lymphocytes. Suppression of Tax synthesis stopped lymphocyte growth and caused cell cycle arrest in the G1 phase. Upon reinduction of tax expression, the arrested cells entered the S phase. This showed that Tax has mitogenic activity, which is required for stimulating the G1- to S-phase transition of immortalized lymphocytes. In mammalian cells, the G1-phase progression is controlled by the serial activation of several cyclin-dependent kinases (Cdks), starting with Cdk4 and Cdk6. In the presence of Tax, both Cdk4 and Cdk6 were activated. The suppression of Tax synthesis, however, resulted in a significant reduction of the Cdk4 and Cdk6 activities but did not influence the expression of Cdk4, Cdk6, or cognate D-type cyclin proteins. These data suggest that Tax induces Cdk4 and Cdk6 activity in primary human T lymphocytes; this Cdk activation is likely to account for the mitogenic Tax effect and for the abnormal T-cell proliferation of HTLV-1-infected lymphocytes.
The human T-cell leukemia/lymphotropic virus type 1 (HTLV-1) induces adult T-cell leukemia (50, 66), a unique clinical disorder of mature CD4+ lymphocytes. The leukemogenic properties of the virus are accompanied by its capacity to change the growth properties of T cells from patients, asymptomatic carriers, and in vitro-infected peripheral blood cell cultures. In contrast to normal T cells, these cells proliferate permanently without antigen stimulation (reviewed in reference 25). The HTLV-induced growth transformation results primarily in interleukin-2 (IL-2)-dependent cell lines, which closely resemble activated and functional T lymphocytes. After prolonged in vitro culture, sequential changes in the expression of proliferation-related functions are observed. These include the eventual conversion to IL-2-independent growth caused by a constitutive stimulation of the IL-2 receptor-associated Jak-STAT pathway (11, 44). This additional change is found associated with the loss of T-cell receptor and T-cell functions and a lack of Lck tyrosine kinase expression (29, 38). Observations made with HTLV-1-transformed cells indicate an abnormal regulation of the cell cycle. These cells contained inactive hyperphosphorylated retinoblastoma (Rb) protein and were resistant to cell cycle arrest induced by transforming growth factor β (28). Additionally, the tumor suppressor p53, although unmutated, showed increased stability (53).
The HTLV-1 genome encodes a regulatory protein, p40tax, that resembles a viral oncogene and thus is believed to be responsible for the transforming features of the virus. Tax induces multiple mesenchymal tumors and leukemia in transgenic mice (26, 47), and it can alter the growth properties of rodent fibroblast cell lines (51, 62) and human T cells (4, 6). In the context of a transformation-defective rhadinovirus vector, Tax can immortalize primary human lymphocytes. Cells immortalized by tax-expressing rhadinoviruses closely resemble HTLV-infected lymphocytes in phenotype and growth behavior (22, 23). Biochemically, p40tax functions as a transcriptional transactivator protein; it stimulates viral transcription and modulates an array of cellular genes by acting on transcription factors CREB (1, 2, 10), NF-κB (33, 58, 59), and p67SRF (17, 18). It enhances the transcription of proto-oncogenes like c-fos and c-jun (17, 18), the genes for the α chain of the IL-2 receptor (IL-2Rα) (12, 30), and several cytokines (27, 37, 46). However, it suppresses the synthesis of the human DNA polymerase β, an enzyme important for DNA repair (31). Recently, Tax was shown to bind to p16INK-4A (61), an inhibitor of cyclin-dependent kinases Cdk4 and Cdk6. These Cdks are essential for the control of the G1-phase progression (8, 52, 54, 56, 63), and they are the first to be activated after cells are stimulated from a quiescent state (40, 41, 43). The kinases are assembled with D-type cyclins in holoenzyme complexes that phosphorylate Rb proteins (14, 34, 43). Interestingly, the negative control of the Cdk4-Cdk6 signalling pathway is frequently impaired or bypassed in transformed and tumor cells (35, 48), implicating that a malfunction in these kinases could result in deregulation of cellular growth.
The mechanism by which Tax influences the growth of transformed primary human T cells is not well understood; it is not even known whether the growth of these cells depends on the presence of Tax. One attractive speculation is that Tax activates Cdks by pulling off INK-4 inhibitors like p16INK-4A and thus acts as a growth factor for the HTLV-infected lymphocytes. To address the growth dysregulation of immortalized primary T cells, which are not accessible by standard DNA transfer techniques, we constructed rhadinovirus recombinants containing a tetracycline-repressible tax gene and immortalized primary human lymphocytes. The proliferation of the immortalized cells was reversibly arrested in the G1 phase of the cell cycle by suppression of tax transcription, thus demonstrating that Tax stimulates the G1- to S-phase transition in immortalized T lymphocytes. We also could show that the activation of cyclin-dependent kinases Cdk4 and Cdk6 was dependent on the presence of Tax. This Cdk activation provides a direct linkage of Tax to the control of the G1 phase of T cells and may account for the mitogenic and immortalizing Tax effect.
MATERIALS AND METHODS
Construction of recombinant rhadinovirus.
The tax sequences were obtained as a BamHI/XhoI fragment from pHIXSLdsph (22) and fused with the tetracycline-regulatable promoter present in pUHG10-3 (21). A SalI/XhoI fragment containing the promoter-tax fusion was subsequently inserted into the SalI site of the recombination vector pRÜneo15-N (22), resulting in pRÜtosG. The sequences for the modified tetracycline repressor (tTA) were derived from the plasmid pUHD15-1 (21) as a XhoI fragment and inserted into the SalI site of pRÜtosG, resulting in the plasmid ptTAX. This recombination plasmid contains the modified tetracycline repressor, the tax gene, a NeoR selection marker, and flanking rhadinoviral sequences. These sequences allowed homologous recombination with the genome of the rhadinovirus herpesvirus saimiri strain 11S4 and directed the tax expression cassettes into the right junction of unique and repetitive sequences.
The generation and purification of the recombinant rhadinovirus herpesvirus saimiri were performed as described previously (7, 24). Briefly, the linearized plasmid ptTAX (2 to 4 μg) and genomic DNA of herpesvirus saimiri strain 11S4 (0.2 to 0.4 μg) were cotransfected into OMK-637 cells, where homologous recombination took place. This cell line is lytically infectable with herpesvirus saimiri. Recombinant viruses were selected by use of the antibiotic G418 and purified by five subsequent plaque isolations. The purity and correct structure of the recombinants were verified by Southern blot analyses as described previously (22). The hybridization probes used were tax cDNA, herpesvirus saimiri sequences derived from the KpnI-D fragment, and the XhoI/BamHI fragment of pUHD15-1. The viral stocks (Hs86-S) obtained were found to contain more than 95% recombinant genomes.
Cell culture and immortalization of primary human T cells.
The T cell line Jurkat (HTLV-1 negative) and the HTLV-1-infected cell line HuT102 were cultured in RPMI 1640 containing 10 to 20% fetal calf serum (FCS), glutamine, and the antibiotics streptomycin and penicillin. The same medium supplemented with IL-2 (20 to 40 U) was used for the propagation of Tesi, NATI-2, and TRI-1 cells (22). The immortalized T cell lines TRI-1 and NATI-2 are derived from cord blood by use of a permanently transcribed tax gene. The viral vector used for the transduction of the constitutive tax gene was the same as the vector used for the insertion of the repressible tax gene. According to growth characteristics and phenotype (CD25 CD45 CD2), TRI-1 and NATI-2 cells closely resemble Tesi cells, which contain recombinant virus Hs86-S. The human CD4+ T cell line SS8BPT (65) and its derivative SS8tetTax were maintained in a mixture (1:1) of RPMI 1640/CG (Vitromex, Vilshofen, Germany) and 20% FCS plus 50 U of IL-2 per ml. Human peripheral blood mononuclear cells were prepared from heparin-treated cord blood by Ficoll-Hypaque (Sigma) density gradient centrifugation, stimulated for 48 h with 5 μg of phytohemagglutinin per ml, and infected with recombinant virus Hs86-S as described previously. Infected peripheral blood mononuclear cells were propagated in RPMI 1640 supplemented with 20% FCS, glutamine, antibiotics, and 20 to 40 U of IL-2 (Boehringer, Mannheim, Germany) per ml.
Characterization of cell lines.
For the detection of high-molecular-weight superhelical DNA in transformed lymphocytes (24), cells were carefully lysed on top of vertical slab gels; cellular DNA was separated electrophoretically into chromosomal, episomal, and linear or degraded fractions. To visualize the recombinant viral sequences, the gels were blotted and hybridized to X-region DNA. Surface markers were detected by monoclonal antibodies binding to human CD4 (Leu-3a), CD8 (Leu-2a), CD45 (anti-HLe-1), CD45 RA, CD45 RO, CD3 (Leu-4), CD25 (anti-Tac), CD28, and CD69 (Leu-23). Tetracycline (1 μg/ml)-treated (7 to 14 days) and untreated cells were stained, washed, and fixed in 1% formaldehyde. The cells were analyzed by flow cytometry with a FACStract analyzer with Lysis II software (Becton Dickinson). To determine the proportions of cells in the G1/G0 (abbreviated G1), S, and G2/M (abbreviated G2) phases in lymphocyte cultures, the DNA content of individual cells was analyzed. Cells (0.8 × 106) were washed, fixed in cold 70% ethanol, and stained with propidium iodide (50 μg of propidium iodide per ml and 100 μg of DNase-free RNase A per ml in phosphate-buffered saline). Flow cytometric analysis was performed with a Coulter Epics Elite analyzer with Multicycle software (Phoenix). The DNA synthesis rate was determined as a measure of cell proliferation. Cells were seeded in triplicate in round-bottom microculture plates at a density of 40,000 to 50,000 cells/100 μl and labelled with 2 μCi of [3H]thymidine ml−1 for 12 to 16 h. Each experiment was repeated a minimum of three times. The cells were harvested, and the radioactivity incorporated in the DNA was quantitated by phosphorimaging (BAS2000; FujiX).
Analysis of gene expression.
For the demonstration of tetracycline-mediated tax suppression, OMK-637 cells were infected with virus stocks diluted 1:10 with minimal essential medium. The permanently growing human T cell line SS8BPT was infected with the recombinant virus (Hs86-S) like the cord blood cells were, but without previous stimulation, and selected by the addition of antibiotic G418. The resulting cell line was designated SS8tetTax. To repress tax expression, the cells were treated with tetracycline (0.01 to 1 μg/ml). tax RNA was identified by Northern blotting as previously described (22). Tax protein and cell cycle control proteins were detected by Western blot analysis. Tetracycline-treated and untreated cells were resuspended in a lysis buffer (50 mM Tris [pH 8.0], 150 mM NaCl, 1% Nonidet P-40, 1 mM sodium vanadate, 10 μg of aprotinin per ml, 10 μg of leupeptin per ml, 5 mM NaF) and incubated for 15 min on ice. Lysates were cleared by centrifugation, and equal amounts of crude cell lysates (30 μg) were separated by gels and transferred to nitrocellulose (Amersham) or Immobilon P (Millipore) membranes. The antibodies used to detect Tax were prepared from hybridoma 168B17-46-34/50 (provided by B. Langton through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID) or anti-Tax rabbit serum. For the detection of cyclin-dependent kinases, cyclins, and retinoblastoma protein, polyclonal rabbit antibodies were purchased from Santa Cruz Biotechnology (anti-Cdk4, anti-Cdk6, anti-Rb), Dianova (anti-cyclin D3), or Pharmingen (anti-p16INK-4A). Bound antibodies were visualized by the enhanced chemiluminescence detection system (Amersham). To demonstrate the repression of Tax function, cells (5 × 106) were electroporated (900-μF charge, 250-V potential) with 10 μg of the Tax-inducible reporter plasmid pU3RI-CAT (23). The cells were harvested 48 h later, and the chloramphenicol acetyltransferase (CAT) reaction was determined as described previously (22).
Determination of cyclin-dependent kinase activity.
Cells were lysed in buffer containing 50 mM Tris-HCl (pH 7.4), 250 mM NaCl, 0.5% Nonidet P-40, 1 mM phenylmethylsulfonyl fluoride, 1 mM sodium orthovanadate, 5 mM NaF, 10 μg of aprotinin ml−1, and 10 μg of leupeptin ml−1 (all protease inhibitors from Sigma). Lysates were frozen, thawed, and clarified. From these extracts, Cdk4 and Cdk6 were detected by immunoblot analysis (30 μg of protein) or immunoprecipitated (300 μg of protein) with anti-Cdk6 and anti-Cdk4 rabbit serum (Santa Cruz Biotechnology). Immune complexes were collected with protein A-agarose (Santa Cruz Biotechnology) and washed four times with a lysis buffer and twice with a kinase buffer (20 mM Tris [pH 7.4], 7.5 mM MgCl2, 1 mM dithiothreitol). For kinase assays, the beads were resuspended in 40 μl of kinase buffer and incubated at 37°C for 30 min in the presence of 30 μM ATP, 5 μCi of [γ-32P]ATP, and 4 μg of glutathione S-transferase–Rb fusion protein. Products were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, followed by autoradiography, and quantitated by phosphorimaging. The Rb substrate was prepared in Escherichia coli from an expression vector that was kindly supplied by Jae U. Jung (32). The glutathione S-transferase fusion protein contains the Rb-coding region from codons 379 to 928.
RESULTS
Construction of recombinant rhadinovirus for tetracycline-repressible tax expression.
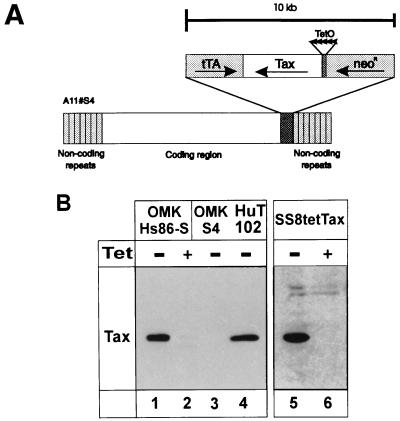
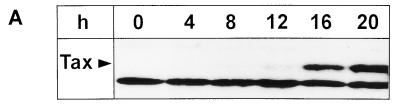
To investigate whether HTLV genes are required not only for inducing immortalization but also for maintaining T-cell proliferation, a recombinant rhadinovirus with repressible tax expression was constructed. This vector efficiently infects primary T lymphocytes, and recombinant vectors persist in T cells as episomes without expressing rhadinoviral genes. To achieve repressible gene expression, we adapted the tetracycline system to an application in the rhadinovirus vector. The tax sequences were cloned under the control of the tTA promoter, combined with the gene encoding the transcriptional activator (tTA), and introduced into a recombination plasmid. The plasmid was cotransfected with infectious virion DNA into permissive tissue cultures, where homologous recombination took place. The vector strain used is a nontransforming, apathogenic deletion variant of the subgroup A herpesvirus saimiri. The wild-type strain from which the vector strain is derived, like all wild-type strains of subgroup A, does not immortalize human cells. The deletion variant has lost some 3.5-kb sequences coding for a protein (STP-A) which transforms rat fibroblasts and induces tumors in nude mice (13, 42). Recombinant viruses were isolated and characterized. Southern blot analysis showed that the viruses contained the expected insert of about 10 kb (Fig. 1A). Subsequently, the recombinant viruses were tested for repressible tax expression. Infected permissive monkey kidney cells (OMK-637) and a human T lymphocyte cell line (SS8tetTax), which is persistently infected with the recombinant virus, were maintained in both the presence and absence of tetracycline. Immunoblot analyses revealed high levels of tax expression in the absence of tetracycline. No detectable Tax protein was present in cells which had been exposed to tetracycline (Fig. 1B). As expected, these differences in Tax protein expression correlate with different amounts of tax RNA detectable in untreated and tetracycline-treated cells (data not shown). These data show that the tTA system is functional within rhadinovirus vectors and that tax expression in this context is efficiently repressible by tetracycline both in permissive and nonpermissive cells.
FIG. 1.
Tetracycline-repressible tax expression from a recombinant rhadinovirus. (A) Insertion of the repressible tax expression cassette into the rhadinovirus vector. The tax expression is controlled by a chimeric promoter containing binding sites (tetracycline operator, TetO) for the artificial transactivator (tTA). The genes coding for Tax, the artificial transactivator (tTA), and the neomycin phosphotransferase (NeoR) were inserted into the right end of the coding sequences of the transformation-defective deletion variant S4 of herpesvirus saimiri strain A11 (A11#S4). (B) Immunoblot analysis of tax expression in lytically and persistently infected cells. Permissive OMK-637 cells were infected in the presence or absence of tetracycline (Tet) with the recombinant virus stock Hs86-S (lanes 1 and 2) or with the vector (S4) alone (lane 3). After the appearance of distinct cytopathic changes, the cells were lysed. Proteins were also extracted from a persistently infected T cell line (SS8tetTax) which had been kept in the presence or absence of tetracycline (lanes 5 and 6) and from HuT102, a cell line infected with HTLV-1 (lane 4). Proteins (20 μg/lane) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, blotted, and reacted with anti-Tax monoclonal antibodies.
Generation of immortalized human T lymphocytes.
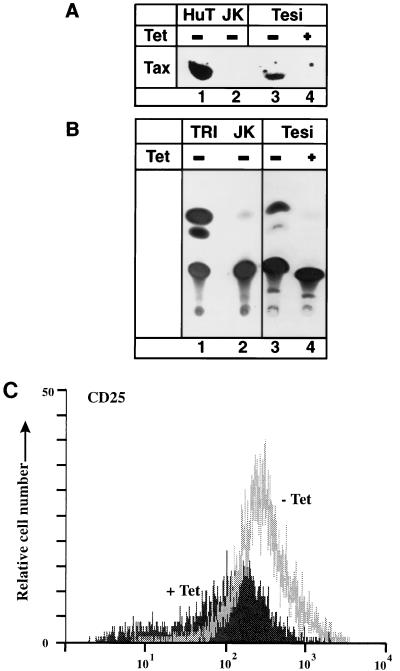
To determine the impact of Tax on T-cell proliferation, it was necessary to introduce the repressible transactivator gene into primary human T cells. To this end, lymphocytes from cord blood were infected with the tax-expressing recombinant virus. As expected from earlier work (22), permanently growing cells could be established only from cells infected with the tax-expressing recombinants and not by infection with the vector strain. These cells (Tesi cells) were in a permanent culture for more than 1 year and thus were considered immortalized. In the presence of exogenous IL-2, the cultures grew with a doubling time of about 1 week. Phenotypically, they resemble activated T-helper lymphocytes since they express CD4, CD25, CD3, CD45, CD69, and FAS (CD95) but no CD8. According to these data, they are very similar to T cells immortalized with other tax-expressing rhadinovirus recombinants or HTLV-1 (22, 56a). The cells immortalized by the repressible tax contain persisting episomal DNA of the recombinant rhadinovirus (data not shown). As expected from earlier experiments, the cultures did not produce infectious recombinant viruses. This was verified by cocultivation with permissive OMK-637 cells. Regulated tax expression was demonstrated by immunoblot analyses (Fig. 2A). In the presence of tetracycline, no Tax expression was detectable. The Tax protein was biologically active since it transactivated the HTLV-1 promoter (Fig. 2B). In accordance with the expectation that Tax stimulates the IL-2Rα promoter, the repression of Tax synthesis was found to correlate strongly with reduced IL-2Rα surface expression on the immortalized T cells (Fig. 2C). These findings show that in the immortalized T cells (Tesi cells), the tax gene is controlled efficiently by tetracycline and that the expressed Tax protein is a functional transactivator of viral and cellular gene expression.
FIG. 2.
Suppression of Tax function by tetracycline in immortalized human T cells. Tesi cells were cultivated in the presence or absence of 1 μg of tetracycline (Tet) per ml. (A) Immunoblot analysis demonstrating suppression of Tax protein expression in the presence of tetracycline by using anti-Tax rabbit serum. Lanes: 1, HuT102 cells; 2, Jurkat cells; 3, Tesi cells; 4, Tesi cells with tetracycline. (B) CAT assay demonstrating suppressed Tax activity in immortalized T cells (Tesi cells). The indicated cells were transfected with 10 μg of the reporter plasmid pU3R1-CAT containing the HTLV-1 promoter which can be stimulated by Tax. Lanes: 1, TRI-1 cells; 2, Jurkat cells; 3, Tesi cells without tetracycline; 4, Tesi cells with 1 μg of tetracycline per ml. The experiment was repeated three times with the same result. (C) Reduced IL-2Rα (CD25) surface expression of immortalized T cells in the presence of tetracycline. Immortalized T cells were stained with anti-CD25–fluorescein isothiocyanate and analyzed by flow cytometry.
Expression of tax is required for the replication of immortalized human lymphocytes.
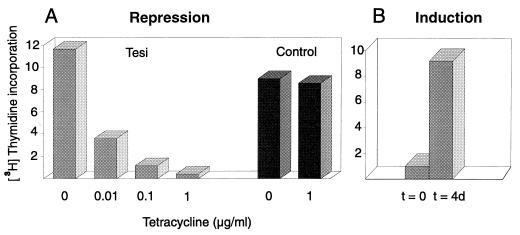
To study the influence of tax expression on T-cell proliferation, tetracycline was added at various concentrations to the immortalized lymphocytes. As a parameter of cell proliferation, the DNA synthesis rate was measured. The addition of tetracycline resulted in a strong, dose-dependent repression of [3H]thymidine incorporation (Fig. 3A). As little as 0.01 μg of tetracycline per ml was sufficient to significantly reduce DNA synthesis. Concentrations of 1 μg/ml prevented DNA replication completely. The addition of 1 μg of tetracycline per ml to the control T cell lines TRI-1, NATI-2, and SS8tetTax, however, did not result in significant changes of [3H]thymidine incorporation (Fig. 3A and data not shown). TRI-1 and NATI-2 cells were immortalized by a constitutively transcribed tax gene transduced by the same virus vector which was used for the generation of Tesi cells. These cells phenotypically closely resemble Tesi cells. The effect of tetracycline on DNA synthesis correlated well with reduced proliferation rates of tetracycline-treated Tesi cell cultures determined by counting cell numbers (data not shown). The tetracycline-mediated antiproliferative effect is reversible and not toxic. Cells suppressed in replication by tetracycline (1 μg/ml) for longer than 3 weeks start to proliferate after removal of this antibiotic (Fig. 3B). In summary, these data indicate that the immortalized T cells require Tax for permanent proliferation.
FIG. 3.
Tax suppression results in a stop of T-cell replication. (A) The replication of immortalized T cells depends on tax gene expression. Tesi cells and a control cell line (NATI-2) were cultivated for two doubling times in the absence or presence of tetracycline (0.01, 0.1, and 1 μg/ml). As a measure of cell proliferation, [3H]thymidine incorporation tests were applied. The radioactivity incorporated into cells was determined as phosphorus-stimulated luminescence per square millimeter with a phosphor imager. The proliferation of Tesi cells was repressed by tetracycline in a dose-dependent manner, whereas the control cell line with constitutive Tax expression (NATI-2) was unaffected. TRI-1 and SS8tetTax behaved like NATI-2. (B) Reversion of growth arrest. The medium used for Tesi cells, which had been growth arrested earlier (t = 0), was depleted of tetracycline, and replication was analyzed 4 days later (t = 4d).
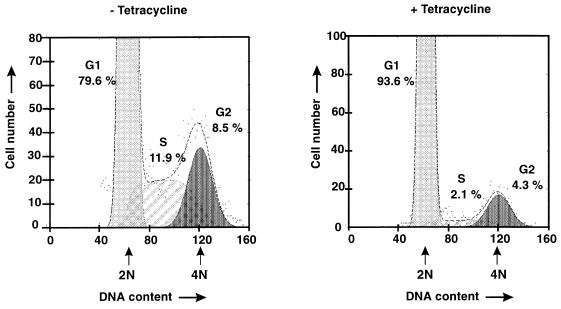
To identify the phase at which the mitogenic Tax signal stimulates the cell cycle, the proportions of cells in the G1, S, and G2 phases were evaluated in both the presence and absence of Tax. Flow cytometric analyses revealed that in unsynchronized cultures, one-fifth of the tax-expressing cells were in the G2 or S phase (Fig. 4). The cultures contained almost the same numbers of cells in the G1 phase (79.5%) as the HTLV-1-transformed cell line HuT-102 did (83.6%). In contrast, cultures which were growth arrested by tax suppression consisted almost completely of cells in the G1 phase (95%). These observations indicate that the suppression of Tax synthesis arrests immortalized cells in the G1 phase of the cell cycle. Our experiments did not hint at a Tax effect on apoptotis in these cultures, since the number of cells with DNA in amounts of less than one genome copy was not significant (data not shown).
FIG. 4.
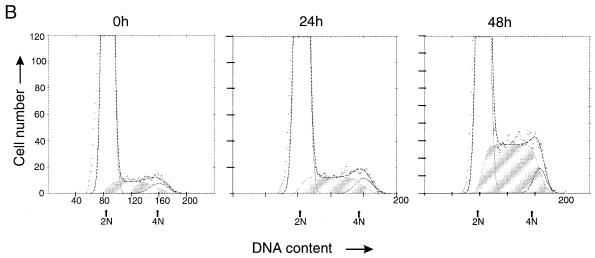
Cell cycle status in proliferating and nonproliferating tetracycline-treated Tesi cells. tax-expressing Tesi cells were cultured in either the presence (+) or absence (−) of tetracycline (1 μg/ml) for three doubling times, fixed, stained with propidium iodide, and subjected to flow cytometry. Apoptotic and dead cells were excluded from the analysis. The dots represent the direct results of the measurements. The lines indicate the relative calculated proportions of cells in the G1 (light grey), S (diagonally hatched), and G2 (dark grey) phases of the cell cycle. The x-axis indicates fluorescence representing the DNA content. Arrows indicate positions of cells with 2N and 4N DNA content.
To study the kinetics of Tax induction and cell cycle progression, tetracycline was removed from Tesi cell cultures arrested in the cell cycle and from SS8tetTax cells. In persistently infected T cells (SS8tetTax), Tax protein was first detected 12 h after tetracycline removal and reached maximal levels 8 h later (Fig. 5A). After 1 day of culture in tetracycline-free medium, a slight increase in the number of Tesi cells in the S-phase fraction was observed; after 2 days, about one-fourth of all cells had entered the S phase (Fig. 5B). This observation is in accordance with [3H]thymidine incorporation tests, which demonstrate the onset of DNA replication between 25 and 43 h after tetracycline removal (data not shown). Based on these observations, the time that Tax required to initiate the S phase is estimated to be 12 to 23 h. These times are similar to those which normal human lymphocytes require to proceed from the early G1 to the S phase (3). After 5 days, the proportions of cells in the G1, S, and G2 phases reached the same steady-state level as that shown in Fig. 4. In summary, these data led us to conclude that the mitogenic Tax signal overcomes a block in the G1 phase of the lymphocytic cell cycle, thus allowing the cells to enter the S phase.
FIG. 5.
S-phase induction by Tax. (A) The kinetics of Tax induction after withdrawal of tetracycline. The tax expression in T cells that are persistently infected with the recombinant rhadinovirus Hs86-S (SS8tetTax) was suppressed by tetracycline. The cells were transferred to normal growth medium. To detect Tax after the times indicated, aliquots were subjected to immunoblot analysis. Tax expression could be detected after 12 h at the earliest. (B) Kinetics of S-phase induction. Tesi cells were treated with tetracycline (1 μg/ml) until DNA synthesis and transition into the S phase had virtually ceased (0 h). After the withdrawal of tetracycline, aliquots were subjected to flow cytometric cell cycle analysis after 24 and 48 h as described in the legend to Fig. 4. The cells in the S phase are indicated by shading. A slight increase in the number of cells in the S phase could be observed at 24 h; a strong increase could be observed after 48 h.
The activities of cyclin-dependent kinases Cdk4 and Cdk6 are increased in the presence of Tax.
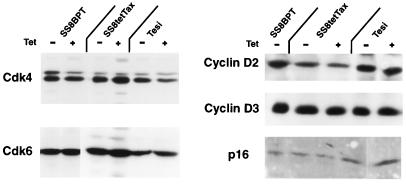
To narrow down the precise events in the G1 phase which are influenced by the Tax protein, we analyzed the activities of the G1-phase-specific cyclin-dependent kinases Cdk4 and Cdk6. These kinases and their cognate cyclins are almost completely absent in quiescent (G0-phase cells); however, they are the first to be activated after entering the cell cycle. The cellular contents of Cdks like Cdk4 and also the levels of the cognate cyclins and the p16 inhibitor are matters of regulation (20, 39, 40, 45). To investigate whether Tax interferes with the control of these proteins, the levels of Cdk4, Cdk6, cyclin D isotypes, and p16INK-4A were determined. Cyclin D1 was not analyzed since it is not present in most T cells (3, 5). The proteins were extracted from Tesi and SS8tetTax cells which had been kept before in the presence and absence of tetracycline to repress or induce, respectively, Tax synthesis. Immunoblot analyses did not reveal major differences in the amounts of cyclins, Cdks, and the Cdk inhibitor (Fig. 6). The expression of G1 cyclins and Cdks in the absence of Tax indicates that growth is arrested in the G1 phase rather than in the G0 phase.
FIG. 6.
Expression of cyclin-dependent kinases 4 and 6 and regulatory cofactors in the immortalized T cells. Tesi cells, the SS8tetTax cells, and the untransduced SS8BPT (control) cells were treated with tetracycline as indicated in Materials and Methods. Cdk4, Cdk6, cyclin D2, cyclin D3, and p16INK-4A were determined by immunoblotting. None of these was modulated in the presence of Tax.
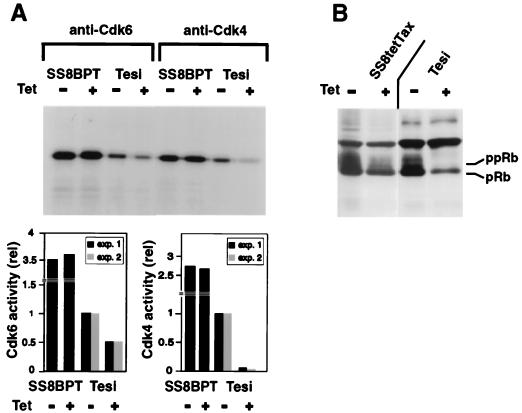
To determine whether the kinase activities of Cdk4 and Cdk6 were affected by Tax, the complexes were immunoprecipitated and in vitro kinase assays were performed with Rb protein used as a substrate. The Cdk activities in the immortalized cord blood (Tesi) cells expressing tax were compared with that in Tesi cells with a tetracycline-repressed tax gene. The results of two independent experiments show a clear correlation between Tax expression and the activity of each of these kinases. In the absence of Tax, the activity of Cdk4 was close to the background and approximately 10-fold stimulated if Tax expression was induced (Fig. 7A). Similarly, the activity of Cdk6 was enhanced in the Tax-immortalized cells. Rb phosphorylation in vivo was examined in the presence and absence of Tax expression. Indeed, the amounts of Cdk4 and Cdk6 activity determined in vitro correlated well with increased amounts of the hyperphosphorylated substrate (Rb) in the cells (Fig. 7B). To determine whether the expression of Tax influences the Cdk activity in T lymphocytes, which proliferate independently of Tax, SS8tetTax cells were analyzed. In standard culture medium which includes supplements of IL-2 and FCS, a minimal reduction in Cdk6 activity was observed if tax was suppressed. Nevertheless, this correlated with a reduced cellular content of hyperphosphorylated Rb protein (Fig. 7B). The Tax effect on Cdk6 was found markedly increased in SS8tetTax cells which had been deprived of IL-2 and FCS for 24 h (data not shown). Under these conditions, Tax also has a moderate stimulatory effect on the proliferation of this T-cell line. These observations suggest that the Tax signal might compensate for the lack of FCS or IL-2 in the activation of the Cdks. In summary, the experiments indicate that the mitogenic signals created by Tax result in activation of both Cdk4 and Cdk6. These observations are consistent with a Tax-mediated INK-4 inactivation.
FIG. 7.
Enhanced activity of Cdk4 and Cdk6 in the presence of Tax. Cells with induced or repressed Tax genes were lysed, and holoenzyme complexes containing Cdk6 or Cdk4 were immunoprecipitated. The kinase activity was assessed in vitro by using recombinant Rb protein as a substrate. (A) Cdk4 and Cdk6 activity of the cell line Tesi in the presence (−Tet) and absence (+Tet) of Tax. Quantification of the kinase activity from two independent experiments is shown graphically at the bottom of panel A. The kinase activities were normalized to the activities in untreated Tesi cells. The T-cell line SS8BPT was used as a tax-negative control. (B) Immunoblot analysis of Rb protein in Tesi and SS8tetTax cells. The upper band represents hyperphosphorylated forms (ppRb), while the lower band represents the hypophosphorylated protein (pRb).
DISCUSSION
To understand how the HTLV-1 transactivator (Tax) protein contributes to the growth regulation of HTLV-1-immortalized T cells, we transduced a conditional tetracycline-repressible tax gene into primary human lymphocytes. This resulted in the immortalization of T cells which require Tax for growth. With Tax present, the T lymphocytes were released from a reversible G1-phase arrest and the cyclin-dependent kinases Cdk4 and Cdk6 were activated. This demonstrates that Tax is required for stimulating the G1-phase progression in immortalized T cells and suggests that the mitogenic Tax effect is mediated by Cdk4 and/or Cdk6.
The repressible tax gene was transduced into primary human lymphocytes by using a rhadinovirus vector, which is appropriate to incorporate three different functional genes (tax, tTA, and neomycin resistance gene) with a total size of about 10 kb. This example shows the usefulness of our vector system for simultaneous transfer of multiple regulated genes into human primary cells. The integration of large stretches of foreign DNA into the vector system is facilitated by the unique genome structure of rhadinoviruses, which have repetitive, potentially replaceable sequences at the ends of their genomes. The recombinant virus is also an example of a functional tetracycline-regulated expression system that can be completely inserted into DNA viruses. The artificial transactivator neither influences the cell cycle (54, 55) nor is tumorigenic in transgenic mice (19). The growth arrest mediated by the addition of tetracycline to cells containing the repressible tax gene is not caused by a toxic effect, since the concentrations applied are far below the toxicity threshold (21). Growth-arrested cells survive for more than 3 weeks in the presence of tetracycline. It is rather improbable that genes of the rhadinovirus contribute to the immortalized phenotype for the following reasons. (i) The only transcription found in human T cells originates in the genomic region, which is deleted in the vector strain used (16). (ii) No viral transcripts were found in T cells infected and immortalized by recombinants constitutively expressing tax (24). (iii) No infectious virus particles are synthesized, as previously shown with several other human lymphocyte lines infected with herpesvirus saimiri recombinants (22, 57). This failure to synthesize virions in human lymphocytic cells correlates with the lack of immediate-early gene expression (16, 57). These genes are essential for the expression of all other classes of viral mRNA in herpesviruses.
The cells immortalized by Tax grow in the presence of IL-2. The moderate reduction of IL-2Rα (CD25) expression on the surface of growth-arrested immortalized cells, which was observed after Tax withdrawal, cannot explain the stimulatory effect of Tax. IL-2Rα is only one component of the IL-2 receptor, and the α chain is not directly involved in signal transduction but rather enhances the affinity of the IL-2 receptor complex to its ligand (64). The remaining enhancing effect of the moderate numbers of α chains on the growth-arrested Tesi cells should be sufficient to mediate the full IL-2 signal. In addition, the large excess of IL-2 in the medium should compensate for the IL-2Rα reduction. These arguments corroborate that Tax can stimulate T-cell growth by a mechanism which is different from the mechanism stimulating IL-2Rα expression.
In contrast to cells that express inducible Tax in the background of established leukemia lines, the immortalized T cells described here are unique since their growth depends on the presence of Tax and expression of the gene encoding Tax can be controlled by tetracycline. Therefore, these cells allow us to correlate the biochemical effects of Tax on cellular gene expression or signalling with cellular growth. By using this system, we found that the activities of the cyclin-dependent kinases Cdk4 and Cdk6 were stimulated in the presence of Tax, which correlates with cellular proliferation. The expression of the Cdks and cyclin D isoforms was unaffected, suggesting that Tax has activated these kinases. Tax can activate Cdk4 by binding to the Cdk inhibitor p16 in vitro (60). This mechanism may also account for the Cdk activation in immortalized primary human T cells. The Cdks binding to D cyclins are rate limiting for the G1-phase progression; interference with their function can cause G1-phase arrest (8, 15, 52, 54, 56, 63). The reduced activity of Cdk4 and Cdk6 thus may explain the growth arrest in phase G1 in the absence of Tax. These data also suggest that the growth-stimulating signal produced by Tax acts on Cdk4 and Cdk6. This conclusion agrees with the observation that the Rb protein of the immortalized Tesi cells is hyperphosphorylated in the presence of Tax. This result is consistent with earlier observations that Rb is continuously hyperphosphorylated in HTLV-1-infected cells (28).
The mechanism by which Tax acts to stimulate cell cycle progression, activation of Cdk4 and Cdk6, differs from that of other tumor virus oncoproteins which bind directly to Rb, such as the adenovirus E1A, the simian virus 40 T antigen, and the human papillomavirus type 16 E7. The Rb-binding proteins have effects similar to those of cellular mutations inactivating the Rb function: both result in down-regulation of Cdk4-Cdk6 activity in transformed and immortalized cells (9, 36, 49). In summary, we have demonstrated for the first time that Tax expression, T-cell growth, and Cdk4 and Cdk6 activities are linked in immortalized primary human T cells. The data hint at a novel strategy of viral oncogenes to overcome the cell cycle control of the Rb tumor suppressor and to induce cell proliferation.
ACKNOWLEDGMENTS
This work was supported by the Deutsche Forschungsgemeinschaft (SFB-466) and Wilhelm Sauder Stiftung.
We are grateful to Edgar Meinl, O. John Semmes, Bryan Cullen, Beatrice Langton, and the AIDS Research and Reference Reagent Program (Division of AIDS, NIAID) for kindly providing the cell line SS8BPT, antisera, and anti-Tax hybridomas. The excellent technical assistance of Claudia Koch is appreciated. We thank Hermann Bujard, Sabine Lang, and Andreas Baur for helpful discussions and Bernhard Fleckenstein for his permanent and generous support of this study.
REFERENCES
- 1.Adya N, Giam C-Z. Distinct regions in human T-cell lymphotropic virus type I Tax mediate interactions with activator protein CREB and basal transcription factors. J Virol. 1995;69:1834–1841. doi: 10.1128/jvi.69.3.1834-1841.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adya N, Zhao L J, Huang W, Boros I, Giam C Z. Expansion of CREB’s DNA recognition specificity by Tax results from interaction with Ala-Ala-Arg at positions 282–284 near the conserved DNA-binding domain of CREB. Proc Natl Acad Sci USA. 1994;91:5642–5646. doi: 10.1073/pnas.91.12.5642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ajchenbaum F, Ando K, DeCaprio J A, Griffin J D. Independent regulation of human D-type cyclin gene expression during G1 phase in primary human T lymphocytes. J Biol Chem. 1993;268:4113–4119. [PubMed] [Google Scholar]
- 4.Akagi T, Ono H, Shimotohno K. Characterization of T cells immortalized by Tax1 of human T-cell leukemia virus type 1. Blood. 1995;86:4243–4249. [PubMed] [Google Scholar]
- 5.Akagi T, Ono H, Shimotohno K. Expression of cell-cycle regulatory genes in HTLV-I infected T-cell lines: possible involvement of Tax1 in the altered expression of cyclin D2, p18Ink4 and p21Waf1/Cip1/Sdi1. Oncogene. 1996;12:1645–1652. [PubMed] [Google Scholar]
- 6.Akagi T, Shimotohno K. Proliferative response of Tax1-transduced primary human T cells to anti-CD3 antibody stimulation by an interleukin-2-independent pathway. J Virol. 1993;67:1211–1217. doi: 10.1128/jvi.67.3.1211-1217.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alt M, Fleckenstein B, Grassmann R. A pair of selectable herpesvirus vectors for simultaneous gene expression in human lymphoid cells. Gene. 1991;102:265–269. doi: 10.1016/0378-1119(91)90088-s. [DOI] [PubMed] [Google Scholar]
- 8.Baldin V, Lukas J, Marcote M J, Pagano M, Draetta G. Cyclin D1 is a nuclear protein required for cell cycle progression in G1. Genes Dev. 1993;7:812–821. doi: 10.1101/gad.7.5.812. [DOI] [PubMed] [Google Scholar]
- 9.Bates S, Parry D, Bonetta L, Vousden K, Dickson C, Peters G. Absence of cyclin D/cdk complexes in cells lacking functional retinoblastoma protein. Oncogene. 1994;9:1633–1640. [PubMed] [Google Scholar]
- 10.Brauweiler A, Garl P, Franklin A A, Giebler H A, Nyborg J K. A molecular mechanism for human T-cell leukemia virus latency and Tax transactivation. J Biol Chem. 1995;270:12814–12822. doi: 10.1074/jbc.270.21.12814. [DOI] [PubMed] [Google Scholar]
- 11.Burton J D, Bamford R N, Peters C, Grant A J, Kurys G, Goldman C K, Brennan J, Roessler E, Waldmann T A. A lymphokine, provisionally designated interleukin T and produced by a human adult T-cell leukemia line, stimulates T-cell proliferation and the induction of lymphokine-activated killer cells. Proc Natl Acad Sci USA. 1994;91:4935–4939. doi: 10.1073/pnas.91.11.4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cross S L, Feinberg M B, Wolf J B, Holbrook N J, Wong Staal F, Leonard W J. Regulation of the human interleukin-2 receptor alpha chain promoter: activation of a nonfunctional promoter by the transactivator gene of HTLV-I. Cell. 1987;49:47–56. doi: 10.1016/0092-8674(87)90754-9. [DOI] [PubMed] [Google Scholar]
- 13.Desrosiers R C, Silva D P, Waldron L M, Letvin N L. Nononcogenic deletion mutants of herpesvirus saimiri are defective for in vitro immortalization. J Virol. 1986;57:701–705. doi: 10.1128/jvi.57.2.701-705.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ewen M E, Sluss H K, Sherr C J, Matsushime H, Kato J, Livingston D M. Functional interactions of the retinoblastoma protein with mammalian D-type cyclins. Cell. 1993;73:487–497. doi: 10.1016/0092-8674(93)90136-e. [DOI] [PubMed] [Google Scholar]
- 15.Ewen M E, Sluss H K, Whitehouse L L, Livingston D M. TGF beta inhibition of Cdk4 synthesis is linked to cell cycle arrest. Cell. 1993;74:1009–1020. doi: 10.1016/0092-8674(93)90723-4. [DOI] [PubMed] [Google Scholar]
- 16.Fickenscher H, Biesinger B, Knappe A, Wittmann S, Fleckenstein B. Regulation of the herpesvirus saimiri oncogene stpC, similar to that of T-cell activation genes, in growth-transformed human T lymphocytes. J Virol. 1996;70:6012–6019. doi: 10.1128/jvi.70.9.6012-6019.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fujii M, Niki T, Mori T, Matsuda T, Matsui M, Nomura N, Seiki M. HTLV-1 Tax induces expression of various immediate early serum responsive genes. Oncogene. 1991;6:1023–1029. [PubMed] [Google Scholar]
- 18.Fujii M, Tsuchiya H, Chuhjo T, Akizawa T, Seiki M. Interaction of HTLV-1 Tax1 with p67SRF causes the aberrant induction of cellular immediate early genes through CArG boxes. Genes Dev. 1992;6:2066–2076. doi: 10.1101/gad.6.11.2066. [DOI] [PubMed] [Google Scholar]
- 19.Furth P A, St. Onge L, Boger H, Gruss P, Gossen M, Kistner A, Bujard H, Hennighausen L. Temporal control of gene expression in transgenic mice by a tetracycline-responsive promoter. Proc Natl Acad Sci USA. 1994;91:9302–9306. doi: 10.1073/pnas.91.20.9302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geng Y, Weinberg R A. Transforming growth factor beta effects on expression of G1 cyclins and cyclin-dependent protein kinases. Proc Natl Acad Sci USA. 1993;90:10315–10319. doi: 10.1073/pnas.90.21.10315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gossen M, Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci USA. 1992;89:5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grassmann R, Berchtold S, Radant I, Alt M, Fleckenstein B, Sodroski J G, Haseltine W A, Ramstedt U. Role of human T-cell leukemia virus type 1 X region proteins in immortalization of primary human lymphocytes in culture. J Virol. 1992;66:4570–4575. doi: 10.1128/jvi.66.7.4570-4575.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grassmann R, Dengler C, Muller Fleckenstein I, Fleckenstein B, McGuire K, Dokhelar M C, Sodroski J G, Haseltine W A. Transformation to continuous growth of primary human T lymphocytes by human T-cell leukemia virus type I X-region genes transduced by a herpesvirus saimiri vector. Proc Natl Acad Sci USA. 1989;86:3351–3355. doi: 10.1073/pnas.86.9.3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grassmann R, Fleckenstein B. Selectable recombinant herpesvirus saimiri is capable of persisting in a human T-cell line. J Virol. 1989;63:1818–1821. doi: 10.1128/jvi.63.4.1818-1821.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grassmann R, Fleckenstein B, Desrosiers R C. Viral transformation of human T-lymphocytes. Adv Cancer Res. 1994;63:211–243. doi: 10.1016/s0065-230x(08)60401-7. [DOI] [PubMed] [Google Scholar]
- 26.Grossman W, Kimata J T, Wong F H, Zutter M, Ratner L. Development of leukemia in mice transgenic for the tax gene of human T-cell leukemia virus type I. Proc Natl Acad Sci USA. 1995;92:1057–1061. doi: 10.1073/pnas.92.4.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Himes S R, Coles L S, Katsikeros R, Lang R K, Shannon M F. HTLV-1 tax activation of the GM-CSF and G-CSF promoters requires the interaction of NF-κB with other transcription factor families. Oncogene. 1993;8:3189–3197. [PubMed] [Google Scholar]
- 28.Hollsberg P, Ausubel L J, Hafler D A. Human T cell lymphotropic virus type I-induced T cell activation. Resistance to TGF-beta 1-induced suppression. J Immunol. 1994;153:566–573. [PubMed] [Google Scholar]
- 29.Inatsuki A, Yasukawa M, Kobayashi Y. Functional alterations of herpes simplex virus-specific CD4+ multifunctional T cell clones following infection with human T lymphotropic virus type I. J Immunol. 1989;143:1327–1333. [PubMed] [Google Scholar]
- 30.Inoue J, Seiki M, Taniguchi T, Tsuru S, Yoshida M. Induction of interleukin 2 receptor gene expression by p40x encoded by human T-cell leukemia virus type 1. EMBO J. 1986;5:2883–2888. doi: 10.1002/j.1460-2075.1986.tb04583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jeang K T, Widen S G, Semmes O J, Wilson S H. HTLV-I trans-activator protein, Tax, is a trans-repressor of the human beta-polymerase gene. Science. 1990;247:1082–1084. doi: 10.1126/science.2309119. [DOI] [PubMed] [Google Scholar]
- 32.Jung J U, Stager M, Desrosiers R C. Virus-encoded cyclin. Mol Cell Biol. 1994;14:7235–7244. doi: 10.1128/mcb.14.11.7235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kanno T, Brown K, Franzoso G, Siebenlist U. Kinetic analysis of human T-cell leukemia virus type I Tax-mediated activation of NF-κB. Mol Cell Biol. 1994;14:6443–6451. doi: 10.1128/mcb.14.10.6443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kato J, Matsushime H, Hiebert S W, Ewen M E, Sherr C J. Direct binding of cyclin D to the retinoblastoma gene product (pRb) and pRb phosphorylation by the cyclin D-dependent kinase CDK4. Genes Dev. 1993;7:331–342. doi: 10.1101/gad.7.3.331. [DOI] [PubMed] [Google Scholar]
- 35.Khatib Z A, Matsushime H, Valentine M, Shapiro D N, Sherr C J, Look A T. Coamplification of the CDK4 gene with MDM2 and GLI in human sarcomas. Cancer Res. 1993;53:5535–5541. [PubMed] [Google Scholar]
- 36.Khleif S N, DeGregori J, Yee C L, Otterson G A, Kaye F J, Nevins J R, Howley P M. Inhibition of cyclin D-CDK4/CDK6 activity is associated with an E2F-mediated induction of cyclin kinase inhibitor activity. Proc Natl Acad Sci USA. 1996;93:4350–4354. doi: 10.1073/pnas.93.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim S J, Kehrl J H, Burton J, Tendler C L, Jeang K T, Danielpour D, Thevenin C, Kim K Y, Sporn M B, Roberts A B. Transactivation of the transforming growth factor beta 1 (TGF-beta 1) gene by human T lymphotropic virus type 1 Tax: a potential mechanism for the increased production of TGF-beta 1 in adult T cell leukemia. J Exp Med. 1990;172:121–129. doi: 10.1084/jem.172.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koga Y, Oh Hori N, Sato H, Yamamoto N, Kimura G, Nomoto K. Absence of transcription of lck (lymphocyte specific protein tyrosine kinase) message in IL-2-independent, HTLV-I-transformed T cell lines. J Immunol. 1989;142:4493–4499. [PubMed] [Google Scholar]
- 39.Lucas J J, Szepesi A, Modiano J F, Domenico J, Gelfand E W. Regulation of synthesis and activity of the PLSTIRE protein (cyclin-dependent kinase 6 (cdk6)), a major cyclin D-associated cdk4 homologue in normal human T lymphocytes. J Immunol. 1995;154:6275–6284. [PubMed] [Google Scholar]
- 40.Matsushime H, Ewen M E, Strom D K, Kato J Y, Hanks S K, Roussel M F, Sherr C J. Identification and properties of an atypical catalytic subunit (p34PSK-J3/cdk4) for mammalian D type G1 cyclins. Cell. 1992;71:323–334. doi: 10.1016/0092-8674(92)90360-o. [DOI] [PubMed] [Google Scholar]
- 41.Matsushime H, Quelle D E, Shurtleff S A, Shibuya M, Sherr C J, Kato J Y. D-type cyclin-dependent kinase activity in mammalian cells. Mol Cell Biol. 1994;14:2066–2076. doi: 10.1128/mcb.14.3.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meinl E, Hohlfeld R, Wekerle H, Fleckenstein B. Immortalization of human T cells by herpesvirus saimiri. Immunol Today. 1995;16:55–58. doi: 10.1016/0167-5699(95)80087-5. [DOI] [PubMed] [Google Scholar]
- 43.Meyerson M, Harlow E. Identification of G1 kinase activity for cdk6, a novel cyclin D partner. Mol Cell Biol. 1994;14:2077–2086. doi: 10.1128/mcb.14.3.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Migone T S, Lin J X, Cereseto A, Mulloy J C, O’Shea J J, Franchini G, Leonard W J. Constitutively activated Jak-STAT pathway in T cells transformed with HTLV-I. Science. 1995;269:79–81. doi: 10.1126/science.7604283. [DOI] [PubMed] [Google Scholar]
- 45.Modiano J F, Domenico J, Szepesi A, Lucas J J, Gelfand E W. Differential requirements for interleukin-2 distinguish the expression and activity of the cyclin-dependent kinases Cdk4 and Cdk2 in human T cells. J Biol Chem. 1994;269:32972–32978. [PubMed] [Google Scholar]
- 46.Mori N, Prager D. Transactivation of the interleukin-1alpha promoter by human T-cell leukemia virus type I and type II Tax proteins. Blood. 1996;87:3410–3417. [PubMed] [Google Scholar]
- 47.Nerenberg M I, Hinrichs S H, Reynolds R K, Khoury G, Jay G. The tat gene of human T-lymphotropic virus type 1 induces mesenchymal tumors in transgenic mice. Science. 1987;237:1324–1329. doi: 10.1126/science.2888190. [DOI] [PubMed] [Google Scholar]
- 48.Okamoto A, Demetrick D J, Spillare E A, Hagiwara K, Hussain S P, Bennett W P, Forrester K, Gerwin B, Serrano M, Beach D H, et al. Mutations and altered expression of p16INK4 in human cancer. Proc Natl Acad Sci USA. 1994;91:11045–11049. doi: 10.1073/pnas.91.23.11045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Parry D, Bates S, Mann D J, Peters G. Lack of cyclin D-Cdk complexes in Rb-negative cells correlates with high levels of p16INK4/MTS1 tumour suppressor gene product. EMBO J. 1995;14:503–511. doi: 10.1002/j.1460-2075.1995.tb07026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Poiesz B J, Ruscetti F W, Gazdar A F, Bunn P A, Minna J D, Gallo R C. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci USA. 1980;77:7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pozzatti R, Vogel J, Jay G. The human T-lymphotropic virus type I tax gene can cooperate with the ras oncogene to induce neoplastic transformation of cells. Mol Cell Biol. 1990;10:413–417. doi: 10.1128/mcb.10.1.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Quelle D E, Ashmun R A, Shurtleff S A, Kato J Y, Bar Sagi D, Roussel M F, Sherr C J. Overexpression of mouse D-type cyclins accelerates G1 phase in rodent fibroblasts. Genes Dev. 1993;7:1559–1571. doi: 10.1101/gad.7.8.1559. [DOI] [PubMed] [Google Scholar]
- 53.Reid R L, Lindholm P F, Mireskandari A, Dittmer J, Brady J N. Stabilization of wild-type p53 in human T-lymphocytes transformed by HTLV-I. Oncogene. 1993;8:3029–3036. [PubMed] [Google Scholar]
- 54.Resnitzky D, Gossen M, Bujard H, Reed S I. Acceleration of the G1/S phase transition by expression of cyclins D1 and E with an inducible system. Mol Cell Biol. 1994;14:1669–1679. doi: 10.1128/mcb.14.3.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Resnitzky D, Hengst L, Reed S I. Cyclin A-associated kinase activity is rate limiting for entrance into S phase and is negatively regulated in G1 by p27Kip1. Mol Cell Biol. 1995;15:4347–4352. doi: 10.1128/mcb.15.8.4347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Resnitzky D, Reed S I. Different roles for cyclins D1 and E in regulation of the G1-to-S transition. Mol Cell Biol. 1995;15:3463–3469. doi: 10.1128/mcb.15.7.3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56a.Rosin, O. Unpublished observations.
- 57.Simmer B, Alt M, Buckreus I, Berthold S, Fleckenstein B, Platzer E, Grassmann R. Persistence of selectable herpesvirus saimiri in various human haematopoietic and epithelial cell lines. J Gen Virol. 1991;72:1953–1958. doi: 10.1099/0022-1317-72-8-1953. [DOI] [PubMed] [Google Scholar]
- 58.Sun S-C, Elwood J, Béraud C, Greene W C. Human T-cell leukemia virus type I Tax activation of NF-κB/Rel involves phosphorylation and degradation of IκBα and RelA (p65)-mediated induction of the c-rel gene. Mol Cell Biol. 1994;14:7377–7384. doi: 10.1128/mcb.14.11.7377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sun S C, Ganchi P A, Ballard D W, Greene W C. NF-kappa B controls expression of inhibitor I kappa B alpha: evidence for an inducible autoregulatory pathway. Science. 1993;259:1912–1915. doi: 10.1126/science.8096091. [DOI] [PubMed] [Google Scholar]
- 60.Suzuki T, Kitao S, Matsushime H, Yoshida M. HTLV-1 Tax protein interacts with cyclin-dependent kinase inhibitor p16INK4A and counteracts its inhibitory activity towards CDK4. EMBO J. 1996;15:1607–1614. [PMC free article] [PubMed] [Google Scholar]
- 61.Suzuki T, Kitao S, Matsushime H, Yoshida M. HTLV-1 Tax protein interacts with cyclin-dependent kinase inhibitor p16INK4A and counteracts its inhibitory activity towards CDK4. EMBO J. 1996;15:1607–1614. [PMC free article] [PubMed] [Google Scholar]
- 62.Tanaka A, Takahashi C, Yamaoka S, Nosaka T, Maki M, Hatanaka M. Oncogenic transformation by the tax gene of human T-cell leukemia virus type I in vitro. Proc Natl Acad Sci USA. 1990;87:1071–1075. doi: 10.1073/pnas.87.3.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van den Heuvel S, Harlow E. Distinct roles for cyclin-dependent kinases in cell cycle control. Science. 1993;262:2050–2054. doi: 10.1126/science.8266103. [DOI] [PubMed] [Google Scholar]
- 64.Waldmann T A. The multi-subunit interleukin-2 receptor. Annu Rev Biochem. 1989;58:875–911. doi: 10.1146/annurev.bi.58.070189.004303. [DOI] [PubMed] [Google Scholar]
- 65.Weber F, Meinl E, Drexler K, Czlonkowska A, Huber S, Fickenscher H, Muller Fleckenstein I, Fleckenstein B, Wekerle H, Hohlfeld R. Transformation of human T-cell clones by herpesvirus saimiri: intact antigen recognition by autonomously growing myelin basic protein-specific T cells. Proc Natl Acad Sci USA. 1993;90:11049–11053. doi: 10.1073/pnas.90.23.11049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yoshida M, Miyoshi I, Hinuma Y. Isolation and characterization of retrovirus from cell lines of human adult T-cell leukemia and its implication in the disease. Proc Natl Acad Sci USA. 1982;79:2031–2035. doi: 10.1073/pnas.79.6.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]