Abstract

N-Acetyl muramic acid (NAM) probes containing alkyne or azide groups are commonly used to investigate aspects of cell wall synthesis because of their small size and ability to incorporate into bacterial peptidoglycan (PG). However, copper-catalyzed alkyne–azide cycloaddition (CuAAC) reactions are not compatible with live cells, and strain-promoted alkyne–azide cycloaddition (SPAAC) reaction rates are modest and, therefore, not as desirable for tracking the temporal alterations of bacterial cell growth, remodeling, and division. Alternatively, the tetrazine-trans-cyclooctene ligation (Tz-TCO), which is the fastest known bioorthogonal reaction and not cytotoxic, allows for rapid live-cell labeling of PG at biologically relevant time scales and concentrations. Previous work to increase reaction kinetics on the PG surface by using tetrazine probes was limited because of low incorporation of the probe. Described here are new approaches to construct a minimalist tetrazine (Tz)-NAM probe utilizing recent advancements in asymmetric tetrazine synthesis. This minimalist Tz-NAM probe was successfully incorporated into pathogenic and commensal bacterial PG where fixed and rapid live-cell, no-wash labeling was successful in both free bacterial cultures and in coculture with human macrophages. Overall, this probe allows for expeditious labeling of bacterial PG, thereby making it an exceptional tool for monitoring PG biosynthesis for the development of new antibiotic screens. The versatility and selectivity of this probe will allow for real-time interrogation of the interactions of bacterial pathogens in a human host and will serve a broader utility for studying glycans in multiple complex biological systems.
Introduction
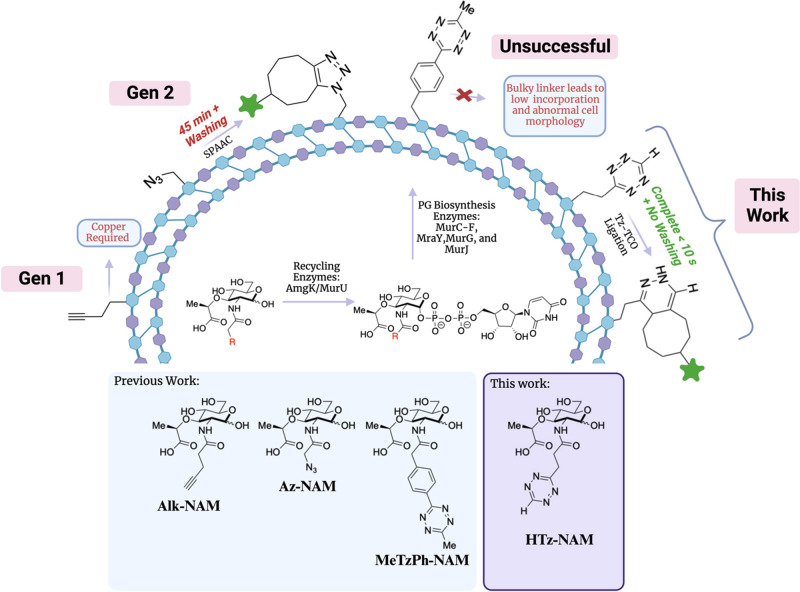
In human health, the bacterial cell wall serves as a molecular calling card for the immune system and is an excellent target for front-line antibiotics.1−3 Peptidoglycan (PG), exclusively found in all bacteria, produces a protective barrier to maintain structural integrity against a variety of environmental insults and osmotic pressure. The PG layer, located just outside the plasma membrane, is a glycopeptide polymer composed of repeating glycan units of N-acetyl muramic acid (NAM) and N-acetyl glucosamine (NAG/GlcNAc). A pentapeptide chain is tethered to the d-lactyl moiety on the 3-position of NAM and consists of l- and d -amino acids. This pentapeptide is then further cross-linked to achieve a three-dimensional macromolecular structure around the cell.4,5 PG biosynthesis is highly conserved across Gram-positive and Gram-negative bacteria and involves over 10 distinct enzymatic processes bringing together NAM, NAG, and peptide building blocks. Enzymes utilized in PG construction have been a major target for therapeutics in the clinic.6,7 The first committed step in the PG biosynthetic pathway is the conversion of uridine diphosphate (UDP)-NAG into UDP-NAM by MurA and MurB. In a stepwise fashion, a series of amino acid ligases (MurC-MurF) then affix the pentapeptide chain onto UDP-NAM, which is temporarily tethered to the membrane by MraY, glycosylated with a NAG residue via MurG, and transported across the membrane by the flippase, MurJ. Finally, mature PG is constructed through the polymerization of lipid II dimers/multimers by transglycosylases, and adjacent peptide chains are cross-linked by transpeptidases. The individual units of the PG are valuable resources to the bacterial cell and several recycling pathways exist to scavenge and then reuse these building blocks. For example, Mayer and co-workers have shown that the Pseudomonas family of bacteria are capable of scavenging UDP-NAM from digested NAM-peptides using recycling enzymes AmgK (N-acetylmuramate/N-acetylglucosamine kinase) and MurU (N-acetylmuramate alpha-1-phosphate uridylyltransferase) as a means to bypass conventional UDP-NAM generation by MurA and MurB (Figure 1).8,9 These multiple biochemical steps—both de novo PG biosynthesis and recycling—present opportunities for interrogation by metabolic labeling strategies.10,11
Figure 1.
Evolution of NAM Probes. NAM derivatives bearing bioorthogonal handles are metabolically incorporated through recycling enzymes, AmgK and MurU, to achieve the corresponding UDP-NAM precursor necessary to shuttle into PG biosynthesis that includes MurC-F, MraY, MurG, and MurJ. Action by transpeptidases and transglycosylases display the bioorthogonal handle in mature PG. The first-generation system involved CuAAC chemistry between Alk-NAM and azide-conjugate fluorophores. A second-generation system utilized the SPAAC reaction of Az-NAM with a cyclooctyne–fluorophore. A third system applied MeTzPh-NAM through a Tz-TCO ligation but was limited by low incorporation and abnormal morphology for probe-incorporated cells. Introduced here is the new probe, HTz-NAM, which can successfully incorporate and undergo Tz-TCO ligation at rapid rates.
Our lab and many others have developed tools that can be used to metabolically and biochemically incorporate probes into the PG polymer through the function of promiscuous transporters and biosynthetic machinery (Figure 1).12−14 Ideally, bioorthogonal reporters for the PG should be small, stable under physiological conditions, and able to support the vitality of the host after incorporation.15−18 Bioorthogonal chemistry has emerged as a powerful tool for the study of biological processes,19,20 cellular imaging,21−27 drug delivery,28−30 glycobiology,31−34 and proteomics.35−38 As we come to further understand the preferences and boundaries of what can be accepted by the PG biosynthetic machinery, we can employ rational design of new chemistries and modifications.
Reaction rate is an essential consideration for live-cell labeling and imaging experiments in bacteria. Slower reaction kinetics necessitate higher concentrations of reacting partners and longer incubation times that can exceed the time scale of both bacterial cell growth and division. Increasing fluorophore concentrations for live-cell imaging can lead to higher off-target labeling and increased background fluorescence, which necessitates extensive washing procedures. Conversely, longer incubation times will miss out on monitoring key temporal aspects of the fast-moving PG synthesis, which occurs within minutes.39,40 In order to optimize live-cell imaging experiments for real-time labeling of PG at biologically relevant concentrations, bioorthogonal reactions with rapid reaction rates are needed.
NAM is solely found in bacteria and, through dedicated recycling enzymes, can be converted into UDP-NAM, the initial building block for PG biosynthesis, which makes it the ideal site for attachment of a bioorthogonal handle.8,9 Previously, we evaluated a library of synthetically accessible NAM probes, including those bearing the bioorthogonal alkyne (Alk-NAM, Figure 1) and azide (Az-NAM, Figure 1) functionalities as tools for investigating PG biosynthesis (Figure 1). The functionalized NAMs could then undergo downstream bioorthogonal reactions to visualize and track PG synthesis and breakdown.41 The copper-catalyzed azide–alkyne cycloaddition (CuAAC) has been applied extensively because of the small reaction partner sizes, but the requirement of a cytotoxic copper catalyst has limited its use in live-cell applications.42 To avoid the use of copper, strain-promoted azide–alkyne cycloaddition (SPAAC) has been employed to image and study glycans through metabolic engineering, albeit with modest live-cell kinetics.43−45
Tetrazine–trans-cyclooctene ligation (Tz-TCO) is a rapid bioorthogonal reaction that proceeds with second-order rate constants of up to 106 M–1 s–1, which allows for visualization of cellular processes at biologically relevant concentrations.46 Applications of Tz-TCO ligation in complex environments through metabolic engineering has been restricted because of limited synthetic accessibility in attaining small Tz and TCO moieties. Notably, the Pires lab has developed tetrazine-bearing d-amino acid reporters that can be metabolically incorporated into bacterial cells by leveraging substrate promiscuity of PG transpeptidases.47−49 However, we note that these probes can be lost during the bacterial growth and remodeling phases; complementary probes that label on the glycan are needed to monitor these dynamic processes.50 Previously, we showed that a NAM derivative bearing a “standard” 3-methyl-6-aryltetrazine (MeTzPh-NAM, Figure 1) was accepted as a substrate for PG recycling and incorporated into the bacterial cell wall. Unfortunately, only a small subpopulation of cells incorporated MeTzPh-NAM, and those that did showed abnormal cell morphologies and diffuse labeling with TCO-fluorophores.51 We hypothesized that this is due to the bulky phenyl linker between the Tz handle and NAM and desired a smaller Tz moiety for glycan labeling.
Recently, we have described versatile methods for the synthesis of tetrazines from ester precursors.52,53 Herein, we describe how these synthetic methods were adapted to construct a “minimalist” tetrazine probe (HTz-NAM, Figure 1) where NAM is attached to the tetrazine by a small 3-carbon linker. This probe is introduced during the first committed step of PG biosynthesis, thereby permitting the entire biosynthetic program to be tracked. Use of HTz-NAM results in high levels of metabolic incorporation into bacterial PG while maintaining the rapid reaction kinetics of the Tz-TCO ligation. The minimalist HTz-NAM probe allows for expansion of the glycobiology toolbox by enabling rapid, real-time, live-cell, no-wash labeling of bacterial PG in both pathogenic and commensal Gram-negative and Gram-positive species. We demonstrate the utility of HTz-NAM in several biologically and immunologically significant applications indicating its versatility.
Results and Discussion
Synthesis of Minimal NAM Probes
2-Aminomuramic acid was prepared on large scale using the streamlined synthesis recently developed in our group,54 and functional groups were introduced via N-acylation of the free amine. HTz-NAM was synthesized as shown in Figure 2. Steglich esterification of monomethyl succinate gave methyl[(3-methyloxetan-3-yl)methyl] succinate (1), which could be converted to thiomethyltetrazine [3, Supporting Information (SI) Figure 1] by a one-pot sequence of BF3·OEt2-promoted oxabicyclo[2.2.2] octyl (OBO) orthoester, condensation with methylthiocarbohydrazide iodide salt (2), and oxidation by phenyliodonium diacetate (PIDA).52 Pd-catalyzed reduction of 3 with triethylsilane followed by PIDA oxidation gave monosubstituted tetrazine (4, SI Figure 2) in 77% yield. Hydrolysis of 4 using trimethyltin hydroxide followed by esterification with pentafluorophenyl trifluoroacetate and diisopropylethylamine yielded the activated HTz-pentafluorophenyl ester (5, SI Figure 3) in 83% yield. We used the pentafluorophenyl (Pfp) ester rather than the N-hydroxysuccinimide ester because of the difficulty of removing N-hydroxysuccinimide from HTz-NAM by chromatography. Coupling 5 with 2-aminomuramic acid afforded the minimalist HTz-NAM in 80% yield.
Figure 2.
(A) Synthesis of HTz-Pfp-ester (5) and HTz-NAM, as well as small molecule fluorescent probes NBD-NAM and 4-DMAPth-NAM. See Supporting Information Figures 1–3 for DSC safety testing for tetrazine precursors 3, 4, and 5. (B) In vitro chemoenzymatic studies for NBD-NAM, 4-DMAPth NAM, and HTz-NAM. (C) Workflow for analyzing HTz-NAM incorporation into EQKU cells. (D) Growth curve for EQKU with varying concentrations of HTz-NAM to determine minimum concentration needed for growth recovery in the presence of a lethal dose of fosfomycin. (E) Lysozyme digestion to confirm HTz-NAM incorporation into mature PG via mass spectrometry analysis of disaccharide fragments.
To test direct incorporation of fluorophores into the glycan, we prepared fluorescent conjugates of 2-aminomuramic acid. Small fluorophores were chosen to maximize the likelihood of acceptance by the PG biosynthetic machinery. Vocadlo and co-workers reported NBD (nitrobenzoxadiazole)-NAG as a probe modality to track O-GlcNAc modified proteins in live cells; here, we adapted their protocol to install this minimal fluorophore on the NAM sugar.55 Briefly, commercially available NBD chloride underwent an SNAr reaction with a β-alanine linker (SI Figure 4). Preparation of the Pfp-activated ester and base-mediated coupling to 2-aminomuramic acid afforded NBD-NAM in 40% yield (Figure 2A). As another alternative, 4-DMAPth (4-dimethylamino phthalimide) has been widely used as a solvatochromic fluorophore for studying protein–protein interactions because of its low-fluorescence quantum yield in polar versus nonpolar environments.56,57 The Imperiali group demonstrated methodology to access the anhydride precursors of this fluorophore type, including 4-DMAPth, which could be linked to amino acids with relative ease.58 Briefly, 4-DMAPth can be prepared using commercially available 4-aminophthalic acid in a two-step process involving reductive amination and subsequent dehydration to yield the anhydride product. The resulting phthalic anhydride then undergoes a dehydrative condensation with β-alanine to form the corresponding phthalimide (SI Figure 5). Pfp trifluoroacetate was then used to form the activated Pfp-ester, which was then coupled to 2-aminomuramic acid to afford 4-DMAPth-NAM in 35% yield (Figure 2A).
Testing Probe Tolerance to Remodeling Workflow
With the newly synthesized probes, their compatibility with the metabolic incorporation system was assayed using a model, engineered strain of Escherichia coli, EQKU, which was able to incorporate a variety of NAM probes into its cell wall.41,51 Briefly, this strain contains a MurQ knockout to limit NAM-6P catabolic processing into NAG. EQKU also contains recycling enzymes AmgK and MurU from the species P. putida, which were encoded into an isopropyl ß-d-1-thiogalactopyranoside (IPTG)-inducible plasmid to enable the incorporation of NAM substrates into mature PG (Figure 2B). First, an in vitro chemoenzymatic assay was employed using purified proteins to observe the turnover of the sugar probes by AmgK into the corresponding α-1-phosphate product and then subsequent conversion by MurU into the UDP-sugar (Figure 2B). AmgK can tolerate a variety of modifications51 and was able to convert all three NAM probes to the corresponding phosphate sugars (Figure 2B). MurU is more restrictive, and the UDP-product of only the HTz-NAM probe was observed (SI Figure 6). Qualitatively, mass spectrometry analysis suggests that AmgK turns over the probes with similar efficiency as previously reported Alk-NAM and Az-NAM.
Following confirmation that relevant UDP-HTz-NAM precursor can be produced chemoenzymatically, its incorporation into whole bacterial PG of EQKU cells was validated (Figure 2C). A growth recovery assay was used with varying concentrations of the HTz-NAM probe (0.06 mM to 6 mM) while under a lethal dose of fosfomycin to determine the minimum concentration of HTz-NAM needed for cell vitality during remodeling. Fosfomycin inhibits the first committed step of de novo PG biosynthesis via MurA,59 which prevents the cell from naturally producing UDP-NAM from UDP-NAG. The HTz-NAM is able to rescue bacterial cell growth at probe concentrations as low as 0.15 mM for at least 6 h, thereby indicating this probe can sustain cell growth in the presence of fosfomycin through the production of UDP-HTz-NAM, which is then shuttled into PG biosynthesis (Figure 2D). To further verify that the probe is directly incorporating into the bacterial PG, remodeled cells were treated with lysozyme to digest the PG into distinct NAG/NAM disaccharide subunits. The digested samples were then analyzed using high-resolution mass spectrometry (HRMS), and the disaccharide fragment containing the Tz moiety was observed (Figure 2E), thereby validating that the HTz-NAM is, indeed, incorporated into the mature polymer.
Visualization of the PG Structure Using HTz-NAM and Kinetics of Tz-TCO Ligation
After confirming that HTz-NAM was incorporated into PG, we next aimed to ensure that tetrazine ligation could be used to visualize the probe in the cell wall. As bioorthogonal coupling partners for HTz-NAM, we used axial-5-hydroxy-trans-cyclooctene (“aTCO”) analogues, which display a favorable combination of fast kinetics and physiochemical properties that enable cell permeability and fast washout.60 aTCO–fluorophore conjugates were synthesized from commercially available aTCO-NHS ester (Sigma-Aldrich). Through remodeling protocols established in our lab, EQKU cells were treated with HTz-NAM for three doubling times (60 min) in the presence of fosfomycin and IPTG (Figure 3A). Following fixation, the remodeled cells were treated with a TCO–5-carboxytetramethylrhodamine (TAMRA) fluorophore conjugate, aTCO-TAMRA, for 15 min. The cells were then visualized with confocal microscopy. Defined, bulk labeling of the cell periphery and FtsZ-septal division ring were observed in comparison with cells treated with NAM and fluorophore (Figure 3B). This labeling is superior to that obtained with the previously reported MeTzPh-NAM probe, which resulted in only select cells being labeled diffusely and nonspecifically with warped and unhealthy morphologies.
Figure 3.
(A) Labeling workflow for PG visualization in EQKU. (B) EQKU PG visualization using either NAM (left) or HTz-NAM (right) with aTCO-TAMRA or aTCO-SiR under fixed (top) or live conditions (bottom), respectively. (C) Tz-TCO kinetics analysis through real-time addition of aTCO-SiR to fixed EQKU cells that had been remodeled to display HTz-NAM in the PG. The kinetics were analyzed starting at 1 s following the 5.6 μM aTCO-SiR addition and represents an average across eight different cell regions across one cell population. See SI Videos 1 and 2, as well as SI Figures 10–13, for experimental details and biological replicate analysis.
This positive result directed us toward live-cell labeling experiments, which were initially attempted by incubating with aTCO-TAMRA. However, only select cells in each field of view were labeled at low concentrations of dye (SI Figure 7). Additionally, the TAMRA fluorophore conjugate was bound nonspecifically to the cells, as the NAM control cells showed significant fluorescence despite extensive washing (SI Figure 8). For these reasons, the fluorogenic aTCO-silarhodamine derivative (aTCO-SiR) was utilized.22,23,61−63 Using aTCO-SiR, rapid and complete labeling of the EQKU cells remodeled with HTz-NAM was observed with minimal background and no wash steps (SI Figure 9).
To measure the kinetics of the labeling reaction on the bacterial PG, confocal microscopy was used to monitor the gain-of-fluorescence in remodeled bacterial cells upon the addition of aTCO-SiR to HTz-NAM remodeled cells. EQKU cells were remodeled with HTz-NAM, fixed, and adhered to the bottom of multichambered well slides. The microscope was focused prior to the reaction using the bright-field and far-red channels. The media was then carefully replaced with a PBS solution of aTCO-SiR (5.6 μM) using a syringe. Analyzing change in fluorescence intensity over time under pseudo-first-order kinetics, the labeling utilizing Tz-TCO occurs on the bacterial PG of these cells with t1/2 = 1.0 ± 0.1 s (SI Videos 1 and 2, Figure 13C, and SI Figures 10–13), which is an improvement from our labeling protocols with CuAAC and SPAAC that required a minimum reaction time of 30–60 min followed by extensive washing protocols. With speeds this fast, this probe can be used to study peptidoglycan biosynthesis in real time at biologically relevant concentrations.
Expanding the Labeling into Commensal and Pathogenic Bacteria
The fast kinetics that we can access using this HTz-NAM probe led us to sample other species of bacteria for assessing the versatility of this probe. The model EQKU strain in the proof-of-concept experiments is a Gram-negative species with a thinner PG layer that is surrounded by a thicker outer membrane coated with lipopolysaccharides (LPS). We speculated that the HTz-NAM would be amenable in labeling strategies applied to a Gram-positive species that lacks an outer membrane. Using Bacillus subtilis-KU (BSKU),41 which has been engineered to harbor amgk and murU in its genome, efficient labeling (Figure 4A) using optimized growth conditions with the NAM probes was observed. The B. subtilis strain was able to be remodeled and fluorescently labeled with either Tz-TCO or CuAAC click conditions; mass spectrometry confirmed the presence of each of these NAM probes, and real-time addition kinetics showed that labeling with aTCO-SiR occurs with t1/2 = 1.1 ± 0.3 s (SI Videos 3 and 4 and SI Figures 14–21).
Figure 4.
Versatility of HTz-NAM remodeling across a variety of bacterial species. (A) Confocal fluorescent images of (left to right) B. subtilus (BSKU), Gram-positive; E. coli strains (DH5α-Tf-KU and RFM795-Tf-KU), Gram-negative; wild-type Pseudomonas species (P. putida and P. aeruginosa). Scale bars, 10 μm. (B) Observation of cell wall detail throughout bacterial growth and division stages. EQKU and DH5α-Tf-KU: aTCO-TAMRA (red) imaged on Zeiss LSM 800. BSKU and RFM795-Tf-KU: aTCO-SiR (pink) imaged on Zeiss LSM 800. P. putida and P. aeruginosa: aTCO-SiR (purple) imaged on Andor Dragonfly 600. Scale bars, 2 μm. (C) P. aeruginosa high-resolution images of no-wash, live labeling with aTCO-SiR. Scale bars, 2 μm. Deconvolution achieved through Imaris software. All images were taken of samples that were performed in biological and technical replicates (see SI Figures 38–92).
Additionally, two strains of E. coli, DH5α-Tf-KU and RFM795-Tf-KU, that utilize AmgK and MurU orthologs from the oral pathogen, Tannerella forsythia, an obligate NAM auxotroph, which seem to convert NAM more efficiently to the prerequisite UDP-NAM building block, were created.45 RFM795 has a defect in its outer membrane protein production that makes it more susceptible to the passage of small molecules and allows for better access to the inner PG layer.64 This leaky E. coli strain, RFM795-Tf-KU, was fully validated to remodel with NAM probes and label with either CuAAC or tetrazine chemistries (SI Figures 22–26), as were the other E. coli strains (Figure 4A,B).
Gratifyingly, when flow cytometry was used to analyze the E. coli population labeled with TCO-AF488 to provide a gross comparison to our standard Alkyne-488 dye previously used for Az-NAM labeling analyses, we observed similar percentages for HTz-NAM and Az-NAM labeling, which suggests comparable probe incorporation (SI Figures 27 and 28). When comparing the HTz-NAM labeled populations to the NAM populations for both E. coli strains and BSKU, we saw statistically significant differences (p < 0.0001) for all three strains remodeled and labeled with HTz-NAM/aTCO-SiR, thereby indicating successful bulk population labeling of these strains compared with the controls (SI Figure 29). This was further confirmed on a single-cell analysis through confocal microscopy, which permitted visualization of the cell wall for all cell types in both fixed and live labeling (Figure 4A) using a variety of fluorophores and corroborated the flow data. It is interesting to note that we found that different bacteria strains labeling is affected by the choice of fluorophore. We note that differences in cell wall architecture play a role in how well the aTCO-SiR dye is able to react with the HTz-NAM in the mature PG, specifically with RFM795 “leaky E. coli” cells that show a significantly higher population of fluorescently labeled cells when compared with their “wild-type” counterpart (SI Figure 30). Expanding the growth curve analysis of the “leaky E. coli” strain RFM showed that lower concentrations of HTz-NAM (100 μM) could sustain bacterial growth (SI Figure 31); these conditions were translated to imaging studies where lower concentrations of HTz-NAM were used for remodeling, and no-wash labeling was achieved with aTCO-SiR (SI Figure 32). These initial glimpses of labeling PG in a variety of bacteria demonstrate that HTz-NAM is an effective tool that can label both Gram-positive and Gram-negative bacterial PGs.
In addition to the genetically modified strains, we desired to expand the labeling with HTz-NAM into strains that naturally recycle PG. The Pseudomonadaceae family of bacteria are adept in the use of PG recycling pathways. Two well-characterized pseudomonads, Pseudomonas putida and Pseudomonas aeruginosa, naturally harbor the PG recycling enzymes, AmgK and MurU.9 Notably, P. aeruginosa is an opportunistic pathogen implicated in many nosocomial infections as a bacterium that is difficult to irradicate. As part of the well-characterized “ESKAPE” nosocomial pathogens, these motile Gram-negative rod-shaped bacteria exhibit multidrug resistance.65−67 There are many virulence factors associated with P. aeruginosa infection, including biofilm formation cell-secreted factors, as well as several anatomical structures, including pili, flagella, and LPS.68 These factors all serve as potential drug targets for improved treatment modalities where new antibiotics are desperately needed to combat these bacteria in the clinic.69−71 It is well known that these bacteria, like others, are able to shed and remodel their PG structures; however, few studies have been able to distinguish PG as a definitive virulence factor during infection.72−76 Additionally, current investigations examining the dynamic changes in PG topology and architecture during infection involve the use of denaturing methods to isolate PG for downstream assaying.77−79 Here, we sought to utilize our fast and robust minimal tetrazine probe to execute dynamic in situ labeling of the PG anatomy in live bacteria to serve as a platform for many potential downstream applications. Previous attempts to label these species resulted in either low incorporation, high background, or unusual morphology and nonspecific labeling when labeled with SPAAC.41,44 Use of the new HTz-NAM and aTCO-SiR labeling strategy resulted in markedly improved labeling and notably less background in the NAM-treated sample in P. putida (Figure 4A, SI Figures 33 and 34). Interestingly, advancing this probe into P. aeruginosa led to remarkably high labeling under basal expression of its AmgK/MurU machinery in no wash samples (Figure 4A, SI Figures 33–37). These results suggest that this new minimal tetrazine probe could be easily applied to detect P. aeruginosa in live patient samples, as cystic fibrosis patients routinely suffer from outgrowth of this bacterium.80−82 This dynamic probe may also serve as a platform to investigate spatial and temporal changes in PG in situ.
To highlight the power and versatility of our new imaging method, we subjected each bacterial strain to detailed imaging analysis to view distinct bacteria growth stages utilizing different TCO–fluorophores and an array of confocal microscopes (SI Figures 38–92). Using HTz-NAM, all of the bacterial growth stages, from early initial growth states where bacteria utilize the GTPase FtsZ to mark and begin PG build up at the future division site (Figure 4B, Early Stage) to large amounts of newly synthesized PG forming the future division site forming the Z-ring (Figure 4B, Mid Stage) to just before separation into two distinct daughter cells (Figure 4B, Very Late Stage), were visualized. In all the growth stages, explicit features, such as the Z-ring, are clearly evident and show the progression and PG architectural changes during bacterial cell division for all of the bacteria species sampled (Figure 4B). Spinning disk confocal imaging of the Pseudomonas strains provides the first glimpses of this organism’s glycan architecture found in the peptidoglycan, which shows the carbohydrate backbone during distinct stages of bacterial cell growth cycle (Figure 4C).83 In the future, we aim to use this labeling strategy as an efficient way to screen for new antibiotics, as Wuo and co-workers have reported for Mycobacterium tuberculosis.84
Development of Bacteria–Macrophage Coculture Conditions
We next wanted to translate to an immunologically relevant system specifically utilizing macrophages, which are an innate immune cell type that engulfs and breaks down bacteria to elicit downstream signal pathways that trigger the immune system.85
Briefly, using a previously established bacterial invasion protocol,41 remodeled E. coli with HTz-NAM were introduced into macrophage culture. After 30 min, cells were treated with the antibiotic, gentamicin, to eliminate any extracellular bacteria. The cells were fixed and labeled with aTCO-TAMRA or aTCO-SiR. Labeled bacteria were observed in macrophages, and nonspecific labeling was limited (Figures 5A, SI Figures 93–95). We also preclicked E. coli and B. subtilis that were remodeled with the tetrazine probe and invaded into macrophages in real time to visualize their engulfment using adapted protocols for invasion (Figure 5B, SI Videos 5 and 6).44 The SiR dye proved to show superior signal-to-background difference, which heightened its potential for no-wash live-cell imaging of bacterial invasions. Previously, we had observed that while SPAAC dyes successfully reacted with intracellular bacteria in live macrophages they would also nonspecifically adhere in the cytosolic space of the macrophage.44 Moving forward, we aim to utilize the live macrophage invasion experiments over a time course to determine how the different bacteria break down over the first few hours following infection as we aim to study their processing into smaller PG fragments that initiate different immune signaling.
Figure 5.
Imaging of bacteria engulfment by macrophages. (A) Fixed labeling of EQKU cells inside macrophages with workflow visualization. EQKU cells were remodeled with HTz-NAM prior to macrophage engulfment. The cells were fixed and then reacted with either aTCO-TAMRA or aTCO-SiR. (B) Live imaging of prelabeled bacteria by macrophages. Macrophages were stained using CellMask Orange (white), and bacteria were labeled with aTCO-SiR (purple) (see SI Figures 93–95 and SI Videos 5 and 6).
Besides visualizing the engulfment of prelabeled bacteria, one of the powerful capabilities of the aTCO-SiR conjugate, as highlighted throughout the paper, is its capability for fluorogenic labeling with fast reaction kinetics.61 To showcase these, we tested the ability to label intracellular bacteria that had already been engulfed into the macrophages (Figure 6). Following the invasion, we added aTCO-SiR (500 nM) to the media of the live cells and immediately watched for uptake and labeling of the internalized bacteria. Within 5 min, the already internalized bacteria in the macrophages were specifically labeled on the cell wall (Figure 6B,C, SI Video 7). This no-wash labeling was able to occur on the live intracellular bacteria cell wall inside of the live macrophages without nonspecific binding and fluorescence of the macrophage, itself, unlike previous attempts with SPAAC labeling. This work can be expanded to selectively label and track specific intracellular pathogens that are known to avoid degradation and burst out of vacuoles to replicate in the cytosol, such as Salmonella and Legionella, to visualize these complex mechanisms in real time.86−88
Figure 6.
Live visualization of Tz-TCO ligation of HTz-NAM-remodeled bacteria engulfed by a macrophage. (A) Workflow for rapid, real-time, live-cell, no-wash labeling of HTz-NAM remodeled EQKU inside of live macrophages. (B) Prior to aTCO-SiR addition, EQKU cells were remodeled with HTz-NAM and engulfed by macrophages that were stained using CellMask Orange (white). (C) Labeling with aTCO-SiR was complete within 5 min. Orthogonal slices of bacterial cell confirming presence inside macrophage (see SI Video 7).
Conclusion
A new tetrazine-functionalized derivative of muramic acid, HTz-NAM, is described as a probe that can be introduced into the bacterial cell wall by the recycling enzymes, AmgK and MurU. Important to the efficacy of HTz-NAM is the minimal size of the tetrazine substituent relative to a previously explored tetrazine-based muramic acid derivative. This probe was incorporated into the PG of a wide variety of bacteria species that were transformed with the recycling enzymes, as well as in wild-type P. putida and P. aeruginosa, which naturally express AmgK and MurU. Bacterial peptidoglycans were visualized through labeling with fluorescent derivatives of aTCO—a trans-cyclooctene with favorable kinetic and physiochemical properties. Rapid labeling is accessible using conjugates of aTCO with TAMRA or with the fluorogenic aTCO-SiR dye that allows for no-wash visualization of bacterial labeling. The kinetics of labeling were measured using confocal microscopy to directly monitor gain-of-fluorescence for single EQKU cells upon the addition of 5.6 μM aTCO-SiR: labeling took place with a half-life of 1.0 ± 0.1 s. The combination of HTz-NAM and fluorescent aTCO derivatives enabled the visualization of the distinct bacteria growth stages across six different strains. This probe was used to label the human pathogen, Pseudomonas aeruginosa, with superior resolution, which detailed critical features of the PG polymer. Additionally, the highly selective nature of this reaction and the fluorogenic property of this dye facilitates real-time addition of aTCO-SiR directly to macrophages during invasion studies without nonspecific binding to the cells. Selective labeling and imaging of the bacterial cell wall was successful both in macrophage cells that were fixed prior to labeling and in macrophages that were labeled with aTCO-SiR and imaged live without washing.
In summary, we developed a new NAM derivative, HTz-NAM, that in combination with aTCO labeling allows for versatile real-time rapid and selective labeling of bacterial PG. Prior tools for labeling the PG backbone relied on slow chemistry that occurred on time scales that exceeded the bacterial doubling time, thereby impeding our ability to investigate distinct changes in PG during biosynthesis, remodeling, and as a result of changes to cell environment. By contrast, labeling with the HTz-NAM/aTCO system is complete within seconds at low fluorophore concentration. This is the first system compatible with live no-wash selective labeling of bacterial PG within host macrophages. We envision numerous applications for the HTz-NAM labeling system in the study of innate immunity in living systems. These minimal probes set the stage for the development of new imaging modalities in the clinic and high-throughput screens for the development of much needed antibiotics.
Acknowledgments
We are thankful for support from the Delaware COBRE program supported by a grant from the National Institute of General Medical Sciences (NIGMS 1 P30 GM110758 and 1 P20 GM104316-01A1). This work was supported by the NIH (U01 CA221230, R21 AI163949, R01 GM138599, and R01 GM132460). We would like to thank Jeff Caplan and the Delaware Biotechnology Institute Bioimaging Center where microscopy access was supported by grants from the NIH-NIGMS (P20 GM103446), the NIGMS (P20 GM139760), and the State of Delaware. Andor Dragonfly was acquired with NIH-NIGMS (S10 OD030321). We would also like to thank William Trout, Christopher am Ende, and the Pfizer DSC safety team; Shi Bai and the UD NMR facility; and PapaNii Asare-Okai, Katherine Martin, and Elijah Hudson from the UD Mass Spectrometry Core Facility. C.L.G. thanks the Dreyfus foundation for support. K.A.W., S.N.H., A.S.H., D.L.M., and A.J. would like to thank the NIH for support through the Chemistry-Biology Interface (CBI) training grant, T32GM133395. L.-M.D.S. would like to thank funding for the University of Delaware Summer Scholars program. J.M.F and A.S.H would like to acknowledge that this research was partially funded by the NSF through the University of Delaware Materials Research Science and Engineering Center, DMR-2011824. Figures were created using BioRender.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.3c13644.
Supplemental figures, synthetic procedures, characterization, experimental details, and biochemical protocols (PDF)
Real-time addition kinetics of Tz-TCO ligation for fixed EQKU remodeled with HTz-NAM-Replicate 1 (MP4)
Real-time addition kinetics of Tz-TCO ligation for fixed EQKU remodeled with HTz-NAM-Replicate 2 (MP4)
Real-time addition kinetics of Tz-TCO ligation for fixed BSKU remodeled with HTz-NAM-Replicate 1 (MP4)
Real-time addition kinetics of Tz-TCO ligation for fixed BSKU remodeled with HTz-NAM-Replicate 2 (MP4)
Live imaging of pre-labeled RFM795-Tf-KU cells engulfed by THP-1 macrophages (MP4)
Live imaging of pre-labeled BSKU cells engulfed by THP-1 macrophages (MP4)
Live-cell no-wash E. coli labeling in macrophages (MP4)
Descriptions of all Supporting Information videos (PDF)
Author Present Address
□ Department of Microbiology, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA 19104, United States
Author Contributions
# A.S.H., S.N.H., and K.A.W. contributed equally to this work.
The authors declare no competing financial interest.
Supplementary Material
References
- Strominger J. L.; Park J. T.; Thompson R. E. Composition of the Cell Wall of Staphylococcus-Aureus - Its Relation to the Mechanism of Action of Penicillin. J. Biol. Chem. 1959, 234 (12), 3263–3268. 10.1016/S0021-9258(18)69662-0. [DOI] [PubMed] [Google Scholar]
- Tipper D. J.; Strominger J. L. Mechanism of Action of Penicillins - a Proposal Based on Their Structural Similarity to Acyl-D-Alanyl-D-Alanine. P Natl. Acad. Sci. USA 1965, 54 (4), 1133. 10.1073/pnas.54.4.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra I.; Roberts M. Tetracycline antibiotics: Mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol Mol. Biol. R 2001, 65 (2), 232. 10.1128/MMBR.65.2.232-260.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmer W.; Blanot D.; de Pedro M. A. Peptidoglycan structure and architecture. FEMS Microbiol Rev. 2008, 32 (2), 149–67. 10.1111/j.1574-6976.2007.00094.x. [DOI] [PubMed] [Google Scholar]
- Schleifer K. H.; Kandler O. Peptidoglycan types of bacterial cell walls and their taxonomic implications. Bacteriol Rev. 1972, 36 (4), 407–77. 10.1128/br.36.4.407-477.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovering A. L.; Safadi S. S.; Strynadka N. C. Structural perspective of peptidoglycan biosynthesis and assembly. Annu. Rev. Biochem. 2012, 81, 451–78. 10.1146/annurev-biochem-061809-112742. [DOI] [PubMed] [Google Scholar]
- Kohanski M. A.; Dwyer D. J.; Collins J. J. How antibiotics kill bacteria: from targets to networks. Nature Reviews Microbiology 2010, 8 (6), 423–435. 10.1038/nrmicro2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gisin J.; Schneider A.; Nagele B.; Borisova M.; Mayer C. A cell wall recycling shortcut that bypasses peptidoglycan de novo biosynthesis. Nat. Chem. Biol. 2013, 9 (8), 491–3. 10.1038/nchembio.1289. [DOI] [PubMed] [Google Scholar]
- Mayer C.; Kluj R. M.; Muhleck M.; Walter A.; Unsleber S.; Hottmann I.; Borisova M. Bacteria’s different ways to recycle their own cell wall. Int. J. Med. Microbiol 2019, 309 (7), 151326. 10.1016/j.ijmm.2019.06.006. [DOI] [PubMed] [Google Scholar]
- Taguchi A.; Kahne D.; Walker S. Chemical tools to characterize peptidoglycan synthases. Curr. Opin Chem. Biol. 2019, 53, 44–50. 10.1016/j.cbpa.2019.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banahene N.; Kavunja H. W.; Swarts B. M. Chemical Reporters for Bacterial Glycans: Development and Applications. Chem. Rev. 2022, 122 (3), 3336–3413. 10.1021/acs.chemrev.1c00729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. R.; Gordon R. A.; Hyland S. N.; Siegrist M. S.; Grimes C. L. Chemical Biology Tools for Examining the Bacterial Cell Wall. Cell Chem. Biol. 2020, 27 (8), 1052–1062. 10.1016/j.chembiol.2020.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radkov A. D.; Hsu Y. P.; Booher G.; VanNieuwenhze M. S. Imaging Bacterial Cell Wall Biosynthesis. Annu. Rev. Biochem. 2018, 87, 991–1014. 10.1146/annurev-biochem-062917-012921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tra V. N.; Dube D. H. Glycans in pathogenic bacteria-potential for targeted covalent therapeutics and imaging agents. Chem. Commun. (Camb) 2014, 50 (36), 4659–73. 10.1039/C4CC00660G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hang H. C.; Yu C.; Kato D. L.; Bertozzi C. R. A metabolic labeling approach toward proteomic analysis of mucin-type O-linked glycosylation. Proc. Natl. Acad. Sci. U. S. A. 2003, 100 (25), 14846–51. 10.1073/pnas.2335201100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sletten E. M.; Bertozzi C. R. Bioorthogonal chemistry: fishing for selectivity in a sea of functionality. Angew. Chem., Int. Ed. Engl. 2009, 48 (38), 6974–98. 10.1002/anie.200900942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scinto S. L.; Bilodeau D. A.; Hincapie R.; Lee W.; Nguyen S. S.; Xu M.; Am Ende C. W.; Finn M. G.; Lang K.; Lin Q.; Pezacki J. P.; Prescher J. A.; Robillard M. S.; Fox J. M. Bioorthogonal chemistry. Nat. Rev. Methods Primers 2021, 1, 30. 10.1038/s43586-021-00028-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson D. M.; Nazarova L. A.; Xie B.; Kamber D. N.; Prescher J. A. Functionalized Cyclopropenes As Bioorthogonal Chemical Reporters. J. Am. Chem. Soc. 2012, 134 (45), 18638–18643. 10.1021/ja3060436. [DOI] [PubMed] [Google Scholar]
- Schumann B.; Malaker S. A.; Wisnovsky S. P.; Debets M. F.; Agbay A. J.; Fernandez D.; Wagner L. J. S.; Lin L.; Li Z.; Choi J.; Fox D. M.; Peh J.; Gray M. A.; Pedram K.; Kohler J. J.; Mrksich M.; Bertozzi C. R. Bump-and-Hole Engineering Identifies Specific Substrates of Glycosyltransferases in Living Cells. Mol. Cell 2020, 78 (5), 824–834. e15 10.1016/j.molcel.2020.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang P. V.; Prescher J. A.; Sletten E. M.; Baskin J. M.; Miller I. A.; Agard N. J.; Lo A.; Bertozzi C. R. Copper-free click chemistry in living animals. P Natl. Acad. Sci. USA 2010, 107 (5), 1821–1826. 10.1073/pnas.0911116107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskin J. M.; Prescher J. A.; Laughlin S. T.; Agard N. J.; Chang P. V.; Miller I. A.; Lo A.; Codelli J. A.; Bertozzi C. R. Copper-free click chemistry for dynamic in vivo imaging. Proc. Natl. Acad. Sci. U. S. A. 2007, 104 (43), 16793–7. 10.1073/pnas.0707090104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shieh P.; Siegrist M. S.; Cullen A. J.; Bertozzi C. R. Imaging bacterial peptidoglycan with near-infrared fluorogenic azide probes. Proc. Natl. Acad. Sci. U. S. A. 2014, 111 (15), 5456–61. 10.1073/pnas.1322727111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemas A.; Xie Y.; Pigga J. E.; Caplan J. L.; Am Ende C. W.; Fox J. M. Catalytic Activation of Bioorthogonal Chemistry with Light (CABL) Enables Rapid, Spatiotemporally Controlled Labeling and No-Wash, Subcellular 3D-Patterning in Live Cells Using Long Wavelength Light. J. Am. Chem. Soc. 2022, 144 (4), 1647–1662. 10.1021/jacs.1c10390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrey H. E.; Judkins J. C.; Am Ende C. W.; Ballard T. E.; Fang Y.; Riccardi K.; Di L.; Guilmette E. R.; Schwartz J. W.; Fox J. M.; Johnson D. S. Systematic Evaluation of Bioorthogonal Reactions in Live Cells with Clickable HaloTag Ligands: Implications for Intracellular Imaging. J. Am. Chem. Soc. 2015, 137 (35), 11461–75. 10.1021/jacs.5b06847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J.; Seckute J.; Cole C. M.; Devaraj N. K. Live-cell imaging of cyclopropene tags with fluorogenic tetrazine cycloadditions. Angew. Chem., Int. Ed. Engl. 2012, 51 (30), 7476–9. 10.1002/anie.201202122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zawada Z.; Guo Z. J.; Oliveira B. L.; Navo C. D.; Li H.; Cal P. M. S. D.; Corzana F.; Jiménez-Osés G.; Bernardes G. J. L. Arylethynyltrifluoroborate Dienophiles for on Demand Activation of IEDDA Reactions. Bioconjugate Chem. 2021, 32 (8), 1812–1822. 10.1021/acs.bioconjchem.1c00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramil C. P.; Dong M. Q.; An P.; Lewandowski T. M.; Yu Z. P.; Miller L. J.; Lin Q. Spirohexene-Tetrazine Ligation Enables Bioorthogonal Labeling of Class B G Protein-Coupled Receptors in Live Cells. J. Am. Chem. Soc. 2017, 139 (38), 13376–13386. 10.1021/jacs.7b05674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossin R.; van Duijnhoven S. M.; Ten Hoeve W.; Janssen H. M.; Kleijn L. H.; Hoeben F. J.; Versteegen R. M.; Robillard M. S. Triggered Drug Release from an Antibody-Drug Conjugate Using Fast ″Click-to-Release″ Chemistry in Mice. Bioconjug Chem. 2016, 27 (7), 1697–706. 10.1021/acs.bioconjchem.6b00231. [DOI] [PubMed] [Google Scholar]
- Mejia Oneto J. M.; Khan I.; Seebald L.; Royzen M. In Vivo Bioorthogonal Chemistry Enables Local Hydrogel and Systemic Pro-Drug To Treat Soft Tissue Sarcoma. ACS Cent Sci. 2016, 2 (7), 476–82. 10.1021/acscentsci.6b00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberger J. E.; Xie Y.; Fang Y.; Lyu X.; Trout W. S.; Dmitrenko O.; Fox J. M. Ligand-Directed Photocatalysts and Far-Red Light Enable Catalytic Bioorthogonal Uncaging inside Live Cells. J. Am. Chem. Soc. 2023, 145 (11), 6067–6078. 10.1021/jacs.2c10655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahal L. K.; Yarema K. J.; Bertozzi C. R. Engineering chemical reactivity on cell surfaces through oligosaccharide biosynthesis. Science 1997, 276 (5315), 1125–8. 10.1126/science.276.5315.1125. [DOI] [PubMed] [Google Scholar]
- Prescher J. A.; Dube D. H.; Bertozzi C. R. Chemical remodelling of cell surfaces in living animals. Nature 2004, 430 (7002), 873–7. 10.1038/nature02791. [DOI] [PubMed] [Google Scholar]
- Bertozzi C. R.; Kiessling L. L. Chemical glycobiology. Science 2001, 291 (5512), 2357–64. 10.1126/science.1059820. [DOI] [PubMed] [Google Scholar]
- Saxon E.; Bertozzi C. R. Cell surface engineering by a modified Staudinger reaction. Science 2000, 287 (5460), 2007–10. 10.1126/science.287.5460.2007. [DOI] [PubMed] [Google Scholar]
- Scinto S. L.; Ekanayake O.; Seneviratne U.; Pigga J. E.; Boyd S. J.; Taylor M. T.; Liu J.; Am Ende C. W.; Rozovsky S.; Fox J. M. Dual-Reactivity trans-Cyclooctenol Probes for Sulfenylation in Live Cells Enable Temporal Control via Bioorthogonal Quenching. J. Am. Chem. Soc. 2019, 141 (28), 10932–10937. 10.1021/jacs.9b01164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallon A. M.; Xu Y.; West G. M.; Am Ende C. W.; Fox J. M. Thiomethyltetrazines Are Reversible Covalent Cysteine Warheads Whose Dynamic Behavior can be ″Switched Off″ via Bioorthogonal Chemistry Inside Live Cells. J. Am. Chem. Soc. 2023, 145 (29), 16069–16080. 10.1021/jacs.3c04444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W.; Lin Z.; Woo C. M.; Baskin J. M. A Chemoproteomics Approach to Profile Phospholipase D-Derived Phosphatidyl Alcohol Interactions. ACS Chem. Biol. 2022, 17 (12), 3276–3283. 10.1021/acschembio.1c00584. [DOI] [PubMed] [Google Scholar]
- Woo C. M.; Felix A.; Zhang L.; Elias J. E.; Bertozzi C. R. Isotope-targeted glycoproteomics (IsoTaG) analysis of sialylated N- and O-glycopeptides on an Orbitrap Fusion Tribrid using azido and alkynyl sugars. Anal Bioanal Chem. 2017, 409 (2), 579–588. 10.1007/s00216-016-9934-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J. T.; Uehara T. How bacteria consume their own exoskeletons (Turnover and recycling of cell wall peptidoglycan). Microbiol Mol. Biol. R 2008, 72 (2), 211–227. 10.1128/MMBR.00027-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reith J.; Mayer C. Peptidoglycan turnover and recycling in Gram-positive bacteria. Appl. Microbiol. Biotechnol. 2011, 92 (1), 1–11. 10.1007/s00253-011-3486-x. [DOI] [PubMed] [Google Scholar]
- Liang H.; DeMeester K. E.; Hou C.-W.; Parent M. A.; Caplan J. L.; Grimes C. L. Metabolic labelling of the carbohydrate core in bacterial peptidoglycan and its applications. Nat. Commun. 2017, 8, 15015. 10.1038/ncomms15015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy D. C.; McKay C. S.; Legault M. C.; Danielson D. C.; Blake J. A.; Pegoraro A. F.; Stolow A.; Mester Z.; Pezacki J. P. Cellular consequences of copper complexes used to catalyze bioorthogonal click reactions. J. Am. Chem. Soc. 2011, 133 (44), 17993–8001. 10.1021/ja2083027. [DOI] [PubMed] [Google Scholar]
- Agard N. J.; Prescher J. A.; Bertozzi C. R. A strain-promoted [3 + 2] azide-alkyne cycloaddition for covalent modification of biomolecules in living systems. J. Am. Chem. Soc. 2004, 126 (46), 15046–7. 10.1021/ja044996f. [DOI] [PubMed] [Google Scholar]
- Wodzanowski K. A.; Caplan J. L.; Kloxin A. M.; Grimes C. L. Multiscale Invasion Assay for Probing Macrophage Response to Gram-Negative Bacteria. Front Chem. 2022, 10, 842602. 10.3389/fchem.2022.842602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wodzanowski K. A.; Hyland S. N.; Chinthamani S.; Sandles L. D.; Honma K.; Sharma A.; Grimes C. L. Investigating Peptidoglycan Recycling Pathways in Tannerella forsythia with N-Acetylmuramic Acid Bioorthogonal Probes. ACS Infect Dis 2022, 8 (9), 1831–1838. 10.1021/acsinfecdis.2c00333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvaraj R.; Fox J. M. trans-Cyclooctene-a stable, voracious dienophile for bioorthogonal labeling. Curr. Opin Chem. Biol. 2013, 17 (5), 753–60. 10.1016/j.cbpa.2013.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pidgeon S. E.; Pires M. M. Metabolic remodeling of bacterial surfaces via tetrazine ligations. Chem. Commun. (Camb) 2015, 51 (51), 10330–3. 10.1039/C5CC01693B. [DOI] [PubMed] [Google Scholar]
- Pidgeon S. E.; Pires M. M. Cell Wall Remodeling of Staphylococcus aureus in Live Caenorhabditis elegans. Bioconjug Chem. 2017, 28 (9), 2310–2315. 10.1021/acs.bioconjchem.7b00363. [DOI] [PubMed] [Google Scholar]
- Apostolos A. J.; Chordia M. D.; Kolli S. H.; Dalesandro B. E.; Rutkowski M. R.; Pires M. M. Real-time non-invasive fluorescence imaging of gut commensal bacteria to detect dynamic changes in the microbiome of live mice. Cell Chem. Biol. 2022, 29, 1721. 10.1016/j.chembiol.2022.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez L.; Espaillat A.; Hermoso J. A.; de Pedro M. A.; Cava F. Peptidoglycan Remodeling by the Coordinated Action of Multispecific Enzymes. Microb Drug Resist 2014, 20 (3), 190–198. 10.1089/mdr.2014.0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMeester K. E.; Liang H.; Jensen M. R.; Jones Z. S.; D’Ambrosio E. A.; Scinto S. L.; Zhou J.; Grimes C. L. Synthesis of Functionalized N-Acetyl Muramic Acids To Probe Bacterial Cell Wall Recycling and Biosynthesis. J. Am. Chem. Soc. 2018, 140 (30), 9458–9465. 10.1021/jacs.8b03304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y.; Fang Y.; Huang Z.; Tallon A. M.; Am Ende C. W.; Fox J. M. Divergent Synthesis of Monosubstituted and Unsymmetrical 3,6-Disubstituted Tetrazines from Carboxylic Ester Precursors. Angew. Chem., Int. Ed. Engl. 2020, 59 (39), 16967–16973. 10.1002/anie.202005569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert W. D.; Fang Y.; Mahapatra S.; Huang Z.; Am Ende C. W.; Fox J. M. Installation of Minimal Tetrazines through Silver-Mediated Liebeskind-Srogl Coupling with Arylboronic Acids. J. Am. Chem. Soc. 2019, 141 (43), 17068–17074. 10.1021/jacs.9b08677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. R.; Wodzanowski K. A.; Santiago C. C.; Hyland S. N.; Follmar J. L.; Asare-Okai P.; Grimes C. L. Protected N-Acetyl Muramic Acid Probes Improve Bacterial Peptidoglycan Incorporation via Metabolic Labeling. ACS Chem. Biol. 2021, 16 (10), 1908–1916. 10.1021/acschembio.1c00268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan H. Y.; Eskandari R.; Shen D.; Zhu Y.; Liu T. W.; Willems L. I.; Alteen M. G.; Madden Z.; Vocadlo D. J. Direct One-Step Fluorescent Labeling of O-GlcNAc-Modified Proteins in Live Cells Using Metabolic Intermediates. J. Am. Chem. Soc. 2018, 140 (45), 15300–15308. 10.1021/jacs.8b08260. [DOI] [PubMed] [Google Scholar]
- Sainlos M.; Imperiali B. Synthesis of anhydride precursors of the environment-sensitive fluorophores 4-DMAP and 6-DMN. Nat. Protoc 2007, 2 (12), 3219–25. 10.1038/nprot.2007.444. [DOI] [PubMed] [Google Scholar]
- Eugenio Vazquez M.; Rothman D. M.; Imperiali B. A new environment-sensitive fluorescent amino acid for Fmoc-based solid phase peptide synthesis. Org. Biomol Chem. 2004, 2 (14), 1965–6. 10.1039/B408001G. [DOI] [PubMed] [Google Scholar]
- Loving G.; Imperiali B. A versatile amino acid analogue of the solvatochromic fluorophore 4-N,N-dimethylamino-1,8-naphthalimide: a powerful tool for the study of dynamic protein interactions. J. Am. Chem. Soc. 2008, 130 (41), 13630–8. 10.1021/ja804754y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver L. L. Fosfomycin: Mechanism and Resistance. Cold Spring Harb Perspect Med. 2017, 7 (2), a025262. 10.1101/cshperspect.a025262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pigga J. E.; Rosenberger J. E.; Jemas A.; Boyd S. J.; Dmitrenko O.; Xie Y. X.; Fox J. M. General, Divergent Platform for Diastereoselective Synthesis of -Cyclooctenes with High Reactivity and Favorable Physiochemical Properties**. Angew. Chem. Int. Edit 2021, 60 (27), 14975–14980. 10.1002/anie.202101483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukinavicius G.; Umezawa K.; Olivier N.; Honigmann A.; Yang G.; Plass T.; Mueller V.; Reymond L.; Correa I. R. Jr; Luo Z. G.; Schultz C.; Lemke E. A.; Heppenstall P.; Eggeling C.; Manley S.; Johnsson K. A near-infrared fluorophore for live-cell super-resolution microscopy of cellular proteins. Nat. Chem. 2013, 5 (2), 132–9. 10.1038/nchem.1546. [DOI] [PubMed] [Google Scholar]
- Koide Y.; Urano Y.; Hanaoka K.; Piao W.; Kusakabe M.; Saito N.; Terai T.; Okabe T.; Nagano T. Development of NIR Fluorescent Dyes Based on Si-rhodamine for in Vivo Imaging. J. Am. Chem. Soc. 2012, 134 (11), 5029–5031. 10.1021/ja210375e. [DOI] [PubMed] [Google Scholar]
- Fu M. Y.; Xiao Y.; Qian X. H.; Zhao D. F.; Xu Y. F. A design concept of long-wavelength fluorescent analogs of rhodamine dyes: replacement of oxygen with silicon atom. Chem. Commun. 2008, (15), 1780–1782. 10.1039/b718544h. [DOI] [PubMed] [Google Scholar]
- Sampson B. A.; Misra R.; Benson S. A. Identification and characterization of a new gene of Escherichia coli K-12 involved in outer membrane permeability. Genetics 1989, 122 (3), 491–501. 10.1093/genetics/122.3.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher H. W.; Talbot G. H.; Bradley J. S.; Edwards J. E.; Gilbert D.; Rice L. B.; Scheld M.; Spellberg B.; Bartlett J. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis 2009, 48 (1), 1–12. 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- Qin S.; Xiao W.; Zhou C.; Pu Q.; Deng X.; Lan L.; Liang H.; Song X.; Wu M. Pseudomonas aeruginosa: pathogenesis, virulence factors, antibiotic resistance, interaction with host, technology advances and emerging therapeutics. Signal Transduct Target Ther 2022, 7 (1), 199. 10.1038/s41392-022-01056-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao C.; Huang X.; Wang Q.; Yao D.; Lu W. Virulence Factors of Pseudomonas Aeruginosa and Antivirulence Strategies to Combat Its Drug Resistance. Front Cell Infect Microbiol 2022, 12, 926758. 10.3389/fcimb.2022.926758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurado-Martín I.; Sainz-Mejías M.; McClean S. An Audacious Pathogen with an Adaptable Arsenal of Virulence Factors. International Journal of Molecular Sciences 2021, 22 (6), 3128. 10.3390/ijms22063128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh C. Where will new antibiotics come from?. Nat. Rev. Microbiol 2003, 1 (1), 65–70. 10.1038/nrmicro727. [DOI] [PubMed] [Google Scholar]
- Torrens G.; Perez-Gallego M.; Moya B.; Munar-Bestard M.; Zamorano L.; Cabot G.; Blazquez J.; Ayala J. A.; Oliver A.; Juan C. Targeting the permeability barrier and peptidoglycan recycling pathways to disarm Pseudomonas aeruginosa against the innate immune system. PLoS One 2017, 12 (7), e0181932 10.1371/journal.pone.0181932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanya D. R. A.; Onesime D.; Vizzarro G.; Jacquier N. Recent advances in therapeutic targets identification and development of treatment strategies towards Pseudomonas aeruginosa infections. BMC Microbiol 2023, 23 (1), 86. 10.1186/s12866-023-02832-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korgaonkar A.; Trivedi U.; Rumbaugh K. P.; Whiteley M. Community surveillance enhances Pseudomonas aeruginosa virulence during polymicrobial infection. Proc. Natl. Acad. Sci. U. S. A. 2013, 110 (3), 1059–64. 10.1073/pnas.1214550110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson E. M.; Shaji Saji N.; Anderson A. C.; Brewer D.; Clarke A. J.; Khursigara C. M. Pseudomonas aeruginosa Alters Peptidoglycan Composition under Nutrient Conditions Resembling Cystic Fibrosis Lung Infections. mSystems 2022, 7 (3), e0015622 10.1128/msystems.00156-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav A. K.; Espaillat A.; Cava F. Bacterial Strategies to Preserve Cell Wall Integrity Against Environmental Threats. Frontiers in Microbiology 2018, 9, 02064. 10.3389/fmicb.2018.02064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobar-Salom M.; Barceló I. M.; Jordana-Lluch E.; Torrens G.; Oliver A.; Juan C. R. Bacterial virulence regulation through soluble peptidoglycan fragments sensing and response: knowledge gaps and therapeutic potential. Fems Microbiology Reviews 2023, 47 (2), fuad010. 10.1093/femsre/fuad010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brott A. S.; Clarke A. J. Peptidoglycan O-Acetylation as a Virulence Factor: Its Effect on Lysozyme in the Innate Immune System. Antibiotics 2019, 8 (3), 94. 10.3390/antibiotics8030094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmer W.; Seligman S. J. Architecture of peptidoglycan: more data and more models. Trends Microbiol 2010, 18 (2), 59–66. 10.1016/j.tim.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Quintela J. C.; Caparros M.; de Pedro M. A. Variability of peptidoglycan structural parameters in gram-negative bacteria. FEMS Microbiol Lett. 1995, 125 (1), 95–100. 10.1016/0378-1097(94)00479-B. [DOI] [PubMed] [Google Scholar]
- Anderson E. M.; Sychantha D.; Brewer D.; Clarke A. J.; Geddes-McAlister J.; Khursigara C. M. Peptidoglycomics reveals compositional changes in peptidoglycan between biofilm- and planktonic-derived Pseudomonas aeruginosa. J. Biol. Chem. 2020, 295 (2), 504–516. 10.1074/jbc.RA119.010505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra S.; Hayes D. Jr; Wozniak D. J.. Cystic Fibrosis and Pseudomonas aeruginosa: the Host-Microbe Interface. Clin Microbiol Rev. 2019, 32 ( (3), ). 10.1128/CMR.00138-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J. C. Pseudomonas aeruginosa in cystic fibrosis: pathogenesis and persistence. Paediatr Respir Rev. 2002, 3 (2), 128–34. 10.1016/S1526-0550(02)00003-3. [DOI] [PubMed] [Google Scholar]
- Jackson L.; Waters V. Factors influencing the acquisition and eradication of early Pseudomonas aeruginosa infection in cystic fibrosis. J. Cyst Fibros 2021, 20 (1), 8–16. 10.1016/j.jcf.2020.10.008. [DOI] [PubMed] [Google Scholar]
- Zhang C.; Reymond L.; Rutschmann O.; Meyer M. A.; Denereaz J.; Qiao J.; Ryckebusch F.; Griffie J.; Stepp W. L.; Manley S. Fluorescent d-Amino Acids for Super-resolution Microscopy of the Bacterial Cell Wall. ACS Chem. Biol. 2022, 17 (9), 2418–2424. 10.1021/acschembio.2c00496. [DOI] [PubMed] [Google Scholar]
- Wuo M. G.; Dulberger C. L.; Brown R. A.; Sturm A.; Ultee E.; Bloom-Ackermann Z.; Choi C.; Garner E. C.; Briegel A.; Hung D. T.; Rubin E. J.; Kiessling L. L.. Antibiotic action revealed by real-time imaging of the mycobacterial membrane. bioRxiv, January 1, 2022, 475452. 10.1101/2022.01.07.475452. [DOI]
- Herskovits A. A.; Auerbuch V.; Portnoy D. A. Bacterial ligands generated in a phagosome are targets of the cytosolic innate immune system. PLoS Pathog 2007, 3 (3), e51 10.1371/journal.ppat.0030051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gog J. R.; Murcia A.; Osterman N.; Restif O.; McKinley T. J.; Sheppard M.; Achouri S.; Wei B.; Mastroeni P.; Wood J. L. N.; Maskell D. J.; Cicuta P.; Bryant C. E. Dynamics of infection of macrophages at the single cell level. J. R Soc. Interface 2012, 9 (75), 2696–2707. 10.1098/rsif.2012.0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin S.; Roy C. R. Host cell processes that influence the intracellular survival of. Cellular Microbiology 2008, 10 (6), 1209–1220. 10.1111/j.1462-5822.2008.01145.x. [DOI] [PubMed] [Google Scholar]
- Horwitz M. A. Formation of a Novel Phagosome by the Legionnaires-Disease Bacterium (Legionella-Pneumophila) in Human-Monocytes. Journal of Experimental Medicine 1983, 158 (4), 1319–1331. 10.1084/jem.158.4.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.