Abstract
Background
The validity of the Apple Watch to measure the heart rate (HR) and oxygen saturation (Spo2) for patients with chronic diseases such as diabetes mellitus (DM), dyslipidemia, and hypertension is still unclear. Therefore, this study aims to investigate the accuracy of the Apple Watch in measuring the Spo2 and HR in patients with chronic diseases.
Methods
Forty-one patients with chronic diseases, including 20 with hypertension, 10 with diabetes, and 11 with dyslipidemia, completed a cross-sectional study. All participants used the Apple Watch against the Polar chest strap and the pulse oximeter at rest and during moderate intensity exercise sessions to measure HR and the SpO2 at rest for 5 minutes, during exercise for 16 minutes, and followed by 3 minutes of rest. The HR was measured during all previous periods, but evaluation of the Spo2 included 5 measures, done only before and after exercise, with a minute interval between each measure.
Results
Overall, a strong correlation exists between measuring the SpO2 using the Apple Watch against the pulse oximeter (Contec) at rest (r = 0.92, p < 0.001) and after exercise (r = 0.86, p < 0.001) in all patients. The HR had a very strong correlation between the Apple Watch and the Polar chest strap (r = 0.99, p < 0.001) in all patients. There was no significant difference (p = 0.76) between the twenty-seven white and fourteen brown-skinned patients.
Conclusion
The Apple Watch is valid to measure the HR and SpO2 in patients with chronic diseases.
Clinical Trial Registration No
Keywords: heart rate, diabetes mellitus, hypertension, dyslipidemia, oxygen saturation
Introduction
Assessment of the heart rate (HR) is an essential variable to determine proper exercise training intensity.1 Utilization of the gold standard 12-lead electrocardiogram (ECG) is considered the most objective HR assessment procedure. A polar chest strap arises as an alternative to the ECG when it is not accessible to use outside the laboratories. The Polar chest strap proved a valid and reliable instrument when tested against the ECG in healthy subjects2–4 and patients with cardiac diseases at rest and during training.5 Monitoring the resting as well as the post-training HRR (heart rate recovery) is essential because it helps predict the cardiopulmonary fitness level as well as the mortality risk among evaluated personnel.6 Chronic diseases such as diabetes, hypertension, and dyslipidemia are increasing worldwide.7
Diabetes mellitus is a rapidly growing non-communicable chronic disease worldwide, becoming one of the most alarming health problems in the 21st century.7 Hypertension prevalence is increasing in the Kingdom of Saudi Arabia (KSA); the rate exceeds 25% among adult Saudi subjects. Monitoring hypertension-related disturbances is an urgent concept to prevent organs damage.8 Recently, a report showed that the prevalence of hypercholesterolemia among the Saudi population ≥ years old is around 12.5%, with males having a higher rate (56.7%) compared to females, who have a lower rate (43.3%).9 Diabetes mellitus, hypertension, and dyslipidemia are comorbidity disorders, so this study focused on these groups of diseases.10
Diabetes mellitus, hypertension and dyslipidemia are comorbidities and risk factors for developing cardiac diseases, so it is important to monitor HR and oxygen saturation (SpO2) in day-to-day monitoring in patients with diabetes mellitus, hypertension, and dyslipidemia to prevent developing cardiac diseases,10–12 but it is difficult to continue monitoring HR and SpO2 in day-to-day monitoring particularly at home, because these measurements require devices available in hospitals for continuous monitoring, smartwatch could compensate for this difficulty by continuously monitoring HR and SpO2 to provide an early diagnosis and intervention to prevent developing cardiac diseases.11
It is also more difficult to continuously measure the HR and SpO2 in diabetic, hypertensive, and dyslipidemia patients due to the effect of vascular disturbances on these diseases compared to healthy subjects. These disturbances could lead to vascular dysfunction in the severe stages of these chronic diseases.13 This study is the first to measure HR and SpO2 in patients with these chronic diseases. SpO2 could be utilized as a biomarker during the evolution of these conditions and can be used to monitor prognosis and intervention-related effects in clinical trials.14
The process of SpO2 evaluation is important because it indirectly reflects the subject’s organ oxygen requirements. The traditional SpO2 measuring procedure is invasive and painful, requiring the collection and assay of an arterial blood sample using a blood gas analyzer, which is considered the gold standard to measure the SpO2, but it can also be measured noninvasively.15 Previous trials proposed evaluating the SpO2 non-invasively using a simple procedure with maximum accuracy.16 Pulse oximetry is a well-known noninvasive instrument used for the monitoring and evaluation of SpO2.17 Routine and frequent screening of patients with chronic diseases is recommended, especially with an age over 50 years and the presence of other comorbidities such as hypertension and hyperlipidemia.18
Recently; the Apple Watch appeared as a new tool to measure HR. All the Apple Watch versions can monitor the HR, but the blood SpO2 can only be measured by the latest 6 and 7 versions. The concept of utilizing the Apple Watch to measure the HR depends on the Photoplethysmography (PPG) non-invasive measurement procedure applied using a wrist-worn watch that continuously monitors both heart beats and blood color changes over time. The PPG is a commonly used approach to measure the HR, cardiac output, blood pressure, and SpO2 in a variety of medical instruments19 that can help discover cardiovascular insults and complications at early stages.20
Implemented new technology and updated features that enable the Apple Watch to monitor HR are available only in the updated series 6 and 7. A very limited number of studies evaluated the validity of the Apple watch against a pulse oximeter in measuring the SpO2 (the SpO2 was evaluated in 23 patients with chronic obstructive pulmonary disease, 61 patients with interstitial lung diseases, and 16 healthy control subjects). The Apple Watch results showed strong positive correlations with those of the pulse oximeter (r = 0.81, P<0.0001). Furthermore; Pipek et al, study recommended testing the Apple Watch’s validity for measuring SpO2 in other groups of patients, such as those with chronic diseases.21
The Apple Watch is a smart instrument with advanced technology that measures the Spo2 and HR, but its validity in patients with chronic diseases, including diabetes mellitus, hypertension, and dyslipidemia, is still unknown. Therefore, this is the first study to evaluate the accuracy and validity of the Apple Watch in measuring SpO2 and HR in patients with chronic diseases, including diabetes mellitus, hypertension, and dyslipidemia.
Methods
A cross-sectional study was conducted, included patients with chronic non-communicable diseases at Umm Al-Qura University Medical Center, Makkah, Saudi Arabia.
Forty-one male patients with chronic non-communicable disease (mean [SD]; age 51 [7] years), including 20 with hypertension, 11 with diabetics, and 10 with dyslipidemia, separately completed the study. The study was approved by the biomedical research ethics committee at Umm Al-Qura University (HAPO-O2-K-012-2022-02-959). The study was registered at clinicaltrail.gov with the registration number (NCT05271864). Twenty-seven patients in this study had white skin (type II) based on the Fitzpatrick scale,22 and fourteen had brown skin (type IV) based on the Fitzpatrick scale,22 with no patient having black skin.
Procedures and Devices
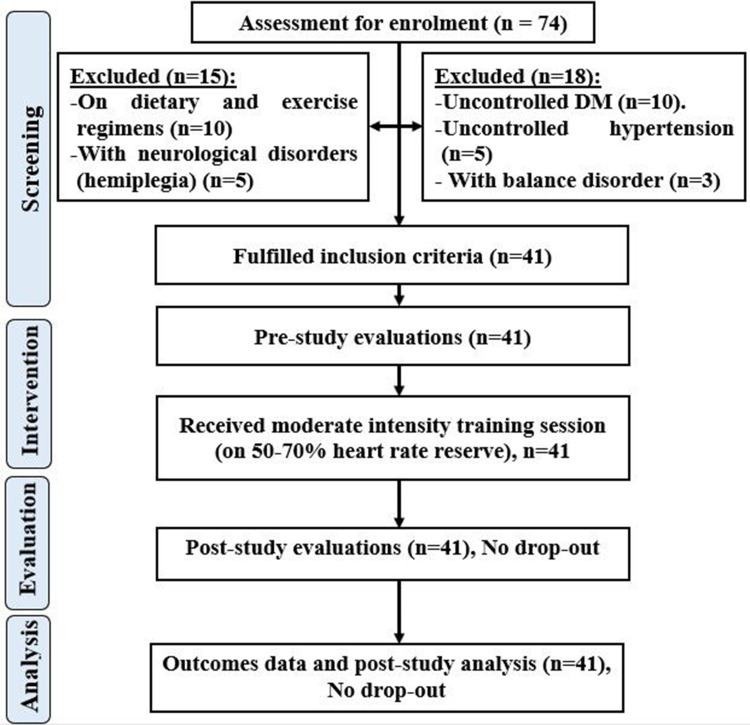
After physician referral, an initial medical screening was done to confirm the eligibility of each patient before starting the study. Only patients in the hypertension group were hypertensive, the rest of the included patients were normotensive. Details of the systolic, diastolic blood pressure and heart rate were added to the patient’s data in Table S1. A patient flow diagram gave details of the patient’s experimental setup (Figure 1). All participants in this study wore the Polar chest strap HR (H10, Polar Electro, OY, Finland) connected to the iPhone (iPhone SE, iPhoneOS 16.1, Apple Inc., California, USA) and the Apple Watch (Series 8, watchOS 9.0, Apple Inc., California, USA) on the left wrist connected to the iPhone (iPhone 11, iPhoneOS 16.1, Apple Inc., California, USA) to measure the heart rate at rest for 5 minutes, followed by moderate intensity exercise [50–70% Heart rate reserve (HRR)], 50 to 70% of HRR was defined as moderate exercise intensity,23 for 16 minutes, and 3 recovery minutes after the exercise session. During the study, all patients rested their hands on the handrails of the bicycle while wearing the devices. The pulse oximeter (CMS50DL, Contec Medical Systems Co., Ltd., China) was used at rest pre and after the exercise session.
Figure 1.
Patient flowchart.
Under resting conditions, the pulse oximeter is fitted on a warm finger while the participant assumes a relaxed setting position. The SpO2 was recorded five times after being stabilized on the pulse oximeter instrument.24,25
The exercise session was about 16 minutes in duration at moderate intensity according to the HRR using the cycle ergometer, preceded by a 3-minute warm-up, and followed by a 3-minute cool-down. The HR was recorded every 30 seconds during the whole period, and the oxygen saturation was recorded five times pre-and-post exercise.
This study followed the principles of the Declaration Helsinki of 1975, revised in 2008. All participants were fully informed about the purpose of the study. Each participant signed a written consent approving his volunteer participation in this study and agreeing to the publication of its results.
Inclusion criteria: eligible participants were patients with chronic diseases such as those with stable diabetes mellitus type 1 or 2, patients with dyslipidemia, and patients with controlled hypertension but not more than 180 mm Hg.
Exclusion criteria: patients with hypoxemia, uncontrolled diabetes mellitus, hypotension, hypothermia, nail polish, uncontrolled hypertension, hyperlipidemia, and vascular dysfunction were excluded from the study. Also, patients with other diseases than those described in the inclusion criteria were also excluded.
Sample Size
A suitable sample size was justified based on the past studies26–33 that used the wearable device, in which the participant numbers ranged from 20 to 60 participants. Suitable sample size was computed using the online G-Power tool (https://download.cnet.com/G-Power/3000-2054_4-10647044.html), considering the effect size of 0.6 (medium effect size), giving a sample size of 39 participants to provide reliable results.34 An extra-number of 2 participants was included to substitute for any participant’s withdrawal or discontinuation.
Data Analysis
All data were entered into JASP statistical version 18.1 for analysis. All data is presented as means and standard deviations. Shapiro–Wilk tests were used to determine the normal distribution of the data. One way ANOVA was used to test the mean difference between groups (hypertension, diabetes, and dyslipidemia). The linear regression and Bland-Altman plots were used to test the level of agreement between devices. The mean difference (MD) and standard deviation of the mean difference (SDD) were calculated to construct the Bland-Altman plots to measure the bias and level of agreement between the devices. Graphs were used in the Bayesian correlation pairwise plots in the JASP to represent the linear regression in HR and SpO2 between different devices.
Pearson correlation was used in cases of normal distribution, or the spearman test was used in cases of non-normal distribution to test the correlation between the Apple Watch and the pulse oximeter for measuring SpO2, and between the Apple Watch and the Polar chest strap to measure HR with a 95% confidence interval (CI). The correlation was intercepted based on the following definitions: r = (r >0.70) was strong, (0.5 to 0.7) was moderate, and r = (<0.5) was week.35 An independent t-test was used to test the difference between white and brown skin color groups for the SpO2 variable at rest. The P value was set at 0.05 for significant results.
Results
Oxygen Saturation (SpO2) at Rest and After Exercise
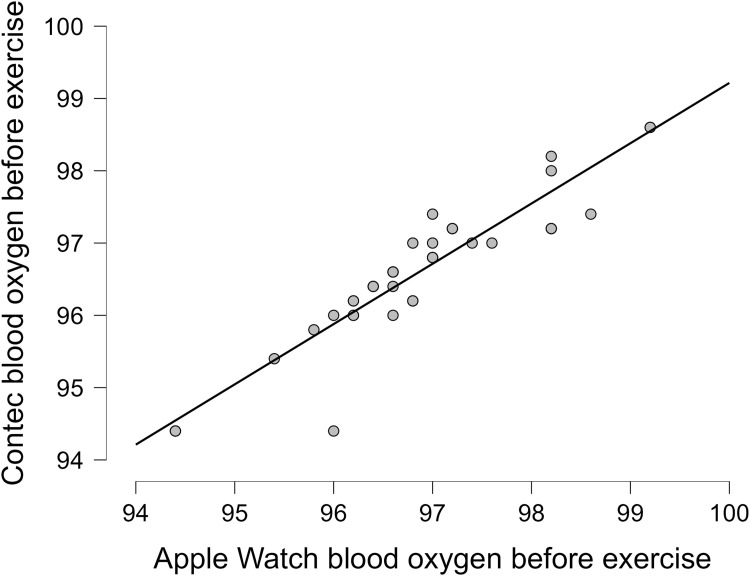
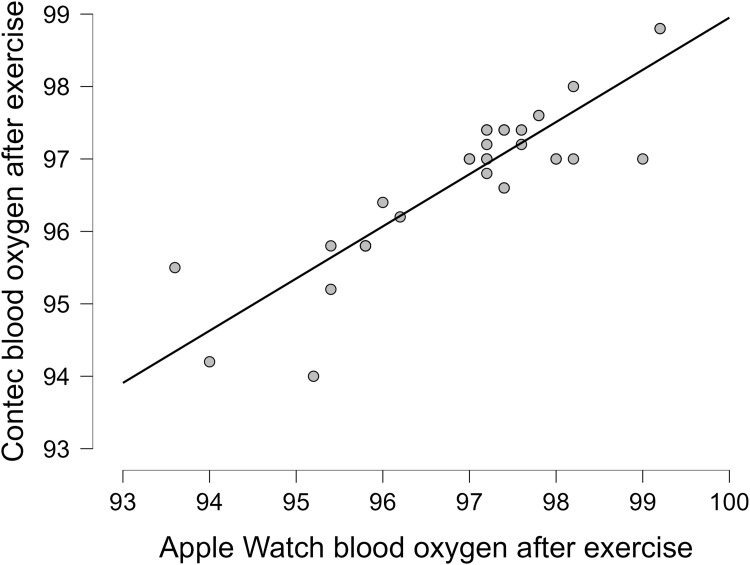
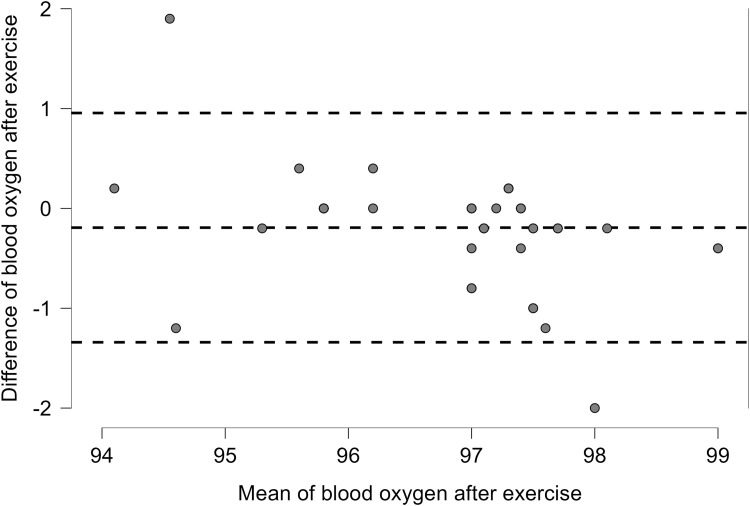
The oxygen saturation (SpO2) from the Apple Watch had a strong correlation to the pulse oximeter (Contec) in all 41 patients at rest and after exercise, with an overall correlation at rest (r = 0.92, p < 0.001) and after exercise (r = 0.86,p < 0.001) (Table 1). More details on the correlations between the Apple Watch and the Contec in each group are revealed in Table 1. More details on the data for the oxygen saturation before and after exercise are present in Table S2. Linear plots showed that the overall correlation for chronic patients between the Apple Watch and the Contec device as the reference device before exercise (Figure 2) and after exercise (Figure 3). Bland Altman plots showed Apple Watch had great variability to measure SpO2 at rest with (Lower LoA–Upper LoA; −0.56 to 1.08) after exercise with (Lower LoA–Upper LoA; −0.97 to 1.35). The mean differences for all patients at rest and after exercise were 0.26 and 0.19, respectively (Figures 4 and 5). There was no significant difference (p = 0.76) between the twenty-seven white (type II in the Fitzpatrick scale) and fourteen brown skin color (type IV in the Fitzpatrick scale) patients on SpO2 before exercise between the Apple Watch and the Contec.
Table 1.
Pearson or Spearman Correlation Between Apple Watch and Pulse Oximeter (Contec) in Patients with Chronic Diseases for Oxygen Saturation at Rest and After Exercise
| Mean (SD) of Practical (Apple Watch) | Mean (SD) of Criterion (Contec) | R (p value) | |
|---|---|---|---|
| SpO2 at rest for hypertension patients | 96.99(1.04) | 96.71(1.06) | 0.88(<0.001) |
| SpO2 at rest for diabetic patients | 96.49(0.95) | 96.27(0.76) | 0.95(<0.001) |
| SpO2 at rest for dyslipidemia patients | 96.88(1.42) | 96.62(1.22) | 0.001(<0.001) |
| SpO2 at rest for all chronic patients | 96.83(1.11) | 96.57(1.00) | 0.92(<0.001) |
| SpO2 after exercise for hypertension patients | 96.86(1.36) | 96.66(1.05) | 0.84(<0.001) |
| SpO2 after exercise for diabetic patients | 97.02(0.76) | 96.89(0.65) | 0.81(<0.001) |
| SpO2 after exercise for dyslipidemia patients | 97.00(1.48) | 96.76(1.27) | 0.97(<0.001) |
| SpO2 after exercise for all chronic patients | 96.94(1.23) | 96.74(1.00) | 0.87(<0.001) |
Abbreviations: R, Pearson or Spearman correlation coefficient; SD, Standard deviation; SpO2, Oxygen saturation.
Figure 2.
Blood oxygen correlation at rest for all patients with chronic diseases.
Figure 3.
Blood oxygen correlation after exercise for all patients with chronic diseases.
Figure 4.
Blood oxygen at rest for all patients with chronic diseases.
Figure 5.
Blood oxygen after exercise for all patients with chronic diseases.
Heart Rate
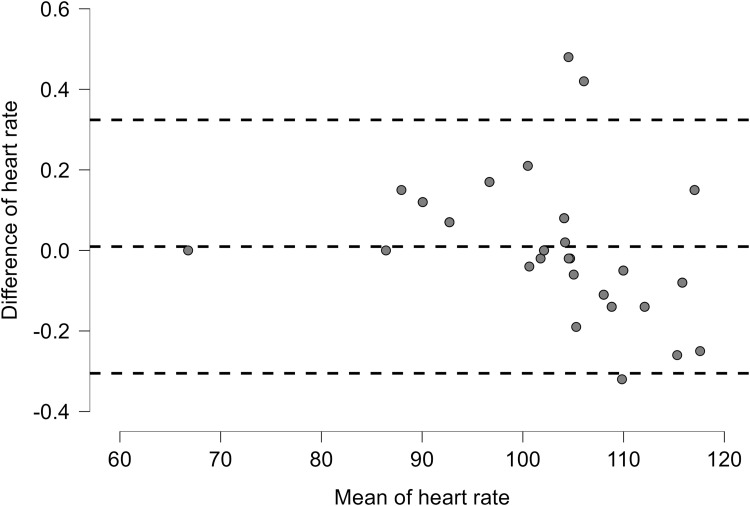
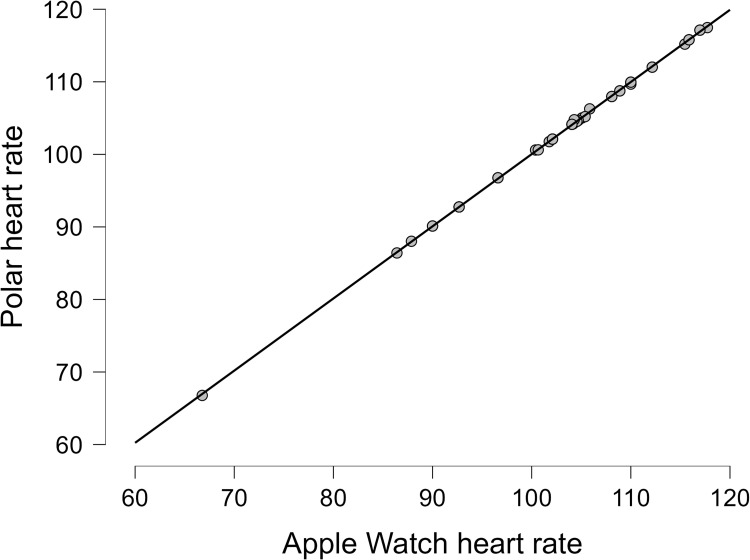
Mean and SD of the heart rate from the Apple Watch and the Polar chest strap during the whole exercise session and resting time before and after exercise are presented in Table 2. Bland Altman plots for heart rate were (Lower LoA–Upper LoA; −0.32 to 0.33) (Figure 3) in all patients with non-combinable chronic diseases. The mean difference in HR for all patients with non-commonable chronic diseases, including hypertension, diabetes, and dyslipidemia, was −0.01 (Figure 6). The correlation between the Apple Watch and Polar chest strap was strong (r = 0.99, p < 0.001) for all patients (Table 2). More details on the correlations between the Apple Watch and the Polar chest strap in each group are revealed in Table 2. More details on the data for the heart rate are present in Table S2. Linear plots showed that the there is an overall significant correlation (r = 0.99, p<0.001) between the Apple Watch and the Polar chest strap device as the reference device in patients with chronic diseases (Figure 7).
Table 2.
Pearson or Spearman Correlation Between Apple Watch and Polar Chest Strap in Patients with Chronic Diseases for HR During Whole Session
| Outcomes | Mean (SD) of Practical (Apple Watch) | Mean (SD) of Criterion (Polar) | R (p value) |
|---|---|---|---|
| HR for Hypertension patients | 102.33(11.68) | 102.35(11.63) | 0.001(<0.001) |
| HR for diabetic patients | 103.58(10.10) | 103.62(10.08) | 0.001(<0.001) |
| HR for dyslipidemia patients | 104.88(7.23) | 104.84(7.10) | 0.001(<0.001) |
| HR for all chronic patients | 103.29(10.15) | 103.30(10.10) | 0.99(<0.001) |
Abbreviations: R, Pearson or Spearman correlation coefficient; HR, Heart rate; SD, Standard deviation.
Figure 6.
Heart rate for all patients with chronic diseases.
Figure 7.
Heart rate correlation for all patients with chronic diseases.
Discussion
This is the first novel study to investigate the accuracy of the Apple Watch in measuring HR and SpO2 in patients with chronic diseases including diabetes, hypertension, and dyslipidemia. Modern medicine continues to continuously update the tools and procedures to effectively face chronic diseases’ impacts. These procedures can effectively facilitate health care service delivery, early diagnosis, patient motivation, and self-care organization.36 The HR and the Spo2 are among the most important health-related technological innovations.
Cardiovascular diseases (CVD) and associated risk factors, including diabetes, hypertension, and hyperlipidemia are among the most common reasons for death globally, affecting the majority of adults older than 60 years old.37
The continuous growth of the wearable devices market is due to rapid development and adaptation as well as low product costs compared to hospital devices such as the ECG.37
The HR is tightly and linearly correlated with the oxygen consumption at a specified time point.38 Monitoring of other vital variables such as the SpO2 is recommended in patients suffering hypoxia to improve their survival;39 in post-operative and post-arrest cardiac patients;40 the pulse oximeter is the most widely used instrument to evaluate the SpO2 because of its accuracy and ease of application.41
In this study, the HR and the SpO2 were evaluated for the first time in the 41 male patients with chronic non-communicable diseases (hypertension, diabetes, and dyslipidemia). The HR was evaluated before, during, and after the moderate-intensity exercise session, while the SpO2 was evaluated before and after the moderate-intensity exercise training session using the Apple Watch compared with the polar chest strap and standard commercial pulse oximeter devices.
The main results of the present study are the close concordance and non-significant differences between the Apple Watch and the standard procedures during evaluation of the Spo2 and the HR in patients with chronic diseases. Results showed a strong correlation between the Apple Watch and the polar chest strap in evaluating HR at rest and during exercise sessions. Results also revealed a strong correlation between the Apple Watch and the standard commercial pulse oximeter device in evaluating the SpO2 in patients with chronic non-communicable diseases before and after exercise sessions. Fortunately, participants skin color yielded non-significant effects on the study outcomes since no obvious variability exists related to the patients’ skin color between the white and brown skin colors (P = 0.76) between the Apple Watch and the Contec before exercise in the SpO2 variable, but the study did not include any patients with dark colors.
The current study findings are largely in agreement with Spaccarotella et al, who evaluated the Spo2 and the HR using the Apple Watch 6 and the standard SpO2 monitoring system in 257 patients with pulmonary (60 patients) or cardiovascular diseases (141 patients), in addition to 56 healthy participants as a control group. They concluded that there was a very good concordance (r = 0.89) in the SpO2 measured by the smartwatch compared to that measured by the standard SpO2 monitoring system (lower limit: −3.49 and upper limit: 3.04), with the difference between the two mean readings being clinically non-significant (p = 0.46), and subgroup comparisons were also non-significant.42
Oxygen saturation is most commonly evaluated in clinical practice by the pulse oximeter device because of its non-invasive nature and ease of use, especially in out-of-clinic situations. The Spo2% is the fraction of oxygen-saturated hemoglobin relative to the total amount of functional hemoglobin. It is important to continuously monitor the SpO2 due to its importance since it is sometimes considered “the fifth vital sign” after the HR, respiratory rate, blood pressure, and temperature.43 The Spo2 is a strong indicator of cardiopulmonary system health and function, and can be used as a guide during the management of chronic disorders.44
Using the updated Apple Watch technology; the level of SpO2 is measured via sensors consisting of 4 LED clusters in addition to 4 photodiodes, all of which are completely incorporated into a redesigned crystal. The red, green, and infrared LEDs are emitting light into the blood stream, while the photodiodes are sensitive to the amount of reflected light to measure the SpO2.42
The main health-related goal of the wearable devices is the monitoring of the body’s vital signs and activity responses in varieties of chronic diseases such as diabetes, dyslipidemia, and hypertension, objectively evaluating patient compliance, and improving the treatment process in general.35 Recent research has evolved the promising role of wearable devices during long-term out-clinic vital signs evaluation. Using a smartwatch application makes it feasible and easy for patients and healthy subjects to continuously evaluate their blood pressure (BP), SpO2, and HR at home.45,46
Home-based evaluation of the vital signs using personal monitoring devices such as Apple Watch is becoming more common and can be considered as effective as clinic-based HR and BP evaluation in predicting cardiac insults, as this can protect some patients with chronic diseases including diabetes, hypertension, and dyslipidemia, who are at risk for developing cardiac diseases,47 from death or some cardiac disease, as some of these patients HR may drop suddenly,48 and in situations when regular HR and BP monitoring and follow-up become challenging and can predispose the patients to serious consequences.45,47
Regarding HR monitoring, the results of the current study were supported by those of the previous reports, which concluded that there was excellent agreement between the HR evaluated by the Apple Watch and the Polar chest strap.35 Falter et al also concluded that utilizing the Apple Watch in evaluating the accuracy of the indoor training HR over the average of 30 seconds is clinically acceptable, the matter that projects to recommend the Apple Watch during cardiac rehabilitation.49
Numerous articles reported the good accuracy of the Apple Watch compared with the gold standard device in monitoring HR in healthy subjects.28,50,51 Weng et al reported the feasibility of utilizing the Apple Watch in monitoring the HR in post-discharge acute myocardial infarction patients affected with type 2 diabetes, hypertension, or hyperlipidemia since they are affected by a slower reduction in their HR relative to their counterparts.52
The results of the current study were augmented by those of Miller et al, who reported a high intraclass correlation coefficient (ICC) for the Apple Watch (0.96 and 0.67), and for the Polar Vantage (0.93 and 0.65).52 Also, excellent agreement (r = 0.98) was observed between the Apple Watch-HR and standard monitoring system-HR (lower limit: −5.84 and upper limit: 5.63), with the difference between the two mean readings being clinically non-significant (p = 0.93), and subgroup comparisons were also non-significant.46
Previous studies positively concluded the accuracy of the photoplethysmography (PPG) technology-based HR evaluation during different activity modes, including those with limited wrist movement and high periodic movements.28,36,47,50 Moreover, previous studies found that the Apple Watch is valid to measure blood oxygen in patients with lung diseases,21 healthy adults with hypoxemia,53 pediatric and adult congenital heart diseases cardiac patients,53 Our results are in alignment with the previous studies, and our study is the first to investigate the validity of the Apple Watch in chronic diseases (diabetes mellitus, hypertension, and dyslipidemia) separately.
Strengths and Limitations
The sample size of this study was suitable to validate the Apple Watch in patients with chronic diseases. Including only males could be one limitation in this study, but no previous studies reported any difference between males and females on measuring the HR or the SpO2 by using the Apple Watch. Limited data regarding the participants activity level as well as short training duration (one session) existed as limiting factors that should be compensated in future studies.
Future research, with the inclusion of different wearable devices, and more variables and patient groups with chronic diseases, is needed to further clarify the accuracy of the wrist-worn devices during the evaluation of the cardiovascular and metabolic variables in those patients.
Conclusions
The Apple Watch used for monitoring the HR and the SpO2 provides satisfactory results when compared with standard evaluation devices. The excellent correlations between the Apple Watch results and the standard evaluation procedures support the concept of increasing its use in clinical practice settings for monitoring the HR and the SpO2 in patients with chronic diseases, particularly those who are at risk for developing cardiac diseases.
Acknowledgments
The authors would like to thank the Deanship of Scientific Research at Umm Al-Qura University for supporting this work by Grant Code: (22UQU4280290DSR01). The authors would also like to thank Dr. Radi Alsafi, Dr. Mohammed Alghamdi, Dr. Yasser Bahakeem, and all staff members at Umm Al-Qura University Medical Center, particularly Samir Yamani and Nasser Alshamrani, for their support and all participants in this study for their time and commitment.
Funding Statement
The authors would like to thank the Deanship of Scientific Research at Umm Al-Qura University for supporting this work by Grant Code: (22UQU4280290DSR01).
Data Sharing Statement
The data that support the findings of this study are available in the Supplementary File.
Author Contributions
All authors made significant and equal contributions to the conception, study design, execution, acquisition of data, analysis, and interpretation. All authors have drafted, written, revised, critically reviewed the article and have agreed on the journal to which it was submitted. All authors have reviewed and agreed on all versions of the article before submission, during revision, the final version accepted for publication, and any significant changes introduced at the proofing stage. All authors agree to take responsibility for the contents of the article.
Disclosure
The authors declare that there is no conflict of interest in this work.
References
- 1.Warren JM, Ekelund U, Besson H, et al. Assessment of physical activity – a review of methodologies with reference to epidemiological research: a report of the exercise physiology section of the European Association of cardiovascular prevention and rehabilitation. Euro J Prevent Cardiol. 2010;17(2):127–139. [DOI] [PubMed] [Google Scholar]
- 2.Lee CM, Gorelick M. Validity of the smarthealth watch to measure heart rate during rest and exercise. Meas Phys Educ Exerc Sci. 2011;15(1):18–25. doi: 10.1080/1091367X.2011.539089 [DOI] [Google Scholar]
- 3.Vanderlei LCM, Silva RA, Pastre CM, et al. Comparison of the Polar S810i monitor and the ECG for the analysis of heart rate variability in the time and frequency domains. Braz J Med Biol Res. 2008;41(10):854–859. doi: 10.1590/S0100-879X2008005000039 [DOI] [PubMed] [Google Scholar]
- 4.Weippert M, Kumar M, Kreuzfeld S, et al. Comparison of three mobile devices for measuring R-R intervals and heart rate variability: polar S810i, Suunto t6 and an ambulatory ECG system. Eur J Appl Physiol. 2010;109(4):779–786. doi: 10.1007/s00421-010-1415-9 [DOI] [PubMed] [Google Scholar]
- 5.Etiwy M, Akhrass Z, Gillinov L, et al. Accuracy of wearable heart rate monitors in cardiac rehabilitation. Cardiovasc Diagn Ther. 2019;9(3):262. doi: 10.21037/cdt.2019.04.08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cole CR, Blackstone EH, Pashkow FJ, et al. Heart-rate recovery immediately after exercise as a predictor of mortality. N Engl J Med. 1999;341(18):1351–1357. doi: 10.1056/NEJM199910283411804 [DOI] [PubMed] [Google Scholar]
- 7.Center of disease control and prevention U.S. (Department of health and human services). National Diabetes Statistics Report, 2020: estimates of Diabetes and Its Burden in the United States 2020. Available from: https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed March 6, 2024.
- 8.Al-Nozha MM, Abdullah M, Arafah MR, et al. Hypertension in Saudi Arabia. Saudi Med J. 2007;28(1):77–84. [PubMed] [Google Scholar]
- 9.Al-Zahrani J, Shubair MM, Al-Ghamdi S, et al. The prevalence of hypercholesterolemia and associated risk factors in Al-Kharj population, Saudi Arabia: a cross-sectional survey. BMC Cardiovasc Disord. 2021;21(1):22. doi: 10.1186/s12872-020-01825-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gazzaz ZJ, Iftikhar R, Jameel T, et al. Association of dyslipidemia and comorbidities with risk factors among diabetic patients: a retrospective analysis. Diabetes Metab Syndr Obes. 2020;13:935–941. doi: 10.2147/DMSO.S235546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Freedson PS, Miller K. Objective monitoring of physical activity using motion sensors and heart rate. Res Q Exerc Sport. 2000;7(2):21–29. doi: 10.1080/02701367.2000.11082782 [DOI] [PubMed] [Google Scholar]
- 12.Khushhal A, Alsubaiei M. Barriers to Establishing Outpatient Cardiac Rehabilitation in the Western Region of Saudi Arabia: a Cross-Sectional Study. J Multidiscip Healthc. 2023;16:653–661. doi: 10.2147/JMDH.S398687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petrie JR, Guzik TJ, Touyz RM. Diabetes, Hypertension, and Cardiovascular Disease: clinical Insights and Vascular Mechanisms. Can J Cardiol. 2018;34(5):575–584. doi: 10.1016/j.cjca.2017.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laursen JC, Jepsen R, Bruun-Rasmussen NE, et al. Blood oxygen saturation is lower in persons with pre-diabetes and screen-detected diabetes compared with non-diabetic individuals: a population-based study of the Lolland-Falster Health Study cohort. Front Epidemiol. 2022;2:1022342. doi: 10.3389/fepid.2022.1022342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sami HM, Kleinman BS, Lonchyna VA. Central venous pulsations associated with a falsely low oxygen saturation measured by pulse oximetry. J Clin Monit. 1991;7(4):309–312. doi: 10.1007/BF01619351 [DOI] [PubMed] [Google Scholar]
- 16.Haroon N, Tiwana MI Design and development of non-invasive prototype to measure pulse rate, blood glucose and oxygen saturation level in arterial blood. Future Technologies Conference. Vancouver, Canada; 2017. [Google Scholar]
- 17.Moloney ED, Kiely JL, McNicholas WT. Controlled oxygen therapy and carbon dioxide retention during exacerbations of chronic obstructive pulmonary disease. Lancet. 2001;357(9255):526–528. doi: 10.1016/S0140-6736(00)04049-6 [DOI] [PubMed] [Google Scholar]
- 18.American Diabetes Association. Peripheral arterial disease in people with diabetes. Diabetes Care. 2003;26(12):3333–3341. doi: 10.2337/diacare.26.12.3333 [DOI] [PubMed] [Google Scholar]
- 19.Allen J. Photoplethysmography and its application in clinical physiological measurement. Physiol Meas. 2007;28(3):R1–R39. doi: 10.1088/0967-3334/28/3/R01 [DOI] [PubMed] [Google Scholar]
- 20.Priyadarsini P, Priyameenakshi K. The non-invasive PPG method of heart rate monitoring in smart phone device. Int J Innov Res Sci Eng Technol. 2015;4:894–898. [Google Scholar]
- 21.Pipek LZ, Nascimento RFV, Acencio MMP, et al. Comparison of SpO2 and heart rate values on Apple Watch and conventional commercial oximeters devices in patients with lung disease. Sci Rep. 2021;11(1):1–7. doi: 10.1038/s41598-021-98453-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tarassenko L, Greenhalgh T Should smartphone apps be used as oximeters? Oxford: COVID-19 Evidence Service; 2020. Available from: https://www.cebm.net/covid-19/question-should-smartphone-apps-beused-as-oximeters-answer-no/. Accessed March 6, 2024.
- 23.Reed JL, Pipe AL. Practical approaches to prescribing physical activity and monitoring exercise intensity. Can J Cardiol. 2016;32(4):514–522. doi: 10.1016/j.cjca.2015.12.024 [DOI] [PubMed] [Google Scholar]
- 24.Luks AM, Swenson ER. Pulse oximetry for monitoring patients with covid-19 at home: potential pitfalls and practical guidance. Ann Am Thorac Soc. 2020;17(9):1040–1046. doi: 10.1513/AnnalsATS.202005-418FR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shcherbina A, Mattsson CM, Waggott D, et al. Accuracy in wrist-worn, sensor-based measurements of heart rate and energy expenditure in a diverse cohort. J Pers Med. 2017;7(2):1–12. doi: 10.3390/jpm7020003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kooiman TJM, Dontje ML, Sprenger SR, et al. Reliability and validity of ten consumer activity trackers. BMC Sports Sci Med Rehabil. 2015;49(4):793–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wallen MP, Gomersall SR, Keating SE, et al. Accuracy of heart rate watches: implications for weight management. PLoS One. 2016;11(5):e0154420. doi: 10.1371/journal.pone.0154420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bai Y, Hibbing P, Mantis C, et al. Comparative evaluation of heart rate-based monitors: apple Watch vs Fitbit Charge HR. J Sports Sci. 2018;36(15):1734–1741. doi: 10.1080/02640414.2017.1412235 [DOI] [PubMed] [Google Scholar]
- 29.Boudreaux B, Hebert E, Hollander D, et al. Validity of wearable activity monitors during cycling and resistance exercise. Med Sci Sports Exerc. 2018;50(3):624–633. doi: 10.1249/MSS.0000000000001471 [DOI] [PubMed] [Google Scholar]
- 30.Chowdhury E, Western M, Nightingale T, et al. Assessment of laboratory and daily energy expenditure estimates from consumer multi-sensor physical activity monitors. PLoS One. 2017;12(2):e0171720. doi: 10.1371/journal.pone.0171720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khushhal A, Nichols S, Evans W, et al. Validity and reliability of the Apple Watch for measuring heart rate during exercise. Sports Med Int Open. 2017;1(6):E206–E211. doi: 10.1055/s-0043-120195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Akoglu H. User’s guide to correlation coefficients. Turk J Emerg Med. 2018;18(3):91–93. doi: 10.1016/j.tjem.2018.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moses JC, Adibi S, Shariful Islam SM, et al. Application of Smartphone Technologies in Disease Monitoring: a Systematic Review. Healthcare. 2021;9(7):889. doi: 10.3390/healthcare9070889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.World Medical Association Declaration of Helsinki. Ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 35.Spaccarotella C, Polimeni A, Mancuso C, et al. Assessment of non-invasive measurements of oxygen saturation and heart rate with an apple smartwatch: comparison with a standard pulse oximeter. J Clin Med. 2022;11(6):1467. doi: 10.3390/jcm11061467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laslett LJ, Alagona P, Clark BA, et al. The worldwide environment of cardiovascular disease: prevalence, diagnosis, therapy, and policy issues: a report from the American College of Cardiology. J Am Coll Cardiol. 2012;60(25):S1–49. doi: 10.1016/j.jacc.2012.11.002 [DOI] [PubMed] [Google Scholar]
- 37.Yetisen AK, Martinez-Hurtado JL, Ünal B, et al. Wearables in Medicine. Adv Mater. 2018;30(33):e1706910. doi: 10.1002/adma.201706910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crisafulli A, Pittau G, Lorrai L, et al. Poor reliability of heart rate monitoring to assess oxygen uptake during field training. Int J Sports Med. 2006;27(1):55–59. doi: 10.1055/s-2005-837504 [DOI] [PubMed] [Google Scholar]
- 39.Porte P. Clinical indications for noninvasive positive pressure ventilation in chronic respiratory failure due to restrictive lung disease, COPD, and nocturnal hypoventilation—A consensus conference report. Chest. 1999;116:521–534. [DOI] [PubMed] [Google Scholar]
- 40.Kilgannon JH, Jones AE, Shapiro NI, et al. Association between arterial hyperoxia following resuscitation from cardiac arrest and in-hospital mortality. JAMA. 2010;303(21):2165–2171. doi: 10.1001/jama.2010.707 [DOI] [PubMed] [Google Scholar]
- 41.Schnapp LM, Cohen NH. Pulse oximetry: uses and abuses. Chest. 1990;98(5):1244–1250. doi: 10.1378/chest.98.5.1244 [DOI] [PubMed] [Google Scholar]
- 42.Mower WR, Sachs C, Nicklin EL, et al. Pulse oximetry as a fifth pediatric vital sign. Pediatrics. 1997;99(5):681–686. doi: 10.1542/peds.99.5.681 [DOI] [PubMed] [Google Scholar]
- 43.Shapiro I, Stein J, MacRae C, et al. Pulse oximetry values from 33,080 participants in the Apple Heart & Movement Study. NPJ Digit Med. 2023;6(1):134. doi: 10.1038/s41746-023-00851-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stergiou GS, Mukkamala R, Avolio A; European Society of Hypertension Working Group on Blood Pressure Monitoring and Cardiovascular Variability. et al. Cuffless blood pressure measuring devices: review and statement by the European society of hypertension working group on blood pressure monitoring and cardiovascular variability. J Hypertens. 2022;40(8):1449–1460. doi: 10.1097/HJH.0000000000003224 [DOI] [PubMed] [Google Scholar]
- 45.Jang Y, Seo JM, Ihm SH, et al. Korean Society of Hypertension. Feasibility, credence, and usefulness of out-of-office cuffless blood pressure monitoring using smartwatch: a population survey. Clin Hypertens. 2023;29(1):15. doi: 10.1186/s40885-023-00242-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cadmus-Bertram L, Gangnon R, Wirkus EJ, et al. Accuracy of heart rate monitoring by some wrist-worn activity trackers. Ann Intern Med. 2017;167(8):607–608. doi: 10.7326/L17-0380 [DOI] [PubMed] [Google Scholar]
- 47.Jacobsen M, Dembek TA, Kobbe G, et al. Noninvasive continuous monitoring of vital signs with wearables: fit for medical use? J Diabetes Sci Technol. 2021;15(1):34–43. doi: 10.1177/1932296820904947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Falter M, Budts W, Goetschalckx K, et al. Accuracy of apple watch measurements for heart rate and energy expenditure in patients with cardiovascular disease: cross-sectional study. JMIR Mhealth Uhealth. 2019;7(3):e11889. doi: 10.2196/11889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang R, Blackburn G, Desai M, et al. Accuracy of wrist-worn heart rate monitors: RESEARCH LETTER. JAMA Cardiol. 2017;2(1):104–105. doi: 10.1001/jamacardio.2016.3340 [DOI] [PubMed] [Google Scholar]
- 50.Gillinov S, Etiwy M, Wang R, et al. Variable Accuracy of wearable heart rate monitors during aerobic exercise. Med Sci Sports Exerc. 2017;49(8):1697–1703. doi: 10.1249/MSS.0000000000001284 [DOI] [PubMed] [Google Scholar]
- 51.Weng D, Ding J, Sharma A, et al. Heart rate trajectories in patients recovering from acute myocardial infarction: a longitudinal analysis of Apple Watch heart rate recordings. Cardiovasc Digit Health J. 2021;2(5):270–281. doi: 10.1016/j.cvdhj.2021.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miller DJ, Sargent C, Roach GD. A validation of six wearable devices for estimating sleep, heart rate and heart rate variability in healthy adults. Sensors. 2022;22(16):6317. doi: 10.3390/s22166317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Windisch P, Schröder C, Förster R, et al. Accuracy of the apple watch oxygen saturation measurement in adults: a systematic review. Cureus. 2023;15(2):e35355. doi: 10.7759/cureus.35355 [DOI] [PMC free article] [PubMed] [Google Scholar]