Abstract
Objective:
Heavy drinking poses serious risks to individuals with HIV, hepatitis C virus (HCV), and especially HIV/HCV coinfection. We adapted the National Institute on Alcohol Abuse and Alcoholism Clinician's Guide to address HIV/HCV coinfection and paired this with the “HealthCall” smartphone app to create an intervention tailored to HIV/HCV. After formative work and pretesting with HIV/HCV coinfected heavy drinkers, we conducted a pilot trial to determine potential of this new intervention for decreasing drinking.
Method:
A sample of 31 HIV/HCV coinfected heavy drinkers were randomly assigned to either intervention (n = 16) or control (n = 15; psychoeducation and brief advice) conditions. All participants completed a 60-day program consisting of approximately 25-minute-long baseline sessions and brief 5–10-minute booster sessions at 30 and 60 days, as well as an assessment-only follow-up at 90 days. Outcomes were measured using the Timeline Followback at baseline, 30, 60, and 90 days. Generalized linear models were used for analysis.
Results:
Intervention participants drank fewer mean drinks per drinking day at 60 days (incidence rate ratio [IRR] = 0.43, p = .03) and 90 days (IRR = 0.34, p < .01). Intervention participants also reported fewer drinking days at 90 days (mean difference = 34.5%; p < .01). Self-efficacy differed between groups during intervention (p < .05).
Conclusions:
Although our sample was small, our results suggested lower drinking among participants who received a modified Clinician's Guide intervention plus use of the smartphone app HealthCall, in comparison with education and advice alone. A larger study is indicated to further examine this brief, disseminable intervention for HIV/HCV coinfected drinkers.
Heavy drinking is harmful for individuals with HIV (Williams et al., 2016). Dangers of drinking with HIV include damage to the liver (Navarro, 2022), medication nonadherence (Azar et al., 2010; including intentional non-adherence; El-Krab & Kalichman, 2021), and poor treatment outcomes (Azar et al., 2010). Hepatitis C virus (HCV) is a viral disease that can cause severe liver damage including cirrhosis, liver failure, and liver cancer (National Institute of Diabetes and Digestive and Kidney Diseases, 2020). Drinking accelerates disease progression in HCV (Xu et al., 2021). For those coinfected with HIV and HCV, HIV accelerates HCV pathogenesis, whereas HCV accelerates HIV disease processes (Gobran et al., 2021). Thus, reducing drinking among HIV/HCV coinfected patients is particularly important.
Several alcohol interventions have been developed to reduce heavy drinking among individuals with HIV, with mixed success (as reviewed by Brown et al., 2013; Madhombiro et al., 2019; Samet & Walley, 2010; Scott-Sheldon et al., 2017). One successful intervention involved motivational interviewing (MI) with use of HealthCall, a technological enhancement to track daily drinking and related behaviors (medication adherence, drug use) for later feedback and discussion (Hasin et al., 2013). HealthCall originally used interactive voice response technology and was later updated to a digital app format (Hasin et al., 2014). Recent research has also supported HealthCall as an effective adjunct to the National Institute on Alcohol Abuse and Alcoholism (NIAAA) Clinician's Guide (2007), a flow-chart guide for clinicians to assess drinking using standard prompts and make guided drinking-reduction recommendations. The Clinician's Guide requires less training and supervision than MI, making it simpler to implement. In a recent analysis, MI plus Health-Call led to greater improvements during treatment, whereas Clinician's Guide plus HealthCall had the most sustained improvements (Hasin et al., 2022).
Until recently, few interventions to reduce risky drinking have targeted HIV/HCV coinfected individuals specifically. Edelman and colleagues used a stepped-care model to encourage abstinence in moderate drinkers with HIV and liver disease (Edelman et al., 2019). Their study showed promise in increasing abstinence but screened out heavy drinkers, a population in need of attention. Another clinical trial targeting HIV/HCV coinfected drinkers conducted by Stein and colleagues compared brief advice to an MI intervention (Stein et al., 2021). This study, which included heavy drinkers, showed similar drinking declines in both groups. This suggests that even brief interventions may help individuals with HIV/HCV decrease drinking. This promising new field requires further study.
In the current study, we tested a brief intervention designed to decrease drinking in HIV/HCV coinfected heavy drinkers. Specifically, we adapted the NIAAA Clinician's Guide for HIV/HCV coinfected individuals and paired it with the HealthCall app. Our pilot study compares Clinician's Guide + HealthCall to education and brief advice (approximating standard of care).
Method
Participants and procedures
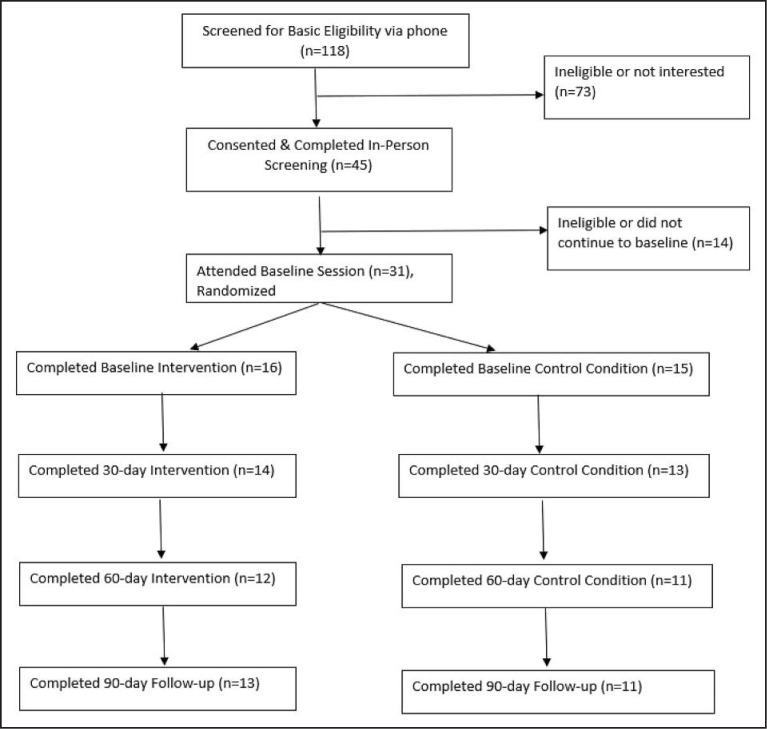
Recruitment and screening. Participants were initially recruited through an infectious disease/comprehensive HIV primary care clinic in a major metropolitan area via passive (poster) and active (recruiter referral) recruitment. (See Figure 1.) When recruitment was exhausted at the clinic, it was opened city-wide via newspaper ads, posters in clinics and HIV service programs, and use of website recruitment (Craigslist, Google Ads, HCV information website). Participants were screened for basic eligibility criteria by phone or recruiter. Eligibility requirements were age 18–99, diagnosis of HIV, present or resolved HCV infection, past-year liver lab results, past-month heavy drinking (4+ drinks on one occasion), ability to read and speak in English, and current HIV care in the New York City area. Participants were excluded if they were participating in a trial of another Health-Call intervention or had plans to move outside of the region during the study. Potentially eligible participants completed informed consent and were further screened for additional exclusionary criteria (suicidality and homicidality, psychosis, or alcohol withdrawal), with referrals given and patients linked promptly to providers in the event of risk. The study received full institutional review board review and approval and was registered on ClinicalTrials.gov (NCT03652675). A sample of 31 HIV/HCV coinfected heavy drinkers were randomly assigned to either intervention (n = 16) or control (n = 15; psychoeducation and brief advice) conditions.
Figure 1.
Participant screening, enrollment, and follow-up. Note: We conducted a blocked randomization, stratified by alcohol use disorder status. Initially, in-person screening and baseline were conducted in separate sessions, with randomization at screening. Four participants were randomized (but not notified) but could not be scheduled for baseline. Per consultation with our biostatistician, given the stratified/blocked design, participants deemed unreachable by our coordinator (blind to condition) yielded their blocked randomization allocation to the next eligible participant.
Baseline. When participants were found to be eligible, our bachelor's-level research coordinator completed the Timeline Followback (TLFB; Sobell, 1995) drinking assessment and administered the Alcohol Use Disorder and Associated Disabilities Interview Schedule alcohol use disorder (AUD) assessment (Grant et al., 2015) to determine AUD status. The research coordinator logged AUD status, and the principal investigator accessed this information to complete a block randomization stratified by AUD status while the research coordinator supervised participant self-assessments. Participants then completed the intervention or control baseline condition with a study counselor. Study counselors held at least a master's degree and were trained on HIV, HCV, and study procedures and interventions. Efforts were made to keep the research coordinator blind to condition throughout the study, although participant self-disclosures or technical issues with smartphones sometimes compromised these efforts. All participants were offered referral brochures to outside care (e.g., Alcoholics Anonymous, hotlines, substance use programs) at baseline and follow-up appointments and were free to seek outside care during study participation.
Follow-up. At the 30- and 60-day follow-up visits, participants completed TLFB with the research coordinator, completed self-assessments, and then completed their follow-up intervention or control condition with the study counselor. At the 90-day follow-up visit, participants completed the TLFB and self-assessments only, with no intervention/control sessions. Because of the emergence of the coronavirus-19 (COVID-19) pandemic, follow-up sessions scheduled during April–July 2020 were conducted remotely via phone and/or online surveys.
Study conditions
Intervention.
DEVELOPMENT AND CONTENT: The intervention consisted of an adaptation of the NIAAA Clinician's Guide (2007) in conjunction with the smartphone application HealthCall (Hasin et al., 2014). The Clinician's Guide was first adapted for HIV by Hasin (Hasin et al., 2022), featuring an added module on HIV medication adherence. We adapted the Clinician's Guide further to address HIV/HCV coinfection. Additions to the Clinician's Guide included psychoeducation on risks of drinking with HIV/HCV coinfection (tailored for treatment status), discussion of adherence to HCV medications, and feedback on liver health using baseline hepatic function panel results (APRI, ALT, Fibroscan, Fibrosure/FibroSpect, liver biopsy). These adaptations were made in consultation with researchers, clinicians, mental health providers, and formative research with five coinfected heavy drinkers. The intervention was pretested for feasibility with an additional five coinfected heavy drinkers. HealthCall is a smartphone app developed by Hasin et al. to help patients monitor drinking, HIV medication adherence, and potential triggers to use (e.g., mood, drug use) through daily self-entries. Feedback graphs are then generated for later discussion.
BASELINE: Participants completed a 20- to 30-minute Clinician's Guide session including psychoeducation, liver function feedback, discussion of drinking and medication adherence, and goal setting. Participants were provided with a study smartphone, oriented to HealthCall, and asked to log in daily for the next 60 days.
30- AND 60-DAY FOLLOW-UPS: Participants came in for assessments and then brief 5- to 10-minute sessions in which they discussed drinking, goals, and adherence, facilitated by feedback graphs generated from HealthCall daily entries. Sessions were audio recorded for supervision.
Control.
BASELINE: The control participants were given and asked to read an educational pamphlet about HIV, HCV, and drinking, providing similar information and exposure time as the intervention condition. They were also given advice that current drinking levels were harmful given their medical conditions and should be decreased.
30- AND 60-DAY FOLLOW-UPS: Participants met with the study counselor for 5–10 minutes during which they were queried about their drinking and given advice to continue reducing their drinking.
Outcome measures
Timeline Followback. Drinking was assessed at each appointment using a 30-day TLFB, administered in person by the trained research coordinator. The TLFB is a widely used measure that has demonstrated reliability (Wray et al., 2016) and validity (Grant et al., 1995). As an accuracy check, 10% of TLFB files were randomly selected and checked for accuracy against audio recordings or notes. Outcome variables included drinks per drinking day (quantity) and percentage days drinking (frequency). To include all data for the small sample, and to recognize abstention as the most desirable outcome, we coded abstainers as having zero drinks per drinking day in calculating drinks per drinking day. However, because zero drinks does not technically count as a drinking day, we also conducted a sensitivity analysis omitting abstainers' data, as a secondary operationalization of this variable. Percentage drinking days was calculated for frequency instead of number of days because of variability in the timeframe of follow-up. Two participants who missed a follow-up but attended the next completed retrospective TLFBs for the missed period.
Self-efficacy. Self-efficacy was measured using the eight-item brief Situational Confidence Questionnaire (Breslin et al., 2000). Participants were asked to rate their percentage confidence in their ability to resist drinking in eight situations using a 100-point visual analog scale. The brief Situational Confidence Questionnaire yields information similar to the 100-item version (Breslin et al., 2000) and shows good reliability and validity (Delaney et al., 2020). Previous research with this scale in an HIV sample showed correlation between higher self-efficacy and success in drinking reduction (Gause et al., 2018).
Readiness-to-change. Readiness-to-change was measured using two widely used readiness-to-change measures: the URICA (University of Rhode Island Change Assessment) and the SOCRATES (Stages of Change Readiness and Treatment Eagerness Scale; Miller, 1996). The URICA is a 24-item scale that uses a Likert response scale. Participants' scores were calculated for Precontemplation, Contemplation, Action, and Maintenance subscales. The final score was calculated by adding the Contemplation, Action, and Maintenance scores, then subtracting the Precontemplation score (UMBC Habits Lab, n.d.). The URICA has been found to have modest but acceptable reliability and good factor validity (Shields & Hufford, 2005). The SOCRATES is a 19-item measure assessing readiness to change in three domains: problem recognition, ambivalence, and taking steps to change behavior. The SOCRATES items have been found to be reliable (Miller, 1996) and to relate to alcohol use levels (Maisto et al., 1999).
Analysis plan
Means and standard deviations were used to describe alcohol-related outcomes (drinks per drinking day and percentage days drank) at each time point and within each treatment group. Changes in alcohol use were analyzed using TLFB data from assessments during treatment (30 and 60 days) and at the 90-day follow-up. Generalized linear mixed models were used to model the effects of time, treatment, and the interaction of time and treatment on the outcomes, using SAS PROC GLIMMIX (SAS Institute Inc., Cary, NC). Models controlled for baseline alcohol use and included random intercepts. Drinks per drinking day was best modeled using a negative binomial distribution, and percentage drinking days was best modeled using a normal distribution. All tests were two sided with p < .05 indicating significance. Incidence rate ratios (IRRs) and associated confidence intervals were used to represent effect sizes of nonnormally distributed variables. IRRs reflect the ratio of expected count (e.g., number of drinks), or rate, of the outcome in the treatment group compared with the reference group at each time point. Similar models were used to assess changes in self-efficacy and readiness to change.
Results
Sample characteristics and retention
Participants were middle aged (M = 56.4 years, SD = 10.4), mostly male (68%), and Black/African American (81%), and 80% reported treated/resolved HCV. Most met AUD criteria (81%). At baseline, they drank on 58% of days (intervention: 54%; control: 62%), with a mean of 5.65 (SD = 3.10) drinks per drinking day (intervention: M = 5.24 [SD = 3.80]; control: M = 6.09 [SD = 2.17]. Retention was 87% at 30 days, 74% at 60 days, and 77% at 90 days (intervention: 88%/75%/81%; control: 87%/73%/73%, respectively).
Drinks per drinking day
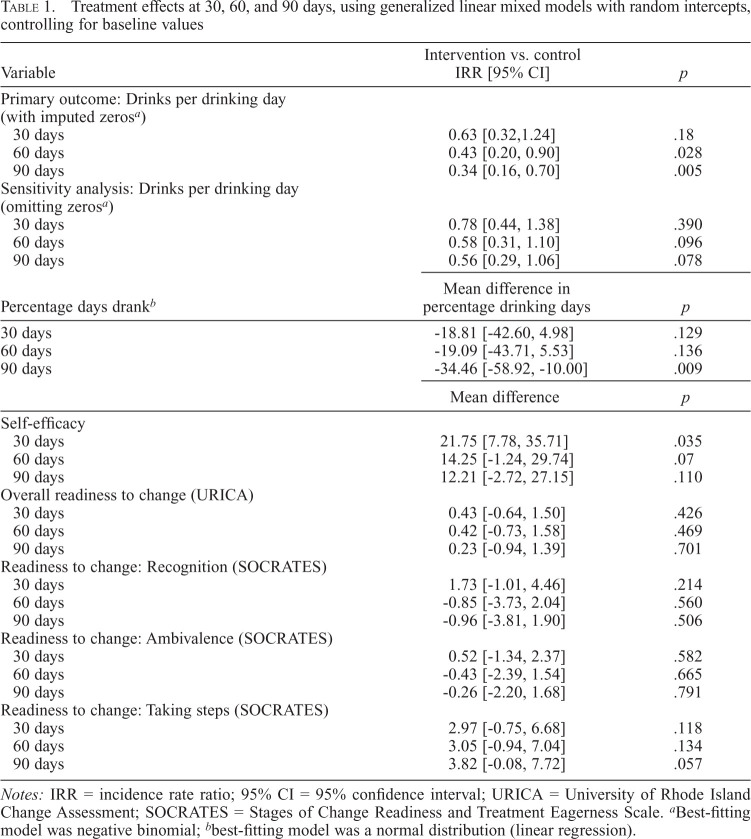
Using the primary drinks per drinking day variable coding abstainers as zero, intervention participants drank fewer mean drinks per drinking day than controls at 60 days (2.6 vs. 5.5, respectively; IRR = 0.43, p = .03) and at 90 days (2.4 vs. 6.0, respectively; IRR = 0.34, p < .01) (Table 1). Using the alternative model of drinks per drinking day excluding abstainers, these effects were marginal at 60 days (IRR = 0.58, p = .096) and 90 days (IRR = 0.56, p = .078).
Table 1.
Treatment effects at 30, 60, and 90 days, using generalized linear mixed models with random intercepts, controlling for baseline values
| Variable | Intervention vs. control IRR [95% CI] | p |
|---|---|---|
| Primary outcome: Drinks per drinking day (with imputed zerosa) | ||
| 30 days | 0.63 [0.32,1.24] | .18 |
| 60 days | 0.43 [0.20, 0.90] | .028 |
| 90 days | 0.34 [0.16, 0.70] | .005 |
| Sensitivity analysis: Drinks per drinking day (omitting zerosa) | ||
| 30 days | 0.78 [0.44, 1.38] | .390 |
| 60 days | 0.58 [0.31, 1.10] | .096 |
| 90 days | 0.56 [0.29, 1.06] | .078 |
| Percentage days drankb | Mean difference in percentage drinking days | p |
|---|---|---|
| 30 days | -18.81 [-42.60, 4.98] | .129 |
| 60 days | -19.09 [-43.71, 5.53] | .136 |
| 90 days | -34.46 [-58.92, -10.00] | .009 |
| Mean difference | p | |
|---|---|---|
| Self-efficacy | ||
| 30 days | 21.75 [7.78, 35.71] | .035 |
| 60 days | 14.25 [-1.24, 29.74] | .07 |
| 90 days | 12.21 [-2.72, 27.15] | .110 |
| Overall readiness to change (URICA) | ||
| 30 days | 0.43 [-0.64, 1.50] | .426 |
| 60 days | 0.42 [-0.73, 1.58] | .469 |
| 90 days | 0.23 [-0.94, 1.39] | .701 |
| Readiness to change: Recognition (SOCRATES) | ||
| 30 days | 1.73 [-1.01, 4.46] | .214 |
| 60 days | -0.85 [-3.73, 2.04] | .560 |
| 90 days | -0.96 [-3.81, 1.90] | .506 |
| Readiness to change: Ambivalence (SOCRATES) | ||
| 30 days | 0.52 [-1.34, 2.37] | .582 |
| 60 days | -0.43 [-2.39, 1.54] | .665 |
| 90 days | -0.26 [-2.20, 1.68] | .791 |
| Readiness to change: Taking steps (SOCRATES) | ||
| 30 days | 2.97 [-0.75, 6.68] | .118 |
| 60 days | 3.05 [-0.94, 7.04] | .134 |
| 90 days | 3.82 [-0.08, 7.72] | .057 |
Notes: IRR = incidence rate ratio; 95% CI = 95% confidence interval; URICA = University of Rhode Island Change Assessment; SOCRATES = Stages of Change Readiness and Treatment Eagerness Scale.
Best-fitting model was negative binomial;
best-fitting model was a normal distribution (linear regression).
Percentage drinking days
Intervention participants reported fewer drinking days at 90 days (M difference = 34.5%; adjusted means = 24.6% vs. 59.1% days; p < .01; Table 1). Differences were not significant at 30 or 60 days.
Self-efficacy
Clinician's Guide + HealthCall participants endorsed higher self-efficacy during intervention (30 days; p < .05), marginally higher self-efficacy at end of intervention (60 days; p = .07), and similar self-efficacy at end of study (90 days; p = .11).
Readiness-to-change
Although the groups did not differ in earlier stages of change, there was a trend toward higher “Readiness to Take Steps” in the Clinician's Guide + HealthCall group at end of study (90 days; p = .06).
Discussion
In this pilot study, HIV/HCV coinfected drinkers reduced drinking more in response to a modified Clinician's Guide intervention plus HealthCall than in response to education and brief advice. This effect was significant when abstainers were included and marginal when they were not, likely because of the small sample and highlighting the importance of this most successful outcome. This may have been related to changes in self-efficacy, although the study was underpowered for mediation analyses. Although readiness to change evidenced no significant differences between study arms, readiness to take steps was marginally higher in Clinician's Guide + HealthCall at 90 days. This study thus suggests that a brief intervention incorporating tracking and feedback may help with risk reduction in this important population.
Interventions for HIV/HCV coinfected drinkers have been limited until recent years, despite risks of drinking on engagement in care and liver-related outcomes for HIV (Azar et al., 2010; Navarro, 2022) and HCV (Xu et al., 2021). However, recent research has shown promise in (a) promoting abstinence using stepped care among HIV-infected moderate drinkers with liver disease (Edelman et al., 2019) and (b) reducing drinking using brief advice or MI among HIV/HCV heavier drinkers (Stein et al., 2021). Our study contributes to this emerging literature by showing that brief structured intervention combined with use of a smartphone app may help reduce drinking in heavy drinkers with HIV/HCV. Our findings echo some successes of similar drinking interventions in HIV (reviewed by Brown et al., 2013; Madhombiro et al., 2019; Samet & Walley, 2010; Scott-Sheldon et al., 2017) and HCV (Sims et al., 2016) mono-infected populations.
Our study has certain limitations. The trial was smaller than the intended n = 60 because of challenges in recruiting HIV/HCV coinfected heavy drinkers. However, the sample size of 31 still resulted in significant findings and provided 80% power to detect differences greater than 3.5 (assuming SD = 3.5) between the groups. The follow-up time was brief. Recruitment challenges necessitated opening recruitment city-wide as opposed to conducting the study within one clinic, which necessitated holding sessions in research offices in lieu of providing in-clinic services. Further, COVID-19 resulted in an unplanned shift from a face-to-face format to a virtual assessment for some follow-ups. The study was underpowered for mediation analyses, leading us to simply assess group differences for self-efficacy and readiness to change. Yet, preliminary findings are promising.
In conclusion, this study is an important step toward improving the health of HIV/HCV coinfected individuals by intervening with harmful drinking. Future research should conduct a larger clinical trial embedded within primary care, providing a well-powered test of an intervention that truly fits into ongoing care. Future researchers should also use mediation analyses and biomarkers.
Acknowledgments
The authors thank the doctors, recruiters, patients, and other clinic staff for their participation, feedback, and help, and all of those who participated in this study. Thank you to Lisa Metsch, Melanie Wall, and Scott Friedman for your mentoring.
Footnotes
This study was funded by National Institutes of Health (NIH) Grant K23AA023753 and by the New York State Psychiatric Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
References
- Azar M. M., Springer S. A., Meyer J. P., Altice F. L. A systematic review of the impact of alcohol use disorders on HIV treatment outcomes, adherence to antiretroviral therapy and health care utilization. Drug and Alcohol Dependence. 2010;112(3):178–193. doi: 10.1016/j.drugalcdep.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslin F. C., Sobell L. C., Sobell M. B., Agrawal S. A comparison of a brief and long version of the Situational Confidence Questionnaire. Behaviour Research and Therapy. 2000;38(12):1211–1220. doi: 10.1016/S0005-7967(99)00152-7. [DOI] [PubMed] [Google Scholar]
- Brown J. L., DeMartini K. S., Sales J. M., Swartzendruber A. L., DiClemente R. J. Interventions to reduce alcohol use among HIV-infected individuals: A review and critique of the literature. Current HIV/AIDS Reports. 2013;10(4):356–370. doi: 10.1007/s11904-013-0174-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney D. J., Bernstein M. H., Harlow L. L., Farrow M., Martin R. A., Stein L. A. R. The Brief Situational Confidence Questionnaire for alcohol: A psychometric assessment with incarcerated youth. Psychological Assessment. 2020;32(3):254–264. doi: 10.1037/pas0000780. [DOI] [PubMed] [Google Scholar]
- Edelman E. J., Maisto S. A., Hansen N. B., Cutter C. J., Dziura J., Deng Y., Fiellin L. E., O'Connor P. G., Bedimo R., Gibert C. L., Marconi V. C., Rimland D., Rodriguez-Barradas M. C., Simberkoff M. S., Tate J. P., Justice A. C., Bryant K. J., Fiellin D. A. Integrated stepped alcohol treatment for patients with HIV and liver disease: A randomized trial. Journal of Substance Abuse Treatment. 2019;106:97–106. doi: 10.1016/j.jsat.2019.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Krab R., Kalichman S. C. Alcohol-antiretroviral therapy interactive toxicity beliefs and intentional medication nonadherence: Review of research with implications for interventions. AIDS and Behavior. 2021;25(S3):251–264. doi: 10.1007/s10461-021-03285-x. [DOI] [PubMed] [Google Scholar]
- Gause N. K., Elliott J. C., Delker E., Stohl M., Hasin D., Aharonovich E. Association between change in self-efficacy to resist drinking and drinking behaviors among an HIV-infected sample: Results from a large randomized controlled trial. Journal of Health Psychology. 2018;23(6):829–839. doi: 10.1177/1359105316664127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobran S. T., Ancuta P., Shoukry N. H. A tale of two viruses: Immunological insights into HCV/HIV coinfection. Frontiers in Immunology. 2021;12:726419. doi: 10.3389/fimmu.2021.726419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant B. F., Goldstein R. B., Smith S. M., Jung J., Zhang H., Chou S. P., Pickering R. P., Ruan W. J., Huang B., Saha T. D., Aivadyan C., Greenstein E., Hasin D. S. The Alcohol Use Disorder and Associated Disabilities Interview Schedule-5 (AUDADIS-5): Reliability of substance use and psychiatric disorder modules in a general population sample. Drug and Alcohol Dependence. 2015;148:27–33. doi: 10.1016/j.drugalcdep.2014.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant K. A., Tonigan J. S., Miller W. R. Comparison of three alcohol consumption measures: A concurrent validity study. Journal of Studies on Alcohol. 1995;56(2):168–172. doi: 10.15288/jsa.1995.56.168. [DOI] [PubMed] [Google Scholar]
- Hasin D. S., Aharonovich E., Greenstein E. HealthCall for the smartphone: Technology enhancement of brief intervention in HIV alcohol dependent patients. Addiction Science & Clinical Practice. 2014;9(1):5. doi: 10.1186/1940-0640-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin D. S., Aharonovich E., O'Leary A., Greenstein E., Pavlicova M., Arunajadai S., Waxman R., Wainberg M., Helzer J., Johnston B. Reducing heavy drinking in HIV primary care: a randomized trial of brief intervention, with and without technological enhancement. Addiction. 2013;108(7):1230–1240. doi: 10.1111/add.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin D. S., Aharonovich E., Zingman B. S., Stohl M., Walsh C., Elliott J. C., Fink D. S., Knox J., Durant S., Menchaca R., Sharma A. HealthCall: A randomized trial assessing a smartphone enhancement of brief interventions to reduce heavy drinking in HIV care. Journal of Substance Abuse Treatment. 2022;138:108733. doi: 10.1016/j.jsat.2022.108733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhombiro M., Musekiwa A., January J., Chingono A., Abas M., Seedat S. Psychological interventions for alcohol use disorders in people living with HIV/AIDS: A systematic review. Systematic Reviews. 2019;8(1):244. doi: 10.1186/s13643-019-1176-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisto S. A., Conigliaro J., McNeil M., Kraemer K., O'Connor M., Kelley M. E. Factor structure of the SOCRATES in a sample of primary care patients. Addictive Behaviors. 1999;24(6):879–892. doi: 10.1016/S0306-4603(99)00047-7. [DOI] [PubMed] [Google Scholar]
- Miller W. R. T., Tonigan J. S. Assessing drinkers' motivation for change: The Stages of Change Readiness and Treatment Eagerness Scale (SOCRATES) Psychology of Addictive Behaviors. 1996;10(2):81–89. doi: 10.1037/0893-164X.10.2.81. [DOI] [Google Scholar]
- National Institute of Diabetes and Digestive and Kidney Diseases. Hepatitis C. 2020 https://www.niddk.nih.gov/health-information/liver-disease/viral-hepatitis/hepatitis-c [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism. Helping patients who drink too much: A clinician's guide. 2007 https://www.samhsa.gov/resource/ebp/helping-patients-who-drink-too-much-clinicians-guide [Google Scholar]
- Navarro J. HIV and liver disease. AIDS Reviews. 2022;25(2):87–96. doi: 10.24875/AIDSRev.M22000052. [DOI] [PubMed] [Google Scholar]
- Samet J. H., Walley A. Y. Interventions targeting HIV-infected risky drinkers: Drops in the bottle. Alcohol Research & Health. 2010;33(3):267–279. [PMC free article] [PubMed] [Google Scholar]
- Scott-Sheldon L. A. J., Carey K. B., Johnson B. T., Carey M. P., the MASH Research Team Behavioral interventions targeting alcohol use among people living with HIV/AIDS: A systematic review and meta-analysis. AIDS and Behavior. 2017;21(S2):126–143. doi: 10.1007/s10461-017-1886-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields A. L., Hufford M. R. Assessing motivation to change among problem drinkers with and without co-occurring major depression. Journal of Psychoactive Drugs. 2005;37(4):401–408. doi: 10.1080/02791072.2005.10399813. [DOI] [PubMed] [Google Scholar]
- Sims O. T., Maynard Q. R., Melton P. A. Behavioral interventions to reduce alcohol use among patients with hepatitis C: A systematic review. Social Work in Public Health. 2016;31(6):565–573. doi: 10.1080/19371918.2016.1160346. [DOI] [PubMed] [Google Scholar]
- Sobell L., Sobell M. Alcohol Timeline Followback (TLFB) user's manual. Toronto, Canada: Addiction Research Foundation; 1995. [Google Scholar]
- Stein M. D., Herman D. S., Kim H. N., Howell A., Lambert A., Madden S., Moitra E., Blevins C. E., Anderson B. J., Taylor L. E., Pinkston M. M. A randomized trial comparing brief advice and motivational interviewing for persons with HIV–HCV co-infection who drink alcohol. AIDS and Behavior. 2021;25(4):1013–1025. doi: 10.1007/s10461-020-03062-2. [DOI] [PubMed] [Google Scholar]
- UMBC Habits Lab. (n.d.) URICA–Readiness Score. https://habitslab.umbc.edu/urica-readiness-score/ [Google Scholar]
- Williams E. C., Hahn J. A., Saitz R., Bryant K., Lira M. C., Samet J. H. Alcohol use and human immunodeficiency virus (HIV) infection: Current knowledge, implications, and future directions. Alcoholism: Clinical and Experimental Research. 2016;40(10):2056–2072. doi: 10.1111/acer.13204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray T. B., Braciszewski J. M., Zywiak W. H., Stout R. L. Examining the reliability of alcohol/drug use and HIV-risk behaviors using Timeline Follow-Back in a pilot sample. Journal of Substance Use. 2016;21(3):294–297. doi: 10.3109/14659891.2015.1018974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H. Q., Wang C. G., Zhou Q., Gao Y. H. Effects of alcohol consumption on viral hepatitis B and C. World Journal of Clinical Cases. 2021;9(33):10052–10063. doi: 10.12998/wjcc.v9.i33.10052. [DOI] [PMC free article] [PubMed] [Google Scholar]