Dear Editor,
Intracerebral hemorrhage (ICH) after deep brain stimulation (DBS) surgery can cause significant morbidity and mortality. The frequency of ICH following DBS is 0.6–3.5% per lead.[1] Whereas ICH that occurs within 24 h of surgery (immediate ICH) is deemed to be a surgery-related complication, etiopathogenic mechanisms of delayed ICH (after 24 h of DBS) are thought to be more complex.[2] Herein, we report two cases of very delayed ICH that occurred months after DBS.
Patient 1
A 65-year-old gentleman with Parkinson's disease (PD) for the past 15 years underwent bilateral subthalamic nucleus (STN) DBS. His immediate postoperative magnetic resonance imaging (MRI) of the brain showed satisfactory electrode placement and no hemorrhage [Figure 1a and b]. Four months later, he developed acute imbalance and swaying to right side for a week. Subsequent MRI of the brain showed a T2-hyperintense collection, with blooming on SWI, measuring 13 mm × 10 mm × 8 mm, near the microelectrode tip in left STN, suggestive of late subacute hematoma [Figure 1c and d]. MR angiogram of the brain was normal. He was hypertensive, well-controlled on Amlodipine 5 mg/day. His physician had also prescribed Aspirin 75 mg/day for primary prevention of cardiovascular diseases, which was withheld a week prior and until a week after DBS. Further probing revealed an incident of mild head trauma (hitting his forehead against a water tap) a few days preceding his symptom onset. Aspirin was subsequently stopped, and the patient gradually improved with conservative management.
Figure 1.
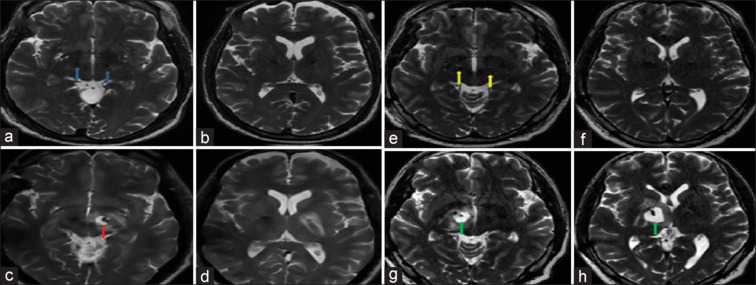
Images (a and b) depict the immediate postoperative T2-weighted sequence of MRI of the brain of patient 1, showing satisfactory electrode placement in bilateral STN with no bleed (blue arrows). Images (c and d) depict T2-weighted hyperintense collection near the tip of the electrode in left STN, suggestive of late subacute hematoma in patient 1 (red arrow) 4 months after DBS. Images (e and f) depict the immediate postoperative T2-weighted sequence of MRI of the brain of patient 2 showing satisfactory electrode placement in bilateral STN with no bleed (yellow arrows). Images (g and h) depict T2-weighted hyperintense collection near the tip of the electrode in right STN, suggestive of late subacute hematoma in patient 2 (green arrow) 2 months after DBS
Patient 2
A 48-year-old hypertensive gentleman suffering from PD of 10 years duration underwent bilateral STN DBS. His immediate postoperative MRI of the brain showed satisfactory electrode placement and no hemorrhage [Figure 1e and f]. Two months later, he presented with increased freezing and swaying to the left. MRI of the brain showed a T2-hyperintense collection, with blooming on SWI, measuring 19 mm × 17 mm × 20 mm, in the right distal peri-lead region, suggestive of late subacute hematoma [Figure 1g and h]. Detailed history revealed trivial fall without head injury 2 days prior to the onset of acute worsening. Blood pressure was fairly well controlled. He was treated with anti-cerebral edema measures and conservative management, with which he recovered and was discharged in 2 days.
Post-DBS ICH has been reported after DBS targeting STN, Globus pallidus interna (GPi), and even nucleus accumbens.[3] Here, we present two patients with very delayed peri-lead ICH after STN DBS, out of our experience of 687 DBS surgeries. Traditionally, post-DBS ICH has been considered delayed if bleeding occurs more than 24 h after the lead insertion. Most delayed ICH reported in the literature have in fact occurred on postoperative day 1 of the procedure.[2] Peri-lead ICH occurring months after DBS is much rarer. Our patients had ICH at 2 and 4 months after the DBS. This is unequivocally delayed and thus rules out direct injury to the vessel by the leads during the lead passage.
Furthermore, although most delayed ICH cases are parenchymal, delayed intracranial bleeding in the form of subdural hemorrhage (SDH) has also been described post DBS.[4,5] However, the pathogenesis of SDH post neurosurgery may not necessarily be related to the DBS procedure.
In our patients, preoperative contrast brain scans did not reveal any vascular malformation. Trajectories for surgery were also planned with utmost caution to avoid vessels. Previously, age, gender, hypertension, aspirin use, and number of microelectrode passes have been reported as risk factors for ICH post DBS.[5] A recent meta-analysis focusing on ICH in DBS showed that patients with post-DBS ICH were approximately 5 years older than those without ICH.[6] In another study, 352 patients with PD implanted with 686 DBS electrodes were analyzed. Eleven patients had ICH. Male gender (10/11) and perioperative hypertension (eight patients had BP >150/100 mmHg) were found to be significantly associated with risk of bleeding. Interestingly, a significantly higher number of patients (7/11) had ICH on the preferred puncture site (side of first puncture for DBS electrode implantation). In addition, ICH was more common after GPi DBS (7.4%) than STN DBS (2.7%) because of the presence of a high number of perforator vessels around GPi.[1]
Although systemic factors such as aspirin use and high BP were present in our patients, the question remains as to why these bleeds were localized to the peri-lead region. We propose that DBS leads elicit local changes in the peri-lead area, which can cause additive effects on the systemic factors, ultimately predisposing the peri-lead area to bleeding in specific situations. Evidence for this hypothesis is derived from post-mortem analysis of two PD brains, 11 years and 12 years after STN-DBS, showing that chronic DBS favors neovascularization.[7]
One important history that was noted in both our patients was trivial fall/trauma preceding the onset of ICH symptoms. We hypothesize that the trauma/fall led to sudden head movement. The densities of the DBS lead and the peri-lead cerebral tissues are different. The sudden head movement most likely caused differential velocity motion of the DBS lead and peri-lead regions. Shearing stress on the surrounding blood vessels due to the differential velocities might have been the most likely cause of ICH [Table 1].
Table 1.
Probable causes of ICH after DBS
| • Venous hypertension (and venous infarction)[8] • Traumatic brain injury (TBI)[9] • Degenerative vasculopathy[10] • Number of microelectrode recording penetrations[11] • Sulcal or ventricular incursion[11] • Delayed erosion of vessel by catheter (in a setting of degenerative vasculopathy)[10] • Damage of the vasculature caused by brain tissue displacement owing to cerebrospinal fluid loss[1] • UnsTable microbleeds from injury to small vessels[12] • Neovascularization[7] • Minor head trauma and recovery[13] (Shear stress because of the difference in density of the lead and surrounding brain tissue) |
In conclusion, we propose the following sequence of events in our patients. DBS leads stimulate peri-lead neovascularization over months. Trivial fall/trauma induces sudden shearing stress on these new vessels. Systemic factors such as high BP/aspirin use coupled with the shearing stress cause the rupture of the peri-lead neovascularity, leading to peri-lead ICH. It is prudent to bear in mind that delayed ICH can occur even after months of DBS, especially in patients who have other risk factors of ICH.
Ethical compliance statement
The authors confirm that the approval of an institution review board was not required for this work. We confirm that we have read the journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Yang C, Qiu Y, Wang J, Wu Y, Hu X, Wu X. Intracranial hemorrhage risk factors of deep brain stimulation for Parkinson's disease: A 2-year follow-up study. J Int Med Res. 2020;48:300060519856747.. doi: 10.1177/0300060519856747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park CK, Jung NY, Kim M, Chang JW. Analysis of delayed intracerebral hemorrhage associated with deep brain stimulation surgery. World Neurosurg. 2017;104:537–44. doi: 10.1016/j.wneu.2017.05.075. [DOI] [PubMed] [Google Scholar]
- 3.Richieri R, Borius PY, Cermolacce M, Millet B, Lançon C, Régis J. A case of recovery after delayed intracranial hemorrhage after deep brain stimulation for treatment-resistant depression. Biol Psychiatry. 2018;83:e11–3.. doi: 10.1016/j.biopsych.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 4.Oyama G, Okun MS, Zesiewicz TA, Tamse T, Romrell J, Zeilman P, et al. Delayed clinical improvement after deep brain stimulation-related subdural hematoma. Report of 4 cases. J Neurosurg. 2011;115:289–94. doi: 10.3171/2011.3.JNS101424. [DOI] [PubMed] [Google Scholar]
- 5.Chung EJ, Kim MS, Kim SJ. Delayed intracranial hemorrhage after deep brain stimulation in two Parkinson's disease patients. J Neurol Sci. 2014;342:202–3. doi: 10.1016/j.jns.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 6.Tiefenbach J, Favi Bocca L, Hogue O, Nero N, Baker KB, Machado AG. Intracranial bleeding in deep brain stimulation surgery: A systematic review and meta-analysis. Stereotact Funct Neurosurg. 2023;101:207–16. doi: 10.1159/000530398. [DOI] [PubMed] [Google Scholar]
- 7.Desmeules F, Munro J, Cottin SC, Noecker A, Gould PV, Saikali S, et al. Post-mortem analysis of Parkinson's disease brains after 11 and 12 years of deep brain stimulation of the subthalamic nucleus. Neuromodulation. 2022;25:S9.. [Google Scholar]
- 8.Morishita T, Okun MS, Burdick A, Jacobson CE, 4th, Foote KD. Cerebral venous infarction: A potentially avoidable complication of deep brain stimulation surgery. Neuromodulation. 2013;16:407–13. doi: 10.1111/ner.12052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nguyen HS, Pahapill PA. Subacute subdural hematoma in a patient with bilateral DBS electrodes. Case Rep Neurol Med. 2015;2015:390727.. doi: 10.1155/2015/390727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu C, Mao G, Williamson R, Whiting D. Delayed intracerebral hemorrhage: A rare complication of deep brain stimulation surgery. Interdisc Neurosurg. 2018;14:135–8. [Google Scholar]
- 11.Zrinzo L, Foltynie T, Limousin P, Hariz MI. Reducing hemorrhagic complications in functional neurosurgery: A large case series and systematic literature review. J Neurosurg. 2012;116:84–94. doi: 10.3171/2011.8.JNS101407. [DOI] [PubMed] [Google Scholar]
- 12.Nowinski WL, Chua BC, Volkau I, Puspitasari F, Marchenko Y, Runge VM, et al. Simulation and assessment of cerebrovascular damage in deep brain stimulation using a stereotactic atlas of vasculature and structure derived from multiple 3- and 7-tesla scans. J Neurosurg. 2010;113:1234–41. doi: 10.3171/2010.2.JNS091528. [DOI] [PubMed] [Google Scholar]
- 13.Walker RB, Grossen AA, O’Neal CM, Conner AK. Delayed hemorrhage following deep brain stimulation device placement in a patient with Parkinson's disease and lupus anticoagulant syndrome: Illustrative case. J Neurosurg Case Lessons. 2022;4:CASE2262. doi: 10.3171/CASE2262. [DOI] [PMC free article] [PubMed] [Google Scholar]


