Abstract
Background & aims
Previous studies have examined the effects of metabolic syndrome (MetS) rather than its severity on race and ethnic disparities in Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD). We used the MetS severity score, a validated sex-race-ethnicity-specific severity measure, to examine the effects of race/ethnicity on the association between MetS severity and MASLD.
Methods
This study included 10,605 adult participants from the Third National Health and Nutrition Examination Survey. The MASLD diagnosis was based on ultrasound findings in patients without excessive alcohol intake or other liver diseases. MetS severity Z-scores were calculated and stratified into four categories low (1st-50th), moderate (>50th-75th), high (>75th-90th), and very high (>90th+)]. Multivariable adjusted logistic regression models with complex survey methods were used to test the effect of MetS severity on MASLD.
Results
The age-adjusted MASLD prevalence was 17.4%, 25.7%, 42.5, and 54.9% in adults with mild, moderate, high, and very high MetS severities, respectively (P-trend <0.001). MetS severity was significantly higher in patients with MASLD than in those without [mean percentile 60th vs. 44th, P<0.001]. Among patients with MASLD, Mexican-American and Black non-Hispanic females had significantly higher age-adjusted MetS severity (68th and 61st, respectively) than White non-Hispanic females 54th, while Black non-Hispanic males had significantly lower MetS severity (56th) than White non-Hispanic males (70th) (P-Interaction = 0.02). Adults with high and very high MetS severity had 2.27 (95% CI:1.70 to 3.03) and 3.12 (95% CI:2.20 to 4.42), respectively, higher adjusted odds of MASLD than those with mild MetS severity.
Conclusions
Racial/ethnic disparities in MetS severity play a pivotal role in the risk of MASLD. Our findings highlight the potential clinical utility of the MetS severity score in identifying at-risk individuals, which will help guide targeted prevention and tailoring management strategies to mitigate the MASLD burden.
1. Introduction
The growing prevalence of sedentary behavior, unhealthy diets, and obesity-induced metabolic syndrome has resulted in an unprecedented surge in the global burden of Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD) [1], formerly known as nonalcoholic fatty liver disease (NAFLD) [2, 3]. MASLD is recognized as the most common cause of chronic liver disease (CLD), affecting an estimated 24% of adults globally [4, 5]. MASLD is characterized by detectable fatty deposits in the liver without chronic viral hepatitis, other etiologies of CLD, steatosis-inducing medications, or excessive alcohol consumption. Metabolic dysfunction-associated steatohepatitis (MASH), formerly known as nonalcoholic steatohepatitis (MASH), is a progressive form of MASLD, as it describes chronic inflammation of hepatocytes with or without fibrosis [6–8]. While most MASLD patients do not require clinical interventions, MASH increases the risk of progression to advanced CLD, including cirrhosis, decompensated cirrhosis, and hepatocellular carcinoma [9, 10].
Due to obesity-induced insulin resistance’s primary function in promoting hepatic steatosis, MASLD is the most common hepatic manifestation of Metabolic Syndrome (MetS) [4, 7, 11–14]. MetS comprises a group of metabolic abnormalities associated with an increased risk of insulin resistance and cardiovascular disease (CVD). The five clinical features of MetS are atherogenic hyperglycemia, dyslipidemia, hypertriglyceridemia, hypertension, and central obesity [15]. The National Heart, Lung, and Blood Institute guidelines for diagnosing MetS utilize a "harmonization definition" of this syndrome. As such, MetS is diagnosed based on a patient presenting abnormalities that exceed prespecified cutoff values for three of the five clinical features of MetS–metabolic abnormalities [16].
Several epidemiological studies have shown that global prevalence varies considerably, with the Middle East at 32%, South America at 30%, and Asia, Europe, and Africa at 27%, 24%, and 13%, respectively [17]. According to a meta-analysis of 34 studies with more than 360,000 unique patients, strong disparities in MASLD prevalence, severity, and prognosis exist between race and ethnic groups within the United States (US) [18]. As such, several studies suggest that Hispanics have the highest incidence of MASLD, with obesity emerging as a central factor in this population. In these studies, African Americans had the lowest MASLD incidence [19–24]. Genetic and metabolic factors have been suggested to underlie the observed racial disparities [25–28], as have incidence rates of insulin resistance and serum triglyceride concentrations [29], but conclusive evidence in support of any model explaining those disparities has yet to be uncovered.
Existing evidence on the natural history of MASLD has focused on identifying disease biomarkers, risk factors, and clinical outcomes [11, 30] and has included MetS diagnosis as a dichotomous risk factor (i.e., present or absent). As the natural history of MASLD is closely related to the component features of MetS, examining the effects of MetS using the current dichotomous definition creates several shortcomings when assessing the effects of MetS on MASLD risk and associated outcomes [31]. First, current guidelines for diagnosing MetS entail the occurrence of any three of the five metabolic abnormalities (i.e., ten possible combinations of metabolic abnormalities). The dichotomous nature of this classification equally treats the effects of any of the five combinations of metabolic abnormalities on the MASLD risk and associated outcomes. Second, MetS diagnosis involves meeting at least three predefined cutoff points for any of the five metabolic abnormalities and neglects the sole and combined values of all MetS features. Third, dichotomous MetS categorization makes it challenging to study and monitor the clinical implications of MetS progression. Fourth, the binary system for MetS definition does not account for racial and sex disparities in MetS severity and their corresponding effects on the risk of MASLD [32].
Assessing MetS by using a continuous measure of disease severity will provide a comprehensive evaluation of its role in MASLD development and progression. The MetS severity score is a validated clinically accessible sex- and race-ethnicity-specific Z-score that encapsulates the combined effects of the nature and severity of all five metabolic abnormalities among adults in the US [33]. The MetS severity score better represents the traditional MetS classification while adjusting for sex and racial/ethnic disparities in the relationship between MetS and cardiometabolic outcomes. The MetS severity score is significantly correlated with pathophysiological biomarkers of MetS, including the Homeostasis Model for Insulin Resistance (HOMA-IR), C-reactive protein (CRP), uric acid, and adiponectin [33, 34]. Multiple studies have also shown that the MetS severity score is significantly associated with long-term risks of cardiovascular disease, type 2 diabetes mellitus, and coronary heart disease [34–37]. Furthermore, the MetS severity score predicts all-cause and cause-specific mortalities in MASLD [31].
We used the MetS severity score to examine the effects of race/ethnicity on the association between MetS severity and MASLD. Understanding the effects of MetS severity on MASLD occurrence will aid clinicians and healthcare professionals in identifying high-risk individuals and in monitoring disease progression. In addition, the results of this study will assist in developing public health interventions to identify the highest priority groups and optimal prevention strategies based on disease severity.
2. Materials and methods
2.1. Data source
This cross-sectional study used data from the Third National Health and Nutrition Examination Survey (NHANES III) between 1988 and 1994. The survey was conducted to assess the health status of the US population, focusing on non-Hispanic Blacks, Mexican Americans, and individuals aged 60 years or older. NHANES III utilized a stratified multistage clustered probability design to sample non-institutionalized members of the US population aged two months or older. The survey included cross-sectional examinations, interview questionnaires, and laboratory sample collection. Of all the participants interviewed, 78% completed the physical examination phase of the survey [38]. The Centers for Disease Control and Prevention (CDC) oversaw the recruitment of all study participants and collection of all consent forms. The consent forms were signed by participants in the survey, and they consented to storing blood specimens for future research. The CDC’s ethics review board approved the NHANES III survey protocol. The study was exempted by the Ohio State University Institutional Review Board because the NHANES III database is a publicly available database that meets the criteria for a limited-use dataset.
2.2. Study sample
The study sample included all the adult participants with valid hepatic steatosis evaluations. Hepatic steatosis was evaluated by three trained ultrasound readers using video-recorded gallbladder ultrasounds during physical examinations of all NHANES III participants aged 20–74 years. Following the initial assessments, all ultrasound readings were re-evaluated and validated by a certified radiologist specializing in hepatic imaging. Hepatic steatosis images were classified as normal, mild, moderate, or severe. The criteria for grading hepatic steatosis included gallbladder wall definition, liver parenchyma degree of brightness, occurrence of deep beam attenuation, presence of liver-to-kidney contrast, and echogenic walls in the small intrahepatic vessels [39].
Of the 16,573 participants aged 20 years or older who attended the survey examination phase, 14,707 qualified for gallbladder ultrasound reading, of which 13,856 had readable ultrasound images [40]. Participants were excluded from the study if they had missing values for exposure, outcome, alcohol intake, ultrasound images, or any of the covariates included in the adjusted analyses. In addition, participants who identified as "Other" on the race/ethnicity questions were also excluded, as the exposure assessment was only available for non-Hispanic whites, non-Hispanic blacks, and Mexican Americans. After implementing all inclusion and exclusion criteria, the final study sample included 10,605 adult participants.
2.3. Exposure
The MetS severity score was the primary exposure variable. The MetS severity score is a validated sex- and race/ethnicity-specific Z-score that captures the relative MetS severity of all five metabolic abnormalities [33]. The score was quantified using Confirmatory Factor Analysis (CFA) with data from the 1999–2010 NHANES databases [33]. All five metabolic features were used in the CFA to construct a summary score that is a continuous representation of the conventional metabolic syndrome characterization. We used individual-level data on high-density lipoprotein (HDL), systolic blood pressure (SBP), waist circumference, triglycerides, and fasting blood glucose to calculate sex- and race/ethnicity-specific MetS severity Z-scores according to the score’s standardized equations [33]. Furthermore, we transformed the MetS severity Z-scores into four percentile-based categories [mild (1st-50th), moderate (>50th-75th), high (>75th-90th), and very high (>90th)].
The National Cholesterol Education Program (NCEP) Adult Treatment Panel III (ATP III) guidelines were used to define the traditional MetS diagnosis. As such, MetS diagnosis was defined by the presence of three of five metabolic factors: (1) hyperglycemia (i.e., fasting blood glucose > 100 mg/dl, or pharmacological treatment), (2) dyslipidemia (i.e., fasting HDL cholesterol level less than 40 mg/dl, men, or 50 mg/dl, women, or pharmacological treatment), (3) hypertriglyceridemia (i.e., fasting triglyceride level over 150 mg/dl, or pharmacological treatment), (4) central obesity (i.e., waist circumference over 40 inches for men, or 35 inches for women), or (5) hypertension (i.e., systolic blood pressure (SBP) over 130 mmHg, or pharmacological treatment) [15].
2.4. Outcome
The primary outcome of the study was the MASLD status. MASLD was identified by the presence of mild, moderate, or severe hepatic steatosis in the absence of excessive drinking (i.e., more than three alcoholic beverages per day for males or more than two alcoholic beverages per day for females), binge drinking (i.e., frequent consumption of five or more alcoholic beverages per day), alcohol consumption restrictions due to illness, positive Hepatitis B virus (HBV) surface antigen test, positive Hepatitis C virus (HCV) RNA Test, or iron overload (i.e., transferrin saturation ≥ 50%). Because metabolic syndrome severity was the main exposure in our study, we did not include cardiometabolic assessment as part of our MASLD definition to avoid potential confounding. Accordingly, our current MASLD definition falls under the cryptogenic steatotic liver disease or possible MASLD categories of the current MASLD nomenclature [1].
2.5. Covariates
Confounder selection was based on prior literature and theoretical rationale. The confounders used in the adjusted multivariable analyses included age, sex, race/ethnicity (white non-Hispanics, black non-Hispanics, or Mexican Americans), education level (< high school, high school, or GED; some college or college degree or higher), access to health insurance (yes or no), alcohol intake (never, former, or current), smoking status (never, former, or current), body mass index (Kg/m2) [underweight (<18.5), healthy weight (≥18.5–25.0<), overweight (≥25.0–30.0<), or obese (≥30)]; abdominal obesity; physical activity (metabolic equivalents per month); healthy eating index percentile; HOMA-IR; aspartate aminotransferase (AST) to alanine aminotransferase (ALT) ratio, and total cholesterol.
2.6. Statistical analyses
The study sample was restricted to participants with non-missing values for exposure, outcome, or any variable used in the adjusted multivariable analyses. We used complex survey methods, including sampling weights, strata, and clusters, to obtain nationally representative estimates for all analyses. To account for the effects of the survey design, Taylor series linearization was used to quantify all the variance values. Missing values related to variance estimation were assumed not to be completely missing at random.
Participant characteristics stratified by race/ethnicity for those with versus without MASLD were examined by testing the difference in means for continuous variables, analysis of variance (ANOVA), and Rao Scott Chi-Square for categorical variables. Age-adjusted mean estimates for clinical characteristics related to MetS were quantified according to sex and race/ethnicity of participants with MASLD. We also evaluated the age-adjusted distributions of MetS severity scores by sex and race/ethnicity for participants with and without MASLD. In addition, we estimated age-adjusted MASLD prevalence by race/ethnicity, sex, and MetS severity Z-score quartile to assess the disease severity distributions.
Multivariable logistic regression models were used to test the association between increased MetS severity and the odds of MASLD. The dose-response relationships between MetS severity and the odds of MASLD were evaluated using the MetS severity score percentiles as a continuous variable, with a three-knot restricted cubic spline (RCS) added to the adjusted logistic regression models. As recommended by Harrell, 2015 [41], the three knots were placed at the 10th, 50th, and 90th of the weighted MetS severity score percentiles for all participants. Wald chi square tests were used to assess the overall and nonlinear associations of the MetS severity score percentiles with the odds of MASLD. Statistical significance was set at P < 0.05. All analyses were performed using SAS software (version 9.4; SAS Institute, Cary, NC, USA). We followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines [42].
3. Results
3.1. Participants baseline characteristics
The baseline characteristics of the adult participants with and without MASLD, according to race/ethnicity, are shown in Table 1. The study sample included 10,605 adult participants from the NHANES III who met the inclusion and exclusion criteria. Independent of race/ethnicity, participants with MASLD were older than their non-MASLD counterparts. Black non-Hispanic and Mexican American adults with MASLD were more likely to be female than those without MASLD. Lower education levels were associated with increased MASLD in white non-Hispanics and Hispanics, but not in Black non-Hispanics. The distributions of alcohol intake and smoking status differed according to the MASLD status, independent of race/ethnicity.
Table 1. Sample characteristics by race/ethnicity and Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD) Status, United States adults, The National Health and Nutrition Examination Survey (NHANES III) 1988–1994 (n = 10,605).
| White, non-Hispanics | Black, non-Hispanics | Mexican Americans | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristics | MASLD | No MASLD | P-value* | MASLD | No MASLD | P-value* | MASLD | No MASLD | P-value* |
| (n = 1,029) | (n = 3,075) | (n = 756) | (n = 2,385) | (n = 1,115) | (n = 2,065) | ||||
| Sex, % (SE) | 0.168 | 0.008 | <0.001 | ||||||
| Male | 46.2 (1.5) | 49.1 (0.9) | 38.1 (2.2) | 44.7 (1.3) | 43.3 (1.4) | 55.5 (1.2) | |||
| Female | 53.8 (1.5) | 50.9 (0.9) | 61.9 (2.2) | 55.3 (1.3) | 56.7 (1.4) | 44.5 (1.2) | |||
| Age, (years) | <0.001 | <0.001 | <0.001 | ||||||
| Median (25th, 75th Percentile) | 44.2 (33.6, 58.1) | 39.4 (29.6, 52.7) | 38.1 (28.7, 51.6) | 35.7 (27.4, 46.6) | 36.7 (28.0, 47.2) | 31.6 (24.6, 41.6) | |||
| Mean (SE) | 46.3 (0.6) | 42.3 (0.5) | 41.2 (0.5) | 39.1 (0.4) | 39.4 (0.6) | 35.4 (0.4) | |||
| Age Group, % (SE) | <0.001 | 0.006 | <0.001 | ||||||
| 18–34 | 26.2 (2.4) | 36.9 (1.4) | 38.7 (2.1) | 44.8 (1.3) | 41 (2.1) | 57.2 (1.7) | |||
| 35–49 | 32.6 (2.5) | 32.8 (1.1) | 32.9 (2.1) | 32.9 (1.1) | 36.6 (1.7) | 27.6 (1.5) | |||
| 49–64 | 25.8 (1.6) | 19.3 (0.8) | 19.1 (1.5) | 15.4 (1.1) | 15.7 (1.3) | 11 (1) | |||
| 65+ | 15.4 (1.1) | 11.1 (0.8) | 9.3 (1.0) | 6.9 (0.7) | 6.7 (0.7) | 4.2 (0.5) | |||
| Education Level, % (SE) | 0.001 | 0.727 | 0.042 | ||||||
| < High School | 18.3 (1.4) | 16.9 (1.1) | 29.2 (2.7) | 28.4 (1.5) | 59.6 (1.8) | 53.5 (1.9) | |||
| High School or GED | 40.5 (1.7) | 34.0 (1.1) | 39.1 (2.7) | 39.1 (1.4) | 23.7 (1.6) | 26.4 (1.2) | |||
| Some College | 19.8 (1.7) | 23.4 (1.1) | 21.9 (1.9) | 20.8 (1.1) | 11.3 (1.1) | 14.6 (1.3) | |||
| College degree or Higher | 21.4 (2.0) | 25.7 (1.2) | 9.8 (1.3) | 11.7 (1.2) | 5.4 (0.8) | 5.4 (0.8) | |||
| Have Health Insurance, % (SE) | 91.7 (0.9) | 89.5 (1.0) | 0.088 | 84.7 (2.1) | 83.6 (1.6) | 0.428 | 62.8 (1.8) | 61.3 (1.9) | 0.443 |
| Alcohol Intake, % (SE) | <0.001 | <0.001 | <0.001 | ||||||
| Never | 13.6 (1.3) | 7.7 (0.8) | 21.4 (1.6) | 16.1 (1.1) | 20.5 (1.7) | 15.2 (1.3) | |||
| Former | 33.9 (1.7) | 30.5 (1.7) | 42.1 (1.9) | 35.4 (1.5) | 34.8 (1.3) | 30.8 (1) | |||
| > 0–1 drinks/day | 42.1 (2.0) | 43.4 (1.6) | 29 (2.3) | 32 (1.4) | 36 (2.9) | 36.4 (1.1) | |||
| > 1 drinks/day** | 10.4 (1.3) | 18.4 (1.1) | 7.6 (0.9) | 16.5 (0.8) | 8.7 (1.3) | 17.6 (1) | |||
| Smoking Status, % (SE) | <0.001 | <0.001 | 0.017 | ||||||
| Never | 44.9 (1.6) | 41.2 (1.3) | 58.6 (2.3) | 49.8 (1.1) | 61.6 (1.5) | 56.2 (1.1) | |||
| Former | 31.8 (1.7) | 27.1 (0.9) | 18.7 (1.5) | 14.9 (0.9) | 20.4 (1.5) | 20.1 (1.2) | |||
| Current | 23.3 (1.5) | 31.7 (1.2) | 22.7 (1.9) | 35.3 (1.1) | 18.0 (1.3) | 23.7 (1.3) | |||
| Body Mass Index (Kg/M2) | <0.001 | <0.001 | <0.001 | ||||||
| Median (25th, 75th Percentile) | 27.8 (24.2, 32.0) | 24.7 (22.2, 27.8) | 28.9 (23.7, 34.6) | 26.2 (23.0, 30.1) | 28.6 (25.4, 32.2) | 25.8 (23.2, 28.7) | |||
| Mean (SE) | 28.6 (0.4) | 25.5 (0.1) | 30.0 (0.4) | 27.1 (0.2) | 29.3 (0.3) | 26.5 (0.1) | |||
| Body Mass Index Category† (Kg/M2), % (SE) | <0.001 | <0.001 | <0.001 | ||||||
| Underweight | 1.9 (0.4) | 2.5 (0.4) | 2.6 (0.6) | 2.2 (0.3) | 0.7 (0.3) | 1.3 (0.4) | |||
| Healthy Weight | 27.1 (2.2) | 49.6 (1.1) | 27.2 (1.8) | 37.3 (1.3) | 22.1 (2.3) | 40.5 (1.1) | |||
| Overweight | 34.1 (1.7) | 32.4 (1.0) | 26.6 (1.3) | 34.2 (0.9) | 36.9 (1.7) | 38.8 (1.1) | |||
| Obese | 36.9 (2.3) | 15.6 (1.0) | 43.6 (1.9) | 26.3 (1.2) | 40.3 (2.3) | 19.5 (1.1) | |||
| Waist to Hip Ratio | <0.001 | <0.001 | <0.001 | ||||||
| Median (25th, 75th Percentile) | 0.93 (0.85, 0.99) | 0.89 (0.83, 0.95) | 0.91 (0.85, 0.97) | 0.88 (0.82, 0.94) | 0.94 (0.88, 0.99) | 0.91 (0.85, 0.96) | |||
| Mean (SE) | 0.93 (0.004) | 0.90 (0.002) | 0.89 (0.004) | 0.91 (0.002) | 0.94 (0.003) | 0.92 (0.002) | |||
| Abdominal Obesity ‡, % (SE) | 75.5 (1.8) | 62.6 (1.3) | <0.001 | 70.3 (1.9) | 57.6 (1.6) | <0.001 | 83.4 (1.2) | 70.1 (1.2) | <0.001 |
| Physical Activity (METs/month) | 0.002 | 0.005 | 0.100 | ||||||
| Median (25th, 75th Percentile) | 62.9 (17.5, 149.5) | 78.8 (23.7, 167.5) | 39.5 (1.1, 124.5) | 52.0 (7.7, 148.8) | 22.6 (0, 104.9) | 34.7 (4.1, 124.8) | |||
| Mean (SE) | 100.6 (4.4) | 118.5 (3.9) | 88.3 (5.3) | 107.9 (4.7) | 78.5 (6.1) | 89.9 (3.8) | |||
| Physically Active, % (SE) | 86.2 (1.3) | 90.7 (0.7) | <0.001 | 75.7 (2.5) | 80.5 (1.2) | 0.035 | 72.1 (2.1) | 76.9 (1.5) | 0.008 |
| Healthy Eating Index | 0.470 | 0.390 | 0.102 | ||||||
| Median (25th, 75th Percentile) | 64.5 (54.1, 74.0) | 63.7 (54.5, 73.2) | 60.2 (50.6, 68.7) | 58.7 (50.2, 67.8) | 65.3 (56.0, 73.9) | 63.8 (55.7, 72.2) | |||
| Mean (SE) | 64.1 (0.5) | 63.8 (0.4) | 59.5 (0.6) | 58.9 (0.2) | 64.8 (0.6) | 63.8 (0.6) | |||
| Healthy Eating Index §, % (SE) | 0.369 | 0.517 | 0.449 | ||||||
| Poor | 17.2 (1.3) | 16.6 (0.9) | 25.4 (2.1) | 27.4 (0.7) | 14.2 (1.6) | 15.3 (1.2) | |||
| Fair | 69.8 (1.8) | 71.9 (0.7) | 70 (2.0) | 67.6 (0.8) | 73.7 (1.6) | 74.2 (1.0) | |||
| Good | 13.0 (1.4) | 11.5 (0.7) | 4.6 (0.7) | 5.1 (0.5) | 12.1 (1.3) | 10.5 (1.2) | |||
| Total Cholesterol (mg/dL) | 0.006 | 0.002 | 0.015 | ||||||
| Median (25th, 75th Percentile) | 204.7 (176.9, 235.6) | 199.0 (173.3, 227.7) | 202.6 (172.8, 231.5) | 192.5 (168.2, 220.8) | 198.2 (170.8, 228.2) | 190.9 (166.2, 219.7) | |||
| Mean (SE) | 208.4 (1.7) | 202.8 (1.0) | 205.6 (1.8) | 197.2 (1.0) | 201.9 (2.4) | 195.2 (1.7) | |||
| HOMA-IR | <0.001 | <0.001 | <0.001 | ||||||
| Median (25th, 75th Percentile) | 2.39 (1.58, 3.90) | 1.62 (1.19, 2.32) | 3.09 (1.80, 5.38) | 2.09 (1.41, 3.18) | 3.06 (1.97, 5.0) | 1.92 (1.32, 3.03) | |||
| Mean (SE) | 3.9 (0.3) | 2.2 (0.08) | 5.2 (0.3) | 3.4 (0.2) | 4.9 (0.4) | 2.8 (0.1) | |||
| AST/ALT Ratio | <0.001 | 0.004 | <0.001 | ||||||
| Median (25th, 75th Percentile) | 1.20 (0.92, 1.54) | 1.35 (1.06, 1.69) | 1.38 (1.09, 1.78) | 1.49 (1.18, 1.89) | 1.05 (0.79, 1.35) | 1.23 (0.95, 1.54) | |||
| Mean (SE) | 1.3 (0.04) | 1.5 (0.08) | 1.5 (0.04) | 1.6 (0.04) | 1.1 (0.02) | 1.3 (0.02) | |||
* Rao-Scott Chi Square P-values for difference in proportions and T-tests P-values for difference in means between adults with versus without Metabolic Dysfunction-Associated Steatotic Liver Disease of the same race/ethnicity
** In MASLD up to 2 drink per days for females and 3 drinks per day for males
† Underweight (< 18.50), Healthy Weight (≥ 18.50–25.00 <), Overweight (≥ 25.00–30.00 <) and Obese (≥ 30)
‡ Waist to Hip Ratio ≥ 0.90 for males or ≥ 0.85 for females
§ Poor < 51%, Fair < 80%, Good ≥ 80%
MET = Metabolic equivalent; AST = Aspartate Aminotransferase; ALT = alanine aminotransferase; % = Weighted Proportion; SE = Standard Error
The prevalence estimates for overweight or morbid obesity were 71.0%, 70.0%, and 77.2, respectively, in white non-Hispanics, black non-Hispanics, and Mexican Americans with MASLD, compared to 48.0%, 60.5%, and 58.3%, respectively, in those without MASLD of the same race/ethnicity. Similarly, participants with MASLD had a 3.1 kg/m2 higher average BMI than those without MASLD. The prevalence of abdominal obesity was also significantly associated with MASLD status in all racial/ethnic groups. The mean values of total cholesterol and HOMA-IR were higher in the MASLD group than in the no MASLD. In contrast, the proportion of physically active participants was lower in the MASLD group than in the no MASLD, independent of race/ethnicity (Table 1).
3.2. MASLD prevalence
The overall prevalence of MASLD was 26.7% (95% CI: 24.3% to 29.1%). Stratified by race/ethnicity, the prevalence of MASLD was 26.7%, 23.3%, and 33.7% in white non-Hispanics, black non-Hispanics, and Mexican Americans, respectively. The prevalence of MASLD was marginally different among males compared to females (25.6% vs. 28.3%; P = 0.06). However, among male participants, the age-adjusted prevalence of MASLD was significantly higher in Mexican Americans than in White non-Hispanics (31.5% vs. 25.9%; P = 0.04). Similarly, Mexican American females had a significantly higher age-adjusted MASLD prevalence than white non-Hispanic females (41.1% vs. 27.6%; P<0.001).
3.3. Metabolic syndrome severity in MASLD
The prevalence of traditionally defined MetS was higher in patients with MASLD than in those without (44.0% vs. 20.4%; P-value <0.001). Black non-Hispanic males and females with MASLD had significantly lower age-adjusted MetS prevalence than white non-Hispanic males and females with MASLD (Table 2). Among females with MASLD, Mexican Americans had a significantly higher prevalence of MetS than White non-Hispanics (35.6% vs. 23.7%; P-value <0.001). An estimated 82.1% of adults with MASLD had at least one feature of traditionally defined MetS, whereas 9.2% met the criteria for all five metabolic components. Stratified by race/ethnicity, the prevalence of at least one metabolic abnormality in participants with MASLD was 82.3%, 77.2%, and 86.9% in White non-Hispanics, Black non-Hispanics, and Mexican Americans, respectively. The age-adjusted average number of metabolic abnormalities was highest in Mexican American females with MASLD (2.1) and lowest in Black non-Hispanic males (1.4).
Table 2. Age adjusted estimates for clinical characteristics related to metabolic syndrome by sex and race/ethnicity, adults with Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD), The National Health and Nutrition Examination Survey (NHANES III) 1988–1994 (n = 3,080).
| Clinical Characteristics | Males | Females | ||||
|---|---|---|---|---|---|---|
| White, non-Hispanics | Black, non-Hispanics | Mexican Americans | White, non-Hispanics | Black, non-Hispanics | Mexican Americans | |
| (n = 1,961) | (n = 1,351) | (n = 1,553) | (n = 2,323) | (n = 1,790) | (n = 1,627) | |
| Number of Metabolic Abnormalities†, Mean (SE) | 1.8 (0.04) | 1.5 (0.04) * | 1.9 (0.05) * | 1.5 (0.06) | 1.8 (0.03) * | 2.1 (0.03) * |
| Metabolic Syndrome‡, % (SE) | 27.9 (0.01) | 21.4 (0.01) * | 31.1 (0.01) | 23.7 (0.01) | 27.7 (0.01) * | 35.6 (0.01) * |
| Central Obesity, % (SE) | 31.1 (0.01) | 26.6 (0.01) * | 31.1 (0.02) | 40.8 (0.01) | 59.5 (0.01) * | 60.4 (0.01) * |
| Waist Circumference (inches), Mean (SE) | 96.6 (0.3) | 93.3 (0.3) * | 96.1 (0.4) | 87.7 (0.5) | 93.9 (0.5) * | 93.1 (0.4) * |
| Hypertriglyceridemia, % (SE) | 40.8 (0.02) | 27.0 (0.01) * | 47.4 (0.02) * | 26.7 (0.01) | 19.6 (0.01) * | 39.6 (0.01) * |
| Triglyceridemia (mg/dL), Mean (SE) | 161.0 (3.9) | 128.5 (2.6) * | 182.1 (4.4) * | 130.8 (3.4) | 111.1 (2.0) * | 153.8 (3.2) * |
| Hypertension, % (SE) | 38.0 (0.01) | 46.1(0.01) * | 36.8 (0.01) | 30.3 (0.01) | 43.3 (0.01) * | 35.5 (0.01) * |
| SBP (mmHg), Mean (SE) | 106.1 (0.8) | 108.6 (0.8) * | 106.0 (0.8) | 87.3 (0.6) | 93.1 (0.6) * | 89.8 (0.6) * |
| Dyslipidemia, % (SE) | 38.2 (0.02) | 24.2 (0.01) * | 37.4 (0.02) | 38.5 (0.02) | 36.3 (0.01) | 47.8 (0.02) * |
| HDL (mg/dL), Mean (SE) | 44.8 (0.5) | 52.1 (0.5) * | 45.3 (0.5) | 55.6 (0.6) | 57.1 (0.5) * | 52.1 (0.5) * |
| Hyperglycemia, % (SE) | 30.4 (0.01) | 30.4 (0.01) | 38.2 (0.02) * | 17.8 (0.01) | 25.9 (0.01) * | 30.5 (0.01) * |
| Plasma Glucose (mg/dL), Mean (SE) | 100.1 (0.6) | 101.9 (0.8) | 105.8 (0.9) * | 95.1 (0.7) | 102.0 (1.5) * | 102.2 (0.7) * |
HDL, High-Density Lipoprotein Cholesterol; SBP, Systolic Blood Pressure
* P-value <0.05 for adults of the same sex in reference to white non-Hispanics
† (1) hyperglycemia (i.e., fasting blood glucose over 100 mg/dl, or pharmacological treatment), (2) dyslipidemia (i.e., fasting HDL cholesterol level less than 40 mg/dl, men, or 50 mg/dl, women, or pharmacological treatment) (3) hypertriglyceridemia (i.e., fasting triglyceride (TG) level over 150 mg/dl, or pharmacological treatment), (4) central obesity (i.e., waist circumference over 40 inches, men, or 35 inches, women), or (5) hypertension (i.e., systolic blood pressure (SBP) over 130 mmHg, or pharmacological treatment)
‡ Three or more metabolic abnormalities
We observed racial/ethnic disparities in the age-adjusted prevalence of all five metabolic abnormalities in male and female patients with MASLD (Table 2). In males with MASLD, the age-adjusted prevalence of central obesity, hypertriglyceridemia, and dyslipidemia was lower in black non-Hispanics than in white non-Hispanics. In contrast, the age-adjusted prevalence of hypertension was higher in male black non-Hispanic patients with MASLD than in their white non-Hispanic counterparts. Mexican American males with MASLD had a significantly higher age-adjusted prevalence of hypertriglyceridemia and hyperglycemia than white non-Hispanic males with MASLD.
Both black non-Hispanic and Mexican American females with MASLD had a higher age-adjusted prevalence of central obesity, hypertension, and hyperglycemia than white non-Hispanic females with MASLD. Furthermore, Mexican American females with MASLD had a higher age-adjusted prevalence of hypertriglyceridemia and dyslipidemia than white non-Hispanic females with MASLD. Similar to Black non-Hispanic males, black non-Hispanic females had a lower age-adjusted hypertriglyceridemia prevalence than white non-Hispanic females with MASLD.
MetS severity was normally distributed in the MASLD and no-MASLD groups. However, the severity of MetS was significantly higher in the patients with MASLD. The overall mean and median MetS severity scores and corresponding percentiles were 0.03 (49th) and -0.07 (47th), respectively. Both the mean and median MetS severity scores were significantly higher in MASLD relative to those without [mean MetS severity Z-score, 0.48 (61st) vs. -0.14 (46th); median MetS severity Z-score, 0.48 (69th) vs. -0.23 (41st)].
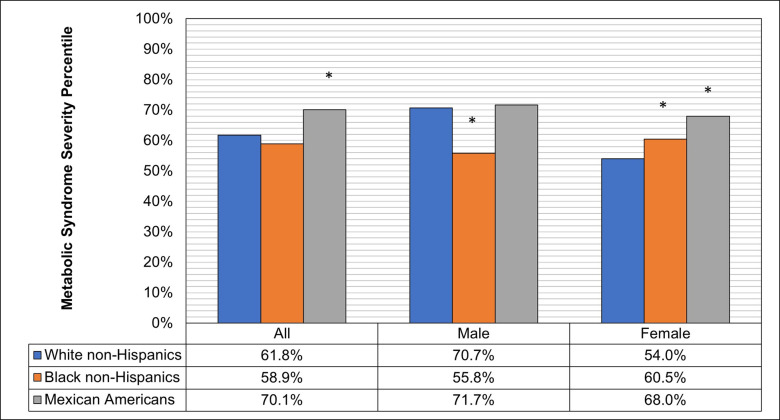
When the sample was stratified by race/ethnicity, and sex, patients with MASLD had significantly higher MetS severity in all comparison groups. Among adults without MASLD, Mexican Americans had the highest severity (53rd) compared with both white non-Hispanics (45th) and black non-Hispanics (43rd). Among participants with MASLD, Mexican American and Black non-Hispanic females had significantly higher MetS severity than White non-Hispanic females (Fig 1). Black non-Hispanic males with MASLD had significantly lower MetS severity (56th percentile) than white non-Hispanic males with MASLD (70th percentile).
Fig 1. Age adjusted mean metabolic syndrome severity percentile by race/ethnicity and sex in adults with Metabolic Dysfunction-Associated Steatotic Liver Disease, The National Health and Nutrition Examination Survey (NHANES III), United States, 1988–1994 (n = 3,088).
* P-value <0.05 for the difference in metabolic syndrome percentile in reference to white non-Hispanics of the same sex.
3.4. MASLD and metabolic syndrome severity
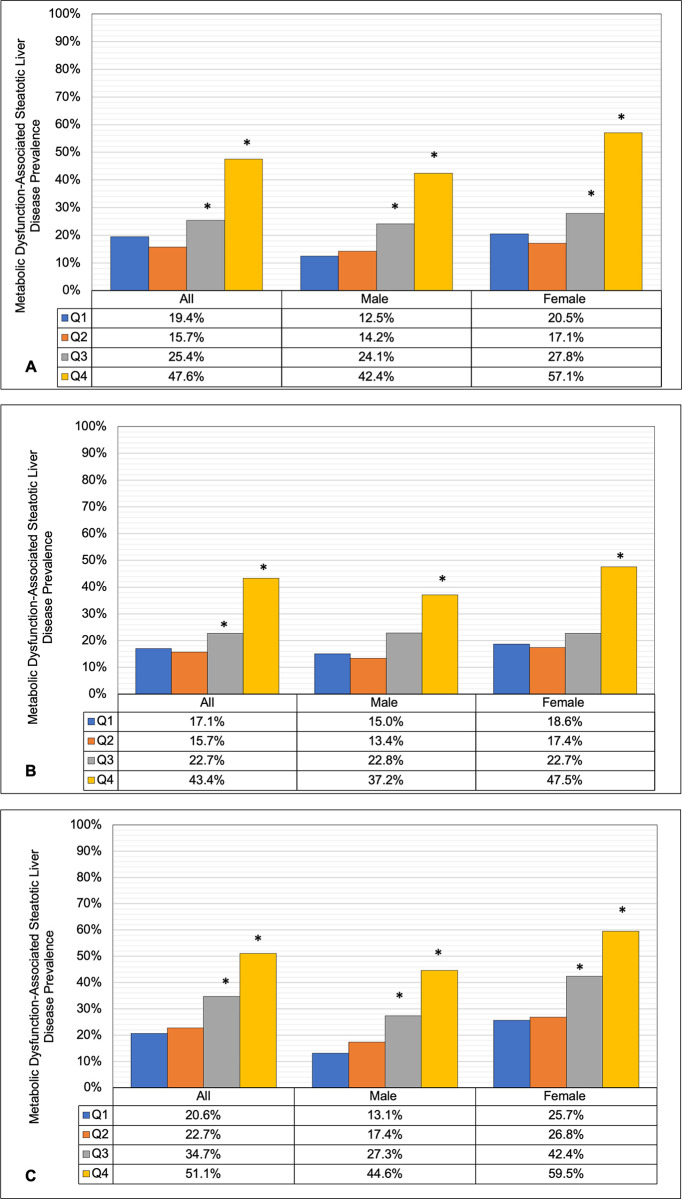
The age-adjusted prevalence of MASLD increased significantly with higher MetS severity group (P-trend <0.001). When the MetS severity score was divided into quartiles, the age-adjusted MASLD prevalence was not significantly different between the first and second MetS severity quartiles (Fig 2A to 2C). In contrast, those in the third and fourth MetS severity quartiles had a significantly higher age-adjusted MASLD prevalence than those in the first severity quartile, independent of race/ethnicity and sex. Similarly, the prevalence of MASLD in the second and third severity quartiles was higher than that in the first quartile among White non-Hispanic and Mexican American males and females (Fig 2A and 2C). In both Black non-Hispanic males and females, only the third severity quartile was significantly higher than the first quartile (Fig 2B).
Fig 2.
Age adjusted Metabolic Dysfunction-Associated Steatotic Liver Disease Prevalence by Sex and Metabolic Syndrome Severity Z-Score Quartile (Q) for A) White, non-Hispanics B) Black, non-Hispanics and C) Mexican Americans, Adult Participants in The National Health and Nutrition Examination Survey (NHANES III), United States, 1988–1994 (n = 10,605). * P-value <0.05 for the difference in age adjusted Metabolic Dysfunction-Associated Steatotic Liver Disease prevalence relative to the first severity quartile.
3.5. Metabolic syndrome severity and the odds of MASLD occurrence
The MetS severity score significantly predicted the occurrence of MASLD in all crude and adjusted regression models. A quartile increase in the MetS severity score was associated with a 36% increase in the adjusted odds for MASLD aOR 1.36 (95% CI:1.17 to 1.57). Similarly, a ten-percentile increase in MetS severity was associated with a 1.15 (95% CI:1.09 to 1.20) higher adjusted odds of MASLD. In the adjusted models, with the severity score included as a categorical variable, adults with high MetS severity had aOR 2.27 (95% CI; 1.70 to 3.03) times the odds of MASLD relative to those with mild MetS severity score (Table 3). Very high MetS severity was associated with 3.12 (95% CI; 2.20 to 4.42) higher adjusted odds of MASLD than mild MetS severity.
Table 3. The association between metabolic syndrome severity and odds of Metabolic Dysfunction-Associated Steatotic Liver Disease Occurrence in United States adults, the National Health and Nutrition Examination Survey (NHANES III) 1988–1994 (n = 10,605).
| Metabolic Syndrome Severity | Unadjusted | Model 1 | Model 2 |
|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Low | Reference | Reference | Reference |
| Moderate | 1.69 (1.36 ⎯ 2.11) | 1.80 (1.45 ⎯ 2.24) | 1.26 (0.97 ⎯ 1.64) |
| High | 3.67 (2.96 ⎯ 4.54) | 3.99 (3.20 ⎯ 4.98) | 2.27 (1.70 ⎯ 3.03) |
| Very High | 6.08 (4.79 ⎯ 7.72) | 6.60 (5.21 ⎯ 8.36) | 3.12 (2.20 ⎯ 4.42) |
Metabolic Syndrome Severity Group, the Metabolic Syndrome Z-score was transformed into four percentiles-based categories [low (0 – 50th), Moderate (>50th– 75th), High (>75th– 90th), and very-high (>90th)]
Model 1 = adjusted for age, sex, and race/ethnicity
Model 2 = Adjusted for age, sex, race/ethnicity, education level, access to health insurance, alcohol intake, smoking status, body mass index, abdominal obesity, physical activity, healthy eating index percentile, HOMA-IR, total cholesterol, and Aspartate Aminotransferase (AST)/alanine aminotransferase (ALT) Ratio
OR; odds ratio, CI; Confidence interval
In the RCS analysis, a significant nonlinear dose-response trend was observed in the relationship between increased odds of MASLD occurrence and higher MetS severity scores in all adjusted models. Generally, the adjusted odds of MASLD increased with higher MetS severity scores relative to the median severity value (50th percentile). Specifically, compared to those with median severity scores, the aOR of MASLD was 1.17 (95% CI; 1.11 to 1.23), 2.05 (95% CI; 1.72 to 2.43), 2.85 (95% CI; 2.23 to 3.65), and 3.38 (95% CI; 2.55 to 4.49), respectively, for adults in the 60th, 80th, 90th, and 95th severity percentiles (Fig 4). In contrast, the adjusted odds of MASLD were significantly lower for those with MetS severity scores below the median value, up to the 43rd percentile.
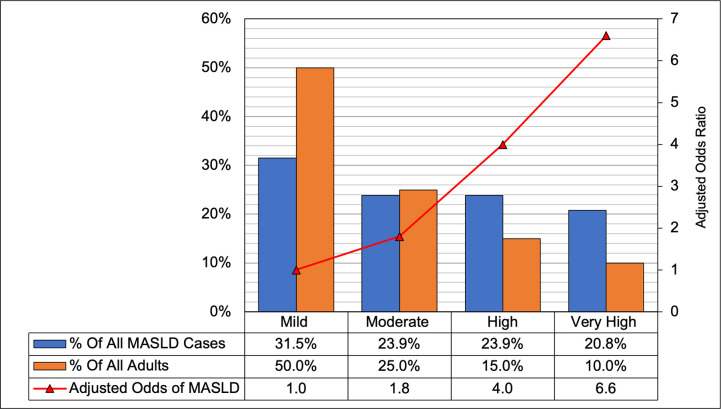
Fig 4. The distribution of metabolic syndrome severity, Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD) prevalence and the adjusted odds of MASLD by metabolic syndrome severity, United States adults, the Third National Health and Nutrition Examination Survey (NHANES III), 1988–1994 (n = 10,605).
* To construct the Metabolic Syndrome Severity Group, the Metabolic Syndrome Z-score was transformed into four percentiles-based categories [low (0 – 50th), Moderate (>50th– 75th), High (>75th– 90th), and very-high (>90th)]. ** MASLD odds ratios in reference to mild severity adjusted for age, sex, and race/ethnicity.
The dose-response relationship between MetS severity and the odds of MASLD was estimated with reference to the 90th severity score percentile (Fig 3B). The adjusted odds of MASLD presence were significantly higher for adults with severity above the 90th percentile relative to those in the 90th percentile. For example, adults in the 95th percentile had aOR 1.18 (95% CI; 1.14 to 1.23) higher odds of MASLD than those in the 90th percentile. In contrast, adults with values below the 90th percentile had significantly lower odds of having MASLD. Relative to those in the 90th percentile, the aOR of MASLD were 0.72 (95% CI; 0.66 to 0.77), 0.35 (95% CI; 0.27 to 0.45), and 0.48 (95% CI; 0.32 to 0.73)] for adults in the 80th, 50th, and 10th percentiles, respectively.
Fig 3.
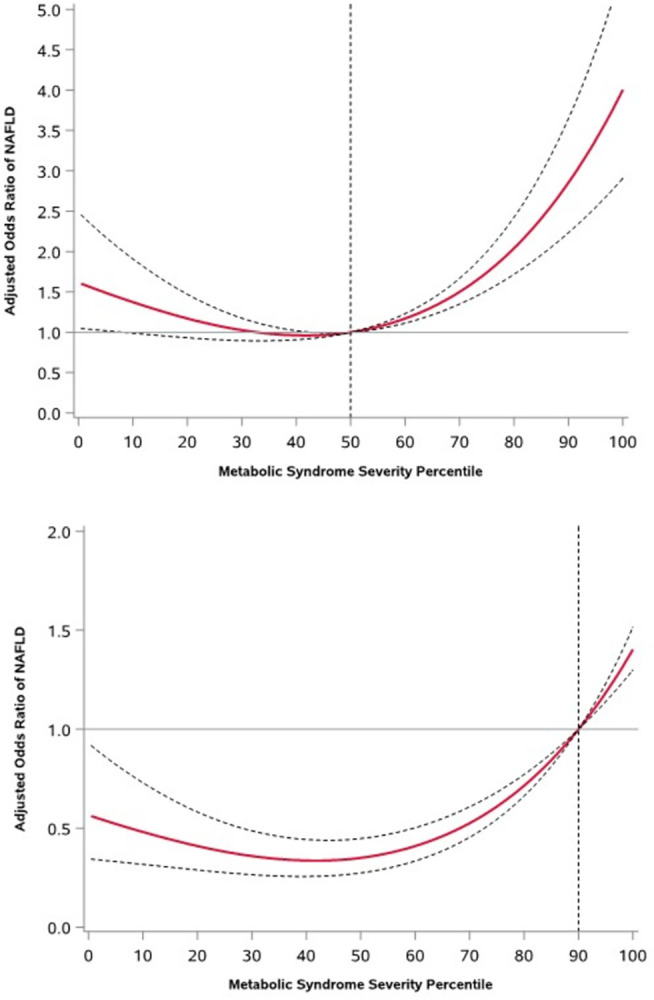
Adjusted Odds Ratios of Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD) Prevalence for Different Metabolic Syndrome Severity Score Percentiles Relative to A) 50th and B) 90th Severity Percentile as the Reference Levels, the National Health and Nutrition Examination Survey (NAHNES III), United States, 1988–1994 (n = 10,605).
3.6. Prevention paradox
The proportion of participants with mild, moderate, high, and very high MetS severity scores was 50%, 25%, 15%, and 10%, respectively. In patients with mild (0th-50th), moderate (>50th-75th), high (>75th-90th), and very high (>90th) MetS severities, the age-adjusted MASLD prevalence was 17.4%, 25.7%, 42.5, and 54.9%, respectively. Approximately 31% of all patients with MASLD had low MetS severity, whereas an estimated 21% had very high severity scores (i.e., 90th or above) (Fig 4). A dose-response relationship was observed, whereby an increase in the MetS severity group, in reference to mild severity, was associated with higher odds of MASLD presence (Fig 4). The highest odds of MASLD were observed for those with very high versus low MetS severity (aOR, 6.60; 95% CI; 5.21 8.36).
4. Discussion
Studies on the link between MetS and MASLD have employed a harmonized disease definition, which encompasses exceeding predefined cutoff values for three out of five metabolic features. This definition accounts for disease occurrence, but equally treats the effects of any combination of metabolic abnormalities on MASLD risk. The traditional definition of MetS also overlooks racial/ethnic and sex disparities in MetS severity and their influence on the risk of MASLD. To bridge this knowledge gap, we used the MetS severity score, a validated sex-race/ethnicity-specific measure, to assess the association between MetS severity and MASLD.
Similar to previous studies, our MASLD cohort was older, with a higher prevalence of morbid obesity and lower physical activity levels than the general US adult population [43, 44]. In MASLD, racial/ethnic disparities were detected in baseline characteristics related to MetS. The prevalence of both MASLD and traditionally defined MetS differed by race/ethnicity, with Mexican American women having the highest disease burden. The severity of MetS was significantly higher in patients with MASLD than in those without. Among those with MASLD, Mexican American male, and female adults had the highest MetS severity compared to white non-Hispanic males and females. The MetS severity score significantly predicted the occurrence of MASLD in all the crude and adjusted models. We also observed nonlinear dose-response relationships between increased adjusted MASLD odds and higher MetS severity scores.
Several studies have examined racial and ethnic MASLD disparities in the context of the prevalence and severity of the individual components of MetS [18, 32, 45–48]. Walker et al. studied waist circumference, blood pressure, triglycerides, HDL cholesterol, and fasting glucose measurements in both adolescents and adults, and found that the odds of MetS varied considerably among white non-Hispanics, black non-Hispanics, and Mexican American groups [48]. The prevalence of high blood pressure, elevated fasting glucose, and insulin resistance was significantly higher in male African Americans than in whites. In females, triglycerides were associated with waist circumference in whites but not in African Americans, while African-American women displayed higher prevalence rates of elevated blood pressure, low HDL, and elevated fasting glucose. Genetic and metabolic factors have been suggested to underlie these disparities [25, 28, 49], as well as the incidence rates of insulin resistance and serum triglyceride concentrations [50, 51]. However, conclusive evidence supporting any model explaining race/ethnic disparities in MASLD is yet to be uncovered. Our study expands on these findings by accounting for the combined effects of all five metabolic features and their severities to explain racial/ethnic disparities in MASLD.
Our findings showed that increased MetS severity was associated with higher nonlinear odds of developing MASLD. This nonlinear relationship between MetS severity and the odds of MASLD helps explain the hepatic progression dynamics in MASLD. The onset of obesity is associated with the excessive accumulation of triglycerides throughout the body. In hepatocytes, increased uptake of triglycerides results in cell-specific lipotoxicity, which elevates the risk of comorbidities such as MASLD [52]. Individuals with high visceral adiposity may suffer from increased plasma-free fatty acids due to impaired insulin function related to peripheral Insulin resistance [53]. Due to the central role that obesity-induced insulin resistance plays in promoting hepatic steatosis, MASLD is regarded as the hepatic manifestation of MetS [4, 7, 11–13, 54, 55]. The prevalence of obesity among MASLD and MASH patients is 51% and 82%, respectively, and the prevalence of MASLD in patients with MetS and diabetes is particularly high [28, 56]. In the absence of MetS or any of its components, the prevalence of MASLD was 6.1% [39].
Our current study aligns with and extends the findings from our previous work on the relationship between MetS severity and mortality risk in patients with NAFLD [31]. In this work, MetS severity was markedly higher in NAFLD patients, indicating a significant predictor for all-cause and cause-specific mortalities. This retrospective cohort study, with a median follow-up of 19.2 years, showed that increases in MetS severity were associated with dose-response increases in biomarkers for cardiovascular disease, insulin resistance, and lipid abnormalities. Additionally, significant nonlinear dose-response relationships were found between increased adjusted mortality risk and higher MetS severity scores. These findings underscore the importance of MetS severity as a driving force for increased mortality risk, thereby reinforcing the need for its incorporation into risk stratification and management strategies for patients with MASLD. Echoing findings from this work, our results further affirm the utility of the MetS severity score as an instrumental clinical tool for the identification and longitudinal monitoring of at-risk patients, which is paramount given the currently limited treatment options for MASLD.
Approximately 55% of all patients with MASLD in our weighted sample had low-to-moderate MetS severity, whereas 21% had very high severity scores (i.e., 90th or above). Therefore, effective prevention measures must combine distinct strategies for high-risk individuals and the general population. Patients with very high MetS severity are likely to seek medical care because of the increased disease severity. Furthermore, high-risk adults are prone to develop advanced symptomatic hepatic conditions, such as MASH, fibrosis, cirrhosis, and HCC. Hence, preventive measures for this group are expected to be secondary and tertiary. Early detection and diagnosis of those at the highest risk will ensure increased survival by mitigating hepatic progression and lowering the elevated risks of CVD and cancer. While pharmaceutical intervention for MASLD must provide substantial clinical benefits at a modest annual price to be cost-effective [57], bariatric surgery has been shown to reduce the risk of cancer and cardiovascular disease in MASLD patients with morbid obesity [58, 59].
A population-wide preventive strategy should employ primary prevention measures, such as promoting a healthy lifestyle and dietary changes, to reduce MetS severity. While the natural history of MASLD might extend beyond the MetS spectrum, those with low-to-moderate severity are less likely to seek MASLD-related medical interventions. Thus, regardless of the causal mechanisms linking low to moderate severity with MASLD, low severity could be viewed as a proxy for a lower likelihood of seeking medical care, resulting in an increased risk of disease progression. From a policy perspective, primary interventions should attempt to shift the mean MetS severity score for patients with MASLD downwards. Because MetS severity predicts a wide array of cardiovascular outcomes, an overall decrease in MetS severity will provide significant public health benefits beyond the MASLD spectrum.
Our study has several limitations. The MASLD assessment was performed using ultrasonography, which could have resulted in misclassification. However, ultrasonography images were assessed by three trained ultrasound readers using standardized reading protocols. Furthermore, due to the lack of data, we could not evaluate the role of MASH, MASH-cirrhosis, and MetS severity. However, MASH and MASH-cirrhosis were associated with higher MetS severity, which is expected to reaffirm our findings on the association between MetS severity and MASLD occurrence. Ascertainment of exposure and baseline characteristics were conducted cross-sectionally, which resulted in nondifferential measurements in the exposed and unexposed groups. Alcohol intake was assessed based on self-reporting, which might have resulted in underestimation.
In conclusion, we have highlighted the pivotal role of MetS severity in driving the risk and racial/ethnic disparities observed in MASLD among adults in the US. The nonlinear dose-response relationship between increased MetS severity and higher odds of MASLD emphasizes the importance of considering disease severity rather than mere presence in understanding MASLD development and progression. Given the distribution of MetS severity in the MASLD spectrum, customized prevention strategies should be considered to address this hepatic epidemic. While the current treatment options for patients with MASLD are limited and indirect, the MetS severity score is a clinically accessible tool that can be used in primary and secondary prevention efforts to reduce the disease burden.
Data Availability
All data used are publicly available from the Center for Disease Control and Prevention at https://wwwn.cdc.gov/nchs/nhanes/nhanes3/default.aspx.
Funding Statement
This study was supported by award number P30CA016058 from the National Cancer Institute.
References
- 1.Rinella ME, Lazarus JV, Ratziu V, Francque SM, Sanyal AJ, Kanwal F, et al. A multi-society Delphi consensus statement on new fatty liver disease nomenclature. Annals of Hepatology. 2023:101133. doi: 10.1016/j.aohep.2023.101133 [DOI] [PubMed] [Google Scholar]
- 2.Bhala N, Jouness RI, Bugianesi E. Epidemiology and natural history of patients with NAFLD. Current pharmaceutical design. 2013;19(29):5169–76. CPD-EPUB-20130204-10 [pii]. doi: 10.2174/13816128113199990336 [DOI] [PubMed] [Google Scholar]
- 3.Setiawan VW, Stram DO, Porcel J, Lu SC, Le Marchand L, Noureddin M. Prevalence of chronic liver disease and cirrhosis by underlying cause in understudied ethnic groups: the multiethnic cohort. Hepatology. 2016;64(6):1969–77. doi: 10.1002/hep.28677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sherif ZA, Saeed A, Ghavimi S, Nouraie S-M, Laiyemo AO, Brim H, Ashktorab H. Global epidemiology of nonalcoholic fatty liver disease and perspectives on US minority populations. Digestive diseases and sciences. 2016;61(5):1214–25. doi: 10.1007/s10620-016-4143-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nature reviews Gastroenterology & hepatology. 2018;15(1):11–20. doi: 10.1038/nrgastro.2017.109 [DOI] [PubMed] [Google Scholar]
- 6.Ludwig J, Viggiano TR, Mcgill DB, Oh B, editors. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clinic Proceedings; 1980. [PubMed] [Google Scholar]
- 7.Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67(1):328–57. doi: 10.1002/hep.29367 [DOI] [PubMed] [Google Scholar]
- 8.Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology. 2012;142(7):1592–609. doi: 10.1053/j.gastro.2012.04.001 [DOI] [PubMed] [Google Scholar]
- 9.Rinella ME. Nonalcoholic fatty liver disease: a systematic review. Jama. 2015;313(22):2263–73. doi: 10.1001/jama.2015.5370 [DOI] [PubMed] [Google Scholar]
- 10.De A, Duseja A. Natural history of simple steatosis or nonalcoholic fatty liver. Journal of Clinical and Experimental Hepatology. 2020;10(3):255–62. doi: 10.1016/j.jceh.2019.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loria P, Marchesini G, Nascimbeni F, Ballestri S, Maurantonio M, Carubbi F, et al. Cardiovascular risk, lipidemic phenotype and steatosis. A comparative analysis of cirrhotic and non-cirrhotic liver disease due to varying etiology. Atherosclerosis. 2014;232(1):99–109. doi: 10.1016/j.atherosclerosis.2013.10.030 [DOI] [PubMed] [Google Scholar]
- 12.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease—Meta‐analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73–84. doi: 10.1002/hep.28431 [DOI] [PubMed] [Google Scholar]
- 13.Yu J, Marsh S, Hu J, Feng W, Wu C. The pathogenesis of nonalcoholic fatty liver disease: interplay between diet, gut microbiota, and genetic background. Gastroenterology research and practice. 2016;2016. doi: 10.1155/2016/2862173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel AH, Peddu D, Amin S, Elsaid MI, Minacapelli CD, Chandler T-M, et al. Nonalcoholic Fatty Liver Disease in Lean/Nonobese and Obese Individuals: A Comprehensive Review on Prevalence, Pathogenesis, Clinical Outcomes, and Treatment. Journal of Clinical and Translational Hepatology. 2023;11(2):502–15. doi: 10.14218/JCTH.2022.00204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang PL. A comprehensive definition for metabolic syndrome. Disease models & mechanisms. 2009;2(5–6):231–7. doi: 10.1242/dmm.001180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kanwar P, Kowdley KV. The Metabolic Syndrome and Its Influence on Nonalcoholic Steatohepatitis. Clinics in liver disease. 2016;20(2):225–43. doi: 10.1016/j.cld.2015.10.002 [DOI] [PubMed] [Google Scholar]
- 17.Carr RM, Oranu A, Khungar V. Nonalcoholic Fatty Liver Disease: Pathophysiology and Management. Gastroenterol Clin North Am. 2016;45(4):639–52. doi: 10.1016/j.gtc.2016.07.003 ; PubMed Central PMCID: PMC5127277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rich NE, Oji S, Mufti AR, Browning JD, Parikh ND, Odewole M, et al. Racial and Ethnic Disparities in Nonalcoholic Fatty Liver Disease Prevalence, Severity, and Outcomes in the United States: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2018;16(2):198–210 e2. doi: 10.1016/j.cgh.2017.09.041 ; PubMed Central PMCID: PMC5794571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303(3):235–41. doi: 10.1001/jama.2009.2014 . [DOI] [PubMed] [Google Scholar]
- 20.Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140(1):124–31. doi: 10.1053/j.gastro.2010.09.038 . [DOI] [PubMed] [Google Scholar]
- 21.Lazo M, Bilal U, Perez-Escamilla R. Epidemiology of NAFLD and Type 2 Diabetes: Health Disparities Among Persons of Hispanic Origin. Curr Diab Rep. 2015;15(12):116. doi: 10.1007/s11892-015-0674-6 . [DOI] [PubMed] [Google Scholar]
- 22.Clark JM, Brancati FL, Diehl AM. The prevalence and etiology of elevated aminotransferase levels in the United States. Am J Gastroenterol. 2003;98(5):960–7. doi: 10.1111/j.1572-0241.2003.07486.x . [DOI] [PubMed] [Google Scholar]
- 23.Weston SR, Leyden W, Murphy R, Bass NM, Bell BP, Manos MM, Terrault NA. Racial and ethnic distribution of nonalcoholic fatty liver in persons with newly diagnosed chronic liver disease. Hepatology. 2005;41(2):372–9. doi: 10.1002/hep.20554 . [DOI] [PubMed] [Google Scholar]
- 24.Ong JP, Younossi ZM. Epidemiology and natural history of NAFLD and NASH. Clin Liver Dis. 2007;11(1):1–16, vii. doi: 10.1016/j.cld.2007.02.009 . [DOI] [PubMed] [Google Scholar]
- 25.Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34(3):274–85. doi: 10.1111/j.1365-2036.2011.04724.x . [DOI] [PubMed] [Google Scholar]
- 26.Argo CK, Caldwell SH. Epidemiology and natural history of non-alcoholic steatohepatitis. Clin Liver Dis. 2009;13(4):511–31. doi: 10.1016/j.cld.2009.07.005 . [DOI] [PubMed] [Google Scholar]
- 27.Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40(6):1387–95. doi: 10.1002/hep.20466 . [DOI] [PubMed] [Google Scholar]
- 28.Portillo-Sanchez P, Bril F, Maximos M, Lomonaco R, Biernacki D, Orsak B, et al. High Prevalence of Nonalcoholic Fatty Liver Disease in Patients With Type 2 Diabetes Mellitus and Normal Plasma Aminotransferase Levels. J Clin Endocrinol Metab. 2015;100(6):2231–8. doi: 10.1210/jc.2015-1966 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Forde KA, Tanapanpanit O, Reddy KR. Hepatitis B and C in African Americans: current status and continued challenges. Clin Gastroenterol Hepatol. 2014;12(5):738–48. doi: 10.1016/j.cgh.2013.06.006 ; PubMed Central PMCID: PMC3947744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen X, Chen Q, Chen L, Zhang P, Xiao J, Wang S. Description and prediction of the development of metabolic syndrome in Dongying City: a longitudinal analysis using the Markov model. BMC Public Health. 2014;14:1033. doi: 10.1186/1471-2458-14-1033 ; PubMed Central PMCID: PMC4209018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elsaid MI, Bridges JF, Li N, Rustgi VK. Metabolic Syndrome Severity Predicts Mortality in Nonalcoholic Fatty Liver Disease. Gastro Hep Advances. 2022;1(3):445–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin SX, Carnethon M, Szklo M, Bertoni A. Racial/ethnic differences in the association of triglycerides with other metabolic syndrome components: the Multi-Ethnic Study of Atherosclerosis. Metabolic syndrome and related disorders. 2011;9(1):35–40. doi: 10.1089/met.2010.0050 ; PubMed Central PMCID: PMC3125569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gurka MJ, Lilly CL, Oliver MN, DeBoer MD. An examination of sex and racial/ethnic differences in the metabolic syndrome among adults: a confirmatory factor analysis and a resulting continuous severity score. Metabolism. 2014;63(2):218–25. doi: 10.1016/j.metabol.2013.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DeBoer MD, Gurka MJ. Clinical utility of metabolic syndrome severity scores: considerations for practitioners. Diabetes, metabolic syndrome and obesity: targets and therapy. 2017;10:65. doi: 10.2147/DMSO.S101624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guo Y, Musani SK, Sims M, Pearson TA, DeBoer MD, Gurka MJ. Assessing the added predictive ability of a metabolic syndrome severity score in predicting incident cardiovascular disease and type 2 diabetes: the Atherosclerosis Risk in Communities Study and Jackson Heart Study. Diabetology & metabolic syndrome. 2018;10(1):42. doi: 10.1186/s13098-018-0344-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gurka MJ, Golden SH, Musani SK, Sims M, Vishnu A, Guo Y, et al. Independent associations between a metabolic syndrome severity score and future diabetes by sex and race: the Atherosclerosis Risk In Communities Study and Jackson Heart Study. Diabetologia. 2017;60(7):1261–70. doi: 10.1007/s00125-017-4267-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gurka MJ, Guo Y, Filipp SL, DeBoer MD. Metabolic syndrome severity is significantly associated with future coronary heart disease in Type 2 diabetes. Cardiovascular diabetology. 2018;17(1):17. doi: 10.1186/s12933-017-0647-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.The National Center for Health Statistics. NHANES III (1988–1994) Description 2018 [cited 2019 02/14]. Available from: https://wwwn.cdc.gov/nchs/nhanes/nhanes3/Default.aspx.
- 39.Jinjuvadia R, Antaki F, Lohia P, Liangpunsakul S. The Association Between Nonalcoholic Fatty Liver Disease and Metabolic Abnormalities in The United States Population. J Clin Gastroenterol. 2017;51(2):160–6. doi: 10.1097/MCG.0000000000000666 ; PubMed Central PMCID: PMC5218881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.The National Center for Health Statistics. Hepatic/Gallbladder Ultrasound and Hepatic Steatosis 2011 [cited 2019 02/14]. Available from: https://wwwn.cdc.gov/nchs/data/nhanes3/34a/HGUHS.htm.
- 41.Harrell FE Jr. Regression modeling strategies: with applications to linear models, logistic and ordinal regression, and survival analysis: Springer; 2015. [Google Scholar]
- 42.Von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. The Lancet. 2007;370(9596):1453–7. [DOI] [PubMed] [Google Scholar]
- 43.Younossi ZM, Paik JM, Al Shabeeb R, Golabi P, Younossi I, Henry L. Are there outcome differences between NAFLD and metabolic-associated fatty liver disease? Hepatology. 2022;76(5):1423–37. Epub 2022/04/02. doi: 10.1002/hep.32499 . [DOI] [PubMed] [Google Scholar]
- 44.Huang Q, Zou X, Wen X, Zhou X, Ji L. NAFLD or MAFLD: Which Has Closer Association With All-Cause and Cause-Specific Mortality?-Results From NHANES III. Front Med (Lausanne). 2021;8:693507. Epub 2021/07/20. doi: 10.3389/fmed.2021.693507 ; PubMed Central PMCID: PMC8280321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shaheen M, Schrode KM, Pan D, Kermah D, Puri V, Zarrinpar A, et al. Sex-specific differences in the association between race/ethnicity and NAFLD among US population. Frontiers in Medicine. 2021:2423. doi: 10.3389/fmed.2021.795421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bonacini M, Kassamali F, Kari S, Barrera NL, Kohla M. Racial differences in prevalence and severity of non-alcoholic fatty liver disease. World Journal of Hepatology. 2021;13(7):763. doi: 10.4254/wjh.v13.i7.763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Agbim U, Carr RM, Pickett-Blakely O, Dagogo-Jack S. Ethnic disparities in adiposity: focus on non-alcoholic fatty liver disease, visceral, and generalized obesity. Current obesity reports. 2019;8:243–54. doi: 10.1007/s13679-019-00349-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Walker S, Gurka M, Oliver M, Johns D, DeBoer M. Racial/ethnic discrepancies in the metabolic syndrome begin in childhood and persist after adjustment for environmental factors. Nutrition, Metabolism and Cardiovascular Diseases. 2012;22(2):141–8. doi: 10.1016/j.numecd.2010.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cavalcante LN, Porto J, Mazo D, Longatto-Filho A, Stefano JT, Lyra AC, et al. African genetic ancestry is associated with lower frequency of PNPLA3 G allele in non-alcoholic fatty liver in an admixed population. Annals of Hepatology. 2022;27(6):100728. doi: 10.1016/j.aohep.2022.100728 [DOI] [PubMed] [Google Scholar]
- 50.Yu SS, Castillo DC, Courville AB, Sumner AE. The triglyceride paradox in people of African descent. Metabolic syndrome and related disorders. 2012;10(2):77–82. doi: 10.1089/met.2011.0108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Browning M, Khoraki J, DeAntonio J, Mazzini G, Mangino M, Siddiqui M, et al. Protective effect of black relative to white race against non-alcoholic fatty liver disease in patients with severe obesity, independent of type 2 diabetes. International journal of obesity. 2018;42(4):926–9. doi: 10.1038/ijo.2017.309 [DOI] [PubMed] [Google Scholar]
- 52.Berk PD, Verna EC. Nonalcoholic fatty liver disease: lipids and insulin resistance. Clinics in liver disease. 2016;20(2):245–62. doi: 10.1016/j.cld.2015.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Boden G, Lebed B, Schatz M, Homko C, Lemieux S. Effects of acute changes of plasma free fatty acids on intramyocellular fat content and insulin resistance in healthy subjects. Diabetes. 2001;50(7):1612–7. doi: 10.2337/diabetes.50.7.1612 [DOI] [PubMed] [Google Scholar]
- 54.Gaglio PJ. Nonalcoholic Fatty Liver Disease. Clinics in liver disease. 2016;20(2):xiii–xiv. doi: 10.1016/j.cld.2016.02.001 [DOI] [PubMed] [Google Scholar]
- 55.Sung K-C, Wild SH, Byrne CD. Development of new fatty liver, or resolution of existing fatty liver, over five years of follow-up, and risk of incident hypertension. Journal of hepatology. 2014;60(5):1040–5. doi: 10.1016/j.jhep.2014.01.009 [DOI] [PubMed] [Google Scholar]
- 56.Chan WK, Tan AT, Vethakkan SR, Tah PC, Vijayananthan A, Goh KL. Non-alcoholic fatty liver disease in diabetics—prevalence and predictive factors in a multiracial hospital clinic population in Malaysia. J Gastroenterol Hepatol. 2013;28(8):1375–83. doi: 10.1111/jgh.12204 . [DOI] [PubMed] [Google Scholar]
- 57.Rustgi VK, Duff SB, Elsaid MI. Cost-effectiveness and potential value of pharmaceutical treatment of nonalcoholic fatty liver disease. Journal of medical economics. 2022;25(1):347–55. doi: 10.1080/13696998.2022.2026702 [DOI] [PubMed] [Google Scholar]
- 58.Elsaid MI, Li Y, Bridges JF, Brock G, Minacapelli CD, Rustgi VK. Association of Bariatric Surgery With Cardiovascular Outcomes in Adults With Severe Obesity and Nonalcoholic Fatty Liver Disease. JAMA Network Open. 2022;5(10):e2235003-e. doi: 10.1001/jamanetworkopen.2022.35003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rustgi VK, Li Y, Gupta K, Minacapelli CD, Bhurwal A, Catalano C, Elsaid MI. Bariatric surgery reduces cancer risk in adults with nonalcoholic fatty liver disease and severe obesity. Gastroenterology. 2021;161(1):171–84. e10. doi: 10.1053/j.gastro.2021.03.021 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data used are publicly available from the Center for Disease Control and Prevention at https://wwwn.cdc.gov/nchs/nhanes/nhanes3/default.aspx.