Abstract
Many host cell surface proteins, including viral receptors, are incorporated into enveloped viruses. To address the functional significance of these host proteins, murine leukemia viruses containing the cellular receptors for Rous sarcoma virus (Tva) or ecotropic murine leukemia virus (MCAT-1) were produced. These receptor-pseudotyped viruses efficiently infect cells expressing the cognate viral envelope glycoproteins, with titers of up to 105 infectious units per milliliter for the Tva pseudotypes. Receptor and viral glycoprotein specificity and functional requirements are maintained, suggesting that receptor pseudotype infection recapitulates events of normal viral entry. The ability of the Tva and MCAT-1 pseudotypes to infect cells efficiently suggests that, in contrast to human immunodeficiency virus type 1 entry, neither of these retroviral receptors requires a coreceptor for membrane fusion. In addition, the ability of receptor pseudotypes to target infected cells suggests that they may be useful therapeutic reagents for directing infection of viral vectors. Receptor-pseudotyped viruses may be useful for identifying new viral receptors or for defining functional requirements of known receptors. Moreover, this work suggests that the production of receptor pseudotypes in vivo could provide a mechanism for expanded viral tropism with potential effects on the pathogenesis and evolution of the virus.
Enveloped viruses initiate the infection of target cells by interacting with specific receptors on the cell surface via the viral envelope glycoproteins. The viral envelope glycoproteins not only bind to the receptor but also catalyze fusion of the viral and host membranes. Receptor availability is often a primary determinant of host range and tissue tropism. Although viruses preferentially incorporate their own glycoproteins, a number of enveloped viruses can expand their tropism by acquiring the envelope glycoproteins of another virus during viral assembly by a process known as phenotypic mixing or pseudotyping. Viral pseudotypes are formed during the coinfection of a cell by two different enveloped viruses or can be generated experimentally by expressing a different viral glycoprotein in cells producing virus. Pseudotype formation in vivo has been postulated to provide a mechanism whereby the pathologic potential of a virus can be modified by coinfection with another virus.
In addition to foreign viral glycoproteins, enveloped viruses can incorporate a number of host surface proteins, including viral receptors, into budding virions (6, 8, 22). For example, class I and class II major histocompatability complex proteins, ICAM-1, ICAM-2, ICAM-3, CR3, CR4, CD43, CD44, CD55, CD59, CD63, and CD71, have all been found in human immunodeficiency virus type 1 (HIV-1) (summarized in reference 3). Similarly, CD55 and CD59 are associated with human cytomegalovirus and also with human T-cell leukemia virus (38). Of interest for the result presented here, the measles virus receptor, CD46, has also been reported in HIV-1 (25). In addition, transient high-level expression in cultured cells causes CD4, the primary cellular receptor for HIV-1, to partition into the membrane of a number of viruses, including retro-, herpes-, and rhabdoviruses (11, 35, 36, 43). It appears that a number of factors influence the efficiency of host protein uptake by enveloped viruses, including the surface density of the glycoprotein, its location within the membrane, or its structural configuration (39, 43).
Although there have been several reports of viral receptors being incorporated into viruses, no functional role for viral receptors in virions has been demonstrated (11, 25, 35, 36, 43). Therefore, we wanted to determine whether viral receptors displayed on the surface of a retrovirus (referred to as receptor pseudotypes) can target the infection of cells expressing the cognate viral glycoproteins. To address this question, we attempted to incorporate the subgroup A Rous sarcoma virus (RSV-A) receptor, Tva, or the ecotropic murine leukemia virus (MLV) receptor, MCAT-1, into MLV virions. Tva was chosen for these experiments because it is a simple type I integral membrane glycoprotein that binds tightly to the RSV-A envelope glycoprotein (EnvA) and has no apparent requirement for additional factors or coreceptors to mediate RSV infection (5, 10, 15). In contrast, MCAT-1 is a multiple-membrane-spanning amino acid transport protein that has physical properties which are quite distinct from those of Tva (1). However, similar to Tva, MCAT-1 does not appear to require additional factors for its viral receptor function. Here we show that both Tva and MCAT-1 are efficiently incorporated into virions and demonstrate that the receptor pseudotypes bearing Tva or MCAT-1 can efficiently infect cells expressing the RSV or MLV glycoproteins, respectively.
MATERIALS AND METHODS
Cell lines and plasmids.
3T3EnvA cells (15) and 293T cells were maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% iron-supplemented calf serum, penicillin (100 U/ml), streptomycin (100 μg/ml), and 2 mM L-glutamine. QT6 cells were maintained in M199 media supplemented with 10% tryptose phosphate broth, 5% fetal bovine serum, 1% chick serum, penicillin (100 U/ml), streptomycin (100 μg/ml), and 2 mM l-glutamine. All sera were heat inactivated.
pCB6 Tva950 (5) and pCB6 Tva* (31) are cytomegalovirus promoter expression plasmids for either the wild type or a mutant form of the subgroup A avian sarcoma and leukosis virus receptor, respectively. pcDNA3 MCAT-1:Flu3 was provided by Jim Cunningham (Harvard University) and expresses a murine cationic amino acid transporter with a triple hemagglutinin epitope tag appended at the 3′ end from a cytomegalovirus promoter. pRR140 and pRR186, provided by Alan Rein (National Cancer Institute, Frederick Cancer Research and Development Center), are plasmids expressing R peptide (−) forms of the Moloney murine leukemia virus and amphotropic Envs, respectively. Other plasmids used include pHit 60, pHit 111, and pHit 123 (37); pHit 456 (9); pCB6 EnvA and pCB6 EnvC (15); pCB6 EnvA Cleavage(−) (16); pCB6 EnvA GPI (17); and pCB6 EnvA A34[A]Q35, which contains an alanine insertion in the putative fusion peptide of the RSV-A envelope protein (2).
Generation of receptor-pseudotype virus.
Receptor-pseudotype virus was generated by an adaptation of the transient three-plasmid retroviral expression system (37). Briefly, 15 μg of pCB6 Tva950, pCB6 Tva*, or pcDNA3 MCAT-1:Flu3 receptor plasmid as well as pHit 60 and pHit 111 was transfected into 6 × 106 293T cells overnight by CaPO4. The medium was changed the next morning. Thirty-six hours posttransfection, the virus-containing medium was clarified by two-step centrifugation at 430 × g and then 2,300 × g. Viral supernatants generated for infections were subsequently aliquoted and stored at −80°C.
Equilibrium density gradient.
293T cells were transiently transfected with plasmids pCB6 Tva950, pHit 60, and pHit 111 as described above. Forty-eight hours posttransfection, viral supernatant was centrifuged through 20% sucrose onto a 60% sucrose cushion in an SW28 rotor at 28,000 rpm for 85 min. The viral band at the 20%/60% sucrose interface was isolated, diluted in phosphate-buffered saline (PBS), and pelleted through 20% sucrose onto another 60% sucrose cushion in an SW41 rotor at 41,000 rpm for 50 min. The viral band was removed, diluted threefold with PBS, layered on top of a 15 to 45% sucrose gradient, and centrifuged in an SW41 rotor at 35,000 rpm for 16 h. One-milliliter fractions were collected, the density was determined with a refractometer (model ABBE-3L; Milton Roy, Rochester, N.Y.), and the linearity of the gradient was confirmed by plotting the density versus fractions. Virions in the fractions were collected by pelleting through 20% sucrose in an SW55 rotor at 55,000 rpm for 10 min and then lysed in RIPA buffer (140 mM NaCl, 10 mM Tris [pH 8.0], 5 mM EDTA, 1% sodium deoxycholate, 1% Triton X-100, 0.1% sodium dodecyl sulfate [SDS]). The fractions were resolved by SDS-polyacrylamide gel electrophoresis (PAGE) (12.5% polyacrylamide), transferred to nitrocellulose, and then analyzed by Western blotting with α-MLV Gag (33) or α-Tva polyclonal sera (32).
Infection assay and Western blot analysis.
QT6 cells were exposed to a replication-competent RSV-A vector carrying an alkaline phosphatase marker gene, RCAS A AP (18), and serially passaged until chronically infected (QT6 AP) as determined by alkaline phosphatase staining (32). For pseudotyped infection, these cells were incubated overnight with 1 ml of receptor-pseudotype virus. The following morning, 2 ml of medium was added. The infection was allowed to proceed for 36 h, after which the cells were fixed with 2% paraformaldehyde and titers were determined by 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) staining infected cells for β-galactosidase (β-Gal) activity (33). 3T3EnvA cells, which stably express EnvA, were similarly infected.
To evaluate the requirements for Env in receptor-pseudotype infection, 6 × 105 293T cells or 4 × 105 QT6 cells were transfected by CaPO4 overnight with 3 μg of env plasmid DNA/well on a six-well plate. The cells were fed the following morning. Twenty-four to 48 h posttransfection, the cells were infected as described above.
QT6 AP (described above), 293T EnvA (transiently expressing EnvA), and 3T3EnvA (described above) target cell EnvA expression levels were evaluated by determining the protein concentration of lysates (Triton lysis buffer; 50 mM Tris [pH 8], 5 mM EDTA [pH 8], 150 mM NaCl, 1% Triton X-100) of each of the three cell types by the Bradford assay. Sixty micrograms of each lysate was resolved on an SDS-PAGE (10% polyacrylamide) gel and analyzed by Western blotting as described above with an α-avian myoblastosis virus polyclonal rabbit serum provided by Tom Matthews (Duke University).
Neutralization assays.
Neutralization was performed by preincubation of receptor-pseudotype MLV(Tva) with α-Tva rabbit polyclonal sera (5) at the indicated dilutions for 30 min at 37°C. The virus-antibody mixture was then used to infect 3T3EnvA cells. After 4 h, the virus inoculum was removed, and the cells were washed twice with PBS and then fed with fresh media containing the appropriate dilution of α-Tva sera. The cells were fixed and stained 48 h postinfection for β-Gal. Percent of infection inhibition was determined by comparing the number of β-Gal-positive cells in treated versus untreated wells.
Blocking MLV(Tva) infection with soluble receptor was achieved by preincubating 3T3EnvA cells with the indicated concentration of purified soluble Tva for 30 min at 25°C. The medium was removed, and MLV(Tva) along with soluble Tva was added to the 3T3EnvA cells. The infection was allowed to proceed for 4 h at 37°C. The viral inoculum was removed, and the cells were washed twice with PBS and then replenished with fresh medium. Titers and percents inhibition were determined as described above.
RESULTS
Incorporation of Tva into MLV virions.
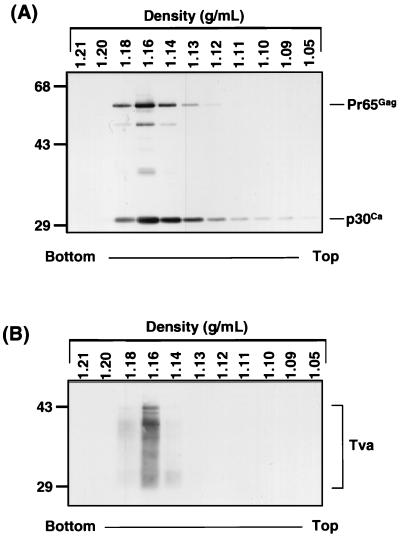
To determine whether receptor pseudotypes could be produced with Tva, a tva expression construct was cotransfected into 293T cells with plasmids expressing MLV gag-pol and an MLV vector genome encoding a β-Gal marker gene. Incorporation of Tva into MLV was assessed by equilibrium density gradient analysis of the transiently produced MLV particles. Virions from the medium of transfected cells were purified twice by centrifugation through 20% sucrose and then sedimented to equilibrium on a 15 to 45% sucrose gradient. Western blot analysis of the gradient fractions revealed that Tva appears to be incorporated into virions, since it cosediments with the MLV Gag proteins p30 and Pr65 (Fig. 1). Furthermore, the virions containing Tva band at a density of 1.16 g of sucrose per ml, as expected for intact retroviral virions under these conditions (19, 43). In parallel experiments in which the MLV Gag-Pol expression vector was omitted, no specific Tva reactivity was detected in any of the sucrose gradient fractions (data not shown). These experiments demonstrate that Tva is indeed incorporated into intact virions, thereby producing MLV(Tva) pseudotypes.
FIG. 1.
Cosedimentation of MLV Gag and Tva in an equilibrium density sucrose gradient. 293T cells were transiently transfected with plasmids pCB6 Tva950, pHit 60, and pHit 111. Forty-eight hours posttransfection, the viral supernatant was centrifuged twice through 20% sucrose onto a 60% sucrose cushion. The viral band was removed, diluted threefold with PBS, layered on top of a 15 to 45% sucrose gradient, and centrifuged to equilibrium. One-milliliter fractions were collected, pelleted through 20% sucrose, and then lysed in RIPA buffer. The fractions were resolved by SDS-PAGE (12.5% polyacrylamide), transferred to nitrocellulose, and then analyzed by Western blotting with anti-Gag antibody (A) (33) or anti-Tva antibody (B) (32). The density of each fraction is displayed. Molecular size standards (in kilodaltons) are indicated on the left.
Infection of cells expressing RSV envelope by MLV(Tva) virions.
The ability of the receptor protein in the MLV(Tva) pseudotypes to mediate infection of host cells was initially evaluated with cells chronically infected with RSV-A (QT6 AP). Quail QT6 cells were infected with a replication-competent RSV vector expressing an alkaline phosphatase reporter gene. After several passages, all the cells were AP positive and thus appeared to be chronically infected. When QT6 AP cells were used as targets for the MLV(Tva) pseudotypes, a titer of 7 × 102 infectious units per milliliter (IU/ml) of receptor-pseudotyped virus stock was obtained (Table 1). QT6 cells that had not been preinfected with the RSV vector were not susceptible to infection by MLV(Tva). These experiments suggest that expression of the viral glycoproteins in the infected cells renders them susceptible to the receptor pseudotypes.
TABLE 1.
MLV (Tva) infection of target cells expressing RSV Env
| Target cell | Titer (IU/ml)a for:
|
|
|---|---|---|
| 293% cells | QT6 cells | |
| Chronically infectedb | ||
| Uninfected | 0 | |
| RSV-A infected | 700 | |
| Transfectedc | ||
| EnvA | 20,000 | 1,800 |
| EnvC | 0 | 0 |
| EnvA cleavage(−) | 18 | 0 |
| EnvA GPI | 0 | 0 |
| EnvA A34[A]Q35 | 22 | 3 |
| Mock | 0 | 0 |
Titers were determined by enumerating β-Gal-positive cells. The data shown are representative of multiple experiments.
QT6 cells were chronically infected with an RSV-A vector, RCAS (A) AP (18).
Transfections are described in Materials and Methods.
To verify that the viral glycoproteins were responsible for the MLV(Tva) infection and to determine the effects of envelope alterations on receptor-pseudotyped infection, cells transiently expressing various RSV envelope glycoproteins were used as targets for infection. QT6 cells and human 293T cells were transiently transfected with a vector expressing the RSV-A envelope glycoprotein. The MLV(Tva) pseudotypes efficiently infected the RSV-A envelope-expressing cells, producing titers averaging 103 and 104 IU/ml in the transfected quail and human cells, respectively (Table 1). The titer of MLV(Tva) pseudotypes could be increased 40-fold by centrifugal concentration of MLV(Tva) (data not shown) (7). Cells not expressing the RSV envelope proteins were not susceptible to infection. Tva binds specifically to subgroup A envelope and will not mediate infection by other subgroups of RSV (5, 10, 15). As expected, transient expression of a subgroup C envelope (EnvC) did not render either quail or human cells susceptible to MLV(Tva) (Table 1).
Although unprocessed retroviral glycoproteins are competent to bind receptor, proteolytic cleavage of the Env precursor to surface and transmembrane subunits is required for full fusogenic activity (14, 23, 27). For RSV, the cleavage-deficient form of EnvA produces viruses with a 4- to 5-log decrease in infectious titer (2). Similarly, viral fusion proteins anchored by a glycosylphosphatidylinositol (GPI) moiety bind receptor but do not mediate full membrane fusion, allowing only partial mixing of the membrane lipids (15, 20, 24, 28, 34, 41). Consistent with the entry defects expected of these envelopes, the GPI-anchored version of EnvA expressed in either 293T or QT6 cells did not mediate MLV(Tva) infection, while a cleavage-deficient form of the RSV-A envelope (EnvA CL−) allowed very low levels of infection by MLV(Tva) in 293T cells (Table 1). Finally, a mutant EnvA (EnvA A34[A]Q35) containing an insertion in the putative fusion peptide of RSV envelope, which dramatically reduces EnvA-mediated entry (2), also severely inhibits MLV(Tva) infection of cells expressing it (Table 1). These experiments demonstrate that infection by viruses carrying a host receptor protein requires a viral envelope glycoprotein on the target cells that specifically binds receptor and is competent to mediate membrane fusion. Indeed, the functional requirements for EnvA appear to be the same whether the envelope protein is on the virion or on the host surface mediating receptor-pseudotype infection.
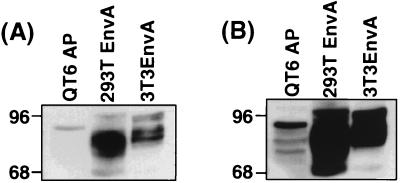
To address the question of whether the level of envelope expression in target cells affected receptor-pseudotype infection, we assessed envelope expression in three target cell lines. Transiently transfected 293T cells express very high levels of EnvA (Fig. 2), whereas EnvA expression in a stable NIH 3T3 cell line is diminished slightly compared to that of 293T cells (15). In contrast, the RSV-infected QT6 cells express much lower levels of EnvA than do either of the other cell types (Fig. 2). As expected, the quail, human, and murine cell lines posttranslationally modify EnvA differentially, leading to slightly different EnvA profiles among the three cell lines, as shown by the results of Western blotting (Fig. 2). Infection of these three cell types roughly paralleled the level of envelope expression with titers of 2 × 104, 2 × 103 to 5 × 103, and 7 × 102 on the 293T, 3T3, and QT6 cells, respectively. Although cells expressing high levels of envelope appear to be better targets, the fact that RSV-infected QT6 cells display significant susceptibility to MLV(Tva) indicates that extremely high levels of envelope protein expression are not required for receptor-pseudotype infection.
FIG. 2.
EnvA expression in MLV(Tva) target cells. Equivalent amounts of protein from cell lysates from recombinant RSV-A chronically infected QT6 cells (QT6 AP), 293T cells transiently transfected with pCB6 EnvA (293T EnvA), and NIH 3T3 cells stably expressing pCB6 EnvA (3T3EnvA) were resolved by SDS-PAGE (10% polyacrylamide), transferred to nitrocellulose, and then analyzed by Western blotting with α-AMV polyclonal sera. (A) Short exposure. (B) Overexposure to better visualize EnvA expression in QT6 AP cells. Molecular size standards (in kilodaltons) are indicated on the left.
Analysis of receptor requirements on virions.
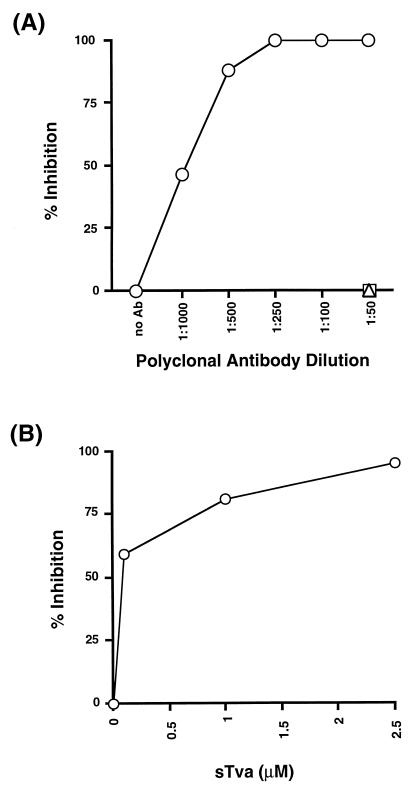
To ensure that the receptor protein incorporated into the virus was responsible for the infectivity of the pseudotypes, we produced virions carrying a nonfunctional mutant of Tva (Tva*). This receptor mutant contains five amino acid substitutions that abrogate its ability to bind envelope or facilitate EnvA-mediated infection of target cells (31). Western blot analysis of virions produced by transient transfection revealed that Tva* was incorporated into MLV pseudotypes at a level which was very similar to that of wild-type Tva; however, the MLV(Tva*) virions were unable to infect 3T3EnvA cells (data not shown). The requirement for receptor was also verified with anti-Tva antibodies to neutralize the receptor-pseudotype infectivity. Infection by the MLV(Tva) virus was neutralized in a dose-dependent manner by treatment of the receptor pseudotypes with anti-Tva antibodies (Fig. 3A). Furthermore, infection by MLV(Tva) could be blocked by competition with a soluble form of the receptor during infection (Fig. 3B). Thus, these results demonstrate that as expected, pseudotype infection requires a functional receptor on the virion.
FIG. 3.
Tva antiserum and soluble Tva inhibit MLV(Tva) infection. (A) Inhibition of infection by antibodies (Ab) to Tva. MLV(Tva) was preincubated with α-Tva (○), α-Ebola GP (□), or prebleed (▵) rabbit polyclonal sera at the indicated dilutions for 30 min at 37°C. The virus-antibody mixture was then used to infect EnvA-expressing NIH 3T3 (3T3EnvA) cells at 37°C. After 4 h, the virus inoculum was removed, and the cells were washed and then replenished with fresh media containing the appropriate dilution of antibody. The cells were fixed and stained 48 h postinfection for β-Gal. Percent inhibition of infection was determined by comparing the number of β-Gal-positive cells in treated wells versus untreated controls. (B) Blocking infection of receptor pseudotypes with soluble receptor. 3T3EnvA cells were preincubated with the indicated concentration of soluble Tva (sTva) for 30 min at 25°C. The medium was removed, and MLV(Tva) along with soluble Tva was added to the 3T3EnvA cells. The infection was allowed to proceed for 4 h at 37°C. The viral inoculum was removed, and the cells were washed and then replenished with fresh medium. Titers and percents inhibition were determined as described for panel A.
MLV receptor also functions in pseudotype virions.
To address the question of whether viral receptors other than Tva could be incorporated into virions and function to direct infection, the ecotropic MLV receptor, MCAT-1, was used to generate receptor pseudotypes. MCAT-1 is an amino acid transporter containing multiple-membrane-spanning domains and is thus quite different from Tva (1, 21). MCAT-1 was coexpressed with MLV Gag-Pol and an MLV vector encoding β-Gal to produce MLV(MCAT) virions. Sucrose gradient purification and Western blot analysis of the virions demonstrated that MCAT-1 was incorporated into MLV particles (data not shown). The MLV(MCAT) pseudotypes were capable of infecting 293T cells transiently expressing the full-length ecotropic MLV envelope protein (MLV Env), with infectious titers ranging from 101 to 102 IU/ml. No infection by the MCAT-1 pseudotypes was seen when mock-transfected cells or 293T cells expressing either amphotropic MLV env or EnvA were used as targets (data not shown). Fusogenicity of MLV Env is dramatically increased by the proteolytic removal of the cytoplasmic tail R peptide during viral budding (29, 30). To address the question of whether the R peptide had the same effect on envelope function during receptor-pseudotyped infection, QT6 cells transiently expressing either full-length or R(−)MLV Env were infected with MLV(MCAT). Compared to QT6 cells expressing full-length MLV Env, the R(−)Env-expressing QT6 cells were 100 to 1,000 times more susceptible to infection by MLV(MCAT), with titers of 0.5 × 104 to 1 × 104 IU/ml. Thus, the MLV Env protein seems to function similarly when mediating receptor pseudotype or typical MLV infection in that the R peptide affects Env fusogenicity. Therefore, similar to Tva, the MCAT pseudotype infection apparently retains the receptor specificity and viral envelope glycoprotein functional requirements of a typical viral infection. Together with our results on Tva, these results with MCAT-1 demonstrate that receptor pseudotypes containing radically different types of receptor proteins can be produced and will direct the infection of target cells expressing the appropriate viral glycoproteins.
DISCUSSION
Our studies directly demonstrate that viral receptors, when incorporated into a budding virion, will direct infection of target cells expressing the cognate viral glycoproteins. Infection by these receptor-pseudotype viruses appears to have specificity and requirements for envelope and receptor function that are similar to those for a normal viral infection except that the geometry of the proteins during binding and membrane fusion is reversed. Furthermore, the titers obtained for the receptor pseudotypes are in general only 10- to 100-fold-lower than titers of envelope pseudotypes we routinely obtain with RSV (2, 33) or Ebola virus envelope pseudotypes of MLV (42). The fact that infection by the receptor pseudotypes appears to be quite efficient suggests that, at least for viruses with an MLV core, a specific interaction between a viral envelope protein and the viral core components is not required for the uncoating of the entering virus and the infection of the target cell. Similar results with pseudotypes having an HIV-1 core suggest that there is also no specific viral glycoprotein requirement for the uncoating of this retrovirus (13). The ability of MLV(Tva) to direct infection was somewhat unexpected, since RSV-A envelope protein does not induce syncytia even when the viral envelope protein and receptor are expressed at very high levels on the cell surface. Therefore, infection by the Tva-pseudotyped virus is unlikely to represent a nonspecific fusion reaction but, rather, suggests that the receptor pseudotypes are duplicating the events of normal viral entry.
Recent experiments by Endres and Hoxie with an HIV-1 pseudotype system demonstrate that the incorporation of CD4 along with either CXCR4 or CCR5 into virions directs infection of HIV-1-infected cells by these receptor pseudotypes and that the infection recapitulates the envelope-receptor specificity and coreceptor requirements of the HIV-1 system (13). The use of Tva and MCAT-1 to produce pseudotypes demonstrates that either of these viral receptors alone appears to be sufficient to direct infection of the target envelope-expressing cells. This result indicates that unlike the lentiviruses HIV-1 and simian immunodeficiency virus, these two oncoretroviruses utilize receptors that act alone and do not require a coreceptor to initiate membrane fusion. While the results of these experiments cannot rule out the possibility of a coreceptor requirement for Tva or MCAT-1, it is unlikely that a coreceptor would be able to nonspecifically incorporate into the virions sufficiently to give the high level of infectivity for the receptor pseudotypes observed, since the incorporation of either Tva or MCAT-1 requires very high levels of receptor expression. In support of the proposed lack of a coreceptor requirement, Tva expression is able to make a wide variety of cells susceptible to RSV vectors (4), suggesting either that the Tva coreceptor is abundantly and widely expressed or that RSV entry does not require a coreceptor. In contrast, previous analysis of MLV infection of MCAT-1-expressing cells suggested that a cofactor was required for ecotropic MLV infection and was limiting in certain cell types (40). However, for the same reasons suggested for Tva, our infection data for the MCAT-1 pseudotypes strongly suggest that a cofactor is not required for this receptor to mediate membrane fusion. Since receptor pseudotypes seem to maintain the correct receptor and/or coreceptor requirements, it is likely that other receptors for enveloped viruses can be tested with this system to determine if the receptor protein is sufficient to direct viral entry. Potential candidates for such analysis include the measles virus receptor, CD46 (12), and the recently identified herpes simplex virus receptor, HVEM (26).
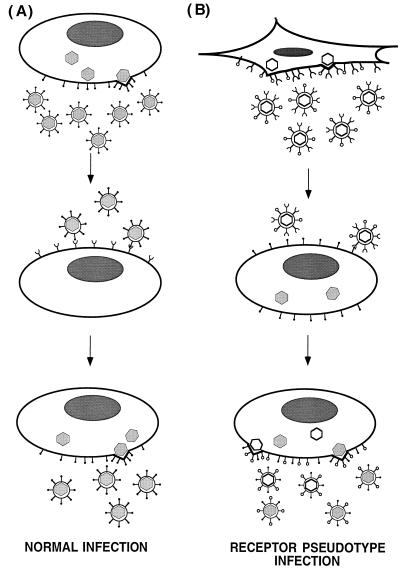
The ability of receptor-pseudotype viruses to infect cells also suggests a biological role for cellular receptors associated with enveloped viruses in expanding the viral host range and perhaps in influencing the course of disease following infection. As shown in the model in Fig. 4, by directing the infection of cells already infected by a different virus, receptor pseudotypes would promote phenotypic mixing of the viruses and further the expansion of the host range of both viruses. Furthermore, the production of receptor pseudotypes might influence viral evolution by providing a mechanism whereby unrelated viruses that normally have different tissue tropisms infect the same cell. Coinfection could allow the exchange of genetic information between the viruses.
FIG. 4.
Model for phenotypic mixing promoted by receptor pseudotypes. (A) Normal infection by enveloped viruses. Virions assemble and bud from the host cell specifically incorporating viral glycoproteins into the virion. The progeny viruses are capable of infecting target cells that express a specific cell surface receptor. (B) Effect of incorporating a viral receptor into virus. Virions bud from the host cell and incorporate a cellular receptor protein into the virion as well as the viral glycoproteins to produce receptor pseudotypes (top). These receptor-pseudotype viruses (white) are capable of infecting cells infected by a different virus (gray) and expressing the cognate viral glycoprotein for the incorporated receptor (middle). These dual-infected cells produce phenotypically mixed virions carrying both viral glycoproteins. • and ○, viral glycoproteins for gray and white viruses, respectively; ∪, receptor for gray virus.
Receptor pseudotypes might be employed as tools to identify viral receptors. They would be especially useful for identifying ubiquitously expressed viral receptors for viruses such as human T-cell leukemia virus, for which no resistant cell lines are available. By incorporating proteins expressed from cDNA libraries into viruses and screening viral envelope-expressing cells for susceptibility to these pseudotypes, cDNAs for ubiquitous viral receptors could be identified. Finally, our data suggest that receptor pseudotypes might prove useful as therapeutic agents for targeting infected cells in vivo.
ACKNOWLEDGMENTS
We thank Mike Endres and Jim Hoxie for communication of unpublished results. We acknowledge Jim Cunningham for supplying the epitope-tagged MCAT-1 clone, Alan Rein for the ecotropic and amphotropic R peptide(−) Env expression plasmids, and Tom Matthews for the rabbit polyclonal α-AMV sera. We also thank Carrie L. Rokos, Robert Doms, and Mike Malim for critical reading of the manuscript and the members of the Bates Laboratory for useful discussions.
This work was supported by grants to P.B. from the National Institutes of Health (CA63531) and the American Heart Association (95015200). J.W.B. is a trainee of grant T32-AI-07325 from the National Institutes of Health.
REFERENCES
- 1.Albritton L M, Tseng L, Scadden D, Cunningham J M. A putative murine ecotropic retrovirus receptor gene encodes a multiple membrane-spanning protein and confers susceptibility to virus infection. Cell. 1989;57:659–666. doi: 10.1016/0092-8674(89)90134-7. [DOI] [PubMed] [Google Scholar]
- 2.Balliet, J. W., and P. Bates. Unpublished data.
- 3.Bastiani L, Laal S, Kim M, Zolla-Pazner S. Host cell-dependent alterations in envelope components of human immunodeficiency virus type 1 virions. J Virol. 1997;71:3444–3450. doi: 10.1128/jvi.71.5.3444-3450.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bates, P. Unpublished observation.
- 5.Bates P, Young J A, Varmus H E. A receptor for subgroup A Rous sarcoma virus is related to the low density lipoprotein receptor. Cell. 1993;74:1043–1051. doi: 10.1016/0092-8674(93)90726-7. [DOI] [PubMed] [Google Scholar]
- 6.Bubbers J E, Lilly F. Selective incorporation of H-2 antigenic determinants into Friend virus particles. Nature. 1977;266:458–459. doi: 10.1038/266458a0. [DOI] [PubMed] [Google Scholar]
- 7.Burns J C, Friedmann T, Driever W, Burrascano M, Yee J K. Vesicular stomatitis virus G glycoprotein pseudotyped retroviral vectors: concentration to very high titer and efficient gene transfer into mammalian and nonmammalian cells. Proc Natl Acad Sci USA. 1993;90:8033–8037. doi: 10.1073/pnas.90.17.8033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calafat J, Janssen H, Demant P, Hilgers J, Zavada J. Specific selection of host cell glycoproteins during assembly of murine leukaemia virus and vesicular stomatitis virus: presence of Thy-1 glycoprotein and absence of H-2, Pgp-1 and T-200 glycoproteins on the envelopes of these virus particles. J Gen Virol. 1983;64:1241–1253. doi: 10.1099/0022-1317-64-6-1241. [DOI] [PubMed] [Google Scholar]
- 9.Cannon P M, Kim N, Kingsman S M, Kingsman A J. Murine leukemia virus-based Tat-inducible long terminal repeat replacement vectors: a new system for anti-human immunodeficiency virus gene therapy. J Virol. 1996;70:8234–8240. doi: 10.1128/jvi.70.11.8234-8240.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Connolly L, Zingler K, Young J A. A soluble form of a receptor for subgroup A avian leukosis and sarcoma viruses (ALSV-A) blocks infection and binds directly to ALSV-A. J Virol. 1994;68:2760–2764. doi: 10.1128/jvi.68.4.2760-2764.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dolter K E, King S R, Holland T C. Incorporation of CD4 into virions by a recombinant herpes simplex virus. J Virol. 1993;67:189–195. doi: 10.1128/jvi.67.1.189-195.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dorig R E, Marcil A, Chopra A, Richardson C D. The human CD46 molecule is a receptor for measles virus (Edmonston strain) Cell. 1993;75:295–305. doi: 10.1016/0092-8674(93)80071-l. [DOI] [PubMed] [Google Scholar]
- 13.Endres, M. J., and J. A. Hoxie. Science, in press.
- 14.Freed E O, Myers D J, Risser R. Mutational analysis of the cleavage sequence of the human immunodeficiency virus type 1 envelope glycoprotein precursor gp160. J Virol. 1989;63:4670–4675. doi: 10.1128/jvi.63.11.4670-4675.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gilbert J M, Bates P, Varmus H E, White J M. The receptor for the subgroup A avian leukosis-sarcoma viruses binds to subgroup A but not to subgroup C envelope glycoprotein. J Virol. 1994;68:5623–5628. doi: 10.1128/jvi.68.9.5623-5628.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gilbert J M, Hernandez L D, Balliet J W, Bates P, White J M. Receptor-induced conformational changes in the subgroup A avian leukosis and sarcoma virus envelope glycoprotein. J Virol. 1995;69:7410–7415. doi: 10.1128/jvi.69.12.7410-7415.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gilbert J M, Hernandez L D, Chernov-Rogan T, White J M. Generation of a water-soluble oligomeric ectodomain of the Rous sarcoma virus envelope glycoprotein. J Virol. 1993;67:6889–6892. doi: 10.1128/jvi.67.11.6889-6892.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hughes S H, Greenhouse J J, Petropoulos C J, Sutrave P. Adaptor plasmids simplify the insertion of foreign DNA into helper-independent retroviral vectors. J Virol. 1987;61:3004–3012. doi: 10.1128/jvi.61.10.3004-3012.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones T A, Blaug G, Hansen M, Barklis E. Assembly of gag-β-galactosidase proteins into retrovirus particles. J Virol. 1990;64:2265–2279. doi: 10.1128/jvi.64.5.2265-2279.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kemble G W, Danieli T, White J M. Lipid-anchored influenza hemagglutinin promotes hemifusion, not complete fusion. Cell. 1994;76:383–391. doi: 10.1016/0092-8674(94)90344-1. [DOI] [PubMed] [Google Scholar]
- 21.Kim J W, Closs E I, Albritton L M, Cunningham J M. Transport of cationic amino acids by the mouse ecotropic retrovirus receptor. Nature. 1991;352:725–728. doi: 10.1038/352725a0. [DOI] [PubMed] [Google Scholar]
- 22.Lodish H F, Porter M. Specific incorporation of host cell surface proteins into budding vesicular stomatitis virus particles. Cell. 1980;19:161–169. doi: 10.1016/0092-8674(80)90397-9. [DOI] [PubMed] [Google Scholar]
- 23.McCune J M, Rabin L B, Feinberg M B, Lieberman M, Kosek J C, Reyes G R, Weissman I L. Endoproteolytic cleavage of gp160 is required for the activation of human immunodeficiency virus. Cell. 1988;53:55–67. doi: 10.1016/0092-8674(88)90487-4. [DOI] [PubMed] [Google Scholar]
- 24.Melikyan G B, White J M, Cohen F S. GPI-anchored influenza hemagglutinin induces hemifusion to both red blood cell and planar bilayer membranes. J Cell Biol. 1995;131:679–691. doi: 10.1083/jcb.131.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Montefiori D C, Cornell R J, Zhou J Y, Zhou J T, Hirsch V M, Johnson P R. Complement control proteins, CD46, CD55, and CD59, as common surface constituents of human and simian immunodeficiency viruses and possible targets for vaccine protection. Virology. 1994;205:82–92. doi: 10.1006/viro.1994.1622. [DOI] [PubMed] [Google Scholar]
- 26.Montgomery R I, Warner M S, Lum B J, Spear P G. Herpes simplex virus-1 entry into cells mediated by a novel member of the TNF/NGF receptor family. Cell. 1996;87:427–436. doi: 10.1016/s0092-8674(00)81363-x. [DOI] [PubMed] [Google Scholar]
- 27.Perez L G, Hunter E. Mutations within the proteolytic cleavage site of the Rous sarcoma virus glycoprotein that block processing to gp85 and gp37. J Virol. 1987;61:1609–1614. doi: 10.1128/jvi.61.5.1609-1614.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ragheb J A, Anderson F. Uncoupled expression of Moloney murine leukemia virus envelope polypeptides SU and TM: a functional analysis of the role of TM domains in viral entry. J Virol. 1994;68:3207–3219. doi: 10.1128/jvi.68.5.3207-3219.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ragheb J A, Anderson W F. pH-independent murine leukemia virus ecotropic envelope-mediated cell fusion: implications for the role of the R peptide and p12E TM in viral entry. J Virol. 1994;68:3220–3231. doi: 10.1128/jvi.68.5.3220-3231.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rein A, Mirro J, Haynes J G, Ernst S M, Nagashima K. Function of the cytoplasmic domain of a retroviral transmembrane protein: p15E-p2E cleavage activates the membrane fusion capability of the murine leukemia virus Env protein. J Virol. 1994;68:1773–1781. doi: 10.1128/jvi.68.3.1773-1781.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rong, L., K. Gendron, B. Strohl, R. Shenoy, R. J. Wool-Lewis, and P. Bates. Submitted for publication. [DOI] [PMC free article] [PubMed]
- 32.Rong L, Bates P. Analysis of the subgroup A avian sarcoma and leukosis virus receptor: the 40-residue, cysteine-rich, low-density lipoprotein receptor repeat motif of Tva is sufficient to mediate viral entry. J Virol. 1995;69:4847–4853. doi: 10.1128/jvi.69.8.4847-4853.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rong L, Edinger A, Bates P. Role of basic residues in the subgroup-determining region of the subgroup A avian sarcoma and leukosis virus envelope in receptor binding and infection. J Virol. 1997;71:3458–3465. doi: 10.1128/jvi.71.5.3458-3465.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salzwedel K, Johnston P B, Roberts S J, Dubay J W, Hunter E. Expression and characterization of glycophospholipid-anchored human immunodeficiency virus type 1 envelope glycoproteins. J Virol. 1993;67:5279–5288. doi: 10.1128/jvi.67.9.5279-5288.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schnell M J, Buonocore L, Kretzschmar E, Johnson E, Rose J K. Foreign glycoproteins expressed from recombinant vesicular stomatitis viruses are incorporated efficiently into virus particles. Proc Natl Acad Sci USA. 1996;93:11359–11365. doi: 10.1073/pnas.93.21.11359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schubert M, Joshi B, Blondel D, Harmison G G. Insertion of the human immunodeficiency virus CD4 receptor into the envelope of vesicular stomatitis virus particles. J Virol. 1992;66:1579–1589. doi: 10.1128/jvi.66.3.1579-1589.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Soneoka Y, Cannon P M, Ramsdale E E, Griffiths J C, Romano G, Kingsman S M, Kingsman A J. A transient three-plasmid expression system for the production of high titer retroviral vectors. Nucleic Acids Res. 1995;23:628–633. doi: 10.1093/nar/23.4.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spear G T, Lurain N S, Parker C J, Ghassemi M, Payne G H, Saifuddin M. Host cell-derived complement control proteins CD55 and CD59 are incorporated into the virions of two unrelated enveloped viruses. Human T cell leukemia/lymphoma virus type I (HTLV-I) and human cytomegalovirus (HCMV) J Immunol. 1995;155:4376–4381. [PubMed] [Google Scholar]
- 39.Suomalainen M, Garoff H. Incorporation of homologous and heterologous proteins into the envelope of Moloney murine leukemia virus. J Virol. 1994;68:4879–4889. doi: 10.1128/jvi.68.8.4879-4889.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang H, Paul R, Burgeson R E, Keene D R, Kabat D. Plasma membrane receptors for ecotropic murine retroviruses require a limiting accessory factor. J Virol. 1991;65:6468–6477. doi: 10.1128/jvi.65.12.6468-6477.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weiss C D, White J M. Characterization of stable Chinese hamster ovary cells expressing wild-type, secreted, and glycosylphosphatidylinositol-anchored human immunodeficiency virus type 1 envelope glycoprotein. J Virol. 1993;67:7060–7066. doi: 10.1128/jvi.67.12.7060-7066.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wool-Lewis, R. J., and P. Bates. Submitted for publication.
- 43.Young J A, Bates P, Willert K, Varmus H E. Efficient incorporation of human CD4 protein into avian leukosis virus particles. Science. 1990;250:1421–1423. doi: 10.1126/science.2175047. [DOI] [PubMed] [Google Scholar]