Opening Vignette
A 65-year-old Chinese woman with a background of diabetes mellitus, hypertension and hyperlipidaemia presented with sudden-onset retrosternal chest tightness that started 1 h ago, and this was associated with dyspnoea and diaphoresis. On examination, her haemodynamics were stable and cardiovascular and respiratory examination were unremarkable. Electrocardiogram (ECG) showed new widespread ST segment depressions in the anterolateral leads, with mildly elevated high-sensitivity troponins at 50 ng/L (reference range: ≤26.2 ng/L). As part of the on-call medical team attending to the patient, you diagnosed her with non-ST segment elevation myocardial infarction (NSTEMI).
DEFINITIONs OF KEY TERMS
Acute chest pain (ACP) refers to pain/discomfort over the anterior thoracic region. The traditional Diamond classification characterises typical angina based on the presence of three cardinal features — substernal chest discomfort, precipitated by exertion and relieved by rest or glyceryl trinitrate (GTN).[1] Ischaemic chest pain is also described as crushing, heavy or tight in character,[2,3] and may radiate to the left arm, neck or jaw.[2] Currently, stratification of chest pain into typical and atypical angina is discouraged due to clinical ambiguity,[2] with the 2021 American Heart Association/American College of Cardiology guidelines proposing the use of ‘cardiac’, ‘possible cardiac’ and ‘noncardiac’ descriptors when assessing the likelihood of ischaemic chest pain based on symptom characteristics, patient’s age and cardiovascular risk factors.[2]
Acute coronary syndrome (ACS) is a subset of unstable/life-threatening forms of ischaemic heart disease (IHD) comprising unstable angina (UA), NSTEMI and ST segment elevation myocardial infarction (STEMI),[4] which typically warrant early coronary evaluation/intervention. In particular, UA is characterised by a crescendo pattern of angina, with increased frequency, duration (>15–20 min) and intensity, or pain at rest or refractory to GTN. Unstable angina is distinguished from NSTEMI by the absence of elevated cardiac biomarkers.
The fourth universal definition of myocardial infarction (MI) describes five aetiological categories of MI [Table S1, Supplemental Digital Appendix],[4] of which type 1 MI (T1MI; atherothrombotic disease) and type 2 MI (T2MI; myocardial oxygen supply/demand mismatch) are most commonly encountered. Diagnosis of MI requires a ≥20% rise and/or fall in troponin levels with at least one value >99th percentile of the upper reference limit, and at least one of the following:[2] (a) Clinical criteria: typical ischaemic symptoms; (b) Electrocardiographic criteria: new ischaemic ECG changes (e.g., new ST–T wave changes or new pathological Q waves); (c) Imaging criteria: imaging evidence of new regional wall motion abnormalities on echocardiography or new loss of viable myocardium on myocardial perfusion imaging (MPI); and (d) Anatomical criteria: identification of intracoronary thrombus on coronary angiography or during autopsy (for T1MI). Unlike MI, myocardial injury occurs when there is isolated hypertroponinaemia, without evidence of myocardial ischaemia.
PREVALENCE AND CAUSES
Acute chest pain accounts for 5%–11% of emergency department (ED) visits,[5,6] of which 5% have ACS[5] and 3% have a missed ACS diagnosis.[7] Chest pain can be broadly classified into cardiac and non-cardiac (e.g., respiratory, mediastinal, gastrointestinal, musculoskeletal/soft tissue) aetiologies [Table S2, Supplemental Digital Appendix], including life-threatening causes such as ACS, aortic dissection, pulmonary embolism, tension pneumothorax and perforated viscus.
CLINICAL APPROACH TO ACUTE CHEST PAIN
History taking
There are two components of history taking for ACP. Firstly, characterise the nature of chest pain using the SOCRATES tool:[8] (a) Site: retrosternal/central (stable angina/ACS, pericarditis, gastro-oesophageal reflux disease [GERD]), right/left anterior chest (lung/pleural pathology, chest wall syndromes), epigastrium (gallstone disease, pancreatitis, peptic ulcer disease [PUD]), costochondral junction (costochondritis); (b) Onset: sudden (ACS, aortic dissection, perforated viscus), gradual (other subacute pathologies); (c) Character: heavy/ crushing/tightness (anginal), pleuritic (pleurisy), sharp/stabbing (pericarditis, pleurisy, neuropathic, chest wall syndromes), burning (GERD, PUD); (d) Radiation: to neck/jaw/arm (ACS), back (aortic dissection, pancreatitis), right shoulder (gallstone disease); (e) Alleviating factors: rest/GTN use (anginal), sitting up and leaning forwards (pericarditis, pancreatitis), antacids)(GERD), analgesia/anti-inflammatory medications (musculoskeletal); (f) Timing: association with meals (GERD, PUD, gallstone disease); (g) Exacerbating factors: exertion/emotional stress (anginal), lying flat (GERD, pericarditis, pancreatitis), deep inspiration (pleurisy), palpation/chest wall movement /coughing (musculoskeletal); and (h) Severity: pain score 1–10.
Secondly, identify associated symptoms, significant negatives and predisposing risk factors to assess the likelihood of clinical differentials. For example, patients with MI may have anginal chest pain associated with breathlessness, palpitations and diaphoresis, with a background of significant cardiovascular risk factors. However, we must also be cognisant of atypical presentations of MI, which are more commonly observed in elderly, female and diabetic patients.[9]
Physical examination
Clinical examination comprises four main objectives. Firstly, haemodynamic assessment is required to identify patients who need urgent resuscitation, stabilisation and escalation of care. For example, the presence of hypotension in MI may be concerning for cardiogenic shock, whereas inter-arm differential systolic blood pressures >20 mmHg may be suggestive of aortic dissection. Secondly, a detailed cardiovascular examination is required to identify complications of myocardial ischaemia, such as acute decompensated heart failure, ventricular septal rupture, ischaemic mitral regurgitation and tachy/bradyarrhythmias. Thirdly, a targeted examination to exclude other differentials should be performed, such as respiratory examination for features of pneumonia/pneumothorax, abdominal examination for peritonism in perforated viscus and chest wall inspection/palpation for musculoskeletal pathologies. Finally, observe for features suggestive of atherosclerotic disease (e.g., Frank’s earlobe crease sign[10]), underlying cardiovascular risk factors (insulin injection marks, xanthelasma/tendon xanthoma, nicotine/tar stains) and microvascular/macrovascular end-organ complications (e.g., previous coronary artery bypass graft and vein harvesting scars, residual hemiplegia from previous stroke, stigmata of end-stage renal disease or presence of dialysis vascular access, signs of peripheral arterial disease, peripheral neuropathy).
INVESTIGATIONS FOR ACUTE CHEST PAIN
Broadly, investigations for ACP include ECG, biomarkers (cardiac enzymes and other laboratory tests) and imaging modalities.
Firstly, ECG is a standard point-of-care test in all patients with ACP. In patients with persistent symptoms or high clinical suspicion of ACS, serial ECG should be repeated to look for evolving ST–T wave changes of myocardial ischaemia. There are classical ECG features of STEMI [Figure S3A, Supplemental Digital Appendix] and STEMI equivalents, including left main coronary artery occlusion [Figure S3B, Supplemental Digital Appendix], Wellen’s syndrome (suggestive of critical proximal left anterior descending [LAD] coronary artery stenosis) [Figure S3C, Supplemental Digital Appendix] and de Winter T waves (suggestive of acute proximal LAD occlusion) [Figure S3D, Supplemental Digital Appendix]. A new-onset left bundle brunch block (LBBB) used to be considered a STEMI or occlusion MI equivalent; however, this finding should be interpreted in the context of clinical and biochemical findings to determine if there is concern of ongoing myocardial ischaemia that warrants urgent reperfusion therapy.[11] In cases of pre-existing LBBB, modified Sgarbossa’s criteria are used to identify STEMI.[12] New-onset right bundle brunch block in occlusion MI may also suggest an occlusion of a proximal septal perforating branch of LAD,[13] which is associated with a large infarct size, higher rates of heart failure, heart block and overall mortality.[14] On the other hand, ECG features of NSTEMI typically include ST depressions and/or T wave inversions [Figure S3E, Supplemental Digital Appendix]. Recently, the Aslanger pattern was described in 6.3% of NSTEMI patients, which portends an inferior occlusive MI, associated with larger infarct size, multivessel disease and higher mortality rates.[15]
Secondly, high-sensitivity cardiac troponins are gold standard biomarkers for diagnosing MI.[4] High-sensitivity troponin I has a higher diagnostic accuracy than troponin T, with sensitivity and specificity >90% when performed on admission.[16] In patients presenting <6 h from the onset of chest pain with a normal first set of high-sensitivity troponins, trending of a second set of troponins at the 3-h mark confers at least 98% negative predictive value for MI.[17] Nonetheless, serial troponin testing beyond the initial two sets may occasionally be helpful to assess for re-infarction in the presence of new/recurrent ischaemic chest pain and/or ECG changes[4] or for prognostication in acute myocarditis.[18]
Thirdly, imaging modalities often include chest radiographs (CXR), point-of-care ultrasound (POCUS) and formal transthoracic echocardiogram. The CXR is useful to assess for cardiopulmonary pathologies such as pulmonary oedema from acute decompensated heart failure, pneumonia or pneumothorax. The POCUS may identify territorial regional wall motion abnormalities suggestive of myocardial ischaemia or reduction in left ventricular ejection fraction,[19] left ventricular apical hypokinesia with basal sparing that may suggest Takotsubo cardiomyopathy in the appropriate context,[20] and look for cardiopulmonary features of aortic dissection,[19] pulmonary embolism, pneumothorax, pneumonia, pericardial and pleural effusion.[21] Advanced imaging modalities include anatomical and functional imaging studies for stable patients with intermediate pretest probability of coronary artery disease (CAD), as well as cardiac magnetic resonance imaging for diagnosis of myocarditis based on Lake Louise criteria.[22]
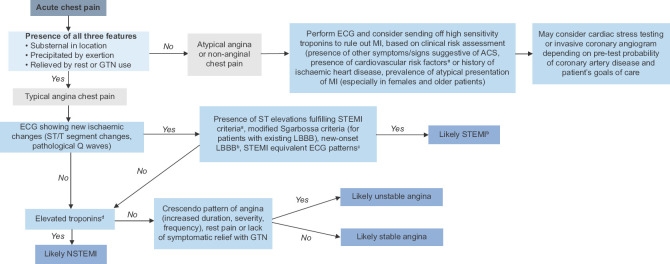
Finally, coronary angiography should be offered to all patients with ACS, in the absence of contraindications, but the timing of coronary evaluation and revascularisation depends on the type and risk stratification of MI. The overall approach to differentiating the causes of ACP is summarised in Table S3 [see Supplemental Digital Appendix], with a summarised algorithm presented in Figure 1.
Figure 1.
Simplified clinical algorithm for acute chest pain. aST segment elevation myocardial infarction (STEMI) electrocardiogram (ECG) criteria: new-onset ST segment elevation in ≥2 contiguous leads (with J-point elevation ≥2.5 mm in men aged <40 years, ≥2 mm in men aged ≥40 years and ≥1.5 mm in women for leads V2–3, and J-point elevation ≥1 mm in all other leads). bNew-onset left bundle brunch block (LBBB) used to be considered STEMI equivalent; however, it is now recognised that a presumed new LBBB should not be interpreted in isolation, but rather in the context of clinical findings and cardiac enzymes to determine its significance. cSTEMI equivalent ECG patterns: left main coronary artery occlusion, Wellen’s syndrome, de Winter’s T waves. dElevated troponins in the context of myocardial ischaemia is defined as ≥20% rise and/or fall in serially trended troponins with at least one value >99th percentile of the upper reference limit. eCardiovascular risk factors: smoking, obesity, diabetes mellitus, hypertension, hyperlipidaemia, family history of premature coronary artery disease (first-degree relative; for men aged <55 years, for women aged <65 years) ACS: acute coronary syndrome, GTN: glyceryl trinitrate, MI: myocardial infarction, NSTEMI: non-ST segment elevation myocardial infarction
DIAGNOSTIC EVALUATION OF CORONARY ARTERY DISEASE
Diagnostic workup for CAD may be performed through invasive or non-invasive coronary evaluation, anatomical or functional imaging, and stress or non-stress testing modalities, depending on the pretest probability of CAD.[2] This is because in low pretest probability cases, an abnormal test is more likely to be a false positive, whereas in high pretest probability situations, a normal test is more likely to be a false negative.[23] Hence, patients with low pretest probability of CAD should avoid cardiac testing, while patients with intermediate pretest probability of CAD may undergo cardiac stress testing (e.g., exercise ECG, stress myocardial perfusion imaging, stress echocardiogram, stress cardiac magnetic resonance imaging) or non-stress cardiac imaging (e.g., computed tomography coronary angiogram), and patients with high pretest likelihood of CAD should directly undergo diagnostic coronary angiogram to detect and quantify the severity of coronary athero-occlusive disease.
Several predictive models exist for risk stratification of patients with suspected CAD, including the updated Diamond–Forrester (UDF) classification,[24] CAD consortium 2[25] and CONFIRM registry scores.[26] In a multicentre study of the utility of the above risk scores in predicting obstructive CAD in patients with suspected CAD detected on computed tomography coronary angiogram, the CAD consortium 2 score had the best discrimination with area under the receiver-operating curve and ability to reclassify low-risk patients at 10% probability threshold.[27] Locally, a novel PRECISE risk score has recently been developed and validated for use in Southeast Asian patient cohorts.[28]
Importantly, cardiac stress testing should be avoided in haemodynamically unstable patients, within 48 h post-MI, in high-risk UA and in the presence of significant cardiac arrhythmias.[2,29] Moreover, decision to work up suspected CAD should take into consideration the patient’s goals of care, benefits and risks of testing, and how the diagnostic findings will change the overall management.
RISK STRATIFICATION OF ACUTE CHEST PAIN
The History, ECG, Age, Risk factors and Troponin (HEART) score[30] is a practical tool in ED settings for risk stratification of patients presenting with ACP into low-risk (0–3), moderate-risk (4–6) and high-risk (7–9) categories to guide disposition and subsequent management of ACP and ACS.
In cases of proven ACS (UA, NSTEMI, STEMI), the Thrombolysis in MI[31,32] and Global Registry of Acute Coronary Events (GRACE)[33] scores scores are useful for predicting clinical outcomes. In particular, a high GRACE score >140 portends poorer prognosis and is considered a high-risk feature in NSTEMI that warrants early intervention within 24 h.[17]
APPROACH TO HYPERTROPONINAEMIA
There are many causes of hypertroponinaemia [Table S4, Supplemental Digital Appendix], as serum troponins can be elevated in any condition that leads to myocardial injury/necrosis, from acute (e.g., sepsis, acute kidney injury, myocarditis) and chronic (e.g., chronic heart failure with elevated left ventricular end-diastolic pressure, chronic kidney disease) myocardial injury to full-blown MI (e.g., T1MI or T2MI).
For simplicity, a ‘three-step approach’ to hypertroponinaemia can be adopted [Figure 2]:
Figure 2.
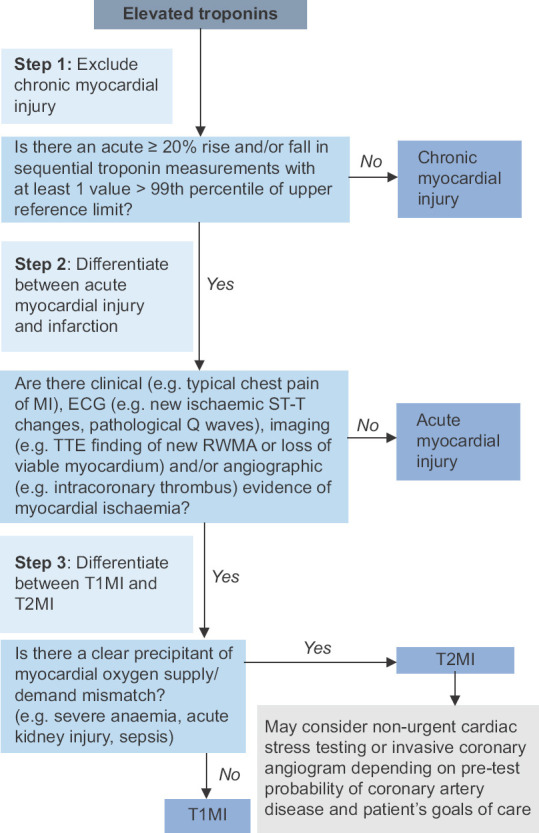
Chart shows a ‘three-step’ approach to hypertroponinaemia. T1MI: type 1 myocardial infarction, T2MI: type 2 myocardial infarction, TTE: transthoracic echocardiogram
Exclude chronic myocardial injury (in the appropriate clinical context, e.g., chronic structural heart disease, chronic kidney disease) by the absence of an acute >20% rise and/or fall in troponin levels on serial measurements.
Differentiate between acute myocardial injury and acute MI by looking for symptoms/ECG/radiological/angiographic changes suggestive of myocardial ischaemia.
In patients with acute MI, differentiate between T1MI and T2MI by looking for clinical precipitants of myocardial oxygen supply/demand mismatch (e.g., severe anaemia, sepsis, acute kidney injury).
In practice, differentiating between T1MI and T2MI is often challenging. In general, T2MI patients are commonly older,[34] female[34,35] and have multiple comorbidities (e.g., renal insufficiency),[34,35,36] whereas T1MI patients are more likely to have prior MI[35] or revascularisation.[34,35] However, both the absolute cardiac troponin levels and percentage change over time are not useful to discriminate between T1MI and T2MI.[37] Coronary angiography is useful to detect coronary atherothrombotic disease with plaque rupture if T1MI is suspected.[36]
In MI cases with clear precipitants of myocardial oxygen supply/demand mismatch and a low pretest probability of T1MI, it is reasonable to treat the underlying clinical event first without pursuing urgent coronary evaluation. Nonetheless, in patients with significant cardiovascular risk factors or known CAD, a low threshold for coronary evaluation is often required, especially if patients develop high-risk features such as recurrent/persistent chest pain, haemodynamic or electrical instability or dynamic ECG changes suggestive of myocardial ischaemia.
In hospitalised patients, common events precipitating myocardial ischaemic imbalance include anaemia, sepsis, renal impairment, cardiac arrhythmias and postoperative state.[36] For example, in severe anaemia, there is reduced oxygen-carrying capacity and myocardial oxygen delivery, while the concomitant hyperdynamic circulation increases myocardial oxygen demand. In sepsis, MI may be inflicted through infective, cytokine and catecholamine-mediated mechanisms.[38] In renal impairment, hypertroponinaemia may be related to clinically silent micro-infarctions or associated left ventricular hypertrophy.[39]
Finally, in unexplained cases of elevated troponins without evidence suggestive of myocardial injury/ischaemia, there is an entity of spurious hypertroponinaemia caused by laboratory assay interference, for instance, due to the presence of heterophile antibodies that may be acquired from iatrogenic (e.g., blood products, monoclonal antibody therapies, vaccinations) and non-iatrogenic (e.g., rheumatoid factor in rheumatoid arthritis and other autoimmune conditions) sources.[40] The possibility of heterophile antibody interference with laboratory troponin assay may be investigated through repeat testing on different analyser platforms or by adding heterophile-blocking reagents.[40]
MANAGEMENT OF ACUTE MYOCARDIAL INFARCTION
In general, management of MI depends on the aetiological subtype (T1MI vs. T2MI) and extent/severity of MI (STEMI vs. NSTEMI).
The management of T1MI (atherothrombotic MI) comprises the following five key components, with differences in the choice of antithrombotic agents and timing of revascularisation depending on the extent of ischaemia (STEMI vs. NSTEMI):
Acute resuscitation and stabilisation: airway, breathing, circulation for the haemodynamically unstable patient, advanced cardiac life support in cardiac arrests, ‘cath lab activation’ for confirmed STEMI and high-risk NSTEMI cases;
Revascularisation therapy: target immediate percutaneous coronary intervention (PCI) (‘door-to-balloon time’) within 90 min for STEMI; target PCI within 72 h for NSTEMI in the absence of high-risk features that may warrant earlier or immediate intervention (e.g., recurrent or persistent chest pain, haemodynamic or electrical instability or presence of dynamic ECG ST–T wave changes suggestive of myocardial ischaemia);[41] in 2018, the TRANSIENT trial studied the entity of ‘transient STEMI’ (i.e., attaining ST segment normalisation and symptomatic relief before definitive revascularisation) and found that these patients generally had small infarct sizes and had no significant differences in major adverse cardiac events between adopting an immediate (as for STEMI) versus delayed (as for NSTEMI) reperfusion strategy;[42]
Symptomatic treatment: sublingual GTN (500 mcg pro re nata, can be repeated 5 min apart) or intravenous GTN (initiate at 15–20 mcg/min, then uptitrate by 10–15 mcg/min at 15–30 min intervals to the desired effect) for symptomatic relief of anginal chest pain;
Guideline-directed medical therapy: (a) dual antiplatelet therapy (DAPT) with aspirin (300 mg loading dose, followed by 100 mg OM maintenance dose) and either clopidogrel (300/600 mg loading dose, followed by 75 mg OM maintenance dose), ticagrelor (180 mg loading dose, followed by 90 mg BD maintenance dose) or prasugrel (60 mg loading dose, followed by 10 mg OM maintenance dose); (b) heparin: consider subcutaneous low-molecular-weight heparin for NSTEMI planned for early PCI (e.g., subcutaneous enoxaparin 1 mg/kg BD, with dose reduction to 1 mg/kg OD if estimated glomerular filtration rate [eGFR] 15–29 mL/min and contraindicated if eGFR <15 mL/min), or intracoronary heparin during PCI; (c) beta-blockers (e.g., carvedilol, metoprolol and bisoprolol); (d) angiotensin converting enzyme inhibitors (ACE-I; e.g., captopril, enalapril and lisinopril); and (e) statins (e.g., atorvastatin);
Post-MI cardiac rehabilitation, preventive health (vaccinations), lifestyle modifications, optimisation of cardiovascular risk factors and longitudinal follow-up.
For T2MI, guideline-directed medical treatment remains fairly scarce. In the presence of clear clinical precipitants of myocardial ischaemic imbalance, management largely involves treating the underlying cause, with the potential role of beta-blockers to reduce myocardial oxygen demand, provided there are no contraindications such as hypotension, bradycardia or acute heart failure.[36] Depending on the patient’s pretest probability of IHD and goals of care, non-urgent cardiac evaluation for underlying CAD may be considered.[36]
TAKE HOME MESSAGES
Acute chest pain can be caused by cardiac and non-cardiac (respiratory, mediastinal, gastrointestinal, musculoskeletal and others, e.g., psychogenic) pathologies.
Approach to chest pain involves clinical characterisation of the chest pain with its associated features and risk factor assessment, with appropriate use of investigations including ECG, cardiac biomarkers and radiological investigations.
Myocardial injury refers to isolated hypertroponinaemia, whereas MI requires clinical, electrocardiographic, imaging or angiographic evidence of acute myocardial ischaemia.
T1MI is caused by acute atherothrombosis in the presence of underlying atherosclerotic coronary disease, whereas T2MI is precipitated by clinical insults that lead to significant myocardial oxygen supply/demand mismatch.
Treatment of MI comprises five key components of acute resuscitation/stabilisation, revascularisation therapy, symptomatic treatment, guideline-directed medical therapy, and post-ACS rehabilitation, follow-up and optimisation of cardiovascular risk factors.
Closing Vignette
The patient was commenced on DAPT (aspirin and ticagrelor) and high-intensity statin. She was also given sublingual GTN with prompt symptomatic relief of chest pain. The following morning, the patient underwent coronary angiogram, which revealed 80% proximal LAD stenosis, and revascularisation was performed with implantation of a drug-eluting stent. Echocardiogram revealed mid-range left ventricular ejection fraction of 45% with regional wall motion abnormalities in the LAD territory. Postprocedure, she was also commenced on other guideline-directed medical therapies including ACE-I and beta-blockers. On discharge, she was given a cardiac rehabilitation appointment, with follow-up at the cardiology clinic.
Financial support and sponsorship
Nil.
Conflicts of interest
See KC is a member of the SMJ Editorial Board and was thus not involved in the peer review and publication decisions of this article.
Supplemental digital content
Appendix at http://links.lww.com/SGMJ/A84
SMC CATEGORY 3B CME PROGRAMME
Online Quiz: https://www.sma.org.sg/cme-programme
Deadline for submission: 6 pm, 15 March 2024
| Question | True | False |
|---|---|---|
| 1. Typical anginal chest pain is classically described as left-sided chest pain that is worse on inspiration or chest wall movements and relieved by analgesia. | ||
|
| ||
| 2. In patients with known history of anginal chest pain, the presence of unstable angina may be characterised by chest pain of increasing frequency, duration and severity, or pain that occurs at rest or does not improve with glyceryl trinitrate (GTN) use. | ||
|
| ||
| 3. Acute coronary syndrome (ACS) is a specific form of ischaemic heart disease that comprises only non-ST segment elevation myocardial infarction (NSTEMI) and ST segment elevation myocardial infarction (STEMI). | ||
|
| ||
| 4. Hypertroponinaemia may be present in both myocardial injury and myocardial infarction (MI). | ||
|
| ||
| 5. The presence of elevated troponins in a patient with end-stage renal failure is always suggestive of an acute cardiac event such as acute MI or acute myocardial injury. | ||
|
| ||
| 6. Approximately 30% of undifferentiated patients who present to the emergency department with acute chest pain (ACP) are found to have ACS. | ||
|
| ||
| 7. Atypical presentations of MI are more commonly observed in females and elderly patients as compared to males and younger patients. | ||
|
| ||
| 8. It is not relevant to measure systolic blood pressure differentials of the two limbs when examining a patient with sudden-onset tearing chest pain that radiates to the back. | ||
|
| ||
| 9. Commonly performed initial investigations in a patient with ACP include electrocardiogram (ECG), cardiac biomarkers and imaging modalities like chest radiograph. | ||
|
| ||
| 10. The presence of a single, elevated troponin value >99th percentile of the upper reference limit is sufficient to diagnose NSTEMI. | ||
|
| ||
| 11. Wellen’s syndrome refers to specific ECG changes that are suggestive of significant right coronary artery stenosis. | ||
|
| ||
| 12. The presence of a new-onset left bundle brunch block on ECG is always equivalent to a STEMI and warrants immediate ‘cath lab activation’ for revascularisation therapy. | ||
|
| ||
| 13. In a patient with ACP, assessing the pretest probability of coronary artery disease can be useful to guide subsequent cardiac diagnostic work up. | ||
|
| ||
| 14. Cardiac stress testing, such as stress myocardial perfusion imaging, should always be performed in a patient with newly diagnosed MI. | ||
|
| ||
| 15. In rare instances, laboratory assay interference may lead to falsely elevated troponin levels. | ||
|
| ||
| 16. In the acute inpatient setting, common clinical precipitants of type 2 MI (T2MI) include anaemia, sepsis, renal impairment, cardiac arrhythmias and postoperative state. | ||
|
| ||
| 17. The presence of heterophile antibodies in the patient’s serum may cause troponin assay interference, leading to false-positive results. | ||
|
| ||
| 18. Management of STEMI involves emergency percutaneous coronary revascularisation and initiation of guideline-directed medical therapy including dual antiplatelet therapy (DAPT), heparin, beta-blocker, angiotension-converting enzyme inhibitors/angiotensin receptor blockers and statin. | ||
|
| ||
| 19. The target ‘door-to-balloon’ time for STEMI is 120 min. | ||
|
| ||
| 20. Treatment of T2MI involves prompt initiation of DAPT (loading and maintenance dose), that usually comprises aspirin with either clopidogrel, ticagrelor or prasugrel. | ||
APPENDIX
Table S1.
Classification of Myocardial Infarction (MI)
a) 4th Universal Definition of MI: aetiological classification (1)
| Category | Description | MI criteria | Treatment | |
|---|---|---|---|---|
|
| ||||
| Troponin criteria | Supporting Evidence of Myocardial Ischaemia | |||
| Type 1 MI | Spontaneous MI due to primary coronary occlusion from acute plaque rupture, erosion causing coronary artery thrombosis | > 20% rise and/or fall of troponin levels with at least one value > 99th percentile of upper reference limit | Clinical symptoms of myocardial ischaemia; new ischaemic ECG changes; imaging evidence of new regional wall motion abnormalities or new loss of viable myocardium or angiographic finding of intra-coronary thrombus | Coronary revascularisation, guideline-directed medical therapy (DAPT +/- heparin, ACEIs, BBs, statins) |
| Type 2 MI | Secondary to a clinical event that causes myocardial oxygen supply/demand mismatch (e.g. anaemia, hypotension/hypertension, cardiac arrhythmias, sepsis) | > 20% rise and/or fall of troponin levels with at least one value > 99th percentile of upper reference limit | Clinical symptoms of myocardial ischaemia; new ischaemic ECG changes; imaging evidence of new regional wall motion abnormalities or new loss of viable myocardium | Treat underlying cause of myocardial ischaemic imbalance, consider judicious use of BBs to reduce myocardial oxygen demand |
| Type 3 MI | Sudden cardiac death (e.g. cardiac arrest) based on clinical symptomatology, electrocardiographic findings or presence of new coronary artery thrombus identified by angiography or at postmortem examination, without the availability of cardiac biomarkers | Not applicable | Clinical symptoms of myocardial ischaemia; new ischaemic ECG changes or presence of ventricular fibrillation; postmortem analyses revealing intra-coronary thrombus | Not applicable |
| Type 4 MI | 4a - MI occurring within 48 hours of PCI | > 20% rise in post- procedural troponins to at least 5x upper reference limit | New ischaemic ECG changes; imaging evidence of new regional wall motion abnormalities or new loss of viable myocardium; angiographic finding of post-procedural flow-limiting complication (coronary thromboembolism, dissection) | Same as for type 1 MI |
| 4b - MI due to coronary in-stent thrombosis (acute -> 0 to 24 hours, subacute - >24 hours to 30 days, late - >30 days to 1 year, very late - more than 1 year) | Same as for type 1 MI | Same as for type 1 MI | Same as for type 1 MI | |
| 4c - MI due to coronary in- stent re-stenosis | Same as for type 1 MI | Same as for type 1 MI | Same as for type 1 MI | |
| Type 5 MI | MI after CABG surgery | > 20% rise in post- procedural troponins to at least 10x upper reference | New ischaemic ECG changes; imaging evidence of new regional wall motion abnormalities; angiographic finding of either graft vessel or native coronary artery occlusion | Same as for type 1 MI |
*ACEIs: ACE inhibitors; BBs: beta blockers; CABG: coronary artery bypass graft; DAPT: dual antiplatelet therapy; MI: myocardial infarction; PCI: percutaneous coronary intervention
b) STEMI vs NSTEMI:
| Category | ECG criteria | Extent of Myocardial Ischaemia | Prognosis |
|---|---|---|---|
| STEMI | New onset ST segment elevation in > 2 contiguous leads (with J-point elevation > 2.5 mm in males < 40 years, > 2 mm in males > 40 years and > 1.5 mm in females for leads V2-3, and J-point elevation > 1 mm in all other leads) (1); or Modified Sgarbossa criteria in patients with existing left bundle brunch block (LBBB) (2); or New-onset LBBB in the appropriate clinical context and biochemical profile | Transmural ischaemia | Poorer short-term outcome and higher inpatient mortality risk |
| NSTEMI | New onset ST segment depression > 0.5 mm in > 2 contiguous leads; and/or New onset T wave inversion > 1 mm in > 2 contiguous leads that have prominent R wave or R/S ratio > 1 (1) | Subendocardial ischaemia | Poorer long-term outcome (possibly contributed by poorer pre-morbid conditions, less likely to be prescribed guideline-directed medical therapy) |
*ECG: electrocardiogram; NSTEMI: non-ST segment elevation myocardial infarction; STEMI: ST segment elevation myocardia infarction
Table S2.
Cardiac and Non-Cardiac Causes of Acute Chest Pain
| Cardiac | |
|---|---|
| Pericardium | Acute pericarditis |
| Myocardium |
Acute coronary syndrome (UA, NSTEMI, STEMI) Stable angina/chronic ischemic heart disease Vasospastic (Prinzmetal’s) angina Coronary microvascular dysfunction Takotsubo (stress) cardiomyopathy Hypertrophic cardiomyopathy Acute myocarditis |
| Valvular heart disease | Aortic stenosis Mitral valve prolapse |
| Non-Cardiac | |
| Respiratory |
Pneumonia Pleuritis Pulmonary embolism Pneumothorax Pulmonary hypertension Asthma/chronic obstructive pulmonary disease (COPD) exacerbation Acute bronchitis Lung cancer Acute chest syndrome (in patients with sickle cell anaemia) |
| Mediastinum | Aortic dissection/aortic aneurysm Mediastinitis Pneumomediastinum Mediastinal masses |
| Gastrointestinal |
Gastroesophageal reflux disease (GERD)/reflux esophagitis Oesophageal motility disorders Boerhaave’s syndrome Peptic ulcer disease Gallstone disease Acute pancreatitis Perforated viscus |
| Musculoskeletal and Soft Tissue Pathologies |
Muscle sprain Rib fractures/trauma Costochondritis (Tietze’s syndrome) Cervical/thoracic spine pathologies (referred pain) Herpes zoster (Shingles) Intercostal neuralgia (post-thoracotomy pain syndrome, post-herpetic neuralgia) Xiphoidalgia |
| Others | Psychogenic (panic attacks, anxiety, somatization disorder) |
Bold: common causes, highlighted: life-threatening causes *NSTEMI: non-ST segment elevation myocardial infarction, STEMI - ST segment elevation myocardial infarction, UA - unstable angina
Figure S3.
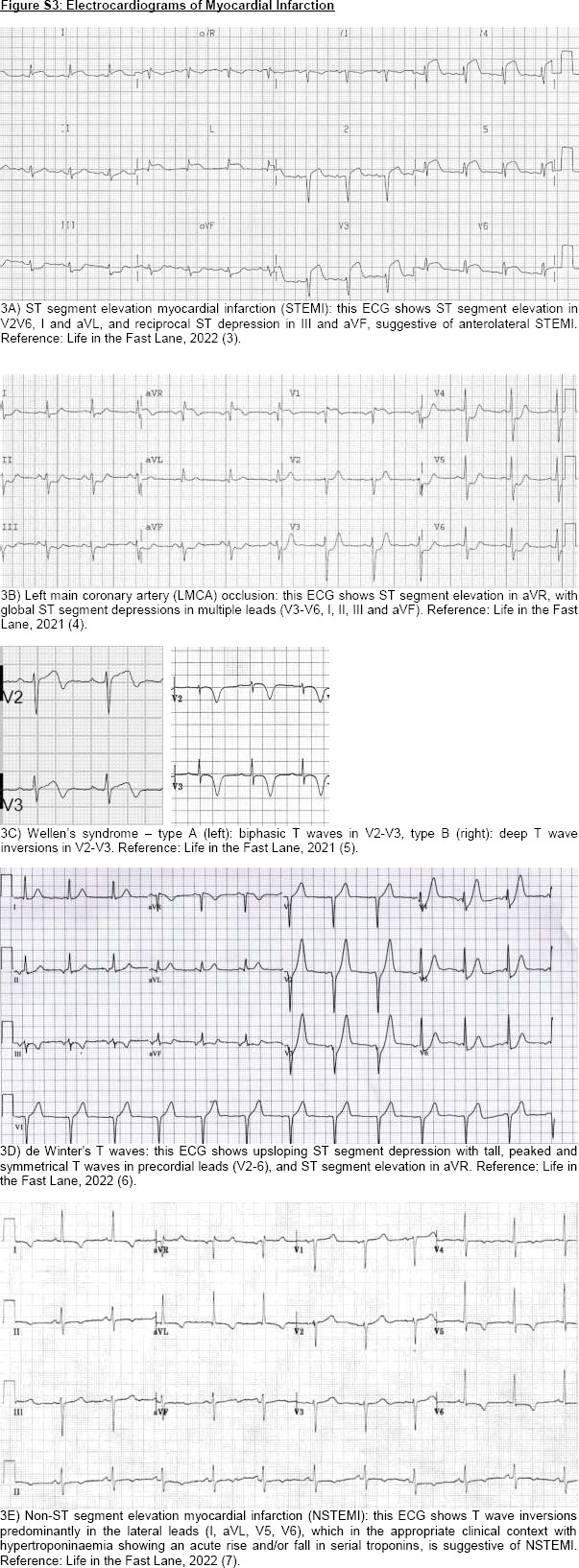
Electrocardiograms of Myocardial Infarction
Table S3.
Clinical, Biochemical, Electrophysiological and Imaging Features to Differentiate Amongst the Common Causes of Acute Chest Pain
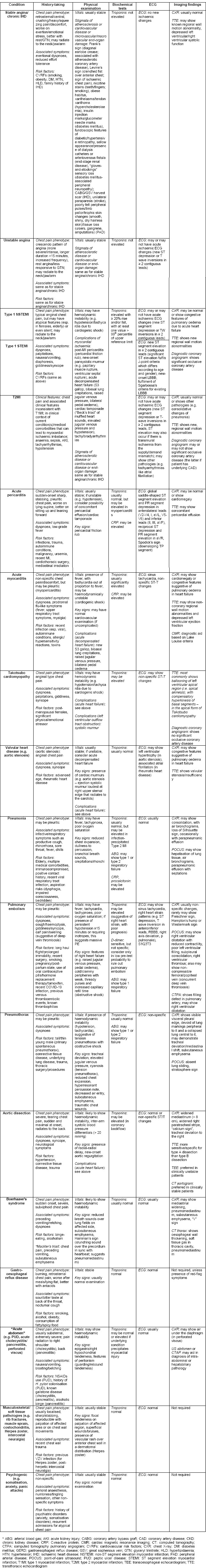
Table S4.
Causes of Hypertroponinaemia
| Causes | |
|---|---|
| Cardiac (coronary artery) | Myocardial infarction |
| Coronary artery vasospasm | |
| Coronary artery dissection | |
| Aortic dissection with coronary extension | |
| Post-percutaneous coronary intervention | |
| Cardiac (noncoronary artery) | Tachyarrhythmias (e.g. atrial fibrillation, supraventricular tachycardia) |
| Myocarditis/myopericarditis | |
| Cardiomyopathies | |
| Infective endocarditis | |
| Infiltrative cardiac disease (e.g. amyloidosis, sarcoidosis) Heart failure (acute, chronic) | |
| Hypertensive crises | |
| Chest trauma/cardiac contusions | |
| Cardiac procedures (e.g. CPR, defibrillation, transvenous pacing, ablation procedures, cardiothoracic surgery) | |
| Cardiotoxins (e.g. anthracyclines, herceptin, recreational drugs such as cocaine) | |
| Non-cardiac | Anaemia |
| Sepsis | |
| VJCUOIO Critical illness | |
| Viral infection (e.g. influenza infection) | |
| Pulmonary embolism | |
| Pulmonary hypertension | |
| Acute respiratory distress syndrome | |
| Chronic obstructive pulmonary disease (COPD) exacerbation Renal impairment (acute, chronic) | |
| Stroke | |
| Subarachnoid haemorrhage | |
| Seizures | |
| Strenuous exercise | |
| Rhabdomyolysis | |
| Burns | |
| False positives | Heterophile antibodies |
| Positive rheumatoid factor | |
| Elevated alkaline phosphatase | |
| Presence of fibrin clots |
References
Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, et al. Fourth Universal Definition of Myocardial Infarction (2018). J Am Coll Cardiol. 2018;72(18):2231–64.
Smith SW, Dodd KW, Henry TD, Dvorak DM, Pearce LA. Diagnosis of ST-elevation myocardial infarction in the presence of left bundle branch block with the ST-elevation to S-wave ratio in a modified sgarbossa rule. Ann Emerg Med. 2012;60(6):766–76.
Burns E, Buttner R. Anterior Myocardial Infarction [Internet]. Life in the Fast Lane. 2022 [cited 2023 Mar 21]. Available from: https://litfl.com/anterior-myocardial-infarction-ecg-library/
Buttner R, Burns E. ST Elevation in aVR [Internet]. Life in the Fast Lane. 2021 [cited 2023 Mar 21]. Available from: https://litfl.com/st-elevation-in-avr/
Cadogan M, Buttner R. Wellens Syndrome [Internet]. Life in the Fast Lane. 2021 [cited 2023 Mar 21]. Available from: https://litfl.com/wellens-syndrome-ecg-library/
Buttner R, Burns E. De Winter T Wave [Internet]. Life in the Fast Lane. 2022 [cited 2023 Mar 21]. Available from: https://litfl.com/de-winter-t-wave/
Burns E, Cadogan M. Myocardial Ischaemia [Internet]. Life in the Fast Lane. 2022 [cited 2023 Mar 21]. Available from: https://litfl.com/myocardial-ischaemia-ecg-library/
REFERENCES
- 1.Diamond GA. A clinically relevant classification of chest discomfort. J Am Coll Cardiol. 1983;1:574–5. doi: 10.1016/s0735-1097(83)80093-x. [DOI] [PubMed] [Google Scholar]
- 2.Gulati M, Levy PD, Mukherjee D, Amsterdam E, Bhatt DL, Birtcher KK, et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR Guideline for the evaluation and diagnosis of chest pain: A report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021;144:e368–454. doi: 10.1161/CIR.0000000000001029. [DOI] [PubMed] [Google Scholar]
- 3.Devon HA, Mirzaei S, Zègre-Hemsey J. Typical and atypical symptoms of acute coronary syndrome: Time to retire the terms? J Am Heart Assoc. 2020;9:1–4. doi: 10.1161/JAHA.119.015539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, et al. Fourth universal definition of myocardial infarction (|y2018) J Am Coll Cardiol. 2018;72:2231–64. doi: 10.1016/j.jacc.2018.08.1038. [DOI] [PubMed] [Google Scholar]
- 5.Hsia RY, Hale Z, Tabas JA. A national study of the prevalence of life-threatening diagnoses in patients with chest pain. JAMA Intern Med. 2016;176:1029–32. doi: 10.1001/jamainternmed.2016.2498. [DOI] [PubMed] [Google Scholar]
- 6.Bjørnsen LP, Naess-Pleym LE, Dale J, Grenne B, Wiseth R. Description of chest pain patients in a Norwegian emergency department. Scand Cardiovasc J. 2019;53:28–34. doi: 10.1080/14017431.2019.1583362. [DOI] [PubMed] [Google Scholar]
- 7.Mokhtari A, Dryver E, Söderholm M, Ekelund U. Diagnostic values of chest pain history, ECG, troponin and clinical gestalt in patients with chest pain and potential acute coronary syndrome assessed in the emergency department. Springerplus. 2015;4:219. doi: 10.1186/s40064-015-0992-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clayton H, Reschak G, Gaynor S, Creamer J. A novel program to assess and manage pain. Medsurg Nurs. 2000;9:318–21. [PubMed] [Google Scholar]
- 9.Čulić V, Eterović D, Mirić D, Silić N. Symptom presentation of acute myocardial infarction: Influence of sex, age, and risk factors. Am Heart J. 2002;144:1012–7. doi: 10.1067/mhj.2002.125625. [DOI] [PubMed] [Google Scholar]
- 10.Frank S. Aural sign of coronary-artery disease. N Engl J Med. 1973;289:327–8. doi: 10.1056/nejm197308092890622. [DOI] [PubMed] [Google Scholar]
- 11.Birnbaum Y, Ye Y, Smith SW, Jneid H. Rapid diagnosis of stemi equivalent in patients with left bundle-branch block: Is it feasible? J Am Heart Assoc. 2021;10:1–4. doi: 10.1161/JAHA.121.023275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith SW, Dodd KW, Henry TD, Dvorak DM, Pearce LA. Diagnosis of ST-elevation myocardial infarction in the presence of left bundle branch block with the ST-elevation to S-wave ratio in a modified sgarbossa rule. Ann Emerg Med. 2012;60:766–76. doi: 10.1016/j.annemergmed.2012.07.119. [DOI] [PubMed] [Google Scholar]
- 13.Strauss DG, Loring Z, Selvester RH, Gerstenblith G, Tomaselli G, Weiss RG, et al. Right, but not left, bundle branch block is associated with large anteroseptal scar. J Am Coll Cardiol. 2013;62:959–67. doi: 10.1016/j.jacc.2013.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang J, Luo H, Kong C, Dong S, Li J, Yu H, et al. Prognostic value of new-onset right bundle-branch block in acute myocardial infarction patients: A systematic review and meta-analysis. Peer J. 2018;2018:1–16. doi: 10.7717/peerj.4497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aslanger E, Yıldırımtürk O, Şimşek B, Sungur A, Cabbar AT, Bozbeyoğlu E, et al. A new electrocardiographic pattern indicating inferior myocardial infarction. J Electrocardiol. 2020;61:41–6. doi: 10.1016/j.jelectrocard.2020.04.008. [DOI] [PubMed] [Google Scholar]
- 16.Keller T, Zeller T, Peetz D, Tzikas S, Roth A, Czyz E, et al. Sensitive troponin I assay in early diagnosis of acute myocardial infarction. N Engl J Med. 2009;361:868–77. doi: 10.1056/NEJMoa0903515. [DOI] [PubMed] [Google Scholar]
- 17.Roffi M, Patrono C, Collet J-P, Mueller C, Valgimigli M, Andreotti F, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2016;37:267–315. doi: 10.1093/eurheartj/ehv408. [DOI] [PubMed] [Google Scholar]
- 18.Liu C, Wang Z, Chen K, Cui G, Chen C, Wang L, et al. The absolute and relative changes in high-sensitivity cardiac troponin I are associated with the in-hospital mortality of patients with fulminant myocarditis. BMC Cardiovasc Disord. 2021;21:1–11. doi: 10.1186/s12872-021-02386-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nishigami K. Point-of-care echocardiography for aortic dissection, pulmonary embolism and acute coronary syndrome in patients with killer chest pain: EASY screening focused on the assessment of effusion, aorta, ventricular size and shape and ventricular asynergy. J Echocardiogr. 2015;13:141–4. doi: 10.1007/s12574-015-0265-1. [DOI] [PubMed] [Google Scholar]
- 20.López Libano J, Alomar LladóL, Zarraga López L. The Takotsubo syndrome: Clinical diagnosis using POCUS. POCUS J. 2022;7:137–9. doi: 10.24908/pocus.v7i1.15296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zanobetti M, Scorpiniti M, Gigli C, Nazerian P, Vanni S, Innocenti F, et al. Point-of-care ultrasonography for evaluation of acute dyspnea in the ED. Chest. 2017;151:1295–301. doi: 10.1016/j.chest.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 22.Ferreira VM, Schulz-Menger J, Holmvang G, Kramer CM, Carbone I, Sechtem U, et al. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: Expert recommendations. J Am Coll Cardiol. 2018;72:3158–76. doi: 10.1016/j.jacc.2018.09.072. [DOI] [PubMed] [Google Scholar]
- 23.Graham IM. Diagnosing coronary artery disease-the diamond and forrester model revisited. Eur Heart J. 2011;32:1311–2. doi: 10.1093/eurheartj/ehr015. [DOI] [PubMed] [Google Scholar]
- 24.Genders TSS, Steyerberg EW, Alkadhi H, Leschka S, Desbiolles L, Nieman K, et al. A clinical prediction rule for the diagnosis of coronary artery disease: Validation, updating, and extension. Eur Heart J. 2011;32:1316–30. doi: 10.1093/eurheartj/ehr014. [DOI] [PubMed] [Google Scholar]
- 25.Genders TSS, Steyerberg EW, Hunink MGM, Nieman K, Galema TW, Mollet NR, et al. Prediction model to estimate presence of coronary artery disease: Retrospective pooled analysis of existing cohorts. BMJ. 2012;344:1–13. doi: 10.1136/bmj.e3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Min JK, Dunning A, Gransar H, Achenbach S, Lin FY, Al-Mallah M, et al. Medical history for prognostic risk assessment and diagnosis of stable patients with suspected coronary artery disease. Am J Med. 2015;128:871–8. doi: 10.1016/j.amjmed.2014.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baskaran L, Danad I, Gransar H, ÓHartaigh B, Schulman-Marcus J, Lin FY, et al. A comparison of the updated Diamond-Forrester, CAD consortium, and CONFIRM history-based risk scores for predicting obstructive coronary artery disease in patients with stable chest pain: The SCOT-HEART coronary CTA cohort. JACC Cardiovasc Imaging. 2019;12:1392–400. doi: 10.1016/j.jcmg.2018.02.020. [DOI] [PubMed] [Google Scholar]
- 28.Wang ZS, Yap J, Koh YLE, Chia SY, Nivedita N, Ang TWA, et al. Predicting coronary artery disease in primary care: Development and validation of a diagnostic risk score for major ethnic groups in Southeast Asia. J Gen Intern Med. 2021;36:1514–24. doi: 10.1007/s11606-021-06701-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Henzlova MJ, Duvall WL, Einstein AJ, Travin MI, Verberne HJ. ASNC imaging guidelines for SPECT nuclear cardiology procedures: Stress, protocols, and tracers. J Nucl Cardiol. 2016;23:606–39. doi: 10.1007/s12350-015-0387-x. [DOI] [PubMed] [Google Scholar]
- 30.Six AJ, Backus BE, Kelder JC. Chest pain in the emergency room: Value of the HEART score. Netherlands Hear J. 2008;16:191–6. doi: 10.1007/BF03086144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Antman EM, Cohen M, Bernink PJLM, McCabe CH, Horacek T, Papuchis G, et al. The TIMI risk score for unstable angina/non–ST elevation MI. JAMA. 2000;284:835. doi: 10.1001/jama.284.7.835. [DOI] [PubMed] [Google Scholar]
- 32.Morrow DA, Antman EM, Charlesworth A, Cairns R, Murphy SA, De Lemos JA, et al. TIMI risk score for ST-elevation myocardial infarction: A convenient, bedside, clinical score for risk assessment at presentation: An intravenous nPA for treatment of infarcting myocardium early II trial substudy. Circulation. 2000;102:2031–7. doi: 10.1161/01.cir.102.17.2031. [DOI] [PubMed] [Google Scholar]
- 33.Wenzl FA, Kraler S, Ambler G, Weston C, Herzog SA, Räber L, et al. Sex-specific evaluation and redevelopment of the GRACE score in non-ST-segment elevation acute coronary syndromes in populations from the UK and Switzerland: A multinational analysis with external cohort validation. Lancet. 2022;400:744–56. doi: 10.1016/S0140-6736(22)01483-0. [DOI] [PubMed] [Google Scholar]
- 34.Raphael CE, Roger VL, Sandoval Y, Singh M, Bell M, Lerman A, et al. Incidence, trends, and outcomes of type 2 myocardial infarction in a community cohort. Circulation. 2020;141:454–63. doi: 10.1161/CIRCULATIONAHA.119.043100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sandoval Y, Thordsen SE, Smith SW, Schulz KM, Murakami Maryann M, Pearce LA, et al. Cardiac troponin changes to distinguish type 1 and type 2 myocardial infarction and 180-day mortality risk. Eur Hear J Acute Cardiovasc Care. 2014;3:317–25. doi: 10.1177/2048872614538411. [DOI] [PubMed] [Google Scholar]
- 36.DeFilippis AP, Chapman AR, Mills NL, De Lemos JA, Arbab-Zadeh A, Newby LK, et al. Assessment and treatment of patients with type 2 myocardial infarction and acute nonischemic myocardial injury. Circulation. 2019;140:1661–78. doi: 10.1161/CIRCULATIONAHA.119.040631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wereski R, Kimenai DM, Taggart C, Doudesis D, Lee KK, Lowry MTH, et al. Cardiac troponin thresholds and kinetics to differentiate myocardial injury and myocardial infarction. Circulation. 2021;144:528–38. doi: 10.1161/CIRCULATIONAHA.121.054302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Drosatos K, Lymperopoulos A, Kennel PJ, Pollak N, Schulze PC, Goldberg IJ. Pathophysiology of sepsis-related cardiac dysfunction: Driven by inflammation, energy mismanagement, or both? Curr Heart Fail Rep. 2015;12:130–40. doi: 10.1007/s11897-014-0247-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Freda BJ, Tang WHW, Van Lente F, Peacock WF, Francis GS. Cardiac troponins in renal insufficiency: Review and clinical implications. J Am Coll Cardiol. 2002;40:2065–71. doi: 10.1016/s0735-1097(02)02608-6. [DOI] [PubMed] [Google Scholar]
- 40.Lakusic N, Merkas IS, Lucinger D, Mahovic D. Heterophile antibodies, false-positive troponin, and acute coronary syndrome: A case report indicating a pitfall in clinical practice. Eur Hear J-Case Rep. 2021:5. doi: 10.1093/ehjcr/ytab018. doi: 10.1093/ehjcr/ytab018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M, Andreotti F, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent st-segment elevation: Task force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC) Eur Heart J. 2016;37:267–315. doi: 10.1093/eurheartj/ehv320. [DOI] [PubMed] [Google Scholar]
- 42.Lemkes JS, Janssens GN, Van Der Hoeven NW, Van De Ven PM, Marques KMJ, Nap A, et al. Timing of revascularization in patients with transient ST-segment elevation myocardial infarction: A randomized clinical trial. Eur Heart J. 2019;40:283–91. doi: 10.1093/eurheartj/ehy651. [DOI] [PubMed] [Google Scholar]