Abstract
Background:
Neoadjuvant administration of immune checkpoint inhibitors (ICIs) combined with chemotherapy demonstrated promising efficacy and manageable safety in locally advanced esophageal squamous cell carcinoma (ESCC). This prospective, single-arm, phase 2 study evaluated the efficacy and safety of neoadjuvant therapy with camrelizumab plus paclitaxel and nedaplatin for 2–4 cycles in ESCC.
Methods:
Patients with locally advanced stage IIa–IIIb ESCC were enrolled in the study and received camrelizumab (200 mg), paclitaxel (155 mg/m2), and nedaplatin (80 mg/m2) intravenously on day one every 3 weeks. Patients underwent surgery after 2–4 cycles of treatment. The primary endpoint was the pathological complete response (pCR) rate. Secondary endpoints included the major pathological response (MPR) rate, R0 resection rate, tumor regression, objective response rate (ORR), and disease-free survival (DFS). Programmed cell death 1 ligand 1 (PD-L1) expression in tumor tissues was measured and quantified using immunohistochemistry staining and combined positive score (CPS), respectively.
Results:
In total, 75 patients were enrolled and received neoadjuvant treatment. Of them, 45 (60%) received two cycles, 18 (24%) received three cycles, and 10 patients (13.3%) received four cycles of neoadjuvant therapy. Ultimately, 62 patients (82.7%) underwent surgery. The patients achieved a pCR of 27.4% (95% CI: 16.9–40.2), an MPR of 45.2% (95% CI: 33.1–59.2), and an ORR of 48.4% (95% CI: 35.5–61.4); all patients had an R0 resection. T and N downstaging occurred in 39 (62.9%) and 19 (30.6%) patients Moreover, patients with CPS ≥10 tended to have enhanced ORR, pCR, and MPR compared to those with CPS <10. Treatment-related adverse events (TRAEs) of grade 1–2 occurred in 59 (78.7%) patients, grade 3 TRAEs in four (5.3%), and one patient (1.3%) experienced a grade 4 TRAE.
Conclusions:
Neoadjuvant camrelizumab combined with chemotherapy showed promising efficacy in locally advanced ESCC, with a manageable safety profile, when administered flexibly in two to four cycles.
Keywords: camrelizumab, chemotherapy, locally advanced resectable esophageal squamous cell carcinoma, neoadjuvant treatment
Introduction
Highlights
A phase 2 study of neoadjuvant chemoimmunotherapy for locally advanced ESCC (n=75).
This study employed a flexible regimen with 2–4 cycles of neoadjuvant therapy.
The pCR and MPR rates were 27.4 and 45.2%, respectively, with manageable safety.
Esophageal cancer (EC) is one of the major health concerns, ranking seventh in cancer incidence and sixth in cancer-related mortality worldwide1. Males are more likely to develop EC than females, with a two-fold to three-fold increase in incidence and mortality1. EC consists of two main histological subtypes: esophageal adenocarcinoma (EAC) and esophageal squamous cell carcinoma (ESCC). ESCC constitutes 90% of EC cases in parts of Asia and sub-Saharan Africa1,2. More than half of the EC-associated morbidity occurs in China, making it the highest disease burden in the country2. In China, risk factors for ESCC may include but are not limited to age, sex, drinking, smoking, and dietary habits such as the consumption of very hot liquids and food, as well as pickled or salted vegetables3.
The randomized phase 3 CROSS and NEOCRTEC5010 trials, which enrolled patients with clinical stage IIb–IIIa resectable locally advanced ESCC, demonstrated that neoadjuvant chemoradiotherapy improved overall survival (OS) compared to surgery alone4–7. The phase 3 randomized OEO2 trial showed neoadjuvant chemotherapy prolonged survival in patients with resectable EC without increased severe toxicity compared to surgery alone8. Additionally, the JCOG9907 trial with clinical stage II or III revealed that neoadjuvant chemotherapy resulted in longer OS, which was superior to adjuvant chemotherapy9. Although neoadjuvant chemoradiotherapy or chemotherapy led to improved survival, the prognosis for ESCC patients is still unsatisfactory, underscoring the need for further research and development of new treatment modalities to improve clinical outcomes.
Chemotherapy drugs can enhance the immunogenicity of the tumor microenvironment (TME) to amplify tumor-specific T cell responses by inducing immunogenic cell death, upregulating antigen presentation, and stimulating the release of damage-associated molecular patterns10. Additionally, chemotherapy drugs can reduce immunosuppression in the TME by targeting immunosuppressive cells such as regulatory T (Treg) cells and myeloid-derived suppressor cells (MDSCs)10. Furthermore, immune checkpoint inhibitors combined with chemotherapy led to superior survival compared to chemotherapy and are recommended as the standard of care as the first-line therapy for ESCC11–13.
The impressive efficacy of the first-line combination therapy of immunotherapy and chemotherapy in advanced ESCC has stimulated interest in exploring their potential in the neoadjuvant setting. Multiple clinical trials have been conducted to evaluate the efficacy and safety of neoadjuvant programmed cell death 1 (PD-1) antibodies in combination with chemotherapy for resectable ESCC. These studies reported pathological complete response (pCR) rates ranging from 20 to 50% (some pCR definitions exclude lymph nodes) and major pathological response (MPR) rates ranging from 42 to 72%. However, limited data are available on long-term clinical outcomes such as OS and progression-free survival (PFS). Most studies included fewer than 50 patients with resectable ESCC14–19. Moreover, determining the optimal duration of neoadjuvant therapy is challenging, considering each patient’s variable physical conditions and compliance. Therefore, tailoring neoadjuvant therapy cycles to individual patient conditions is a valuable area of exploration.
Camrelizumab is a humanized monoclonal antibody that binds to PD-1. Several clinical trials showed the therapeutic benefit of camrelizumab plus chemotherapy in the first-line setting for advanced or metastatic ESCC13 and in the neoadjuvant setting for locally advanced ESCC20–22. Moreover, the CMISG1701 trial and JCOG 1109 NExT trial showed that chemoradiotherapy did not significantly prolong OS compared to neoadjuvant chemotherapy23,24. Therefore, this phase 2 study employed a flexible neoadjuvant regimen of two to four cycles, combining chemotherapy and camrelizumab, considering individual patient differences in physical condition, response to neoadjuvant therapy, and surgical compliance. The study aimed to evaluate the efficacy of this regimen by assessing pathological response, radiographic response, and its safety profile.
Methods
Study design
The study was designed as a prospective, open-label, single-arm, phase 2 trial and involved neoadjuvant treatment with camrelizumab in combination with chemotherapy for patients with locally advanced resectable ESCC. Recruitment started from 2 June 2020, to 1 July 2022. The study adhered to the Declaration of Helsinki as well as the Good Clinical Practice Guidelines. The study protocol was approved by the ethics committee, and all patients provided written informed consent before participating in the study. The work has been reported in line with the strengthening the reporting of cohort, cross-sectional and case–control studies in surgery (STROCSS) criteria25 (Supplemental Digital Content 1, http://links.lww.com/JS9/B506).
Participants
Eligible patients, aged between 18 and 70 years, were diagnosed with locally advanced stage IIa–IIIb ESCC according to the AJCC 8th edition TNM staging system. Enrolled patients possessed an Eastern Cooperative Oncology Group (ECOG) performance status of 0-1, had measurable lesions based on the Response Evaluation Criteria in Solid Tumors (RECIST) 1.1, had sufficient organ function, and were anticipated to survive beyond three months. Patients were excluded if they had active, suspected, or known autoimmune diseases. Moreover, those with previously or concurrently other malignancies (excluding properly treated nonmelanoma skin cancer, carcinoma in situ of the cervix, and papillary carcinoma of the thyroid) or any other factors that may impact the participant’s safety or the compliance of the trial, were also excluded.
Procedures
The study utilized a neoadjuvant combination treatment consisting of camrelizumab at a dose of 200 mg, paclitaxel at a dose of 155 mg/m2, and nedaplatin at a dose of 80 mg/m2. Participants received all the agents via intravenous infusion on the first day, every 3 weeks. The combination therapy was administered for 2–4 cycles, and tumor response assessments were conducted after 2nd cycle of neoadjuvant treatment and before surgery according to the RECIST 1.1 criteria. After patients were treated for at least two neoadjuvant therapy cycles, the investigators decided whether to proceed with surgical operation while considering the patient’s wishes. Adverse events (AEs) were monitored for 90 days following the final dose of drug administration or until 30 days after surgery. AEs were assessed using the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE version 5.0). Patients received adjuvant therapy based on the investigators’ judgment.
Outcomes
pCR was adopted as the primary endpoint, and the second endpoints comprised MPR, R0 resection rate, tumor regression, objective response rate (ORR), disease control rate (DCR), and disease-free survival (DFS). pCR was referred to as the absence of residual tumor cells in the primary tumor and lymph nodes. The presence of 10% or fewer residual tumor cells in the primary tumor and lymph nodes was considered MPR. DFS was defined as the time between the surgery and local or distant recurrence or death from any cause, whichever occurred first. The four-tier College of American Pathologists grading system was used to assess the tumor regression.
Immunohistochemistry
The expression of PD-L1 was detected by immunohistochemistry (IHC) in ESCC specimens. All esophageal tumor tissues were fixed in formalin with 10% neutral buffer, embedded in paraffin, and sliced into 4-micron serial sections. The ready-to-use PD-L1 antibody was purchased from AmoyDx Company (Xiamen, China). According to the manufacturer’s instructions, the high-pressure repaired samples were incubated with antibodies overnight at 4°C. Visualization with DAB staining indicated the membrane localization and expression levels of PD-L1 in the intratumoral cells, and nuclei were counterstained using Haematoxylin. A combined positive score (CPS) was adopted to quantify PD-L1 expression levels26. CPS, varying from 1 to 100, was defined as a percent score [(the sum of PD-L1-positive surviving tumor cells and immune cells (lymphocytes and macrophages)/surviving tumor cells] in the sample. Two pathologists blinded to patients’ information evaluated and scored the PD-L1 stainings under a 20× objective lens. ESCC samples with CPS ≥1 were considered PD-L1 positive, while those with CPS <1 were negative27. Tumors with CPS ≥10, as determined by PD-L1 IHC 22C3 pharmDx (Agilent Technologies), have been approved to aid in screening ESCC patients eligible for pembrolizumab, which was supported by the phase III KEYNOTE-181 study28. Therefore, patients were also divided into CPS <10 and CPS ≥10 groups.
Statistical analysis
Statistically, a minimum of 56 patients were required to detect an increase in pCR from 20 to 40% with a power of 90% at a one-sided significance level of 2.5%, using an exact binomial test. If presuming a dropout rate of 20%, 70 patients should be enrolled. For continuous variables, the median and range were calculated. For categorical variables, the number and percentage of patients in each category were calculated. The 95% CI was calculated using the Clopper–Pearson method. Exploratory subgroup analyses of pathological and radiographic responses were performed based on the following baseline characteristics (unplanned): ECOG performance status (0 vs 1), smoking history (yes vs no), drinking history (yes vs no), tumor location (middle vs lower), clinical T stage (T2 vs T3), clinical N stage (N0 vs N1), and clinical stage (II vs III). Pearson’s correlation coefficient was used to determine correlations between radiological response and tumor regression. Statistical analyses were performed using the Statistical Analysis System (SAS) 9.2 version. We set the level of statistical significance at 5% without further notification.
Results
Patient characteristics
In total, 75 patients were included in this study and received neoadjuvant treatment. Most of these patients were male (97.3%) and had an ECOG performance status of 0 (70.7%). Most patients presented with clinically staged T3 tumors (73.3%) and lymph node metastasis (61.3%). Furthermore, more than half of the patients reported a smoking and drinking history, and their tumors were located in the lower esophagus. Tumor samples from 61 patients were assessed for PD-L1 expression. Twenty-five patients (33.3%) had a CPS <1, while CPS ≥1 was observed in 36 patients (48%) (Table 1).
Table 1.
Baseline characteristics.
| Patients (n=75) | |
|---|---|
| Age (years) | |
| Median (range) | 62 (48, 74) |
| Sex, n (%) | |
| Male | 73 (97.3) |
| Female | 2 (2.7) |
| ECOG performance status, n (%) | |
| 0 | 53 (70.7) |
| 1 | 22 (29.3%) |
| Smoking, n (%) | |
| Never | 34 (45.3) |
| Former or current | 41 (54.7) |
| Drinking, n (%) | |
| Never | 32 (42.7) |
| Former or current | 43 (57.3) |
| Tumor location, n (%) | |
| Upper | 4 (5.3) |
| Middle | 30 (40) |
| Lower | 41 (54.7) |
| Clinical T stage, n (%) | |
| T2 | 20 (26.7) |
| T3 | 55 (73.3) |
| Clinical N stage, n (%) | |
| N0 | 29 (38.7) |
| N1 | 35 (46.7) |
| N2 | 11 (14.6) |
| Clinical stage (AJCC, 8th edition), n (%) | |
| II | 37 (49.3) |
| III | 38 (50.7) |
| Tumor length, mm | |
| Median (range) | 50 (9.13, 130) |
| PD-L1 CPS expression, n (%) | |
| CPS <1 | 25 (33.3) |
| CPS ≥1 | 36 (48.0) |
| CPS <10 | 41 (54.7) |
| CPS ≥10 | 20 (26.7) |
| Unknown | 14 (18.7) |
Completion of treatment
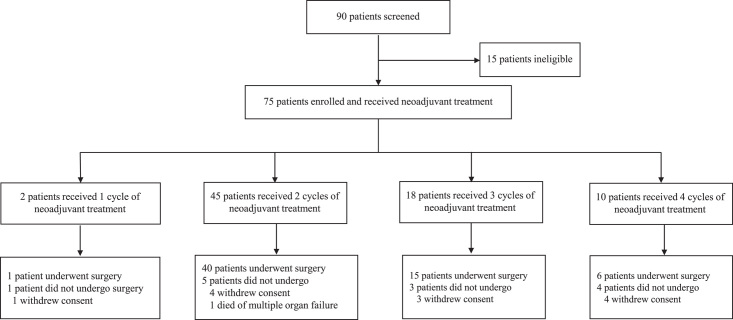
Of the 45 patients who received two cycles of neoadjuvant treatment, 40 underwent surgery, while the remaining five discontinued due to withdrawal of informed consent (n=4) or death from multiple organ failure (n=1). Eighteen patients finished three cycles of neoadjuvant treatment; among them, 15 patients received surgery, and three withdrew consent. Six of the 10 patients achieving four cycles of neoadjuvant therapy underwent surgery; besides, one patient had a surgical operation outside the trial center, and four withdrew from the trial. Additionally, one patient underwent surgery after one cycle of neoadjuvant treatment at his request. In total, 62 (82.7%) of the 75 enrolled patients were subjected to surgical resections (Fig. 1).
Figure 1.
Study flowchart.
Radiographic response and pathological response
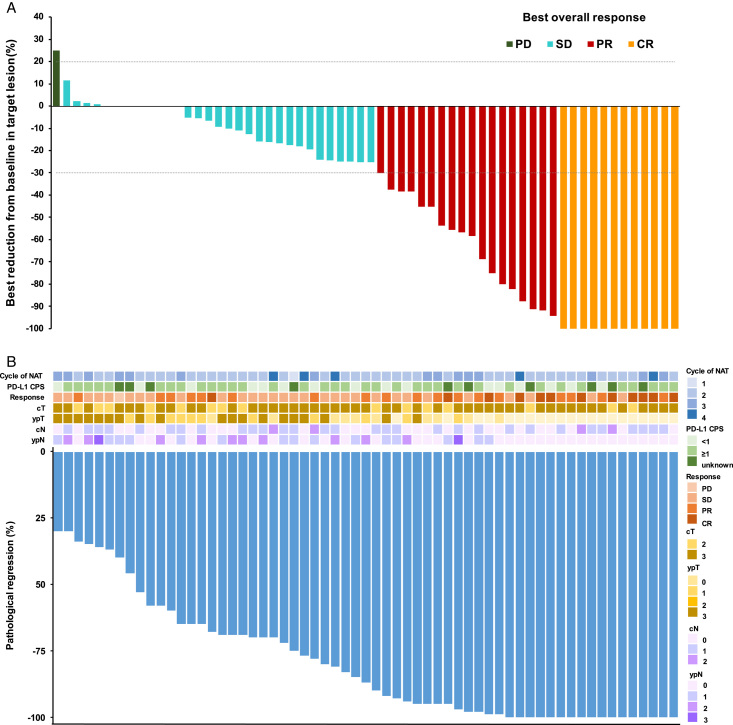
Among 75 patients, the ORR was 48.4% (95% CI: 35.5–61.4), and the DCR was 98.4% (Table 2). After one cycle of neoadjuvant treatment in line with RECIST 1.1 criteria, one patient achieved stable disease (SD). Out of 40 patients who received two cycles of treatment, nine (22.5%), thirteen (32.5%), and eighteen (45%) achieved complete response (CR), partial response (PR), and SD, respectively. In terms of the fifteen patients who received three cycles, one (6.7%) achieved CR, five (33.3%) achieved PR, eight (53.3%) achieved SD, and one (6.7%) experienced progressive disease (PD). Among six patients who received four cycles, two (33.3%) and four (66.7%) achieved CR and SD, respectively. A waterfall plot was used to show the best radiographic responses of ESCC patients (Fig. 2A).
Table 2.
Radiographic response and pathological response.
| The first cycle (n=1) | The second cycle (n=40) | The third cycle (n=15) | The fourth cycle (n=6) | Total | |
|---|---|---|---|---|---|
| Radiographic response, n (%) | |||||
| Complete response | 0 | 9 (22.5) | 1 (6.7) | 2 (33.3) | 12 (19.4) |
| Partial response | 0 | 13 (32.5) | 5 (33.3) | 0 | 18 (29) |
| Stable disease | 1 (100) | 18 (45) | 8 (53.3) | 4 (66.7) | 31 (50) |
| Progressive disease | 0 | 0 | 1 (6.7) | 0 | 1 (1.6) |
| Objective response | 0 | 22 (55) | 6 (40) | 2 (33.3) | 30 (48.4) |
| Disease control | 1 (100) | 40 (100) | 14 (93.3) | 6 (100) | 61 (98.4) |
| Pathological response, n (%) | |||||
| pCR | 0 | 12 (30) | 3 (20) | 2 (33.3) | 17 (27.4) |
| MPR | 0 | 19 (47.5) | 7 (46.7) | 2 (33.3) | 28 (45.2) |
| TRG 0 | 0 | 12 (30) | 3 (20) | 2 (33.3) | 17 (27.4) |
| TRG 1 | 0 | 6 (15) | 5 (33.3) | 0 | 11 (17.7) |
| TRG 2 | 1 (100) | 12 (30) | 5 (33.3) | 1 (16.7) | 19 (30.6) |
| TRG 3 | 0 | 10 (25) | 2 (13.3) | 2 (33.3) | 14 (22.6) |
As one patient who received four cycles of neoadjuvant treatment underwent surgery outside the trial center, the results of the pathological assessment were not obtained.
pCR, pathological complete response; MPR, major pathological response; TRG, tumor regression grade, assessed by the four-tier College of American Pathologists grading system.
Figure 2.
Radiographic response and pathological response for patients who underwent surgery. A Waterfall plot of best radiographic response by RECIST 1.1 in 62 patients, B Waterfall plot of pathological tumor regression in 61 patients. As one patient who received four cycles of neoadjuvant treatment underwent surgery outside the trial center, the results of the pathological assessment were not obtained. CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease. NAT, neoadjuvant therapy.
Among 62 patients who underwent surgery, the pCR rate was 27.4% (95% CI: 16.9–40.2), and the MPR rate was 45.2% (95% CI: 33.1–59.2) (Table 2). In addition, 17 patients (27.4%) had pCR, including 12 patients (30%) after two cycles, three (20%) after three cycles, and two (33.3%) after four cycles of treatment. Furthermore, 19 (47.5%), seven (46.7%), and two (33.3%) achieved MPR. Detailed tumor regression results are shown in a waterfall plot (Fig. 2B), including the cycle of neoadjuvant therapy, PD-L1 CPS score, radiographic response, and clinical stage (cT, ypT, cN, and ypN). Thirty-nine patients (62.9%) achieved T downstaging, and 19 (30.6%) achieved N downstaging, with 21 (33.9%) showing pathological T0 and 34 (54.8%) reaching N0.
In addition, there were no significant differences in ORR, pCR, and MPR between subgroups based on ECOG performance status (0 vs 1), smoking history (yes vs no), drinking history (yes vs no), tumor location (middle vs lower), clinical T stage (T2 vs T3), clinical N stage (N0 vs N1), and clinical stage (II vs III) (Supplementary Table 1, Supplemental Digital Content 2, http://links.lww.com/JS9/B507).
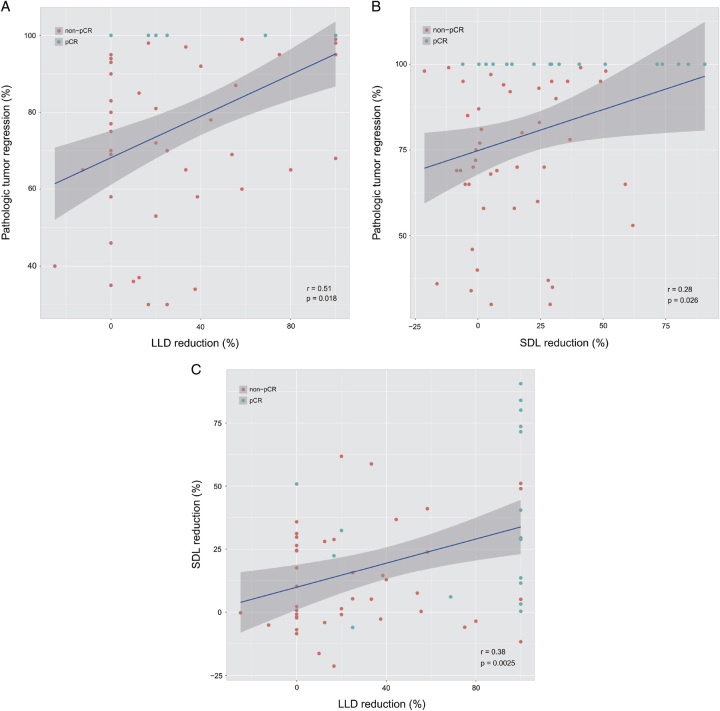
Correlation analysis showed that pathological tumor regression was positively correlated with the reduction in lesion longest diameter (LLD) and short diameter of the largest lesion (SDL) (both P<0.05, Fig. 3A, B). Furthermore, the reduction in LLD was positively correlated with the decrease in SDL (P<0.05) (Fig. 3C).
Figure 3.
Correlation between radiographic response related parameters and pathological tumor regression. A LLD reduction was positively correlated with pathological tumor regression (P=0.018). B SDL reduction was positively correlated with pathological tumor regression (P=0.026). C LLD reduction was positively correlated with SDL reduction (P=0.0025). LLD, lesion longest diameter; SDL, short diameter of the largest.
PD-L1 expression and clinical responses
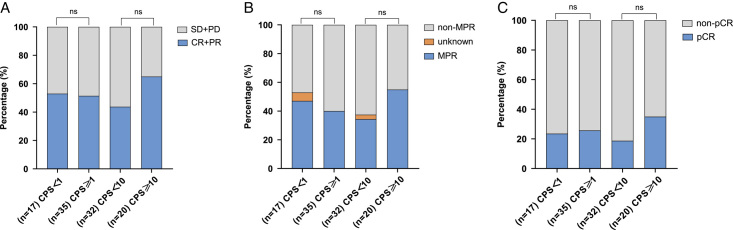
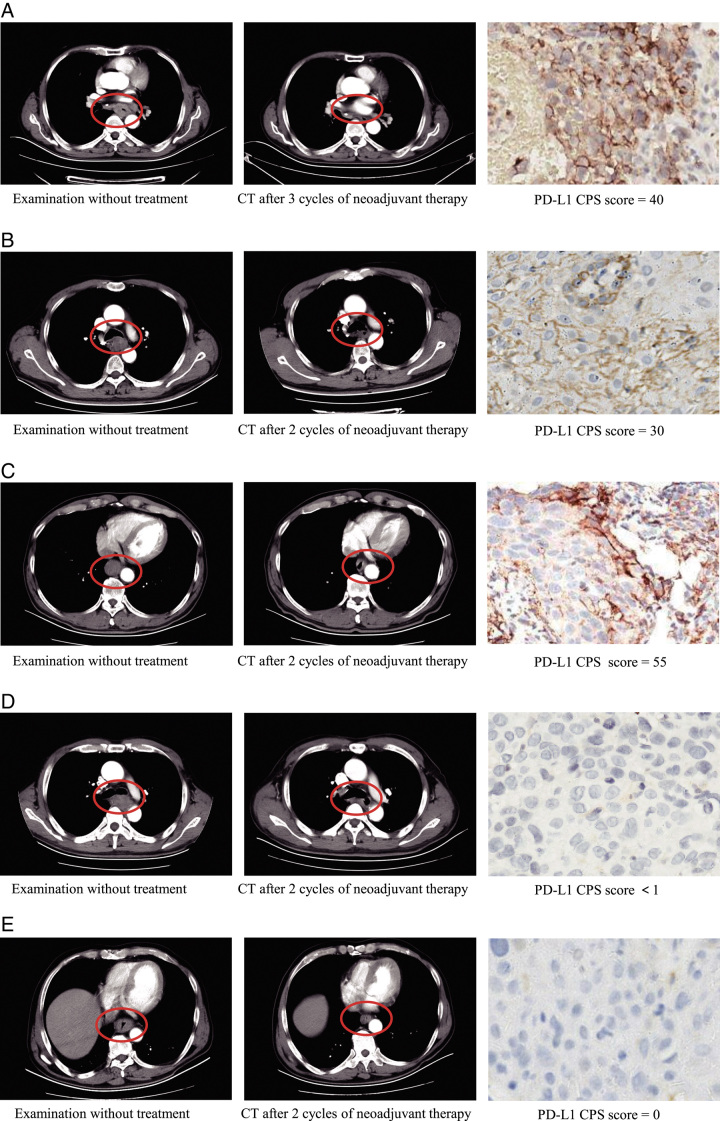
Among patients who underwent surgery, 17 had PD-L1 CPS <1, and 35 had PD-L1 CPS ≥1. ESCC patients with PD-L1 CPS <1 group had an ORR of 52.9%, a pCR of 23.5%, and an MPR of 47.1%. In the PD-L1 CPS ≥1 group, the ORR was 51.4%, pCR was 25.7%, and MPR was 40%. Moreover, when the PD-L1 CPS cutoff value of 10 was applied, ORR, pCR, and MPR were higher in CPS ≥10 than in CPS <10 groups, but a statistically significant difference was not achieved between the two groups with the χ2test (Fig. 4, Supplementary Table 1, Supplemental Digital Content 2, http://links.lww.com/JS9/B507). Representative radiological response images from five patients with PD-L1 CPS scores of 0, <1, 30, 40, and 55 were presented in Figure 5. Patients A–C achieved PR, while patients D–E achieved SD. Patient A achieved MPR, whereas patients B–E had viable tumor cell proportions of 31, 13, 15, and 47%, respectively. These results warrant further validation by studies including more participants and endpoints (e.g. event-free survival, DFS, and OS).
Figure 4.
Analyses of (A) the objective response rate, (B) major pathological response (MPR) and (C) pathological complete response (pCR) for subgroups based on PD-L1 expression. CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease.
Figure 5.
Representative cases. Patient A, with a PD-L1 CPS score of 40, achieved partial response (PR) after three cycles of neoadjuvant therapy and eventually reached major pathological response (MPR). Patient B, with a PD-L1 CPS score of 30, achieved PR after 2 cycles of neoadjuvant therapy, with 31% viable tumor cells remaining. Patient C, with a PD-L1 CPS score of 55, achieved PR after 2 cycles of neoadjuvant therapy, with 13% viable tumor cells remaining. Patient D (PD-L1 CPS score of <1) achieved stable disease (SD) after 2 cycles of neoadjuvant therapy, with a residue of 15% viable tumor cells. Patient E (PD-L1 CPS score of 0) achieved SD after 2 cycles of neoadjuvant therapy, with 47% viable tumor cell residue.
Surgery outcomes
Among 62 patients who underwent surgery, the median interval between the last neoadjuvant treatment and a surgical operation was 39.5 days (range 28–118). Twenty-one (33.9%) patients underwent robotic surgery, 40 (64.5%) received laparoscopic surgery, and one (1.6%) had thoracotomy. All patients had R0 resection. The median operation time was 362.5 min (range 180–567), and the median postoperative hospital stay was 10 days (range 6–41). Postoperative complications were observed in 29 patients (46.8%), including 19 cases of atelectasis (30.6%), 15 cases of pneumonia (24.2%), and 15 cases of pleural effusion (24.2%) (Supplementary Table 2, Supplemental Digital Content 2, http://links.lww.com/JS9/B507).
Safety
Among 75 patients, 59 (78.7%) experienced grade 1–2 treatment-related adverse events (TRAEs), four (5.3%) suffered from grade 3 TRAEs, and one (1.3%) was subjected to a grade 4 TRAE. The most frequent grade 1–2 TRAEs were alopecia (49.3%), reactive cutaneous capillary endothelial proliferation (46.7%), and asthenia (40%). The most common grade 3 TRAEs were anemia (5.3%) and thrombocytopenia (2.7%) (Table 3). Tragically, one patient died due to multiple organ failure after receiving two cycles of neoadjuvant treatment, and one died due to respiratory failure after surgery.
Table 3.
Treatment-related adverse events.
| Treatment-related adverse events, n (%) | Grades 1–2 | Grade 3 | Grade 4 |
|---|---|---|---|
| Any adverse events | 59 (78.7) | 4 (5.3) | 1 (1.3) |
| Alopecia | 37 (49.3) | 0 | 0 |
| Reactive cutaneous capillary endothelial proliferation | 35 (46.7) | 0 | 0 |
| Fatigue | 30 (40) | 0 | 0 |
| Anorexia | 25 (33.3) | 0 | 0 |
| Arthritis | 25 (33.3) | 0 | 0 |
| Nausea | 22 (29.3) | 0 | 0 |
| Myalgia | 22 (29.3) | 0 | 0 |
| Pruritus | 22 (29.3) | 0 | 0 |
| Vomiting | 15 (20) | 0 | 0 |
| Peripheral sensory nerve disorders | 12 (16) | 0 | 0 |
| Increased γ-glutamyl transpeptidase | 13 (17.3) | 0 | 0 |
| Anemia | 13 (17.3) | 4 (5.3) | 0 |
| Decreased platelet count | 7 (9.3) | 2 (2.7) | 0 |
| Alanine aminotransferase increased | 10 (13.3) | 1 (1.3) | 0 |
| Diarrhea | 8 (10.7) | 0 | 0 |
| Insomnia | 8 (10.7) | 0 | 0 |
| Rash | 8 (10.7) | 0 | 0 |
| Aspartate aminotransferase increased | 9 (12) | 1 (1.3) | 0 |
| Thyroid dysfunction | 13 (17.3) | 0 | 0 |
| Headache | 7 (9.3) | 0 | 0 |
| Dizziness | 6 (8) | 0 | 0 |
| Hyperbilirubinemia | 12 (16) | 1 (1.3) | 0 |
| Lactate dehydrogenase (LDH)/ Alkaline phosphatase (AKP) increased | 5 (6.7) | 0 | 0 |
| Dyspepsia | 5 (6.7) | 0 | 0 |
| Decreased white blood cell count | 4 (5.3) | 0 | 0 |
| Decreased lymphocyte count | 4 (5.3) | 0 | 0 |
| Constipation | 3 (4%) | 0 | 0 |
| Decreased neutrophil count | 2 (2.7) | 0 | 1 (1.3) |
| Abdominal pain | 2 (2.7) | 0 | 0 |
| Fever | 2 (2.7) | 0 | 0 |
| Abdominal distension | 1 (1.3) | 0 | 0 |
| Edema | 1 (1.3) | 0 | 0 |
| Hypertension | 1 (1.3) | 0 | 0 |
Discussion
This phase 2 trial aimed to assess the efficacy and safety of neoadjuvant camrelizumab combined with chemotherapy for locally advanced ESCC, with patients having the option to receive 2–4 cycles of treatment. The study revealed that 27.4% of patients achieved a pCR (ypT0N0), while 45.2% achieved an MPR. We did not meet the primary endpoint of this study, having expected an increase in the pCR rate from 20 to 40%; however, the pCR rate was higher than that reported in other neoadjuvant chemotherapy studies, where rates of 4 and 3.8% were observed8,29. Furthermore, the safety profile of the treatment was manageable. These findings suggest that 2–4 cycles of neoadjuvant camrelizumab combined with chemotherapy are feasible in clinical practice.
Neoadjuvant chemoimmunotherapy studies for locally advanced ESCC typically involve two cycles of treatment. One study using sintilimab combined with paclitaxel and liposomal carboplatin reported a pCR (ypT0) of 22.2% and an MPR of 44.4%14. In contrast, other studies reported a ypT0N0 of 31.4% with camrelizumab combined with nab-paclitaxel and cisplatin (NIC-ESCC2019 study) and a ypT0N0 of 29.1 and an MPR of 49.1% with toripalimab combined with albumin-bound (nab) paclitaxel and S-116,19. Additionally, two studies using three cycles of neoadjuvant chemoimmunotherapy, one using pembrolizumab in combination with traditional two-drug chemotherapy (PEN-ICE study) and the other using tislelizumab together with carboplatin and nab-paclitaxel, reported ypT0 rates of 46.2 and 50%, and MPR rates of 69.2 and 72%, respectively15,17. In a study of four cycles of socazolimab combined with nab-paclitaxel and cisplatin, 41.4 and 69.0% of patients achieved ypT0N0 and MPR, respectively18. A longer duration of neoadjuvant therapy seems to improve pathological response. However, in this study, patients who received two, three, and four cycles of neoadjuvant treatment had pCR rates of 30% (12/40), 20% (3/15), and 33.3% (2/6), respectively, while the MPR rates were 47.5% (19/40), 46.7% (7/15), and 33.3% (2/6). Given that these analyses were conducted on small sample sizes, further investigation is needed to determine whether longer durations of neoadjuvant therapy lead to better pathological responses while considering patients’ ability to tolerate the treatment.
In the middle of 2020, Kojima demonstrated that while used as second-line therapy, pembrolizumab was superior to chemotherapy in prolonging OS in patients with advanced EC having PD-L1 CPS ≥10 in a randomized phase III KEYNOTE-181 study28. Moreover, PD-L1 IHC 22C3 pharmDx is approved by the FDA to identify patients suitable for pembrolizumab monotherapy or combination therapies for several types of cancer, including nonsmall cell lung cancer (NSCLC), head and neck squamous cell carcinoma (HNSCC), triple-negative breast cancer (TNBC), and ESCC30. On the contrary, the ESCORT study demonstrated that as second-line therapy, camrelizumab conferred a survival benefit in advanced or metastatic ESCC patients compared to chemotherapy, irrespective of PD-L1 expression31. Herein, we also attempted to determine the association between PD-L1 expression and clinical responses (i.e. ORR, pCR, and MPR). We observed no significant differences in pCR and MPR between the PD-L1 CPS <1 and PD-L1 CPS ≥1 groups. Interestingly, pCR and MPR were numerically higher in the group with PD-L1 CPS ≥10 than in the group with PD-L1 CPS <10. Our findings align with several studies performed in locally advanced ESCC with PD-1 inhibitors plus chemotherapy used as neoadjuvant treatment17,20,21. For instance, Liu and colleagues demonstrated that there was no significant association between PD-L1 expression levels and pCR in a study of 60 ESCC patients administrated with camrelizumab and chemotherapy (nab-paclitaxel and carboplatin)21. Yang et al.20 reported similar results with the same neoadjuvant regimes in ESCC patients (n=23). Recently, Yan et al.17 also detected no significant association between clinical responses and a combination of tislelizumab and chemotherapy in neoadjuvant therapy for resectable ESCC (n=45). Several reasons may help to explain why our study failed to verify the predictive value of PD-L1 CPS scoring on clinical response in ESCC. First, we used different PD-1 antibodies from other studies. Second, the III KEYNOTE-181 study concluded based on OS instead of ORR, pCR, and MPR28. Therefore, we will continue to follow-up with the patients and use OS as the primary endpoint when possible. Third, compared to sample sizes over 600 in the phase III KEYNOTE-181 study28, the number of patients in the current study is relatively few, and more patients should be enrolled to increase the statistical power. Fourth, unlike the enrollment of advanced EC patients in the KEYNOTE-181 study in which pembrolizumab was used as second-line therapy28, the PD-1 inhibitor and chemotherapy were used as neoadjuvant treatment in local resectable ESCC. Overall, no definitive biomarkers are currently available for accurately predicting pathological response for locally resectable patients treated with a neoadjuvant PD-1 inhibitor and chemotherapy. Extensive, larger-scale studies are required urgently to address this crucial question.
Toxicity was a notable concern during the study, and the toxicity profile was consistent with the known effects of camrelizumab combined with chemotherapy, with no new signals observed16,20,21. In this study, most patients (78.7%) experienced grade 1-2 TRAEs, while 5.3 and 3.3% experienced grades 3 and 4 TRAEs, respectively. Among patients treated with camrelizumab plus nab-paclitaxel and cisplatin, 75% had any grade TRAEs, and 10.7% had grade 3 TRAEs16. For patients receiving camrelizumab plus nab-paclitaxel and carboplatin, 96.7% experienced TRAEs, and 56.7% had grade 3 or worse TRAEs21. This TRAE incidence was higher than that observed in the present study, possibly due to the more intensive treatment received by patients in that study. Nab-paclitaxel was given at a dose of 100 mg/m2 on days 1, 8, and 15 during each cycle21, while patients in this study received paclitaxel via intravenous infusion at a dose of 155 mg/m2 on day 1 in each cycle. Furthermore, the surgical profile, including intraoperative blood loss and median postoperative hospital stay, was consistent with previous neoadjuvant camrelizumab combined with chemotherapy studies16,20,21. However, pulmonary complications were more frequently observed in this study, with one patient dying of respiratory failure. Similar pulmonary complications were reported in two other neoadjuvant chemoimmunotherapy studies, including one patient who died of pneumonia and another who died of acute respiratory failure15,21. These findings highlight the need for a more cautious assessment of the risk of pulmonary complications during treatment and more careful monitoring and immediate management of such complications.
This study had some limitations that should be considered when interpreting its findings. First, the single-arm design of the study did not include a control group, which limits the ability to make comparisons with standard treatment options. Second, although the study included more patients than previous publications regarding administrating neoadjuvant chemoimmunotherapy to local resectable ESCC patients, the small sample size may impact the study’s statistical power and the generalizability of the results. Although this study did not reach its primary endpoint, the pCR rate was comparable to other studies of neoadjuvant chemoimmunotherapy. Third, a limitation of this clinical study was the over-representation of male patients. While it is well-established that ~70% of EC cases occur in men, the over-representation of male patients in this study may also be attributed to the higher incidence of males with EC in our study center. Fourth, OS was not predefined as an endpoint, although it is a crucial measure of neoadjuvant therapy efficacy. Finally, the long-term outcomes of DFS and OS will require further follow-up.
In conclusion, the administration of neoadjuvant chemoimmunotherapy in clinical practice should consider several factors, including treatment response and the patient’s physical condition. The results of this single-arm phase 2 study suggest that 2–4 cycles of neoadjuvant camrelizumab combined with paclitaxel and nedaplatin were effective and had a manageable safety profile for locally advanced ESCC. This study suggests that the duration of neoadjuvant therapy may be personalized based on individual patient characteristics in clinical practice. These findings may provide valuable insights for optimizing neoadjuvant chemoimmunotherapy in managing locally advanced ESCC.
Ethical approval
Ethical approval was obtained from the ethics committee institutional review board of the Harbin Medical University Cancer Hospital (2020-50-IIT), and written informed consent was obtained from all patients.
Consent
Written informed consent was obtained from the patient for publication of this paper. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Sources of funding
This research was not sponsored or supported by any institution or funding.
Author contribution
J.M., Y.Y., and J.Z.: conception and design; L.Z. and C.F.: administrative support; X.W. and X.L.: patient enrollment; Y.X. and H.J.: acquisition of data; J.M., X.L., and X.W.: data analysis and interpretation; J.M. and J.Z.: manuscript writing and revision; J.M. and J.Z.: supervision. All the authors read and approved the final manuscript.
Conflicts of interest disclosure
The authors declare no conflicts of interest.
Research registration unique identifying number (UIN)
Name of the registry: Chinese Clinical Trial Registry.
Unique identifying number or registration ID: ChiCTR2000033761.
Hyperlink to your specific registration (must be publicly accessible and will be checked): https://www.chictr.org.cn/showproj.html?proj=55022.
Guarantor
Jianqun Ma and Jinhong Zhu.
Data availability statement
Data are available from the corresponding author upon reasonable request.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Supplementary Material
Acknowledgements
The authors sincerely thank every team member in the Esophageal Mediastinal Ward of Thoracic Surgery, Harbin Medical University Cancer Hospital, for their efforts and contributions to this study. The authors also thank all the patients, their families, and the institutions for supporting this study.
Footnotes
Yingnan Yang, Jinfeng Zhang, and Hongxue Meng contributed equally to this work.
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal’s website, www.lww.com/international-journal-of-surgery.
Published online 4 December 2023
Contributor Information
Yingnan Yang, Email: yangyingnanltx@163.com.
Jinfeng Zhang, Email: mrzhangjinfeng@126.com.
Hongxue Meng, Email: menghongxue@hrbmu.edu.cn.
Xiaodong Ling, Email: xiaodongling@hrbmu.edu.cn.
Xiaoyuan Wang, Email: 346650985@qq.com.
Yanzhong Xin, Email: 12913283@qq.com.
Hao Jiang, Email: jiangh1128@163.com.
Luquan Zhang, Email: dabiao1989@126.com.
Chengyuan Fang, Email: cbcdtz@163.com.
Hao Liang, Email: lianghaoshaw@126.com.
Jianqun Ma, Email: jianqunma@hrbmu.edu.cn.
Jinhong Zhu, Email: zhujinhong@hrbmu.edu.cn.
References
- 1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209–249. [DOI] [PubMed] [Google Scholar]
- 2. Morgan E, Soerjomataram I, Rumgay H, et al. The global landscape of esophageal squamous cell carcinoma and esophageal adenocarcinoma incidence and mortality in 2020 and projections to 2040: new estimates from GLOBOCAN 2020. Gastroenterology 2022;163:649–58.e2. [DOI] [PubMed] [Google Scholar]
- 3. Shen Y, Xie S, Zhao L, et al. Estimating individualized absolute risk for esophageal squamous cell carcinoma: a population-based study in high-risk areas of China. Front Oncol 2020;10:598603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074–2084. [DOI] [PubMed] [Google Scholar]
- 5. Eyck BM, van Lanschot JJB, Hulshof M, et al. Ten-year outcome of neoadjuvant chemoradiotherapy plus surgery for esophageal cancer: the randomized controlled CROSS trial. J Clin Oncol 2021;39:1995–2004. [DOI] [PubMed] [Google Scholar]
- 6. Yang H, Liu H, Chen Y, et al. Neoadjuvant chemoradiotherapy followed by surgery versus surgery alone for locally advanced squamous cell carcinoma of the esophagus (NEOCRTEC5010): a phase III multicenter, randomized, open-label clinical trial. J Clin Oncol 2018;36:2796–2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yang H, Liu H, Chen Y, et al. Long-term efficacy of neoadjuvant chemoradiotherapy plus surgery for the treatment of locally advanced esophageal squamous cell carcinoma: the NEOCRTEC5010 randomized clinical trial. JAMA Surg 2021;156:721–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Surgical resection with or without preoperative chemotherapy in oesophageal cancer: a randomised controlled trial. Lancet 2002;359:1727–1733. [DOI] [PubMed] [Google Scholar]
- 9. Ando N, Kato H, Igaki H, et al. A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907). Ann Surg Oncol 2012;19:68–74. [DOI] [PubMed] [Google Scholar]
- 10. Galluzzi L, Humeau J, Buqué A, et al. Immunostimulation with chemotherapy in the era of immune checkpoint inhibitors. Nat Rev Clin Oncol 2020;17:725–741. [DOI] [PubMed] [Google Scholar]
- 11. Doki Y, Ajani JA, Kato K, et al. Nivolumab combination therapy in advanced esophageal squamous-cell carcinoma. N Engl J Med 2022;386:449–462. [DOI] [PubMed] [Google Scholar]
- 12. Sun JM, Shen L, Shah MA, et al. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Lancet 2021;398:759–771. [DOI] [PubMed] [Google Scholar]
- 13. Luo H, Lu J, Bai Y, et al. Effect of camrelizumab vs placebo added to chemotherapy on survival and progression-free survival in patients with advanced or metastatic esophageal squamous cell carcinoma: the ESCORT-1st randomized clinical trial. JAMA 2021;326:916–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang Z, Ye J, Li H, et al. Neoadjuvant sintilimab and chemotherapy in patients with resectable esophageal squamous cell carcinoma: a prospective, single-arm, phase 2 trial. Front Immunol 2022;13:1031171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Duan H, Shao C, Pan M, et al. Neoadjuvant pembrolizumab and chemotherapy in resectable esophageal cancer: an open-label, single-arm study (PEN-ICE). Front Immunol 2022;13:849984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu J, Li J, Lin W, et al. Neoadjuvant camrelizumab plus chemotherapy for resectable, locally advanced esophageal squamous cell carcinoma (NIC-ESCC2019): a multicenter, phase 2 study. Int J Cancer 2022;151:128–137. [DOI] [PubMed] [Google Scholar]
- 17. Yan X, Duan H, Ni Y, et al. Tislelizumab combined with chemotherapy as neoadjuvant therapy for surgically resectable esophageal cancer: a prospective, single-arm, phase II study (TD-NICE). Int J Surg 2022;103:106680. [DOI] [PubMed] [Google Scholar]
- 18. Li Y, Zhou A, Liu S, et al. Comparing a PD-L1 inhibitor plus chemotherapy to chemotherapy alone in neoadjuvant therapy for locally advanced ESCC: a randomized Phase II clinical trial : a randomized clinical trial of neoadjuvant therapy for ESCC. BMC Med 2023;21:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang G, Yuan J, Pan C, et al. Multi-omics analysis uncovers tumor ecosystem dynamics during neoadjuvant toripalimab plus nab-paclitaxel and S-1 for esophageal squamous cell carcinoma: a single-center, open-label, single-arm phase 2 trial. EBioMedicine 2023;90:104515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yang W, Xing X, Yeung SJ, et al. Neoadjuvant programmed cell death 1 blockade combined with chemotherapy for resectable esophageal squamous cell carcinoma. J Immunother Cancer 2022;10:e003497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu J, Yang Y, Liu Z, et al. Multicenter, single-arm, phase II trial of camrelizumab and chemotherapy as neoadjuvant treatment for locally advanced esophageal squamous cell carcinoma. J Immunother Cancer 2022;10:e004291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhang B, Zhao H, Wu X, et al. Perioperative outcomes of neoadjuvant chemotherapy plus camrelizumab compared with chemotherapy alone and chemoradiotherapy for locally advanced esophageal squamous cell cancer. Front Immunol 2023;14:1066527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tang H, Wang H, Fang Y, et al. Neoadjuvant chemoradiotherapy versus neoadjuvant chemotherapy followed by minimally invasive esophagectomy for locally advanced esophageal squamous cell carcinoma: a prospective multicenter randomized clinical trial. Ann Oncol 2023;34:163–172. [DOI] [PubMed] [Google Scholar]
- 24. Kato K, Ito Y, Daiko H, et al. A randomized controlled phase III trial comparing two chemotherapy regimen and chemoradiotherapy regimen as neoadjuvant treatment for locally advanced esophageal cancer, JCOG1109 NExT study. J Clin Oncol 2022;40(4_suppl):238. [Google Scholar]
- 25. Mathew G, Agha R, Albrecht J, et al. STROCSS 2021: strengthening the reporting of cohort, cross-sectional and case-control studies in surgery. Int J Surg 2021;96:106165. [DOI] [PubMed] [Google Scholar]
- 26. Zhang C, Zhang G, Xue L, et al. Patterns and prognostic values of programmed cell death-ligand 1 expression and CD8+ T-cell infiltration in small cell carcinoma of the esophagus: a retrospective analysis of 34 years of National Cancer Center data in China. Int J Surg 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kulangara K, Zhang N, Corigliano E, et al. Clinical utility of the combined positive score for programmed death ligand-1 expression and the approval of pembrolizumab for treatment of gastric cancer. Arch Pathol Lab Med 2019;143:330–337. [DOI] [PubMed] [Google Scholar]
- 28. Kojima T, Shah MA, Muro K, et al. Randomized phase III KEYNOTE-181 study of pembrolizumab versus chemotherapy in advanced esophageal cancer. J Clin Oncol 2020;38:4138–4148. [DOI] [PubMed] [Google Scholar]
- 29. Wang H, Tang H, Fang Y, et al. Morbidity and mortality of patients who underwent minimally invasive esophagectomy after neoadjuvant chemoradiotherapy vs neoadjuvant chemotherapy for locally advanced esophageal squamous cell carcinoma: a randomized clinical trial. JAMA Surg 2021;156:444–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vainer G, Huang L, Emancipator K, et al. Equivalence of laboratory-developed test and PD-L1 IHC 22C3 pharmDx across all combined positive score indications. PLoS One 2023;18:e0285764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Huang J, Xu J, Chen Y, et al. Camrelizumab versus investigator’s choice of chemotherapy as second-line therapy for advanced or metastatic oesophageal squamous cell carcinoma (ESCORT): a multicentre, randomised, open-label, phase 3 study. Lancet Oncol 2020;21:832–842. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available from the corresponding author upon reasonable request.