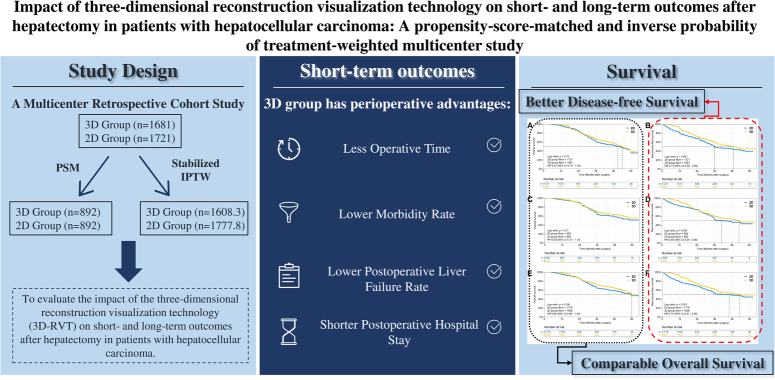
Abstract
Background:
Three-dimensional reconstruction visualization technology (3D-RVT) is an important tool in the preoperative assessment of patients undergoing liver resection. However, it is not clear whether this technique can improve short-term and long-term outcomes in patients with hepatocellular carcinoma (HCC) compared with two-dimensional (2D) imaging.
Method:
A total of 3402 patients from five centers were consecutively enrolled from January 2016 to December 2020, and grouped based on the use of 3D-RVT or 2D imaging for preoperative assessment. Baseline characteristics were balanced using propensity score matching (PSM, 1:1) and stabilized inverse probability of treatment‐weighting (IPTW) to reduce potential selection bias. The perioperative outcomes, long-term overall survival (OS), and recurrence-free survival (RFS) were compared between the two groups. Cox-regression analysis was used to identify the risk factors associated with RFS.
Results:
A total of 1681 patients underwent 3D-RVT assessment before hepatectomy (3D group), while 1721 patients used 2D assessment (2D group). The PSM cohort included 892 patient pairs. In the IPTW cohort, there were 1608.3 patients in the 3D group and 1777.9 patients in the 2D group. In both cohorts, the 3D group had shorter operation times, lower morbidity and liver failure rates, as well as shorter postoperative hospital stays. The 3D group had more margins ≥10 mm and better RFS than the 2D group. The presence of tumors with a diameter ≥5 cm, intraoperative blood transfusion and multiple tumors were identified as independent risk factors for RFS, while 3D assessment and anatomical resection were independent protective factors.
Conclusion:
In this multicenter study, perioperative outcomes and RFS of HCC patients following 3D-RVT assessment were significantly different from those following 2D imaging assessment. Thus, 3D-RVT may be a feasible alternative assessment method before hepatectomy for these patients.
Keywords: hepatectomy, hepatocellular carcinoma, three-dimensional reconstruction visualization technology, two-dimensional imaging, preoperative assessment
Introduction
Highlights
The effect of three-dimensional reconstruction visualization technology (3D-RVT) on short-term and long-term outcomes in hepatectomy for hepatocellular carcinoma is unknown.
This is the first multicenter study to compare the 3D-RVT with two-dimensional imaging.
This study uses both propensity score matching and stabilized inverse probability of treatment‐weighted methods to balance the baseline characteristics.
3D-RVT may be a more reliable method for assessment prior to hepatectomy.
Currently, surgery is still the main treatment option for hepatocellular carcinoma (HCC)1–3. However, the complex hepatic structure and anatomical variation, as well as tumor invasion of the intrahepatic vessels all increase the difficulty of HCC surgery4,5. In addition, preserving sufficient functional liver volume is also of great significance6,7. Therefore, preoperative plans and safety assessments are particularly important in liver surgery for HCC.
In recent years, three-dimensional reconstruction visualization technology (3D-RVT) has been gradually introduced for precise preoperative surgical planning8,9. 3D-RVT can convert two-dimensional (2D) MRI or computed tomography (CT) images into three-dimensional (3D) models10, which can intuitively and clearly show surgeons various anatomical structures inside the liver. Moreover, surgeons can also use 3D reconstruction software to calculate the liver volume and simulate the surgical process, thus enabling accurate surgical planning11–13. Several studies have shown that 3D-RVT provides certain perioperative benefits to patients undergoing liver resection, but the results have been inconsistent due to the heterogeneity of study designs and insufficient sample sizes14–20. What is more, the efficacy of 3D-RVT for HCC surgery and the benefit it provides to patients remains controversial, especially in terms of long-term outcomes, and evidence-based confirmation from multicenter, large-sample studies is still lacking. This study retrospectively analyzed the clinicopathological data of patients with HCC admitted to five high-volume liver surgery centers in China to evaluate the short-term and long-term outcomes of 3D-RVT for hepatectomy.
Methodology
Study design and patient enrollment
A multicenter retrospective study was conducted between January 2016 and December 2020, at the following five high-volume hospitals: the Eastern Hepatobiliary Surgery Hospital (EHSH), Zhujiang Hospital (ZJH), Anhui Provincial Hospital (APH), Mengchao Hepatobiliary Hospital (MCHH), and Shengjing Hospital (SJH). According to the principles promulgated in the Declaration of Helsinki, informed consent to permit their data to be used for clinical research was obtained from all patients, either at the time of hospital admission or before surgery. The clinical and pathological data of the patients were collected at various centers, after which they were collated and analyzed centrally at Zhujiang Hospital. This study was approved by the Institutional Review Board of the Zhujiang Hospital of Guangzhou, China (Approval No: 2020-KY-040-02), and registered on the Research Registry under the unique identifying number researchregistry9804. The study is reported in line with the strengthening the reporting of cohort studies in surgery (STROCSS) criteria21 (Supplemental Digital Content 8, http://links.lww.com/JS9/B718).
All of the enrolled patients met the following criteria: (1) age 18–75 years; (2) preoperative diagnosis of HCC by CT/MRI; (3) the diagnosis of HCC was confirmed by an experienced pathologist after the operation. Exclusion criteria included: (1) hyperthyroidism; (2) severe cardiac or pulmonary disease; (3) significant renal failure (creatinine >400 μmol/l); (4) pregnancy; (5) history of previous major abdominal surgery or other malignant diseases (local ablation before surgery, transcatheter arterial chemoembolization before surgery, or other neoadjuvant therapy); (6) presence of extrahepatic metastases or major vascular invasion; (7) greater than 3 tumor nodules; (8) treated with palliative resection, that is, macroscopically (R2) positive margins; (9) missing data on prognostic variables; and (10) followed up for less than 12 months but alive at the last follow-up after surgery. The patients were divided into 3D and 2D groups based on the use of 3D-RVT or 2D imaging.
Survey of surgical skills of enrolled surgeons
To reduce the potential bias of the study results due to differences in surgical skills between surgeons at different centers, we conducted a survey of the level of surgical skill of surgeons with previous experience of open hepatectomy and who had performed at least 50 laparoscopic hepatectomies per year in oncology patients, including (1) written evidence of the number of previous cases performed by the participating surgeon from the record room, and (2) provision of four case videos of laparoscopic hepatectomies performed within the previous 2 months for blinded review of the surgical videos. A blinded review committee consisting of three hepatobiliary surgeons from tertiary care centers not involved in this study was formed to assess eligibility. Unedited laparoscopic hepatectomy videos were randomly assigned to the reviewers. The surgical technique and degree of tumor cure were assessed by two reviewers, with additional review by a third expert if the two reviewers disagreed. Qualified surgeons who met the selection criteria were deemed eligible for this study. Subsequently, cases from these physicians were selected for inclusion in this retrospective analysis.
Preoperative assessment
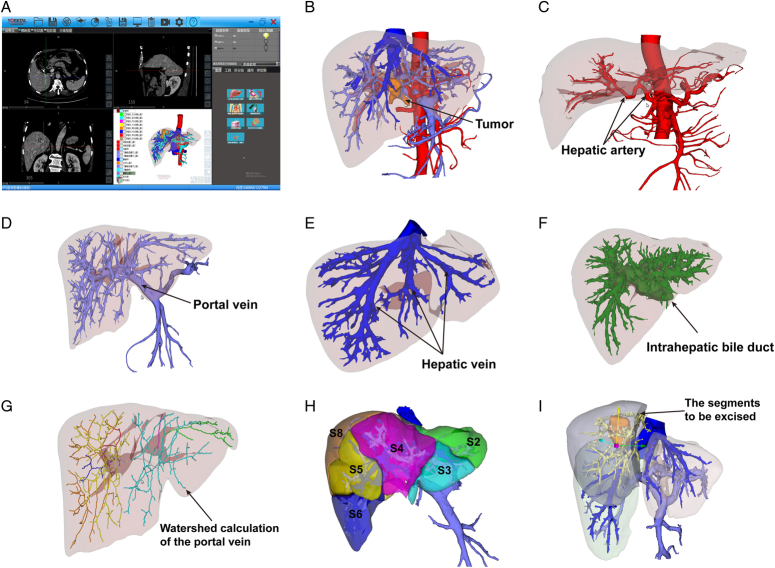
Routine preoperative investigations included medical history, hepatitis testing, full blood counts, liver and renal function tests, serum alpha-fetoprotein (AFP), and coagulation tests. Imaging studies included abdominal ultrasonography (US), contrast-enhanced CT and/or MRI to assess the resectability of the tumor. The difference was that the lesions, vessels, bile ducts, and liver in the 3D group were reconstructed preoperatively using 3D-RVT-based software, and a virtual surgical resection was performed (Fig. 1). The five centers discussed and agreed on the operation of the 3D-RVT beforehand, and the specific procedures and standards were based on an expert consensus developed by several countries12. All cases were discussed in weekly multidisciplinary treatment (MDT) meetings to achieve a consensus on tumor resectability.
Figure 1.
The various application of three-dimensional reconstruction visualization technology (3D-RVT). (A) the 3D-RVT software; (B) the three-dimensional model based on CT image reconstruction; (C) hepatic artery analysis; (D) portal vein analysis; (E) hepatic vein analysis; (F) intrahepatic bile duct analysis; (G) portal vein territory analysis; (H) individualized liver segmentation; and (I) simulated surgery.
Surgical procedures
Hepatectomy was performed as an open or laparoscopic procedure according to the surgeon’s assessment and the patient’s preference. A brief survey of surgical technique and intraoperative management was conducted before the start of the study. Anatomical resection (AR) was defined as the complete removal of the liver parenchyma confined within the corresponding portal territory, or at least one Couinaud segment that contains the tumor together with the related portal vein branch, hepatic vein, and the corresponding hepatic territory22,23. Nonanatomical resection (NAR), also known as partial resection or parenchyma-sparing resection, involves a less extensive hepatectomy compared with AR and aims to achieve minimum sufficient margins while preserving as much liver parenchyma as possible, regardless of the border of the anatomical segments. The surgeon chose to perform AR or NAR after a comprehensive evaluation of the patient’s condition. The Pringle maneuver was routinely performed during surgery in cycles of 15/5 min of clamping/unclamping. Anesthesia was managed in all centers using a restrictive intravenous rehydration approach combined with low central venous pressure during liver transection.
Follow-up and recurrence management
Follow-up included serum AFP, liver function, abdominal ultrasound, enhanced CT examination and lung examination every 2–3 months in the first year, and every 6 months thereafter until the patient died or withdrew from the follow-up. The diagnosis of tumor recurrence was based on elevated serum alpha-fetoprotein (AFP) levels and characteristic imaging findings. Once tumor recurrence was identified, aggressive treatment such as renewed hepatic resection, radiofrequency ablation, transarterial chemoembolization (TACE), or sorafenib was given depending on the patient’s general condition, liver function reserve, and pattern of tumor recurrence.
Overall survival (OS) was defined as the time between surgery and the date of death, or the time when the patient was last seen alive at follow-up. Recurrence-free survival (RFS) was defined as the time interval between surgery and the first diagnosis of HCC recurrence or the last follow-up. The deadline for follow-up in all centers was December 2021.
Clinicopathological variables and study outcomes
Preoperative variables included patient age, sex, cirrhosis, hepatitis, serum alanine aminotransferase (ALT), serum total bilirubin (TBIL), serum albumin (ALB), AFP, platelet count (PLT), hemoglobin (Hb), Child-Pugh classification, and prothrombin time (PT). The final diagnosis of HCC was confirmed by histopathological examination, and microvascular invasion (MVI) was determined by histopathological examination of the resected surgical specimens. Tumor-related variables included largest tumor diameter, number of tumors, MVI, and tumor differentiation. Surgical type (LH or open hepatectomy), type of hepatectomy (AR or NAR) and extent of hepatectomy (minor or major) were retrieved from the electronic medical records system. A major hepatectomy was defined as a hepatectomy of three or more Couinaud segments24.
The short-term outcomes included intraoperative parameters and postoperative outcomes. Operation-related variables included the duration of surgery, intraoperative blood loss, and blood transfusion. Postoperative outcomes mainly included postoperative complications, recovery results, and postoperative hospital stay (POHS), as well as pathological evaluation of the surgical margin. The complications included postoperative bile leakage (POBL), pleural effusion, ascites, abdominal bleeding, incision infection, abdominal infection, pulmonary infection, and posthepatectomy liver failure (PHLF). POBL was defined according to the 2010 International Study Group of Liver Surgery (ISGLS)25. PHLF was defined as a serum total bilirubin level exceeding 50 μmol/l and prothrombin time lower than 50% on postoperative day 526. Morbidity was graded according to Clavien–Dindo surgical complications27. In addition, liver function indexes were recorded on the first, third, and fifth days after operation (POD1, POD3, and POD5). Readmissions within 30 days and the 90-day mortality rate were also recorded. We identified the incidence and patterns of recurrence and long-term survival outcomes, including OS and RFS.
Statistical analysis
The Kolmogorov–Smirnov method was used to test the normality of continuous data, which were compared using a two-sided t-test for normally distributed parameters, expressed as the mean±SD, or the Wilcoxon–Mann–Whitney test for non-normally distributed parameters, expressed as the median (interquartile range). Comparisons between groups of counted data were made using the χ2 test or Fisher’s exact test. To balance the differences in baseline characteristics due to possible selection bias between patients in the 3D and 2D groups across the five centers, propensity score matching (PSM) and stabilized inverse probability of treatment weighting (IPTW) were used. Variables entered into the propensity model included age, sex, hepatitis, cirrhosis, Child-Pugh classification, AFP, ALT, TBIL, ALB, Hb, PLT, PT, maximum tumor diameter, number of tumors, degree of differentiation, MVI, surgical type, type of hepatectomy, and extent of hepatectomy. The propensity scores were calculated using a multivariate logistic regression model, and the PSM analysis was performed using a 1:1 nearest neighbor matching algorithm with a caliper of 0.03 without replacement. For the stabilized IPTW method, a pseudo-population was created by weighting the inverse probability of a patient based on the propensity score. This model preserved the size of the study cohort, no study participants were removed, had advantages over the PSM method, and reduced the occurrence of false positives compared with IPTW28.
The standardized mean difference (SMD) was calculated to assess the balance between the two groups at baseline, and SMD ≤0.1 indicated the best balance. Kaplan–Meier curves were used to analyze OS and RFS, and log-rank tests were used for comparisons. Univariate regression analysis was used to explore potential risk factors associated with RFS. The statistically significant variables in univariate analysis and other variables considered to have an influence on the outcome were further incorporated into multivariate analysis. The threshold of statistical significance was set at P<0.05. Data were analyzed using R Studio for Windows version 4.0.5 (R Studio Inc.) and SPSS Statistics Software version 25.0 (IBM Corp.).
Results
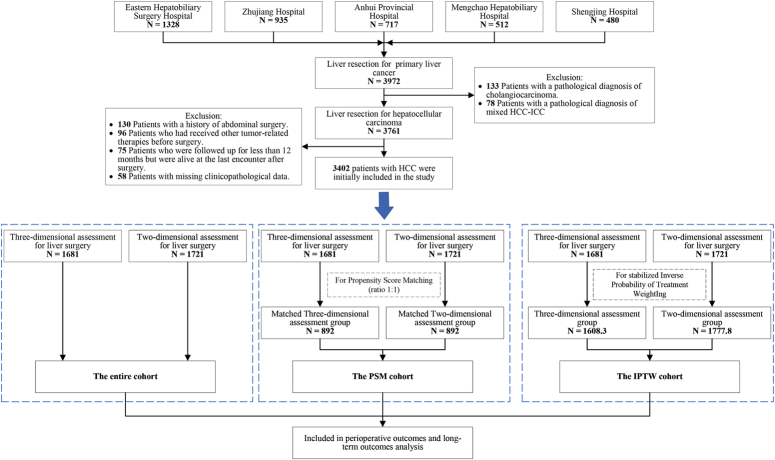
Between 2016 and 2020, 3972 patients underwent curative hepatectomy in the participating centers, of which 3402 met the inclusion criteria and were available for further analysis. Among them, 1681 patients received 3D-RVT preoperatively (3D group), while the remaining 1721 underwent 2D imaging assessment (2D group). (See Tables, Supplemental Digital Content 1, http://links.lww.com/JS9/B711, which shows the institutional distribution of these patients and the distribution of the year of surgery.) The PSM created 892 patient pairs who underwent 3D and 2D assessment, while IPTW created 1608.3 standardized patients who underwent 3D assessment and1777.8 who underwent 2D assessment (Fig. 2).
Figure 2.
Flow diagram of participant selection.
Patient characteristics
The demographics, clinical characteristics, and tumor-related variables of patients from the entire cohort, PSM and IPTW cohorts are summarized in Table 1. Compared to the 2D group, the 3D group had a higher proportion of individuals with cirrhosis (48.2 vs. 42.1%, P<0.001), AFP≥400 ng/ml (44.1 vs. 26.3%, P<0.001), ALT≥45 U/l (40.9 vs. 23.9%, P<0.001), Hb<100 g/l (5.5 vs. 3.3%, P=0.002), PLT<100 ×109/l (9.3 vs. 3.9%, P<0.001), LH type (40.2 vs. 18.1%, P<0.001), AR type (87.7 vs. 62.1%, P<0.001), major hepatectomy (73.4 vs. 34.2%, P<0.001) and tumor diameter≥5 cm (57.6 vs. 50%, P<0.001), but a lower proportion of male patients (75.4 vs. 84.7%, P<0.001), hepatitis (62.7 vs. 78.5%, P<0.001), TBIL≥17.5 μmol/l (31.0 vs. 38.5%, P<0.001), ALB<35 g/l (11.5 vs. 18.8%, P<0.001), PT≥13.5 s (20.4 vs. 26.5%, P<0.001), multiple tumors (10.4 vs. 20.0%, P<0.001), poor differentiation (25.3 vs. 34.6%, P<0.001), and positive MVI (34.3 vs. 41.9%, P<0.001).
Table 1.
Demographic and clinical characteristics of patients stratified by 3D-RVT assessment use.
| The entire cohort | The PSM cohort | The stabilized IPTW cohort | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristics | 3D group (n=1681) | 2D group (n=1721) | SMD | P | 3D group (n=892) | 2D group (n=892) | SMD | P | 3D group (n=1608.3) | 2D group (n=1777.8) | SMD | P |
| Age (year)a | 54.0 (45.0–61.0) | 55.0 (47.0–63.0) | 0.111 | 0.002 | 54.0 (45.0–62.0) | 54.0 (45.0–62.0) | 0.018 | 0.708 | 54.0 (46.0–61.0) | 54.0 (45.0–62.0) | 0.011 | 0.206 |
| Sex, male | 1267 (75.4) | 1457 (84.7) | 0.234 | <0.001 | 702 (78.7) | 716 (80.3) | 0.036 | 0.412 | 1286.0 (80.0) | 1419.3 (79.8) | 0.003 | 0.932 |
| Hepatitis | 1054 (62.7) | 1351 (78.5) | 0.352 | <0.001 | 631 (70.7) | 635 (71.2) | 0.009 | 0.835 | 1123.7 (69.9) | 1252.5 (70.5) | 0.013 | 0.707 |
| Cirrhosis | 811 (48.2) | 724 (42.1) | 0.124 | <0.001 | 420 (47.1) | 409 (45.9) | 0.025 | 0.602 | 721.2 (44.8) | 777.9 (43.8) | 0.022 | 0.533 |
| Child-Pugh | 0.017 | 0.629 | 0.000 | 1.000 | 0.017 | 0.655 | ||||||
| Class A | 1669 (99.3) | 1711 (99.4) | 887 (99.4) | 887 (99.4) | 1597.9 (99.4) | 1768.6 (99.5) | ||||||
| Class B | 12 (0.7) | 10 (0.6) | 5 (0.6) | 5 (0.6) | 10.4 (0.6) | 9.2 (0.5) | ||||||
| AFP (ng/ml) | 0.378 | <0.001 | 0.041 | 0.375 | 0.034 | 0.351 | ||||||
| <400 | 940 (55.9) | 1268 (73.7) | 560 (62.8) | 578 (64.8) | 1019.9 (63.4) | 1156.3 (65.0) | ||||||
| ≥400 | 741 (44.1) | 453 (26.3) | 332 (37.2) | 314 (35.2) | 588.4 (36.6) | 621.5 (35.0) | ||||||
| ALT (U/l) | 0.368 | <0.001 | 0.027 | 0.550 | 0.026 | 0.456 | ||||||
| <45 | 994 (59.1) | 1309 (76.1) | 580 (65.0) | 592 (66.4) | 1009.0 (62.7) | 1092.8 (61.5) | ||||||
| ≥45 | 687 (40.9) | 412 (23.9) | 312 (35.0) | 300 (33.6) | 599.3 (37.3) | 685.0 (38.5) | ||||||
| TBIL (μmol/l) | 0.159 | <0.001 | 0.036 | 0.453 | 0.008 | 0.827 | ||||||
| <17.5 | 1160 (69.0) | 1058 (61.5) | 598 (67.0) | 583 (65.4) | 1059.5 (65.9) | 1177.6 (66.2) | ||||||
| ≥17.5 | 521 (31.0) | 663 (38.5) | 294 (33.0) | 309 (34.6) | 548.8 (34.1) | 600.2 (33.8) | ||||||
| ALB (g/l) | 0.204 | <0.001 | 0.056 | 0.280 | 0.003 | 0.963 | ||||||
| <35 | 194 (11.5) | 324 (18.8) | 120 (13.5) | 136 (15.2) | 265.3 (16.5) | 291.3 (16.4) | ||||||
| ≥35 | 1487 (88.5) | 1397 (81.2) | 772 (86.5) | 756 (84.8) | 1343.0 (83.5) | 1486.5 (83.6) | ||||||
| Hb (g/l) | 0.106 | 0.002 | 0.000 | 1.000 | 0.027 | 0.491 | ||||||
| <100 | 92 (5.5) | 57 (3.3) | 35 (3.9) | 35 (3.9) | 80.6 (5.0) | 99.8 (5.6) | ||||||
| ≥100 | 1589 (94.5) | 1664 (96.7) | 857 (96.1) | 857 (96.1) | 1527.7 (95.0) | 1677.9 (94.4) | ||||||
| PLT (×109/l) | 0.220 | <0.001 | 0.042 | 0.277 | 0.006 | 0.896 | ||||||
| <100 | 157 (9.3) | 67 (3.9) | 60 (6.7) | 49 (5.5) | 118.8 (7.4) | 134.0 (7.5) | ||||||
| ≥100 | 1524 (90.7) | 1654 (96.1) | 832 (93.3) | 843 (94.5) | 1489.5 (92.6) | 1643.8 (92.5) | ||||||
| PT (s) | 0.144 | <0.001 | 0.011 | 0.827 | 0.004 | 0.938 | ||||||
| <13.5 | 1338 (79.6) | 1265 (73.5) | 666 (74.7) | 670 (75.1) | 1172.2 (72.9) | 1292.4 (72.7) | ||||||
| ≥13.5 | 343 (20.4) | 456 (26.5) | 226 (25.3) | 222 (24.9) | 436.1 (27.1) | 485.4 (27.3) | ||||||
| Surgical type | 0.501 | <0.001 | 0.057 | 0.194 | 0.024 | 0.507 | ||||||
| LH | 676 (40.2) | 312 (18.1) | 274 (30.7) | 249 (27.9) | 506.6 (31.5) | 579.6 (32.6) | ||||||
| OH | 1005 (59.8) | 1409 (81.9) | 618 (69.3) | 643 (72.1) | 1101.7 (68.5) | 1198.2 (67.4) | ||||||
| Type of hepatectomy | 0.620 | <0.001 | 0.010 | 0.856 | 0.061 | 0.079 | ||||||
| AR | 1475 (87.7) | 1068 (62.1) | 722 (80.9) | 725 (81.3) | 1258.2 (78.2) | 1345.3 (75.7) | ||||||
| NAR | 206 (12.3) | 653 (37.9) | 170 (19.1) | 167 (18.7) | 350.1 (21.8) | 432.5 (24.3) | ||||||
| Extent of hepatectomyb | 0.855 | <0.001 | 0.000 | 1.000 | 0.037 | 0.299 | ||||||
| Major | 1234 (73.4) | 589 (34.2) | 530 (59.4) | 530 (59.4) | 904.1 (56.2) | 966.8 (54.4) | ||||||
| Minor | 447 (26.6) | 1132 (65.8) | 362 (40.6) | 362 (40.6) | 704.2 (43.8) | 811.0 (45.6) | ||||||
| Diameter (cm)c | 0.153 | <0.001 | 0.025 | 0.600 | 0.004 | 0.918 | ||||||
| <5 | 712 (42.4) | 860 (50.0) | 392 (43.9) | 403 (45.2) | 743.6 (46.2) | 818.3 (46.0) | ||||||
| ≥5 | 969 (57.6) | 861 (50.0) | 500 (56.1) | 489 (54.8) | 864.7 (53.8) | 959.5 (54.0) | ||||||
| Tumor number | 0.269 | <0.001 | 0.048 | 0.397 | 0.002 | 0.963 | ||||||
| Single | 1506 (89.6) | 1377 (80.0) | 759 (85.1) | 746 (83.6) | 1349.0 (83.9) | 1492.4 (83.9) | ||||||
| Multiple | 175 (10.4) | 344 (20.0) | 133 (14.9) | 146 (16.4) | 259.3 (16.1) | 285.4 (16.1) | ||||||
| Differentiation | 0.204 | <0.001 | 0.023 | 0.643 | 0.033 | 0.349 | ||||||
| Well-moderate | 1255 (74.7) | 1125 (65.4) | 625 (70.1) | 616 (69.1) | 1133.3 (70.5) | 1226.0 (69.0) | ||||||
| Poor | 426 (25.3) | 596 (34.6) | 267 (29.9) | 276 (30.9) | 475.0 (29.5) | 551.8 (31.0) | ||||||
| MVI | 0.156 | <0.001 | 0.019 | 0.695 | 0.038 | 0.285 | ||||||
| Negative | 1104 (65.7) | 1000 (58.1) | 559 (62.7) | 567 (63.6) | 1027.0 (63.9) | 1102.5 (62.0) | ||||||
| Positive | 577 (34.3) | 721 (41.9) | 333 (37.3) | 325 (36.4) | 581.3 (36.1) | 675.3 (38.0) | ||||||
Values in parentheses are percentages unless indicated otherwise.
Values are median (i.q.r.).
Major liver resection was defined as removal of three or more liver segments.
Diameter refers to the maximum tumor diameter that can be obtained at the time of preoperative evaluation.
2D, two-dimensional; 3D, three-dimensional; 3D-RVT, three-dimensional reconstruction visualization technology; AFP, α-fetoprotein; ALB, albumin; ALT, alanine aminotransferase; AR, anatomical resection; Hb, hemoglobin; IPTW, inverse probability of treatment weighting; LH, laparoscopic hepatectomy; MVI, microvascular invasion; NAR, nonanatomical resection; OH, open hepatectomy; PLT, platelet; PSM, propensity score matching; PT, prothrombin time; SMD, standardized mean difference; TBIL, total bilirubin.
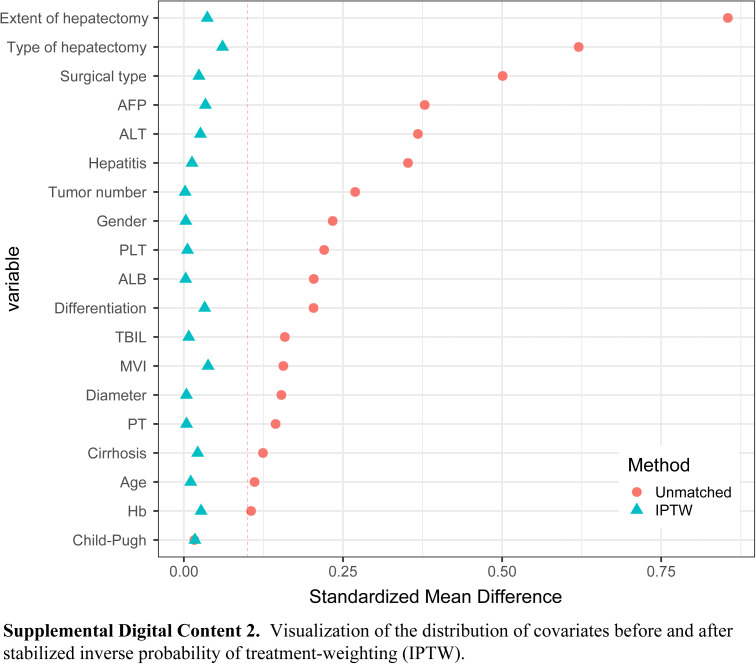
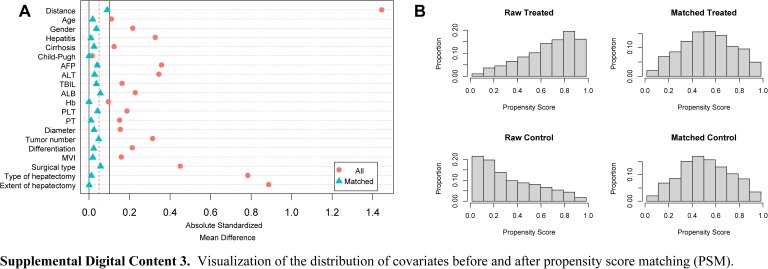
Covariate distributions between patients in the 2D and 3D groups were found to be balanced after conditioning based on the propensity score. There were no significant differences between the two groups in the PSM and IPTW cohorts (all SMD<0.1). (See Figures, Supplemental Digital Content 2, http://links.lww.com/JS9/B712 and Supplemental Digital Content 3, http://links.lww.com/JS9/B713, which demonstrate the visualization of the SMD before and after PSM and IPTW.)
Detailed information on the tumor node metastasis (TNM) classification, Barcelona Clinic Liver Cancer (BCLC) staging, and China liver cancer (CNLC) staging data for the 3402 patients in this study can be found in the supplementary materials (see Tables, Supplemental Digital Content 1, http://links.lww.com/JS9/B711, which shows the staging details of the patients in the three cohorts).
Comparison of short-term outcomes
The comparison of perioperative and postoperative outcomes between patients in the 3D and 2D groups in the entire cohort, PSM, and IPTW cohorts is shown in Table 2.
Table 2.
Comparisons for perioperative outcomes, follow-up, and recurrence patterns between 3D and 2D groups in unweighted sample, propensity score-matched sample, and inverse probability of treatment-weighted sample.
| The entire cohort | The PSM cohort | The stabilized IPTW cohort | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristics | 3D group (n=1681) | 2D group (n=1721) | P | 3D group (n=892) | 2D group (n=892) | P | 3D group (n=1608.3) | 2D group (n=1777.8) | P |
| Operation time (min)* | 180.0 (120.0–300.0) | 240.0 (160.0–310.0) | <0.001 | 165.0 (120.0–240.0) | 260.0 (200.0–330.0) | <0.001 | 180.0 (120.0–300.0) | 260.0 (180.0–330.0) | <0.001 |
| Blood loss (ml)* | 200.0 (100.0–300.0) | 200.0 (100.0–300.0) | 0.195 | 200.0 (100.0–300.0) | 200.0 (100.0–300.0) | 0.027 a | 200.0 (100.0–300.0) | 200.0 (100.0–300.0) | 0.655 |
| Blood transfusion | 321 (19.1) | 340 (19.8) | 0.627 | 184 (20.6) | 188 (21.1) | 0.816 | 309.8 (19.3) | 417.2 (23.5) | 0.003 |
| Morbidity | 307 (18.3) | 605 (35.2) | <0.001 | 184 (20.6) | 246 (27.6) | 0.001 | 339.0 (21.1) | 582.8 (32.8) | <0.001 |
| Complications | – | – | – | ||||||
| Grade I or II | 173 (10.3) | 457 (26.6) | 106 (57.6) | 150 (61.0) | 203.0 (12.6) | 416.3 (23.4) | |||
| Grade III or IV or V | 134 (8.0) | 148 (8.6) | 78 (42.4) | 96 (39.0) | 136.0 (8.5) | 166.5 (9.4) | |||
| Liver failure | 39 (2.3) | 73 (4.2) | 0.002 | 21 (2.4) | 46 (5.2) | 0.002 | 33.0 (2.1) | 82.6 (4.7) | <0.001 |
| POHS (day)* | 8.0 (7.0–10.0) | 8.0 (7.0–11.0) | 0.240 | 8.0 (7.0–9.0) | 8.0 (7.0–11.0) | <0.001 b | 8.0 (7.0–9.0) | 8.0 (7.0–11.0) | <0.001 c |
| 30-day readmission | 37 (2.2) | 75 (4.4) | <0.001 | 20 (2.2) | 25 (2.8) | 0.450 | 26.8 (1.7) | 59.9 (3.4) | 0.002 |
| Resection margin ≥10 mm | 1339 (79.7) | 1226 (71.2) | <0.001 | 703 (78.8) | 603 (67.6) | <0.001 | 1262.1 (78.5) | 1228.7 (69.1) | <0.001 |
| Death during follow-up | 363 (21.6) | 380 (22.1) | 0.732 | 178 (20.0) | 184 (20.6) | 0.724 | 315.3 (19.6) | 353.2 (19.9) | 0.863 |
| Recurrence | 494 (29.4) | 619 (36.0) | <0.001 | 276 (30.9) | 300 (33.6) | 0.224 | 478.8 (29.8) | 616.2 (34.6) | 0.003 |
| Recurrence pattern | – | – | – | ||||||
| Intrahepatic | 245 (14.6) | 278 (16.2) | 143 (16.0) | 139 (15.6) | 251.8 (15.7) | 271.2 (15.3) | |||
| Extrahepatic | 158 (9.4) | 203 (11.8) | 104 (11.7) | 108 (12.1) | 162.2 (10.1) | 214.9 (12.1) | |||
| Both | 91 (5.4) | 138 (8.0) | 29 (3.3) | 53 (5.9) | 64.8 (4.0) | 130.2 (7.3) | |||
Values in parentheses are percentages unless indicated otherwise.
values are median (i.q.r.).
The mean±SD of intraoperative blood loss for 3D group and 2D group were 237.3±160.4, 298.8±434.3 ml, respectively.
The mean±SD of postoperative hospital stay for 3D group and 2D group were 7.9±1.5, 9.3±4.6 days, respectively.
The mean±SD of postoperative hospital stay for 3D group and 2D group were 8.3±2.1, 9.5±4.4 days, respectively.
2D, two-dimensional; 3D, three-dimensional; IPTW, inverse probability of treatment weighting; POHS, postoperative hospital stay; PSM, propensity score matching.
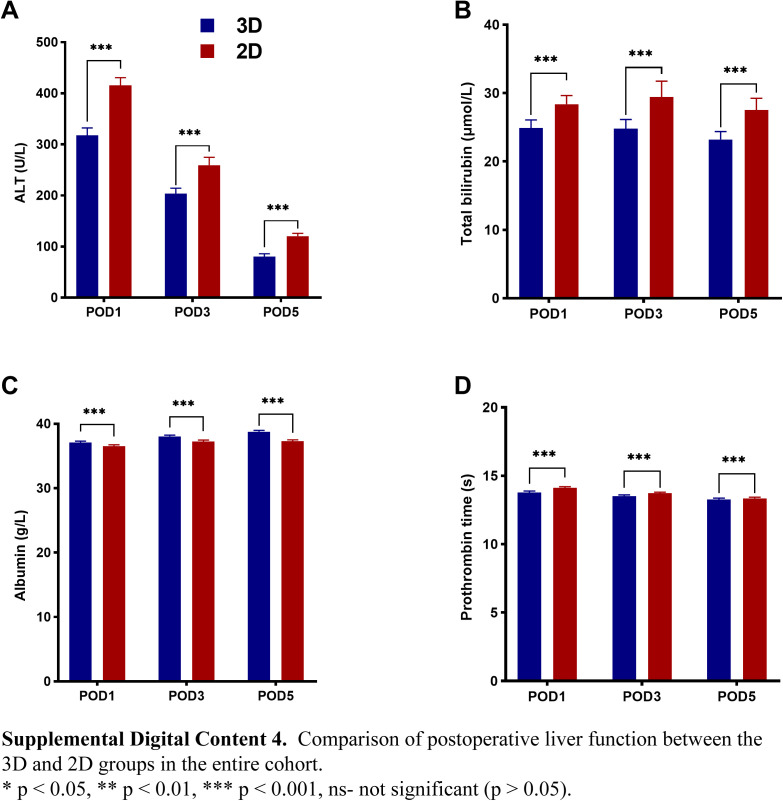
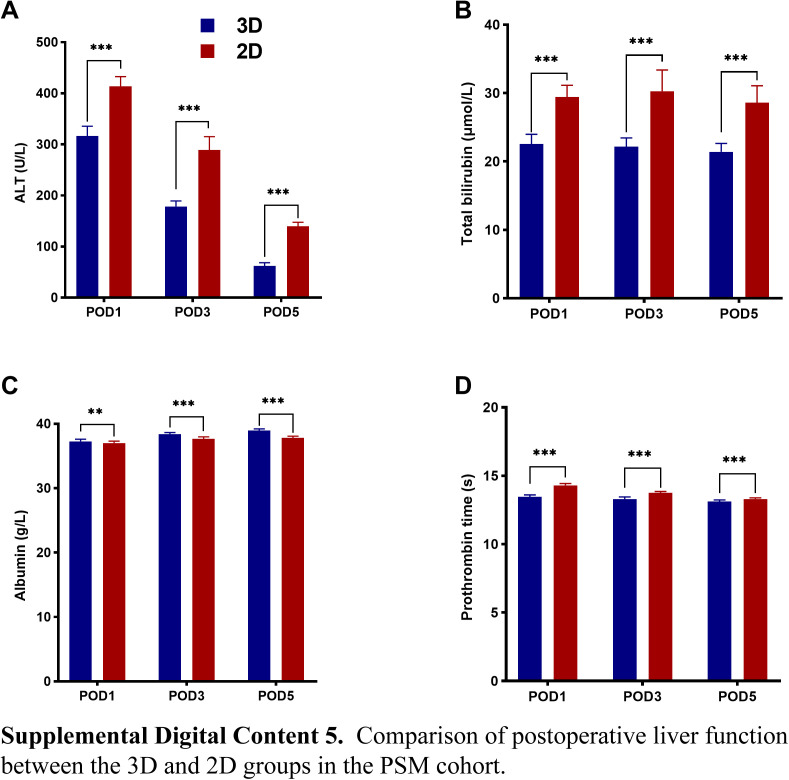
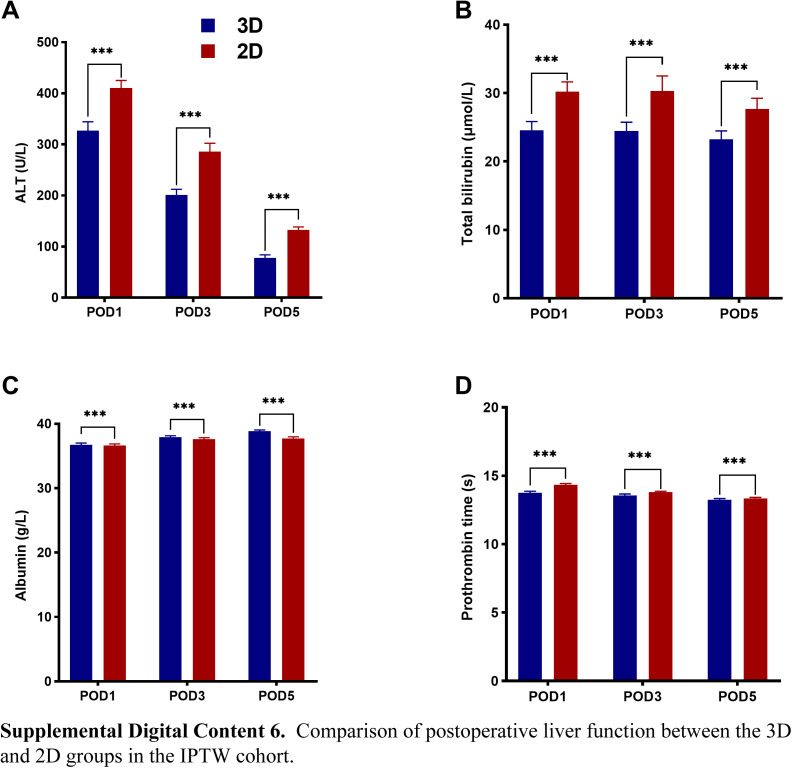
In the PSM and IPTW cohorts, the median operation time among patients in the 3D group was lower than in the 2D group [PSM cohort: 165.0 (120.0–240.0) vs. 260.0 (200.0–330.0) min, P<0.001; and IPTW cohort: 180.0 (120.0–300.0) vs. 260.0 (180.0–330.0), P<0.001]. Furthermore, the patients in the 3D group had lower morbidity (PSM cohort: 20.6 vs. 27.6%, P=0.001; and IPTW cohort: 21.1 vs. 32.8%, P<0.001), and PHLF proportion (PSM cohort: 2.4 vs. 5.2%, P=0.002; and IPTW cohort: 2.1 vs. 4.7%, P<0.001). In addition, the median POHS following hepatectomy was shorter in the 3D group [PSM cohort: 8.0 (7.0–9.0) vs. 8.0 (7.0–11.0) days, P<0.001; and IPTW cohort: 8.0 (7.0–9.0) vs. 8.0 (7.0–11.0) days, P<0.001], and the mean POHS were 7.9 vs. 9.3 days, and 8.3 vs. 9.5 days, respectively. The levels of ALT, TBIL, and PT on POD1, POD3, and POD5 were lower in the 3D group than in the 2D group (P<0.001). Furthermore, the average serum ALB levels were higher in the 3D group (P<0.001) (see Figure, Supplemental Digital Content 4–6 http://links.lww.com/JS9/B714, http://links.lww.com/JS9/B715, http://links.lww.com/JS9/B716, which summarizes the postoperative liver function data from the three cohorts).
The 3D group had a higher proportion of individuals with resection margin ≥10 mm (PSM cohort: 78.8 vs. 67.6%, P<0.001; and IPTW cohort: 78.5 vs. 69.1%, P<0.001).
Neither group had a perioperative death (within 90 days after surgery). Blood loss, intraoperative transfusion rates and 30-day unplanned readmission rates in both groups suggested inconsistent outcomes in the two cohorts. The instrumental variable analysis showed that the use of 3D-RVT has the potential to reduce blood loss and transfusion rates, but not 30-day readmission rates. (See Word file, Supplemental Digital Content 7, http://links.lww.com/JS9/B717, which shows the instrumental variable analysis for additional evaluation.)
Comparison of long-term outcomes
After discharge from the hospital, surgical patients were followed up through outpatient clinics, telephone calls, or WeChat. The median follow-up time in the 3D and 2D groups was 23.0 (17.0–31.0) vs. 22.0 (18.0–27.0) days in the entire cohort, and 26.0 (19.0–33.0) vs. 23.0 (20.0–33.0) days in the PSM cohort, respectively.
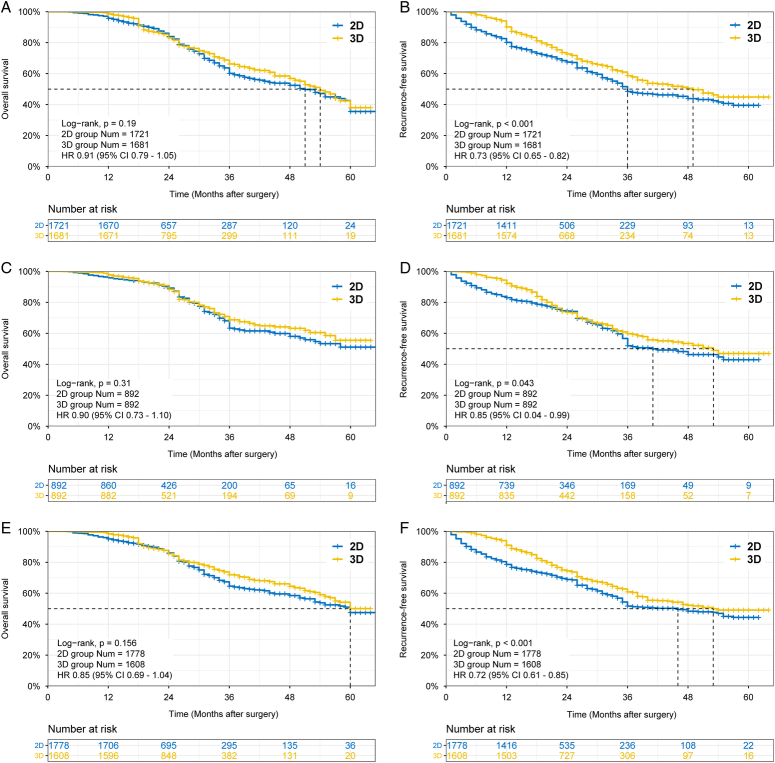
The OS and RFS curves for the three cohorts are shown in Figure 3. Mortality during follow-up was comparable between the two groups (PSM cohort: 20.0 vs. 20.6%, P=0.724; and IPTW cohort: 19.6 vs. 19.9%, P=0.863). In the PSM cohort, the 3-year and 5-year OS rates were 68.8 vs. 63.4% and 55.5 vs. 51.1% for the 3D and 2D groups, respectively (P=0.31). In the IPTW cohort, the 3-year and 5-year OS rates in the 3D and 2D groups were 71.9 vs. 64.6%, 50.1 vs. 47.5%, respectively (P=0.156).
Figure 3.
Survival analysis of patients undergoing hepatectomy in the 3D group versus 2D group in the three cohorts. (A) Comparison of OS between the two groups in the entire cohort (P=0.19); (B) Comparison of RFS between the two groups in the entire cohort (P<0.001); (C) Comparison of OS between the two groups in the PSM cohort (P=0.31); (D) Comparison of RFS between the two groups in the PSM cohort (P=0.043); (E) Comparison of OS between the two groups in the IPTW cohort (P=0.156); and (F) Comparison of RFS between the two groups in the IPTW cohort (P<0.001). 3D, three-dimensional; 2D, two-dimensional; OS, overall survival; RFS, recurrence-free survival.
However, the 3D group presented a lower recurrence rate than the 2D group [30.9 vs. 33.6% in the PSM cohort (P=0.224); and 29.8 vs. 34.6% in the IPTW cohort (P=0.003)]. The instrumental variable analysis showed that the use of 3D-RVT may reduce recurrence rates. The RFS was better in the 3D group than in the 2D group. In the PSM cohort, the 3-year and 5-year RFS rates were higher for the 3D group (59.8 vs. 52.0%, and 46.9 vs. 42.9%, respectively; P=0.043). Similarly, the IPTW cohort showed higher 3-year and 5-year RFS rates for the 3D group (60.9 vs. 51.7%, and 49.1 vs. 44.4%, respectively; P<0.001). In terms of recurrence pattern, intrahepatic recurrence was the most common.
Prognostic factors associated with RFS
Univariate and multivariate Cox-regression analyses of prognostic factors associated with RFS in the entire cohort, PSM, and IPTW cohorts are shown in Tables 3–5.
Table 3.
Univariate and multivariate analysis of recurrence-free survival for HCC patients in the entire cohort.
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| Characteristics | HR | 95% CI | P | HR | 95% CI | P |
| Group (3D vs. 2D) | 0.72 | (0.64–0.81) | <0.001 | 0.78 | (0.69–0.89) | <0.001 |
| Age (≥60 vs. <60 years) | 0.90 | (0.79–1.02) | 0.085 | |||
| Sex (Female vs. Male) | 0.88 | (0.75–1.02) | 0.093 | |||
| Hepatitis (Presence vs. Absence) | 1.04 | (0.91–1.18) | 0.580 | |||
| Cirrhosis (Yes vs. No) | 1.02 | (0.91–1.15) | 0.691 | |||
| AFP (≥400 vs. <400 ng/ml) | 0.93 | (0.82–1.05) | 0.251 | |||
| Surgical approach (OH vs. LH) | 0.94 | (0.82–1.07) | 0.369 | |||
| Resection approach (AR vs. NAR) | 0.65 | (0.57–0.74) | <0.001 | 0.68 | (0.59–0.77) | <0.001 |
| Blood transfusion (Yes vs. No) | 1.33 | (1.16–1.52) | <0.001 | 1.28 | (1.12–1.47) | <0.001 |
| Resection margin (≥10 vs. <10 mm) | 1.02 | (0.89–1.17) | 0.776 | |||
| Differentiation (Poor vs. Well-Moderated) | 1.07 | (0.94–1.22) | 0.282 | |||
| MVI (Positive vs. Negative) | 1.11 | (0.99–1.25) | 0.082 | |||
| Tumor diameter (≥5 vs. <5 cm)a | 1.29 | (1.15–1.46) | <0.001 | 1.31 | (1.16–1.48) | <0.001 |
| Tumor number (Multiple vs. Single) | 1.26 | (1.09–1.47) | 0.003 | 1.19 | (1.02–1.39) | 0.028 |
Tumor diameter refers to the maximum tumor diameter that can be obtained at the time of preoperative evaluation.
2D, two-dimensional; 3D, three-dimensional; AFP, α-fetoprotein; AR, anatomical resection; HR, hazard ratio; LH, laparoscopic hepatectomy; MVI, microvascular invasion; NAR, nonanatomical resection; OH, open hepatectomy.
Table 5.
Univariate and multivariate analysis of recurrence-free survival for HCC patients in the stabilized IPTW cohort.
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| Characteristics | HR | 95% CI | P | HR | 95% CI | P |
| Group (3D vs.2D) | 0.72 | (0.61–0.85) | <0.001 | 0.71 | (0.61–0.84) | <0.001 |
| Age (≥60 vs. <60 y) | 0.85 | (0.71–1.02) | 0.078 | |||
| Sex (Female vs. Male) | 0.85 | (0.68–1.06) | 0.149 | |||
| Hepatitis (Presence vs. Absence) | 1.04 | (0.86–1.25) | 0.713 | |||
| Cirrhosis (Yes vs. No) | 1.08 | (0.91–1.27) | 0.388 | |||
| AFP (≥400 vs. <400 ng/mL) | 1.06 | (0.88–1.27) | 0.562 | |||
| Surgical approach (OH vs. LH) | 0.88 | (0.71–1.08) | 0.204 | |||
| Resection approach (AR vs. NAR) | 0.81 | (0.68–0.96) | 0.018 | 0.75 | (0.64–0.89) | 0.001 |
| Blood transfusion (Yes vs. No) | 1.24 | (1.01–1.54) | 0.045 | 1.19 | (0.97–1.46) | 0.091 |
| Resection margin (≥10 vs. <10 mm) | 1.01 | (0.82–1.26) | 0.896 | |||
| Differentiation (Poor vs. Well-Moderated) | 0.95 | (0.78–1.14) | 0.563 | |||
| MVI (Positive vs. Negative) | 1.04 | (0.87–1.24) | 0.674 | |||
| Tumor diameter (≥5 vs. <5 cm)a | 1.47 | (1.24–1.74) | <0.001 | 1.49 | (1.25–1.78) | <0.001 |
| Tumor number (Multiple vs. Single) | 1.33 | (1.07–1.66) | 0.011 | 1.33 | (1.07–1.65) | 0.010 |
Tumor diameter refers to the maximum tumor diameter that can be obtained at the time of preoperative evaluation.
2D, two-dimensional; 3D, three-dimensional; AFP, α-fetoprotein; AR, anatomical resection; HR, hazard ratio; IPTW, inverse probability of treatment weighting; LH, laparoscopic hepatectomy; MVI, microvascular invasion; NAR, nonanatomical resection; OH, open hepatectomy.
Table 4.
Univariate and multivariate analysis of recurrence-free survival for HCC patients in the PSM cohort.
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| Characteristics | HR | 95% CI | P | HR | 95% CI | P |
| Group (3D vs.2D) | 0.84 | (0.72–0.99) | 0.043 | 0.82 | (0.70–0.97) | 0.020 |
| Age (≥60 vs. <60 years) | 0.87 | (0.73–1.03) | 0.109 | |||
| Sex (Female vs. Male) | 0.93 | (0.76–1.15) | 0.511 | |||
| Hepatitis (Presence vs. Absence) | 1.14 | (0.94–1.37) | 0.185 | |||
| Cirrhosis (Yes vs. No) | 1.04 | (0.88–1.23) | 0.623 | |||
| AFP (≥400 vs. <400 ng/mL) | 1.06 | (0.89–1.25) | 0.533 | |||
| Surgical approach (OH vs. LH) | 0.91 | (0.76–1.09) | 0.309 | |||
| Resection approach (AR vs. NAR) | 0.76 | (0.62–0.92) | 0.006 | 0.72 | (0.59–0.88) | 0.001 |
| Blood transfusion (Yes vs. No) | 1.27 | (1.06–1.53) | 0.011 | 1.22 | (1.01–1.47) | 0.039 |
| Resection margin (≥10 vs. <10 mm) | 1.04 | (0.86–1.26) | 0.681 | |||
| Differentiation (Poor vs. Well-Moderated) | 1.18 | (0.99–1.40) | 0.066 | |||
| MVI (Positive vs. Negative) | 1.09 | (0.92–1.29) | 0.330 | |||
| Tumor diameter (≥5 vs. <5 cm)a | 1.54 | (1.30–1.83) | <0.001 | 1.55 | (1.30–1.84) | <0.001 |
| Tumor number (Multiple vs. Single) | 1.11 | (0.90–1.38) | 0.327 | |||
Tumor diameter refers to the maximum tumor diameter that can be obtained at the time of preoperative evaluation.
2D, two-dimensional; 3D, three-dimensional; AFP, α-fetoprotein; AR, anatomical resection; HR, hazard ratio; LH, laparoscopic hepatectomy; MVI, microvascular invasion; NAR, nonanatomical resection; OH, open hepatectomy; PSM, propensity score matching.
Multivariate analysis revealed that preoperative 3D assessment (in the entire cohort: HR=0.78, 95% CI: 0.69–0.89, P<0.001; PSM cohort: HR=0.82, 95% CI: 0.70–0.97, P=0.020; IPTW cohort: HR=0.71, 95% CI: 0.61–0.84, P<0.001) and anatomical resection (in the entire cohort: HR=0.68, 95% CI: 0.59–0.77, P<0.001; PSM cohort: HR=0.72, 95% CI: 0.59–0.88, P=0.001; IPTW cohort: HR=0.75, 95% CI: 0.64–0.89, P=0.001) were protective factors for RFS.
Conversely, tumor diameter ≥5 cm (in the entire cohort: HR=1.31, 95% CI: 1.16–1.48, P<0.001; PSM cohort: HR=1.55, 95% CI: 1.30–1.84, P<0.001; IPTW cohort: HR=1.49, 95% CI: 1.25–1.78, P<0.001) was associated with poorer RFS.
In addition, intraoperative blood transfusion was suggested to be a possible risk factor for RFS in both the entire cohort and the PSM cohort (in the entire cohort: HR=1.28, 95% CI: 1.12–1.47, P<0.001; PSM cohort: HR=1.22, 95% CI: 1.01–1.47, P=0.039). In addition, multiple tumors may be associated with poorer RFS in both the entire cohort and the IPTW cohort (in the entire cohort: HR=1.19, 95% CI: 1.02–1.39, P=0.028; IPTW cohort: HR=1.33, 95% CI: 1.07–1.65, P=0.010).
Subgroup analysis based on the tumor characteristics or surgical type
Perioperative outcomes and recurrence rates were compared between the 3D and 2D groups within several subgroups stratified by tumor diameter (<3 cm, >10 cm, or other), number of tumors (single or multiple) and type of surgery (OH or LH). The OS and RFS were also compared between the two groups within each subgroup. Notably, propensity models were recalculated within each subgroup and a stabilized IPTW method was used to balance baseline characteristics between the 3D and 2D groups. Baseline variables were balanced between the 3D and 2D groups in all subgroups (P>0.05). The 3D group showed relatively better perioperative outcomes and lower recurrence rates in all subgroups, although there were some discrepancies in some subgroups. The detailed results can be seen in the supplementary materials (see Word file, Supplemental Digital Content 7, http://links.lww.com/JS9/B717, which shows the perioperative outcomes, recurrence rates, and long-term prognostic outcomes for the 3D and 2D groups within each subgroup).
Discussion
Hepatectomy remains a challenging procedure because of the complex and variable intrahepatic anatomy9,29–32. In the past, surgeons conducted preoperative evaluation using two-dimensional images such as CT and MRI33,34. They converted these 2D images into 3D models in their mind, a process that requires great experience. Expert surgeons are often able to complete the 2D to 3D transformation quickly and accurately in their minds for reliable surgical planning, but surgeons who have not yet reached the expert level may be more prone to miscalculation, especially when facing anatomical variants. The 3D-RVT can provide a 3D anatomical image, which to some extent reduces the need for 2D to 3D transformation in the surgeon’s mind and helps the surgeon plan the operation accurately35–38. The 3D-RVT technique plays four main roles in hepatectomy: 1) It provides a detailed 3D, rotating model of the liver anatomy, showing the mutual spatial location of the tumor, liver and intrahepatic structures; 2) It allows the systematic summarization and classification of variant types of the portal vein, hepatic artery, hepatic veins, and intrahepatic bile ducts, improving the surgeon’s understanding of the complexity and variability of the liver39–41; 3) It enables detailed portal vein territory analysis and individualized liver segmentation to divide the liver into subsegment units and guide AR12,42,43; 4) It enables virtual hepatectomy planning, whereby the software can calculate the residual liver volume and assess intact blood flow to the remnant liver11,17,44,45. It is worth noting that unlike most centers that only rely on imaging specialists for reconstruction10, our centers use a surgeon-led, human-computer interaction protocol for reconstruction and surgical planning46. This process leads to a deeper understanding of individual anatomical information, especially in the case of anatomical variants and complex surgical planning. As the surgeon must continuously conceptualize the simulated surgical scenario in their mind during this calibration process, it will also continuously improve the surgeon’s preoperative assessment and surgical planning skills, potentially easing the hepatectomy learning curve. We hoped that this distinctive protocol for 3D-RVT application would lead to better short-term and long-term results in the 3D group in this study. The results of a multicenter retrospective study can be affected by a number of confounding factors, so we chose the propensity score method for baseline balancing. Since PSM will lead to the loss of some cases, we also chose the stabilized IPTW method, which creates a pseudo-population but potentially reduces the false-positive rate compared to the general IPTW. Simultaneous use of these two PS methods thus increases the confidence of the results of this study. To our knowledge, this is the first multicenter study to investigate the impact of 3D-RVT on liver resection in patients with HCC.
In this study, both the PSM and IPTW cohorts suggested that the operation time was significantly shorter in the 3D group, which may be attributed to the following reasons. First, the length of surgery is related to the complexity of the surgery itself and the surgeon’s operating skills and experience. During surgery, the surgeons sometimes need to think about the image to obtain more information as a surgical guide, they need to reconstruct the stereoscopic image in their mind, which may take extra time, especially for young surgeons. Furthermore, there is subjective bias in the understanding of the same 2D image by different physicians, even those with the same title and experience. The reconstructed image in the surgeon’s mind can vary due to differences in understanding, which can affect the intraoperative management of important vessels and even lead to prolonged operation time due to the occurrence of unintended bleeding. The 3D-RVT enables surgeons with varying levels of expertise to discuss a unified model, even during surgery, resulting in fewer unforeseen complications and therefore shorter operation times.
The reduction of morbidity and the incidence of PHLF in the 3D group can be attributed to several factors. 1) Accidental intraoperative vascular and biliary injuries are a major cause of increased postoperative morbidity. Here, the 3D-RVT allows for more detailed and accurate preoperative planning, enabling the surgeon to avoid vital vessels, bile ducts or vessels at risk of bleeding when selecting the surgical access. It also offers a more intuitive understanding of the patient’s intrahepatic ductal alignment, all of which has the potential to reduce the incidence of bleeding or bile leakage, particularly in patients with ductal variants. 2) The topology of the liver is altered during hepatectomy, resulting in changes of the spatial relationships among some anatomical structures, which can make identification of the ducts more difficult. Some cautious surgeons may determine the relationship between blood vessels and bile ducts by reviewing CT/MRI images during surgery, but this approach is time-consuming and subjective. By contrast, 3D-RVT provides anatomical information from any angle during the procedure, effectively avoiding hemorrhage due to injury to major hepatic vessels or bile leakage due to injury to major bile ducts. 3) As PHLF is often caused by insufficient remnant liver volume, the liver volume calculation and virtual hepatectomy provided by 3D-RVT software allows for a safer procedure17,18. What is more, 3D-RVT allows the preoperative determination of the integrity of the remnant liver inflow and outflow tracts during surgical planning, which in turn reduces PHLF caused by remnant liver congestion (RLC) or remnant liver ischemia (RLI)45,47. The reduction of bleeding and bile leaks shortens the operation time, reduces morbidity, speeds up the recovery, and may shorten postoperative hospital stay, as suggested in both the PSM and IPTW cohorts.
Long-term outcomes are crucial for patients with HCC and play a key role in the selection of surgical approaches1,2. There was no significant difference in OS between the two groups in this study. Since subsequent therapy such as radiotherapy, transcatheter arterial chemoembolization, hepatic arterial infusion chemotherapy, multikinase inhibitors and immune checkpoint inhibitors can also be used to prolong the OS of patients with tumor recurrence after hepatectomy, this study, like many others, recorded a high 5-year survival rate of 40–60% after surgery31,48. However, the 3D group showed better RFS than the 2D group in all cohorts, which may be related to several factors as follows: 1) Previous studies have shown that AR for HCC results in better oncological outcomes and improved RFS compared to NAR49. In this study, the differences in liver resection modalities between the two groups were balanced using the PS method in the baseline data, and the proportion of AR was comparable in the PSM and IPTW cohorts. The 2D group divided the liver segments according to the Couinaud method, not the portal territory, which cannot completely remove the liver parenchyma of the portal vein territory, and there is a possibility of leaving minute cancerous foci in the territory. However, 3D-RVT has the potential to enable total excision of the liver parenchyma by relying on analysis of the portal vein, which can eliminate minute cancerous foci and reduce the likelihood of recurrence. 2) It has been suggested that laparoscopic manipulation can limit tumor dissemination by reducing tumor turnover. Some studies suggested that LH reduces immunosuppression, thus potentially reducing the rate of tumor recurrence and offering a better prognosis in some studies50,51. However, the limited field of view of laparoscopy can lead to disorientation, resulting in incomplete resection of the tumor or inadvertent vascular injury and ultimately tumor spread. With the full use of 3D-RVT, the understanding of the complexity and variability of intrahepatic anatomy is also improving, enhancing the surgeon’s ability to maintain correct direction of transection and complete the resection of the tumor during the procedure. 3) Some studies have shown that wide-margin hepatectomy (≥10 mm) is superior to narrow-margin hepatectomy (<10 mm), especially in patients with preoperatively predictable MVI52,53. In the present study, more wide margins (≥10 mm) were achieved in the 3D group, which may have played a role in the better RFS. To our knowledge, there are few reports on the impact of 3D-RVT on long-term outcomes. Li et al.17 demonstrated that patients undergoing preoperative 3D evaluation before extensive hepatectomy achieved higher 3-year OS and RFS. Mise et al.11 reported better RFS in the virtual hepatectomy group, but only in HCC patients with impaired liver function and limited disease, while there was no difference in the overall comparison. However, these reports were limited by small sample sizes and potential heterogeneity, which precluded them from reaching a more precise conclusion.
The results of multivariate analysis in our study showed that tumors with a diameter ≥5 cm and multiple tumors were significant independent risk factors reducing RFS, which was consistent with the results of previous studies54–56. Notably, intraoperative blood transfusion was also a risk factor associated with recurrence. It has been demonstrated that red blood cell transfusion promotes cancer recurrence, which may be related to the potential triggering of antitumor immune mechanisms underlying allogeneic perioperative transfusions57. In addition, the lymphocyte component of blood products used for transfusion may also reduce patient immunity, leading to HCC recurrence58. This provides a new perspective on HCC recurrence and may reveal why 3D-RVT may contribute to better RFS in HCC patients. The results of the multivariate analyses did not suggest AFP and MVI as risk factors. This may be due to the fact that the patients included in this study were only from the eastern population and the sample size was reduced during the case screening process, which led to different results of multivariate analyses in different studies59,60. In addition, the different variables included in the model may have contributed to this result61,62.
The present study has some limitations. First, different follow-up times in the two groups of patients and retrospective study design were major limitations in the current study. The study may have suffered from selection bias and potential confounding because patients were not randomly assigned. The feasibility of surgery also depends on the surgeon’s technical skills and confidence in surgery, which also leads to differences in the selection of patients for surgery in different centers to a certain extent. Secondly, this study lacked a standardized postoperative treatment protocol, and there were subtle differences in postoperative treatment protocols at each institution. Nevertheless, since each institution participating in this study has good guideline compliance, we believe that this difference has a negligible impact on the results. Third, we did not focus on OS in the risk factor analyses. Since the wide choice of treatments available after recurrence, hence the findings from OS may not reflect the correlation between 3D-RVT and long-term outcomes in HCC patients. Finally, although 3D-RVT may lead to better RFS, due to the complex recurrence mechanism of HCC, the relationship of 3D-RVT with different recurrence patterns was not further analyzed in this study.
In conclusion, we conducted a broad clinical study based on data from five centers in China, which demonstrated that preoperative 3D-RVT for HCC hepatectomy helps achieve precise tumor resection, reduce intraoperative hepatic vascular injury, shorten the operation time, decrease the incidence of postoperative complications, and accelerate postoperative patient recovery, while potentially also improving RFS.
Ethical approval
The study was approved by the Institutional Review Board of the Zhujiang Hospital of Guangzhou, China (No: 2020-KY-040-02). According to the principles promulgated in the Declaration of Helsinki, informed consent to permit their data to be used for clinical research was obtained from all the patients, either at hospital admission or before surgery.
Sources of funding
This work was supported by the National Natural Science Foundation of China Mathematics Tianyuan Foundation (grant number 12026602); the National Natural Science Foundation of China (grant number 82272132); the promotion and application of digital minimally invasive navigation complex hepatectomy (grant number 20230322152307666); and promotion and application of tumor 3D visualization combined with molecular fluorescence imaging technology (grant number 20230319214525105).
Author contribution
X.Z., W.L., J.Y., and C.F.: contributed to the concept of the study; X.Z., H.T., and Y.D.: designed the study; Y.D., Y.Z., J.Y., and F.X.: contributed to the data collection; X.Z., Y.D., and Y.Z.: contributed to the data analysis and data interpretation; X.Z. and H.T.: drafted the manuscript; J.Z., W.J., J.L., C.D., N.X., N.Z., W.Z., and W.L.: contributed to critically reviewed and revised the manuscript; J.Y. and C.F.: provided research funding. All the authors reviewed the manuscript for important intellectual content and approved the final manuscript. X.Z., H.T., Y.D., and Y.Z.: contributed equally to this work as co-first authors.
Conflicts of interest disclosure
The authors declare that there are no competing interests.
Research registration unique identifying number (UIN)
Name of the registry: ClinicalTrials.gov (https://clinicaltrials.gov/) Research Registry (https://www.researchregistry.com/).
Unique Identifying number or registration ID: NCT05118451 researchregistry9804.
Hyperlink to your specific registration (must be publicly accessible and will be checked):https://clinicaltrials.gov/study/NCT05118451; https://researchregistry.knack.com/research-registry#home/registrationdetails/657a584a2dfd0d00267e9bd7/ This is the real-world retrospective cohort of a prospective RCT study (NCT05118451). Data from this cohort were used with patient informed consent and institutional ethical approval.
Guarantor
Chihua Fang and Jian Yang.
Data availability statement
Data are available on reasonable request to the corresponding author (fangchihua@smu.edu.cn or yangjian486@126.com).
Provenance and peer review
Not commissioned, externally peer-reviewed.
Supplementary Material
Supplementary Material
Footnotes
Xiaojun Zeng, Haisu Tao, Yanchen Dong, and Yuwei Zhang are shared first authors.
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal’s website, www.lww.com/international-journal-of-surgery.
Published online 19 January 2024
Contributor Information
Xiaojun Zeng, Email: smuzxj@hotmail.com.
Haisu Tao, Email: taohaisu_dr@126.com.
Yanchen Dong, Email: nmtdyc@163.com.
Yuwei Zhang, Email: zzzyw333@gmail.com.
Junying Yang, Email: yangjy2041@163.com.
Feichao Xuan, Email: xuanfeichao123@163.com.
Jian Zhou, Email: zhou.jian@zs-hospital.sh.cn.
Weidong Jia, Email: jwd1968@ustc.edu.cn.
Jingfeng Liu, Email: drjingfeng@126.com.
Chaoliu Dai, Email: daicl@sj-hospital.org.
Haoyu Hu, Email: 237621921@qq.com.
Nan Xiang, Email: zjyyxn@126.com.
Ning Zeng, Email: chen_ning16@foxmail.com.
Weiping Zhou, Email: ehphwp@126.com.
Wanyee Lau, Email: josephlau@surgery.cuhk.edu.hk.
Jian Yang, Email: yangjian486@126.com.
Chihua Fang, Email: fangchihua@smu.edu.cn.
References
- 1. Vogel A, Meyer T, Sapisochin G, et al. Hepatocellular carcinoma. Lancet 2022;400:1345–1362. [DOI] [PubMed] [Google Scholar]
- 2. Zhou J, Sun H, Wang Z, et al. Guidelines for the Diagnosis and Treatment of Hepatocellular Carcinoma (2019 Edition). Liver Cancer 2020;9:682–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vibert E, Schwartz M, Olthoff KM. Advances in resection and transplantation for hepatocellular carcinoma. J Hepatol 2020;72:262–276. [DOI] [PubMed] [Google Scholar]
- 4. Yang T, Lin S, Xie Q, et al. Impact of 3D printing technology on the comprehension of surgical liver anatomy. Surg Endosc 2019;33:411–417. [DOI] [PubMed] [Google Scholar]
- 5. Jiang J, Pei L, Jiang R. Clinical efficacy and safety of 3D vascular reconstruction combined with 3D navigation in laparoscopic hepatectomy: systematic review and meta-analysis. J Gastrointest Oncol 2022;13:1215–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. van Mierlo KM, Schaap FG, Dejong CH, et al. Liver resection for cancer: new developments in prediction, prevention and management of postresectional liver failure. J Hepatol 2016;65:1217–1231. [DOI] [PubMed] [Google Scholar]
- 7. Inoue Y, Fujii K, Ishii M, et al. Volumetric and functional regeneration of remnant liver after hepatectomy. J Gastrointest Surg 2019;23:914–921. [DOI] [PubMed] [Google Scholar]
- 8. Fang C, Zhang P, Qi X. Digital and intelligent liver surgery in the new era: prospects and dilemmas. EBioMedicine 2019;41:693–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen H, He Y, Jia W. Precise hepatectomy in the intelligent digital era. Int J Biol Sci 2020;16:365–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang Y, Cao D, Chen SL, et al. Current trends in three-dimensional visualization and real-time navigation as well as robot-assisted technologies in hepatobiliary surgery. World J Gastrointest Surg 2021;13:904–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mise Y, Hasegawa K, Satou S, et al. How has virtual hepatectomy changed the practice of liver surgery? experience of 1194 virtual hepatectomy before liver resection and living donor liver transplantation. Ann Surg 2018;268:127–133. [DOI] [PubMed] [Google Scholar]
- 12. Fang CH, An J, Bruno A, et al. Consensus recommendations of three-dimensional visualization for diagnosis and management of liver diseases. Hepatol Int 2020;14:437–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ni ZK, Lin D, Wang ZQ, et al. Precision liver resection: three-dimensional reconstruction combined with fluorescence laparoscopic imaging. Surg Innov 2021;28:71–78. [DOI] [PubMed] [Google Scholar]
- 14. Okuda Y, Taura K, Seo S, et al. Usefulness of operative planning based on 3-dimensional CT cholangiography for biliary malignancies. Surgery 2015;158:1261–1271. [DOI] [PubMed] [Google Scholar]
- 15. Nakayama K, Oshiro Y, Miyamoto R, et al. The effect of three-dimensional preoperative simulation on liver surgery. World J Surg 2017;41:1840–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhao D, Lau WY, Zhou W, et al. Impact of three-dimensional visualization technology on surgical strategies in complex hepatic cancer. Biosci Trends 2018;12:476–483. [DOI] [PubMed] [Google Scholar]
- 17. Li P, Wang M, Yang Y, et al. Preoperative three-dimensional versus two-dimensional evaluation in assessment of patients undergoing major liver resection for hepatocellular carcinoma: a propensity score matching study. Ann Transl Med 2020;8:182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang SM, Huang ZW, Cai L, et al. Three-dimensional versus two-dimensional video-assisted hepatectomy for liver disease: a meta-analysis of clinical data. Article Videosurgery Miniinvasive Tec 2021;16:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sheng W, Yuan C, Wu L, et al. Clinical application of a three-dimensional reconstruction technique for complex liver cancer resection. Surg Endosc 2022;36:3246–3253. [DOI] [PubMed] [Google Scholar]
- 20. Liu Y, Wang Q, Du B, et al. A meta-analysis of the three-dimensional reconstruction visualization technology for hepatectomy. Asian J Surg 2023;46:669–676. [DOI] [PubMed] [Google Scholar]
- 21. Mathew G, Agha R, Albrecht J, et al. STROCSS 2021: strengthening the reporting of cohort, cross-sectional and case-control studies in surgery. Int J Surg 2021;96:106165. [DOI] [PubMed] [Google Scholar]
- 22. Wakabayashi G, Cherqui D, Geller DA, et al. The Tokyo 2020 terminology of liver anatomy and resections: updates of the Brisbane 2000 system. J Hepatobiliary Pancreat Sci 2022;29:6–15. [DOI] [PubMed] [Google Scholar]
- 23. Lalmahomed ZS, Ayez N, van der Pool AE, et al. Anatomical versus nonanatomical resection of colorectal liver metastases: is there a difference in surgical and oncological outcome? World J Surg 2011;35:656–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Strasberg SM. Nomenclature of hepatic anatomy and resections: a review of the Brisbane 2000 system. J Hepatobiliary Pancreat Surg 2005;12:351–355. [DOI] [PubMed] [Google Scholar]
- 25. Koch M, Garden OJ, Padbury R, et al. Bile leakage after hepatobiliary and pancreatic surgery: a definition and grading of severity by the International Study Group of Liver Surgery. Surgery 2011;149:680–688. [DOI] [PubMed] [Google Scholar]
- 26. Balzan S, Belghiti J, Farges O, et al. The “50-50 criteria” on postoperative day 5: an accurate predictor of liver failure and death after hepatectomy. Ann Surg 2005;242:824–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Xu S, Ross C, Raebel MA, et al. Use of stabilized inverse propensity scores as weights to directly estimate relative risk and its confidence intervals. Value Health 2010;13:273–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wu Z, Tang H, Wang L, et al. Postoperative survival analysis of hepatocellular carcinoma patients with liver cirrhosis based on propensity score matching. BMC Surg 2022;22:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chen R, Fang C, Yang J. ASO author reflections: laparoscopic in situ anatomical mesohepatectomy for solitary massive HCC using combined intrafascial and extrafascial approaches with indocyanine green navigation: a new era of digital intelligent liver surgery. Ann Surg Oncol 2022;29:2041–2042. [DOI] [PubMed] [Google Scholar]
- 31. Llovet JM, Kelley RK, Villanueva A, et al. Hepatocellular carcinoma. Nat Rev Dis Primers 2021;7:6. [DOI] [PubMed] [Google Scholar]
- 32. Saito S, Yamanaka J, Miura K, et al. A novel 3D hepatectomy simulation based on liver circulation: application to liver resection and transplantation. Hepatology 2005;41:1297–1304. [DOI] [PubMed] [Google Scholar]
- 33. Maluccio M, Covey A. Recent progress in understanding, diagnosing, and treating hepatocellular carcinoma. CA Cancer J Clin 2012;62:394–399. [DOI] [PubMed] [Google Scholar]
- 34. Hanna GB, Shimi SM, Cuschieri A. Randomised study of influence of two-dimensional versus three-dimensional imaging on performance of laparoscopic cholecystectomy. Lancet 1998;351:248–251. [DOI] [PubMed] [Google Scholar]
- 35. Fang CH, Tao HS, Yang J, et al. Impact of three-dimensional reconstruction technique in the operation planning of centrally located hepatocellular carcinoma. J Am Coll Surg 2015;220:28–37. [DOI] [PubMed] [Google Scholar]
- 36. He YB, Bai L, Aji T, et al. Application of 3D reconstruction for surgical treatment of hepatic alveolar echinococcosis. World J Gastroenterol 2015;21:10200–10207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wei XB, Xu J, Li N, et al. The role of three-dimensional imaging in optimizing diagnosis, classification and surgical treatment of hepatocellular carcinoma with portal vein tumor thrombus. HPB (Oxford) 2016;18:287–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zeng X, Zhu W, Lin W, et al. Laparoscopic anatomical extended right posterior sectionectomy using virtual liver segment projection navigation and indocyanine green fluorescence imaging. Ann Surg Oncol 2023;30:375–376. [DOI] [PubMed] [Google Scholar]
- 39. Yan J, Feng H, Wang H, et al. Hepatic artery classification based on three-dimensional CT. Br J Surg 2020;107:906–916. [DOI] [PubMed] [Google Scholar]
- 40. Watanabe A, Yoshizumi T, Harimoto N, et al. Right hepatic venous system variation in living donors: a three-dimensional CT analysis. Br J Surg 2020;107:1192–1198. [DOI] [PubMed] [Google Scholar]
- 41. Fang C, You J, Lau W, et al. Anatomical variations of hepatic veins: three-dimensional computed tomography scans of 200 subjects. World J Surg 2012;36:120–124. [DOI] [PubMed] [Google Scholar]
- 42. Majumder P, Singh A, Wang Z, et al. Surface-fill hydrogel attenuates the oncogenic signature of complex anatomical surface cancer in a single application. Nat Nanotechnol 2021;16:1251–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ichida H, Imamura H, Yoshioka R, et al. Re-evaluation of the Couinaud classification for segmental anatomy of the right liver, with particular attention to the relevance of cranio-caudal boundaries. Surgery 2021;169:333–340. [DOI] [PubMed] [Google Scholar]
- 44. Alirr OI, Rahni AAA. Survey on liver tumour resection planning system: steps, techniques, and parameters. J Digit Imaging 2020;33:304–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Li XL, Xu B, Zhu XD, et al. Simulation of portal/hepatic vein associated remnant liver ischemia/congestion by three-dimensional visualization technology based on preoperative CT scan. Ann Transl Med 2021;9:756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tao H, Fang C, Yang J. ASO author reflections: laparoscopic anatomical segment 8 resection using digital intelligent liver surgery technologies: the combination of multiple navigation approaches. Ann Surg Oncol 2023;30:7388–7390. [DOI] [PubMed] [Google Scholar]
- 47. Cho JY, Han HS, Choi Y, et al. Association of remnant liver ischemia with early recurrence and poor survival after liver resection in patients with hepatocellular carcinoma. JAMA Surg 2017;152:386–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet 2018;391:1301–1314. [DOI] [PubMed] [Google Scholar]
- 49. Liao K, Yang K, Cao L, et al. Laparoscopic anatomical versus non-anatomical hepatectomy in the treatment of hepatocellular carcinoma: a randomised controlled trial. journal article; randomized controlled trial. Int J Surg 2022;102:106652. [DOI] [PubMed] [Google Scholar]
- 50. Sun Q, Zhang X, Gong X, et al. Survival analysis between laparoscopic and open hepatectomy for hepatocellular carcinoma: a meta-analysis based on reconstructed time-to-event data. Hepatol Int 2021;15:1215–1235. [DOI] [PubMed] [Google Scholar]
- 51. Chopra SS, Haacke N, Meisel C, et al. Postoperative immunosuppression after open and laparoscopic liver resection: assessment of cellular immune function and monocytic HLA-DR expression. Jsls 2013;17:615–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yang P, Si A, Yang J, et al. A wide-margin liver resection improves long-term outcomes for patients with HBV-related hepatocellular carcinoma with microvascular invasion. Surgery 2019;165:721–730. [DOI] [PubMed] [Google Scholar]
- 53. Shi M, Guo RP, Lin XJ, et al. Partial hepatectomy with wide versus narrow resection margin for solitary hepatocellular carcinoma: a prospective randomized trial. Ann Surg 2007;245:36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yang SY, Yan ML, Duan YF, et al. Perioperative and long-term survival outcomes of laparoscopic versus laparotomic hepatectomy for BCLC stages 0-A hepatocellular carcinoma patients associated with or without microvascular invasion: a multicenter, propensity score matching analysis. Hepatol Int 2022;16:892–905. [DOI] [PubMed] [Google Scholar]
- 55. Liang B, Yao S, Zhou J, et al. Liver resection versus radiofrequency ablation for hepatitis B virus-related small hepatocellular carcinoma. J Hepatocell Carcinoma 2018;5:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zhou YM, Zhang XF, Li B, et al. Postoperative complications affect early recurrence of hepatocellular carcinoma after curative resection. BMC Cancer 2015;15:689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Gong Y, Tang Y, Xue Y, et al. Impact of intraoperative allogenic and autologous transfusion on immune function and prognosis in patients with hepatocellular carcinoma. Medicine (Baltimore) 2020;99:e22568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zhao M, Duan X, Han X, et al. Sarcopenia and systemic inflammation response index predict response to systemic therapy for hepatocellular carcinoma and are associated with immune cells. Front Oncol 2022;12:854096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Leffondre K, Jager KJ, Boucquemont J, et al. Representation of exposures in regression analysis and interpretation of regression coefficients: basic concepts and pitfalls. Nephrol Dial Transplant 2014;29:1806–1814. [DOI] [PubMed] [Google Scholar]
- 60. Abd ElHafeez S, D’Arrigo G, Leonardis D, et al. Methods to analyze time-to-event data: the Cox Regression Analysis. Oxid Med Cell Longev 2021;2021:1302811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Chen Z, Yang F, Ge L, et al. Outcomes of renal cell carcinoma with associated venous tumor thrombus: experience from a large cohort and short time span in a single center. BMC Cancer 2021;21:766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Li L, Li X, Li W, et al. Prognostic models for outcome prediction in patients with advanced hepatocellular carcinoma treated by systemic therapy: a systematic review and critical appraisal. BMC Cancer 2022;22:750. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available on reasonable request to the corresponding author (fangchihua@smu.edu.cn or yangjian486@126.com).