Abstract
Background:
Carbon dioxide gas-induced pneumoperitoneum might be the reason for the shorter postoperative survival of patients with malignant tumors. Whether CO2 gas-induced pneumothorax has unfavorable impacts on the surgical and oncological outcomes of minimally invasive esophagectomy remains unclear.
Methods:
Between 2010 and 2016, a total of 998 patients with squamous cell carcinoma of the esophagus who received video-assisted surgery were registered from three large-volume medical centers. The overall survival (OS) and disease-free survival (DFS) were compared after using propensity score-matched and inverse probability-weighted methods. In addition, the tumor-relapse state was evaluated, and the relapse pattern was compared.
Results:
A total of 422 and 576 minimally invasive esophagectomies with intraoperative one-lung ventilation and CO2-induced pneumothorax were enrolled, respectively. The 5-year OS and DFS were similar between the CO2-induced pneumothorax (64.2% and 64.7%) and one-lung ventilation (65.3% and 62.4%) groups following propensity matching. The inverse probability weighting revealed similarly equal survival results in the two groups. The 5-year relapse rates were 35.1% and 30.6% in the one-lung ventilation and CO2-induced pneumothorax groups, respectively. Moreover, the relapse patterns were not significantly different between the two groups.
Conclusion:
The results of this study suggested that the use of intraoperative one-lung ventilation and CO2-induced pneumothorax have similar oncological outcomes; therefore, the two methods are both viable options in esophagectomy.
Keywords: artificial pneumothorax, carbon dioxide, esophageal cancer, surgery
Introduction
Highlights
The study performed a large, multicenter analysis using propensity score matching and inverse probability of treatment weight models.
The long-term effects of carbon dioxide pneumothorax and traditional one-lung ventilation methods were similar in minimally invasive esophagectomy.
The application of carbon dioxide pneumothorax ventilation methods is not correlated with esophageal squamous cell carcinoma relapse or different relapse patterns.
Carbon dioxide gas-induced pneumothorax has no unfavored impacts on the surgical and oncological outcomes of minimally invasive esophagectomy.
Compared with open esophagectomy, minimally invasive approaches are preferable for operable esophageal cancer patients due to their short-term surgical benefits1 and comparable long-term outcomes2. Carbon dioxide gas-induced pneumothorax (CO2 pneumothorax) and one-lung ventilation are two major methods to maintain lung collapse and therefore ensure successful procedures in the chest cavity. Intraoperative CO2 pneumothorax is similar to artificial pneumoperitoneum but with lower gas pressures of 6–12 mmHg3. Although CO2 pneumothorax and one-lung ventilation can both provide equal surgical visualization during surgery for esophageal cancer, the influx of CO2 might be one of the factors affecting tumor cell spillage and metastasis. In the LACC (Laparoscopic Approach to Cervical Cancer) trial, CO2-induced pneumoperitoneum may have been the reason for the shorter overall survival (OS) resulting from minimally invasive radical hysterectomy4. Previous studies suggested that insufflation of CO2 could result in tumor cell metastasis5 and may induce tumor spillage into the peritoneal cavity6. Therefore, it is hypothesized that CO2 gas-induced pneumothorax might have unfavorable impacts on the oncological outcomes of minimally invasive esophagectomy.
Additionally, the order of surgical procedures is different in CO2 pneumothorax compared with the one-lung ventilation setting. Using CO2 pneumothorax, the esophagus with a cutting margin and circumferential resection margin is usually left temporarily in the chest for later removal in cervical or abdominal procedures; however, the isolated esophagus can be removed from the chest cavity immediately when using the one-lung ventilation technique. Studies have suggested that the rate of positive esophageal cancer is 8.6–83.1%7 in circumferential resection margins. When applying CO2 pneumothorax, the extended exposure of esophageal samples might result in tumor cell dissemination in the pleural cavity and therefore trigger tumor relapse inside the chest, which has unfavorable effects on prognosis.
In this initial study, data from three high-volume medical centers from southeast China were analyzed to compare the oncological impacts of CO2 pneumothorax versus one-lung ventilation. OS and DFS were compared by using propensity score matching and inverse probability of treatment propensity score weighting. Additionally, the tumor-relapse state and pattern were evaluated.
Methods
Patient cohort
Between 2010 and 2016, patients with a primary diagnosis of pathologically confirmed esophageal squamous cell carcinoma (ESCC) were retrospectively reviewed from three high-volume (>400 esophagectomies per year) clinical centers in southeast China (names of medical centers are omitted temporally due to the double-blind peer review policy). The esophagectomies were performed by experienced surgical teams.
The inclusive criteria were: (1) patients with pathologically diagnosed ESCC; (2) the surgical procedure was minimally invasive McKeown esophagectomy with extended 2-field lymph node dissection. Patients were excluded if: (1) they received neoadjuvant therapies; (2) patients had synchronous or metachronous second malignant cancer. The work has been reported in line with the STROCSS criteria8.
Covariates
General characteristics, including case ID, name, age, sex, body mass index (BMI), cancer history, pathological stage, margin state, adjuvant therapies, use of CO2 pneumothorax or one-lung ventilation, and follow-up, were retrospectively collected. The tumors and harvested lymph nodes were restaged according to the 7th edition of the American Joint Committee on Cancer (AJCC) staging system.
Objectives
The primary objective of this study was to compare the OS and DFS of surgically managed ESCC patients who underwent CO2 pneumothorax or one-lung ventilation. A secondary objective was to determine the impacts of CO2 pneumothorax and one-lung ventilation methods on tumor relapse both inside and outside the pleural cavity.
CO2 pneumothorax and one-lung ventilation techniques
All patients were operated under general anesthesia with combined inhalation and intravenous methods. During one-lung ventilation, a double-lumen endotracheal tube was inserted, and the correct position was assured by auscultation and fiberoptic bronchoscopy before and after the patient was in the lateral decubitus position. Mechanical ventilation with a tidal volume (TV) of 6–8 ml/kg and respiratory rate of 12–14 bpm was used during one-lung ventilation. The FiO2% and respiratory rate were adjusted to maintain normocapnia (partial arterial carbon dioxide pressure between 35 and 45 mmHg) and oxygen saturation (over 90%).
When using CO2 pneumothorax, a single-lumen endotracheal tube was inserted. The CO2 insufflation (CO2 pressure =6–8 mmHg)-induced artificial pneumothorax allowed two-lung ventilation during surgery and provided good exposure as well. The ventilation parameters were similar to those in the one-lung ventilation setting: a TV of 6–8 ml/kg and a respiratory rate of 12–14 bpm. The FiO2 and respiratory rate were adjusted to maintain normocapnia and oxygen saturation.
Follow-ups
Patients were followed every 3 months postoperatively for the first 2 years and then every 6 months for 3–5 years. Additionally, annual follow-up visits were conducted 5 years postoperatively. The OS was calculated from the date of first diagnosis to the date of death or the last follow-up. The time from R0 resection to disease recurrence or death was considered DFS. Patients who lost to follow-up or who survived until the last follow-up were regarded as censored.
Statistical analysis
Distributions of patient characteristics and perioperative surgical outcomes in the one-lung ventilation or CO2 pneumothorax groups were calculated using Pearson’s chi-square (χ 2) or Fisher’s exact test for categorical data and the Wilcoxon test for continuous data. All tests were two-sided, and values of P<0.05 were considered statistically significant.
The survival result of a pilot cohort of 626 patients (208 in one-lung ventilation and 418 in CO2 pneumothorax groups) show that 5-year OS rates were 65.7% and 56.3% in CO2 pneumothorax and one-lung ventilation groups. The sample size calculation was then performed by ‘pwr’ package in R software using the ‘two proportions’ method. The significant level (α) and power were set as 0.05 and 0.8, respectively. As a result, the number of patients required in each group should be 421.
Two models, propensity score and inverse probability of treatment weight (IPTW), were constructed to balance patient characteristics. The 1:1 propensity score matching was performed by using the ‘MatchIt’ package in R software. The propensity score was calculated using the logistic regression model and the ‘nearest’ method. Based on the propensity score, we used IPTW, which was calculated as 1/(propensity score) in the CO2 pneumothorax group and 1/(1-propensity score) in the one-lung ventilation group. The difference between groups is further evaluated by standardized mean difference (SMD) both before and after IPTW. The cohort with SMD less than 10% and P-value over 0.05 is regarded as well-adjusted.
Candidate variables included all variables considered to be correlated with the prognosis of ESCC, or significantly different in variance estimations among patients who underwent one-lung ventilation or CO2 pneumothorax (with a threshold of P<0.10) by univariable analysis. Finally, the covariates included in the propensity score matching were age, sex, smoking status, alcohol consumption status, pT stage, pN stage, and adjuvant therapy.
Results
Patient characteristics
According to the inclusion and exclusion criteria, 998 ESCC patients were treated with minimally invasive McKeown esophagectomy, of whom 576 (57.7%) underwent CO2 pneumothorax and 422 (42.3%) underwent one-lung ventilation. The distribution of cases was 375 (37.6%), 196 (19.6%), and 427 (42.8%) from the three hospitals, respectively. No cases were converted from intraoperative CO2 pneumothorax to one-lung ventilation. The unmatched baseline characteristics of the cohort are listed in Table 1. Patients in the one-lung ventilation group had a significantly earlier pT stage (P<0.001). The R0 resection rates were 99.1% and 99.5% in the one-lung ventilation and CO2 pneumothorax group, respectively. The patients tended to be elderly and had a relatively late pN stage in the one-lung ventilation group, whereas a higher percentage of smoking and alcohol-drinking patients were in the one-lung ventilation group (Table 1).
Table 1.
Patient characteristics in CO2 pneumothorax and one-lung ventilation groups both before and after inverse probability matching.
| Before matching | After matching | |||||
|---|---|---|---|---|---|---|
| Characteristics | One-lung ventilation (N=422 ) | CO2 pneumothorax (N=576) | P | One-lung ventilation (N=1008) | CO2 pneumothorax (N=995) | P |
| Age (mean±SDa) | 60.4±8.2 | 61.3±8.7 | 0.077 | 61.0±8.1 | 61.0±8.6 | 0.959 |
| Gender (M/F) | 311 (73.7)/111 (26.3) | 422 (73.3)/154 (26.7) | 0.936 | 755 (74.9)/253 (25.1) | 729 (73.3)/266 (26.7) | 0.628 |
| pT stages | <0.001 | 0.995 | ||||
| Tis | 15 (3.6) | 137 (23.8) | 162 (16.1) | 152 (15.3) | ||
| T1 | 94 (22.3) | 57 (9.9) | 151 (15.0) | 149 (15.0) | ||
| T2 | 67 (15.9) | 134 (23.3) | 204 (20.2) | 202 (20.3) | ||
| T3 | 243 (57.6) | 240 (41.7) | 482 (47.8) | 481 (48.3) | ||
| T4a | 3 (0.7) | 8 (1.4) | 9 (0.9) | 11 (1.1) | ||
| pN stages | 0.128 | 0.968 | ||||
| N0 | 225 (53.3) | 97 (42.0) | 591 (58.6) | 566 (56.9) | ||
| N1 | 115 (27.3) | 89 (38.5) | 254 (25.2) | 262 (26.3) | ||
| N2 | 68 (16.1) | 44 (19.0) | 138 (13.7) | 142 (14.2) | ||
| N3 | 14 (3.3) | 11 (1.9) | 25 (2.5) | 25 (2.5) | ||
| Smoking (Yes/No) | 194 (46.0)/228 (54.0) | 238 (41.3)/338 (58.7) | 0.161 | 448 (44.4)/560 (55.6) | 428 (43.0)/567 (57.0) | 0.700 |
| Alcohol (Yes/No) | 126 (29.9)/296 (70.1) | 135 (23.4)/441 (76.6) | 0.027 | 249 (24.7)/759 (75.3) | 251 (25.2)/744 (74.8) | 0.867 |
| R0 resection (Yes/No) | 4 (0.9)/418 (99.1) | 3 (0.5)/573 (99.5) | 0.465 | 6 (0.6)/1002 (99.4) | 3 (0.3)/992 (99.7) | 0.507 |
| Adjuvant therapies (Yes/No) | 86 (20.4)/336 (79.6) | 132 (22.9)/444 (77.1) | 0.459 | 215 (21.3)/793 (78.7) | 220 (22.1)/775 (77.9) | 0.677 |
SD, standard deviation.
To make a reliable comparison, the propensity score and IPTW were constructed. The covariates included in the propensity score matching were age, sex, smoking status, alcohol consumption status, pT stage, pN stage, and adjuvant therapy. The balanced baseline characteristics after matching are shown in Table 1.
Ventilation methods and long-term survival
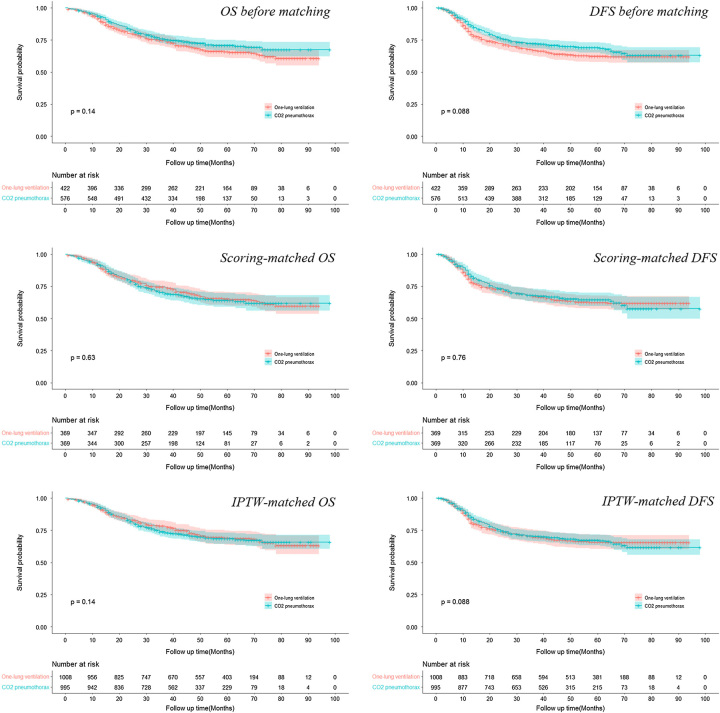
The median follow-up of the patient cohort was 55.2 months. The overall 5-year survival rates and corresponding P values are shown in Table 2. In the propensity score matching model, both the 5-year OS and DFS showed no significant difference between the CO2 pneumothorax and one-lung ventilation groups by Cox proportional hazard regression analysis (OS: HR= 1.06, 95% CI, 0.83–1.37; DFS: HR= 0.96, 95% CI, 0.75–1.23). In addition, the IPTW model also suggested similar outcomes (OS: HR= 1.06, 95% CI, 0.95–1.35; DFS: HR= 0.98, 95% CI, 0.77–1.26), which indicated the comparable oncological impacts of CO2 pneumothorax versus one-lung ventilation methods. Survival curves of propensity score matching and IPTW models depicted no significant difference between the two groups as well (Fig. 1).
Table 2.
The 5-year OS and DFS of patients received CO2 pneumothorax versus one-lung ventilation before and after matching.
| Variables | CO2 pneumothorax(%) | One-lung ventilation (%) | P |
|---|---|---|---|
| Before matching (95% CI) | |||
| OS | 70.8 (66.8–75.0) | 65.8 (61.2–70.8) | 0.140 |
| DFS | 69.0 (65.1–73.2) | 62.4 (57.8–67.5) | 0.088 |
| Propensity score matching (95% CI) | |||
| OS | 64.7 (59.7–70.2) | 65.3 (60.3–70.6) | 0.63 |
| DFS | 64.2 (59.0–69.7) | 62.4 (57.5–67.8) | 0.76 |
| IPTW (95% CI) | |||
| OS | 68.7 (64.5–73.1) | 69.1 (63.9–74.8) | 0.140 |
| DFS | 67.3 (63.2–71.8) | 65.8 (60.6–71.6) | 0.088 |
DFS, disease-free survival; OS, overall survival.
Figure 1.
The survival curves of patients before and after propensity score matching and inverse probability of treatment weight matching. DFS, disease-free survival; OS, overall survival.
Effects of ventilation method on ESCC relapse
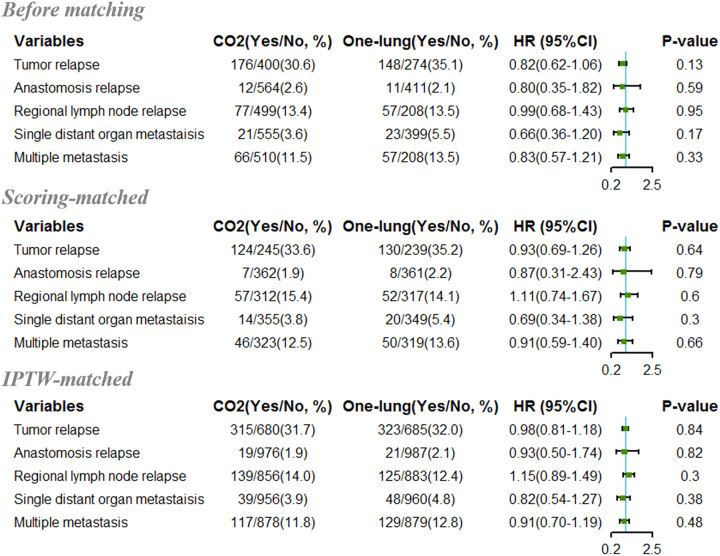
A total of 324 (32.5%) patients, including 148 (35.1%) and 176 (30.6%) patients in the one-lung ventilation and CO2-induced pneumothorax groups, respectively, suffered from tumor recurrence or metastasis following radical esophagectomy in the study cohort. All relapses were further classified into three categories based on the first identified tumor relapse site: (1) anastomosis relapse, (2) regional lymph node relapse, and (3) single distant organ metastasis9. Nevertheless, patients with multiple sites of recurrence at the first diagnosis were also included and therefore classified as the fourth category as multiple metastases. The total number of cases with relapse was 23 (2.3%), 134 (13.4%), 44 (4.4%), and 123 (12.3%) for anastomosis, regional lymph node, single distant organ relapse, and multiple metastasis, respectively. The following logistic analysis revealed that the application of two ventilation methods was not correlated with different ESCC relapse patterns (Fig. 2).
Figure 2.
The forest plot showing hazard ratios of CO2 pneumothorax versus one-lung ventilation for esophageal squamous cell carcinoma patients who received esophagectomy.
Discussions
In general, special ventilation methods must be applied to keep the surgical side of the lung compressed for ease of procedures in thoracic operations. CO2 gas pressure in abdominal oncological operations may be involved in tumor cell spillage and worse long-term survival5,6. Therefore, the oncological impacts should be considered before using the CO2 pneumothorax ventilation method. In this study, we performed a multicenter analysis using propensity score matching and IPTW models to compare the long-term effects of CO2 pneumothorax with those of the traditional one-lung ventilation method.
Our results conflict with the findings in the LACC trial, which indicated that usage of CO2-induced pneumoperitoneum might be the reason for shorter OS resulting from minimally invasive radical hysterectomy4. Although the mechanisms of CO2-induced tumor metastasis or relapse were not the objective of our study, we presume that the possible reason for the different outcomes could be that the CO2 pressures were lower, ranging from 6 to 8 mmHg in thoracic surgery. In contrast, the insufflation pressures generally range from 12 to 15 mmHg in abdominal operations10. By simulating the thoracoscopic CO2 pneumothorax environment in vitro, Jiang et al.3 suggested that only higher-pressure CO2 pneumothorax (over 12 mmHg) significantly increased esophageal cancer cell invasion and metastasis, while a pressure lower than 8 mmHg was clinically safe. Our study investigated the oncological effects of CO2 pneumothorax in ESCC, which is essential to translate these in-vitro results into clinical recommendations.
Additionally, our findings suggested that CO2 pneumothorax was neither associated with the risk of tumor relapse nor with relapse in different patterns. The correlations between CO2 insufflation and tumor relapse can be found in previous publications. Kuntz et al.11 suggested that a lower pH level secondary to CO2 pneumoperitoneum destroyed the defensive function of the peritoneum and therefore explained the higher incidence of port-site metastasis of oncological laparoscopic surgery. However, there were no port-site metastases in our cohort, and one patient suffered from pleural dissemination and malignant effusion. However, the patient was in the one-lung ventilation group. Therefore, our study did not have enough data to support the relationship between CO2 pneumothorax and pleural metastasis. In addition, although evidence could not be found in previous studies, tumor cells can presumably have higher chances of reaching the lymphovascular system after mediastinal lymph node dissection, which damages the normal barrier of the pleural membrane. Notably, the gross esophageal sample will stay in the chest cavity longer when using CO2 pneumothorax. However, the correlation analysis between CO2 pneumothorax and tumor relapse in the lymph nodes or distant organs was negative, which suggested that the hypothesis that CO2 pneumothorax may lower the risk of ESCC metastasis could be false.
DFS and OS are two common endpoints applied in evaluating the outcomes of radical surgery for malignancies. According to Clinical Trial Endpoints for the Approval of Cancer Drugs and Biologics Guidance for Industry of FDA, DFS usually presents greater difference compared with OS between treatment and control groups. Greater difference would actually require less sample size and shorter follow-up time12. When calculating the sample size, the difference of DFS was 10.1% (62.9% and 52.8% in CO2 pneumothorax and one-lung ventilation groups), which was indeed greater than 9.4% in OS (65.7% and 56.3% in CO2 pneumothorax and one-lung ventilation groups), and resulted in a smaller sample size requirement. The 5-year OS, rather than DFS, was therefore applied in sample size calculation to further ensure the reliability of the results.
Propensity score matching can effectively balance the potential confounding factors for further analysis. Although different opinions exist regarding the kinds of covariates that should be selected into the matching model, it is more important to balance prognostically correlated variables than other covariates that affect treatment selection but have no influence on the outcome13. In addition, a study showed that further inclusion of variables that did not affect outcome can introduce bias14. In the present study, we first included all covariates that were distributed differently between the two groups and then considered variables that influence prognosis after esophagectomy. Therefore, age, adjuvant therapies, R0 resection, and pathological T and N stages were all included in the propensity score model. The effect size indices of matched characteristics were balanced.
Retrospective studies have certain inherent limitations. Despite the use of matching, the effects of unobserved cofounding variables remained in the comparison. Obviously, our study was only concerned the oncological effects of CO2 pneumothorax on ESCC; however, the response of other tumors to CO2 pressure may be different. All these limitations should be considered when interpreting the results. Further large-scale prospective randomized trials are necessary to confirm our results.
In summary, the study demonstrates that CO2 pneumothorax is comparable to the traditional one-lung ventilation method in terms of the long-term survival outcomes and risk of tumor relapse in different patterns. Therefore, the two methods are both viable options in esophagectomy.
Ethical approval
The study was approved by the IRB of Sun Yat-sen University Cancer Center (Approval Number: B2022-593-01).
Consent
The study was approved by the IRB of Sun Yat-sen University Cancer Center and patients’ consents were waived.
Sources of funding
Natural and Science Foundation of China (No. 82002469).
Author contribution
J.C.: conceptualization, methodology, software, and writing – original draft preparation; H.J.: data curation, conceptualization, and data validation; B.Z.: data curation, conceptualization, data validation, and writing – reviewing and editing; T.Z.: data curation; H.W.: data validation; Y.L.: data curation and data validation; W.G.: data curation and writing – original draft preparation; P.W.: data validation and writing – reviewing and editing; C.C.: methodology, writing – reviewing and editing, and supervision; L.T.: conceptualization, writing – reviewing and editing, and investigation; J.F.: conceptualization, methodology, investigation, and writing – reviewing and editing.
Conflicts of interest disclosure
No authors reported any conflicts of interest.
Research registration unique identifying number (UIN)
Name of the registry: Research Data.
Unique identifying number or registration ID: 2302250001.
Hyperlink to your specific registration (must be publicly accessible and will be checked): www.researchdata.org.cn
Guarantor
Dr Jianhua Fu.
Data availability statement
Data in our submission can be accessible upon reasonable requirements for research purposes.
Provenance and peer review
None.
Footnotes
Junying Chen, Heng Jiao, and Bin Zheng contributed equally to this work and therefore share the first co-authorship.
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Published online 4 December 2023
Contributor Information
Junying Chen, Email: chenjuny@sysucc.org.cn.
Jiao Heng, Email: jiao.heng@zs-hospital.sh.cn.
Bin Zheng, Email: lacustrian@163.com.
Taidui Zeng, Email: zengtaidui@163.com.
Hao Wang, Email: wang.hao@zs-hospital.sh.cn.
Peizong Wang, Email: wangpz@sysucc.org.cn.
Yaobin Lin, Email: linyaob@sysucc.org.cn.
Wuyou Gao, Email: gaowy@sysucc.org.cn.
Chun Chen, Email: chenchun0209@163.com.
Lijie Tan, Email: tan.lijie@zs-hospital.sh.cn.
Jianhua Fu, Email: fujh@sysucc.org.cn.
References
- 1. Biere SS, van Berge Henegouwen MI, Maas KW, et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet 2012;379:1887–1892. [DOI] [PubMed] [Google Scholar]
- 2. Straatman J, van der Wielen N, Cuesta MA, et al. Minimally invasive versus open esophageal resection: three-year follow-up of the previously reported randomized controlled trial: the TIME trial. Ann Surg 2017;266:232–236. [DOI] [PubMed] [Google Scholar]
- 3. Jiang T, Lin M, Zhan C, et al. High-pressure artificial pneumothorax promotes invasion and metastasis of oesophageal cancer cells. Interact Cardiovasc Thorac Surg 2019;29:275–282. [DOI] [PubMed] [Google Scholar]
- 4. Ramirez PT, Frumovitz M, Pareja R, et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. New Engl J Med 2018;379:1895–1904. [DOI] [PubMed] [Google Scholar]
- 5. Lin F, Pan L, Li L, et al. Effects of a simulated CO2 pneumoperitoneum environment on the proliferation, apoptosis, and metastasis of cervical cancer cells in vitro. Med Sci Monit 2014;20:2497–2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kong TW, Chang SJ, Piao X, et al. Patterns of recurrence and survival after abdominal versus laparoscopic/robotic radical hysterectomy in patients with early cervical cancer. J Obstet Gynaecol Res 2016;42:77–86. [DOI] [PubMed] [Google Scholar]
- 7. Karstens KF, Izbicki JR, Reeh M. Does the margin matter in esophageal cancer. Dig Surg 2018;35:196–203. [DOI] [PubMed] [Google Scholar]
- 8. Mathew G, Agha R, Albrecht J, et al. STROCSS 2021: strengthening the reporting of cohort, cross-sectional and case–control studies in surgery. Int J Surg 2021;96:106165. [DOI] [PubMed] [Google Scholar]
- 9. Chen JY, Liu QW, Zhang SS, et al. Prophylactic thoracic duct ligation is associated with poor prognosis and regional lymph node relapse in esophageal squamous cell carcinoma. J Surg Oncol 2020;122:336–343. [DOI] [PubMed] [Google Scholar]
- 10. Cai Z, Malbrain ML, Sun J, et al. Does elevated intra-abdominal pressure during laparoscopic colorectal surgery cause acute gastrointestinal injury? Wideochir Inne Tech Maloinwazyjne 2015;10:161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kuntz C, Wunsch A, Bodeker C, et al. Effect of pressure and gas type on intraabdominal, subcutaneous, and blood pH in laparoscopy. Surg Endosc 2000;14:367–371. [DOI] [PubMed] [Google Scholar]
- 12. McLeod C, Norman R, Litton E, et al. Choosing primary endpoints for clinical trials of health care interventions. Contemp Clin Trials Commun 2019;16:100486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Austin PC, Grootendorst P, Anderson GM. A comparison of the ability of different propensity score models to balance measured variables between treated and untreated subjects: a Monte Carlo study. Stat Med 2007;26:734–753. [DOI] [PubMed] [Google Scholar]
- 14. Myers JA, Rassen JA, Gagne JJ, et al. Effects of adjusting for instrumental variables on bias and precision of effect estimates. Am J Epidemiol 2011;174:1213–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data in our submission can be accessible upon reasonable requirements for research purposes.