Abstract
Sperm capacitation, crucial for fertilization, occurs in the female reproductive tract and can be replicated in vitro using a medium rich in bicarbonate, calcium, and albumin. These components trigger the cAMP-PKA signaling cascade, proposed to promote hyperpolarization of the mouse sperm plasma membrane through activation of SLO3 K+ channel. Hyperpolarization is a hallmark of capacitation: proper membrane hyperpolarization renders higher in vitro fertilizing ability, while Slo3 KO mice are infertile. However, the precise regulation of SLO3 opening remains elusive. Our study challenges the involvement of PKA in this event and reveals the role of Na+/H+ exchangers. During capacitation, calcium increase through CatSper channels activates NHE1, while cAMP directly stimulates the sperm-specific NHE, collectively promoting the alkalinization threshold needed for SLO3 opening. Hyperpolarization then feeds back Na+/H+ activity. Our work is supported by pharmacology, and a plethora of KO mouse models, and proposes a novel pathway leading to hyperpolarization.
Teaser
Alkalinization of sperm cytoplasm activates potassium channels to hyperpolarize the plasma membrane in a PKA independent cascade.
Introduction.
The sperm capacitation process in mammalian sperm involves a complex cascade of events that unfolds within the female reproductive tract upon ejaculation (for a review see1). These events are vital for enabling sperm to successfully fertilize the egg and are replicated in vitro using a specialized capacitating media. These media contain various components, including energy sources, bicarbonate, Ca2+, and albumin. At the molecular level, capacitation is hallmarked by critical processes such as sperm membrane potential (Em) hyperpolarization and intracellular alkalinization2–4. Genetic and pharmacological investigations have unveiled specific transporters that underlie the ionic fluxes involved in such changes. Among them, the atypical sperm-specific Na+/H+ exchanger (SLC9C1 aka sNHE) and the SLO3 K+ channel have emerged as key players. Their indispensability for capacitation is underscored by the fact that mice deficient in these genes exhibit infertility, albeit without discernible abnormalities in other physiological aspects, as these proteins are uniquely expressed in sperm 2,5,6. Given the importance of sperm Em hyperpolarization associated to SLO3 currents, it is intriguing that the molecular events leading to SLO3 opening have not been completely revealed yet, besides knowing that SLO3 responds to alkalinization.
The principal membrane H+ transporters responsible for mediating H+ efflux in response to the rising extracellular pH within the female reproductive tract are Na+/H+ exchangers (NHEs) (for a review see 7). Three groups identify NHE isoforms in mammalian sperm: NHE1 and NHE5 (SLC9A subgroup), NHA1 and NHA2 (SLC9B subgroup), and sNHE as a member of the SLC9C subgroup, with the recent identification of SLC9C2 in at least rat and human sperm8,9. Their roles and significance in sperm physiology remain an active area of research.
The localization of NHE1 to the sperm midpiece is established 10, but its precise contribution to sperm fertility has been somewhat enigmatic. Notably, the breeding outcomes of NHE1-deficient mice have provided intriguing insights. While mating between NHE1 KO males and females yielded no successful breeding, a litter could be carried to term when Nhe1+/− (HET) males mated with NHE1 KO females 11. As for NHE5, its functional importance in sperm physiology is yet to be elucidated, though it has been also localized to the midpiece of mouse sperm 10. Disruptions in the NHA exchangers provoked subfertility in single Nha1 or Nha2 KO males, whereas double KO males were rendered completely infertile, marked by severely compromised sperm motility. These phenotypes were associated with attenuated cAMP synthesis by soluble adenylyl cyclase (sAC) and reduced expression of the full-length sAC isoform 12. Addition of cell-permeable cAMP analogs rescued sperm motility defects, while fertility defects seemed to arise from deficient acrosome reaction 13. Regarding sNHE, it is localized to the principal piece of the sperm flagellum. It was found to affect sperm motility, a phenotype amenable to rescue through the addition of cell-permeable cAMP analogs 6. The presence of a cyclic nucleotide-binding domain (CNBD) and a putative voltage-sensor motif on sNHE suggests its potential regulation by cyclic nucleotides and changes in Em. It has been suggested that cAMP modulates intracellular pH (pHi) by regulating sNHE activity 14,15. While these advances have broadened our understanding of key cellular processes that orchestrate capacitation, many questions remain unanswered regarding the precise physiological roles of these transporters (and even their presence) in sperm function.
In this article, we investigate the role of cAMP and its downstream targets in sperm alkalinization that drives hyperpolarization of Em during capacitation. Unexpectedly, inhibition of PKA catalytic activity did not impair Em hyperpolarization despite sAC activity being necessary for this change in Em. Non-capacitated sperm exposed to cAMP permeable analogs underwent Em hyperpolarization. Our results using a battery of genetic mouse models and pharmacology demonstrate the critical role of NHE1 and sNHE in the pathway leading to Em hyperpolarization, through pH modulation by Ca2+ and cAMP, respectively.
RESULTS
PKA catalytic activity is not required for Em hyperpolarization
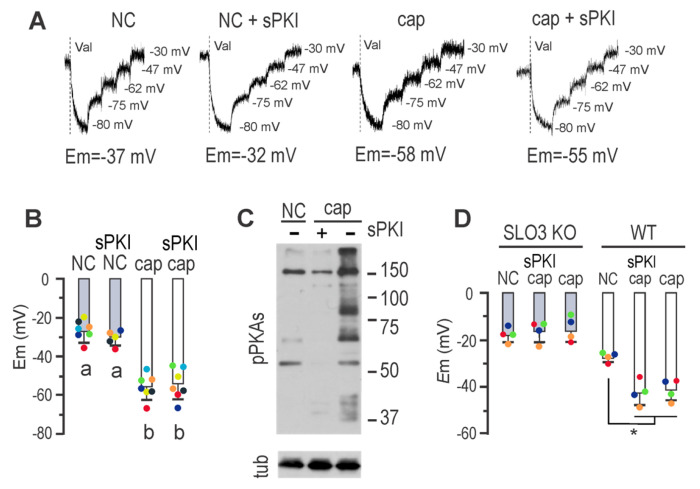
One of the initial events in the capacitation-signaling pathway involves the bicarbonate-induced stimulation of sAC, leading to increased cAMP synthesis, which activates PKA 16. Two pieces of evidence support PKA’s role in mouse sperm Em hyperpolarization: 1) the PKA inhibitor H-89 prevented Em hyperpolarization, when present during capacitation, and 2) Em hyperpolarization increased with bicarbonate in a concentration-dependent manner 17. To gain insights into this pathway, we attempted to inhibit Em hyperpolarization using the synthetic and permeable PKA inhibitor peptide sPKI, known to effectively and specifically inhibit its catalytic activity 18. Surprisingly, and in contrast to the effect of H-89, sperm Em hyperpolarization associated to capacitation was not inhibited by sPKI (Fig. 1A and B). As a control we show that sPKI did not affect Em in NC sperm (Fig. 1A and B). We verified the effective blockade of PKA activity by sPKI through immunoblotting analysis using antibodies against phosphorylated PKA substrates (pPKAs) 19 (Fig. 1C). Furthermore, Em in capacitating medium containing sPKI depended on SLO3 activity, as cells did not hyperpolarize in the presence of BaCl2 (Sup. Fig. 1), an effective blocker of SLO3 currents 20. As an additional control, sperm from Slo3 null mice did not hyperpolarize in the presence of sPKI, further disregarding the possibility of non-physiological hyperpolarization caused by sPKI (Fig. 1D).
FIGURE 1. Mouse sperm Em hyperpolarization can dispense PKA catalytic activity.
A, Fluorescence traces showing the values of the sperm Em obtained after sperm incubation in either non-capacitating or capacitating conditions containing or not 15 μM sPKI for 60 min. Each experiment displays its calibration curve and the estimated Em value. B, Summary of Em measurements of sperm incubated in conditions depicted in A (mean ± SEM; n ≥ 5); different letters indicate statistically significant differences (p<0.001). C, Sperm were incubated for 60 min in non-capacitating or capacitating medium containing or not 15 μM sPKI. Each condition was processed for western blot analysis with a monoclonal anti-pPKAs antibody. Membrane was stripped and analyzed for the presence of tubulin using anti-β-tub (clone E7). D, Sperm Em measurements obtained after 60 min incubation of either Slo3 KO (gray boxes) or WT sperm (white boxes) in either non-capacitating or capacitating medium containing or not 15 μM sPKI (mean ± SEM; n = 4; * p<0.001). Each colored dot represents the value for each independent sample.
cAMP drives Em hyperpolarization
Non-capacitating media were supplemented with the permeable cAMP analogue 8Br-cAMP, known to stimulate phosphorylation of PKA substrate 21. 8Br-cAMP promoted Em hyperpolarization, which sPKI did not inhibit (Fig. 2A). We further inhibited cAMP production during capacitation using the potent and selective sAC inhibitor TDI-10229 22. As shown in figure 2B, TDI-10229 prevented Em hyperpolarization when added to capacitating media. The addition of 8Br-cAMP overcame the inhibitory effect of TDI-10229, further supporting the role of cAMP in Em hyperpolarization, independently of PKA catalytic activity.
FIGURE 2. Mouse sperm Em hyperpolarization is cAMP regulated and sNHE dependent.
A, Sperm Em obtained after incubation in either non-capacitating or capacitating conditions containing 500 μM 8Br-cAMP alone or in addition to15 μM sPKI for 60 min. Results are expressed as a normalization of percentage of hyperpolarization considering mean NC and cap values as 0% and 100%, respectively (mean ± SEM; n=4). Different letters indicate statistically significant differences (p<0.001). B, Sperm Em obtained after incubation in either capacitating (with either 10 μM TDI10229 alone or in combination with 500 μM 8Br-cAMP) or non-capacitating conditions containing or not 500 μM 8Br-cAMP for 60 min. Results are expressed as a normalized percentage of hyperpolarization considering NC mean and cap mean values as 0% and 100%, respectively (mean ± SEM; n=9). Different letters indicate statistically significant differences (p<0.001). Each colored dot represents the value for each independent sample. C, Sperm were incubated for 60 min in non-capacitating media containing increasing concentrations of 6Bnz-cAMP, as indicated. Each condition was processed for western blot analysis with a monoclonal anti-pPKAs antibody. The membrane was stripped and analyzed for the presence of tubulin using anti-β-tub (clone E7). D, Summary of densitometry analysis of sperm cells incubated as in A (mean ± SEM; n=3). Different letters indicate statistically significant differences (p<0.05). E, Em measurements of sperm incubated in non-capacitating conditions containing either 30 μM 8-pCPT-2’-O-Me-cAMP (8pCPT), 50 nM 6Bnz-cAMP, 500 μM 8Br-cAMP or 500 μM dibutyryl-cAMP (db-cAMP) for 60 min (mean ± SEM; n = 3). Different letters indicate statistically significant differences (p<0.001). F, Summary of Em sperm measurements from sNhe KO (gray boxes) or WT mice (black boxes) obtained after incubation in either capacitating or non-capacitating conditions containing 500 μM 8Br-cAMP or 10 mM NH4Cl for 60 min (mean ± SEM; n = 5; * p<0.05).
cAMP targets during promotion of Em hyperpolarization
The second messenger cAMP has three primary effectors: PKA, the exchange protein activated by cAMP (EPAC, a guanine-nucleotide-exchange factor), and the cyclic nucleotide binding domains found in cyclic nucleotide-gated ion channels (for a comprehensive review, see 1). To shed light on the signaling pathway through which cAMP promotes sperm Em hyperpolarization, we selected specific agonists of cAMP targets, including the widely used 8-pCPT-2’-O-Me-cAMP to activate EPAC and the membrane-permeant PKA selective agonist N6-Benzyladenosine-cAMP (6Bnz-cAMP). To select an appropriate concentration of 6Bnz-cAMP, we initially exposed sperm to increasing concentrations of the PKA agonist for 60 minutes in non-capacitating media to assess PKA activity, as indicated by the in vivo phosphorylation of PKA substrates. Western blots using anti-pPKAs antibodies revealed that a concentration of 50 nM 6Bnz-cAMP induced a saturating phosphorylation of PKA substrates (Figs. 2C and D) and used hereafter to stimulate PKA. Then, the roles of EPAC and PKA in Em were then addressed by direct stimulation. As previously shown, 50 μM 8-pCPT-2’-O-Me-cAMP was used for direct stimulation of EPAC23. While both general cAMPs analogues db-cAMP and 8Br-cAMP promoted Em hyperpolarization, neither specific activation of EPAC with 8-pCPT-2’-O-Me-cAMP nor of PKA with 6Bnz-cAMP induced this Em shift (Fig. 2E), ruling out the direct involvement of EPAC and PKA in this process.
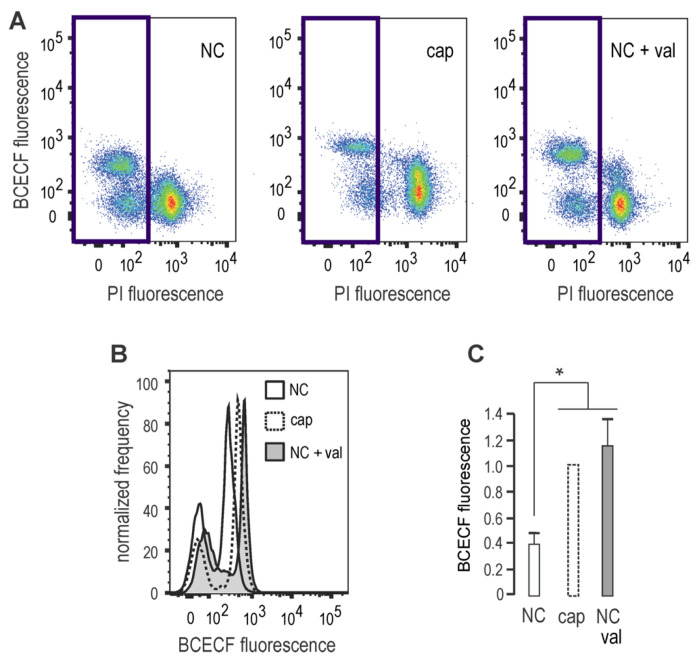
The presence of a cyclic nucleotide binding domain in sNHE suggests that its function may be regulated by cAMP. As pharmacological inhibitors of sNHE are not yet available, we analyzed Em hyperpolarization in sperm from sNHE null mice. Figure 2F demonstrates that sperm from sNhe KO mice did not undergo hyperpolarization when incubated in capacitating conditions for 60 minutes. Gain of function was not observed in the presence of the agonist 8Br-cAMP, which could be attributed to the absence of the cyclic nucleotide binding domain harbored by sNHE. Alkalinization induced by NH4Cl addition promoted Em hyperpolarization in both WT and sNHE KO sperm, confirming the functional response of SLO3 channels in this KO model. Interestingly, previous research showed that the sAC inhibitor TDI-10229 blocked pHi increase associated with capacitation 22. As mentioned, sNHE is proposed to be also regulated by Em through a voltage-sensor domain (VSD), which would be part of a positive feedback loop 24. To further support previous work, we analyzed the effect of Em on pHi, through the stimulation of Em hyperpolarization with the K+ ionophore, valinomycin 25. Non-capacitated sperm incubated with valinomycin showed an increase of pHi similar to that observed in capacitated cells, as evidence by fluorescence increase of BCECF loaded sperm (Fig. 3A–C), in agreement with previous results 26. Of note, this effect on pHi was absent in sNHE deficient mice26.
FIGURE 3. Em hyperpolarization induces intracellular alkalinization.
Sperm were incubated in either non-capacitating or capacitating media in the absence (DMSO) or presence of 1 μM Valinomycin (Val). A, Representative BCECF versus PI two dimensional fluorescence dot plot analysis. Blue square shows non-PI stained sperm. B, Histogram analysis depicting normalized frequency of sperm, and BCECF fluorescence performed in live sperm populations. C, Normalized median fluorescence intensity of BCECF compared to capacitating condition (mean ± SEM, n=4, *p < 0.05).
Role of Na+/H+ exchangers in sperm Em hyperpolarization
sNHE is not the sole exchanger present in mouse sperm. To explore the role of other Na+/H+ exchangers in Em hyperpolarization, we employed the potent inhibitor 5-(N,N-dimethyl)-amiloride (DMA), which targets members of the SLC9A sub-family, including NHE1, NHE2, and NHE3, with Ki values of 0.02, 0.25, and 14 μM, respectively, and negligible effects on NHE4, NHE5, and NHE7 27. DMA, which does not directly affect SLO3 or CatSper channels as recently shown28, was added at different concentrations to capacitating media to address its effect on Em hyperpolarization. Figure 4A shows a concentration-dependent inhibition of Em hyperpolarization. A concentration of 1 μM DMA significantly inhibited hyperpolarization. Of note, 10 μM DMA did not affect the phosphorylation of PKA substrates (Fig. 4B), thus excluding an effect on cAMP synthesis. Considering that NHE1, NHE5, NHA1, NHA2 and sNHE are known to be present in mouse sperm plasma membrane, and that DMA inhibits NHE1, NHE2, and NHE3, then NHE1 might be the primary target of DMA in these cells 27. In line with these findings, recent research demonstrated that DMA reduced K+ currents in mouse sperm by impairing the regulation of pHi 28. Along this line, figure 4C shows that DMA inhibited alkalinization associated to capacitation in mouse sperm.
FIGURE 4. NHE1 activity through Ca2+ stimulation is conducive to Em hyperpolarization.
A, Em obtained after sperm incubation in either non-capacitating or capacitating conditions containing different concentrations of dimethyl-amiloride (DMA) as indicated, for 60 min (mean ± SEM; n = 4; * p<0.001). B, Sperm were incubated for 60 min in non-capacitating or in capacitating medium in the presence or absence of 10 <M DMA. Each condition was processed for western blot analysis with a monoclonal anti-pPKAs antibody. C, Sperm were incubated for 60 min in non-capacitating or capacitating medium containing or not 10 μM TDI-10229 (TDI) or 1 μM DMA. Different letters indicate statistically significant differences (p<0.001). D, Em obtained after sperm incubation in either non-capacitating or capacitating conditions containing different concentrations of cariporide, as indicated, for 60 min. Different letters indicate statistically significant differences (mean ± SEM; n = 4; * p<0.001). E, Em obtained after sperm incubation in non-capacitating conditions in the presence or not of 500 μM 8Br-cAMP (8Br), or in capacitating conditions. As specified, capacitating conditions were supplemented with either 1 μM DMA (DMA), or 10 μM cariporide (carip) for 60 min. As indicated, these conditions were also supplemented with 500 μM 8Br-cAMP or 10 mM NH4Cl (mean ± SEM; n = 4; * p<0.01). F, Sperm Em from either CatSper1 KO (left panel) or WT mice (right panel) were obtained after sperm incubation in either non-capacitating (with boxes) or capacitating conditions (grey boxes) containing or not either 500 μM 8Br-cAMP or 10 mM NH4Cl for 60 min (mean ± SEM; n = 5; * p<0.005).
A second inhibitor, cariporide, which selectively targets NHE1 29, was also tested. Figure 4D shows that it effectively inhibited Em hyperpolarization when added to capacitating media at 10 μM. The effects of both cariporide and DMA could be bypassed by the addition of either 8Br-cAMP that directly stimulates sNHE or NH4Cl that directly stimulates SLO3 (Fig. 4E).
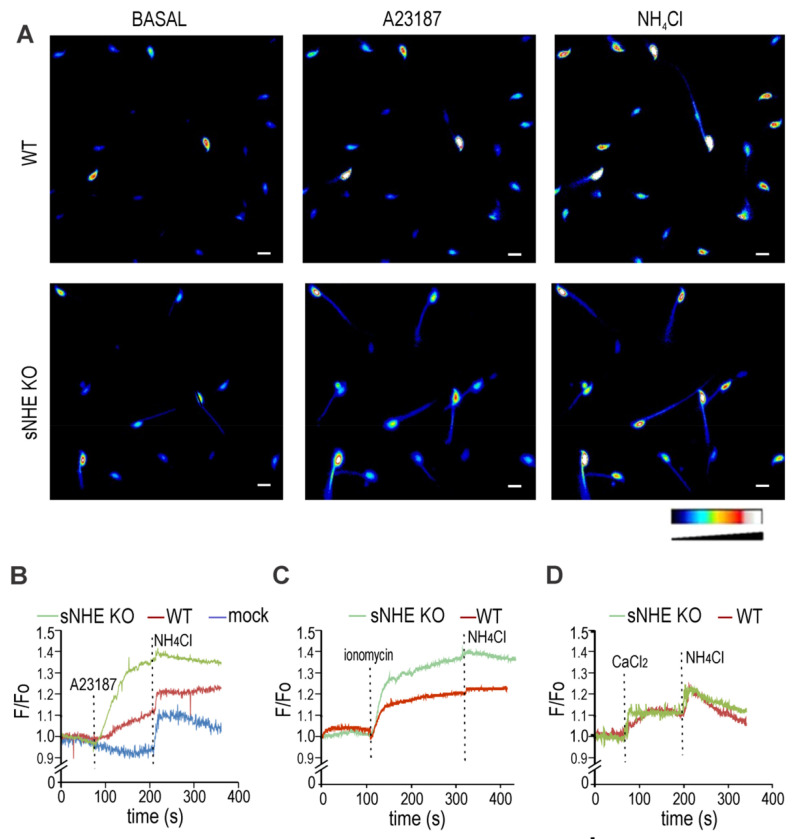
NHE1 possesses a C-terminal domain with extensive disordered regions 30, which binds calmodulin in the presence of Ca2+ 31. In other cell types, this binding induces an alkaline shift in the pHi sensitivity of NHE1, resulting in its activation at less acidic pHi 32. Therefore, the increase in intracellular Ca2+ associated with capacitation could activate NHE1, leading to an increase in pHi. To investigate the role of Ca2+ in Em hyperpolarization, we assessed Em in the CatSper1 KO model. Figure 4F demonstrates that sperm from CatSper1 KO mice exhibited deficient Em hyperpolarization, which could be restored by the addition of either 8Br-cAMP or NH4Cl, similar to the effects observed when inhibiting NHE1 by either DMA or cariporide (see Fig. 4E). Accordingly, Figure 5 (A–B) shows that the Ca2+ ionophore A23187 promoted pHi increase in sperm loaded with the pH sensitive dye BCECF. Treatment with Ca2+ ionophore A23187 promoted a pH increase also in sNHE KO, but to a greater extent in sNHE KO than in WT sperm, probably through over-expression of NHE1. As a control, NH4Cl was used to evoke pHi increase in both WT and sNHE KO sperm. Same effect was observed when sperm were challenged with ionomycin, as a second Ca2+ ionophore (Fig. 5C). Even though Ca2+ ionophores are used to increase intracellular Ca2+, it can be argued that they also promote pharmacological pHi increase due to H+ extrusion, as ionophores exchange Ca2+ for H+. Thus, as a second approach, cells were incubated in media without added Ca2+ salts (which still contain micromolar concentrations of Ca2+33 ), and challenged with 1.7 mM Ca2+ (Fig. 5D). As before, pHi increase could be clearly evidenced, both in WT and sNHE KO sperm, further substantiating the role of Ca2+ on pHi increase and supporting the lack of hyperpolarization in CatSper KO sperm.
FIGURE 5. Intracellular Ca2+ increase promotes cytoplasmic alkalinization in sperm cells.
Non-capacitated sperm cells were loaded with 0.5 μM BCECF-AM for 30 min before smearing onto laminin-precoated coverslips to record fluorescence. A, Representative fluorescence images of WT (upper panels) and sNhe KO (lower panels) sperm exposed to 10 μM of the ionophore A23187, followed by 10 mM NH4Cl. Reference bar for fluorescence intensity is depicted. Scale bar is equal to 10 μm. B, Summary average traces (8 cells in each trace) of experiments performed in A, including a mock treatment on WT sperm performed with DMSO instead of A23187. C, Summary average traces (8 cells in each trace) of either WT or sNhe KO sperm were exposed to 10 μM ionomycin followed by 20 mM NH4Cl. D, Summary average traces (8 cells in each trace) of sperm incubated in nominal zero Ca2+ (no added Ca2+ salts) challenged with 1.7 mM CaCl2 and followed by 20 mM NH4Cl.
Altogether, these findings indicate that cAMP is responsible for Em hyperpolarization, which includes an increase in pHI mediated by NHE exchangers, independent of PKA catalytic activity, and ultimately promoting SLO3 channel opening.
DISCUSSION
The mammalian sperm-specific K+ channel, SLO3, plays a pivotal role in processes leading to sperm capacitation. Slo3 KO mice are unable to undergo a physiologically stimulated acrosome reaction and are consequently infertile 2,34, underscoring the significance of investigating sperm Em hyperpolarization. However, the regulation of this channel during capacitation remains poorly understood.
Experiments conducted by Escoffier et al 35 demonstrated that Em hyperpolarization associated to capacitation was inhibited by H-89. It is worth noting that H-89 is now recognized for its non-specific effects 36. On the other hand, synthetic short peptides of PKI, such as PKI-(14-22)-amide (sPKI), have gained wide acceptance as pharmaceutical agents for selectively inhibiting PKA activity 37, demonstrating a high level of specificity. Our observations showed that sperm capacitated in the presence of sPKI, while displaying inhibition of PKA substrates phosphorylation, exhibited hyperpolarized Em. Furthermore, inhibition of sAC by TDI-10229 22 blocked Em hyperpolarization, consistent with its impact on impairing pHi alkalinization 22. When the permeable analogue 8Br-cAMP was introduced to non-capacitated sperm, it induced Em hyperpolarization, a finding that aligns with previous reports involving a different cAMP analogue 35. This supports the role of cAMP in the road of activating SLO3 channels and excludes PKA as indispensable.
The mouse SLO3 channel was found to be activated by intracellular alkalinization 38, though the precise mechanism of its modulation by pHi remains unresolved (for an in-depth review, see 39). An important regulator of SLO3 is the leucine-rich-repeat-containing protein 52 (LRRC52) 40. The significance of LRRC52 for SLO3 activity was demonstrated in Lrrc52 KO mice, where alkalinization failed to hyperpolarize sperm Em to the same extent as in WT sperm, suggesting a crucial role for LRCC52 in SLO3 response to alkalinization 39. In this regard, Na+/H+ exchangers (NHEs) have emerged as potential contributors to sperm alkalinization during capacitation. NHEs are responsible for regulating the pH of different cell compartments in a variety of cell types. Of particular importance in sperm physiology, sNHE is located in the principal piece of the sperm flagellum and possesses a cyclic nucleotide-binding domain 41. Sperm from sNHE null mice did not undergo capacitation associated hyperactivation 6, although this phenotype was restored by the addition of permeable cAMP analogues. Similar to slc9c1 KO, disruption of either NHA1 or NHA2 resulted in a reduced sperm motility phenotype, which was also rescued by incubating sperm with cAMP analogues, pointing towards the role of NHE exchangers in proper regulation of sAC activity and/or expression 12. However, our findings herein demonstrate that cAMP analogues did not restore Em hyperpolarization in sNHE null sperm. Despite the ability of cAMP addition to restore motility in sNHE null sperm 6,41, it did not reinstate Em hyperpolarization, indicating the involvement of sNHE in this pathway, likely through its cyclic nucleotide-binding domain. Recently, a human case was reported in which a mutation in sNHE resulted in a deletion in the cyclic nucleotide-binding domain. These sperm, lacking a functional cyclic nucleotide-binding domain, exhibited asthenozoospermia and infertility, indicating its importance also in human sperm 42.
Physiological modulation of the NHE family has been a subject of study for many years, mainly through pharmacology. Modified analogues of amiloride were designed to enhance specificity toward NHEs. DMA bears a double substitution of the 5-amino group nitrogen which increases its potency and selectivity toward NHE1. Our results demonstrated that DMA produced a robust inhibition of Em hyperpolarization when present in capacitating media. Cariporide, a non-related amiloride inhibitor of NHE1, with negligible effects on either Na+/Ca2+ exchangers or ENaCs 43, also inhibited Em hyperpolarization. In all instances, Em hyperpolarization could be reinstated by the addition of permeable cAMP analogues. Therefore, these results indicate the participation of both sNHE and NHE1 in triggering Em hyperpolarization of mouse sperm. cAMP possibly acts through the CNBD present in sNHE to increase pHi, triggering the opening of SLO3. This Em hyperpolarization, in turn, stimulates a positive feedback loop onto sNHE, as previously suggested 24,26. On the other hand, intracellular Ca2+ might activate NHE1 through its extensive disordered regions in the long cytoplasmic C-terminal domain 30,31. It has been proposed that this binding allows activation of NHE1 at a less acidic pHi 32. Therefore, upon the increase in intracellular Ca2+ associated with capacitation, NHE1 could drive pHi to a more alkaline state. In human sperm, NHE1 has been recently shown to be expressed at low amounts 44. Thus, possible differences in the control of intracellular pH between mouse and human can be expected.
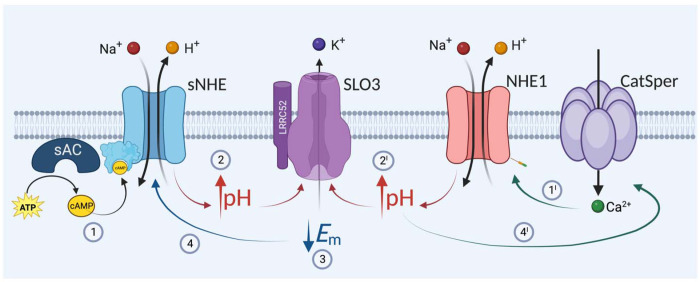
Considering these findings, we propose a mechanism that regulates pHi and Em hyperpolarization in mouse sperm, in which NHE1 and sNHE act synergistically (Fig. 6). Synthesis of cAMP induces alkalinization via sNHE, which, together with the action of NHE1 driven by intracellular Ca2+ increase, promotes the necessary alkalinization to increase the conductance of SLO3. In turn, Em hyperpolarization stimulates a positive feedback loop that further activates sNHE.
Figure 6. Working model proposing a dual action of sNHE and NHE1 on sperm Em hyperpolarization.
(1) Synthesis of cAMP induces alkalinization via sNHE, which, together with the action of NHE1 driven by intracellular Ca2+ increase (1’), promote the necessary alkalinization (2 and 2’) to increase the conductance of SLO3 (3). In turn, Em hyperpolarization stimulates a positive feedback loop that further activates sNHE (4). It is worth noting that the sole action of sNHE (inhibition of NHE1) or of NHE1 (in the case of sNhe KO) is not sufficient, under physiological conditions, to promote SLO3 opening.
This work paves the way for the study of the role of NHEs in mammalian sperm, considering their pivotal role in capacitation. These insights into the regulatory network of sperm capacitation contribute to our understanding of the fundamental processes that underlie fertilization competence, offering new perspectives for future research in this field.
Materials and Methods
Experimental Design
C57BL/6 male mature (10–13 weeks-old) male mice (wild type, Catsper1 KO 5, Slo3 KO 2 and sNhe KO 41) were used. In all cases, mice housing and all experimental procedures were conducted in accordance with the corresponding Institutional animal care guidelines, reviewed and approved by the Ethical Committees of the Instituto de Biología y Medicina Experimental, Buenos Aires, Argentina #32/2021, Animal Care and Use Committee of the Facultad de Ciencias Bioquímicas y Farmacéuticas de Rosario (UNR), Argentina (#380/2023) and of the Instituto de Biotecnología, UNAM, Mexico. The Guide for Care and Use of Laboratory Animals approved by the National Institutes of Health (NIH) was strictly met. In all cases, sperm were prepared as detailed below, using high grade reagents, as follows: Bovine serum albumin (BSA, fatty acid-free), cariporide (HOE-642), isobutilmetilxantina (IBMX) and 2’-O-dibutiril adenosín monofosfato-3’,5’ cíclico (db-AMPc), carbonyl cyanide 3-chlorophenylhydrazone (CCCP), dimethyl sulfoxide, Ca2+ ionophore A23187 and ionomycin were purchased from Sigma (St. Louis, MO). PKI 14–22 amide myristoylated (sPKI) was obtained from Tocris. 3-amino-N-(aminoiminomethyl)-6-chloro-5-(dimethylamino)-2-pyrazinecarboxamide monohydrochloride (DMA), 8-bromo-cyclic 3’,5’-(hydrogen phosphate)-adenosine monosodium salt (8Br-cAMP), (N-benzoyl-adenosine cyclic 3’,5’-(hydrogen phosphate) 6Bnz-cAMP), monosodium salt, and 8-[(4-chlorophenyl)thio]-2’-O-methyl-adenosine cyclic 3’,5’-hydrogen phosphate (8pCPT-2-O’-methyl cAMP), monosodium salt and valinomycin were purchased from Cayman Chemicals (Ann Arbor, MI). Anti-phospho-PKA substrates (anti-pPKAs) (clone 100G7E) antibodies and Horseradish peroxidase-conjugated anti-mouse and anti-rabbit IgG were purchased from Cell Signaling Technology (Danvers,MA). β-tubulin (clone E7) was purchased by Developmental Studies Hybridoma Bank. The sAC inhibitor TDI-10229 was kindly provided by Drs Levin and Buck, Department of Pharmacology, Weill Cornell Medicine, New York City, USA. 3,3-dipropylthiadicarbocyanine iodide (DiSC3(5)), BCECF-AM and pluronic acid from Invitrogen, Thermo Fisher Scientific (Waltham, MA, USA); while propidium iodide from Santa Cruz Biotechnology (Dallas, TX, USA). 3,3-dipropylthiadicarbocyanine iodide (DiSC3(5)), BCECF-AM and pluronic acid were dissolved in DMSO; propidium iodide was dissolved in hexa-distilled water.
Sperm preparation
Cauda epididymal mouse sperm were collected from adult male mice (10 –13 weeks old). Each minced cauda epididymis was placed in 600 μl of HEPES-buffered TYH medium (H-TYH) containing 119.3 mM NaCl, 4.7 mM KCl, 1.2 mM KH2PO4, 1.2 mM MgSO4, 5.6 mM glucose, 0.5 mM sodium pyruvate, 1.7 mM Ca2+, and 20 mM HEPES (pH 7.3), accounting for H-TYH medium (“NC medium”). After 15 min of incubation at 37°C (swim-out), epididymides were removed and the suspension was adjusted with NC medium to a final concentration of 1–2 × 107 cells/ml. For capacitation, BSA and NaHCO3 were added to final concentrations of 5 mg/ml and 20 mM respectively (“cap medium”) and incubated at 37°C for at least 1 h, or the indicated period.
SDS-PAGE and immunoblotting
After treatment, sperm were collected by centrifugation, washed in 1 ml of PBS, resuspended in Laemmli sample buffer without β-mercaptoethanol, and boiled for 5 min. After centrifugation, 5% β-mercaptoethanol was added to the supernatants and boiled again for 5 min. Protein extracts equivalent to 1-2 × 106 sperm per lane were subjected to SDS-PAGE and electro-transferred to PVDF membranes (Bio-Rad) at 250 mA for 60 min on ice. Membranes were blocked with 3% BSA in TBS containing 0.1% Tween-20 (T-TBS). Antibodies were diluted in T-TBS containing 1% BSA as follows: 1/3,000 for anti-pPKAs and 1/10,000 for anti-β-tubulin. Secondary antibodies were diluted 1/10,000 in T-TBS and developed using an enhanced chemiluminescence detection kit (ECL Kallium Biolumina) according to manufacturer’s instructions. When necessary, PVDF membranes were stripped at 60 °C for 15 min in 2% SDS, 0.74% β-mercaptoethanol, and 62.5 mM Tris (pH 6.5) and washed 6 times, 5 min each time, in T-TBS. In all experiments, molecular masses were expressed in kilodaltons (kDa).
Membrane potential assay in cell populations.
Sperm Em changes were assessed using DiSC3(5), as previously described 45. After treatment, cells were loaded with 1 μM of the membrane-potential-sensitive dye DiSC3(5) (Molecular Probes) for 2 min. Sperm were transferred to a gently stirred cuvette at 37°C, and the fluorescence was monitored with a Cary Eclipse fluorescence spectrophotometer (Agilent, CA) at 620/670 nm excitation/emission wavelengths. CCCP (0.5 μM) was added as uncoupler of oxidative phosphorylation to avoid mitochondrial contribution to the recorded Em. Recordings were initiated when steady-state fluorescence was reached and calibration was performed at the end of each measure by adding 1 μM valinomycin and sequential additions of KCl for internal calibration curves, as previously described 46. Sperm Em was obtained from the initial fluorescence (measured as Arbitrary Fluorescence Units) by linearly interpolating it in the theoretical Em values from the calibration curve against arbitrary fluorescence units of each trace. This internal calibration for each determination compensates for variables that influence the absolute fluorescence values.
Determination of pHi by flow cytometry
Sperm pHi changes were assessed using BCECF-AM as previously described 47. After incubation in the appropriate medium, samples were centrifuged at 400 x g for 4 min at room temperature and resuspended in 200 μl of NC H-TYH medium containing 0.5 μM BCECF-AM for 20 min at 37°C. Samples were washed again and resuspended in 50 μl of NC H-TYH medium. Before collecting data, 3 μM of propidium iodide was added to monitor viability. Data were recorded as individual cellular events using a MACSQuant Analyzer cytometer (Miltenyi Biotec, Germany). Side-scatter area (SSC-A) and forward-scatter area (FSC-A) data were collected from 20,000 events per sample in order to define sperm population as previously described 17. In all cases, doublet exclusion was performed analyzing two-dimensional dot plot FSC-A vs FSC-H. Positive cells for BCECF were collected using fluorescein isothiocyanate (FITC; 530/30) filter together with peridinin chlorophyll protein complex (PerCP; 670LP) filter. Although the two indicators had minimal emission overlap, compensation was done. For calibration curves, samples were split and resuspended with high K+ buffered solutions at pH 6.3, 6.5, 7.0, 7.4, or 8.0 (1.2 mM MgSO4, 1.6mM CaCl2, 23.8 mM HEPES, 2.78 mM glucose, 3.38 mM sodium pyruvate, 120 mM KCl; pH previously adjusted with NaOH) and 5 μM nigericin was added to equilibrate intracellular and extracellular pH. Data were analyzed using FlowJo software (V10.0.7).
Analysis of pHi by single cell imaging
Sperm were loaded with the fluorescent pHi indicator as described in the previous sections. Cells were later adhered to 1 mg/ml laminin-precoated coverslips, allowing their flagella to move continuously. The coverslip was mounted on a chamber (Harvard Apparatus) and placed on the stage of an inverted microscope (Eclipse TE 300; Nikon). Fluorescence illumination was supplied by a Luxeon V Star Lambertian Cyan LED (Lumileds Lighting LLC) attached to a custom-built stroboscopic control box. The LED was mounted into a FlashCube40 assembly with a dichroic mirror (M40-DC400; Rapp Opto Electronic; bandwidths: excitation, 450–490 nm; dichroic mirror 505 nm; and emission, 520–560 nm). The LED output was synchronized to the Exposure Out signal of an iXon 888 CCD camera via the control box to produce a single flash of 2-ms duration per individual exposure. The camera exposure time was set equivalent to the flash duration (2 ms). Images were collected every 500 ms using iQ software (Andor Technology).
Statistical analysis
Data are expressed as mean ± standard error of the mean (SEM) of at least three independent experiments for all determinations. Statistical analyses were performed using the GraphPad Prism 6 software (La Jolla, CA). Studenťs t test was used to compare mean values between control and tested groups, while differences between mean values of multiple groups were analyzed by oneway analysis of variance (ANOVA) with multiple comparison tests. Significance is indicated in the figure legends.
Supplementary Material
Acknowledgments
We are thankful to Yoloxochitl Sánchez Guevara for technical support with the sNhe KO mouse strain.
Funding:
Agencia Nacional de Promoción Científica y Tecnológica Grant PICT 2017-3217 (DarioK)
Agencia Nacional de Promoción Cientáfica y Tecnológica Grant PICT 2019-1779 (DarioK)
Agencia Nacional de Promoción Científica y Tecnológica Grant PICT 2021-A-0102 (DarioK)
Dirección General de Asuntos del Personal Académico of the Universidad Nacional Autónoma de México Grant PAPIIT IN207122 (CT)
Dirección General de Asuntos del Personal Académico of the Universidad Nacional Autónoma de México Grant PAPIIT IN200919 (AD)
Consejo Nacional de Ciencia y Tecnología from Mexico Grant CF-2023-I-291 (AD)
National Institutes of Health grant RO1HD038082-17A1 (AD)
National Institutes of Health grant R01HD069631 (CMS)
National Institutes of Health grant R01HD106968 (DiegoK)
Footnotes
Competing interest
Authors declare no competing interests.
Data and materials availability
All data are available in the main text or the supplementary materials.
REFERENCES
- 1.Stival C. et al. Sperm capacitation and acrosome reaction in mammalian sperm. Adv Anat Embryol Cell Biol 220, 93–106 (2016). [DOI] [PubMed] [Google Scholar]
- 2.Santi C. M. et al. The SLO3 sperm-specific potassium channel plays a vital role in male fertility. FEBS Lett 584, 1041–1046 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matamoros-Volante A. & Trevino C. L. Capacitation-associated alkalization in human sperm is differentially controlled at the subcellular level. J Cell Sci 133, (2020). [DOI] [PubMed] [Google Scholar]
- 4.Baro Graf C. et al. Membrane Potential Assessment by Fluorimetry as a Predictor Tool of Human Sperm Fertilizing Capacity. Front Cell Dev Biol 7, 383 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ren D. et al. A sperm ion channel required for sperm motility and male fertility. Nature 413, 603–609 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang D. et al. A sperm-specific Na+/H+ exchanger (sNHE) is critical for expression and in vivo bicarbonate regulation of the soluble adenylyl cyclase (sAC). Proc Natl Acad Sci U S A 104, 9325–9330 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nishigaki T. et al. Intracellular pH in sperm physiology. Biochemical and Biophysical Research Communications vol. 450 1149–1158 Preprint at 10.1016/j.bbrc.2014.05.100 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gardner C. C. & James P. F. Na+/H+ Exchangers (NHEs) in Mammalian Sperm: Essential Contributors to Male Fertility. Int. J. Mol. Sci. 24, 14981 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gardner C. C. & James P. F. The SLC9C2 Gene Product (Na+/H+ Exchanger Isoform 11; NHE11) Is a Testis-Specific Protein Localized to the Head of Mature Mammalian Sperm. Int J Mol Sci 24, (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Woo A. L., James P. F. & Lingrel J. B. Roles of the Na,K-ATPase α4 isoform and the Na+/H+ exchanger in sperm motility. Mol Reprod Dev 62, 348–356 (2002). [DOI] [PubMed] [Google Scholar]
- 11.Bell S. M. et al. Targeted disruption of the murine Nhe1 locus induces ataxia, growth retardation, and seizures. Am J Physiol Cell Physiol 276, (1999). [DOI] [PubMed] [Google Scholar]
- 12.Chen S. R. et al. Sodium–hydrogen exchanger NHA1 and NHA2 control sperm motility and male fertility. Cell Death Dis 7, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balbach M. et al. Molecular Mechanism Underlying the Action of Zona-pellucida Glycoproteins on Mouse Sperm. Front Cell Dev Biol 8, 572735 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Windler F. et al. The solute carrier SLC9C1 is a Na+/H+-exchanger gated by an S4-type voltage-sensor and cyclic-nucleotide binding. Nat Commun 9, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lishko P. V et al. The control of male fertility by spermatozoan ion channels. Annu Rev Physiol 74, 453–475 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baro Graf C. et al. Everything you ever wanted to know about PKA regulation and its involvement in mammalian sperm capacitation. Mol Cell Endocrinol 518, 110992 (2020). [DOI] [PubMed] [Google Scholar]
- 17.Escoffier J., Krapf D., Navarrete F., Darszon A. & Visconti P. E. Flow cytometry analysis reveals a decrease in intracellular sodium during sperm capacitation. J Cell Sci 125, 473–85 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weeks K. L. et al. Protein Kinase Inhibitor Peptide as a Tool to Specifically Inhibit Protein Kinase A. Frontiers in Physiology | www.frontiersin.org 1, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krapf D. et al. Inhibition of Ser/Thr phosphatases induces capacitation-associated signaling in the presence of Src kinase inhibitors. Journal of Biological Chemistry 285, 7977–7985 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wrighton D. C., Muench S. P. & Lippiat J. D. Mechanism of inhibition of mouse Slo3 (KCa5.1) potassium channels by quinine, quinidine and barium. Br J Pharmacol 172, 4355–4363 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wertheimer E. et al. Compartmentalization of distinct cAMP signaling pathways in mammalian sperm. Journal of Biological Chemistry 288, 35307–35320 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Balbach M. et al. Soluble adenylyl cyclase inhibition prevents human sperm functions essential for fertilization. Mol Hum Reprod 27, 1–13 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lucchesi O., Ruete M. C., Bustos M. A., Quevedo M. F. & Tomes C. N. The signaling module cAMP/Epac/Rap1/PLCε/IP3 mobilizes acrosomal calcium during sperm exocytosis. Biochim Biophys Acta Mol Cell Res 1863, 544–561 (2016). [DOI] [PubMed] [Google Scholar]
- 24.Chávez J. C. et al. SLO3 K+ channels control calcium entry through CATSPER channels in sperm. Journal of Biological Chemistry 289, 32266–32275 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Graf C. B. et al. Determination of a robust assay for human sperm membrane potential analysis. Front Cell Dev Biol 7, 101 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hernández-Garduño S. et al. Hyperpolarization induces cytosolic alkalization of mouse sperm flagellum probably through sperm Na+/H+ exchanger. Reproduction 164, 125–134 (2022). [DOI] [PubMed] [Google Scholar]
- 27.Masereel B., Pochet L. & Laeckmann D. An overview of inhibitors of Na+/H+ exchanger. European Journal of Medicinal Chemistry vol. 38 547–554 Preprint at 10.1016/S0223-5234(03)00100-4 (2003). [DOI] [PubMed] [Google Scholar]
- 28.Kang H. et al. Na+/H+ exchangers involve in regulating the ph-sensitive ion channels in mouse sperm. Int J Mol Sci 22, 1–13 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muzzachi S. et al. Effect of cariporide on ram sperm pH regulation and motility: possible role of NHE1. Reproduction 155, 433–445 (2018). [DOI] [PubMed] [Google Scholar]
- 30.Nørholm A.-B. et al. The Intracellular Distal Tail of the Na+/H+ Exchanger NHE1 Is Intrinsically Disordered: Implications for NHE1 Trafficking. Biochemistry 50, 3469–3480 (2011). [DOI] [PubMed] [Google Scholar]
- 31.Köster S., Pavkov-Keller T., Kühlbrandt W. & Yildiz O. Structure of human Na +/H + exchanger NHE1 regulatory region in complex with calmodulin and Ca 2+. Journal of Biological Chemistry 286, 40954–40961 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wakabayashi S., Bertrand B., Ikeda T., Pouysségur J. & Shigekawa M. Mutation of calmodulin-binding site renders the Na+/H+ exchanger (NHE1) highly H+-sensitive and Ca2+ regulation-defective. Journal of Biological Chemistry 269, 13710–13715 (1994). [PubMed] [Google Scholar]
- 33.Navarrete F. A. et al. Biphasic role of calcium in mouse sperm capacitation signaling pathways. J Cell Physiol 230, 1758–1769 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zeng X. H., Yang C., Kim S. T., Lingle C. J. & Xia X. M. Deletion of the Slo3 gene abolishes alkalizationactivated K+ current in mouse spermatozoa. Proc Natl Acad Sci U S A 108, 5879–5884 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Escoffier J. et al. Flow cytometry analysis reveals that only a subpopulation of mouse sperm undergoes hyperpolarization during capacitation. Biol Reprod 92, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Limbutara K., Kelleher A., Yang C. R., Raghuram V. & Knepper M. A. Phosphorylation Changes in Response to Kinase Inhibitor H89 in PKA-Null Cells. Sci Rep 9, 1–10 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen Y. & Sabatini B. L. The Kinase Specificity of Protein Kinase Inhibitor Peptide. Front Pharmacol 12, 632815 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Navarro B., Kirichok Y. & Clapham D. E. KSper, a pH-sensitive K+ current that controls sperm membrane potential. Proc Natl Acad Sci U S A 104, 7688 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lyon M. D. et al. SLO3: A Conserved Regulator of Sperm Membrane Potential. International Journal of Molecular Sciences 2023, Vol. 24, Page 11205 24, 11205 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dolan J. et al. The extracellular Leucine-rich repeat superfamily; a comparative survey and analysis of evolutionary relationships and expression patterns. BMC Genomics 8, 320 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang D., King S. M., Quill T. A., Doolittle L. K. & Garbers D. L. A new sperm-specific Na+/H+ exchanger required for sperm motility and fertility. Nat Cell Biol 5, 1117–1122 (2003). [DOI] [PubMed] [Google Scholar]
- 42.Cavarocchi E. et al. The sodium/proton exchanger SLC9C1 (sNHE) is essential for human sperm motility and fertility. Clin Genet 99, 684–693 (2021). [DOI] [PubMed] [Google Scholar]
- 43.Scholz W. et al. Protective effects of HOE642, a selective sodium-hydrogen exchange subtype 1 inhibitor, on cardiac ischaemia and reperfusion. Cardiovasc Res 29, 260 (1995). [PubMed] [Google Scholar]
- 44.Grahn E. et al. Control of intracellular pH and bicarbonate by CO2 diffusion into human sperm. Nat Commun 14, (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ritagliati C., Baro Graf C., Stival C. & Krapf D. Regulation mechanisms and implications of sperm membrane hyperpolarization. Mech Dev 154, 33–43 (2018). [DOI] [PubMed] [Google Scholar]
- 46.Ritagliati C. et al. Lysine acetylation modulates mouse sperm capacitation. Sci Rep 8, 1–14 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Luque G. M. et al. Cdc42 localized in the CatSper signaling complex regulates cAMP-dependent pathways in mouse sperm. FASEB Journal 35, e21723 (2021). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available in the main text or the supplementary materials.