Abstract
Human herpesvirus 8 (HHV-8) is the probable viral etiologic agent for Kaposi’s sarcoma. The HHV-8 genome encodes viral interferon regulatory factor (vIRF), a gene product that has homology to the IRF family of transcription factors. We demonstrate that vIRF inhibits responses to type I and type II interferons and blocks IRF-1-mediated transcription. vIRF does not compete with IRF-1 for binding to DNA or complex directly with IRF-1. The ability of vIRF to block IRF-1-mediated transcription is independent of the DNA binding domains of both vIRF and IRF-1. These data suggest that vIRF may contribute to viral pathogenesis and cellular transformation by interfering with interferon- and IRF-1-mediated gene expression through a novel mechanism.
Human herpesvirus 8 (HHV-8), also referred to as Kaposi’s sarcoma (KS) herpesvirus, is a recently identified gammaherpesvirus that is associated with and most likely the etiologic agent of KS (6, 7). In KS lesions, HHV-8 infects endothelial cells (3), the likely precursor of KS, and it has been detected in virtually all KS lesions from all known risk groups (6–8, 27), in all primary effusion lymphomas (4), and in many cases of Castleman’s disease (31). Sequence analysis of the HHV-8 genome reveals at least 81 open reading frames that potentially code for viral gene products (29). In addition to many genes shared with other herpesviruses, HHV-8 contains genes whose products have homology to cellular proteins, such as macrophage inflammatory protein 1, cyclin D, interleukin 6 (IL-6), and Bcl-2 (5, 29). Among these viral genes is a gene within open reading frame K9, which encodes a 449-amino-acid protein that has homology to the interferon (IFN) regulatory factor (IRF) family of transcription factors and has thus been named viral IRF (vIRF) (25, 29). Although vIRF has only a 13% overall identity to human IRF family proteins, much of its homology is localized to a region of the N terminus which is homologous to the IRF DNA binding motif. For example, the N-terminal region of vIRF has a portion with 70% identity to the IRF binding motif found in the interferon consensus sequence binding protein (ICSBP) (29).
The IRF family of transcription factors are cellular DNA binding proteins that act as activators or repressors of promoters containing variations on the IRF binding sequence (19, 33). Family members include IRF-1 (ISGF-2) (13), IRF-2 (21), IRF-3 (1, 15), IRF-4 (lymphocyte-specific IRF) (16), ICSBP (9), and ISGF-3γ(p48) (34). IRF-1 is an IFN-inducible transcription factor that was originally identified by its ability to positively regulate the IFN-β promoter through the PRD I motif (13, 14). Deletion analysis of IRF-1 reveals that the N terminus contains a DNA binding domain, while the C terminus has the ability to transactivate gene expression (24). IRF-2 contains a DNA binding domain similar to that of IRF-1 and binds to the same IRF consensus element (17). Unlike IRF-1, however, IRF-2 lacks a strong transactivation domain in its C terminus and acts as an inhibitor of IRF-1-mediated transcription. The ability of IRF-2 deletion mutants to inhibit IRF-1 correlates directly with the ability to bind DNA (24), indicating that DNA binding is essential to IRF-2-mediated inhibition of IRF-1. Thus, IRF-2 can act as a negative regulator of IRF-1-mediated transcription by competing for and occupying IRF binding sites with a protein that is not a strong transactivator.
Because vIRF has homology to IRF family members, we explored the ability of vIRF to regulate transcription both in response to IFNs and in response to IRF-1. We demonstrate that vIRF blocks induction of an IFN-responsive reporter construct, (PRD I)4-CAT, in response to either IFN-α or IFN-γ. Furthermore, vIRF blocks induction of (PRD I)4-CAT by IRF-1. We demonstrate that there is specificity in this inhibition since induction of the NF-κB-responsive reporter, (PRD II)4-CAT, is not blocked by vIRF. In these studies, we explored the role of DNA binding and interactions with IRF-1 in the ability of vIRF to inhibit IRF-1-mediated transactivation of (PRD I)4-CAT. We demonstrate that the inhibition of IRF-1-mediated transactivation by vIRF differs from the inhibition that occurs in response to IRF-2. We demonstrate that in vitro-translated vIRF neither directly binds to the IRF consensus nor complexes with IRF-1. Furthermore, the putative DNA binding domain of vIRF is not necessary for its inhibitory effect. These studies suggest a novel mechanism by which this viral homolog inhibits IRF-1-mediated transcriptional activation.
MATERIALS AND METHODS
Tissue culture.
Human umbilical vein endothelial cells (HUVECs) were isolated from human umbilical veins that had been cannulated, perfused with Hanks balanced salt solution to remove blood, and then incubated with 1% collagenase for 15 min at 37°C. After removal of collagenase, cells were cultured in medium 199 supplemented with 20% fetal calf serum (GIBCO BRL Life Technologies, Inc.), 16 U of heparin (ESI Pharmaceuticals, Cherry Hill, N.J.) per ml, 25 mM HEPES buffer, 100 μg of endothelial mitogen (Biomedical Technologies, Inc., Stoughton, Mass.) per ml, 2 mM l-glutamine, 100 U of penicillin per ml, and 100 U of streptomycin per ml and grown at 37°C on tissue culture plates coated with 0.1% gelatin. Cells were passaged at confluency by splitting them 1:4 and were used within the first six passages.
Preparation of vIRF expression vectors.
An expression vector for vIRF was constructed by amplifying the coding region for vIRF by using DNA from the HHV-8-infected cell line BCBL-1. The 5′ PCR primer 5′-CGGGATCCAGCCATGGACCCAGGCCAAAGACCGAAC-3′ was engineered to contain a Kozak consensus site and a BamHI site, and the 3′ PCR primer 5′-CGGAATTCTTATTGCATGGCATCCCATAACGG-3′ was engineered to contain an EcoRI site. These were thermocycled for 40 rounds with Pfu polymerase (Stratagene). An N-terminal truncation of the vIRF product lacking amino acids 1 to 150 was obtained by PCR amplification of cloned vIRF DNA with primers 5′-CGGGATCCAGCCATGGACGCCTCGTTTAAAGGCACCAGG-3′ and 5′-CGGAATTCTTATTGCATGGCATCCCATAACGG-3′. The PCR products were ligated into the BamHI and EcoRI sites of pDNA 3.1(+) (Invitrogen) to allow eukaryotic expression under the control of the cytomegalovirus immediate-early promoter/enhancer.
Preparation of GAL4-IRF fusion construct.
The plasmid Gal4(1–147) contains a sequence encoding an N terminally 9E10 c-myc epitope-tagged DNA binding domain of the yeast GAL4 protein consisting of amino acids 1 to 147, which was inserted into the plasmid pCO2 downstream of a cytomegalovirus promoter/enhancer. GAL4-IRFt contains a fragment of IRF-1 spanning amino acids 105 to 325 fused to the C-terminal side of the DNA binding domain of GAL4 inserted into pCO2.
Transfection.
HUVECs were transfected by a modification of the DEAE-dextran method. Cells (about 90% confluent) were transfected with 10 μg of total plasmid in 568 μl of phosphate-buffered saline containing 0.5 mg of DEAE-dextran per ml. After a 30-min incubation, complete HUVEC medium (6 ml) containing 8 μM chloroquine was added, and cells were incubated for an additional 2.5 h prior to a 2-min shock with 10% dimethyl sulfoxide (DMSO) in phosphate-buffered saline. Cellular extracts standardized for protein concentration were assayed for chloramphenicol acetyltransferase (CAT) activity by assessing the acetylation of [14C]chloramphenicol by separation by silica gel thin-layer chromatography (23).
In vitro translation.
Recombinant IRF-1, IRF-2, and vIRF were prepared by in vitro translation in a reticulocyte lysate system (Promega TNT system) with T7 polymerase and plasmids in which the coding region for IRF-1, IRF-2, or vIRF was downstream of a T7 promoter.
Gel shift analysis.
Electrophoretic mobility shift assay (EMSA) analysis was performed by incubating equal amounts of recombinant IRF-1, IRF-2, or vIRF with radiolabeled PRD I DNA consisting of four tandem IRF binding elements of the same sequence as that found in (PRD I)4-CAT. The PRD I DNA probe was synthesized as a single template of the sequence GATCCAAGTGAAAGTGAAAGTGAAAGTGAGATC along with the complementary primer GATCTCACTTT. Templates and primers were annealed and radiolabeled with [32P]dCTP by using Klenow fragment as previously described (26). Unradiolabeled DNA probes were generated by substituting nonradioactive dCTP. Binding reactions were carried out as previously described (26). When present, nonradiolabeled specific (PRD I) and nonspecific (κB) competitors were present in 10-fold excesses. Binding complexes were resolved by 4% nondenaturing polyacrylamide gel electrophoresis in Tris-glycine buffer and visualized by autoradiography.
RESULTS
vIRF interferes with signaling by both type I and type II IFNs.
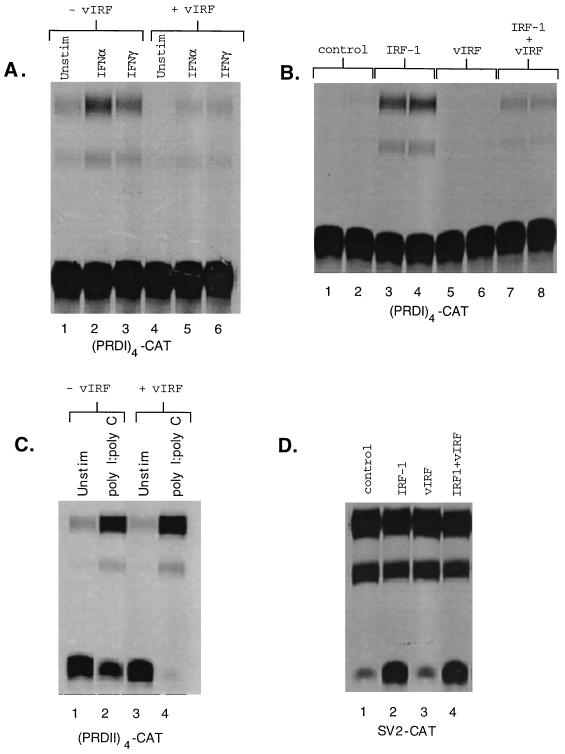
Since many of the IRF family members are involved in regulating responses to both type I and type II IFNs, we examined the ability of vIRF to modulate induction of an IFN-sensitive reporter, (PRD I)4-CAT (10), by either IFN-α or IFN-γ. Transient transfections were performed in HUVECs, and cell extracts were assayed for CAT activity (23). Both IFN-α and IFN-γ induced CAT activity (Fig. 1A, lanes 2 and 3) that was blocked by cotransfection with an expression vector for vIRF (Fig. 1A, lanes 5 and 6).
FIG. 1.
Effect of vIRF on gene induction by IFNs and IRF-1. (A) HUVECs were cotransfected with a total of 10 μg of plasmid consisting of 7 μg of (PRD I)4-CAT (10) and 3 μg of vIRF expression vector or empty expression vector. After a 24-h recovery following DMSO shock, cells were induced with IFN-α (1,000 U/ml) or IFN-γ (250 U/ml) for 18 h. Cellular extracts standardized for protein concentration were assayed for CAT activity by assessing the acetylation of [14C]chloramphenicol by separation by silica gel chromatography (23). (B) HUVECs were cotransfected with 7 μg of (PRD I)4-CAT and either 3 μg of empty expression vector (control), 1.5 μg of expression vector for either IRF-1 or vIRF with 1.5 μg of empty expression vector, or a combination of both 1.5 μg of vIRF and 1.5 μg of IRF-1. Cells were incubated for 48 h after the DMSO shock, and cellular extracts standardized for protein concentration were assayed for CAT activity. (C) HUVECs were transfected with 10 μg of plasmid consisting of 7 μg of (PRD II)4-CAT (10) and 3 μg of vIRF or empty expression vector. After a 24-h recovery following the DMSO shock, cells were incubated with poly(I) · poly(C) (100 μg/ml) for 18 h, and cellular extracts standardized for protein concentration were assayed for CAT activity. (D) HUVECs were cotransfected with 7 μg of SV2-CAT and with 3 μg of empty expression vector (control) or with 3 μg of expression vectors for IRF-1, vIRF, or a combination of IRF-1 and vIRF. Cells were incubated for 48 h after the DMSO shock, and cellular extracts standardized for protein concentration were assayed for CAT activity.
vIRF inhibits IRF-1-mediated transcription.
Since vIRF inhibited induction of (PRD I)4-CAT by IFN-α and IFN-γ, we hypothesized that vIRF may target a transcription factor common to both pathways. Although IFN-α and IFN-γ interact with distinct receptors and activate distinct pathways, they share the ability to induce expression of IRF-1. The ability of vIRF to affect IRF-1-mediated transcription was assessed by cotransfecting HUVECs with (PRD I)4-CAT along with an expression vector for IRF-1, vIRF, or a combination of both. Cotransfection of (PRD I)4-CAT with an expression vector for IRF-1 resulted in an approximately 16-fold induction of CAT activity (Fig. 1B, lanes 3 and 4), which was reduced to a 2.5-fold induction by the coexpression of vIRF (Fig. 1B, lanes 7 and 8). vIRF alone did not induce CAT activity above the control level (Fig. 1B, lanes 5 and 6). Thus, vIRF interfered with IRF-1-mediated transcription and did not itself drive expression of an IRF-1-responsive reporter. This inhibition of IRF-1 by vIRF has been demonstrated in four separate experiments, with duplicate determinations in two of the experiments. The range of inhibition was 80 to 90% (mean ± standard deviation = 85.6% ± 4.3%) for all six determinations.
The effect of vIRF on an NF-κB-responsive reporter was examined by using (PRD II)4-CAT, an NF-κB-responsive reporter in which (PRD II)4 was substituted for the (PRD I)4 element in (PRD I)4-CAT (10). vIRF did not inhibit the induction of (PRD II)4-CAT by poly(I) · poly(C) (Fig. 1C), a potent activator of NF-κB in endothelial cells (26), indicating that vIRF does not repress transcription in response to an inducible transcription factor distinct from IRF-1. The ability of vIRF to inhibit IRF-1-mediated induction of (PRD I)4-CAT was not due to an alteration in transfectional efficiency or interference with CAT expression at some point other than transcription, since transfection of a constitutive promoter-reporter construct, SV2-CAT, resulted in CAT expression that was not inhibited by vIRF (Fig. 1D). Taken together, these data demonstrate that vIRF blocks gene induction by type I and type II IFNs and most likely does so at least in part by specifically interfering with IRF-1-mediated transcription.
The putative DNA binding domain from vIRF is not necessary for inhibition of IRF-1-mediated transcription.
Sequence analysis of vIRF demonstrates the greatest homology to IRF family members in the N-terminal region, where the DNA binding domains are located. In order to test whether the putative vIRF DNA binding domain was necessary for inhibition of IRF-1-mediated gene induction, an N-terminal truncation mutant (NTvIRF) in which the 150 amino acids containing the putative DNA binding domain were removed was constructed. The truncated vIRF retained the ability to inhibit IRF-1-mediated transcription of (PRD I)4-CAT, and this inhibition was equivalent to that of full-length vIRF (Fig. 2). This demonstrates that unlike IRF-2, vIRF does not require its putative DNA binding domain for inhibition of IRF-1-mediated transcription, suggesting that vIRF inhibits IRF-1-mediated transcription by a mechanism other than competing with IRF-1 for binding of IRF elements.
FIG. 2.
The vIRF DNA binding domain is not necessary for inhibition of IRF-1-mediated transcription. An expression vector in which the N-terminal 150 amino acids containing the putative DNA binding site (black box in panel C) of vIRF were deleted was created. The effects of vIRF and NTvIRF on IRF-1-mediated transcription were assessed by cotransfection with a (PRD I)4-CAT reporter gene. Cells were incubated for 48 h after completion of transfection, and cellular extracts standardized for protein concentration were assayed for CAT activity. vIRF and NTvIRF were comparably effective at inhibiting IRF-1-mediated transcription. All experiments were done in duplicate. Panel A shows the mean fold induction ± the standard error from three determinations.
In vitro-translated vIRF does not bind to the IRF consensus element.
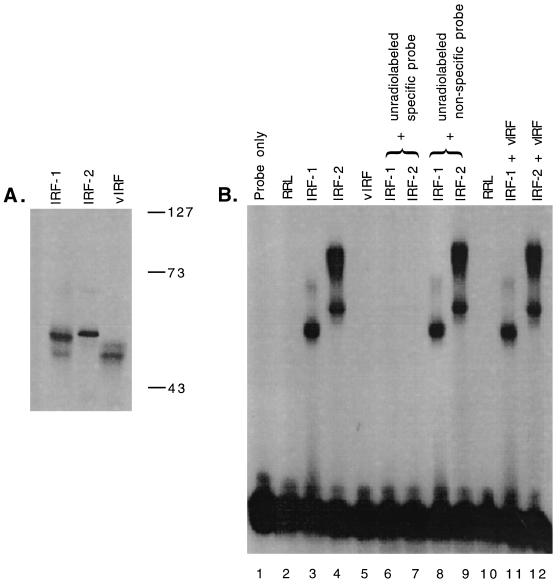
In vitro-translated vIRF that was 35S labeled migrated at a mobility consistent with its predicted molecular weight of 48,465 (Fig. 3A, lane 3). It migrated faster than in vitro-translated IRF-1 and IRF-2, both of which are known to migrate slower than predicted based on their molecular weights (24). In order to directly test the ability of vIRF to bind an IRF DNA element, EMSAs in which equal amounts of in vitro-translated IRF-1, IRF-2, and vIRF were incubated with radiolabeled DNA corresponding to four tandem IRF binding sequences found in (PRD I)4-CAT were performed. While the addition of IRF-1 or IRF-2 resulted in discrete band shifts (Fig. 3B, lanes 3 and 4), no detectable shift was observed upon the addition of vIRF (Fig. 3B, lane 5). This demonstrates that vIRF did not bind to IRF elements under conditions in which both IRF-1 and IRF-2 bound. Specificity of the bands shifted by both IRF-1 and IRF-2 was confirmed by competition with specific and nonspecific nonradiolabeled DNA (Fig. 3B, lanes 6 to 9). Several distinct PRD I binding complexes that contained IRF-2 were seen, most likely as a consequence of the four tandem IRF binding sites. Although there were two bands observed in the in vitro-translated vIRF, both of these bands appeared to have intact N-terminal sequences, based on Western blot analysis of vIRF that had an epitope tag at its N terminus (data not shown). This suggests that both bands of vIRF seen in Fig. 3 had an intact N terminus and hence a DNA binding domain. EMSAs with low-ionic-strength gel separation, performed to address the possibility that vIRF-DNA complexes were sensitive to the Tris-glycine system, yielded identical results (data not shown). In addition, coincubation of vIRF with either IRF-1 or IRF-2 failed to alter band intensity, indicating that vIRF did not alter the ability of IRF-1 or IRF-2 to bind DNA (Fig. 3B, lanes 11 and 12). These data suggest that vIRF inhibits IRF-1 by a mechanism that does not involve direct binding to the IRF DNA binding element.
FIG. 3.
Effect of vIRF on DNA binding. (A) Translations were carried out in the presence of [35S]methionine, and translation products were resolved by sodium dodecyl sulfate–10% polyacrylamide gel electrophoresis and detected by autoradiography. Translation products were quantified by phosphorimager (Bio-Rad) analysis, and concentrations were determined by adjustment to methionine content. Numbers at the right are molecular size markers (in kilodaltons). (B) EMSA analysis was performed by incubating equal amounts of IRF-1, IRF-2, or vIRF with radiolabeled PRD I DNA consisting of four tandem IRF binding elements of the sequence found in (PRD I)4-CAT. RRL, rabbit reticulocyte lysate in which empty vector without an IRF-coding sequence was used in the translation reaction. When present, nonradiolabeled specific (PRD I) and nonspecific (κB) competitors were present in 10-fold excesses. Binding complexes were resolved by 4% nondenaturing polyacrylamide gel electrophoresis and visualized by autoradiography.
vIRF does not complex directly to IRF-1.
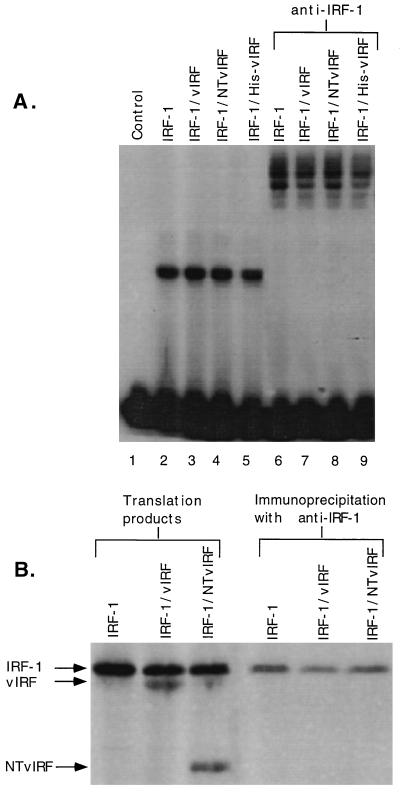
The possibility that vIRF may complex directly to IRF-1 was assessed by cotranslating IRF-1 with vIRF or NTvIRF. EMSA analysis of the cotranslated products resulted in a single band from IRF-1 that was unaltered in mobility by cotranslation with vIRF, NTvIRF, or N-terminally epitope-tagged vIRF (His-vIRF), suggesting that vIRF was not complexing with DNA-bound IRF-1 (Fig. 4A). Furthermore, supershift patterns with anti-IRF-1 resulted in identical banding patterns (Fig. 4A, lanes 6 to 9). In addition, His-vIRF retained the ability to inhibit the induction of (PRD I)4-CAT by IRF-1 (data not shown), and cotranslated IRF-1 and His-vIRF resulted in a band shift that did not supershift with an antibody directed against the His-vIRF recombinant epitope (data not shown). Immunoprecipitations of all samples tested failed to yield any band other than IRF-1 (Fig. 4B, lanes 4 to 6), indicating that vIRF and IRF-1 did not associate in vitro. Overexposure of Fig. 4B failed to reveal any immunoprecipitate other than IRF-1 (data not shown). These data indicate that in vitro-translated vIRF does not complex with IRF-1 in the soluble or DNA-bound state.
FIG. 4.
Effect of cotranslation of vIRF with IRF-1. IRF-1 was translated alone or in combination with vIRF, NTvIRF, or His-vIRF by using the in vitro translation system as described in Materials and Methods. (A) EMSA analysis was performed by incubating equal volumes of translation products with radiolabeled PRD I DNA. Supershift analysis was carried out with antibody specific for IRF-1 (PKI-1). Complexes were resolved by 4% nondenaturing polyacrylamide gel electrophoresis and visualized by autoradiography. (B) 35S-methionine-labeled protein products were obtained by in vitro translation of IRF-1 individually or by cotranslation of IRF-1 with either vIRF or NTvIRF. Comparable amounts of IRF-1 were present under all conditions, and bands reflecting the levels of IRF-1, vIRF, and NTvIRF were resolved by sodium dedecyl sulfate-polyacrylamide gel electrophoresis. The translation products were subjected to immunoprecipitation (32) with protein A-Sepharose 6MB beads (Pharmacia) and polyclonal anti-IRF-1 (PKI-1). Comparison of translation products pre- and postimmunoprecipitation demonstrated that IRF-1, but not vIRF or NTvIRF, was recovered with antibodies to IRF-1.
vIRF targets the transactivation domain of IRF-1.
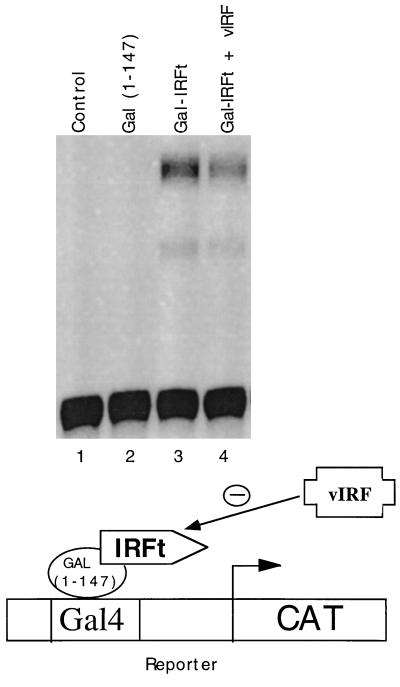
To test if vIRF was targeting the transactivation domain of IRF-1, the fusion construct GAL4-IRF1t, in which IRF-1’s transactivation domain was fused to the DNA binding domain from the yeast transcription factor GAL4, was created. The ability of this fusion protein to transactivate a GAL4-CAT reporter is dependent on GAL4-mediated DNA binding and IRF-1-mediated transactivation. Transfection of HUVECs with GAL4-IRF1t resulted in induction of a GAL4-responsive CAT reporter (Fig. 5, lane 3). This induction was inhibited 30% by cotransfection with expression vector for vIRF (Fig. 5, lane 4). The induction of CAT activity by GAL4-IRF1t was not due to sequences in the GAL4 DNA binding domain [GAL4(1–147]), since the DNA binding domain alone was unable to induce CAT activity (Fig. 5, lane 2).
FIG. 5.
Effect of vIRF on GAL4-IRFt-mediated transcription. An expression vector was constructed for a fusion protein (GAL4-IRFt) that contained the DNA binding domain from GAL4 and the transactivation domain from IRF-1. HUVECs that were cotransfected with 7 μg of GAL4-CAT reporter plasmid (11) and 3 μg of expression vector were assessed for CAT activity 48 h following transfection. Expression of GAL4-IRFt induced GAL4-CAT reporter activity, whereas neither an empty expression vector (control) nor an expression vector containing the GAL4 DNA binding domain [GAL4(1–147)] but lacking the IRF-1 transactivation domain induced CAT activity. Coexpression of vIRF with GAL-IRFt caused a 30% reduction in the level of CAT reporter activity that was induced by GAL-IRFt. This experiment is a representative example and has been reproduced multiple times.
DISCUSSION
Taken together, the above-described data indicate that vIRF is capable of inhibiting responses to IFN-α, IFN-γ, and IRF-1 at the transcriptional level. The observations that vIRF does not bind to an IRF element as determined by EMSA analysis and that the vIRF putative DNA binding domain is not necessary for inhibition suggest that vIRF blocks IRF-1-mediated transcription by a mechanism that does not involve direct binding to the IRF DNA binding element. The lack of detectable DNA binding by in vitro-translated vIRF could be a consequence of the need for in vivo folding or posttranslational modifications. However, our observation that truncated vIRF missing the putative DNA binding domain also maintains IRF-1-inhibitory activity suggests a mechanism of action distinct from DNA binding. The inhibition by vIRF of the transactivation domain of IRF-1 fused to the GAL4 DNA binding domain further supports the hypothesis that DNA binding by vIRF is not required for its inhibitory action. Furthermore, in vitro-translated vIRF does not alter the mobility of IRF-1 band shifts by EMSA or coimmunoprecipitate with IRF-1, suggesting that it does not directly bind to IRF-1. Thus, vIRF may interfere with the ability of IRF-1 to transactivate by a mechanism that interferes with IRF-1 modification or targets components of the transactivation process other than IRF-1.
Both IFN-α and IFN-γ activate multiple transcription factors in addition to IRF-1. IFN-α most notably activates ISGF-3, which binds to IFN-stimulated response elements (ISREs) via the ISGF-3γ (p48) component (22, 34). The ISRE contains an IRF-1 binding site, but ISGF-3γ requires additional flanking base pairs to bind DNA. IFN-γ activates p91-p91 homodimers (gamma interferon-activated factor) (30) that bind the gamma-activated sequence element. Although gamma-activated sequence has no sequence similarity to IRF binding sites, p91-p91-p48 complexes that bind the ISRE in response to IFN-γ have been detected in some cell types. We have shown that vIRF specifically blocks IRF-1-mediated transcription. Whether it has any effect on the function of other IFN-induced transcription factors is under investigation.
The ability of vIRF to block responses to IFNs and to IRF-1 in endothelial cells may be of particular relevance to KS since the HHV-8-infected spindle cells in KS lesions appear to be of endothelial origin. Interfering with IRF-1-mediated gene induction would be beneficial to HHV-8 in multiple ways. IRF-1 is involved in the induction of gene products that play a role in antiviral responses such as IFN-β (28), RNA-activated protein kinase (2), 2′,5′-oligodenylate synthetase (28), and major histocompatibility complex class I molecules (28). In addition, IRF-1 plays a role in cellular responses to cytokines involved in antiviral and inflammatory immune responses, including IFN-α, IFN-β (12), IFN-γ, tumor necrosis factor alpha (12), IL-1β (12), and IL-6 (20). Thus, by inhibiting IRF-1, HHV-8 can potentially interfere with antiviral immunology at numerous points. Furthermore, IRF-1 has been shown to be a tumor suppressor, and inhibitors of IRF-1 can function as oncogenes (18). Thus, the HHV-8 vIRF gene product can potentially play a role in neoplastic transformation of HHV-8-infected cells. Neoplasms must have both disregulated growth patterns and the ability to evade immune detection and/or destruction. Since vIRF can potentially aid in both of these capacities, it may play a fundamental role in HHV-8-mediated oncogenesis.
ACKNOWLEDGMENTS
We thank Tom Maniatis for (PRD I)4-CAT and (PRD II)4-CAT vectors and Peter King for IRF-1 antibody and useful input. We especially thank David Jollow and Florence Roan for helpful conversations and advice and Lani L. L. Paxton for critical review of the manuscript.
This work was supported in part by NIH grants RO1 CA60345, RO1 CA67382, and P30AR42687 to M.K.O. and by a Wellcome Trust university award to S.G.
REFERENCES
- 1.Au W C, Moore P A, Lowther W, Juang Y T, Pitha P M. Identification of a member of the interferon regulatory factor family that binds to the interferon-stimulated response element and activates expression of interferon-induced genes. Proc Natl Acad Sci USA. 1995;92:11657–11661. doi: 10.1073/pnas.92.25.11657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beretta L, Gabbay M, Berger R, Hanash S M, Sonenberg N. Expression of the protein kinase PKR is modulated by IRF-1 and is reduced in 5q-associated leukemias. Oncogene. 1996;12:1593–1596. [PubMed] [Google Scholar]
- 3.Boshoff C, Schulz T F, Kennedy M M, Graham A K, Fisher C, Thomas A, McGee J O, Weiss R A, O’Leary J J. Kaposi’s sarcoma-associated herpesvirus infects endothelial and spindle cells. Nat Med. 1995;1:1274–1278. doi: 10.1038/nm1295-1274. [DOI] [PubMed] [Google Scholar]
- 4.Cesarman E, Chang Y, Moore P S, Said J W, Knowles D M. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N Engl J Med. 1995;332:1186–1191. doi: 10.1056/NEJM199505043321802. [DOI] [PubMed] [Google Scholar]
- 5.Cesarman E, Nador R G, Bai F, Bohenzky R A, Russo J J, Moore P S, Chang Y, Knowles D M. Kaposi’s sarcoma-associated herpesvirus contains G protein-coupled receptor and cyclin D homologs which are expressed in Kaposi’s sarcoma and malignant lymphoma. J Virol. 1996;70:8218–8223. doi: 10.1128/jvi.70.11.8218-8223.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang Y, Cesarman E, Pessin M S, Lee F, Culpepper J, Knowles D M, Moore P S. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcoma. Science. 1994;266:1865–1869. doi: 10.1126/science.7997879. [DOI] [PubMed] [Google Scholar]
- 7.Chang Y, Moore P S. Kaposi’s sarcoma (KS)-associated herpesvirus and its role in KS. Infect Agents Dis. 1996;5:215–222. [PubMed] [Google Scholar]
- 8.Chang Y, Ziegler J, Wabinga H, Katangole-Mbidde E, Boshoff C, Schulz T, Whitby D, Maddalena D, Jaffe H W, Weiss R A, Moore P S. Kaposi’s sarcoma-associated herpesvirus and Kaposi’s sarcoma in Africa. Uganda Kaposi’s Sarcoma Study Group Arch Intern Med. 1996;156:202–204. [PubMed] [Google Scholar]
- 9.Driggers P H, Ennist D L, Gleason S L, Mak W H, Marks M S, Levi B Z, Flanagan J R, Appella E, Ozato K. An interferon gamma-regulated protein that binds the interferon-inducible enhancer element of major histocompatibility complex class I genes. Proc Natl Acad Sci USA. 1990;87:3743–3747. doi: 10.1073/pnas.87.10.3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fan C-M, Maniatis T. Two different virus-inducible elements are required for human b-interferon gene regulation. EMBO J. 1989;8:101–110. doi: 10.1002/j.1460-2075.1989.tb03353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flint K J, Jones N C. Differential regulation of three members of the ATF/CREB family of DNA-binding proteins. Oncogene. 1991;6:2019–2026. [PubMed] [Google Scholar]
- 12.Fujita T, Reis L F, Watanabe N, Kimura Y, Taniguchi T, Vilcek J. Induction of the transcription factor IRF-1 and interferon-beta mRNAs by cytokines and activators of second-messenger pathways. Proc Natl Acad Sci USA. 1989;86:9936–9940. doi: 10.1073/pnas.86.24.9936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fujita T, Sakakibara J, Sudo Y, Miyamoto M, Kimura Y, Taniguchi T. Evidence for a nuclear factor(s), IRF-1, mediating induction and silencing properties to human IFN-beta gene regulatory elements. EMBO J. 1988;7:3397–3405. doi: 10.1002/j.1460-2075.1988.tb03213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goodbourn S, Maniatis T. Overlapping positive and negative regulatory domains of the human beta-interferon gene. Proc Natl Acad Sci USA. 1988;85:1447–1451. doi: 10.1073/pnas.85.5.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grant C E, Vasa M Z, Deeley R G. cIRF-3, a new member of the interferon regulatory factor (IRF) family that is rapidly and transiently induced by dsRNA. Nucleic Acids Res. 1995;23:2137–2146. doi: 10.1093/nar/23.12.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grossman A, Mittrucker H W, Nicholl J, Suzuki A, Chung S, Antonio L, Suggs S, Sutherland G R, Siderovski D P, Mak T W. Cloning of human lymphocyte-specific interferon regulatory factor (hLSIRF/hIRF4) and mapping of the gene to 6p23-p25. Genomics. 1996;37:229–233. doi: 10.1006/geno.1996.0547. [DOI] [PubMed] [Google Scholar]
- 17.Harada H, Fujita T, Miyamoto M, Kimura Y, Maruyama M, Furia A, Miyata T, Taniguchi T. Structurally similar but functionally distinct factors, IRF-1 and IRF-2, bind to the same regulatory elements of IFN and IFN-inducible genes. Cell. 1989;58:729–739. doi: 10.1016/0092-8674(89)90107-4. [DOI] [PubMed] [Google Scholar]
- 18.Harada H, Kitagawa M, Tanaka N, Yamamoto H, Harada K, Ishihara M, Taniguchi T. Anti-oncogenic and oncogenic potentials of interferon regulatory factors-1 and -2. Science. 1993;259:971–974. doi: 10.1126/science.8438157. [DOI] [PubMed] [Google Scholar]
- 19.Harada H, Takahashi E I, Itoh S, Harada K, Hori T-A, Taniguchi T. Structure and regulation of the human interferon regulatory factor 1 (IRF-1) and IRF-2 genes: implications for a gene network in the interferon system. Mol Cell Biol. 1994;14:1500–1509. doi: 10.1128/mcb.14.2.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harroch S, Gothelf Y, Watanabe N, Revel M, Chebath J. Interleukin-6 activates and regulates transcription factors of the interferon regulatory factor family in M1 cells. J Biol Chem. 1993;268:9092–9097. [PubMed] [Google Scholar]
- 21.Itoh S, Harada H, Fujita T, Mimura T, Taniguchi T. Sequence of a cDNA coding for human IRF-2. Nucleic Acids Res. 1989;17:8372. doi: 10.1093/nar/17.20.8372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kessler D S, Levy D E, Darnell J E., Jr Two interferon-induced nuclear factors bind a single promoter element in interferon-stimulated genes. Proc Natl Acad Sci USA. 1988;85:8521–8525. doi: 10.1073/pnas.85.22.8521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kingston R, Sheen J. Reporter system using chloramphenicol acetyltransferase. In: Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman G J, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley and Sons; 1989. pp. 9.6.2–9.6.9. [Google Scholar]
- 24.Lin R, Mustafa A, Nguyen H, Gewert D, Hiscott J. Mutational analysis of interferon (IFN) regulatory factors 1 and 2. Effects on the induction of IFN-beta gene expression. J Biol Chem. 1994;269:17542–17549. [PubMed] [Google Scholar]
- 25.Moore P S, Boshoff C, Weiss R A, Chang Y. Molecular mimicry of human cytokine and cytokine response pathway genes by KSHV. Science. 1996;274:1739–1744. doi: 10.1126/science.274.5293.1739. [DOI] [PubMed] [Google Scholar]
- 26.Offermann M K, Zimring J, Mellits K H, Hagan M K, Shaw R, Medford R M, Mathews M B, Goodbourn S, Jagus R. Activation of the double-stranded-RNA-activated protein kinase and induction of vascular cell adhesion molecule-1 by poly (I) · poly (C) in endothelial cells. Eur J Biochem. 1995;232:28–36. doi: 10.1111/j.1432-1033.1995.tb20777.x. [DOI] [PubMed] [Google Scholar]
- 27.Rady P L, Yen A, Rollefson J L, Orengo I, Bruce S, Hughes T K, Tyring S K. Herpesvirus-like DNA sequences in non-Kaposi’s sarcoma skin lesions of transplant patients. Lancet. 1995;345:1339–1340. doi: 10.1016/s0140-6736(95)92538-4. [DOI] [PubMed] [Google Scholar]
- 28.Reis L F, Harada H, Wolchok J D, Taniguchi T, Vilcek J. Critical role of a common transcription factor, IRF-1, in the regulation of IFN-beta and IFN-inducible genes. EMBO J. 1992;11:185–193. doi: 10.1002/j.1460-2075.1992.tb05041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Russo J J, Bohenzky R A, Chien M C, Chen J, Yan M, Maddalena D, Parry J P, Peruzzi D, Edelman I S, Chang Y, Moore P S. Nucleotide sequence of the Kaposi’s sarcoma-associated herpesvirus (HHV8) Proc Natl Acad Sci USA. 1996;93:14862–14867. doi: 10.1073/pnas.93.25.14862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shuai K, Schindler C, Prezioso V, Darnell J J. Activation of transcription by IFN-gamma: tyrosine phosphorylation of a 91-kD DNA binding protein. Science. 1992;258:1808–1812. doi: 10.1126/science.1281555. [DOI] [PubMed] [Google Scholar]
- 31.Soulier J, Grollet L, Oksenhendler E, Cacoub P, Cazals-Hatem D, Babinet P, d’Agay M F, Clauvel J P, Raphael M, Degos L, et al. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman’s disease. Blood. 1995;86:1276–1280. [PubMed] [Google Scholar]
- 32.Springer T. Purification of proteins by precipitation. In: Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley and Sons; 1989. pp. 10.16.1–10.16.11. [Google Scholar]
- 33.Tanaka N, Kawakami T, Taniguchi T. Recognition DNA sequences of interferon regulatory factor 1 (IRF-1) and IRF-2, regulators of cell growth and the interferon system. Mol Cell Biol. 1993;13:4531–4538. doi: 10.1128/mcb.13.8.4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Veals S A, Schindler C, Leonard D, Fu X-Y, Aebersold R, Darnell J E, Jr, Levy D E. Subunit of an alpha-interferon-responsive transcription factor is related to interferon regulatory factor and Myb families of DNA-binding proteins. Mol Cell Biol. 1992;12:3315–3324. doi: 10.1128/mcb.12.8.3315. [DOI] [PMC free article] [PubMed] [Google Scholar]