Abstract
Purpose:
Proof-of-principle human studies suggest that transcranial direct current stimulation (tDCS) over the left dorsolateral prefrontal cortex (L-DLPFC) may improve the clinical symptoms of depression. This multicenter study (N=35) tested remotely supervised and repeated daily multichannel tDCS delivered at home designed to target the L-DLPFC in a group of patients with major depressive disorder (MDD). The main objectives of the study were to assess the feasibility and safety of home-based, remotely-supervised tDCS for patients with MDD. As an exploratory aim, we also aimed to evaluate the efficacy. The primary efficacy measure for this study was the median percent change from baseline to the end of the 4-week post-treatment follow-up period in the observer-rated Montgomery-Asberg Depression Mood Rating Scale (MADRS).
Methods:
For each study participant, this open-label feasibility telemedicine pilot study involved 37 at-home stimulation sessions (30 minutes each) of multichannel tDCS targeting L-DLPFC administered over eight weeks, with a follow-up period of 4 weeks following the final stimulation session. The stimulation montage (electrode positions and currents) was optimized by employing computational models using available structural data from a similar population (group optimization). Conducted entirely remotely, the study employed the MADRS for assessment at baseline, at weeks 4 and 8 during treatment, and at 4-week follow-up visits.
Results:
The population who completed all study visits consisted of 34 patients (85.3% women and 14.7% men) with a mean age of 59 years, a diagnosis of MDD according to DSM-V criteria, and a MADRS score ≥20 at the time of study enrolment. At baseline, the mean time since MDD diagnosis was 24.0 (SD 19.1) months. Nearly 90% of the participants (n=29) completed the full course of 37 stimulation sessions at home, covering both the acute and taper phases. No detrimental effects were observed, and no participants had suicidal ideation and/or behavior, whether at baseline during treatment or during the four weeks post-treatment. The study observed a Median percentage MADRS score reduction of 64.5% (48.6, 72.4). Over 70% of participants (n=24) showed a ≥ 50% improvement in MADRS scores from baseline to the last visit (4 weeks post-treatment), with a response rate (RR) of 72.7%. Secondary measures, including the Quick Inventory of Depressive Symptomatology-Self Report (QUIDS-SR) and the Quality of Life Enjoyment and Satisfaction Questionnaire-Short Form (Q-LES-Q-SF), reflected similar improvements. The mean (SD) and the median difference between the final visit and baseline for the Q-LES-Q-SF score were 27.9 (13.8) and 26.8 (17.9, 35.7), respectively.
Conclusions:
These results suggest that remotely supervised and supported home-based tDCS is safe and feasible, and antidepressant efficacy motivates further appropriately controlled clinical studies.
1. Introduction
Major depressive disorder (MDD) is a pervasive and debilitating mental health condition that affects millions of individuals worldwide (Kupfer, 2012). The overall prevalence of depressive disorders in Europe is estimated to be 6% and higher in women (8%) than in men (5%) (Arias, 2021), possibly due to differences in biopsychosocial, psychological, and environmental factors (Kuehner, 2017). Furthermore, recent evidence indicates a rising incidence in youth (Liu, 2020), with MDD-afflicted adolescents up to thirty times more likely to commit suicide (Stringaris, 2017). MDD is characterized by a persistent first-person experience of sadness, hopelessness, and a lack of interest or pleasure in activities, with 30% of patients with treatment-resistant depression attempting suicide at least once in their lives. Beyond the devastating impact on personal well-being, MDD carries substantial economic costs, including healthcare expenses and reduced work productivity (Adorjan & Falkai, 2019).
About 20–40% of patients do not benefit sufficiently from conventional antidepressant therapies, including trials of medication and psychotherapy (Greden, 2001). Pharmacological treatments have limited efficacy, side effects are common (Carvalho, 2016), and one-third of patients are medication-resistant (Rush, 2006) and experience recurrent depressive episodes (Nemeroff, 2007). For patients with treatment-resistant MDD, several neuromodulation strategies offer potential relief, such as electroconvulsive therapy (ECT) and repetitive transcranial magnetic stimulation (rTMS) (Hyde, 2022). While these treatments can be effective, they often come with significant costs, potential side effects, and the need for complex equipment and highly trained staff, making them less accessible in regions lacking specialized facilities. This accessibility issue is particularly problematic for elderly populations who face additional mobility restrictions and require assistance and support to access outpatient clinic services. Indeed, it is estimated that approximately 15% of the elderly (aged > 65) experience clinically significant depressive symptoms (Blazer, 2003), which can lead to increased morbidity and early mortality (Schulz, 2002). Additionally, older age significantly predicts a more challenging progression of depression (Mitchell & Subramanian, 2005), including a lower likelihood of treatment response (Licht-Strunck, 2007; Tedeschini, 2011), reduced prospects for functional recovery (Little, 1998), and increased risk of relapse (Beekman, 2002).
In our earlier study (Cappon, 2022), we investigated the feasibility of an innovative protocol where tDCS is administered at home for older adults with MDD (patient participants), supported by a caregiver (N=5). This investigation included a remotely hosted training program to equip caregivers with the necessary knowledge and skills to administer tDCS at home, eliminating the need for lab visits (Cappon, 2022). Based on this preliminary work, we conducted a larger pilot study of subject and subject-administrator device utilization, remotely supervised and supported home-based tDCS for antidepressant treatment of adult patients aged 22 and older with MDD who had failed to get satisfactory improvement from at least one prior antidepressant medication in the current episode.
tDCS
tDCS is a method for noninvasive brain stimulation based on decades-old observations that neuronal firing is modulated by low-amplitude electrical direct current (DC). When applied to the cerebral cortex, cathodal DC suppresses neuronal firing (Creutzfedt, 1962; Nitsche, 2000), while anodal DC increases neuronal firing and leads to increased excitability in the targeted cortex. More precisely, the present understanding indicates that the electric field associated with tDCS currents by Ohm’s law is responsible for the depolarization or hyperpolarization of the soma membrane of elongated neurons (Pyramidal cells) and possibly, others to a lesser extent (Molaee-Ardekani, 2013; Galan, 2023), depending on the direction of the field relative to the orientation of the cells (Ruffini, 2013, 2014, 2018): the electric field component normal to the cortical surface will depolarize pyramidal neurons if it is pointing “inward” at that location (from apical dendrite to soma), and vice-versa. With multichannel tDCS, it is possible to choose the position of the electrodes and currents to optimize stimulation at a chosen target map involving one or more regions (a cortical network). Low-intensity, controlled currents (typically ~1 mA and <4 mA) are applied through scalp electrodes in repeated 20–60 min sessions. The resulting subtle but persistent modulation of neuronal activity is believed to lead to plastic effects derived from Hebbian mechanisms (Ruffini, 2018). Notably, tDCS-generated electric fields can interact with functional brain networks, thus enabling modulation of brain connectivity related to mood disorders and MDD.
When appropriate guidelines are followed, tDCS is safe and extremely well tolerated (Antal, 2017) both in the clinic and in remotely supervised home tDCS (Pilloni, 2023; Woodham, 2024). Hundreds of tDCS trials have demonstrated the technique to be well-tolerated and safe. tDCS units are also inexpensive and lightweight. The electrical supply can be derived from conventional or rechargeable batteries. The scalp electrodes can be fastened in seconds. tDCS can be combined easily with other therapies, such as those that may be required for the resuscitation of an acutely injured patient.
There has been a fairly large number of studies, including randomized, sham-controlled clinical trials (RCTs) on the effects of tDCS in MDD. Results have been variable and, in part, discrepant. For example, Brunoni et al. (2017) found tDCS to have similar efficacy to antidepressant medications, while Loo et al. (2018) found no efficacy of real tDCS over sham in MDD. Nonetheless, several meta-analyses have concluded that tDCS is effective for MDD (Mutz, 2018; Brunoni, 2016). Razza et al. (2020) completed a systematic review of all studies of tDCS for the treatment of acute major depressive episodes completed up to January 2020. They included all randomized, sham-controlled clinical trials (RCTs) enrolling participants with an acute depressive episode, a total of 23 RCTs with 1,092 participants. They found that active tDCS was superior to sham regarding endpoint depression scores, response, and remission rates. Moreover, active tDCS was safe with a side-effect profile comparable to sham. Moffa et al. (2020) also recently published an individual patient data (IPD) meta-analysis evaluating the efficacy and acceptability of tDCS for the treatment of acute major depressive episodes. The IPD meta-analysis is more accurate in estimating the efficacy of an intervention and also superior to the aggregated data approach for obtaining predictors of treatment outcomes since it uses the raw data of each participant collected from each study (Riley, 2010). Moffa (2020) included data from all published placebo-controlled trials on tDCS as the only intervention in MDD conducted until December 2018. This included 9 eligible studies with a total of 572 participants. They found active tDCS to be significantly superior to sham for an antidepressant response (30.9% vs. 18.9% respectively; OR = 1.96), remission (19.9% vs. 11.7%, OR = 1.94), and depression improvement (effect size β = 0.31). Moreover, they found a consistent, continuous clinical improvement after the end of the tDCS treatment course. It is noteworthy that the clinical efficacy was substantially higher in the studies where the tDCS course was longer (3–4 weeks versus 1–2 weeks). Zhang (2023) conducted a comprehensive meta-analysis to evaluate the antidepressant efficacy of transcranial direct current stimulation (tDCS) as a nonpharmacological treatment for depression. By reviewing randomized controlled trials up to December 30, 2020, the analysis included 27 studies with a total of 1204 patients, comparing 653 patients receiving active tDCS treatment to 551 receiving sham tDCS. The results indicated that active tDCS significantly improved depressive symptoms over sham treatments, with a moderate effect size (g = 0.46). Although active tDCS showed superiority in increasing response and remission rates, these differences were not statistically significant. Dropout rates between active and sham tDCS groups were similar, suggesting comparable tolerability. The findings suggest that tDCS, particularly with specific parameters such as a 2 mA stimulation current for 30-minute sessions and in patients not on antidepressants, holds promise as a treatment modality for depressive episodes.
The variability in the literature on the antidepressant effects of tDCS may reflect differences in patient selection as well as in the tDCS protocol. Longer courses of treatment seem particularly important to ensure sustained, lasting benefits. Consistent with the current understanding of mechanisms of action, tDCS antidepressant effects may involve long-term neuroplastic changes that take time to develop and may, in fact, continue to evolve and mature even after the tDCS treatment course has ended. This makes long treatment courses with maintenance phases important and home-based interventions appealing. Importantly, across all studies, active tDCS has been well tolerated, and there have been no significant adverse or side effects.
tDCS at home
As a relatively simple and portable technology, tDCS is particularly well suited for remotely supervised, home-based treatment. Several equipment manufacturers have developed tDCS systems for remotely supervised, home-based use, where the treatment is administered by the patient or an administrator. Treatment parameters, scheduling, and use can be monitored remotely by clinic or research staff. To date, this has been piloted for the treatment of a number of conditions, including neuropathic pain (Garcia-Larrea, 2019), auditory hallucinations in schizophrenia (Andrade, 2013), multiple sclerosis (Charvet, 2017; Charvet, 2018; Kasschau, 2016; Kasschau, 2015), Mal de Debarquement Syndrome (Cha, 2016), Parkinson’s disease (Agarwal, 2018; Dobbs, 2018), trigeminal neuralgia (Hagenacker, 2014), vascular dementia (André, 2016), Prader-Willi syndrome (Azevedo, 2017), and, recently, MDD (Charvet, 2023; Woodham, 2024) with promising results.
Palm et al. (2018) completed a systematic review of all available evidence on home use of tDCS until May 2017. They identified 22 original research papers, trial protocols, or trial registrations involving home-use tDCS, mostly as an add-on intervention to cognitive or physiotherapeutic intervention. They showed that treatment adherence was high and side effects minimal, and thus, they concluded that remotely controlled and supervised home-used tDCS was feasible and promising. The experience with home-use tDCS has continued to grow since then.
In the setting of depression, Clayton et al. (2018) reported a case of one patient with comorbid multiple sclerosis and recurrent depressive episodes who received a course of remotely supervised tDCS following ECT treatment. Fatigue and mood ratings improved. More recently, Alonzo et al. (2019) completed a proof-of-principle, open-label trial in 34 participants suffering from MDD who were taught to self-administer 20–28 tDCS sessions (2 mA, 30 min, F3-anode and F8-cathode montage according to 10–20 EEG placement) over 4 weeks followed by a taper phase of 4 sessions 1 week apart. Participants were initially monitored via video link for a few days and then through the completion of an online treatment diary. One participant withdrew from the study due to too many missed sessions. The remaining 33 participants completed 93% of the scheduled sessions in the initial 4-week phase. Ten of the thirteen participants who qualified for the maintenance phase opted to continue. Mood improved significantly from baseline (27.5 on MADRS) to 1 month after the end of acute treatment (MADRS 15.5; p < 0.001). Side effects reported across 1,149 sessions were minimal, primarily mild to moderate tingling or burning/heat sensation during stimulation and redness at the electrode sites. This study provides clear, initial evidence that home-based, remotely supervised tDCS treatment is feasible for depressed patients and offers a potentially effective intervention.
Recently, Cappon et al. (2022) investigated the feasibility of the protocol used in our study, where tDCS is administered within the homes of older adults with MDD (patient participants) with the help of a study companion (i.e., caregiver). The study, designed by us during the COVID crisis, explored the feasibility of a remotely-hosted training program to avoid visiting the lab. We also employed a newly developed multi-channel tDCS system with real-time monitoring designed to guarantee the safety and efficacy of home-based tDCS. They found that the home-based, remotely-supervised, study companion administered, multi-channel tDCS protocol for older adults with MDD was feasible and safe, paving the way for the larger study described here.
In the study by Charvet (2023), home tDCS was evaluated as a novel therapeutic approach for major depression through an observational clinical trial. This trial involved 16 participants with moderate-to-severe major depressive episodes who underwent 28 sessions of left anodal dorsolateral prefrontal cortex tDCS over six weeks, followed by a tapering phase of weekly sessions for an additional four weeks. There were no serious or treatment-limiting adverse events caused by the tDCS intervention, and no participant experienced an increase in depression or suicidality that warranted treatment discontinuation or additional intervention. The findings revealed a significant reduction in depressive symptoms as early as week 2, with continuous improvement noted at each subsequent biweekly assessment. By the end of the acute intervention, responder and remission rates were 75% and 63%, respectively, which increased to 88% and 81% following the tapering period.
Finally, in a recent study by Woodham (2024), tDCS using large rubber electrodes with sponges (23 cm2) with anode over F3 and cathode over F4 in the 10/20 EEG system was evaluated as a home-based treatment for MDD in a fully remote, multisite, double-blind, placebo-controlled, randomized superiority trial conducted in the UK and USA. The study enrolled adults aged 18 years and older who were diagnosed with MDD based on DSM-5 criteria and were experiencing a current depressive episode of at least moderate severity, as indicated by a score of 16 or higher on the 17-item Hamilton Depression Rating Scale (HDRS), but did not have a history of treatment-resistant depression. The study’s protocol included a 10-week blinded phase, consisting of five tDCS sessions per week for the first three weeks, followed by three sessions per week for the subsequent seven weeks. This was followed by a 10-week open-label phase. The tDCS treatment featured 30-minute sessions, where active tDCS was administered at 2 mA and sham tDCS at 0 mA, both with brief ramping up and down phases to mimic the sensation of the active device. A total of 174 participants with MDD were randomized into either the active treatment group (n=87; mean age 37.1 ± 11.1 years) or the sham treatment group (n=87; mean age 38.3 ± 10.9 years). The results revealed a significant improvement in the HDRS scores in the active treatment group, with a mean reduction of 9.4 ± 6.25 points, compared to a mean reduction of 7.1 ± 6.10 points in the sham treatment group (95% CI 0.5 to 4.0, p = 0.012). With regard to MADRS ratings, the active tDCS treatment arm showed a significant improvement from baseline to week 10, with a mean improvement of 11.3 ± 8.81 relative to the sham treatment of 7.7 ± 8.47 (p= 0.006). The effects were evident at week 10, supporting a recent individual patient data analysis, which found that tDCS effect sizes continue to increase up to 10 weeks as compared to sham stimulation (Nikolin, 2023). Safety was monitored using real-time assessments through video conference and the availability of a dedicated study number with 24-hour access to researchers. There were no significant differences in the rates of discontinuation between the active (n=13) and sham (n=12) groups. There were no serious adverse events related to the device and no incidents of serious suicide risk. The findings of this study add to the data suggesting that home-based tDCS, when supervised remotely, represents a feasible, acceptable, and safe first-line treatment for MDD without a history of treatment-resistant depression, highlighting the importance of continued effective safety monitoring.
2. Methods
Participants
Inclusion criteria included a diagnosis of MDD according to the Diagnostic and Statistical Manual of Mental Disorders (DSM-V), as determined via a telehealth interview with a study site psychiatrist or study staff physician with experience in the management of MDD, 22 years or older as of the date of study enrolment, experiencing a major depressive episode of at least four weeks duration, and a MADRS score ≥20 at the time of study enrolment. Participants also had to be taking at least one medication approved by the FDA for the treatment of depression (except bupropion) whose dose had remained unchanged for four weeks prior to study enrolment. In addition, participants had to identify and designate one or more adults (persons aged 22 or older) as ‘Administrator/s.’ These individuals had to be willing, able, and formally agree to administer the home-based tDCS, be accessible to the study staff, reporting any safety concerns, potential protocol violations, and any other study-related matters. Subjects also needed access to a wireless internet (Wi-Fi) connection where the study treatments were administered. An accurate and current accounting of the study treatments for each subject was maintained on an ongoing basis by the device interface within the NE portal. Participants were recruited from five centers in the United States (3 in Florida, 1 in Oklahoma, and 1 in Georgia).
Exclusion criteria included any DSM-V-defined psychotic disorder in the three months preceding the date of study enrolment, high suicide risk (active suicidal ideation) assessed on C-SSRS, history of clinically defined medically significant neurological disorder, skin lesions on the scalp at the proposed electrode sites, any cranial metal implants (excluding ≦1 mm thick epicranial titanium skull plates and dental fillings) or medical devices (i.e., cardiac pacemaker, deep brain stimulator, medication infusion pump, cochlear implant, vagus nerve stimulator), previous surgeries opening the skull leaving skull defects capable of allowing the insertion of a cylinder with a radius greater or equal to 5 mm. Participants on antidepressant medications were allowed to enter the trial provided that the medication dose remained unchanged for four weeks prior to enrolment in the trial and there was no planned dose change for the duration of the trial. Bupropion was excluded.
The study (NCT05205915, clinicaltrials.gov) was approved by the WCG-IRB, and written informed consent was obtained from each participant prior to the start of study-specific procedures. Because of the nature of this study, consent was obtained electronically online. Information was provided both verbally and in writing, and subjects (or their legal representatives) had ample opportunity to inquire about the details of the study.
To establish preliminary evidence of the safety, tolerability, and feasibility of home administration, results from other home studies (e.g., Alonzo, 2019) suggested that approximately 30 subjects were appropriate. Formal sample size calculation was not applicable. Finally, the assessment of the clinical investigation variables considered in this clinical investigation did not require any specific equipment.
The study was conducted according to the Declaration of Helsinki, Protection of Human Volunteers (21 CFR 50), Safety reporting in clinical investigations of medical devices under Regulation (EU) 2017/745, Institutional Review Boards (21 CFR 56), Obligations of Clinical Investigators (21 CFR 812), and Clinical Investigation of Medical Devices for Human Subjects – Good Clinical Practice (ISO 14155:2020). The clinical investigation was approved by the FDA (protocol number: NE-02, version 5 dated January 22nd, 2022 (FDA approval letter RE: G160208/S010 dated March 3, 2022) and WCG- IRB on January 31st, 2022).
Protocol
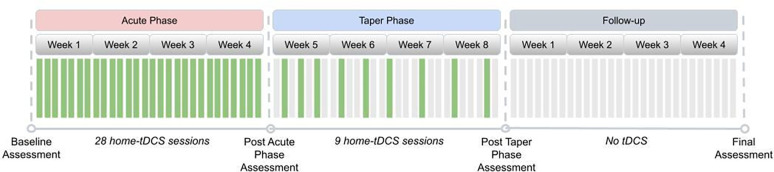
This study was conducted on a “virtual” basis, and all visits were remote. The treatment course (see Figure 1) consisted of an acute phase of 28 tDCS sessions conducted daily (7 days per week) over four weeks, consistent with the protocol of Alonzo et al. (2019). This was motivated by the results of Brunoni et al. (2016) and the meta-analysis of Moffa et al. (2020), which found a positive association between increased tDCS ‘dose’ and treatment efficacy. After that, participants underwent a taper phase of an additional nine sessions of tDCS applied in progressively decreasing frequency until day #60 of the study as follows: (i) Three tDCS sessions once every other day, (ii) three tDCS sessions once every third day, (iii) three tDCS sessions once every fourth day.
Figure 1. Study Design.
The design included an Acute Phase with 28 home tDCS sessions followed by a Taper phase during four weeks. Assessments were all remote.
An incomplete session was defined as one that discontinued stimulation prior to 100% completion and could be repeated within 24 hours if less than 75% of the session was delivered to the subject. A missed session (0% stimulation delivered) was defined as an anticipated session that did not occur within 24 hours of the assigned date/time.
The Montgomery-Asberg Depression Rating Scale (MADRS) (Montgomery & Asberg, 1979) was completed at baseline, approximately at days #28 and #56 of treatment, and at the end of the 4-week follow-up period.
Multichannel tDCS montage
Stimulation was applied using the Starstim device, with current delivered via four Starstim Pi electrodes (circular Ag/AgCl electrodes using gel with a contact area of 3.14 cm2) embedded in the cap. All study subjects used the same fixed montage (electrode locations and currents).
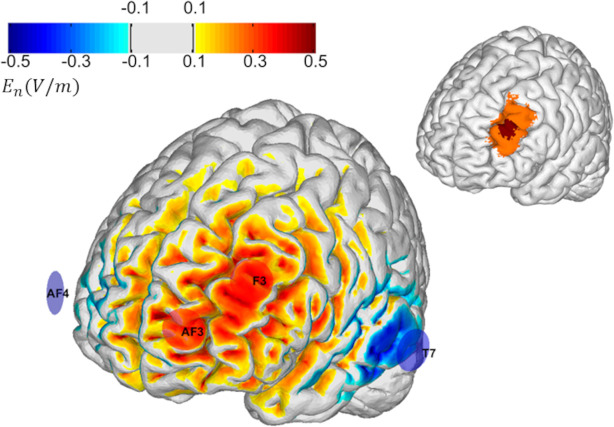
The left dorsolateral prefrontal cortex (L-DLPFC) has been consistently related to depression symptomatology (Mayberg, 2001; Pizzagalli, 2011). Specifically, the L-DLPFC is hypoactive in depression, and an increase in activity is associated with antidepressant response. The stimulation target for this study is shown in Figure 2. This target region was selected because it encompasses many clinically validated transcranial magnetic stimulation (TMS) targets for refractory MDD, including those proposed by Fox (2012), Mir-Moghtdaei (2015), Herbsman (2009), Rusjan (2010), and Fitzgerald (2009).
Figure 2. Left: Montage design with group optimization.
The figure’s inset shows the target, which consists of a central region (in red) surrounded by a buffer region of lower weights (in orange). The selected group optimized montage consisted of 4 electrodes: two anodes located over the target (AF3 and F3) and cathodes located further away (T7 and AF4). The color scale represents the normal component (normal to the cortical surface with red/blue denoting inward/outward E-field normal component) of the E-field induced by the optimized montage in the cortical surface (in V/m).
Consequently, we designed the multichannel tDCS montage with the maximal normal component of the electrical field targeting the L-DLPFC (excitatory, with the component pointing from CSF into gray matter) with minimal off-target stimulation and for administration via four NG Pistim electrodes using the Starstim®-Home system.
To create a well-targeted but non-personalized montage appropriate for use across many subjects, we used the Stimweaver® algorithm (Ruffini, 2014) with Group Optimization (Salvador, 2021). The Stimweaver® algorithm searches the space of electrode locations and currents to match the produced electric field with the desired weighted target map, minimizing an Objective Function that reflects the error of the match for a particular subject. In Group Optimization, the Objective Function is an average of the Objective Function from many subjects in an anatomically representative MRI dataset. In this particular case, we performed a group optimization over 27 healthy patients with an age range between 18 and 93 (55±25 years old). Biophysical volume conducting head models were calculated for each subject in the dataset using the methods summarized in Mercadal et al. (2022). The target map for the optimization was created in MNI space (using the Colin head model, Miranda et al., 2013) and mapped to each individual’s cortical surface by using affine registration to MNI space.
The target map was created using the methods summarized in Cappon et al. (2022), and it consisted of a central hot spot with higher weight surrounded by a buffer region with lower weight. The target En-field (the component of the E-field normal to the cortical surface) in this target region was defined as 0.75 V/m (weight 10). The rest of the cortical surface was assigned to a 0.0 V/m target En with a lower weight (2). The montage was constrained to a maximum of four stimulation electrodes. The currents were limited to 1.7 mA max per electrode (in absolute value) and 4.0 mA for the total injected current (here defined as the sum of current in all the anodes), well below the recommended safety limits (Antal, 2017).
The restriction on the maximum number of channels was imposed to ensure the ease of set-up. The electrode positions found were AF3 and F3 (anodes) as well as T7 and AF4 (cathodes), according to the 10–20 EEG system (Figure 2). The average En-field normal on the target produced by this montage ranged from 0.07 V/m to 0.26 V/m (0.13±0.04 V/m). In the rest of the non-involved cortex, it remained low: −0.002±0.001. For all participants, the current intensity was ramped up over 30 s, then sustained at the stimulation intensity for 30 min, and then ramped down over 30 seconds.
Home tDCS system
This study used the Starstim Home Kit (Neuroelectrics, see Figure 2). Neuroelectrics developed this system for home-based tDCS, effectively overcoming previous challenges with other forms of tDCS and used in several studies, e.g., Garcia-Larrea 2019 (NCT02346396). The Starstim Home Kit uses Neuroelectrics’ Starstim system with additional features that allow researchers and clinicians to “prescribe” and monitor home-based tDCS to end users. The user can communicate in real-time with remote study staff via video-conferencing during device training and during the first three use sessions. The Starstim system includes an EEG-like headcap with holes located where small electrodes can be attached and secured in place in the correct position on the scalp. These electrode holes are color- and number-coded so that electrode leads with corresponding colors in the tDCS device are appropriately attached to the corresponding electrodes, eliminating the potential for accidental mismatching of the electrodes and the leads. The Starstim®-Home Kit further incorporates a smart tablet wirelessly connected to the internet.
In more detail, the system includes 1) Necbox, the portable wireless tDCS device that applies brain stimulation; 2) Neoprene headcap: electrode positioner on which the relevant electrode positions are marked on the headcap with different colors; 3) Color-coded electrode cables: marked with the same colors as the headcap and with numbers visible on the software interface; 4) Pistim (3.14 cm2) Ag/AgCl electrodes; 5) Tablet with HomeApp: a user interface that guides patients throughout the session and ensures correct delivery of the treatment. 6) Neuroelectrics Portal: web interface for investigators to schedule treatment sessions and monitor compliance in real-time.
The tablet allowed the study companions and patient participants to initiate the tDCS sessions, receive specific step-by-step instructions needed to complete the tDCS administration process and record any side effects via custom-developed questionnaires on the tablet. The table provides simplified instructions and step-by-step touchscreen prompts for the participant. This process has been designed for ease of use, even for individuals who are not computer savvy. The tablet automatically runs an impedance check before and during the delivery of the tDCS current and blocks the stimulation if the electrode impedance reaches above 20 kΩ. Moreover, the tablet has a manual abort function for the participant to stop the stimulation if they are experiencing any discomfort or pain. The research staff are notified if this occurs and reach out to the participant to resolve the situation. The tablet further interfaces with another component of the Starstim®-Home Kit called the Neuroelectrics Portal, which the research staff can use to schedule a specific time slot when the execution of the tDCS sessions is allowed. If the stimulation is attempted outside of this time slot, the tablet will inform the participant that the stimulation is currently unavailable and indicate when the next time slot is scheduled. The tablet further allows the study staff to remotely monitor patient participant progression through each session, side effects, and treatment compliance. This portal also ensures that all the stimulation parameters, including stimulation intensity, stimulation duration, and number of sessions, are pre-configured into the system and cannot be adjusted by study companions or patient participants.
Finally, following earlier work described in Cappon et al. (2023), we developed a training and supervision program to accompany the Starstim Home Kit. Study staff members used these training materials to train subjects and administrators on the proposed use of the device. Study staff members monitored treatment sessions until the subject-administrator pairs demonstrated proficiency in all treatment-related procedures, typically through the first three sessions. At the end of each treatment period, the study staff continued to stay in touch with the subject-administrator pairs and inquire about their use of treatment sessions.
Clinical measures
The main purpose of this study was to obtain preliminary data in advance of a larger clinical trial designed to test whether repeated, daily sessions of at-home transcranial direct current stimulation (tDCS) are feasible and safe and explore if this approach can lead to a clinically significant improvement in patients with MDD.
The NE cloud portal provided information related to electrode impedance, tDCS progress, and tDCS session interruption or termination, whether voluntary or due to a technical issue. These metrics were used to assess feasibility (number of interrupted sessions, missed sessions). Adverse Event collection occurred at the time of informed consent, and any Serious Adverse Events were evaluated as the primary safety endpoint.
The primary efficacy measure for this study was the median percentage change from baseline to the end of the 4-week post-treatment period in the observer-rated Montgomery-Asberg Depression Mood Rating Scale (MADRS, Montgomery & Asberg, 1979). The secondary outcome measures were: a) Response rate calculated for the study subjects, where clinically significant response was defined as ≥ 50% improvement in MADRS score from baseline to the 4-week follow-up, b) Percentage change in MADRS score from baseline to the end of week 4 of treatment, to the end of week 8 of treatment, and to the end of the 4-week follow-up period was calculated for all study subjects, c) Change from baseline to 4-week follow-up in the participant-rated Quick Inventory of Depressive Symptomatology (QIDS-SR) (Rush, 2003) administered at the same time points as the MADRS, d) Change from baseline to 4-week follow-up in the Quality of Life Enjoyment and Satisfaction Questionnaire Short Form (Q-LES-Q-SF) (Endicott, 1993), administered at the same time points as the MADRS.
Statistical analysis
This was an open-label pilot feasibility telemedicine study. This pilot study involved a total of 37 at-home stimulation sessions (30 minutes each) of multichannel excitatory tDCS targeting the L-DLPFC administered over eight weeks, with a follow-up period of 4 weeks following the final stimulation session.
The following populations of analysis were used: a) Safety population (SAF): All participants who have undergone transcranial direct current stimulation at least once (including incomplete stimulation sessions); b) Intention-to-treat (ITT): All participants who have signed the Informed Consent form; c) Per protocol (PP): All participants who have completed at least 75% of the 37 tDCS sessions, have had the final MADRS score recorded and have no major protocol deviations.
For the primary objective analysis, the efficacy measure is the median percentage change (MPC) from baseline to the end of the 4-week post-treatment period in the observer-rated Montgomery-Asberg Depression Mood Rating Scale (MADRS). A descriptive analysis of the MADRS at each visit, baseline, week 4, week 8, and at the 4-week post-treatment visit, is also presented. This analysis was performed for both the ITT and the PP sets.
3. Results
Participants
The study was conducted according to the Declaration of Helsinki, Protection of Human Volunteers (21 CFR 50), Safety reporting in clinical investigations of medical devices under Regulation (EU) 2017/745, Institutional Review Boards (21 CFR 56), Obligations of Clinical Investigators (21 CFR 812), and Clinical Investigation of Medical Devices for Human Subjects – Good Clinical Practice (ISO 14155:2020). The clinical investigation was approved by the FDA (protocol number: NE-02, version 5 dated January 22nd, 2022 (FDA approval letter RE: G160208/S010 dated March 3, 2022) and WCG- IRB on January 31st, 2022).
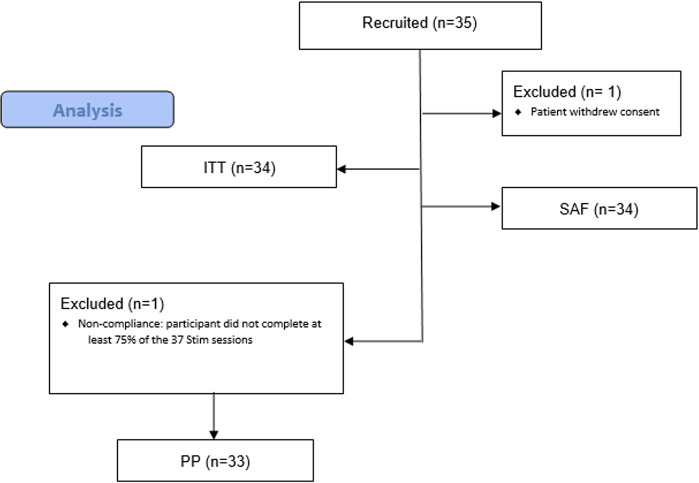
The total valid sample included 35 patients. Figure 3 shows a flowchart of patients recruited and the number and reasons for the exclusion of each population during the study.
Figure 3.
CONSORT flow diagram.
At baseline, study ITT population participants (n=34) were aged between 24 and 78 years and were primarily female (85.3%).
Regarding concomitant psychiatric medications, more than one-third of the patients (12 (35.3%)) were on Sertraline, six (17.6%) were on Citalopram. Three patients (8.8%) were on Duloxetine, three (8.8%) on Memantine, 3 (8.8%) on Quetiapine, and three (8.8%) on Trazodone.
Safety and Adverse Event Monitoring
Concerning safety, no detrimental effects were observed for the patients. Noteworthy, as measured with the C-SSSRS, no participants had suicidal ideation and/or behavior, whether at baseline during treatment or at four weeks post-treatment.
Protocol deviations were evaluated for any trends or patterns that would require additional corrective actions or submissions. All of them were minor, and none resulted in an adverse event or required patient discontinuation from the study. Only 5 (15%) patients experienced adverse events during the study. None of them were reported as serious. Two unexpected adverse events were reported in one patient (3%), and eight adverse device events were reported in four patients (12%). Likewise, no serious adverse device events were reported. Tables for adverse events characteristics and adverse events by subject are available in the Safety section of the SAR document.
Feasibility and compliance
Nearly 90% of the patients (n=29) completed the 37 stimulation sessions at home during the acute and taper phases. Four (12%) patients missed one session. Regarding completeness, 2 (6%) reported sessions with >100% completeness, and 6 (18%) reported sessions with less than 75% of completeness.
Efficacy
The mean (SD) difference between the final visit and baseline for the MADRS score was −19.8 (8.6) for both ITT and the PP population datasets. The primary endpoint (median percentage change in the MADRS score) was 64.5% (48.6%, 72.4%) in both populations.
In more than 70% of patients (n=24), an improvement ≥ 50% was observed in the MADRS score from baseline to the last visit (4 weeks post-treatment, see Figure 4). The calculated response rate (RR) was 73%. Finally, improvement was observed from baseline to the end of the study (4 weeks post-treatment) for the QIDS-SR and the Q-LES-Q-SF scores. The mean (SD) and the median (IQR) difference between the final visit and baseline for the Q-LES-Q-SF score were 27.9 (13.8) and 26.8 (17.9, 35.7), respectively.
Figure 4: Comprehensive depiction of treatment response over time in patients with Major Depressive Disorder (MDD).
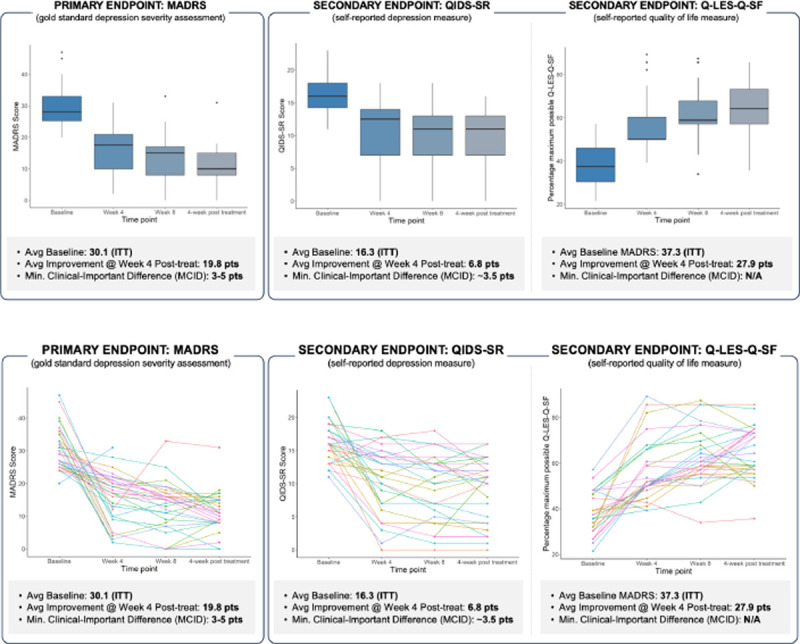
Top-left panel: Boxplot illustrating the distribution of MADRS scores at baseline and at weeks 4, 8, and 12. Top-right panel: Longitudinal trajectories of individual patient MADRS scores, indicating varied response patterns over the treatment course. Bottom panel: individual participant changes in MADRS scores from baseline, displayed at week 4, week 8, and at the 4-week post-treatment follow-up (final visit). The data collectively underscore the heterogeneity in treatment response and the progressive nature of symptom reduction over time.
4. Discussion
This early feasibility study has demonstrated the feasibility and safety of a home-based, remotely-supervised tDCS intervention with the Starstim device in patients with MDD and obtained valuable efficacy data in planning randomized, controlled, more extensive clinical trials. A single-arm prospective multicentre study with 35 MDD patients was carried out. The population who completed all study visits consisted of 34 patients (85% women and 15% men) with a mean age of 60 years and a MADRS score ≥20 at the time of study enrolment. The sample was a representative subset of the MDD population and reflects some of the characteristics of a larger group that could benefit from home-based tDCS.
Regarding primary objectives, for feasibility, nearly 90% of the patients (n=29) completed the 37 home stimulation sessions throughout the acute and taper phase at home. These positive results confirm the feasibility of the Starstim Home device and provide crucial information that should be considered for further pivotal studies.
Concerning safety, no detrimental effects were observed for the patients with the use of the IMD. Noteworthy, as measured with the C-SSSRS, no participants had suicidal ideation and/or behavior, whether at baseline during treatment or at four weeks post-treatment.
In terms of efficacy, the median percentage reduction of the MADRS score was 64.5% (48.6, 72.4), and the mean (SD) difference between the final visit and baseline for the MADRS score was −19.8 (8.6) for both the ITT and the PP population datasets. This can be tentatively compared to results in earlier studies (Wang et al., 2019), as well as the results in Woodham (2024), where the active tDCS treatment arm showed a significant improvement from baseline to week 10, with a change of mean score of −11.3 ± 8.8 relative to sham treatment 7.7 ± 8.5. The results in this study are also similar to those in the active arm in the recent placebo-controlled study by Salehinejad et al. (2024).
Likewise, concerning secondary objectives, in more than 70% of patients (n=24), an improvement of ≥ 50% was observed in the MADRS score from baseline to the last visit (4 weeks post-treatment). The calculated response rate (RR) was 72.7%. Along the same lines, improvement was observed from baseline to the end of the study (4 weeks post-treatment) for the QIDS-SR and the Q-LES-Q-SF scores. The mean (SD) and the median (IQR) difference between the final visit and baseline for the Q-LES-Q-SF score were 27.9 (13.8) and 26.8 (17.9, 35.7), respectively.
Protocol deviations were evaluated for any trends or patterns that would require additional corrective actions or submissions. All of them were minor, and none resulted in an adverse event or required patient discontinuation from the study.
Considering the good performance of the home-based device plus the overall improvement in depression rating scales (MADRS), symptomatology, and satisfaction questionnaires, it can be said that the developed solution deployed using the Starstim home system was well-accepted and useful for the patients and that it presumably fulfills an unmet need. The Starstim portable technology proved relatively simple to use and exhibited outstanding performance with a good safety profile. Pending larger controlled studies, this study provides early substantial evidence that home-based, remotely supervised, and supported tDCS treatment is feasible for depressed patients and offers a potentially effective intervention. Therefore, this tool could play a significant and outstanding role in applying knowledge to improve the health and healthcare of MDD patients.
Limitations
The design of this feasibility study did not include a control group. This type of study design may not have been optimal to assess significant differences in the proposed outcomes. The lack of a control group makes it difficult to argue potential time-dependent changes or to relate changes just to the investigational medical device intervention. However, in comparison with similar studies, the effect size results are very promising.
However, this study is one of few of its kind in which a home intervention is being assessed for its impact on MDD well-being. The incremental development of innovative/breakthrough health technologies takes a long time, during which innovation will have to successfully go through testing and evidence generation before it can be launched. As part of this process, early feasibility studies provide the opportunity to capture relevant additional information for the intended use from a real-world setting that would not be possible in non-clinical studies (i.e., bench testing and animal studies) at a very early stage.
Further studies are needed to assess the impact of a digital intervention on MDD, with a longer follow-up period, including a control group and a larger sample size. However, this proof of concept was planned to verify whether the Starstim portable technology was feasible and could achieve the desired outcome, and this has been convincingly shown in a real-world setting.
5. Conclusions
This pilot open-label study has demonstrated the feasibility of an innovative home-based, remotely supervised, study companion-led, multi-channel tDCS intervention for older adults suffering from MDD and provided useful safety and efficacy data for the design of a larger randomized controlled at-home study.
Acknowledgments.
We thank the PIs from the commercial sites in the study for their invaluable contribution to the success of the project and the Clinical Research team at Neuroelectrics for their professionalism and perseverance.
Footnotes
Conflicts of interest. Drs. Thais Baleeiro, Francesca Castaldo, and Ricardo Salvador work for Neuroelectrics, a company dedicated to creating brain stimulation solutions. Dr. Giulio Ruffini works for and is a co-founder of Neuroelectrics and holds several patents in model-driven non-invasive brain stimulation. Dr. A. Pascual-Leone is partly supported by grants from the National Institutes of Health (R01AG076708, R01AG059089, R03AG072233, and P01 AG031720), and BrightFocus Foundation. Dr. A. Pascual-Leone serves as a paid member of the scientific advisory boards for Neuroelectrics, Magstim Inc., TetraNeuron, Skin2Neuron, MedRhythms, and Hearts Radiant. He is co-founder of TI solutions and co-founder and chief medical officer of Linus Health. Dr. A. Pascual-Leone is listed as an inventor on several issued and pending patents on the real-time integration of transcranial magnetic stimulation with electroencephalography and magnetic resonance imaging, and applications of noninvasive brain stimulation in various neurological disorders, as well as digital biomarkers of cognition and digital assessments for early diagnosis of dementia.
References
- Adorjan K, Falkai P. Premature mortality, causes of death, and mental disorders. Lancet. 2019. Nov 16;394(10211):1784–1786. doi: 10.1016/S0140-6736(19)32521-8. Epub 2019 Oct 24. [DOI] [PubMed] [Google Scholar]
- Agarwal S., Pawlak N., Cucca A., Sharma K., Dobbs B., Shaw M., Charvet L., Biagioni M.J., 2018. Remotely-supervised transcranial direct current stimulation paired with cognitive training in Parkinson’s disease: an open-label study. Clin. Neurosci. 57, 51–57. [DOI] [PubMed] [Google Scholar]
- Alonzo A., et al. , 2019. Pilot trial of home-administered transcranial direct current stimulation for the treatment of depression. J. Affect. Disord. 252, 475–483. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association, 2000. Diagnostic and Statistical Manual of Mental Disorders, fourth ed., Text Revision,. American Psychiatric Association Publishing, Washington, DC. [Google Scholar]
- Andrade C., 2013. Once- to twice-daily, 3-year domiciliary maintenance transcranial direct current stimulation for severe, disabling, clozapine-refractory continuous auditory hallucinations in schizophrenia. J. ECT 29, 239–242. [DOI] [PubMed] [Google Scholar]
- André S., Heinrich S., Kayser F., Menzler K., Kesselring J., Khader P.H., Lefaucheur J.P., Mylius V., 2016. At-home tDCS of the left dorsolateral prefrontal cortex improves visual short-term memory in mild vascular dementia. J. Neurol. Sci. 369, 185–190. [DOI] [PubMed] [Google Scholar]
- Antal A., et al. , 2017. Low intensity transcranial electric stimulation: safety, ethical, legal regulatory and application guidelines. Clin. Neurophysiol. 128 (9), 1774–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias de la Torre J, Vilagut G, Ronaldson A, Serrano-Blanco A, Martín V, Peters M, Valderas JM, Dregan A, Alonso J. Prevalence and variability of current depressive disorder in 27 European countries: a population-based study. Lancet Public Health. 2021. Oct;6(10):e729–e738. doi: 10.1016/S2468-2667(21)00047-5. Epub 2021 May 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azevedo C., Gomes J.S., Trevizol A.P., Dias A.M, Cordeiro Q., 2017. At-home transcranial direct current stimulation in Prader-Willi syndrome with severe intellectual disability: a case study. J ECT 33, e29–e30. [DOI] [PubMed] [Google Scholar]
- Beck A.T., Ward C.H., Mendelson M., Mock J., Erbaugh J., 1961. An inventory for measuring depression. Arch. Gen. Psychiatry 4, 561–571. [DOI] [PubMed] [Google Scholar]
- Beekman AT, Geerlings SW, Deeg DJ, Smit JH, Schoevers RS, de Beurs E, Braam AW, Penninx BW, van Tilburg W. The natural history of late-life depression: a 6-year prospective study in the community. Arch Gen Psychiatry 2002; 59: 605–611 [DOI] [PubMed] [Google Scholar]
- Bikson et al. , (2016): “Safety of transcranial Direct Current Stimulation: Evidence Based Update 2016”, Brain Stimul. 2016. Sep-Oct;9(5):641–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikson M., et al. , Safety of transcranial direct current stimulation: evidence-based update 2016. Brain Stimulation, 2016. 9(5): p. 641–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikson M., Grossman P., Thomas C., Zannou A.L., Jiang J., Adnan T., Mourdoukoutas A.P., Kronberg G., Truong D., Boggio P., Brunoni A.R., Charvet L., Fregni F., Fritsch B., Gillick B., Hamilton R.H., Hampstead B.M., Jankord R., Kirton A., Knotkova H., Liebetanz D., Liu A., Loo C., Nitsche M., Reis J., Richardson J.D., Rotenberg A., Turkeltaub P.E., Woods A.J., 2016. Safety of transcranial direct current stimulation: evidence-based update 2016. Brain Stimul. 9, 641–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazer DG. Depression in late life: review and commentary. J Gerontol A Biol Sci Med Sci 2003; 58: 249–265 [DOI] [PubMed] [Google Scholar]
- Boggio P.S., et al. , A randomized, double-blind clinical trial on the efficacy of cortical direct current stimulation for the treatment of major depression. International Journal of Neuropsychopharmacology, 2008. 11(2): p. 249–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunoni A.R., Moffa A.H., Fregni F., Palm U., Padberg F., Blumberger D.M., Daskalakis Z.J., Bennabi D., Haffen E., Alonzo A., Loo C.K., 2016. Transcranial direct current stimulation for acute major depressive episodes: meta-analysis of individual patient data. Br. J. Psychiatry 208, 522–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunoni A.R., Moffa A.H., Sampaio-Junior B., Borrione L., Moreno M.L., Fernandes R.A., Veronezi B.P., Nogueira B.S., Aparicio L.V.M., Razza L.B., Chamorro R., Tort L.C., Fraguas R., Lotufo P.A., Gattaz W.F., Fregni F., Benseñor I.M., 2017. Trial of electrical direct-current therapy versus escitalopram for depression. N. Engl. J. Med. 376, 2523–2533. [DOI] [PubMed] [Google Scholar]
- Brunoni A.R., Moffa A.H., Sampaio-Júnior B., Gálvez V., Loo C.K., 2017b. Treatment-emergent mania/hypomania during antidepressant treatment with transcranial direct current stimulation (tDCS): a systematic review and meta-analysis. Brain Stimul. 10, 260–262. [DOI] [PubMed] [Google Scholar]
- Davide Cappon, den Boer Tim Jordan Caleb, Wanting Yu, Alexander Lo, Nicole LaGanke, Biagi Maria Chiara Skorupinski Pawel, Giulio Ruffini, Oscar Morales, Eran Metzger, Bradley Manor, Alvaro Pascual-Leone, Safety and Feasibility of Tele-Supervised Home-Based Transcranial Direct Current Stimulation for Major Depressive Disorder, Frontiers in Aging Neuroscience, 13, 2022, doi: 10.3389/fnagi.2021.765370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho A.F., et al. , 2016. The safety, tolerability and risks associated with the use of newer generation antidepressant drugs: a critical review of the literature. Psychother. Psychosom. 85 (5), 270–288. [DOI] [PubMed] [Google Scholar]
- Charvet L.E., Kasschau M., Datta A., Knotkova H., Stevens M.C., Alonzo A., Loo C., Krull K.R., Bikson M., 2015. Remotely-supervised transcranial direct current stimulation (tDCS) for clinical trials: guidelines for technology and protocols. Front. Syst. Neurosci. 9, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charvet L.E., Dobbs B., Shaw M.T., Bikson M., Datta A., Krupp L.B., 2017. Remotely supervised transcranial direct current stimulation for the treatment of fatigue in multiple sclerosis: results from a randomized, sham-controlled trial. Mult. Scler. J. 24, 1760–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charvet L.E., Shaw M.T., Dobbs B., Frontario A., Sherman K., Bikson M., Datta A., Krupp L., Zenaipour E., Kasschau M., 2018. Remotely supervised transcranial direct current stimulation increases the benefit of at-home cognitive training in multiple sclerosis. Neuromodulation 21, 383–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charvet L, George A, Charlson E, Lustberg M, Vogel-Eyny A, Eilam-Stock T, Cho H, Best P, Fernandez L, Datta A, Bikson M, Nazim K, Pilloni G. Home-administered transcranial direct current stimulation is a feasible intervention for depression: an observational cohort study. Front Psychiatry. 2023. Aug 22;14:1199773. doi: 10.3389/fpsyt.2023.1199773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton A.M., Howard J., Dobbs B., Shaw M.T., Charvet L.E., 2018. Remotely supervised transcranial direct current stimulation after ECT improves mood and cognition in a patient with multiple sclerosis: a case study. J. Ect 34, e15. [DOI] [PubMed] [Google Scholar]
- Creutzfeldt O.D., Fromm G.H., and Kapp H., Influence of transcortical DC currents on cortical neuronal activity. Experimental neurology, 1962. 5(6): p. 436–452. [DOI] [PubMed] [Google Scholar]
- Dobbs B., Pawlak N., Biagioni M., Agarwal S., Shaw M, Pilloni G., Bikson M., Datta A., Charvet L., 2018. Generalizing remotely supervised transcranial direct current stimulation (tDCS): feasibility and benefit in Parkinson’s disease. J. Neuroeng. Rehabil. 15, 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endicott J., Nee J., Harrison W., Blumenthal R., 1993. Quality of life enjoyment and satisfaction questionnaire: a new measure. Psychopharmacol. Bull. 29, 321–326. [PubMed] [Google Scholar]
- Feeser M., Prehn K., Kazzer P., Mungee A., Bajbouj M., 2014. Transcranial direct current stimulation enhances cognitive control during emotion regulation. Brain. Stimul. 7, 105–112. [DOI] [PubMed] [Google Scholar]
- Ferrucci R., et al. , Transcranial direct current stimulation in severe, drug-resistant major depression. Journal of affective disorders, 2009. 118(1): p. 215–219. [DOI] [PubMed] [Google Scholar]
- Fitzgerald PB, Hoy K, McQueen S, Maller JJ, Herring S, Segrave R, Bailey M, Been G, Kulkarni J, Daskalakis ZJ. A randomized trial of rTMS targeted with MRI based neuro-navigation in treatment-resistant depression. Neuropsychopharmacology. 2009. Apr;34(5):1255–62. doi: 10.1038/npp.2008.233. Epub 2009 Jan 14. [DOI] [PubMed] [Google Scholar]
- Fox M. D., Buckner R. L., White M. P., Greicius M. D., and Pascual-Leone A. (2012). Efficacy of transcranial magnetic stimulation targets for depression is related to intrinsic functional connectivity with the subgenual cingulate. Biol. Psychiatry 72, 595–603. doi: 10.1016/j.biopsych.2012.04.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galan-Gadea A, Salvador R, Bartolomei F, Wendling F, Ruffini G. Spherical harmonics representation of the steady-state membrane potential shift induced by tDCS in realistic neuron models. J Neural Eng. 2023. Mar 7;20(2). doi: 10.1088/1741-2552/acbabd. [DOI] [PubMed] [Google Scholar]
- Garcia-Larrea L, Perchet C, Hagiwara K, André-Obadia N, At-Home Cortical Stimulation for Neuropathic Pain: a Feasibility Study with Initial Clinical Results, Neurotherapeutics. 2019. Oct;16(4):1198–1209. doi: 10.1007/s13311-019-00734-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greden JF. The burden of disease for treatment-resistant depression. J Clin Psychiatry. 2001;62 Suppl 16:26–31. Review. [PubMed] [Google Scholar]
- Greden JF. The burden of recurrent depression: causes, consequences, and future prospects., J Clin Psychiatry. 2001;62 Suppl 22:5–9. Review. [PubMed] [Google Scholar]
- Gutiérrez-Rojas L, Porras-Segovia A, Dunne H, Andrade-González N, Cervilla JA. Prevalence and correlates of major depressive disorder: a systematic review. Braz J Psychiatry. 2020. Nov-Dec;42(6):657–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenacker T., Bude V., Naegel S., Holle D., Katsarava Z., Diener H.C., Obermann M., 2014. Patient-conducted anodal transcranial direct current stimulation of the motor cortex alleviates pain in trigeminal neuralgia. J. Headache Pain 15, 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbsman T, Avery D, Ramsey D, Holtzheimer P, Wadjik C, Hardaway F, Haynor D, George MS, Nahas Z. More lateral and anterior prefrontal coil location is associated with better repetitive transcranial magnetic stimulation antidepressant response. Biol Psychiatry. 2009. Sep 1;66(5):509–15. doi: 10.1016/j.biopsych.2009.04.034. Epub 2009 Jul 9. [DOI] [PubMed] [Google Scholar]
- Hyde J, Carr H, Kelley N, Seneviratne R, Reed C, Parlatini V, Garner M, Solmi M, Rosson S, Cortese S, Brandt V. Efficacy of neurostimulation across mental disorders: systematic review and meta-analysis of 208 randomized controlled trials. Mol Psychiatry. 2022. Jun;27(6):2709–2719. doi: 10.1038/s41380-022-01524-8. Epub 2022 Apr 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasschau M., Reisner J., Sherman K., Bikson M., Datta A., Charvet L., 2016. Transcranial direct current stimulation is feasible for remotely supervised home delivery in multiple sclerosis. Neuromodulation 19, 824–831. [DOI] [PubMed] [Google Scholar]
- Kasschau M., Sherman K., Haider L., Frontario A., Shaw M., Datta A., Bikson M., Charvet L., 2015. A protocol for the use of remotely-supervised transcranial direct current stimulation (tDCS) in multiple sclerosis (MS). J. Vis. Exp., e53542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehner C. Why is depression more common among women than among men? Lancet Psychiatry. 2017. Feb;4(2):146–158. doi: 10.1016/S2215-0366(16)30263-2. Epub 2016 Nov 15. [DOI] [PubMed] [Google Scholar]
- Kupfer D.J., Frank E., Phillips M.L., 2012. Major depressive disorder: new clinical, neurobiological, and treatment perspectives. Lancet 379 (9820), 1045–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licht-Strunk E, van der Windt DA, van Marwijk HW, de Haan M, Beekman AT. The prognosis of depression in older patients in general practice and the community. A systematic review. Fam Pract 2007; 24: 168–180 [DOI] [PubMed] [Google Scholar]
- Little JT, Reynolds CF, Dew MA, Frank E, Begley AE, Miller MD, Cornes C, Mazumdar S, Perel JM, Kupfer DJ. How common is resistance to treatment in recurrent, nonpsychotic geriatric depression? Am J Psychiatry 1998; 155: 1035–1038 [DOI] [PubMed] [Google Scholar]
- Liu CH, Zhang E, Wong GTF, Hyun S, Hahm HC. Factors associated with depression, anxiety, and PTSD symptomatology during the COVID-19 pandemic: Clinical implications for U.S. young adult mental health. Psychiatry Res. 2020. Aug;290:113172. doi: 10.1016/j.psychres.2020.113172. Epub 2020 Jun 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo C.K., Alonzo A., Martin D., Mitchell P.B., Galvez V., Sachdev P, 2012. Transcranial direct current stimulation for depression: 3-week, randomised, sham-controlled trial. Br. J. Psychiatry 200, 52–59. [DOI] [PubMed] [Google Scholar]
- Loo C.K., et al. , A double-blind, sham-controlled trial of transcranial direct current stimulation for the treatment of depression. International Journal of Neuropsychopharmacology, 2010. 13(1): p. 61–69. [DOI] [PubMed] [Google Scholar]
- Loo C.K., Husain M.M., McDonald W.M., Aaronson S., O’Reardon J.P., Alonzo A., Weickert C.S., Martin D.M., McClintock S.M., Mohan A., Lisanby S.H., 2018. International randomized-controlled trial of transcranial Direct Current Stimulation in depression. Brain Stimul. 11, 125–133. [DOI] [PubMed] [Google Scholar]
- Mayberg H. (2001). “Depression and frontal-subcortical circuits: focus on prefrontal-limbic interactions” in Frontal-Subcortical Circuits in Psychiatric and Neurological Disorders, eds Lichter D. G. and Cummings J. L. (New York, NY: Guilford Press; ), 177–206. [Google Scholar]
- Medical Device Coordination Group Document. MDCG 2021–6 Regulation ( EU ) 2017 / 745 – Questions & Answers regarding clinical investigation. 2021;1–19. [Google Scholar]
- Nilsson Mary E., et al. , February 2013. Columbia–Suicide Severity Rating Scale Scoring and Data Analysis Guide. Version 2.0. https://cssrs.columbia.edu/wpcontent/uploads/ScoringandDataAnalysisGuide-for-Clinical-Trials-1.pdf [Google Scholar]
- Mercadal B., Salvador R., Biagi M. C., Bartolomei F., Wendling F., & Ruffini G. (2022). Modeling implanted metals in electrical stimulation applications. Journal of Neural Engineering. 10.1088/1741-2552/ac55ae [DOI] [PubMed] [Google Scholar]
- Mir-Moghtadaei A, Caballero R, Fried P, Fox MD, Lee K, Giacobbe P, Daskalakis ZJ, Blumberger DM, Downar J. Concordance Between BeamF3 and MRI-neuronavigated Target Sites for Repetitive Transcranial Magnetic Stimulation of the Left Dorsolateral Prefrontal Cortex. Brain Stimul. 2015. Sep-Oct;8(5):965–73. doi: 10.1016/j.brs.2015.05.008. Epub 2015 May 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda et al. (2013) The electric field in the cortex during transcranial current stimulation. NeuroImage 70 (2013) 48–58, 10.1016/j.neuroimage.2012.12.034 [DOI] [PubMed] [Google Scholar]
- Mitchell AJ, Subramaniam H. Prognosis of depression in old age compared to middle age: a systematic review of comparative studies. Am J Psychiatry 2005; 162: 1588–1601 [DOI: 10.1176/appi.ajp.162.9.1588] [DOI] [PubMed] [Google Scholar]
- Moffa AH, Martin D, Alonzo A, Bennabi D, Blumberger DM, Benseñor IM, Daskalakis Z, Fregni F, Haffen E, Lisanby SH, Padberg F, Palm U, Razza LB, Sampaio-Jr B, Loo C, Brunoni AR. Efficacy and acceptability of transcranial direct current stimulation (tDCS) for major depressive disorder: An individual patient data meta-analysis. [DOI] [PubMed]
- Molaee-Ardekani B, Márquez-Ruiz J, Merlet I, Leal-Campanario R, Gruart A, Sánchez-Campusano R, Birot G, Ruffini G, Delgado-García JM, Wendling F. Effects of transcranial Direct Current Stimulation (tDCS) on cortical activity: a computational modeling study. Brain Stimul. 2013. Jan;6(1):25–39. doi: 10.1016/j.brs.2011.12.006. Epub 2012 Feb 28. [DOI] [PubMed] [Google Scholar]
- Montgomery S.A., Asberg M., 1979. A new depression scale designed to be sensitive to change. Br. J. Psychiatry 134, 382–389. [DOI] [PubMed] [Google Scholar]
- Mutz J., Edgcumbe D.R., Brunoni A.R., Fu C.H.Y., 2018. Efficacy and acceptability of non-invasive brain stimulation for the treatment of adult unipolar and bipolar depression: a systematic review and meta-analysis of randomised sham-controlled trials. Neurosci. Biobehav. Rev. 92, 291–303. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB. Prevalence and management of treatment-resistant depression. J Clin Psychiatry. 2007;68 Suppl 8:17–25. Review. [PubMed] [Google Scholar]
- Nikolin S, Moffa A, Razza L, Martin D, Brunoni A, Palm U, Padberg F, Bennabi D, Haffen E, Blumberger DM, Salehinejad MA, Loo CK. Time-course of the tDCS antidepressant effect: An individual participant data meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2023. Jul 13;125:110752. doi: 10.1016/j.pnpbp.2023.110752. Epub 2023 Mar 16. [DOI] [PubMed] [Google Scholar]
- Nitsche M.A. and Paulus W., Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. The Journal of Physiology, 2000. 527(3): p. 633–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche M.A., et al. , Safety criteria for transcranial direct current stimulation (tDCS) in humans. Clinical Neurophysiology, 2003. 114(11): p. 2220–2222. [DOI] [PubMed] [Google Scholar]
- Opitz A., et al. , (2016). “Spatiotemporal structure of intracranial electric fields induced by transcranial electric stimulation in humans and nonhuman primates”, Scientific Reports 6:31236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palm U., Kumpf U., Behler N., Wulf L., Kirsch B., Wörsching J., Keeser D., Hasan A., Padberg F., 2018. Home use, remotely supervised, and remotely controlled transcranial direct current stimulation: a systematic review of the available evidence. Neuromodulation 21, 323–333. [DOI] [PubMed] [Google Scholar]
- Pizzagalli D. A. (2011). Frontocingulate dysfunction in depression: toward biomarkers of treatment response. Neuropsychopharmacology 36, 183–206. doi: 10.1038/npp.2010.166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razza LB, Palumbo P, Moffa AH, Carvalho AF, Solmi M, Loo CK, Brunoni AR. A systematic review and meta-analysis on the effects of transcranial direct current stimulation in depressive episodes. Depress Anxiety. 2020. Feb 26. doi: 10.1002/da.23004. [Epub ahead of print] Review. [DOI] [PubMed] [Google Scholar]
- Riley R.D., Lambert P.C., Abo-Zaid G., 2010. Meta-analysis of individual participant data: rationale, conduct, and reporting. BMJ 340, c221. [DOI] [PubMed] [Google Scholar]
- Ruffini G (2013), Wendling F, Merlet I, Molaee-Ardekani B, Mekonnen A, Salvador R, Soria-Frisch A, Grau C, Dunne S, Miranda PC. Transcranial current brain stimulation (tCS): models and technologies. IEEE Trans Neural Syst Rehabil Eng. 2013. May;21(3):333–45. doi: 10.1109/TNSRE.2012.2200046. [DOI] [PubMed] [Google Scholar]
- Ruffini G (2014), Fox M.D., Ripolles O., Miranda P.C. and Pascual-Leone A., 2014. Optimization of multifocal transcranial current stimulation for weighted cortical pattern targeting from realistic modeling of electric fields. Neuroimage, 89, pp.216–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffini G (2018), Fabrice Wendling, Roser Sanchez-Todo, Emiliano Santarnecchi, Targeting brain networks with multichannel transcranial current stimulation (tCS), Current Opinion in Biomedical Engineering, Volume 8, 2018, Pages 70–77, ISSN 2468–4511, 10.1016/j.cobme.2018.11.001. [DOI] [Google Scholar]
- Rush A.J., Trivedi M.H., Ibrahim H.M., Carmody T.J., Arnow B., Klein D.N., Markowitz J.C., Ninan P.T., Kornstein S., Manber R., Thase M.E., Kocsis J.H., Keller M.B., 2003. The 16-item quick inventory of depressive symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol. Psychiatry 54, 573–583. [DOI] [PubMed] [Google Scholar]
- Rush A.J., Trivedi M.H., Wisniewski S.R., Nierenberg A.A., Stewart J.W., Warden D., Niederehe G., Thase M.E., Lavori P.W., Lebowitz B.D., McGrath P.J., Rosenbaum J.F., Sackeim H.A., Kupfer D.J., Luther J., Fava M., 2006. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D Report. Am. J. Psychiatry 163, 1905–1917. [DOI] [PubMed] [Google Scholar]
- Rusjan PM, Barr MS, Farzan F, Arenovich T, Maller JJ, Fitzgerald PB, Daskalakis ZJ. Optimal transcranial magnetic stimulation coil placement for targeting the dorsolateral prefrontal cortex using novel magnetic resonance image-guided neuronavigation. Hum Brain Mapp. 2010. Nov;31(11):1643–52. doi: 10.1002/hbm.20964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehinejad M. A., Abdi M., Dadashi M., Rostami R., Salvador R., Ruffini G., & Nitsche M. (2023). Optimized HD-tDCS protocol for clinical use in patients with major depressive disorder. Brain Stimulation: Basic, Translational, and Clinical Research in Neuromodulation, 16(1), 213. 10.1016/j.brs.2023.01.294 [DOI] [Google Scholar]
- Salehinejad M., Abdi M., Dadashi M., Zolghadriha A., Salvador R., Ruffini G., & Nitsche M. A. (2024, March 2). Optimized multichannel tDCS protocol for clinical use in patients with major depressive disorder: A randomized, controlled trial. 10.31219/osf.io/tms4h [DOI] [Google Scholar]
- Salvador R., Biagi M. C., Manor B. and Ruffini G., 2021. Group Level Montage Optimization in Transcranial Electrical Stimulation. Brain Stimulation 14 (6): 1646. 10.1016/j.brs.2021.10.185. [DOI] [Google Scholar]
- Schulz R, Drayer RA, Rollman BL. Depression as a risk factor for non-suicide mortality in the elderly. Biol Psychiatry 2002; 52: 205–225 [DOI: 10.1016/S0006-3223(02)01423-3] [DOI] [PubMed] [Google Scholar]
- Sheehan D.V., Lecrubier Y., Sheehan K.H., Amorim P., Janavs J., Weiller E., Hergueta T., Baker R., Dunbar G.C., 1998. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry 59 (Suppl 20)), 22–33 quiz 34–57. [PubMed] [Google Scholar]
- Stringaris A. (2017), Editorial: What is depression?. J Child Psychol Psychiatr, 58: 1287–1289. 10.1111/jcpp.12844 [DOI] [PubMed] [Google Scholar]
- Tedeschini E, Levkovitz Y, Iovieno N, Ameral VE, Nelson JC, Papakostas GI. Efficacy of antidepressants for late-life depression: a meta-analysis and meta-regression of placebo-controlled randomized trials. J Clin Psychiatry 2011; 72: 1660–1668 [DOI: 10.4088/JCP.10r06531] [DOI] [PubMed] [Google Scholar]
- Wang Y. Transcranial direct current stimulation for the treatment of major depressive disorder: A meta-analysis of randomized controlled trials. Psychiatry Res. 2019. Jun;276:186–190. doi: 10.1016/j.psychres.2019.05.012. Epub 2019 May 7. [DOI] [PubMed] [Google Scholar]
- Woodham R.D., Selvaraj Sudhakar, Lajmi Nahed, Hobday Harriet, Sheehan Gabrielle, Ghazi-Noori Ali-Reza, Lagerberg Peter J., Rizvi Maheen, Kwon Sarah S., Orhii Paulette, Maislin David, Hernandez Lucia, Machado-Vieira Rodrigo, Soares Jair C., Young Allan H., Fu Cynthia H.Y., Home-based transcranial direct current stimulation RCT in major depression, medRxiv 2023.11.27.23299059; doi: 10.1101/2023.11.27.23299059 [DOI] [PubMed] [Google Scholar]
- Zhang R, Lam CLM, Peng X, Zhang D, Zhang C, Huang R, Lee TMC. Efficacy and acceptability of transcranial direct current stimulation for treating depression: A meta-analysis of randomized controlled trials. Neurosci Biobehav Rev. 2021. Jul;126:481–490. doi: 10.1016/j.neubiorev.2021.03.026. Epub 2021 Mar 28. [DOI] [PubMed] [Google Scholar]