Abstract
Dedicated genetic pathways regulate cysteine homeostasis. For example, high levels of cysteine activate cysteine dioxygenase, a key enzyme in cysteine catabolism in most animal and many fungal species. The mechanism by which cysteine dioxygenase is regulated is largely unknown. In an unbiased genetic screen for mutations that activate cysteine dioxygenase (cdo-1) in the nematode Caenorhabditis elegans, we isolated loss-of-function mutations in rhy-1 and egl-9, which encode proteins that negatively regulate the stability or activity of the oxygen-sensing hypoxia inducible transcription factor (hif-1). EGL-9 and HIF-1 are core members of the conserved eukaryotic hypoxia response. However, we demonstrate that the mechanism of HIF-1-mediated induction of cdo-1 is largely independent of EGL-9 prolyl hydroxylase activity and the von Hippel-Lindau E3 ubiquitin ligase, the classical hypoxia signaling pathway components. We demonstrate that C. elegans cdo-1 is transcriptionally activated by high levels of cysteine and hif-1. hif-1-dependent activation of cdo-1 occurs downstream of an H2S-sensing pathway that includes rhy-1, cysl-1, and egl-9. cdo-1 transcription is primarily activated in the hypodermis where it is also sufficient to drive sulfur amino acid metabolism. Thus, the regulation of cdo-1 by hif-1 reveals a negative feedback loop that maintains cysteine homeostasis. High levels of cysteine stimulate the production of an H2S signal. H2S then acts through the rhy-1/cysl-1/egl-9 signaling pathway to increase HIF-1-mediated transcription of cdo-1, promoting degradation of cysteine via CDO-1.
Research organism: C. elegans
eLife digest
Proteins are large molecules in our cells that perform various roles, from acting as channels through which nutrients can enter the cell, to forming structural assemblies that help the cell keep its shape. Proteins are formed of chains of building blocks called amino acids. There are 20 common amino acids, each with a different ‘side chain’ that confers it with specific features.
Cysteine is one of these 20 amino acids. Its side chain has a ‘thiol’ group, made up of a sulfur atom and a hydrogen atom. This thiol group is very reactive, and it is an essential building block of enzymes (proteins that speed up chemical reactions within the cell), structural proteins and signaling molecules. While cysteine is an essential amino acid for the cell to function, excess cysteine can be toxic. The concentration of cysteine in animal cells is tightly regulated by an enzyme called cysteine dioxygenase.
This enzyme is implicated in two rare conditions that affect metabolism, where the product of cysteine dioxygenase is a key driver of disease severity. Additionally, cysteine dioxygenase acts as a tumor suppressor gene, and its activity becomes blocked in diverse cancers. Understanding how cysteine dioxygenase is regulated may be important for research into these conditions.
While it has been shown that excess cysteine drives the production and activity of cysteine dioxygenase, how the cell detects high levels of cysteine remained unknown. Warnhoff et al. sought to resolve this question using the roundworm Caenorhabditis elegans. First, the scientists demonstrated that, like in mammals, high levels of cysteine drive the production of cysteine dioxygenase in C. elegans. Next, the researchers used an approach called an unbiased genetic screening to find genes that induce cysteine dioxygenase production when they are mutated. These experiments revealed that the protein HIF-1 can drive the production of cysteine dioxygenase when it is activated by a pathway that senses hydrogen sulfide gas.
Based on these results, Warnhoff et al. propose that high levels of cysteine lead to the production of hydrogen sulfide gas that in turn drives the production of cysteine dioxygenase via HIF-1 activation of gene expression.
The results reported by Warnhoff et al. suggest that modulating HIF-1 signaling could control the activity of cysteine dioxygenase. This information could be used in the future to develop therapies for molybdenum cofactor deficiency, isolated sulfite oxidase deficiency and several types of cancer. However, first it will be necessary to demonstrate that the same signaling pathway is active in humans.
Introduction
Cysteine is a sulfur-containing amino acid that mediates many oxidation/reduction reactions of proteins, is the redox center of the abundant antioxidant tripeptide glutathione which also serves as a major cysteine reserve, and is essential for iron-sulfur cluster assembly in the mitochondrion (Wu et al., 2004; Zheng et al., 1993). Cysteine residues in many proteins are in close proximity in the primary or folded protein sequence and are oxidized in the endoplasmic reticulum to form intra- and interprotein disulfide linkages, most commonly in secreted proteins which mediate intercellular signaling and defense (Noiva, 1994; Raina and Missiakas, 1997; Rietsch and Beckwith, 1998). In many enzymes, the reactivity of the cysteine sulfur is key for the coordination of metals such as zinc or iron, which support protein structure and catalytic activity (Giles et al., 2003; Tainer et al., 1991; Miller et al., 1985). Cysteine is also a key source of hydrogen sulfide (H2S), a volatile signaling molecule (Singh and Banerjee, 2011). While cysteine has these essential functions, excess cysteine is also toxic. High levels of cysteine impair mitochondrial respiration by disrupting iron homeostasis (Hughes et al., 2020), acts as a neural excitotoxin (Olney et al., 1990), and promotes the formation of toxic levels of hydrogen sulfide gas (Singh and Banerjee, 2011; Evans, 1967; Truong et al., 2006). Given this balance between essential and toxic, cysteine homeostasis is key for the health of cells and organisms.
CDO1-mediated oxidation is the primary pathway of cysteine catabolism when sulfur amino acid (methionine or cysteine) availability is normal or high (Bella et al., 1996). The dipeptide cystathionine is a key intermediate in this pathway. Cystathionine is catabolized by cystathionase (CTH-2 in Caenorhabditis elegans, CTH in mammals) producing cysteine and α-ketobutyrate. Cysteine is further oxidized using dissolved atmospheric dioxygen to cysteinesulfinate by cysteine dioxygenase (CDO-1 in C. elegans, CDO1 in mammals; Figure 1A; Stipanuk, 2004). The further oxidation of cysteinesulfinate downstream of CDO-1 generates highly toxic sulfites that are normally oxidized to more benign sulfate by sulfite oxidase (Figure 1A).
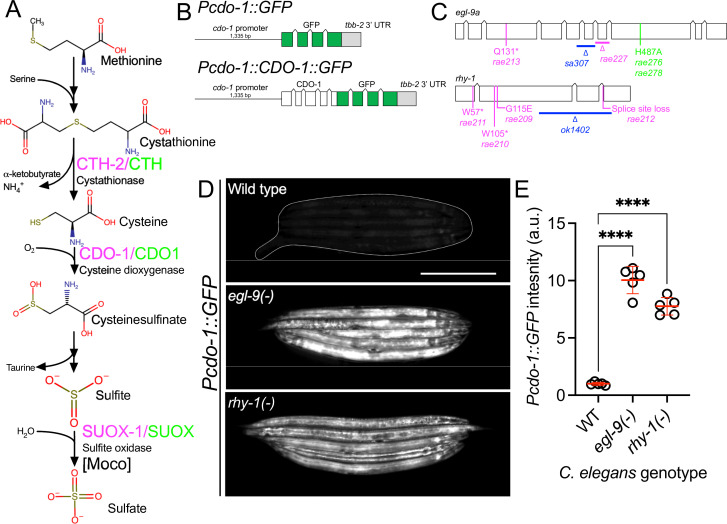
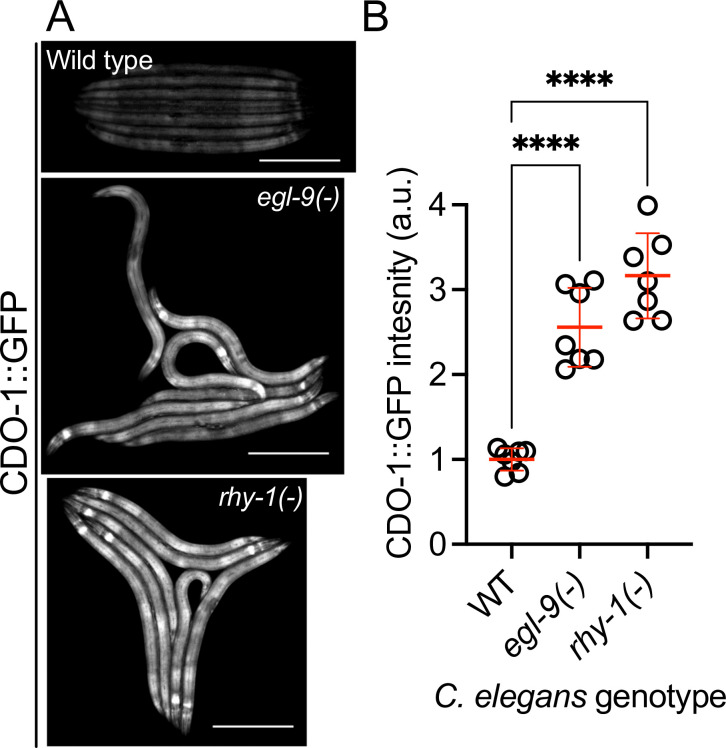
Figure 1. egl-9 and rhy-1 inhibit cdo-1 transcription.
(A) Pathway for sulfur amino acid metabolism beginning with methionine. We highlight the roles of cystathionase (CTH-2/CTH), cysteine dioxygenase (CDO-1/CDO1), and the Moco-requiring sulfite oxidase enzyme (SUOX-1/SUOX). C. elegans enzymes (magenta) and their human homologs (green) are displayed. (B) Pcdo-1::GFP promoter fusion (upper) and Pcdo-1::CDO-1::GFP C-terminal protein fusion (lower) transgenes used in this work are displayed. Boxes indicate exons, connecting lines indicate introns. The cdo-1 promoter is shown as a straight line. (C) egl-9a and rhy-1 gene structures. Boxes indicate exons and connecting lines are introns. Colored annotations indicate mutations generated or used in our work. Magenta; chemically-induced mutations that activated Pcdo-1::CDO-1::GFP fusion protein. Blue; reference null alleles isolated independent of our work. Green; CRISPR/Cas9-generated mutation that inactivates the prolyl hydroxylase domain of EGL-9. (D) Expression of Pcdo-1::GFP transgene is displayed for wild-type, egl-9(sa307), and rhy-1(ok1402) C. elegans animals at the L4 stage. Scale bar is 250 μm. White dotted line outlines animals with basal GFP expression. For GFP imaging, exposure time was 100ms. (E) Quantification of GFP expression displayed in (D). Individual datapoints are shown (circles) as are the mean and standard deviation (red lines). n is 5 individuals per genotype. Data are normalized so that wild-type expression of Pcdo-1::GFP is 1 arbitrary unit (a.u.). ****, p<0.0001, ordinary one-way ANOVA with Dunnett’s post hoc analysis.
Figure 1—figure supplement 1. The Pcdo-1::CDO-1::GFP transgene encodes a functional cysteine dioxygenase enzyme.
As a critical player in cysteine homeostasis, CDO1 is a highly regulated enzyme: the activity and abundance of CDO1 increase dramatically in cells and animals fed excess cysteine and methionine (Stipanuk and Ueki, 2011). CDO1 levels are governed both transcriptionally and post-translationally via proteasomal degradation (Dominy et al., 2006a; Dominy et al., 2006b; Stipanuk et al., 2004; Lee et al., 2004; Kwon and Stipanuk, 2001). However, the molecular players that sense high levels of cysteine and promote CDO1 activation remain incompletely defined.
Understanding these regulatory mechanisms is an important goal as CDO1 dysfunction is implicated in disease. CDO1 is a tumor suppressor whose activity is silenced in diverse cancers via promoter methylation, a potential biomarker of tumor grade and progression (Kojima et al., 2018; Yamashita et al., 2018; Brait et al., 2012). Decreased CDO1 activity may support tumor cell growth by reducing reactive oxygen species and decreasing drug susceptibility (Kang et al., 2019; Jeschke et al., 2013; Hao et al., 2017; Ma et al., 2022).
Using the nematode C. elegans, we have previously shown that CDO-1 is a key player in the pathophysiology of two fatal inborn errors of metabolism; isolated sulfite oxidase deficiency (ISOD) and molybdenum cofactor deficiency (MoCD; Duran et al., 1978; Mudd et al., 1967). Molybdenum cofactor (Moco) is an essential 520 Dalton prosthetic group synthesized from GTP by a conserved multistep biosynthetic pathway that is present in about 2/3 of bacterial genomes and nearly all eukaryotic genomes (Schwarz et al., 2009; Zhang and Gladyshev, 2008). C. elegans can either retrieve Moco synthesized by the bacteria it consumes or can synthesize Moco de novo using its own Moco biosynthetic pathway (Warnhoff and Ruvkun, 2019). C. elegans strains carrying mutations in genes encoding Moco biosynthetic enzymes (moc) and feeding on wild-type E. coli develop normally. Yet, these same moc-mutant C. elegans when fed on Moco-deficient E. coli as their sole nutritional source are inviable, arresting development at an early larval stage. A saturated genetic selection for mutations that suppress this inviability identified multiple independent mutations in cth-2 or cdo-1, genes which encode enzymes in the cysteine biosynthetic and degradation pathway. The toxic sulfites produced downstream of CDO-1 are normally oxidized to more benign sulfate by Moco-requiring sulfite oxidase (SUOX-1 in C. elegans, SUOX in mammals) an essential enzyme in C. elegans and humans (Figure 1A; Mudd et al., 1967; Warnhoff and Ruvkun, 2019). Therefore, loss of cdo-1 or cth-2 suppresses the lethality caused by both Moco and sulfite oxidase deficiencies in C. elegans by preventing the production of toxic sulfites (Warnhoff and Ruvkun, 2019; Warnhoff et al., 2021; Snoozy et al., 2022). Thus, understanding the fundamental mechanisms that govern the levels and activity of CDO-1 is critical to generating new therapeutic hypotheses to treat these diseases.
To define genes that regulate cdo-1 levels and activity, we performed an unbiased genetic screen in the nematode C. elegans to identify mutations that increase the expression or abundance of a Pcdo-1::CDO-1::GFP reporter transgene. We identified multiple independent loss-of-function mutations in two genes, egl-9 and rhy-1, that dramatically increase expression of this Pcdo-1::CDO-1::GFP transgene. These enzymes act in the hypoxia and H2S-sensing pathway, and we demonstrate that the conserved hypoxia-inducible transcription factor (HIF-1) activates cdo-1 transcription in this pathway (Shen et al., 2006; Budde and Roth, 2011; Ma et al., 2012). We further show that high levels of cysteine promote cdo-1 transcription, and that hif-1 and cysl-1 (another component of the H2S-sensing pathway) are required for viability under high cysteine conditions. We demonstrate that transcriptional activation of cdo-1 via HIF-1 promotes CDO-1 activity and establish the C. elegans hypodermis as a key tissue of CDO-1 activation and function. Unexpectedly, we find that cdo-1 regulation is governed by a HIF-1 pathway largely independent of EGL-9 prolyl hydroxylase activity and von Hippel-Lindau (VHL-1), the canonical O2 -sensing pathway (Epstein et al., 2001; Ivan et al., 2001). These data establish a new connection between the HIF-1/H2S-sensing pathway and sulfur amino acid catabolism governed by CDO-1.
Results
egl-9 and rhy-1 negatively regulate cdo-1 transcription
To identify regulators of CDO-1 expression or activity, we engineered a transgene expressing a C-terminal green fluorescent protein (GFP) fusion to the full-length CDO-1 protein driven by the native cdo-1 promoter (Pcdo-1::CDO-1::GFP, Figure 1B). Transgenic animals were generated by integrating the Pcdo-1::CDO-1::GFP fusion protein into the C. elegans genome (Frøkjær-Jensen et al., 2014). The Pcdo-1::CDO-1::GFP fusion protein was functional and rescued a cdo-1 loss-of-function mutation: the Pcdo-1::CDO-1::GFP fusion protein reverses the suppression of Moco-deficient lethality caused by cdo-1 loss of function (Figure 1—figure supplement 1). The reanimation of Moco-deficient lethality by the transgene depends on CDO-1 enzymatic activity because a transgene expressing an active-site mutant Pcdo-1::CDO-1[C85Y]::GFP does not rescue the cdo-1 mutant phenotype (Figure 1—figure supplement 1; Warnhoff and Ruvkun, 2019; McCoy et al., 2006). Thus, the Pcdo-1::CDO-1::GFP transgenic fusion protein is functional, suggesting that its expression pattern reflects endogenous protein expression, localization, and levels.
We performed a mutagenesis screen to identify genes that control the expression or accumulation of CDO-1 protein. Specifically, we performed an EMS chemical mutagenesis of C. elegans and screened in the F2 generation, after newly induced random mutations were allowed to become homozygous, for mutations that caused increased GFP accumulation by the Pcdo-1::CDO-1::GFP reporter transgene (Brenner, 1974). Using whole-genome sequencing, we determined that two independently isolated mutants carried distinct mutations in egl-9 and four other independently isolated mutants had unique mutations in rhy-1 (Figure 1C). The presence of multiple independent alleles suggests these mutations in egl-9 or rhy-1 are causative for the increased Pcdo-1::CDO-1::GFP expression or accumulation observed. egl-9 encodes the O2 -sensing prolyl hydroxylase and orthologue of mammalian EglN1. rhy-1 encodes the regulator of hypoxia inducible transcription factor, an enzyme with homology to membrane-bound O-acyltransferases (Shen et al., 2006; Epstein et al., 2001). The genetic screen produced nonsense alleles of both egl-9 and rhy-1 suggesting the increased Pcdo-1::CDO-1::GFP expression or accumulation is caused by loss of egl-9 or rhy-1 function (Figure 1C). Inactivation of egl-9 or rhy-1 activates a transcriptional program mediated by the hypoxia inducible transcription factor, HIF-1 (Shen et al., 2006; Epstein et al., 2001). To determine if egl-9 or rhy-1 regulate cdo-1 transcription, we engineered a separate reporter construct where only GFP (rather than the full length CDO-1::GFP fusion protein) is transcribed by the cdo-1 promoter (Pcdo-1::GFP, Figure 1B). This Pcdo-1::GFP transgene was introduced into strains with independently isolated egl-9(sa307) and rhy-1(ok1402) null reference alleles (Shen et al., 2006; Darby et al., 1999). The egl-9(sa307) and rhy-1(ok1402) mutations dramatically induce expression of GFP driven by the Pcdo-1::GFP transcriptional reporter transgene (Figure 1D and E). The activation of cdo-1 transcription by independently isolated egl-9 or rhy-1 mutations demonstrates that the egl-9 and rhy-1 mutations isolated in our screen for the induction or accumulation of Pcdo-1::CDO-1::GFP are the causative genetic lesions. Furthermore, these data demonstrate that egl-9 and rhy-1 are necessary for the normal transcriptional repression of cdo-1.
HIF-1 directly activates cdo-1 transcription downstream of the rhy-1, cysl-1, egl-9 genetic pathway
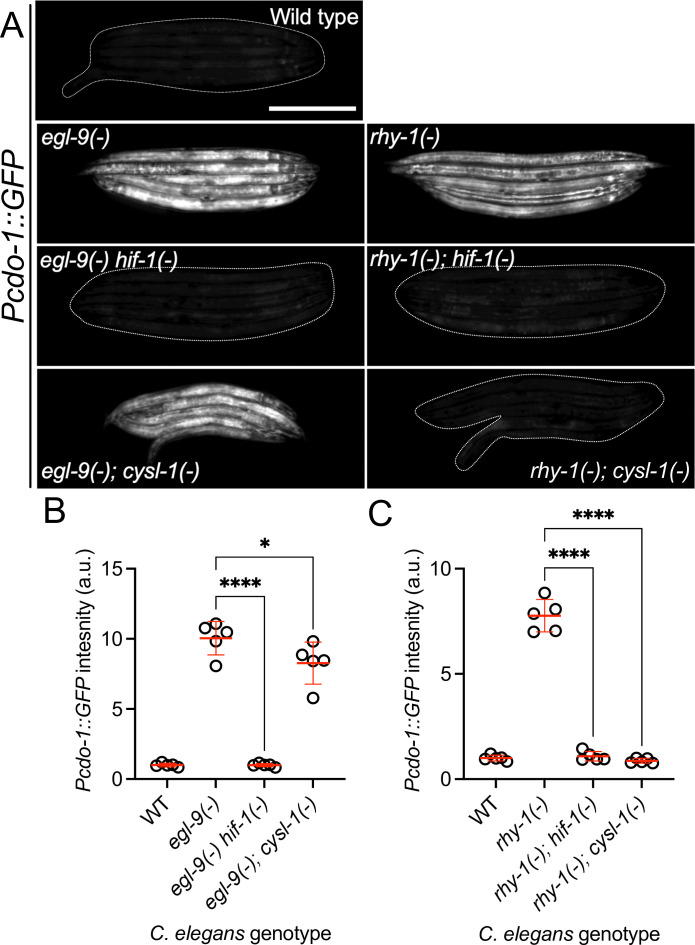
rhy-1 and egl-9 act in a pathway that regulates the abundance and activity of the HIF-1 transcription factor (Shen et al., 2006; Ma et al., 2012). The activity of rhy-1 is most upstream in the pathway and negatively regulates the activity of cysl-1, which encodes a cysteine synthase-like enzyme of probable algal origin (Wang et al., 2022). CYSL-1 directly binds to and inhibits EGL-9 in an H2S-modulated manner (Ma et al., 2012). EGL-9 uses molecular oxygen as well as an α-ketoglutarate cofactor to directly inhibit HIF-1 via prolyl hydroxylation, which recruits the VHL-1 ubiquitin ligase to ubiquitinate HIF-1, targeting it for degradation by the proteasome (Figure 5A) (Salceda and Caro, 1997; Huang et al., 1998; Sutter et al., 2000). Given that loss-of-function mutations in rhy-1 or egl-9 activate cdo-1 transcription, we tested if cdo-1 transcription is activated by HIF-1 as an output of this hypoxia/H2S-sensing pathway. We performed epistasis studies using null mutations that inhibit the activity of hif-1 (cysl-1(ok762) and hif-1(ia4)) or activate hif-1 (rhy-1(ok1402) and egl-9(sa307)). The induction of Pcdo-1::GFP by egl-9 inactivation was dependent upon the activity of hif-1, but not on the activity of cysl-1 (Figure 2A and B). In contrast, induction of Pcdo-1::GFP by rhy-1 inactivation was dependent upon the activity of both hif-1 and cysl-1 (Figure 2A and C). These results reveal a genetic pathway whereby rhy-1, cysl-1, and egl-9 function in a negative-regulatory cascade to control the activity of HIF-1 which transcriptionally activates cdo-1. Our epistasis studies of cdo-1 transcriptional regulation by HIF-1 align well with previous analyses of this genetic pathway in the context of transcription of cysl-2 (a paralog of cysl-1) and the ‘O2-ON response’ (Ma et al., 2012).
Figure 2. cdo-1 transcription is activated by HIF-1 downstream of RHY-1, CYSL-1, and EGL-9.
(A) Expression of the Pcdo-1::GFP transgene is displayed for wild-type, egl-9(sa307), egl-9(sa307) hif-1(ia4) double mutant, egl-9(sa307); cysl-1(ok762) double mutant, rhy-1(ok1402), rhy-1(ok1402); hif-1(ia4) double mutant, and rhy-1(ok1402); cysl-1(ok762) double mutant C. elegans animals at the L4 stage of development. Scale bar is 250 μm. White dotted line outlines animals with basal GFP expression. For GFP imaging, exposure time was 100ms. (B, C) Quantification of the data displayed in (A). Individual datapoints are shown (circles) as are the mean and standard deviation (red lines). n is 5 individuals per genotype. Data are normalized so that wild-type expression of Pcdo-1::GFP is 1 arbitrary unit (a.u.). *, p<0.05, ****, p<0.0001, ordinary one-way ANOVA with Dunnett’s post hoc analysis. Note, wild-type, egl-9(-), and rhy-1(-) images in panel A and quantification of Pcdo-1::GFP in panels B and C are identical to the data presented in (Figure 1D and E). They are re-displayed here to allow for clear comparisons to the double mutant strains of interest.
To demonstrate that HIF-1 activates transcription of endogenous cdo-1, we explored published RNA-sequencing data of wild-type, egl-9(-), and egl-9(-) hif-1(-) mutant animals (Pender and Horvitz, 2018). egl-9(-) mutant C. elegans display an eightfold increase in cdo-1 mRNA compared to wild type. This induction was dependent on hif-1; a hif-1(-) mutation completely suppressed the induction of cdo-1 mRNA caused by an egl-9(-) mutation (Pender and Horvitz, 2018). These RNA-seq data confirm our findings using the Pcdo-1::GFP transcriptional reporter that HIF-1 is a transcriptional activator of cdo-1. ChIP-seq data of HIF-1 performed by the modERN project show that HIF-1 directly binds the cdo-1 promoter (peak from –1165 to –714 base pairs 5’ to the cdo-1 ATG start codon; Kudron et al., 2018; Vora et al., 2022). This HIF-1 binding site contains three copies of the HIF-binding motif (5’-RCGTG-3’; Kaelin and Ratcliffe, 2008). Thus, cdo-1 is a downstream effector of HIF-1 and is likely a direct transcriptional target of HIF-1.
High levels of cysteine promote cdo-1 transcription and cause lethality in cysl-1 and hif-1 mutant animals
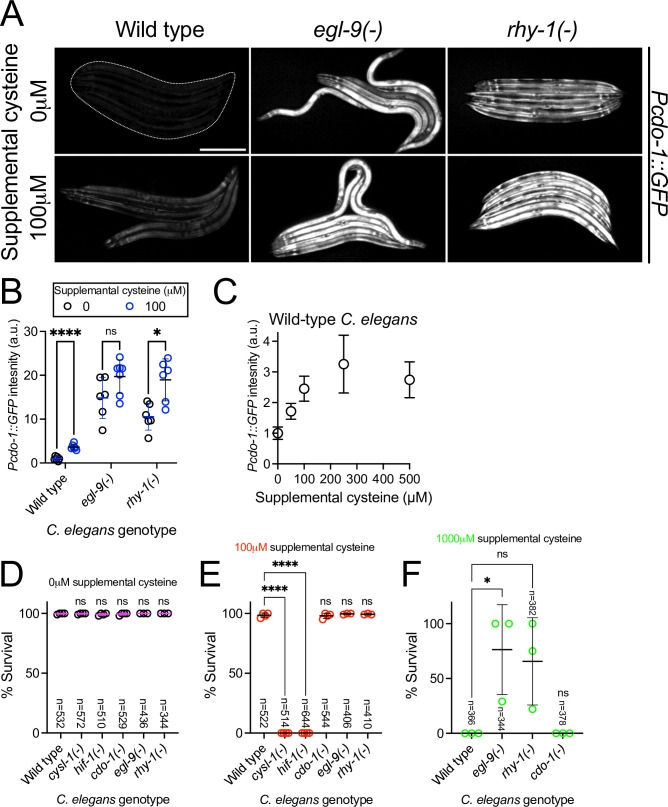
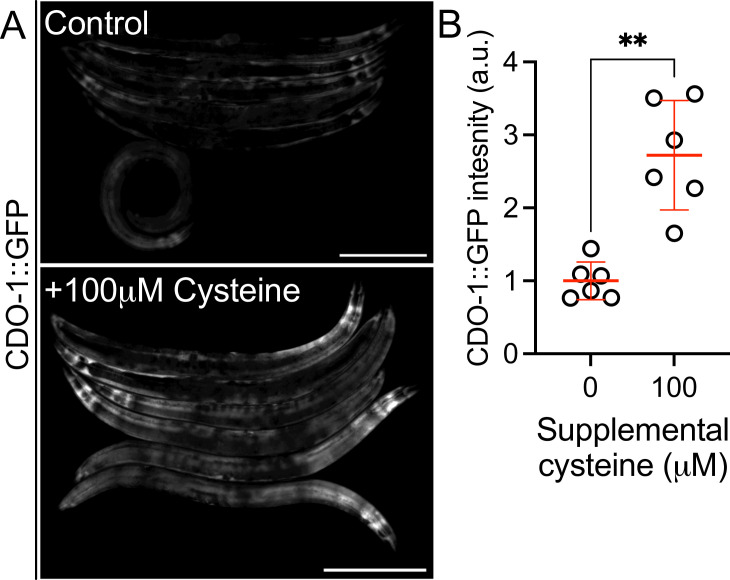
Mammalian CDO1 levels and activity are highly induced by dietary cysteine (Bella et al., 1996; Stipanuk, 2004; Stipanuk and Ueki, 2011). To determine if this homeostatic response is conserved in C. elegans, we exposed transgenic C. elegans carrying the Pcdo-1::GFP transcriptional reporter to high supplemental cysteine. Like our egl-9 and rhy-1 loss-of-function mutations, high levels of cysteine promoted cdo-1 transcription (Figure 3A–C). Despite the significant 3.6-fold induction of Pcdo-1::GFP caused by 100 μM supplemental cysteine, we note that this induction is not as dramatic as the induction caused by null mutations in egl-9 or rhy-1. This perhaps reflects the animals’ ability to buffer environmental cysteine which is bypassed by genetic intervention.
Figure 3. High levels of cysteine activate cdo-1 transcription and are lethal to hif-1 and cysl-1 mutant animals.
(A) Expression of the Pcdo-1::GFP transgene is displayed for wild-type, egl-9(sa307), and rhy-1(ok1402) young-adult C. elegans exposed to 0 or 100 μM supplemental cysteine. Scale bar is 250 μm. White dotted line outlines animals with basal GFP expression. For GFP imaging, exposure time was 100ms. Supplemental cysteine did not impact the mortality of the animals being imaged. (B) Quantification of the data displayed in (A). Individual datapoints are shown (circles) as are the mean and standard deviation (black lines). n is 6 or 7 individuals per genotype. Data are normalized so that expression of Pcdo-1::GFP in wild-type C. elegans exposed to 0 μM supplemental cysteine is equal to 1 arbitrary unit (a.u.). *, p<0.05, ****, p<0.0001, multiple unpaired t test with Welch’s correction. (C) Quantification of the Pcdo-1::GFP expression is displayed for wild-type young-adult C. elegans exposed to 0, 50, 100, 250, or 500 μM supplemental cysteine. Mean and standard deviation are displayed. n is 6 or 7 individuals per concentration of supplemental cysteine. (D–F) The percentage of animals that survive overnight exposure to (D) 0, (E) 100, or (F) 1000 μM supplemental cysteine. Individual datapoints (circles) represent biological replicates. Three or four biological replicates were performed for each experiment and the total individuals scored amongst all replicates is displayed (n). *, p<0.05, ****, p<0.0001, ordinary one-way ANOVA with Dunnett’s post hoc analysis. ns indicates no significant difference was identified.
We hypothesized that cysteine might activate cdo-1 transcription through the RHY-1/CYSL-1/EGL-9/HIF-1 pathway. In this pathway, cysl-1 and hif-1 act to promote cdo-1 transcription. Thus, we sought to test whether cysl-1 or hif-1 were necessary for the induction of Pcdo-1::GFP by high levels of cysteine. However, this experiment was not possible as we observed 100% lethality in cysl-1(-) and hif-1(-) mutant animals exposed to 100 μM supplemental cysteine, a cysteine concentration at which wild-type animals are healthy (Figure 3E). Importantly, wild-type, cysl-1(-) and hif-1(-) animals were all healthy under control conditions without supplemental cysteine (Figure 3D). While this phenotype limits our ability to establish the role of cysl-1 and hif-1 in the induction of cdo-1 by high levels of cysteine, these data demonstrate that cysl-1 and hif-1 are necessary for survival under high cysteine conditions. Given the requirement of hif-1 for survival in high levels of cysteine, we hypothesized that mutations in egl-9 or rhy-1 that activate hif-1 might promote cysteine resistance. Indeed, we found that egl-9(-) and rhy-1(-) mutant C. elegans were partially viable when exposed to 1000 μM supplemental cysteine, a concentration that causes 100% lethality in wild-type animals (Figure 3F). Thus, egl-9 and rhy-1 are negative regulators cysteine tolerance. Taken together, these data demonstrate a critical physiological role for the RHY-1/CYSL-1/EGL-9/HIF-1 pathway in promoting cysteine homeostasis.
To further test the interaction between high levels of cysteine and the RHY-1/CYSL-1/EGL-9/HIF-1 pathway, we exposed egl-9(-); Pcdo-1::GFP mutant animals to control or high levels of supplemental cysteine. We reasoned that if cysteine and egl-9 loss of function promote Pcdo-1::GFP accumulation in the same pathway, then their effects should not be additive. Consistent with this hypothesis, we saw no difference in Pcdo-1::GFP expression in egl-9(-) mutant animals exposed to 0 or 100 μM supplemental cysteine (Figure 3A and B). We also tested the ability of supplemental cysteine to further activate Pcdo-1::GFP expression in rhy-1(-) mutant animals. In contrast to our results with egl-9(-), we observed that high levels of cysteine caused a significant induction of Pcdo-1::GFP in the rhy-1(-) mutant background (Figure 3A and B). These data suggest that cysteine acts in a pathway with egl-9 but operates in parallel to the function of rhy-1.
Given the established role of CDO-1 in cysteine catabolism, we tested whether cdo-1(-) mutants were also sensitive to high levels of cysteine. cdo-1(-) mutant animals were not sensitive to high levels of supplemental cysteine compared to the wild type (Figure 3D–F). Thus, cdo-1 is not necessary for survival under high cysteine conditions. This suggests the existence of alternate pathways that promote cysteine homeostasis. Given the role of the RHY-1/CYSL-1/EGL-9/HIF-1 pathway in promoting cysteine homeostasis, we propose that HIF-1 activates pathways (in addition to cdo-1) that promote survival under high cysteine conditions.
Activated CDO-1 accumulates and is functional in the hypodermis
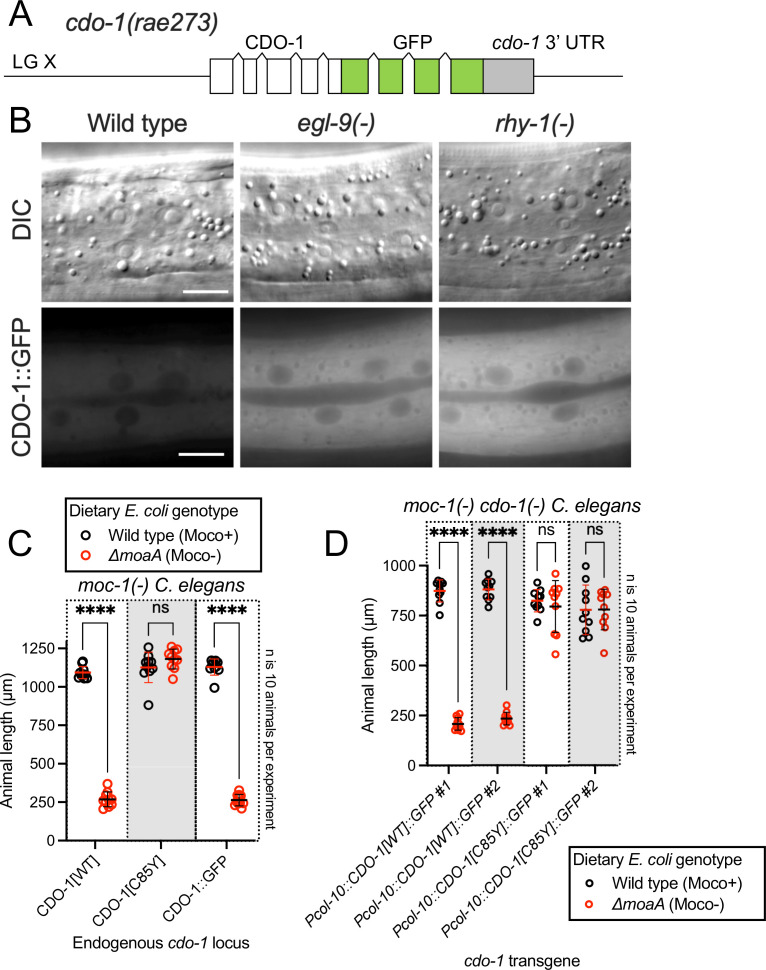
We sought to identify the site of action of CDO-1. To observe CDO-1 localization, we used CRISPR/Cas9 to insert the GFP open-reading frame into the endogenous cdo-1 locus, replacing the native cdo-1 stop codon. This cdo-1(rae273) allele encodes a C-terminal tagged ‘CDO-1::GFP’ fusion protein from the native cdo-1 genomic locus (Figure 4A). To determine if CDO-1::GFP was functional, we combined the CDO-1::GFP fusion protein with a null mutation in moc-1, a gene that is essential for C. elegans Moco biosynthesis (Warnhoff and Ruvkun, 2019). We then observed the growth of moc-1(-) CDO-1::GFP C. elegans on wild-type and Moco-deficient E. coli. The moc-1(-) mutant C. elegans expressing CDO-1::GFP from the native cdo-1 locus arrest during larval development when fed Moco-deficient E. coli, but not when fed Moco-producing E. coli (Figure 4C). This lethality is caused by the CDO-1-mediated production of sulfites which are only toxic when C. elegans is Moco deficient, and demonstrates that the CDO-1::GFP fusion protein is functional (Warnhoff and Ruvkun, 2019). These data suggest that the CDO-1::GFP expression we observe is physiologically relevant.
Figure 4. Hypodermal CDO-1 accumulates in the cytoplasm when egl-9 or rhy-1 are inactive and is sufficient to promote sulfur amino acid metabolism.
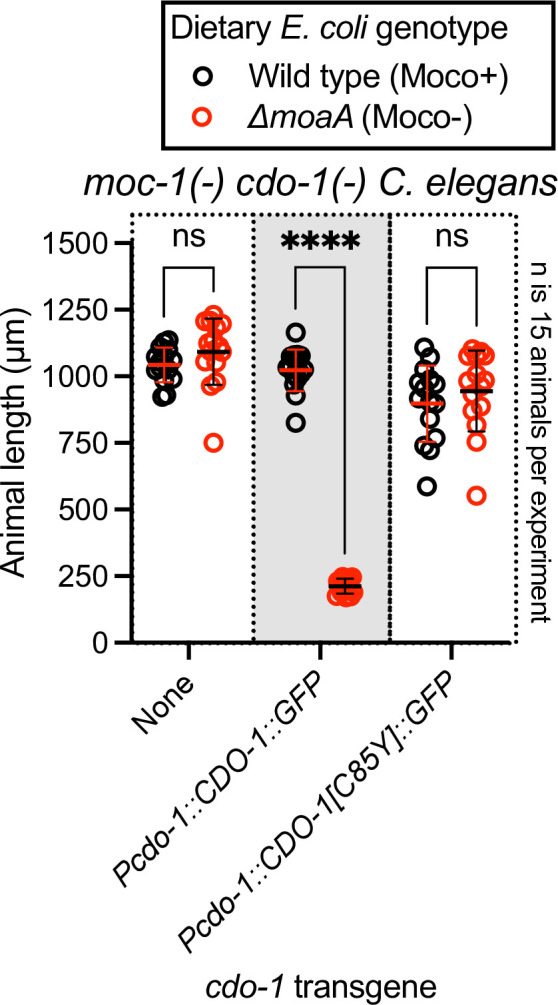
(A) Diagram of cdo-1(rae273), a CRISPR/Cas9-generated allele with GFP inserted into the cdo-1 gene, creating a functional C-terminal CDO-1::GFP fusion protein expressed from the native cdo-1 locus. (B) Differential interference contrast (DIC) and fluorescence imaging are shown for wild-type, egl-9(sa307), and rhy-1(ok1402) C. elegans expressing CDO-1::GFP encoded by cdo-1(rae273). Scale bar is 10 μm. For GFP imaging, exposure time was 200 ms. An anterior segment of the Hyp7 hypodermal cell is displayed. CDO-1::GFP accumulates in the cytoplasm and is excluded from the nuclei. (C) moc-1(ok366), moc-1(ok366) cdo-1(mg622), and moc-1(ok366) cdo-1(rae273) animals were cultured from synchronized L1 larvae for 72 hr on wild-type (black, Moco+) or ΔmoaA mutant (red, Moco-) E. coli. (D) moc-1(ok366) cdo-1(mg622) double mutant animals expressing Pcol-10::CDO-1::GFP or Pcol-10::CDO-1[C85Y]::GFP transgenes were cultured for 48 hr on wild-type (black, Moco+) or ΔmoaA mutant (red, Moco-) E. coli. Two independently derived strains were tested for each transgene. For panels C and D, animal lengths were determined for each condition. Individual datapoints are shown (circles) as are the mean and standard deviation. Sample size (n) is 10 individuals for each experiment. ****, p<0.0001, multiple unpaired t test with Welch’s correction. ns indicates no significant difference was identified.
Figure 4—figure supplement 1. A functional CDO-1::GFP fusion protein is induced by loss of egl-9 or rhy-1.
Figure 4—figure supplement 2. CDO-1::GFP encoded by cdo-1(rae273) is induced by supplemental cysteine.
When wild-type animals expressing CDO-1::GFP were grown under standard culture conditions, we observed CDO-1::GFP expression in multiple tissues, including prominent expression in the hypodermis (Figure 4B, Figure 4—figure supplement 1). We tested if CDO-1::GFP fusion protein levels were affected by inactivation of egl-9 or rhy-1. We generated egl-9(-); CDO-1::GFP and rhy-1(-); CDO-1::GFP animals, and assayed expression of CDO-1::GFP. We found that CDO-1::GFP fusion protein levels, encoded by cdo-1(rae273), were increased by egl-9(-) or rhy-1(-) mutations (Figure 4B, Figure 4—figure supplement 1). This is consistent with our studies using Pcdo-1::CDO-1::GFP and Pcdo-1::GFP transgenes. Furthermore, high levels of cysteine also promoted accumulation of CDO-1::GFP (Figure 4—figure supplement 2). In all scenarios, the hypodermis was the most prominent site of CDO-1::GFP accumulation. Specifically, we found that CDO-1::GFP was expressed in the cytoplasm of Hyp7, the major C. elegans hypodermal cell (Figure 4B).
Based on the expression pattern of CDO-1::GFP encoded by cdo-1(rae273), we hypothesized that CDO-1 acts in the hypodermis to promote sulfur amino acid metabolism. To test this hypothesis, we engineered a cdo-1 rescue construct in which cdo-1 is expressed exclusively in the hypodermis under the control of a hypoderm-specific collagen (col-10) promoter (Pcol-10::CDO-1::GFP; Hong et al., 2000). Collagens are expressed exclusively in the hypodermis of nematodes, with a periodic induction in phase with the almost diurnal molting cycle (Meeuse et al., 2020). Multiple independent transgenic C. elegans strains were generated by integrating the Pcol-10::CDO-1::GFP construct into the C. elegans genome and tested for rescue of the cdo-1(-) mutant suppression of Moco-deficient larval arrest (Frøkjær-Jensen et al., 2014). Thus, tissue-specific complementation of the cdo-1 mutation would regenerate a lethal arrest phenotype caused by Moco deficiency. We found that multiple independent transgenic strains of cdo-1(-) moc-1(-) double mutant animals carrying the Pcol-10::CDO-1::GFP transgene displayed a larval arrest phenotype when fed a Moco-deficient diet (Figure 4D). These data demonstrated that hypodermal-specific expression of cdo-1 is sufficient to rescue the cdo-1(-) mutant suppression of Moco-deficient lethality. This rescue was dependent upon the enzymatic activity of CDO-1 as an active site variant of this transgene (Pcol-10::CDO-1[C85Y]::GFP) did not rescue the suppressed larval arrest of cdo-1(-) moc-1(-) double mutant animals fed Moco-deficient diets (Figure 4D). Taken together, our analyses of CDO-1::GFP expression demonstrate that CDO-1 is expressed, and that expression is regulated, in multiple tissues, principal among them being the hypodermis and Hyp7 cell. Our tissue-specific rescue data demonstrate that hypodermal expression of cdo-1 is sufficient to promote cysteine catabolism and suggest that the hypodermis is a critical tissue for sulfur amino acid metabolism. However, we cannot exclude the possibility that CDO-1 also acts in other cells and tissues as well.
HIF-1 promotes CDO-1 activity downstream of the H2S-sensing pathway
We sought to determine the physiological impact of cdo-1 transcriptional activation by HIF-1. We reasoned mutations that activate HIF-1 and increase cdo-1 transcription may cause increased CDO-1 activity. CDO-1 sits at a critical metabolic node in the degradation of the sulfur amino acids cysteine and methionine (Figure 1A). A key byproduct of sulfur amino acid metabolism and CDO-1 is sulfite, a reactive toxin that is detoxified by the Moco-requiring sulfite oxidase (SUOX-1). Null mutations in suox-1 cause larval lethality. However, animals carrying the suox-1(gk738847) hypomorphic allele are healthy under standard culture conditions (Warnhoff and Ruvkun, 2019; Warnhoff et al., 2021). suox-1(gk738847) mutant animals display only 4% SUOX-1 activity compared to wild type and are exquisitely sensitive to sulfite stress (Oliphant et al., 2023). Thus, the suox-1(gk738847) mutation creates a sensitized genetic background to probe for increases in endogenous sulfite production. To test if increased cdo-1 transcription would impact the growth of suox-1-comprimised animals, we combined the egl-9 null mutation, which promotes HIF-1 activity and cdo-1 transcription, with the suox-1(gk738847) allele. While egl-9(-) and suox-1(gk738847) single mutant animals are healthy under standard culture conditions, the egl-9(-); suox-1(gk738847) double mutant animals are extremely sick and require significantly more days to exhaust their E. coli food source under standard culture conditions (Table 1). These data establish a synthetic genetic interaction between these loci. To determine the role of sulfur amino acid metabolism in the egl-9(-); suox-1(gk738847) synthetic sickness phenotype, we engineered egl-9(-); cdo-1(-) suox-1(gk738847) and cth-2(-); egl-9(-); suox-1(gk738847) triple mutant animals. The egl-9; suox-1 synthetic sickness phenotype was suppressed by inactivating mutations in cdo-1 or cth-2 which block the endogenous production of sulfite (Table 1). These data demonstrate that the deleterious activity of the egl-9(-) mutation in a suox-1(gk738847) background requires functional sulfur amino acid metabolism.
Table 1. Growth of C. elegans strains on standard laboratory conditions.
C. elegans strains and their corresponding mutations are displayed. For each strain, 5 L4-stage animals were seeded onto standard NGM petri dishes seeded with a monoculture of E. coli OP50. Petri dishes were monitored until the animals (and their progeny) depleted the lawn of E. coli. This was recorded as ‘days for population to starve petri dish’. The average of these experiments is displayed for each C. elegans strain as is the standard deviation (SD) and the number of biological replicates (n). Significant differences in population growth were determined by appropriate comparisons to either suox-1(gk738847) (GR2269) or egl-9(sa307); suox-1(gk738847) (USD421) using an ordinary one-way ANOVA with Dunnett’s post hoc analysis. No test indicates a statistical comparison was not made. Note, the data for USD421 are displayed twice in the table to allow for ease of comparison.
| C. elegans strain | Genetic locus 1 | Genetic locus 2 | Genetic locus 3 | Days for population to starve petri dish ±SD (n) | Significant difference compared to GR2269 (Adjusted p Value) |
|---|---|---|---|---|---|
| N2 | Wild type | Wild type | Wild type | 5±1 (8) | No test |
| GR2269 | Wild type | suox-1(gk738847) | Wild type | 8±1 (4) | No test |
| JT307 | egl-9(sa307) | Wild type | Wild type | 7±1 (5) | No test |
| USD421 | egl-9(sa307) | suox-1(gk738847) | Wild type | 19±5 (11) | 0.0003 (***) |
| USD926 | egl-9(rae276) | Wild type | Wild type | 6±1 (3) | No test |
| USD937 | egl-9(rae276) | suox-1(gk738847) | Wild type | 9±1 (3) | 0.93 (ns) |
| USD512 | rhy-1(ok1402) | Wild type | Wild type | 6±0 (4) | No test |
| USD414 | rhy-1(ok1402) | suox-1(gk738847) | Wild type | 7±0 (4) | 0.99 (ns) |
| CB5602 | vhl-1(ok161) | Wild type | Wild type | 6±0 (4) | No test |
| USD422 | vhl-1(ok161) | suox-1(gk738847) | Wild type | 8±1 (4) | 0.99 (ns) |
| C. elegans strain | Genetic locus 1 | Genetic locus 2 | Genetic locus 3 | Days for population to starve petri dish ±SD (n) | Significant difference compared to USD421 (Adjusted p value) |
| USD421 | egl-9(sa307) | suox-1(gk738847) | Wild type | 19±5 (11) | No test |
| USD430 | egl-9(sa307) | suox-1(gk738847) | cdo-1(mg622) | 7±0 (6) | <0.0001 (****) |
| USD433 | egl-9(sa307) | suox-1(gk738847) | cth-2(mg599) | 9±1 (4) | <0.0001 (****) |
| USD432 | egl-9(sa307) | suox-1(gk738847) | rhy-1(ok1402) | 7±1 (4) | <0.0001 (****) |
| USD434 | egl-9(sa307) | suox-1(gk738847) | cysl-1(ok762) | 16±1 (9) | 0.1 (ns) |
| USD431 | egl-9(sa307) | suox-1(gk738847) | hif-1(ia4) | 9±1 (6) | <0.0001 (****) |
To determine the role of the H2S-sensing pathway in the synthetic sickness phenotype displayed by egl-9(-); suox-1(gk738847) double mutant animals, we introduced null alleles of cysl-1 or hif-1 into the egl-9(-); suox-1(gk738847) double mutant. The egl-9; suox-1 synthetic sickness phenotype was dependent upon hif-1 but not cysl-1 (Table 1). These results are consistent with our proposed genetic pathway and support the model that transcriptional activation of cdo-1 by HIF-1 causes increased CDO-1 activity and increased flux of sulfur amino acids through their catabolic pathway.
Loss of rhy-1 also strongly activates cdo-1 transcription. We hypothesized that rhy-1(-); suox-1(gk738847) double mutant animals would display a synthetic sickness phenotype like egl-9(-); suox-1(gk738847) double mutant animals. However, based upon their ability to exhaust their E. coli food source under standard culture conditions, rhy-1(-); suox-1(gk738847) double mutant animals were just as healthy as either rhy-1(-) or suox-1(gk738847) single mutant C. elegans (Table 1). These data suggest that increasing cdo-1 transcription alone is not sufficient to promote sulfite production via CDO-1. In addition to the role played by rhy-1 in the regulation of HIF-1 activity, rhy-1 itself is a transcriptional target of HIF-1. Loss of egl-9 activity induces rhy-1 mRNA ~50-fold in a hif-1-dependent manner (Pender and Horvitz, 2018). Given this potent transcriptional activation, we wondered if rhy-1 might play an additional role downstream of HIF-1 in the regulation of sulfur amino acid metabolism and sulfite production. To test this hypothesis, we engineered rhy-1(-); egl-9(-); suox-1(gk738847) triple mutant animals. To our surprise, the rhy-1(-); egl-9(-); suox-1(gk738847) triple mutant animals were healthy, demonstrating that rhy-1 was necessary for the deleterious activity of the egl-9(-) mutation in a suox-1(gk738847) background (Table 1). These genetic data suggest a dual role for rhy-1 in the control of sulfur amino acid metabolism; first as a component of a regulatory cascade that controls the activity of HIF-1 and second as a functional downstream effector of HIF-1 that is required for sulfur amino acid metabolism.
This is not the first description of a rhy-1 role downstream of hif-1. Overexpression of a rhy-1-encoding transgene suppresses the lethality of a hif-1(-) mutant during HSS stress (Horsman et al., 2019). These data establish RHY-1 as both a regulator and effector of HIF-1. How RHY-1, a predicted membrane-bound O-acyltransferase, molecularly executes these dual roles remains to be explored.
EGL-9 prolyl hydroxylase activity and VHL-1 are largely dispensable in the regulation of CDO-1
EGL-9 inhibits HIF-1 through its prolyl hydroxylase domain that hydroxylates HIF-1 proline 621 (Figure 5A; Epstein et al., 2001). To evaluate the impact of the EGL-9 prolyl hydroxylase domain on the regulation of cdo-1, we generated a prolyl hydroxylase domain-inactive egl-9 mutation using CRISPR/Cas9. We engineered an egl-9 mutation that substitutes an alanine in place of histidine 487 (H487A) (Figure 1C). Histidine 487 of EGL-9 is highly conserved and catalytically essential in the prolyl hydroxylase domain active site (Figure 5B; Pan et al., 2007; Shao et al., 2009). To evaluate the impact of an inactive EGL-9 prolyl hydroxylase domain on the transcription of cdo-1, we engineered a C. elegans strain carrying the egl-9(H487A) mutation with the Pcdo-1::GFP transcriptional reporter. egl-9(H487A) caused a modest increase in Pcdo-1::GFP accumulation in the C. elegans intestine, suggesting that the EGL-9 prolyl hydroxylase domain is necessary to repress cdo-1 transcription in the intestine (Figure 5C and D). However, the activation of Pcdo-1::GFP by the egl-9(H487A) mutation was markedly less when compared to Pcdo-1::GFP activation caused by an egl-9(-) null mutation (Figure 5C and D). These data suggest that EGL-9 has a prolyl hydroxylase domain-independent activity that is responsible for repressing cdo-1 transcription.
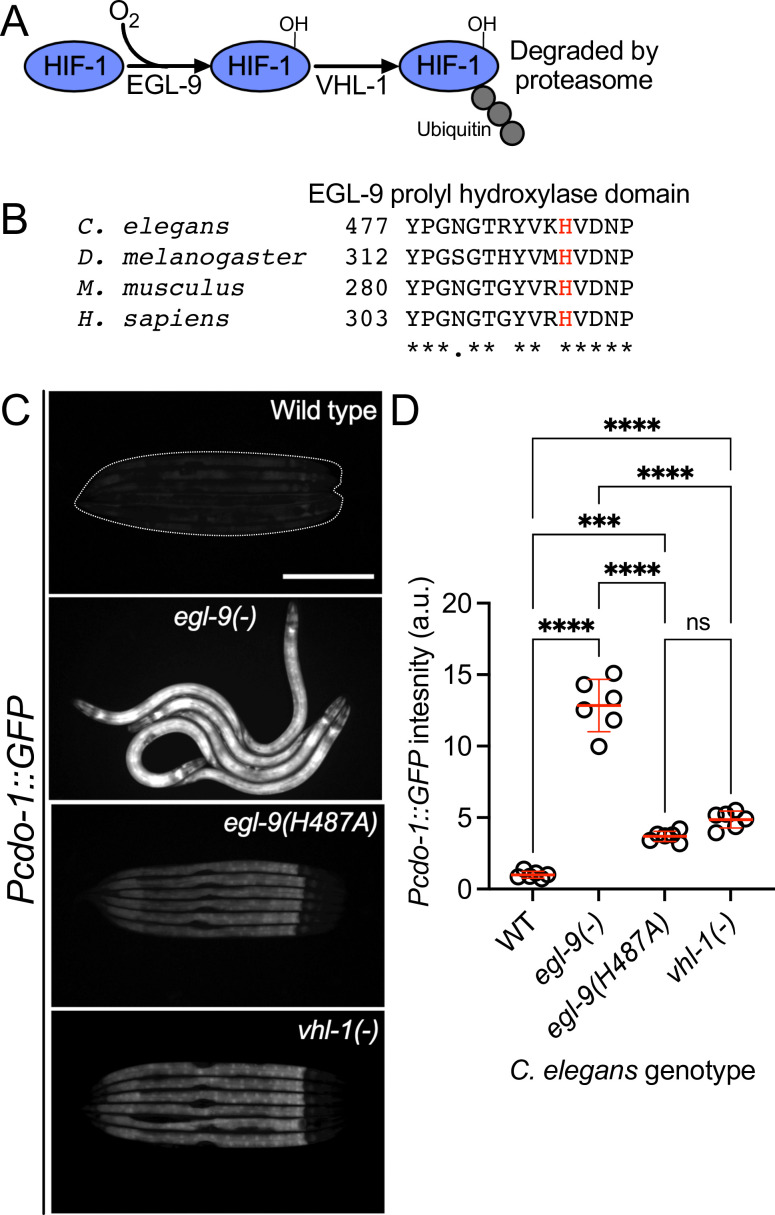
Figure 5. egl-9 inhibits cdo-1 transcription in a largely prolyl-hydroxylase and VHL-1-independent manner.
(A) The pathway of HIF-1 processing during normoxia is displayed. EGL-9 uses O2 as a substrate to hydroxylate (-OH) HIF-1 on specific proline residues. Prolyl hydroxylated HIF-1 is bound by VHL-1 which facilitates HIF-1 polyubiquitination and targets HIF-1 for degradation by the proteasome. (B) Amino acid alignment of the EGL-9 prolyl hydroxylase domain from C. elegans, D. melanogaster, M. musculus, and H. sapiens. ‘*’ indicate perfect amino acid conservation while ‘.’ indicates weak similarity amongst species compared. Highlighted (red) is the catalytically essential histidine 487 residue in C. elegans. Alignment was performed using Clustal Omega (EMBL-EBI). (C) Expression of Pcdo-1::GFP promoter fusion transgene is displayed for wild-type, egl-9(sa307, -), egl-9(rae276, H487A), and vhl-1(ok161) C. elegans animals at the L4 stage of development. Scale bar is 250 μm. White dotted line outlines animals with basal GFP expression. For GFP imaging, exposure time was 500ms. (D) Quantification of the data displayed in (C). Individual datapoints are shown (circles) as are the mean and standard deviation (red lines). n is 6 individuals per genotype. Data are normalized so that wild-type expression of Pcdo-1::GFP is 1 arbitrary unit (a.u.). ***, p<0.001, ****, p<0.0001, ordinary one-way ANOVA with Tukey’s multiple comparisons test. ns indicates no significant difference was identified.
EGL-9 hydroxylates specific proline residues on HIF-1. These hydroxylated proline residues are recognized by the von Hippel-Lindau E3 ubiquitin ligase (VHL-1). VHL-1-mediated ubiquitination promotes degradation of HIF-1 by the proteasome (Figure 5A; Ivan et al., 2001). Thus, the EGL-9 prolyl hydroxylase domain and VHL-1 act in a pathway to regulate HIF-1. To determine the role of VHL-1 in regulating cdo-1, we engineered a C. elegans strain carrying the vhl-1(-) null mutation with our Pcdo-1::GFP reporter. vhl-1 inactivation also caused a modest increase in Pcdo-1::GFP expression in the C. elegans intestine, suggesting that vhl-1 is necessary to repress cdo-1 transcription (Figure 5C and D). However, activation of Pcdo-1::GFP by the vhl-1(-) mutation was less than activation caused by an egl-9(-) null mutation (Figure 5C and D). These data suggest that EGL-9 has a VHL-1-independent activity that is responsible for repressing cdo-1 transcription.
To evaluate the impact of inactivating the EGL-9 prolyl hydroxylase domain or VHL-1 on cysteine metabolism, we again employed the suox-1(gk738847) hypomorphic mutation that sensitizes animals to increases in sulfite. We engineered egl-9(H487A); suox-1(gk738847) and vhl-1(-) suox-1(gk738847) double mutant animals and evaluated the health of those strains. In contrast to egl-9(-); suox-1(gk738847) double mutant animals which are extremely sick, egl-9(H487A); suox-1(gk738847) and vhl-1(-) suox-1(gk738847) double mutant animals are healthy (Table 1). These genetic data suggest that neither the EGL-9 prolyl hydroxylase domain nor VHL-1 are necessary to repress cysteine catabolism/sulfite production. However, it is plausible that the egl-9(H487) or vhl-1(-) mutations modestly activate cysteine metabolism, likely proportional to their activation of the Pcdo-1::GFP transgene (Figure 5C and D), and that this activation is not sufficient to produce enough sulfites to negatively impact the growth of suox-1(gk738847) mutant animals.
Discussion
CDO-1 is a physiologically relevant effector of HIF-1
The hypoxia-inducible factor HIF-1 is a master regulator of the cellular response to hypoxia. It activates the transcription of many genes and pathways that are critical to maintain metabolic homeostasis in the face of low O2. For example, mammalian HIF1α induces the hematopoietic growth hormone erythropoietin, glucose transport and glycolysis, as well as lactate dehydrogenase (Semenza and Wang, 1992; Bashan et al., 1992; Loike et al., 1992; Firth et al., 1994). The nematode C. elegans encounters a range of O2 tensions in its natural habitat of rotting material: as microbial abundance increases, O2 levels decrease from atmospheric levels (~21% O2). In fact, C. elegans prefers 5–12% O2, perhaps because hypoxia predicts abundant bacterial food sources (Gray et al., 2004). Members of the HIF-1 pathway and its targets have emerged from genetic studies of C. elegans (Epstein et al., 2001). For instance, C. elegans HIF-1 promotes H2S homeostasis by inducing transcription of the mitochondrial sulfide quinone oxidoreductase (sqrd-1), detoxifying H2S (Budde and Roth, 2011).
We sought to define genes that regulate the levels and activity of cysteine dioxygenase (CDO-1), a critical regulator of cysteine homeostasis (Bella et al., 1996; Stipanuk, 2004; Stipanuk and Ueki, 2011). Taking an unbiased genetic approach in C. elegans, we found that cdo-1 was highly regulated by HIF-1 downstream of a signaling pathway that includes rhy-1, cysl-1, and egl-9. We demonstrated that HIF-1 promotes cdo-1 transcription, accumulation of CDO-1 protein, and increased CDO-1 activity. Loss of rhy-1 or egl-9 activate hif-1 to in turn strongly induce the cdo-1 promoter. The pathway for activation of cdo-1 also requires cysl-1, which functions downstream of rhy-1 and upstream of egl-9. Based on ChIP-Seq studies, HIF-1 directly binds to the cdo-1 promoter (Kudron et al., 2018). Interestingly, mammalian CDO1 is regulated at both the transcriptional and post-transcriptional level in response to high dietary cysteine (Dominy et al., 2006a; Dominy et al., 2006b; Stipanuk et al., 2004; Lee et al., 2004; Kwon and Stipanuk, 2001). Our studies in the nematode C. elegans suggest that CDO1 transcription in mammals might be governed by HIF1α in response to changes in cellular cysteine. Importantly, all members of the RHY-1/CYSL-1/EGL-9 pathway in C. elegans have homologs encoded by mammalian genomes (Ma et al., 2012). Given the conservation of these proteins, future studies may show that similar cysteine and H2S-responsive signaling pathways operate in mammals.
We also show that the transcriptional activation of cdo-1 by HIF-1 promotes CDO-1 enzymatic activity. Genetic activation of cdo-1 by loss of egl-9 causes severe sickness in a mutant with reduced sulfite oxidase activity, an activity required to cope with the toxic sulfite produced via CDO-1. This synthetic sickness is dependent upon an intact HIF-1 signaling pathway and a functioning sulfur amino acid metabolism pathway, as the dramatic sickness of an egl-9; suox-1 double mutant is suppressed by loss of hif-1, cth-2, or cdo-1. These genetic results validate the intersection of HIF-1 and CDO-1 in converging biological regulatory pathways and encourage further exploration of this regulatory node. The potential physiological relevance of the connection between HIF-1 signaling and cysteine metabolism is discussed below.
It was initially surprising that the canonical hypoxia-sensing transcription factor, that is activated by relatively low O2 tensions, induces transcription of cdo-1, which encodes an oxidase that requires dissolved O2 to oxidize cysteine. Both EGL-9 and CDO-1 are dioxygenases, requiring O2 as a substrate to catalyze their chemical reactions. EGL-9 functions as an O2 sensor, using its O2 substrate to hydroxylate HIF-1 at relatively high O2 partial pressures, targeting it for degradation. EGL-9 is uniquely poised to sense deviations from the normally high physiological O2 concentrations given its high Km for O2 (Hirsilä et al., 2003; Dao et al., 2009; Ehrismann et al., 2007; Li et al., 2023; Losman et al., 2020). While it is not known if mammalian CDO1 or C. elegans CDO-1 dioxygenases are active at lower O2 tensions than EGL-9, it is possible, even probable, that the set point for activation of HIF-1 by the failure of EGL-9 prolyl hydroxylation, is at a higher O2 tension than the Km of CDO-1 for oxidation of cysteine. In this way, CDO-1 could still coordinate cysteine catabolism while the cell is experiencing hypoxia and EGL-9 is unable to hydroxylate HIF-1.
Evidence for distinct pathways of HIF-1 activation by hypoxia and cysteine/H2S
The hypoxia-signaling pathway is defined by EGL-9-dependent prolyl hydroxylation of HIF-1. Hydroxylated HIF-1 is then targeted for degradation via VHL-1-mediated ubiquitination (Epstein et al., 2001; Ivan et al., 2001; Kaelin and Ratcliffe, 2008). However, multiple lines of evidence, reinforced by our work, demonstrate VHL-1- and prolyl hydroxylase-independent activity of EGL-9. egl-9(-) null mutant C. elegans accumulate HIF-1 protein and display increased transcription of many genes, including nhr-57, an established target of HIF-1 (Shen et al., 2006). In rescue experiments of an egl-9(-) null mutant, Shao et al., 2009 demonstrate that a wild-type egl-9 transgene restores normal HIF-1 protein levels and HIF-1 transcription. However, rescue experiments with a prolyl hydroxylase domain-inactive egl-9(H487A) transgene do not correct the accumulation of HIF-1 protein and only partially reduce the HIF-1 transcriptional output (Shao et al., 2009). Through our studies of cdo-1 transcription, we demonstrate that an egl-9(H487A) mutant incompletely activates HIF-1 transcription when compared to an egl-9(-) null mutation (Figure 5C and D). Taken together, we conclude EGL-9 has activity independent of its prolyl hydroxylase domain, mirroring and supporting previous work (Shao et al., 2009).
In studies of the HIF-1-dependent Pnhr-57::GFP transcriptional reporter, (Shen et al., 2006) observed that egl-9(-) null mutants promote HIF-1 transcription more than a vhl-1(-) mutant (Shen et al., 2006). We observe this same distinction between egl-9 and vhl-1 mutations with our Pcdo-1::GFP reporter (Figure 5C and D). This difference in transcriptional activity is not explained by HIF-1 protein levels as HIF-1 protein accumulated equally in egl-9(-) and vhl-1(-) null mutants (Shen et al., 2006). This observation suggests that EGL-9 represses both HIF-1 levels and activity. This study also notes that vhl-1 represses HIF-1 transcription in the intestine while egl-9 acts in a wider array of tissues including the intestine and the hypodermis (Shen et al., 2006). Budde and Roth, 2010 also demonstrate that loss of vhl-1 promotes HIF-1 transcription in the C. elegans intestine while total loss of egl-9 promotes HIF-1 transcription in multiple tissues including the intestine and the hypodermis (Budde and Roth, 2010). Our data expand upon these observations by demonstrating that loss of the EGL-9 prolyl hydroxylase domain promotes cdo-1 transcription in the intestine alone, mirroring the loss of vhl-1 (Figure 5C). Budde and Roth, 2010 additionally demonstrate that physiologically relevant stimuli also elicit a tissue-specific transcriptional response: hypoxia promotes HIF-1 transcription in the intestine while H2S promotes HIF-1 transcription in the hypodermis. Importantly, H2S promotes hypodermal HIF-1 transcription in a vhl-1(-) mutant, demonstrating a VHL-1-independent pathway for H2S activation of HIF-1 (Budde and Roth, 2010). We demonstrate that high levels of cysteine promote cdo-1 transcription in the hypodermis. Taken together with our data, these studies suggest two distinct pathways for activating HIF-1 transcription: (i) a hypoxia-sensing pathway that is dependent upon vhl-1 and the EGL-9 prolyl hydroxylase domain and promotes HIF-1 activity in the intestine and (ii) an H2S-sensing pathway that is independent of vhl-1 and the EGL-9 prolyl hydroxylase domain and promotes HIF-1 activity in the hypodermis.
The focus of CDO-1 expression and regulation of sulfite production in the hypoderm may be due to the demand in sulfur metabolism as well as oxygen-dependent hydroxylation of collagens during the C. elegans molting cycle. Many collagen genes are expressed before each larval molt and many collagen prolines are hydroxylated, like particular HIF-1 prolines, and their cysteines form disulfides during collagen assembly. The large demand for the amino acid cysteine in the many collagen genes expressed at high levels during a molt may challenge cysteine homeostasis in the hypodermis (Meeuse et al., 2020).
The genetic details of the H2S-sensing pathway were solidified through studies of rhy-1 in C. elegans. Ma et al., 2012 demonstrate that hif-1 repression via rhy-1 requires the activity of cysl-1, a gene encoding a cysteine synthase-like protein (Ma et al., 2012). They further demonstrate that high H2S promotes a physical interaction between CYSL-1 and EGL-9, resulting in the inactivation of EGL-9 and increased HIF-1 activity. Together, these studies suggest distinct genetic regulators of EGL-9/HIF-1 signaling: rhy-1 and cysl-1 govern the H2S-sensing pathway while vhl-1 mediates the hypoxia-sensing pathway. These pathways are distinct in their requirement for the EGL-9 prolyl hydroxylase domain.
A negative feedback loop senses high cysteine/H2S, promotes CDO-1 activity, and maintains cysteine homeostasis
Why would the RHY-1/CYSL-1/EGL-9/HIF-1 H2S-sensing pathway control the levels and activity of cysteine dioxygenase? We speculate this intersection facilitates a homeostatic pathway allowing C. elegans to sense and respond to cysteine level. We propose that H2S acts as a gaseous signaling molecule to promote cysteine catabolism. H2S activates HIF-1 in the hypodermis by promoting the CYSL-1-mediated inactivation of EGL-9 (Ma et al., 2012). We show that high levels of cysteine similarly induce cdo-1 transcription in the hypodermis. Our genetic data demonstrate that cdo-1 is induced by the same genetic pathway that senses H2S in C. elegans and CDO-1 acts in the hypodermis, the major site of H2S-induced transcription. Furthermore, H2S induces endogenous cdo-1 transcription more than threefold while cdo-1 mRNA levels do not change when C. elegans are exposed to hypoxia (Miller et al., 2011; Shen et al., 2005). Thus, it is likely that H2S promotes cdo-1 transcription through RHY-1, CYSL-1, EGL-9, and HIF-1. H2S is a reasonable small molecule signal to alert cells to high levels of cysteine. Excess cysteine results in the production of H2S mediated by multiple enzymes including cystathionase (CTH), cystathionine β-synthase (CBS), and 3-mercaptopyruvate sulfurtransferase (MST; Singh and Banerjee, 2011; Jurkowska et al., 2014). For example, CTH activity within the carotid body produces H2S that modifies the mammalian response to hypoxia (Peng et al., 2010). We speculate that excess cysteine in C. elegans promotes the enzymatic production of H2S which activates HIF-1 via the RHY-1/CYSL-1/EGL-9 signaling pathway. In our homeostatic model, H2S-activated HIF-1 would then induce cdo-1 transcription, promoting CDO-1 activity and the catabolism of the high-cysteine trigger (Figure 6). Supporting this model, cysl-1(-) and hif-1(-) mutant C. elegans cannot survive in a high cysteine environment, demonstrating their central role in promoting cysteine homeostasis.
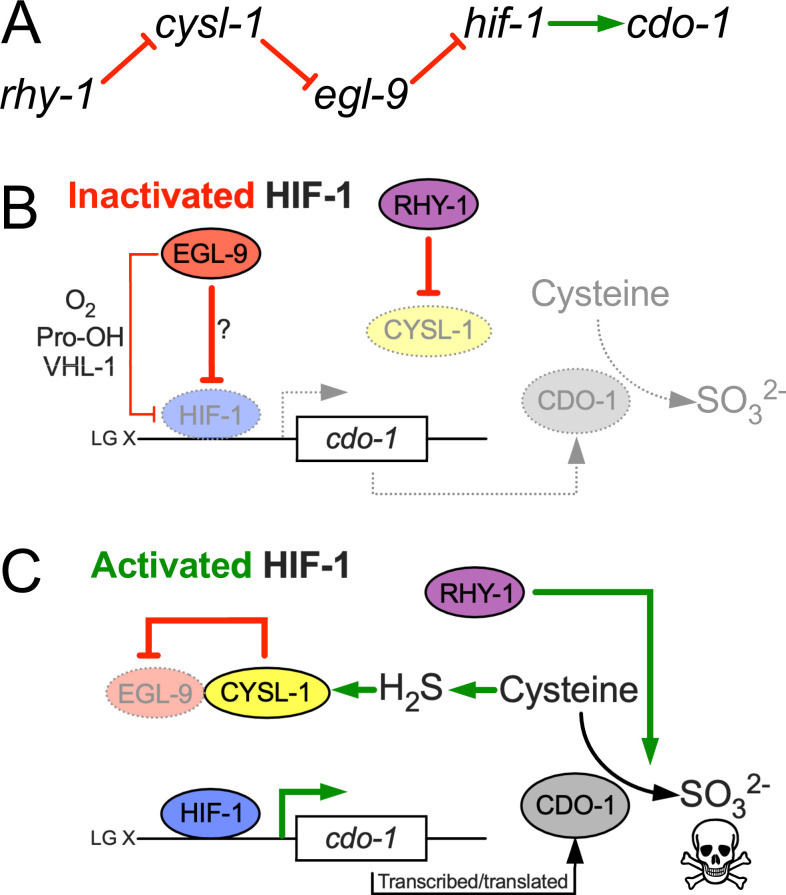
Figure 6. Model for the regulation of cysteine metabolism by HIF-1.
(A) Proposed genetic pathway for the regulation of cdo-1. rhy-1, cysl-1, and egl-9 act in a negative-regulatory cascade to control activity of the HIF-1 transcription factor, which activates transcription of cdo-1. (B) Under basal conditions (inactivated HIF-1), EGL-9 negatively regulates HIF-1 through 2 distinct pathways; one pathway is dependent upon O2, prolyl hydroxylation (Pro-OH), and VHL-1, while the second acts independently of these canonical factors. Under these conditions cdo-1 transcription is kept at basal levels and cysteine catabolism is not induced. (C) During conditions where HIF-1 is activated (high H2S), CYSL-1 directly binds and inhibits EGL-9, preventing HIF-1 inactivation. Active HIF-1 binds the cdo-1 promoter, driving transcription and promoting CDO-1 protein accumulation. High CDO-1 levels promote the catabolism of cysteine leading to production of sulfites (SO32-) that are toxic during Moco or SUOX-1 deficiency. HIF-1-induced cysteine catabolism requires the activity of rhy-1.
Importantly, the human ortholog of EGL-9 (EglN1) has previously been implicated as a cysteine sensor in the context of triple negative breast cancer (TNBC) (Briggs et al., 2016). This study determined that HIF1α accumulates in TNBC cells even during normoxia. Briggs et al., 2016 demonstrate that L-glutamate secretion via TNBC cells suppresses HIF1α prolyl hydroxylation, stabilizing HIF1α. L-glutamate secretion is mediated via the glutamate/cystine antiporter, xCT (Bannai and Kitamura, 1980; Sato et al., 1999). L-glutamate secretion inhibits xCT and a concomitant decrease in intracellular cystine/cysteine was observed. The authors propose that low intracellular cystine/cysteine produces oxidizing conditions that oxidize specific EglN1 cysteine residues, inhibiting the activity of EglN1. Thus, Briggs et al., 2016 propose EglN1 as a cysteine sensor whose activity is promoted by cysteine.
Our work in C. elegans also strongly suggests a role for EGL-9 in sensing and responding to cysteine. We show that high levels of cysteine promote transcription of the HIF-1 target gene cdo-1, and that this induction is not additive with a null mutation in egl-9, suggesting cysteine and egl-9 act in a pathway. Therefore, we propose that in C. elegans high levels of cysteine inhibit the activity of EGL-9, the opposite effect observed in TNBC cells. Furthermore, our genetic studies demonstrate that regulation of cdo-1 transcription occurs largely independent of the EGL-9 prolyl hydroxylase domain and VHL-1, while the mechanism proposed by Briggs et al., 2016 suggests that the stabilization of HIF1α by L-glutamate is correlated with increased prolyl hydroxylation. Despite these differences, it seems likely that the roles for C. elegans EGL-9 and human EglN1 in sensing cysteine are connected. However, additional studies are required to determine if there is an evolutionary relationship between these cysteine-sensing mechanisms.
Members of the H2S-sensing pathway have also been implicated in the C. elegans response to infection (Darby et al., 1999; Burton et al., 2021). Many secreted proteins that mediate intercellular signaling or innate immune responses to pathogens are cysteine-rich and form disulfide bonds in the endoplasmic reticulum before protein secretion (Gibbs et al., 2008; Frand et al., 2000). Levels of free cysteine may fall during the massive inductions of cysteine-rich secreted proteins in development or immune defense, as well as during the synthesis of collagens in the periodic molting cycle (Meeuse et al., 2020; Mizuki and Kasahara, 1992). The oxidation of so many cysteines to disulfides in the endoplasmic reticulum might locally lower O2 levels preventing EGL-9 hydroxylation of HIF-1. Thus, the intersection between HIF-1 and cysteine homeostasis we have uncovered may contribute to a regulatory axis in cell-cell signaling during development and in immune function.
Methods
General methods and strains
C. elegans were cultured using established protocols (Brenner, 1974). Briefly, animals were cultured at 20 °C on nematode growth media (NGM) seeded with wild-type E. coli (OP50). The wild-type strain of C. elegans was Bristol N2. Additional E. coli strains used in this work were BW25113 (Wild type, Moco+) and JW0764-2 (ΔmoaA753::kan, Moco-; Baba et al., 2006).
C. elegans mutant and transgenic strains used in this work are listed here. When previously published, sources of strains are referenced. Unless a reference is provided, all strains were generated in this study.
Non-transgenic C. elegans
ZG31, hif-1(ia4) V (Jiang et al., 2001)
JT307, egl-9(sa307) V (Darby et al., 1999)
GR2254, moc-1(ok366) X (Warnhoff and Ruvkun, 2019)
GR2260, cdo-1(mg622) X (Warnhoff and Ruvkun, 2019)
GR2261, cdo-1(mg622) moc-1(ok366) X (Warnhoff and Ruvkun, 2019)
GR2269, suox-1(gk738847) X (Warnhoff and Ruvkun, 2019)
CB5602, vhl-1(ok161) X (Epstein et al., 2001)
USD410, cysl-1(ok762) X, outcrossed 3 x for this work
USD414, rhy-1(ok1402) II; suox-1(gk738847) X
USD421, egl-9(sa307) V; suox-1(gk738847) X
USD422, vhl-1(ok161) suox-1(gk738847) X
USD430, egl-9(sa307) V; cdo-1(mg622) suox-1(gk738847) X
USD431, hif-1(ia4) egl-9(sa307) V; suox-1(gk738847) X
USD432, rhy-1(ok1402) II; egl-9(sa307) V; suox-1(gk738847) X
USD433, cth-2(mg599) II; egl-9(sa307) V; suox-1(gk738847) X
USD434, egl-9(sa307) X; cysl-1(ok762) suox-1(gk738847) X
USD512, rhy-1(ok1402) II, outcrossed 4 x for this work
USD706, unc-119(ed3) III; cdo-1(mg622) moc-1(ok366) X
USD920, cdo-1(rae273) moc-1(ok366) X
USD921, egl-9(sa307) V; cdo-1(rae273) X
USD922, rhy-1(ok1402) II; cdo-1(rae273) X
USD937, egl-9(rae276) V; suox-1(gk738847) X
MiniMos transgenic lines
USD531, unc-119(ed3) III; raeTi1 [Pcdo-1::CDO-1::GFP unc-119(+)]
USD719, unc-119(ed3) III; cdo-1(mg622) moc-1(ok366); raeTi14 [Pcdo-1::CDO-1(C85Y)::GFP unc-119(+)]
USD720, unc-119(ed3) III; raeTi15 [Pcdo-1::GFP unc-119(+)]
USD730, rhy-1(ok1402) II; unc-119(ed3) III; raeTi15 [Pcdo-1::GFP unc-119(+)]
USD733, unc-119(ed3) III; egl-9(sa307) V; raeTi15 [Pcdo-1::GFP unc-119(+)]
USD739, unc-119(ed3) III; cdo-1(mg622) moc-1(ok366) X; raeTi1 [Pcdo-1::CDO-1::GFP unc-119(+)]
USD766, unc-119(ed3) III; cdo-1(mg622) moc-1(ok366) X; raeTi32 [Pcol-10::CDO-1::GFP unc-119(+)]
USD767, unc-119(ed3) III; cdo-1(mg622) moc-1(ok366) X; raeTi33 [Pcol-10::CDO-1::GFP unc-119(+)]
USD776, rhy-1(ok1402) II; unc-119(ed3) III; hif-1(ia4) V; raeTi15 [Pcdo-1::GFP unc-119(+)]
USD777, unc-119(ed3) III; egl-9(sa307) hif-1(ia4) V; raeTi15 [Pcdo-1::GFP unc-119(+)]
USD780, rhy-1(ok1402) II; unc-119(ed3) III; cysl-1(ok762) X; raeTi15 [Pcdo-1::GFP unc-119(+)]
USD787, unc-119(ed3) III; egl-9(sa307) V; cysl-1(ok762) X; raeTi15 [Pcdo-1::GFP unc-119(+)]
USD808, unc-119(ed3) III; cdo-1(mg622) moc-1(ok366) X; raeTi40 [Pcol-10::CDO-1[C85Y]::GFP unc-119(+)]
USD810, unc-119(ed3) III; cdo-1(mg622) moc-1(ok366) X; raeTi41 [Pcol-10::CDO-1[C85Y]::GFP unc-119(+)]
USD940, unc-119(ed3) III; vhl-1(ok161) X; raeTi15 [Pcdo-1::GFP unc-119(+)]
USD1160, unc-119(ed3) III; cysl-1(ok762) X; raeTi15 [Pcdo-1::GFP unc-119(+)]
USD1161, unc-119(ed3) III; hif-1(ia4) V; raeTi15 [Pcdo-1::GFP unc-119(+)]
EMS-derived strains
USD659, unc-119(ed3) III; egl-9(rae213) V; raeTi1 [Pcdo-1::CDO-1::GFP unc-119(+)]
USD674, unc-119(ed3) III; egl-9(rae227) V; raeTi1 [Pcdo-1::CDO-1::GFP unc-119(+)]
USD655, rhy-1(rae209) II; unc-119(ed3) III; raeTi1 [Pcdo-1::CDO-1::GFP unc-119(+)]
USD656, rhy-1(rae210) II; unc-119(ed3) III; raeTi1 [Pcdo-1::CDO-1::GFP unc-119(+)]
USD657, rhy-1(rae211) II; unc-119(ed3) III; raeTi1 [Pcdo-1::CDO-1::GFP unc-119(+)]
USD658, rhy-1(rae212) II; unc-119(ed3) III; raeTi1 [Pcdo-1::CDO-1::GFP unc-119(+)]
CRISPR/Cas9-derived strains
USD914, cdo-1(rae273) X, CDO-1::GFP
USD926, egl-9(rae276) V, EGL-9[H487A]
USD928, unc-119(ed3) III; egl-9(rae278) V; raeTi15 [Pcdo-1::GFP unc-119(+)]
MiniMos transgenesis
Cloning of original plasmid constructs was performed using isothermal/Gibson assembly (Gibson et al., 2009). All MiniMos constructs were assembled in pNL43, which is derived from pCFJ909, a gift from Erik Jorgensen (Addgene plasmid #44480; Lehrbach and Ruvkun, 2016). Details about plasmid construction are described below. MiniMos transgenic animals were generated using established protocols that rescue the unc-119(ed3) Unc phenotype (Frøkjær-Jensen et al., 2014).
To generate a construct that expressed CDO-1 under the control of its native promoter, we cloned the wild-type cdo-1 genomic locus from 1,335 base pairs upstream of the cdo-1 ATG start codon to (and including) codon 190 encoding the final CDO-1 amino acid prior to the TAA stop codon. This wild-type genomic sequence was fused in frame with a C-terminal GFP and tbb-2 3’UTR (376 bp downstream of the tbb-2 stop codon) in the pNL43 plasmid backbone. This plasmid is called pKW24 (Pcdo-1::CDO-1::GFP).
To generate pKW44, a construct encoding the active site mutant transgene Pcdo-1::CDO-1(C85Y)::GFP, we performed Q5 site-directed mutagenesis on pKW24, following manufacturer’s instructions (New England Biolabs). In pKW44, codon 85 was mutated from TGC (cysteine) to TAC (tyrosine).
To generate pKW45 (Pcdo-1::GFP), the 1,335 base pair cdo-1 promoter was amplified and fused directly to the GFP coding sequence. Both fragments were derived from pKW24, excluding the cdo-1 coding sequence and introns.
pKW49 is a construct driving cdo-1 expression from the hypodermal-specific col-10 promoter (Pcol-10::CDO-1::GFP) (Hong et al., 2000). The col-10 promoter (1,126 base pairs upstream of the col-10 start codon) was amplified and fused upstream of the cdo-1 ATG start codon in pKW24, replacing the native cdo-1 promoter. pKW53 [Pcol-10::CDO-1(C85Y)::GFP] was engineered using the same Q5-site-directed mutagenesis strategy as was described for pKW44. However, this mutagenesis used pKW49 as the template plasmid.
Chemical mutagenesis and whole genome sequencing
To define C. elegans gene activities that were necessary for the control of cdo-1 levels, we carried out a chemical mutagenesis screen for animals that accumulate CDO-1 protein. To visualize CDO-1 levels, we engineered USD531, a transgenic C. elegans strain carrying the raeTi1 [pKW24, Pcdo-1::CDO-1::GFP] transgene. USD531 transgenic C. elegans were mutagenized with ethyl methanesulfonate (EMS) using established protocols (Brenner, 1974). F2 generation animals were manually screened, and mutant isolates were collected that displayed high accumulation of Pcdo-1::CDO-1::GFP. We demanded that mutant strains of interest were viable and fertile.
We followed established protocols to identify EMS-induced mutations in our strains of interest (Lehrbach et al., 2017). Briefly, whole genomic DNA was prepared from C. elegans using the Gentra Puregene Tissue Kit (Qiagen) and genomic DNA libraries were prepared using the NEBNext genomic DNA library construction kit (New England Biolabs). DNA libraries were sequenced on an Illumina Hi-Seq and deep sequencing reads were analyzed using standard methods on Galaxy, a web-based platform for computational analyses ( Galaxy Community, 2022). Briefly, sequencing reads were trimmed and aligned to the WBcel235 C. elegans reference genome (Bolger et al., 2014; Li and Durbin, 2010). Variations from the reference genome and the putative impact of those variations were annotated and extracted for analysis (Wilm et al., 2012; Cingolani et al., 2012a; Cingolani et al., 2012b). Here, we report the analysis of 6 new mutant strains (USD655, USD656, USD657, USD658, USD659, and USD674). Among these mutant strains, we found two unique mutations in egl-9 (USD659 and USD674) and four unique mutations in rhy-1 (USD655, USD656, USD657, USD658). The allele names and molecular identity of these new egl-9 and rhy-1 mutations are specified in Figure 1C. These genes were prioritized based on the isolation of multiple independent alleles and their established functions in a common pathway, the hypoxia and H2S-sensing pathway (Shen et al., 2006; Budde and Roth, 2011; Ma et al., 2012). Whole genome sequencing data for these C. elegans strains have been deposited at the NIH Sequence Read Archive (SRA) under accession PRJNA1063314.
Genome engineering by CRISPR/Cas9
We followed standard protocols to perform CRISPR/Cas9 genome engineering of cdo-1 and egl-9 genomic loci in C. elegans (Ghanta and Mello, 2020; Arribere et al., 2014; Paix et al., 2017; Dokshin et al., 2018). Essential details of the CRISPR/Cas9-generated reagents in this work are described below.
We used homology-directed repair to generate cdo-1(rae273) [CDO-1::GFP]. The guide RNA was 5’-gactacagaggatctaagaa-3’ (crRNA, IDT). The GFP donor double-stranded DNA (dsDNA) was amplified from pCFJ2249 using primers that contained roughly 40 bp of homology to cdo-1 flanking the desired insertion site (Aljohani et al., 2020). The primers used to generate the donor dsDNA were: 5’-gtacggcaagaaagttgactacagaggatctaagaataatagtactagcggtggcagtgg-3’ and 5’-agaatcaacacgttattacattgagggatatgttgtttacttgtagagctcgtccattcc-3’. Glycine 189 of CDO-1 was removed by design to eliminate the PAM site and prevent cleavage of the donor dsDNA.
The egl-9(rae276) and egl-9(rae278) [EGL-9(H487A)] alleles were also generated by homology-directed repair using the same combination of guide RNA and single-stranded oligodeoxynucleotide (ssODN) donor. The guide RNA was 5’-tgtgaagcatgtagataatc-3’ (crRNA, IDT) The ssODN donor was 5’-gcttgccatctatcctggaaatggaactcgttatgtgaaggctgtagacaatccagtaaaagatggaagatgtataaccactatttattactg-3’ (Ultramer, IDT). Successful editing resulted in altering the coding sequence of EGL-9 to encode for an alanine rather than the catalytically essential histidine at position 487. We also used synonymous mutations to introduce an AccI restriction site that is helpful for genotyping the rae276 and rae278 mutant alleles.
C. elegans growth assays
To assay developmental rates, C. elegans were synchronized at the first stage of larval development. To synchronize animals, embryos were harvested from gravid adult animals via treatment with a bleach and sodium hydroxide solution. Embryos were then incubated overnight in M9 solution causing them to hatch and arrest development at the L1 stage (Stiernagle, 2006). Synchronized L1 animals were cultured on NGM seeded with BW25113 (Wild type, Moco+) or JW0764-2 (ΔmoaA753::kan, Moco-) E. coli. Animals were cultured for 48 or 72 hr (specified in the appropriate figure legends), and live animals were imaged as described below. Animal length was measured from tip of head to the end of the tail.
To determine qualitative ‘health’ of various C. elegans strains, we assayed the ability of these strains to consume all E. coli food provided on an NGM petri dish. For this experiment, dietary E. coli was produced via overnight culture in liquid LB in a 37 °C shaking incubator. A total of 200 μl of this E. coli was seeded onto NGM petri dishes and allowed to dry, producing nearly identical lawns and growth environments. Then, 5 L4 animals of a strain of interest were introduced onto these NGM petri dishes seeded with OP50. For each experiment, petri dishes were monitored daily and scored when all E. coli was consumed by the population of animals. This assay is beneficial because it integrates many life-history measures (i.e. developmental rate, brood size, embryonic viability, etc.) into a single simple assay that can be scaled and applied to many C. elegans strains in parallel.
Cysteine exposure
To determine the impact of supplemental cysteine on expression of cdo-1 and animal viability, we exposed various C. elegans strains to 0, 50, 100, 250, 500, or 1000 μM supplemental cysteine. C. elegans strains were synchronously grown on NGM media supplemented with E. coli OP50 at 20 °C until reaching the L4 stage of development. Live L4 animals were then collected from the petri dishes and cultured in liquid M9 media containing 4 X concentrated E. coli OP50 with or without supplemental cysteine. These liquid cultures were gently rocked at 20 °C overnight. Post exposure, GFP imaging was performed as described in the Microscopy section of the materials and methods. Alternatively, animals exposed overnight to 0, 100, or 1000 μM supplemental cysteine were scored for viability after being seeded onto NGM petri dishes. Animals were determined to be alive if they responded to mechanical stimulus.
Microscopy
Low-magnification bright field and fluorescence images (imaging GFP simultaneously in multiple animals) were collected using a Zeiss AxioZoom V16 microscope equipped with a Hamamatsu Orca flash 4.0 digital camera using Zen software (Zeiss). For experiments with supplemental cysteine, low magnification bright field and fluorescence images were collected using a Nikon SMZ25 microscope equipped with a Hamamatsu Orca flash 4.0 digital camera using NIS-Elements software (Nikon). High magnification differential interference contrast (DIC) and GFP fluorescence images (imaging CDO-1::GFP encoded by cdo-1(rae273)) were collected using Zeiss AxioImager Z1 microscope equipped with a Zeiss AxioCam HRc digital camera using Zen software (Zeiss). All images were processed and analyzed using ImageJ software (NIH). All imaging was performed on live animals paralyzed using 20 mM sodium azide. For all fluorescence images shown within the same figure panel, images were collected using the same exposure time and processed identically. To quantify GFP expression, the average pixel intensity was determined within a set transverse section immediately anterior to the developing vulva. Background pixel intensity was determined in a set region of interest distinct from the C. elegans samples and was subtracted from the sample measurements.
Acknowledgements
Some C. elegans strains were provided by the CGC, which is funded by the NIH Office of Research Infrastructure Programs (P40 OD010440). Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under award numbers R01 GM044619 (to GR) and R35 GM146871 (to KW).
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Contributor Information
Kurt Warnhoff, Email: kurt.warnhoff@sanfordhealth.org.
Pankaj Kapahi, Buck Institute for Research on Aging, United States.
Piali Sengupta, Brandeis University, United States.
Funding Information
This paper was supported by the following grants:
National Institute of General Medical Sciences R35 GM146871 to Kurt Warnhoff.
National Institute of General Medical Sciences R01 GM044619 to Gary Ruvkun.
Additional information
Competing interests
No competing interests declared.
Author contributions
Conceptualization, Data curation, Supervision, Funding acquisition, Investigation, Methodology, Writing – original draft, Writing – review and editing.
Investigation, Methodology.
Investigation, Methodology.
Investigation, Methodology.
Conceptualization, Supervision, Funding acquisition, Writing – original draft, Writing – review and editing.
Additional files
Data availability
All C. elegans strains, bacterial strains, and plasmids are described in the Methods section and are available from the corresponding authors with no restrictions. Source data have been deposited on Dryad and can be accessed at https://doi.org/10.5061/dryad.kd51c5bdk. Whole genome sequencing data have been deposited at the NIH BioProject under accession PRJNA1063314.
The following datasets were generated:
Warnhoff K, Bhattacharya S, Snoozy J, Breen P, Ruvkun G. 2024. Hypoxia-inducible factor induces cysteine dioxygenase and promotes cysteine homeostasis in Caenorhabditis elegans. NCBI BioProject. PRJNA1063314
Warnhoff K, Bhattacharya S, Snoozy J, Breen P, Ruvkun G. 2024. Data from: Hypoxia-inducible factor induces cysteine dioxygenase and promotes cysteine homeostasis in Caenorhabditis elegans. Dryad Digital Repository.
References
- Aljohani MD, El Mouridi S, Priyadarshini M, Vargas-Velazquez AM, Frøkjær-Jensen C. Engineering rules that minimize germline silencing of transgenes in simple extrachromosomal arrays in C. elegans. Nature Communications. 2020;11:6300. doi: 10.1038/s41467-020-19898-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arribere JA, Bell RT, Fu BXH, Artiles KL, Hartman PS, Fire AZ. Efficient marker-free recovery of custom genetic modifications with CRISPR/Cas9 in Caenorhabditis elegans. Genetics. 2014;198:837–846. doi: 10.1534/genetics.114.169730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, Datsenko KA, Tomita M, Wanner BL, Mori H. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Molecular Systems Biology. 2006;2:2006.0008. doi: 10.1038/msb4100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannai S, Kitamura E. Transport interaction of L-cystine and L-glutamate in human diploid fibroblasts in culture. The Journal of Biological Chemistry. 1980;255:2372–2376. [PubMed] [Google Scholar]
- Bashan N, Burdett E, Hundal HS, Klip A. Regulation of glucose transport and GLUT1 glucose transporter expression by O2 in muscle cells in culture. The American Journal of Physiology. 1992;262:C682–C690. doi: 10.1152/ajpcell.1992.262.3.C682. [DOI] [PubMed] [Google Scholar]
- Bella DL, Kwon YH, Stipanuk MH. Variations in dietary protein but not in dietary fat plus cellulose or carbohydrate levels affect cysteine metabolism in rat isolated hepatocytes. The Journal of Nutrition. 1996;126:2179–2187. doi: 10.1093/jn/126.9.2179. [DOI] [PubMed] [Google Scholar]
- Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brait M, Ling S, Nagpal JK, Chang X, Park HL, Lee J, Okamura J, Yamashita K, Sidransky D, Kim MS. Cysteine dioxygenase 1 is a tumor suppressor gene silenced by promoter methylation in multiple human cancers. PLOS ONE. 2012;7:e44951. doi: 10.1371/journal.pone.0044951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs KJ, Koivunen P, Cao S, Backus KM, Olenchock BA, Patel H, Zhang Q, Signoretti S, Gerfen GJ, Richardson AL, Witkiewicz AK, Cravatt BF, Clardy J, Kaelin WG. Paracrine induction of HIF by glutamate in breast cancer: eglN1 senses cysteine. Cell. 2016;166:126–139. doi: 10.1016/j.cell.2016.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budde MW, Roth MB. Hydrogen sulfide increases hypoxia-inducible factor-1 activity independently of von hippel-lindau tumor suppressor-1 in C. elegans. Molecular Biology of the Cell. 2010;21:212–217. doi: 10.1091/mbc.e09-03-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budde MW, Roth MB. The response of Caenorhabditis elegans to hydrogen sulfide and hydrogen cyanide. Genetics. 2011;189:521–532. doi: 10.1534/genetics.111.129841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton NO, Willis A, Fisher K, Braukmann F, Price J, Stevens L, Baugh LR, Reinke A, Miska EA. Intergenerational adaptations to stress are evolutionarily conserved, stress-specific, and have deleterious trade-offs. eLife. 2021;10:e73425. doi: 10.7554/eLife.73425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cingolani P, Patel VM, Coon M, Nguyen T, Land SJ, Ruden DM, Lu X. Using Drosophila melanogaster as a model for genotoxic chemical mutational studies with a new program, snpSift. Frontiers in Genetics. 2012a;3:35. doi: 10.3389/fgene.2012.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cingolani P, Platts A, Wang LL, Coon M, Nguyen T, Wang L, Land SJ, Lu X, Ruden DM. A program for annotating and predicting the effects of single nucleotide polymorphisms, snpEff: sNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly. 2012b;6:80–92. doi: 10.4161/fly.19695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dao JH, Kurzeja RJM, Morachis JM, Veith H, Lewis J, Yu V, Tegley CM, Tagari P. Kinetic characterization and identification of a novel inhibitor of hypoxia-inducible factor prolyl hydroxylase 2 using a time-resolved fluorescence resonance energy transfer-based assay technology. Analytical Biochemistry. 2009;384:213–223. doi: 10.1016/j.ab.2008.09.052. [DOI] [PubMed] [Google Scholar]
- Darby C, Cosma CL, Thomas JH, Manoil C. Lethal paralysis of Caenorhabditis elegans by Pseudomonas aeruginosa. PNAS. 1999;96:15202–15207. doi: 10.1073/pnas.96.26.15202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dokshin GA, Ghanta KS, Piscopo KM, Mello CC. Robust genome editing with short single-stranded and long, partially single-stranded DNA donors in Caenorhabditis elegans. Genetics. 2018;210:781–787. doi: 10.1534/genetics.118.301532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominy JE, Hirschberger LL, Coloso RM, Stipanuk MH. In vivo regulation of cysteine dioxygenase via the ubiquitin-26S proteasome system. Advances in Experimental Medicine and Biology. 2006a;583:37–47. doi: 10.1007/978-0-387-33504-9_4. [DOI] [PubMed] [Google Scholar]
- Dominy JE, Hirschberger LL, Coloso RM, Stipanuk MH. Regulation of cysteine dioxygenase degradation is mediated by intracellular cysteine levels and the ubiquitin-26 S proteasome system in the living rat. The Biochemical Journal. 2006b;394:267–273. doi: 10.1042/BJ20051510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duran M, Beemer FA, van de Heiden C, Korteland J, de Bree PK, Brink M, Wadman SK, Lombeck I. Combined deficiency of xanthine oxidase and sulphite oxidase: a defect of molybdenum metabolism or transport? Journal of Inherited Metabolic Disease. 1978;1:175–178. doi: 10.1007/BF01805591. [DOI] [PubMed] [Google Scholar]
- Ehrismann D, Flashman E, Genn DN, Mathioudakis N, Hewitson KS, Ratcliffe PJ, Schofield CJ. Studies on the activity of the hypoxia-inducible-factor hydroxylases using an oxygen consumption assay. The Biochemical Journal. 2007;401:227–234. doi: 10.1042/BJ20061151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O’Rourke J, Mole DR, Mukherji M, Metzen E, Wilson MI, Dhanda A, Tian YM, Masson N, Hamilton DL, Jaakkola P, Barstead R, Hodgkin J, Maxwell PH, Pugh CW, Schofield CJ, Ratcliffe PJ. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001;107:43–54. doi: 10.1016/s0092-8674(01)00507-4. [DOI] [PubMed] [Google Scholar]
- Evans CL. The toxicity of hydrogen sulphide and other sulphides. Quarterly Journal of Experimental Physiology and Cognate Medical Sciences. 1967;52:231–248. doi: 10.1113/expphysiol.1967.sp001909. [DOI] [PubMed] [Google Scholar]
- Firth JD, Ebert BL, Pugh CW, Ratcliffe PJ. Oxygen-regulated control elements in the phosphoglycerate kinase 1 and lactate dehydrogenase a genes: similarities with the erythropoietin 3’ enhancer. PNAS. 1994;91:6496–6500. doi: 10.1073/pnas.91.14.6496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frand AR, Cuozzo JW, Kaiser CA. Pathways for protein disulphide bond formation. Trends in Cell Biology. 2000;10:203–210. doi: 10.1016/s0962-8924(00)01745-1. [DOI] [PubMed] [Google Scholar]
- Frøkjær-Jensen C, Davis MW, Sarov M, Taylor J, Flibotte S, LaBella M, Pozniakovsky A, Moerman DG, Jorgensen EM. Random and targeted transgene insertion in Caenorhabditis elegans using a modified Mos1 transposon. Nature Methods. 2014;11:529–534. doi: 10.1038/nmeth.2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galaxy Community The Galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2022 update. Nucleic Acids Research. 2022;50:8999. doi: 10.1093/nar/gkac610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghanta KS, Mello CC. Melting dsdna donor molecules greatly improves precision genome editing in Caenorhabditis elegans. Genetics. 2020;216:643–650. doi: 10.1534/genetics.120.303564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs GM, Roelants K, O’Bryan MK. The CAP superfamily: cysteine-rich secretory proteins, antigen 5, and pathogenesis-related 1 proteins--roles in reproduction, cancer, and immune defense. Endocrine Reviews. 2008;29:865–897. doi: 10.1210/er.2008-0032. [DOI] [PubMed] [Google Scholar]
- Gibson DG, Young L, Chuang RY, Venter JC, Hutchison CA, Smith HO. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nature Methods. 2009;6:343–345. doi: 10.1038/nmeth.1318. [DOI] [PubMed] [Google Scholar]
- Giles NM, Watts AB, Giles GI, Fry FH, Littlechild JA, Jacob C. Metal and redox modulation of cysteine protein function. Chemistry & Biology. 2003;10:677–693. doi: 10.1016/s1074-5521(03)00174-1. [DOI] [PubMed] [Google Scholar]
- Gray JM, Karow DS, Lu H, Chang AJ, Chang JS, Ellis RE, Marletta MA, Bargmann CI. Oxygen sensation and social feeding mediated by a C. elegans guanylate cyclase homologue. Nature. 2004;430:317–322. doi: 10.1038/nature02714. [DOI] [PubMed] [Google Scholar]
- Hao S, Yu J, He W, Huang Q, Zhao Y, Liang B, Zhang S, Wen Z, Dong S, Rao J, Liao W, Shi M. Cysteine dioxygenase 1 mediates erastin-induced ferroptosis in human gastric cancer cells. Neoplasia. 2017;19:1022–1032. doi: 10.1016/j.neo.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsilä M, Koivunen P, Günzler V, Kivirikko KI, Myllyharju J. Characterization of the human prolyl 4-hydroxylases that modify the hypoxia-inducible factor. The Journal of Biological Chemistry. 2003;278:30772–30780. doi: 10.1074/jbc.M304982200. [DOI] [PubMed] [Google Scholar]
- Hong Y, Lee RC, Ambros V. Structure and function analysis of LIN-14, a temporal regulator of postembryonic developmental events in Caenorhabditis elegans. Molecular and Cellular Biology. 2000;20:2285–2295. doi: 10.1128/MCB.20.6.2285-2295.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsman JW, Heinis FI, Miller DL. A novel mechanism to prevent h(2)s toxicity in Caenorhabditis elegans. Genetics. 2019;213:481–490. doi: 10.1534/genetics.119.302326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang LE, Gu J, Schau M, Bunn HF. Regulation of hypoxia-inducible factor 1alpha is mediated by an O2-dependent degradation domain via the ubiquitin-proteasome pathway. PNAS. 1998;95:7987–7992. doi: 10.1073/pnas.95.14.7987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes CE, Coody TK, Jeong M-Y, Berg JA, Winge DR, Hughes AL. Cysteine toxicity drives age-related mitochondrial decline by altering iron homeostasis. Cell. 2020;180:296–310. doi: 10.1016/j.cell.2019.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kaelin WG. HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292:464–468. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- Jeschke J, O’Hagan HM, Zhang W, Vatapalli R, Calmon MF, Danilova L, Nelkenbrecher C, Van Neste L, Bijsmans ITGW, Van Engeland M, Gabrielson E, Schuebel KE, Winterpacht A, Baylin SB, Herman JG, Ahuja N. Frequent inactivation of cysteine dioxygenase type 1 contributes to survival of breast cancer cells and resistance to anthracyclines. Clinical Cancer Research. 2013;19:3201–3211. doi: 10.1158/1078-0432.CCR-12-3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Guo R, Powell-Coffman JA. The Caenorhabditis elegans hif-1 gene encodes a bhlh-pas protein that is required for adaptation to hypoxia. PNAS. 2001;98:7916–7921. doi: 10.1073/pnas.141234698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurkowska H, Roman HB, Hirschberger LL, Sasakura K, Nagano T, Hanaoka K, Krijt J, Stipanuk MH. Primary hepatocytes from mice lacking cysteine dioxygenase show increased cysteine concentrations and higher rates of metabolism of cysteine to hydrogen sulfide and thiosulfate. Amino Acids. 2014;46:1353–1365. doi: 10.1007/s00726-014-1700-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaelin WG, Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Molecular Cell. 2008;30:393–402. doi: 10.1016/j.molcel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Kang YP, Torrente L, Falzone A, Elkins CM, Liu M, Asara JM, Dibble CC, DeNicola GM. Cysteine dioxygenase 1 is a metabolic liability for non-small cell lung cancer. eLife. 2019;8:e45572. doi: 10.7554/eLife.45572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima K, Nakamura T, Ohbu M, Katoh H, Ooizumi Y, Igarashi K, Ishii S, Tanaka T, Yokoi K, Nishizawa N, Yokota K, Kosaka Y, Sato T, Watanabe M, Yamashita K. Cysteine dioxygenase type 1 (CDO1) gene promoter methylation during the adenoma-carcinoma sequence in colorectal cancer. PLOS ONE. 2018;13:e0194785. doi: 10.1371/journal.pone.0194785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudron MM, Victorsen A, Gevirtzman L, Hillier LW, Fisher WW, Vafeados D, Kirkey M, Hammonds AS, Gersch J, Ammouri H, Wall ML, Moran J, Steffen D, Szynkarek M, Seabrook-Sturgis S, Jameel N, Kadaba M, Patton J, Terrell R, Corson M, Durham TJ, Park S, Samanta S, Han M, Xu J, Yan KK, Celniker SE, White KP, Ma L, Gerstein M, Reinke V, Waterston RH. The modern resource: genome-wide binding profiles for hundreds of Drosophila and Caenorhabditis elegans transcription factors. Genetics. 2018;208:937–949. doi: 10.1534/genetics.117.300657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon YH, Stipanuk MH. Cysteine regulates expression of cysteine dioxygenase and gamma-glutamylcysteine synthetase in cultured rat hepatocytes. American Journal of Physiology. Endocrinology and Metabolism. 2001;280:E804–E815. doi: 10.1152/ajpendo.2001.280.5.E804. [DOI] [PubMed] [Google Scholar]
- Lee JI, Londono M, Hirschberger LL, Stipanuk MH. Regulation of cysteine dioxygenase and gamma-glutamylcysteine synthetase is associated with hepatic cysteine level. The Journal of Nutritional Biochemistry. 2004;15:112–122. doi: 10.1016/j.jnutbio.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Lehrbach NJ, Ruvkun G. Proteasome dysfunction triggers activation of SKN-1A/Nrf1 by the aspartic protease DDI-1. eLife. 2016;5:e17721. doi: 10.7554/eLife.17721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrbach NJ, Ji F, Sadreyev R. Next-generation sequencing for identification of EMS-induced mutations in Caenorhabditis elegans. Current Protocols in Molecular Biology. 2017;117:7. doi: 10.1002/cpmb.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Durbin R. Fast and accurate long-read alignment with burrows-wheeler transform. Bioinformatics. 2010;26:589–595. doi: 10.1093/bioinformatics/btp698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Shen S, Bickler P, Jacobson MP, Wu LF, Altschuler SJ. Searching for molecular hypoxia sensors among oxygen-dependent enzymes. eLife. 2023;12:e87705. doi: 10.7554/eLife.87705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loike JD, Cao L, Brett J, Ogawa S, Silverstein SC, Stern D. Hypoxia induces glucose transporter expression in endothelial cells. The American Journal of Physiology. 1992;263:C326–C333. doi: 10.1152/ajpcell.1992.263.2.C326. [DOI] [PubMed] [Google Scholar]
- Losman JA, Koivunen P, Kaelin WG. 2-Oxoglutarate-dependent dioxygenases in cancer. Nature Reviews. Cancer. 2020;20:710–726. doi: 10.1038/s41568-020-00303-3. [DOI] [PubMed] [Google Scholar]
- Ma DK, Vozdek R, Bhatla N, Horvitz HR. CYSL-1 interacts with the O2-sensing hydroxylase EGL-9 to promote H2S-modulated hypoxia-induced behavioral plasticity in C. elegans. Neuron. 2012;73:925–940. doi: 10.1016/j.neuron.2011.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma G, Zhao Z, Qu Y, Cai F, Liu S, Liang H, Zhang R, Deng J. Cysteine dioxygenase 1 attenuates the proliferation via inducing oxidative stress and integrated stress response in gastric cancer cells. Cell Death Discovery. 2022;8:493. doi: 10.1038/s41420-022-01277-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy JG, Bailey LJ, Bitto E, Bingman CA, Aceti DJ, Fox BG, Phillips GN. Structure and mechanism of mouse cysteine dioxygenase. PNAS. 2006;103:3084–3089. doi: 10.1073/pnas.0509262103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeuse MW, Hauser YP, Morales Moya LJ, Hendriks GJ, Eglinger J, Bogaarts G, Tsiairis C, Großhans H. Developmental function and state transitions of a gene expression oscillator in Caenorhabditis elegans. Molecular Systems Biology. 2020;16:e9498. doi: 10.15252/msb.20209498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J, McLachlan AD, Klug A. Repetitive zinc-binding domains in the protein transcription factor IIIA from Xenopus oocytes. The EMBO Journal. 1985;4:1609–1614. doi: 10.1002/j.1460-2075.1985.tb03825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DL, Budde MW, Roth MB. HIF-1 and SKN-1 coordinate the transcriptional response to hydrogen sulfide in Caenorhabditis elegans. PLOS ONE. 2011;6:e25476. doi: 10.1371/journal.pone.0025476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuki N, Kasahara M. Mouse submandibular glands express an androgen-regulated transcript encoding an acidic epididymal glycoprotein-like molecule. Molecular and Cellular Endocrinology. 1992;89:25–32. doi: 10.1016/0303-7207(92)90207-m. [DOI] [PubMed] [Google Scholar]
- Mudd SH, Irreverre F, Laster L. Sulfite oxidase deficiency in man: demonstration of the enzymatic defect. Science. 1967;156:1599–1602. doi: 10.1126/science.156.3782.1599. [DOI] [PubMed] [Google Scholar]
- Noiva R. Enzymatic catalysis of disulfide formation. Protein Expression and Purification. 1994;5:1–13. doi: 10.1006/prep.1994.1001. [DOI] [PubMed] [Google Scholar]
- Oliphant KD, Fettig RR, Snoozy J, Mendel RR, Warnhoff K. Obtaining the necessary molybdenum cofactor for sulfite oxidase activity in the nematode Caenorhabditis elegans surprisingly involves a dietary source. The Journal of Biological Chemistry. 2023;299:102736. doi: 10.1016/j.jbc.2022.102736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olney JW, Zorumski C, Price MT, Labruyere J. L-cysteine, a bicarbonate-sensitive endogenous excitotoxin. Science. 1990;248:596–599. doi: 10.1126/science.2185543. [DOI] [PubMed] [Google Scholar]
- Paix A, Folkmann A, Seydoux G. Precision genome editing using CRISPR-Cas9 and linear repair templates in C. elegans. Methods. 2017;121–122:86–93. doi: 10.1016/j.ymeth.2017.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y, Mansfield KD, Bertozzi CC, Rudenko V, Chan DA, Giaccia AJ, Simon MC. Multiple factors affecting cellular redox status and energy metabolism modulate hypoxia-inducible factor prolyl hydroxylase activity in vivo and in vitro. Molecular and Cellular Biology. 2007;27:912–925. doi: 10.1128/MCB.01223-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pender CL, Horvitz HR. Hypoxia-inducible factor cell non-autonomously regulates C. elegans stress responses and behavior via a nuclear receptor. eLife. 2018;7:e36828. doi: 10.7554/eLife.36828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y-J, Nanduri J, Raghuraman G, Souvannakitti D, Gadalla MM, Kumar GK, Snyder SH, Prabhakar NR. H2S mediates O2 sensing in the carotid body. PNAS. 2010;107:10719–10724. doi: 10.1073/pnas.1005866107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raina S, Missiakas D. Making and breaking disulfide bonds. Annual Review of Microbiology. 1997;51:179–202. doi: 10.1146/annurev.micro.51.1.179. [DOI] [PubMed] [Google Scholar]
- Rietsch A, Beckwith J. The genetics of disulfide bond metabolism. Annual Review of Genetics. 1998;32:163–184. doi: 10.1146/annurev.genet.32.1.163. [DOI] [PubMed] [Google Scholar]
- Salceda S, Caro J. Hypoxia-inducible factor 1alpha (HIF-1alpha) protein is rapidly degraded by the ubiquitin-proteasome system under normoxic conditions: its stabilization by hypoxia depends on redox-induced changes. The Journal of Biological Chemistry. 1997;272:22642–22647. doi: 10.1074/jbc.272.36.22642. [DOI] [PubMed] [Google Scholar]
- Sato H, Tamba M, Ishii T, Bannai S. Cloning and expression of a plasma membrane cystine/glutamate exchange transporter composed of two distinct proteins. The Journal of Biological Chemistry. 1999;274:11455–11458. doi: 10.1074/jbc.274.17.11455. [DOI] [PubMed] [Google Scholar]
- Schwarz G, Mendel RR, Ribbe MW. Molybdenum cofactors, enzymes and pathways. Nature. 2009;460:839–847. doi: 10.1038/nature08302. [DOI] [PubMed] [Google Scholar]
- Semenza GL, Wang GL. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Molecular and Cellular Biology. 1992;12:5447–5454. doi: 10.1128/mcb.12.12.5447-5454.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Z, Zhang Y, Powell-Coffman JA. Two distinct roles for EGL-9 in the regulation of HIF-1-mediated gene expression in Caenorhabditis elegans. Genetics. 2009;183:821–829. doi: 10.1534/genetics.109.107284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen C, Nettleton D, Jiang M, Kim SK, Powell-Coffman JA. Roles of the HIF-1 hypoxia-inducible factor during hypoxia response in Caenorhabditis elegans. The Journal of Biological Chemistry. 2005;280:20580–20588. doi: 10.1074/jbc.M501894200. [DOI] [PubMed] [Google Scholar]
- Shen C, Shao Z, Powell-Coffman JA. The Caenorhabditis elegans rhy-1 gene inhibits HIF-1 hypoxia-inducible factor activity in a negative feedback loop that does not include vhl-1. Genetics. 2006;174:1205–1214. doi: 10.1534/genetics.106.063594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S, Banerjee R. PLP-dependent H(2)S biogenesis. Biochimica et Biophysica Acta. 2011;1814:1518–1527. doi: 10.1016/j.bbapap.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snoozy J, Breen PC, Ruvkun G, Warnhoff K. Warnhoff, moc-6/MOCS2A is necessary for molybdenum cofactor synthesis in C. elegans. MicroPubl Biol. 2022;1:531. doi: 10.17912/micropub.biology.000531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiernagle T. Maintenance of C. elegans. WormBook. 2006;10:1–11. doi: 10.1895/wormbook.1.101.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stipanuk MH. Sulfur amino acid metabolism: pathways for production and removal of homocysteine and cysteine. Annual Review of Nutrition. 2004;24:539–577. doi: 10.1146/annurev.nutr.24.012003.132418. [DOI] [PubMed] [Google Scholar]
- Stipanuk MH, Hirschberger LL, Londono MP, Cresenzi CL, Yu AF. The ubiquitin-proteasome system is responsible for cysteine-responsive regulation of cysteine dioxygenase concentration in liver. American Journal of Physiology. Endocrinology and Metabolism. 2004;286:E439–E448. doi: 10.1152/ajpendo.00336.2003. [DOI] [PubMed] [Google Scholar]
- Stipanuk MH, Ueki I. Dealing with methionine/homocysteine sulfur: cysteine metabolism to taurine and inorganic sulfur. Journal of Inherited Metabolic Disease. 2011;34:17–32. doi: 10.1007/s10545-009-9006-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter CH, Laughner E, Semenza GL. Hypoxia-inducible factor 1alpha protein expression is controlled by oxygen-regulated ubiquitination that is disrupted by deletions and missense mutations. PNAS. 2000;97:4748–4753. doi: 10.1073/pnas.080072497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tainer JA, Roberts VA, Getzoff ED. Metal-binding sites in proteins. Current Opinion in Biotechnology. 1991;2:582–591. doi: 10.1016/0958-1669(91)90084-i. [DOI] [PubMed] [Google Scholar]
- Truong DH, Eghbal MA, Hindmarsh W, Roth SH, O’Brien PJ. Molecular mechanisms of hydrogen sulfide toxicity. Drug Metabolism Reviews. 2006;38:733–744. doi: 10.1080/03602530600959607. [DOI] [PubMed] [Google Scholar]
- Vora M, Pyonteck SM, Popovitchenko T, Matlack TL, Prashar A, Kane NS, Favate J, Shah P, Rongo C. The hypoxia response pathway promotes PEP carboxykinase and gluconeogenesis in C. elegans. Nature Communications. 2022;13:6168. doi: 10.1038/s41467-022-33849-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Pandey T, Long Y, Delgado-Rodriguez SE, Daugherty MD, Ma DK. Co-opted genes of algal origin protect C. elegans against cyanogenic toxins. Current Biology. 2022;32:4941–4948. doi: 10.1016/j.cub.2022.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnhoff K, Ruvkun G. Molybdenum cofactor transfer from bacteria to nematode mediates sulfite detoxification. Nature Chemical Biology. 2019;15:480–488. doi: 10.1038/s41589-019-0249-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnhoff K, Hercher TW, Mendel RR, Ruvkun G. Protein-bound molybdenum cofactor is bioavailable and rescues molybdenum cofactor-deficient C. elegans. Genes & Development. 2021;35:212–217. doi: 10.1101/gad.345579.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilm A, Aw PPK, Bertrand D, Yeo GHT, Ong SH, Wong CH, Khor CC, Petric R, Hibberd ML, Nagarajan N. LoFreq: a sequence-quality aware, ultra-sensitive variant caller for uncovering cell-population heterogeneity from high-throughput sequencing datasets. Nucleic Acids Research. 2012;40:11189–11201. doi: 10.1093/nar/gks918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Fang Y-Z, Yang S, Lupton JR, Turner ND. Glutathione metabolism and its implications for health. The Journal of Nutrition. 2004;134:489–492. doi: 10.1093/jn/134.3.489. [DOI] [PubMed] [Google Scholar]
- Yamashita K, Hosoda K, Nishizawa N, Katoh H, Watanabe M. Epigenetic biomarkers of promoter DNA methylation in the new era of cancer treatment. Cancer Science. 2018;109:3695–3706. doi: 10.1111/cas.13812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Gladyshev VN. Molybdoproteomes and evolution of molybdenum utilization. Journal of Molecular Biology. 2008;379:881–899. doi: 10.1016/j.jmb.2008.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L, White RH, Cash VL, Jack RF, Dean DR. Cysteine desulfurase activity indicates a role for NIFS in metallocluster biosynthesis. PNAS. 1993;90:2754–2758. doi: 10.1073/pnas.90.7.2754. [DOI] [PMC free article] [PubMed] [Google Scholar]